Development of pH-Responsive N-benzyl-N-O-succinyl Chitosan Micelles Loaded with a Curcumin Analog (Cyqualone) for Treatment of Colon Cancer
Abstract
1. Introduction
2. Results and Discussion
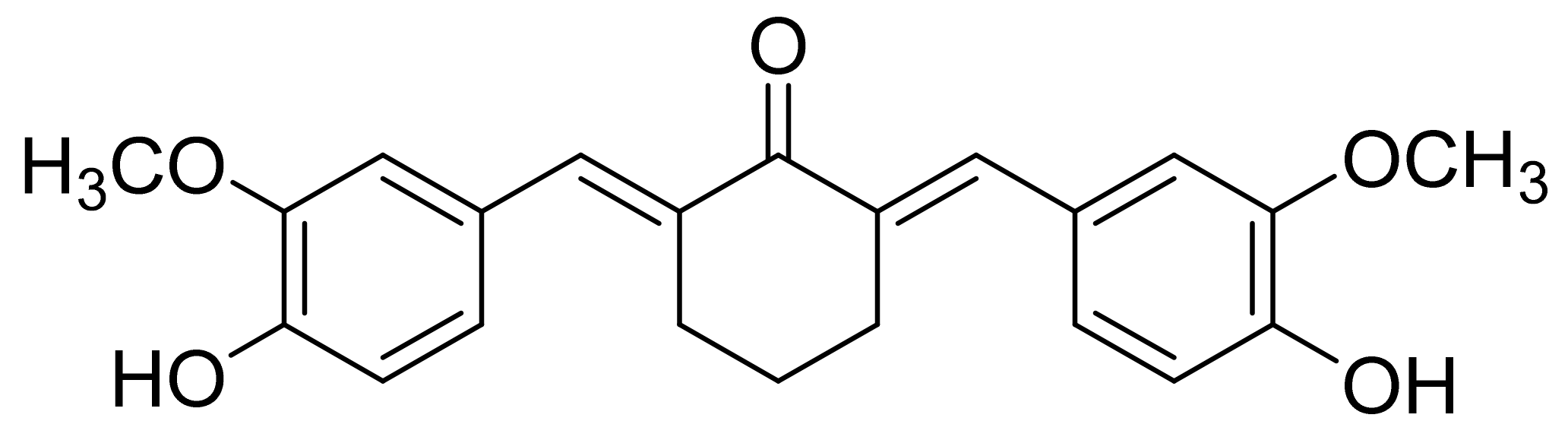
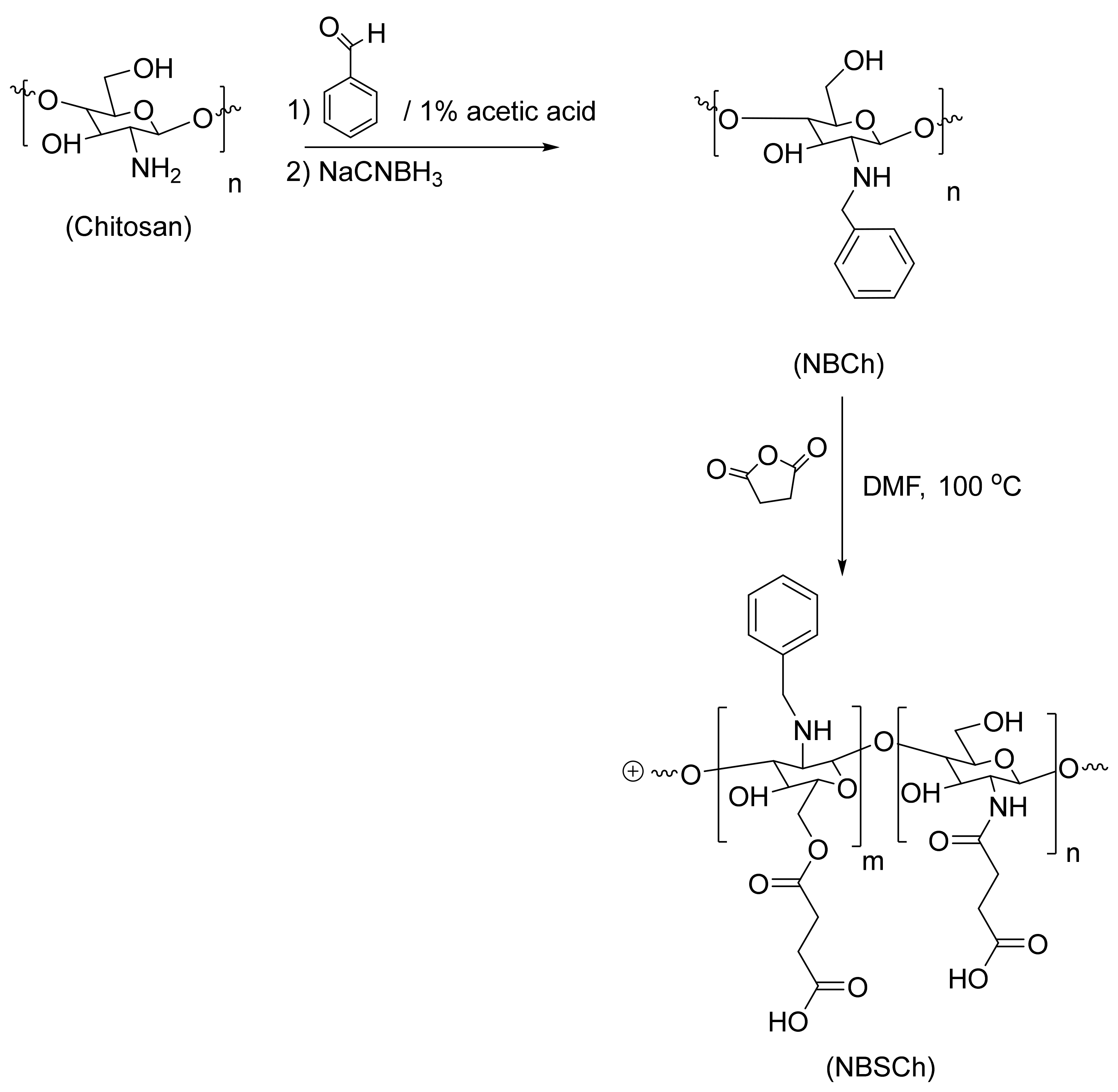
2.1. Synthesis and Characterization of N-benzyl-N,O-succinyl Chitosan (NBSCh)
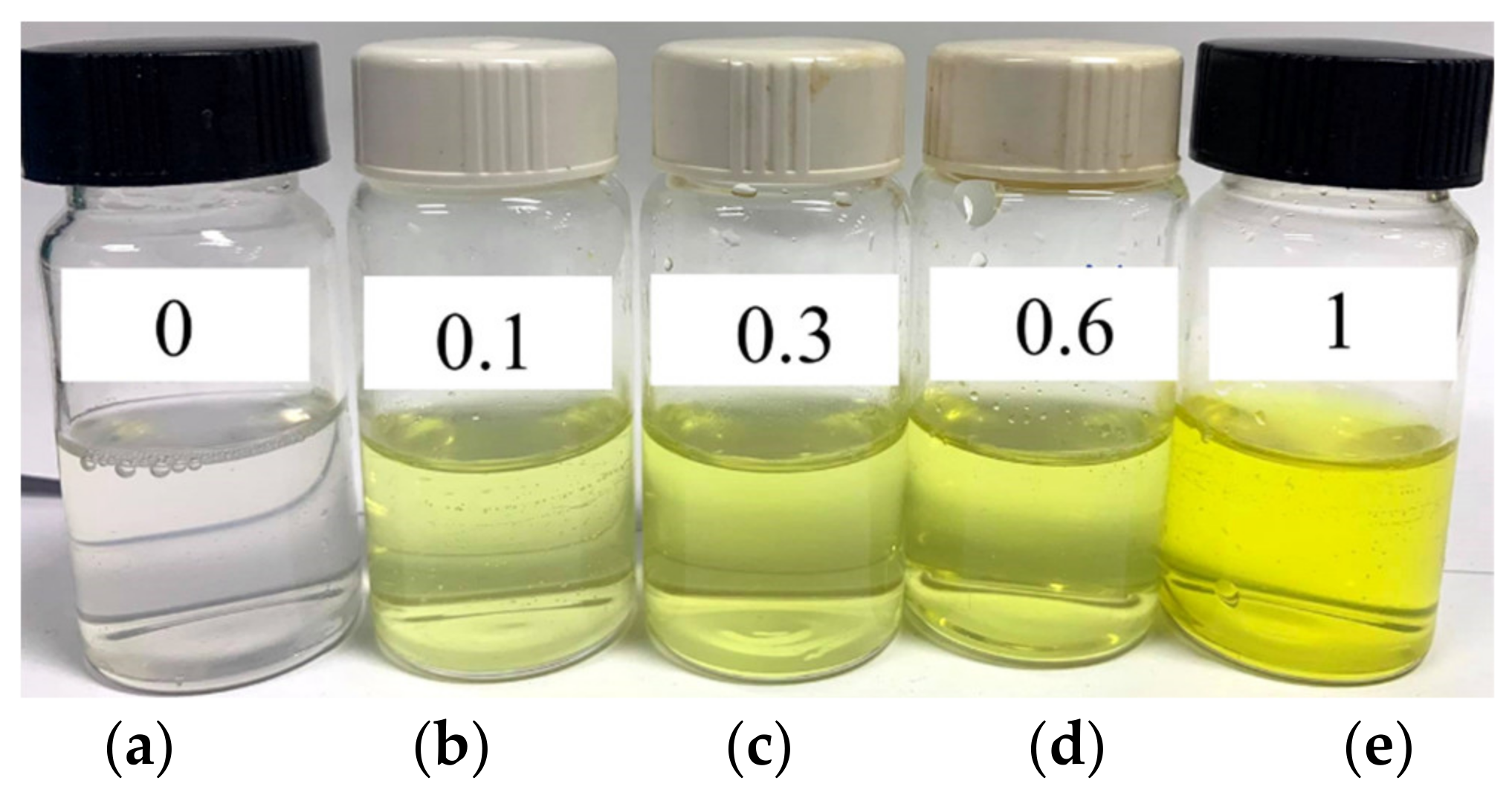
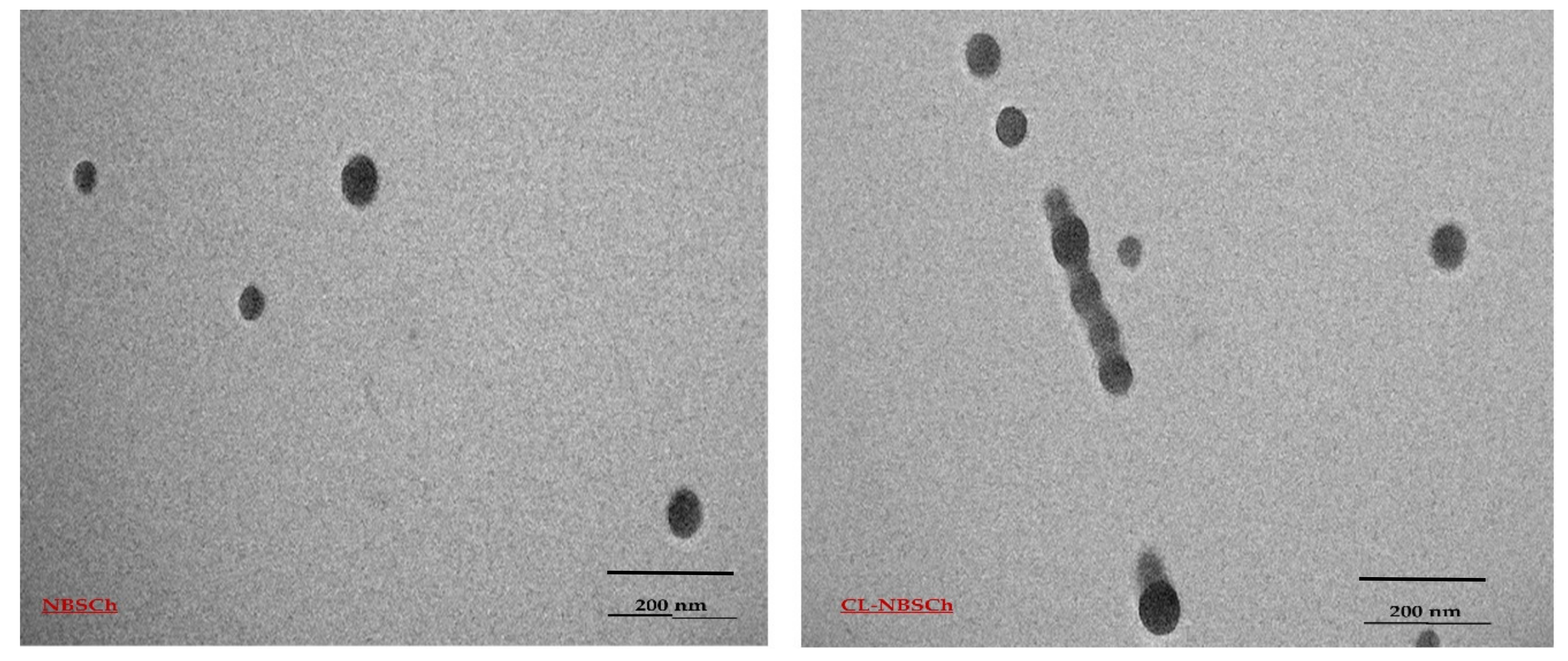
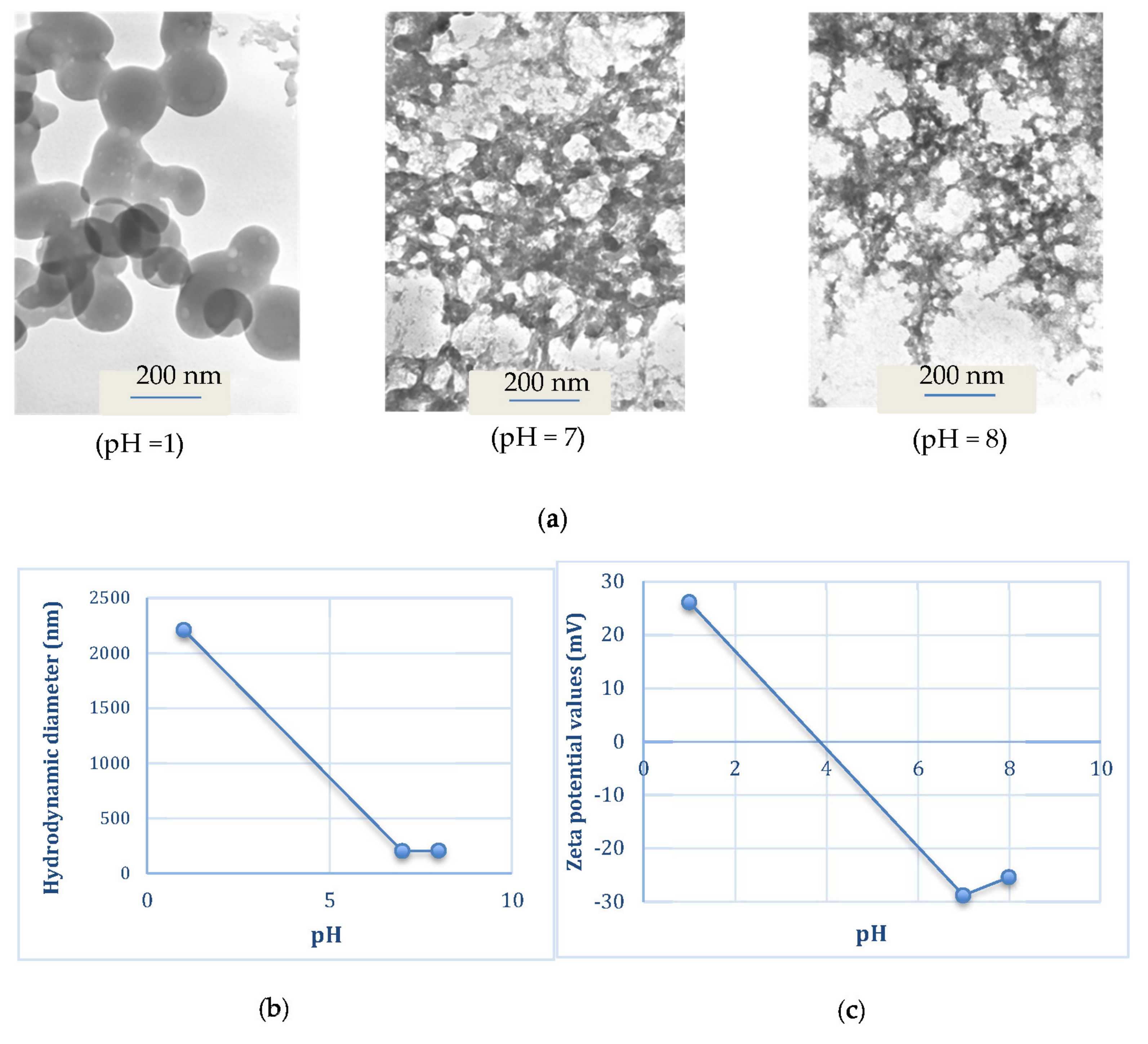
2.2. Preparation and Characterization of Micelles
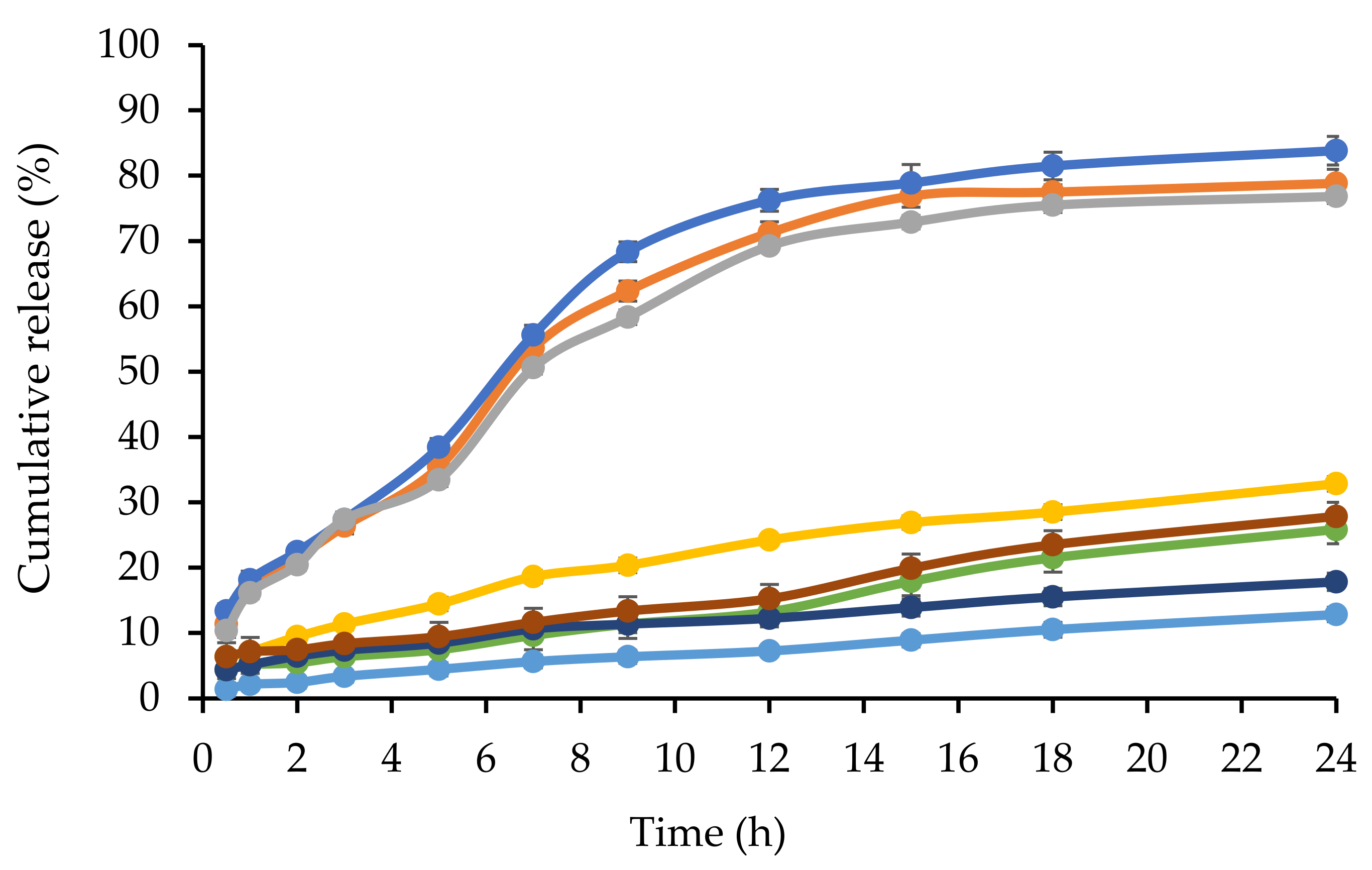
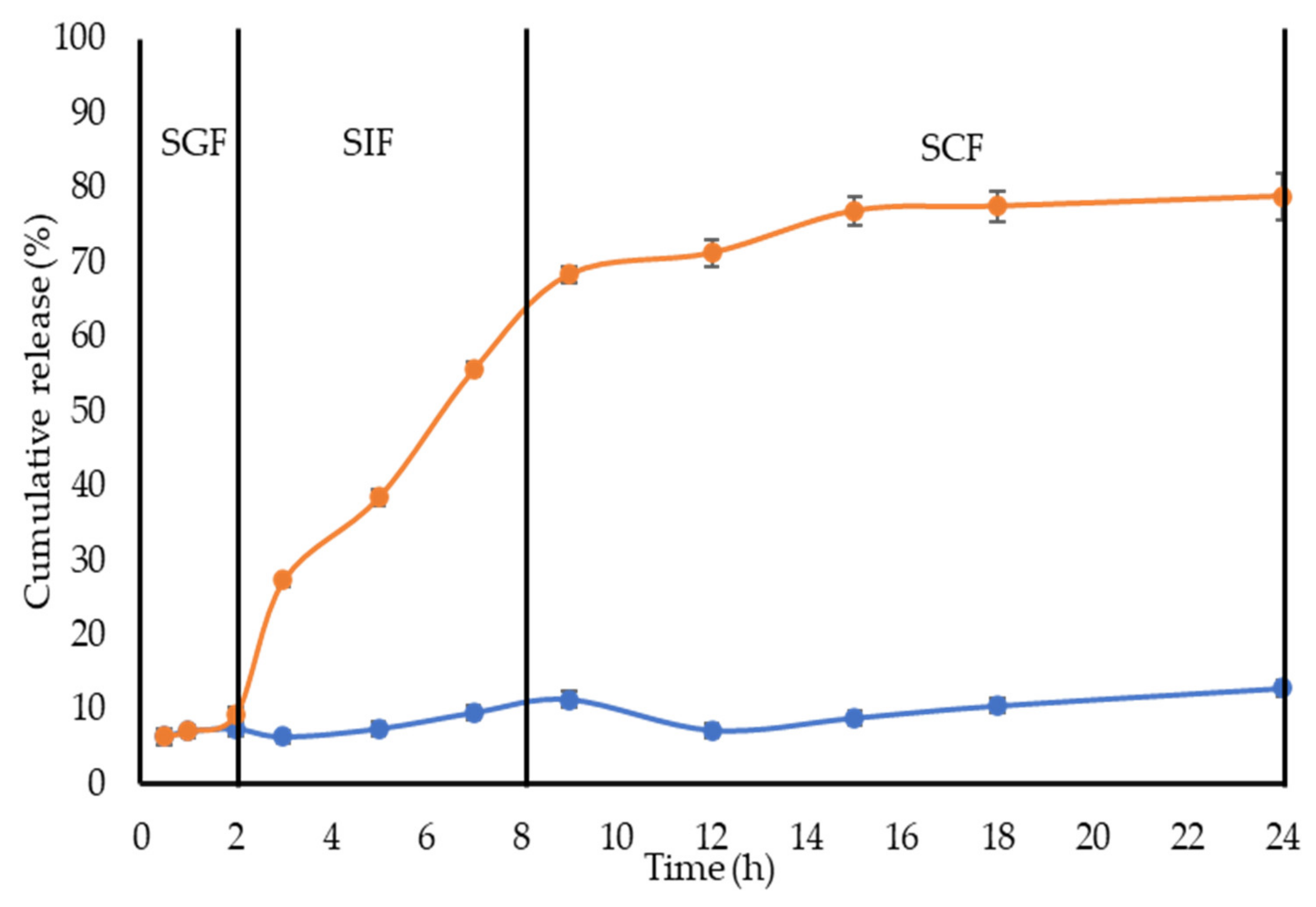
2.3. In Vitro Release of CL from NBSCh Micelles
2.4. Effect of Temperature on the Stability of CL-Loaded NBSCh Micelle Powders
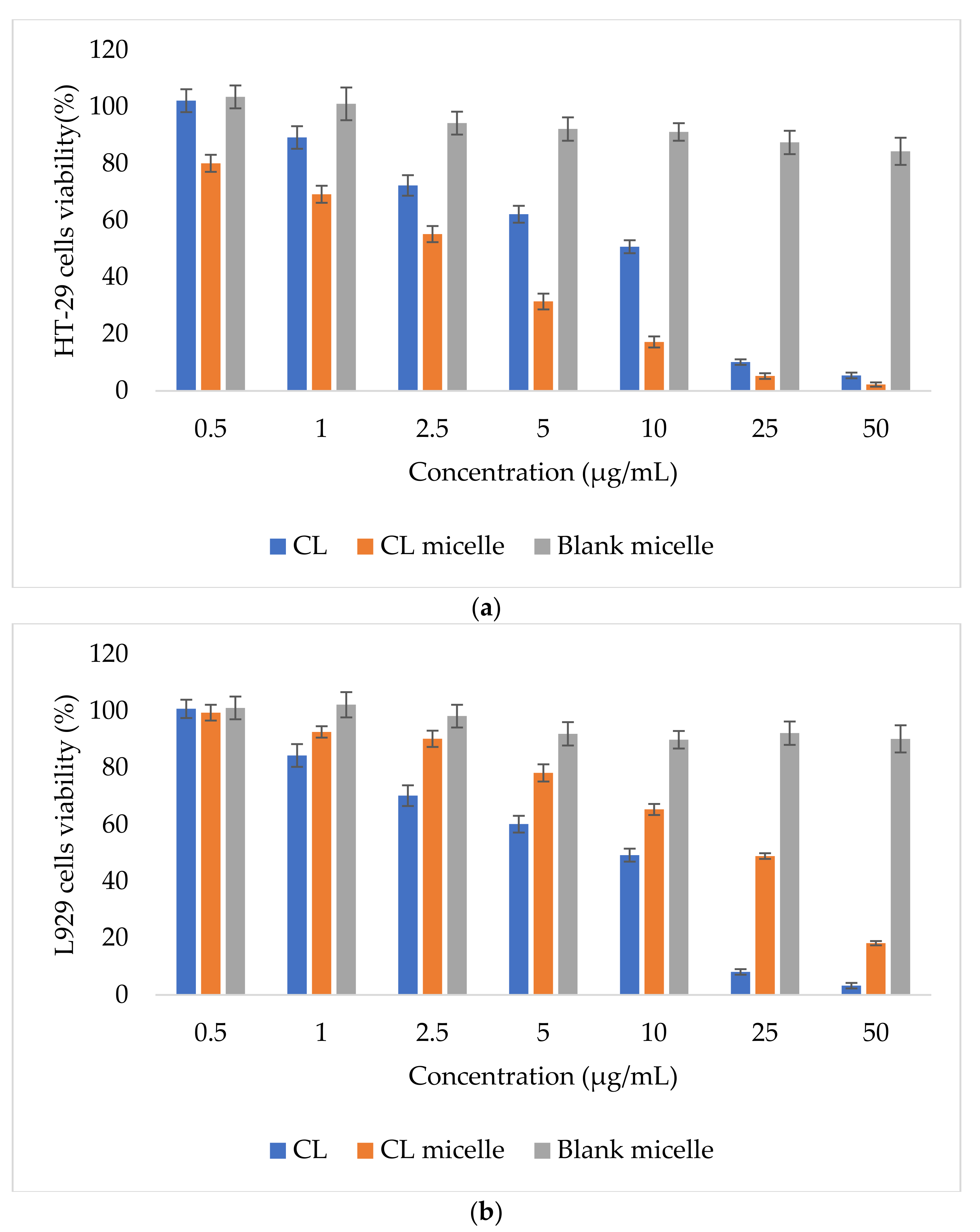
2.5. CL-Loaded NBSCh Micelles Were Selective and Cytotoxic to HT-29 Colon Cancer Cells
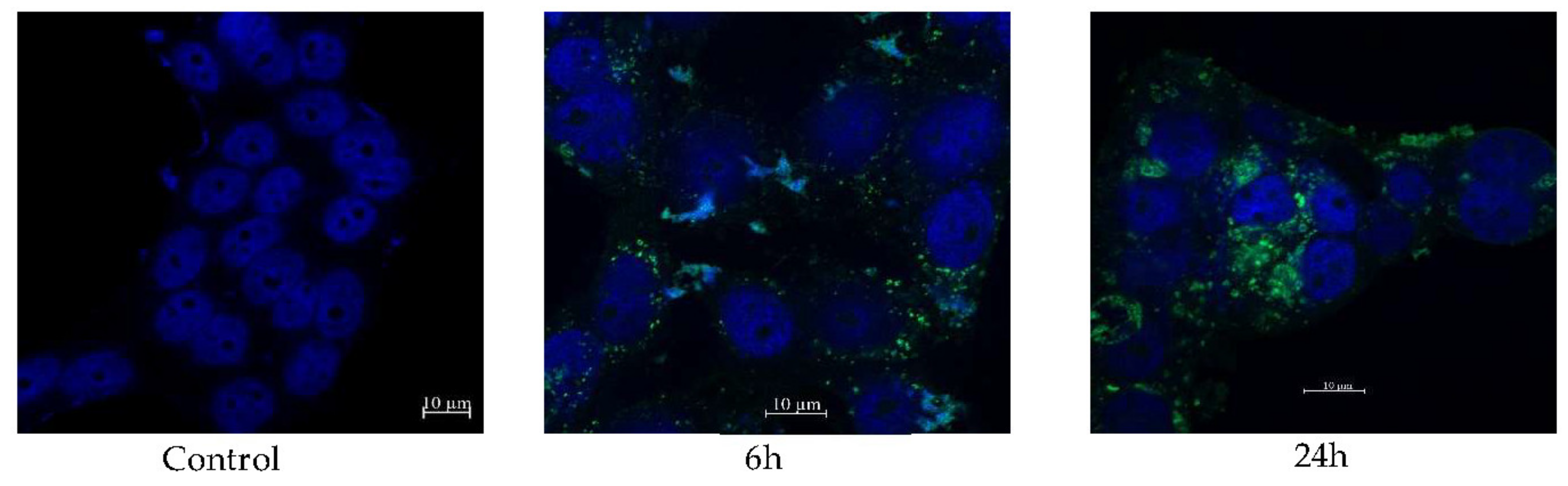
2.6. Cellular Uptake of CL-Loaded NBSCh Micelles
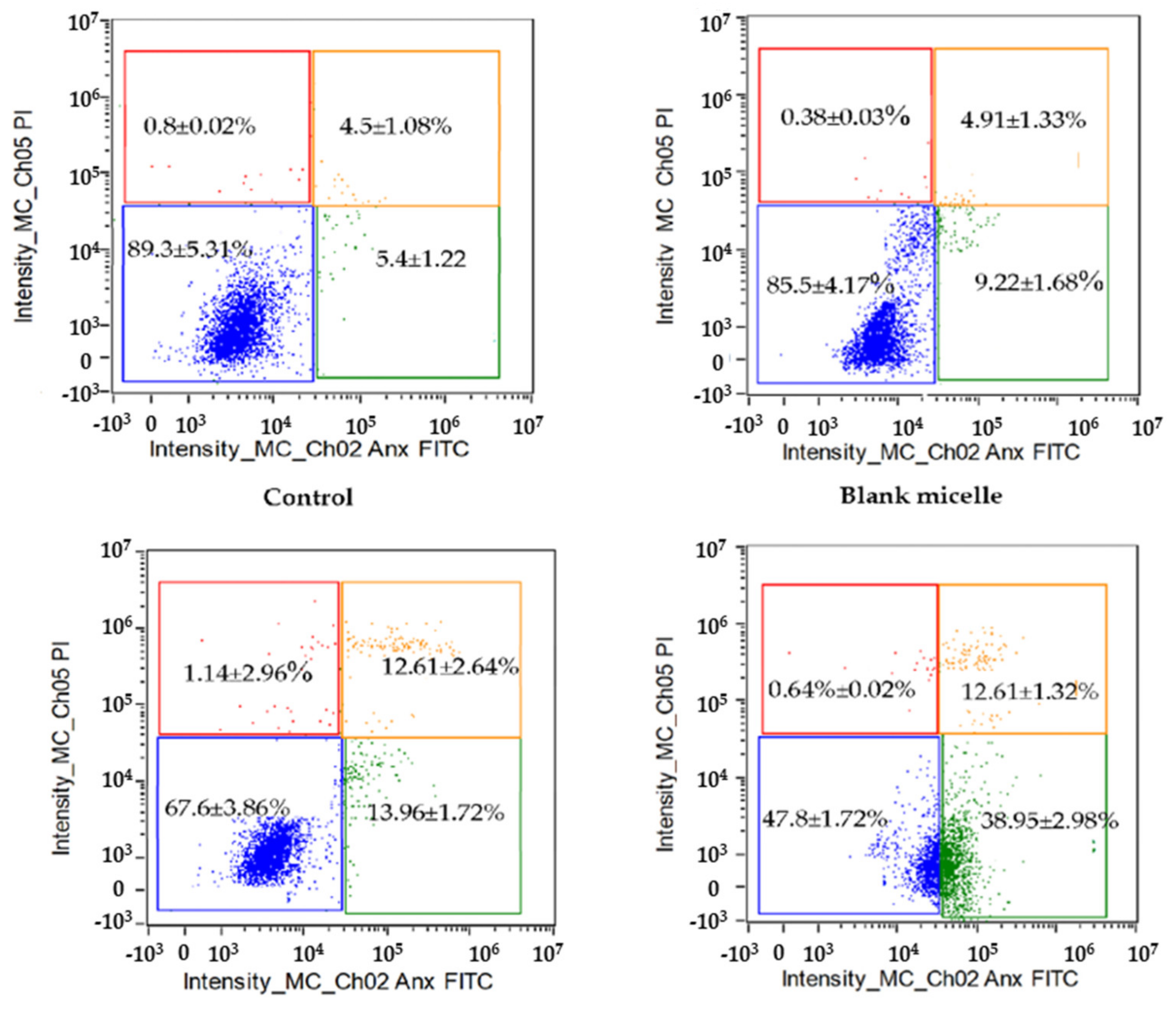
2.7. CL-Loaded NBSCh Micelles Promoted Early Apoptosis in HT-29 Cancer Cells
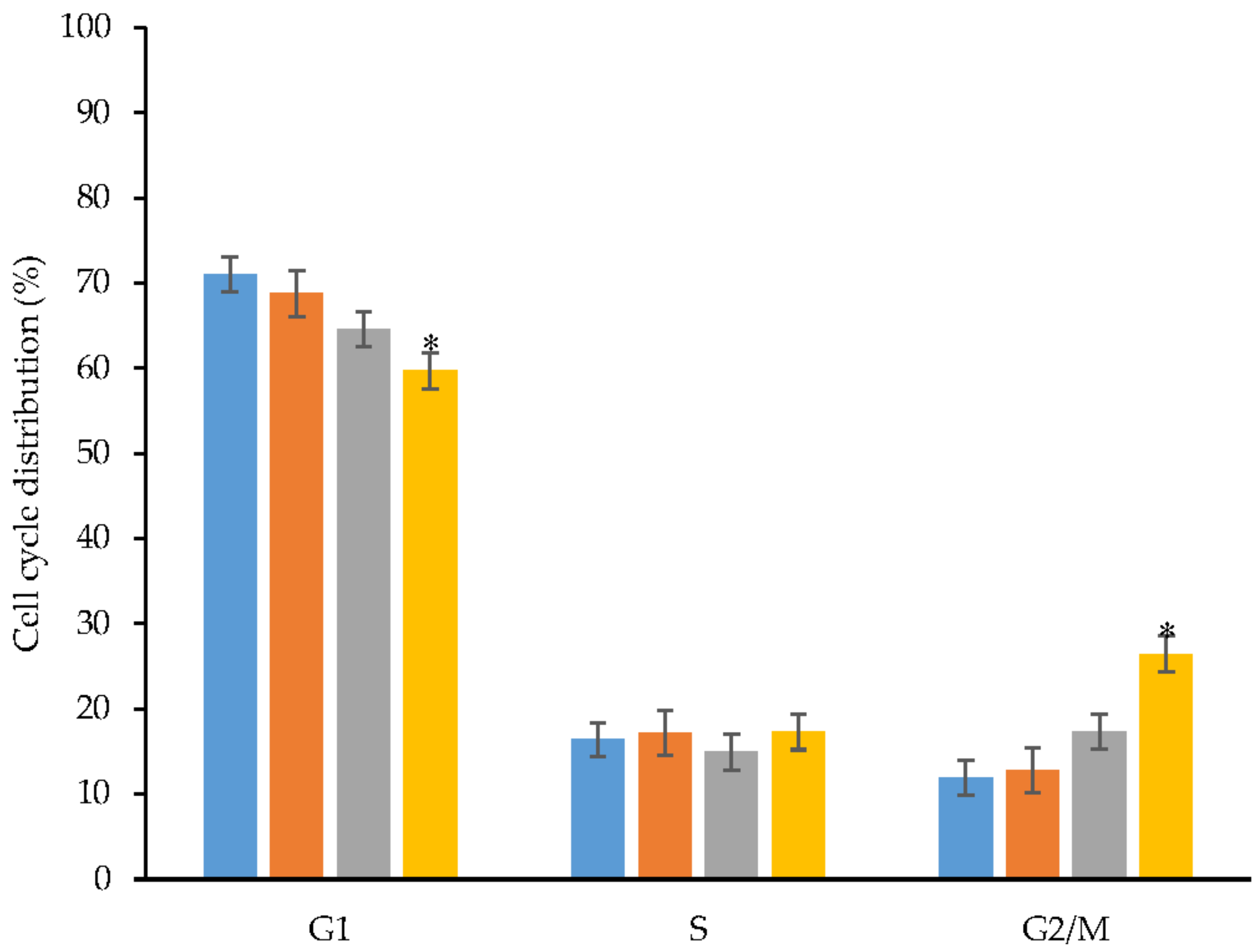
2.8. Cell Cycle for Antiproliferative Effect of CL and CL-Loaded Micelles on HT-29 Human Colon Cancer Cells
3. Materials and Methods
3.1. Chemicals
3.2. Reagents and Cell Lines for Cytotoxicity Studies
3.3. Synthesis of N-benzyl-N,O-succinyl Chitosan (NBSCh)
3.4. Preparation of Blank NBSCh Micelles and CL-Loaded NBSCh Micelles
3.5. Determination of Critical Micelle Concentration (CMC)
3.6. Physicochemical Characterization of CL-Loaded NBSCh Micelles
3.6.1. Size and Zeta Potential Measurements
3.6.2. Micellar Morphology Determination
3.7. The Entrapment Efficiency (EE) and Loading Capacity (LC) of CL in NBSCh Micelles
3.8. In Vitro Release of CL from NBSCh Micelles
3.9. Determination of Stability of Freeze-Dried CL-Loaded NBSCh Micelle
3.10. Anticancer Activity of CL-Loaded NBSCh Micelle
3.10.1. Determination of In Vitro Cytotoxicity of CL-Loaded NBSCh Micelles
