Nanomaterials-Based Novel Immune Strategies in Clinical Translation for Cancer Therapy
Abstract
1. Introduction
2. Cancer and the Role of the Immune System
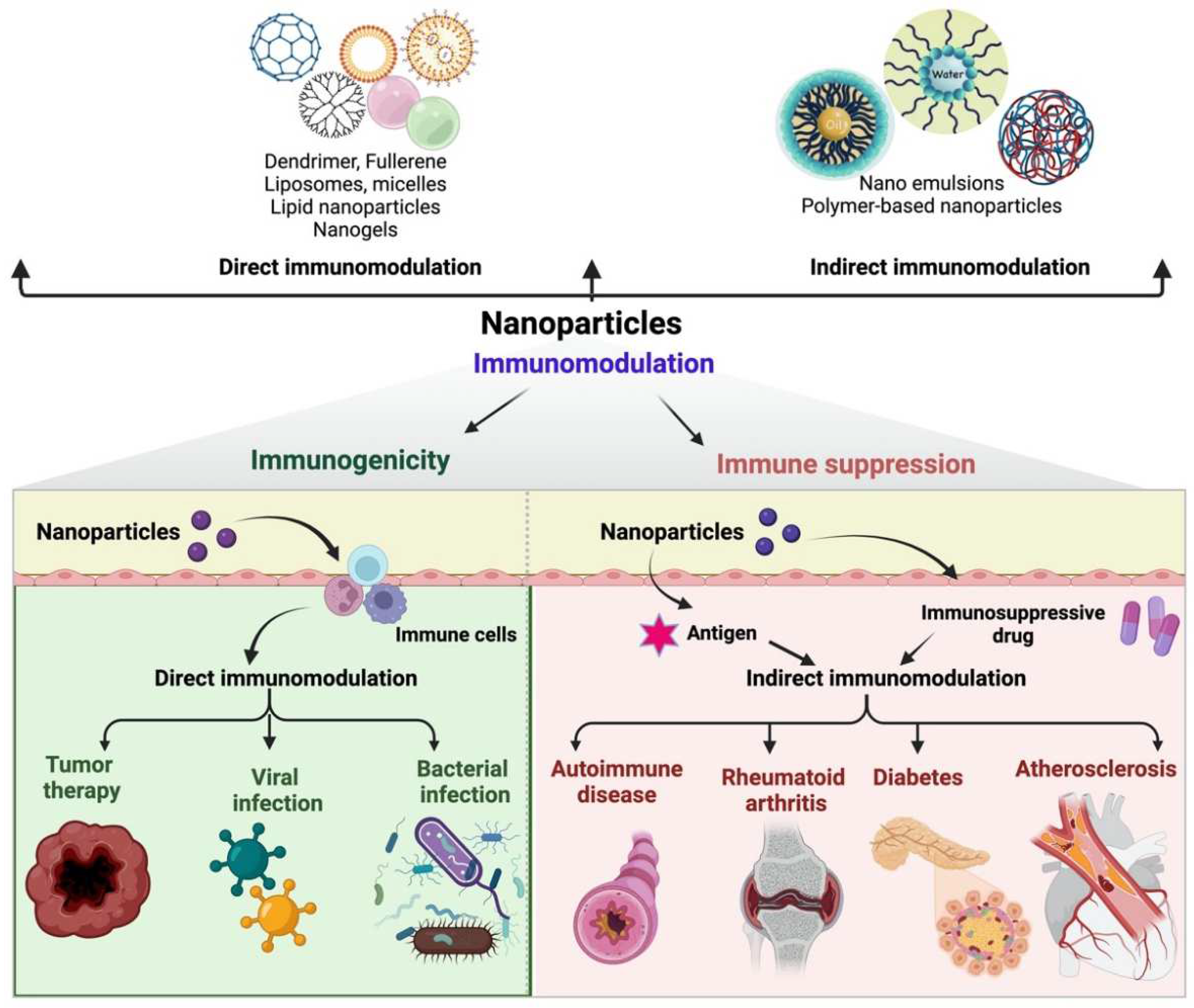
3. Nanomaterials for Indirect and Direct Immunomodulation
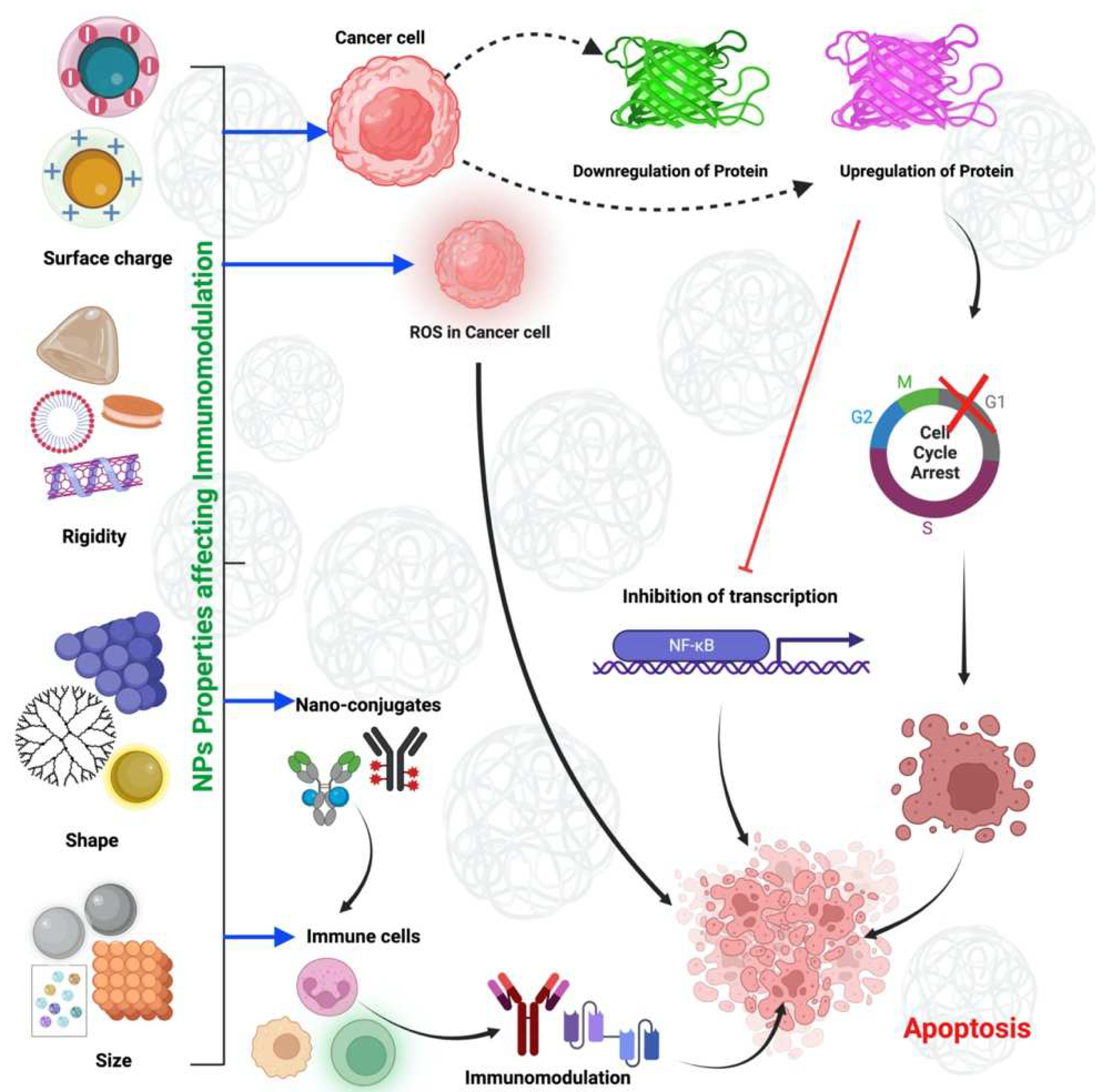
4. Physicochemical Properties of Nanomaterials and Their Impact on the Immune System
4.1. Size-Dependent Immunomodulation of NMs
4.2. Immunomodulation of NMs by Shape
4.3. Immunomodulation of NMs by Rigidity
4.4. Immunomodulation of NMs by Surface Charge
5. Advantages and Disadvantages of the Different Types of Nanocarriers
6. Nanomaterial-Based Immunotherapy
7. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lakshmi Narendra, B.; Eshvendar Reddy, K.; Shantikumar, S.; Ramakrishna, S. Immune System: A Double-Edged Sword in Cancer. Inflamm. Res. 2013, 62, 823–834. [Google Scholar] [CrossRef] [PubMed]
- De Visser, K.E.; Eichten, A.; Coussens, L.M. Paradoxical Roles of the Immune System during Cancer Development. Nat. Rev. Cancer 2006, 6, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Houghton, A.N. Cancer Antigens: Immune Recognition of Self and Altered Self. J. Exp. Med. 1994, 180, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cao, X. Immunosuppressive Cells in Tumor Immune Escape and Metastasis. J. Mol. Med. 2016, 94, 509–522. [Google Scholar] [CrossRef]
- Muenst, S.; Läubli, H.; Soysal, S.D.; Zippelius, A.; Tzankov, A.; Hoeller, S. The Immune System and Cancer Evasion Strategies: Therapeutic Concepts. J. Intern. Med. 2016, 279, 541–562. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. The Tumor Microenvironment and Its Role in Promoting Tumor Growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef]
- Lei, X.; Lei, Y.; Li, J.K.; Du, W.X.; Li, R.G.; Yang, J.; Li, J.; Li, F.; Tan, H.B. Immune Cells within the Tumor Microenvironment: Biological Functions and Roles in Cancer Immunotherapy. Cancer Lett. 2020, 470, 126–133. [Google Scholar] [CrossRef]
- Kitamura, T.; Qian, B.Z.; Pollard, J.W. Immune Cell Promotion of Metastasis. Nat. Rev. Immunol. 2015, 15, 73–86. [Google Scholar] [CrossRef]
- Ferrari, S.; Rovati, B.; Porta, C.; Alessandrino, P.E.; Bertolini, A.; Collovà, E.; Riccardi, A.; Danova, M. Lack of Dendritic Cell Mobilization into the Peripheral Blood of Cancer Patients Following Standard- or High-Dose Chemotherapy plus Granulocyte-Colony Stimulating Factor. Cancer Immunol. Immunother. 2003, 52, 359–366. [Google Scholar] [CrossRef]
- Wertel, I.; Polak, G.; Barczyński, B.; Kotarski, J. Subpopulations of Peripheral Blood Dendritic Cells during Chemotherapy of Ovarian Cancer. Ginekol. Pol. 2007, 78, 768–771. [Google Scholar]
- Krienke, C.; Kolb, L.; Diken, E.; Streuber, M.; Kirchhoff, S.; Bukur, T.; Akilli-Öztürk, Ö.; Kranz, L.M.; Berger, H.; Petschenka, J.; et al. A Noninflammatory mRNA Vaccine for Treatment of Experimental Autoimmune Encephalomyelitis. Science 2021, 371, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Irvine, D.J.; Hanson, M.C.; Rakhra, K.; Tokatlian, T. Synthetic Nanoparticles for Vaccines and Immunotherapy. Chem. Rev. 2015, 115, 11109–11146. [Google Scholar] [CrossRef] [PubMed]
- Sunshine, J.C.; Perica, K.; Schneck, J.P.; Green, J.J. Particle Shape Dependence of CD8+ T Cell Activation by Artificial Antigen Presenting Cells. Biomaterials 2014, 35, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Wahab, S.; Alshahrani, M.Y.; Ahmad, M.F.; Abbas, H. Current Trends and Future Perspectives of Nanomedicine for the Management of Colon Cancer. Eur. J. Pharmacol. 2021, 910, 174464. [Google Scholar] [CrossRef]
- Dhara, V.; Shetty, S.S.; de Arruda, J.A.A.; Silva, T.A.; Russo, R.C.; Shetty, N.J.; Pidaparthi, M.; Wollenberg, B.; Rao, V.U.S.; Gopinath, T.P.S. Decoding the Influence of the Immune System and Immunotherapy Targets on Carcinomas: A Hidden Prism in Oral Cancer Therapy. Disease-a-Month 2022, 69, 101353. [Google Scholar] [CrossRef]
