Protective Effects of Omega-3 Supplementation against Doxorubicin-Induced Deleterious Effects on the Liver and Kidneys of Rats
Abstract
1. Introduction
2. Results
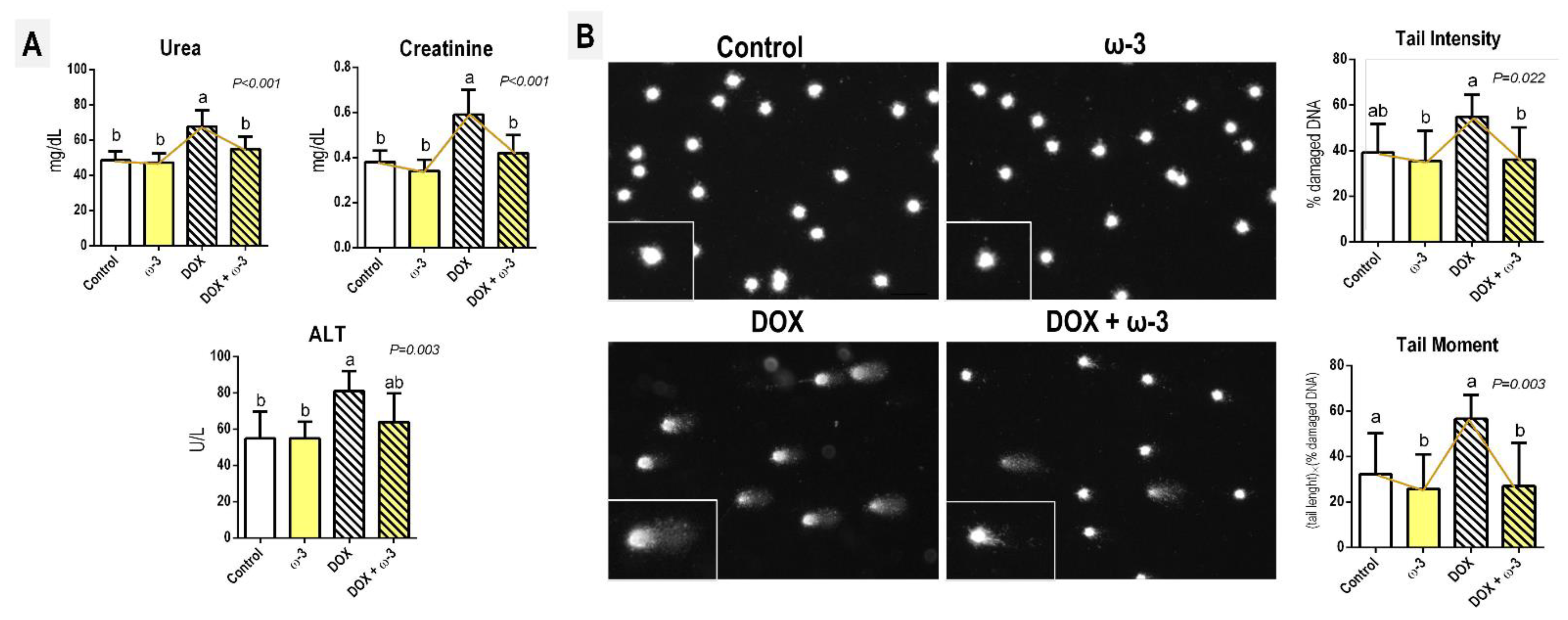
2.1. The ω-3 Supplementation Attenuates a DOX-Induced Increase in Kidney Damage Markers
2.2. The ω-3 Supplementation Attenuates a DOX-Induced Increase in Blood Genotoxicity
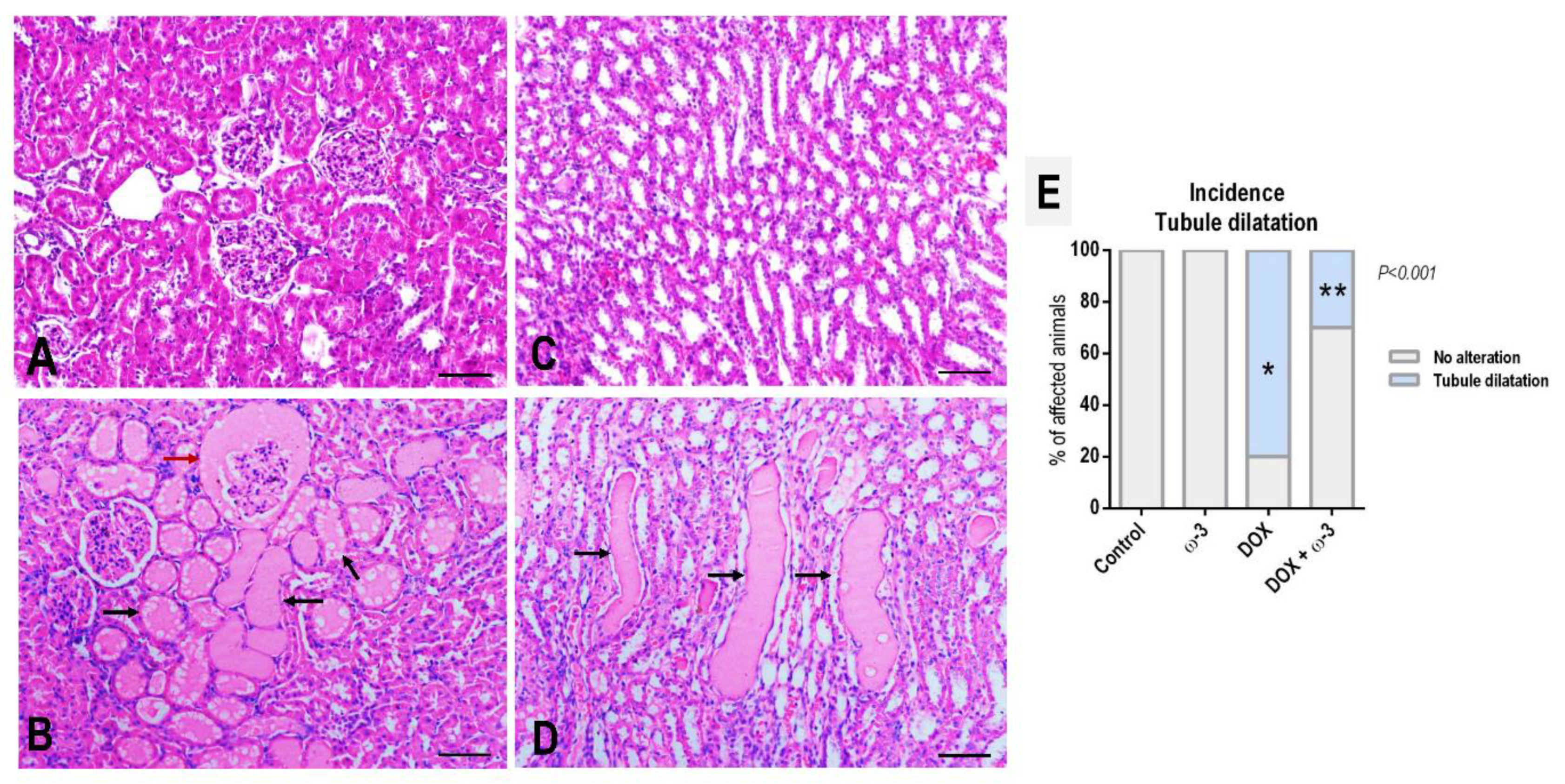
2.3. The ω-3 Supplementation Reduced DOX-Induced Kidney Tubule Dilatation
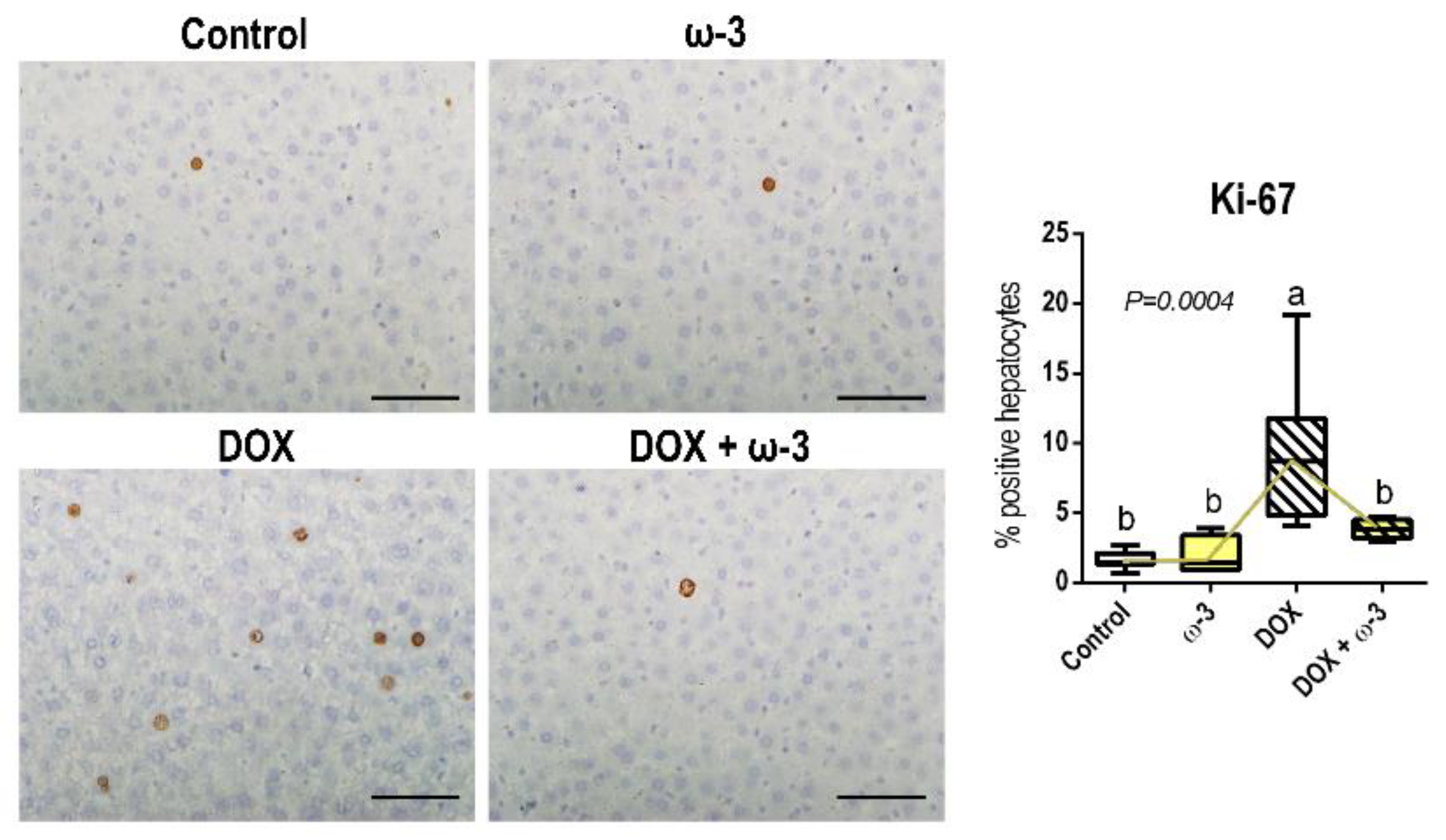
2.4. The ω-3 Supplementation Reduced a DOX-Induced Increase in Hepatocyte Proliferation
2.5. The ω-3 Supplementation Reduced a DOX-Induced Increase in Hepatic p65 Protein
3. Discussion
4. Materials and Methods
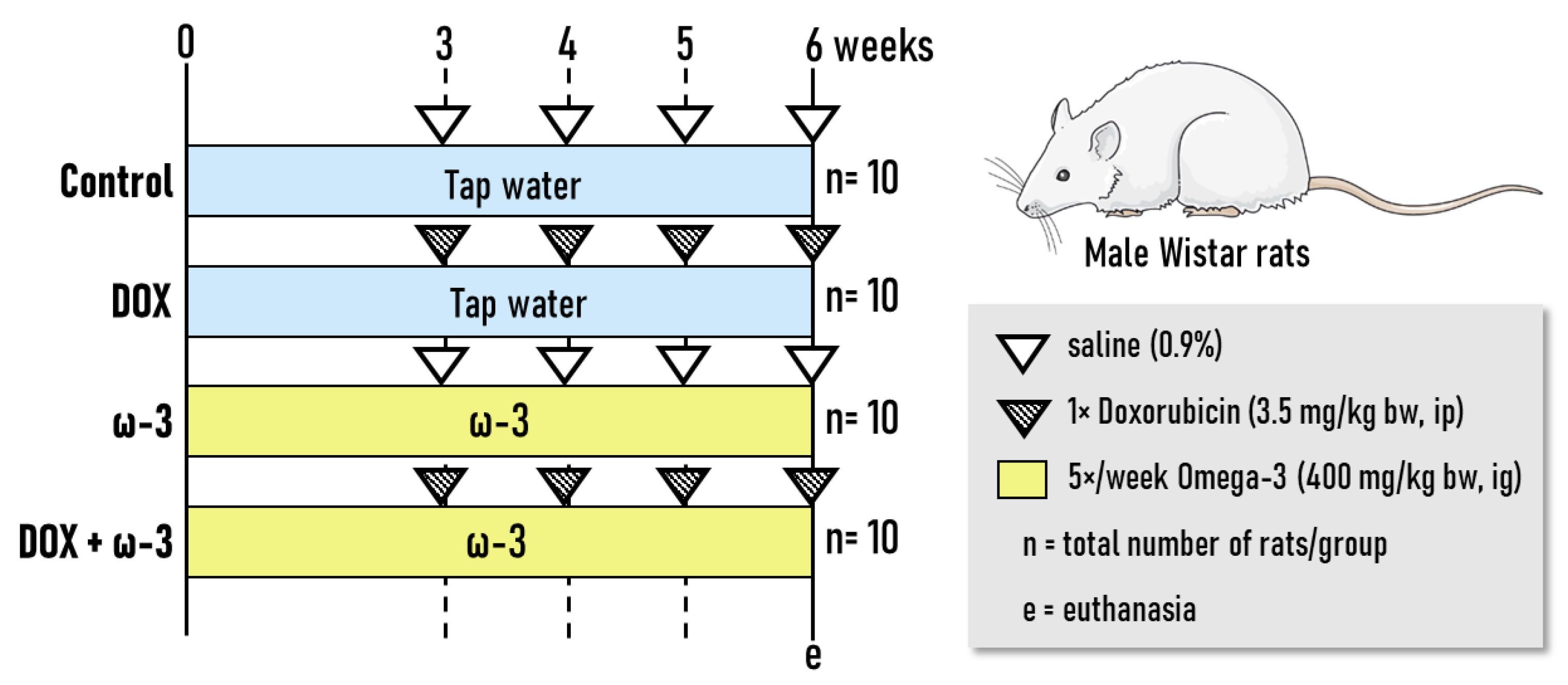
4.1. Experimental Design
4.2. Genotoxicity Assessment
4.3. ALT, Creatinine and Urea Serum Determination
4.4. Histopathological Evaluation and Collagen Morphometry
4.5. Immunohistochemistry for Ki-67
4.6. Immunoblot
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- World Health Organization (WHO). Global Health Estimates 2020: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2019. WHO. 2020. Available online: who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death (accessed on 20 November 2021).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Volkova, M.; Russell, R. Anthracycline Cardiotoxicity: Prevalence, Pathogenesis and Treatment. Curr. Cardiol. Rev. 2012, 7, 214–220. [Google Scholar] [CrossRef]
- van der Zanden, S.Y.; Qiao, X.; Neefjes, J. New insights into the activities and toxicities of the old anticancer drug doxorubicin. FEBS J. 2021, 288, 6095–6111. [Google Scholar] [CrossRef]
- Swain, S.M.; Whaley, F.S.; Ewer, M.S. Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials. Cancer 2003, 97, 2869–2879. [Google Scholar] [CrossRef]
- Damodar, G.; Smitha, T.; Gopinath, S.; Vijayakumar, S.; Rao, Y. An evaluation of hepatotoxicity in breast cancer patients receiving injection doxorubicin. Ann. Med. Health Sci. Res. 2014, 4, 74. [Google Scholar] [CrossRef] [PubMed]
