Enhanced Ammonia Decomposition by Tuning the Support Properties of Ni/GdxCe1-xO2-δ at 600 °C
Abstract
1. Introduction
2. Results
2.1. Characterizations
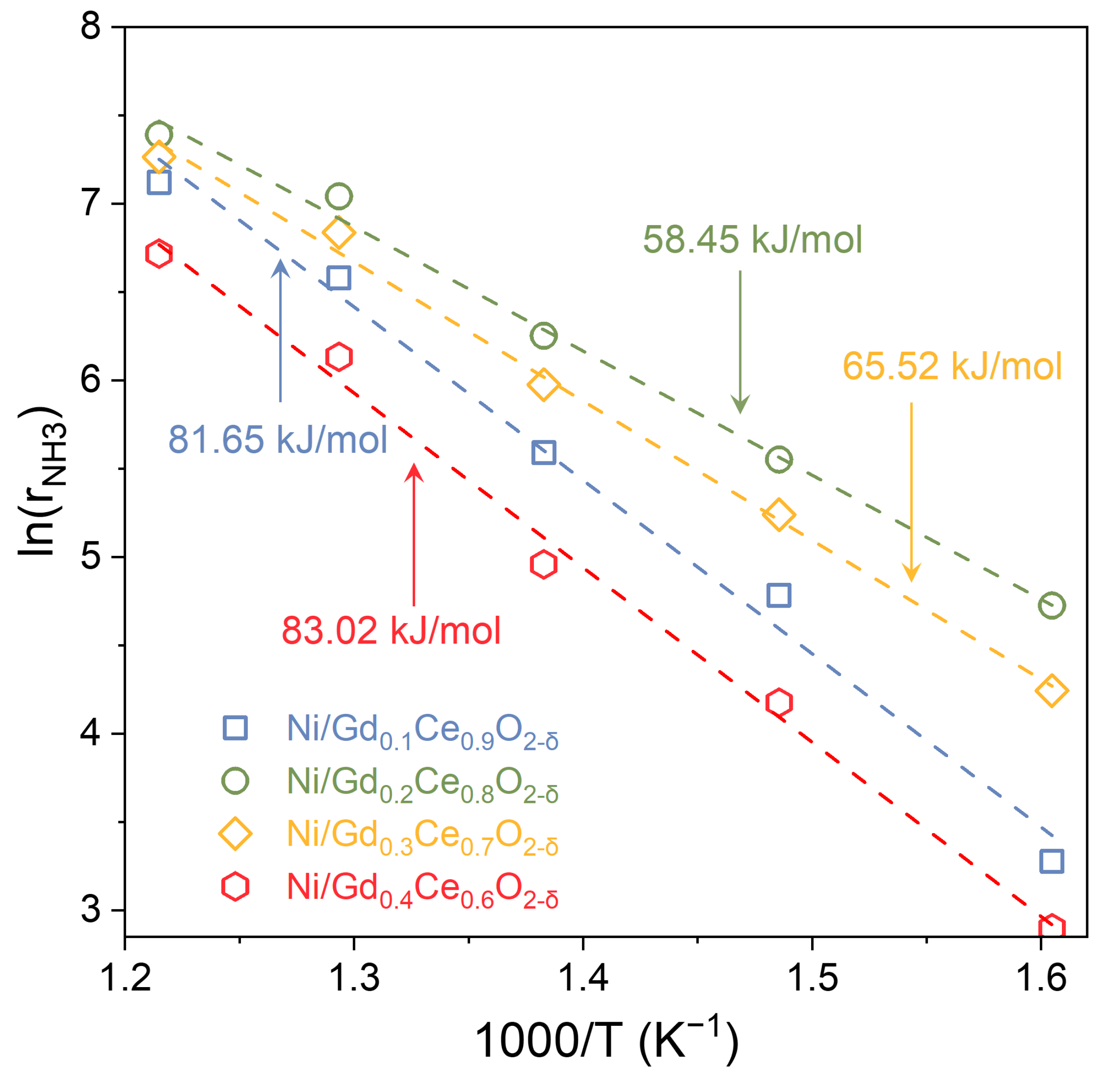
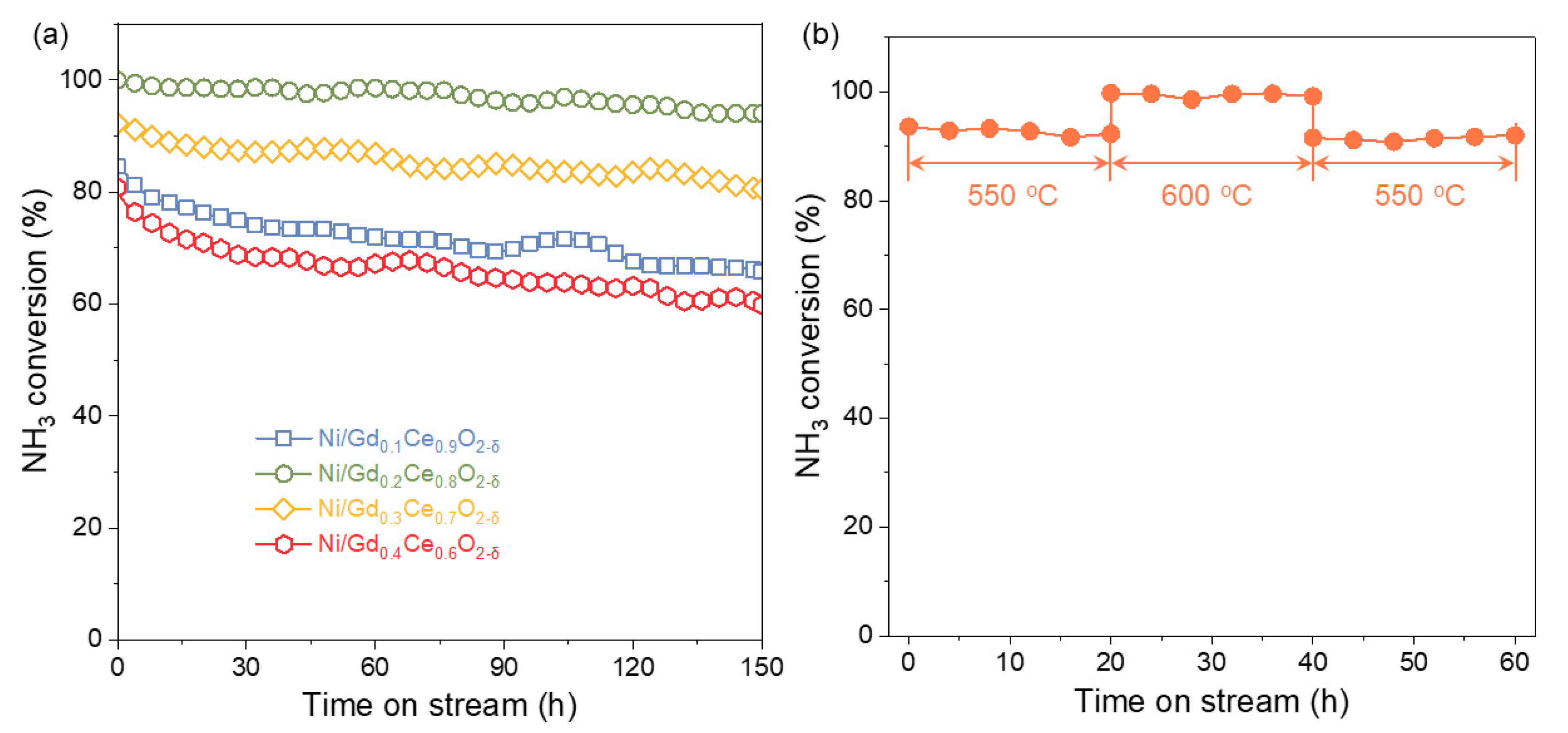
2.2. Ammonia Decomposition Performance
2.3. Mechanical Investigation
2.3.1. DFT Calculation
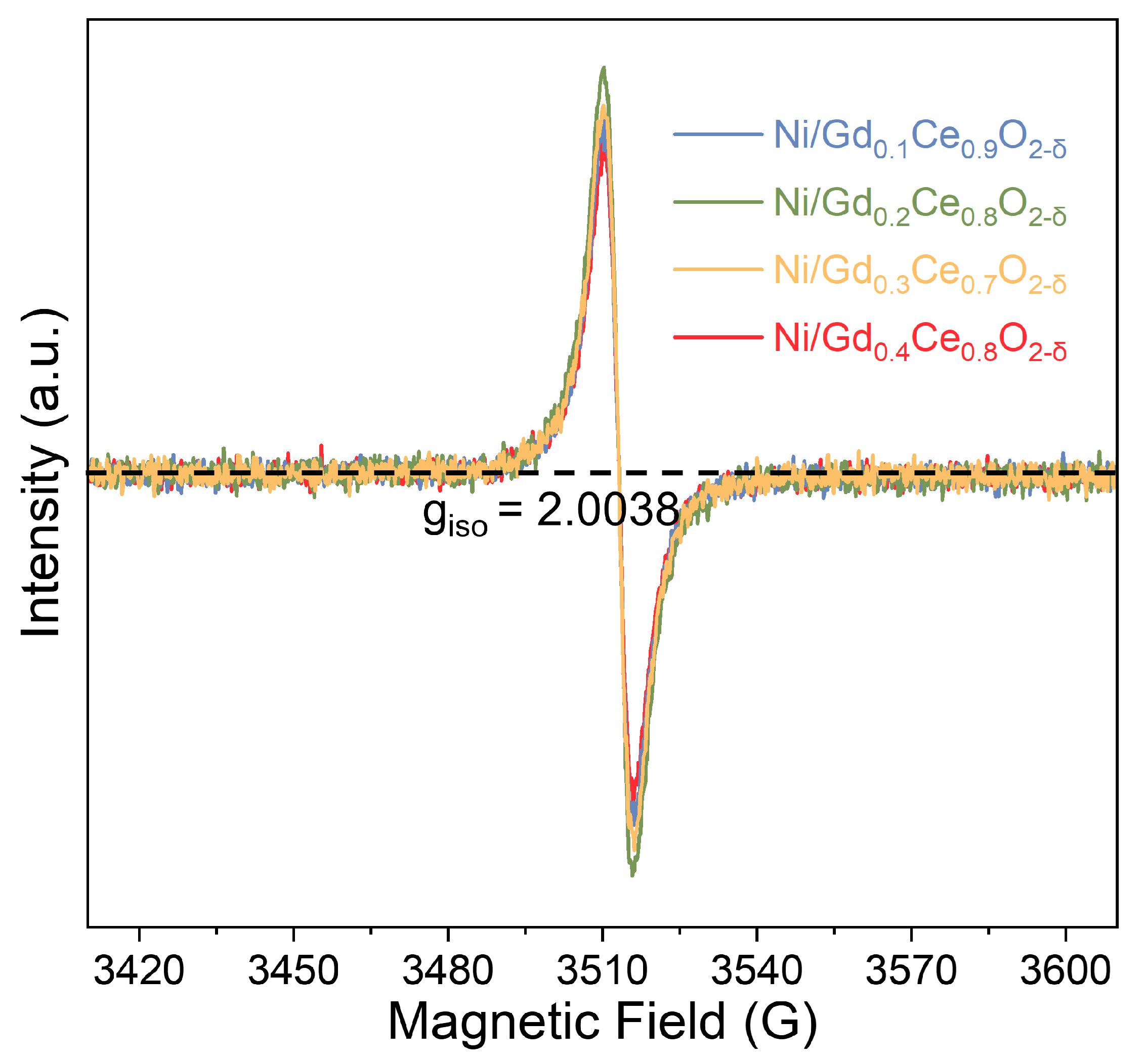
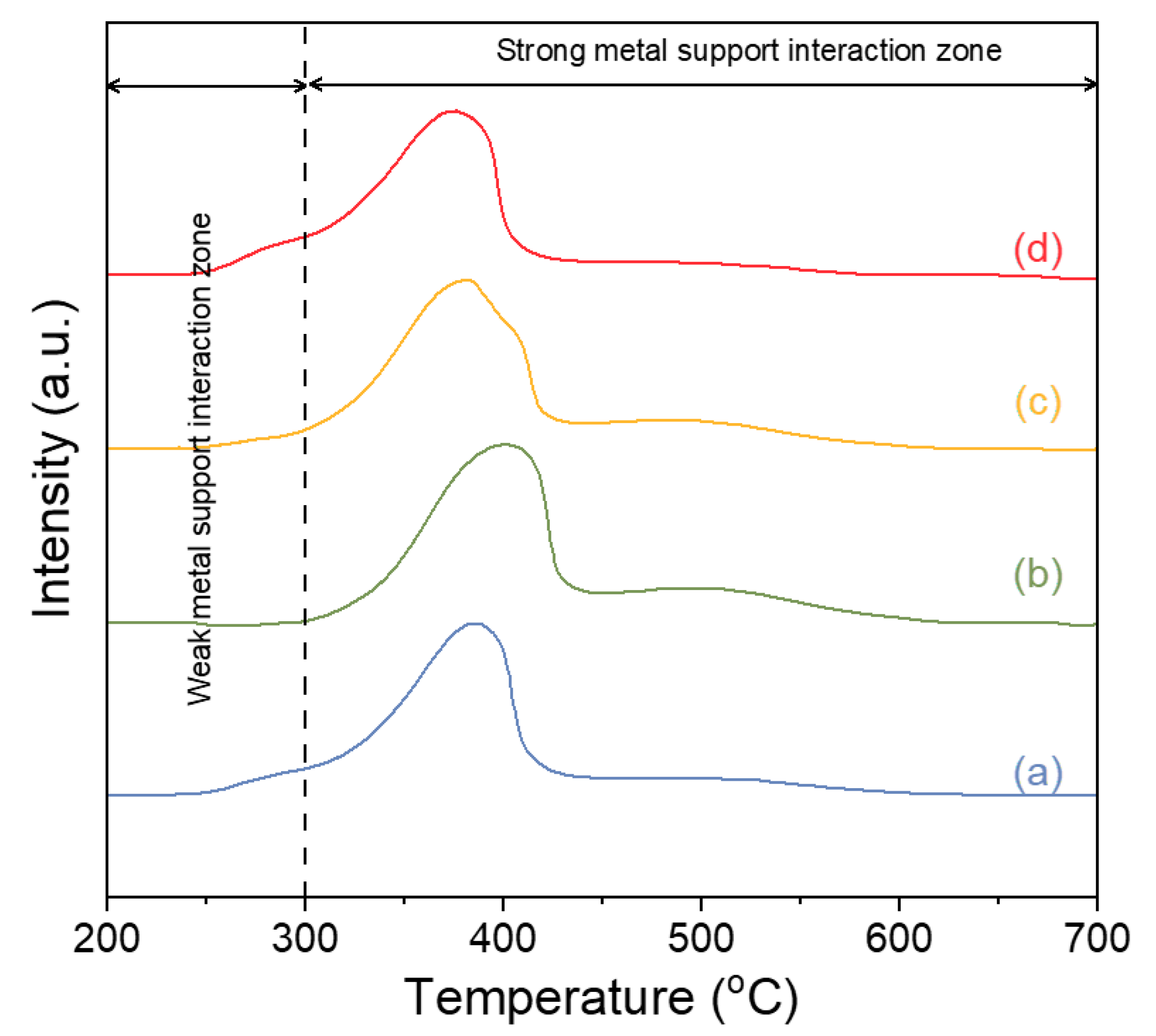
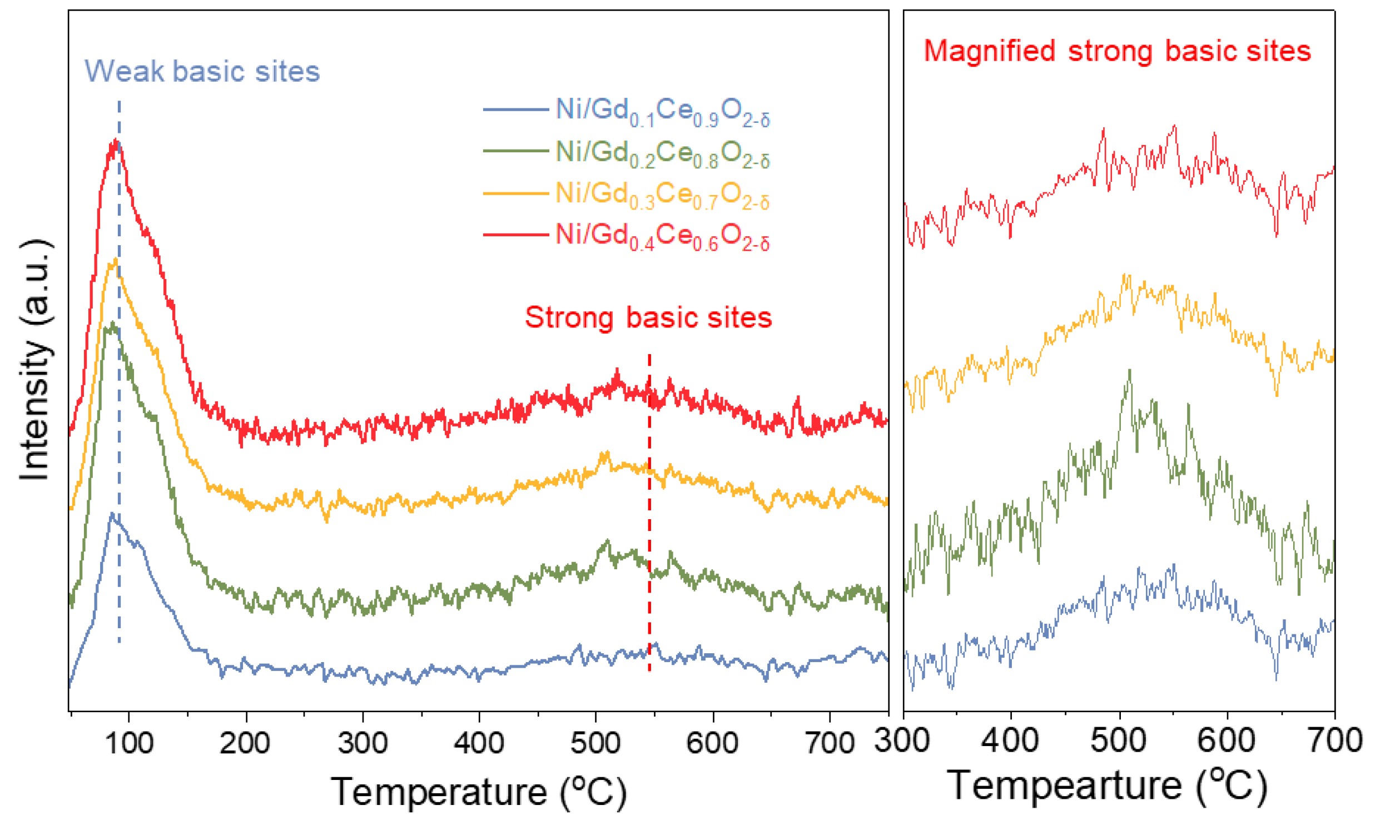
2.3.2. Surface Properties of the Catalysts
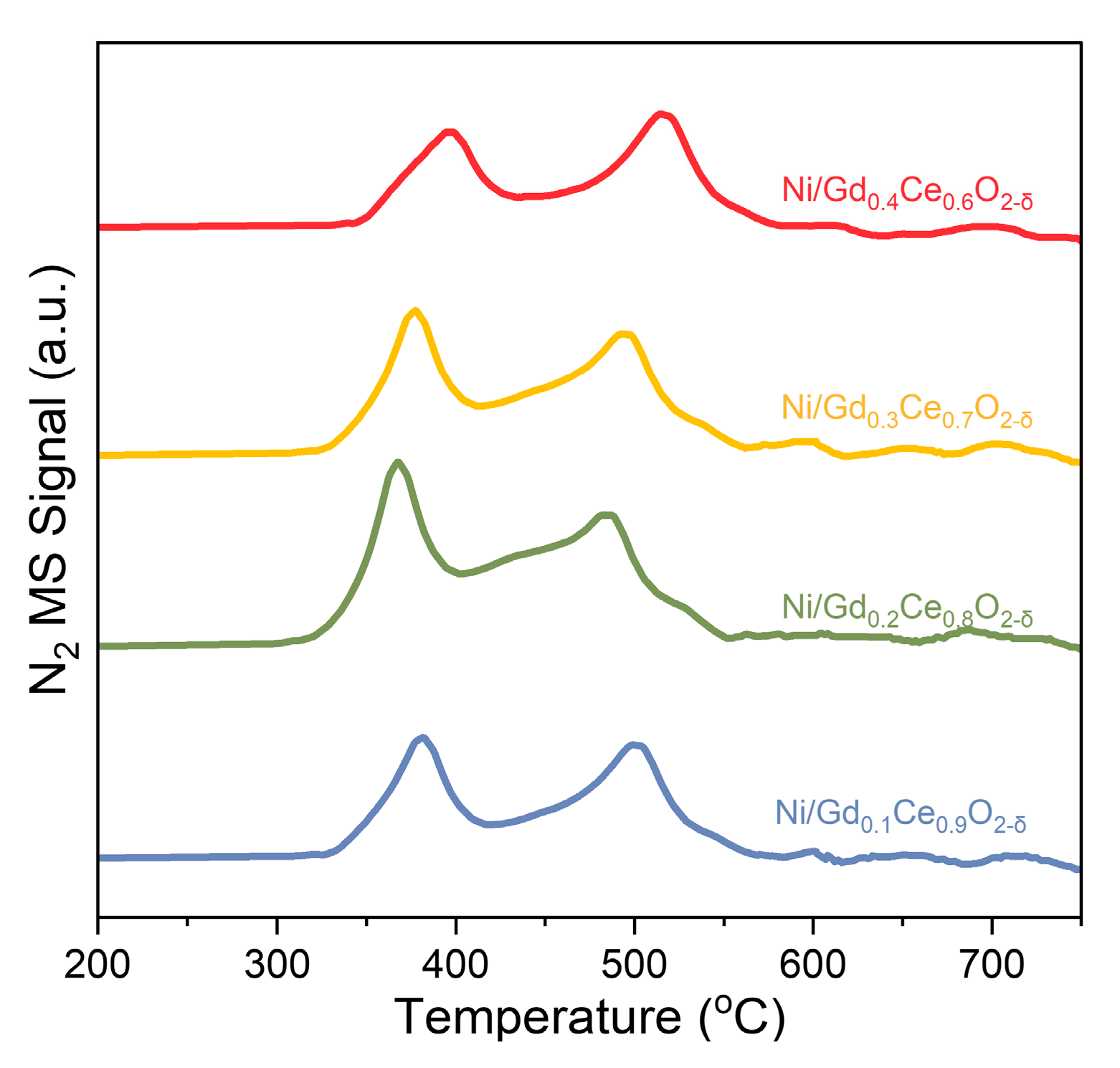
2.3.3. NH3 TPSR
3. Materials and Methods
3.1. Preparation of the Catalysts
3.2. Characterizations
3.3. Computational Details
3.4. Experiment Setup
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nishiyama, H.; Yamada, T.; Nakabayashi, M.; Maehara, Y.; Yamaguchi, M.; Kuromiya, Y.; Nagatsuma, Y.; Tokudome, H.; Akiyama, S.; Watanabe, T.; et al. Photocatalytic solar hydrogen production from water on a 100-m2 scale. Nature 2021, 598, 304–307. [Google Scholar] [CrossRef]
- Scheffe, J.R.; Allendorf, M.D.; Coker, E.N.; Jacobs, B.W.; McDaniel, A.H.; Weimer, A.W. Hydrogen production via chemical looping redox cycles using atomic layer deposition-synthesized iron oxide and cobalt ferrites. Chem. Mater. 2011, 23, 2030–2038. [Google Scholar] [CrossRef]
