Abstract
To gain insight into the effect of Si/C arrangement on the molecular framework of strained polyhedral compounds, dodecahedrane analogues containing equal numbers of carbon and silicon (Si/C equally mixed dodecahedrane analogues) were investigated using the ab initio molecular orbital method. There are 1648 isomers for which the Si/C arrangement on the molecular framework is different. Based on the geometry optimization of all the isomers as well as the carbon and silicon analogues, the characteristics of the structure, relative energy, and strain energy of the Si/C equally mixed compounds are presented. Then, important factors controlling the relative energy, such as strain energy, are proposed through regression analysis. Also discussed is the correlation between the relative energy and the indices of Si/C dispersion, such as the number of skeletal C–Si single bonds and condensed five-membered rings constituting the polyhedral structure.
1. Introduction
Tetrahedrane, cubane, and dodecahedrane are known as saturated hydrocarbons with the structure of a regular polyhedron of Plato [1]. These compounds have long been an attractive subject for many experimental and theoretical studies because of scientific curiosity about their highly symmetric structure and the significant strain energy brought about by the deformed sp3 structure. Every polyhedral compound has already been synthesized. Tetrahedrane has been synthesized as derivatives with bulky substituents, while cubane and dodecahedrane have been obtained as genuine hydrocarbons, CXHX(X = 8,20) [2,3,4,5,6,7,8,9,10]. Dodecahedrane, the target of the present study, has a highly symmetric structure (the point group of C20H20 is Ih) and is known as the completely hydrogen-saturated dodecahedral fullerene, which is the smallest fullerene [11,12]. Persiladodecahedrane (also called persilafullerane), the structure of which encapsulates a Cl− anion, has recently been synthesized by Bamberg et al. [13,14]
Among the polyhedral compounds, tetrahedrane and cubane are highly strained due to the condensed three- or four-membered rings in their molecular skeletons. The strain energy of dodecahedrane, however, is remarkably lower than those of tetrahedrane and cubane as the molecule’s structure is constructed from less strained five-membered rings [15]. Paquette’s group has experimentally determined the strain energy of dodecahedrane to be 61.4 kcal mol−1 [16,17], which is significantly smaller than the strain energies of tetrahedrane (140.0 kcal mol−1) and cubane (154.7 kcal mol−1) [18].
For the theoretical studies, Nagase et al. suggested that a small polyhedral hydrocarbon’s strain energy decreases with the substitution of the skeletal carbons by the heavier group 14 elements, especially for the cages containing four–membered rings, through the ab initio molecular orbital method [19,20]. Earley systematically investigated the strain energy of 10 types of polyhedral compounds, and the heavier group 14 elements substituted analogues by ab initio molecular orbital calculations [15]. The author suggested that the strain energies of polyhedral compounds are affected by the inner condensed ring’s strain, and the former can be approximately assessed as the sum of the latter.
Recently, compounds with a structure containing both carbon and silicon in the molecular skeleton have attracted attention. For Si/C mixed unsaturated compounds, Si-doped fullerene analogues in which the skeletal carbons are substituted by silicons have been confirmed by photoionization mass spectroscopy [21,22,23]. Furthermore, we revealed that some of the C and Si alternately mixed annulenes (benzene and cyclooctatetraene analogues, and so on) have unique characteristics that are completely different from those of the carbon and silicon congeners [24,25,26]. On the other hand, silaadamantanes and silicon carbides, both of which are known as functional materials, are the typical examples of the Si/C mixed saturated compounds. Fotooh et al. investigated the HOMO–LUMO gap changes brought about by Si substitutions in adamantane [27]. Miranda et al. obtained the thermodynamic quantities of silaadamantane with theoretical calculations and compared them with those of adamantane and persilaadamantane [28]. Silicon carbides with diamond-type crystal structures made of only C–Si bonds have been utilized in a wide variety of fields because of their unique semiconductor and mechanical characteristics. They are expected to be the next generation of power semiconductors of the.
For saturated cyclic or polyhedral compounds, the Si/C mixing effect on the angle strain, which is one of their important characteristics, is interesting. However, there are only a few theoretical studies for two-element-mixing analogues of tetrahedrane and cubane [29,30]. In this situation, our fragment structure energy (FSE) analysis demonstrated that Si/C alternately mixing contributes to the stabilization of the systems in the ideal (with no angle strain) sp3 environment as well as the sp2 environment, which was not observed for the mixing of carbon or the other heavier group 14 elements [31].