3.10.2. Determination of Cellular Uptake of NBSCh Micelles
3.10.3. Cell Apoptosis Study
3.10.4. Cell Cycle Analysis for Antiproliferative Effect of CL and CL-Loaded Micelles
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Gao, X.; Zheng, F.; Guo, G.; Liu, X.; Fan, R.; Qian, Z.-Y.; Huang, N.; Wei, Y.-Q. Improving the anti-colon cancer activity of curcumin with biodegradable nano-micelles. J. Mater. Chem. B 2013, 1, 5778–5790. [Google Scholar] [CrossRef] [PubMed]
- Krasteva, N.; Georgieva, M. Promising Therapeutic Strategies for Colorectal Cancer Treatment Based on Nanomaterials. Pharmaceutics 2022, 14, 1213. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Xie, H.; Shen, W.; Shao, L.; Zeng, L.; Huang, X.; Zhu, Q.; Zhai, X.; Li, K.; Qiu, Z.; et al. The Synergism of Natural Compounds and Conventional Therapeutics against Colorectal Cancer Progression and Metastasis. Front. Biosci.-Landmark 2022, 27, 263. [Google Scholar] [CrossRef]
- DeRidder, L.; Rubinson, D.A.; Langer, R.; Traverso, G. The past, present, and future of chemotherapy with a focus on individualization of drug dosing. J. Control. Release 2022, 352, 840–860. [Google Scholar] [CrossRef] [PubMed]
- Ranjbari, J.; Mokhtarzadeh, A.; Alibakhshi, A.; Tabarzad, M.; Hejazi, M.; Ramezani, M. Anti-cancer drug delivery using carbohydrate-based polymers. Curr. Pharm. Des. 2017, 23, 6019–6032. [Google Scholar] [CrossRef]
- Valerii, M.C.; Benaglia, M.; Caggiano, C.; Papi, A.; Strillacci, A.; Lazzarini, G.; Campieri, M.; Gionchetti, P.; Rizzello, F.; Spisni, E. Drug delivery by polymeric micelles: An in vitro and in vivo study to deliver lipophilic substances to colonocytes and selectively target inflamed colon. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 675–685. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Large, D.E.; Soucy, J.R.; Hebert, J.; Auguste, D.T. Advances in Receptor-Mediated, Tumor-Targeted Drug Delivery. Adv. Ther. 2019, 2, 1800091. [Google Scholar] [CrossRef]