- Fang, X.; Wang, X.; Ouyang, L.; Zhang, L.; Sun, S.; Liang, Y.; Luo, Y.; Zheng, D.; Kang, T.; Liu, Q.; et al. Amorphous Co-Mo-B Film: A High-Active Electrocatalyst for Hydrogen Generation in Alkaline Seawater. Molecules 2022, 27, 7617. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Y.; Yuan, L.; Zhang, M.; Zhang, C. Scandium Decoration of Boron Doped Porous Graphene for High-Capacity Hydrogen Storage. Molecules 2019, 24, 2382. [Google Scholar] [CrossRef]
- Shao, Z.; Li, Y.; Liu, C.; Ai, W.; Luo, S.-P.; Liu, Q. Reversible interconversion between methanol-diamine and diamide for hydrogen storage based on manganese catalyzed (de)hydrogenation. Nat. Commun. 2020, 11, 591. [Google Scholar] [CrossRef] [PubMed]
- Klerke, A.; Christensen, C.H.; Nørskov, J.K.; Vegge, T. Ammonia for hydrogen storage: Challenges and opportunities. J. Mater. Chem. 2008, 18, 2304–2310. [Google Scholar] [CrossRef]
- Puszkiel, J.; Gasnier, A.; Amica, G.; Gennari, F. Tuning LiBH4 for Hydrogen Storage: Destabilization, Additive, and Nanoconfinement Approaches. Molecules 2020, 25, 163. [Google Scholar] [CrossRef]
- Li, D.-H.; Li, Q.-M.; Qi, S.-L.; Qin, H.-C.; Liang, X.-Q.; Li, L. Theoretical Study of Hydrogen Production from Ammonia Borane Catalyzed by Metal and Non-Metal Diatom-Doped Cobalt Phosphide. Molecules 2022, 27, 8206. [Google Scholar] [CrossRef]
- Akbayrak, S.; Özkar, S. Ammonia borane as hydrogen storage materials. Int. J. Hydrogen Energy 2018, 43, 18592–18606. [Google Scholar] [CrossRef]
