Different Extraction Approaches for the Analysis of Melatonin from Cabernet Sauvignon and Feteasca Neagra Wines Using a Validated HPLC-FL Method
Abstract
1. Introduction
2. Results and Discussion
2.1. Validation and Uncertainty Estimation of HPLC-FL Method
2.2. Different Extraction Methods of Melatonin from Wine
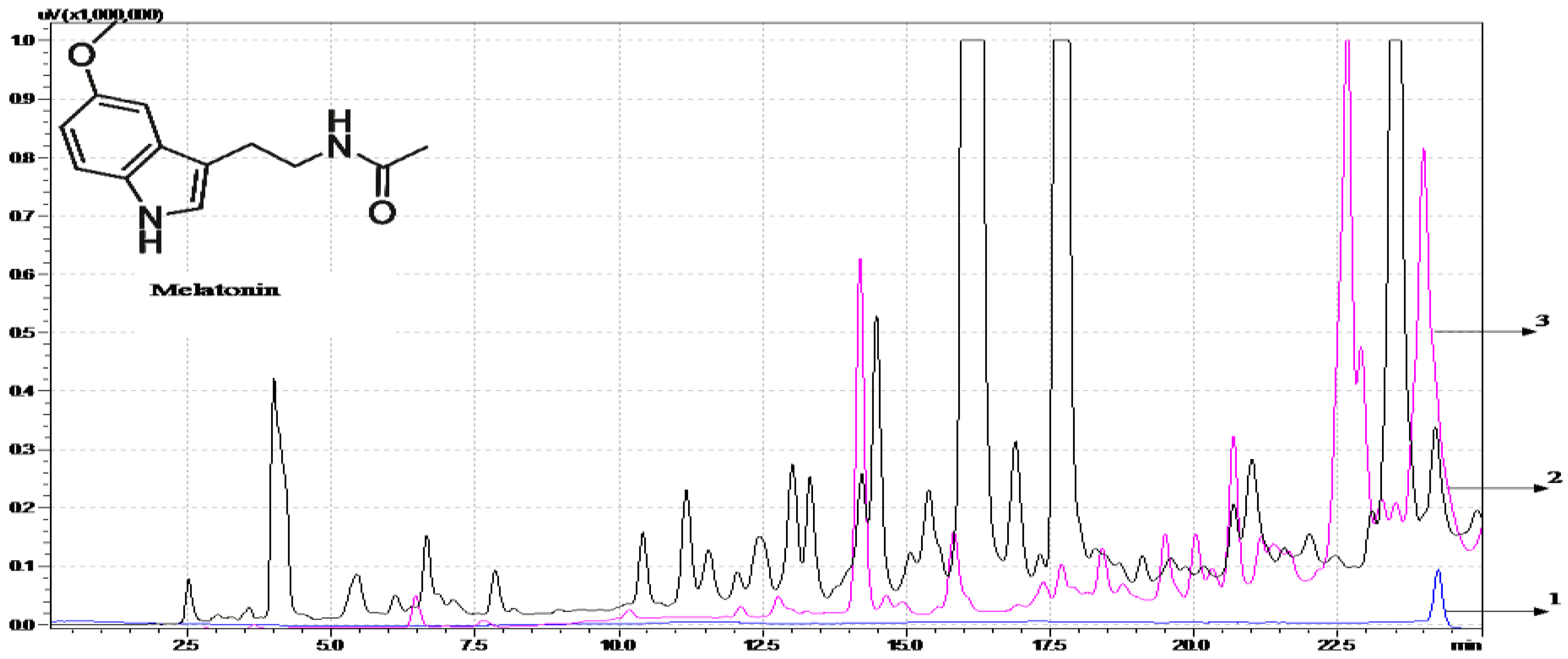
2.3. DLLME-HPLC-FL Method Application in Wine Samples
3. Materials and Methods
3.1. Reagents
3.2. Wine Sampling
3.3. SPE Method
3.4. QuEChERS Method
3.5. DLLME Method
3.6. HPLC Analysis
3.7. Measurement Uncertainty
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin as a natural ally against oxidative stress: A physicochemical examination. J. Pineal. Res. 2011, 51, 1–16. [Google Scholar] [CrossRef]
- Hacışevki, A.; Baba, B. An Overview of Melatonin as an Antioxidant Molecule: A Biochemical Approach. In Melatonin-Molecular Biology, Clinical and Pharmaceutical Approaches, 1st ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Mercolini, L.; Addolorata Saracino, M.; Bugamelli, F.; Ferranti, A.; Malaguti, M.; Hrelia, S.; Raggi, M.A. HPLC-Fl analysis of melatonin and resveratrol isomers in wine using an SPE procedure. J. Separ. Sci. 2008, 31, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yue, Q.; Bian, F.; Zhai, H.; Yao, Y. Melatonin Treatment Enhances the Polyphenol Content and Antioxidant Capacity of Red Wine. Hortic. Plant J. 2018, 4, 44–50. [Google Scholar] [CrossRef]
- Fernandez-Mar, M.I.; Mateos, R.; García-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Bioactive compounds in wine: Resveratrol, hydroxytyrosol and melatonin: A review. Food Chem. 2021, 130, 797–813. [Google Scholar] [CrossRef]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine Polyphenol Content and Its Influence on Wine Quality and Properties: A Review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef]
- El-Sayed, G.O.; Amin, A.S. Voltammetric Determination of Melatonin in Tablet Dosage Forms and Human Serum. Lat. Am. J. Pharm. 2010, 29, 1235–1240. [Google Scholar]
- Sae-Teaw, M.; Johns, J.; Johns, N.P.; Subongkot, S. Serum melatonin levels and antioxidant capacities after consumption of pineapple, orange, or banana by healthy male volunteers. J. Pineal. Res. 2013, 55, 58–64. [Google Scholar] [CrossRef]
- Stage, P.W.; Sombra, L.L.; Messina, G.; Martinez, L.D.; Silya, M.F. Determination of melatonin in wine and plant extracts by capillary electrochromatography with immobilized carboxylic multi-walled carbon nanotubes as stationary phase. Electrophoresis 2010, 31, 2242–2248. [Google Scholar] [CrossRef]
