Nanoparticle-Imprinted Silica Gel for the Size-Selective Capture of Silver Ultrafine Nanoparticles from Water
Abstract
1. Introduction
2. Results and Discussion
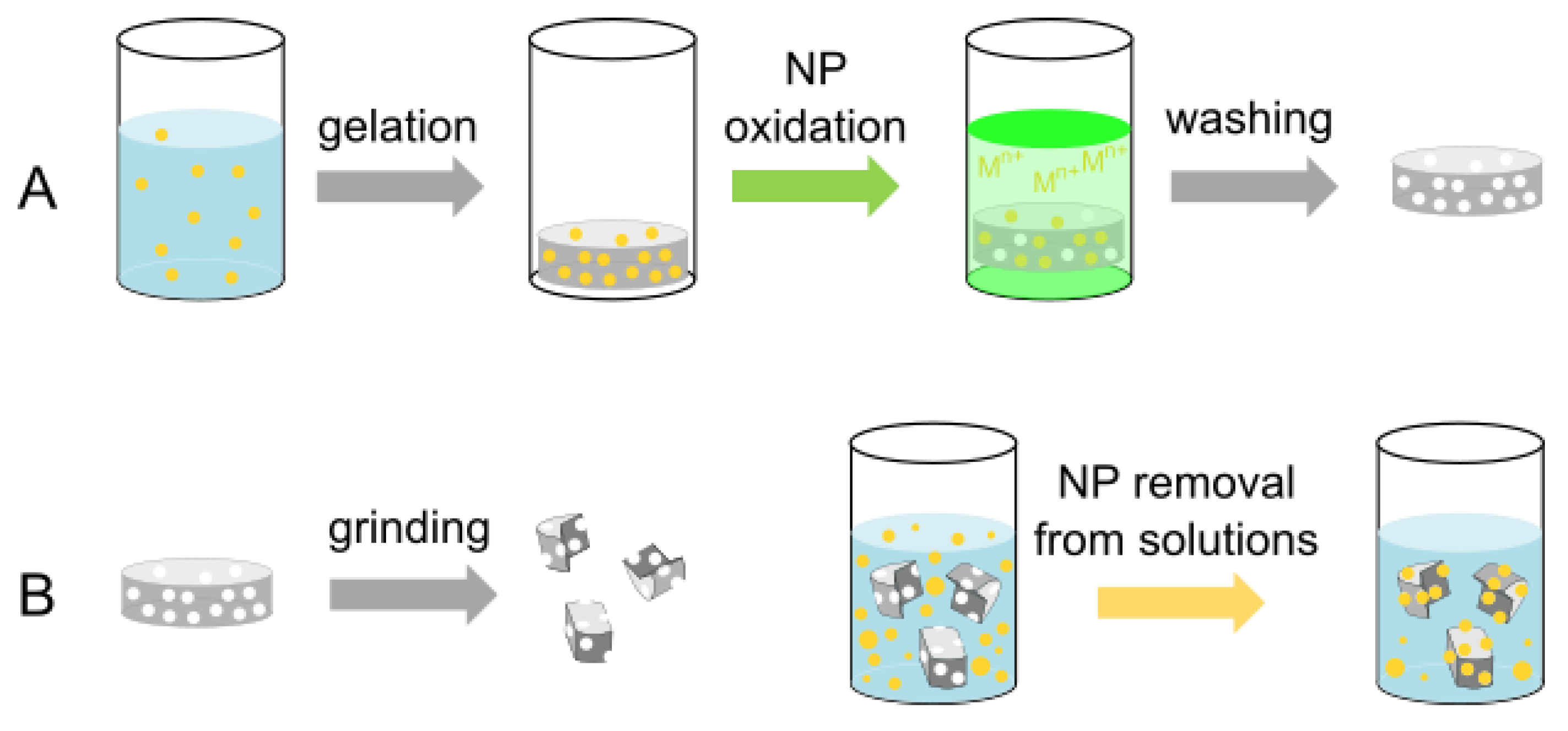
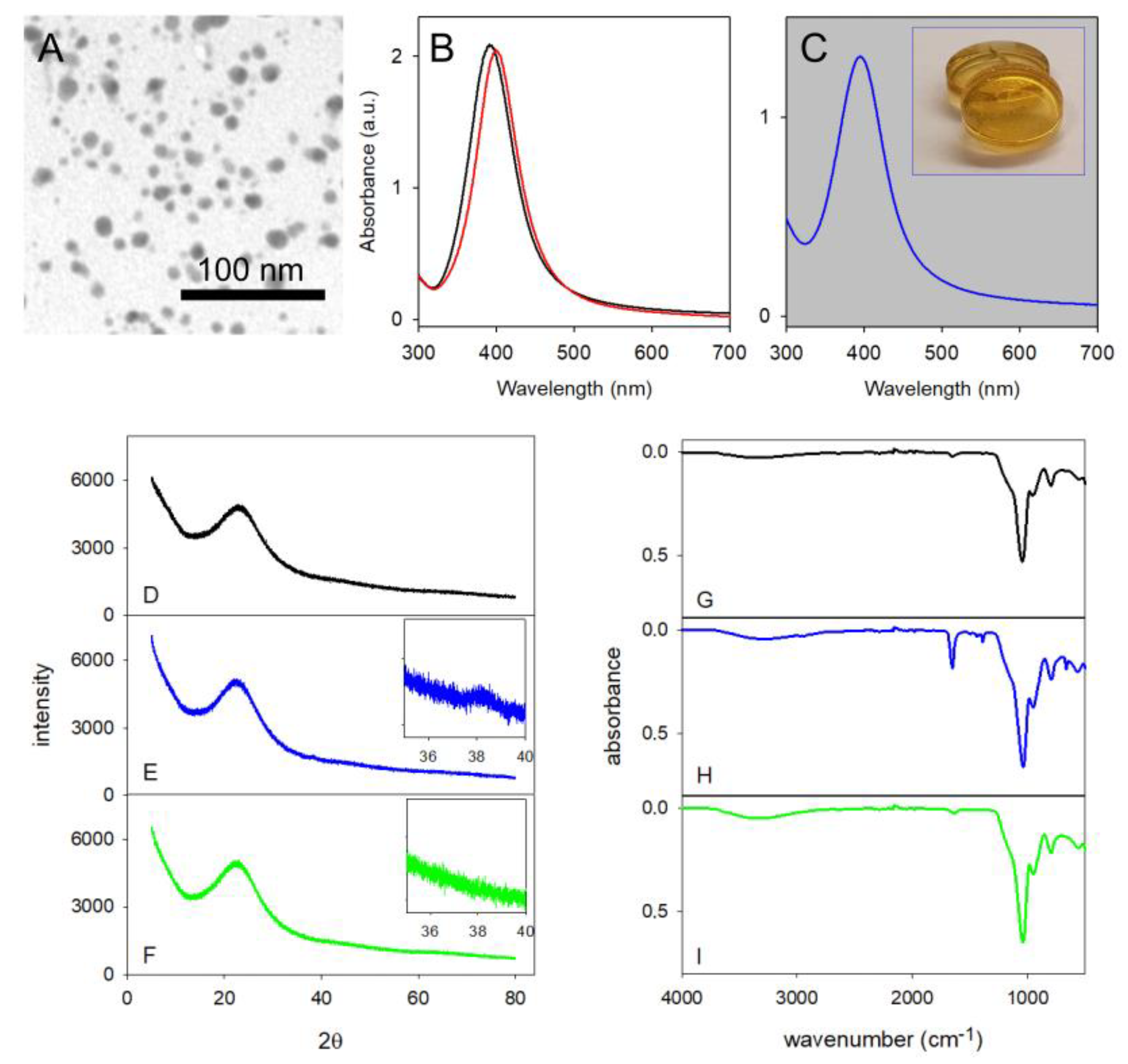
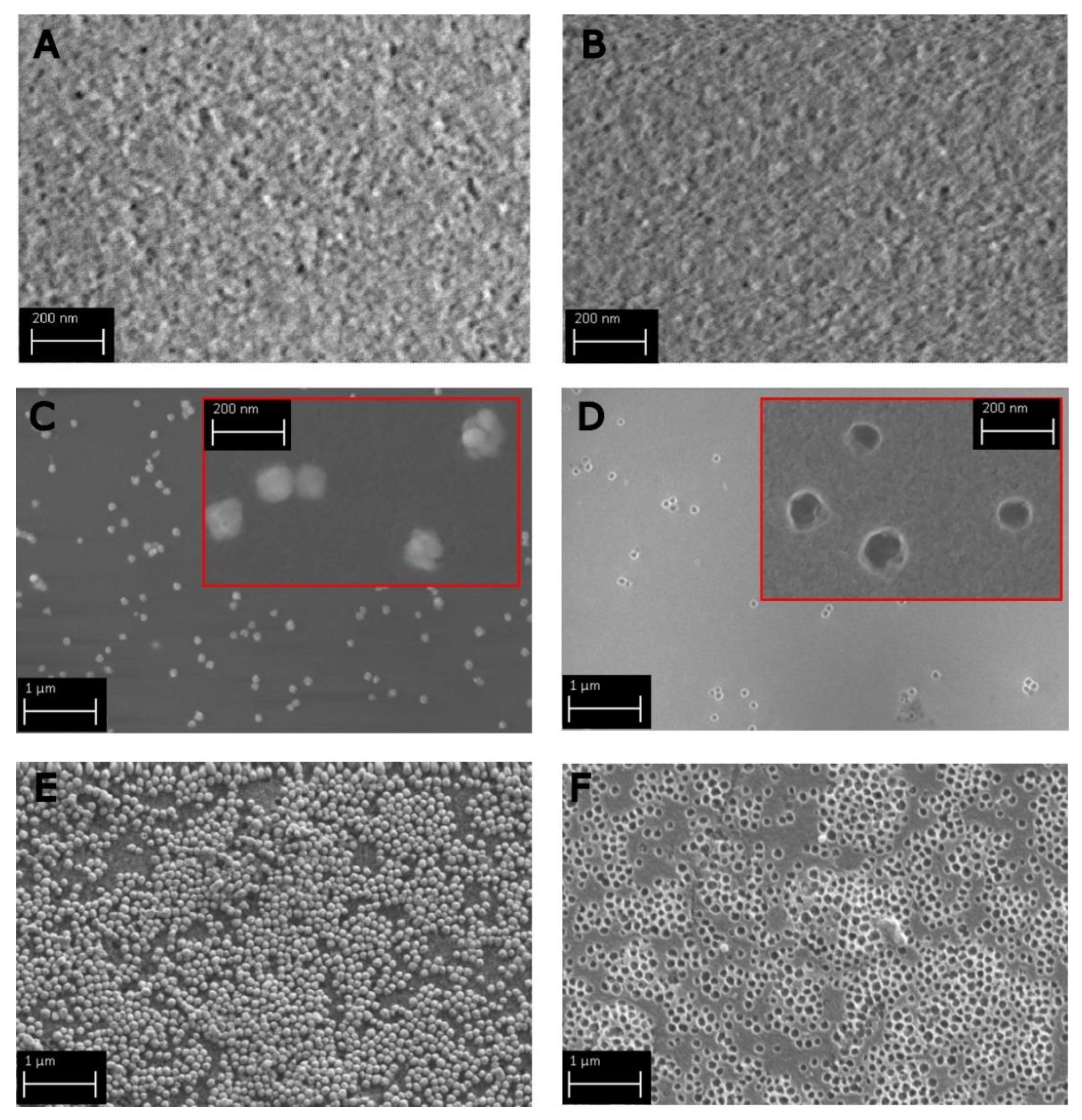
2.1. Silica Monoliths with Embedded 8 nm Ag-ufNP
2.2. Silica Monoliths with Embedded 18 nm and 115 nm Au-ufNP
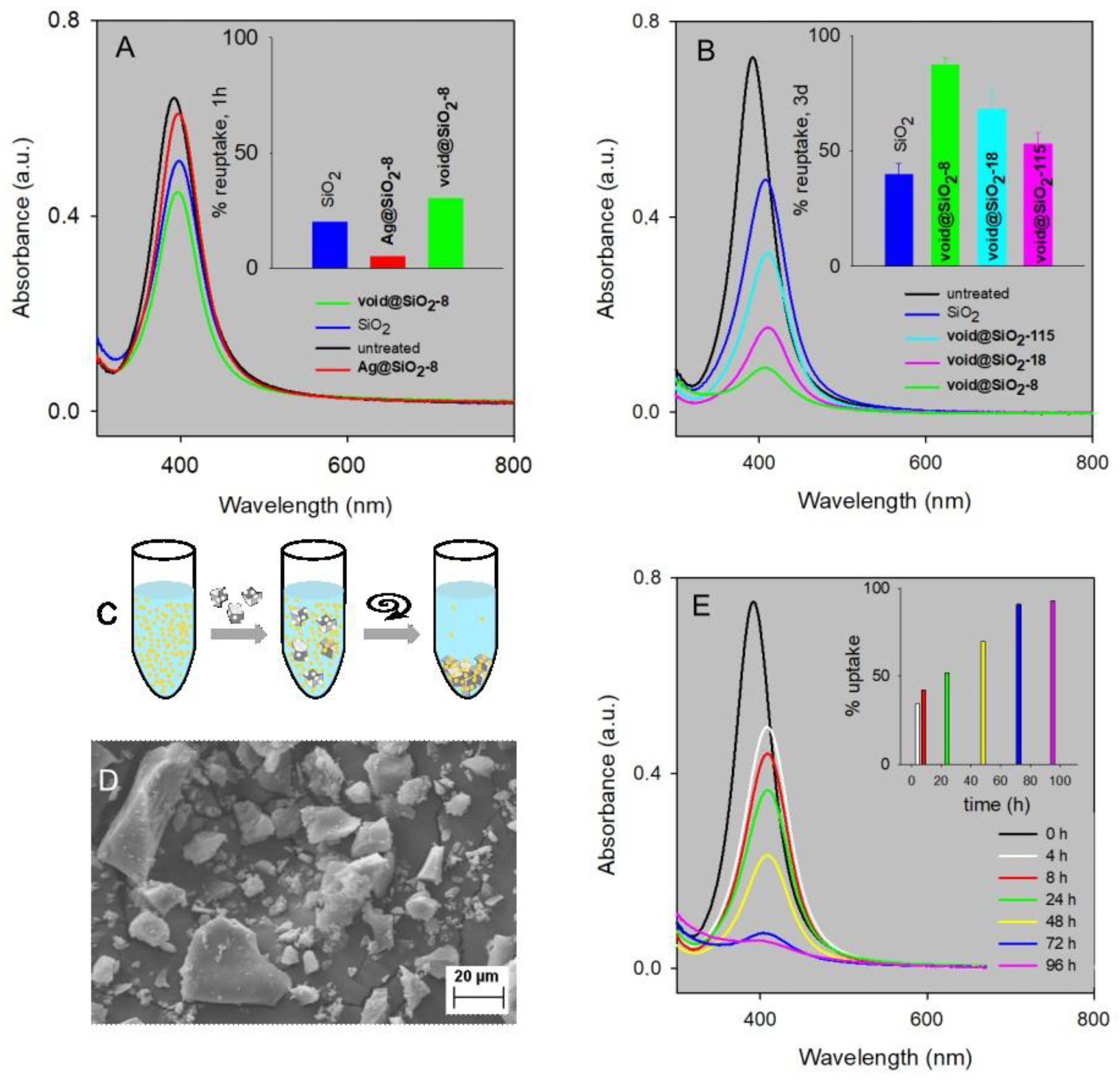
2.3. Void Imprinted Silica Gel
2.4. Uptake of 8 nm Ag-ufNP
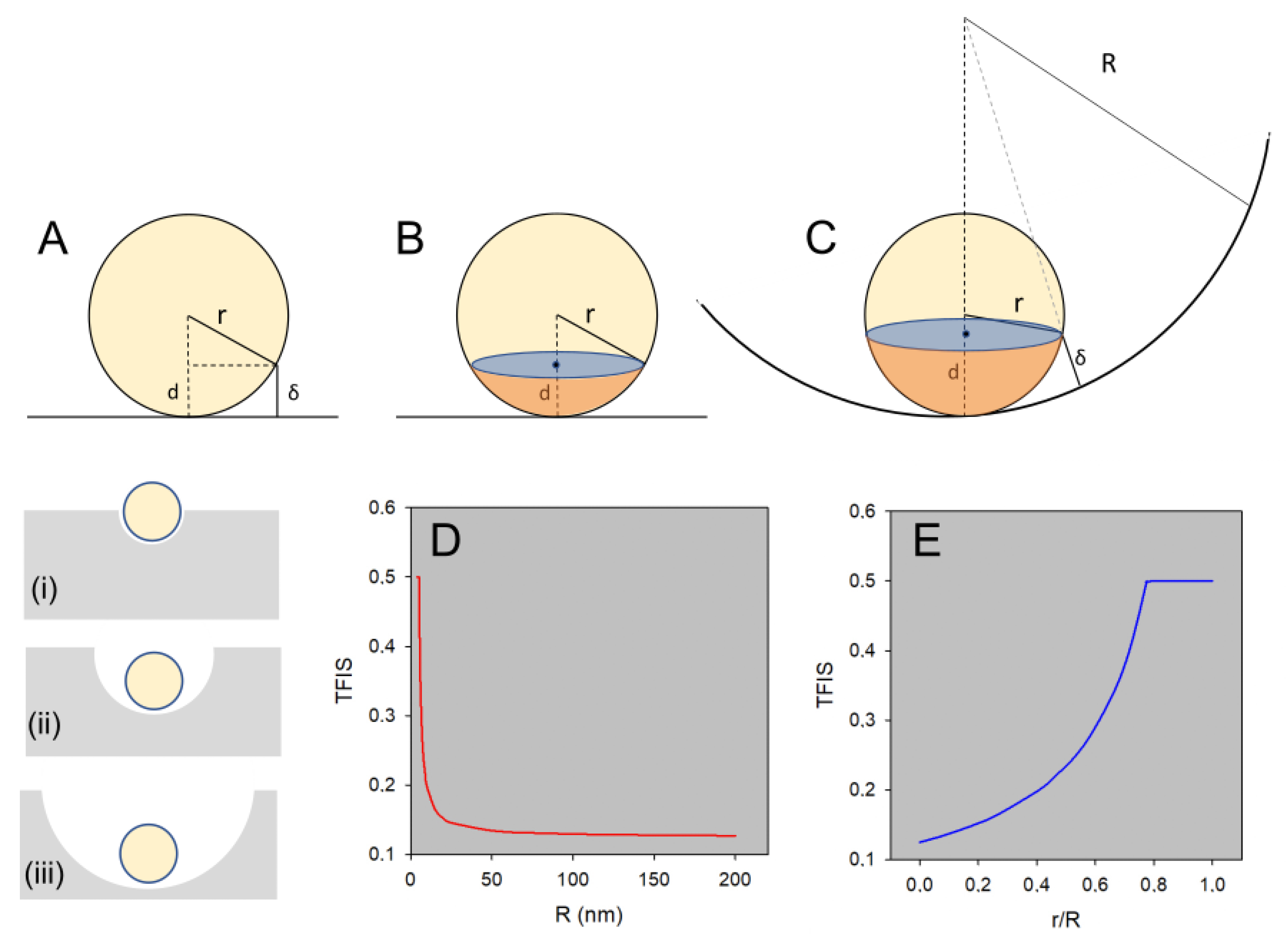
2.5. Size Selectivity
3. Materials and Methods
3.1. Materials
3.2. Methods and Instrumentation
3.3. Syntheses
3.3.1. Ag-ufNP, d = 8 nm
3.3.2. Au-ufNP, d = 18 nm
3.3.3. AuNP, d = 115 nm
3.3.4. Coating Nanoparticles with PEG Thiols
3.3.5. Preparation of NP-Containing Silica Gel Monoliths (Ag@SiO2-8, Au@SiO2-18, Au@SiO2-115)
3.3.6. Preparation of Silica Gel Monoliths without NP
3.3.7. Removal of Ag-ufNP (8 nm), Au-ufNP (18 nm) and AuNP (115 nm) from Silica Gel Monoliths
3.3.8. Preparation of void@SiO2-8, void@SiO2-18 and void@SiO2-115 Powders
3.3.9. Reuptake of Ag-ufNP (8 nm) by void@SiO2-8, void@SiO2-18, void@SiO2-115 and SiO2 Powders
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sanderson, P.; Delgado-Saborit, J.M.; Harrison, R.M. A review of chemical and physical characterisation of atmospheric metallic nanoparticles. Atmos. Environ. 2014, 94, 353–365. [Google Scholar]
