Experimental Study on Electrochemical Desulfurization of Coal Liquefaction Residue
Abstract
1. Introduction
2. Results and Discussion
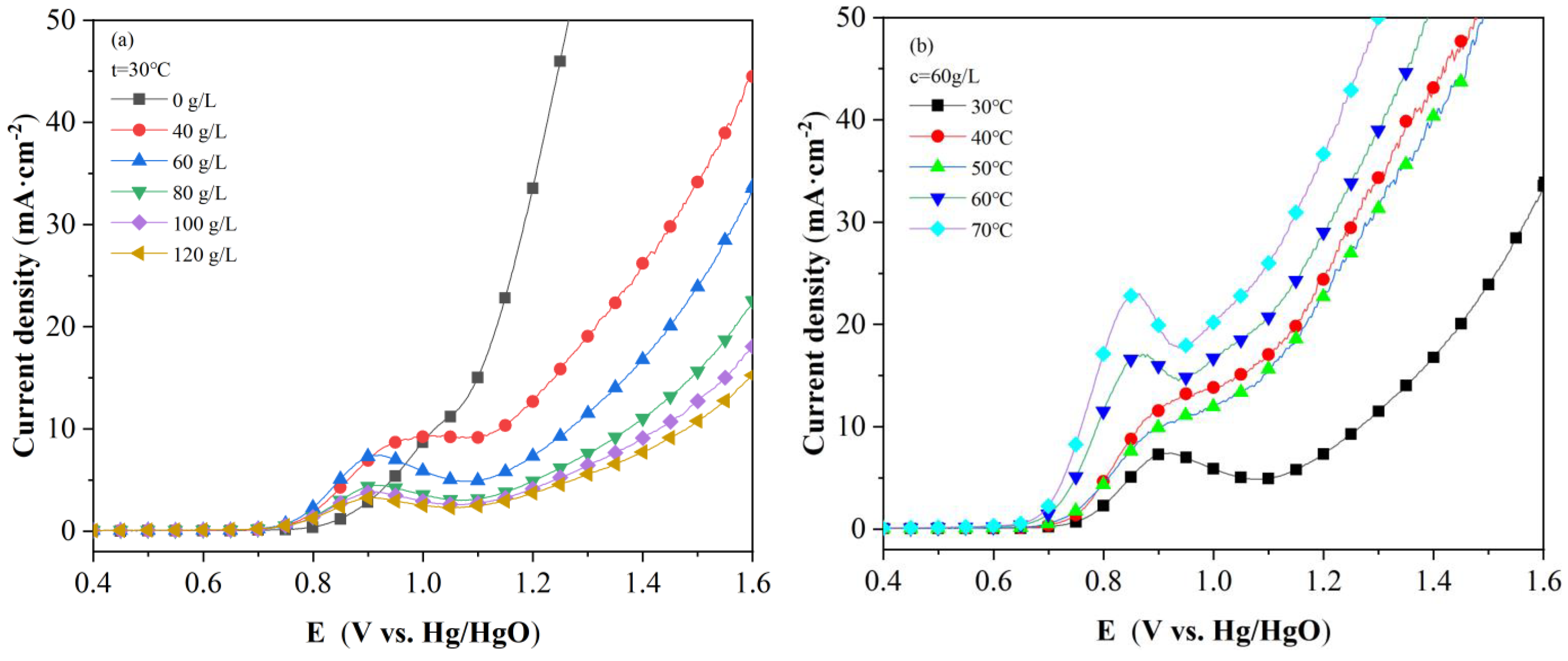
2.1. LSV Polarization Curve
2.2. Reaction Dynamics
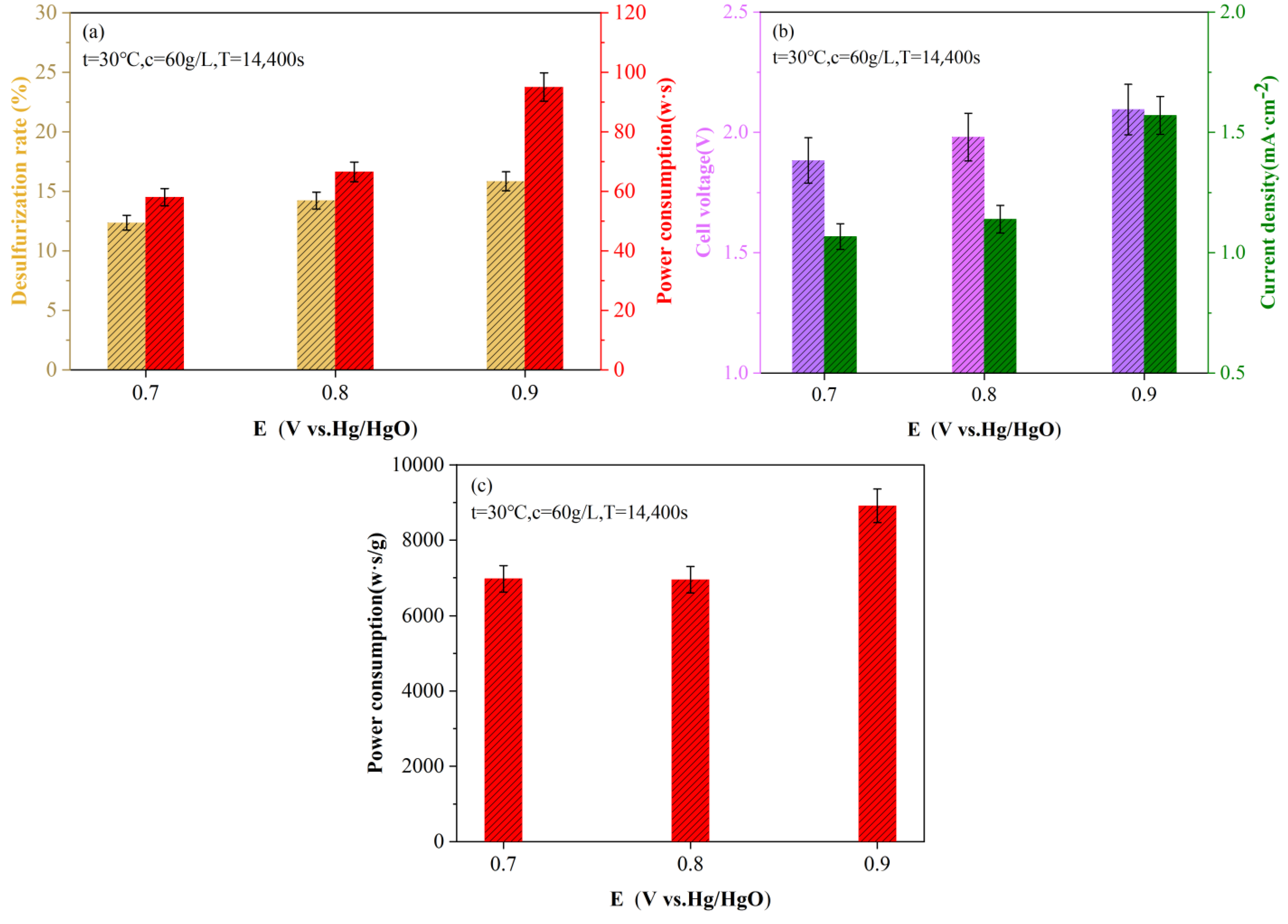
2.3. Electrolytic Desulfurization Efficiency and Desulfurization Energy Consumption
