Chondroitin Sulfate from Oreochromis niloticus Waste Reduces Leukocyte Influx in an Acute Peritonitis Model
Abstract
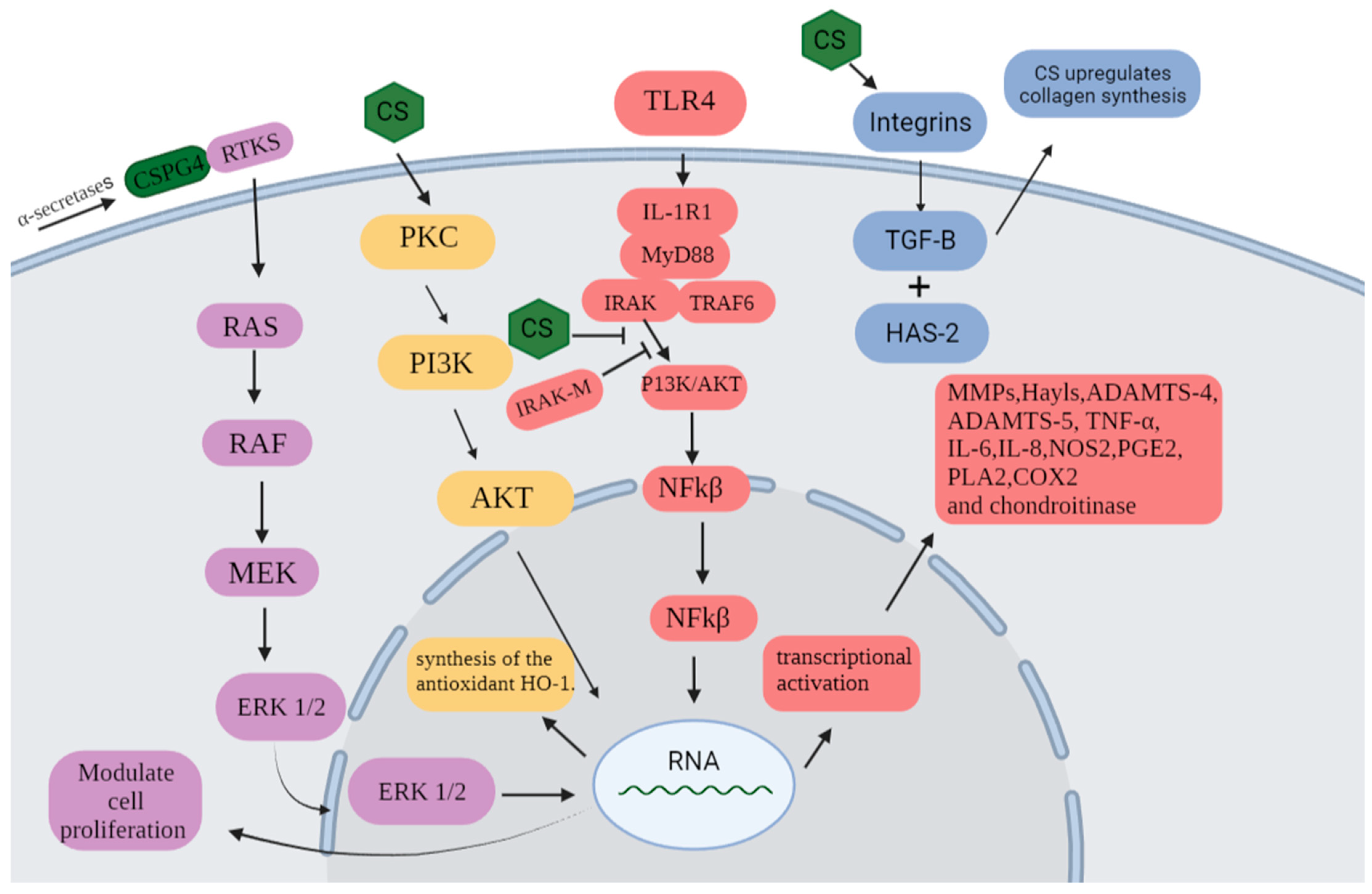
1. Introduction
2. Results and Discussion
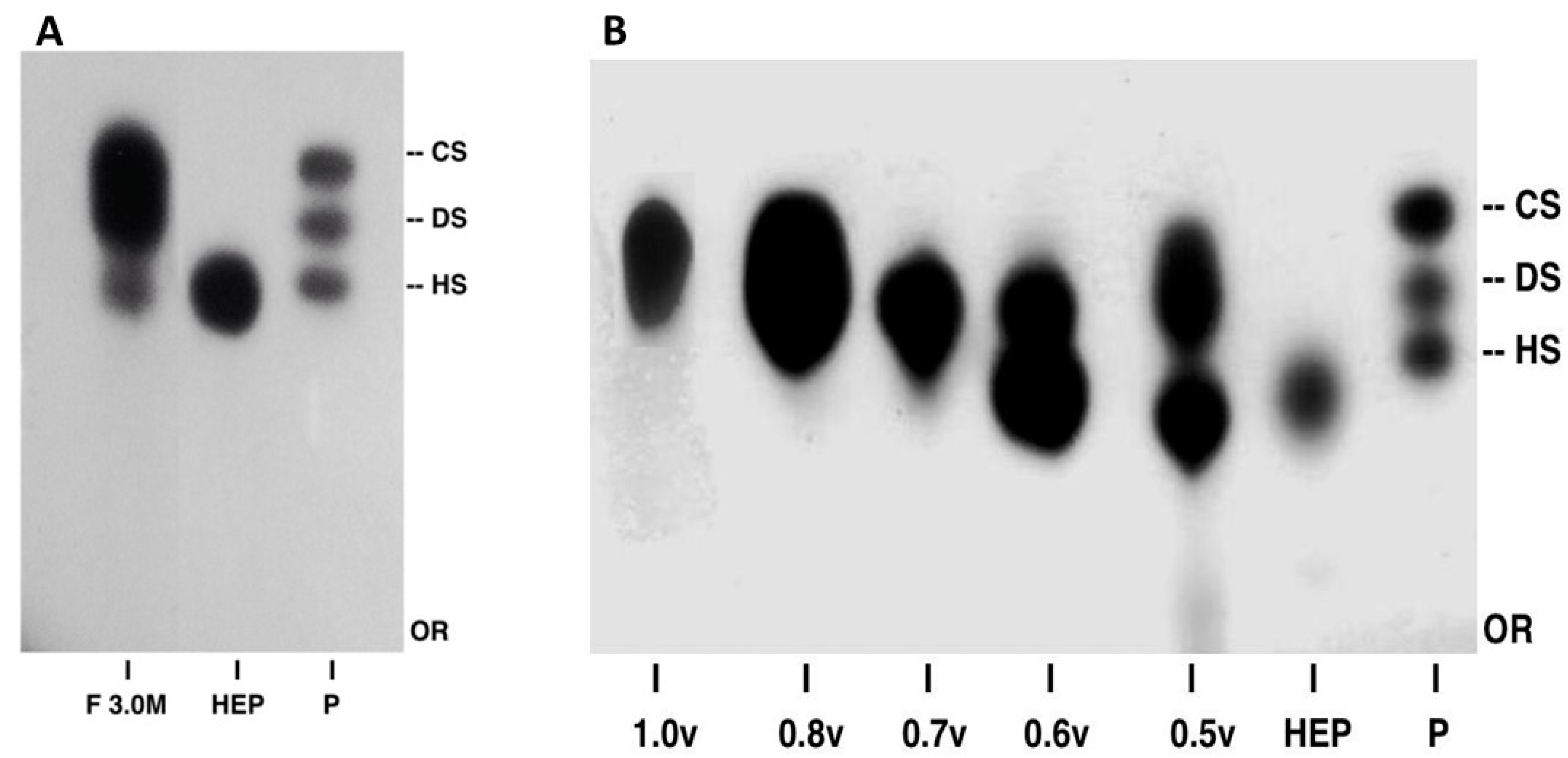
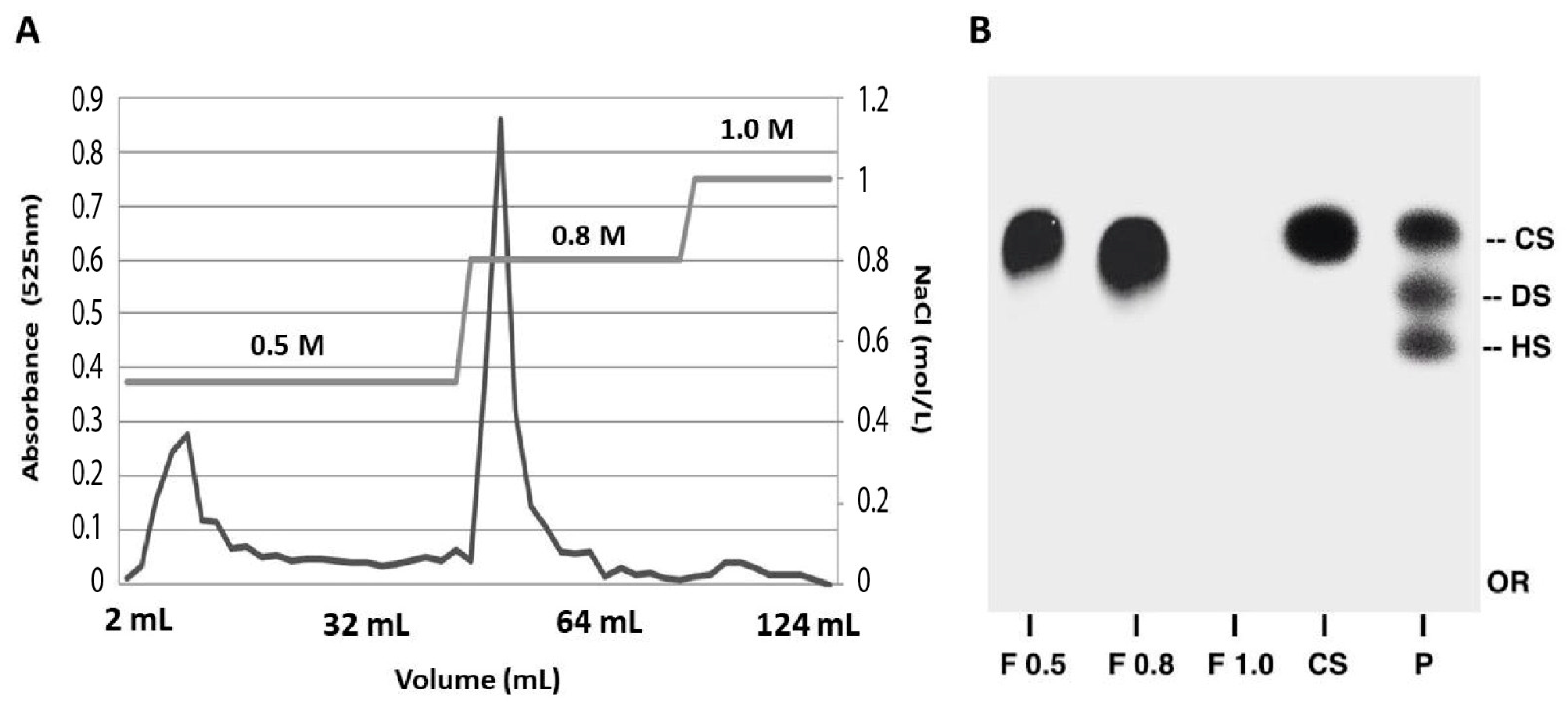
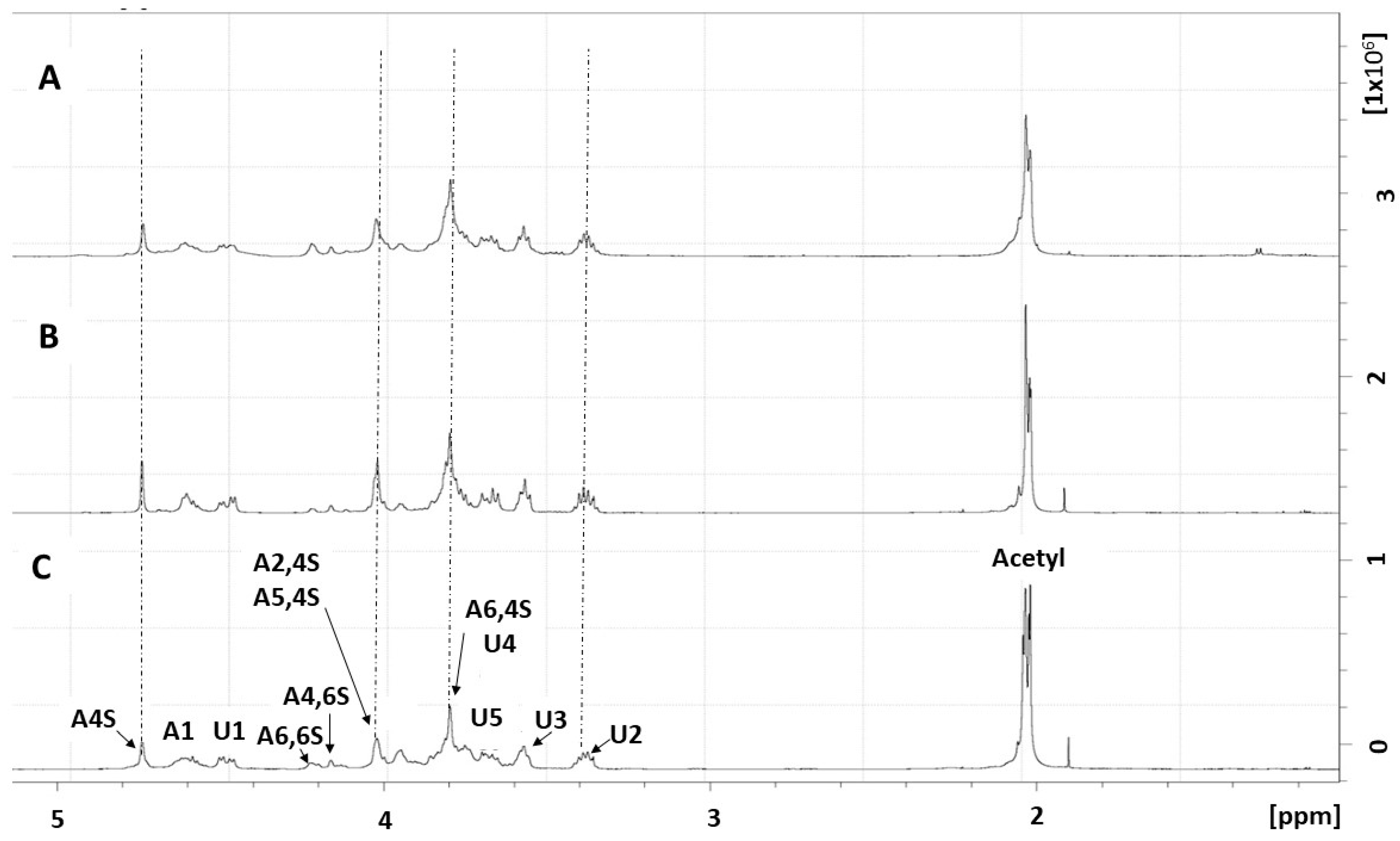
2.1. Purification and Structural Characterization of Chondroitin from O. nilotic
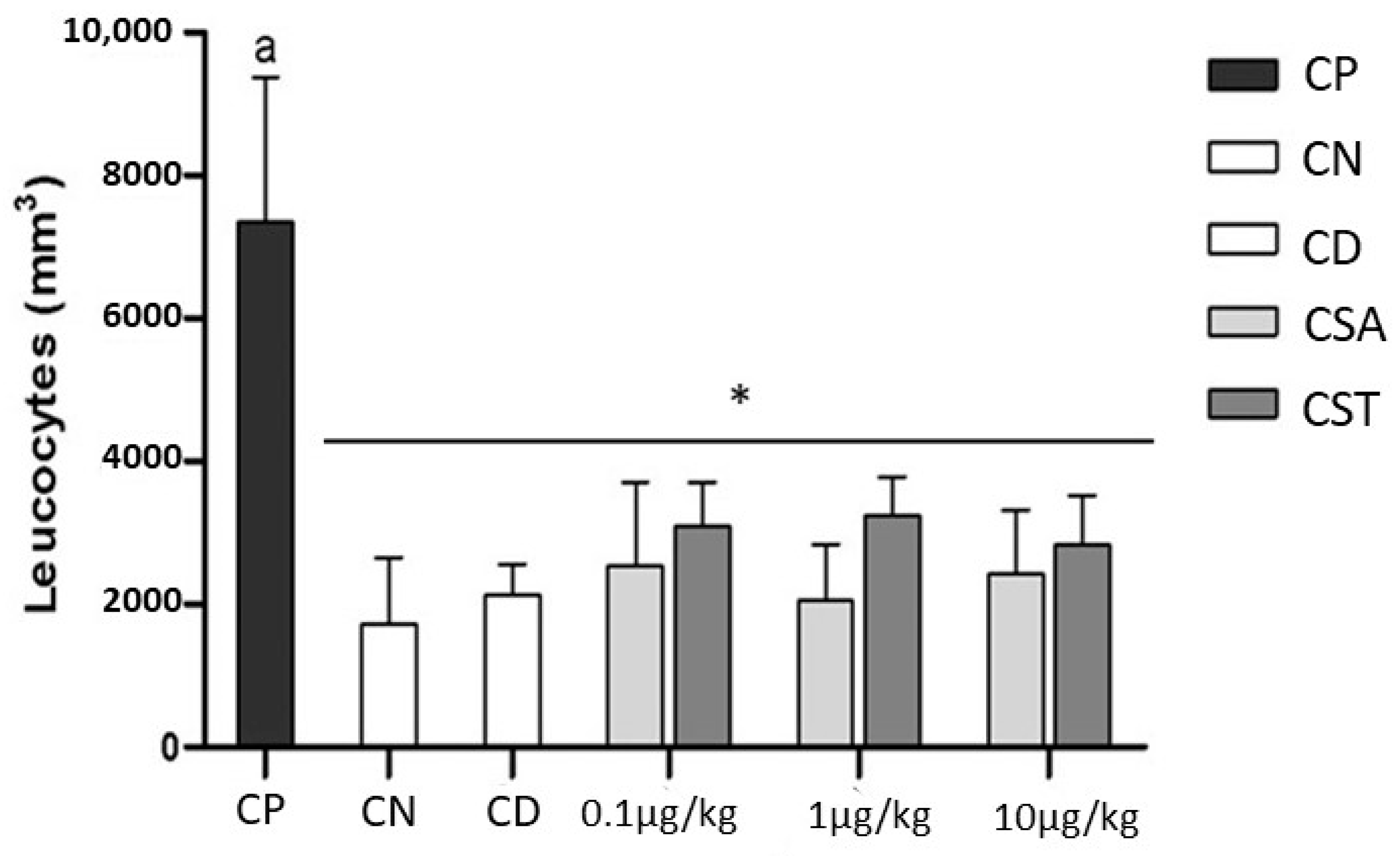
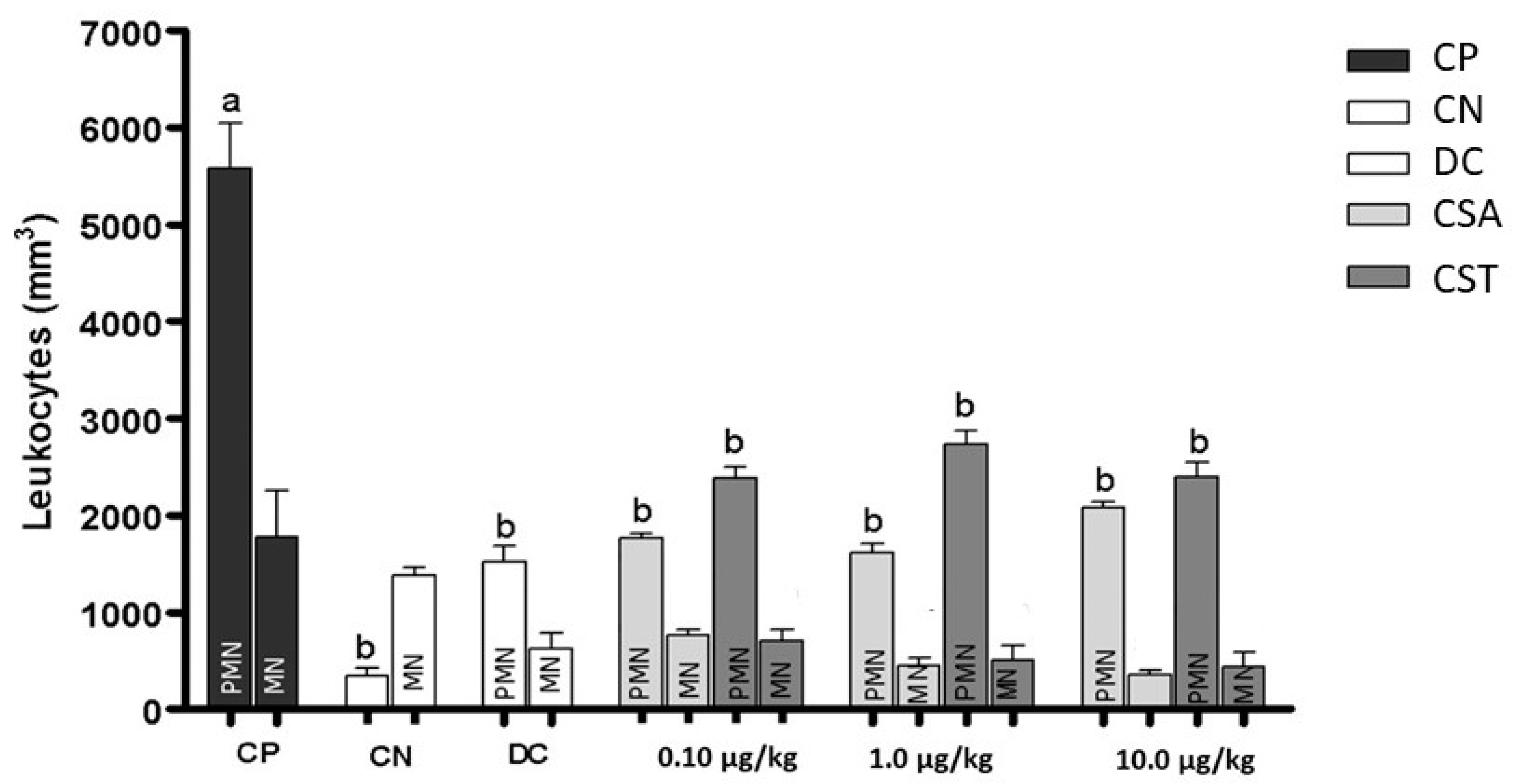
2.2. Effect of Chondroitin from O. nilotic in Leukocyte Influx in Acute Peritonitis Models
3. Materials and Methods
3.1. Samples/Materials
3.2. Animals
3.3. Extraction and Purification of Chondroitin Sulfate
3.4. Agarose Gel Electrophoresis
3.5. NMR Spectroscopy
3.6. Effect of Chondroitin Sulfate from Tilapia on Inflammation
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- FAO. The State of World Fisheries and Aquaculture 2022; FAO: Rome, Italy, 2022; ISBN 978-92-5-136364-5. [Google Scholar]
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M. A 20-Year Retrospective Review of Global Aquaculture. Nature 2021, 591, 551–563. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020; FAO: Rome, Italy, 2020; ISBN 978-92-5-132692-3. [Google Scholar]
- Gallo, N.; Natali, M.L.; Quarta, A.; Gaballo, A.; Terzi, A.; Sibillano, T.; Giannini, C.; De Benedetto, G.E.; Lunetti, P.; Capobianco, L.; et al. Aquaponics-Derived Tilapia Skin Collagen for Biomaterials Development. Polymers 2022, 14, 1865. [Google Scholar] [CrossRef]
- Abdallah, M.M.; Fernández, N.; Matias, A.A.; do Rosário Bronze, M. Hyaluronic Acid and Chondroitin Sulfate from Marine and Terrestrial Sources: Extraction and Purification Methods. Carbohydr. Polym. 2020, 243, 116441. [Google Scholar] [CrossRef]