- Chau, R.M.W.; Patel, B.A. Determination of serotonin, melatonin and metabolites in gastrointestinal tissue using high-performance liquid chromatography with electrochemical detection. Biomed. Chromatogr. 2009, 23, 175–181. [Google Scholar] [CrossRef]
- Boccalandro, H.E.; González, C.V.; Wunderlin, D.A.; Silva, M.F. Melatonin levels, determined by LC-ESI-MS/MS, fluctuate during the day/night cycle in Vitis vinifera cv Malbec: Evidence of its antioxidant role in fruits. J. Pineal. Res. 2011, 51, 226–232. [Google Scholar] [CrossRef]
- Carneiro, A.F.; Carneiro, C.N.; Gomez, F.J.V.; Spisso, A.; Silva, M.F.; Minho, L.A.C.; dos Santos, W.N.L.; Dias, F.D. Doehlert matrix for the optimization of ultrasound dispersive liquid–liquid microextraction of melatonin in Argentine and Brazilian wine samples. Microchem. J. 2020, 159, 105313. [Google Scholar] [CrossRef]
- Mercolini, L.; Mandrioli, R.; Raggi, M.A. Content of melatonin and other antioxidants in grape-related foodstuffs: Measurement using a MEPS-HPLC-FL method. J. Pineal Res. 2012, 53, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Viegas, O.; Esteves, C.; Rocha, J.; Melo, A.; Ferreira, I.M.P.L.V.O. Simultaneous determination of melatonin and trans-resveratrol in wine by dispersive liquid–liquid microextraction followed by HPLC-FLD. Food Chem. 2021, 339, 128091. [Google Scholar] [CrossRef]
- Carneiro, C.N.; Gomez, F.J.V.; Spisso, A.; Silva, M.F.; Azcarate, S.M.; Dias, F.D. Geographical characterization of South America wines based on their phenolic and melatonin composition: An exploratory analysis. Microchem. J. 2020, 158, 105240. [Google Scholar] [CrossRef]
- Albu, C.; Radu, L.E.; Radu, G.L. Assessment of Melatonin and Its Precursors Content by a HPLC-MS/MS Method from Different Romanian Wines. ACS Omega 2020, 5, 27254–27260. [Google Scholar] [CrossRef]
- Rodriguez-Naranjo, M.I.; Gil-Izquierdo, A.; Troncoso, A.M.; Cantos, E.; Garcia-Parrilla, M.C. Melatonin: A new bioactive compound in wine. J. Food. Compos. Anal. 2011, 24, 603–608. [Google Scholar] [CrossRef]
- Fracassetti, D.; Vigentini, I.; Lo Faro, A.F.F.; De Nisi, P.; Foschino, R.; Tirelli, A.; Orioli, M.; Iriti, M. Assessment of Tryptophan, Tryptophan Ethylester, and Melatonin Derivatives in Red Wine by SPE-HPLC-FL and SPE-HPLC-MS Methods. Foods 2019, 8, 99. [Google Scholar] [CrossRef]
- Marhuenda, J.; Villano, D.; Arcusa, R.; Zafrilla, P. Melatonin in Wine and Beer: Beneficial Effects. Molecules 2021, 26, 343. [Google Scholar] [CrossRef] [PubMed]
