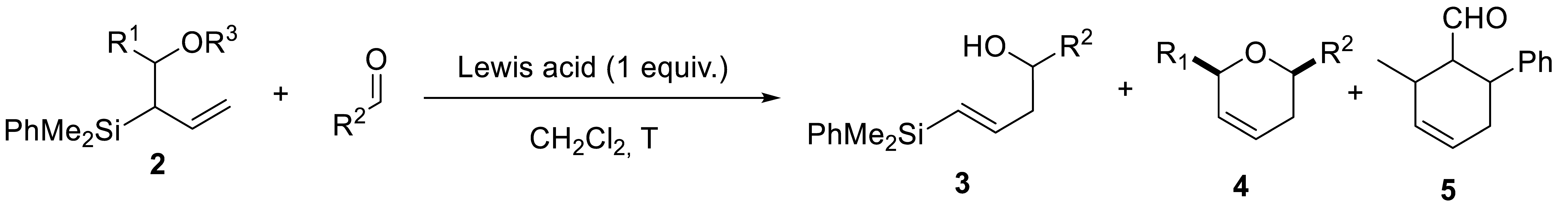
Abstract
A convenient regioselective synthesis of allyl- and vinylsilyl alcohols, from a common precursor, was described, by selecting the appropriate reaction conditions. Allyl- and vinylsilyl alcohols were tested in silyl-Prins cyclizations for the preparation of disubstituted oxygenated heterocycles in a one-pot sequential reaction. The methodology was sensitive to the structure of the starting alkenylsilyl alcohol and reaction conditions, with competitive pathways observed (particularly for allylsilyl alcohols), such as Peterson elimination and oxonia-Cope reactions. However, the use of vinylsilyl alcohols allowed the preparation of differently disubstituted cis-2,6-dihydropyrans in moderate to good yields. Computational studies support the proposed mechanism.
1. Introduction
Heterocyclic compounds are some of the most common chemical structures found in nature. Among them, functionalized oxygen heterocycles have attracted the attention of scientists, due to their presence in a wide number of biologically active natural products [1,2]. Tremendous efforts have been made to develop synthetic methodologies which can contribute to the construction of these cores. Particularly, intramolecular Prins cyclization has been established as a powerful tool in organic synthesis, for the construction of five- to eight-membered oxacycles, serving as key intermediates for the total synthesis of a variety of natural products [3]. This methodology involves the reaction of an alkenol with an aldehyde, in the presence of a Brønsted or Lewis acid. The general accepted mechanism involves the generation of an initial oxocarbenium ion intermediate, which can subsequently evolve through an endo-dig cyclization. Then, the corresponding cyclic carbocation can be trapped with a nucleophile, present in the reaction medium. High stereoselectivities are usually obtained, due to the equatorial disposition of substituents in chairlike transition states (TS).
Due to the importance of these types of oxacycles in drug discovery, new chemical modifications have been studied in recent decades, to refine and improve their synthesis. The so-called silyl-Prins cyclization, involves the participation of electron-rich alkenes, such as allyl- or vinylsilanes, and shows better selectivity towards the formation of single products, due to the stabilization of the carbocation β to silicon [4,5,6]. Although this methodology has been reported for some allylsilyl [7,8,9,10] and vinylsinyl alcohols [11,12,13,14,15], more efforts are required to increase the reaction scope and facilitate the access to novel 2,6-disubstituted dihydropyrane derivatives. Based on our experience in silyl-Prins cyclizations [16,17,18], in this work we investigate the access to either allyl- or vinylsilyl homoallylic alcohols from a common allyl(dimethyl)phenylsilane, and the possibilities of participation of the resulting substrates in silyl-Prins cyclizations (Scheme 1). Considering previously reported methodologies, as well as our model substrate, we anticipate some challenges that must be overcome:
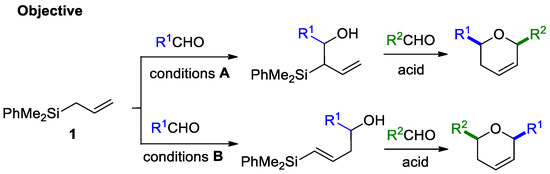
Scheme 1.
Regioselective formation of allyl- and vinyl alcohols and subsequent Prins cyclization.
- (a)
- Selective methodologies are needed to obtain exclusively allyl- or vinyl silylalcohols from a common allylsilane precursor (conditions A and B, Scheme 1).
- (b)
- Alternative reaction pathways may interfere in the silyl-Prins cyclization reaction outcome [19], generating complex mixtures due to the formation of highly reactive intermediates.
Our first goal, is the regioselective synthesis of new allyl- and E-vinylsilyl homoallylic alcohols, from a common allylsilane 1. Once these reaction conditions have been optimized, attempts to achieve silyl-Prins cyclization from both derivatives are evaluated, in order to compare the selectivity and the scope of both convergent processes. In addition, we study (both by computational and experimental methods) the challenge of favoring silyl-Prins cyclization over alternative reaction pathways.
2. Results
2.1. Synthesis of Allyl- and Vinylsilyl Alcohols
We started our investigations synthesizing allyl(diphenyl)silane 1. This substrate was prepared with the in situ formation of an organozinc reagent from allyl bromide, which was subsequently added to a solution of chloro(dimethyl)phenylsilane in THF at 0 °C (Section 3.2.2) [20]. The desired derivative 1 was obtained in a 95% yield and could be used without any further purification. With 1 in hand, we focused our attention on the regioselective synthesis of allyl- and vinylsilyl alcohols 2 and 3. The acidic character of the protons α to the silicon atom, could be used to generate an α-silylcarbanion, that was in equilibrium with a γ-silylcarbanion. Then, subsequent addition of an aldehyde under controlled conditions exclusively generated the desired derivatives (2 or 3).
Allylsilyl alcohols 2 were obtained when the incorporation of the electrophile occured though the α-position. Thus, the reaction of 1 with n-BuLi, in the presence of N-tetramethylethylendiamine (TMEDA), generated an intermediate silylcarbanion I. Ti(OiPr)4 was subsequently added as an auxiliary reagent, to block the γ-position and promote the selective addition through the α-position (Scheme 2). In this manner, various homoallylic alcohols 2 were selectively prepared in moderate yields, by addition of either alkyl- or arylaldehyde coupling partners (2a–c, 35–55%). The reaction takes places with total diastereoselectivity, obtaining exclusively anti products [21].
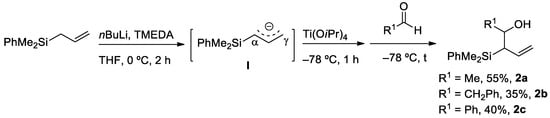
Scheme 2.
Regioselective synthesis of allylsilyl alcohols 2.
Fortunately, in the absence of the auxiliary reagent Ti(OiPr)4, the intermediate silylcarbanion I, reacts with aldehydes through the desired γ-position, which is the least hindered one, providing the regioisomeric vinylsilyl alcohols 3. Representative examples were obtained with both alkyl- and aryl aldehydes, in moderate yields (Scheme 3). With these alternative protocols we were able to prepare a series of compounds 2 and 3, ready to be used in silyl-Prins cyclization reactions.
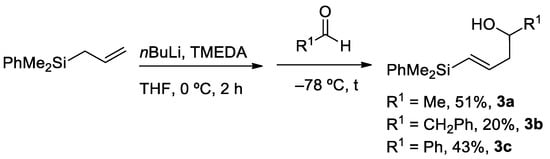
Scheme 3.
Regioselective synthesis of vinylsilyl alcohols 3.