- Yemm, K.E.; Alwan, L.M.; Malik, A.B.; Salazar, L.G. Renal toxicity with liposomal doxorubicin in metastatic breast cancer. J. Oncol. Pharm. Pract. 2019, 25, 1738–1742. [Google Scholar] [CrossRef] [PubMed]
- Kameo, S.Y.; Ramos, M.J.O.; Lima, R.B.; Amorim, B.F.; Costa, J.D.S.; Marinho, P.M.L.; Sawada, N.O.; Silva, G.M. Hematotoxicity and functional impacts related to chemotherapy with doxorubicin and cyclophosphamide for invasive ductal breast carcinoma: A study in clinical records. J. Health Biol. Sci. 2021, 9, 1. [Google Scholar] [CrossRef]
- Sleijfer, S.; Rizzo, E.; Litière, S.; Mathijssen, R.H.J.; Judson, I.R.; Gelderblom, H.; Van Der Graaf, W.T.A.; Gronchi, A. Predictors for doxorubicin-induced hematological toxicity and its association with outcome in advanced soft tissue sarcoma patients; a retrospective analysis of the EORTC-soft tissue and bone sarcoma group database. Acta Oncol. 2018, 57, 1117–1126. [Google Scholar] [CrossRef]
- Schein, C.F.; Marques, A.R.; Vargas, C.L.; Kirsten, V.R. Efeitos colaterais da quimioterapia em pacientes oncológicos hospitalizados. Discip. Sci. Saúde 2006, 7, 101–107. [Google Scholar]
- Sun, X.P.; Wan, L.L.; Yang, Q.J.; Huo, Y.; Han, Y.L.; Guo, C. Scutellarin protects against doxorubicin-induced acute cardiotoxicity and regulates its accumulation in the heart. Arch. Pharm. Res. 2017, 40, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Jacevic, V.; Djordjevic, A.; Srdjenovic, B.; Milic-Tores, V.; Segrt, Z.; Dragojevic-Simic, V.; Kuca, K. Fullerenol nanoparticles prevents doxorubicin-induced acute hepatotoxicity in rats. Exp. Mol. Pathol. 2017, 102, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Ye, J.; Qin, Z.; Ding, X. Protective effects of madecassoside against Doxorubicin-induced nephrotoxicity in vivo and in vitro. Sci. Rep. 2015, 5, 18314. [Google Scholar] [CrossRef] [PubMed]
