N-Doped Carbon Fibers Derived from Porous Wood Fibers Encapsulated in a Zeolitic Imidazolate Framework as an Electrode Material for Supercapacitors
Abstract
1. Introduction
2. Results and Discussion
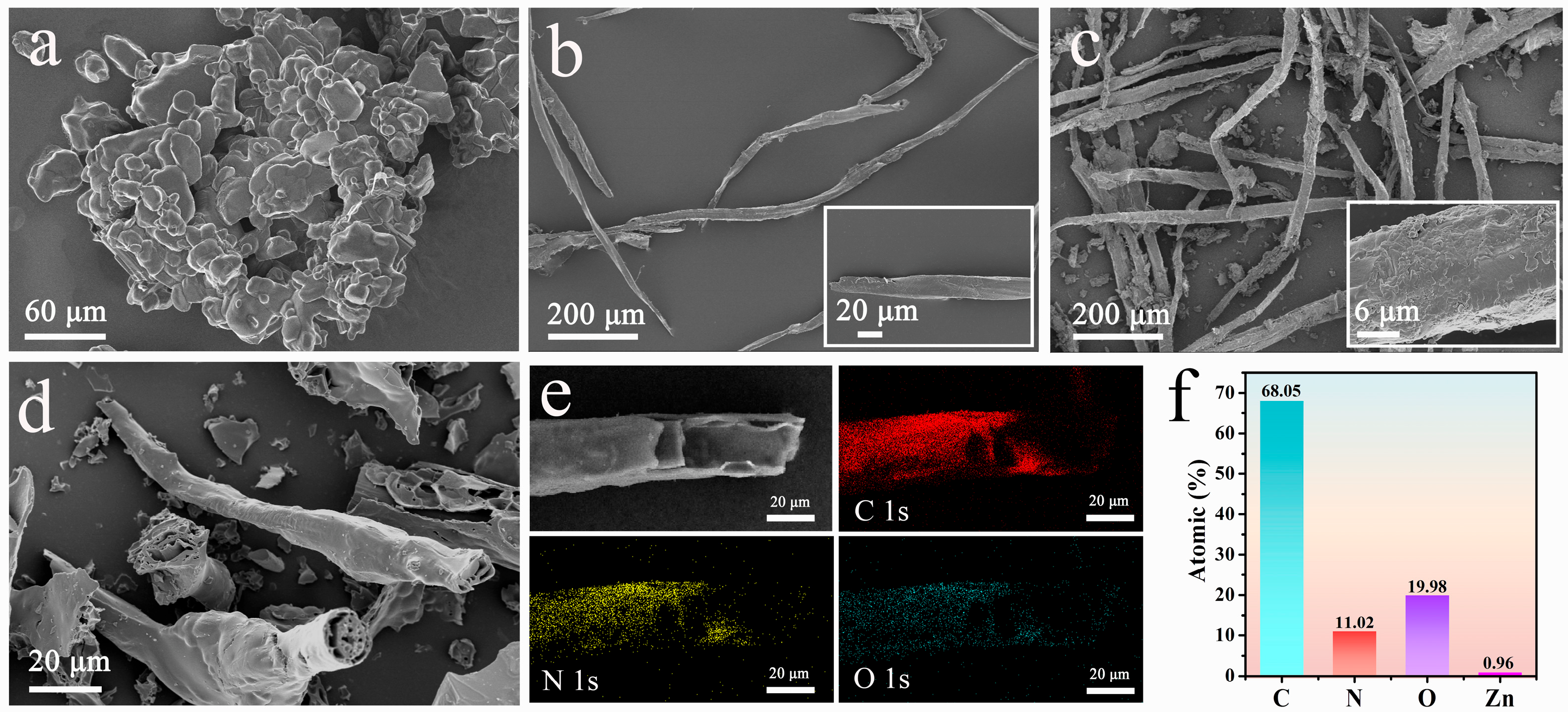
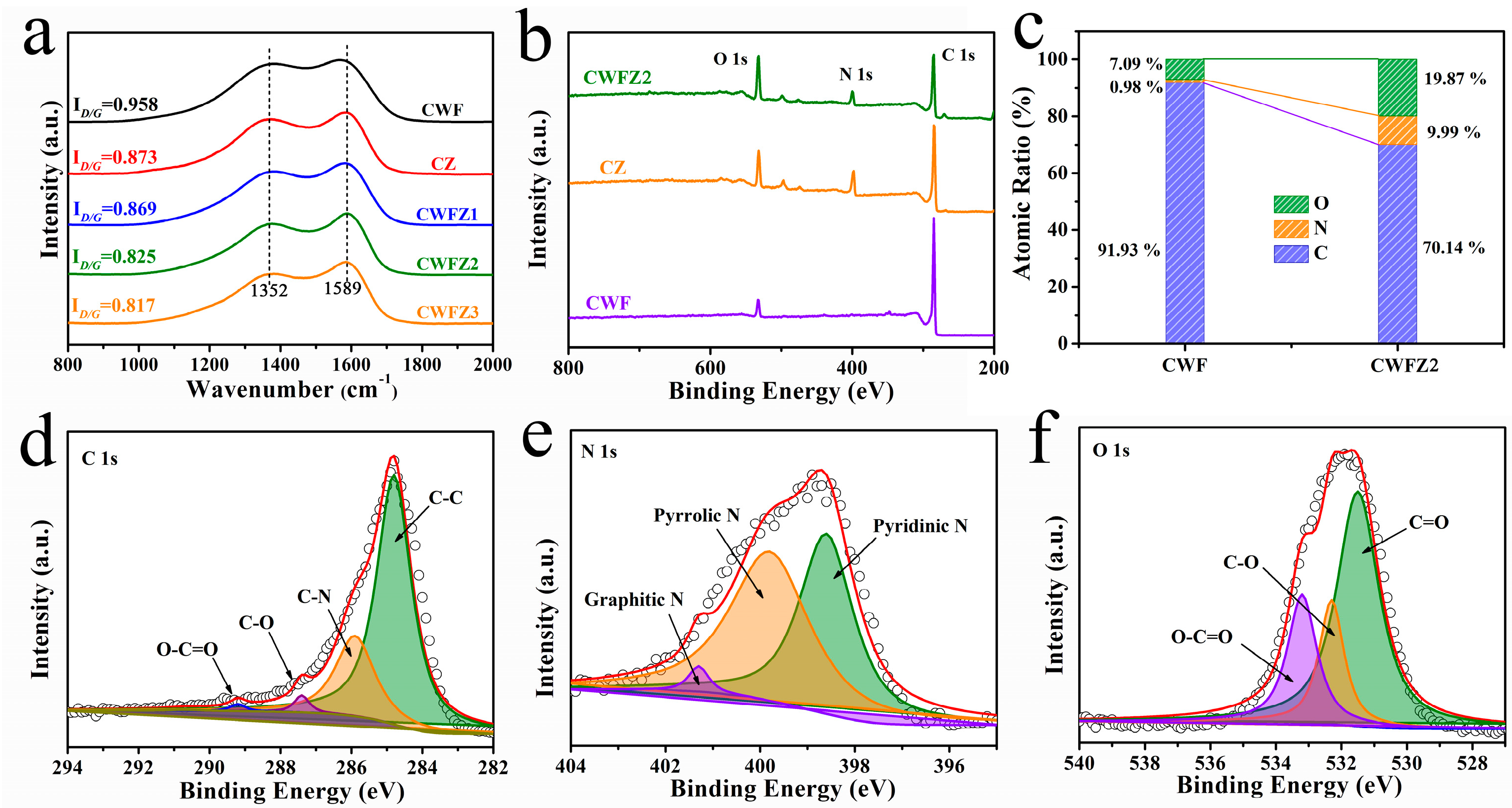
2.1. The Morphology and Structure of Different Samples
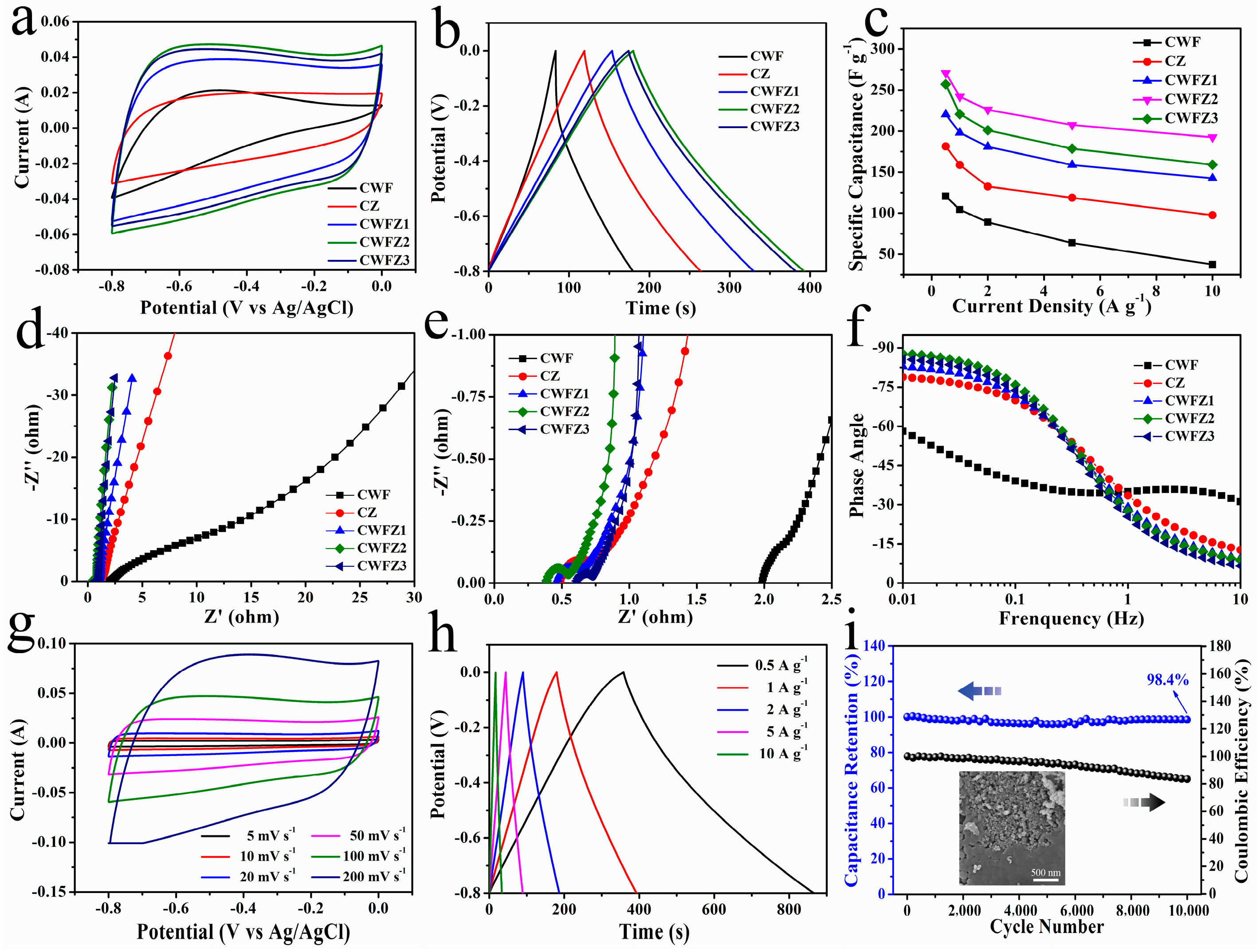
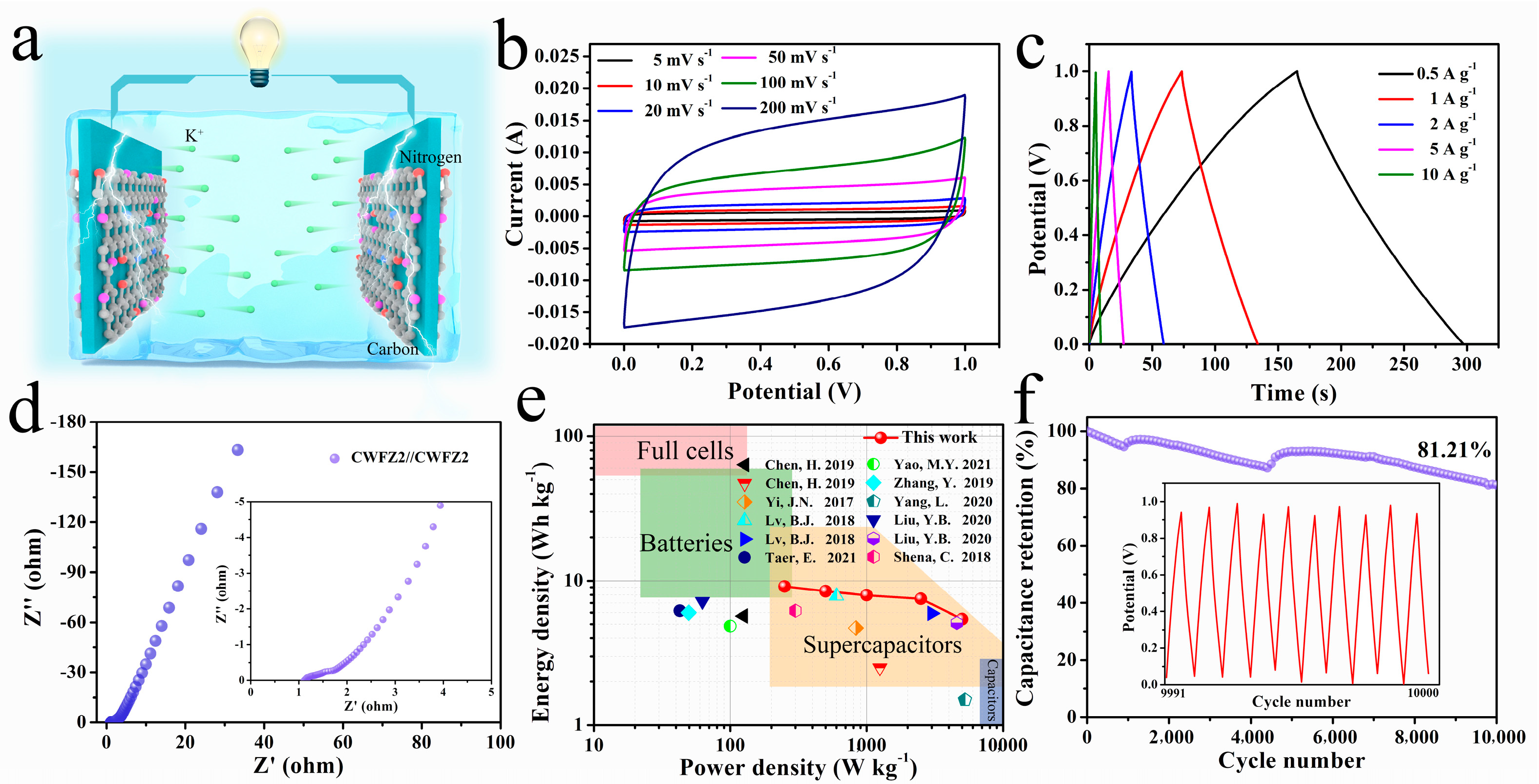
2.2. The Electrochemical Performances of Different Samples
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of WF@ZIF-8 Composite
3.2.2. Preparation of WF@ZIF-8 Carbon Fibers
3.2.3. Characterizations
3.2.4. Electrochemical Performances
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ager, J.W.; Lapkin, A.A. Chemical storage of renewable energy. Science 2018, 360, 707–708. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.Q.; Yao, W.J.; He, X.L.; Song, T.Y.; Tang, Y.B. Mixed polyanionic compounds as positive electrodes for low-cost electrochemical energy storage. Angew. Chem. Int. Edit. 2020, 59, 9255–9262. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Zhou, Y.L.; Yan, W.; Luo, L.C.; Deng, J.P.; Du, G.B.; Fan., M.Z.; Zhao, W.G. Design and construction of hierarchical sea urchin-like NiCo-LDH@ACF composites for high-performance supercapacitors. Ind. Crops Prod. 2021, 171, 113900. [Google Scholar] [CrossRef]
- Heo, Y.J.; Lee, J.W.; Son, Y.R.; Lee, J.H.; Yeo, C.S.; Lam, T.D.; Park, S.Y.; Park, S.J.; Sinh, L.H.; Shin, M.K. Large-scale conductive yarns based on twistable korean traditional paper for supercapacitor applications: Toward high-performance paper supercapacitors. Adv. Energy Mater. 2018, 8, 1801854. [Google Scholar] [CrossRef]
- Xu, M.M.; Wang, A.Q.; Xiang, Y.; Ejaz, A.; Niu, J.F. Self-template bagasse-based porous carbons for high performance supercapacitors. Ind. Crops Prod. 2022, 171, 114291. [Google Scholar] [CrossRef]
- Chen, W.M.; Li, Z.; Jiang, F.; Luo, M.; Yang, K.; Zhang, D.T.; Xu, W.W.; Liu, C.Z.; Zhou, X.Y. Water evaporation triggered self-assembly of MXene on non-carbonized wood with well-aligned channels as size-customizable free-standing electrode for supercapacitors. Energy Environ. Mater. 2022, in press. [Google Scholar] [CrossRef]