- Laurienzo, P. Marine Polysaccharides in Pharmaceutical Applications: An Overview. Mar. Drugs 2010, 8, 2435–2465. [Google Scholar] [CrossRef]
- Mohamed, S.; Coombe, D. Heparin Mimetics: Their Therapeutic Potential. Pharmaceuticals 2017, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, L.H.C.; Vasconcelos, B.M.F.; Silva, M.B.; Souza-Junior, A.A.; Chavante, S.F.; Andrade, G.P.V. Chondroitin Sulfate from Fish Waste Exhibits Strong Intracellular Antioxidant Potential. Braz. J. Med. Biol. Res. 2021, 54, e10730. [Google Scholar] [CrossRef]
- Francos-Quijorna, I.; Sánchez-Petidier, M.; Burnside, E.R.; Badea, S.R.; Torres-Espin, A.; Marshall, L.; de Winter, F.; Verhaagen, J.; Moreno-Manzano, V.; Bradbury, E.J. Chondroitin Sulfate Proteoglycans Prevent Immune Cell Phenotypic Conversion and Inflammation Resolution via TLR4 in Rodent Models of Spinal Cord Injury. Nat. Commun. 2022, 13, 2933. [Google Scholar] [CrossRef]
- Bishnoi, M.; Jain, A.; Hurkat, P.; Jain, S.K. Chondroitin Sulphate: A Focus on Osteoarthritis. Glycoconj. J. 2016, 33, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Habuchi, O. Functions of Chondroitin/Dermatan Sulfate Containing GalNAc4,6-Disulfate. Glycobiology 2022, 32, 664–678. [Google Scholar] [CrossRef]
- Bai, Z.; Qu, Y.; Shi, L.; Li, X.; Yang, Z.; Ji, M.; Hou, P. Identification of a Germline CSPG4 Variation in a Family with Neurofibromatosis Type 1-like Phenotype. Cell Death Dis. 2021, 12, 765. [Google Scholar] [CrossRef]
- Egea, J.; García, A.G.; Verges, J.; Montell, E.; López, M.G. Antioxidant, Antiinflammatory and Neuroprotective Actions of Chondroitin Sulfate and Proteoglycans. Osteoarthr. Cartil. 2010, 18, S24–S27. [Google Scholar] [CrossRef]
- Bauerova, K.; Ponist, S.; Kuncirova, V.; Mihalova, D.; Paulovicova, E.; Volpi, N. Chondroitin Sulfate Effect on Induced Arthritis in Rats. Osteoarthr. Cartil. 2011, 19, 1373–1379. [Google Scholar] [CrossRef]