- Pugazhendhi, A.; Edison, T.N.J.I.; Velmurugan, B.K.; Jacob, J.A.; Karuppusamy, I. Toxicity of Doxorubicin (Dox) to different experimental organ systems. Life Sci. 2018, 200, 26–30. [Google Scholar] [CrossRef]
- Varela-López, A.; Battino, M.; Navarro-Hortal, M.D.; Giampieri, F.; Forbes-Hernández, T.Y.; Romero-Márquez, J.M.; Collado, R.; Quiles, J.L. An update on the mechanisms related to cell death and toxicity of doxorubicin and the protective role of nutrients. Food Chem. Toxicol. 2019, 134, 110834. [Google Scholar] [CrossRef]
- DeFilippis, A.P.; Blaha, M.J.; Jacobson, T.A. Omega-3 fatty acids for cardiovascular disease prevention. Curr. Treat. Options Cardiovasc. Med. 2010, 12, 365–380. [Google Scholar] [CrossRef]
- Calder, P.C. Mechanisms of action of (n-3) fatty acids. J. Nutr. 2012, 142, 592S–599S. [Google Scholar] [CrossRef]
- Teng, L.; Shao, L.; Zhao, Y.; Yu, X.; Zhang, D.; Zhang, H. The beneficial effect of n-3 polyunsaturated fatty acids on doxorubicin-induced chronic heart failure in rats. J. Int. Med. Res. 2010, 38, 940–948. [Google Scholar] [CrossRef]
- Uygur, R.; Aktas, C.; Tulubas, F.; Alpsoy, S.; Topcu, B.; Ozen, O.A. Cardioprotective effects of fish omega-3 fatty acids on doxorubicin-induced cardiotoxicity in rats. Hum. Exp. Toxicol. 2014, 33, 435–445. [Google Scholar] [CrossRef]
- Tulubas, F.; Gurel, A.; Oran, M.; Topcu, B.; Caglar, V.; Uygur, E. The protective effects of ω-3 fatty acids on doxorubicin-induced hepatotoxicity and nephrotoxicity in rats. Toxicol. Ind. Health 2015, 31, 638–644. [Google Scholar] [CrossRef]
- Lódi, M.; Bánhegyi, V.; Bódi, B.; Gyöngyösi, A.; Kovács, Á.; Árokszállási, A.; Hamdani, N.; Fagyas, M.; Édes, I.; Csanádi, Z.; et al. Prophylactic, single-drug cardioprotection in a comparative, experimental study of doxorubicin-induced cardiomyopathy. J. Transl. Med. 2020, 18, 470. [Google Scholar] [CrossRef] [PubMed]