- Heuser, S.; Yang, N.J.; Hof, F.; Schulte, A.; Schönherr, H.; Jiang, X. 3D 3C-SiC/graphene hybrid nanolaminate films for high-performance supercapacitors. Small 2018, 14, 1801857. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Irfan, O.M.; Moustafa, H.M. H3PO4/KOH activation agent for high performance rice husk activated carbon electrode in acidic media supercapacitors. Molecules 2023, 28, 296. [Google Scholar] [CrossRef]
- Samal, R.; Bhat, M.; Kapse, S.; Thapa, R.; Late, D.J.; Routa, C.S. Enhanced energy storage performance and theoretical studies of 3D cuboidal manganese diselenides embedded multiwalled carbon nanotubes composite. J. Colloid Interf. Sci. 2021, 598, 500–510. [Google Scholar] [CrossRef]
- Saini, S.; Chand, P.; Joshi, A. Biomass derived carbon for supercapacitor applications: Review. J. Energy Storage 2021, 39, 102646. [Google Scholar] [CrossRef]
- Xiong, W.; Ouyang, J.; Wang, X.; Hua, Z.; Zhao, L.; Li, M.; Lu, Y.; Yin, W.; Liu, G.; Zhou, C.; et al. Semi-embedding Zn-Co3O4 derived from hybrid ZIFs into wood-derived carbon for high-performance supercapacitors. Mater. Molecules 2022, 27, 8572. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.W.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-based supercapacitors produced by activation of graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Ehrnst, Y.; Ahmed, H.; Komljenovic, R.; Massahud, E.; Shepelin, N.A.; Sherrell, P.C.; Ellis, A.V.; Rezk, A.R.; Yeo, L.Y. Acoustotemplating: Rapid synthesis of freestanding quasi-2D MOF/graphene oxide heterostructures for supercapacitor applications. J. Mater. Chem. A 2022, 10, 7058–7072. [Google Scholar] [CrossRef]
- Gayathri, S.; Arunkumar, P.; Han, J.H. Scanty graphene-driven phase control and heteroatom functionalization of ZIF-67-derived CoP-draped N-doped carbon/graphene as a hybrid electrode for high-performance asymmetric supercapacitor. J. Colloid Interf. Sci. 2021, 582, 1136–1148. [Google Scholar] [CrossRef]