- Heal, M.R.; Kumar, P.; Harrison, R.M. Particles, air quality, policy and health. Coord. Chem. Rev. 2012, 41, 6606–6630. [Google Scholar]
- Blaser, S.A.; Scheringer, M.; MacLeod, M.; Hungerbühler, K. Estimation of cumulative aquatic exposure and risk due to silver: Contribution of nano-functionalized plastics and textiles. Sci. Total Environ. 2008, 390, 396–409. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, N.; Cummin, E. Ranking initial environmental and human health risk resulting from environmentally relevant nanomaterials. J. Environ. Sci. Health A 2010, 45, 992–1007. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, A.J.; Østerskov Jensen, A.C.; Kling, K.I.; Nørgaard, A.; Brinch, A.; Christensen, F.; Jensen, K.A. Quantitative material releases from products and articles containing manufactured nanomaterials: Towards a release library. Nanoimpact 2017, 5, 119–132. [Google Scholar] [CrossRef]
- Park, B.; Donaldson, K.; Duffin, R.; Tran, L.; Kelly, F.; Mudway, I.; Morin, J.-P.; Guest, R.; Jenkinson, P.; Samaras, Z. Hazard and risk assessment of a nanoparticulate cerium oxide-based diesel fuel additive—A case study. Inhal. Toxicol. 2008, 20, 547–566. [Google Scholar] [CrossRef]
- Keller, A.A.; Lazareva, A. Predicted releases of engineered nanomaterials: From global to regional to local. Environ. Sci. Technol. Lett. 2014, 1, 65–70. [Google Scholar] [CrossRef]