- Mishra, A.P.; Chandra, S.; Tiwari, R.; Srivastava, A.; Tiwari, G. Therapeutic potential of prodrugs towards targeted drug delivery. Open Med. Chem. J. 2018, 12, 111. [Google Scholar] [CrossRef]
- Muhamad, N.; Plengsuriyakarn, T.; Na-Bangchang, K. Application of active targeting nanoparticle delivery system for chemotherapeutic drugs and traditional/herbal medicines in cancer therapy: A systematic review. Int. J. Nanomed. 2018, 13, 3921. [Google Scholar] [CrossRef]
- Vong, L.B.; Nagasaki, Y. (Eds.) Redox Polymeric Nanoparticle as an Effective Oral Nanotherapeutics for Inflammatory Bowel Disease and Cancer. In Proceedings of the International Conference on the Development of Biomedical Engineering in Vietnam, Ho Chi Minh, Vietnam, 27–29 June 2018; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Barua, S.; Mitragotri, S. Challenges associated with Penetration of Nanoparticles across Cell and Tissue Barriers: A Review of Current Status and Future Prospects. Nano Today 2014, 9, 223–243. [Google Scholar] [CrossRef]
- Lai, S.K.; Wang, Y.-Y.; Hanes, J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 2009, 61, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Le Garrec, D.; Ranger, M.; Leroux, J.-C. Micelles in anticancer drug delivery. Am. J. Drug Deliv. 2004, 2, 15–42. [Google Scholar] [CrossRef]
- Lachowicz, D.; Karabasz, A.; Bzowska, M.; Szuwarzyński, M.; Karewicz, A.; Nowakowska, M. Blood-compatible, stable micelles of sodium alginate–curcumin bioconjugate for anti-cancer applications. Eur. Polym. J. 2019, 113, 208–219. [Google Scholar] [CrossRef]
- Uchegbu, I.F.; Carlos, M.; McKay, C.; Hou, X.; Schätzlein, A.G. Chitosan amphiphiles provide new drug delivery opportunities. Polym. Int. 2014, 63, 1145–1153. [Google Scholar] [CrossRef]
