Abstract
The Cannabis genus of plants has been widely used in different cultures for various purposes. It is separated into three main species: sativa, indica, and ruderalis. In ancient practices, the plant was used as a multipurpose crop and valued for its fiber, food, and medicinal uses. Since methodologies for the extraction, processing, and identification of components have become available, medical, and food applications have been increasing, allowing potential development in the pharmaceutical and healthy functional food industries. Although the growing legalization and adoption of cannabis for the treatment of diseases are key factors pushing the growth of its market, the biggest challenge is to obtain higher-quality products in a time- and cost-effective fashion, making the process of extraction and separation an essential step. Latin American countries exhibit great knowledge of extraction technologies; nevertheless, it is still necessary to verify whether production costs are economically profitable. In addition, there has been an increase in commercial cannabis products that may or may not be allowed, with or without quality fact sheets, which can pose health risks. Hence, legalization is mandatory and urgent for the rest of Latin American countries. In this article, the phytochemical compounds (cannabinoids, terpenes, and phenolic compounds), the current status of legalization, extraction techniques, and research advances in cannabis in Latin America are reviewed.
1. Introduction
The Cannabis genus of plants has been widely used in diverse cultures for various purposes. Its origin is believed to have arisen in Central Asia and quickly spread throughout Europe and America [1]; it is separated into three main species, sativa, indica, and ruderalis, with different varieties or artificial crosses produced to enhance certain effects. The global Cannabis sativa market, including essential oils, foods, personal-care products, and medical formulations, has gained much attention over the last few years due to the favorable regulatory framework. Undoubtedly, the enormous interest in cannabis cultivation derives from the well-known pharmacological properties of cannabinoids and terpenes biosynthesized by plants [2]. The term “Cannabis” defines the dry or fresh leaves and flowers of Cannabis sativa or Cannabis indica plants.
The name “cannabinoid” has then been associated with the biological profile of the psychotropic constituent of marijuana, Δ9-tetrahydrocannabinol (THC), found within almost 200 known cannabinoids [3]. Another major component of Cannabis is cannabidiol (CBD), which has excellent medicinal utility. Specifically, CBD is considered for therapeutic use and does not generate psychotropic effects. In addition, to plant Cannabis sativa, two types of cannabinoids—synthetic cannabinoids (e.g., WIN55212-2) and endogenous cannabinoids (eCB), anandamide (ANA) and 2-arachidonoylglycerol (2-AG)—are known [4]. The active components of Cannabis sativa mimic the effects of endocannabinoids by activating specific cannabinoid receptors, particularly CB1 and CB2, and regulating a broad spectrum of physiological functions in which an alteration could potentially cause a variety of effects, namely, analgesic, neuroprotective, antiemetic, anticonvulsant, anti-inflammatory, and antispasmodic, and their therapeutic potential has been shown in cancer, epilepsy, sclerosis, neuropathic and chronic pain, spinal cord injury, Parkinson’s and Alzheimer’s diseases, post-traumatic stress disorder and anxiety, schizophrenia, and pulmonary disease [5]. Cannabis compounds have been used in other product developments and launched in international markets.
Among Cannabis sativa L. species, hemp and marijuana are plants with differing morphologies, chemical compositions, and marketed products. Usually, marijuana is cultivated with the purpose of producing THC, and its strains can be artificially manipulated to provide a high potency of this psychoactive molecule. New marijuana strains have been developed as hybrid varieties to increase specific characteristics (terpenes and CBD-specific content), differentiate the strain, or increase the drug’s effectiveness. Each marijuana strain can contain between 10 and 30% THC, 33 times more powerful than hemp. A low level of CBD is no longer characteristic of marijuana, as strains such as Dinamed are circulating on the market, with up to 14% CBD and about 0.5% THC (https://www.dinafem.org/es/dinamed-cbd/(accessed on 10 February 2023)).
Hemp is defined as the stems, seeds, and flowers whose harvest can produce oil, food, paper, fiber textiles, foodstuffs, building materials, and even topical ointments. In contrast to marijuana, hemp is rich in CBD molecules and contains at least 0.3% THC, decreasing the psychoactive effects. Therefore, it has been decriminalized in many countries to take advantage of its industrial uses. The World Anti-Doping Agency (WADA) changed its regulations by eliminating CBD from prohibited substances, thus allowing athletes to use CBD oils and derivatives without any repercussions. The U.S. Food and Drug Administration (FDA) has not approved the use of Cannabis as a medical treatment for different ailments [6], however isolated THC and CBD pharmaceuticals are licensed and approved [7]. Some FDA-approved drug products are Epidiolex (cannabidiol) and three synthetic cannabis-related drug products: Marinol (dronabinol), Syndros (dronabinol), and Cesamet (nabilone). Another treatment called Sativex® is used (in Canada and a few European countries, including the United Kingdom, Germany, and Spain) as an adjunctive treatment for the symptomatic relief of neuropathic pain in multiple sclerosis [8].
The legal aspects related to the prohibition and criminalization of the consumption, use, and production of Cannabis have been a barrier to generating scientific and technological knowledge in Latin America. Lately, it has been changed, and several countries have legalized its medicinal and medical use. In this sense, the diversification of food and medical cannabis products has been developed, and technological processes considering their extraction, production, and formulation have been promoted. Developing high-efficiency extraction and purification techniques and optimizing their operating conditions at the pilot scale are essential for scaling up the industrial production of the main bioactive compounds in C. sativa [9].
Efficient, safe, and cost-effective extraction and purification technologies must be developed and optimized to improve the yield and selectivity of CBD and terpenes and ensure the purity and safety of the extract for food applications [10]. Extensive scientific research is needed to provide evidence of the benefits for health and safety in the consumption of phytocannabinoids, which, depending on the doses and purity of the components, will help to establish regulations for consumption in each country, first for the development of products with CBD and later those with THC.
It is essential to highlight that, in many Latin American countries, cannabis legalization requests are in progress as an alternative solution to illegal traffic and associated crime. Argentina, Brazil, Chile, Colombia, Ecuador, and Peru are permissive for cultivation for personal or medical use in small amounts. By 2022, all countries in Latin America had regulated the medical cannabis industry, except for Mexico, Honduras, Nicaragua, and El Salvador [11].
Different authorities have implemented four different models of legal cannabis production and supply: (1) taxed commercial supply, where licensed farms supply licensed retail outlets (followed by some US states, including Colorado, Washington state, Alaska, and Massachusetts); (2) government supply, where the government hires a limited number of farms and controls the supply through these outlets (Uruguay follows this model, along with two other models); (3) home growth permission, where there are no taxes or sales outlets (this is followed by Washington DC and Uruguay as well); (4) Social Clubs, in which groups of people can grow it in a collective and consume it without any taxes or sales outlets (this is the third model that Uruguay follows) [12]. Based on a recent market analysis, in 2019, the global cannabis market (regulated and illicit) was estimated at USD 344 billion, with 263 million consumers annually [13].
In this sense, the present document is organized with an emphasis on the technical extraction processing description of the main Cannabis components, the current impact of Cannabis research in Latin America, and finally, an overview of the economic effect of scaled-up processing and future value-added chains.
2. Data Collection
The information used in writing this review article comes from the analysis of relevant documents reported in trusted literature sources, such as primary data, books, and national or international journals, published from January 2000 until December 2022. The prior references were mainly cited from sources such as Scopus, Science Direct, Google Scholar, ResearchGate, Web of Science, and other published sources with the following keywords: cannabis components, cannabis extraction processing, Latin American research, health benefits, and market potential. Furthermore, data searches were also conducted using different online platforms. This review article does not have any inclusion criteria. Finally, the search was only limited to articles published in English, but information about the market and legislation was found in other languages.
3. Cannabis Extract: Varieties and Phytochemical Composition
The genus Cannabis, which is a member of the Cannabaceae family, is one of the oldest domesticated crops in the world due to its multiple applications, especially its healing properties [1]. It is an annual and dioecious plant, meaning it can be male or female. Furthermore, environmental factors, such as the appropriate photoperiod and a low temperature, can induce pollen production in female flowers, which leads to the development of feminized seeds [14].
Its evolution, taxonomic classification, and phylogenetic connections remain poorly understood. These shortcomings stem from scarce research due to legal and government restrictions, which have resulted in the high heterozygosity observed in Cannabis genomes today [14].
In the genus Cannabis, the exact number of species that have resulted from extensive hybridization and subsequent rehybridization of their original botanical descriptors is controversial. The genus classification includes three species with distinct phenotypic differences: Cannabis sativa, a tall and less furcate plant with long and narrow leaves, Cannabis indica Lam (Lamarck), a short and highly branched plant with broader leaves, and Cannabis ruderalis, a short plant with less branching and small and thick leaves [14].
Cannabis sativa is an economically important genus that provides protein, oil-rich seeds, and long and short fibers for industrial applications (building materials, textiles, or paper). A wide variety of secondary metabolites are found, such as terpenoids and cannabinoids, which are of interest to different industries [15]. The plant allows the acquisition of a large amount of lignocellulosic biomass in a brief time, which is why it is considered an abundant renewable source from which biopolymers, resistant fibers [16], and textiles can be obtained, and its use in feeding livestock has even been described [17].
Hemp powder’s antibacterial activity was also investigated against Escherichia coli. The hemp powder inhibited bacterial growth, and antibacterial agents were linked to the chemical composition of bast fibers (free and esterified sterols, triterpenes, β-sitosterol, and β-amyrin, with the presence of lignin, phenolic compounds, alkaloids, and cannabinoids) [18].
Despite scientific research on Cannabis being restricted by the Single Convention on Narcotic Drugs of 1961, nowadays, legislation in several countries allows its potential use for medical applications by using methodologies and technologies to study its composition and develop better extraction processing methods to obtain compounds for medical formulations.
3.1. Cannabis Phytochemical Composition
Phytochemical components of Cannabis sp. are represented by cannabinoids, flavones, and terpenes, which have been the object of study in research works. Forty years ago, Turner et al. [19] reported almost 421 total compounds. More than 560 components have been identified in Cannabis, considered natural product phytocompounds [20]. Among these components, 120 are in the typical C21 group of compounds, known as total cannabinoids, including their analogs and transformation products. Other components include nitrogenous compounds (27), amino acids (18), proteins (3), enzymes (6), glycoproteins (2), sugars and related compounds (34), hydrocarbons (50), simple alcohols (7), simple aldehydes (12), simple ketones (13), simple acids (20), fatty acids (23), simple esters (12), lactones (1), steroids (11), terpenes (120), non-cannabinoid phenols (25), vitamins (1), pigments (2), and elements (9).
3.1.1. Cannabinoids
Cannabinoids represent more than 20% of secondary metabolites isolated from the cannabis plant [21]. Cannabinoids belong to the chemical class of terpene phenolics, widely distributed in nature. These metabolites are produced by, stored in, and secreted from the glandular trichomes of female flowers as a defense mechanism [22]. Trichomes are epidermal protrusions that line plants’ leaves, braces, and stems. However, their location in other parts of the plant is possible, although to a lesser extent (Figure 1), e.g., seeds, leaves, roots, and pollen [16,23]. Livingston et al. [24] concluded that revealing the particular and uncommon properties of these economically and biotechnologically important structures provides new opportunities to optimize the harvest time and extraction processing for the obtention of cannabinoids.