- Chaikittisilp, W.; Hu, M.; Wang, H.J.; Huang, H.S.; Fujita, T.; Wu, K.C.W.; Chen, L.C.; Yamauchi, Y.; Ariga, K. Nanoporous carbons through direct carbonization of a zeolitic imidazolate framework for supercapacitor electrodes. Chem. Commun. 2012, 48, 7259–7261. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.L.; Wu, J.X.; Zhang, W.; Tan, Y.Y.; Gao, J.; Zhao, J.C.; Tang, B. The calcined zeolitic imidazolate framework-8 (ZIF-8) under different conditions as electrode for supercapacitor applications. J. Solid State Electr. 2014, 18, 3203–3207. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, X.P.; Wei, F.X.; Sui, Y.W.; Qi, J.Q. Controllable synthesis of ZIF-derived nano-hexahedron porous carbon for supercapacitor electrodes. Mater. Lett. 2020, 258, 126761. [Google Scholar] [CrossRef]
- Ata, M.S.; Poon, R.; Syed, A.M.; Milne, J.; Zhitomirsky, I. New developments in non-covalent surface modification, dispersion and electrophoretic deposition of carbon nanotubes. Carbon 2018, 130, 584–598. [Google Scholar] [CrossRef]
- Wang, Y.F.; Chen, B.W.; Zhang, Y.; Fu, L.J.; Zhu, Y.S.; Zhang, L.X.; Wu, Y.P. ZIF-8@MWCNT-derived carbon composite as electrode of high performance for supercapacitor. Electrochim. Acta 2016, 213, 252–259. [Google Scholar] [CrossRef]
- Wang, M.; Yang, J.; Jia, K.L.; Liu, S.Y.; Hu, C.; Qiu, J.S. Boosting supercapacitor performance of graphene by coupling with nitrogen-doped hollow carbon frameworks. Chem. Eur. J. 2020, 26, 2897–2903. [Google Scholar] [CrossRef]
- Wang, D.H.; Chen, Y.; Wang, H.Q.; Zhao, P.H.; Liu, W.; Wang, Y.Z.; Yang, J.L. N-doped porous carbon anchoring on carbon nanotubes derived from ZIF-8/polypyrrole nanotubes for superior supercapacitor electrodes. Appl. Surf. Sci. 2018, 457, 1018–1024. [Google Scholar] [CrossRef]
- Zhang, D.L.; Zhang, J.H.; Pan, M.D.; Wang, Y.; Sun, T. Necklace-like C-ZIF-8@MWCNTs fabricated by electrochemical deposition towards enhanced supercapacitor. J. Alloys Compd. 2021, 853, 157368. [Google Scholar] [CrossRef]
- Tu, K.K.; Puertolas, B.; Vidal, M.A.; Wang, Y.R.; Sun, J.G.; Traber, J.; Burgert, I.; Pérez-Ramírez, J.; Keplinger, T. Green synthesis of hierarchical metal-organic framework/wood functional composites with superior mechanical properties. Adv. Sci. 2020, 7, 1902897. [Google Scholar] [CrossRef]
