Abstract
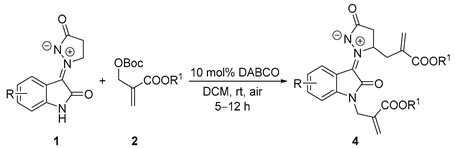
Allylation of N-unsubstituted isatin N,N′-cyclic azomethine imines with Morita-Baylis-Hillman carbonates in the presence of 1–10 mol% DABCO in DCM at room temperature, rapidly gave N-allylated and N, β-diallylated isatin N,N′-cyclic azomethine imine 1,3-dipoles in moderate to high yields. The reaction features mild reaction conditions, easily practical operation, and short reaction times in most cases. Furthermore, the alkylated products were transformed into novel bicyclic spiropyrrolidine oxoindole derivatives through the [3+2] or [3+3]-cycloaddition with maleimides or Knoevenagel adducts.
1. Introduction
Heterocycles are privileged structural units that are frequently encountered in biologically active natural products as well as in pharmaceuticals and agrochemicals [1,2,3]. In particular, the pyrazole-ring skeletons, including pyrazolone and pyrazolidinone, are the core skeletons in many biologically active compounds. For example, edaravone (I) was used as a free radical scavenger for the treatment of amyotrophic lateral sclerosis (ALS) (Figure 1) [4]. Antipyrine (II) showed analgesic and antipyretic activities [5], and the analogue aminophenazone (III) also demonstrated antipyretic and anti-inflammatory activities [6]. In addition, Metamizole (IV) has been considered the strongest antipyretic drug for perioperative and cancer pain [7]. Eltrombopag (V) was used for the treatment of low blood platelet counts in adults with idiopathic chronic immune thrombocytopenia [8]. Furthermore, sulfamazone (VI) was regarded as a drug candidate for anti-inflammatory activity [9]. Therefore, the exploration of practical and efficient methods for the synthesis of dinitrogen-fused heterocycles has attracted extensive attention in the field of organic chemistry and pharmacology. Moreover, 3-substituted oxindoles are also heterocyclic frameworks and have widely existed in bioactive molecules [10,11,12,13,14,15,16]. Various methods of constructing 3-substituted oxindoles have been reported [17,18,19].
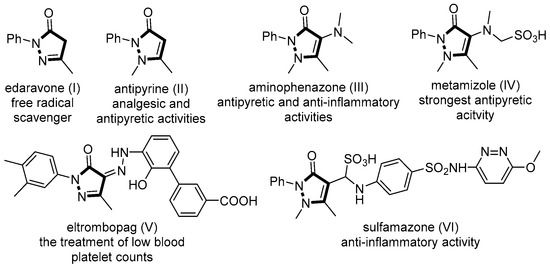
Figure 1.
Representatives of bioactive dinitrogen-fused heterocycles (I–IV).
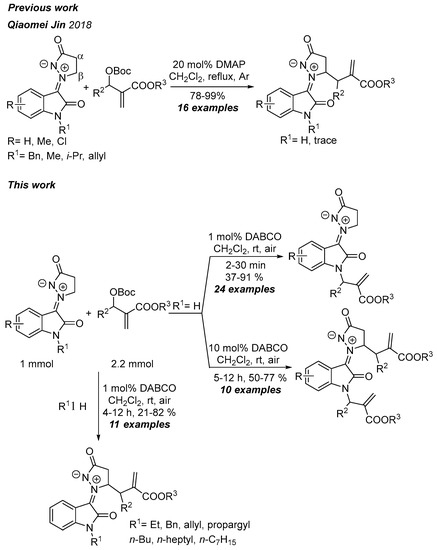
Very recently, Jin and co-workers [20] envisioned a direct pathway to access 3,3-spiropiperidine oxindoles via the [3+3]-annulation of the isatin N,N′-cyclic azomethine imine 1,3-dipole with Morita-Baylis-Hillman (MBH) carbonates in tertiary amines or phosphines. However, the authors did not find the above products but observed the allylation of isatin N,N′-cyclic azomethine imines with MBH carbonates catalyzed by 4-dimethylaminopyridine (DMAP), which generated 16 corresponding products in excellent yields (78–99%) (Scheme 1). There was a disadvantage when N-unsubstituted isatin N,N′-cyclic azomethine imine was employed as a substrate, as the reaction only afforded a trace amount of allylated product. To date, the reactions of isatin N,N′-cyclic azomethine imines were rarely studied and demonstrated by only a few examples [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36]. Therefore, it is urgent to explore the new reaction of isatin N,N′-cyclic azomethine imines. MBH adducts contain the structural moieties of allylic alcohols or amines, Michael acceptors, and electron-withdrawing groups, which makes them valuable substrates for various types of reactions such as Michael addition, allylic substitution, cycloaddition reaction, Friedel-Crafts reaction, Claisen rearrangement, etc. [37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58]. Based on our previous studies of 1,3-dipolar cycloaddition, Michael addition of azomethine ylides and azomethine imines and palladium-catalyzed tandem reaction to construct 3,3-disubstituted indolinones [59,60,61,62,63,64,65,66,67,68], herein we report the mono-/diallylation of isatin N,N′-cyclic azomethine imines from the condensation of isatin and pyrazolidones with MBH carbonates.
Scheme 1.
Mono- or diallylation of N,N′-cyclic azomethine imine 1,3-dipoles with MBH carbonates. Qiaomei Jin 2018 [20].
2. Results and Discussion
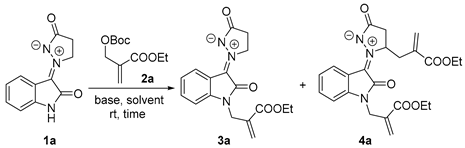
Before starting this work, we found that N-unprotected isatin N,N′-cyclic azomethine imine 1a reacted with MBH carbonate 2a in the presence of 5 mol% DMAP in dichloromethane (DCM) at room temperature in 45 min via N- and Cβ-allylation; this gave the corresponding 3a and 4a, respectively, in 24% and 17% yields (Table 1, entry 1). Jin’s group [20] reported that 1a reacted with 2a, only to obtain a trace amount of β-allylated product in the presence of 20 mol% DMAP in DCM at refluxing (3a or 4a was not observed). The above result encouraged us to explore and develop N-and β-allylation as a supplement to their approach.

Table 1.
The condition optimization of the model reaction a.