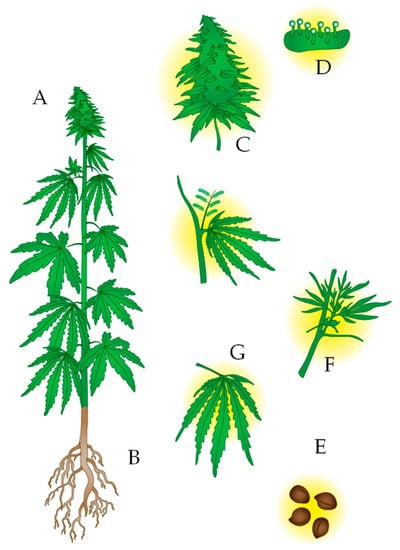
Figure 1.
Representation of a Cannabis sativa plant. (A). The plant is usually tall and not highly branched, with long and narrow leaves. Depending on whether it is the drug type or fiber type, the stem can be thin or thick, respectively. (B). It has fasciculate roots. (C). The flower, in the case of female plants, develops in the upper part of the plant. Male plants do not have a flower. (D). Glandular trichomes store secondary metabolites, both cannabinoids and terpenes or phenolic compounds. (E). From the seeds, the oil can be extracted, and there are high contents of THC and CBD in their bark since they are in contact with the leaves of the plant. (F). Stem. (G). Leaves.
Cannabinoids can be produced by employing two metabolic pathways that have already been studied: the polyketide pathway, which gives rise to olivetolic acid (OLA), and the methylerythritol phosphate pathway (MEP), which leads to the synthesis of geranyl diphosphate (GPP) [14]. In 1999, Pate proposed the term phytocannabinoids for C21 compounds produced by cannabis [25]. One hundred twenty cannabinoids have been found, and the main active constituent is Δ9-tetrahydrocannabinol (Δ9 -THC), generated in the leaves and flower sprouts of the plant. C. sativa L. with a Δ9-THC content of less than 0.3% is cultivated as hemp, and Cannabis containing more than 0.3 percent THC is considered a medical marijuana product [26]. Cannabinoids have been classified into 11 types: (-)-Δ9-trans-tetrahydrocannabinol (Δ9-THC), (-)-Δ8-trans-tetrahydrocannabinol (Δ8-THC), cannabigerol (CBG), cannabichromene (CBC), cannabidiol (CBD), cannabinodiol (CBND), cannabielsoin (CBE), cannabicyclol (CBL), cannabinol (CBN), and cannabitriol (CBT). Some of them, such as cannabidiol (CBD), cannabichromene (CBC), cannabigerol (CBG), and cannabinol (CBN), have demonstrated non-psychoactive effects but with demonstrated pharmacological activity. The presence of terpenes and flavonoids in Cannabis extracts improves the biological activities of the cannabinoids [27].
THC is the principal psychoactive substance and is abundant in Cannabis drug-type plants. It induces feelings of euphoria, anxiety, paranoia, and cognitive deficits. However, its medicinal benefits include helping to relieve nausea caused by specific cancer treatments and exerting an anti-inflammatory effect [28]. On the other hand, CBD has analgesic and neuroprotective effects and properties that relieve discomfort in people with cancer and diabetes. Differences in CBD–THC ratios delineate three types of classes: type I (ratio < 0.5), type II (ratio 0.5–3.0), and type III (ratio > 3.0) [1,14]. The concentrations of these compounds and others, such as terpene phenolics, are dependent on the age, type of tissue, variety, and environmental and growth conditions, such as nutrients, humidity, and the photoperiod of the plant [16].
3.1.2. Terpenes
Terpenes can be easily extracted from the raw material by using steam distillation. They are also called essential or volatile oil. They have been used as an anti-inflammatory in the respiratory and digestive tract and have become economically significant in flavors and fragrances. Terpenes are lipophilic compounds that can pass through membranes quickly. They can alter the THC pharmacokinetics by permeating the blood–brain barrier, functioning as a permeating agent, and modulating the affinity of THC for the CB1 receptor. Therefore, they present a broad range of medical properties depending on the terpene [29]. Likewise, they are responsible for the smell and taste of different varieties of Cannabis. Over 120 terpenoid compounds have been identified in the plant; mono- and sesquiterpenes, with 10 and 15 carbons, respectively, are detected in the flowers, roots, and leaves. Triterpenes, with 30 carbons, have been detected in the roots and fibers of hemp, as well as in the oil of Cannabis seeds [16] (Table 1). It is necessary to further characterize terpenes and terpene profiles from Cannabis since robust analytical standards are lacking, and some terpene compounds are still unknown. The terpene composition in cannabis-extracted oil or resin is dependent upon genetic, environmental, and evolutionary factors, as well as differences between individual plants [29,30].

Table 1.
Terpenes found in Cannabis sativa. Classification according to the number of carbons, the part of the plant where they are located, and some of their pharmacological effects.
3.1.3. Phenolic Compounds
The phenylpropanoid pathway in the cytoplasm produces phenolic compounds, which are subsequently transported in the vacuole or deposited on the cell wall. Although the flavonoid pathway has been extensively studied in various plants, there are no specific data on flavonoid biosynthesis in Cannabis. In general, in plants, phenolic compounds can act as antioxidants under certain physiological conditions, protecting plants from oxidative stress. In humans, it has been shown that there is a correlation between the intake of phenolic compounds in the diet and a lower incidence of chronic diseases such as cancer and cardiovascular and neurodegenerative diseases [31], but these positive health effects cannot be totally understood because the compounds are poorly bioavailable.
In C. sativa, thirty-four flavonoids have been isolated, which can be categorized into seven bare chemical skeletons: ethylated, glycosylated (C or O glycosides), prenylated, or geranylated [32]. Most of these are flavones (apigenin and luteolin), flavonols (kaempferol and quercetin), aglycones, or glycosides [33,34]. In C. sativa, cannflavin A, B, and C have been isolated and represent hemp-specific methylated isoprenoid flavones. It is known to possess anti-inflammatory action [35]. In Central Italy, quercetin, naringenin, and naringin were identified and quantified in a hydroalcoholic extract from hemp inflorescences of monoecious cultivars [36].
Another class of polyphenolics, dihydrostilbenoids, have been isolated from Cannabis, with canniprene being the primary representative [37]. In C. sativa, higher flavonoid content has been reported in the leaves than in the other plant tissues, and the concentration seems to decrease with plant tissue age. Thus, higher flavonoid content is found in young cannabis plants [33].
4. Current Status of Cannabis Extraction Techniques
The extraction methods used to obtain cannabinoids and other bioactive ingredients represent a critical and economically important step, mainly when they can be used as pharmaceutical, cosmetic, and food product ingredients. This step is not understood and needs to be reviewed. Furthermore, from the laboratory scale to the pilot or industrial scale, there are several variables to consider to select the best extraction method (purity yield), which include variety selection, cultivation, harvesting, extraction technique, plant material pre-treatment, etc. [38].
4.1. Current Conventional and Emerging Technologies for Cannabinoid Extraction Processing
Before extracting cannabinoids and other biocompounds from Cannabis, previous stages need to be considered. These include selecting, drying, pulverizing, and sieving the plant material [39]. Another treatment (before or after extraction) consists of decarboxylation to convert acid cannabinoids to their neutral psychoactive forms by heating at 100–150 °C for 15–120 min [40].
Traditional methodologies for extracting cannabinoids from cannabis materials utilize volatile solvents (mainly ethanol), liquid propane or butane, supercritical CO2 (SC-CO2), or ice water. Several medicinal cannabis extract procedures use alcohol as a solvent because of its high efficiency and food-grade quality. However, solvents such as butane, propane, or hexane are of limited use because they can produce an uncontrolled hazardous environment.
Some laboratory, pilot plant, and industrial extraction processes have been developed to obtain and improve the quality and purity of the main cannabinoids or terpenes, and some examples of extraction techniques are described in this section and Table 2.

Table 2.
Cannabis plant: examples of different technical extraction processing methods, equipment, and efficiency.
Currently, the most potent type of oil extract is called Full-Extract Cannabis Oil (FECO). It has the highest concentration of cannabinoids, usually composing between 50 and 80 percent of the weight. Other less potent extracts are tinctures, which are liquid extractions made primarily with alcohol, glycerin, olive oil, or coconut [52].
4.1.1. Butane Hash Oil Extraction (BHO)
Using liquid butane gas to extract cannabinoids from Cannabis flowers has made it possible to obtain a product called butane hash oil (BHO) with a THC concentration higher than that found in the flowers [53]. Between 1993 and 2015, the THC content in seized BHO samples had maximum concentrations of up to 90% compared to the 37.1% obtained in cannabis flower samples [54]. BHO is also known as “amber”, “dab”, “glass”, “honey”, “shatter”, or “wax”. The extraction process consists of dissolving cannabinoids and terpenes from the plant material using a hydrocarbon, most commonly butane, to obtain a concentrate in which the most significant component is THC. Butane is non-polar. Thus, the extraction process cannot extract some soluble compounds, such as chlorophylls or alkaloids. Butane gas (highly flammable and volatile) permeates the air and can be ignited by a flame source (Figure 2). After extraction processing, butane is purged by evaporation to obtain BHO [55]. Like other extraction processes, decarboxylation and winterization are essential steps to obtain BHO [39]. Butane extraction can be risky if there is no closed-loop system to recover and recycle butane, a highly volatile and flammable compound that accumulates in closed spaces [55,56].
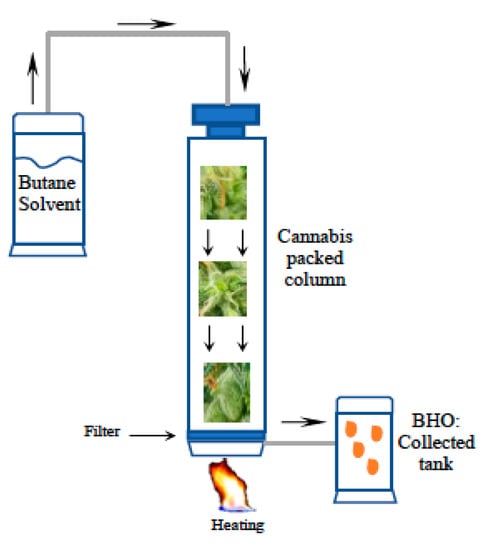
Figure 2.
Butane Hash Oil extraction system. Cannabis in put into a column or extraction cylinder and butane solvent is passed through, heated a low temperature to extract terpenes and cannabinoids. The extract is then filtered and recovered into a new cylinder.
Non-commercial extraction or home use can quickly adapt BHO for extraction processing. However, it can be dangerous if extraction areas are not in optimally ventilated to eliminate butane vapor accumulation. On the other hand, the BHO obtained may contain a certain amount of butane, methacrolein, and benzene (products of the degradation of terpenes at elevated temperatures), which may cause acute lung injury and pulmonary edema [57]. Regarding patent research, when using the term “butane hash oil”, 32 patents were found in 2022 on the Scopus platform.