- Xia, Q.Q.; Chen, C.J.; Li, T.; He, S.M.; Gao, J.L.; Wang, X.Z.; Hu, L.B. Solar-assisted fabrication of large-scale, patternable transparent wood. Sci. Adv. 2021, 7, eabd7342. [Google Scholar] [CrossRef]
- Cheng, H.Y.; Meng, J.K.; Wu, G.; Chen, S. Hierarchical micro-mesoporous carbon-frameworks-based hybrid nanofibers for high-density capacitive energy storage. Angew. Chem. Int. Edit. 2019, 131, 17626–17634. [Google Scholar] [CrossRef]
- Li, Z.W.; An, Y.F.; Dong, S.Y.; Chen, C.J.; Wu, L.Y.; Sun, Y.; Gang, X.G. Progress on zinc ion hybrid supercapacitors: Insights and challenges. Energy Storage Mater. 2020, 31, 252–266. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.X.; Wang, H.F.; Jiao, X.Y.; Ouyang, Y.; Xia, X.F.; Lei, W.; Hao, Q.L. ZIF-8 nanocrystals derived N-doped carbon decorated graphene sheets for symmetric supercapacitors. Electrochim. Acta 2018, 289, 494–502. [Google Scholar] [CrossRef]
- Wang, H.W.; Zhang, Y.; Ang, H.X.; Zhang, Y.Q.; Tan, H.T.; Zhang, Y.F.; Guo, Y.Y.; Franklin, J.B.; Wu, X.L.; Srinivasan, M.; et al. A high-energy lithium-ion capacitor by integration of a 3D interconnected titanium carbide nanoparticle chain anode with a pyridine-derived porous nitrogen-doped carbon cathode. Adv. Funct. Mater. 2016, 26, 3082–3093. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, Y.; Zhou, M.; Tan, S.J.; Peymanfar, R.; Aslibeiki, B.; Ji, G.B. Ultrabroad microwave absorption ability and infrared stealth property of nano-micro CuS@rGO lightweight aerogels. Nano-Micro Lett. 2022, 14, 53. [Google Scholar] [CrossRef]
- Sha, J.; Li, Y.; Salvatierra, R.V.; Wang, T.; Dong, P.; Ji, Y.; Lee, S.; Zhang, C.; Smith, R.H.; Ajayan, P.M.; et al. Three-dimensional printed grapheme foams. ACS Nano 2017, 11, 6860–6867. [Google Scholar] [CrossRef]
- Sun, Y.; Xue, J.J.; Li, Z.W.; Ding, B.; An, Y.F.; Zang, S.; Dou, H.; Jiang, J.M.; Zhang, X.G. Rational design of ZIF-8 assimilated hierarchical porous carbon nanofibers as binder-free electrodes for supercapacitors. J. Electroanal. Chem. 2021, 895, 115471. [Google Scholar] [CrossRef]