2.2. Study of the Silyl-Prins Cyclization with Allylsilyl Alcohols 2
With various allylsilyl alcohols 2 in hand, we screened a series of reaction conditions, to achieve the desired silyl-Prins cyclization.
We started our investigations with derivative 2a (R1 = Me), which was treated with various Lewis acids (1.2 equiv.) at different reaction temperatures (Table 1, entries 1–4). Unfortunately, we did not observe the formation of the desired oxacycle 4, obtaining complex mixtures, and identifying product 3 at low temperature (Table 1, entry 1). The formation of vinylsilyl alcohol 3, would imply a competitive oxonia-Cope reaction of intermediate II, instead of the desired cyclization step (Scheme 4). To minimize side-reactions, we decided to use less reactive aldehydes, such as (E)-3-phenylprop-2-enal (Table 1, entry 5). However, 1H-NMR analysis of the reaction crude, showed a complex mixture, in which unreacted aldehyde was observed, but neither the starting material nor oxonia-Cope products could be identified. This result suggests the possibility of an alternative reaction pathway from 2, such as a Peterson elimination, which would provide a volatile 1,3-pentadiene, probably lost in the rotavapor system (Scheme 4). To further study the occurrence of this competitive reaction, we set up the reaction with allylsilyl alcohols 2b (R1 = CH2Ph) and 2c (R1 = Ph) (Table 1, entries 6–9). Gratifyingly, a detailed analysis of the reaction mixture now showed the presence of cyclic products (4a or 4i), together with 3a (Table 1, entries 6 and 7), which suggested an initial oxonia-Cope reaction, followed by subsequent cyclization. In order to try to overcome secondary reactions, we turned our attention to the use of trimethylsilyl ester derivatives as starting materials (Table 1, entries 10–12) [22]. Unfortunately, no reaction, or complex mixtures, was observed (Table 1, entries 10 and 11), although an interesting new product 5 was identified and characterized (Table 1, entry 12). The formation of this carbocycle can be explained through a Diels–Alder reaction of the 1,3-diene produced in the Peterson elimination and the corresponding aldehyde ((E)-3-phenylprop-2-enal), which corroborates the previously proposed Peterson elimination pathway (Scheme 4).

Table 1.
Screening of the reaction conditions.
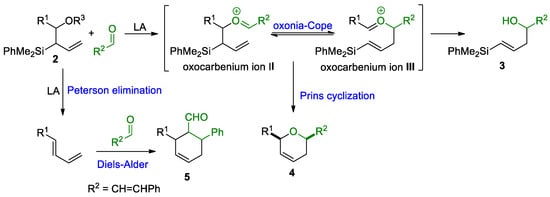
Scheme 4.
Reaction pathways from allylsilyl alcohols 2.
As shown before, the reaction of different allylsilyl alcohols with aldehydes, in the presence of Lewis acids, turned out to be very challenging, due to the different behavior of the implicated substrates, as well as the variety of competitive reaction pathways. However, we were able to identify and isolate key products for a better understanding of the implicated intermediates, that will help in the development of future silyl-Prins cyclizations. Next, and considering previous data, we focused our attention on the study of the silyl-Prins cyclization of vinylsilyl alcohols 3.
2.3. Silyl-Prins Cyclization Study of Vinylsilyl Alcohols 3
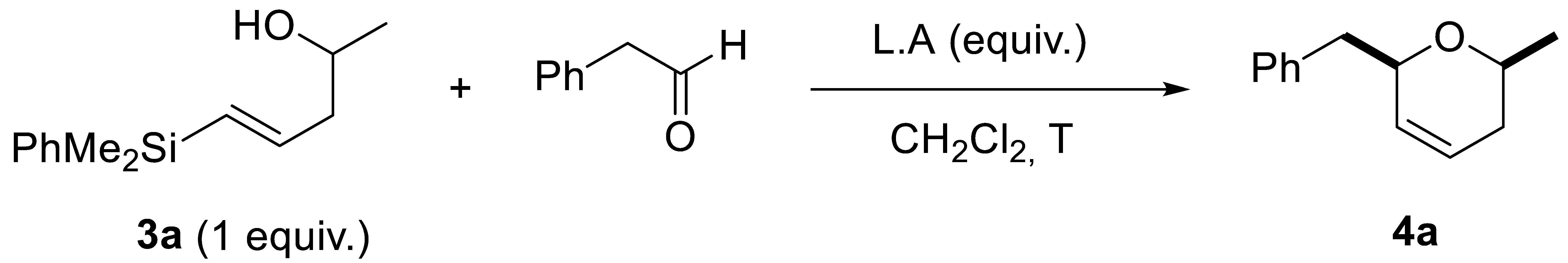
First of all, we screened different conditions and Lewis acids, in order to find the optimized conditions for the cyclization, using as a key model derivative 3a. As shown in Table 2, complex mixtures were obtained at 0 °C when BF3·OEt2 or TMSCl were used as Lewis acids (Table 2, entries 1–4). Gratifyingly, a cis-2,6-disubstituted dihydropyrane derivative 4a was obtained when 1 equiv. of TMSOTf was used at −78 °C (Table 2, entries 5–7). Analysis of the reaction crude, showed very good diastereoselectivity for cis-4a (cis:trans, 90:10), which was isolated in a 48% yield (Table 2, entry 6). Increasing the equivalents of the aldehyde, or reducing the amount of the Lewis acid, provided lower yields (Table 2, entries 5 and 7).

Table 2.
Screening of the reaction conditions.
Having determined the optimal conditions, we studied the reaction scope for other vinylsilyl alcohols and aldehydes (Scheme 5). As shown, the reaction of (E)-vinylsilyl alcohols bearing an alkylic R1 group, with alkylic aldehydes, provides, in moderate to good yields, the desired disubstituted dihydropyrans (4a–4g). Interestingly, although the reaction of allylsilyl alcohols 2 with aldehydes failed to give the desired oxacycles (Table 2), we now were able to circumvent this difficulty by choosing the appropriate substituents in the vinylsilyl alcohols and the aldehyde (for instance, dihydropyran 4b could be obtained using the homologated aldehyde partner). It was also possible to install a phenylethylene group in the dihydropyrane with good diastereoselectivity (dr > 95:5), with the preparation of compound 4f (79% yield). However, the presence of a hydroxy group in the aromatic ring of the aldehyde (when the corresponding 3-(p-hydroxyphenyl)propionaldehyde was used) significantly affected the yield of the reaction (4g, 43%). Other interesting functional groups, such as an isopropylester, could also be installed successfully (4h, 46% yield). An interesting aspect of this reaction, is the excellent diastereoselectivity observed in most cases for the formation of the cis-2,6-disubstituted dihydropyran. On the other hand, less reactive aromatic or vinylic aldehydes produced complex mixtures, in which it was possible to isolate symmetric tetrahydropyrans, with identical substituents attached at the 2,6-positions (4i, 37% yield). This is in accordance with our previous results on the reaction with vinylsilyl alcohols, in which an alternative sigmatropic [3,3] oxonia-Cope transposition pathway was more favored. Similarly, vinylsilyl alcohols with an aryl R1 group, provided complex reaction mixtures.
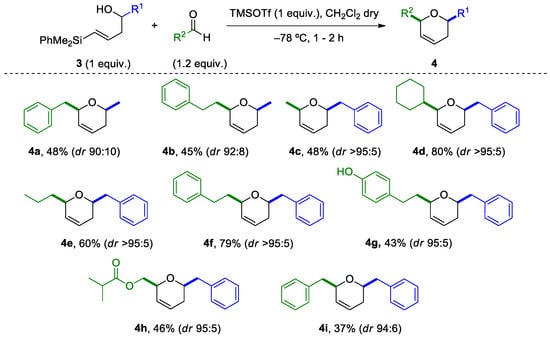
Scheme 5.