- Yu, S.-J.-; Yin, Y.-G.; Liu, J.-F. Silver nanoparticles in the environment. Environ. Sci. Process. Impacts 2013, 15, 78–92. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Deshmukh, S.P.; Patil, S.M.; Mullani, S.B.; Delekar, S.D. Silver nanoparticles as an effective disinfectant: A review. Mater. Sci. Eng. C 2019, 97, 954–965. [Google Scholar] [CrossRef]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial silver nanomaterials. Coord. Chem. Rev. 2018, 357, 1–17. [Google Scholar] [CrossRef]
- Mueller, N.C.; Nowack, B. Exposure Modeling of Engineered Nanoparticles in the Environment. Environ. Sci. Technol. 2008, 42, 4447–4453. [Google Scholar] [CrossRef] [PubMed]
- Pallavicini, P.; Taglietti, A.; Dacarro, G.; Diaz-Fernandez, Y.A.; Galli, M.; Grisoli, P.; Patrini, M.; Santucci De Magistris, G.; Zanoni, R. Self-assembled monolayers of silver nanoparticles firmly grafted on glass surfaces: Low Ag+ release for an efficient antibacterial activity. J. Colloid Interfaces Sci. 2010, 350, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Taglietti, A.; Arciola, C.R.; D’Agostino, A.; Dacarro, G.; Montanaro, L.; Campoccia, D.; Cucca, L.; Vercellino, M.; Poggi, A.; Pallavicini, P.; et al. Antibiofilm activity of a monolayer of silver nanoparticles anchored to an amino-silanized glass surface. Biomaterials 2014, 35, 1779–1788. [Google Scholar] [CrossRef]