The goal of the present study was to explore the Si/C mixing effects on the structure and properties such as strain energy of polyhedral compounds through quantum chemical calculation. For the purpose, we decided to investigate the Si/C equally mixed dodecahedral analogues, C10Si10H20. The main reason is that the number of the isomers with different Si/C arrangements is the largest [32,33,34], 1648, among a series of Si/C mixed dodecahedrane analogues, CXSi20–XH20(X = 0,1,2, … ,20), so the trend in the effect based on large amounts of data is considered to be rather reliable. First, the structure of all the isomers was determined, and then the relative energy and strain energy were compared not only for the Si/C mixed analogues but also for dodecahedrane (C20H20) and persiladodecahedrane (Si20H20). In addition, regression analysis was carried out to examine the correlations between the relative energy, strain energy, and indices of the Si/C arrangement.
2. Methods
The geometries of all molecules were fully optimized at the Hartree–Fock (HF) [35] and the second-order Møller–Plesset perturbation (MP2) [36,37] level with the 6-31G(d) basis set [38]. Then, normal mode vibrational analysis was performed to confirm the molecular characteristics as an equilibrium structure. As a result, all molecules investigated here were found to be equilibrium structures at the HF/6-31G(d) level. For some specific molecules, the analysis was carried out at the MP2/6-31G(d) level as well, and they were confirmed to be equilibrium structures at both levels of calculations.
Strain energy (SE) was estimated from the homodesmotic reaction energy (∆E), described by the following equation [39,40]:
C10Si10H20 is the energy of Si/C equally mixed dodecahedrane analogues, while C2H6, CSiH6, and Si2H6 are, respectively, the energies of ethane, methyl silane, and disilane. On the other hand, nCC, nCSi, and nSiSi are, respectively, the numbers of C–C bonds, C–Si bonds, and Si–Si bonds. The first term of the right side of Equation 1 represents the energy of Si/C mixed isobutane analogues that comes from each vertex atom and the three atoms connected with that atom. Therefore, the naA coefficients are different for every isomer because they depend on the arrangement of Si and C atoms in the framework, and nCC, nCSi, and nSiSi are determined from naA. The details are in the Supplementary Materials.
Furthermore, single- and multiple-regression analyses were carried out to assess the contribution of crucial factors controlling the relative stability of the isomers. All calculations were performed with the Gaussian 16 program package [41].
On the other hand, 1648 Si/C arrangements of the isomers were obtained with our own program. The details are presented in the Supplementary Materials.
3. Structures
Seven isomers (a–g) with different Si/C arrangements are depicted in Figure 1. Among the 1648 isomers, they are especially characteristic for their relative energy (RE), strain energy (SE), and the number of C–Si bonds (NC–Si).
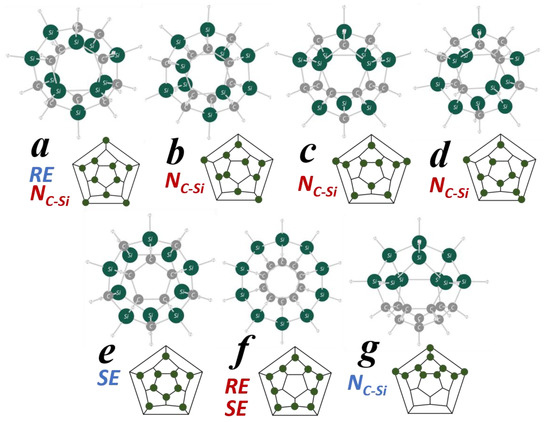
Figure 1.
The MP2/6-31G(d) optimized structure and polyhedral graph of seven isomers of Si/C equally mixed dodecahedrane analogues of C10Si10H20. Green circles show Si and gray ones are C in the structures, while green dots represent Si in the graphs. For the RE, SE, and NC–Si, red characters indicate that the property is the largest among all 1648 of the isomers, while blue ones indicate the smallest property.
The properties are compared among all the 1648 isomers together with some other items in Table 1. Isomers a–d have 24 C–Si bonds, which is the largest NC–Si among all the isomers. The dodecahedral framework consists of 30 sides (bonds), so for a–d, 80% of the bonds are C–Si bonds. In contrast, the NC–Si of g is the smallest, as it is only six. As NC–Si is considered to be an index of dispersion of C and Si in the molecular framework, the two elements are considerably delocalized in a–d, while they are extremely localized in g, which is obvious from the figure.

Table 1.
Several important properties of the Si/C equally mixed dodecahedrane isomers, dodecahedrane, and the silicon analogues at the MP2/6-31G(d) level.
The isomer a has the lowest electronic energy at the present level of theory. Therefore, RE is the energy of each isomer relative to that of a. As shown in Table 1, however, it is almost the same for a–d. In fact, we found that the order of the relative stability can change at other levels of theory, but the energy differences are still small (see Table S2 in the Supplementary Materials). A histogram of the number of isomers from the RE is shown in Figure 2. As in the case of a–d, it was found that there exist many isomers in between the tiny energy range (5.0 kcal mol−1) except for f, suggesting this is one of the characteristics of the Si/C equally mixed dodecahedrane analogues. On the other hand, e, with the second-largest NC–Si (20), has the smallest SE, 29.9 kcal mol−1. It is noteworthy that the RE of e is also close to those of a–d, which have the largest NC–Si.