- Ravi, H.; Kurrey, N.; Manabe, Y.; Sugawara, T.; Baskaran, V. Polymeric chitosan-glycolipid nanocarriers for an effective delivery of marine carotenoid fucoxanthin for induction of apoptosis in human colon cancer cells (Caco-2 cells). Mater. Sci. Eng. C 2018, 91, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Sajomsang, W.; Gonil, P.; Saesoo, S.; Ruktanonchai, U.R.; Srinuanchai, W.; Puttipipatkhachorn, S. Synthesis and anticervical cancer activity of novel pH responsive micelles for oral curcumin delivery. Int. J. Pharm. 2014, 477, 261–272. [Google Scholar] [CrossRef]
- Meng, B.; Li, J.; Cao, H. Antioxidant and anti-inflammatory activities of curcumin on diabetes mellitus and its complications. Curr. Pharm. Des. 2013, 19, 2101–2113. [Google Scholar] [PubMed]
- Noorafshan, A.; Ashkani-Esfahani, S. A review of therapeutic effects of curcumin. Curr. Pharm. Des. 2013, 19, 2032–2046. [Google Scholar]
- He, G.; Feng, C.; Vinothkumar, R.; Chen, W.; Dai, X.; Chen, X.; Ye, Q.; Qiu, C.; Zhou, H.; Wang, Y.; et al. Curcumin analog EF24 induces apoptosis via ROS-dependent mitochondrial dysfunction in human colorectal cancer cells. Cancer Chemother. Pharmacol. 2016, 78, 1151–1161. [Google Scholar] [CrossRef]
- Mapoung, S.; Pitchakarn, P.; Yodkeeree, S.; Ovatlarnporn, C.; Sakorn, N.; Limtrakul, P. Chemosensitizing effects of synthetic curcumin analogs on human multi-drug resistance leukemic cells. Chem.-Biol. Interact. 2016, 244, 140–148. [Google Scholar] [CrossRef]
- Mapoung, S.; Suzuki, S.; Fuji, S.; Naiki-Ito, A.; Kato, H.; Yodkeeree, S.; Ovatlarnporn, C.; Takahashi, S.; Dejkriengkraikul, P.L. Cyclohexanone curcumin analogs inhibit the progression of castration-resistant prostate cancer in vitro and in vivo. Cancer Sci. 2019, 110, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Markaverich, B.M.; Schauweker, T.H.; Gregory, R.R.; Varma, M.; Kittrell, F.S.; Medina, D.; Varma, R.S. Nuclear type II sites and malignant cell proliferation: Inhibition by 2,6-bis-benzylidenecyclohexanones. Cancer Res. 1992, 52, 2482–2488. [Google Scholar]
