Recent Progress on Nanocarriers for Topical-Mediated RNAi Strategies for Crop Protection—A Review
Abstract
1. Introduction
2. Non-Transformative RNAi Strategies
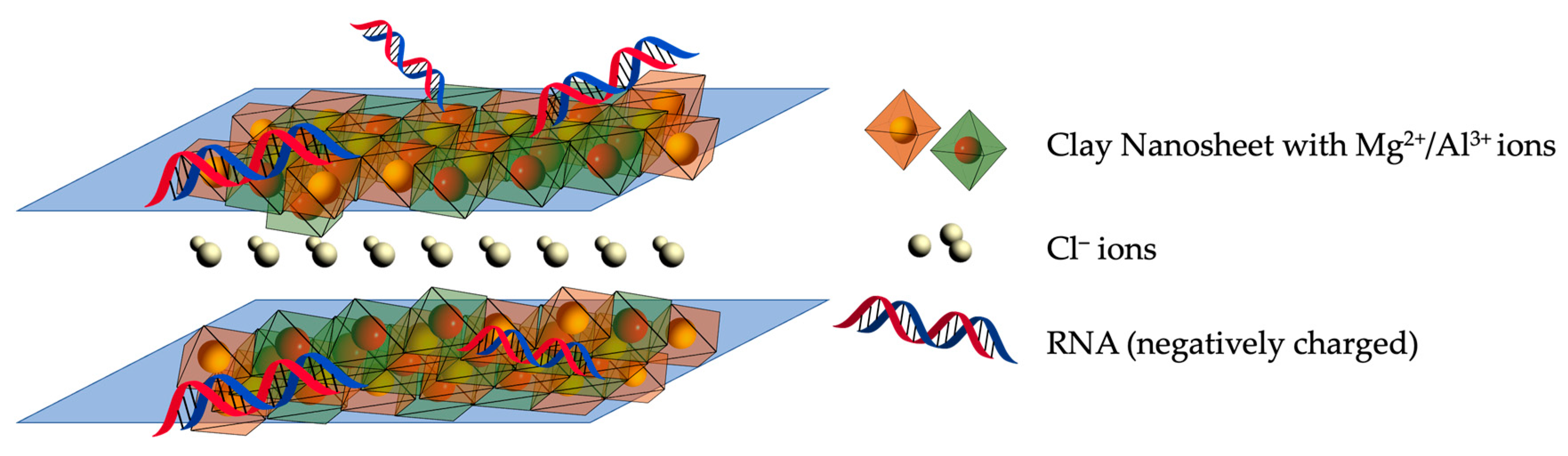
2.1. Layered Double Hydroxide (LDH)
2.2. Carbon Dots (CDs)
2.3. Carbon Nanotubes
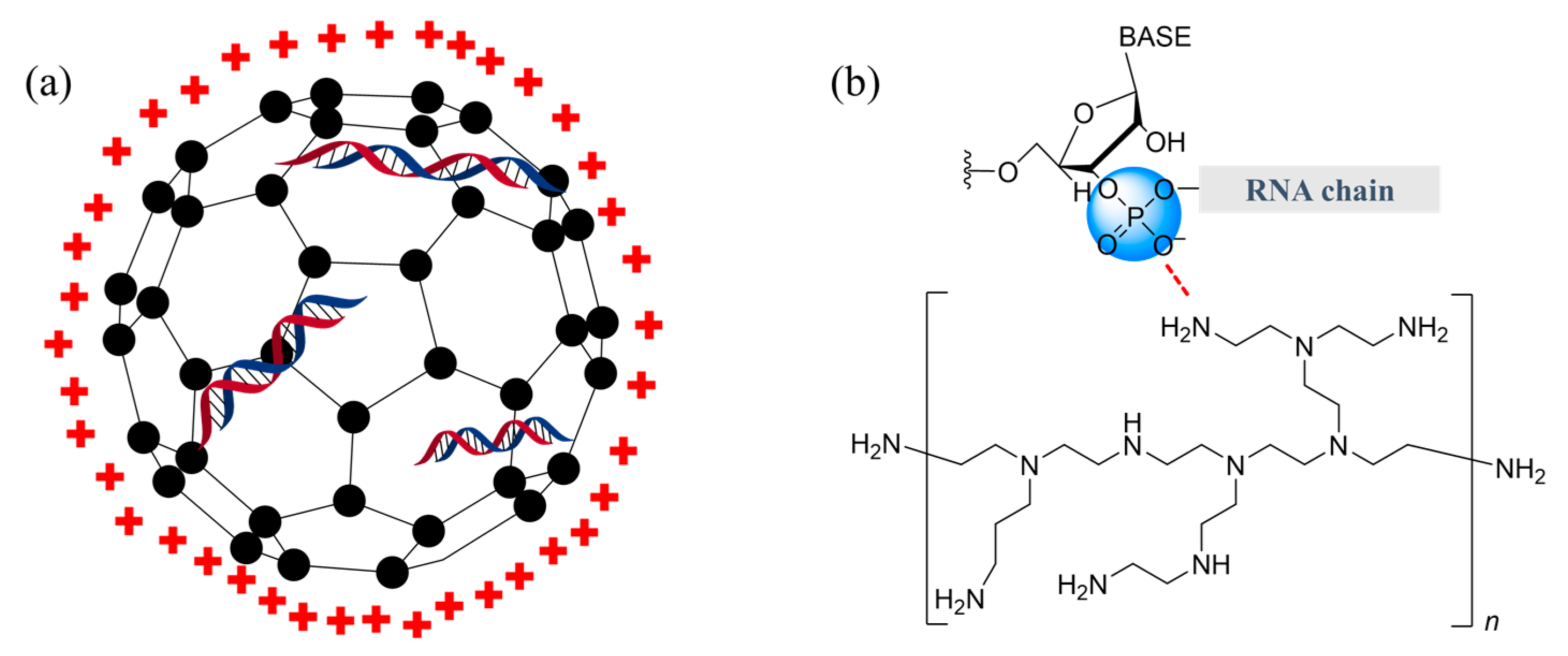
2.4. Chitosan
2.5. Peptides
2.6. Gold Nanoparticles
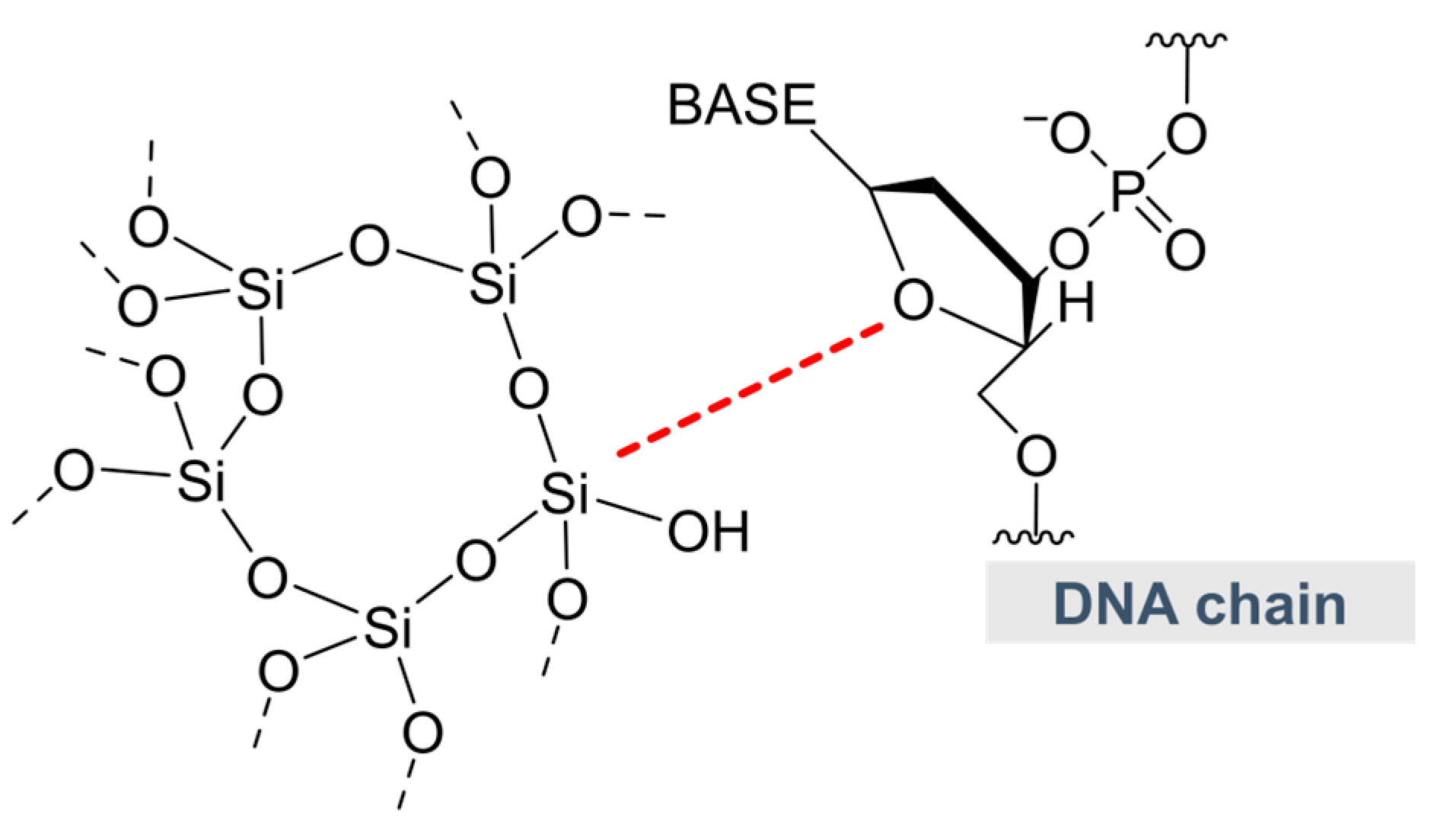
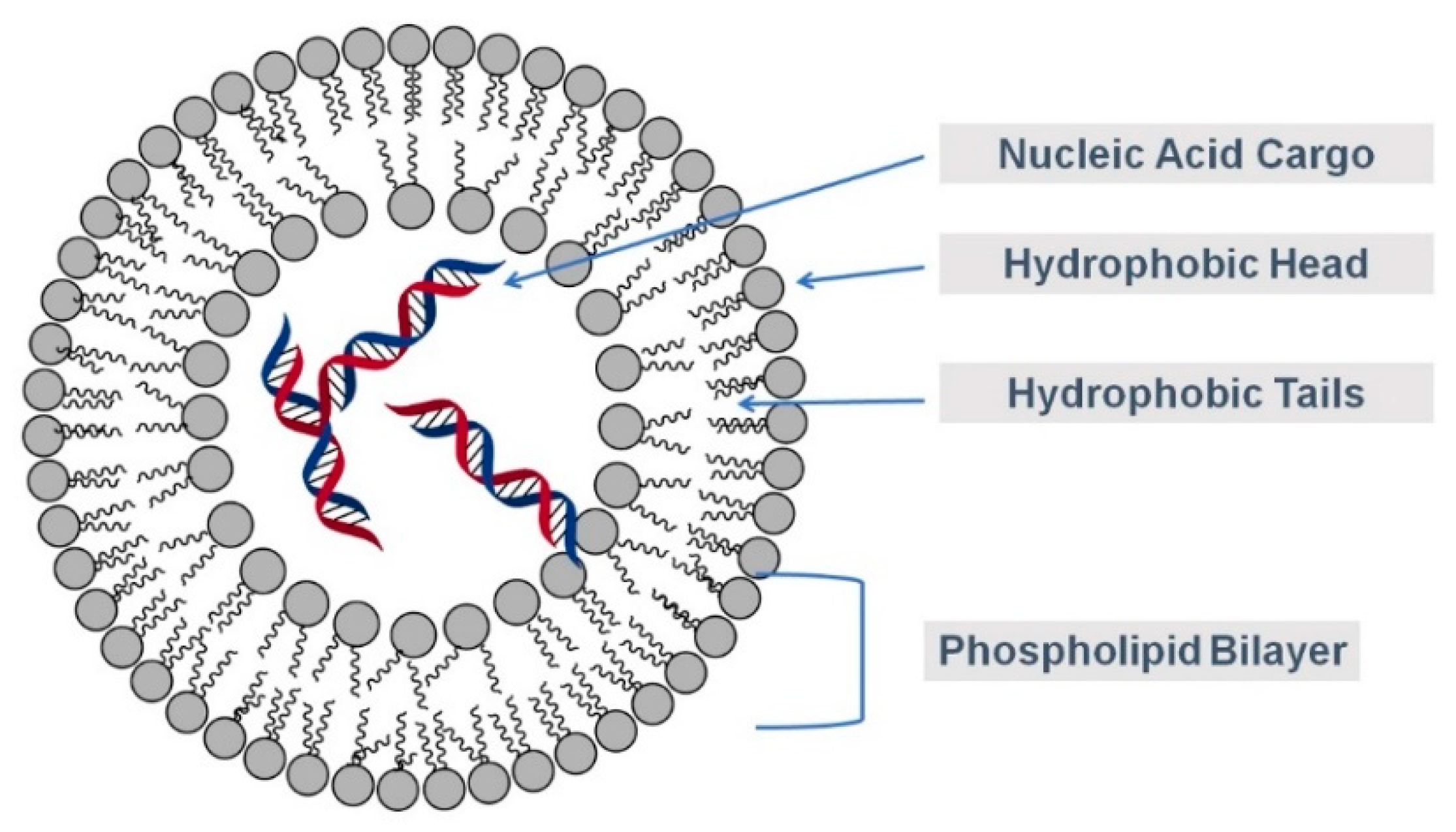
2.7. Other Potential Carriers—Silica and Liposomes
3. Potential Risks, Safety Concerns and Limitations
4. Conclusions and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. World Population Prospects: The 2017 Revision, Key Findings and Advance Tables; Department of Economic and Social Affairs; United Nations: New York, NY, USA, 2017. [Google Scholar]
- Douglas, A.E. Strategies for Enhanced Crop Resistance to Insect Pests. Annu. Rev. Plant Biol. 2018, 69, 637–660. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. The Pesticides Use Database Includes Data on the Use of Major Pesticide Groups (Insecticides, Herbicides, Fungicides, Plant Growth Regulators and Rodenticides) and of Relevant Chemical Families; FAO: Rome, Italy, 2019. [Google Scholar]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An Extensive Review on the Consequences of Chemical Pesticides on Human Health and Environment. J. Clean. Prod. 2021, 283, 124657. [Google Scholar] [CrossRef]
- Boedeker, W.; Watts, M.; Clausing, P.; Marquez, E. The global distribution of acute unintentional pesticide poisoning: Estimations based on a systematic review. BMC Public Health. 2020, 20, 1875. [Google Scholar] [CrossRef]
- Romeis, J.; Naronjo, S.E.; Meissle, M.; Shelton, A.M. Genetically engineered crops help support conservation biological control. Biol. Control 2019, 130, 136–154. [Google Scholar] [CrossRef]
- Schulz, R.; Bub, S.; Petschick, L.L.; Stehle, S.; Wolfram, J. Applied pesticide toxicity shifts toward plants and invertebrates, even in GM crops. Science 2021, 372, 81–84. [Google Scholar] [CrossRef]
- AgbioInvestor. Time and Cost to Develop a New GM Trait. Available online: https://croplife.org/wp-content/uploads/2022/05/AgbioInvestor-Trait-RD-Branded-Report-Final-20220512.pdf (accessed on 20 October 2022).
