Green Inhibition of Corrosion of Aluminium Alloy 5083 by Artemisia annua L. Extract in Artificial Seawater
Abstract
1. Introduction
2. Results
2.1. High-Performance Liquid Chromatography
2.2. Electrochemical Tests
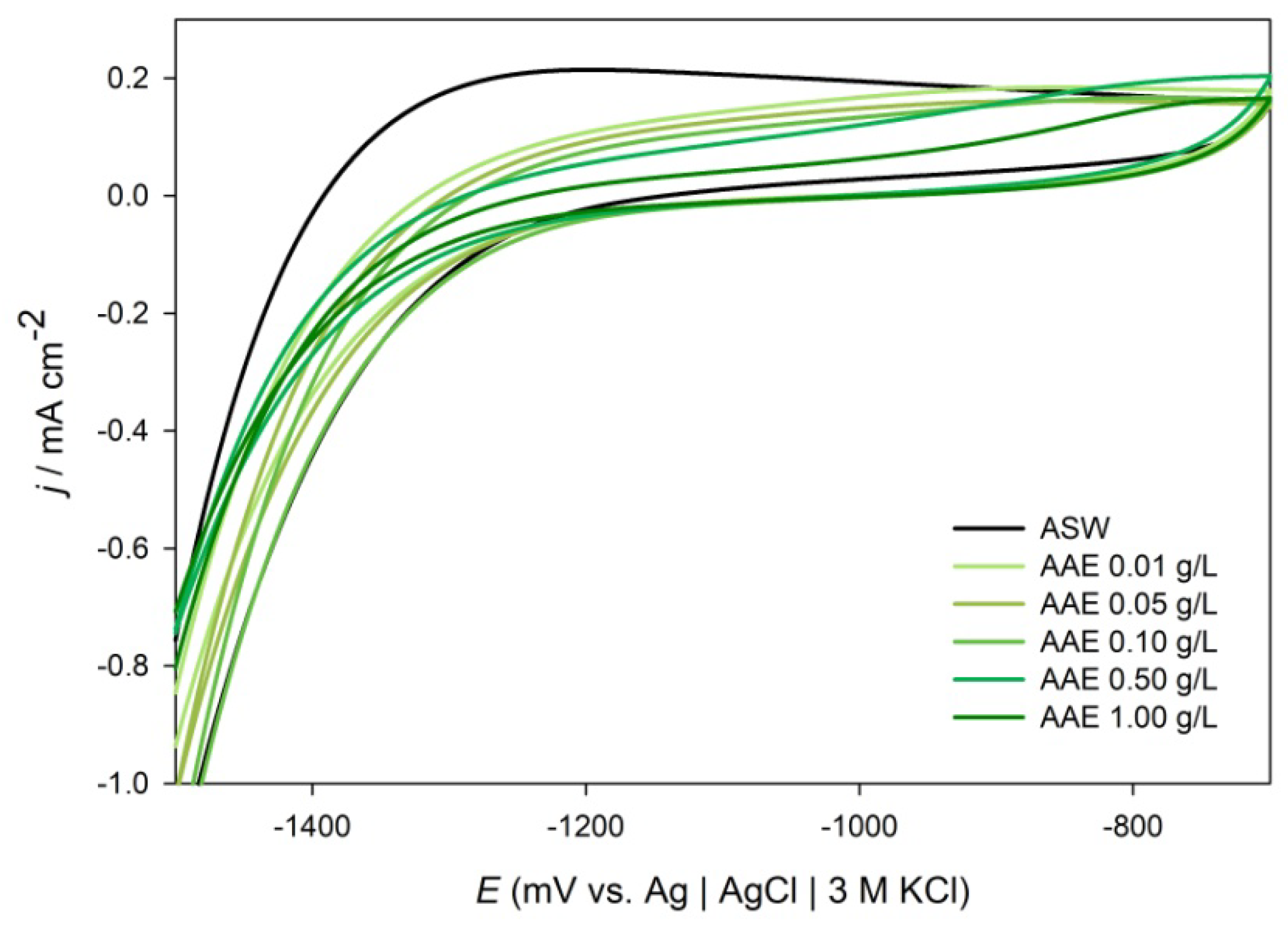
2.2.1. Cyclic Voltammetry
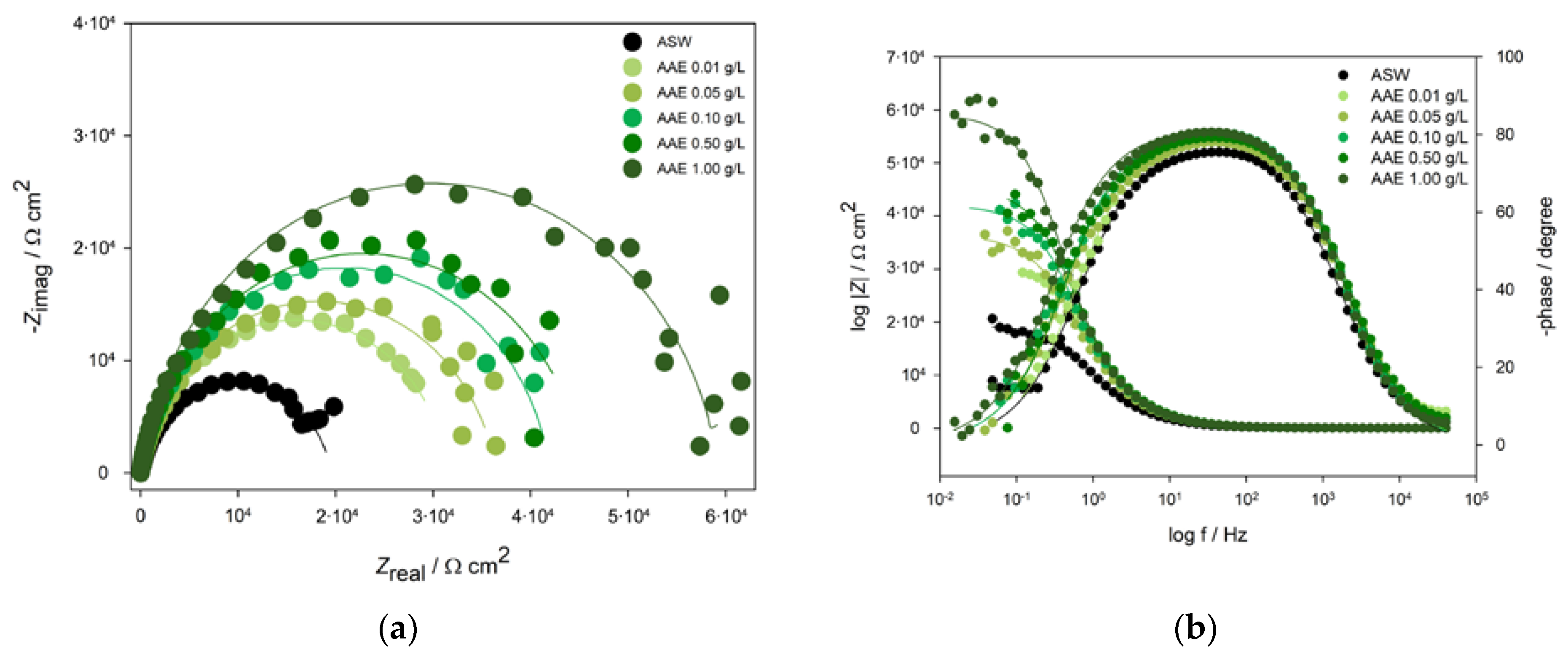
2.2.2. Electrochemical Impedance Spectroscopy
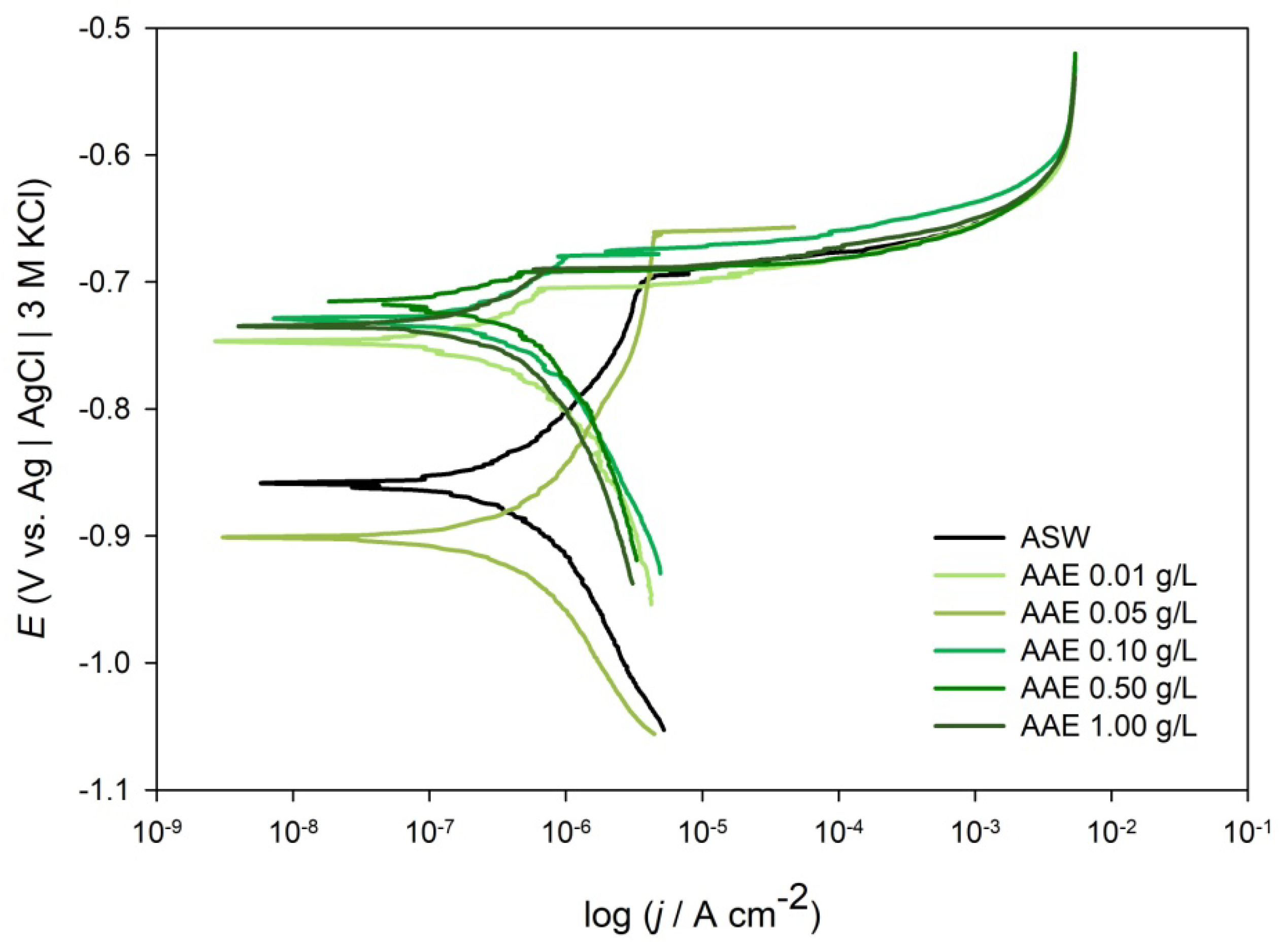
2.2.3. Potentiodynamic Polarization
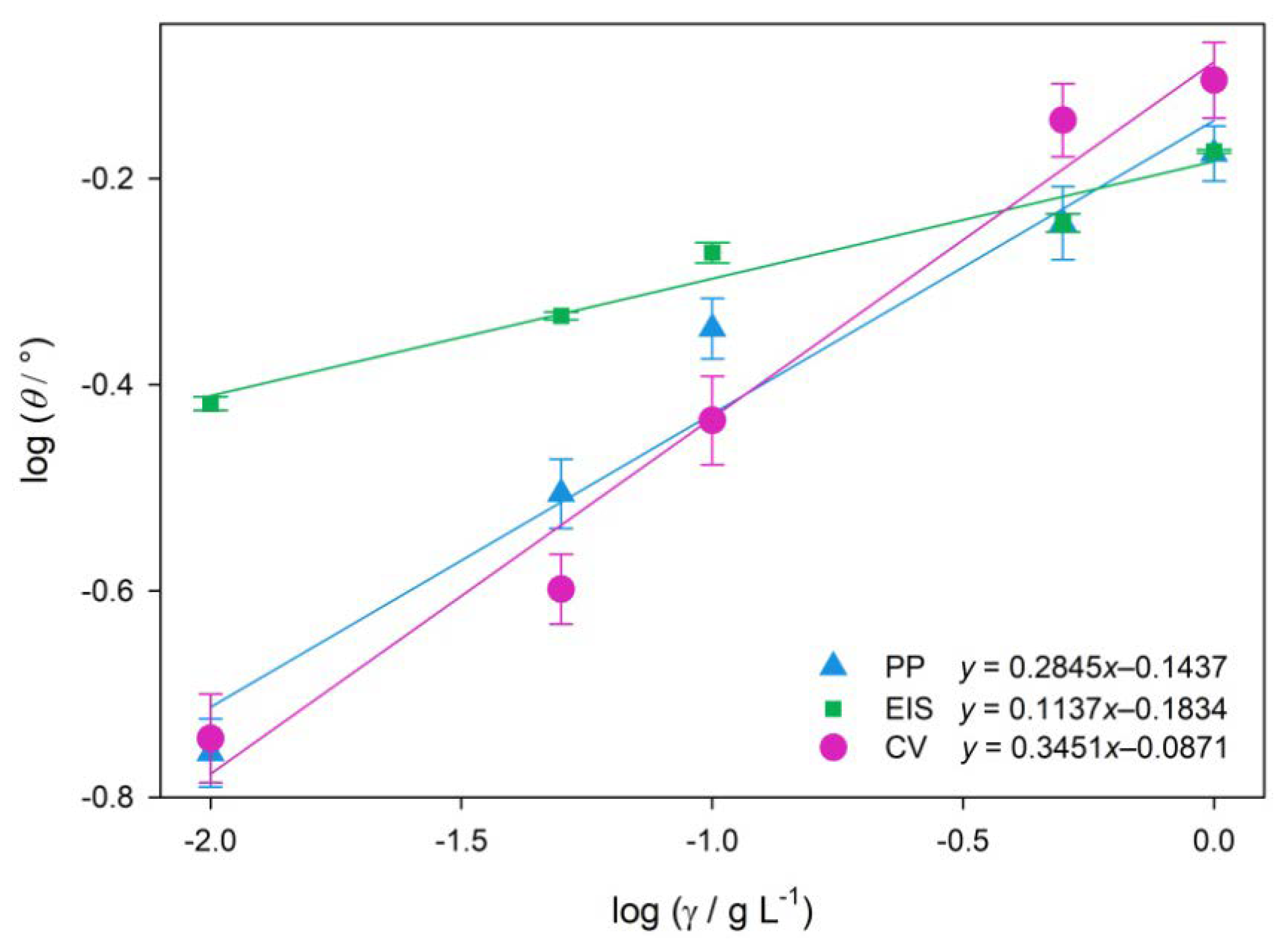
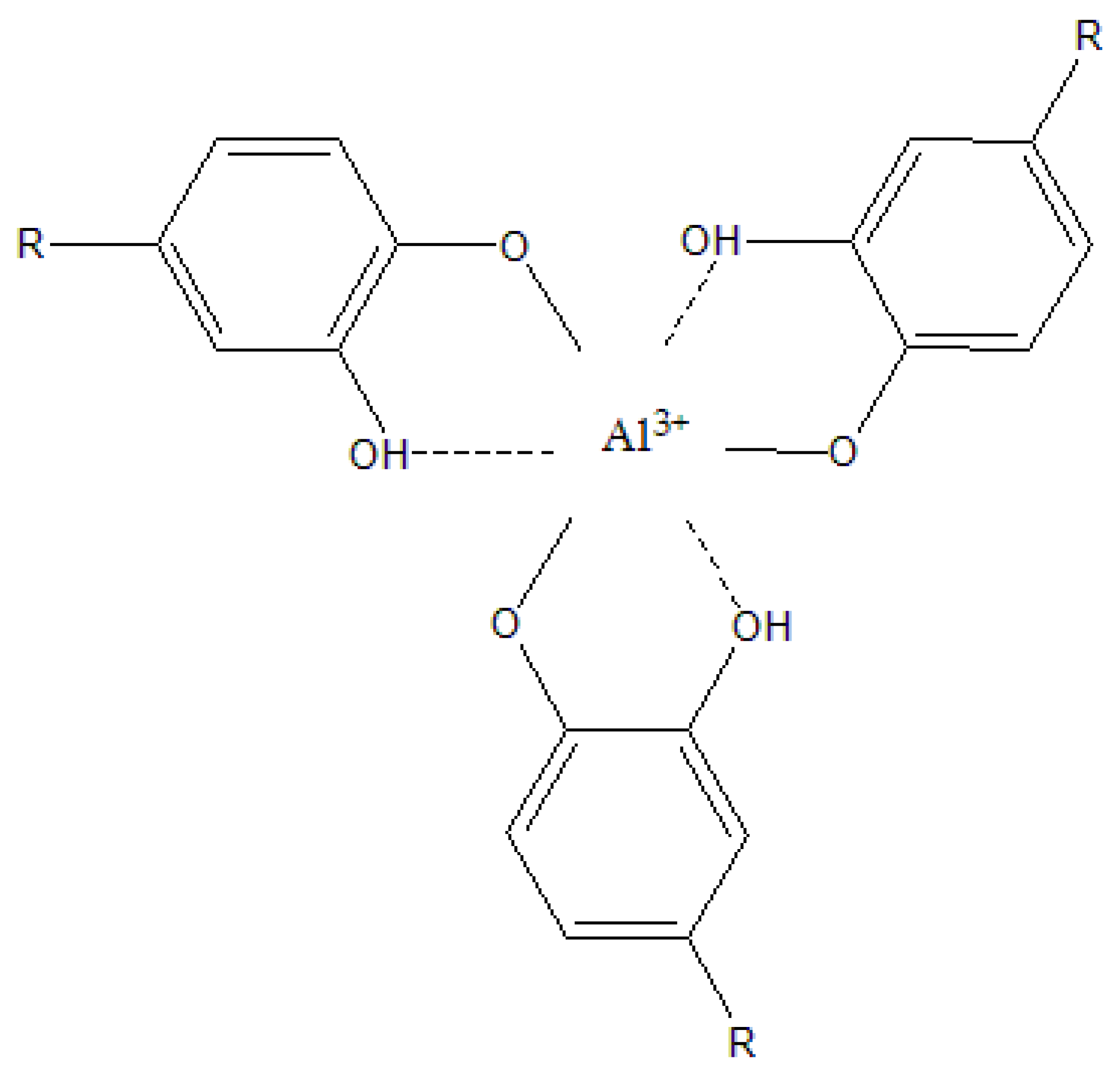
2.3. Adsorption Mechanism
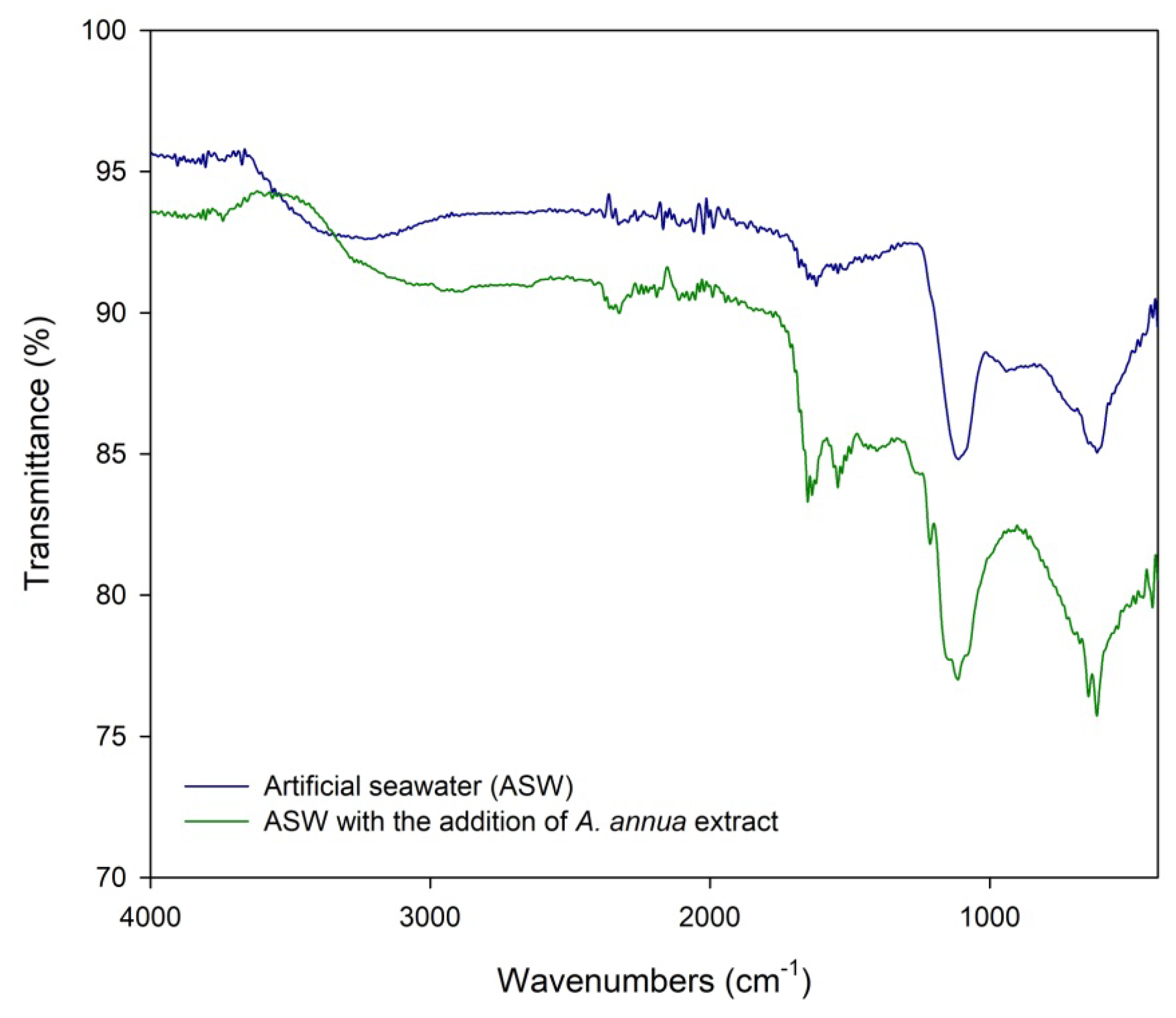
2.4. Surface Film Characterization
3. Discussion
4. Materials and Methods
4.1. The Working Electrode and Working Electrolyte
4.2. Collection and Preparation of Plant Material
4.3. High-Performance Liquid Chromatography Analysis
4.4. Electrochemical Tests
4.4.1. Cyclic Voltammetry
4.4.2. Electrochemical Impedance Spectroscopy
4.4.3. Potentiodynamic Polarization
4.5. Adsorption Mechanism
4.6. Surface Film Characterization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Maxwell, S.R. Prospects for the use of antioxidant therapies. Drugs 1995, 49, 345–361. [Google Scholar] [CrossRef]
- Sies, H. Oxidative eustress: On constant alert for redox homeostasis. Redox Biol. 2021, 41, 101867. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic Stress and Reactive Oxygen Species: Generation, Signaling, and Defense Mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.A.; Pérez De La Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Godoy, J.A.; Rios, J.A.; Picón-Pagès, P.; Herrera-Fernández, V.; Swaby, B.; Crepin, G.; Vicente, R.; Fernández-Fernández, J.M.; Muñoz, F.J. Mitostasis, Calcium and Free Radicals in Health, Aging and Neurodegeneration. Biomolecules 2021, 11, 1012. [Google Scholar] [CrossRef]
- Harman, D. Aging: Overview. Ann. N. Y. Acad. Sci. 2001, 928, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Odinga, E.S.; Waigi, M.G.; Gudda, F.O.; Wang, J.; Yang, B.; Hu, X.; Li, S.; Gao, Y. Occurrence, formation, environmental fate and risks of environmentally persistent free radicals in biochars. Environ. Int. 2020, 134, 105172. [Google Scholar] [CrossRef] [PubMed]