- Volpi, N. Therapeutic Applications of Glycosaminoglycans. CMC 2006, 13, 1799–1810. [Google Scholar] [CrossRef] [PubMed]
- Pecchi, E.; Priam, S.; Mladenovic, Z.; Gosset, M.; Saurel, A.-S.; Aguilar, L.; Berenbaum, F.; Jacques, C. A Potential Role of Chondroitin Sulfate on Bone in Osteoarthritis: Inhibition of Prostaglandin E2 and Matrix Metalloproteinases Synthesis in Interleukin-1β- Stimulated Osteoblasts. Osteoarthr. Cartil. 2012, 20, 127–135. [Google Scholar] [CrossRef]
- Chen, S.; Chen, W.; Chen, Y.; Mo, X.; Fan, C. Chondroitin Sulfate Modified 3D Porous Electrospun Nanofiber Scaffolds Promote Cartilage Regeneration. Mater. Sci. Eng. C 2021, 118, 111312. [Google Scholar] [CrossRef] [PubMed]
- Guan, T.; Ding, L.-G.; Lu, B.-Y.; Guo, J.-Y.; Wu, M.-Y.; Tan, Z.-Q.; Hou, S.-Z. Combined Administration of Curcumin and Chondroitin Sulfate Alleviates Cartilage Injury and Inflammation via NF-ΚB Pathway in Knee Osteoarthritis Rats. Front. Pharmacol. 2022, 13, 882304. [Google Scholar] [CrossRef]
- Stellavato, A.; Restaino, O.F.; Vassallo, V.; Cassese, E.; Finamore, R.; Ruosi, C.; Schiraldi, C. Chondroitin Sulfate in USA Dietary Supplements in Comparison to Pharma Grade Products: Analytical Fingerprint and Potential Anti-Inflammatory Effect on Human Osteoartritic Chondrocytes and Synoviocytes. Pharmaceutics 2021, 13, 737. [Google Scholar] [CrossRef]
- Presa, F.B.; Marques, M.L.M.; Viana, R.L.S.; Nobre, L.T.D.B.; Costa, L.S.; Rocha, H.A.O. The protective role of sulfated poly-saccharides from green seaweed Udotea flabellum in cells exposed to oxidative damage. Mar. Drugs 2018, 20, 135. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.S.; Cavalcante, R.S.; Palhares, L.C.G.F.; Hughes, A.J.; Andrade, G.P.V.; Yates, E.A.; Nader, H.B.; Lima, M.A.; Chavante, S.F. A Non-Hemorrhagic Hybrid Heparin/Heparan Sulfate with Anticoagulant Potential. Carbohydr. Polym. 2014, 99, 372–378. [Google Scholar] [CrossRef]