2.4. Mechanism Analysis of Electrolytic Oxidation Desulfurization
2.4.1. Chemical Bond Analysis of Liquefied Residue
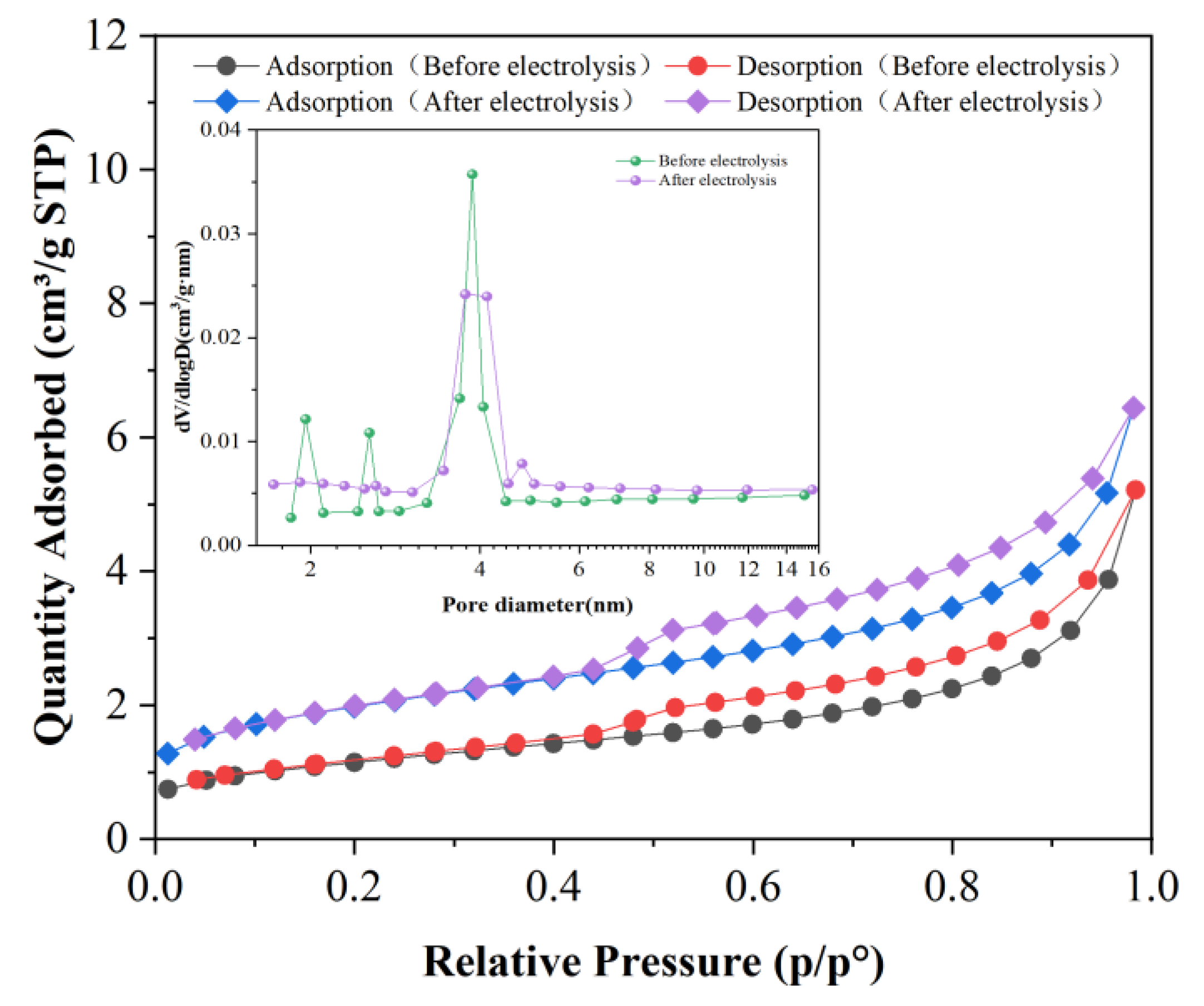
2.4.2. Surface Morphology and Pore Structure of Liquefied Residue
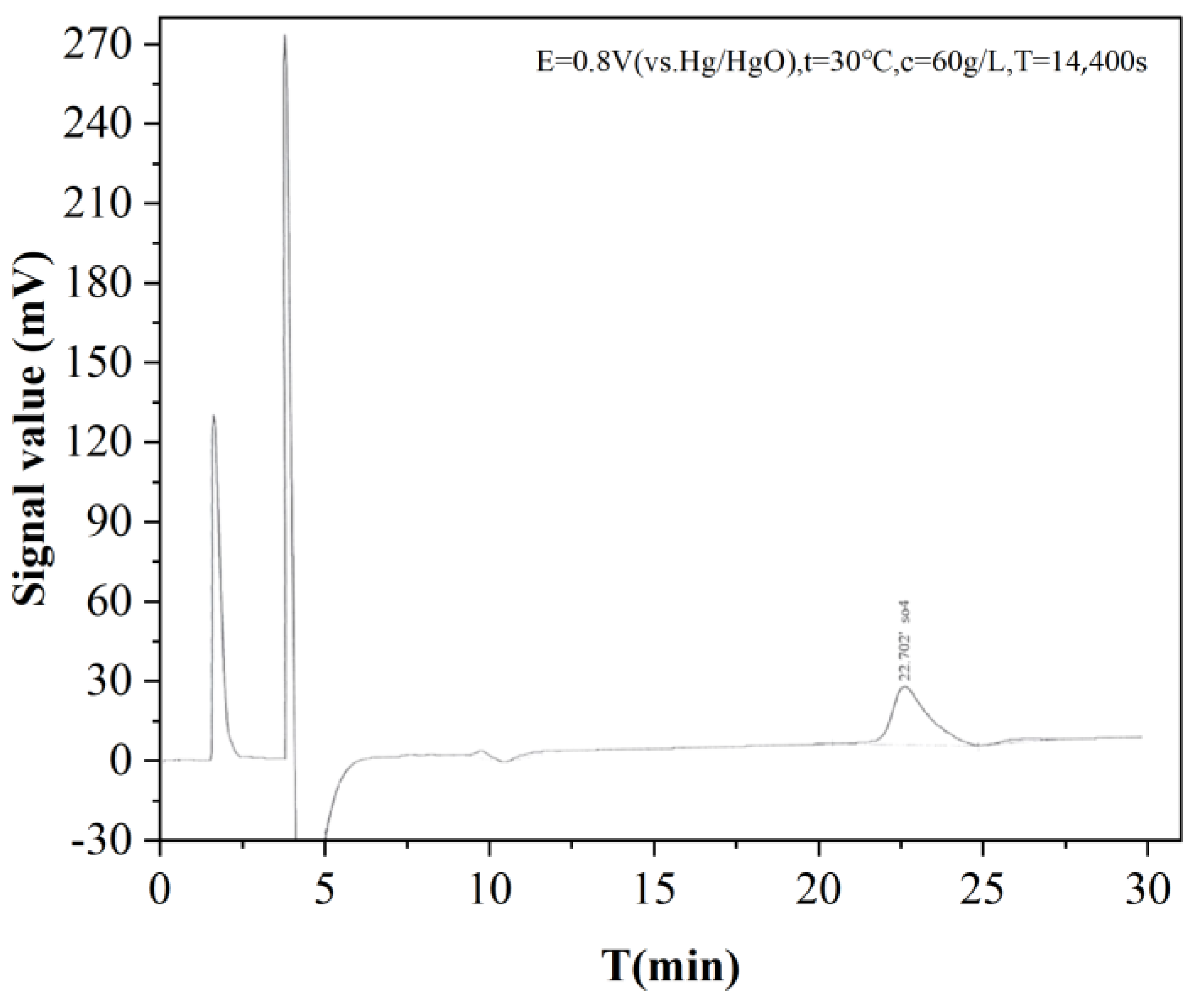
2.4.3. Electrolyte Ion Analysis
3. Experimental Part
3.1. Raw Material
3.2. Experimental Apparatus and Methods
3.2.1. Desulfurization Rate and Kinetic Calculation
- ρ—Desulfurization rate, %;
- S0—S content in raw material liquefaction residue, %;
- S1—S content in liquefaction residue after electrolysis, %.
- j—current density, A/cm2;
- Ea—activation energy, J/mol;
- f—pre-exponential factor;
- R—gas molar constant, 8.314 J/mol·K;
- T—thermodynamic temperature, K.
3.2.2. Calculation of Energy Consumption of Per Unit Sulfur Removal
- E—cumulative electricity consumption, W·s/g;
- U’—real-time electrolyzer voltage, V;
- I—real-time current, A;
- t—electrolysis time, 1 s;
- ms—sulfur removal amount, g.