- Bebber, D.P.; Ramotowski, M.A.T.; Gurr, S.J. Crop pests and pathogens move polewards in a warming world. Nat. Clim. Chang. 2013, 3, 985–988. [Google Scholar] [CrossRef]
- Ratcliff, F.; Harrison, B.D.; Baulcombe, D.C. A Similarity Between Viral Defense and Gene Silencing in Plants. Science 1997, 276, 1558–1560. [Google Scholar] [CrossRef]
- Mat Jalaluddin, N.S.; Othman, R.Y.; Harikrishna, J.A. Global trends in research and commercialization of exogenous and endogenous RNAi technologies for crops. Crit. Rev. Biotechnol. 2019, 39, 67–78. [Google Scholar] [CrossRef]
- Tenllado, F.; Diaz-Ruiz, J.R. Double-Stranded RNA-mediated interference with plant virus infection. J. Virol. 2001, 75, 12288–12297. [Google Scholar] [CrossRef]
- Qiao, L.; Lan, C.; Capriotti, L.; Ah-Fong, A.; Sanchez, J.N.; Hamby, R.; Heller, J.; Zhao, H.; Glass, N.L.; Judelson, H.S.; et al. Spray-Induced gene silencing for disease control is dependent on the efficiency of pathogen RNA uptake. Plant Biotechnol. J. 2021, 19, 1756–1768. [Google Scholar] [CrossRef]
- Micura, R. Small Interfering RNAs and Their Chemical Synthesis. Angew. Chem. Int. Ed. 2002, 41, 2265–2269. [Google Scholar] [CrossRef]
- Bachman, P.; Fischer, J.; Song, Z.; Urbanczyk-Wochniak, E.; Watson, G. Environmental Fate and Dissipation of Applied dsRNA in Soil, Aquatic Systems, and Plants. Front. Plant Sci. 2020, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Parker, K.M.; Borrero, V.B.; van Leeuwen, D.M.; Lever, M.A.; Mateescu, B.; Sander, M. Environmental Fate of RNA Interference Pesticides: Adsorption and Degradation of Double-Stranded RNA Molecules in Agricultural Soils. Environ. Sci. Technol. 2019, 53, 3027–3036. [Google Scholar] [CrossRef]
- Numata, K.; Ohtani, M.; Yoshizumi, T.; Demura, T.; Kodama, Y. Local gene silencing in plants via synthetic dsRNA and carrier peptide. Plant Biotechnol. J. 2014, 12, 1027–1034. [Google Scholar] [CrossRef]
- Mitter, N.; Worrall, E.A.; Robinson, K.E.; Li, P.; Jain, R.G.; Taochy, C.; Fletcher, S.J.; Carroll, B.J.; Lu, G.Q.M.; Xu, Z.P. Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat. Plants 2017, 3, 16207. [Google Scholar] [CrossRef]
- Cunningham, F.J.; Goh, N.S.; Demirer, G.S.; Matos, J.L.; Landry, M.P. Nanoparticle-Mediated Delivery towards Advancing Plant Genetic Engineering. Trends Biotechnol. 2018, 36, 882–897. [Google Scholar] [CrossRef]
- Schwab, F.; Zhai, G.; Kern, M.; Turner, A.; Schnoor, J.L.; Wiesner, M.R. Barriers, pathways and processes for uptake, translocation and accumulation of nanomaterials in plants--Critical review. Nanotoxicology 2016, 10, 257–278. [Google Scholar] [CrossRef] [PubMed]
- Joga, M.R.; Zotti, M.J.; Smagghe, G.; Christiaens, O. RNAi Efficiency, Systemic Properties, and Novel Delivery Methods for Pest Insect Control: What We Know So Far. Front. Physiol. 2016, 7, 553. [Google Scholar] [CrossRef]
- Bennett, M.; Deikman, J.; Hendrix, B.; Iandolino, A. Barriers to Efficient Foliar Uptake of dsRNA and Molecular Barriers to dsRNA Activity in Plant Cells. Front. Plant Sci. 2020, 11, 816. [Google Scholar] [CrossRef]
- Christiaens, O.; Petek, M.; Smagghe, G.; Taning, C.N.T. The Use of Nanocarriers to Improve the Efficiency of RNAi-Based Pesticides in Agriculture. In Nanopesticides; Fraceto, L.F., S.S. de Castro, V.L., Grillo, R., Ávila, D., Caixeta Oliveira, H., Lima, R., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Pugsley, C.E.; Isaac, R.E.; Warren, N.J.; Cayre, O.J. Recent Advances in Engineered Nanoparticles for RNAi-Mediated Crop Protection Against Insect Pests. Front. Agro. 2021, 3, 652981. [Google Scholar] [CrossRef]
- Yong, J.; Zhang, R.; Bi, S.; Li, P.; Sun, L.; Mitter, N.; Carroll, B.J.; Xu, Z.P. Sheet-Like clay nanoparticles deliver RNA into developing pollen to efficiently silence a target gene. Plant Physiol. 2021, 187, 886–899. [Google Scholar] [CrossRef]
- Mosa, M.A.; Youssef, K. Topical delivery of host induced RNAi silencing by layered double hydroxide nanosheets: An efficient tool to decipher pathogenicity gene function of Fusarium crown and root rot in tomato. Physiol. Mol. Plant Pathol. 2021, 115, 101684. [Google Scholar] [CrossRef]
- Schwartz, S.H.; Hendrix, B.; Hoffer, P.; Sanders, R.A.; Zheng, W. Carbon Dots for Efficient Small Interfering RNA Delivery and Gene Silencing in Plants. Plant Physiol. 2020, 184, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Avital, A.; Muzika, N.S.; Persky, Z.; Bar, G.; Michaeli, Y.; Fridman, Y.; Karny, A.; Shklover, J.; Shainsky, J.; Savaldi-Goldstein, S.; et al. Foliar Delivery of siRNA Particles for Treating Viral Infections in Agricultural Grapevines. Adv. Funct. Mater. 2021, 31, 2101003. [Google Scholar] [CrossRef] [PubMed]
- Demirer, G.S.; Zhang, H.; Goh, N.S.; Pinals, R.L.; Chang, R.; Landry, M.P. Carbon nanocarriers deliver siRNA to intact plant cells for efficient gene knockdown. Sci. Adv. 2020, 6, eaaz0495. [Google Scholar] [CrossRef] [PubMed]