4.1.2. Soxhlet Extraction (SE)
Soxhlet extraction is another process developed to obtain essential compounds from the cannabis plant. This method uses solvents, is rapid and efficient, and is suitable for large-scale operations. The process consists of heating the plant material under reflux, and the main solvent used is ethanol [58]. Ethanol is added to a container of Cannabis to react in heating and reflux conditions for at least one hour [59]. The resulting extract is then clarified to separate the solids from the solution saturated with cannabinoids and terpenes. The saturated ethanol solution is then boiled to concentrate the cannabinoids. For industrial capacity, this system can be adapted. However, it can be pretty costly. It is necessary to balance the technical and economic benefits that can be realized by implementing this extraction technology [58]. It is essential to mention that ethanol vapor can be recovered using various strategies or distillation steps. The extraction solvent also can be used as a first step for subsequent extraction (supercritical CO2, microwave heating, etc).
4.1.3. Cold Press Extraction (CPE)
Hemp seeds are high-value components, with approximately 25–35% lipids, 20–25% proteins, 20–30% carbohydrates, 10–15% insoluble fibers, and numerous natural source minerals [60]. This technique has been used to extract fatty acids and bioactive compounds from cannabis seed oil. When the seed is clean and ready for extraction, it is ground in mills, cylinders, or spurs to break the seeds, and the oil comes out. These mechanical pressing extraction systems are considered cold extraction methods. Pressing is a standard method that uses low-cost and simple technology. In cold pressing, the seeds pass through a low-speed and low-pressure press, whose internal temperature can be kept below 50 °C. However, when a screw press is used, it is heated to improve the extraction yield, which can produce some pleasant sensory characteristics in the extracted oil [61].
The semi-defatted paste or oil sludge resulting from the pressing process may be required for further solvent extraction processing or processed a second time with a press with high pressure and speed that separates the oil from the remaining seed paste for the recovery of the remaining oil [62]. In general, this process has the advantage of preserving the properties of the oils, although with a lower yield.
4.1.4. Supercritical CO2 Extraction (SC-CO2)
Supercritical CO2 has been used to extract high-added-value compounds such as nutraceuticals and pharmaceuticals. Additionally, toxic compounds such as pesticides are removed from solid and liquid matrices [63]. Supercritical CO2 extraction has been used to obtain a high-ԛuality product without any toxic residue by the Cannabis industry over the last 22 years [64,65].
Extraction with SC-CO2 consists of the use of CO2 at a pressure and temperature higher than its critical values or critical points (31.3 °C and 72.9 atm), which causes it to present properties of a liquid and dense gas at the same time, facilitating the extraction and separation of the dissolved solute from SC-CO2 after decompression [66]. Supercritical CO2 has been used to extract hemp (C. sativa) seed oil, obtaining a greater quantity of tocopherols and a lower quantity of pigment than extraction by Soxhlet and cold press processing [67].
Briefly, the process includes the industrial hemp material, followed by a treatment that reduces the particle size (cutting or grinding). Then, the cut material is settled in a pressure vessel, and SC-CO2 is passed into the container. SC-CO2 is preferably maintained at 40–100 °C and 150–300 bar pressure. After that, the CO2-dissolved compounds are collected in a lower-pressure container and precipitate as CBD crude extract. The crude extract may contain cannabidiol acid (CBD A), and it is possible to convert CBD A to CBD by decarboxylation using heating (100–120 °C). The crude CBD extract can be subjected to a purification step.
According to Benner et al. [68], there is considerable variability in the temperature (113 to 140 °C) and pressure ranges (3000 to 5000 PSI) that different extractor facilities use for the process. These conditions can affect the quality and yield of cannabinoids, terpenes, and flavonoids. Greater temperatures increase the risk of denaturing terpenes and increase waxes and resins, which decrease the overall extract quality. It may be possible that applying pulses of ethanol (co-solvent) improves the extraction speed with low consumption of the co-solvent and CO2 in strains of C. sativa L. with a low concentration of cannabinoids [41].
The use of ethanol as a co-solvent improves the extraction process. In addition, the decarboxylation of cannabinoids (increased solubility in SC-CO2) and winterization (to remove waxes present in the flowers) are still crucial steps that enhance the extraction of THC and CBD [69]. Additionally, using other solvents or re-using ethanol to minimize operating costs is possible. The SC-CO2 process is presented in International PCT Publication WO2016/153347, which is directed at a cannabidiol isolate from industrial hemp for use in pharmaceutical or cosmetic preparations.
The first study, which was reported by Rochfort et al. [70], included an optimized CO2 supercritical fluid extraction (SFE) process protocol without a co-solvent for a large amount (15 kg) of medicinal cannabis bud material for a pharmaceutical product. The highest extraction (7.1%) was produced under a high flow rate (150 g/min), with a long extraction time (10 h) at high pressure (320 bar). A comparative study between the cannabinoid and terpene concentrations before and after SC-CO2 extraction resulted in a significant reduction in the terpenes and a significant increase in the THC and CBD contents in the final extract.
The main advantages of SC-CO2 seem to be a clean, safe (non-flammable), and cost-effective alternative to obtain cannabis extract. Nowadays, many equipment suppliers can be easily identified. However, one of their disadvantages is the excessive cost of the equipment. A typical large-scale unit can cost from USD 300,000 to USD 10 million [68].
4.1.5. Microwave-Assisted Extraction (MAE)
Microwaves have been used as a heating method to extract organic compounds such as polycyclic aromatic hydrocarbons, polychlorinated biphenyls, pesticides, herbicides, and phenols from several types of matrices [71,72]. Microwave-assisted extraction (MAE) consists of heating the polar-solvent–sample mixture by ionic conduction and dipole rotation mechanisms through the application of an electromagnetic field (300 MHz to 300 GHz), which reduces the processing time and the solvent volume used [73]. Microwave-assisted extraction (MAE) has been used successfully to extract compounds of interest from plants [74]. During the MAE of hemp inflorescences, an aqueous residue and the residual deterpenated plant biomass remain in the reactor as by-products, being a valuable source of flavonoids and phytocannabinoids [75].
The variation in parameters such as the solvent concentration, extraction time, and solid–liquid ratio (S/L) has been shown to influence variables such as the yield, IC50, EC50, CBD, THC, total flavonoids (TF), and total phenols (TP) from C. sativa [42]. In recent research work, the influence of variables on the MAE efficiency (microwave irradiation power, extraction time, and added water) was evaluated to obtain high-quality products: essential oil, an aqueous extract rich in polyphenols, and phytocannabinoids [76]. On the other hand, microwaves at 915 MHz have been established in continuous operation on an industrial scale for the extraction of cannabis compounds. It is possible to process larger volumes of matter in less time with a high extraction efficiency (>95%) [77]; despite its positive characteristics, the potential for use at an industrial scale is limited.
4.1.6. Ultrasound-Assisted Extraction (UAE)
Ultrasound (>20 kHz) has been used to assist extraction. Bubble cavitation facilitates the extraction of polysaccharides, edible oils, essential oils, proteins, peptides, pigments, and other bioactive molecules in diverse matrices [78]. The cavitation process generates changes in temperature and pressure at a bubble level, facilitating the transfer of matter and reducing the volume of the solvent and the extraction time [79]. Ultrasound-assisted extraction (UAE) has been used to extract bioactive compounds from C. sativa L., and optimizing the process using 80% methanol, 15 min, and 130 W improved the extraction of THC and CBG from C. sativa L. [47]. In another study, the optimized extraction conditions for volatile compounds and cannabinoids were 3 s−1 cycles, an 80% amplitude, 5 min of sonication, and a 1:1 isopropanol/cyclohexane mixture [80]. However, the same study discusses the disadvantage of ultrasound in separating terpenes from cannabinoids compared to using SC-CO2 and ethanol as a co-solvent. Compared to MAE, the UAE method produces better THC and CBD extraction yields [71].
4.1.7. Pressurized Liquid Extraction (PLE, ASE, HSPE)
Pressurized liquid extraction (PLE), accelerated solvent extraction (ASE), or high-pressure solvent extraction (HSPE) is considered a green solid–liquid extraction technique for sustainable bioactive compounds from natural sources. PLE is conducted by applying heat and pressure to the extraction solvent to which the sample is subjected. The extraction rate and efficiency are related to the sample solubility, solvent, rate of mass transfer, extractability of compounds, pressure, and temperature [81].
Recently, Olejar et al. [82] developed a method for the thermo-chemical conversion of acidic cannabinoids during pressurized liquid extraction to isolate cannabidiol. An optimized PLE method using water for thermo-chemical conversion before an ethanol extraction step was evaluated. The extraction process combined with purification by flash chromatography and liquid–liquid extraction yields cannabidiol crystals of greater than 90% purity. In other research work, the influence of temperature, pressure, extraction time, and the number of cycles for the PLE of cannabinoids from hemp was studied [46].
PLE has also been evaluated for CBD extraction from hemp threshing residue with ethanol, where the solvent mass-consumption and operation time were decreased compared to a supercritical fluid extraction technique [83].
All these results and conditions show that this technology could have the potential for cannabinoid extraction from various parts of the plant.
4.1.8. Deep Eutectic Solvent Extraction (DESs)
Advanced research extraction processing and applications to obtain different molecules have attracted extensive attention, with a new generation of green solvents that can be used. The deep eutectic solvent (DES) has been evaluated and was reported to have properties like those of ionic liquids. For the first time, Křížek et al. [84] evaluated the effect of hydrophobic DESs based on menthol and natural carboxylic acids on the efficiency with which they extracted phytocannabinoids (THC, CBD, THCA, and CBDA) from the cannabis plant material. The DES mixture of menthol–acetic acid (1:1 M ratio) showed excellent extraction efficiency and was a non-toxic and biodegradable alternative to organic solvent for phytocannabinoid extraction. Furthermore, these solvents have advantages such as low toxicity, low precursor cost, simple synthesis, little volatility, and high biodegradability [51].
4.1.9. Emerging Extraction Technologies
For cannabis extraction, other extraction techniques can be considered, such as extraction, enzyme-assisted extraction (EAE), microwave distillation (MD), pulsed electric field (PEF), emerging subcritical-butane-based, and emerging solvent-free extraction techniques.
For example, an emerging solvent-free extraction, namely, a new solvent-free method for extracting the full spectrum of phytocannabinoids, terpenes, and flavonoids from the whole cannabis/hemp plant, was patented by [68]. Furthermore, n-butane has been a more than valid alternative to n-hexane and petroleum ether for the extraction of lipophilic natural products. In the case of Cannabis, this method also has wide use [85]. The subcritical-butane-based extraction solvent for cannabinoids from hemp inflorescences was reported for the first time by Fiorito et al. [86]. The pulsed electric field (PEF), a nonthermal technology, has been applied to extract oil from Cannabis seeds with promising results. Higher extraction efficiency improved the oil quality with less thermal degradation [87,88] Combining two or more technologies can be considered to obtain a better Cannabis or bioactive compound extract.
Higher bioactive compound or cannabinoid yields can also be obtained using emerging extraction techniques combined with fast and efficient separation. Kitrytė et al. [89] evaluated a multistep biorefining technology for isolating valuable phytocannabinoids and antioxidant fractions from industrial hemp threshing residues via the consecutive application of SFE-CO2, PLE, and EAE.
Although these emerging technologies (EAE, PEF, MD, and SFE-CO2) are expensive, they could be excellent options for considering the potential and effective extraction results of phytochemicals from Cannabis.
