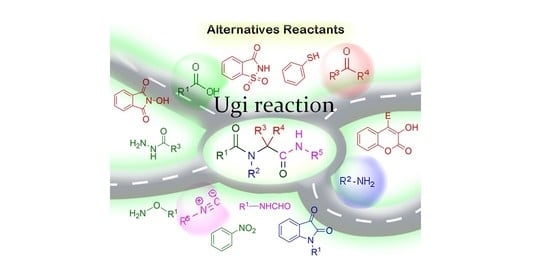
Ugi Four-Component Reactions Using Alternative Reactants
Abstract
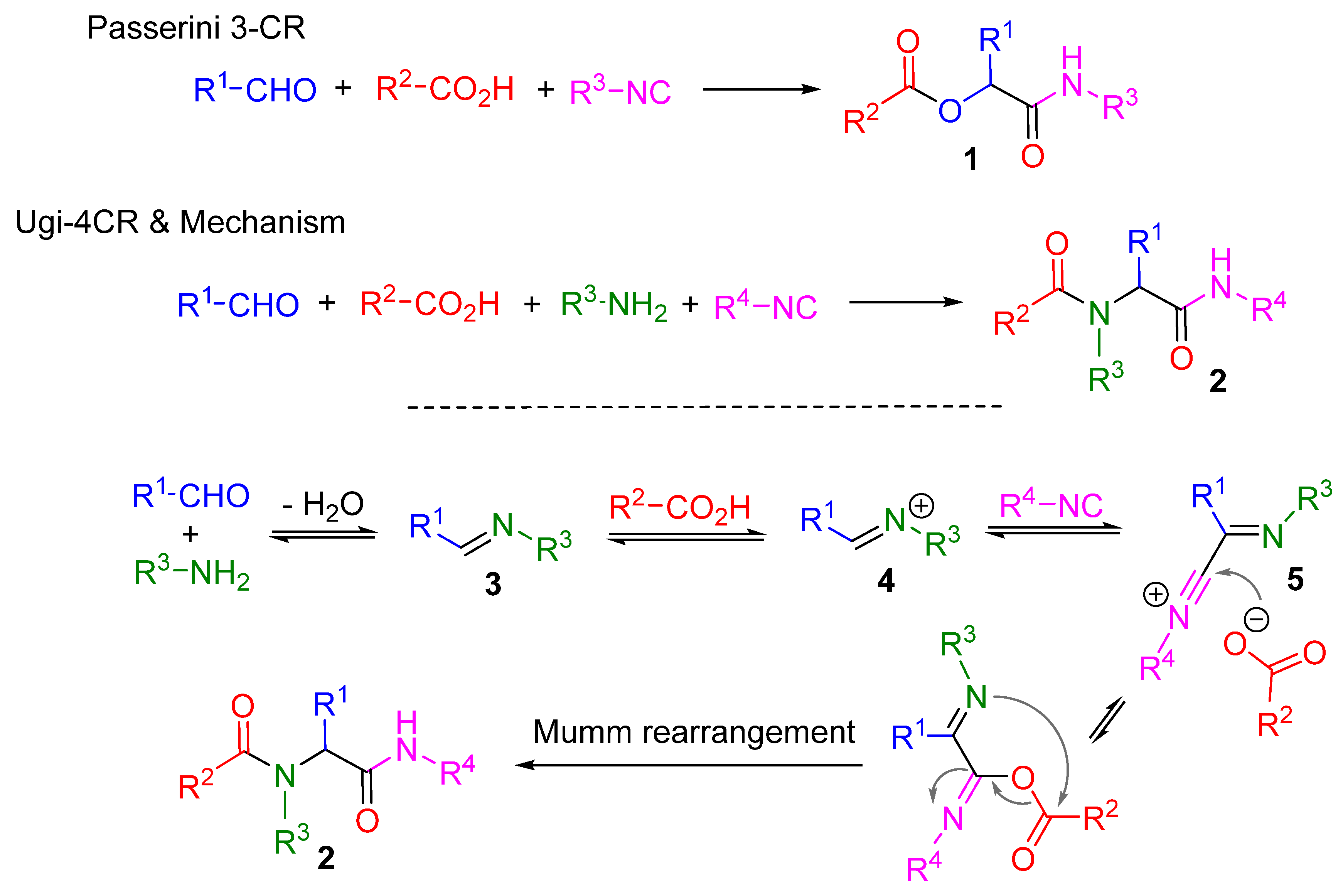
1. Introduction
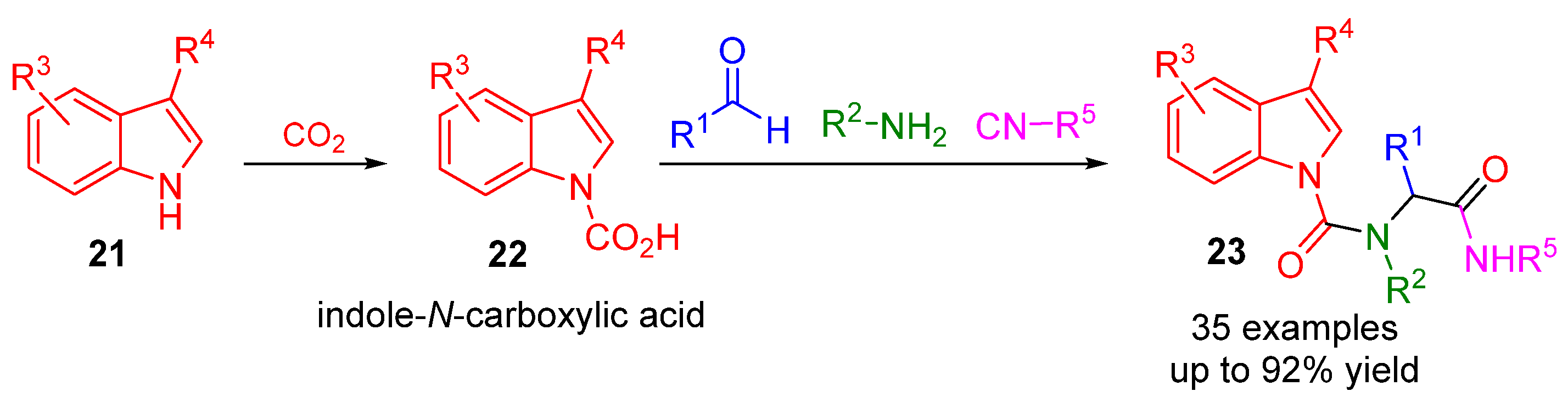
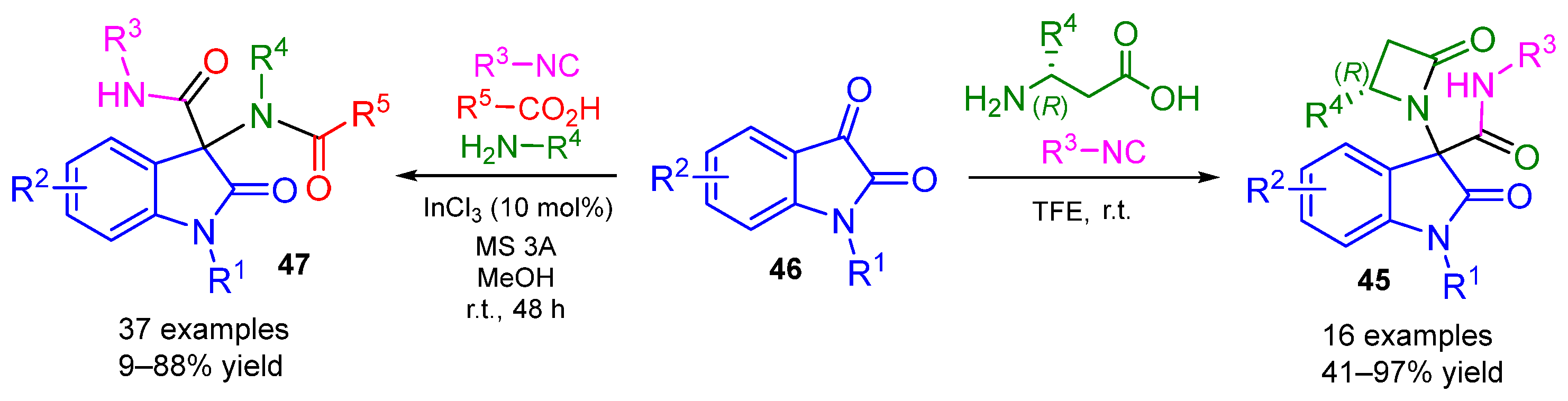
2. Alternative Components for Carboxylic Acids
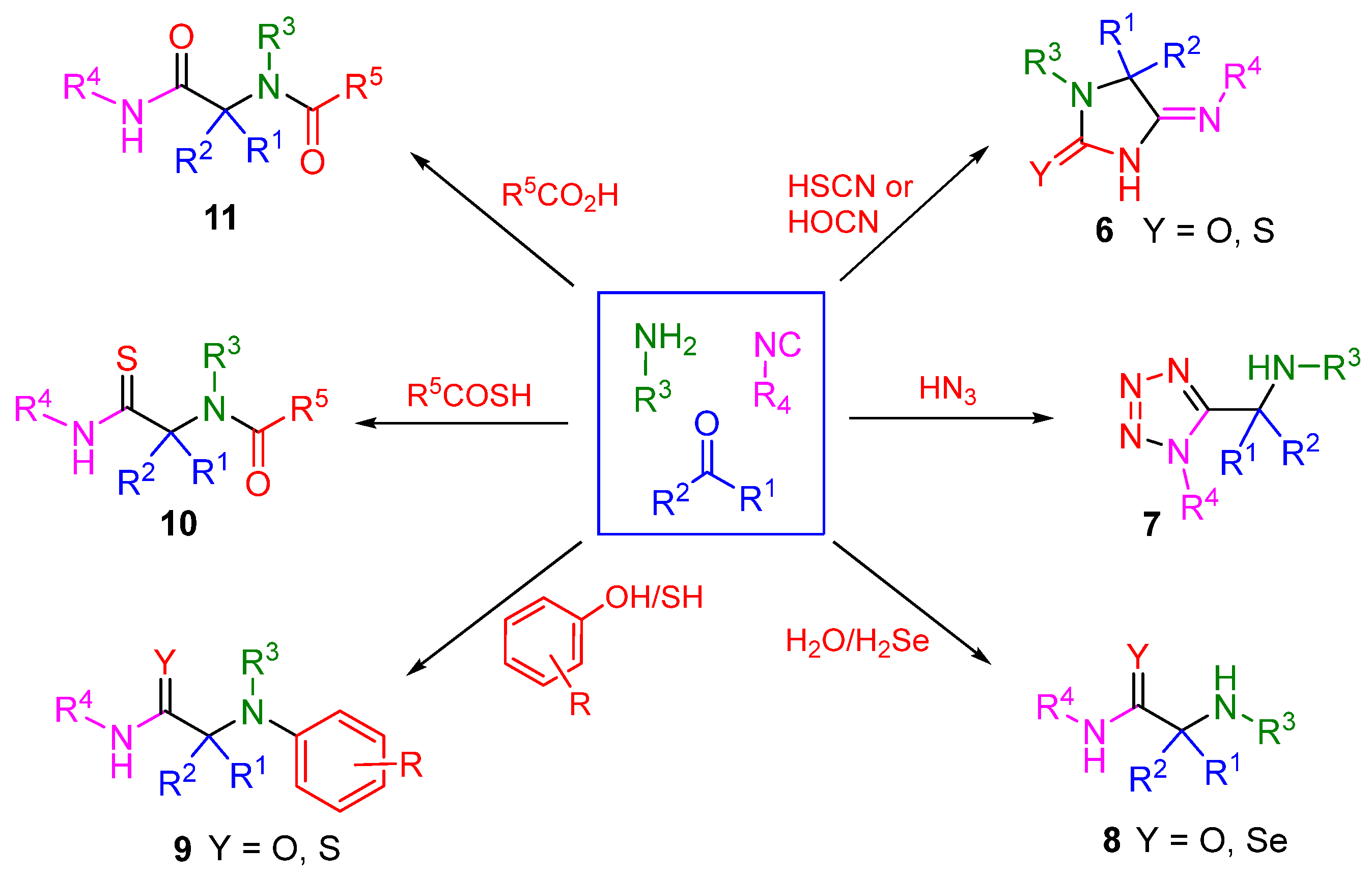
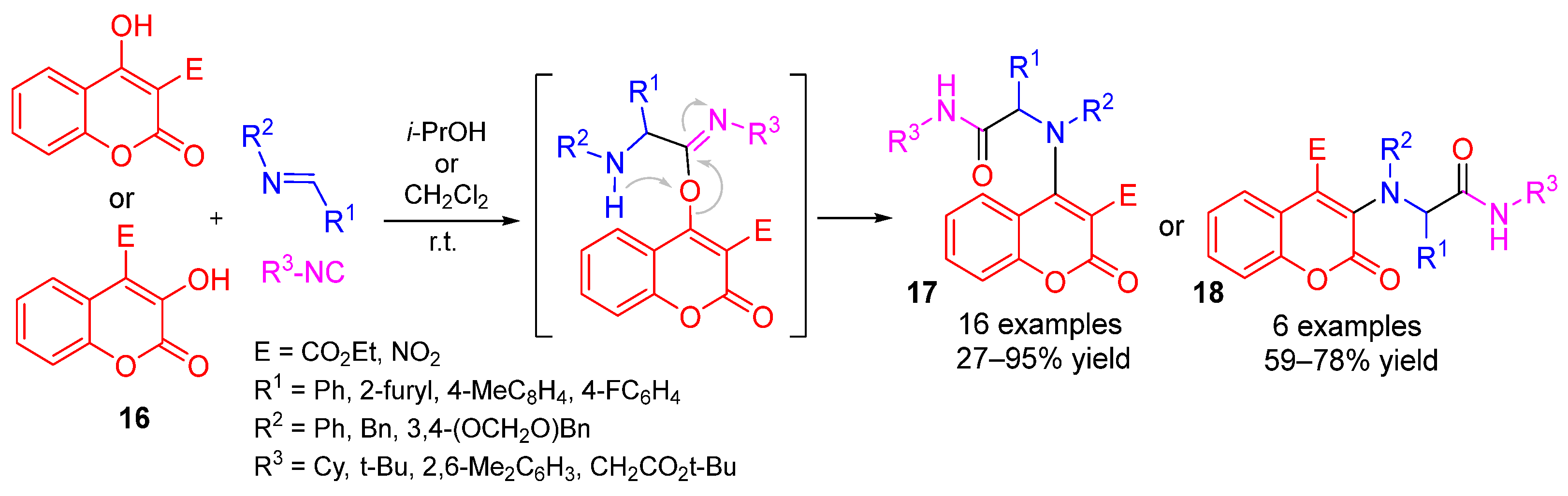
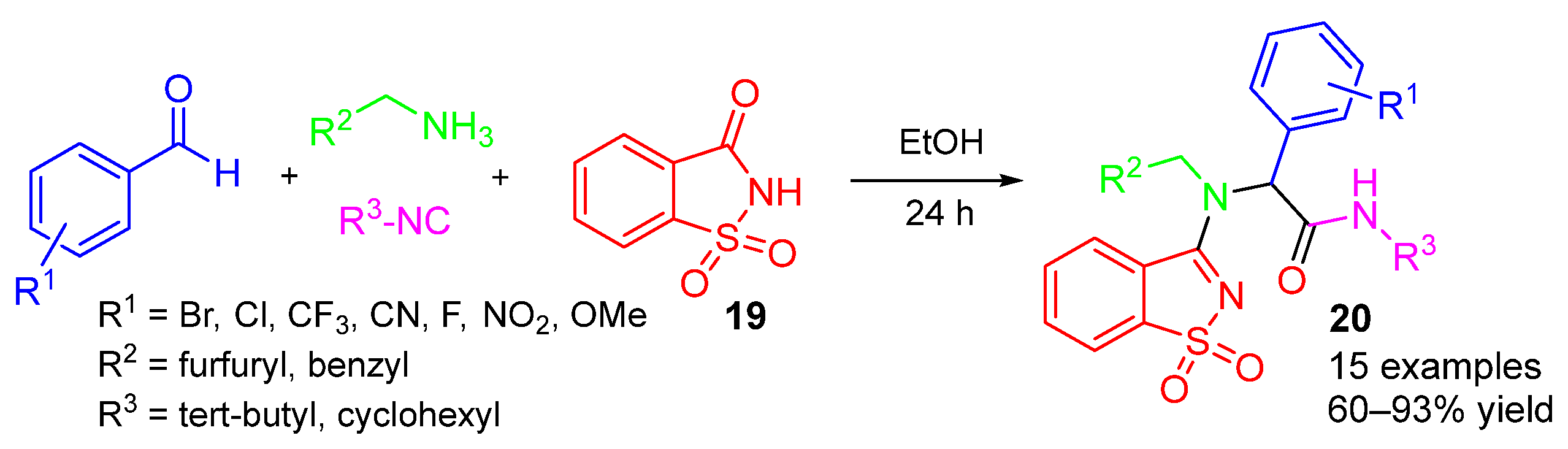
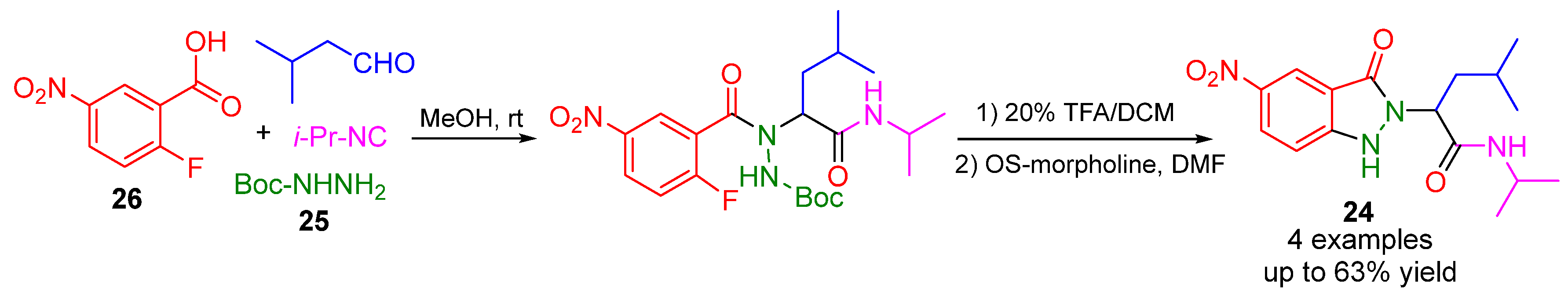
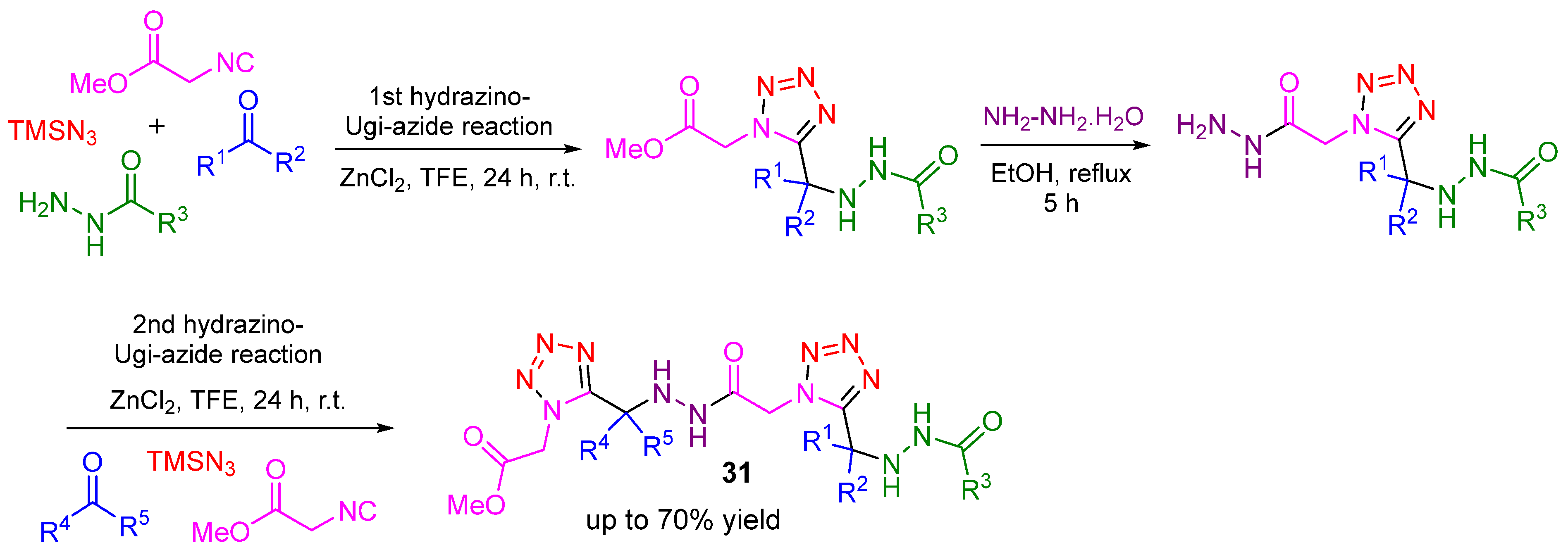
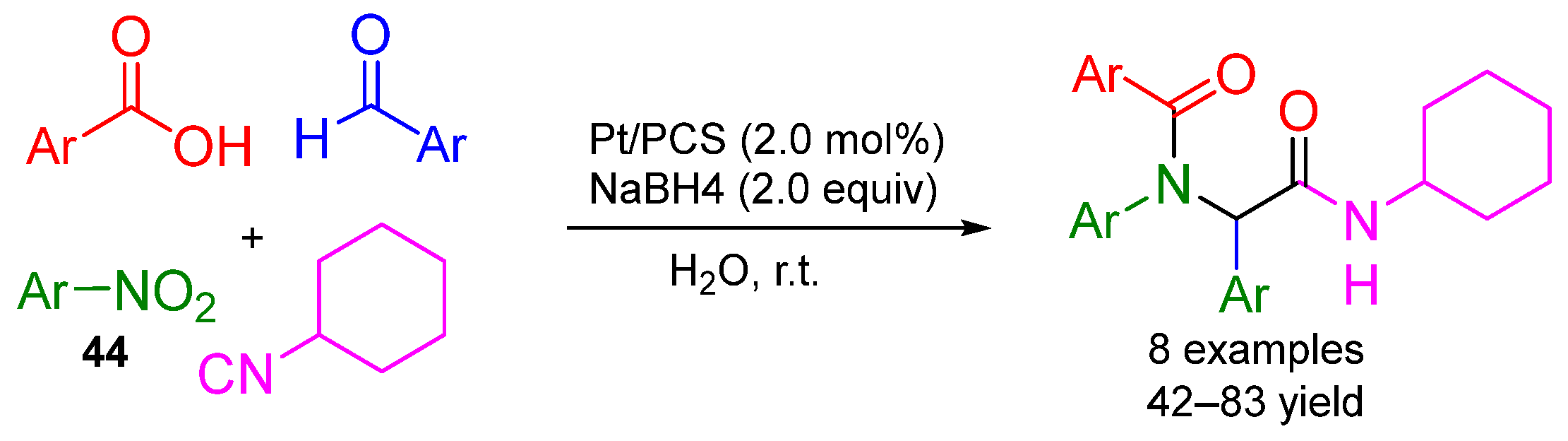
3. Alternative Components for Amines
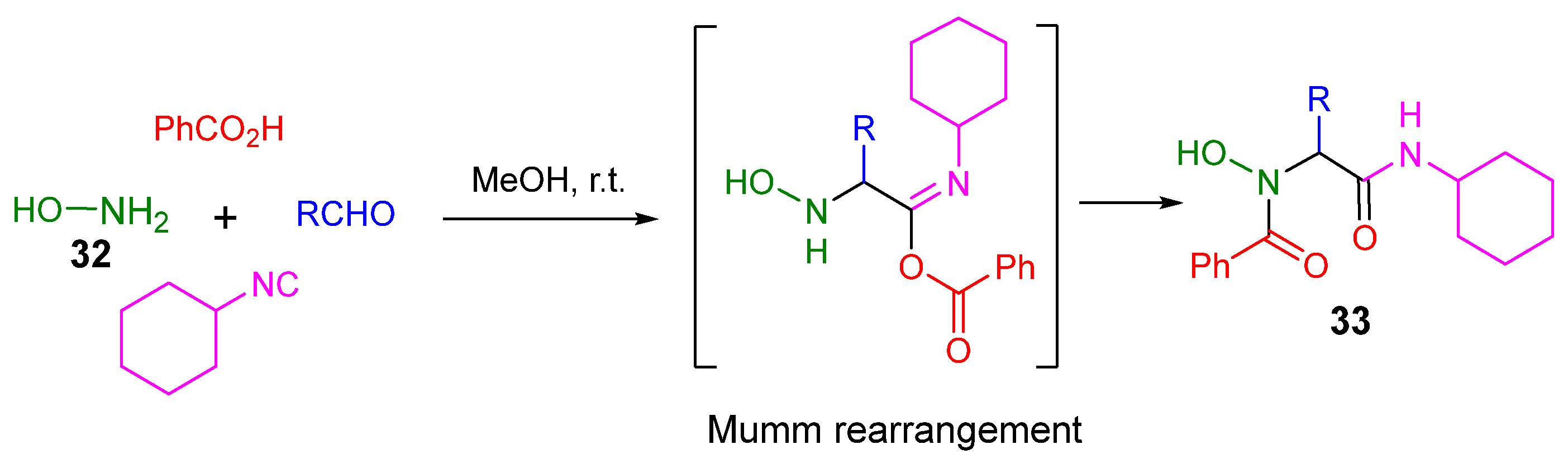
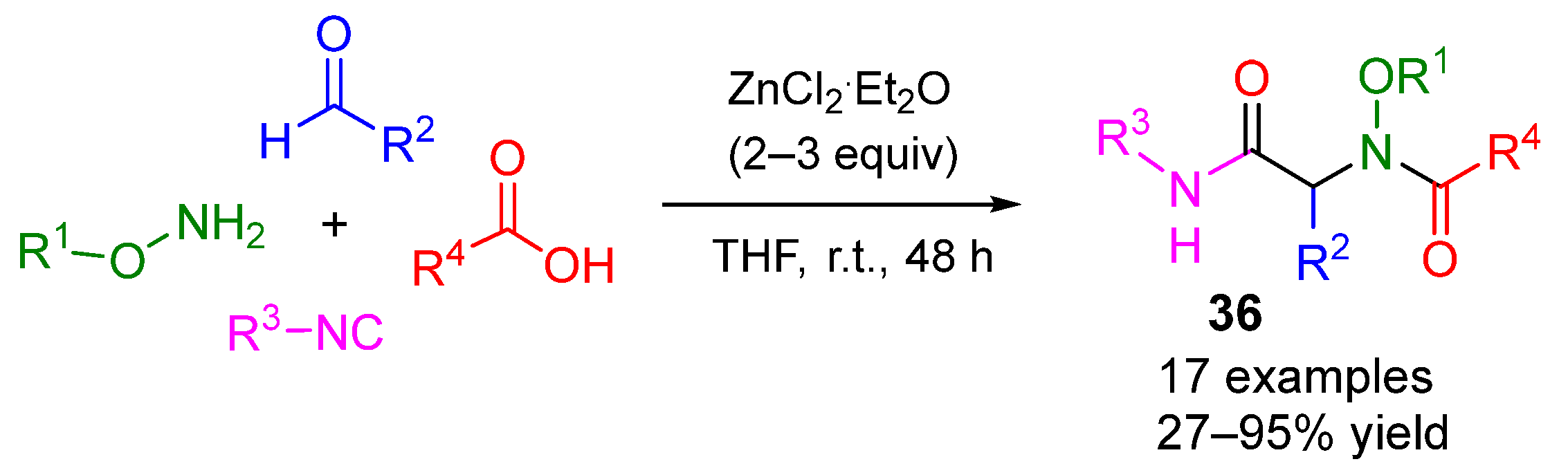
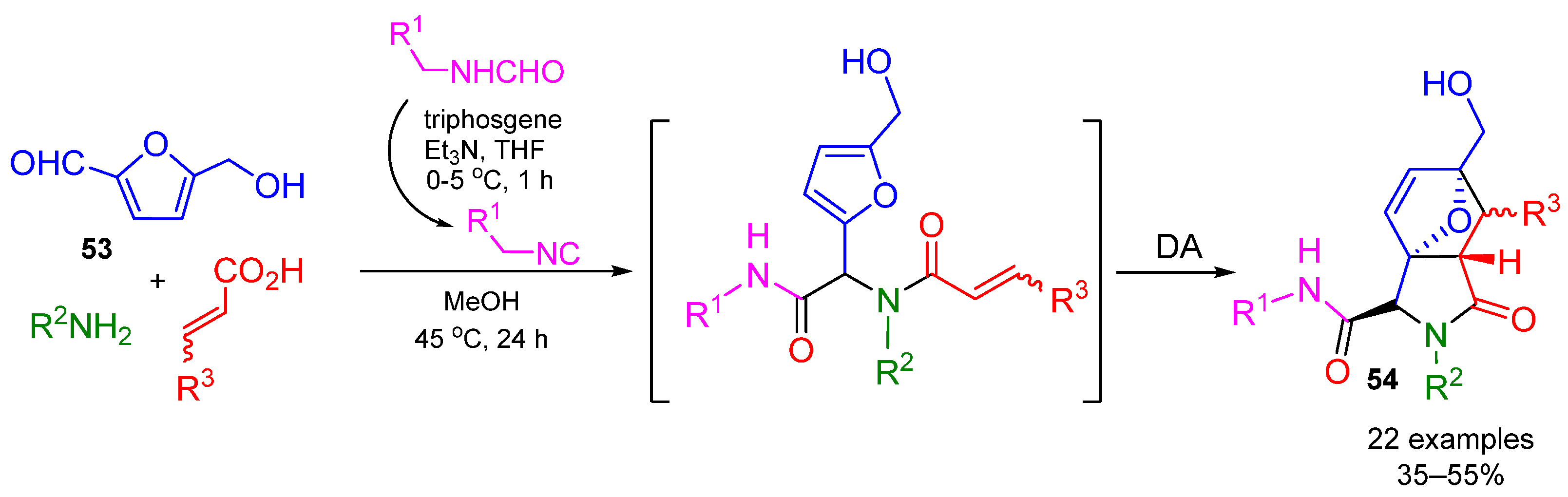
4. Alternative Components for Aldehydes
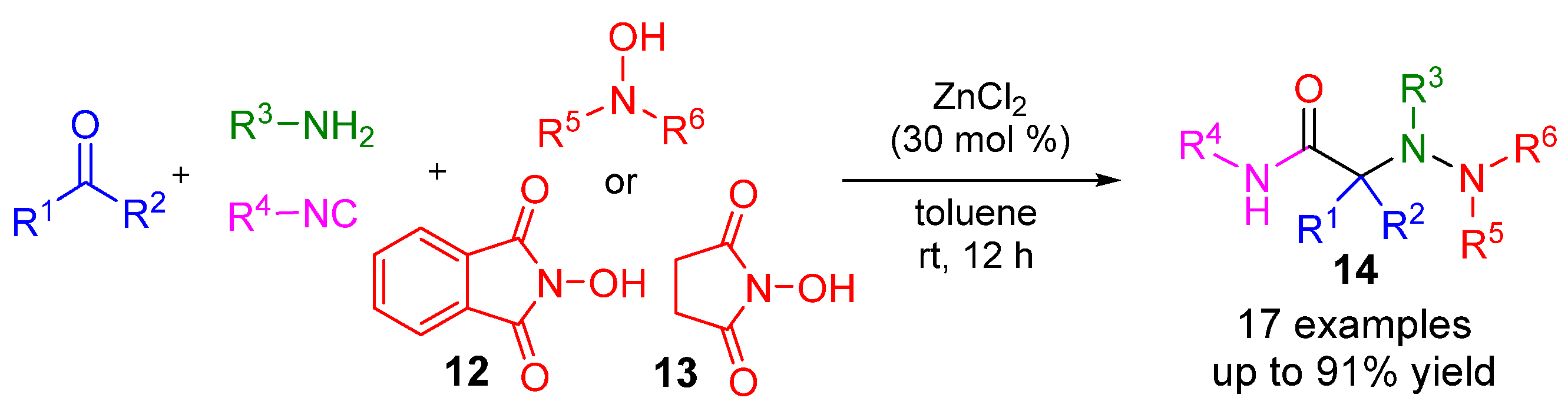
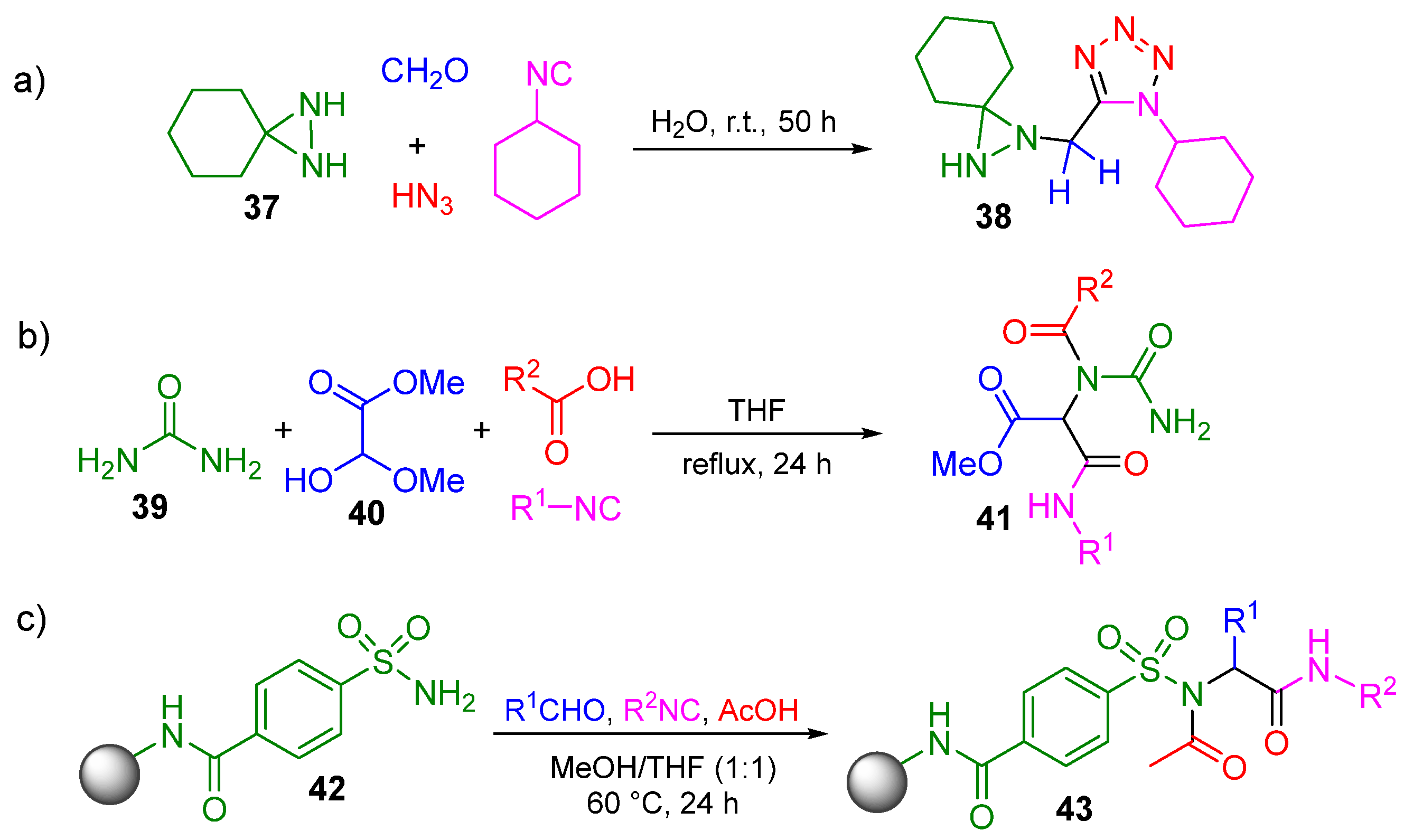
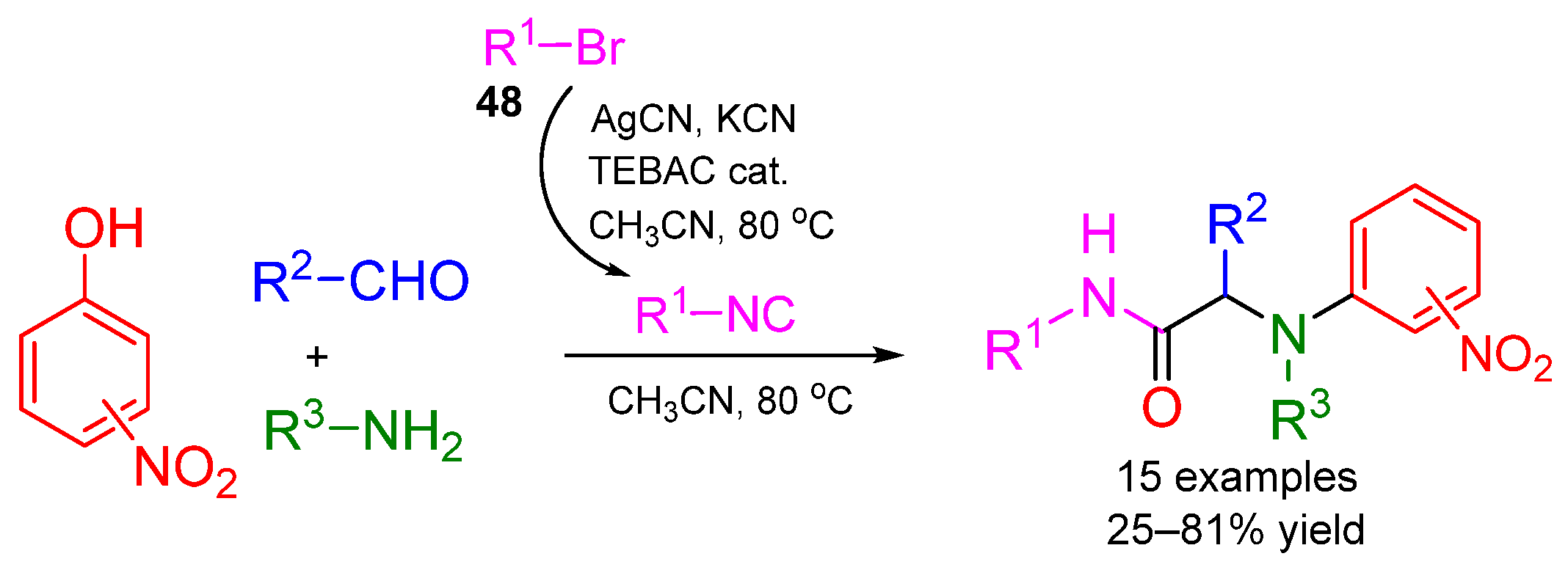
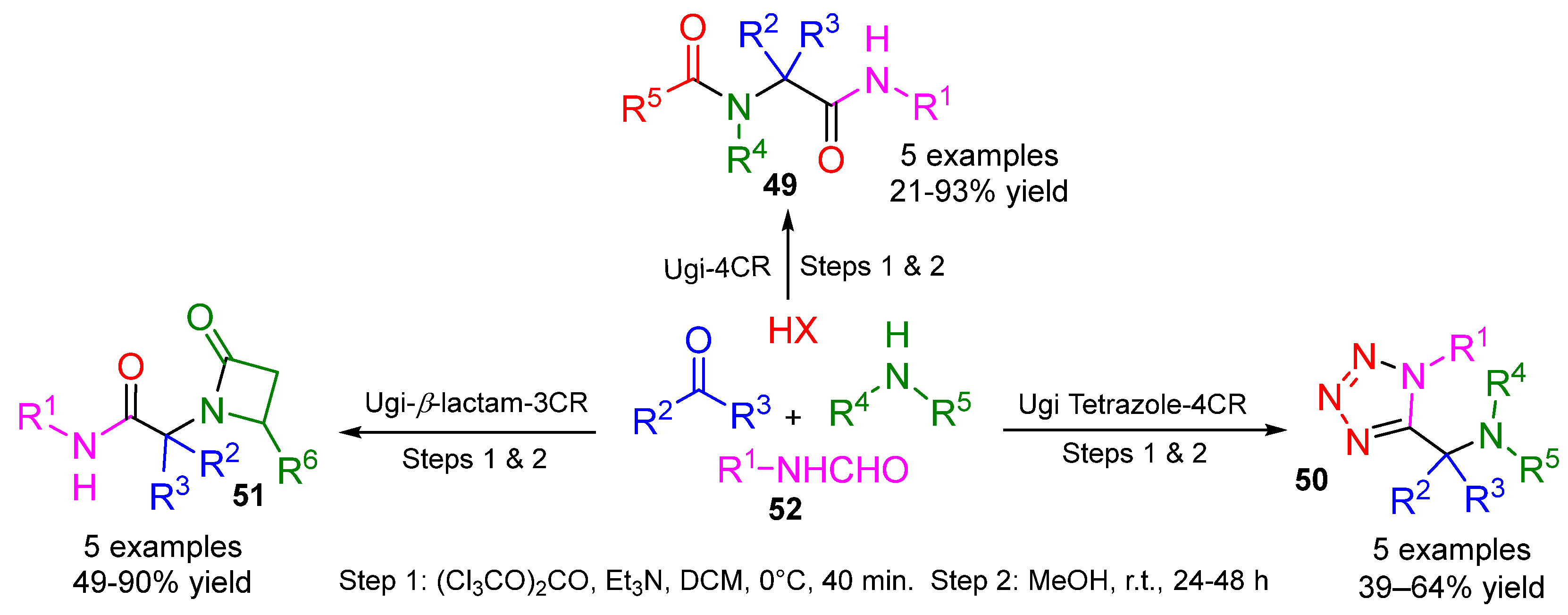
5. Alternative Components for Isocyanides
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhi, S.; Ma, X.; Zhang, W. Consecutive Multicomponent Reactions for the Synthesis of Complex Molecules. Org. Biomol. Chem. 2019, 17, 7632–7650. [Google Scholar] [CrossRef]
- Ma, X.; Zhi, S.; Zhang, W. Recent Developments on Five-Component Reactions. Molecules 2021, 26, 1986. [Google Scholar] [CrossRef]
- Domling, A.; Wang, W.; Wang, K. Chemistry and Biology of Multicomponent Reactions. Chem. Rev. 2012, 112, 3083–3135. [Google Scholar] [CrossRef]
- Hooshmand, S.E.; Yazdani, H.; Hulme, C. Six-Component Reactions and Beyond: The Nuts and Bolts. Eur. J. Org. Chem. 2022, 2022, e202200569. [Google Scholar] [CrossRef]
- Kar, S.; Sanderson, H.; Roy, K.; Benfenati, E.; Leszczynski, J. Green Chemistry in the Synthesis of Pharmaceuticals. Chem. Rev. 2022, 122, 3637–3710. [Google Scholar] [CrossRef]
- Chen, M.-N.; Mo, L.-P.; Cui, Z.-S.; Zhang, Z.-H. Magnetic Nanocatalysts: Synthesis and Application in Multicomponent Reactions. Curr. Opin. Green Sustain. Chem. 2019, 15, 27–37. [Google Scholar] [CrossRef]
- Neto, B.A.D.; Rocha, R.O.; Rodrigues, M.O. Catalytic Approaches to Multicomponent Reactions: A Critical Review and Perspectives on the Roles of Catalysis. Molecules 2021, 27, 132. [Google Scholar] [CrossRef]
- Ruijter, E.; Scheffelaar, R.; Orru, R.V.A. Multicomponent Reaction Design in the Quest for Molecular Complexity and Diversity. Angew. Chem. Int. Ed. 2011, 50, 6234–6246. [Google Scholar] [CrossRef]
- Dömling, A. Innovations and Inventions: Why Was the Ugi Reaction Discovered Only 37 Years after the Passerini Reaction? J. Org. Chem. 2022. [Google Scholar] [CrossRef]