- Frazier, K.S.; Seely, J.C.; Hard, G.C.; Betton, G.; Burnett, R.; Nakatsuji, S.; Nishikawa, A.; Durchfeld-Meyer, B.; Bube, A. Proliferative and Nonproliferative Lesions of the Rat and Mouse Urinary System. Toxicol. Pathol. 2012, 40, 14S–86S. [Google Scholar] [CrossRef] [PubMed]
- Litterst, C.L.; Collins, J.M.; Lowe, M.C.; Arnold, S.T.; Powell, D.M.; Guarino, A.M. Local and systemic toxicity resulting from large-volume ip administration of doxorubicin in the rat. Cancer Treat. Rep. 1982, 66, 157–161. [Google Scholar] [PubMed]
- Nejak-Bowen, K.; Moghe, A.; Cornuet, P.; Preziosi, M.; Nagarajan, S.; Monga, S.P. Role and regulation of p65/β-catenin association during liver injury and regeneration: A “complex” relationship. Gene Expr. 2017, 17, 219–235. [Google Scholar] [CrossRef] [PubMed]
- Adıyaman, M.Ş.; Adıyaman, Ö.A.; Dağlı, A.F.; Karahan, M.Z.; Kaya, İ.; Dağlı, M.N. Effects of grapeseed extract on doxorubicin-induced cardiotoxicity in rats. Herz 2021, 46, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, D.; Ozturk, E.; Kaymak, E.; Akin, A.T.; Yakan, B. Thymoquinone attenuates doxorubicin-cardiotoxicity in rats. J. Biochem. Mol. Toxicol. 2021, 35, e22618. [Google Scholar] [CrossRef]
- Ibrahim Fouad, G.; Ahmed, K.A. The protective impact of berberine against doxorubicin-induced nephrotoxicity in rats. Tissue Cell 2021, 73, 101612. [Google Scholar] [CrossRef]
- Verschoor, A.J.; Litière, S.; Marréaud, S.; Judson, I.; Toulmonde, M.; Wardelmann, E.; LeCesne, A.; Gelderblom, H. Survival of soft tissue sarcoma patients after completing six cycles of first-line anthracycline containing treatment: An EORTC-STBSG database study. Clin. Sarcoma Res. 2020, 10, 18. [Google Scholar] [CrossRef]
- Baltali, E.; Altundaǧ, M.K.; Güler, N.; Özişik, Y.; Firat, D.; Baran, I.; Tekuzman, G. Paclitaxel and doxorubicin combination in the first-line treatment of metastatic breast cancer. Tumori 2002, 88, 200–203. [Google Scholar] [CrossRef]
- Sangweni, N.F.; Gabuza, K.; Huisamen, B.; Mabasa, L.; van Vuuren, D.; Johnson, R. Molecular insights into the pathophysiology of doxorubicin-induced cardiotoxicity: A graphical representation. Arch. Toxicol. 2022, 96, 1541–1550. [Google Scholar] [CrossRef]