3.3. Experimental Characterization Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Li, J.; Yang, J.; Liu, Z. Hydro-treatment of a direct coal liquefaction residue and its components. Catal. Today 2008, 130, 389–394. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Lai, S.; Dong, H.; Chen, X.; Wang, X.; Nie, Y.; Sheng, Y.; Zhang, S. Ionic liquids to extract valuable components from direct coal liquefaction residues. Fuel 2012, 94, 617–619. [Google Scholar] [CrossRef]
- Chu, X.; Li, W.; Li, B.; Chen, H. Sulfur transfers from pyrolysis and gasification of direct liquefaction residue of Shenhua coal. Fuel 2008, 87, 211–215. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, L.; Hu, H.; Xun, Y. Effect of composition in coal liquefaction residue on catalytic activity of the resultant carbon for methane decomposition. Fuel 2012, 96, 462–468. [Google Scholar] [CrossRef]
- An, H.; Liu, Z.; Fang, X.; Feng, Z.; Duan, X. Kinetic study of CO2 gasification on coal char and extraction residue char from direct coal liquefaction. Int. J. Energy Res. 2021, 45, 3750–3760. [Google Scholar] [CrossRef]
- Khare, S.; Dell’Amico, M. An overview of solid-liquid separation of residues from coal liquefaction processes. Can. J. Chem. Eng. 2012, 91, 324–331. [Google Scholar] [CrossRef]
- Chu, X.J.; Wang, Y.G.; Zhao, L.H. Research on Pyrolysis Kinetics of Shenhua Coal and Direct Liquefaction Residue. Adv. Mater. Res. 2012, 550, 2758–2762. [Google Scholar] [CrossRef]
- Lv, D.; Yuchi, W.; Bai, Z.; Bai, J.; Kong, L.; Guo, Z.; Yan, J.; Li, W. An approach for utilization of direct coal liquefaction residue: Blending with low-rank coal to prepare slurries for gasification. Fuel 2015, 145, 143–150. [Google Scholar] [CrossRef]
- Han, L.; Zhu, C.-C.; Yao, Q.; Li, P.; Wu, G.-G.; Meng, X.-L.; Zhang, B.; Li, X.; Bai, Z.-Q.; Li, W. In-situ catalytic gasification of sodium-rich direct coal liquefaction residue under CO2 atmosphere and kinetic analysis of gasification reaction process. J. Energy Inst. 2022, 101, 233–242. [Google Scholar] [CrossRef]
- Yan, J.; He, J.; Yang, Q.; Bai, Z.; Lei, Z.; Li, Z.; Xue, C.; Wang, Z.; Ren, S.; Kang, S.; et al. A study of gasification behavior of residues from mild coal hydro-liquefaction. Fuel 2021, 293, 120456. [Google Scholar] [CrossRef]
- Xu, J.; Bai, Z.; Li, Z.; Guo, Z.; Hao, P.; Bai, J.; Li, W. Interactions during co-pyrolysis of direct coal liquefaction residue with lignite and the kinetic analysis. Fuel 2018, 215, 438–445. [Google Scholar] [CrossRef]
- Xu, L.; Tang, M.; Liu, B.; Ma, X.; Zhang, Y.; Argyle, M.D.; Fan, M. Pyrolysis characteristics and kinetics of residue from China Shenhua industrial direct coal liquefaction plant. Thermochim. Acta 2014, 589, 1–10. [Google Scholar] [CrossRef]
- Tian, B.; Gao, J.; Guo, Q.; Zhu, X.; Zhang, H.; Zhu, W.; Hao, X.; Yang, C.; Yang, Y.; Li, Y.-W. Process study and CO2 emission reduction analysis of coal liquefaction residue fluidized bed pyrolysis. Front. Energy Res. 2022, 10, 965047. [Google Scholar] [CrossRef]
- Huang, X.; Yang, Z.; Liu, M.; He, J.; Li, L.; Cui, C.; Huang, Z.; Wang, S.; Yan, D. Release characteristics of organic pollutants during co-firing coal liquefaction residue in a circulating fluidized bed boiler. Fuel 2023, 332, 125958. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Yang, C.; Cao, Y.; Su, X.; Tahir, M.U. Self-template porous carbon by direct activation of high-ash coal liquefaction residue for high-rate supercapacitor electrodes. Int. J. Energy Res. 2020, 45, 4782–4792. [Google Scholar] [CrossRef]
- Qin, F.; Li, Q.; Tang, T.; Zhu, J.; Gan, X.; Chen, Y.; Li, Y.; Zhang, S.; Huang, X.; Jia, D. Functional carbon dots from a mild oxidation of coal liquefaction residue. Fuel 2022, 322, 124216. [Google Scholar] [CrossRef]