- Ugi, I. Versuche Mit Isonitrilen. Angewandte Chemie-International Edition; Wiley-VCH Verlag GmbH: Berlin, Germany, 1959; Volume 71, p. 386. [Google Scholar]
- Gazzotti, S.; Rainoldi, G.; Silvani, A. Exploitation of the Ugi–Joullié Reaction in Drug Discovery and Development. Expert Opin. Drug Discov. 2019, 14, 639–652. [Google Scholar] [CrossRef]
- Zhang, W. Green Synthesis of Heterocycles Via MCRs. In Multicomponent Reactions towards Heterocycles: Concepts and Applications; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2022; Chapter 5; pp. 163–209. [Google Scholar] [CrossRef]
- Lei, J.; Meng, J.-P.; Tang, D.-Y.; Frett, B.; Chen, Z.-Z.; Xu, Z.-G. Recent Advances in the Development of Polycyclic Skeletons via Ugi Reaction Cascades. Mol. Divers. 2018, 22, 503–516. [Google Scholar] [CrossRef]
- Shaabani, A.; Hooshmand, S.E. Diversity-Oriented Catalyst-Free Synthesis of Pseudopeptides Containing Rhodanine Scaffolds via a One-Pot Sequential Isocyanide-Based Six-Component Reactions in Water Using Ultrasound Irradiation. Ultrason. Sonochem. 2018, 40, 84–90. [Google Scholar] [CrossRef]
- Lambruschini, C.; Moni, L.; Banfi, L. Diastereoselectivity in Passerini Reactions of Chiral Aldehydes and in Ugi Reactions of Chiral Cyclic Imines. Eur. J. Org. Chem. 2020, 2020, 3766–3778. [Google Scholar] [CrossRef]
- Tripolitsiotis, N.P.; Thomaidi, M.; Neochoritis, C.G. The Ugi Three-Component Reaction; a Valuable Tool in Modern Organic Synthesis. Eur. J. Org. Chem. 2020, 2020, 6525–6554. [Google Scholar] [CrossRef]
- Insuasty, D.; Castillo, J.; Becerra, D.; Rojas, H.; Abonia, R. Synthesis of Biologically Active Molecules through Multicomponent Reactions. Molecules 2020, 25, 505. [Google Scholar] [CrossRef]
- Zakharova, E.A.; Shmatova, O.I.; Kutovaya, I.V.; Khrustalev, V.N.; Nenajdenko, V.G. Synthesis of Macrocyclic Peptidomimetics via the Ugi-Click-Strategy. Org. Biomol. Chem. 2019, 17, 3433–3445. [Google Scholar] [CrossRef]
- Yang, B.; Zhao, Y.; Wei, Y.; Fu, C.; Tao, L. The Ugi Reaction in Polymer Chemistry: Syntheses, Applications and Perspectives. Polym. Chem. 2015, 6, 8233–8239. [Google Scholar] [CrossRef]
- Tomohara, K.; Ohashi, N.; Uchida, T.; Nose, T. Synthesis of Natural Product Hybrids by the Ugi Reaction in Complex Media Containing Plant Extracts. Sci. Rep. 2022, 12, 15568. [Google Scholar] [CrossRef]
- Touré, B.B.; Hall, D.G. Natural Product Synthesis Using Multicomponent Reaction Strategies. Chem. Rev. 2009, 109, 4439–4486. [Google Scholar] [CrossRef]
- Fouad, M.A.; Abdel-Hamid, H.; Ayoup, M.S. Two Decades of Recent Advances of Ugi Reactions: Synthetic and Pharmaceutical Applications. RSC Adv. 2020, 10, 42644–42681. [Google Scholar] [CrossRef]