- Wraith, D. Autoimmunity: Antigen-Specific Immunotherapy. Nature 2016, 530, 422–423. [Google Scholar] [CrossRef] [PubMed]
- Milling, L.; Zhang, Y.; Irvine, D.J. Delivering Safer Immunotherapies for Cancer. Adv. Drug Deliv. Rev. 2017, 114, 79–101. [Google Scholar] [CrossRef]
- Parkin, J.; Cohen, B. An Overview of the Immune System. Lancet 2001, 357, 1777–1789. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.V.; Deng, M.; Ting, J.P.Y. The NLRP3 Inflammasome: Molecular Activation and Regulation to Therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Callaway, J.B.; Ting, J.P.Y. Inflammasomes: Mechanism of Action, Role in Disease, and Therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef]
- Muñoz-Planillo, R.; Kuffa, P.; Martínez-Colón, G.; Smith, B.L.; Rajendiran, T.M.; Núñez, G. K+ Efflux Is the Common Trigger of NLRP3 Inflammasome Activation by Bacterial Toxins and Particulate Matter. Immunity 2013, 38, 1142–1153. [Google Scholar] [CrossRef] [PubMed]
- Wahab, S.; Hussain, A. Cytokines as Targets for Immunomodulation. Int. J. Pharm. Pharm. Sci. 2013, 5, 60–64. [Google Scholar]
- Moslehi, M.; Moazamiyanfar, R.; Dakkali, M.S.; Rezaei, S.; Rastegar-Pouyani, N.; Jafarzadeh, E.; Mouludi, K.; Khodamoradi, E.; Taeb, S.; Najafi, M. Modulation of the Immune System by Melatonin; Implications for Cancer Therapy. Int. Immunopharmacol. 2022, 108, 108890. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Tuli, H.S.; Yerer, M.B.; Sharma, A.; Sak, K.; Srivastava, S.; Pandey, A.; Garg, V.K.; Sethi, G.; Bishayee, A. Natural Product-Based Nanoformulations for Cancer Therapy: Opportunities and Challenges. Semin. Cancer Biol. 2021, 69, 5–23. [Google Scholar] [CrossRef]
- Schirrmacher, V. From Chemotherapy to Biological Therapy: A Review of Novel Concepts to Reduce the Side Effects of Systemic Cancer Treatment (Review). Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Gun, S.Y.; Lee, S.W.L.; Sieow, J.L.; Wong, S.C. Targeting Immune Cells for Cancer Therapy. Redox Biol. 2019, 25, 101174. [Google Scholar] [CrossRef] [PubMed]
- Stolfi, C.; Troncone, E.; Marafini, I.; Monteleone, G. Role of Tgf-Beta and Smad7 in Gut Inflammation, Fibrosis and Cancer. Biomolecules 2021, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Rockson, S.G.; Keeley, V.; Kilbreath, S.; Szuba, A.; Towers, A. Cancer-Associated Secondary Lymphoedema. Nat. Rev. Dis. Primers 2019, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Grizzi, F.; Bianchi, P.; Malesci, A.; Laghi, L. Prognostic Value of Innate and Adaptive Immunity in Colorectal Cancer. World J. Gastroenterol. 2013, 19, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Wahab, S.; Ahmad, M.P.; Hussain, A.; Qadir, S.F.A. Nanomaterials for the Delivery of Herbal Bioactive Compounds. Curr. Nanosci. 2021, 18, 425–441. [Google Scholar] [CrossRef]
- Ahmad, I.; Alshahrani, M.Y.; Wahab, S.; Al-Harbi, A.I.; Nisar, N.; Alraey, Y.; Alqahtani, A.; Mir, M.A.; Irfan, S.; Saeed, M. Zinc Oxide Nanoparticle: An Effective Antibacterial Agent against Pathogenic Bacterial Isolates. J. King Saud Univ. Sci. 2022, 34, 102110. [Google Scholar] [CrossRef]
- Gorjikhah, F.; Davaran, S.; Salehi, R.; Bakhtiari, M.; Hasanzadeh, A.; Panahi, Y.; Emamverdy, M.; Akbarzadeh, A. Improving “Lab-on-a-Chip” Techniques Using Biomedical Nanotechnology: A Review. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1609–1614. [Google Scholar] [CrossRef] [PubMed]
- El-Ansary, A.; Faddah, L.M. Nanoparticles as Biochemical Sensors. Nanotechnol. Sci. Appl. 2010, 3, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Farokhzad, O.C. Nanotechnology for Drug Delivery: The Perfect Partnership. Expert Opin. Drug Deliv. 2008, 5, 927–929. [Google Scholar] [CrossRef] [PubMed]
- European Commission. European Commission Recommendations on the Definition of Nanomaterial. Off. J. Eur. Union 2010, 24, 6. [Google Scholar]
- Sheet, N.F. Nanotechnology Fact Sheet. 2014. Available online: https://www.fda.gov/science-research/nanotechnology-programs-fda/nanotechnology-fact-sheet (accessed on 10 December 2022).