Reaction scope of the silyl-Prins cyclization.
2.4. Reaction Mechanism Study
The reaction of allylsilyl alcohols turned out to be more challenging than the corresponding one with E-vinylsilyl alcohols. Very likely, this is due to the concurrence of more favored alternative pathways, such as oxonia-Cope transposition and Peterson elimination. In the case of E-vinylsilyl alcohols, cis-dihydropyrane derivatives were selectively obtained through a silyl-Prins cyclization reaction. A plausible mechanism for this reaction would start with the reaction of E-vinylsilyl alcohol 3 with the aldehyde, in the presence of a Lewis acid, to generate an oxocarbenium ion III (Scheme 6). Then, a 6-endo-dig cyclization would take place, to afford intermediate IV (stabilized by the silicon atom), followed by a subsequent desilylation reaction, to afford final product 4 [23]. This process can be considered a tandem reaction, in which TMSOTf promotes the formation of intermediate III and the elimination step. The stereocontrol can be explained through the preferential pseudoequatorial conformation of substituents R1 and R2 in the transition state, to achieve minimal repulsion.
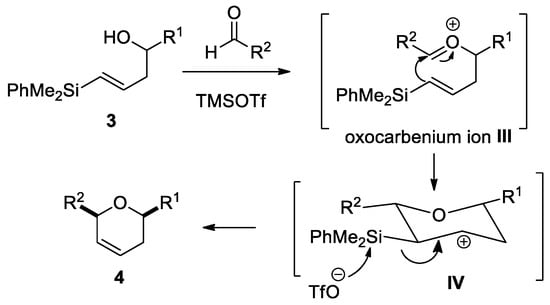
Scheme 6.
Mechanism proposal of the silyl-Prins cyclization.
In order to gain insight into the reaction mechanism and the high stereoselectivity observed, a computational study of the silyl-Prins cyclization has been performed at a fundamental level. In this work, the Amsterdam Density Functional 2017.01 (ADF) [24,25] software package was used to perform DFT calculations. The geometries were fully optimized without symmetry constraints, using the combination of the gradient corrections of the Becke exchange functional and Perdew correlation functional, which use the Vosko–Wilk–Nusair exchange-correlation potential reported by Becke (1998) and Perdew (1986) (BP86-D3) [26,27,28], in combination with the latest version of Grimme dispersion correction [29]. Triple-ζ Slater-type orbitals (STO), were used to describe the valence shells. The solvation environment was treated with the Conductor-like Screening Model (COSMO) [30], considering dichloromethane (ɛ = 8.9) in order to reproduce the experimental conditions performed in our lab. Analytical frequencies were computed, to characterize the stationary points and calculate the free energies (standard state T = 298.15 K, p = 1 atm). The transition state was followed after a fractional displacement of the imaginary vibrational mode, to determine the reaction path to reactant(s) and product(s). Molecular renderings were made with Chemcraft [31].
In Figure 1, the reaction profile of the 6-endo silyl-Prins cyclization towards dihydropyran 4a is shown. In this study, only the approximation between the two carbon atoms that yields a chair conformation for the oxygenated heterocycle is described, since no transition state has been found for a boat conformation. The results obtained, indicate that the reaction shows a very low energy profile, with an energy barrier for the transition state of 2.7 kcal/mol. In such a transition state, the distance between the C atoms which will be bonded in the cyclization step, corresponds to 1.95 Å. Following the imaginary vibrational mode to cyclic intermediate IV (tetrahydropyranyl carbocation), the minimum presents a Gibbs free energy value of 1.6 kcal/mol, which corresponds to an energy difference with the transition state of only 1.1 kcal/mol. This low energy profile is consistent with the experimental results concerning the reversibility of the silyl-Prins cyclization, which could follow other competitive pathways. Furthermore, we carried out computational studies for the R isomer (C atom that holds the methyl group); geometries of the reactants and products were fully optimized. The obtained results, show that for the R isomer, where the methyl is in axial arrangement, both ΔH and ΔG energies present higher values in comparison with the S isomer (see Supporting Information, Figure S1). In addition, for the R isomer, the rotational freedom allowed by the sigma bonds prior to cyclization could lead to another conformation, where the bulky groups (methyl and benzyl) are placed in equatorial arrangement, which explains the preferential formation of cis-2,6-disubstituted derivatives 4 (see Supporting Information, Scheme S1).

Figure 1.
Reaction scheme for silyl-Prins cyclization. Gibbs free energies are provided in kcal·mol−1, and distances in angstroms (Å).
3. Materials and Methods
3.1. General Remarks
Unless otherwise noted, experiments were carried out with dry solvents under a nitrogen atmosphere. Dichloromethane was dried with preactivated molecular sieves. Flash column chromatography was performed using Silica Gel 60 (230–400 mesh ASTM). Thin-layer chromatography (TLC) was performed using an aluminum backed plate, pre-coated with silica gel (0.20 mm, silica gel 60), with a fluorescent indicator (254 nm) from Macherey. NMR spectra were recorded at the nuclear magnetic resonance service of the Laboratory of Instrumental Techniques (L.T.I., www.laboratoriotecnicasinstrumentales.es), University of Valladolid, at Varian 400 MHz (1H, 399.85 MHz; 13C, 100.61 MHz) and Varian 500 MHz (1H, 500.12 MHz; 13C, 100.61 MHz), with the spectrometers at room temperature (25 °C). Chemical shifts (δ) were reported in parts per million (ppm), relative to the residual solvent peaks recorded, rounded to the nearest 0.01 for 1H-NMR and 0.1 for 13C-NMR (reference: CDCl3 [1H: 7.26, 13C: 77.2]). Spin–spin coupling constants (J) in 1H-NMR were given in Hz, to the nearest 0.1 Hz, and peak multiplicity was indicated as follows s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), and br (broad). 13C-NMR were recorded with complete proton decoupling. Carbon types, structure assignments and attribution of peaks were determined from two-dimensional correlation experiments (HSQC, COSY, and HMBC). Relative stereochemistry was assigned based on the 2D-NOE experiments. High-resolution mass spectra (HRMS) were measured at the mass spectrometry service of the Laboratory of Instrumental Techniques, University of Valladolid, on a UPLC-MS system (UPLC: Waters ACQUITY H-class UPLC; MS: Bruker Maxis Impact) by electrospray ionization (ESI positive and negative).
3.2. Synthesis of Allyl(diphenyl)silane 1
A solution of 2.99 g (45 mmol, 1.5 equiv.) of zinc, in 25 mL THF (1.2 M), was cooled to 0 °C under a nitrogen atmosphere. Then, allyl bromide (45 mmol, 1.5 equiv.) was added, and after five minutes under vigorous stirring, 5.03 mL of phenyldimethylchlorosilane (30 mmol, 1.0 equiv.) was added dropwise. When the starting materials had been consumed, it was hydrolyzed with 20 mL of NH4Cl sat. The phases were then separated, extracting the aqueous phase three times with diethyl ether. The organic phases were combined, washed with NaCl sat., and dried over anhydrous Na2SO4. The solvent was then evaporated under reduced pressure. The crude mixture was analyzed by NMR and then purified by column chromatography in silica gel, using hexane, providing allylsilane 1 in quantitative yield. This (1) was obtained as a colorless oil in 95% chemical yield (5.02 g from 30 mmol of PhMe2SiCl). 1H-NMR (500 MHz, CDCl3) δ 7.52–7.47 (m, 2H, Ar-H), 7.36–7.32 (m, 3H, Ar-H), 5.83–5.71 (m, 1H, HC=), 4.87 (d, Jtrans = 17.0 Hz, 1H, =CHH), 4.83 (d, Jcis = 10.4 Hz, 1H, = CHH), 1.74 (d, J = 8.0 Hz, 2H, CH2), 0.27 (s, 6H, (CH3)2- Si).