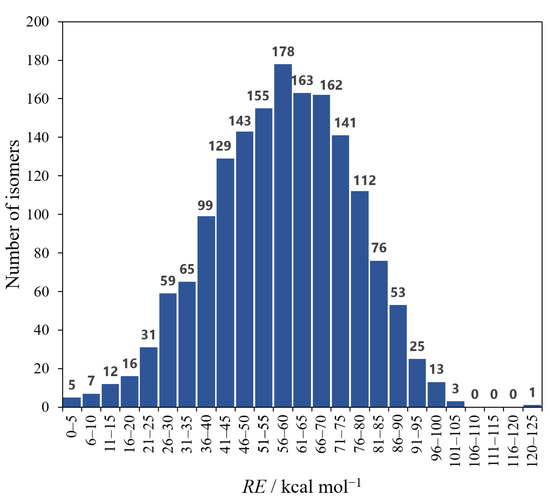
Figure 2.
Histogram of the number of isomers and RE. The class range of RE is 5.0 kcal mol−1.
The SE of the Si/C mixed isomers is between those of dodecahedrane and persiladodecahedrane but rather close to that of the carbon analogue. Incidentally, the SE of the silicon analogue estimated by Earley is much larger than ours, suggesting that the effect of electron correlation might be important for the estimation of the strain energy of the silicon analogues consisting of flexible Si–Si bonds (see Table S3 in the Supplementary Materials). The isomer f has the largest SE among all the 1648 isomers, and its SE is even larger than that of dodecahedrane. In fact, it was found that in addition to f, there are eight more isomers that have a larger SE than dodecahedrane. Obviously, RE seems to be proportional to SE among the Si/C mixed isomers.
Table 2 shows each bond length for the isomers mentioned above. The C–C and Si–Si bond lengths of dodecahedrane and the silicon analogue are also shown for comparison. The calculated lengths of the C–C bonds of C20H20 and the Si–Si bonds of Cl−@Si20H20 agree well with their respective experimental values. The encapsulated Cl− does not seem to have any effect on the dodecahedral structure. In addition, every bond length except for that of the C–Si bond of Si/C equally mixed isomers is not much different from those in C20H20 and Si20H20. The C–Si bond lengths of a–e are close to those of methylsilane CH3SiH3 (1.88 Å) obtained at the same level of theory, suggesting the bond length is almost not influenced by the polyhedral structure. On the other hand, the corresponding bond lengths of f and g are longer than those of the other isomers, so the relatively large strain energy might affect the C–Si distance and relative stability.

Table 2.
Five kinds of bond length (Å) of dodecahedrane analogues at the MP2/6-31G(d) level.
Finally, the HOMO–LUMO energy gap of the dodecahedrane analogues was examined. For comparison, the energy gap of diamond-type molecules with molecular sizes similar to those of dodecahedranes is also shown in Table 3 as the model of silicon carbide. C30H40 and Si30H40 are the model compounds of the diamond-type crystal terminated by hydrogens and the silicon analogue, respectively, while C10Si20H40 and C20Si10H40 are the two types of Si/C mixed congeners [31]. The frontier molecular orbitals of the seven isomers a to f, dodecahedrane, and persiladodecahedrane are displayed in Figures S2–S10 of the Supplementary Materials. As the table shows, the energy gaps of the Si/C mixed analogues are halfway between those of dodecahedrane and persiladodecahedrane, except for isomer f. This can be explained by the fact that the frontier MOs of f tend to have large coefficients, especially on the long Si–Si σ-bond sequence (see Figure S7), so the energy gap becomes close to that of σ and σ* of Si–Si bonds in Si20H20, which is known as theσ-conjugation of Si–Si bonds. However, a surprising thing is that the gap is rather smaller than that of the silicon analogue. Incidentally, there are eight more isomers whose HOMO–LUMO gap is smaller than that of Si20H20. Long Si–Si bond sequences such as those in isomer f are also seen in their structures (see Figure S12 in the Supplementary Materials). An interesting thing is that the SE of these isomers is larger than that of dodecahedrane, as mentioned above. The HOMO–LUMO gap of isomer g with the branched Si–Si bonds is also small compared with that of the other isomers, probably for the same reason.

Table 3.
Energy level of HOMO and LUMO and their energy gap of the Si/C equally mixed dodecahedrane isomers, dodecahedrane, and the silicon analogues at the MP2/6-31G(d) level. For the other molecules [31], see the text.