4.2. Purification Step
Some products can use the oil or aqueous extract to obtain products such as cannabis oils, vaporization substances, emulsions, food additives, edibles, drinks, and oral sprays. For example, medical or therapeutic products require beyond 99% purity.
Thus, once the extraction process has been carried out, the extract may contain non-specific lipid-soluble materials or waxes, which can be removed by a traditional purification process such as winterization. Winterization consists of the ethanol solubilization of the resin, followed by precipitation at 20 °C and wax filtration. Subsequently, ethanol and other residual solvents can be removed by evaporation at 40–60 °C [39].
Depending on the desired final product, the extracts can be subjected to additional purification steps, such as vacuum distillation or short-path distillation (molecular distillation) to produce a pure cannabinoid (90%) [90] or centrifuge chromatography to isolate cannabinoids with high-purity content (99%) [90,91].
For example, Cannabis oil can be further fractionated into distillate and a residue by molecular distillation, also known as “short-path” distillation. Molecular distillation is used to separate and purify heat-sensitive compounds and high-molecular-weight compounds and is identified as a “safe method” [92,93,94].
This method is based on high vacuum pressure (1–0.001 mbar), which allows the molecules that escape from the evaporation surface to reach the surface and condense before colliding with each other [95,96]. The vacuum condition allows a short residence time and a small holdup volume [97]. According to the equipment used, molecular distillation can be of the centrifugal type or the falling film type, depending on whether the liquid to be distilled is distributed as a thin film on the evaporation surface through a laminar flow or a centrifugal force, respectively [98,99] (Figure 3). Molecular distillation has also been part of post-extraction refinement processes to obtain distillate cannabinoids (90%) [91,100,101]. The CBD crude extract can be subjected to thin-film evaporation to produce a refined extract. Molecular distillation is preferably performed at a temperature within 100–160 °C.
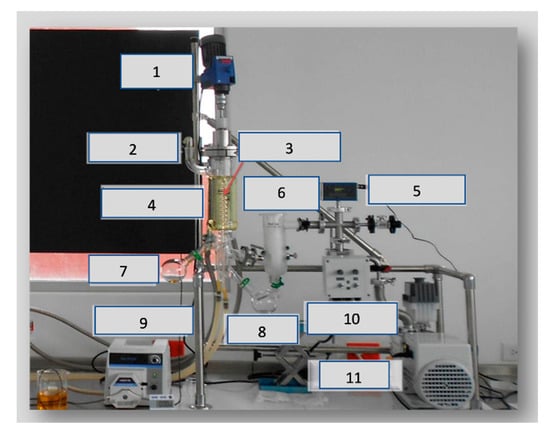
Figure 3.
Lab-scale InCon ICL-04A short-path distillation. 1. Mixer; 2. feeding; 3. condenser; 4. evaporator; 5. vacuum gauge; 6. vacuum trap; 7. residue; 8. distilled; 9. peristaltic pump; 10. pump; 11. vacuum pump.
Thus, different technological and experimental design strategies can be used for cannabinoid extraction and production. It is essential to note that the final application and the compound of interest define the most efficient process to obtain the desired product [102].
5. Current Research on Extraction Processing in Latin America
Worldwide, the industrial and scientific interest in Cannabis has been increasing. Therefore, rapid and accurate methodologies for the quality extraction of the main components will be crucial. Cannabinoid extraction can be associated with compound–matrix interactions, which depend on the variety, harvesting, environmental factors, and selected extraction technique. The extraction efficiency is also influenced by operational parameters, such as the temperature, time, solvent, physicochemical properties, extraction technique, and Cannabis strains.
To date, Cannabis and cannabis formulations of several varieties of medical marijuana concentrate, including edibles, are being marketed without testing regulations. As Cannabis is a natural product that can be susceptible to pests and microbial deterioration (bacterial and fungal attack), careful extraction and quality control must exclude these and other potentially dangerous contaminants from medicinal products [103].
The leading research of extraction technologies for Cannabis has been widely performed in Europe and the USA. However, there is interest in these technologies and commercialization opportunities in Latin America, especially in Brazil, Uruguay, Chile, Colombia, and Mexico, where cannabis legalization requests are in progress to eliminate illegal trafficking while obtaining beneficial health impacts.
It is evident that there is a need for more original research and designs of extraction technologies and equipment from developing countries. However, in the last few years, the increase in publications has been undeniable, influenced by research and development in Europe and North America. Several publications from the Scopus database (2000–2022) are shown in Figure 4. The worldwide research demonstrates that the leading cannabis extraction technology studied is solvent extraction, followed by ultrasound-assisted extraction (UAE) and supercritical extraction (SC-CO2). When these results are compared to the primary extraction techniques in Latina America, solvents and supercritical extraction are the most studied in these countries.
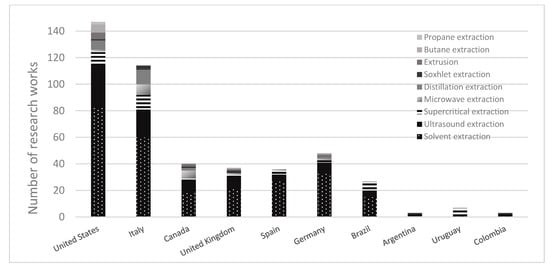
Figure 4.
Worldwide publications of cannabinoid extraction technologies.
In Brazil, legislation indicates that growing Cannabis is still forbidden. It is only permitted with legal authorization. However, developing technologies to obtain cannabinoid extracts with health benefits represents an active area of research. Brazilian research includes extraction technologies evaluated in various plant parts to obtain cannabinoids, fatty acids, and terpenes. Some of them have used pressurized n-propane as a solvent for the extraction of hemp oils as an alternative to improve extraction efficiency without changing their nutritional qualities. n-Propane demonstrated improved oil solubility and extraction. When the studies are aimed at producing, characterizing, and quantifying extracts enriched in cannabinoids, solvent extraction by using maceration (methanol, diethyl ether) is commonly used. When essential oils and cannabinoids (CBD, Δ9-THC, and CBN) are the target compounds, pure SC-CO2 with ethanol as a co-solvent has been preferred as an extraction technique (see Table 3).
In research works developed in Colombia, cannabidiol was extracted at up to 66% from the original biomass using a semi-continuous lixiviation process with absolute ethanol as the solvent and a constant temperature and stirring speed. This study provided a more efficient extraction method compared to Soxhlet extraction (efficiency of 10.5% vs. 11.07%, respectively) and with a meager initial investment compared to other techniques, such as SC-CO2 [104] In 2022, Vega and Dávila [105] studied the extraction time, particle size, and solid–solvent ratio using Soxhlet extraction (ethanol as a solvent) to extract phenolic compounds from the leaves and stems of Cannabis sativa L. These parts of the plant are considered residual biomass with a reliable source of bioactive compound extracts and attractive economic and environmental advantages. Similar to Brazilian research, maceration (using ethanol) and SC-CO2 extraction for comparison were evaluated and reported as two of the most used methods to obtain cannabinoid extracts for medical purposes. The SC-CO2 extraction conditions led to the highest total THC and CBD recovery [106].
The pharmacological and medicinal potential of cannabinoids has been a topic of interest in Uruguay and Brazilian universities. To demonstrate this effect on various human tumor cells, extracts of Cannabis flowers were obtained by SC-CO2 (ethanol as a co-solvent) at low temperatures and without organic solvents using a previous decarboxylation step. Using this extraction technique at 40 MPa and 50 °C resulted in a high yield and high Δ9-THC content. The extracts with high concentrations of neutral cannabinoids showed high antitumor activity in cervical cancer cell lines [69].
The first country in the world to regulate the production of Cannabis for recreational, medicinal, and industrial use was Uruguay. In Uruguay, CBD is the main export product for medical Cannabis. Then, the market will demand significant amounts of CBD in the next few years. Thus, a regulatory point of view concerning the health, medicinal, and nutritional properties of industrial cannabis products has been necessary. In 2021, Uruguayan research concluded that it is crucial to consider the standardization of chemical profiles of Cannabis sativa extracts used in medicinal cannabis oil. These profiles could be modified due to different extraction and purification conditions, such as the temperature (solvent evaporation process) or chemical reactions due to oxygen. These chemical changes must be carefully considered in the development of medicinal oils.
Argentina and Mexico are waiting for new regulations to generate new horizons to initiate investigations of medicinal Cannabis products that demonstrate safety and evidence in treating different pathologies, carry out studies in experimental models, advance the genetics of the plant, and consolidate production. In agreement with the extraction techniques used in Latin America, Cannabis sativa L. extracts with a high concentration of Δ9-tetrahydrocannabinolic acid (THCA) and Δ9-tetrahydrocannabinol (THC) were obtained by supercritical carbon dioxide (SC-CO2) extraction in Argentina. The extraction yield was highly dependent on the pressure and raw material quality composition, and a process extraction efficiency as high as 92% was achieved [41].
In México, in the same situation as Brazil, public research is not permitted without legal authorization. Thus, only a few review papers were identified. One of them reviewed state-of-the-art Cannabis patents and included specialized/secondary metabolite isolation methods, synthesis, production, genes, development of medicaments, drug delivery systems, equipment, machines, industrial processes, plant culture techniques, and improved plant varieties [107]. In another review, the term “Tetrahydroevolution of cannabis” was suggested by the author, where this cannabis tetrahydroevolution can be divided into three main dimensions: (1) the neo-production of Cannabis, (2) the neo-refining of raw materials, and (3) the neo-presentation of products derived from cannabis [108]. These dimensions have been referred to for the recent superior cannabis development reported in the last several decades compared to its preceding quality. A third paper reviewed and discussed the latest development works aiming at the innovative extraction of cannabinoids and purification of CBD using traditional, emerging, and synergistic extraction techniques and strategies and their relevant outcomes [109] (Table 3).
This analysis shows opportunities for Cannabis in Latin American research and development. Data from the Scopus database highlight the extraction methods used by Latin American countries over the last ten years of research. These techniques have been used to optimize the conditions to obtain metabolites from other plant materials, such as corn, seeds, oil extract, nut shells, marine microalgae, etc. [110,111,112,113,114] (Figure 5). This information is critical to appreciating the technological potential to implement these methodologies for cannabis research. However, extraction technologies tailored to Cannabis in some Latin American countries have been discontinued by their legislation.


Table 3.
Contribution to research on cannabis extraction technologies by Latin American countries.
Table 3.