3.3. Synthesis of Allylsilyl Alcohols 2
A solution of 1.5 g (8.5 mmol, 1.0 eq.) of allylsilane 1, in 15 mL THF (0.57 M), was cooled to 0 °C (under nitrogen). Next, 6.4 mL n-BuLi (10.2 mmol, 1.1 eq.) and 2.1 mL TMEDA (14.0 mmol, 1.65 eq.) were added dropwise. The mixture was stirred at this temperature for 2 h. Then, the reaction as cooled down to −78 °C and 2.52 mL Ti(iOPr)4 (8.5 mmol, 1.0 eq.) was added. After 1 h, the corresponding aldehyde (10.2 mmol, 1.2 eq.) was added dropwise to the solution. When the starting materials had been consumed (control by TCL), it was hydrolyzed with 15 mL of NH4Cl sat. The phases were then separated, extracting the aqueous phase three times with diethyl ether. The organic phases were combined, washed with NaCl sat., and dried over anhydrous Na2SO4. The solvent was then evaporated under reduced pressure. The crude mixture was analyzed by NMR and then purified by column chromatography in silica gel, using a mixtures of hexane-ethyl acetate (10:1), yielding allylsilyl alcohols 2.
3-(dimethyl(phenyl)silyl)pent-4-en-2-ol (2a) was obtained following the general procedure 3.3, as a yellow oil, in 55% chemical yield (3.028 g from 22.7 mmol of 1). 1H-NMR (500 MHz, CDCl3) δ 7.56–7.49 (m, 2H, Ar-H), 7.39–7.32 (m, 3H, Ar-H), 5.81 (dt, J = 17.2, 10.4 Hz, 1H, HC=), 5.09 (dd, J = 10.4, 1.7 Hz, 1H, =CHH), 4.96 (ddd, J = 17.2, 2.0, 0.7 Hz, 1H, =CHH), 4.00–3.87 (m, 1H, HC-OH), 1.85 (dd, J = 10.4, 6.0 Hz, 1H, HC), 1.52 (br s, 1H, OH), 1.11 (d, J = 6.3 Hz, 3H, CH3), 0.35 (s, 3H, Si-CH3), 0.32 (s, 3H, Si-CH3). 13C-NMR (101 MHz, CDCl3) δ 138.0 (C), 135.9 (HC=), 134.1 (CH), 129.2 (CH), 127.9 (CH), 116.1 (=CH2), 67.6 (HC-OH), 44.7 (CH), 23.6 (CH3), −3.0 (Si-CH3), −3.8 (Si-CH3).
3-(dimethyl(phenyl)silyl)-1-phenylpent-4-en-2-ol (2b) was obtained following the general procedure 3.3, as a yellow oil, in 33% chemical yield (826 mg from 8.5 mmol of 1). 1H-NMR (500 MHz, CDCl3) δ 7.56–7.49 (m, 2H, Ar-H), 7.37–7.31 (m, 3H, Ar-H), 7.29–7.24 (m, 3H, Ar-H), 7.23–7.17 (m, 1H, Ar-H), 7.12–7.08 (m, 2H, Ar-H), 5.97 (dt, J = 17.1, 10.5 Hz, 1H, HC=), 5.11 (dd, J = 10.4, 2.1 Hz, 1H =CHH), 4.92 (ddd, J = 17.1, 2.1, 0.5 Hz, 1H, =CHH), 4.01–3.90 (m, 1H, HC-OH), 2.70–2.65 (m, 2H, Ph-CH2), 1.96 (dd, J = 10.4, 3.5 Hz, 1H, CH), 1.45 (dd, J = 3.5, 1.1 Hz, 1H, OH), 0.36 (s, 3H, Si-CH3), 0.32 (s, 3H, Si-CH3). 13C-NMR (101 MHz, CDCl3) δ 139.1 (C), 138.0 (C), 135.1 (HC=), 134.2 (CH), 129.4 (CH), 129.2 (CH), 128.6 (CH), 127.9 (CH), 126.4 (CH), 115.7 (=CH2), 72.6 (HC-OH), 44.0 (CH2), 41.6 (CH), −3.4 (Si-CH3), −3.8 (Si-CH3).
2-(dimethyl(phenyl)silyl)-1-phenylbut-3-en-1-ol (2c) was obtained following the general procedure 3.3, as a yellow oil, in 49% chemical yield (1.18 g from 8.5 mmol of 1). 1H-NMR (500 MHz, CDCl3) δ 7.48–7.42 (m, 2H, Ar-H), 7.37–7.31 (m, 3H, Ar-H), 7.29–7.25 (m, 2H, Ar-H), 7.24–7.20 (m, 3H, Ar-H), 5.88 (dt, J = 17.2, 10.4 Hz, 1H, HC=), 5.05 (dd, J = 10.4, 1.8 Hz, 1H, =CHH), 4.90 (dd, J = 17.2, 1.8 Hz, 1H, =CHH), 4.75 (dd, J = 6.9, 2.7 Hz, 1H, HC-OH), 2.26 (dd, J = 10.4, 6.9 Hz, 1H, HC), 2.03 (d, J = 2.7 Hz, 1H, OH), 0.19 (s, 3H, Si-CH3), 0.08 (s, 3H, Si-CH3). 13C-NMR (101 MHz, CDCl3) δ 143.8 (C), 137.5 (C), 135.8 (HC=), 134.3 (CH), 129.2 (CH), 128.3 (CH), 127.8 (CH), 127.7 (CH), 126.8 (CH), 116.4 (=CH2), 74.3 (HC-OH), 45.1 (CH), −3.3 (Si-CH3), −3.9 (Si-CH3).
3.4. Synthesis of Vinylsilyl Alcohols 3
A solution of 1.5 g (8.5 mmol, 1.0 equiv.) of allylsilane 1 in 15 mL THF (0.57 M) was cooled to 0 °C under a nitrogen atmosphere. Next, 6.4 mL of n-BuLi (10.2 mmol, 1.1 equiv.) and 2.1 mL TMEDA (14.0 mmol, 1.65 equiv.) were added dropwise. The mixture was stirred at this temperature for 2 h. Then, the reaction was cooled down to −78 °C and the corresponding aldehyde (10.2 mmol, 1.2 equiv.) was added dropwise to the solution. When the starting materials had been consumed (control by TLC), it was hydrolyzed with 15 mL of NH4Cl sat. The phases were then separated, extracting the aqueous phase three times with diethyl ether. The organic phases were combined, washed with NaCl sat., and dried over anhydrous Na2SO4. The solvent was then evaporated under reduced pressure. The crude mixture was analyzed by NMR and then purified by column chromatography in silica gel, using a mixtures of hexane-ethyl acetate (8:1), yielding E-vinylsilyl alcohols 3.