For the model molecules of silicon carbide, the observations were similar to those for the dodecahedrane analogues, though the energy gap of the Si/C mixed dodecahedranes is about 1 eV smaller than the average of the two types of the Si/C alternately mixed diamond-type molecules (12.9 eV, see Table 3). The small HOMO–LUMO energy gap can be considered to be one of the requirements of a molecule as a good semiconductor. In this sense, some isomers of the Si/C equally mixed dodecahedranes were found to have the potential for such functional materials.
As a result, Si/C mixed dodecahedrane analogues such as isomer f might be semiconductor candidates depending on the Si/C arrangement in their molecular framework. Moreover, this is an interesting example of a Si/C arrangement significantly affecting a molecular property.
4. Regression Analyses for the RE of Isomers
In the previous section, it was revealed that some important factors such as strain energy (SE) and the number of C–Si bonds (NC–Si) affect the relative energy (RE) of every isomer. Therefore, we tried to determine how important each factor is for the relative stability of all the 1648 isomers using regression analyses.
4.1. Relative Energy vs. Strain Energy
Figure 3 shows the correlation of RE against SE. The correlation coefficient (R) and the coefficient of determination (R2) are, respectively, 0.9432 and 0.8897, suggesting a considerably strong correlation between RE and SE. Therefore, regarding the strain energy, the Si/C equally mixed dodecahedranes seem to have a common characteristic as strained polyhedral compounds. Furthermore, it is obvious from the figure that isomer f, with the largest SE as well as RE among all the isomers, is specifically unique.
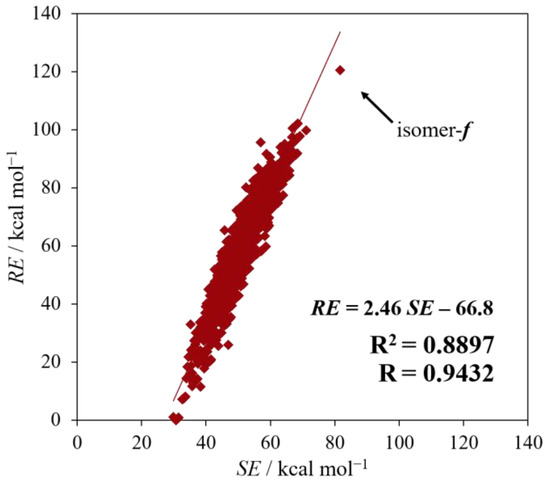
Figure 3.
The results of regression analysis between SE and RE. The arrow indicates isomer f.
4.2. Relative Energy vs. the Number of C–Si Bonds
As shown in the preceding section, the most stable isomers have the largest NC–Si, which means that a structure with delocalized C and Si tends to be relatively stable. However, as seen from the R and R2 in Figure 4, there is a weak correlation between NC–Si and RE, and it is not that high compared with that with SE. This result is considered to be evidence showing the superiority of SE over NC–Si with regard to the impact on the RE.
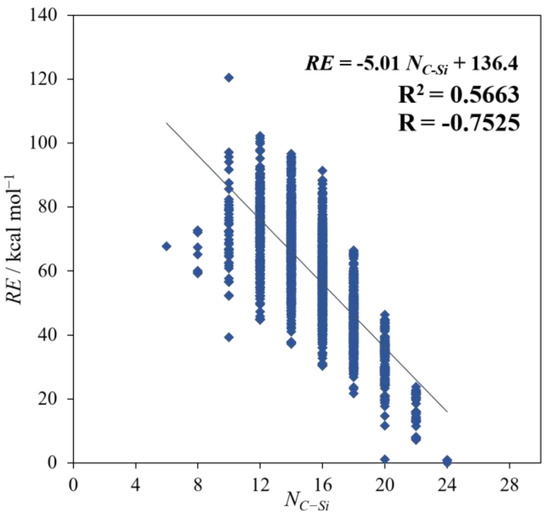
Figure 4.
The plots of RE against NC–Si.
4.3. Relative Energy vs. Five-Membered Rings of the Molecular Skeletons
Some correlation between RE and NC–Si was expected from the fact that a group of isomers with the smallest RE tend to have the larger NC–Si. However, according to the regression analysis described in Figure 4, the correlation was found to not be as high as noted above. Therefore, to look at the dispersion of C and Si from another point of view, we tried to focus on the twelve five−membered rings (5MRs) constituting the molecular framework of the Si/C equally mixed dodecahedrane analogues.
As shown in Figure 5, there are eight types of 5MRs depending on the Si/C arrangement of the pentagonal vertexes. The extent of the Si/C delocalization/localization is estimated by the comparison of the two types of C2Si3 (5MR−3 and 4) and Si3C2 (5MR−5 and 6). For both cases, C and Si are more delocalized in the latter than the former, as seen from Figure 5.