Contribution to research on cannabis extraction technologies by Latin American countries.
| Country | Biomass Form | Target Compound | Extraction Technology | Conditions | References |
|---|---|---|---|---|---|
| Brazil | Seeds | Fatty acids Tocopherols β−Carotene | Pressurized n-propane | Temperature: 40, 50, and 60 °C Pressure: 6, 8, and 10 MPa | [115] |
| General biomass: forensic samples | THC/CBD | Solvent extraction: Dynamic maceration methanol, and diethyl ether | Heating | [116] | |
| Hybrid flowers (2 varieties) | Cannabinoids | Green solvent extraction: supercritical carbon dioxide (SC-CO2) | Temperature: 50, 60, 70 60 °C Pressure: 200, 300 bar Co-solvent: 0, 10% EtOH | [50] | |
| Standard | CBD | Supercritical carbon dioxide (SC-CO2) | Temperature: 315, 326, 334 K Pressure: 11.3–19.4 MPa | [117] | |
| Colombia | Cannabis with fully ripe inflorescence | THC | Supercritical fluid extraction (SFE) using CO2–ethanol | Pressure: 15–33 MPa Temperature: 40–80 °C Co-solvent: (0–5%) EtOH | [118] |
| General biomass (flowers, leaves, stems, and other parts) | Cannabinoids (CBD, CBDA, CBC, CBG, THC) | Soxhlet extraction compared to semi-continuous lixiviation process | Soxhlet extraction: 2 h, solvent/biomass ratio of 6:1 Single-stage extractions: ethanol (40 g and 2 g of biomass) Temperature: 40 and 19 °C | [104] | |
| Stems and leaves | Phenolic-rich extracts | Ethanol solvent extraction | EtOH 96% | [105] | |
| Uruguay | Female inflorescences | THC/CBD Cannabinoids | Solvent extraction (short maceration) and supercritical fluid extraction (SC-CO2) | Temperature: 60, 70 °C Pressure: 200, 300 bar Co-solvent: 0, 10% EtOH | [106] |
| Flowers | CBD/THC | Green solvent extraction: supercritical carbon dioxide (SC-CO2) | Temperature: 50 and 70 °C Pressure: 22 and 40 MPa | [69] | |
| Argentina | Cannabis sativa extracts | Terpenoids, CBD/THC | Soxhlet and maceration extraction | Temperature: 40 and 70 °C to dryness under reduced pressure | [119] |
| Cannabis sativa extracts | THCA, THC | Supercritical carbon dioxide (SC-CO2) | Pressure: 17, 24, and 34 MPa Temperature: 328 K | [41] | |
| Mexico | Reviews | [107,109] |
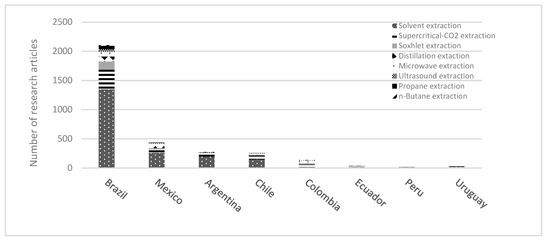
Figure 5.
Extraction processing techniques in research articles contributed by Latin American countries.
6. Future of Commercialization and Perspective on the Value-Added Chain in Latin America
A potential niche for a boost in the cannabis derivative market is the biomedical area. Before its current illicit substance status, Cannabis had been used for hundred years for medical purposes. In the US, Cannabis was described in the United States Pharmacopoeia for the first time in 1850 and then widely used as a patent drug during the 19th and early 20th centuries [120].
The global medical cannabis market increased in value from USD 8.28 billion in 2017 to approximately USD 9 billion in 2020, with expected values of USD 28.07 billion in 2024, and it is expected to reach nearly USD 50 billion by 2028 [121,122]. According to another report, The Road Map to a $57 Billion Worldwide Market, legal cannabis worldwide is expected to hit USD 57 billion by 2027. The same report indicated that Germany is ready to become the leader of the European cannabis market, followed by Italy, with USD 1.2 billion in sales by 2027. In South America, the medical cannabis market may grow from USD 125 million in 2018 to USD 776 million in 2027, led by Brazil, Argentina, Peru, and Uruguay [123].
According to this information, the cannabis market forecast is economically strong. Nevertheless, there are still obstacles in the development of medicinal Cannabis. For example, the United States, Canada, and the United Kingdom designated USD 1.56 billion between 2000 and 2018 for research funding. Half of the budget was spent on understanding the potential harms of the recreational drug, approximately 20-fold the amount paid for cannabis research and development in the therapeutic area [124].
Considering health problems, cannabis oil, which is rich in medical properties, is booming, as the demand from patients with chronic illnesses, such as Parkinson’s disease, cancer, Alzheimer’s disease, and other neurological disorders, is increasing worldwide [125]. However, not all countries permit their commercialization. Thus, an increase in illegal products marketed as products for medical use or therapeutic purposes is found in these countries, especially in the Latin American market.
According to research and development, demand in the current value-added chain is mandatory. The existing value chain only has permitted crops (spread, flowering, and harvesting), distribution in legal markets, and finally, sale in local markets.
In Latin America, however, the perspective on the value-added chain for cannabis must include advanced crops, an official distribution channel (considering a legal market), retail sales (local and online markets), and research and development (human resource capabilities, extraction equipment, physical infrastructure) to obtain high-quality products for medical, food, or cosmetic purposes (Figure 6).
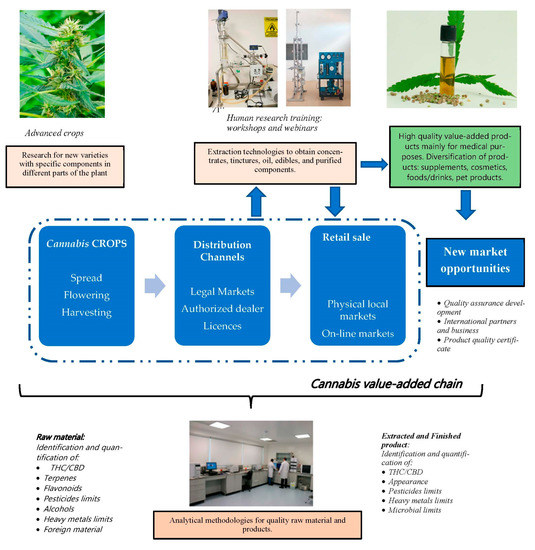
Figure 6.
Perspectives on Cannabis value-added chain in Latin America include research on extractions techniques for cannabinoids to obtain diversification of high-quality products.
Analytical and technological tools are also necessary for industrial manufacturers to offer a safe product, improve quality, and extend product lines to diversify and open new market segments to find a more successful business model in Latin American countries.
7. Conclusions and Future Perspectives
Cannabis compounds are essential in pharmaceuticals for the treatment of depression, anxiety, insomnia, epilepsy, and seizures or as ingredients in healthy functional foods (oil, tinctures, cookies, ice cream, chocolates, butter, brownies, cooking oil, jellies, beverages, capsules, pills, etc.). In general, research and development, especially on the cannabis extraction topic, is increasing due to these positive health benefits and the local economic impact of cannabis products.
In addition, obtaining higher-quality cannabis products represents higher prices. The correct selection of the extraction and separation processes is essential, with this step being one of the biggest challenges. Latin American countries exhibit profound knowledge and availability of extraction technologies for cannabis extraction. In this sense, it is necessary to estimate the production cost to evaluate whether they are economically profitable, at least at the laboratory scale, and to obtain a final desirable product.
In Latin America, there is also an increase in commercial cannabis extract or cannabis products that may or me not be allowed, with or without quality fact sheets, which can pose health risks. Hence, legalization is mandatory and urgent for the rest of Latin American countries.
This action will allow the innovation, research, and development of cannabis products according to the value-added chain (from the raw material to the final product) expected for Latin American countries. Furthermore, it will allow the evaluation of more selective, efficient, and effective extraction and processing technologies to obtain high-quality cannabis extracts and to enable the development of new formulations with added value for medical purposes.
Author Contributions
Writing—original draft preparation, Á.S.-J.; conceptualization and writing—review and editing, J.A.G.-F.; investigation and references, G.A.C.-H.; extraction technology summary, A.D.P.; market research and economic impact, E.B.-A.; funding acquisition—J.A.G.-F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to Programa Verano Delfín 2021 for the scholarship granted to Daniela Robles Vences and Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco (CIATEJ) for the support given for the development of the review.
Conflicts of Interest
The authors declare that they have no known competing commercial interest or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
ECS, endocannabinoid system; CNS, central nervous system; CB1R, cannabinoid receptor 1; CB2R, cannabinoid receptor 2; eCB, endogenous cannabinoids; ANA, anandamide; 2-AG, 2-arachidonoylglycerol; CB1, CB2, cannabinoid receptors; WADA, World Anti-Doping Agency; FDA, Food and Drug Administration; OLA, olivetolic acid; MEP, methylerythritol phosphate pathway; GPP, geranyl diphosphate; THC, tetrahydrocannabinol; THCA, Δ9-tetrahydrocannabinolic acid; CBD, cannabidiol; Δ9-THC, Δ9-tetrahydrocannabinol; Δ8-THC, (-)-Δ8-trans-tetrahydrocannabinol; CBG, cannabigerol; CBC, cannabichromene; CBND, cannabinodiol; CBE, cannabielsoin, CBL, cannabicyclol; CBN, cannabinol; CBT, cannabitriol; CBD A, cannabidiol acid; SC-CO2, supercritical CO2; FECO, Full-Extract Cannabis Oil; BHO, butane hash oil; CPE, cold press extraction, SFE, supercritical fluid extraction; MAE, microwave-assisted extraction; S/L, solid–liquid ratio; TF, total flavonoids; TP, total phenols; UAE, ultrasound-assisted extraction; PLE, pressurized liquid extraction; ASE, accelerated solvent extraction; HSPE, high-pressure solvent extraction; DESs, deep eutectic solvent extraction; EAE, enzyme-assisted extraction; PEF, pulsed electric field; MD, microwave distillation.
References
- Hurgobin, B.; Tamiru-Oli, M.; Welling, M.T.; Doblin, M.S.; Bacic, A.; Whelan, J.; Lewsey, M.G. Recent Advances in Cannabis Sativa Genomics Research. New Phytol. 2021, 230, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Micalizzi, G.; Vento, F.; Alibrando, F.; Donnarumma, D.; Dugo, P.; Mondello, L. Cannabis sativa L.: A Comprehensive Review on the Analytical Methodologies for Cannabinoids and Terpenes Characterization. J. Chromatogr. A 2021, 1637, 461864. [Google Scholar] [CrossRef]
- Lumír, L.; Ondřej, O.; Hanuš, H.; Meyer, S.M.; Muñoz, E.; Muñoz, M.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A Unified Critical Inventory. Nat. Prod. Rep. 2016, 33, 1347–1448. [Google Scholar] [CrossRef]
- Wu, J. Cannabis, Cannabinoid Receptors, and Endocannabinoid System: Yesterday, Today, and Tomorrow. Acta Pharmacol. Sin. 2019, 40, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Kis, B.; Ifrim, F.C.; Buda, V.; Avram, S.; Pavel, I.Z.; Antal, D.; Paunescu, V.; Dehelean, C.A.; Ardelean, F.; Diaconeasa, Z.; et al. Cannabidiol—From Plant to Human Body: A Promising Bioactive Molecule with Multi-Target Effects in Cancer. Int. J. Mol. Sci. 2019, 20, 5905. [Google Scholar] [CrossRef]
- FDA and Cannabis: Research and Drug Approval Process | FDA. Available online: https://www.fda.gov/news-events/public-health-focus/fda-and-cannabis-research-and-drug-approval-process (accessed on 15 March 2021).