- Lamb, K.E.; Dolan, M.D.; Kennedy, D.F. Ammonia for hydrogen storage; A review of catalytic ammonia decomposition and hydrogen separation and purification. Int. J. Hydrogen Energy 2019, 44, 3580–3593. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, E.D. Ammonia Decomposition over Ru/SiO2 Catalysts. Catalysts 2022, 12, 1203. [Google Scholar] [CrossRef]
- Hu, Z.-P.; Weng, C.-C.; Yuan, G.-G.; Lv, X.-W.; Yuan, Z.-Y. Ni nanoparticles supported on mica for efficient decomposition of ammonia to COx-free hydrogen. Int. J. Hydrogen Energy 2018, 43, 9663–9676. [Google Scholar] [CrossRef]
- Lu, B.; Li, L.; Ren, M.; Liu, Y.; Zhang, Y.; Xu, X.; Wang, X.; Qiu, H. Ammonia decomposition over iron-based catalyst: Exploring the hidden active phase. Appl. Catal. B 2022, 314, 121475. [Google Scholar] [CrossRef]
- Pinzón, M.; Sánchez-Sánchez, A.; Romero, A.; de la Osa, A.R.; Sánchez, P. Self-combustion Ni and Co-based perovskites as catalyst precursors for ammonia decomposition. Effect of Ce and Mg doping. Fuel 2022, 323, 124384. [Google Scholar] [CrossRef]
- Okura, K.; Miyazaki, K.; Muroyama, H.; Matsui, T.; Eguchi, K. Ammonia decomposition over Ni catalysts supported on perovskite-type oxides for the on-site generation of hydrogen. RSC Adv. 2018, 8, 32102–32110. [Google Scholar] [CrossRef]
- Duan, X.; Qian, G.; Fan, C.; Zhu, Y.; Zhou, X.; Chen, D.; Yuan, W. First-principles calculations of ammonia decomposition on Ni (110) surface. Surf. Sci. 2012, 606, 549–553. [Google Scholar] [CrossRef]