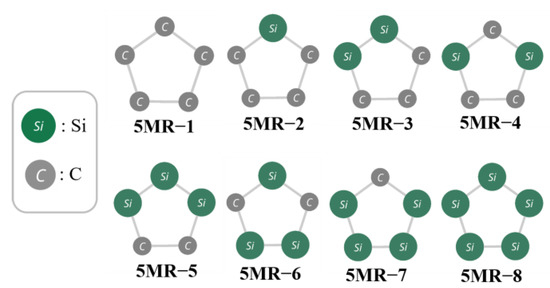
Figure 5.
All types of 5−membered rings (5MRs) in Si/C mixed dodecahedrane analogues.
Figure 6 shows the correlation between the number of each 5MR (N5MR−X; X = 1−8) and RE. For every 5MR, the number is limited because of the restriction on the number of C and Si being 10 each in the dodecahedral framework. For example, as the number of 5MR, 0−3 are only allowed for 5MR−1 and 5MR−8, while those are 0−5 for 5MR−2 and 5MR−7, and so on. Among them, the coefficients of correlation for N5MR−3 and N5MR−5 (“localized” group) are about 0.6, so these two types of 5MR have a weakly positive correlation with RE, while there are weakly negative correlations (R ≈ −0.6) between RE and 5MR−4,6 (“delocalized” group). These results suggest that isomers consisting of the Si/C delocalized 5MR are relatively stable, though the correlation is not high.
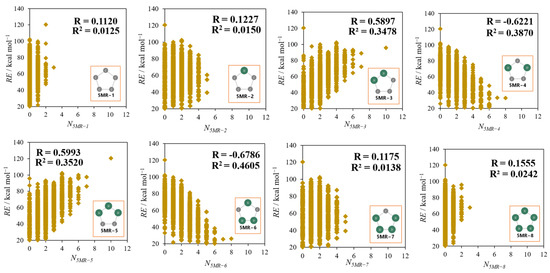
Figure 6.
The correlations between RE and N5MR−X for the 8 types of 5MR.
Next, we also examined the correlation between RE and the sum of the N5MR−X of groups consisting of two kinds of 5MR having the same tendency, 5MR−3, 5 (“localized” group) and 5MR−4, 6 (“delocalized” group). As shown in Figure 7a, RE has a stronger correlation with the sum of N5MR−3 and N5MR−5 in comparison with that of the respective single 5MR cases mentioned above. Likewise, the sum of N5MR−4 and N5MR−6 has a stronger negative correlation with RE, as seen in Figure 7b. Therefore, these results indicate that isomers with the structure in which C and Si are rather delocalized in 5MRs tend to be stable, and the same is true for the number of C–Si bonds shown in Figure 4.
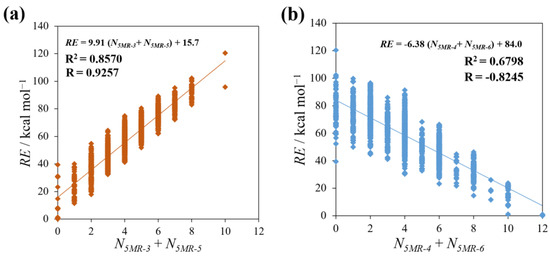
Figure 7.
The plots between RE and the number of the sum of two 5MRs that have a similar correlation for RE: (a) 5MR−3 + 5MR−5 (“localized” group) and (b) 5MR−4 + 5MR−6 (“delocalized” group).
From the investigation in this section, it was found that the specific five−membered rings considerably affect the relative stability of the isomers.
4.4. Multiple Regression Analysis of SE and NC−Si
We examined the three important properties SE, NC–Si, and N5MR−X correlated with the RE of the isomers as discussed above. So, our next concern was quantitatively comparing each contribution to RE. Apparently, SE has a strong correlation, but that of the others is rather mild. Furthermore, NC–Si and N5MR−X (the sum of N5MR−3 and N5MR−5, etc.) are not considered to be factors completely independent of each other, as both are related to the dispersion of C and Si in the molecular framework. Furthermore, both variables have some correlation with SE, the coefficient of correlation being −0.6178 for NC–Si and 0.8897 for the sum of N5MR−3 and N5MR−5 (see Figure S13). It is not appropriate to select variables having a strong correlation with each other. Then, we chose NC–Si, with a smaller correlation with SE, as an index of the delocalization of C and Si and performed a multiple regression analysis of SE and NC–Si.
The multiple regression equation for this study was as follows:
REs = asSE SEs − asCSi NsC–Si
REs is a response variable for standardized RE. SEs and NsC–Si are, respectively, the explanatory variables for standardized SE and NC–Si; and asSE and asCSi are the coefficients, respectively.
The details are provided in the Supplementary Materials.