Subsequently, we found that DABCO, instead of DMAP, quickly gave N-allylated product 3a with a satisfactory yield in the same condition (entry 2). To improve the yield and regioselectivity of the reaction, the reaction conditions were optimized. First, the solvents were investigated. In the chloroalkanes, both chloroform and dichloroethane (DCE), the reactions led to inferior results in contrast with DCM (entries 3 and 4). The aprotic polar solvents, dimethylsulfoxide (DMSO), N,N-dimethylformamide (DMF) and N,N-dimethylacetamide (DMA), also gave poor yields and regioselectivities. Other solvents, such as ethyl acetate, acetonitrile (ACN), and ethers (for example, diethyl ether, tetrahydrofuran (THF), and dioxane), led to unsatisfying results. Therefore, DCM was selected as the best solvent. Second, various organic and inorganic bases were screened. When common tertiary amines were used, including triethylamine (TEA), diisopropylethylamine (DIPEA), and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), the yield of the N-allylated product was lower than using DMAP. In inorganic bases, NaOH, KOH, and NaH, only 7–13% yields were obtained, while in Na2CO3, K2CO3, and Cs2CO3, the reaction did not work at all. Triphenylphosphine made the reaction yield diallylated product 4a with poor yield (26%). Combined with the above results, DABCO was selected as the base. Next, the loading amounts of DABCO were screened. When a 1 mol% loading amount was used, the yield was better than the 5 mol% loading amounts (entry 24), in which 10 mol% loading amounts conversely gave an inferior yield (entry 25). In addition, the concentration of the reaction and equivalent of MBH carbonate 2a were also screened to find that the reaction gave the best yield (91%) in the presence of 2.2 equivalent 2a. When the reaction time was extended to 7 h and 10 mol% DABCO was used, only the diallylated product 4a was formed in 77% yield. The optimal reaction condition for monoallylation was established, and the desired product could be obtained in 91% yield when using isatin N,N′-cyclic azomethine imine 1a (1 equiv.), MBH carbonate 2a (2.2 equiv.), and catalyst DABCO (1 mmol%) in DCM at rt for 30 min (entry 28). The optimal reaction condition for diallylation afforded 77% of the product yield when using isatin N,N′-cyclic azomethine imine 1a (1 equiv.), MBH carbonate 2a (2.2 equiv.), and catalyst DABCO (10 mmol%) in DCM at rt for 7 h (entry 33).
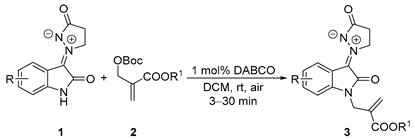
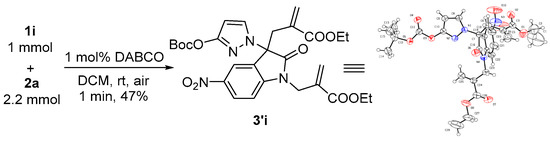
After establishing the optimal reaction conditions, a wide range of different substituted aryl isatin N,N′-cyclic azomethine imines have been explored for this nucleophilic substitution reaction. As summarized in Table 2, various substituent groups employed on the isatin moiety of 1 could be tolerated which afforded the desired products with moderate to excellent yields (49–91%) (Table 2, entries 1–8), except for 5-nitro isatin N,N′-cyclic azomethine imine 1d (entry 9). The reaction of 1a with 2a under a 1 mmol scale with the same yield (91%) compared with under 0.5 mmol at the most optimal conditions. It is worth noting that the corresponding products 3a could be afforded in 84% yield (1.28 g) when istain N,N′-cyclic azomethine imine 1a was scaled up to 4.65 mmol. The substituent patterns on the benzene ring of azomethine imines had a vital impact on the yields. Overall, the yields dropped off, whether it is electron-withdrawing or electron-donating groups, particularly the 7-CF3 group (entry 9). To our surprise, 5-nitro isatin N,N′-cyclic azomethine imine reacted with 2a, to give C3- and N-diallylated product 3′i, but not 3i within a short time (1 min) (Scheme 2). The structure of 3′i was confirmed unambiguously by single-crystal X-ray diffraction [69]. Various MBH carbonates (R1 = Me, n-Pr, n-Bu, and t-Bu) also reacted smoothly, in which the yields were 40–80%.

Table 2.
Synthesis of N-allylated products 3 from isatin N,N′-cyclic azomethine imine 1 and MBH carbonates 2 a.
Scheme 2.
The reaction of 5-nitro N,N′-cyclic azomethine imine 1i with MBH carbonate 2a.
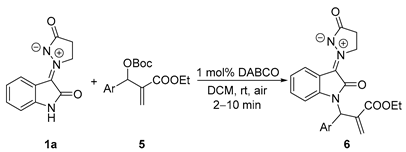
Subsequently, the generality of the allylation was further demonstrated using various aryl MBH carbonates. As outlined in Table 3, it is regrettable that all the yields of examples were not better than that of the model reaction, regardless of electron-donating groups and electron-withdrawing groups in phenyl. All the results showed that these reactions gave a complex when the aryl groups of MBH carbonates were 4-MeOC6H4, 4-FC6H4, 2-BrC6H4, and 2-NO2C6H4 (entries 7, 10, 14, and 17). These reactions led to the desired products in low yields or with an inseparable by-product (see Supporting Information) when aryl groups were 2-MeC6H4, 2-MeOC6H4, 2-FC6H4, and 2-ClC6H4 (entries 2, 5, 8, and 11). To our surprise, 3-thiophenyl MBH carbonate also afforded the diallylated product 6′t (Scheme 3), similarly to that of isatin N,N′-cyclic azomethine imine bearing a 5-NO2 group in benzene ring (Scheme 3).

Table 3.
Synthesis of N-allylated products 6 from isatin N,N′-cyclic azomethine imine 1 and MBH carbonates 5 a.
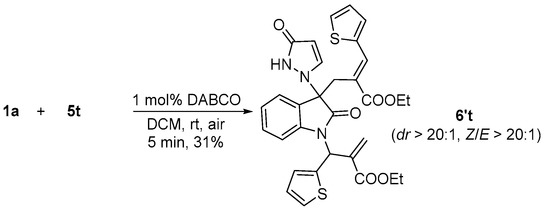
Scheme 3.
The reaction of 5-nitro N,N′-cyclic azomethine imine 1a with thiophenyl MBH carbonate 5t.