- Lucentini, I.; García Colli, G.; Luzi, C.; Serrano, I.; Soler, L.; Divins, N.J.; Martínez, O.M.; Llorca, J. Modelling and simulation of catalytic ammonia decomposition over Ni-Ru deposited on 3D-printed CeO2. Chem. Eng. J. 2022, 427, 131756. [Google Scholar] [CrossRef]
- Chellappa, A.S.; Fischer, C.M.; Thomson, W.J. Ammonia decomposition kinetics over Ni-Pt/Al2O3 for PEM fuel cell applications. Appl. Catal. A 2002, 227, 231–240. [Google Scholar] [CrossRef]
- Fu, E.; Qiu, Y.; Lu, H.; Wang, S.; Liu, L.; Feng, H.; Yang, Y.; Wu, Z.; Xie, Y.; Gong, F.; et al. Enhanced NH3 decomposition for H2 production over bimetallic M(M=Co, Fe, Cu)Ni/Al2O3. Fuel Process. Technol. 2021, 221, 106945. [Google Scholar] [CrossRef]
- Huang, C.; Yu, Y.; Tang, X.; Liu, Z.; Zhang, J.; Ye, C.; Ye, Y.; Zhang, R. Hydrogen generation by ammonia decomposition over Co/CeO2 catalyst: Influence of support morphologies. Appl. Surf. Sci. 2020, 532, 147335. [Google Scholar] [CrossRef]
- Deng, Q.-F.; Zhang, H.; Hou, X.-X.; Ren, T.-Z.; Yuan, Z.-Y. High-surface-area Ce0.8Zr0.2O2 solid solutions supported Ni catalysts for ammonia decomposition to hydrogen. Int. J. Hydrogen Energy 2012, 37, 15901–15907. [Google Scholar] [CrossRef]
- Torrente-Murciano, L.; Hill, A.K.; Bell, T.E. Ammonia decomposition over cobalt/carbon catalysts—Effect of carbon support and electron donating promoter on activity. Catal. Today 2017, 286, 131–140. [Google Scholar] [CrossRef]
- Li, X.-K.; Ji, W.-J.; Zhao, J.; Wang, S.-J.; Au, C.-T. Ammonia decomposition over Ru and Ni catalysts supported on fumed SiO2, MCM-41, and SBA-15. J. Catal. 2005, 236, 181–189. [Google Scholar] [CrossRef]