- Laisney, J.; Gurusamy, D.; Baddar, Z.E.; Palli, S.R.; Unrine, J.M. RNAi in Spodoptera frugiperda Sf9 Cells via Nanomaterial Mediated Delivery of dsRNA: A Comparison of Poly-l-arginine Polyplexes and Poly-l-arginine-Functionalized Au Nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 25645–25657. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, Y.; Xu, D.; Goh, N.S.; Demirer, G.S.; Cestellos-Blanco, S.; Chen, Y.; Landry, M.P.; Yang, P. Gold-Nanocluster-Mediated delivery of siRNA to intact plant cells for efficient gene knockdown. Nano Lett. 2021, 21, 5859–5866. [Google Scholar] [CrossRef] [PubMed]
- Petrônio, M.S.; Barros-Alexandrino, T.T.; Lima, A.M.; Assis, O.B.G.; Nagata, A.K.I.; Nakasu, E.Y.T.; Tiera, M.J.; Pilon, L. Physicochemical and Toxicity Investigation of Chitosan-based dsRNA Nanocarrier Formation. Biointerface Res. Appl. Chem. 2022, 12, 5266–5279. [Google Scholar]
- Ma, Z.; Zhang, Y.; Li, M.; Chao, Z.; Du, X.; Yan, S.; Shen, J. A first greenhouse application of bacteria-expressed and nanocarrier-delivered RNA pesticide for Myzus persicae control. J. Pest Sci. 2022, 96, 181–193. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Z.Z.; Zhou, H.; Chao, Z.J.; Yan, S.; Shen, J. Nanocarrier-Delivered dsRNA suppresses wing development of green peach aphids. Insect Sci. 2022, 29, 669–682. [Google Scholar] [CrossRef]
- Ladewig, K.; Niebert, M.; Xu, Z.P.; Gray, P.P.; Lu, G.Q. Efficient siRNA delivery to mammalian cells using layered double hydroxide nanoparticles. Biomaterials 2010, 31, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Wang, J.; Wang, Q.; O’Hare, D.; Wan, Y. Layered Double Hydroxide Nanotransporter for Molecule Delivery to Intact Plant Cells. Sci. Rep. 2016, 6, 26738. [Google Scholar] [CrossRef] [PubMed]
- Dalakouras, A.; Wassenegger, M.; Dadami, E.; Ganopoulos, I.; Pappas, M.L.; Papadopoulou, K. Genetically Modified Organism-Free RNA Interference: Exogenous Application of RNA Molecules in Plants. Plant Physiol. 2020, 182, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Uslu, V.V.; Bassler, A.; Krczal, G.; Wassenegger, M. High-Pressure-Sprayed Double Stranded RNA Does Not Induce RNA Interference of a Reporter Gene. Front. Plant Sci. 2020, 11, 534391. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Guo, H.; Chen, G.; Ma, C.; Xing, B. Distribution of different surface modified carbon dots in pumpkin seedlings. Sci. Rep. 2018, 8, 7991. [Google Scholar] [CrossRef]
- Li, W.; Zheng, Y.; Zhang, H.; Liu, Z.; Su, W.; Chen, S.; Liu, Y.; Zhuang, J.; Lei, B. Phytotoxicity, Uptake, and Translocation of Fluorescent Carbon Dots in Mung Bean Plants. ACS Appl. Mater. Interfaces 2016, 8, 19939–19945. [Google Scholar] [CrossRef]
- Li, H.; Huang, J.; Lu, F.; Liu, Y.; Song, Y.; Sun, Y.; Zhong, J.; Huang, H.; Wang, Y.; Li, S.; et al. Impacts of Carbon Dots on Rice Plants: Boosting the Growth and Improving the Disease Resistance. ACS Appl. Bio Mater. 2018, 1, 663–672. [Google Scholar] [CrossRef]
- Carpita, N.; Sabularse, D.; Montezinos, D.; Delmer, D.P. Determination of the pore size of cell walls of living plant cells. Science 1979, 205, 1144–1147. [Google Scholar] [CrossRef]
- Kozielski, K.L.; Tzeng, S.Y.; Green, J.J. Bioengineered nanoparticles for siRNA delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 449–468. [Google Scholar] [CrossRef]
- Wang, J.W.; Cunningham, F.J.; Goh, N.S.; Boozarpour, N.N.; Pham, M.; Landry, M.P. Nanoparticles for protein delivery in planta. Curr. Opin. Plant Biol. 2021, 60, 102052. [Google Scholar] [CrossRef]
- Edwards, C.H.; Christie, C.R.; Masotti, A.; Celluzzi, A.; Caporali, A.; Campbell, E.M. Dendrimer-Coated carbon nanotubes deliver dsRNA and increase the efficacy of gene knockdown in the red flour beetle Tribolium castaneum. Sci. Rep. 2020, 10, 12422. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, B.; Wang, Q.; Shi, X.; Xiao, Z.; Lin, J.; Fang, X. Carbon Nanotubes as Molecular Transporters for Walled Plant Cells. Nano Lett. 2009, 9, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Serag, M.F.; Kaji, N.; Gaillard, C.; Okamoto, Y.; Terasaka, K.; Jabasini, M.; Tokeshi, M.; Mizukami, H.; Bianco, A.; Baba, Y. Trafficking and subcellular localization of multiwalled carbon nanotubes in plant cells. ACS Nano 2011, 5, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Burlaka, O.M.; Pirko, Y.V.; Yemets, A.I.; Blume, Y.B. Plant Genetic Transformation Using Carbon Nanotubes for DNA Delivery. Cytol. Genet. 2015, 49, 349–357. [Google Scholar] [CrossRef]