- PDQ Integrative. Alternative, and Complementary Therapies Editorial Board. Cannabis and Cannabinoids (PDQ®): Health Professional Version. In Cancer Information Summaries; National Cancer Institute: Frederick, MD, USA, 2002. [Google Scholar]
- Elsaid, S.; Kloiber, S.; le Foll, B. Effects of Cannabidiol (CBD) in Neuropsychiatric Disorders: A Review of Pre-Clinical and Clinical Findings. Prog. Mol. Biol. Transl. Sci. 2019, 167, 25–75. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, H.Y.; Li, S.H.; Ma, W.; Wu, D.T.; Li, H.B.; Xiao, A.P.; Liu, L.L.; Zhu, F.; Gan, R.Y. Cannabis sativa Bioactive Compounds and Their Extraction, Separation, Purification, and Identification Technologies: An Updated Review. TrAC Trends Anal. Chem. 2022, 149, 116554. [Google Scholar] [CrossRef]
- Chen, C.; Pan, Z. Cannabidiol and Terpenes from Hemp–Ingredients for Future Foods and Processing Technologies. J. Future Foods 2021, 1, 113–127. [Google Scholar] [CrossRef]
- Understanding the Cannabis Laws in Latin America | Sounds and Colours. Available online: https://soundsandcolours.com/subjects/travel/understanding-the-cannabis-laws-in-latin-america-64296/ (accessed on 30 January 2023).
- Cannabis Policy: Status and Recent Developments. Available online: https://www.emcdda.europa.eu/publications/topic-overviews/cannabis-policy/html_en (accessed on 30 January 2023).
- Global Cannabis Report. 2019 Industry Outlook. Available online: https://ml.globenewswire.com/Resource/Download/b3594bc2-048e-4a1c-98bf-469320daa663 (accessed on 8 March 2023).
- Henry, P.; Khatodia, S.; Kapoor, K.; Gonzales, B.; Middleton, A.; Hong, K.; Hilyard, A.; Johnson, S.; Allen, D.; Chester, Z.; et al. A Single Nucleotide Polymorphism Assay Sheds Light on the Extent and Distribution of Genetic Diversity, Population Structure and Functional Basis of Key Traits in Cultivated North American Cannabis. J. Cannabis Res. 2020, 2, 26. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Davis, A.; Kumar, S.K.; Murray, B.; Zheljazkov, V.D. Industrial Hemp (Cannabis sativa Subsp. Sativa) as an Emerging Source for Value-Added Functional Food Ingredients and Nutraceuticals. Molecules 2020, 25, 4078. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis Sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Kleinhenz, M.D.; Magnin, G.; Lin, Z.; Griffin, J.; Kleinhenz, K.E.; Montgomery, S.; Curtis, A.; Martin, M.; Coetzee, J.F. Plasma Concentrations of Eleven Cannabinoids in Cattle Following Oral Administration of Industrial Hemp (Cannabis sativa). Sci. Rep. 2020, 10, 12753. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.A.; Wang, J.; Warner, P.; Wang, H. Antibacterial Properties of Hemp Hurd Powder against E. coli. J. Appl. Polym. Sci. 2015, 132, 1–6. [Google Scholar] [CrossRef]
- Turner, C.E.; Elsohly, M.A.; Boeren, E.G. Constituents of Cannabis sativa L. XVII. A Review of the Natural Constituents. J. Nat. Prod. 1980, 43, 169–234. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Slade, D. Chemical Constituents of Marijuana: The Complex Mixture of Natural Cannabinoids. Life Sci. 2005, 78, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Lata, H.; Khan, I.A.; ElSohly, M.A. Cannabis sativa L.: Botany and Horticulture. In Cannabis sativa L.-Botany and Biotechnology; Chandra, S., Lata, H., ElSohly, M.A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 79–100. [Google Scholar] [CrossRef]
- Tanney, C.A.S.; Backer, R.; Geitmann, A.; Smith, D.L. Cannabis Glandular Trichomes: A Cellular Metabolite Factory. Front. Plant Sci. 2021, 12, 721986. [Google Scholar] [CrossRef] [PubMed]
- Desaulniers Brousseau, V.; Wu, B.-S.; MacPherson, S.; Morello, V.; Lefsrud, M. Cannabinoids and Terpenes: How Production of Photo-Protectants Can Be Manipulated to Enhance Cannabis sativa L. Phytochemistry. Front. Plant Sci. 2021, 12, 1035. [Google Scholar] [CrossRef]
- Livingston, S.J.; Quilichini, T.D.; Booth, J.K.; Wong, D.C.J.; Rensing, K.H.; Laflamme-Yonkman, J.; Castellarin, S.D.; Bohlmann, J.; Page, J.E.; Samuels, A.L. Cannabis Glandular Trichomes Alter Morphology and Metabolite Content during Flower Maturation. Plant J. 2020, 101, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Pate, D. Chemical Ecology of Cannabis. J. Internat. Hemp Assoc. 1994, 22, 32–37. [Google Scholar]
- Hilderbrand, R.L. Hemp & Cannabidiol: What Is a Medicine? Mo. Med. 2018, 115, 306–309. [Google Scholar]
- Brenneisen, R. Chemistry and Analysis of Phytocannabinoids and Other Cannabis Constituents. In Marijuana and the Cannabinoids; Humana Press: Totowa, NJ, USA, 2007; pp. 17–49. [Google Scholar] [CrossRef]
- Sawtelle, L.; Holle, L.M. Use of Cannabis and Cannabinoids in Patients With Cancer. Ann. Pharmacother. 2021, 55, 870–890. [Google Scholar] [CrossRef]
- Booth, J.K.; Bohlmann, J. Terpenes in Cannabis sativa–From Plant Genome to Humans. Plant Sci. 2019, 284, 67–72. [Google Scholar] [CrossRef]
- Hazekamp, A.; Fischedick, J.T. Cannabis-From Cultivar to Chemovar. Drug Test. Anal. 2012, 4, 660–667. [Google Scholar] [CrossRef]
- Artz, I.C.; Hollman, P.C. Polyphenols and Disease Risk in Epidemiologic Studies. Am. J. Clin. Nutr. 2005, 81, 317S2013325S. [Google Scholar] [CrossRef]
- Radwan, M.M.; Chandra, S.; Gul, S.; ElSohly, M.A. Cannabinoids, Phenolics, Terpenes and Alkaloids of Cannabis. Molecules 2021, 26, 2774. [Google Scholar] [CrossRef]
- Flores-Sanchez, I.J.; Verpoorte, R. Secondary Metabolism in Cannabis. Phytochem. Rev. 2008, 7, 615–639. [Google Scholar] [CrossRef]
- Radwan, M.; Ross, S.; Slade, D.; Ahmed, S.; Zulfiqar, F.; ElSohly, M. Isolation and Characterization of New Cannabis Constituents from a High Potency Variety. Planta Med. 2008, 74, 267–272. [Google Scholar] [CrossRef]
- Bautista, J.L.; Yu, S.; Tian, L. Flavonoids in Cannabis Sativa: Biosynthesis, Bioactivities, and Biotechnology. ACS Omega 2021, 6, 5119–5123. [Google Scholar] [CrossRef] [PubMed]
- di Giacomo, V.; Recinella, L.; Chiavaroli, A.; Orlando, G.; Cataldi, A.; Rapino, M.; Di Valerio, V.; Politi, M.; Antolini, M.D.; Acquaviva, A.; et al. Metabolomic Profile and Antioxidant/Anti-Inflammatory Effects of Industrial Hemp Water Extract in Fibroblasts, Keratinocytes and Isolated Mouse Skin Specimens. Antioxidants 2021, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Pollastro, F.; Minassi, A.; Fresu, L.G. Cannabis Phenolics and Their Bioactivities. Curr. Med. Chem. 2018, 25, 1160–1185. [Google Scholar] [CrossRef] [PubMed]
- Al Ubeed, H.M.S.; Bhuyan, D.J.; Alsherbiny, M.A.; Basu, A.; Vuong, Q.V. A Comprehensive Review on the Techniques for Extraction of Bioactive Compounds from Medicinal Cannabis. Molecules 2022, 27, 604. [Google Scholar] [CrossRef] [PubMed]
- Giroud, C.; de Cesare, M.; Berthet, A.; Varlet, V.; Concha-Lozano, N.; Favrat, B. E-Cigarettes: A Review of New Trends in Cannabis Use. Int. J. Environ. Res. Public Health 2015, 12, 9988–10008. [Google Scholar] [CrossRef] [PubMed]
- Flockhart, I.; Gary, W.; Dring, S.; Archer, L. Method of Preparing Cannabidiol from Plant Material. U.S. Patent US20060167283A1, 23 September 2003. [Google Scholar]
- Rovetto, L.J.; Aieta, N.V. Supercritical Carbon Dioxide Extraction of Cannabinoids from Cannabis sativa L. J. Supercrit. Fluids 2017, 129, 16–27. [Google Scholar] [CrossRef]
- Drinić, Z.; Vladić, J.; Koren, A.; Zeremski, T.; Stojanov, N.; Kiprovski, B.; Vidović, S. Microwave-assisted Extraction of Cannabinoids and Antioxidants from Cannabis sativa Aerial Parts and Process Modeling. J. Chem. Technol. Biotechnol. 2020, 95, 831–839. [Google Scholar] [CrossRef]
- Karğılı, U.; Aytaç, E. Supercritical Fluid Extraction of Cannabinoids (THC and CBD) from Four Different Strains of Cannabis Grown in Different Regions. J. Supercrit. Fluids 2021, 179, 105410. [Google Scholar] [CrossRef]
- Qamar, S.; Torres, Y.J.M.; Parekh, H.S.; Robert Falconer, J. Extraction of Medicinal Cannabinoids through Supercritical Carbon Dioxide Technologies: A Review. J. Chromatogr. B 2021, 1167, 122581. [Google Scholar] [CrossRef]
- Kornpointer, C.; Martinez, A.S.; Schnürch, M.; Halbwirth, H.; Schröder, K. Combined Ionic Liquid and Supercritical Carbon Dioxide Based Dynamic Extraction of Six Cannabinoids from Cannabis sativa L. Green Chem. 2021, 23, 10079–10089. [Google Scholar] [CrossRef] [PubMed]
- Serna-Loaiza, S.; Adamcyk, J.; Beisl, S.; Kornpointner, C.; Halbwirth, H.; Friedl, A. Pressurized Liquid Extraction of Cannabinoids from Hemp Processing Residues: Evaluation of the Influencing Variables. Processes 2020, 8, 1334. [Google Scholar] [CrossRef]
- Agarwal, C.; Máthé, K.; Hofmann, T.; Csóka, L. Ultrasound-Assisted Extraction of Cannabinoids from Cannabis sativa L. Optimized by Response Surface Methodology. J. Food Sci. 2018, 83, 700–710. [Google Scholar] [CrossRef]
- Leiman, K.; Colomo, L.; Armenta, S.; de la Guardia, M.; Esteve-Turrillas, F.A. Fast Extraction of Cannabinoids in Marijuana Samples by Using Hard-Cap Espresso Machines. Talanta 2018, 190, 321–326. [Google Scholar] [CrossRef]