- Nadal Rodríguez, P.; Ghashghaei, O.; Bagán, A.; Escolano, C.; Lavilla, R. Heterocycle-Based Multicomponent Reactions in Drug Discovery: From Hit Finding to Rational Design. Biomedicines 2022, 10, 1488. [Google Scholar] [CrossRef]
- Ruijter, E.; Orru, R.V.A. Multicomponent Reactions—Opportunities for the Pharmaceutical Industry. Drug Discov. Today Technol. 2013, 10, e15–e20. [Google Scholar] [CrossRef]
- Zarganes-Tzitzikas, T.; Neochoritis, C.G.; Dömling, A. Atorvastatin (Lipitor) by MCR. ACS Med. Chem. Lett. 2019, 10, 389–392. [Google Scholar] [CrossRef]
- Afshari, R.; Shaabani, A. Materials Functionalization with Multicomponent Reactions: State of the Art. ACS Comb. Sci. 2018, 20, 499–528. [Google Scholar] [CrossRef]
- Yazdani, H.; Hooshmand, S.E.; Stenzel, M.H. Fusion of Cellulose and Multicomponent Reactions: Benign by Design. ACS Sustain. Chem. Eng. 2022, 10, 4359–4373. [Google Scholar] [CrossRef]
- Sepahvand, H.; Heravi, M.M.; Saber, M.; Hooshmand, S.E. Techniques and Support Materials for Enzyme Immobilization Using Ugi Multicomponent Reaction: An Overview. J. Iran. Chem. Soc. 2021, 19, 2115–2130. [Google Scholar] [CrossRef]
- Shaabani, A.; Afshari, R. Magnetic Ugi-Functionalized Graphene Oxide Complexed with Copper Nanoparticles: Efficient Catalyst toward Ullman Coupling Reaction in Deep Eutectic Solvents. J. Colloid Interface Sci. 2018, 510, 384–394. [Google Scholar] [CrossRef]
- Méndez, Y.; Chang, J.; Humpierre, A.R.; Zanuy, A.; Garrido, R.; Vasco, A.V.; Pedroso, J.; Santana, D.; Rodríguez, L.M.; García-Rivera, D. Multicomponent Polysaccharide–Protein Bioconjugation in the Development of Antibacterial Glycoconjugate Vaccine Candidates. Chem. Sci. 2018, 9, 2581–2588. [Google Scholar] [CrossRef]
- Rezaei, A.; Akhavan, O.; Hashemi, E.; Shamsara, M. Toward Chemical Perfection of Graphene-Based Gene Carrier via Ugi Multicomponent Assembly Process. Biomacromolecules 2016, 17, 2963–2971. [Google Scholar] [CrossRef]
- Reguera, L.; Méndez, Y.; Humpierre, A.R.; Valdés, O.; Rivera, D.G. Multicomponent Reactions in Ligation and Bioconjugation Chemistry. Acc. Chem. Res. 2018, 51, 1475–1486. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, Y.; Liu, G.; Wei, Y.; Wu, Y.; Tao, L. Antibacterial Self-Healing Hydrogel via the Ugi Reaction. ACS Appl. Polym. Mater. 2019, 2, 404–410. [Google Scholar] [CrossRef]
- Humpierre, A.R.; Zanuy, A.; Saenz, M.; Garrido, R.; Vasco, A.V.; Pérez-Nicado, R.; Soroa-Milán, Y.; Santana-Mederos, D.; Westermann, B.; Vérez-Bencomo, V. Expanding the Scope of Ugi Multicomponent Bioconjugation to Produce Pneumococcal Multivalent Glycoconjugates as Vaccine Candidates. Bioconjug. Chem. 2020, 31, 2231–2240. [Google Scholar] [CrossRef]