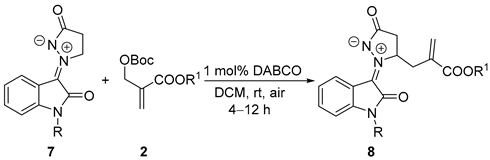
Various N-substituted isatin N,N′-cyclic azomethine imines 8 (R = alkyl, allyl, Bn, and propargyl) that were not used for testing by Jin’s group except for 8c, could also react with MBH-carbonate 2a with moderate to excellent yields (Table 4, entries 2–11) in our optimal condition. It is surprising that N-methyl isatin N,N′-cyclic azomethine imine 7a hardly reacted with 2a in the standard condition (entry 1). Among them, N-Bn isatin N,N′-cyclic azomethine imine could offer the desired product with a good yield (75%), though not as high as the yield (92%) reported by Jin’s group (entry 3). The reaction of N-allyl isatin N,N′-cyclic azomethine imine 7d gave the best result (82% yield), using 2a as a partner (entry 4). However, N-propargyl isatin N,N′-cyclic azomethine imine only gave a 20% yield, because of some side reactions (entry 5).

Table 4.
Synthesis of N,β-allylated products 8 from N-substituted isatin N,N′-cyclic azomethine imine 6 and MBH carbonates 2 a.
To expand the application of the reaction, isatin N,N′-cyclic azomethine imines 1 reacted with MBH carbonates 2 in the presence of 10 mol% DABCO and prolonged the reaction time, which afforded diallylated products 4 in 41–77% yield (Table 5). On the whole, the yields of all reactions were not high, except for 4e and 4i. The possible reason is that the prolonged reaction time leads to increasing side reactions.

Table 5.
Synthesis of N,β-diallylated products 4 from isatin N,N′-cyclic azomethine imine 1 and MBH carbonates 2 a.
The reaction of α-methyl isatin N,N′-cyclic azomethine imine 9 with MBH carbonate 2a was tested, which successfully obtained a corresponding product 10 in excellent yield (84%) within 2 min (Scheme 4). Meanwhile, β-phenyl isatin N,N′-cyclic azomethine imines 11 could also obtain the desired product 12 with a satisfied yield (77%) within 10 min.
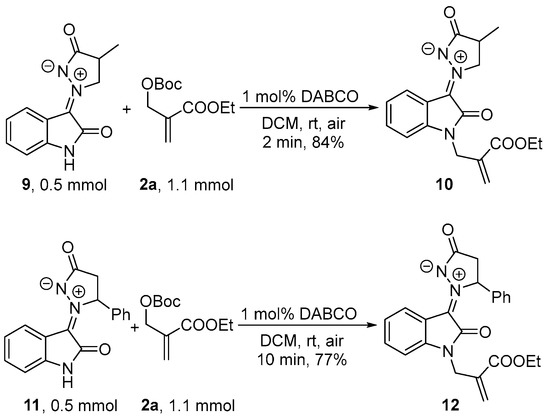
Scheme 4.
The reaction of α-methyl and β-phenyl N,N′-cyclic azomethine imines 9 and 11 with MBH carbonate 2a.
The N-allylated product 3a exhibited a potentially wide application in organic synthesis (Scheme 5). For example, the Michael addition of 3a with β-nitrostyrene in the presence of DABCO provided 3,3-disubstituted oxindole 13 in 37% yield with >20:1 dr, while no product was obtained in the condition reported by Wang’s group [22]. The [3+3] cycloaddition of 3a with Knoevenagel adduct under K2CO3/DCE could afford spiropyridazine oxoindole 14 in 60% yield with >20:1 dr, which the [3+2] cycloaddition of 3a with maleimide also produced tricyclic spiropyrrolidine oxoindole 15 in 91% yield with >20:1 dr. Finally, 3a could be converted into diallylated product 4a with moderate yield (64%).
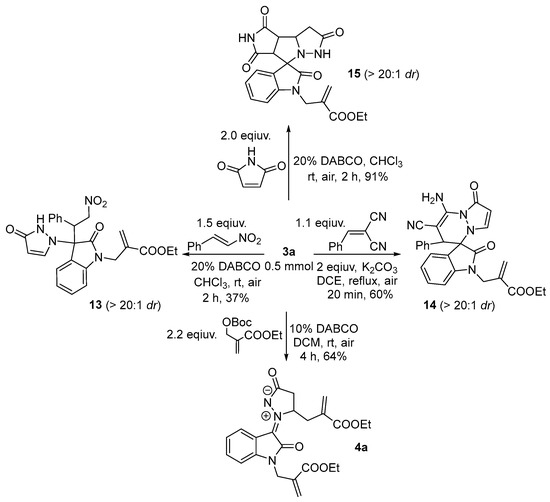
Scheme 5.
Transformation of 3a.
Based on the literature reports [20], our results, and X-ray analysis, a plausible mechanism is proposed for the formation of 3a, 3′I, and 4a (Scheme 6). First, isatin N,N′-cyclic azomethine imine 1 reacted with MBH carbonate 2 in the presence of DABCO, to obtain N-alkylated products 3 or 6. The resonance form In-A of 3 or 6 quickly tautomerized to the delocalized intermediate In-B under DABCO. Second, pyrazolenone intermediate In-C could be generated from In-B, then promote the isatin carbanion to react with MBH carbonate 2 through β-allylation, with the corresponding product 4 obtained. Moreover, when R was a nitro group, the delocalized intermediate In-B preferred to proceed with C3-allylation, followed by a Boc-protected reaction of the hydroxy group, to achieve N- and C3-diallylated product 3′i.
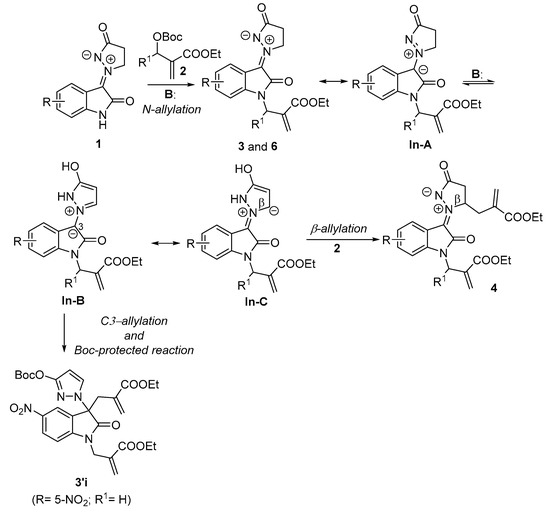
Scheme 6.