- Markaverich, B.M.; Vijjeswarapu, M. Multiple sites of type II site ligand (luteolin and BMHPC) regulation of gene expression in PC-3 cells. Int. J. Biomed. Sci. 2012, 8, 219. [Google Scholar] [PubMed]
- Revalde, J.L.; Li, Y.; Wijeratne, T.S.; Bugde, P.; Hawkins, B.C.; Rosengren, R.J.; Paxton, J.W. Curcumin and its cyclohexanone analogue inhibited human Equilibrative nucleoside transporter 1 (ENT1) in pancreatic cancer cells. Eur. J. Pharmacol. 2017, 803, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Yadav, B.; Taurin, S.; Rosengren, R.J.; Schumacher, M.; Diederich, M.; Somers-Edgar, T.J.; Larsen, L. Synthesis and cytotoxic potential of heterocyclic cyclohexanone analogues of curcumin. Bioorganic Med. Chem. 2010, 18, 6701–6707. [Google Scholar] [CrossRef]
- Ban, C.; Jo, M.; Park, Y.H.; Kim, J.H.; Han, J.Y.; Lee, K.W.; Kweon, D.-H.; Choi, Y.J. Enhancing the oral bioavailability of curcumin using solid lipid nanoparticles. Food Chem. 2020, 302, 125328. [Google Scholar] [CrossRef]
- Eskandari, Z.; Bahadori, F.; Altikatoğlu Yapaöz, M.; Yenign, V.; Koçyiğit, A.; Onyuksel, H. NF-kappa B inhibition activity of curcumin-loaded sterically stabilized micelles and its up-regulator effect on enhancement of cytotoxicity of a new nano-pirarubicin formulation in the treatment of breast cancer. Rec. Nat. Prod. 2019, 13, 390–404. [Google Scholar] [CrossRef]
- Momekova, D.; Ugrinova, I.; Slavkova, M.; Momekov, G.; Grancharov, G.; Gancheva, V.; Petrov, P.D. Superior proapoptotic activity of curcumin-loaded mixed block copolymer micelles with mitochondrial targeting properties. Biomater. Sci. 2018, 6, 3309–3317. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Cao, S.; Li, Y.; Xiao, P.; Huang, Z.; Li, H.; Ma, Y. Internalization and subcellular transport mechanisms of different curcumin loaded nanocarriers across Caco-2 cell model. J. Drug Deliv. Sci. Technol. 2019, 52, 660–669. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Sun, C.-Y.; Adu-Frimpong, M.; Yu, J.-N.; Xu, X.