- Qiu, Y.; Fu, E.; Gong, F.; Xiao, R. Catalyst support effect on ammonia decomposition over Ni/MgAl2O4 towards hydrogen production. Int. J. Hydrogen Energy 2022, 47, 5044–5052. [Google Scholar] [CrossRef]
- Gu, Y.; Ma, Y.; Long, Z.; Zhao, S.; Wang, Y.; Zhang, W. One-pot synthesis of supported Ni@Al2O3 catalysts with uniform small-sized Ni for hydrogen generation via ammonia decomposition. Int. J. Hydrogen Energy 2021, 46, 4045–4054. [Google Scholar] [CrossRef]
- Zeng, D.; Kang, F.; Qiu, Y.; Cui, D.; Li, M.; Ma, L.; Zhang, S.; Xiao, R. Iron oxides with gadolinium-doped cerium oxides as active supports for chemical looping hydrogen production. Chem. Eng. J. 2020, 396, 125153. [Google Scholar] [CrossRef]
- Tang, H.; Wang, Y.; Zhang, W.; Liu, Z.; Li, L.; Han, W.; Li, Y. Catalytic activity of Ru supported on SmCeOx for ammonia decomposition: The effect of Sm doping. J. Solid State Chem. 2021, 295, 121946. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Kavadiya, S.; He, X.; Wang, W.-N.; Karakocak, B.B.; Lin, Y.-C.; Berezin, M.Y.; Biswas, P. Engineering stable Pt nanoparticles and oxygen vacancies on defective TiO2 via introducing strong electronic metal–support interaction for efficient CO2 photoreduction. Chem. Eng. J. 2020, 389, 123450. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, Y.; Hou, F.; Wu, C.; Pan, L.; Zou, J.; Wang, L.; Zhang, X.; Liu, G.; Li, G. Engineering oxygen vacancies and nickel dispersion on CeO2 by Pr doping for highly stable ethanol steam reforming. Appl. Catal. B 2019, 258, 117940. [Google Scholar] [CrossRef]