The plausible reaction mechanism.
3. Materials and Methods
3.1. General Methods
All reactions were carried out without strict water-free and oxygen-free conditions. All solvents and reagents were obtained from commercial suppliers and were directly used for reactions without further purification unless otherwise stated. When the reactions were performed at the condition of NaH, DCM was pre-dried with CaH2. Flash chromatography was performed using silica gel (200–300 mesh). Reactions were monitored by TLC or/and colour changes of the reaction solution. Visualization was achieved under a UV lamp (254 nm and 365 nm), I2, and by developing the plates with phosphomolybdic acid. 1H and 13C NMR were recorded on 400 and 600 MHz NMR spectrometers with tetramethylsilane (TMS) as the internal standard. The chemical shift values were corrected to 7.26 ppm (1H NMR) and 77.16 ppm (13C NMR) for CDCl3. IR spectra were acquired on an FT-IR spectrometer and are reported in wavenumbers (cm−1). High-resolution mass spectra were obtained using electrospray ionization (ESI). 1H NMR splitting patterns are designated as singlet (s), double (d), broad singlet (br s), triplet (t), quartet (q), doublet of doublets (dd), multiples (m), etc. Coupling constants (J) are reported in Hertz (Hz).
3.2. Preparation of Intermediates
Pyrazolidine-3-ones were obtained by the reaction of hydrazone monohydrate with methyl acrylate in ethanol under refluxing conditions [21]. All isatin N,N′-cyclic azomethine imines 1 were prepared by the condensation of isatins and the above pyrazolidone in menthol under 45 °C or a refluxing condition [21]. All MBH carbonates 2 were prepared by two-step reactions, including the Morta–Maylis–Hillman reaction (1 equiv. DABCO/1 equiv. aldehyde/1.5 equiv. acrylate/1:1 dioxane:H2O or THF/2–3 days) [70] and the formation of an O-Boc derivative (0.1 equiv. DMAP/1 equiv. MBH alcohol/1.5 equiv.Boc2O/DCM/rt/overnight), with 22–64% total yields [71].
3.3. General Procedure for Condition Optimization
A 10 mL tube was charged with isatin N,N′-cyclic azomethine imine 1a (0.5 mmol, 1.0 equiv.), MBH carbonate 2a (0.55–1.65 mmol, 1.1–3.3 equiv.), base (0.005–0.1 mmol, 1–20 mol%), and solvent (1.0–4.0 mL). The suspended solution was vigorously stirred at rt-reflux, and then the base was added. When the suspension reaction liquid became completely clear, the reaction had finished. The solution was added by 5 mL H2O and 15 mL brine before the resulting mixture was extracted with DCM (5 × 10 mL). The combined organic layers were dry with Na2SO4, filtered, and concentrated. The residue was purified by flash silica gel chromatography eluated with EtOAc:PE (1:5 to 1:1) to afford the corresponding products 3a and/or 4a.
3.4. General Procedure for Typical Procedure for Monoallylation
A tube (25 mL) was charged with isatin N,N′-cyclic azomethine imine 1a (1.0 mmol, 1.0 equiv.), MBH carbonate 2a (2.2 mmol, 2.2 equiv.), and DCM (4 mL). The suspended solution was vigorously stirred at rt, and then DABCO (0.01 mmol, 0.01 equiv., 1 mol%) was added. When the reaction mixture became clear the reaction finished (2–30 min). The solution was added by 10 mL H2O and 30 mL brine before the resulting mixture was extracted with DCM (5 × 20 mL). The combined organic layers were dried with Na2SO4, filtered, and concentrated. The residue was purified by flash silica gel chromatography eluated with EtOAc:PE (1:5 to 1:1) to afford the corresponding monoallylated products 3, 6, 8, 10, and 12.
3.5. General Procedure for Typical Procedure for Dialkylation
A tube (25 mL) was charged with isatin N,N′-cyclic azomethine imine 1a (1.0 mmol, 1.0 equiv.), MBH carbonate 2a (2.2 mmol, 2.2 equiv.), and DCM (4 mL). The suspended solution was vigorously stirred at rt, and then DABCO (0.1 mmol, 0.1 equiv., 10 mol%) was added. When the reaction mixture became clear the reaction finished (5–12 h). The solution was added by 10 mL H2O and 30 mL brine before the resulting mixture was extracted with DCM (5 × 20 mL). The combined organic layers were dried with Na2SO4, filtered, and concentrated. The residue was purified by flash silica gel chromatography eluated with EtOAc:PE (1:5 to 1:1) to afford the corresponding diallylated products 4.
3.6. Deriverziation of 3a
DABCO (11 mg, 0.10 mmol, 0.2 equiv.) was added to a solution of 3a (164 mg, 0.5 mmol, 1.0 equiv) and β-nitrostyrenes (224 mg, 1.5 mmol, 1.5 equiv) in CHCl3 (2.0 mL) at rt. The mixture was stirred at rt for 2.0 h. The resulting mixture was a saturated NH4Cl solution (10 mL). The aqueous solution was extracted with EtOAc (3 × 15 mL). The combined organic layers were dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by flash silica gel chromatography eluated with petroleum ether:EtOAc (3:1 to 1:1) to furnish Michael adduct 13.
K2CO3 (138 mg, 1 mmol, 2.0 equiv.) was added to a solution of 3a (164 mg, 0.5 mmol, 1.0 equiv) and 2-benzylidenemalononitrile (85 mg, 0.55 mmol, 1.1 equiv) in DCE (2.0 mL) at rt. The mixture was stirred at 83 °C for 20 min. The resulting mixture was a saturated NH4Cl solution (10 mL). The aqueous solution was extracted with DCM (3 × 15 mL). The combined organic layers were dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by flash silica gel chromatography eluated with petroleum ether:EtOAc (5:1 to 1:1) to furnish cycloadduct 14.
DABCO (11 mg, 0.10 mmol, 0.2 equiv.) was added to a solution of 3a (164 mg, 0.5 mmol, 1.0 equiv) and maleimide (97 mg, 1.0 mmol, 2.0 equiv) in CHCl3 (2.0 mL) at rt. The mixture was stirred at rt for 2.0 h. The resulting mixture was a saturated NH4Cl solution (10 mL). The aqueous solution was extracted with EtOAc (3 × 15 mL). The combined organic layers were dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by flash silica gel chromatography eluated with petroleum ether:EtOAc (3:1 to 1:1) to furnish cycloadduct 15.