- Matser, A.M.; Krebbers, B.; Berg, R.W.v.d.; Bartels, P.V. Advantages of high pressure sterilisation on quality of food products. Trends Food Sci. Technol. 2004, 15, 79–85. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Novel antioxidants in food quality preservation and health promotion. Eur. J. Lipid Sci. Technol. 2010, 112, 930–940. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative Stress, Plant Natural Antioxidants, and Obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Santos, M.J.M.C.; Silva, L.K.R.; Pereira, L.C.L.; Santos, I.A.; Lannes, S.C.d.S.; Silva, M.V.d. Natural antioxidants used in meat products: A brief review. Meat Sci. 2019, 148, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Vaiserman, A.; Koliada, A.; Zayachkivska, A.; Lushchak, O. Nanodelivery of Natural Antioxidants: An Anti-aging Perspective. Front. Bioeng. Biotechnol. 2020, 7, 447. [Google Scholar] [CrossRef] [PubMed]
- Al-Juhaimi, F.; Ghafoor, K.; Özcan, M.M.; Jahurul, M.H.A.; Babiker, E.E.; Jinap, S.; Sahena, F.; Sharifudin, M.S.; Zaidul, I.S.M. Effect of various food processing and handling methods on preservation of natural antioxidants in fruits and vegetables. J. Food Sci. Technol. 2018, 55, 3872–3880. [Google Scholar] [CrossRef]
- Anand, S.; Sati, N. Artificial Preservatives and their harmful effects: Looking toward nature for safer alternatives. Int. J. Pharm. Sci. Res. 2013, 4, 2496–2501. [Google Scholar] [CrossRef]
- Chiang, Y.-F.; Chen, H.-Y.; Ali, M.; Shieh, T.-M.; Huang, Y.-J.; Wang, K.-L.; Chang, H.-Y.; Huang, T.-C.; Hong, Y.-H.; Hsia, S.-M. The Role of Cell Proliferation and Extracellular Matrix Accumulation Induced by Food Additive Butylated Hydroxytoluene in Uterine Leiomyoma. Nutrients 2021, 13, 3074. [Google Scholar] [CrossRef] [PubMed]
- Choe, S.; Yang, K. Toxicological studies of antioxidants, butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA). Korean J. Food Sci. Technol. 1982, 14, 283–288. [Google Scholar]