- Chavante, S.F.; Santos, E.A.; Oliveira, F.W.; Guerrini, M.; Torri, G.; Casu, B.; Dietrich, C.P.; Nader, H.B. A Novel Heparan Sulphate with High Degree of N-Sulphation and High Heparin Cofactor-II Activity from the Brine Shrimp Artemia Franciscana. Int. J. Biol. Macromol. 2000, 27, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Maccari, F.; Ferrarini, F.; Volpi, N. Structural Characterization of Chondroitin Sulfate from Sturgeon Bone. Carbohydr. Res. 2010, 345, 1575–1580. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shen, Q.; Zhang, C.; Jia, W.; Han, L.; Yu, Q. Chicken Leg Bone as a Source of Chondroitin Sulfate. Carbohydr. Polym. 2019, 207, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Ai, C.; Guo, L.; Fu, Y.; Cao, C.; Song, S. Characteristic Oligosaccharides Released from Acid Hydrolysis for the Structural Analysis of Chondroitin Sulfate. Carbohydr. Res. 2017, 449, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Pietrasik, Z.; Ozimek, L.; Betti, M. Extraction, Isolation and Analysis of Chondroitin Sulfate from Broiler Chicken Biomass. Process Biochem. 2012, 47, 1909–1918. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, F.; Linhardt, R.J. Analysis of the Glycosaminoglycan Chains of Proteoglycans. J. Histochem. Cytochem. 2021, 69, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Mucci, A. 1H and 13C Nuclear Magnetic Resonance Identification and Characterization of Components of Chondroitin Sulfates of Various Origin. Carbohydr. Polym. 2000, 41, 37–45. [Google Scholar] [CrossRef]