- Liu, M.Y.; Zhu, F.; Cao, W.S.; Song, W.H.; Liu, J.X.; Feng, X.C.; Li, Z.; Cao, Y.Z.; Wang, P.F.; Niu, J. Multifunctional sulfate-assistant synthesis of seaweed-like N,S-doped carbons as highperformance anodes for K-ion capacitors. J. Mater. Chem. A 2022, 10, 9612–9620. [Google Scholar] [CrossRef]
- Zhu, F.; Cao, W.S.; Song, W.H.; Peng, J.Y.; Yang, N.; Niu, J.; Wang, F. Biomass-derived carbon prepared through a quadruple-functional-salt approach for application in K-ion capacitors. Chem. Eng. J. 2022, 449, 137561. [Google Scholar] [CrossRef]
- Wang, M.X.; Zhang, J.; Yi, X.B.; Zhao, X.F.; Liu, B.X.; Liu, X.C. Nitrogen-doped hierarchical porous carbon derived from ZIF-8 supported on carbon aerogels with advanced performance for supercapacitor. Appl. Surf. Sci. 2020, 507, 145166. [Google Scholar] [CrossRef]
- Song, Z.R.; Zhang, G.Y.; Deng, X.L.; Tian, Y.; Xiao, X.H.; Deng, W.T.; Hou, H.S.; Zou, G.Q.; Ji, X.B. Strongly coupled interfacial engineering inspired by robotic arms enable high-performance sodium-ion capacitors. Adv. Funct. Mater. 2022, 32, 2205453. [Google Scholar] [CrossRef]
- Etogo, C.A.; Huang, H.W.; Hong, H.; Liu, G.X.; Zhang, L. Metal-organic-frameworksengaged formation of Co0.85Se@C nanoboxes embedded in carbon nanofibers film for enhanced potassium-ion storage. Energy Storage Mater. 2020, 24, 167–176. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, C.; Ji, G.; Hu, R.; Zheng, J. An all-in-one supercapacitor with high stretchability via a facial strategy. J. Mater. Chem. A 2020, 8, 8255–8261. [Google Scholar] [CrossRef]
- Niu, J.; Shao, R.; Liu, M.Y.; Liang, J.J.; Zhang, Z.P.; Dou, M.L.; Huang, Y.Q.; Wang, F. Porous carbon electrodes with battery-capacitive storage features for high performance Li-ion capacitors. Energy Storage Mater. 2018, 12, 145–152. [Google Scholar] [CrossRef]
- Zhao, Y.H.; He, X.Y.; Chen, R.R.; Liu, Q.; Liu, J.Y.; Yu, J.; Li, J.Q.; Zhang, H.S.; Dong, H.X.; Zhang, M.L.; et al. A flexible all-solid-state asymmetric supercapacitors based on hierarchical carbon cloth@CoMoO4@NiCo layered double hydroxide core-shell heterostructures. Chem. Eng. J. 2018, 352, 29–38. [Google Scholar] [CrossRef]