A tube (25 mL) was charged with 3a (1.0 mmol, 1.0 equiv.), MBH carbonate 2a (2.2 mmol, 2.2 equiv.), and DCM (4 mL). The suspended solution was vigorously stirred at rt, and then DABCO (0.1 mmol, 0.1 equiv., 10 mol%) was added. When the reaction mixture became clear the reaction finished (5–12 h). The solution was added by 10 mL H2O and 30 mL brine before the resulting mixture was extracted with DCM (5 × 20 mL). The combined organic layers were dried with Na2SO4, filtered, and concentrated. The residue was purified by flash silica gel chromatography eluated with EtOAc:PE (1:5 to 1:1) to afford the corresponding allylated adduct 4.
4. Conclusions
In summary, we have developed a general method of DABCO-catalyzed mono-/diallylation of isatin N,N′-cyclic azomethine imine 1,3-dipoles with MBH carbonates. Various mono and diallyl isatin N,N′-cyclic azomethine imines are afforded in moderate to excellent yields (21–91%). All the synthesized compounds 3, 3′i, 4, 6, 6′t, 8, 10, 12, 13, 14, and 15 were confirmed through 1H and 13C NMR, IR, and HMRS technologies (see Supplementary Materials). Furthermore, product 3a can be transformed into functionalized compounds by cycloaddition and Michael to demonstrate the synthetic utilities. Further exploration and application of this reaction in organic synthesis are ongoing in our laboratory.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28073002/s1, Figure S1: The phenomenon of the reaction and TLC; Data for all new compounds; Copes of NMR for all new compounds; Copes of HRMS for all new compounds; Copes of data of X-ray crystal structure for 3′i.
Author Contributions
Writing—original draft preparation, G.Y. (Guizhou Yue); writing—review and editing, C.Y. and H.C.; methodology, G.Y. (Guizhou Yue) and J.F.; conducting the experiments, Q.W., S.L., G.Y. (Guosheng Yang), X.Z. and X.Y.; validation, Q.W.; supervision, G.Y. (Guizhou Yue) and L.Z.; funding acquisition, G.Y. (Guizhou Yue); mass spectrometry, C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Sichuan Science and Technology Program (No. 2020YFH0129), the National Key R&D Program of China (No. 2019YFD1002100), and the Program Sichuan Veterinary Medicine and Drug Innovation Group of China Agricultural Research System (No. SCCXTD-2020-18).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Guizhou Covalent Bond Bochuang Technology Co., Ltd., for the NMR analysis.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 3, 3′i, 4, 6, 6′t, 8, 10, 12, 13, 14 and 15 are available from the authors.
References
- Eicher, T.; Hauptmann, S. The Chemistry of Heterocycles, 2nd ed.; Wiley-VCH: Weinheim, German, 2003. [Google Scholar]
- Varvounis, G.; Fiamegos, Y.; Pilidis, G. Pyrazol-3-ones part 1: Synthesis and applications. Adv. Heterocycl. Chem. 2001, 80, 75–165. [Google Scholar]
- Elguero, J. Pyrazoles: Comprehensive Heterocyclic Chemistry, 2nd ed.; Katritzky, A.R., Rees, C.W., Scriven, E.F.V., Eds.; Elsevier: Oxford, UK, 1996; Volume 3, pp. 1–75. [Google Scholar]
- Zhao, Z.; Dai, X.; Li, C.; Wang, X.; Tian, J.; Feng, Y.; Xie, J.; Ma, C.; Nie, Z.; Fan, P.; et al. Pyrazolone structural motif in medicinal chemistry: Retrospect and prospect. Eur. J. Med. Chem. 2020, 186, 111893. [Google Scholar] [CrossRef]
- Lapchak, P.A. A critical assessment of edaravone acute ischemic stroke efficacy trials: Is edaravone an effective neuroprotective therapy? Expert Opin. Pharmacother. 2010, 11, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Meiattini, F.; Prencipe, L.; Bardelli, F.; Giannini, G.; Tarli, P. The 4-hydroxybenzoate/4-aminophenazone chromogenic system used in the enzymic determination of serum cholesterol. Clin. Chem. 1978, 24, 2161–2165. [Google Scholar] [CrossRef] [PubMed]
- Bussel, J.B.; Cheng, G.; Saleh, M.N.; Psaila, B.; Kovaleva, L.; Meddeb, B.; Kloczko, J.; Hassani, H.; Mayer, B.; Stone, N.L.; et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N. Engl. J. Med. 2007, 357, 2237–2247. [Google Scholar] [CrossRef]
- Freitag, F.G.; Cady, R.; DiSerio, F.; Elkind, A.; Gallagher, R.M.; Goldstein, J.; Klapper, J.A.; Rapoport, A.M.; Sadowsky, C.; Saper, J.R.; et al. Comparative study of a combination of isometheptene mucate, dichloralphenazone with acetaminophen and sumatriptan succinate in the treatment of migraine. Headache 2001, 41, 391–398. [Google Scholar] [CrossRef]
- Pecenco GL, A.A.; Bacciardi, M. Sulphenazone in pediatric practice. Case studies. Minerva Pediatr. 1982, 34, 39–43. [Google Scholar]
- Dounay, A.B.; Overman, L.E. The asymmetric intramolecular Heck reaction in natural product total synthesis. Chem. Rev. 2003, 103, 2945–2963. [Google Scholar] [CrossRef]
- Marti, C.; Carreira, E.M. Construction of Spiro[pyrrolidine-3, 3′-oxindoles]-recent applications to the synthesis of oxindole alkaloids. Eur. J. Org. Chem. 2003, 12, 2209–2219. [Google Scholar] [CrossRef]
- Galliford, C.V.; Scheidt, K.A. Pyrrolidinyl-spirooxindole natural products as inspirations for the development of potential therapeutic agents. Angew. Chem. Int. Ed. 2007, 46, 8748–8758. [Google Scholar] [CrossRef]