-N. Glutathione-sensitive PEGylated curcumin prodrug nanomicelles: Preparation, characterization, cellular uptake and bioavailability evaluation. Int. J. Pharm. 2019, 555, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Rauf Khan, A.; Fu, M.; Zhai, Y.; Ji, J.; Bobrovskaya, L.; Zhai, G. Advances in curcumin-loaded nanopreparations: Improving bioavailability and overcoming inherent drawbacks. J. Drug Target. 2019, 27, 917–931. [Google Scholar] [CrossRef]
- Zheng, B.; Zhang, X.; Peng, S.; McClements, D.J. Impact of curcumin delivery system format on bioaccessibility: Nanocrystals, nanoemulsion droplets, and natural oil bodies. Food Funct. 2019, 10, 4339–4349. [Google Scholar] [CrossRef]
- Woraphatphadung, T.; Sajomsang, W.; Rojanarata, T.; Akkaramongkolporn, P.; Ngawhirunpat, T.; Opanasopit, P. Preparation and characterization of N-benzyl-N, O-succinyl chitosan polymeric micelles for solubilization of poorly soluble non-steroidal anti-inflammatory drugs. Trop. J. Pharm. Res. 2017, 16, 2349–2357. [Google Scholar] [CrossRef]
- Woraphatphadung, T.; Sajomsang, W.; Rojanarata, T.; Ngawhirunpat, T.; Tonglairoum, P.; Opanasopit, P. Development of Chitosan-Based pH-Sensitive Polymeric Micelles Containing Curcumin for Colon-Targeted Drug Delivery. AAPS PharmSciTech 2018, 19, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Popovici, C.; Popa, M.; Sunel, V.; Atanase, L.I.; Ichim, D.L. Drug delivery systems based on Pluronic micelles with antimicrobial activity. Polymers 2022, 14, 3007. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Kang, Y.; Hollett, G.; Chen, X.; Zhao, W.; Gu, Z.; Wu, J. Polymeric nanoparticles for colon cancer therapy: Overview and perspectives. J. Mater. Chem. B 2016, 4, 7779–7792. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, H.; Liu, Z.; Chen, B. Effects of major parameters of nanoparticles on their physical and chemical properties and recent application of nanodrug delivery system in targeted chemotherapy. Int. J. Nanomed. 2017, 12, 8483. [Google Scholar] [CrossRef]