- Muroyama, H.; Saburi, C.; Matsui, T.; Eguchi, K. Ammonia decomposition over Ni/La2O3 catalyst for on-site generation of hydrogen. Appl. Catal. A 2012, 443–444, 119–124. [Google Scholar] [CrossRef]
- He, H.; Jiang, H.; Yang, F.; Liu, J.; Zhang, W.; Jin, M.; Li, Z. Bimetallic NixCo10-x/CeO2 as highly active catalysts to enhance mid-temperature ammonia decomposition: Kinetics and synergies. Int. J. Hydrogen Energy 2023, 48, 5030–5041. [Google Scholar] [CrossRef]
- Ju, X.; Liu, L.; Zhang, X.; Feng, J.; He, T.; Chen, P. Highly Efficient Ru/MgO Catalyst with Surface-Enriched Basic Sites for Production of Hydrogen from Ammonia Decomposition. ChemCatChem 2019, 11, 4161–4170. [Google Scholar] [CrossRef]
- Podila, S.; Driss, H.; Zaman, S.F.; Ali, A.M.; Al-Zahrani, A.A.; Daous, M.A.; Petrov, L.A. Effect of preparation methods on the catalyst performance of Co/MgLa mixed oxide catalyst for COx-free hydrogen production by ammonia decomposition. Int. J. Hydrogen Energy 2017, 42, 24213–24221. [Google Scholar] [CrossRef]
- Wang, Z.; Cai, Z.; Wei, Z. Highly Active Ruthenium Catalyst Supported on Barium Hexaaluminate for Ammonia Decomposition to COx-Free Hydrogen. ACS Sustain. Chem. Eng. 2019, 7, 8226–8235. [Google Scholar] [CrossRef]