- Ganem, B. Strategies for Innovation in Multicomponent Reaction Design. Acc. Chem. Res. 2009, 42, 463–472. [Google Scholar] [CrossRef]
- Angélica de Fátima, S.B.; Dos Santos, V.A.; Andrade, C.K.Z. Synthesis of Acylhydrazino-Peptomers, a New Class of Peptidomimetics, by Consecutive Ugi and Hydrazino-Ugi Reactions. Beilstein J. Org. Chem. 2016, 12, 2865–2872. [Google Scholar] [CrossRef]
- Foley, C.; Shaw, A.; Hulme, C. Two-Step Route to Diverse N-Functionalized Peptidomimetic-like Isatins through an Oxidation/Intramolecular Oxidative-Amidation Cascade of Ugi Azide and Ugi Three-Component Reaction Products. Org. Lett. 2016, 18, 4904–4907. [Google Scholar] [CrossRef]
- Sharma, U.K.; Sharma, N.; Vachhani, D.D.; Van der Eycken, E.V. Metal-Mediated Post-Ugi Transformations for the Construction of Diverse Heterocyclic Scaffolds. Chem. Soc. Rev. 2015, 44, 1836–1860. [Google Scholar] [CrossRef]
- Zarganes-Tzitzikas, T.; Chandgude, A.L.; Dömling, A. Multicomponent Reactions, Union of MCRs and Beyond. Chem. Rec. 2015, 15, 981–996. [Google Scholar] [CrossRef]
- Rodrigues, M.O.; Eberlin, M.N.; Neto, B.A.D. How and Why to Investigate Multicomponent Reactions Mechanisms? A Critical Review. Chem. Rec. 2021, 21, 2762–2781. [Google Scholar] [CrossRef]
- Cores, Á.; Clerigué, J.; Orocio-Rodríguez, E.; Menéndez, J.C. Multicomponent Reactions for the Synthesis of Active Pharmaceutical Ingredients. Pharmaceuticals 2022, 15, 9. [Google Scholar] [CrossRef]
- Dömling, A.; Ugi, I. Multicomponent Reactions with Isocyanides. Angew. Chem. Int. Ed. 2000, 39, 3168–3210. [Google Scholar] [CrossRef]
- Neochoritis, C.G.; Zhao, T.; Dömling, A. Tetrazoles via Multicomponent Reactions. Chem. Rev. 2019, 119, 1970–2042. [Google Scholar] [CrossRef]
- Barthelon, A.; El Kaïm, L.; Gizolme, M.; Grimaud, L. Thiols in Ugi- and Passerini–Smiles-Type Couplings. Eur. J. Org. Chem. 2008, 2008, 5974–5987. [Google Scholar] [CrossRef]
- El Kaïm, L.; Grimaud, L. The Ugi–Smiles and Passerini–Smiles Couplings: A Story About Phenols in Isocyanide-Based Multicomponent Reactions. Eur. J. Org. Chem. 2014, 2014, 7749–7762. [Google Scholar] [CrossRef]
- El Kaim, L.; Grimaud, L. Beyond the Ugi Reaction: Less Conventional Interactions between Isocyanides and Iminium Species. Tetrahedron 2009, 65, 2153–2171. [Google Scholar] [CrossRef]
- Pelliccia, S.; Alfano, I.A.; Galli, U.; Novellino, E.; Giustiniano, M.; Tron, G.C. α-Amino Acids as Synthons in the Ugi-5-Centers-4-Components Reaction: Chemistry and Applications. Symmetry 2019, 11, 798. [Google Scholar] [CrossRef]