- Golestanipour, A.; Nikkhah, M.; Aalami, A.; Hosseinkhani, S. Gene Delivery to Tobacco Root Cells with Single-Walled Carbon Nanotubes and Cell-Penetrating Fusogenic Peptides. Mol. Biotechnol. 2018, 60, 863–878. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.Y.; Lew, T.T.S.; Sweeney, C.J.; Koman, V.B.; Wong, M.H.; Bohmert-Tatarev, K.; Snell, K.D.; Seo, J.S.; Chua, N.H.; Strano, M.S. Chloroplast-selective gene delivery and expression in planta using chitosan-complexed single-walled carbon nanotube carriers. Nat. Nanotechnol. 2019, 14, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan nanoparticle based delivery systems for sustainable agriculture. Int. J. Biol. Macromol. 2015, 77, 36–51. [Google Scholar] [CrossRef]
- Dhandapani, R.K.; Gurusamy, D.; Howell, J.L.; Palli, S.R. Development of CS-TPP-dsRNA nanoparticles to enhance RNAi efficiency in the yellow fever mosquito, Aedes aegypti. Sci. Rep. 2019, 9, 8775. [Google Scholar] [CrossRef]
- Gurusamy, D.; Mogilicherla, K.; Palli, S.R. Chitosan nanoparticles help double-stranded RNA escape from endosomes and improve RNA interference in the fall armyworm, Spodoptera frugiperda. Arch. Insect. Biochem. Physiol. 2020, 104, e21677. [Google Scholar] [CrossRef]
- Lichtenberg, S.S.; Tsyusko, O.V.; Palli, S.R.; Unrine, J.M. Uptake and Bioactivity of Chitosan/Double-Stranded RNA Polyplex Nanoparticles in Caenorhabditis elegans. Environ. Sci. Technol. 2019, 53, 3832–3840. [Google Scholar] [CrossRef]
- Unnamalai, N.; Kang, B.G.; Lee, W.S. Cationic oligopeptide-mediated delivery of dsRNA for post-transcriptional gene silencing in plant cells. FEBS Lett. 2004, 566, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, M.; Yoshizumi, T.; Sudesh, K.; Kodama, Y.; Keiji, N. Double-Stranded DNA introduction into intact plants using peptide–DNA complexes. Plant Biotechnol. J. 2015, 32, 39–45. [Google Scholar] [CrossRef]
- Thagun, C.; Chuah, J.; Numata, K. Targeted Gene Delivery into Various Plastids Mediated by Clustered Cell-Penetrating and Chloroplast-Targeting Peptides. Adv. Sci. 2019, 6, 1902064. [Google Scholar] [CrossRef] [PubMed]
- Avila, L.A.; Chandrasekar, R.; Wilkinson, K.E.; Balthazor, J.; Heerman, M.; Bechard, J.; Brown, S.; Park, Y.; Dhar, S.; Reeck, G.R.; et al. Delivery of lethal dsRNAs in insect diets by branched amphiphilic peptide capsules. J. Control. Release 2018, 273, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.; Wupperfeld, D.; Morales, D.; Reich, N. Shape Matters: Gold Nanoparticle Shape Impacts the Biological Activity of siRNA Delivery. Bioconjug. Chem. 2019, 30, 853–860. [Google Scholar] [CrossRef]
- Kim, H.J.; Takemoto, H.; Yi, Y.; Zheng, M.; Maeda, Y.; Chaya, H.; Hayashi, K.; Mi, P.; Pittella, F.; Christie, R.J.; et al. Precise Engineering of siRNA Delivery Vehicles to Tumors Using Polyion Complexes and Gold Nanoparticles. Acs Nano. 2014, 8, 8979–8991. [Google Scholar] [CrossRef]
- Elhaj Baddar, Z.; Gurusamy, D.; Laisney, J.; Tripathi, P.; Palli, S.R.; Unrine, J.M. Polymer-coated hydroxyapatite nanocarrier for double-stranded RNA delivery. J. Agric. Food Chem. 2020, 68, 6811–6818. [Google Scholar] [CrossRef]
- Buchman, J.T.; Elmer, W.H.; Ma, C.; Landy, K.M.; White, J.C.; Haynes, C.L. Chitosan-Coated Mesoporous Silica Nanoparticle Treatment of Citrullus lanatus (Watermelon): Enhanced Fungal Disease Suppression and Modulated Expression of Stress-Related Genes. ASC Sustain. Chem. Eng. 2019, 7, 19649–19659. [Google Scholar] [CrossRef]
- Chung, T.H.; Wu, S.H.; Yao, M.; Lu, C.W.; Lin, Y.S.; Hung, Y.; Mou, C.Y.; Chen, Y.C.; Huang, D.M. The effect of surface charge on the uptake and biological function of mesoporous silica nanoparticles in 3T3-L1 cells and human mesenchymal stem cells. Biomaterials 2007, 28, 2959–2966. [Google Scholar] [CrossRef]
- Lu, F.; Wu, S.H.; Hung, Y.; Mou, C.Y. Size effect on cell uptake in well-suspended, uniform mesoporous silica nanoparticles. Small 2009, 5, 1408–1413. [Google Scholar] [CrossRef]
- Chang, F.P.; Kuang, L.Y.; Huang, C.A.; Jane, W.N.; Hung, Y.; Hsing, Y.C.; Mou, C.Y. A simple plant gene delivery system using mesoporous silica nanoparticles as carriers. J. Mater. Chem. B 2013, 1, 5279–5287. [Google Scholar] [CrossRef] [PubMed]
- Hajiahmadi, Z.; Shirzadian-Khorramabad, R.; Kazemzad, M.; Sohani, M.M. Enhancement of tomato resistance to Tuta absoluta using a new efficient mesoporous silica nanoparticle-mediated plant transient gene expression approach. Sci. Hortic. 2019, 243, 367–375. [Google Scholar] [CrossRef]
- Sun, D.; Hussain, H.I.; Yi, Z.; Siegele, R.; Cresswell, T.; Kong, L.; Cahill, D.M. Uptake and cellular distribution, in four plant species, of fluorescently labeled mesoporous silica nanoparticles. Plant Cell Rep. 2014, 33, 1389–1402. [Google Scholar] [CrossRef] [PubMed]
- Torney, F.; Trewyn, B.G.; Lin, V.S.; Wang, K. Mesoporous silica nanoparticles deliver DNA and chemicals into plants. Nat. Nanotechnol. 2007, 2, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Hussain, H.I.; Yi, Z.; Rookes, J.E.; Kong, L.; Cahill, D.M. Delivery of Abscisic Acid to Plants Using Glutathione Responsive Mesoporous Silica Nanoparticles. J. Nanosci. Nanotechnol. 2018, 18, 1615–1625. [Google Scholar] [CrossRef]