- Zhang, L.; Gu, F.X.; Chan, J.M.; Wang, A.Z.; Langer, R.S.; Farokhzad, O.C. Nanoparticles in Medicine: Therapeutic Applications and Developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef]
- Kubackova, J.; Zbytovska, J.; Holas, O. Nanomaterials for Direct and Indirect Immunomodulation: A Review of Applications. Eur. J. Pharm. Sci. 2020, 142, 105139. [Google Scholar] [CrossRef]
- Hirn, S.; Semmler-Behnke, M.; Schleh, C.; Wenk, A.; Lipka, J.; Schäffler, M.; Takenaka, S.; Möller, W.; Schmid, G.; Simon, U.; et al. Particle Size-Dependent and Surface Charge-Dependent Biodistribution of Gold Nanoparticles after Intravenous Administration. Eur. J. Pharm. Biopharm. 2011, 77, 407–416. [Google Scholar] [CrossRef]
- Rosalia, R.A.; Silva, A.L.; Camps, M.; Allam, A.; Jiskoot, W.; Van Der Burg, S.H.; Ossendorp, F.; Oostendorp, J. Efficient Ex Vivo Induction of T Cells with Potent Anti-Tumor Activity by Protein Antigen Encapsulated in Nanoparticles. Cancer Immunol. Immunother. 2013, 62, 1161–1173. [Google Scholar] [CrossRef]
- Almeida, J.P.M.; Lin, A.Y.; Langsner, R.J.; Eckels, P.; Foster, A.E.; Drezek, R.A. In Vivo Immune Cell Distribution of Gold Nanoparticles in Naïve and Tumor Bearing Mice. Small 2014, 10, 812–819. [Google Scholar] [CrossRef]
- Alshahrani, M.Y.; Rafi, Z.; Alabdallah, N.M.; Shoaib, A.; Ahmad, I.; Asiri, M.; Zaman, G.S.; Wahab, S.; Saeed, M.; Khan, S. A Comparative Antibacterial, Antioxidant, and Antineoplastic Potential of Rauwolfia Serpentina (L.) Leaf Extract with Its Biologically Synthesized Gold Nanoparticles (r-Aunps). Plants 2021, 10, 2278. [Google Scholar] [CrossRef] [PubMed]
- Hardy, C.L.; LeMasurier, J.S.; Mohamud, R.; Yao, J.; Xiang, S.D.; Rolland, J.M.; O’Hehir, R.E.; Plebanski, M. Differential Uptake of Nanoparticles and Microparticles by Pulmonary APC Subsets Induces Discrete Immunological Imprints. J. Immunol. 2013, 191, 5278–5290. [Google Scholar] [CrossRef] [PubMed]
- Boraschi, D.; Italiani, P.; Palomba, R.; Decuzzi, P.; Duschl, A.; Fadeel, B.; Moghimi, S.M. Nanoparticles and Innate Immunity: New Perspectives on Host Defence. Semin. Immunol. 2017, 34, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Moyano, D.F.; Liu, Y.; Peer, D.; Rotello, V.M. Modulation of Immune Response Using Engineered Nanoparticle Surfaces. Small 2016, 12, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Kutscher, H.L.; Chao, P.; Deshmukh, M.; Singh, Y.; Hu, P.; Joseph, L.B.; Reimer, D.C.; Stein, S.; Laskin, D.L.; Sinko, P.J. Threshold Size for Optimal Passive Pulmonary Targeting and Retention of Rigid Microparticles in Rats. J. Control. Release 2010, 143, 31–37. [Google Scholar] [CrossRef]
- Faraji, A.H.; Wipf, P. Nanoparticles in Cellular Drug Delivery. Bioorg. Med. Chem. 2009, 17, 2950–2962. [Google Scholar] [CrossRef]
- Champion, J.A.; Walker, A.; Mitragotri, S. Role of Particle Size in Phagocytosis of Polymeric Microspheres. Pharm. Res. 2008, 25, 1815–1821. [Google Scholar] [CrossRef]
- Champion, J.A.; Mitragotri, S. Role of Target Geometry in Phagocytosis. Proc. Natl. Acad. Sci. USA 2006, 103, 4930–4934. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Impact of Particle Elasticity on Particle-Based Drug Delivery Systems. Adv. Drug Deliv. Rev. 2017, 108, 51–67. [Google Scholar] [CrossRef]
- Getts, D.R.; Shea, L.D.; Miller, S.D.; King, N.J.C. Harnessing Nanoparticles for Immune Modulation. Trends Immunol. 2015, 36, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; Xiao, Z.; Valencia, P.M.; Radovic-Moreno, A.F.; Farokhzad, O.C. Targeted Polymeric Therapeutic Nanoparticles: Design, Development and Clinical Translation. Chem. Soc. Rev. 2012, 41, 2971–3010. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.E.; Wang, Y.Y.; Yang, Q.; Hoang, T.; Chattopadhyay, S.; Hoen, T.; Ensign, L.M.; Nunn, K.L.; Schroeder, H.; McCallen, J.; et al. Anti-PEG Antibodies Alter the Mobility and Biodistribution of Densely PEGylated Nanoparticles in Mucus. Acta Biomater. 2016, 43, 61–70. [Google Scholar] [CrossRef]
- Bagalkot, V.; Deiuliis, J.A.; Rajagopalan, S.; Maiseyeu, A. “Eat Me” Imaging and Therapy. Adv. Drug Deliv. Rev. 2016, 99, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Ramos, G.C.; Fernandes, D.; Charão, C.T.; Souza, D.G.; Teixeira, M.M.; Assreuy, J. Apoptotic Mimicry: Phosphatidylserine Liposomes Reduce Inflammation through Activation of Peroxisome Proliferator-Activated Receptors (PPARs) in Vivo. Br. J. Pharmacol. 2007, 151, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Even-Or, O.; Xu, X.; van Rosmalen, M.; Lim, L.; Gadde, S.; Farokhzad, O.C.; Fisher, E.A. Nanoparticles Containing a Liver X Receptor Agonist Inhibit Inflammation and Atherosclerosis. Adv. Healthc. Mater. 2015, 4, 228–236. [Google Scholar] [CrossRef]
- Konduru, N.V.; Tyurina, Y.Y.; Feng, W.; Basova, L.V.; Belikova, N.A.; Bayir, H.; Clark, K.; Rubin, M.; Stolz, D.; Vallhov, H.; et al. Phosphatidylserine Targets Single-Walled Carbon Nanotubes to Professional Phagocytes in Vitro and in Vivo. PLoS One 2009, 4, e4398. [Google Scholar] [CrossRef]
- Weissleder, R.; Kelly, K.; Sun, E.Y.; Shtatland, T.; Josephson, L. Cell-Specific Targeting of Nanoparticles by Multivalent Attachment of Small Molecules. Nat. Biotechnol. 2005, 23, 1418–1423. [Google Scholar] [CrossRef]
- Duan, X.; Chan, C.; Lin, W. Nanoparticle-Mediated Immunogenic Cell Death Enables and Potentiates Cancer Immunotherapy. Angew. Chem. Int. Ed. 2019, 58, 670–680. [Google Scholar] [CrossRef]
- Guo, J.; Yu, Z.; Das, M.; Huang, L. Nano Codelivery of Oxaliplatin and Folinic Acid Achieves Synergistic Chemo-Immunotherapy with 5-Fluorouracil for Colorectal Cancer and Liver Metastasis. ACS Nano 2020, 14, 5075–5089. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Chan, C.; Han, W.; Guo, N.; Weichselbaum, R.R.; Lin, W. Immunostimulatory Nanomedicines Synergize with Checkpoint Blockade Immunotherapy to Eradicate Colorectal Tumors. Nat. Commun. 2019, 10, 1899. [Google Scholar] [CrossRef] [PubMed]
- Wahab, S.; Alsayari, A.; Bin Muhsinah, A.; Ahmad, I.; Hussain, M.S.; Mallick, J. Cirsilineol Inhibits the Proliferation of Human Prostate Cancer Cells by Inducing Reactive Oxygen Species (ROS)-Mediated Apoptosis. Evid.-Based Complement. Altern. Med. 2022, 2022, 7975664. [Google Scholar] [CrossRef]
- Yang, Y.W.; Luo, W.H. Cellular Biodistribution of Polymeric Nanoparticles in the Immune System. J. Control. Release 2016, 227, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, Y.; Zhang, L.; Huang, L. Nanoparticle-Delivered Transforming Growth Factor-β SiRNA Enhances Vaccination against Advanced Melanoma by Modifying Tumor Microenvironment. ACS Nano 2014, 8, 3636–3645. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Liu, Q.; Lin, C.M.; Luo, C.; Wang, Y.; Liu, L.; Yin, W.; Hu, S.; Kim, W.Y.; Huang, L. Targeting Tumor-Associated Fibroblasts for Therapeutic Delivery in Desmoplastic Tumors. Cancer Res. 2017, 77, 719–731. [Google Scholar] [CrossRef]
- Xia, X.; Mai, J.; Xu, R.; Perez, J.E.T.; Guevara, M.L.; Shen, Q.; Mu, C.; Tung, H.Y.; Corry, D.B.; Evans, S.E.; et al. Porous Silicon Microparticle Potentiates Anti-Tumor Immunity by Enhancing Cross-Presentation and Inducing Type I Interferon Response. Cell Rep. 2015, 11, 957–966. [Google Scholar] [CrossRef]
- Zhu, S.; Niu, M.; O’Mary, H.; Cui, Z. Targeting of Tumor-Associated Macrophages Made Possible by PEG-Sheddable, Mannose-Modified Nanoparticles. Mol. Pharm. 2013, 10, 3525–3530. [Google Scholar] [CrossRef]
- Qiu, F.; Becker, K.W.; Knight, F.C.; Baljon, J.J.; Sevimli, S.; Shae, D.; Gilchuk, P.; Joyce, S.; Wilson, J.T. Poly(Propylacrylic Acid)-Peptide Nanoplexes as a Platform for Enhancing the Immunogenicity of Neoantigen Cancer Vaccines. Biomaterials 2018, 182, 82–91. [Google Scholar] [CrossRef]