(E)-5-(dimethyl(phenyl)silyl)pent-4-en-2-ol (3a) was obtained following the general procedure 3.4, as a yellow oil, in 51% chemical yield (1.18 g from 11.4 mmol of 1). 1H-NMR (500 MHz, CDCl3) δ 7.53–7.50 (m, 2H, Ar-H), 7.37–7.33 (m, 3H, Ar-H), 6.11 (dt, J = 18.6, 6.7 Hz, 1H, =CH), 5.91 (dt, J = 18.6, 1.2 Hz, 1H, HC=), 3.93–3.84 (m, 1H, HC-OH), 2.40–2.33 (m, 1H, CHH), 2.32–2.25 (m, 1H, CHH), 1.21 (d, J = 6.2 Hz, 3H, CH3), 0.35 (s, 6H, (CH3)2-Si). 13C-NMR (101 MHz, CDCl3) δ 144.8 (=CH), 138.9 (C), 133.9 (CH), 132.3 (HC=), 129.1 (C-H), 127.9 (CH), 67.0 (HC-OH), 46.9 (CH2), 23.0 (CH3), −2.4 (CH3)-Si), −2.5. (CH3)-Si).
(E)-5-(dimethyl(phenyl)silyl)-1-phenylpent-4-en-2-ol (3b) was obtained following the general procedure 3.4, as a yellow oil, in 20% chemical yield (500 mg from 8.5 mmol of 1). 1H-NMR (500 MHz, CDCl3) δ 7.55–7.50 (m, 2H, Ar-H), 7.38–7.35 (m, 3H, Ar-H), 7.33–7.29 (m, 2H, Ar-H), 7.25–7.20 (m, 3H, Ar-H), 6.17 (dt, J = 18.7, 6.5 Hz, 1H, =CH), 5.94 (d, J = 18.7 Hz, 1H, HC=), 3.97–3.89 (m,1H, HC-OH), 2.82 (dd, J = 13.5, 5.1 Hz, 1H, CHH-Ph), 2.73 (dd, J = 13.5, 7.9 Hz, 1H, CHH-Ph), 2.48–2.41 (m, 1H, CHH), 2.38–2.29 (m, 1H, CHH), 1.66 (br s, 1H, OH), 0.35 (s, 6H, Si-(CH3)2). 13C-NMR (101 MHz, CDCl3) δ 144.7 (=CH), 138.9 (C), 138.5 (C), 133.9 (CH), 132.4 (HC=), 129.6 (CH), 129.1 (CH), 128.7 (CH), 127.9 (CH), 126.6 (CH), 71.7 (HC-OH), 44.4 (CH2), 43.5, (CH2-Ph), −2.4 (Si-(CH3)2).
(E)-4-(dimethyl(phenyl)silyl)-1-phenylbut-3-en-1-ol (3c) was obtained following the general procedure 3.4, as a yellow oil, in 43% chemical yield (890 mg from 7.4 mmol of 1). 1H-NMR (500 MHz, CDCl3) δ 7.50–7.44 (m, 3H, Ar-H), 7.37–7.32 (m, 7H, Ar-H), 6.11 (dt, J = 18.6, 6.7 Hz, 1H, =CH), 5.91 (dt, J = 18.6, 1.0 Hz, 1H, HC=), 4.81–4.75 (m, 1H, HC-OH), 2.61 (t, J = 6.7 Hz, 2H, CH2), 2.00 (br s, 1H, OH), 0.32 (s, 3H, (Si-CH3)2). 13C-NMR (101 MHz, CDCl3) δ 144.4 (=CH), 144.0 (C), 138.8 (C), 133.9 (CH), 132.7 (HC=), 129.1 (CH), 128.5 (CH), 127.9 (CH), 127.7 (CH), 126.0 (CH), 73.5 (HC-OH), 47.0 (CH2), −2.4 ((Si-CH3)2).
3.5. TMSOTf-Promoted Cyclization Reaction to Afford Dihydropyranes 4
A solution of 73 mg (0.33 mmol, 1.0 equiv.) of the E-vinylsilyl alcohol 3a and the corresponding aldehyde (0.40 mmol, 1.2 equiv.), in 7 mL dichloromethane (0.05 M), was cooled to −78 °C under a nitrogen atmosphere. Then, TMSOTf (0.33 mmol, 1.0 equiv.) was added dropwise. The mixture was stirred at this temperature for 1 or 2 h while monitored by TLC. When the starting materials had been consumed, it was hydrolyzed with 6 mL of saturated NaHCO3. The phases were then separated, extracting the aqueous phase three times with dichloromethane. The organic phases were combined, washed with NaCl sat., and dried over anhydrous Na2SO4. The solvent was then evaporated under reduced pressure. The crude mixture was analyzed by NMR and then purified by column chromatography in silica gel, using mixtures of hexane-ethyl acetate, yielding dihydropyrans 4.
(2R*,6S*)-6-benzyl-2-methyl-3,6-dihydro-2H-pyran (4a) was obtained following the general procedure 3.5, as a yellow oil, in 48% chemical yield (30 mg from 0.33 mmol of 3a). 1H-NMR (500 MHz, CDCl3) δ 7.30–7.27 (m, 2H, Ar-H), 7.25–7.20 (m, 3H, Ar-H), 5.80–5.76 (m, 1H, HC=), 5.62–5.57 (m, 1H, =CH), 4.38–4.32 (m, 1H, O-CH), 3.73–3.66 (m, 1H, HC-O), 3.01 (dd, J = 13.5, 6.3 Hz, 1H, Ph-CHH), 2.68 (dd, J = 13.5, 8.1 Hz, 1H, Ph-CHH), 1.98–1.93 (m, 2H, CH2), 1.25 (d, J = 6.2 Hz, 3H, CH3). 13C-NMR (101 MHz, CDCl3) δ 138.4 (C), 129.8 (CH), 129.2 (=CH), 128.3 (CH), 126.3 (CH), 125.1 (HC=), 75.9 (O-CH), 70.2 (CH-O), 42.3 (Ph-CH2), 30.8 (CH2), 21.5 (CH3). HRMS (ESI+) m/z calc. for C13H16NaO ([M+Na]+): 211.1093, found 211.1097 [13].
(2R*,6S*)-2-methyl-6-phenethyl-3,6-dihydro-2H-pyran (4b) was obtained following the general procedure 3.5, as a yellow oil, in 45% chemical yield (30 mg from 0.33 mmol of 3a). 1H-NMR (500 MHz, CDCl3) δ 7.30–7.27 (m, 2H, Ar-H), 7.23–7.15 (m, 3H, Ar-H), 5.84–5.78 (m, 1H, HC=), 5.65–5.61 (m, 1H, =CH), 4.17–4.10 (m, 1H, O-CH), 3.73–3.64 (m, 1H, HC-O), 2.80–2.70 (m, 2H, CH2-CH2-Ph), 2.00–1.94 (m, 2H, CH2), 1.87–1.80 (m, 2H, CH2-CH2-Ph), 1.26 (d, J = 5.8 Hz, 3H, CH3). 13C-NMR (101 MHz, CDCl3) δ 142.5 (C), 130.2 (CH), 128.7 (=CH), 128.4 (CH), 126.3 (CH), 125.2 (HC=), 74.1 (O-CH), 70.1 (CH-O), 37.3 (CH2-CH2-Ph), 33.1 (CH2), 31.4 (CH2-CH2-Ph), 21.9 (CH3). HRMS (ESI+) m/z calc. for C14H18NaO ([M+Na]+): 225.1250, found 225.1254. [32].