- Zhou, Q.H.; Chang, J.; Jiang, Y.T.; Wei, T.; Sheng, L.Z.; Fan, Z.J. Fast charge rate supercapacitors based on nitrogen-doped aligned carbon nanosheet networks. Electrochim. Acta 2017, 251, 91–98. [Google Scholar] [CrossRef]
- Li, Y.Y.; Chen, Z.M.; Zhang, J.N.; Xu, Q. Dual tuning of 1 D heteroatoms doped porous carbon nanoarchitectures for supercapacitors: The role of balanced P/N doping and core@shell nano-networks. RSC Adv. 2016, 6, 9180–9185. [Google Scholar] [CrossRef]
- Cai, T.L.; Kuang, L.W.; Wang, C.; Jin, C.D.; Wang, Z.; Sun, Q.F. Cellulose as an adhesive for the synthesis of carbon aerogel with a 3D hierarchical network structure for capacitive energy storage. ChemElectroChem 2019, 6, 2586–2594. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, M.K.; Cai, Y.Y.; Zhao, G.Z.; Xie, Y.; Zhang, L.; Zhu, G.; Pan, L.K. Scalable synthesis of strutted nitrogen doped hierarchical porous carbon nanosheets for supercapacitors with both high gravimetric and volumetric performances. Carbon 2021, 179, 458–468. [Google Scholar] [CrossRef]
- Fan, P.D.; Ren, J.; Pang, K.L.; Cheng, Y.; Wu, X.; Zhang, Z.G.; Ren, J.K.; Huang, W.; Song, R. Cellulose solvent assisted, one-step pyrolysis to fabricate heteroatoms-doped porous carbons for electrode materials of supercapacitors. ACS Sustain. Chem. Eng. 2018, 6, 7715–7724. [Google Scholar] [CrossRef]
- Shang, Z.; An, X.Y.; Liu, L.Q.; Yang, J.; Zhang, W.; Dai, H.Q.; Cao, H.B.; Xu, Q.L.; Liu, H.B.; Ni, Y.H. Chitin nanofibers as versatile bio-templates of zeolitic imidazolate frameworks for N-doped hierarchically porous carbon electrodes for supercapacitor. Carbohyd. Polym. 2021, 251, 117107. [Google Scholar] [CrossRef]
- Lee, B.M.; Jeong, C.U.; Hong, S.K.; Yun, J.M.; Choi, J.H. Eco-friendly fabrication of porous carbon monoliths from water-soluble carboxymethyl cellulose for supercapacitor applications. J. Ind. Eng. Chem. 2020, 82, 367–373. [Google Scholar] [CrossRef]
- Zhu, W.W.; Wang, H.; Zhao, R.; Yang, M.Y.; Liu, Y.; Yan, D.M. In situ fabrication of nitrogen doped porous carbon nanorods derived from metalorganic frameworks and its application as supercapacitor electrodes. J. Solid State Chem. 2019, 277, 100–106. [Google Scholar] [CrossRef]