- Gulla, S.K.; Rao, B.R.; Moku, G.; Jinka, S.; Nimmu, N.V.; Khalid, S.; Patra, C.R.; Chaudhuri, A. In Vivo Targeting of DNA Vaccines to Dendritic Cells Using Functionalized Gold Nanoparticles. Biomater. Sci. 2019, 7, 773–788. [Google Scholar] [CrossRef]
- Roy, A.; Chandra, S.; Mamilapally, S.; Upadhyay, P.; Bhaskar, S. Anticancer and Immunostimulatory Activity by Conjugate of Paclitaxel and Non-Toxic Derivative of Lps for Combined Chemo-Immunotherapy. Pharm. Res. 2012, 29, 2294–2309. [Google Scholar] [CrossRef] [PubMed]
- Seth, A.; Gholami Derami, H.; Gupta, P.; Wang, Z.; Rathi, P.; Gupta, R.; Cao, T.; Morrissey, J.J.; Singamaneni, S. Polydopamine-Mesoporous Silica Core-Shell Nanoparticles for Combined Photothermal Immunotherapy. ACS Appl. Mater. Interfaces 2020, 12, 42499–42510. [Google Scholar] [CrossRef] [PubMed]
- Colzani, B.; Pandolfi, L.; Hoti, A.; Iovene, P.A.; Natalello, A.; Avvakumova, S.; Colombo, M.; Prosperi, D. Investigation of Antitumor Activities of Trastuzumab Delivered by PLGA Nanoparticles. Int. J. Nanomed. 2018, 13, 957–973. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Liu, Y.; Xu, C.F.; Shen, S.; Sun, R.; Du, X.J.; Xia, J.X.; Zhu, Y.H.; Wang, J. Restoring Anti-Tumor Functions of T Cells via Nanoparticle-Mediated Immune Checkpoint Modulation. J. Control. Release 2016, 231, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Zheng, P.; Jiang, F.; Zhao, Y.; Wang, M.; Chang, M.; Ma, P.; Lin, J. MnOx Nanospikes as Nanoadjuvants and Immunogenic Cell Death Drugs with Enhanced Antitumor Immunity and Antimetastatic Effect. Angew. Chem. Int. Ed. 2020, 59, 16381–16384. [Google Scholar] [CrossRef]
- Chung, C.K.; Fransen, M.F.; van der Maaden, K.; Campos, Y.; García-Couce, J.; Kralisch, D.; Chan, A.; Ossendorp, F.; Cruz, L.J. Thermosensitive Hydrogels as Sustained Drug Delivery System for CTLA-4 Checkpoint Blocking Antibodies. J. Control. Release 2020, 323, 1–11. [Google Scholar] [CrossRef]
- Chiang, C.S.; Lin, Y.J.; Lee, R.; Lai, Y.H.; Cheng, H.W.; Hsieh, C.H.; Shyu, W.C.; Chen, S.Y. Combination of Fucoidan-Based Magnetic Nanoparticles and Immunomodulators Enhances Tumour-Localized Immunotherapy. Nat. Nanotechnol. 2018, 13, 746–754. [Google Scholar] [CrossRef]
- Yu, X.; Gao, D.; Gao, L.; Lai, J.; Zhang, C.; Zhao, Y.; Zhong, L.; Jia, B.; Wang, F.; Chen, X.; et al. Inhibiting Metastasis and Preventing Tumor Relapse by Triggering Host Immunity with Tumor-Targeted Photodynamic Therapy Using Photosensitizer-Loaded Functional Nanographenes. ACS Nano 2017, 11, 10147–10158. [Google Scholar] [CrossRef]
- Cheung, A.S.; Zhang, D.K.Y.; Koshy, S.T.; Mooney, D.J. Scaffolds That Mimic Antigen-Presenting Cells Enable Ex Vivo Expansion of Primary T Cells. Nat. Biotechnol. 2018, 36, 160–169. [Google Scholar] [CrossRef]
- Hani, U.; Osmani, R.A.M.; Yasmin, S.; Gowda, B.H.J.; Ather, H.; Ansari, M.Y.; Siddiqua, A.; Ghazwani, M.; Al Fatease, A.; Alamri, A.H.; et al. Novel Drug Delivery Systems as an Emerging Platform for Stomach Cancer Therapy. Pharmaceutics 2022, 14, 1576. [Google Scholar] [CrossRef]
- Roach, K.A.; Stefaniak, A.B.; Roberts, J.R. Metal Nanomaterials: Immune Effects and Implications of Physicochemical Properties on Sensitization, Elicitation, and Exacerbation of Allergic Disease. J. Immunotoxicol. 2019, 16, 87–124. [Google Scholar] [CrossRef] [PubMed]
- Ball, R.L.; Hajj, K.A.; Vizelman, J.; Bajaj, P.; Whitehead, K.A. Lipid Nanoparticle Formulations for Enhanced Co-Delivery of SiRNA and MRNA. Nano Lett. 2018, 18, 3814–3822. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Rho, S.; Stiles, W.R.; Hu, S.; Baek, Y.; Hwang, D.W.; Kashiwagi, S.; Kim, M.S.; Choi, H.S. Size-Dependent EPR Effect of Polymeric Nanoparticles on Tumor Targeting. Adv. Healthc. Mater. 2020, 9, 1236–1249. [Google Scholar] [CrossRef] [PubMed]
- Wahab, S.; Muzammil, K.; Nasir, N.; Khan, M.S.; Ahmad, M.F.; Khalid, M.; Ahmad, W.; Dawria, A.; Reddy, L.K.V.; Busayli, A.M. Review Advancement and New Trends in Analysis of Pesticide Residues in Food: A Comprehensive Review. Plants 2022, 11, 1106. [Google Scholar] [CrossRef]
- Ilinskaya, A.N.; Dobrovolskaia, M.A. Immunosuppressive and Anti-Inflammatory Properties of Engineered Nanomaterials. Br. J. Pharmacol. 2014, 3988–4000. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, X.; Bian, Y.; Wang, S.; Chai, Q.; Guo, Z.; Wang, Z.; Zhu, P.; Peng, H.; Yan, X.; et al. Dual-Targeting Nanoparticle Vaccine Elicits a Therapeutic Antibody Response against Chronic Hepatitis B. Nat. Nanotechnol. 2020, 15, 406–416. [Google Scholar] [CrossRef]
- Smith, D.M.; Simon, J.K.; Baker, J.R. Applications of Nanotechnology for Immunology. Nat. Rev. Immunol. 2013, 13, 592–605. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Wahab, S.; Ahmad, I.; Irfan, S.; Baig, M.H.; Farouk, A.-E.; Dong, J.-J. Use of Natural Compounds as a Potential Therapeutic Agent Against COVID-19. Curr. Pharm. Des. 2021, 27, 1144–1152. [Google Scholar] [CrossRef]
- Shen, S.; Dai, H.; Fei, Z.; Chai, Y.; Hao, Y.; Fan, Q.; Dong, Z.; Zhu, Y.; Xu, J.; Ma, Q.; et al. Immunosuppressive Nanoparticles for Management of Immune-Related Adverse Events in Liver. ACS Nano 2021, 15, 9111–9125. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.M.J.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte Membrane-Camouflaged Polymeric Nanoparticles as a Biomimetic Delivery Platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.M.J.; Fang, R.H.; Wang, K.C.; Luk, B.T.; Thamphiwatana, S.; Dehaini, D.; Nguyen, P.; Angsantikul, P.; Wen, C.H.; Kroll, A.V.; et al. Nanoparticle Biointerfacing by Platelet Membrane Cloaking. Nature 2015, 526, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Visalakshan, R.M.; Macgregor, M.N.; Sasidharan, S.; Ghazaryan, A.; Mierczynska-Vasilev, A.M.; Morsbach, S.; Mailänder, V.; Landfester, K.; Hayball, J.D.; Vasilev, K. Biomaterial Surface Hydrophobicity-Mediated Serum Protein Adsorption and Immune Responses. ACS Appl. Mater. Interfaces 2019, 11, 27615–27623. [Google Scholar] [CrossRef]
- Moyano, D.F.; Goldsmith, M.; Solfiell, D.J.; Landesman-Milo, D.; Miranda, O.R.; Peer, D.; Rotello, V.M. Nanoparticle Hydrophobicity Dictates Immune Response. J. Am. Chem. Soc. 2012, 134, 3965–3967. [Google Scholar] [CrossRef]
- Park, J.; Zhang, Y.; Saito, E.; Gurczynski, S.J.; Moore, B.B.; Cummings, B.J.; Anderson, A.J.; Shea, L.D. Intravascular Innate Immune Cells Reprogrammed via Intravenous Nanoparticles to Promote Functional Recovery after Spinal Cord Injury. Proc. Natl. Acad. Sci. USA 2019, 116, 14947–14954. [Google Scholar] [CrossRef]
- Min, Y.; Roche, K.C.; Tian, S.; Eblan, M.J.; McKinnon, K.P.; Caster, J.M.; Chai, S.; Herring, L.E.; Zhang, L.; Zhang, T.; et al. Antigen-Capturing Nanoparticles Improve the Abscopal Effect and Cancer Immunotherapy. Nat. Nanotechnol. 2017, 12, 877–882. [Google Scholar] [CrossRef]
- Falagan-Lotsch, P.; Grzincic, E.M.; Murphy, C.J. One Low-Dose Exposure of Gold Nanoparticles Induces Long-Term Changes in Human Cells. Proc. Natl. Acad. Sci. USA 2016, 113, 13318–13323. [Google Scholar] [CrossRef]