(2S*,6S*)-2-benzyl-6-methyl-3,6-dihydro-2H-pyran (4c) was obtained following the general procedure 3.5, as a yellow oil, in 48% chemical yield (22 mg from 0.24 mmol of 3b). 1H-NMR (500 MHz, CDCl3) δ 7.31–7.26 (m, 2H, Ar-H), 7.25–7.18 (m, 3H, Ar-H), 5.76–5.70 (m, 1H, HC=), 5.63–5.56 (m, 1H, =CH), 4.28–4.20 (m, 1H, O-CH), 3.82–3.75 (m, 1H, HC-O), 3.01 (dd, J = 13.6, 6.5 Hz, 1H, Ph-CHH), 2.69 (dd, J = 13.6, 7.0 Hz, 1H, Ph-CHH), 2.07–1.96 (m, 1H, CHH), 1.90–1.81 (m, 1H, CHH), 1.24 (d, J = 6.8 Hz, 3H, CH3). 13C-NMR (101 MHz, CDCl3) δ 138.7 (C), 131.6 (=CH), 129.5 (CH), 128.3 (CH), 126.3 (CH), 124.1 (HC=), 75.1 (CH-O), 71.3 (O-CH), 42.7 (Ph-CH2), 33.0 (CH2), 21.5 (CH3). HRMS (ESI+) m/z calc. for C13H16NaO ([M+Na]+): 211.1093, found 211.1097.
(2S*,6S*)-2-benzyl-6-cyclohexyl-3,6-dihydro-2H-pyran (4d) was obtained following the general procedure 3.5, as a yellow oil, in 80% chemical yield (49 mg from 0.24 mmol of 3b). 1H-NMR (500 MHz, CDCl3) δ 7.32–7.17 (m, 5H, Ar-H), 5.81–5.73 (m, 1H, HC=), 5.68–5.62 (m, 1H, =CH), 3.92–3.85 (m, 1H, O-CH), 3.77–3.68 (m, 1H, HC-O), 2.95 (dd, J = 13.7, 6.8 Hz, 1H, Ph-CHH), 2.68 (dd, J = 13.7, 6.1 Hz, 1H, Ph-CHH), 2.05–1.93 (m, 1H, CHHcycle), 1.90–1.82 (m, 1H, CHHcycle), 1.82–1.61 (m, 5H, CH2), 1.50–1.39 (m, 1H, CH), 1.31–1.01 (m, 5H, CH2c). 13C-NMR (101 MHz, CDCl3) δ 139.1 (C), 129.6 (CH), 129.1 (=CH), 128.2 (CH), 126.1 (CH), 125.0 (HC=), 79.2 (O-CH), 74.8 (HC-O), 42.9 (CH), 42.7 (CH2-Ph), 31.2 (CH2cycle), 28.8 (CH2), 28.3 (CH2), 26.8 (CH2), 26.5 (CH2), 26.5 (CH2). HRMS (ESI+) m/z calc. for C18H24NaO ([M+Na]+): 279.1719, found 279.1722.
(2S*,6S*)-2-benzyl-6-propyl-3,6-dihydro-2H-pyran (4e) was obtained following the general procedure 3.5, as a yellow oil, in 60% chemical yield (31 mg from 0.24 mmol of 3b). 1H RMN (500 MHz, CDCl3) δ 7.32–7.18 (m, 5H, Ar-H), 5.79–5.71 (m, 1H, HC=), 5.65–5.58 (m, 1H, =CH), 4.13–4.04 (m, 1H, O-CH), 3.80–3.70 (m, 1H, HC-O), 2.98 (dd, J = 13.7, 6.9 Hz, 1H, Ph-CHH), 2.70 (dd, J = 13.7, 6.2 Hz, 1H, Ph-CHH), 2.10–1.98 (m, 1H, CHH), 1.92–1.82 (m, 1H, CHH), 1.60–1.37 (m, 4H, CH2CH2), 0.92 (t, J = 6.9 Hz, 3H, CH3). 13C-NMR (101 MHz, CDCl3) δ 139.0 (C), 130.8 (=CH), 129.6 (CH), 128.3 (CH), 126.2 (CH), 124.5 (HC=), 75.0 (HC-O), 74.9 (O-CH), 42.6 (Ar-CH2), 37.9 (CH2), 31.2 (CH2), 18.6 (CH2), 14.2 (CH3). HRMS (ESI+) m/z calc. for C15H20NaO ([M+Na]+): 239.1406, found 239.1410.
(2S*,6S*)-2-benzyl-6-phenethyl-3,6-dihydro-2H-pyran (4f) was obtained following the general procedure 3.5, as a yellow oil, in 79% chemical yield (53 mg from 0.24 mmol of 3b). 1H-NMR (500 MHz, CDCl3) δ 7.34–7.28 (m, 4H, Ar-H), 7.25–7.20 (m, 3H, Ar-H), 7.17–7.13 (m, 1H, Ar-H), 7.07–7.04 (m, 2H, Ar-H), 5.80–5.75 (m, 1H, HC=), 5.61–5.56 (m, 1H, =CH), 4.02–3.97 (m, 1H, O-CH), 3.78–3.72 (m, 1H, HC-O), 2.96 (dd, J = 13.7, 7.8 Hz, 1H, Ph-CHH), 2.80–2.67 (m, 3H, Ph-CHH; CH2-CH2-Ph), 2.12–2.04 (m, 1H, CHH), 1.97–1.90 (m, 1H, CHH), 1.87–1.73 (m, 2H, CH2-Ph). 13C-NMR (101 MHz, CDCl3) δ 142.3 (C), 139.1 (C), 130.6 (=CH), 129.6 (CH), 128.8 (CH), 128.4 (CH), 128.3 (CH), 126.3 (CH), 125.7 (CH), 124.9 (HC=), 75.1 (CH-O), 73.7 (O-CH), 42.7 (Ph-CH2), 37.3 (CH2-Ph), 31.4 (CH2-CH2-Ph), 31.3 (CH2). HRMS (ESI+) m/z calc. for C20H22NaO ([M+Na]+): 301.1563, found 301.1564.
4-(2-((2S*,6S*)-6-benzyl-5,6-dihydro-2H-pyran-2-yl)ethyl)phenol (4g) was obtained following the general procedure 3.5, as a yellow oil, in 43% chemical yield (30 mg from 0.24 mmol of 3b). 1H-NMR (500 MHz, CDCl3) δ 7.34–7.27 (m, 5H, Ar-H), 7.25–7.21 (m, 1H, Ar-H), 6.88 (d, J = 8.6 Hz, 2H, Ar-H), 6.67 (d, J = 8.6 Hz, 2H, Ar-H), 5.80–5.73 (m, 1H, HC=), 5.60–5.53 (m, 1H, =CH), 4.49 (s, 1H, OH), 3.99–3.91 (m, 1H, O-CH), 3.77–3.70 (m, 1H, HC-O), 2.94 (dd, J = 13.4, 7.3 Hz, 1H, Ph-CHH), 2.76 (dd, J = 13.4, 5.8 Hz, 1H, Ph-CHH), 2.71–2.59 (m, 2H, CH2-CH2-Ar), 2.12–2.02 (m, 1H, CHH), 1.98–1.90 (m, 1H, CHH), 1.83–1.74 (m, 1H, CHH-CH2-Ar), 1.73–1.65 (m, 1H, CHH-CH2-Ar). 13C-NMR (101 MHz, CDCl3) δ 153.5 (C), 139.2 (C), 134.5 (C), 130.6 (=CH), 129.9 (CH), 129.6 (CH), 128.3 (CH), 126.3 (CH), 124.8 (HC=), 115.1 (CH), 75.1 (CH-O), 73.6 (O-CH), 42.7 (Ph-CH2), 37.5 (CH2-CH2-Ar), 31.3 (CH2), 30.4 (CH2-CH2-Ar). HRMS (ESI+) m/z calc. for C20H22NaO2 ([M+Na]+): 317.1512, found 317.1513.