- Pateiro, M.; Gómez-Salazar, J.A.; Jaime-Patlán, M.; Sosa-Morales, M.E.; Lorenzo, J.M. Plant Extracts Obtained with Green Solvents as Natural Antioxidants in Fresh Meat Products. Antioxidants 2021, 10, 181. [Google Scholar] [CrossRef]
- de Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef]
- Gupta, V.K.; Sharma, S.K. Plants as natural antioxidants. Indian J. Nat. Prod. Resour. 2006, 5, 326–334. [Google Scholar]
- Naik, S. Antioxidants and their role in biological functions: An overview. Indian Drugs 2003, 40, 501–508. [Google Scholar] [CrossRef]
- Pratt, D.E. Natural Antioxidants from Plant Material. In Phenolic Compounds in Food and Their Effects on Health II; American Chemical Society: Washington, DC, USA, 1992; Volume 507, pp. 54–71. [Google Scholar]
- Amarowicz, R.; Pegg, R.B. Natural antioxidants of plant origin. Adv. Food Nutr. Res. 2019, 90, 1–81. [Google Scholar] [CrossRef] [PubMed]
- Leopoldini, M.; Marino, T.; Russo, N.; Toscano, M. Antioxidant Properties of Phenolic Compounds: H-Atom versus Electron Transfer Mechanism. J. Phys. Chem. A 2004, 108, 4916–4922. [Google Scholar] [CrossRef]
- Wright, J.S.; Johnson, E.R.; DiLabio, G.A. Predicting the Activity of Phenolic Antioxidants: Theoretical Method, Analysis of Substituent Effects, and Application to Major Families of Antioxidants. J. Am. Chem. Soc. 2001, 123, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Ko, X.; Sharma, S. Adsorption and Self-Assembly of Surfactants on Metal-Water Interfaces. J. Phys. Chem. B 2017, 121, 10364–10370. [Google Scholar] [CrossRef]
- Khokhar, S.; Owusu Apenten, R.K. Iron binding characteristics of phenolic compounds: Some tentative structure–activity relations. Food Chem. 2003, 81, 133–140. [Google Scholar] [CrossRef]
- Zulkifli, F.; Ali, N.A.; Yusof, M.S.M.; Khairul, W.M.; Rahamathullah, R.; Isa, M.I.N.; Wan Nik, W.B. The Effect of Concentration of Lawsonia inermis as a Corrosion Inhibitor for Aluminum Alloy in Seawater. Adv. Phys. Chem. 2017, 2017, 8521623. [Google Scholar] [CrossRef]
- Kovačević, N.; Kokalj, A. The relation between adsorption bonding and corrosion inhibition of azole molecules on copper. Corros. Sci. 2013, 73, 7–17. [Google Scholar] [CrossRef]