- Li, P.Y.; Bearoff, F.; Zhu, P.; Fan, Z.; Zhu, Y.; Fan, M.; Cort, L.; Kambayashi, T.; Blankenhorn, E.P.; Cheng, H. PEGylation Enables Subcutaneously Administered Nanoparticles to Induce Antigen-Specific Immune Tolerance. J. Control. Release 2021, 331, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, X.; Yuan, P.; Jin, R.; Bao, L.; Qiu, X.; Liu, S.; Liu, T.; Gooding, J.J.; Chen, W.J.; et al. Modular Immune-Homeostatic Microparticles Promote Immune Tolerance in Mouse Autoimmune Models. Sci. Transl. Med. 2021, 13, eaaw9668. [Google Scholar] [CrossRef]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H.; et al. Systemic RNA Delivery to Dendritic Cells Exploits Antiviral Defence for Cancer Immunotherapy. Nature 2016, 534, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, J.; Bedocs, P.; Rozsnyay, Z.; Weiszhár, Z.; Urbanics, R.; Rosivall, L.; Cohen, R.; Garbuzenko, O.; Báthori, G.; Tóth, M.; et al. Liposome-Induced Complement Activation and Related Cardiopulmonary Distress in Pigs: Factors Promoting Reactogenicity of Doxil and AmBisome. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, P.; Yin, Y.; Cai, X.; Huang, Z.; Chen, J.; Dong, L.; Zhang, J. The Promotion of Type 1 T Helper Cell Responses to Cationic Polymers in Vivo via Toll-like Receptor-4 Mediated IL-12 Secretion. Biomaterials 2010, 31, 8172–8180. [Google Scholar] [CrossRef]
- Srijampa, S.; Buddhisa, S.; Ngernpimai, S.; Sangiamdee, D.; Chompoosor, A.; Tippayawat, P. Effects of Gold Nanoparticles with Different Surface Charges on Cellular Internalization and Cytokine Responses in Monocytes. Bionanoscience 2019, 9, 580–586. [Google Scholar] [CrossRef]
- Fromen, C.A.; Robbins, G.R.; Shen, T.W.; Kai, M.P.; Ting, J.P.Y.; De Simone, J.M. Controlled Analysis of Nanoparticle Charge on Mucosal and Systemic Antibody Responses Following Pulmonary Immunization. Proc. Natl. Acad. Sci. USA 2015, 112, 488–493. [Google Scholar] [CrossRef]
- Mou, Y.; Xing, Y.; Ren, H.; Cui, Z.; Zhang, Y.; Yu, G.; Urba, W.J.; Hu, Q.; Hu, H. The Effect of Superparamagnetic Iron Oxide Nanoparticle Surface Charge on Antigen Cross-Presentation. Nanoscale Res. Lett. 2017, 12, 1–8. [Google Scholar] [CrossRef]
- Hui, Y.; Wibowo, D.; Liu, Y.; Ran, R.; Wang, H.F.; Seth, A.; Middelberg, A.P.J.; Zhao, C.X. Understanding the Effects of Nanocapsular Mechanical Property on Passive and Active Tumor Targeting. ACS Nano 2018, 12, 2846–2857. [Google Scholar] [CrossRef]
- Sosale, N.G.; Rouhiparkouhi, T.; Bradshaw, A.M.; Dimova, R.; Lipowsky, R.; Discher, D.E. Cell Rigidity and Shape Override CD47′s “Self”-Signaling in Phagocytosis by Hyperactivating Myosin-II. Blood 2015, 125, 542–552. [Google Scholar] [CrossRef]
- Xia, Y.; Wei, J.; Du, Y.; Wan, T.; Ma, X.; An, W.; Guo, A.; Miao, C.; Yue, H.; Li, S.; et al. Exploiting the Pliability and Lateral Mobility of Pickering Emulsion for Enhanced Vaccination. Nat. Mater. 2018, 17, 187–194. [Google Scholar] [CrossRef]
- Merkel, T.J.; Jones, S.W.; Herlihy, K.P.; Kersey, F.R.; Shields, A.R.; Napier, M.; Luft, J.C.; Wu, H.; Zamboni, W.C.; Wang, A.Z.; et al. Using Mechanobiological Mimicry of Red Blood Cells to Extend Circulation Times of Hydrogel Microparticles. Proc. Natl. Acad. Sci. USA 2011, 108, 586–591. [Google Scholar] [CrossRef]
- Hasani-Sadrabadi, M.M.; Majedi, F.S.; Bensinger, S.J.; Wu, B.M.; Bouchard, L.S.; Weiss, P.S.; Moshaverinia, A. Mechanobiological Mimicry of Helper T Lymphocytes to Evaluate Cell–Biomaterials Crosstalk. Adv. Mater. 2018, 30, 1706780. [Google Scholar] [CrossRef]
- Schudel, A.; Francis, D.M.; Thomas, S.N. Material Design for Lymph Node Drug Delivery. Nat. Rev. Mater. 2019, 4, 415–428. [Google Scholar] [CrossRef]
- Son, S.; Nam, J.; Zenkov, I.; Ochyl, L.J.; Xu, Y.; Scheetz, L.; Shi, J.; Farokhzad, O.C.; Moon, J.J. Sugar-Nanocapsules Imprinted with Microbial Molecular Patterns for MRNA Vaccination. Nano Lett. 2020, 20, 1499–1509. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Li, H.; Ma, M.; Fu, J.; Dong, Y.; Guo, P. Size, Shape, and Sequence-Dependent Immunogenicity of RNA Nanoparticles. Mol. Ther. Nucleic Acids 2017, 9, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.; Halman, J.R.; Shah, A.B.; Khisamutdinov, E.F.; Dobrovolskaia, M.A.; Afonin, K.A. Structure and Composition Define Immunorecognition of Nucleic Acid Nanoparticles. Nano Lett. 2018, 18, 4309–4321. [Google Scholar] [CrossRef] [PubMed]
- Safari, H.; Kelley, W.J.; Saito, E.; Kaczorowski, N.; Carethers, L.; Shea, L.D.; Eniola-Adefeso, O.; Eniola-Adefeso, O. Neutrophils Preferentially Phagocytose Elongated Particles-An Opportunity for Selective Targeting in Acute Inflammatory Diseases. Sci. Adv. 2020, 6, eaba1474. [Google Scholar] [CrossRef]
- Chen, X.; Yan, Y.; Müllner, M.; Ping, Y.; Cui, J.; Kempe, K.; Cortez-Jugo, C.; Caruso, F. Shape-Dependent Activation of Cytokine Secretion by Polymer Capsules in Human Monocyte-Derived Macrophages. Biomacromolecules 2016, 17, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, H.J.; Hang, T.; Yu, Y.; Liu, G.; He, G.; Xiao, S.; Yang, B.-r.; Yang, C.; Liu, F.; et al. Physical Activation of Innate Immunity by Spiky Particles. Nat. Nanotechnol. 2018, 13, 1078–1086. [Google Scholar] [CrossRef]
- Getts, D.R.; Martin, A.J.; Mccarthy, D.P.; Terry, R.L.; Hunter, Z.N.; Yap, W.T.; Getts, M.T.; Pleiss, M.; Luo, X.; King, N.J.C.; et al. Microparticles Bearing Encephalitogenic Peptides Induce T-Cell Tolerance and Ameliorate Experimental Autoimmune Encephalomyelitis. Nat. Biotechnol. 2012, 30, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Kanchan, V.; Panda, A.K. Interactions of Antigen-Loaded Polylactide Particles with Macrophages and Their Correlation with the Immune Response. Biomaterials 2007, 28, 5344–5357. [Google Scholar] [CrossRef]
- Taylor, M.J.; Husain, K.; Gartner, Z.J.; Mayor, S.; Vale, R.D. A DNA-Based T Cell Receptor Reveals a Role for Receptor Clustering in Ligand Discrimination. Cell 2017, 169, 108–119.e20. [Google Scholar] [CrossRef]
- Hickey, J.W.; Vicente, F.P.; Howard, G.P.; Mao, H.Q.; Schneck, J.P. Biologically Inspired Design of Nanoparticle Artificial Antigen-Presenting Cells for Immunomodulation. Nano Lett. 2017, 17, 7045–7054. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.; Lucas, M.; Sousa, A.; Soares, T.; Ribeiro, D.; Carvalho, F.; Fernandes, E. Small-Size Silver Nanoparticles Stimulate Neutrophil Oxidative Burst through an Increase of Intracellular Calcium Levels. World Acad. Sci. J. 2020, 2, 1. [Google Scholar] [CrossRef]
- Kim, S.; Oh, W.K.; Jeong, Y.S.; Hong, J.Y.; Cho, B.R.; Hahn, J.S.; Jang, J. Cytotoxicity of, and Innate Immune Response to, Size-Controlled Polypyrrole Nanoparticles in Mammalian Cells. Biomaterials 2011, 32, 2342–2350. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.K.; Kim, S.; Choi, M.; Kim, C.; Jeong, Y.S.; Cho, B.R.; Hahn, J.S.; Jang, J. Cellular Uptake, Cytotoxicity, and Innate Immune Response of Silica-Titania Hollow Nanoparticles Based on Size and Surface Functionality. ACS Nano 2010, 4, 5301–5313. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Kostarelos, K.; Fadeel, B. Cytokine Profiling of Primary Human Macrophages Exposed to Endotoxin-Free Graphene Oxide: Size-Independent NLRP3 Inflammasome Activation. Adv. Healthc. Mater. 2018, 7, 1700815. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhang, Q.; Fan, S.; Zhang, A.; Liu, B.; Hong, Y.; Guo, J.; Cui, D.; Song, J. The Vacuolization of Macrophages Induced by Large Amounts of Inorganic Nanoparticle Uptake to Enhance the Immune Response. Nanoscale 2019, 11, 22849–22859. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lim, D.H.; Lim, H.J.; Kwon, T.; Choi, J.S.; Jeong, S.; Choi, I.H.; Cheon, J. Size Dependent Macrophage Responses and Toxicological Effects of Ag Nanoparticles. Chem. Commun. 2011, 47, 4382–4384. [Google Scholar] [CrossRef]
- Kinaret, P.A.S.; Scala, G.; Federico, A.; Sund, J.; Greco, D. Carbon Nanomaterials Promote M1/M2 Macrophage Activation. Small 2020, 16, 1907609. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Yao, C.; Yin, X.; Li, C.; Huang, Y.; Wu, M.; Wang, B.; Guo, X.; Wang, Y.; Wu, M. Size, Shape, and Protein Corona Determine Cellular Uptake and Removal Mechanisms of Gold Nanoparticles. Small 2018, 14, 1801451. [Google Scholar] [CrossRef]