- Xu, L.; Lu, Y.; Yalikun, N.; Shi, C.; Wang, H.; Xu, Y.; Liu, J. Preparation and Application of Coal-Liquefaction-Residue-Based Carbon Material. Processes 2022, 10, 2455. [Google Scholar] [CrossRef]
- Luo, H.; Li, T.; Wang, F.; Cui, K. Study on the effect of ash in coal liquefaction residue on the preparation of high-density carbon materials by induced polycondensation. Energy Sources Recovery Util. Environ. Eff. 2022, 44, 340–352. [Google Scholar] [CrossRef]
- Zhou, Y.; Albijanic, B.; Tadesse, B.; Wang, Y.; Yang, J.; Zhu, X. Desulphurization of coals of different ranks in the presence of slimes by reverse flotation. Energy Rep. 2019, 5, 1316–1323. [Google Scholar] [CrossRef]
- Cao, D.; Xu, X.; Jiang, S. Ultrasound-electrochemistry enhanced flotation and desulphurization for fine coal. Sep. Purif. Technol. 2020, 258, 117968. [Google Scholar] [CrossRef]
- Nawaz, Z.; Naveed, S.; Shaikh, N.A.; Ali, A. Bio-desulphurization of Lakhra coal by effective microoganisms (EM) technology. J. Chem. Soc. Pak. 2006, 28, 611–618. [Google Scholar]
- Ma, M.; Wang, W.; Zhang, K. Occurrence Characteristics of Fine-Grained Pyrite in Coal and Its Scaling Effect on Flotation Desulfurization. ACS Omega 2022, 7, 42467–42481. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Sun, T.; Jia, J.; Lou, Z. Mild Process for Reductive Desulfurization of Diesel Fuel Using Sodium Borohydride in Situ Generated via Sodium Metaborate Electroreduction. Ind. Eng. Chem. Res. 2013, 52, 7660–7667. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, Y.; Zhong, Q.; Li, F.; Huang, J.; Wang, B. Reduction and Desulfurization of Petroleum Coke in Ammonia and Their Thermodynamics. Energy Fuels 2016, 30, 3385–3391. [Google Scholar] [CrossRef]
- Tang, X.-D.; Hu, T.; Li, J.-J.; Wang, F.; Qing, D.-Y. Desulfurization of Kerosene by the Electrochemical Oxidation and Extraction Process. Energy Fuels 2015, 29, 2097–2103. [Google Scholar] [CrossRef]
- Coughlin, R.W.; Farooque, M. Hydrogen production from coal, water and electrons. Nature 1979, 279, 301–303. [Google Scholar] [CrossRef]
- Farooque, M.; Coughlin, R.W. Electrochemical gasification of coal (investigation of operating conditions and variables). Fuel 1979, 58, 705–712. [Google Scholar] [CrossRef]
- Shen, Y.; Sun, T.; Jia, J. A novel desulphurization process of coal water slurry via sodium metaborate electroreduction in the alkaline system. Fuel 2012, 96, 250–256. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, X.; Sun, T.; Jia, J. Recent advances of sodium borohydride reduction in coal water slurry desulfurization: Integration of chemical and electrochemical reduction. RSC Adv. 2012, 2, 8867–8882. [Google Scholar] [CrossRef]
- Ying, Z.; Geng, Z.; Zheng, X.; Dou, B.; Cui, G. Enhancing biochar oxidation reaction with the mediator of Fe2+/Fe3+ or NO2−/NO3− for efficient hydrogen production through biochar-assisted water electrolysis. Energy Convers. Manag. 2021, 244, 114523. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Y.; Hu, Y.; Wang, Z.; Chen, J.; Niu, X.; Li, Y.; Gong, X. HO selective cleavage Fe S bond for FeS2 electrolysis in alkaline solution. Electrochim. Acta 2019, 306, 327–338. [Google Scholar] [CrossRef]
- Rehman, F.; Ahmad, S.W.; Zafar, M.S.; Ahmad, S.; Zia-Ul-Haq, M. Parametric Optimization of Coal Desulfurization Through Alkaline Leaching. Pol. J. Chem. Technol. 2018, 20, 103–109. [Google Scholar] [CrossRef]
- Wapner, P.G.; Lalvani, S.B.; Awad, G. Organic sulfur removal from coal by electrolysis in alkaline media. Fuel Process. Technol. 1988, 18, 25–36. [Google Scholar] [CrossRef]
- Zeng, X.; Xiao, X.; Li, Y.; Chen, J.; Wang, H. Deep desulfurization of liquid fuels with molecular oxygen through graphene photocatalytic oxidation. Appl. Catal. Environ. 2017, 209, 98–109. [Google Scholar] [CrossRef]