- Yousef-tabar-Miri, L.; Hosseinjani-Pirdehi, H.; Akrami, A.; Hallajian, S. Recent investigations in the synthesis of spirooxindole derivatives by Iranian researchers. J. Iran. Chem. Soc. 2020, 17, 2179–2231. [Google Scholar] [CrossRef]
- Koguchi, Y.; Kohno, J.; Nishio, M.; Takahashi, K.; Okuda, T.; Ohnuki, T.; Komatsubara, S. TMC-95A, B, C, and D, novel proteasome inhibitors produced by Apiospora montagnei Sacc. TC 1093 taxonomy, production, isolation, and biological activities. J. Antibiotics. 2000, 53, 105–109. [Google Scholar]
- Tokunaga, T.; Hume, W.E.; Nagamine, J.; Kawamura, T.; Taiji, M.; Nagata, R. Structure–activity relationships of the oxindole growth hormone secretagogues. Bioorg. Med. Chem. Lett. 2005, 15, 1789–1792. [Google Scholar] [CrossRef] [PubMed]
- Fer-nandes, P.D.; Zardo, R.S.; Figueiredo, G.S.M.; Silva, B.V.; Pinto, A.C. Anti-inflammatory properties of convolutamydine A and two structural analogues. Life Sci. 2014, 116, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Chimni, S.S.; Mahajan, S.; Kumar, A. Stereoselective synthesis of 3-amino-2-oxindoles from isatin imines: New scaffolds for bioactivity evaluation. RSC Adv. 2015, 5, 52481–52496. [Google Scholar] [CrossRef]
- Pellissier, H. Synthesis of chiral 3-substituted 3-amino-2-oxindoles through enantioselective catalytic nucleophilic additions to isatin imines. Beilstein J. Org. Chem. 2018, 14, 1349–1369. [Google Scholar] [CrossRef]
- Kaur, J.; Chimni, S.S. Catalytic synthesis of 3-aminooxindoles via addition to isatin imine: An update. Org. Biomol. Chem. 2018, 16, 3328–3347. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, J.; Jin, Q. DMAP-catalyzed alkylation of isatin N,N′-cyclic azomethine imine 1,3-dipoles with Morita-Baylis-Hillman carbonates. New J. Chem. 2018, 42, 7025–7029. [Google Scholar] [CrossRef]
- Wang, X.; Yang, P.; Zhang, Y.; Tang, C.-Z.; Tian, F.; Peng, L.; Wang, L.-X. Isatin N,N′-cyclic azomethine imine 1,3-dipole and abnormal [3+2]-cycloaddition with maleimide in the presence of 1,4-diazabicyclo[2.2.2]octane. Org. Lett. 2017, 19, 646–649. [Google Scholar] [CrossRef]
- Wang, X.; Wu, L.; Yang, P.; Song, X.-J.; Ren, H.-X.; Peng, L.; Wang, L.-X. Isatin N,N′-cyclic azomethine imine 1,3-dipole and base catalyzed Michael addition with β-nitrostyrene via C3 umpolung of oxindole. Org. Lett. 2017, 19, 3051–3054. [Google Scholar] [CrossRef]
- Jin, Q.; Zhang, J.; Jiang, C.; Zhang, D.; Gao, M.; Hu, S. Self [3 + 4] cycloadditions of isatin N,N′-cyclic azomethine imine 1,3-dipole with N-(o-chloromethyl)aryl amides. J. Org. Chem. 2018, 83, 8410–8416. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, F.M.; Eslami, M.; Siahpoosh, A.; Golfam, H. A diastereoselective construction of functionalized dihydro-pyridazine based spirooxindole scaffold via C-3 umpolung of isatin N,N′-cyclic azomethine imine. New J. Chem. 2019, 43, 10318–10323. [Google Scholar] [CrossRef]
- Wang, Q.-H.; Zhu, Z.-X.; Huang, T.; Wu, M.-S. Base catalyzed unexpected rearrangement of isatin-derived N,N′-cyclic azomethine imines and Michael addition to hindered vinylidene bisphosphonates: Access to 3,3-disubstituted oxindole-fused pyrazolidin-3-one derivatives containing bisphosphonates. Tetrahedron 2019, 75, 416–421. [Google Scholar] [CrossRef]
- Meerakrishna, R.S.; Suresh, S.S.; Athira, M.; Choutipalli, V.S.K.; Shanmugam, P. Diverse reactivity of isatin based N,N′-cyclic azomethine imine dipoles with arynes: Synthesis of 1′-methyl-2′-oxospiro [indene-1,3′-indolines] and 3-aryl-3-pyrazol-2-oxindoles. New. J. Chem. 2020, 44, 11593–11601. [Google Scholar] [CrossRef]
- Jin, Q.; Zhang, D.; Zhang, J. A [3+2] cycloaddition/C-arylation of isatin N,N′-cyclic azomethine imine 1,3-dipole with arynes. RSC Adv. 2020, 10, 30620–30623. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, G.; Chen, Z.; Wu, M.; Wang, J.; Trigoura, L.; Guo, H.; Xing, Y.; Sun, S. Synthesis of spiro(indoline-3,1-pyrazolo[1,2-a ]pyrazoles) by 1,3-dipolar cycloadditions of isatin N,N′-cyclic azomethine imines with alkynes. J. Heterocycl. Chem. 2020, 57, 2044–2047. [Google Scholar] [CrossRef]
- Song, X.J.; Ren, H.X.; Xiang, M.; Li, C.Y.; Tian, F.; Wang, L.X. Base catalyzed abnormal [3+2]-cycloaddition between isatin N,N′-cyclic azomethine imine 1,3-dipole and 3-methyleneoxindole for the one-step construction of tetracyclic bispirooxindoles. J. Org. Chem. 2020, 85, 3921–3928. [Google Scholar] [CrossRef]
- Fang, Q.-Y.; Jin, H.-S.; Wang, R.-B.; Zhao, L.-M. A role for isatin azomethine imines as a dipolarphile in cycloaddition reactions. Org. Lett. 2020, 22, 7358–7362. [Google Scholar] [CrossRef]
- Kartikey, K.D.D.; Reddy, M.S.; Chowhan, L.R. Isatin N,N′-cyclic azomethine imine 1, 3-dipole mediated regio and diastereoselective synthesis of isoxazole-containing spirooxindoles by an abnormal [3+2] cycloaddition. Tetrahedron Lett. 2020, 61, 152664. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Yang, T.; Chen, R.; Ma, X.; Liu, H.; Wang, K.-K. 1,3-dipolar cycloaddition of isatin N,N′-cyclic azomethine imines with α,β-unsaturated aldehydes catalyzed by DBU in water. RSC Adv. 2020, 10, 24288–24292. [Google Scholar] [CrossRef]