- Devarajan, P.V.; Jain, S. Targeted Drug Delivery: Concepts and Design; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Cheng, W.; Gu, L.; Ren, W.; Liu, Y. Stimuli-responsive polymers for anti-cancer drug delivery. Mater. Sci. Eng. C 2014, 45, 600–608. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered nanoparticles for drug delivery in cancer therapy. Nanomater. Neoplasms 2021, 53, 31–142. [Google Scholar]
- Xu, G.; Shi, H.; Ren, L.; Gou, H.; Gong, D.; Gao, X.; Huang, N. Enhancing the anti-colon cancer activity of quercetin by self-assembled micelles. Int. J. Nanomed. 2015, 10, 2051. [Google Scholar]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Bhuket, P.R.N.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan/alginate nanoparticles as a promising approach for oral delivery of curcumin diglutaric acid for cancer treatment. Mater. Sci. Eng. C 2018, 93, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Akbar, M.U.; Zia, K.M.; Nazir, A.; Iqbal, J.; Ejaz, S.A.; Akash, M.S.H. Pluronic-based mixed polymeric micelles enhance the therapeutic potential of curcumin. AAPS PharmSciTech 2018, 19, 2719–2739. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.-L.; Kuo, H.-P.; Johnson, A.; Wu, L.-C.; Chang, K.L.B. Curcumin-Loaded Mesoporous Silica Nanoparticles Markedly Enhanced Cytotoxicity in Hepatocellular Carcinoma Cells. Int. J. Mol. Sci. 2019, 20, 2918. [Google Scholar] [CrossRef] [PubMed]
- Amanlou, N.; Parsa, M.; Rostamizadeh, K.; Sadighian, S.; Moghaddam, F. Enhanced cytotoxic activity of curcumin on cancer cell lines by incorporating into gold/chitosan nanogels. Mater. Chem. Phys. 2019, 226, 151–157. [Google Scholar] [CrossRef]

| Sample | %C | %H | %N | %O | C/N | DSB | DSS |
|---|---|---|---|---|---|---|---|
| Chitosan | 49.9 | 7.57 | 7.01 | 35.52 | 7.12 | - | - |
| NBCh | 60.22 | 10.62 | 5.49 | 23.67 | 10.97 | 0.55 | - |
| NBSCh | 56.89 | 9.97 | 3.91 | 29.23 | 15.83 | 0.55 | 1.02 |