- Shi, S.; Chen, Y.; Luo, Z.; Nie, G.; Dai, Y. Role of oxidative stress and inflammation-related signaling pathways in doxorubicin-induced cardiomyopathy. Cell Commun. Signal 2023, 21, 61. [Google Scholar] [CrossRef] [PubMed]
- Sheibani, M.; Azizi, Y.; Shayan, M.; Nezamoleslami, S.; Eslami, F.; Farjoo, M.H.; Dehpour, A.R. Doxorubicin-Induced Cardiotoxicity: An Overview on Pre-clinical Therapeutic Approaches. Cardiovasc. Toxicol. 2022, 22, 292–310. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S.; Lingappa, N.; Mayrovitz, H. Potential Therapeutic Treatments for Doxorubicin-Induced Cardiomyopathy. Cureus 2022, 14, e21154. [Google Scholar] [CrossRef] [PubMed]
- Moini Jazani, A.; Arabzadeh, A.; Haghi-Aminjan, H.; Nasimi Doost Azgomi, R. The role of ginseng derivatives against chemotherapy-induced cardiotoxicity: A systematic review of non-clinical studies. Front. Cardiovasc. Med. 2023, 10, 1022360. [Google Scholar] [CrossRef]
- Sobiborowicz-Sadowska, A.M.; Kamińska, K.; Cudnoch-Jędrzejewska, A. Neprilysin Inhibition in the Prevention of Anthracycline-Induced Cardiotoxicity. Cancers 2023, 15, 312. [Google Scholar] [CrossRef]
- Favreau-Lessard, A.J.; Blaszyk, H.; Jones, M.A.; Sawyer, D.B.; Pinz, I.M. Systemic and cardiac susceptibility of immune compromised mice to doxorubicin. Cardio-Oncology 2019, 5, 2. [Google Scholar] [CrossRef]
- Vrignaud, P.; Londos-Gagliardi, D.; Robert, J. Hepatic metabolism of doxorubicin in mice and rats. Eur. J. Drug Metab. Pharmacokinet. 1986, 11, 101–105. [Google Scholar] [CrossRef]
- Ballet, F.; Vrignaud, P.; Robert, J.; Rey, C.; Poupon, R. Hepatic extraction, metabolism and biliary excretion of doxorubicin in the isolated prefused rat liver. Cancer Chemother. Pharmacol. 1987, 19, 240–245. [Google Scholar] [CrossRef]
- Hilmer, S.N.; Cogger, V.C.; Muller, M.; Couteur, D.G. The hepatic pharmacokinetics of doxorubicin and liposomal doxorubicin. Drug Metab. Disp. 2004, 32, 794–799. [Google Scholar] [CrossRef]