- Tao, Y.; Tao, Y. Ugi Reaction of Amino Acids: From Facile Synthesis of Polypeptoids to Sequence-Defined Macromolecules. Macromol. Rapid Commun. 2021, 42, 2000515. [Google Scholar] [CrossRef]
- Chandgude, A.L.; Dömling, A. N-Hydroxyimide Ugi Reaction toward α-Hydrazino Amides. Org. Lett. 2017, 19, 1228–1231. [Google Scholar] [CrossRef]
- Mercalli, V.; Nyadanu, A.; Cordier, M.; Tron, G.C.; Grimaud, L.; El Kaim, L. N–N Bond Formation in Ugi Processes: From Nitric Acid to Libraries of Nitramines. Chem. Commun. 2017, 53, 2118–2121. [Google Scholar] [CrossRef]
- Neo, A.G.; Castellano, T.G.; Marcos, C.F. Enol-Ugi Reaction of Hydroxycoumarins: Straightforward Synthesis of Amino Acid Derived Coumarin Enamines. Synthesis 2015, 47, 2431–2438. [Google Scholar] [CrossRef]
- Aknin, K.; Gauriot, M.; Totobenazara, J.; Deguine, N.; Deprez-Poulain, R.; Deprez, B.; Charton, J. Squaric Acid Is a Suitable Building-Block in 4C-Ugi Reaction: Access to Original Bivalent Compounds. Tetrahedron Lett. 2012, 53, 458–461. [Google Scholar] [CrossRef]
- Ramezanpour, S.; Bigdeli, Z.; Alavijeh, N.S.; Rominger, F. Application of Saccharin as an Acidic Partner in the Ugi Reaction for the One-Pot Synthesis of 3-Iminosaccharins. Synlett 2017, 28, 1214–1218. [Google Scholar] [CrossRef]
- Gelman, M.; Massarano, T.; Lavi, R.; Byk, G. A New Multicomponent Reaction MCR4 for the Synthesis of Analogs of Staurosporine. Curr. Org. Chem. 2018, 22, 505–517. [Google Scholar] [CrossRef]
- Massarano, T.; Mazir, A.; Lavi, R.; Byk, G. Solid-Phase Multicomponent Synthesis of 3-Substituted Isoindolinones Generates New Cell-Penetrating Probes as Drug Carriers. ChemMedChem 2020, 15, 833–838. [Google Scholar] [CrossRef]
- Shaabani, A.; Hooshmand, S.E. Isocyanide and Meldrum’s Acid-Based Multicomponent Reactions in Diversity-Oriented Synthesis: From a Serendipitous Discovery towards Valuable Synthetic Approaches. RSC Adv. 2016, 6, 58142–58159. [Google Scholar] [CrossRef]
- Moghaddam, F.M.; Goudarzi, M.; Chamani, F.; Dezag, H.M. A New Mumm-Type Rearrangement with Dithiocarbamates via Isocyanide-Based Multicomponent Reaction under Ultrasound Irradiation: Synthesis of Polysubstituted Pyrrolidine Compounds. New J. Chem. 2020, 44, 9699–9702. [Google Scholar] [CrossRef]
- Janvier, P.; Bienaymé, H.; Zhu, J. A Five-Component Synthesis of Hexasubstituted Benzene. Angew. Chem. Int. Ed. 2002, 41, 4291–4294. [Google Scholar] [CrossRef]
- Zeng, L.; Sajiki, H.; Cui, S. Multicomponent Ugi Reaction of Indole-N-Carboxylic Acids: Expeditious Access to Indole Carboxamide Amino Amides. Org. Lett. 2019, 21, 5269–5272. [Google Scholar] [CrossRef]