- Jiang, M.; Cao, X.P.; Zhu, D.D.; Duan, Y.X.; Zhang, J.M. Hierarchically porous N-doped carbon derived from ZIF-8 nanocomposites for electrochemical applications. Electrochim. Acta 2016, 196, 699–707. [Google Scholar] [CrossRef]
- Wang, X.W.; Sun, G.Z.; Routh, P.; Kim, D.H.; Huang, W.; Chen, P. Heteroatom-doped grapheme materials: Syntheses, properties and applications. Chem. Soc. Rev. 2014, 43, 7067–7098. [Google Scholar] [CrossRef]
- Eftekhari, A. The mechanism of ultrafast supercapacitors. J. Mater. Chem. A 2018, 6, 2866–2876. [Google Scholar] [CrossRef]
- Chen, H.; Liu, T.; Mou, J.R.; Zhang, W.J.; Jiang, Z.J.; Liu, J.; Huang, J.L.; Liu, M.L. Free-standing N-self-doped carbon nanofiber aerogels for high-performance all-solidstate supercapacitors. Nano Energy 2019, 63, 103836. [Google Scholar] [CrossRef]
- Yi, J.N.; Qing, Y.; Wu, C.T.; Zeng, Y.X.; Wu, Y.Q.; Lu, X.H.; Tong, Y.X. Lignocellulose-derived porous phosphorus-doped carbon as advanced electrode for supercapacitors. J. Power Sources 2017, 351, 130–137. [Google Scholar] [CrossRef]
- Lv, B.J.; Li, P.P.; Liu, Y.; Lin, S.S.; Gao, B.F.; Lin, B.Z. Nitrogen and phosphorus co-doped carbon hollow spheres derived from polypyrrole for high-performance supercapacitor electrodes. Appl. Surf. Sci. 2018, 435, 169–175. [Google Scholar] [CrossRef]
- Taer, E.; Yusra, D.A.; Amri, A.; Taslim, R.; Putri, A. The synthesis of activated carbon made from banana stem fibers as the supercapacitor electrodes. Mater. Today Proc. 2021, 44, 3346–3349. [Google Scholar] [CrossRef]
- Yao, M.Y.; Zhao, X.; Zhang, Q.H.; Zhang, Y.F.; Wang, Y. Polyaniline nanowires aligned on MOFs-derived nanoporous carbon as high-performance electrodes for supercapacitor. Electrochim. Acta 2021, 390, 138804. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Zhang, P.X.; Wang, J.; Xu, M.; Deng, Q.; Zeng, Z.L.; Deng, S.G. Ultra-high surface area and nitrogen-rich porous carbons prepared by a low temperature activation method with superior gas selective adsorption and outstanding supercapacitance performance. Chem. Eng. J. 2019, 355, 309–319. [Google Scholar] [CrossRef]