- Prasanna, P.L.; Renu, K.; Valsala Gopalakrishnan, A. New molecular and biochemical insights of doxorubicin-induced hepatotoxicity. Life Sci. 2020, 250, 117599. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, C.; Fan, S.; Wu, S.; Yang, F.; Fang, Z.; Fu, H.; Li, Y. Omega-3 polyunsaturated fatty acid attenuates the inflammatory response by modulating microglia polarization through SIRT1-mediated deacetylation of the HMGB1/NF-ΚB pathway following experimental traumatic brain injury. J. Neuroinflamm. 2018, 15, 116. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yang, B.; Ji, R.; Xu, W.; Mai, K.; Ai, Q. Omega-3 polyunsaturated fatty acids alleviate hepatic steatosis-induced inflammation through Sirt1-mediated nuclear translocation of NF-κB p65 subunit in hepatocytes of large yellow croaker (Larmichthys crocea). Fish Shellfish Immunol. 2017, 71, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Harahap, Y.; Ardiningsih, P.; Winarti, A.C.; Purwanto, D.J. Analysis of the doxorubicin and doxorubicinol in the plasma of breast cancer patients for monitoring the toxicity of doxorubicin. Drug Des. Dev. Ther. 2020, 14, 3469–3475. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Zhai, J.; Hu, T.; Gao, H.; Tao, L.; Zhang, Y.; Song, Y.; Zhang, S. Dioscorea bulbifera L. delays the excretion of doxorubicin and aggravates doxorubicin-induced cardiotoxicity and nephrotoxicity by inhibiting the expression of P-glycoprotein in mice liver and kidney. Xenobiotica 2019, 49, 1116–1125. [Google Scholar] [CrossRef]
- Saad, S.Y.; Najjar, T.A.; Al-Rikabi, A.C. The preventive role of deferoxamine against acute doxorubicin-induced cardiac, renal and hepatic toxicity in rats. Pharmacol. Res. 2001, 43, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fan, P.; Zhu, L.; Zhuang, W.; Jiang, L.; Zhang, H.; Huang, H. Enhanced in vitro antitumor efficacy of a polyunsaturated fatty acid-conjugated pH-responsive self-assembled ion-pairing liposome-encapsulated prodrug. Nanotechnology 2020, 31, 155101. [Google Scholar] [CrossRef]