- Marzorati, S.; Friscione, D.; Picchi, E.; Verotta, L. Cannabidiol from Inflorescences of Cannabis sativa L.: Green Extraction and Purification Processes. Ind. Crops Prod. 2020, 155, 112816. [Google Scholar] [CrossRef]
- Grijó, D.R.; Vieitez Osorio, I.A.; Cardozo-Filho, L. Supercritical Extraction Strategies Using CO2 and Ethanol to Obtain Cannabinoid Compounds from Cannabis Hybrid Flowers. J. CO2 Util. 2018, 28, 174–180. [Google Scholar] [CrossRef]
- Cai, C.; Yu, W.; Wang, C.; Liu, L.; Li, F.; Tan, Z. Green Extraction of Cannabidiol from Industrial Hemp (Cannabis sativa L.) Using Deep Eutectic Solvents Coupled with Further Enrichment and Recovery by Macroporous Resin. J. Mol. Liq. 2019, 287, 110957. [Google Scholar] [CrossRef]
- Dach, J.; Moore, E.A.; Kander, J. Cannabis Extracts in Medicine: The Promise of Benefits in Seizure Disorders, Cancer, and Other Conditions, 1st ed.; McFarland & Company, Inc.: Jefferson, NC, USA, 2015. [Google Scholar]
- Loflin, M.; Earleywine, M. A New Method of Cannabis Ingestion: The Dangers of Dabs? Addict. Behav. 2014, 39, 1430–1433. [Google Scholar] [CrossRef]
- Bell, C.; Slim, J.; Flaten, H.K.; Lindberg, G.; Arek, W.; Monte, A.A. Butane Hash Oil Burns Associated with Marijuana Liberalization in Colorado. J. Med. Toxicol. 2015, 11, 422–425. [Google Scholar] [CrossRef]
- Al-Zouabi, I.; Stogner, J.M.; Miller, B.L.; Lane, E.S. Butane Hash Oil and Dabbing: Insights into Use, Amateur Production Techniques, and Potential Harm Mitigation. Subst. Abuse Rehabil. 2018, 9, 91–101. [Google Scholar] [CrossRef]
- Miller, B.L.; Stogner, J.M.; Miller, J.M. Exploring Butane Hash Oil Use: A Research Note. J. Psychoact. Drugs 2016, 48, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.P.; Zechar, K. Lung Injury from Inhaling Butane Hash Oil Mimics Pneumonia. Respir. Med. Case Rep. 2019, 26, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Luque de Castro, M.D.; Priego-Capote, F. Soxhlet Extraction: Past and Present Panacea. J. Chromatogr. A 2010, 1217, 2383–2389. [Google Scholar] [CrossRef]
- Lewis-Bakker, M.M.; Yang, Y.; Vyawahare, R.; Kotra, L.P. Extractions of Medical Cannabis Cultivars and the Role of Decarboxylation in Optimal Receptor Responses. Cannabis Cannabinoid Res. 2019, 4, 183–194. [Google Scholar] [CrossRef]
- Oomah, B.D.; Busson, M.; Godfrey, D.V.; Drover, J.C.G. Characteristics of Hemp (Cannabis sativa L.) Seed Oil. Food Chem. 2002, 76, 33–43. [Google Scholar] [CrossRef]
- Savoire, R.; Lanoisellé, J.L.; Vorobiev, E. Mechanical Continuous Oil Expression from Oilseeds: A Review. Food Bioproc. Tech. 2013, 6, 1–16. [Google Scholar] [CrossRef]
- Bogaert, L.; Mathieu, H.; Mhemdi, H.; Vorobiev, E. Characterization of Oilseeds Mechanical Expression in an Instrumented Pilot Screw Press. Ind. Crops Prod. 2018, 121, 106–113. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Supercritical Fluid Extraction and Fractionation of Natural Matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Moreno, T.; Montanes, F.; Tallon, S.J.; Fenton, T.; King, J.W. Extraction of Cannabinoids from Hemp (Cannabis sativa L.) Using High Pressure Solvents: An Overview of Different Processing Options. J. Supercrit. Fluids 2020, 161, 104850. [Google Scholar] [CrossRef]
- Gregory, L.H. Essential DIY Cannabis Concentrates: Readers Basic Guide to Original Methods for Marijuana Extracts, Oils and Concentrates; Independently Published, Ed.; 2020. Available online: https://oceanofpdf.com/authors/lisa-h-gregory-ph-d/pdf-epub-essential-diy-cannabis-concentrates-readers-basic-guide-to-original-methods-for-marijuana-extracts-oils-and-concentrates-download/ (accessed on 15 March 2021).
- Hedrick, J.L.; Mulcahey, L.J.; Taylor, L.T. Supercritical Fluid Extraction. Mikrochim. Acta 1992, 108, 115–132. [Google Scholar] [CrossRef]
- Aladić, K.; Jarni, K.; Barbir, T.; Vidović, S.; Vladić, J.; Bilić, M.; Jokić, S. Supercritical CO2 Extraction of Hemp (Cannabis sativa L.) Seed Oil. Ind. Crops Prod. 2015, 76, 472–478. [Google Scholar] [CrossRef]
- Benner, J.D.; McGiffin, D.B.; Douglas, L.M.; Delmarva Hemp LLC. Method for Solvent-Free Extraction and Concentration of Full Spectrum of Cannabinoids in a Carrier Oil. U.S. Patent 11,291,699, 5 April 2020. [Google Scholar]
- Ribeiro Grijó, D.; Vieitez Osorio, I.A.; Cardozo-Filho, L. Supercritical Extraction Strategies Using CO2 and Ethanol to Obtain Cannabinoid Compounds from Cannabis Hybrid Flowers. J. CO2 Util. 2019, 30, 241–248. [Google Scholar] [CrossRef]
- Rochfort, S.; Isbel, A.; Ezernieks, V.; Elkins, A.; Vincent, D.; Deseo, M.A.; Spangenberg, G.C. Utilisation of Design of Experiments Approach to Optimise Supercritical Fluid Extraction of Medicinal Cannabis. Sci. Rep. 2020, 10, 9124. [Google Scholar] [CrossRef]
- De Vita, D.; Madia, V.N.; Tudino, V.; Saccoliti, F.; De Leo, A.; Messore, A.; Roscilli, P.; Botto, A.; Pindinello, I.; Santilli, G.; et al. Comparison of Different Methods for the Extraction of Cannabinoids from Cannabis. Nat. Prod. Res. 2020, 34, 2952–2958. [Google Scholar] [CrossRef] [PubMed]
- Letellier, M.; Budzinski, H. Microwave Assisted Extraction of Organic Compounds. Analusis 1999, 27, 259–270. [Google Scholar] [CrossRef]
- Bagade, S.B.; Patil, M. Recent Advances in Microwave Assisted Extraction of Bioactive Compounds from Complex Herbal Samples: A Review. Crit. Rev. Anal. Chem. 2021, 51, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.-H.; Yusoff, R.; Ngoh, G.-C.; Kung, F.W.-L. Microwave-Assisted Extractions of Active Ingredients from Plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef]
- Gunjević, V.; Grillo, G.; Carnaroglio, D.; Binello, A.; Barge, A.; Cravotto, G. Selective Recovery of Terpenes, Polyphenols and Cannabinoids from Cannabis sativa L. Inflorescences under Microwaves. Ind. Crops Prod. 2021, 162, 113247. [Google Scholar] [CrossRef]
- Mazzara, E.; Carletti, R.; Petrelli, R.; Mustafa, A.M.; Caprioli, G.; Fiorini, D.; Scortichini, S.; Dall’Acqua, S.; Sut, S.; Nuñez, S.; et al. Green Extraction of Hemp (Cannabis sativa L.) Using Microwave Method for Recovery of Three Valuable Fractions (Essential Oil, Phenolic Compounds and Cannabinoids): A Central Composite Design Optimization Study. J. Sci. Food Agric. 2022, 102, 6220–6235. [Google Scholar] [CrossRef]
- Radoiu, M.; Kaur, H.; Bakowska-Barczak, A.; Splinter, S. Microwave-Assisted Industrial Scale Cannabis Extraction. Technologies 2020, 8, 45. [Google Scholar] [CrossRef]
- Tiwari, B.K. Ultrasound: A Clean, Green Extraction Technology. TrAC Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Albero, B.; Tadeo, J.L.; Pérez, R.A. Ultrasound-Assisted Extraction of Organic Contaminants. TrAC Trends Anal. Chem. 2019, 118, 739–750. [Google Scholar] [CrossRef]
- Omar, J.; Olivares, M.; Alzaga, M.; Etxebarria, N. Optimisation and Characterisation of Marihuana Extracts Obtained by Supercritical Fluid Extraction and Focused Ultrasound Extraction and Retention Time Locking GC-MS. J. Sep. Sci. 2013, 36, 1397–1404. [Google Scholar] [CrossRef]
- Rehman, M.U.; Abdullah; Khan, F.; Niaz, K. Introduction to Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; Volume 1. [Google Scholar] [CrossRef]
- Olejar, K.J.; Hatfield, J.; Arellano, C.J.; Gurau, A.T.; Seifried, D.; Heuvel, B.V.; Kinney, C.A. Thermo-Chemical Conversion of Cannabis Biomass and Extraction by Pressurized Liquid Extraction for the Isolation of Cannabidiol. Ind. Crops Prod. 2021, 170, 113771. [Google Scholar] [CrossRef]
- Béri, J.; Nagy, S.; Kovács, Á.K.; Vági, E.; Székely, E. Pressurized Liquid Extraction of Hemp Residue and Purification of the Extract with Liquid-Liquid Extraction. Period. Polytech. Chem. Eng. 2022, 66, 82–90. [Google Scholar] [CrossRef]
- Křížek, T.; Bursová, M.; Horsley, R.; Kuchař, M.; Tůma, P.; Čabala, R.; Hložek, T. Menthol-Based Hydrophobic Deep Eutectic Solvents: Towards Greener and Efficient Extraction of Phytocannabinoids. J. Clean. Prod. 2018, 193, 391–396. [Google Scholar] [CrossRef]
- Rapinel, V.; Rombaut, N.; Rakotomanomana, N.; Vallageas, A.; Cravotto, G.; Chemat, F. An Original Approach for Lipophilic Natural Products Extraction: Use of Liquefied n-Butane as Alternative Solvent to n-Hexane. LWT-Food Sci. Technol. 2017, 85, 524–533. [Google Scholar] [CrossRef]
- Fiorito, S.; Epifano, F.; Palumbo, L.; Collevecchio, C.; Genovese, S. A Subcritical Butane-Based Extraction of Non-Psychoactive Cannabinoids from Hemp Inflorescences. Ind. Crops Prod. 2022, 183, 114955. [Google Scholar] [CrossRef]
- Haji-Moradkhani, A.; Rezaei, R.; Moghimi, M. Optimization of Pulsed Electric Field-assisted Oil Extraction from Cannabis Seeds. J. Food Process Eng. 2019, 42, e13028. [Google Scholar] [CrossRef]
- Boussetta, N.; Soichi, E.; Lanoisellé, J.-L.; Vorobiev, E. Valorization of Oilseed Residues: Extraction of Polyphenols from Flaxseed Hulls by Pulsed Electric Fields. Ind. Crops Prod. 2014, 52, 347–353. [Google Scholar] [CrossRef]
- Kitrytė, V.; Bagdonaitė, D.; Rimantas Venskutonis, P. Biorefining of Industrial Hemp (Cannabis sativa L.) Threshing Residues into Cannabinoid and Antioxidant Fractions by Supercritical Carbon Dioxide, Pressurized Liquid and Enzyme-Assisted Extractions. Food Chem. 2018, 267, 420–429. [Google Scholar] [CrossRef]