| Sample ID | Concentration of Loaded CL (mg/mL) | Size (nm) | PDI | Zeta Potential (mV) | %EE | %LC |
|---|---|---|---|---|---|---|
| Blank micelles | - | 60.1 ± 0.7 | 0.096 ± 0.008 | −29.3 ± 0.3 | - | - |
| 0.1-CL-micelles | 0.1 | 59.0 ± 0.7 | 0.172 ± 0.018 | −27.5 ± 1.4 | 44.4 ± 1.1 | 4.25 ± 1.1 |
| 0.3-CL-micelles | 0.3 | 59.8 ± 0.6 | 0.186 ± 0.030 | −30.3 ± 0.5 | 46.3 ± 1.0 | 12.20 ± 1.2 |
| 0.6-CL-micelles | 0.6 | 62.4 ± 0.9 | 0.236 ± 0.022 | −31.0 ± 0.6 | 45.1 ± 1.9 | 21.30 ± 1.5 |
| 1-CL-micelles | 1 | 64.7 ± 0.7 | 0.272 ± 0.028 | −30.3 ± 0.5 | 48.7 ± 1.3 | 32.75 ± 1.6 |
| Sample | HT-29 | L929 | Selectivity |
|---|---|---|---|
| IC50 (µg/mL) | IC50 (µg/mL) | (Fold) | |
| Cyqualone (CL) | 10.6 ± 1.14 | 10.2 ± 1.01 * | 0.97 * |
| CL-NBSCh micelles | 3.4 ± 0.82 * | 24.3 ± 2.23 | 7.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sripetthong, S.; Eze, F.N.; Sajomsang, W.; Ovatlarnporn, C. Development of pH-Responsive N-benzyl-N-O-succinyl Chitosan Micelles Loaded with a Curcumin Analog (Cyqualone) for Treatment of Colon Cancer. Molecules 2023, 28, 2693. https://doi.org/10.3390/molecules28062693
Sripetthong S, Eze FN, Sajomsang W, Ovatlarnporn C. Development of pH-Responsive N-benzyl-N-O-succinyl Chitosan Micelles Loaded with a Curcumin Analog (Cyqualone) for Treatment of Colon Cancer. Molecules. 2023; 28(6):2693. https://doi.org/10.3390/molecules28062693
Chicago/Turabian StyleSripetthong, Sasikarn, Fredrick Nwude Eze, Warayuth Sajomsang, and Chitchamai Ovatlarnporn. 2023. "Development of pH-Responsive N-benzyl-N-O-succinyl Chitosan Micelles Loaded with a Curcumin Analog (Cyqualone) for Treatment of Colon Cancer" Molecules 28, no. 6: 2693. https://doi.org/10.3390/molecules28062693
APA StyleSripetthong, S., Eze, F. N., Sajomsang, W., & Ovatlarnporn, C. (2023). Development of pH-Responsive N-benzyl-N-O-succinyl Chitosan Micelles Loaded with a Curcumin Analog (Cyqualone) for Treatment of Colon Cancer. Molecules, 28(6), 2693. https://doi.org/10.3390/molecules28062693