As a result, the following Equation (3) was obtained.
REs = 0.77 SEs − 0.27NsC–Si
Based on this analysis, the determination coefficient was confirmed to be extremely high, 0.9363.
The coefficient of SEs is about three times larger than that of NsC–Si at the present level of calculations, suggesting that the strain energy is a major factor controlling the relative stability of the isomers of the Si/C equally mixed dodecahedrane analogues.
5. Conclusions
The effect of half-silicon substitution of the skeletal carbons of dodecahedrane on the structure and some properties, such as strain energy, was investigated through the ab initio molecular orbital method. There are 1648 isomers of the Si/C equally mixed dodecahedrane, in which Si/C arrangement energies are very close. Among them, however, the least stable isomer involving a long Si sequence in the molecular structure was found to have remarkably high relative energy and strain energy. The strain energies and HOMO–LUMO gaps of the Si/C mixed compounds are intermediate between those of dodecahedrane and the per-silicon analogues, except for a few isomers. These isomers seem to be considerably unique among all the Si/C equally mixed dodecahedrane analogues. The relative stability of all the isomers tends to be high when the strain energy is low, and the C and Si are quite delocalized in the dodecahedral framework.
According to the regression analysis, the strain energy was found to be highly correlated with the relative energy. Also examined were the contributions of the number of C–Si bonds and the condensed five-membered rings in the dodecahedral skeleton. Both are expected to have some important effect on the relative energy as they can be the indexes to assess the Si/C dispersion, but the correlation with the relative energy was rather weak. Furthermore, standardized multiple regression analysis revealed that the impact of the strain energy on the relative energy is greater than that of the number of C–Si bonds.
From the present study, the properties of Si/C equally mixed dodecahedranes were found to greatly depend on the Si/C arrangement: the silicon substitution, in other words. Therefore, molecular designs that take the results into account may contribute to the development of a new functional material such as silicon carbide. Investigations of Si/C mixed dodecahedranes with other Si/C ratios are underway.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28062769/s1, Table S1. The number of isomers for the all kinds of Si/C mixed dodecahedrane analogues; Table S2. The REs at several levels of theory; Table S3. The strain energies at several levels of theory; Table S4. The properties of the nine isomers at the MP2/6-31G(d); Figure S1. The first step of the program; Figure S2. isomer-a; Figure S3. isomer-b; Figure S4. isomer-c; Figure S5. isomer-d; Figure S6. isomer-e; Figure S7. isomer-f; Figure S8. isomer-g; Figure S9. Dodecahedrane; Figure S10. Persiladodecahedrane; Figure S11. The histogram against HOMO–LUMO gap at the MP2/6-31G(d); Figure S12. The MP2/6-31G(d) optimized structures and polyhedral graphs of the nine isomers whose HOMO–LUMO gaps below that of Si20H20; Figure S13. The correlation against SE.
Author Contributions
Theoretical calculations, T.U. and T.N.; statistical analysis, T.U.; writing—original draft preparation, T.U.; writing—review and editing, T.N., T.K. and M.H.; visualization, T.U.; supervision, T.K.; project administration, T.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JST SPRING, grant number JPMJSP 2146.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are contained within the article and are also available in the Supplementary Materials.
Acknowledgments
We are grateful to Tatsuya Nagao and Hideyuki Itabashi at Gunma University for their valuable comments and beneficial discussion.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Unno, M. Substituted Polyhedral Silicon and Germanium Clusters. In Functional Molecular Silicon Compounds II: Low Oxidation States; Springer: Berlin/Heidelberg, Germany, 2014; Volume 156, pp. 49–84. [Google Scholar]