- Di Muzio, L.; Paolicelli, P.; Trilli, J.; Petralito, S.; Carriero, V.C.; Brandelli, C.; Spano, M.; Sobolev, A.P.; Mannina, L.; Casadei, M.A. Insights into the Reaction of Chondroitin Sulfate with Glycidyl Methacrylate: 1D and 2D NMR Investigation. Carbohydr. Polym. 2022, 296, 119916. [Google Scholar] [CrossRef]
- Mourão, P.A.S.; Pereira, M.S.; Pavão, M.S.G.; Mulloy, B.; Tollefsen, D.M.; Mowinckel, M.-C.; Abildgaard, U. Structure and Anticoagulant Activity of a Fucosylated Chondroitin Sulfate from Echinoderm. J. Biol. Chem. 1996, 271, 23973–23984. [Google Scholar] [CrossRef] [PubMed]
- Volpi, N. Quality of Different Chondroitin Sulfate Preparations in Relation to Their Therapeutic Activity. J. Pharm. Pharmacol. 2010, 61, 1271–1280. [Google Scholar] [CrossRef]
- Flangea, C.; Serb, A.; Schiopu, C.; Tudor, S.; Sisu, E.; Seidler, D.; Zamfir, A. Discrimination of GalNAc (4S/6S) Sulfation Sites in Chondroitin Sulfate Disaccharides by Chip-Based Nanoelectrospray Multistage Mass Spectrometry. Open Chem. 2009, 7, 752–759. [Google Scholar] [CrossRef]
- Santos, G.R.; Porto, A.C.; Soares, P.A.; Vilanova, E.; Mourão, P.A. Exploring the Structure of Fucosylated Chondroitin Sulfate through Bottom-up Nuclear Magnetic Resonance and Electrospray Ionization-High-Resolution Mass Spectrometry Approaches. Glycobiology 2017, 27, 625–634. [Google Scholar] [CrossRef]
- Cañas, N.; Gorina, R.; Planas, A.M.; Vergés, J.; Montell, E.; García, A.G.; López, M.G. Chondroitin Sulfate Inhibits Lipopolysaccharide-Induced Inflammation in Rat Astrocytes by Preventing Nuclear Factor Kappa B Activation. Neuroscience 2010, 167, 872–879. [Google Scholar] [CrossRef]