- Pallavicini, P.; Dacarro, G.; Taglietti, A. Self-Assembled monolayers of silver nanoparticles: From intrinsic to switchable inorganic antibacterial surfaces. Eur. J. Inorg. Chem. 2018, 2018, 4846–4855. [Google Scholar] [CrossRef]
- Pallavicini, P.; Bassi, B.; Chirico, G.; Collini, M.; Dacarro, G.; Fratini, E.; Grisoli, P.; Patrini, M.; Sironi, L.; Taglietti, A.; et al. Modular approach for bimodal antibacterial surfaces combining photo-switchable activity and sustained biocidal release. Sci. Rep. 2017, 7, 5259. [Google Scholar] [CrossRef]
- Bright, R.M.; Musick, M.D.; Natan, M.J. Preparation and Characterization of Ag Colloid Monolayers. Langmuir 1998, 14, 5695–5701. [Google Scholar] [CrossRef]
- Pallavicini, P.; Preti, L.; De Vita, L.; Dacarro, G.; Diaz Fernandez, Y.A.; Merli, D.; Rossi, S.; Taglietti, A.; Vigani, B. Fast dissolution of silver nanoparticles at physiological pH. J. Colloids Interfaces Sci. 2020, 563, 177–188. [Google Scholar] [CrossRef]
- Truesdale, G.A.; Downing, A.L. Solubility of oxygen in water. Nature 1954, 173, 1236. [Google Scholar] [CrossRef]
- Liu, J.; Hurt, R.H. Ion release kinetics and particle persistence in aqueous nanosilver colloids. Environ. Sci. Technol. 2010, 44, 2169–2175. [Google Scholar] [CrossRef]
- Cennamo, N.; Donà, A.; Pallavicini, P.; D’Agostino, G.; Dacarro, G.; Zeni, L.; Pesavento, M. Sensitive detection of 2,4,6-trinitrotoluene by tridimensionalmonitoring of molecularly imprinted polymer with optical fiber andfive-branched gold nanostars. Sensor Actuators B Chem. 2015, 208, 291–298. [Google Scholar] [CrossRef]