- Xue, H.; Ren, W.; Denkinger, M.; Schlotzer, W.; Wischmeyer, P.E. Nutritional modulation of cardiotoxicity and anticancer efficacy related to doxorubicin chemotherapy by glutamine and ω-3 polyunsaturated fatty acids. J. Parenter Enteral Nutr. 2015, 40, 52–66. [Google Scholar] [CrossRef]
- Celik Samanci, T.; Gökcimen, A.; Kilic Eren, M.; Gürses, K.M.; Pilevneli, H.; Kuyucu, Y. Effects of bone marrow-derived mesenchymal stem cells on doxorubicin-induced liver injury in rats. J. Biochem. Mol. Toxicol. 2022, 36, e22985. [Google Scholar] [CrossRef] [PubMed]
- Spivak, M.; Bubnov, R.; Yemets, I.; Lazarenko, L.; Timoshok, N.; Vorobieva, A.; Mohnatyy, S.; Ulberg, Z.; Reznichenko, L.; Grusina, T.; et al. Doxorubicin dose for congestive heart failure modeling and the use of general ultrasound equipment for evaluation in rats. Longitudinal in vivo study. Med. Ultrason. 2013, 15, 23–28. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The arrive guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.C.; Sasaki, Y.F. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Kumaravel, T.S.; Jha, A.N. Reliable Comet assay measurements for detecting DNA damage induced by ionising radiation and chemicals. Mutat. Res. 2006, 605, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Thoolen, B.; Ten Kate, F.J.W.; van Diest, P.J.; Malarkey, D.E.; Elmore, S.A.; Maronpot, R.R. Comparative histomorphological review of rat and human hepatocellular proliferative lesions. J. Toxicol. Pathol. 2012, 25, 189–199. [Google Scholar] [CrossRef]


| Parameters | Groups/Treatments | p Value | |||
|---|---|---|---|---|---|
| Control | ω-3 | DOX | DOX + ω-3 | ||
| N | 10 | 10 | 10 | 10 | |
| Initial (g) | 279.18 ± 25.47 | 278.1 ± 22.23 | 280.67 ± 30.56 | 279.5 ± 24.55 | =0.997 |
| Final (g) | 412.04 ± 40.29 a | 416.05 ± 36.52 a | 283.21 ± 43.07 b | 314.9 ± 30.91 b | <0.001 |