- Karny, A.; Zinger, A.; Kajal, A.; Shainsky-Roitman, J.; Schroeder, A. Therapeutic nanoparticles penetrate leaves and deliver nutrients to agricultural crops. Sci. Rep. 2018, 8, 7589. [Google Scholar] [CrossRef] [PubMed]
- Wytinck, N.; Manchur, C.L.; Li, V.H.; Whyard, S.; Belmonte, M.F. dsRNA Uptake in Plant Pests and Pathogens: Insights into RNAi-Based Insect and Fungal Control Technology. Plants 2020, 9, 1780. [Google Scholar] [CrossRef] [PubMed]
- Alghuthaymi, M.A.; Ahmad, A.; Khan, Z.; Khan, S.H.; Ahmed, F.K.; Faiz, S.; Nepovimova, E.; Kuča, K.; Abd-Elsalam, K.A. Exosome/Liposome-like Nanoparticles: New Carriers for CRISPR Genome Editing in Plants. Int. J. Mol. Sci. 2021, 22, 7456. [Google Scholar] [CrossRef]
- US-EPA. White Paper on RNAi Technology as a Pesticide: Problem Formulation for Human Health and Ecological Risk Assessment; US-EPA: Washington, DC, USA, 2013. [Google Scholar]
- OECD. Considerations for the Environmental Risk Assessment of the Application of Sprayed or Externally Applied ds-RNA-Based Pesticides; OECD: Paris, France, 2020. [Google Scholar]
- Singh, J.K.D.; Mat Jalaluddin, N.S.; Sanan-Mishra, N.; Harikrishna, J.A. Genetic modification in Malaysia and India: Current regulatory framework and the special case of non-transformative RNAi in agriculture. Plant Cell Rep. 2019, 38, 1449–1463. [Google Scholar] [CrossRef]
- Romeis, J.; Widmer, F. Assessing the Risks of Topically Applied dsRNA-Based Products to Non-target Arthropods. Front. Plant Sci. 2020, 11, 679. [Google Scholar] [CrossRef]
- Perrimon, N.; Mathey-Prevot, B. Matter arising: Off-targets and genome-scale RNAi screens in Drosophila. Fly 2007, 1, 1–5. [Google Scholar] [CrossRef]
- Schmidt, E.E.; Pelz, O.; Buhlmann, S.; Kerr, G.; Horn, T.; Boutros, M. GenomeRNAi: A database for cell-based and in vivo RNAi phenotypes, 2013 update. Nucleic Acids Res. 2013, 41, D1021–D1026. [Google Scholar] [CrossRef]
- Yamada, T.; Morishita, S. Accelerated off-target search algorithm for siRNA. Bioinformatics 2005, 21, 1316–1324. [Google Scholar] [CrossRef]
- Good, R.T.; Varghese, T.; Golz, J.F.; Russell, D.A.; Papanicolaou, A.; Edwards, O.; Robin, C. OfftargetFinder: A web tool for species-specific RNAi design. Bioinformatics 2016, 32, 1232–1234. [Google Scholar] [CrossRef]
- Luck, S.; Kreszies, T.; Strickert, M.; Schweizer, P.; Kuhlmann, M.; Douchkov, D. siRNA-Finder (si-Fi) Software for RNAi-Target Design and Off-Target Prediction. Front. Plant Sci. 2019, 10, 1023. [Google Scholar] [CrossRef]
- Ahmed, F.; Senthil-Kumar, M.; Dai, X.; Ramu, V.S.; Lee, S.; Mysore, K.S.; Zhao, P.X. pssRNAit: A Web Server for Designing Effective and Specific Plant siRNAs with Genome-Wide Off-Target Assessment. Plant Physiol. 2020, 184, 65–81. [Google Scholar] [CrossRef]
- Sciabola, S.; Xi, H.; Cruz, D.; Cao, Q.; Lawrence, C.; Zhang, T.; Rotstein, S.; Hughes, J.D.; Caffrey, D.R.; Stanton, R.V. PFRED: A computational platform for siRNA and antisense oligonucleotides design. PLoS ONE 2021, 16, e0238753. [Google Scholar] [CrossRef] [PubMed]
- Casacuberta, J.M.; Devos, Y.; du Jardin, P.; Ramon, M.; Vaucheret, H.; Nogué, F. Biotechnological uses of RNAi in plants: Risk assessment considerations. Trends Biotechnol. 2015, 33, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Kaksonen, M.; Roux, A. Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2018, 19, 313–326. [Google Scholar] [CrossRef]
- Wytinck, N.; Sullivan, D.S.; Biggar, K.T.; Crisostomo, L.; Pelka, P.; Belmonte, M.F.; Whyard, S. Clathrin mediated endocytosis is involved in the uptake of exogenous double-stranded RNA in the white mold phytopathogen Sclerotinia sclerotiorum. Sci. Rep. 2020, 10, 12773. [Google Scholar] [CrossRef] [PubMed]
- The Environmental Protection Authority of New Zealand. Determining Whether Eukaryotic Cell Lines Treated with Double-Stranded RNA are Genetically Modified Organisms; The Environmental Protection Authority of New Zealand: Wellington, New Zealand, 2018. [Google Scholar]
- De Schutter, K.; Taning, C.N.T.; Daele, L.V.; Van Damme, E.J.M.; Dubruel, P.; Smagghe, G. RNAi-based Biocontrol Products: Market Status, Regulatory Aspects and Risk Assessment. Front. Insect Sci. 2022, 1, 818037. [Google Scholar] [CrossRef]
- Bramlett, M.; Plaetinck, G.; Maienfisch, P. RNA-based Biocontrols—A New Paradigm in Crop Protection. Engineering 2020, 6, 522–527. [Google Scholar] [CrossRef]
- Islam, M.T.; Davis, Z.; Chen, L.; Englaender, J.; Zomorodi, S.; Frank, J.; Bartlett, K.; Somers, E.; Carballo, S.M.; Kester, M.; et al. Minicell-Based fungal RNAi delivery for sustainable crop protection. Microb. Biotechnol. 2021, 14, 1847–1856. [Google Scholar] [CrossRef] [PubMed]
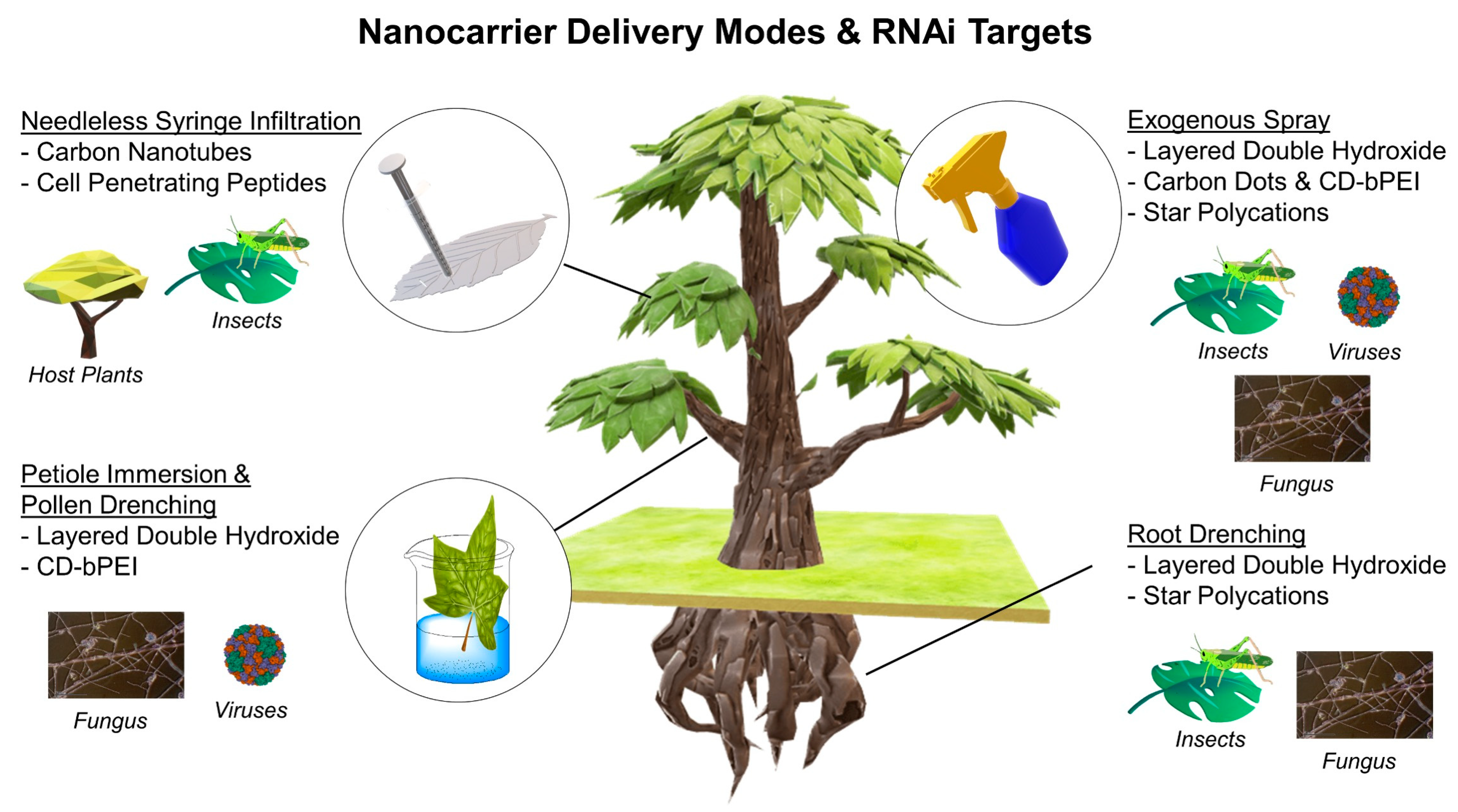


| No | Delivery Vehicle | Synthesis Method a | Surface Modification | Particle Sizes | RNA Size | Ref. |
|---|---|---|---|---|---|---|