- Krylov, V.B.; Grachev, A.A.; Ustyuzhanina, N.E.; Ushakova, N.A.; Preobrazhenskaya, M.E.; Kozlova, N.I.; Portsel, M.N.; Konovalova, I.N.; Novikov, V.Y.; Siebert, H.-C.; et al. Preliminary Structural Characterization, Anti-Inflammatory and Anticoagulant Activities of Chondroitin Sulfates from Marine Fish Cartilage. Russ. Chem. Bull. 2011, 60, 746–753. [Google Scholar] [CrossRef]
- Crespo, D.; Asher, R.A.; Lin, R.; Rhodes, K.E.; Fawcett, J.W. How Does Chondroitinase Promote Functional Recovery in the Damaged CNS? Exp. Neurol. 2007, 206, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Sugahara, K. Potential Therapeutic Application of Chondroitin Sulfate/Dermatan Sulfate. CDDT 2008, 5, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Lee, A.; Choi, K.; Kim, K.; Youn, I.; Trippel, S.B.; Panitch, A. Biomimetic Aggrecan Reduces Cartilage Extracellular Matrix from Degradation and Lowers Catabolic Activity in Ex Vivo and in Vivo Models. Macromol. Biosci. 2013, 13, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Peng, X.; Lin, J.; Zhang, Y.; Tian, X.; Zhan, L.; Zhao, G. The Therapeutic Potential of Chondroitin Sulfate in Aspergillus Fumigatus Keratitis. Mol. Immunol. 2022, 147, 50–61. [Google Scholar] [CrossRef]
- Singh, S.; Singh, T.G.; Singh, M.; Najda, A.; Nurzyńska-Wierdak, R.; Almeer, R.; Kamel, M.; Abdel-Daim, M.M. Anticonvulsive Effects of Chondroitin Sulfate on Pilocarpine and Pentylenetetrazole Induced Epileptogenesis in Mice. Molecules 2021, 26, 6773. [Google Scholar] [CrossRef]
- Yeung, Y.T.; Aziz, F.; Guerrero-Castilla, A.; Arguelles, S. Signaling Pathways in Inflammation and Anti-Inflammatory Therapies. CPD 2018, 24, 1449–1484. [Google Scholar] [CrossRef]
- Barros-Gomes, J.A.C.; Nascimento, D.L.A.; Silveira, A.C.R.; Silva, R.K.; Gomes, D.L.; Melo, K.R.T.; Almeida-Lima, J.; Camara, R.B.G.; Silva, N.B.; Rocha, H.A.O. In Vivo Evaluation of the Antioxidant Activity and Protective Action of the Seaweed Gracilaria Birdiae. Oxid. Med. Cell. Longev. 2018, 2018, 9354296. [Google Scholar] [CrossRef]