- Zinner, G.; Kliegel, W. Zur Kenntnis Der Ugi-Reaktion Mit Hydrazinen, I. Arch. Pharm. 1966, 299, 746–756. [Google Scholar] [CrossRef]
- Krasavin, M.; Bushkova, E.; Parchinsky, V.; Shumsky, A. Hydrazinopeptide Motifs Synthesized via the Ugi Reaction: An Insight into the Secondary Structure. Synthesis 2010, 2010, 933–942. [Google Scholar] [CrossRef]
- Krasavin, M. Amine (Imine) Component Surrogates in the Ugi Reaction and Related Isocyanide-Based Multicomponent Reactions. In Isocyanide Chemistry: Applications in Synthesis and Material Science; John Wiley & Sons: Hoboken, NJ, USA, 2012; Chapter 6; pp. 195–231. [Google Scholar] [CrossRef]
- Bushkova, E.; Parchinsky, V.; Krasavin, M. Efficient Entry into Hydrazinopeptide-like Structures via Sequential Ugi Reactions. Mol. Divers. 2010, 14, 493. [Google Scholar] [CrossRef]
- Tempest, P.; Ma, V.; Kelly, M.G.; Jones, W.; Hulme, C. MCC/SNAr Methodology. Part 1: Novel Access to a Range of Heterocyclic Cores. Tetrahedron Lett. 2001, 42, 4963–4968. [Google Scholar] [CrossRef]
- Patil, P.; Zhang, J.; Kurpiewska, K.; Kalinowska-Tłuścik, J.; Dömling, A. Hydrazine in the Ugi Tetrazole Reaction. Synthesis 2016, 48, 1122–1130. [Google Scholar] [CrossRef]
- Wang, Y.; Patil, P.; Kurpiewska, K.; Kalinowska-Tluscik, J.; Dömling, A. Two Cycles with One Catch: Hydrazine in Ugi 4-CR and Its Postcyclizations. ACS Comb. Sci. 2017, 19, 193–198. [Google Scholar] [CrossRef]
- Angélica de Fátima, S.B.; Dos Santos, V.A.; Andrade, C.K.Z. Consecutive Hydrazino-Ugi-Azide Reactions: Synthesis of Acylhydrazines Bearing 1, 5-Disubstituted Tetrazoles. Beilstein J. Org. Chem. 2017, 13, 2596–2602. [Google Scholar] [CrossRef]
- Zinner, G.; Moderhack, D.; Kliegel, W. Hydroxylamin-Derivate, XXXVII. Hydroxylamine in Der Vierkomponenten-Kondensation Nach Ugi. Chem. Ber. 1969, 102, 2536–2546. [Google Scholar] [CrossRef]
- Moderhack, D. Unerwarteter Verlauf Der Ugi-Reaktion Mit N-Alkylhydroxylaminen. Justus Liebigs Ann. Chem. 1973, 1973, 359–364. [Google Scholar] [CrossRef]
- Zinner, G.; Moderhack, D.; Hantelmann, O.; Bock, W. Hydroxylamine in Der Vierkomponenten-Kondensation Nach Ugi, II. Chem. Ber. 1974, 107, 2947–2955. [Google Scholar] [CrossRef]
- Basso, A.; Banfi, L.; Guanti, G.; Riva, R.; Riu, A. Ugi Multicomponent Reaction with Hydroxylamines: An Efficient Route to Hydroxamic Acid Derivatives. Tetrahedron Lett. 2004, 45, 6109–6111. [Google Scholar] [CrossRef]
- Basso, A.; Banfi, L.; Guanti, G.; Riva, R. One-Pot Synthesis of α-Acyloxyaminoamides via Nitrones as Imine Surrogates in the Ugi MCR. Tetrahedron Lett. 2005, 46, 8003–8006. [Google Scholar] [CrossRef]