| 1 | Layered Double Hydroxide (LDH) | Co-precipitation | Mg3Al–NO3-LDH | 80 to 300 nm | 330 bp and 977 bp dsRNA hairpin | [18] |
| Co-precipitation | Mg3Al–NO3-LDH | 50 to 120 nm | 300 bp dsRNA | [25] | ||
| Co-precipitation | Mg3Al–NO3-LDH | 30 to 90 nm | 30–40 bp dsRNA | [26] | ||
| 2 | Carbon Dot (CD) | Solvothermal | Branched Polyethyleneimine | 2.7 to 3.9 nm | 22 nt siRNA | [27] |
| CD-Branched Polyethylenimine (bPEI) | Hydrothermal | Lipid modification (addition of 1,2-epoxytetradecane) | 220 nm | 250 bp dsRNA | [28] | |
| 3 | Carbon Nanotube (CNT) | HiPco | Not reported | 776 nm (length), 1.567 nm (height) | 19 nt siRNA | [29] |
| 4 | Cell-penetrating peptide (CPP) (i.e., Bp100) | Chemical synthesis | Polycation (KH)9 | 100 to 300 nm | 456 bp dsRNA | [17] |
| 5 | Gold (Au) Nanoparticle | Chemical synthesis | Poly-l-arginine | 60 to 100 nm | 355 bp dsRNA | [30] |
| Chemical synthesis | Polyethyleneimine | 6 to 30 nm | 21 bp siRNA | [31] | ||
| 6 | Chitosan Nanoparticle | Chemical synthesis | Hydrochloric acid (HCl) | 73.25 nm | 40 bp DNA producing 21 nt ssRNA | [32] |
| 7 | Star Polycation (SPc) | Chemical synthesis | hpRNA-SPc | Not reported | 331, 333, 413 and 508 bp hairpin dsRNA | [33] |
| dsRNA-SPc | Not reported | 359 and 489 bp dsRNA | [34] |
| No | Delivery Vehicle | Mode of Delivery | Target/Effect | Exposure | Durability | Efficacy | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Layered Double Hydroxide (LDH) | Topical application on A. thaliana leaves or spray atomizer on V. unguiculata and N. tabacum | Viruses. Silences replicase gene of PMMoV and target gene of CMV | 200 μL samples of 15 μg CMV2b-dsRNA–LDH, sprayed at day 0 only | Partial degradation was observed for naked dsRNAs after 2 min while dsRNA-LDHs remain intact | LDH-only treated plants developed more necrotic lesions compared to dsRNA-LDH at the same time points (Day 1 and 5). LDH-dsRNA offered higher protection against the virus at 20 days post spraying | [18] |
| S. lycopersicum pollen drenching | Virus. Silences target gene of CMV | Concentrations of LDH-50 and dsRNA were 100 and 10 mg/L. Treatment is up to 7 days | Complete degradation for naked dsRNAs after 10 min while dsRNA-LDHs remain intact | Treatment for 3 days with LDH–dsRNA led to a 16.7% decrease in GUS protein activity. No significant changes were observed with naked dsRNAs alone after treatment for 7 days | [25] | ||
| Leave spray, petiole adsorption or root dripping | Fungus. Silences FoCYP51, FoChs1 and FoEF2 genes of Fusarium oxysporum | Leaves spray & petioles adsorption: 300 μg of dsRNAs in 3 mL of ddH2O per plant Root dipping: 3 μg of dsRNA in 3 mL of nano solution per plant | Degradation of naked dsRNA began after 1 min and completed after 10 min. dsRNA bounded LDH is still intact after 1 h of incubation | Disease severity that was observed for leaves spray (10%), petioles adsorption (15%) and dipping roots (35%) | [26] | ||
| 2 | Carbon Dots (CD) | Low-pressure spray | Host Plant. Silencing GFP transgenes and endogenous genes in N. benthamiana and S. ycopersicum | Concentration of siRNA/CD is 12 ng/μL, and is sprayed on plants at Days 1, 7 and 14 | Complete degradation of naked dsRNAs in 15 min. dsRNA-CDs remain intact after a 60-minincubation | A 79% reduction was observed in the phenotypic tissues at Day 5 after treatment. Bleaching phenotype persisted up to 20 days after treatment | [27] |
| 3 | CD-Branched Polyethylenimine (CD- bPEI) | Leave spray and petiole immersion | Virus. Silences RNA polymerase and coat protein genes of Grapevine leafroll associated virus-3 (GLRaV-3) | A 0.00092 g/mL and translatedinto a 32x dilution factor | Degradation of naked dsRNAs began after 2 h while dsRNA-CDs-bPEI remain intact | Virus titre decreased over three weeks after a single-dose administration, but multiple doses are needed to improve fruit quality | [28] |
| 4 | Carbon Nanotube (CNT) | Needleless syringe infiltration on leaves of N. benthamiana | Host Plant. Silences mGFP5 transgenes in leaves | Concentrations: siRNA (100 nM) SWNT (2 mg/liter) | Degradation (94%) of naked dsRNAs after 6 h. dsRNA-SNWT degradation (30%) after 6 h | Gene silencing efficiency was up to 95% within 1 day after infiltration | [29] |
| 5 | Cell-penetrating peptides (CPP) (i.e., Bp100) | Needleless syringe infiltration on A. thaliana leaves | Insect. Silence GFP and firefly luciferase genes | 100 μL of the dsRNA-peptide, incubated for up to 36 h | Naked dsRNAs were slightly degraded after 12 h while the dsRNA-peptides remain intact | No silencing effects was observed for naked dsRNAs while genetic down-regulation was observed for dsRNA-peptides within 12 h and up to 36 h | [17] |
| 6 | Gold Nanoparticle | Not tested on plants (insect cell assay only) | Insect. Silences Luciferase gene in Spodopteria frugiperda | dsRNA (50 μg/mL) | dsRNA-Au showed better endosomal escape compared to dsRNA alone. | Up to 58% reduction of the luciferase activity for dsRNA-Au compared to dsRNA alone | [30] |