- Kipari, T.; Watson, S.; Houlberg, K.; Lepage, S.; Hughes, J.; Cailhier, J.-F. Lymphocytes Modulate Peritoneal Leukocyte Recruitment in Peritonitis. Inflamm. Res. 2009, 58, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, K.; Mikami, T.; Uyama, T.; Mizuguchi, S.; Nomura, K.; Kitagawa, H. Recent Advances in the Structural Biology of Chondroitin Sulfate and Dermatan Sulfate. Curr. Opin. Struct. Biol. 2003, 13, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Moser, M.; Sperandio, M. The Molecular Basis of Leukocyte Recruitment and Its Deficiencies. Mol. Immunol. 2013, 55, 49–58. [Google Scholar] [CrossRef]
- Parish, C.R. The Role of Heparan Sulphate in Inflammation. Nat. Rev. Immunol. 2006, 6, 633–643. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving Inflammation: Dual Anti-Inflammatory and pro-Resolution Lipid Mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The Nuclear Factor NF- B Pathway in Inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Geering, B.; Stoeckle, C.; Conus, S.; Simon, H.-U. Living and Dying for Inflammation: Neutrophils, Eosinophils, Basophils. Trends Immunol. 2013, 34, 398–409. [Google Scholar] [CrossRef]
- Vodovotz, Y.; Constantine, G.; Faeder, J.; Mi, Q.; Rubin, J.; Bartels, J.; Sarkar, J.; Squires, R.H.; Okonkwo, D.O.; Gerlach, J.; et al. Translational Systems Approaches to the Biology of Inflammation and Healing. Immunopharmacol. Immunotoxicol. 2010, 32, 181–195. [Google Scholar] [CrossRef]
- Jaques, L.B.; Balueux, R.E.; Dietrich, C.P.; Kavanagh, L.W. A Microelectrophoresis Method for Heparin. Can. J. Physiol. Pharmacol. 1968, 46, 351–360. [Google Scholar] [CrossRef]