((2R*,6S*)-6-benzyl-5,6-dihydro-2H-pyran-2-yl)methyl propionate (4h) was obtained following the general procedure 3.5, as a yellow oil, in 46% chemical yield (30 mg from 0.24 mmol of 3b). 1H-NMR (500 MHz, CDCl3) δ 8 7.31–7.17 (m, 5H, Ar-H), 5.92–5.85 (m, 1H, HC=), 5.63–5.57 (m, 1H, =CH), 4.39–4.32 (m, 1H, O-CH), 4.15 (dd, J = 11.4, 6.1 Hz, 2H, HHC-O), 4.11 (dd, J = 11.4, 4.8 Hz, 2H, HHC-O), 3.83–3.74 (m, 1H, HC-O), 2.78 (dd, J = 13.8, 7.0 Hz, 1H, Ph-CHH), 2.72 (dd, J = 13.8, 6.2 Hz, 1H, Ph-CHH), 2.62–2.54 (m, 1H, CH), 2.11–2.01 (m, 1H, CHH), 1.96–1.87 (m, 1H, CHH), 1.17 (d, J = 7.0 Hz, 3H, CH3), 1.16 (d, J = 7.0 Hz, 3H, CH3). 13C-NMR (101 MHz, CDCl3) δ 177.1 (C), 138.6 (C), 129.5 (CH), 128.3 (CH), 127.1 (HC=), 126.3 (=CH), 126.1 (CH), 74.8 (CH-O), 73.4 (O-CH), 66.2 (CH2-O), 42.4 (Ar-CH2), 34.1 (CH), 30.8 (CH2), 19.2 (CH3), 19.1 (CH3). HRMS (ESI+) m/z calc. for C18H24NaO ([M+Na]+): 279.1719, found 279.1722.
(2S*,6S*)-2,6-dibenzyl-3,6-dihydro-2H-pyran (4i) was obtained following the general procedure 3.5, as a yellow oil, in 37% chemical yield (23 mg from 0.24 mmol of 3b). 1H-NMR (400 MHz, CDCl3) δ 7.30–7.27 (m, 2H, Ar-H), 7.25–7.17 (m, 8H, Ar-H), 5.79–5.74 (m, 1H, HC=), 5.64–5.60 (m, 1H, =CH), 4.35–4.28 (m, 1H, O-CH), 3.78–3.72 (m, 1H, HC-O), 2.99–2.92 (m, 2H, Ph-CHH + HHC-Ph), 2.76–2.67 (m, 2H, Ph-CHH + HHC-Ph), 2.09–2.00 (m, 1H, CHHcycle), 1.93–1.86 (m, 1H, CHHcycle). 13C-NMR (101 MHz, CDCl3) δ 138.8 (C), 138.6 (C), 129.7 (HC=), 129.6 (CH), 128.3 (CH), 126.3 (CH), 126.1 (CH), 125.1 (=CH), 76.0 (O-CH), 75.1 (HC-O), 42.6 (Ph-CH2), 42.2 (CH2-Ph), 31.0 (CH2). HRMS (ESI+) m/z calc. for C19H20NaO ([M+Na]+): 287.1406, found 287.1406 [33].
3.6. BF3·OEt2-Promoted Diels Alder Reaction to Afford Derivatives 5
A solution of 60 mg (0.20 mmol, 1.0 equiv.) of protected allylsilyl alcohol 3a and 30 μL trans-cinnamaldehyde (0.36 mmol, 1.2 equiv.), in 6 mL dichloromethane (0.05 M), was cooled to 0 °C under a nitrogen atmosphere. Then, BF3·OEt2 (0.36 mmol, 1.2 equiv.) was added dropwise. The mixture was stirred at this temperature for 4 h while being monitored by TLC. When the starting materials had been consumed, it was hydrolyzed with 5 mL of saturated NaHCO3. The phases were then separated, extracting the aqueous phase three times with dichloromethane. The organic phases were combined, washed with NaCl sat., and dried over anhydrous Na2SO4. The solvent was then evaporated under reduced pressure. The crude mixture was analyzed by NMR and then purified by column chromatography in silica gel, using a mixture of hexane-ethyl acetate (20:1), affording aldehyde 5.
3-methyl-1,2,3,6-tetrahydro-[1,1’-biphenyl]-2-carbaldehyde (5) was obtained as a yellow oil, in 43% chemical yield (13 mg from 0.20 mmol of 3a). 1H-NMR (400 MHz, CDCl3) δ 9.62 (d, J = 3.8 Hz, 1H, CHO), 7.33–7.28 (m, 2H, Ar-H), 7.25–7.20 (m, 1H, Ar-H), 5.83–5.72 (m, 2H, HC=CH), 3.36–3.28 (m, 1H, HC-Ph), 2.84–2.77 (m, 1H, HC-CHO), 2.68 2.57 (m, 1H, HC-CH3), 2.48–2.37 (m, 1H, CHH), 2.27–2.17 (m, 1H, CHH), 1.10 (d, J = 6.7 Hz, 3H, CH3). 13C-NMR (101 MHz, CDCl3) δ 206.1 (CHO), 144.0 (C), 131.9 (=CH), 128.9 (CH), 127.8 (CH), 126.8 (CH), 126.0 (HC=), 55.1 (HC-CHO), 37.0 (HC-Ph), 32.8 (CH2), 29.9 (HC-CH3), 17.0 (CH3). HRMS (ESI+) m/z calc. for C14H15O ([M-H]−): 199.1128, found 199.1124.
4. Conclusions
Allyl(phenyldimethyl)silane 1 (obtained in a quantitative synthesis without any further purification steps) is a versatile substrate that has been used to prepare, in a regioselective manner, either allyl- or vinylsilyl alcohols, choosing the appropriate reaction protocols. Thus, E-vinylsilyl alcohols were suitable coupling agents in silyl-Prins cyclizations, with highly reactive alkyl aldehydes in the presence of TMSOTf. These Prins cyclization reactions allowed the preparation of a battery of cis-2,6-disubstituted dihydropyrane derivatives, in moderate to good yields, and with good to excellent diastereoselectivities (>90:10). The cyclization reaction turned out to be very sensitive to different reaction conditions, particularly for allylsilyl alcohols and non-activated aldehydes, in which we could identify and isolate side-products resulting from oxonia-Cope [34] transposition and Peterson elimination processes. Computational studies showed a very low energy profile for the cyclization of the oxygenated heterocycle, indicating that such cyclization is a reversible process from a thermodynamic point of view.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28073080/s1, with contains the 1H- and 13C-NMR spectra of the synthetized compounds, and Cartesian coordinates for theoretical studies. Figure S1. Relative energies for Reactants and products for S isomer and R isomer respectively computed at BP86-D3 triple-z theoretical level. Scheme S1. Sequence of consecutive rotations for sigma bonds that place bulky groups in equatorial arrangement for R isomer.
Author Contributions
Conceptualization, A.B.; methodology, L.F.P.; software, Á.S.-G.; validation, L.F.P. and A.B.; investigation, L.F.P.; data curation, L.F.P.; writing—original draft preparation, E.L.; writing—review and editing, E.L. and A.B.; supervision, A.B.; project administration, A.B.; funding acquisition, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Junta de Castilla y León, grant number VA294-P18 and Ministerio de Ciencia e Innovación (PID2020-116076RJI00/AEI/10.13039/501100011033).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data can be found in supporting information.