- Bajus, S.; Agel, F.; Kusche, M.; Ní Bhriain, N.; Wasserscheid, P. Alkali hydroxide-modified Ru/γ-Al2O3 catalysts for ammonia decomposition. Appl. Catal. A 2016, 510, 189–195. [Google Scholar] [CrossRef]
- Wang, B.; Kong, H.; Wang, H.; Wang, Y.; Hu, X. Kinetic and thermodynamic analyses of mid/low-temperature ammonia decomposition in solar-driven hydrogen permeation membrane reactor. Int. J. Hydrogen Energy 2019, 44, 26874–26887. [Google Scholar] [CrossRef]



| Crystallite Size (nm) | Lattice Constant (Å) | |
|---|---|---|
| Ni/Gd0.1Ce0.9O2-δ | 58 | 5.15 |
| Ni/Gd0.2Ce0.8O2-δ | 60 | 5.17 |
| Ni/Gd0.3Ce0.7O2-δ | 57 | 5.20 |
| Ni/Gd0.4Ce0.6O2-δ | 59 | 5.23 |
| Catalysts | Surface Area a | Actual Ni Loading b | Ni Dispersion c | Ni Particle Size c |
|---|---|---|---|---|
| m2/g | wt% | % | nm | |
| Ni/Gd0.1Ce0.9O2-δ | 121.4 | 9.85 | 15.8 | 8.7 |
| Ni/Gd0.2Ce0.8O2-δ | 120.6 | 9.93 | 20.7 | 8.3 |
| Ni/Gd0.3Ce0.7O2-δ | 124.3 | 9.79 | 17.1 | 8.1 |
| Ni/Gd0.4Ce0.6O2-δ | 119.5 | 9.85 | 14.8 | 8.9 |
| Catalysts | Temperature | GHSV | NH3 Conversion | Hydrogen Production Rate | References |
|---|---|---|---|---|---|
| °C | ml/(gcat.h) | % | mmol/(gcat.h) | ||
| Ni/Al2O3 | 700 | 30,000 | 79.2 | 1591.1 | [19] |
| FeNi/Al2O3 | 700 | 30,000 | 15.7 | 315.4 | [19] |
| CoNi/Al2O3 | 700 | 30,000 | 100 | 2008.9 | [19] |
| CuNi/Al2O3 | 700 | 30,000 | 34.7 | 697.1 | [19] |
| Ni/ZrO2 | 650 | 6000 | 34.8 | 139.8 | [30] |
| Ni/TiO2 | 650 | 6000 | 83.1 | 333.9 | [30] |
| Ni/CeO2 | 650 | 6000 | 89.7 | 360.4 | [30] |
| Ni/La2O3 | 650 | 6000 | 100 | 401.8 | [30] |