- Zinner, G.; Bock, W. Notiz Über Die UGI-Reaktion Mit Diaziridinen. Arch. Pharm. 1973, 306, 94–96. [Google Scholar]
- Zychlinski, A.; Ugi, I. MCR. Part 9. A New and Easy Way for the Preparation of Piperazine-2-keto-carboxamides. Heterocycles 1998, 49, 29–32. [Google Scholar]
- Campian, E.; Lou, B.; Saneii, H. Solid-Phase Synthesis of α-Sulfonylamino Amide Derivatives Based on Ugi-Type Condensation Reaction Using Sulfonamides as Amine Input. Tetrahedron Lett. 2002, 43, 8467–8470. [Google Scholar] [CrossRef]
- Hamzavi, S.F.; Jamili, S.; Yousefzadi, M.; Mashinchian Moradi, A.; Amrollahi Biuki, N. Immobilization of Platinum Nanoparticles on the Functionalized Chitosan Particles: An Efficient Catalyst for Reduction of Nitro Compounds and Tandem Reductive Ugi Reactions. Mol. Divers. 2020, 24, 985–995. [Google Scholar] [CrossRef]
- Rainoldi, G.; Lesma, G.; Picozzi, C.; Presti, L.L.; Silvani, A. One Step Access to Oxindole-Based β-Lactams through Ugi Four-Center Three-Component Reaction. RSC Adv. 2018, 8, 34903–34910. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, M.; Chadha, N.; Silakari, O. Oxindole: A Chemical Prism Carrying Plethora of Therapeutic Benefits. Eur. J. Med. Chem. 2016, 123, 858–894. [Google Scholar] [CrossRef]
- Brandao, P.; Lopez, O.; Leitzbach, L.; Stark, H.; Fernandez-Bolanos, J.G.; Burke, A.J.; Pineiro, M. Ugi Reaction Synthesis of Oxindole–Lactam Hybrids as Selective Butyrylcholinesterase Inhibitors. ACS Med. Chem. Lett. 2021, 12, 1718–1725. [Google Scholar] [CrossRef]
- Brandão, P.; Puerta, A.; Padrón, J.M.; Kuznetsov, M.L.; Burke, A.J.; Pineiro, M. Ugi Adducts of Isatin as Promising Antiproliferative Agents with Druglike Properties. Asian J. Org. Chem. 2021, 10, 3434–3455. [Google Scholar] [CrossRef]
- Liu, J.; Yang, T.; Wang, Z.-P.; Wang, P.-L.; Feng, J.; Ding, S.-Y.; Wang, W. Pyrimidazole-Based Covalent Organic Frameworks: Integrating Functionality and Ultrastability via Isocyanide Chemistry. J. Am. Chem. Soc. 2020, 142, 20956–20961. [Google Scholar] [CrossRef]
- Kaur, T.; Wadhwa, P.; Bagchi, S.; Sharma, A. Isocyanide Based [4+1] Cycloaddition Reactions: An Indispensable Tool in Multi-Component Reactions (MCRs). Chem. Commun. 2016, 52, 6958–6976. [Google Scholar] [CrossRef]
- Shaabani, A.; Mohammadian, R.; Afshari, R.; Hooshmand, S.E.; Nazeri, M.T.; Javanbakht, S. The Status of Isocyanide-Based Multi-Component Reactions in Iran (2010–2018). Mol. Divers. 2021, 25, 1145–1210. [Google Scholar] [CrossRef]
- Eckert, H. Diversity Oriented Syntheses of Conventional Heterocycles by Smart Multi Component Reactions (MCRs) of the Last Decade. Molecules 2012, 17, 1074–1102. [Google Scholar] [CrossRef]