- Maier, G.; Pfriem, S.; Schafer, U.; Matusch, R. Tetra-tert-butyltetrahedrane. Angew. Chem. Int. Ed. Engl. 1978, 17, 520–521. [Google Scholar] [CrossRef]
- Maier, G.; Neudert, J.; Wolf, O.; Pappusch, P.; Sekiguchi, A.; Tanaka, M.; Matsuo, T. Tetrakis (trimethylsilyl)tetrahedrane. J. Am. Chem. Soc. 2002, 124, 13819–13826. [Google Scholar] [CrossRef]
- Tanaka, M.; Sekiguhi, A. Hexakis(trimethylsilyl)tetrahedranyltetrahedrane. Angew. Chem. Int. Ed. Engl. 2005, 44, 5751. [Google Scholar] [CrossRef]
- Nakamoto, M.; Inagaki, Y.; Nishina, M.; Sekiguchi, A. Perfluoroaryltetrahedranes: Tetrahedranes with Extended σ-π Conjugation. J. Am. Chem. Soc. 2009, 131, 3172–3173. [Google Scholar] [CrossRef] [PubMed]
- Eaton, P.E.; Cole, T.W. Cubane. J. Am. Chem. Soc. 1964, 86, 3157–3158. [Google Scholar] [CrossRef]
- Ternansky, R.J.; Balogh, D.W.; Paquette, L.A. Dodecahedrane. J. Am. Chem. Soc. 1982, 104, 4503–4504. [Google Scholar] [CrossRef]
- Paquette, L.A.; Ternansky, R.J.; Balogh, D.W.; Taylor, W.J. Total synthesis of a monosubstituted dodecahedrane. The methyl derivative. J. Am. Chem. Soc. 1983, 105, 5441–5446. [Google Scholar] [CrossRef]
- Paquette, L.A.; Ternansky, R.J.; Balogh, D.W.; Kentgen, G. Total synthesis of dodecahedrane. J. Am. Chem. Soc. 1983, 105, 5446–5450. [Google Scholar] [CrossRef]
- Gallucci, J.C.; Doecke, C.W.; Paquette, L.A. X-ray structure analysis of the pentagonal dodecahedrane hydrocarbon (CH)20. J. Am. Chem. Soc. 1986, 108, 1343–1344. [Google Scholar] [CrossRef]
- Prinzbach, H.; Weller, A.; Landenberger, P.; Wahl, F.; Worth, J.; Scott, L.T.; Gelmont, M.; Olevano, D.; von Issendorff, B. Gas-phase production and photoelectron spectroscopy of the smallest fullerene, C20. Nature 2000, 407, 60–63. [Google Scholar] [CrossRef]
- Jarrold, M.F. The smallest fullerene. Nature 2000, 407, 26–27. [Google Scholar] [CrossRef]
- Bamberg, M.; Bamberg, M.; Bursch, M.; Hansen, A.; Brandl, M.; Sentis, G.; Kunze, L.; Bolte, M.; Lerner, H.W.; Grimme, S.; et al. [Cl@Si20H20]−: Parent Siladodecahedrane with Endohedral Chloride Ion. J. Am. Chem. Soc. 2021, 143, 10865–10871. [Google Scholar] [CrossRef] [PubMed]
- Tillmann, J.; Wender, J.H.; Bahr, U.; Bolte, M.; Lerner, H.-W.; Holthausen, M.C.; Wagner, M. One-Step Synthesis of a [20]-Silafullerane with an Endohedral Chloride Ion. Angew. Chem. Int. Ed. 2015, 54, 5429–5433. [Google Scholar] [CrossRef]
- Earley, C.W. Ab Initio Investigation of Strain in Group 14 Polyhedrane Clusters (MnHn: N = 4, 6, 8, 10,12, 16, 20, 24). J. Phys. Chem. A 2000, 104, 6622–6627. [Google Scholar] [CrossRef]
- Beckhaus, H.-D.; Ruchardt, C.; Lagerwall, D.R.; Paquette, L.A.; Wahl, F.; Prinzbach, H. Experimental Enthalpies of Formation and Strain Energies for the Caged C20H20 Pagodane and Dodecahedrane Frameworks. J. Am. Chem. Soc. 1994, 116, 11775–11778. [Google Scholar] [CrossRef]
- Beckhaus, H.-D.; Ruchardt, C.; Lagerwall, D.R.; Paquette, L.A.; Wahl, F.; Prinzbach, H. Experimental Enthalpies of Formation and Strain Energies for the Caged C20H20 Pagodane and Dodecahedrane Frameworks. [Erratum to document cited in CA122:80644]. J. Am. Chem. Soc. 1995, 117, 8885. [Google Scholar] [CrossRef]
- Wiberg, K.B. The Concept of Strain in Organic Chemistry. Angew. Chem. Int. Ed. Engl. 1986, 25, 312–322. [Google Scholar] [CrossRef]
- Nagase, S.; Nakano, M.; Kudo, T. Strain and structures in the silicon analogues of tetrahedrane, prismane, and cubane. A theoretical study. J. Chem. Soc. Chem. Commun. 1987, 60–62. [Google Scholar] [CrossRef]
- Nagase, S. Much Less Strained Cubane Analogues with Si, Ge, Sn, and Pb Skeletons. Angew. Chem. Int. Ed. Engl. 1989, 28, 329–330. [Google Scholar] [CrossRef]
- Ray, C.; Pellarin, M.; Lermé, J.L.; Vialle, J.L.; Broyer, M.; Blase, X.; Mélinon, P.; Kéghélian, P.; Perez, A. Synthesis and Structure of Silicon-doped Heterofullerenes. Phys. Rev. Lett. 1998, 80, 5365–5368. [Google Scholar] [CrossRef]