- Gong, X.; Wang, Z.; Zhao, L.; Zhang, S.; Wang, D.; Wang, M. Competition of Oxygen Evolution and Desulfurization for Bauxite Electrolysis. Ind. Eng. Chem. Res. 2017, 56, 6136–6144. [Google Scholar] [CrossRef]
- Chuang, K.C.; Markuszewski, R.; Wheelock, T.D. Desulfurization of coal by oxidation in alkaline solutions. Fuel Process. Technol. 1983, 7, 43–57. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, Y.; Gong, X.; Wang, Z.; Zhang, S.; Wang, M. Intensified hydrogen production and desulfurization at elevated temperature and pressure during coal electrolysis. Electrochim. Acta 2018, 284, 560–568. [Google Scholar] [CrossRef]
- Qu, J.; Xie, H.; Song, Q.; Ning, Z.; Zhao, H.; Yin, H. Electrochemical desulfurization of solid copper sulfides in strongly alkaline solutions. Electrochem. Commun. 2018, 92, 14–18. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, J.; Wang, Z.; Liu, J.; Qian, G.; Wang, D.; Gong, X. Review of electrochemical oxidation desulfurization for fuels and minerals. Fuel 2021, 305, 121562. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, Y.; Hu, Y.; Wang, Z.; Liu, J.; Qian, G.; Gong, X. Pressure intensified HO evolution from OER and electrolysis desulfurization. Electrochim. Acta 2019, 318, 202–210. [Google Scholar] [CrossRef]
- Zhang, T.; Hu, Y.; Zhou, Y.; Gong, X.; Wang, Z.; Zhang, S.; Wang, M. Oxygen Reduction Reaction from Water Electrolysis Intensified by Pressure and O2−Oxidation Desulfurization. J. Electrochem. Soc. 2018, 165, E139–E147. [Google Scholar] [CrossRef]
- Han, H.L.; Zhou, L.W.; Liu, K.; Lu, Z.H.; Luo, N. A process optimization on high temperature electrolytic desulfurization in hot metal pretreatment using magnesit-based desulfurizer. Metalurgija 2020, 59, 295–298. [Google Scholar]
- Gong, X.; Ge, L.; Wang, Z.; Zhuang, S.; Wang, Y.; Ren, L.; Wang, M. Desulfurization from Bauxite Water Slurry (BWS) Electrolysis. Met. Mater. Trans. 2015, 47, 649–656. [Google Scholar] [CrossRef]




| Sample | Relative Content/% | ||||||
|---|---|---|---|---|---|---|---|
| Satellites | Thiophene | R-S-R R-SH | R-SOX-R | C | C-O | O-C = O | |
| Before electrolysis | 46.36 | 18.90 | 34.74 | 0 | 88.24 | 7.49 | 4.27 |
| After electrolysis | 35.27 | 11.94 | 16.19 | 36.60 | 87.46 | 8.40 | 4.14 |
| Sample | Surface Area /(m2·g−1) | Pore Volume /(cm3 g −1) | Average Pore Diameter d/nm |
|---|---|---|---|
| Before electrolysis | 3.9825 | 0.005073 | 8.1044 |
| After electrolysis | 6.7625 | 0.007413 | 5.8892 |
| Sample | Proximate Analysis Wad/% | Ultimate Analysis Wdaf/% | |||||||
|---|---|---|---|---|---|---|---|---|---|
| M | A | V | FC | C | H | N | O * | S | |
| SH-DCLR | 0.04 | 15.73 | 40.53 | 50.10 | 76.17 | 4.38 | 0.74 | 16.90 | 1.81 |
| Sample | Sulfur Content/% | |||
|---|---|---|---|---|
| Total | Organic | Pyrite | Sulfate | |
| SH-DCLR | 1.87 | 1.81 | 0.06 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, J.; Zhang, Y.; Li, N.; Bai, R.; Liu, Q.; Zhou, X. Experimental Study on Electrochemical Desulfurization of Coal Liquefaction Residue. Molecules 2023, 28, 2749. https://doi.org/10.3390/molecules28062749
Fan J, Zhang Y, Li N, Bai R, Liu Q, Zhou X. Experimental Study on Electrochemical Desulfurization of Coal Liquefaction Residue. Molecules. 2023; 28(6):2749. https://doi.org/10.3390/molecules28062749
Chicago/Turabian StyleFan, Jianming, Yongfeng Zhang, Na Li, Ruzhan Bai, Qi Liu, and Xing Zhou. 2023. "Experimental Study on Electrochemical Desulfurization of Coal Liquefaction Residue" Molecules 28, no. 6: 2749. https://doi.org/10.3390/molecules28062749
APA StyleFan, J., Zhang, Y., Li, N., Bai, R., Liu, Q., & Zhou, X. (2023). Experimental Study on Electrochemical Desulfurization of Coal Liquefaction Residue. Molecules, 28(6), 2749. https://doi.org/10.3390/molecules28062749