- Udensi, S.C.; Ekpe, O.E.; Nnanna, L.A. Corrosion inhibition performance of low cost and eco-friendly Treculia africana leaves extract on aluminium alloy AA7075-T7351 in 2.86% NaCl solutions. Sci. Afr. 2021, 12, e00791. [Google Scholar] [CrossRef]
- Raghavendra, N. Green Compounds to Attenuate Aluminum Corrosion in HCl Activation: A Necessity Review. Chem. Afr. 2020, 3, 21–34. [Google Scholar] [CrossRef]
- Alrefaee, S.H.; Rhee, K.Y.; Verma, C.; Quraishi, M.A.; Ebenso, E.E. Challenges and advantages of using plant extract as inhibitors in modern corrosion inhibition systems: Recent advancements. J. Mol. Liq. 2021, 321, 114666. [Google Scholar] [CrossRef]
- Martinović, I.; Pilić, Z.; Zlatić, G.; Barišić, M.; Čelan, S. Corrosion Inhibition of Aluminium by Alchemilla vulgaris L. Extract in 3% NaCl Solution. Croat. Chem. Acta 2021, 94, 103–109. [Google Scholar] [CrossRef]
- Salleh, S.Z.; Yusoff, A.H.; Zakaria, S.K.; Taib, M.A.A.; Abu Seman, A.; Masri, M.N.; Mohamad, M.; Mamat, S.; Ahmad Sobri, S.; Ali, A.; et al. Plant extracts as green corrosion inhibitor for ferrous metal alloys: A review. J. Clean. Prod. 2021, 304, 127030. [Google Scholar] [CrossRef]
- Pilic, Z.; Martinovic, I.; Pavlinovic, M.; Zlatic, G. Effect of Helichrysum italicum on the Electrochemical Corrosion Behaviour of Iron in Simulated Acid Rain Solution. Croat. Chem. Acta 2019, 92, 79–86. [Google Scholar] [CrossRef]
- Boudalia, M.; Fernández-Domene, R.M.; Tabyaoui, M.; Bellaouchou, A.; Guenbour, A.; García-Antón, J. Green approach to corrosion inhibition of stainless steel in phosphoric acid of Artemesia herba albamedium using plant extract. J. Mater. Res. Technol. 2019, 8, 5763–5773. [Google Scholar] [CrossRef]
- Pilić, Z.; Dragičević, I.; Martinović, I. The anti-corrosion behaviour of Satureja montana L. extract on iron in NaCl solution. Open Chem. 2019, 17, 1087–1094. [Google Scholar] [CrossRef]
- Motamedi, M.; Ramezanzadeh, B.; Mahdavian, M. Corrosion inhibition properties of a green hybrid pigment based on Pr-Urtica Dioica plant extract. J. Ind. Eng. Chem. 2018, 66, 116–125. [Google Scholar] [CrossRef]