- Cennamo, N.; D’Agostino, G.; Donà, A.; Dacarro, G.; Pallavicini, P.; Pesavento, M.; Zeni, L. Localized Surface Plasmon Resonance with Five-Branched Gold Nanostars in a Plastic Optical Fiber for Bio-Chemical Sensor Implementation. Sensors 2013, 13, 14676–14686. [Google Scholar] [CrossRef] [PubMed]
- Grisoli, P.; De Vita, L.; Milanese, C.; Taglietti, A.; Diaz Fernandez, Y.; Bouzin, M.; D’Alfonso, L.-; Sironi, L.; Rossi, S.; Vigani, B.; et al. PVA Films with Mixed Silver Nanoparticles and Gold Nanostars for Intrinsic and Photothermal Antibacterial Action. Nanomaterials 2021, 11, 1387. [Google Scholar] [CrossRef] [PubMed]
- Kraus-Ophir, S.; Witt, J.; Wittstock, G.; Mandler, D. Nanoparticle-Imprinted Polymers for Size-Selective Recognition of Nanoparticles. Angew. Chem. Int. Ed. 2014, 53, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Bruchiel-Spanier, N.; Giordano, G.; Vakahi, A.; Guglielmi, M.; Mandler, D. Electrochemically Deposited Sol–Gel Based Nanoparticle-Imprinted Matrices for the Size-Selective Detection of Gold Nanoparticles. ACS Appl. Nano Mater. 2018, 1, 5612–5619. [Google Scholar] [CrossRef]
- Zelikovich, D.; Dery, S.; Bruchiel-Spanier, N.; Tal, N.; Savchenko, P.; Gross, E.; Mandler, D. Shell−Matrix Interaction in Nanoparticle-Imprinted Matrices: Implications for Selective Nanoparticle Detection and Separation. ACS Appl. Nano Mater. 2021, 4, 10819–10827. [Google Scholar] [CrossRef]