- Wang, X.; Cui, X.; Zhao, Y.; Chen, C. Nano-Bio Interactions: The Implication of Size-Dependent Biological Effects of Nanomaterials. Sci. China Life Sci. 2020, 63, 1168–1182. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Tang, P.S.; Chan, W.C.W. The Effect of Nanoparticle Size, Shape, and Surface Chemistry on Biological Systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Jiang, X.; Das, A.; Zhou, Q.; Yu, M.; Jin, R.; Zheng, J. Glomerular Barrier Behaves as an Atomically Precise Bandpass Filter in a Sub-Nanometre Regime. Nat. Nanotechnol. 2017, 12, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.T.; Van Der Vlies, A.J.; Simeoni, E.; Angeli, V.; Randolph, G.J.; O’Neil, C.P.; Lee, L.K.; Swartz, M.A.; Hubbell, J.A. Exploiting Lymphatic Transport and Complement Activation in Nanoparticle Vaccines. Nat. Biotechnol. 2007, 25, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Talamini, L.; Violatto, M.B.; Cai, Q.; Monopoli, M.P.; Kantner, K.; Krpetić, Ž.; Perez-Potti, A.; Cookman, J.; Garry, D.; Silveira, C.P.; et al. Influence of Size and Shape on the Anatomical Distribution of Endotoxin-Free Gold Nanoparticles. ACS Nano 2017, 11, 5519–5529. [Google Scholar] [CrossRef]
- Fan, Z.; Zhu, P.; Zhu, Y.; Wu, K.; Li, C.Y.; Cheng, H. Engineering Long-Circulating Nanomaterial Delivery Systems. Curr. Opin. Biotechnol. 2020, 66, 131–139. [Google Scholar] [CrossRef]
- Kolishetti, N.; Dhar, S.; Valencia, P.M.; Lin, L.Q.; Karnik, R.; Lippard, S.J.; Langer, R.; Farokhzad, O.C. Engineering of Self-Assembled Nanoparticle Platform for Precisely Controlled Combination Drug Therapy. Proc. Natl. Acad. Sci. USA 2010, 107, 17939–17944. [Google Scholar] [CrossRef]
- Qi, H.; Zhou, H.; Tang, Q.; Lee, J.Y.; Fan, Z.; Kim, S.; Staub, M.C.; Zhou, T.; Mei, S.; Han, L.; et al. Block Copolymer Crystalsomes with an Ultrathin Shell to Extend Blood Circulation Time. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Kettler, K.; Giannakou, C.; de Jong, W.H.; Hendriks, A.J.; Krystek, P. Uptake of Silver Nanoparticles by Monocytic THP-1 Cells Depends on Particle Size and Presence of Serum Proteins. J. Nanopart. Res. 2016, 18, 1–9. [Google Scholar] [CrossRef]
- Smith, M.J.; Brown, J.M.; Zamboni, W.C.; Walker, N.J. From Immunotoxicity to Nanotherapy: The Effects of Nanomaterials on the Immune System. Toxicol. Sci. 2014, 138, 249–255. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic Potential of Materials at the Nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; McNeil, S.E. Immunological Properties of Engineered Nanomaterials. In Nanoscience and Technology: A Collection of Reviews from Nature Journals; World Scientific Publishing Co.: Hackensack, NJ, USA, 2009; pp. 278–287. ISBN 9789814287005. [Google Scholar]
- Geng, Y.; Dalhaimer, P.; Cai, S.; Tsai, R.; Tewari, M.; Minko, T.; Discher, D.E. Shape Effects of Filaments versus Spherical Particles in Flow and Drug Delivery. Nat. Nanotechnol. 2007, 2, 249–255. [Google Scholar] [CrossRef] [PubMed]
- van Pomeren, M.; Peijnenburg, W.J.G.M.; Vlieg, R.C.; van Noort, S.J.T.; Vijver, M.G. The Biodistribution and Immuno-Responses of Differently Shaped Non-Modified Gold Particles in Zebrafish Embryos. Nanotoxicology 2019, 13, 558–571. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.K.; Kim, S.; Yoon, H.; Jang, J. Shape-Dependent Cytotoxicity and Proinflammatory Response of Poly(3,4-Ethylenedioxythiophene) Nanomaterials. Small 2010, 6, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Frey, M.; Bobbala, S.; Karabin, N.; Scott, E. Influences of Nanocarrier Morphology on Therapeutic Immunomodulation. Nanomedicine 2018, 13, 1795–1811. [Google Scholar] [CrossRef]
- Solis, A.G.; Bielecki, P.; Steach, H.R.; Sharma, L.; Harman, C.C.D.; Yun, S.; de Zoete, M.R.; Warnock, J.N.; To, S.D.F.; York, A.G.; et al. Mechanosensation of Cyclical Force by PIEZO1 Is Essential for Innate Immunity. Nature 2019, 573, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Chen, W.; Lou, J.; Rittase, W.; Li, K. Mechanosensing through Immunoreceptors. Nat. Immunol. 2019, 20, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Müllner, M.; Dodds, S.J.; Nguyen, T.H.; Senyschyn, D.; Porter, C.J.H.; Boyd, B.J.; Caruso, F. Size and Rigidity of Cylindrical Polymer Brushes Dictate Long Circulating Properties in Vivo. ACS Nano 2015, 9, 1294–1304. [Google Scholar] [CrossRef]
- Cifuentes-Rius, A.; Boase, N.R.B.; Font, I.; Coronas, N.; Ramos-Perez, V.; Thurecht, K.J.; Borrós, S. In Vivo Fate of Carbon Nanotubes with Different Physicochemical Properties for Gene Delivery Applications. ACS Appl. Mater. Interfaces 2017, 9, 11461–11471. [Google Scholar] [CrossRef]
- Kuai, R.; Ochyl, L.J.; Bahjat, K.S.; Schwendeman, A.; Moon, J.J. Designer Vaccine Nanodiscs for Personalized Cancer Immunotherapy. Nat. Mater. 2017, 16, 489–498. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Feng, S.S. Effects of Particle Size and Surface Modification on Cellular Uptake and Biodistribution of Polymeric Nanoparticles for Drug Delivery. Pharm. Res. 2013, 30, 2512–2522. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Li, Y.; Luo, J.; Lee, J.S.; Xiao, W.; Gonik, A.M.; Agarwal, R.G.; Lam, K.S. The Effect of Surface Charge on in Vivo Biodistribution of PEG-Oligocholic Acid Based Micellar Nanoparticles. Biomaterials 2011, 32, 3435–3446. [Google Scholar] [CrossRef] [PubMed]
- Nangia, S.; Sureshkumar, R. Effects of Nanoparticle Charge and Shape Anisotropy on Translocation through Cell Membranes. Langmuir 2012, 28, 17666–17671. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Standley, S.M.; Goh, S.L.; Fréchet, J.M.J. Enhanced Antigen Presentation and Immunostimulation of Dendritic Cells Using Acid-Degradable Cationic Nanoparticles. J. Control. Release 2005, 105, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Fytianos, K.; Chortarea, S.; Rodriguez-Lorenzo, L.; Blank, F.; Von Garnier, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Aerosol Delivery of Functionalized Gold Nanoparticles Target and Activate Dendritic Cells in a 3D Lung Cellular Model. ACS Nano 2017, 11, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Lunov, O.; Syrovets, T.; Loos, C.; Beil, J.; Delacher, M.; Tron, K.; Nienhaus, G.U.; Musyanovych, A.; Mailänder, V.; Landfester, K.; et al. Differential Uptake of Functionalized Polystyrene Nanoparticles by Human Macrophages and a Monocytic Cell Line. ACS Nano 2011, 5, 1657–1669. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Wei, T.; Farbiak, L.; Johnson, L.T.; Dilliard, S.A.; Siegwart, D.J. Selective Organ Targeting (SORT) Nanoparticles for Tissue-Specific MRNA Delivery and CRISPR–Cas Gene Editing. Nat. Nanotechnol. 2020, 15, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Gan, Y.J.; Iqbal, S.; Jiang, W.; Yuan, Y.Y.; Wang, J. Delivery of Tacrolimus with Cationic Lipid-Assisted Nanoparticles for Ulcerative Colitis Therapy. Biomater. Sci. 2018, 6, 1916–1922. [Google Scholar] [CrossRef]
- Li, Y.; Yang, H.Y.; Thambi, T.; Park, J.H.; Lee, D.S. Charge-Convertible Polymers for Improved Tumor Targeting and Enhanced Therapy. Biomaterials 2019, 217, 119299. [Google Scholar] [CrossRef]
- Elci, S.G.; Jiang, Y.; Yan, B.; Kim, S.T.; Saha, K.; Moyano, D.F.; Yesilbag Tonga, G.; Jackson, L.C.; Rotello, V.M.; Vachet, R.W. Surface Charge Controls the Suborgan Biodistributions of Gold Nanoparticles. ACS Nano 2016, 10, 5536–5542. [Google Scholar] [CrossRef]
- Cesta, M.F. Normal Structure, Function, and Histology of the Spleen. Toxicol. Pathol. 2006, 34, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Gowd, V.; Ahmad, A.; Tarique, M.; Suhail, M.; Zughaibi, T.A.; Tabrez, S.; Khan, R. Advancement of Cancer Immunotherapy Using Nanoparticles-Based Nanomedicine. Semin. Cancer Biol. 2022, 86, 624–644. [Google Scholar] [CrossRef]