- Chaudhary, S.; Tak, R.K. Natural corrosion inhibition and adsorption characteristics of tribulus terrestris plant extract on aluminium in hydrochloric acid environment. Biointerface Res. Appl. Chem. 2022, 12, 2603–2617. [Google Scholar] [CrossRef]
- Pilić, Z.; Martinović, I.; Zlatić, G. Electrochemical behaviour of iron in simulated acid rain in presence of Achillea millefolium L. Int. J. Electrochem. Sci. 2018, 13, 5151–5163. [Google Scholar] [CrossRef]
- Chung, I.-M.; Malathy, R.; Priyadharshini, R.; Hemapriya, V.; Kim, S.-H.; Prabakaran, M. Inhibition of mild steel corrosion using Magnolia kobus extract in sulphuric acid medium. Mater. Today Commun. 2020, 25, 101687. [Google Scholar] [CrossRef]
- Zlatić, G.; Arapović, A.; Martinović, I.; Martinović Bevanda, A.; Bošković, P.; Prkić, A.; Paut, A.; Vukušić, T. Antioxidant Capacity of Herzegovinian Wildflowers Evaluated by UV–VIS and Cyclic Voltammetry Analysis. Molecules 2022, 27, 5466. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Cao, S.; Qiu, F.; Zhang, B. Traditional application and modern pharmacological research of Artemisia annua L. Pharmacol. Ther. 2020, 216, 107650. [Google Scholar] [CrossRef] [PubMed]
- Ekiert, H.; Świątkowska, J.; Klin, P.; Rzepiela, A.; Szopa, A. Artemisia annua–Importance in Traditional Medicine and Current State of Knowledge on the Chemistry, Biological Activity and Possible Applications. Planta Med. 2021, 87, 584–599. [Google Scholar] [CrossRef] [PubMed]
- Mirbehbahani, F.S.; Hejazi, F.; Najmoddin, N.; Asefnejad, A. Artemisia annua L. as a promising medicinal plant for powerful wound healing applications. Prog. Biomater. 2020, 9, 139–151. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Wenli, S.; Cheng, Q. Exploring Artemisia annua L., artemisinin and its derivatives, from traditional Chinese wonder medicinal science. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 1719–1741. [Google Scholar] [CrossRef]
- The Plant List Version 1.1. Available online: http://www.theplantlist.org/ (accessed on 2 October 2022).