- Dugheri, S.; Marrubini, G.; Mucci, N.; Cappelli, G.; Bonari, A.; Pompilio, I.; Trevisani, L.; Arcangeli, G. A review of micro-solid-phase extraction techniques and devices applied in sample pretreatment coupled with chromatographic analysis. Acta Chromatogr. 2021, 33, 99–111. [Google Scholar] [CrossRef]
- Wang, X.; You, J.; Liu, A.L.; Qi, X.; Li, D.H.; Zhao, Y.; Zhang, Y.X.; Zhang, L.X.; Zhang, X.R.; Li, P.W. Variation in Melatonin Contents and Genetic Dissection of Melatonin Biosynthesis in Sesame. Plants 2022, 11, 2005. [Google Scholar] [CrossRef] [PubMed]
- Su, M.C.; Cheng, Y.Y.; Zhang, C.C.; Zhu, D.; Jia, M.; Zhang, Q.Y.; Wu, H.X.; Chen, G. Determination of the levels of tryptophan and 12 metabolites in milk by liquid chromatography-tandem mass spectrometry with the QuEChERS method. J. Dairy Sci. 2020, 103, 9851–9859. [Google Scholar] [CrossRef]
- Albu, C.; Radu, G.L. Development and Application of a HPLC-PDA-FL Method for the Determination of Melatonin and its Precursors in Infant Formulas. Food Anal. Methods 2018, 11, 951–958. [Google Scholar] [CrossRef]
- AOAC. Appendix F: Guidelines for Standard Method Performance Requirements (SMPR). In AOAC Official Methods of Analysis; AOAC: Rockville, MD, USA, 2012. [Google Scholar]
- Eurachem. The Fitness for Purpose of Analytical Methods. In A Laboratory Guide to Method Validation and Related Topics; Eurachem: London, UK, 2014. [Google Scholar]
- Eurachem. Eurachem/CITAC Guide: Quantifying Uncertainty in Analytical Measurement, 3rd ed.; Eurachem: London, UK, 2012; ISBN 9780948926303. [Google Scholar]
- Sun, R.; Wang, Y.; He, H.; Wan, Y.; Li, L.; Sha, J.; Jiang, G.; Li, T.; Ren, B. Solubility measurement, solubility behavior analysis and thermodynamic modelling of melatonin in twelve pure solvents from 278.15 K to 323.15 K. J. Mol. Liq. 2020, 319, 114139. [Google Scholar] [CrossRef]
- Ye, T.; Yin, X.; Yu, L.; Zheng, S.J.; Cai, W.J.; Wu, Y.; Feng, Y.Q. Metabolic analysis of the melatonin biosynthesis pathway using chemical labeling coupled with liquid chromatography-mass spectrometry. J. Pineal Res. 2019, 66, e12531. [Google Scholar] [CrossRef] [PubMed]
| Compound | λex nm | λem nm | tR min. | The Linear Regression Equation ng mL−1 | R | Linearity of Response ng mL−1 | LoD ng mL−1 | LoQ ng mL−1 |
|---|---|---|---|---|---|---|---|---|
| Melatonin | 285 | 340 | 23.58 | A = 219403.4 × C − 248.5414 | 0.9996 | 1–25 | 0.01 | 0.05 |
| Analyte | Date | Conc. ng mL−1 | Peak Areas | CV | tR | CV |
|---|---|---|---|---|---|---|