| Ni10/CeO2 | 650 | 30,000 | 31.0 | 622.8 | [31] |
| Ni7.5Co2.5/CeO2 | 650 | 30,000 | 31.0 | 622.8 | [31] |
| Ni2.5Co7.5/CeO2 | 650 | 30,000 | 31.0 | 622.8 | [31] |
| Co10/CeO2 | 650 | 30,000 | 31.0 | 622.8 | [31] |
| Ni/Gd0.1Ce0.9O2-δ | 600 | 30,000 | 84.6 | 1699.6 | This work |
| Ni/Gd0.2Ce0.8O2-δ | 600 | 30,000 | 100 | 2008.9 | This work |
| Ni/Gd0.3Ce0.7O2-δ | 600 | 30,000 | 92.3 | 1854.2 | This work |
| Ni/Gd0.4Ce0.6O2-δ | 600 | 30,000 | 80.8 | 1623.2 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, H.; Chen, C.; Bian, C.; Ren, J.; Liu, J.; Huang, W. Enhanced Ammonia Decomposition by Tuning the Support Properties of Ni/GdxCe1-xO2-δ at 600 °C. Molecules 2023, 28, 2750. https://doi.org/10.3390/molecules28062750
He H, Chen C, Bian C, Ren J, Liu J, Huang W. Enhanced Ammonia Decomposition by Tuning the Support Properties of Ni/GdxCe1-xO2-δ at 600 °C. Molecules. 2023; 28(6):2750. https://doi.org/10.3390/molecules28062750
Chicago/Turabian StyleHe, Haihua, Chonglai Chen, Chaoqun Bian, Junhua Ren, Jiajia Liu, and Wei Huang. 2023. "Enhanced Ammonia Decomposition by Tuning the Support Properties of Ni/GdxCe1-xO2-δ at 600 °C" Molecules 28, no. 6: 2750. https://doi.org/10.3390/molecules28062750
APA StyleHe, H., Chen, C., Bian, C., Ren, J., Liu, J., & Huang, W. (2023). Enhanced Ammonia Decomposition by Tuning the Support Properties of Ni/GdxCe1-xO2-δ at 600 °C. Molecules, 28(6), 2750. https://doi.org/10.3390/molecules28062750