- Song, X.J.; Ren, H.X.; Xiang, M.; Li, C.Y.; Zou, Y.; Li, X.; Huang, Z.C.; Tian, F.; Wang, L.X. Organocatalytic enantioselective Michael addition between 3-(3-hydroxy-1H-pyrazol-1-yl)oxindole and β-nitrostyrene for the preparation of chiral disubstituted oxindoles. J. Org. Chem. 2020, 85, 9290–9300. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Huang, Z.-C.; Xiang, M.; Li, C.-Y.; Li, X.; Tian, F.; Wang, L.-X. Spiro scaffold chiral organocatalyst of 3,2′-pyrrolidinyl spiro-oxindole amine and its catalytic evaluation in the enantioselective aldol condensation between 3-(3-hydroxy-1H-pyrazol-1-yl)-oxindole and paraformaldehyde. J. Org. Chem. 2021, 86, 17371–17379. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Wu, S.; Xu, H. Organocatalytic asymmetric [3+3] annulation of isatin N,N′-cyclic azomethine imines with enals: Efficient approach to functionalized spiro N-heterocyclic oxindoles. Chin. Chem. Lett. 2021, 32, 672–675. [Google Scholar] [CrossRef]
- Borah, B.; Chowhan, R.L. Recent updates on the stereoselective synthesis of structurally functionalized spiro-oxindoles mediated by isatin N,N′-cyclic azomethine imine 1, 3-dipoles. Tetrahedron Lett. 2022, 104, 154014. [Google Scholar] [CrossRef]
- Rios, R. Organocatalytic enantioselective methodologies using Morita–Baylis–Hillman carbonates and acetates. Catal. Sci. Technol. 2012, 2, 267–278. [Google Scholar] [CrossRef]
- Xie, P.; Huang, Y. Morita–Baylis–Hillman adduct derivatives (MBHADs): Versatile reactivity in Lewis base-promoted annulation. Org. Biomol. Chem. 2015, 13, 8578–8595. [Google Scholar] [CrossRef]
- Zhong, N.-J.; Wang, Y.-Z.; Cheng, L.; Wang, D.; Liu, L. Recent advances in the annulation of Morita–Baylis–Hillman adducts. Org. Biomol. Chem. 2018, 16, 5214–5227. [Google Scholar] [CrossRef]
- Chen, Z.C.; Chen, Z.; Du, W.; Chen, Y.C. Transformations of modified Morita–Baylis–Hillman adducts from isatins catalyzed by Lewis bases. Chem. Rec. 2019, 20, 541–555. [Google Scholar] [CrossRef]
- Suresh, A.; Lal, S.; Namboothiri, I.N.N. Regio-and stereoselective synthesis of functionalized and fused heterocycles from Morita–Baylis–Hillman adducts of dicyclopentadienone. Org. Biomol. Chem. 2022, 20, 2271–2281. [Google Scholar] [CrossRef]
- Pareek, A.; Sivanandan, S.T.; Bhagat, S.; Namboothiri, I.N.N. [3+2]-Annulation of oxindolinyl-malononitriles with Morita–Baylis–Hillman acetates of nitroalkenes for the regio- and diastereoselective synthesis of spirocyclopentane-indolinones. Tetrahedron 2022, 108, 132650. [Google Scholar] [CrossRef]
- Ma, J.; Gao, B.; Song, G.; Zhang, R.; Wang, Q.; Ye, Z.; Chen, W.-W.; Zhao, B. Asymmetric α-Allylation of Glycinate with Switched Chemoselectivity Enabled by Customized Bifunctional Pyridoxal Catalysts. Angew. Chem. Int. Ed. 2022, 61, e202200850. [Google Scholar]
- Lin, J.; Zhu, Y.; Cai, W.; Huang, Y. Phosphine-mediated sequential [2+4]/[2+3] annulation to construct pyrroloquinolines. Org. Lett. 2022, 24, 1593–1597. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wang, J.; Zheng, J.; Luo, Q.-Q.; Leng, H.; Zheng, S.; Peng, C.; Han, B.; Zhan, G. Organocatalytic (5+1) benzannulation of Morita–Baylis–Hillman carbonates: Synthesis of multisubstituted 4-benzylidene pyrazolones. New J. Chem. 2022, 46, 11617–11622. [Google Scholar] [CrossRef]
- Dabaria, K.K.; Bai, R.; Jat, P.K.; Badsara, S.S. Atom-economical, catalyst-free hydrosulfonation of densely functionalized alkenes: Access to oxindole-containing sulfones. New J. Chem. 2022, 46, 12905–12909. [Google Scholar] [CrossRef]
- He, X.-H.; Fu, X.-J.; Zhan, G.; Zhang, N.; Li, X.; Zhu, H.-P.; Peng, C.; He, G.; Han, B. Organocatalytic asymmetric synthesis of multifunctionalized α-carboline-spirooxindole hybrids that suppressed proliferation in colorectal cancer cells. Org. Chem. Front. 2022, 9, 1048–1055. [Google Scholar] [CrossRef]
- Ni, N.; Chen, J.; Ding, S.; Cheng, D.; Li, X.; Xu, X. Synthesis of Acrylonitrile Derivatives via Visible Light-induced Coupling Reaction of Morita-Baylis-Hillman Adducts with Tertiary Amines and α-Trimethylsilyl Amines. Asian J. Org. Chem. 2022, 11, e202100747. [Google Scholar] [CrossRef]
- Cho, C.-W.; Kong, J.-R.; Krische, M.J. Phosphine-catalyzed regiospecific allylic amination and dynamic kinetic resolution of Morita− Baylis− Hillman acetates. Org. Lett. 2004, 6, 1337–1339. [Google Scholar] [CrossRef]
- Du, Y.; Han, X.; Lu, X. Alkaloids-catalyzed regio-and enantioselective allylic nucleophilic substitution of tert-butyl carbonate of the Morita–Baylis–Hillman products. Tetrahedron Lett. 2004, 45, 4967–4971. [Google Scholar] [CrossRef]
- Zhang, T.-Z.; Dai, L.-X.; Hou, X.-L. Enantioselective allylic substitution of Morita–Baylis–Hillman adducts catalyzed by planar chiral [2.2] paracyclophane monophosphines. Tetrahedron Asymm. 2007, 18, 1990–1994. [Google Scholar] [CrossRef]