- King, J.W. The Relationship between Cannabis/Hemp Use in Foods and Processing Methodology. Curr. Opin. Food Sci. 2019, 28, 32–40. [Google Scholar] [CrossRef]
- Rutz, A. CPC Distribution Chromatography of Cannabinoids. U.S. Patent 10,568,863B2, 26 February 2016. [Google Scholar]
- Ito, V.M.; Martins, P.F.; Batistella, C.B.; Maciel Filho, R.; Maciel, M.R.W. Natural Compounds Obtained through Centrifugal Molecular Distillation. Appl. Biochem. Biotechnol. 2006, 129–132, 716–726. [Google Scholar] [CrossRef]
- Lim, X.Y.; Tan, T.Y.C.; Rosli, S.H.M.; Saat, M.N.F.; Ali, S.S.; Mohamed, A.F.S. Cannabis sativa Subsp. Sativa’s Pharmacological Properties and Health Effects: A Scoping Review of Current Evidence. PLoS ONE 2021, 16, e0245471. [Google Scholar] [CrossRef] [PubMed]
- Mezza, G.N.; Borgarello, A.V.; Grosso, N.R.; Fernandez, H.; Pramparo, M.C.; Gayol, M.F. Antioxidant Activity of Rosemary Essential Oil Fractions Obtained by Molecular Distillation and Their Effect on Oxidative Stability of Sunflower Oil. Food Chem. 2018, 242, 9–15. [Google Scholar] [CrossRef]
- Lin, S.W.; Yoo, C.K. Short-path Distillation of Palm Olein and Characterization of Products. Eur. J. Lipid Sci. Technol. 2009, 111, 142–147. [Google Scholar] [CrossRef]
- Wang, S.; Gu, Y.; Liu, Q.; Yao, Y.; Guo, Z.; Luo, Z.; Cen, K. Separation of Bio-Oil by Molecular Distillation. Fuel Process. Technol. 2009, 90, 738–745. [Google Scholar] [CrossRef]
- Tovar, L.P.; Pinto, G.M.F.; Wolf-Maciel, M.R.; Batistella, C.B.; Maciel-Filho, R. Short-Path-Distillation Process of Lemongrass Essential Oil: Physicochemical Characterization and Assessment Quality of the Distillate and the Residue Products. Ind. Eng. Chem. Res. 2011, 50, 8185–8194. [Google Scholar] [CrossRef]
- Batistella, C.B.; Maciel, M.R.W. Modeling, Simulation and Analysis of Molecular Distillators: Centrifugal and Falling Film. Comput. Chem. Eng. 1996, 20, S19–S24. [Google Scholar] [CrossRef]
- Cermak, S.C.; Isbell, T.A. Pilot-Plant Distillation of Meadowfoam Fatty Acids. Ind. Crops Prod. 2002, 15, 145–154. [Google Scholar] [CrossRef]
- Hacke, A.C.M.; Lima, D.; de Costa, F.; Deshmukh, K.; Li, N.; Chow, A.M.; Marques, J.A.; Pereira, R.P.; Kerman, K. Probing the Antioxidant Activity of Δ 9 -Tetrahydrocannabinol and Cannabidiol in Cannabis sativa Extracts. Analyst 2019, 144, 4952–4961. [Google Scholar] [CrossRef] [PubMed]
- Blake, A.; Nahtigal, I. The Evolving Landscape of Cannabis Edibles. Curr. Opin. Food Sci. 2019, 28, 25–31. [Google Scholar] [CrossRef]
- Rasera, G.B.; Ohara, A.; de Castro, R.J.S. Innovative and Emerging Applications of Cannabis in Food and Beverage Products: From an Illicit Drug to a Potential Ingredient for Health Promotion. Trends Food Sci. Technol. 2021, 115, 31–41. [Google Scholar] [CrossRef]
- Thomas, B.F.; ElSohly, M.A. Analytical Methods in Formulation Development and Manufacturing. In The Analytical Chemistry of Cannabis; Brian, T.F., Ed.; Elsevier Science & Technology Books: Oxford, UK, 2016; pp. 63–81. [Google Scholar] [CrossRef]
- Buitrago-Suescún, O.Y.; Santaella-Serrano, M.A. Semicontinuous Lixiviation Process for Compound Extraction from Cannabis sativa Grown in Colombia. Ing. Investig. 2021, 42, e91616. [Google Scholar] [CrossRef]
- Vega, G.A.; Dávila, J.A. Use of Non-Psychoactive Residual Biomass from Cannabis sativa L. for Obtaining Phenolic Rich-Extracts with Antioxidant Capacity. Nat. Prod. Res. 2022, 36, 4193–4199. [Google Scholar] [CrossRef]
- Fernández, S.; Carreras, T.; Castro, R.; Perelmuter, K.; Giorgi, V.; Vila, A.; Rosales, A.; Pazos, M.; Moyna, G.; Carrera, I.; et al. A Comparative Study of Supercritical Fluid and Ethanol Extracts of Cannabis Inflorescences: Chemical Profile and Biological Activity. J. Supercrit. Fluids 2021, 179, 105385. [Google Scholar] [CrossRef]
- Flores-Sanchez, I.J.; Ramos-Valdivia, A.C. A Review from Patents Inspired by the Genus Cannabis. Phytochem. Rev. 2017, 16, 639–675. [Google Scholar] [CrossRef]
- Vinasco-Barco, J.A. The Tetrahydroevolution of Cannabis. A Reference to Production, Refining and Current Presentation of Cannabis. Cult. Drog. 2020, 25, 61–89. [Google Scholar] [CrossRef]
- Lustenberger, S.; Boczkaj, G.; Castro-Muñoz, R. Cannabinoids: Challenges, Opportunities and Current Techniques towards Its Extraction and Purification for Edibles. Food Biosci. 2022, 49, 101835. [Google Scholar] [CrossRef]
- Menezes, E.G.O.; de Souza e Silva, A.P.; de Sousa, K.R.P.; de Azevedo, F.; Morais, R.M.; de Carvalho Junior, R.N. Development of an Innovative Strategy Capable of Describing the Large-Scale Extraction of Tucumã-of-Pará Oil (Astrocaryum Vulgare Mart.) Using Supercritical CO2 as Solvent. J. Supercrit. Fluids 2023, 193, 105825. [Google Scholar] [CrossRef]
- Martínez-Padilla, L.P.; Hernández-Rojas, F.S.; Sosa-Herrera, M.G.; Juliano, P. Novel Application of Ultrasound and Microwave-Assisted Methods for Aqueous Extraction of Coconut Oil and Proteins. J. Food Sci. Technol. 2022, 59, 3857–3866. [Google Scholar] [CrossRef] [PubMed]
- Cruz Reina, L.J.; López, G.D.; Durán-Aranguren, D.D.; Quiroga, I.; Carazzone, C.; Sierra, R. Compressed Fluids and Soxhlet Extraction for the Valorization of Compounds from Colombian Cashew (Anacardium occidentale) Nut Shells Aimed at a Cosmetic Application. J. Supercrit. Fluids 2023, 192, 105808. [Google Scholar] [CrossRef]
- Feller, R.; Matos, Â.P.; Mazzutti, S.; Moecke, E.H.S.; Tres, M.V.; Derner, R.B.; Oliveira, J.V.; Junior, A.F. Polyunsaturated Ω-3 and Ω-6 Fatty Acids, Total Carotenoids and Antioxidant Activity of Three Marine Microalgae Extracts Obtained by Supercritical CO2 and Subcritical n-Butane. J. Supercrit. Fluids 2018, 133, 437–443. [Google Scholar] [CrossRef]
- Ampofo, J.; Ngadi, M. Ultrasound-Assisted Processing: Science, Technology and Challenges for the Plant-Based Protein Industry. Ultrason. Sonochem. 2022, 84, 105955. [Google Scholar] [CrossRef]
- Grijó, D.R.; Piva, G.K.; Osorio, I.V.; Cardozo-Filho, L. Hemp (Cannabis sativa L.) Seed Oil Extraction with Pressurized n-Propane and Supercritical Carbon Dioxide. J. Supercrit. Fluids 2019, 143, 268–274. [Google Scholar] [CrossRef]
- Milanez, P.R.; da Silva, F.M.R.; Scussel, R.; de Melo, M.E.; Martins, A.B.; Machado-de-Ávila, R.A.; Barlow, J.W.; Feuser, P.E.; Rigo, F.K.; de Aguiar Amaral, P. Cannabis Extracts and Their Cytotoxic Effects on Human Erythrocytes, Fibroblasts, and Murine Melanoma. Rev. Bras. Farmacogn. 2021, 31, 750–761. [Google Scholar] [CrossRef]
- Ribeiro Grijó, D.; Olivo, J.E.; Curty, O.; Lima, M. Analysis of the Different Solubility Data of Cannabidiol in Supercritical Carbon Dioxide Described in the Literature. Braz. J. Chem. Eng. 2022, 39, 225–234. [Google Scholar] [CrossRef]
- Gallo-Molina, A.C.; Castro-Vargas, H.I.; Garzón-Méndez, W.F.; Martínez Ramírez, J.A.; Rivera Monroy, Z.J.; King, J.W.; Parada-Alfonso, F. Extraction, Isolation and Purification of Tetrahydrocannabinol from the Cannabis sativa L. Plant Using Supercritical Fluid Extraction and Solid Phase Extraction. J. Supercrit. Fluids 2019, 146, 208–216. [Google Scholar] [CrossRef]
- Pegoraro, C.N.; Nutter, D.; Thevenon, M.; Ramirez, C.L. Chemical Profiles of Cannabis sativa Medicinal Oil Using Different Extraction and Concentration Methods. Nat. Prod. Res. 2021, 35, 2249–2252. [Google Scholar] [CrossRef]
- Bridgeman, M.B.; Abazia, D.T. Medicinal Cannabis: History, Pharmacology, And Implications for the Acute Care Setting. Pharm. Ther. 2017, 42, 180–188. [Google Scholar]
- Energias Market Research. Global Medical Marijuana Offering Business Opportunity in. Available online: https://www.globenewswire.com/news-release/2018/03/01/1402007/0/en/Global-Medical-Marijuana-Offering-Business-Opportunity-in-Medical-Industry-and-is-projected-to-Reach-USD-28-07-billion-by-2024-Energias-Market-Research-Pvt-Ltd.html (accessed on 30 November 2021).
- Wood, L. Global Medical Cannabis Market Forecast to 2028-Escalating Government Funding for Spreading Awareness and Exploring Medicinal Benefits of Cannabis-ResearchAndMarkets.com | Business Wire. Available online: https://www.businesswire.com/news/home/20210519005520/en/Global-Medical-Cannabis-Market-Forecast-to-2028---Escalating-Government-Funding-for-Spreading-Awareness-and-Exploring-Medicinal-Benefits-of-Cannabis---ResearchAndMarkets.com (accessed on 15 March 2023).
- Arcview Market Research; BDS Analytics. The Roadmap to a $57 Billion Worldwide Market EXECUTIVE SUMMARY. Available online: https://shop.bdsa.com/wp-content/uploads/2019/06/Roadmap-Exec-Summ.pdf (accessed on 28 November 2021).
- O’Grady, C. Cannabis Research Database Shows How U.S. Funding Focuses on Harms of the Drug. Science 2020, 27. [Google Scholar] [CrossRef]
- Grand View Research. Medical Marijuana Market Size & Share Report, 2030. Available online: https://www.grandviewresearch.com/industry-analysis/medical-marijuana-market (accessed on 15 March 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).