- Ashjari, M.; Garmroodi, M.; Ahrari, F.; Yousefi, M.; Mohammadi, M. Soluble Enzyme Cross-Linking via Multi-Component Reactions: A New Generation of Cross-Linked Enzymes. Chem. Commun. 2020, 56, 9683–9686. [Google Scholar] [CrossRef]
- Collet, J.W.; Roose, T.R.; Ruijter, E.; Maes, B.U.W.; Orru, R.V.A. Base Metal Catalyzed Isocyanide Insertions. Angew. Chem. Int. Ed. 2020, 59, 540–558. [Google Scholar] [CrossRef]
- Váradi, A.; Palmer, T.C.; Notis Dardashti, R.; Majumdar, S. Isocyanide-Based Multicomponent Reactions for the Synthesis of Heterocycles. Molecules 2015, 21, 19. [Google Scholar] [CrossRef]
- Bhat, S.I.; Kigga, M.; Heravi, M.M. Multicomponent Reactions Based on In Situ Generated Isocyanides for the Construction of Heterocycles. Chem. Heterocycl. Compd. 2021, 57, 709–719. [Google Scholar] [CrossRef]
- El Kaim, L.; Grimaud, L.; Schiltz, A. Isocyanide-Based Multicomponent Reaction ‘without’Isocyanides. Synlett 2009, 2009, 1401–1404. [Google Scholar] [CrossRef]
- El Kaïm, L.; Grimaud, L.; Schiltz, A. “Isocyanide-Free” Ugi Reactions. Org. Biomol. Chem. 2009, 7, 3024–3026. [Google Scholar] [CrossRef]
- Sharma, S.; Maurya, R.A.; Min, K.; Jeong, G.; Kim, D. Odorless Isocyanide Chemistry: An Integrated Microfluidic System for a Multistep Reaction Sequence. Angew. Chem. Int. Ed. 2013, 52, 7564–7568. [Google Scholar] [CrossRef]
- Neochoritis, C.G.; Stotani, S.; Mishra, B.; Dömling, A. Efficient Isocyanide-Less Isocyanide-Based Multicomponent Reactions. Org. Lett. 2015, 17, 2002–2005. [Google Scholar] [CrossRef]
- Neochoritis, C.G.; Zarganes-Tzitzikas, T.; Stotani, S.; Domling, A.; Herdtweck, E.; Khoury, K.; Dömling, A. Leuckart–Wallach Route toward Isocyanides and Some Applications. ACS Comb. Sci. 2015, 17, 493–499. [Google Scholar] [CrossRef]
- Golubev, P.; Pankova, A.; Krasavin, M. “Isocyanide-Less” Ugi/Intramolecular Diels-Alder Reaction of 5-Hydroxymethylfurfural. Tetrahedron Lett. 2019, 60, 1578–1581. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hooshmand, S.E.; Zhang, W. Ugi Four-Component Reactions Using Alternative Reactants. Molecules 2023, 28, 1642. https://doi.org/10.3390/molecules28041642
Hooshmand SE, Zhang W. Ugi Four-Component Reactions Using Alternative Reactants. Molecules. 2023; 28(4):1642. https://doi.org/10.3390/molecules28041642
Chicago/Turabian StyleHooshmand, Seyyed Emad, and Wei Zhang. 2023. "Ugi Four-Component Reactions Using Alternative Reactants" Molecules 28, no. 4: 1642. https://doi.org/10.3390/molecules28041642
APA StyleHooshmand, S. E., & Zhang, W. (2023). Ugi Four-Component Reactions Using Alternative Reactants. Molecules, 28(4), 1642. https://doi.org/10.3390/molecules28041642