| Gain (g) | 132.85 ± 19.38 a | 137.95 ± 17.28 a | 1.11 ± 37.2 b | 35.4 ± 27.28 b | <0.001 |
| Absolute liver weight (g) | 13.69 ± 1.14 a | 14.26 ± 1.68 a | 10.84 ± 2.34 b | 13.11 ± 1.13 a | <0.001 |
| Relative liver weight (%) | 3.33 ± 0.15 a | 3.42 ± 0.25 a | 3.81 ± 0.46 b | 4.18 ± 0.36 b | <0.001 |
| Absolute right kidney weight (g) | 1.44 ± 0.11 | 1.35 ± 0.14 | 1.23 ± 0.34 | 1.24 ± 0.21 | =0.107 |
| Relative right kidney weight (%) | 0.35 ± 0.04 a | 0.32 ± 0.02 a | 0.44 ± 0.10 b | 0.39 ± 0.05 ab | <0.001 |
| Absolute left kidney weight (g) | 1.46 ± 0.15 a | 1.34 ± 0.14 ab | 1.15 ± 0.47 b | 1.26 ± 0.19 ab | =0.027 |
| Relative left kidney weight (%) | 0.35 ± 0.03 a | 0.32 ± 0.02 a | 0.41 ± 0.15 b | 0.40 ± 0.05 b | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espírito Santo, S.G.; Monte, M.G.; Polegato, B.F.; Barbisan, L.F.; Romualdo, G.R. Protective Effects of Omega-3 Supplementation against Doxorubicin-Induced Deleterious Effects on the Liver and Kidneys of Rats. Molecules 2023, 28, 3004. https://doi.org/10.3390/molecules28073004
Espírito Santo SG, Monte MG, Polegato BF, Barbisan LF, Romualdo GR. Protective Effects of Omega-3 Supplementation against Doxorubicin-Induced Deleterious Effects on the Liver and Kidneys of Rats. Molecules. 2023; 28(7):3004. https://doi.org/10.3390/molecules28073004
Chicago/Turabian StyleEspírito Santo, Sara Gomes, Marina Gaiato Monte, Bertha Furlan Polegato, Luís Fernando Barbisan, and Guilherme Ribeiro Romualdo. 2023. "Protective Effects of Omega-3 Supplementation against Doxorubicin-Induced Deleterious Effects on the Liver and Kidneys of Rats" Molecules 28, no. 7: 3004. https://doi.org/10.3390/molecules28073004
APA StyleEspírito Santo, S. G., Monte, M. G., Polegato, B. F., Barbisan, L. F., & Romualdo, G. R. (2023). Protective Effects of Omega-3 Supplementation against Doxorubicin-Induced Deleterious Effects on the Liver and Kidneys of Rats. Molecules, 28(7), 3004. https://doi.org/10.3390/molecules28073004