| Melatonin | 22.06 | 2.5 | 1,183,246 | 0.23% | 23.61 | 0.09% |
| 1,184,829 | 23.56 | |||||
| 1,179,878 | 23.58 | |||||
| 1,183,979 | 23.58 | |||||
| 1,178,708 | 23.60 | |||||
| 5 | 1,420,635 | 0.97% | 23.59 | 0.11% | ||
| 1,431,014 | 23.59 | |||||
| 1,438,296 | 23.60 | |||||
| 1,456,557 | 23.62 | |||||
| 1,446,875 | 23.65 |
| Analyte | Conc. ng mL−1 | Date | Conc.ng mL−1 | Conc. ng mL−1 | CV |
|---|---|---|---|---|---|
| Melatonin | 10 | 24.06 | 10.14 | 9.98 ± 0.09 | 0.97% |
| 9.99 | |||||
| 10.04 | |||||
| 10.05 | |||||
| 9.89 | |||||
| 29.07 | 9.95 | ||||
| 10.09 | |||||
| 10.12 | |||||
| 9.92 | |||||
| 9.85 | |||||
| 06.10 | 9.97 | ||||
| 10.06 | |||||
| 9.89 | |||||
| 9.83 | |||||
| 10.02 |
| Extraction Method | Wine Concentration ng mL−1 | Conc. Melatonin Added ng mL−1 | Conc. Wine + Melatonin Obtained ng mL−1 | Extraction Yield (%) |
|---|---|---|---|---|
| SPE | 1.25 | 1 | 1.10 ± 0.02 | 49 |
| 10 | 6.52 ± 0.04 | 58 | ||
| 25 | 16.27 ± 0.06 | 62 | ||
| QuEChERS | 1.25 | 1 | 1.55 ± 0.01 | 69 |
| 10 | 9.00 ± 0.08 | 80 | ||
| 25 | 20.47 ± 0.1 | 78 | ||
| DLLME | 1.25 | 1 | 1.96 ± 0.03 | 87 |
| 10 | 10.24 ± 0.08 | 91 | ||
| 25 | 24.41 ± 0.12 | 93 |
| Wine Varieties | Melatonin ng mL−1 | |
|---|---|---|
| Punchdown Method | Pumping Over Method | |
| Feteasca Neagra | 0.74 ± 0.06 | 1.09 ± 0.09 |
| Cabernet Sauvignon | 0.84 ± 0.06 | 1.36 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eremia, S.A.V.; Albu, C.; Radu, G.L.; Ion, M. Different Extraction Approaches for the Analysis of Melatonin from Cabernet Sauvignon and Feteasca Neagra Wines Using a Validated HPLC-FL Method. Molecules 2023, 28, 2768. https://doi.org/10.3390/molecules28062768
Eremia SAV, Albu C, Radu GL, Ion M. Different Extraction Approaches for the Analysis of Melatonin from Cabernet Sauvignon and Feteasca Neagra Wines Using a Validated HPLC-FL Method. Molecules. 2023; 28(6):2768. https://doi.org/10.3390/molecules28062768
Chicago/Turabian StyleEremia, Sandra A. V., Camelia Albu, Gabriel L. Radu, and Marian Ion. 2023. "Different Extraction Approaches for the Analysis of Melatonin from Cabernet Sauvignon and Feteasca Neagra Wines Using a Validated HPLC-FL Method" Molecules 28, no. 6: 2768. https://doi.org/10.3390/molecules28062768
APA StyleEremia, S. A. V., Albu, C., Radu, G. L., & Ion, M. (2023). Different Extraction Approaches for the Analysis of Melatonin from Cabernet Sauvignon and Feteasca Neagra Wines Using a Validated HPLC-FL Method. Molecules, 28(6), 2768. https://doi.org/10.3390/molecules28062768