- Carbonara, T.; Pascale, R.; Argentieri, M.P.; Papadia, P.; Fanizzi, F.P.; Villanova, L.; Avato, P. Phytochemical analysis of a herbal tea from Artemisia annua L. J. Pharm. Biomed. Anal. 2012, 62, 79–86. [Google Scholar] [CrossRef]
- Suberu, J.O.; Gorka, A.P.; Jacobs, L.; Roepe, P.D.; Sullivan, N.; Barker, G.C.; Lapkin, A.A. Anti-plasmodial polyvalent interactions in Artemisia annua L. aqueous extract–possible synergistic and resistance mechanisms. PLoS ONE 2013, 8, e80790. [Google Scholar] [CrossRef]
- Ijsseling, F. General guidelines for corrosion testing of materials for marine applications: Literature review on sea water as test environment. Br. Corros. J. 1989, 24, 53–78. [Google Scholar] [CrossRef]
- Davis, J.R. Corrosion of Aluminum and Aluminum Alloys; ASM International: Almere, The Netherlands, 1999. [Google Scholar]
- Ashkenazi, D. How aluminum changed the world: A metallurgical revolution through technological and cultural perspectives. Technol. Forecast. Soc. Change 2019, 143, 101–113. [Google Scholar] [CrossRef]
- Sharma, A.K.; Bhandari, R.; Aherwar, A.; Rimašauskienė, R.; Pinca-Bretotean, C. A study of advancement in application opportunities of aluminum metal matrix composites. Mater. Today Proc. 2020, 26, 2419–2424. [Google Scholar] [CrossRef]
- Guan, F.; Zhai, X.; Duan, J.; Zhang, J.; Li, K.; Hou, B. Influence of sulfate-reducing bacteria on the corrosion behavior of 5052 aluminum alloy. Surf. Coat. Technol. 2017, 316, 171–179. [Google Scholar] [CrossRef]
- San, N.O.; Nazır, H.; Dönmez, G. Evaluation of microbiologically influenced corrosion inhibition on Ni–Co alloy coatings by Aeromonas salmonicida and Clavibacter michiganensis. Corros. Sci. 2012, 65, 113–118. [Google Scholar] [CrossRef]
- Hamulić, D.; Rodič, P.; Poberžnik, M.; Jereb, M.; Kovač, J.; Milošev, I. The effect of the methyl and ethyl group of the acrylate precursor in hybrid silane coatings used for corrosion protection of aluminium alloy 7075-T6. Coatings 2020, 10, 172. [Google Scholar] [CrossRef]
- Buchheit, R. A compilation of corrosion potentials reported for intermetallic phases in aluminum alloys. J. Electrochem. Soc. 1995, 142, 3994. [Google Scholar] [CrossRef]
- Muralidharan, S.; Dinakar, N. A comparative investigation on weld metal properties of similar and dissimilar TIG welded joints on Al-Mg alloys. Mater. Today Proc. 2022, 62, 2217–2223. [Google Scholar] [CrossRef]
- Jiao, Z.-h.; Lang, L.-h.; Zhao, X.-n. 5A06-O aluminium–magnesium alloy sheet warm hydroforming and optimization of process parameters. Trans. Nonferrous Met. Soc. China 2021, 31, 2939–2948. [Google Scholar] [CrossRef]
- Cinto, C.; Sreejith, P. Development of High Strength to Weight Ratio Aluminium–Magnesium Alloy with Enhanced Corrosion Resistance. IRJET 2019, 6, 1010–1032. [Google Scholar]
- Hasenay, D.; Šeruga, M. The growth kinetics and properties of potentiodynamically formed thin oxide films on aluminium in citric acid solutions. J. Appl. Electrochem. 2007, 37, 1001–1008. [Google Scholar] [CrossRef]
- Gudić, S.; Radošević, J.; Krpan-Lisica, D.; Kliškić, M. Anodic film growth on aluminium and Al–Sn alloys in borate buffer solutions. Electrochim. Acta 2001, 46, 2515–2526. [Google Scholar] [CrossRef]
- Pilic, Z.; Martinovic, I.; Yan, Y.; Li, W.; Cai, L.; Hau, B. A comparative study on the electrochemical behaviour of aluminium and 8090 Al-Li-Cu-Mg alloy in acid rain solution. Int. J. Electrochem. Sci. 2017, 12, 3576–3588. [Google Scholar] [CrossRef]
- Salghi, R.; Jodeh, S.; Ebenso, E.E. Inhibition of C-steel corrosion by green tea extract in hydrochloric solution. Int. J. Electrochem. Sci. 2017, 12, 3283–3295. [Google Scholar] [CrossRef]
- Pilić, Z.; Martinović, I. Effect of Helichrysum italicum on the corrosion of copper in simulated acid rain solution. Chem. Biochem. Eng. Q. 2019, 33, 449–457. [Google Scholar] [CrossRef]
- Fouda, A.; Gadow, H.; Abd Elal, E.; El-Tantawy, M. Corrosion Inhibition of Aluminium by Rice Straw Extract in 2 M Hydrochloric Acid Solution. J. Bio-Tribo-Corros. 2021, 7, 102. [Google Scholar] [CrossRef]
- Eldesoky, A.; Diab, M.; El-Sonbati, A.; Salam, S. Anti-corrosive properties of new eco-friendly dimethylamino compounds on C-steel corrosion in 2 M HCl. Int. J. Electrochem. Sci. 2017, 12, 4215–4237. [Google Scholar] [CrossRef]
- Halambek, J.; Berković, K.; Vorkapić-Furač, J. Laurus nobilis L. oil as green corrosion inhibitor for aluminium and AA5754 aluminium alloy in 3% NaCl solution. Mater. Chem. Phys. 2013, 137, 788–795. [Google Scholar] [CrossRef]
- Pereira, S.S.d.A.A.; Pegas, M.M.; Fernandez, T.L.; Magalhaes, M.; Schöntag, T.G.; Lago, D.C.; de Senna, L.F.; D’Elia, E. Inhibitory action of aqueous garlic peel extract on the corrosion of carbon steel in HCl solution. Corros. Sci. 2012, 65, 360–366. [Google Scholar] [CrossRef]
- Lekbach, Y.; Bennouna, F.; El Abed, S.; Balouiri, M.; El Azzouzi, M.; Aouniti, A.; Ibnsouda Koraichi, S. Green Corrosion Inhibition and Adsorption Behaviour of Cistus ladanifer Extract on 304L Stainless Steel in Hydrochloric Acid Solution. Arab. J. Sci. Eng. 2021, 46, 103–113. [Google Scholar] [CrossRef]