- Zhang, S.-J.; Cui, H.-L.; Jiang, K.; Li, R.; Ding, Z.-Y.; Chen, Y.-C. Enantioselective allylic amination of Morita–Baylis–Hillman carbonates catalysed by modified cinchona alkaloids. Eur. J. Org. Chem. 2009, 2009, 5804–5809. [Google Scholar] [CrossRef]
- Zhao, M.-X.; Chen, M.-X.; Tang, W.-H.; Wei, D.-K.; Dai, T.-L.; Shi, M. Cinchona alkaloid catalyzed regio- and enantioselective allylic amination of Morita–Baylis–Hillman carbonates with isatins. Eur. J. Org. Chem. 2012, 2012, 3598–3606. [Google Scholar] [CrossRef]
- Zhu, L.; Hu, H.; Qi, L.; Zheng, Y.; Zhong, W. Enantioselective allylic substitution of Morita–Baylis–Hillman adducts catalyzed by chiral bifunctional ferrocenylphosphines. Eur. J. Org. Chem. 2016, 2016, 2139–2144. [Google Scholar] [CrossRef]
- Zi, Y.; Lange, M.; Schultz, C.; Vilotijevic, I. Latent nucleophiles in Lewis base catalyzed enantioselective N-allylations of N-Heterocycles. Angew. Chem. Int. Ed. 2019, 58, 10727–10731. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Yin, T.; Feng, A.; Hu, Y.; Yu, C.; Li, T.; Yao, C. Base-promoted regiodivergent allylation of N-acylhydrazones with Morita–Baylis–Hillman carbonates by tuning the catalyst. Org. Biomol. Chem. 2019, 17, 5283–5293. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.R.; Mohammed, S.Z.; Kumaraswamy, P.; Kajare, R.C.; Patil, A.D.; Ganga, V.S.R.; Ramaraju, A.; Sridhar, B. A strategy for the synthesis of bicyclic fused cyclopentenones from MBH-carbonates of propiolaldehydes. Synthesis 2022, 54, 3623–3630. [Google Scholar] [CrossRef]
- Wang, S.; Gao, Y.; Song, S.; Li, X.; Zhang, Z.; Xiang, J.B.; Zheng, L. Lewis base catalyzed allylation reaction of N-aryl amides with Morita–Baylis–Hillman carbonates. Tetrahedron 2022, 120, 132903. [Google Scholar] [CrossRef]
- Chen, Z.; Yue, G.; Lu, C.; Yang, G. Synthesis of a library of indolizines using poly (ethylene glycol) as soluble support. Synlett 2004, 2004, 1231–1234. [Google Scholar] [CrossRef]
- Yue, G.; Wan, Y.; Song, S.; Yang, G.; Chen, Z. Synthesis of a library of benzoindolizines using poly (ethylene glycol) as soluble support. Bioorg. Med. Chem. Lett. 2005, 15, 453–458. [Google Scholar] [CrossRef]
- Yue, G.; Chen, Z.; Yang, G. Synthesis of a library of 1,2,3,7-tetrasubstituted indolizines using poly(ethylene glycol) as soluble support. J. Heterocycl. Chem. 2006, 43, 781–786. [Google Scholar] [CrossRef]
- Yue, G.-Z.; Huang, Q.-M.; Zou, P. Recent development in the synthesis of indolizines. Chin. J. Org. Chem. 2007, 27, 1060–1068. (In Chinese) [Google Scholar]
- Yue, G.; Wu, Y.; Dou, Z.; Chen, H.; Yin, Z.; Song, X.; He, C.; Wang, X.; Feng, J.; Zhang, Z.; et al. Synthesis of spiropyrrolidine oxindoles via Ag-catalyzed stereo- and regioselective 1,3-diploar cycloaddition of indole-based azomethine ylides with chalcones. New J. Chem. 2018, 42, 20024–20031. [Google Scholar] [CrossRef]
- Yue, G.; Dou, Z.; Zhou, Z.; Zhang, L.; Feng, J.; Chen, H.; Yin, Z.; Song, X.; Liang, X.; Wang, X.; et al. Rapid abnormal [3+2]-cycloaddition of isatin N,N′-cyclic azomethine imine 1,3-dipoles with chalcones. New J. Chem. 2020, 44, 8813–8817. [Google Scholar] [CrossRef]
- Yue, G.; Liu, B. Research progress on [3+n] (n≥3) cycloaddition of 1,3-diploes. Chin. J. Org. Chem. 2020, 40, 3132–3153. (In Chinese) [Google Scholar] [CrossRef]
- Yue, G.; Li, S.; Jiang, D.; Ding, G.; Feng, J.; Chen, H.; Yang, C.; Yin, Z.; Song, X.; Liang, X.; et al. Syntheses of 3,3-disubstituted dihydrobenzofurans, indolines, indolinones and isochromanes by palladium-catalyzed tandem reaction using Pd(PPh3)2Cl2/(±)-BINAP as a catalytic system. Catalysts 2020, 10, 1084. [Google Scholar] [CrossRef]
- Yue, G.; Jiang, D.; Dou, Z.; Li, S.; Feng, J.; Zhang, L.; Chen, H.; Yang, C.; Yin, Z.; Song, X.; et al. Rapid umpolung Michael addition of isatin N,N′-cyclic azomethine imine 1,3-dipoles with chalcones. New J. Chem. 2021, 45, 11712–11718. [Google Scholar] [CrossRef]
- Yang, G.; Li, S.; Wang, Q.; Chen, H.; Yang, C.; Yin, Z.; Song, X.; Zhang, L.; Lu, C.; Yue, G. K2CO3-promoted formal [3+3]-cycloaddition of N-unsubstituted isatin N,N′-cyclic azomethine imine 1,3-dipoles with Knoevenagel adducts. Molecules 2023, 28, 1034. [Google Scholar] [CrossRef]
- CCDC 2108657 Contain the Supplementary Crystallographic Data for Compound 3′i. Available online: www:Ccdc.cam.ac.uk/data_request/cif (accessed on 15 August 2021).
- Camilo, N.S.; Santos, H.; Zeoly, L.A.; Fernandes, F.S.; Rodrigues, M.T.; Silva, T.S.; Lima, S.R.; Serafim, J.C.; de Oliveira, A.S.B.; Carpanez, A.G.; et al. An improved protocol for the Morita–Baylis–Hillman Reaction allows unprecedented broad synthetic scope. Eur. J. Org. Chem. 2022, 2022, e202101448. [Google Scholar] [CrossRef]
- Basel, B.; Hassner, A. Di-tert-butyl dicarbonate and 4-(dimethylamino)pyridine revisited. Their reactions with amines and alcohols. J. Org. Chem. 2000, 65, 6368–6380. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).