- Seneviratne, J.; Cox, J.A. Sol-gel materials for the solid phase extraction of metals from aqueous solution. Talanta 2000, 52, 801–806. [Google Scholar] [CrossRef]
- Adachi, T.; Sakka, S. The role of N, N-dimethylformamide, a DCCA, in the formation of silica-gel monoliths by sol-gel method. J. Non-Cryst. Solids 1988, 99, 118–128. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich Method for Gold Nanoparticle Synthesis Revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef] [PubMed]
- Pallavicini, P.; De Vita, L.; Merlin, F.; Milanese, C.; Borzenkov, M.; Taglietti, A.; Chirico, G. Suitable polymeric coatings to avoid localized surface plasmon resonance hybridization in printed patterns of photothermally responsive gold nanoinks. Molecules 2020, 25, 2499. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, J.; Zhang, X.; Liu, Y.; Zhou, D.; Sun, H.; Zhang, H.; Yang, B. Controllable Synthesis of Stable Urchin-like Gold Nanoparticles Using Hydroquinone to Tune the Reactivity of Gold Chloride. J. Phys. Chem. C 2011, 115, 3630–3637. [Google Scholar] [CrossRef]
- Gao, X.; Lu, Y.; He, S.; Li, X.; Chen, W. Colorimetric detection of iron ions (III) based on the highly sensitive plasmonic response of the N-acetyl-l-cysteine-stabilized silver nanoparticles. Anal. Chim. Acta 2015, 879, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Cychosz, K.A.; Thommes, M. Progress in the Physisorption Characterization of Nanoporous Gas Storage Materials. Engineering 2018, 4, 559–566. [Google Scholar] [CrossRef]
- Leong, Y.K. Yield stress and zeta potential of nanoparticulate silica dispersions under the influence of adsorbed hydrolysis products of metal ions—Cu(II), Al(III) and Th(IV). J. Colloid Interface Sci. 2005, 292, 557–566. [Google Scholar] [CrossRef]

| B.E.T. Specific Surface Area (SSA) (m2/g) | SSA Contributed by Micropores (%) | Average Pore Radius Calculated at P/P0 = 0.99 (nm) | Pore Radius by BJH (nm) | |
|---|---|---|---|---|
| Ag@SiO2-8 (monolith) | 609.9 (26) | 2.08 (0.22) | 1.49 (0.05) | 1.71 (0.02) |
| void@SiO2-8 (monolith) | 657.2 (29) | 20.07 (0.81) | 1.35 (0.02) | 1.62 (0.08) |
| Ag@SiO2-8 (powder) | 492.7 | 43.77 | 1.17 | 1.53 |
| void@SiO2-8 (powder) | 669.4 | 46.12 | 1.16 | 1.53 |
| Au@SiO2-115 (monolith) | 600.5 | 72.85 | 0.97 | 1.53 |
| void@SiO2-115 (monolith) | 663.4 | 74.03 | 0.98 | 1.53 |
| SiO2 (monolith) | 724.9 (27) | 54.1 (11) | 1.21 (0.11) | 1.65 (0.85) |
| Total Ag (M) | % Uptaken Ag | Uptaken Mass (μg) | Uptaken NP Number | % Occupied Cavities |
|---|---|---|---|---|
| 5.8 × 10−5 | 86% | 53.7 | 1.91 × 1013 | 5.3 |
| 1.16 × 10−4 | 73% | 91.5 | 3.25 × 1013 | 9.1 |
| 2.9 × 10−4 | 65% | 203.9 | 7.25 × 1013 | 20.2 |