- Caraglia, M.; Marra, M.; Misso, G.; Lamberti, M.; Salzano, G.; De Rosa, G.; Abbruzzese, A. Tumour-Specific Uptake of Anti-Cancer Drugs: The Future Is Here. Curr. Drug Metab. 2011, 13, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Madhunapantula, S.V.; Robertson, G.P. Toxicological Considerations When Creating Nanoparticle-Based Drugs and Drug Delivery Systems. Expert Opin. Drug Metab. Toxicol. 2012, 8, 47–69. [Google Scholar] [CrossRef] [PubMed]
- Savolainen, K.; Alenius, H.; Norppa, H.; Pylkkänen, L.; Tuomi, T.; Kasper, G. Risk Assessment of Engineered Nanomaterials and Nanotechnologies-A Review. Toxicology 2010, 269, 92–104. [Google Scholar] [CrossRef]
- Sun, D. Nanoparticles Are the Future of Medicine–Researchers Are Experimenting with New Ways to Design Tiny Particle Treatments for Cancer. Available online: https://theconversation.com/nanoparticles-are-the-future-of-medicine-researchers-are-experimenting-with-new-ways-to-design-tiny-particle-treatments-for-cancer-180009 (accessed on 10 December 2022).
- Yavuz, B.; Pehlivan, S.B.; Vural, I.; Ünlü, N. In Vitro/In Vivo Evaluation of Dexamethasone - PAMAM Dendrimer Complexes for Retinal Drug Delivery. J. Pharm. Sci. 2015, 104, 3814–3823. [Google Scholar] [CrossRef]
- Madaan, K.; Kumar, S.; Poonia, N.; Lather, V.; Pandita, D. Dendrimers in Drug Delivery and Targeting: Drug-Dendrimer Interactions and Toxicity Issues. J. Pharm. Bioallied Sci. 2014, 6, 139–150. [Google Scholar] [CrossRef]
- Lynn Kirkpatrick, D.; Weiss, M.; Naumov, A.; Bartholomeusz, G.; Bruce Weisman, R.; Gliko, O. Carbon Nanotubes: Solution for the Therapeutic Delivery of SiRNA? Materials 2012, 5, 278–301. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, M.; Liu, C. Advances in Nanotechnology-Based Immunotherapy for Glioblastoma. Front. Immunol. 2022, 13, 1890. [Google Scholar] [CrossRef]
- Luo, L.; Shu, R.; Wu, A. Nanomaterial-Based Cancer Immunotherapy. J. Mater. Chem. B 2017, 5, 5517–5531. [Google Scholar] [CrossRef]
- Cruz, L.J.; Tacken, P.J.; Fokkink, R.; Joosten, B.; Stuart, M.C.; Albericio, F.; Torensma, R.; Figdor, C.G. Targeted PLGA Nano- but Not Microparticles Specifically Deliver Antigen to Human Dendritic Cells via DC-SIGN in Vitro. J. Control. Release 2010, 144, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Toyota, H.; Yanase, N.; Yoshimoto, T.; Harada, M.; Kat, Y.; Mizuguchi, J. Vaccination with OVA-Bound Nanoparticles Encapsulating IL-7 Inhibits the Growth of OVA-Expressing E.G7 Tumor Cells in Vivo. Oncol. Rep. 2015, 33, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.N.; Vokali, E.; Lund, A.W.; Hubbell, J.A.; Swartz, M.A. Targeting the Tumor-Draining Lymph Node with Adjuvanted Nanoparticles Reshapes the Anti-Tumor Immune Response. Biomaterials 2014, 35, 814–824. [Google Scholar] [CrossRef]
- De Titta, A.; Ballester, M.; Julier, Z.; Nembrini, C.; Jeanbart, L.; Van Der Vlies, A.J.; Swartz, M.A.; Hubbell, J.A. Nanoparticle Conjugation of CpG Enhances Adjuvancy for Cellular Immunity and Memory Recall at Low Dose. Proc. Natl. Acad. Sci. USA 2013, 110, 19902–19907. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Kwak, M.; Chauhan, P.S.; Puranik, N.; Lee, P.C.W.; Jin, J.O. Cancer Immunotherapy by Immune Checkpoint Blockade and Its Advanced Application Using Bio-Nanomaterials. Semin. Cancer Biol. 2022, 86, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Reda, M.; Ngamcherdtrakul, W.; Nelson, M.A.; Siriwon, N.; Wang, R.; Zaidan, H.Y.; Bejan, D.S.; Reda, S.; Hoang, N.H.; Crumrine, N.A.; et al. Development of a Nanoparticle-Based Immunotherapy Targeting PD-L1 and PLK1 for Lung Cancer Treatment. Nat. Commun. 2022, 13, 1–11. [Google Scholar] [CrossRef]
- Le, Q.V.; Yang, G.; Wu, Y.; Jang, H.W.; Shokouhimehr, M.; Oh, Y.K. Nanomaterials for Modulating Innate Immune Cells in Cancer Immunotherapy. Asian J. Pharm. Sci. 2019, 14, 16–29. [Google Scholar] [CrossRef]
- Shams, F.; Golchin, A.; Azari, A.; Mohammadi Amirabad, L.; Zarein, F.; Khosravi, A.; Ardeshirylajimi, A. Nanotechnology-Based Products for Cancer Immunotherapy. Mol. Biol. Rep. 2021, 49, 1389–1412. [Google Scholar] [CrossRef]
- Li, Z.; Tan, S.; Li, S.; Shen, Q.; Wang, K. Cancer Drug Delivery in the Nano Era: An Overview and Perspectives (Review). Oncol. Rep. 2017, 38, 611–624. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Yadav, D.; Tayal, S.; Jin, J.-O. Therapeutic Advancements in the Management of Diabetes Mellitus with Special Reference to Nanotechnology. Curr. Pharm. Des. 2020, 26, 4909–4916. [Google Scholar] [CrossRef]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective Use of Nanocarriers as Drug Delivery Systems for the Treatment of Selected Tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Zhang, Z. The Application of Nanotechnology in Immune Checkpoint Blockade for Cancer Treatment. J. Control. Release 2018, 290, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery Technologies for Cancer Immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef] [PubMed]

| Nanomaterial Composition, Carrier | Payloads | Therapy | Properties | Outcomes | References |
|---|---|---|---|---|---|
| Lipid-calcium-phosphate NP, liposome-protamine-hyaluronic acid NP | Trp 2 peptide, CpG oligonucleotide, siRNA | Cytokines or chemokines modulation | Down-regulate TGF-β, increase CD8+ T cells levels, decrease Treg cells level | Dramatically increase levels of tumor-infiltrating CD8+ T cells and decrease Treg cells level | [66] |
| Lipid-coated protamine DNA complexes | Plasmid DNA encoding TNF-related apoptosis- TRAIL-inducing ligand protein | Cellular modulation | Generate approximately 70% of TAFs as sTRAIL-producing cells | Nanoparticles to modify tumor-associated fibroblasts (TAFs) as an effective strategy to treat desmoplastic cancers | [67] |
| Porous silicon microparticle | HER2 antigen | DC-based vaccine | High IFN-I and MHC II levels cause CD11c+ DC infiltration | PSM stimulate DC-based cancer immunotherapy | [68] |
| Mannose-modified PLGA nanoplatforms | mannose | Cellular modulation | Deplete M2 TAMs | Lower uptake by regular macrophages | [69] |
| pH-responsive poly (propylacrylic acid) nanocomplex | α-galactosylceramide (α-GalCer) | Peptide-based vaccine | Improve antigen-specific CD8+ T cells responses | Peptide/pPAA nanoplexes are a simple way to increase CD8+ T cell responses to peptide antigens | [70] |
| Au-SGSH nanocomplex | Melanoma antigen (MART1)-encoded DNA vaccine | Nucleic acid-based vaccine | Increase the levels of TNF-α and induce a large amount of CD11c+ DC infiltration | Potential use for in vivo DC-targeted genetic immunization against cancer | [71] |
| PLGA NPs | TLR-4 and PTX agonist | Chemotherapy-induced ICD | Increase APCs and T cells’ activation ability | Increased cancer-fighting power, fewer side effects, and simplified administration | [72] |
| Multifunctional near-infrared (NIR)-responsive core-shell nanoparticles | gardiquimod | PTT-induced immunotherapy | Activate and increase tumor infiltration of CD8+ T and DCs cells, release TAAs | Photothermal immunotherapeutic potential | [73] |
| Trastuzumab-loaded polyacid nanoparticles | trastuzumab | Tumor-targeted antibody therapy | Signaling transduction and cell-mediated cytotoxicity | PLGA NPs may include TZ and conventional chemotherapeutics | [74] |
| Poly (ethylene glycol)-block-poly (d,l-lactide) copolymer | CTLA4 small interfering RNA (siRNA) | - | Stimulate T cell activation proliferation by silencing the CTLA4 molecules | Efficient cancer immunotherapy with nanoparticles for melanoma | [75] |