- Xie, X.; Rivier, A.-S.; Zakrzewicz, A.; Bernimoulin, M.; Zeng, X.-L.; Wessel, H.P.; Schapira, M.; Spertini, O. Inhibition of Selectin-Mediated Cell Adhesion and Prevention of Acute Inflammation by Nonanticoagulant Sulfated Saccharides. J. Biol. Chem. 2000, 275, 34818–34825. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.B.; Pinto, L.d.L.d.S.; Medeiros, L.H.; Souza, A.A., Jr.; Chavante, S.F.; Filgueira, L.G.A.; Camara, R.B.G.; Sassaki, G.L.; Rocha, H.A.O.; Andrade, G.P.V. Chondroitin Sulfate from Oreochromis niloticus Waste Reduces Leukocyte Influx in an Acute Peritonitis Model. Molecules 2023, 28, 3082. https://doi.org/10.3390/molecules28073082
Silva MB, Pinto LdLdS, Medeiros LH, Souza AA Jr., Chavante SF, Filgueira LGA, Camara RBG, Sassaki GL, Rocha HAO, Andrade GPV. Chondroitin Sulfate from Oreochromis niloticus Waste Reduces Leukocyte Influx in an Acute Peritonitis Model. Molecules. 2023; 28(7):3082. https://doi.org/10.3390/molecules28073082
Chicago/Turabian StyleSilva, Marianna Barros, Lívia de Lourdes de Sousa Pinto, Luiz Henrique Medeiros, Airton Araújo Souza, Jr., Suely Ferreira Chavante, Luciana Guimarães Alves Filgueira, Rafael Barros Gomes Camara, Guilherme Lanzi Sassaki, Hugo Alexandre Oliveira Rocha, and Giulianna Paiva Viana Andrade. 2023. "Chondroitin Sulfate from Oreochromis niloticus Waste Reduces Leukocyte Influx in an Acute Peritonitis Model" Molecules 28, no. 7: 3082. https://doi.org/10.3390/molecules28073082
APA StyleSilva, M. B., Pinto, L. d. L. d. S., Medeiros, L. H., Souza, A. A., Jr., Chavante, S. F., Filgueira, L. G. A., Camara, R. B. G., Sassaki, G. L., Rocha, H. A. O., & Andrade, G. P. V. (2023). Chondroitin Sulfate from Oreochromis niloticus Waste Reduces Leukocyte Influx in an Acute Peritonitis Model. Molecules, 28(7), 3082. https://doi.org/10.3390/molecules28073082