- Yang, L.; Wu, D.L.; Wang, T.; Jia, D.Z. B/N Co-doped carbon nanosheets derived from the self-assembly of hitosan-amino acids gels for greatly improved supercapacitor performances. ACS Appl. Mater. Interfaces 2020, 12, 18692–18704. [Google Scholar] [CrossRef]
- Liu, Y.B.; Huang, G.X.; Li, Y.Y.; Yao, Y.H.; Zhang, F.M.; Xing, B.L.; Zhang, C.X. N-O-S Co-doped hierarchical porous carbons derived from calcium lignosulfonate for high-performance supercapacitors. Energ. Fuel. 2020, 34, 3909–3922. [Google Scholar] [CrossRef]
- Shena, C.; Lia, R.Z.; Yan, L.J.; Shi, Y.X.; Guo, H.T.; Zhang, J.H.; Lin, Y.; Zhang, Z.K.; Gong, Y.Y.; Niu, L.Y. Rational design of activated carbon nitride materials for symmetric supercapacitor applications. Appl. Surf. Sci. 2018, 455, 841–848. [Google Scholar] [CrossRef]


| Sample | Wood Fibers | Zn(NO3)2·6H2O | 2-MeIm |
|---|---|---|---|
| WFZ1 | 0.5 g | 0.5 g | 0.5 g |
| WFZ2 | 0.5 g | 0.5 g | 1 g |
| WFZ3 | 1 g | 0.5 g | 1 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Qing, Y.; Wang, D.; Li, L.; Wu, Y. N-Doped Carbon Fibers Derived from Porous Wood Fibers Encapsulated in a Zeolitic Imidazolate Framework as an Electrode Material for Supercapacitors. Molecules 2023, 28, 3081. https://doi.org/10.3390/molecules28073081
Zhang Z, Qing Y, Wang D, Li L, Wu Y. N-Doped Carbon Fibers Derived from Porous Wood Fibers Encapsulated in a Zeolitic Imidazolate Framework as an Electrode Material for Supercapacitors. Molecules. 2023; 28(7):3081. https://doi.org/10.3390/molecules28073081
Chicago/Turabian StyleZhang, Zhen, Yan Qing, Delong Wang, Lei Li, and Yiqiang Wu. 2023. "N-Doped Carbon Fibers Derived from Porous Wood Fibers Encapsulated in a Zeolitic Imidazolate Framework as an Electrode Material for Supercapacitors" Molecules 28, no. 7: 3081. https://doi.org/10.3390/molecules28073081
APA StyleZhang, Z., Qing, Y., Wang, D., Li, L., & Wu, Y. (2023). N-Doped Carbon Fibers Derived from Porous Wood Fibers Encapsulated in a Zeolitic Imidazolate Framework as an Electrode Material for Supercapacitors. Molecules, 28(7), 3081. https://doi.org/10.3390/molecules28073081