| 5.8 × 10−4 | 60% | 591.1 | 2.10 × 1014 | 58.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pallavicini, P.; Preti, L.; Protopapa, M.L.; Carbone, D.; Capodieci, L.; Diaz Fernandez, Y.A.; Milanese, C.; Taglietti, A.; Doveri, L. Nanoparticle-Imprinted Silica Gel for the Size-Selective Capture of Silver Ultrafine Nanoparticles from Water. Molecules 2023, 28, 4026. https://doi.org/10.3390/molecules28104026
Pallavicini P, Preti L, Protopapa ML, Carbone D, Capodieci L, Diaz Fernandez YA, Milanese C, Taglietti A, Doveri L. Nanoparticle-Imprinted Silica Gel for the Size-Selective Capture of Silver Ultrafine Nanoparticles from Water. Molecules. 2023; 28(10):4026. https://doi.org/10.3390/molecules28104026
Chicago/Turabian StylePallavicini, Piersandro, Luca Preti, Maria L. Protopapa, Daniela Carbone, Laura Capodieci, Yuri A. Diaz Fernandez, Chiara Milanese, Angelo Taglietti, and Lavinia Doveri. 2023. "Nanoparticle-Imprinted Silica Gel for the Size-Selective Capture of Silver Ultrafine Nanoparticles from Water" Molecules 28, no. 10: 4026. https://doi.org/10.3390/molecules28104026
APA StylePallavicini, P., Preti, L., Protopapa, M. L., Carbone, D., Capodieci, L., Diaz Fernandez, Y. A., Milanese, C., Taglietti, A., & Doveri, L. (2023). Nanoparticle-Imprinted Silica Gel for the Size-Selective Capture of Silver Ultrafine Nanoparticles from Water. Molecules, 28(10), 4026. https://doi.org/10.3390/molecules28104026