- Mahalakshmi, P.; Rajendran, S.; Nandhini, G.; Joycee, S.; Vijaya, N.; Umasankareswari, T.; Renuga, D.N. Inhibition of corrosion of mild steel in sea water by an aqueous extract of turmeric powder. Int. J. Corros. Scale Inhib. 2020, 9, 706–725. [Google Scholar] [CrossRef]
- Brown, T. The infrared carbonyl absorption in some p-quinones and related substances. Spectrochim. Acta 1962, 18, 1065–1071. [Google Scholar] [CrossRef]
- Boumaza, A.; Djelloul, A.; Guerrab, F. Specific signatures of α-alumina powders prepared by calcination of boehmite or gibbsite. Powder Technol. 2010, 201, 177–180. [Google Scholar] [CrossRef]
- Hariharan, M.; Varghese, N.; Cherian, A.B.; Sreenivasan, P.V.; Paul, J.; Asmy Antony, K.A. Synthesis and Characterisation of CaCO3 (Calcite) Nano Particles from Cockle Shells Using Chitosan as Precursor. Int. J. Sci. Res. Publ. 2014, 4, 1–5. [Google Scholar]
- Favaro, L.; Boumaza, A.; Roy, P.; Lédion, J.; Sattonnay, G.; Brubach, J.B.; Huntz, A.M.; Tétot, R. Experimental and ab initio infrared study of χ-, κ- and α-aluminas formed from gibbsite. J. Solid State Chem. 2010, 183, 901–908. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Niaei, A.; Salari, D. Production of γ-Al2O3 from Kaolin. Open J. Phys. Chem. 2011, 1, 23–27. [Google Scholar] [CrossRef]
- Romero Toledo, R.; Ruiz Santoyo, V.; Moncada Sánchez, C.D.; Martínes Rosales, M. Effect of aluminum precursor on physicochemical properties of Al2O3 by hydrolysis/precipitation method. Nova Sci. 2018, 10, 83–99. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.; Xu, Y.; Han, B.; Wang, Y.; Liu, X.; Yan, Z. Synthesis and characterization of mesoporous Si-modified alumina with high thermal stability. Microporous Mesoporous Mater. 2017, 238, 84–89. [Google Scholar] [CrossRef]
- Farahmandjou, M.; Khodadadi, A.; Yaghoubi, M. Low Concentration Iron-Doped Alumina (Fe/Al2O3) Nanoparticles Using Co-Precipitation Method. J. Supercond. Nov. Magn. 2020, 33, 3425–3432. [Google Scholar] [CrossRef]
- Khodadadi, A.; Farahmandjou, M.; Yaghoubi, M. Investigation on synthesis and characterization of Fe-doped Al2O3 nanocrystals by new sol–gel precursors. Mater. Res. Express 2018, 6, 025029. [Google Scholar] [CrossRef]
- Zhu, L.; Sun, C.; Chen, L.; Lu, X.; Li, S.; Ye, G.; Liu, L. Influences of NH4F additive and calcination time on the morphological evolution of α-Al2O3 from a milled γ-Al2O3 precursor. Z. Naturforsch. B 2017, 72, 665–670. [Google Scholar] [CrossRef]
- Prashanth, P.A.; Raveendra, R.S.; Hari Krishna, R.; Ananda, S.; Bhagya, N.P.; Nagabhushana, B.M.; Lingaraju, K.; Raja Naika, H. Synthesis, characterizations, antibacterial and photoluminescence studies of solution combustion-derived α-Al2O3 nanoparticles. J. Asian Ceram. Soc. 2015, 3, 345–351. [Google Scholar] [CrossRef]
- Aman, Y.; Rossignol, C.; Garnier, V.; Djurado, E. Low temperature synthesis of ultrafine non vermicular α-alumina from aerosol decomposition of aluminum nitrates salts. J. Eur. Ceram. Soc. 2013, 33, 1917–1928. [Google Scholar] [CrossRef]
- Rabu, R.; Jewena, N.; Das, S.; Khandaker, J.; Ahmed, F. Synthesis of metal-oxide (Al2O3) nanoparticles by using autoclave for the efficient absorption of heavy metal ions. J. Nanomater. Mol. Nanotechnol. 2020, 9, 6. [Google Scholar]
- Tang, B.; Ge, J.; Zhuo, L.; Wang, G.; Niu, J.; Shi, Z.; Dong, Y. A Facile and Controllable Synthesis of γ-Al2O3 Nanostructures without a Surfactant. Eur. J. Inorg. Chem. 2005, 2005, 4366–4369. [Google Scholar] [CrossRef]
- Abdel-Gawad, S.A.; Osman, W.M.; Fekry, A.M. Characterization and corrosion behavior of anodized Aluminum alloys for military industries applications in artificial seawater. Surf. Interfaces 2019, 14, 314–323. [Google Scholar] [CrossRef]
- Świsłocka, R.; Kalinowska, M.; Ferenc, W.; Sarzyński, J.; Lewandowski, W. Spectroscopic and magnetic properties of Cu(II) complexes with selected biologically important ligands. Open Chem. 2012, 10, 1095–1105. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, T.; Zhu, M. A comparison of the surface properties of lignin and sulfonated lignins by FTIR spectroscopy and wicking technique. Colloids Surf. A Physicochem. Eng. Asp. 2008, 320, 57–60. [Google Scholar] [CrossRef]
- Qin, Y.; Yang, D.; Guo, W.; Qiu, X. Investigation of grafted sulfonated alkali lignin polymer as dispersant in coal-water slurry. J. Ind. Eng. Chem. 2015, 27, 192–200. [Google Scholar] [CrossRef]
- Islamović, S.; Korać, F.; Ostojić, J.; Kezo, M.; Gutić, S.; Koštroman, L.; Halilović, A. Corrosion Characteristics of Raw and Anodised Aluminium. Kem. Ind. Čas. Kem. Kem. Inž. Hrvat. 2013, 62, 241–246. [Google Scholar]
- Liang, Z.; Jiang, K.; Zhang, T.; Dou, Z. Corrosion behavior of Cu–Sn bronze alloys in simulated archeological soil media. Mater. Corros. 2019, 71, 617–627. [Google Scholar] [CrossRef]