| Needleless syringe infiltration on mGFP5 N. benthamiana leaves without needle | Host Plant. Silences mGFP5 transgenes in N. benthamiana leaves | 100 ng of siRNA | Complete degradation was observed after 30 min of incubation for naked dsRNAs while dsRNA-Gold NP remain intact | No upregulation of NbrbohB suggests low to no stress to plant tissues | [31] | ||
| 7 | Chitosan Nanoparticle | Not tested | Virus. Silences coat protein gene of Tomato mosaicvirus | 200 μg/mL of the dsRNA-chitosan. | Not reported | dsRNA-chitosan has low toxicity with no inhibitory effects on root development | [32] |
| 8 | Star Polycation (SPc) | Spray on oilseed rapes leaves infested with Myzus persicae using pneumatic water sprayer | Insect. Silences essential genes. ATP-A: 413 bp, LOC111039523; ATP-d: 383 bp, LOC111041166; ATP-G: 301 bp, LOC111040044 of M. persicae | 0.2 μL dsRNA/SPc formulation sprayed at Day 0 | Complete degradation was observed for naked dsRNAs in 12.5% of aphid hemolymph after 1.5 h while dsRNA-SPc remain intact. | Control efficacy was 61% on Day 3 after treatment with SPc-dsRNA and maintained at 50% until Day 6. | [33] |
| Root drenching | Insect. Silencing M. persicae vestigial (vg) & Ultrabithorax (Ubx) genes involved in wing formation. | Exposing radish seedling to 200 μL dsRNA/SPc formulation at Day 0 prior to M. persica transplantation | Not reported | About 40% of M. persica developed effective wings when both dsRNA-genes were used | [34] |
| Application Number | Priority Date | Legal Status | Assignee | Invention Details |
|---|---|---|---|---|
| US15/579,120 | 03.06.2015 | Granted in US (2020) | Monsanto Technology LLC | Composition: Polynucleotide, particulate and osmolyte Delivery: Abrading a surface of a plant with a particulate, followed by applying an RNA onto the plant surface |
| US15/579,125 | 02.06.2015 | Granted in US (2021), EP (2021) | Monsanto Technology LLC | Composition: Polynucleotide, at least one lipase enzyme, one or more osmolytes, surfactants, abrasives or any combination Delivery: Applying lipase enzyme, osmolytes, and surfactants, followed by an RNA onto the plant surface |
| US16/062,008 | 14.12.2015 | Granted in US (2021) EP (2021) | Monsanto Technology LLC | Composition: Polynucleotide targeting gene of flea beetle and cross-linked cationic polysaccharide Delivery: Applying onto a seed, plant surface or foliar spray |
| US61/748,095 | 01.01.2013 | Granted in AU (2019), CN (2019), US (2018) | AB Seeds Ltd./Monsanto Technology LLC | Delivery: Soaking ungerminated seed with a solution comprising a concentration of between 0.005 and 1.5 pg/pL of the dsRNA molecule, followed by drying the seed |
| US16/583,863 | 26.09.2018 | Granted in US (2021) | Greenlight Biosciences Inc | Composition: dsRNA targeting Leptinotarsa decemlineata Inhibitor of Apoptosis (IAP) gene Delivery: Spray, fog, seed treatment, drench, drip irrigation, in furrow, insect diet, or bait |
| US15/752,274 | 13.08.2015 | Pending | Forrest Innovations Ltd. | Composition: Polynucleotide and at least one cell wall degrading enzyme, a nucleic acid condensing agent, a transfection reagent, a surfactant, and a cuticle penetrating agent |
| US14/381,045 | 06.03.2014 | Granted in JP (2020) US (2020) | RIKEN | Composition: Polynucleotide and a carrier peptide containing a cell-penetrating sequence and a penetrating polycationic sequence |
| Applicationnumber | Priority Date | Legal Status | Assignee | Invention Details |
| US15/106,548 | 20.12.2013 | Granted in AU (2018), CA (2021), EP (2019), ES (2020), US (2020) | University of Queensland | Composition: dsRNA and layered double hydroxide with a charge ratio is 2:1 to 1:20 Delivery: Spray |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mat Jalaluddin, N.S.; Asem, M.; Harikrishna, J.A.; Ahmad Fuaad, A.A.H. Recent Progress on Nanocarriers for Topical-Mediated RNAi Strategies for Crop Protection—A Review. Molecules 2023, 28, 2700. https://doi.org/10.3390/molecules28062700
Mat Jalaluddin NS, Asem M, Harikrishna JA, Ahmad Fuaad AAH. Recent Progress on Nanocarriers for Topical-Mediated RNAi Strategies for Crop Protection—A Review. Molecules. 2023; 28(6):2700. https://doi.org/10.3390/molecules28062700
Chicago/Turabian StyleMat Jalaluddin, Nurzatil Sharleeza, Maimunah Asem, Jennifer Ann Harikrishna, and Abdullah Al Hadi Ahmad Fuaad. 2023. "Recent Progress on Nanocarriers for Topical-Mediated RNAi Strategies for Crop Protection—A Review" Molecules 28, no. 6: 2700. https://doi.org/10.3390/molecules28062700
APA StyleMat Jalaluddin, N. S., Asem, M., Harikrishna, J. A., & Ahmad Fuaad, A. A. H. (2023). Recent Progress on Nanocarriers for Topical-Mediated RNAi Strategies for Crop Protection—A Review. Molecules, 28(6), 2700. https://doi.org/10.3390/molecules28062700