- Billas, I.M.L.; Tast, F.; Branz, W.; Malinowski, N.; Heinebrodt, M.; Martin, T.P.; Boeroa, M.; Massobriob, C.; Parrinello, M. Experimental and computational studies of Si-doped fullerenes. Eur. Phys. J. D 1999, 9, 337–340. [Google Scholar] [CrossRef]
- Pellarin, M.; Ray, C.; Lermé, J.; Vialle, J.L.; Broyer, M.J. Photolysis experiments on SiC mixed clusters: From silicon carbide clusters to silicon doped fullerenes. J. Chem. Phys. 1999, 110, 6927–6938. [Google Scholar]
- Kudo, T.; Schmidt, M.W.; Matsunaga, N. Ab Initio Molecular Orbital Study of the First Four Si/C Alternately Substituted Annulenes. J. Phys. Chem. A 2019, 123, 4588–4598. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Mesuda, A.; Kudo, T. Theoretical Study of the Si/C Mixed Benzenes and Their Major Valence Isomers. Organometallics 2020, 39, 3041–3049. [Google Scholar] [CrossRef]
- Nakamura, T.; Kudo, T. The Planarity of the Heteroatom Analogues of Benzene: Energy Component Analysis and the Planarization of Hexasilabenzene. J. Comput. Chem. 2019, 40, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Fotooh, F.K.; Atashparvar, M. Theoretical Study of the Effect of Simultaneous Doping with Silicon, on Structure and Electronic Properties of Adamantane. Russ. J. Phys. Chem. B 2019, 13, 1–8. [Google Scholar] [CrossRef]
- Miranda, W.D.S.A.; Coutinho, S.S.; Tavares, M.S.; Moreira, E.; Azevedo, D.L. Ab initio vibrational and thermodynamic properties of adamantane, sila-adamantane (Si10H16), and C9Si1H16 isomers. J. Mol. Struct. 2016, 1122, 299–308. [Google Scholar] [CrossRef]
- Rayne, S.; Forest, K. Structures, enthalpies of formation, and ionization energies for the parent and binary mixed carbon, silicon, nitrogen, and phosphorus cubane derivatives: A G4MP2 theoretical study. Nat Prec. 2010. [Google Scholar] [CrossRef]
- Rayne, S.; Forest, K. Binary mixed carbon, silicon, nitrogen, and phosphorus cubane derivatives as potential high energy materials. Comput. Theor. Chem. 2016, 1080, 10–15. [Google Scholar] [CrossRef]
- Nakamura, T.; Uchiyama, T.; Kudo, T. Comparison of group 14 elements in sp3 and sp2 environmentby fragment structure energy analysis. J. Comput. Chem. 2021, 42, 1817–1825. [Google Scholar] [CrossRef]
- Wahl, F.; Wörth, J.; Prinzbach, H. The Pagodane Route to Dodecahedranes: An Improved Approach to the C20H20 Parent Framework; Partial and Total Functionalizations-Does C20-Fullerene Exist? Angew Chem. Int. Ed. Engl. 1993, 32, 1722–1726. [Google Scholar] [CrossRef]
- Fujita, S. Unit Subduced Cycle Indices with and without Chirality Fittingness for Ih Group. An Application to Systematic Enumeration of Dodecahedrane Derivatives. Bull. Chem. Soc. Jpn. 1990, 63, 2759–2769. [Google Scholar] [CrossRef]
- Nemba, R.M. The Denumerants of Icosahedral Group for Symmetry Itemized Enumeration of Coisomeric Dodecahedrane Derivatives and Heteroanalogues. II. MATCH Commun. Math. Comput. Chem. 2021, 86, 85–120. [Google Scholar]
- Roothaan, C.C.J. New Developments in Molecular Orbital Theory. Rev. Mod. Phys. 1951, 23, 69–89. [Google Scholar] [CrossRef]
- Møller, C.; Plesset, M.S. Note on an Approximation Treatment for Many-Electron Systems. Phys. Rev. 1934, 46, 618–622. [Google Scholar] [CrossRef]
- Head-Gordon, M.; Pople, J.A.; Frisch, M.J. MP2 energy evaluation by direct methods. Chem. Phys. Lett. 1988, 153, 503–506. [Google Scholar] [CrossRef]
- Hariharan, P.C.; Pople, J.A. The influence of polarization functions on molecular orbital hydrogenation energies. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- George, P.; Trachtman, M.; Bock, C.W.; Brett, A.M. An alternative approach to the problem of assessing destabilization energies (strain energies) in cyclic hydrocarbons. Tetrahedron 1976, 32, 317–323. [Google Scholar] [CrossRef]
- Wheeler, S.E.; Houk, K.N.; Schleyer, P.V.R.; Allen, W.D. A Hierarchy of Homodesmotic Reactions for Thermochemistry. J. Am. Chem. Soc. 2009, 131, 2547–2560. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).