- Lekbach, Y.; Li, Z.; Xu, D.; El Abed, S.; Dong, Y.; Liu, D.; Gu, T.; Koraichi, S.I.; Yang, K.; Wang, F. Salvia officinalis extract mitigates the microbiologically influenced corrosion of 304L stainless steel by Pseudomonas aeruginosa biofilm. Bioelectrochemistry 2019, 128, 193–203. [Google Scholar] [CrossRef]
- Deyab, M. Corrosion inhibition of aluminum in biodiesel by ethanol extracts of Rosemary leaves. J. Taiwan Inst. Chem. Eng. 2016, 58, 536–541. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M.; Obot, I.B.; Suleiman, R.K. A critical review on the recent studies on plant biomaterials as corrosion inhibitors for industrial metals. J. Ind. Eng. Chem. 2019, 76, 91–115. [Google Scholar] [CrossRef]
- Chellouli, M.; Chebabe, D.; Dermaj, A.; Erramli, H.; Bettach, N.; Hajjaji, N.; Casaletto, M.P.; Cirrincione, C.; Privitera, A.; Srhiri, A. Corrosion inhibition of iron in acidic solution by a green formulation derived from Nigella sativa L. Electrochim. Acta 2016, 204, 50–59. [Google Scholar] [CrossRef]
- Kokalj, A. Corrosion inhibitors: Physisorbed or chemisorbed? Corros. Sci. 2022, 196, 109939. [Google Scholar] [CrossRef]
- Hotta, H.; Ueda, M.; Nagano, S.; Tsujino, Y.; Koyama, J.; Osakai, T. Mechanistic study of the oxidation of caffeic acid by digital simulation of cyclic voltammograms. Anal. Biochem. 2002, 303, 66–72. [Google Scholar] [CrossRef]
- Trabelsi, S.K.; Tahar, N.B.; Trabelsi, B.; Abdelhedi, R. Electrochemical Oxidation of Ferulic Acid in Aqueous Solutions at Gold Oxide and Lead Dioxide Electrodes. J. Appl. Electrochem. 2005, 35, 967–973. [Google Scholar] [CrossRef]
- John, M.; Gumbinger, H.G.; Winterhoff, H. Oxidation products of caffeic acid as model substances for the antigonadotropic activity of plant extracts. Planta Med. 1990, 56, 14–18. [Google Scholar] [CrossRef]
- Dickson, A.G. Thermodynamics of the dissociation of boric acid in synthetic seawater from 273.15 to 318.15 K. Deep Sea Res. Part A Oceanogr. Res. Pap. 1990, 37, 755–766. [Google Scholar] [CrossRef]
- Čulum, D. Hemijski Sastav i Biološka Aktivnost Odabranih Ljekovitih i Endemičnih Biljaka BiH. Ph.D. Thesis, University of Sarajevo, Sarajevo, Bosnia and Herzegovina, 2022. [Google Scholar]
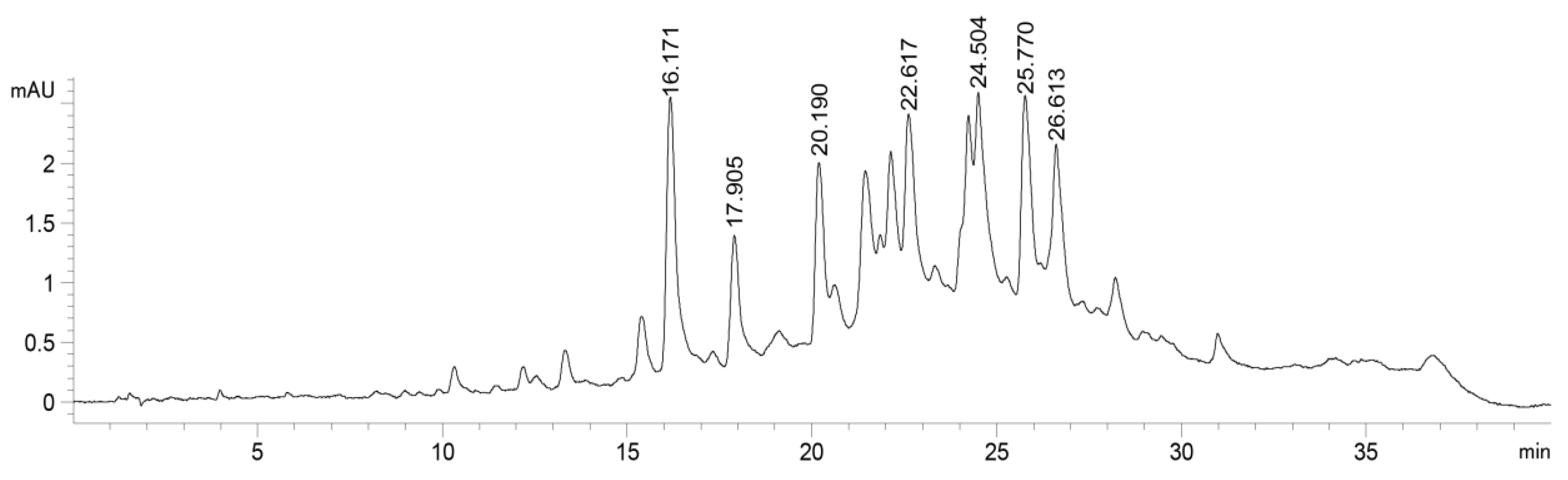

| Rt (min) | Compound | Molecular Formula | γ (ppm) | LOD (ppm) | LOQ (ppm) |
|---|---|---|---|---|---|
| 16.171 | Chlorogenic acid | C16H18O9 | 28.64 ± 0.55 | 4.05 | 12.28 |
| 17.905 | Caffeic acid | C9H8O4 | 7.70 ± 0.08 | 0.58 | 1.75 |
| γ (g L−1) | QA (µC cm−2) | d (nm) | θ | η (%) |
|---|---|---|---|---|
| 0 | 36.4 | 0.99 | − | − |
| 0.01 | 29.8 | 0.81 | 0.181 | 18.1 |
| 0.05 | 27.2 | 0.75 | 0.252 | 25.2 |
| 0.10 | 23.0 | 0.63 | 0.368 | 36.8 |
| 0.50 | 10.2 | 0.28 | 0.719 | 71.9 |
| 1.00 | 7.8 | 0.21 | 0.786 | 78.6 |
| γ | R | R1 | CPE1 × 10−6 | n1 | θ | η |
|---|---|---|---|---|---|---|
| g L−1 | Ω cm−2 | kΩ cm−2 | Ω−1sn cm−2 | % | ||
| 0 | 19.3 | 19.53 | 14.9 | 0.879 | – | – |
| 0.01 | 19.6 | 31.59 | 9.4 | 0.905 | 0.382 | 38.2 |
| 0.05 | 19.6 | 36.47 | 11.4 | 0.887 | 0.464 | 46.4 |
| 0.10 | 18.3 | 41.97 | 9.9 | 0.912 | 0.534 | 53.4 |
| 0.50 | 19.6 | 45.57 | 9.3 | 0.902 | 0.571 | 57.1 |
| 1.00 | 19.3 | 59.29 | 9.9 | 0.912 | 0.670 | 67.0 |
| γ | Ecorr | jcorr | βa | βc | θ | η |
|---|---|---|---|---|---|---|
| g L−1 | V | μA cm−2 | mV dec−1 | mV dec−1 | % | |
| 0 | –0.859 | 0.673 | 0.197 | 0.238 | – | – |
| 0.01 | –0.735 | 0.556 | 0.167 | 0.228 | 0.175 | 17.5 |
| 0.05 | –0.800 | 0.463 | 0.141 | 0.171 | 0.312 | 31.2 |
| 0.10 | –0.727 | 0.369 | 0.107 | 0.124 | 0.451 | 45.1 |
| 0.50 | –0.716 | 0.289 | 0.081 | 0.145 | 0.571 | 57.1 |
| 1.00 | –0.735 | 0.224 | 0.077 | 0.102 | 0.667 | 66.7 |
| AAE Concentration (g L−1) | Dissolved Al3+ (μg L−1 cm−2) | Corrosion Rate (μg cm−2 h−1) | η (%) |
|---|---|---|---|
| 0 (1 h) | 15.30 | 0.765 | – |
| 1.00 (1 h) | 3.09 | 0.155 | 79.73 |
| 0 (24 h) | 17.85 | 0.037 | – |
| 1.00 (24 h) | 15.45 | 0.032 | 13.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zlatić, G.; Martinović, I.; Pilić, Z.; Paut, A.; Mitar, I.; Prkić, A.; Čulum, D. Green Inhibition of Corrosion of Aluminium Alloy 5083 by Artemisia annua L. Extract in Artificial Seawater. Molecules 2023, 28, 2898. https://doi.org/10.3390/molecules28072898
Zlatić G, Martinović I, Pilić Z, Paut A, Mitar I, Prkić A, Čulum D. Green Inhibition of Corrosion of Aluminium Alloy 5083 by Artemisia annua L. Extract in Artificial Seawater. Molecules. 2023; 28(7):2898. https://doi.org/10.3390/molecules28072898
Chicago/Turabian StyleZlatić, Gloria, Ivana Martinović, Zora Pilić, Andrea Paut, Ivana Mitar, Ante Prkić, and Dušan Čulum. 2023. "Green Inhibition of Corrosion of Aluminium Alloy 5083 by Artemisia annua L. Extract in Artificial Seawater" Molecules 28, no. 7: 2898. https://doi.org/10.3390/molecules28072898
APA StyleZlatić, G., Martinović, I., Pilić, Z., Paut, A., Mitar, I., Prkić, A., & Čulum, D. (2023). Green Inhibition of Corrosion of Aluminium Alloy 5083 by Artemisia annua L. Extract in Artificial Seawater. Molecules, 28(7), 2898. https://doi.org/10.3390/molecules28072898