Acknowledgments
L.F.P. acknowledges a predoctoral grant, funded by the “Junta de Castilla y León”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nasir, N.M.; Ermanis, K.; Clarke, P.A. Strategies for the construction of tetrahydropyran rings in the synthesis of natural products. Org. Biomol. Chem. 2014, 12, 3323–3335. [Google Scholar] [CrossRef] [PubMed]
- Fuwa, H. Contemporary Strategies for the Synthesis of Tetrahydropyran Derivatives: Application to Total Synthesis of Neopeltolide, a Marine Macrolide Natural Product. Mar. Drugs 2016, 14, 65. [Google Scholar] [CrossRef] [PubMed]
- Reyes, E.; Prieto, L.; Uria, U.; Carrillo, L.; Vicario, J.L. Recent Advances in the Prins Reaction. ACS Omega 2022, 7, 31621–31627. [Google Scholar] [CrossRef]
- Díez-Poza, C.; Barbero, A. Synthesis of O− And N− Heterocycles by Silyl-Prins Cyclization of Allylsilanes. Eur. J. Org. Chem. 2017, 32, 4651–4665. [Google Scholar] [CrossRef]
- Pastor, I.M.; Yus, M. Focused Update on the Prins Reaction and the Prins Cyclization. Curr. Org. Chem. 2012, 16, 1277–1312. [Google Scholar] [CrossRef]
- Díez-Poza, C.; Barbero, H.; Díez-Varga, A.; Barbero, A. The Silyl-Prins Reaction as an Emerging Method for the Synthesis of Heterocycles in Progress in Heterocyclic Chemistry. In Progress of Heterocyclic Chemistry; Gribble, G.W., Joule, J.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 30, pp. 13–41. [Google Scholar]
- Su, Q.; Panek, J.S. Total Synthesis of (-)-Apicularen A. J. Am. Chem. Soc. 2004, 126, 2425–2430. [Google Scholar] [CrossRef]
- Kjellgren, J.; Szabó, K.J. Synthesis of stereodefined vinyl-tetrahydropyran and vinyl-octahydrochromene derivatives via acetization-cyclization of allylsilanes with aldehydes. Origin of the high stereoselectivity. Tetrahedron Lett. 2002, 43, 1123–1126. [Google Scholar] [CrossRef]
- Keck, G.E.; Kraft, M.B.; Truong, A.P.; Li, W.; Sánchez, C.C.; Kedei, N.; Lewin, N.E.; Blumberg, P.M. Convergent Assembly of Highly Potent Analogues of Bryostatin 1 via Pyran Annulation: Bryostatin Look-Alikes that Mimic Phorbol Ester Function. J. Am. Chem. Soc. 2008, 130, 6660–6661. [Google Scholar] [CrossRef]
- Sánchez, C.C.; Keck, G.E. Total Synthesis of (+)-Dactylolide. Org. Lett. 2005, 7, 3053–3056. [Google Scholar] [CrossRef]
- Overman, L.E.; Castaneda, A.; Blumenkopf, T.A. Acetal-initiated cyclizations of vinylsilanes: A general synthesis of allylically unsaturated oxacyclics. J. Am. Chem. Soc. 1986, 108, 1303–1304. [Google Scholar]
- Semeyn, C.; Blaauw, R.H.; Hiemstra, H.; Speckamp, W.N. Stereoselective Synthesis of Dihydropyrans via Vinylsilane-Terminated Cyclizations of Ester-Substituted Oxycarbenium Ion Intermediates. J. Org. Chem. 1997, 62, 3426–3427. [Google Scholar] [CrossRef]
- Dobbs, A.P.; Martinović, S. The Silyl-Prins reaction: A novel method for the synthesis of dihydropyrans. Tetrahedron Lett. 2002, 43, 7055–7057. [Google Scholar] [CrossRef]
- Lian, Y.; Hinkle, R.J. BiBr3-Initiated Tandem Addition/Silyl-Prins Reactions to 2,6-disubstituted Dihydropyrans. J. Org. Chem. 2006, 71, 7071–7074. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Wang, K.; Gao, L.; Song, Z. Chrial crotyl geminal bis(silane): A useful reagent for asymmetric Sakurai allylation by selective desilylation-enabled chirality transfer. Chem. Commun. 2017, 53, 3078–3081. [Google Scholar]
- Barbero, A.; Diez-Varga, A.; Pulido, F.J.; González-Ortega, A. Synthesis of Azepane Derivatives by Silyl-aza-Prins Cyclization of Allylsilyl Amines: Influence of the Catalyst in the Outcome of the Reaction. Org. Lett. 2016, 18, 1972–1975. [Google Scholar] [CrossRef]
- Barbero, A.; Díez-Varga, A.; Herrero, M.; Pulido, F.J. From Silylated Trishomoallylic Alcohols to Dioxaspiroundecades or Oxocanes: Catalyst and Substitution Influence. J. Org. Chem. 2016, 81, 2704–2712. [Google Scholar] [CrossRef]
- Barbero, A.; Díez-Poza, C. Unexpected Dominio Silyl-Prins/Aryl Migration Process from Geminal Vinylsilyl alcohols. Org. Lett. 2021, 23, 8385–8389. [Google Scholar]
- Alder, R.W.; Carta, F.; Reed, C.A.; Stoyanova, I.; Willis, C.L. Searching for intermediates in Silyl Prins cyclizations: The 2-oxa—5-adamantyl carbocation. Org. Biomol. Chem. 2010, 8, 1551–1559. [Google Scholar] [CrossRef]
- Ramesh, R.; Reddy, D.S. Zinc mediated allylations of chlorosilanes promoted by ultrasound: Synthesis of novel constrained sila aminoacids. Org. Biomol. Chem. 2014, 12, 4093–4097. [Google Scholar] [CrossRef]
- Chen, M.; Roush, W.R. Enantioselective synthesis of syn- and anti-β-hydroxiallylsilanes via allenes hydroboration-aldehyde allylboration reactions. Org. Lett. 2011, 13, 1992–1995. [Google Scholar] [CrossRef]
- Karimi, B.; Golshani, B. Mild and Highly Efficient Method for the silylation of Alcohols Using Hexamethyldisilazane Catalyzed by Iodine Under Nearly Neutral Reaction Conditions. J. Org. Chem. 2000, 65, 7228–7230. [Google Scholar] [CrossRef] [PubMed]
- Pulido, F.J.; Barbero, A.; Val, P.; Díez, A.; González-Ortega, A. Efficiency of Acid- and Mercury-Catalyzed Cyclization Reactions in the Synthesis of Tetrahydrofurans from Allylsilyl Alcohols. Eur. J. Org. Chem. 2012, 2012, 5350–5356. [Google Scholar] [CrossRef]
- Te Velde, G.; Bickelhaupt, F.M.; van Gisbergen, S.J.A.; Fonseca Guerra, C.; Baerends, E.J.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931–967. [Google Scholar] [CrossRef]
- ADF2013. SCM, Theoretical Chemistry. Available online: http://www.scm.com (accessed on 29 January 2023).
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: A critical analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H.A. Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Klamt, A.; Schuurmann, G. COSMO: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans. 1993, 2, 799–805. [Google Scholar] [CrossRef]
- Chemcraft. Available online: http://www.chemcraftprog.com (accessed on 29 January 2023).
- Markó, I.E.; Dobbs, A.P.; Scheirmann, V.; Chellé, F.; Bayston, D.J. Concise and Stereocontrolled Assembly of Substituted Dihydropyrans: Synthetic Studies Towards the trans-dioxadecalin Subunit of Okadaic Acid. Tetrahedron Lett. 1997, 38, 2899–2902. [Google Scholar]
- Viswanathan, G.S.; Yang, J.; Li, C.-J. A Novel Stereoselective Cyclization to Functionalized Dihydropyrans. Org. Lett. 1999, 1, 993–995. [Google Scholar] [CrossRef]
- Jasti, R.; Rychnovsky, S.D. Racemization in Prins Cyclization Reactions. J. Am. Chem. Soc. 2006, 128, 13640–13648. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).