| RGD-modified single-walled carbon nanotube as artificial tobacco mosaic virus | doxorubicin | Oncolytic virotherapy | Cytomembrane penetration and endoplasmic reticulum disruption cause Ca2+ release | Induce robust composite oncolytic processes, including cytomembrane penetration | |
| MnOx nanospikes | Ovalbumin | Protein-based vaccine | Secretion levels of IL-6 and TNF-α | Effectively inhibit primary/distal tumor growth and tumor metastasis | [76] |
| Poloxamer 407 | Anti-CTLA4 antibodies | Anti-CTLA4 therapy | Decrease systemic antibody levels | Effectively slow down tumor growth, whilst significantly reducing serum anti-CTLA-4 levels | [77] |
| Fucoidan-dextran-based magnetic nanomedicine | Anti-CD3, anti-CD28, anti-PD-L1 | Anti-PD-1/PD-L1 therapy | Decrease the chaotic distribution of anti-PD-L1 and decrease the toxic effects caused by off-target effects. | Potential of integrating anti-PD-L1 and T cell activators | [78] |
| Photosensitizer (HPPH)-coated αvβ6-targeting peptide-functionalized graphene oxide | Photosensitizer | PDT (photodynamic therapy)-induced ICD | Increase cytotoxic CD8+ T lymphocytes infiltration | PDT using GO(HPPH)-PEG-HK may ablate primary tumors | [79] |
| Fluid lipid bilayer supported by mesoporous silica micro-rods | IL-2, anti-CD28, anti-CD3 | ACT | T cell polyclonal growth is increased two to tenfold | APC-ms enables antigen-specific expansion of rare cytotoxic T cell subpopulations | [80] |
| Nanomaterial Composition | Parameter of Nanomaterial | Model | Immune Cells | Outcomes | References |
|---|---|---|---|---|---|
| Poly lactic-co-glycolic acid nanoparticles (MSC-PD-L1+ NPs) | Surface: mesenchymal stem cell membrane | In vivo: intravenous administration | T cells and macrophages | This strategy has been shown to potentially treat various cancers’ immunotherapy-associated irAE in clinical applications. | [92] |
| Biodegradable polymeric nanoparticles | Surface: natural erythrocyte membranes | In vivo: intravenous administration | Macrophages | After 72 h after receiving the particle injection, the biodistribution analysis found considerable particle retention in the blood. | [93] |
| Polymeric nanoparticles | Surface: plasma membrane of human platelets | In vivo: intravenous administration | Macrophages | Platelet-mimetic nanoparticles enhanced therapeutic efficacy. | [94] |
| Plasma polymerization | Surface: hydrophobic, hydrophilic groups | In vitro in vivo: intravenous administration | Macrophages, monocytes, and splenocytes | Surface modifications were made to modulate serum protein adsorption and to achieve the desirable innate immune response to implanted biomaterials and devices. | [95] |
| Gold nanoparticles (Au NPs) | Surface: inverse phosphocholine lipids | In vivo: intravenous administration | Neutrophils | It has demonstrated the importance of hydrophobicity in IS activation. | [96] |
| Polymeric nanoparticles | Surface: Poly-ethylene-alt-maleic anhydride | In vivo: intravenous administration | Macrophages, neutrophils, monocytes | These particles might be used in trauma and to treat inflammatory diseases. | [97] |
| Antigen-capturing nanoparticles (AC-NPs) | Surface: MalAC | In vivo: intratumorally | T cells and DCs | This model might be used for cancer immunotherapy. | [98] |
| Au NPs | Surface: PEG | In vitro | Human dermal fibroblast | This increases the level of IL-6. | [99] |
| PLGA | Surface: PEG | In vivo: subcutaneous injection | Neutrophils and DCs | This induced immune tolerance through subcutaneous administration. | [100] |
| Mesoporous silica | Surface: thiol, amino, and PEG | In vivo: intravenous injection | Macrophages and T cells | This increases TGF-β and T cells. | [101] |
| Lipoplexes | Charge: negatively | In vivo: intravenous administration | Macrophages and plasmacytoid DCs | This increases the release of IFNα and DC maturation. | [102] |
| Liposome; polyglutamic acid; chitosan | Charge: negatively | In vitro | Complement and platelet system | This increases complement activation and P-selection. | [103] |
| Cationic polymers | Charge: positively | In vivo: intraperitoneal injection | Peritoneal macrophages and spleen cells | These increase the level of TNFα, IL-12, and Th1 responses. | [104] |
| Gold nanoparticles | Charge: positively | In vitro | U937 cells and human lymphoma cell line | These increase the production of IL-6. | [105] |
| Cationic nanohydrogel | Charge: positively | In vivo: pulmonary immunization | T cells, B cells, and DCs | This increases activated CD4+ T, Germinal center B cells expansion, and activated DCs. | |
| Lipid nanoparticles | Charge: positively | In vitro In vivo: intravenous administration | Bone marrow-derived dendritic cells, cytotoxic T lymphocytes, and CD11b- cells | These increase ROS generation and CCL2 expression, type I interferon response, Th1 cytokines expression (IL-2, IFNγ, TNFα), and CD8+ T cell response. | [106] |
| Superparamagnetic iron oxide | Charge: positively | In vitro | DCs | This increases the antigen cross-presentation. | [107] |
| Stiff-nanocapsules | Rigidity: silica | In vitro | RAW264.7 cells, Murine macrophage cell line | These increase cellular uptake. | [108] |
| PLGA | Rigidity: soft-emulsion droplets | In vivo: subcutaneous vaccination | DCs | This increases DCs and CD86+. | [109,110] |
| Hydrogel | Rigidity: | In vivo: intravenous administration | Spleen cells | This increases the spleen retention. | [111] |
| Lipid-coated alginate | Rigidity: soft-microparticles with low modulus | In vitro | CD8+ T cells | This increases the activated CD8+ T cells. | [112] |
| Polysaccharides | Rigidity: soft-hollow capsules | In vivo: subcutaneous injection | T cells and DCs | These increase the activation of T cells and DCs and increase lymph node targeting. | [113,114] |
| Polymeric particles | Shape: tetrahedron | In vitro In vivo: intravenous injection | Peripheral blood mononuclear cells, RAW264.7 cells, murine macrophage cell line | These increase the level of IL-6, TNF-α, and IFN response. | [115,116] |
| Polymeric particles | Shape: spherical | In vitro In vivo: intravenous injection | Neutrophils in normal and encephalomyelitis-inflamed mouse blood | These decrease cellular uptake. | [117] |
| Polymer capsules | Shape: rod | In vitro | Human monocyte-derived macrophages | These increase the level of IL-8, TNF-α. | [118] |
| TiO2 microparticles | Shape: spike | In vitro | Bone marrow-derived macrophages and dendritic cells | These increase CD40, IL-1β, and IFN-γ. | [119] |
| Antigen-decorated microparticles | Size: 500 nm diameter | In vivo: intravenous injection | T cells | These increase long-term T cell tolerance, T cell anergy, and regulatory T cell activation. | [120] |
| Antigen-loaded polylactide particles | Size: 200–600 nm | In vivo: intramuscular injection | J774A.1 cells, murine alveolar macrophage cell line | These decrease Th2-type immune response, IL-4, and MHC-II expression and antibody titers. | [121] |
| Superparamagnetic iron oxide | Size: 50 nm | In vitro | Human CD8+ T cells | The activation of T cells occurs. | [80,122,123] |
| Small-size silver nanoparticles | Size: 5, 10, and 50 nm | In vitro | Human neutrophil | These increase ROS, NADPH oxidase, and intracellular calcium. | [124] |
| Polypyrrole nanoparticles | Size: 5 nm | In vitro | J774A.1 cells and murine alveolar macrophage cell line | These increase IL-6, IL-1, and TNF-α. | [125] |
| Polypyrrole nanoparticles | Size: 20, 40, 60, 80, and 100 nm | In vitro | J774A.1 cells and murine alveolar macrophage cell line | These decrease CD86 and increase CD40, CD80. | [126] |
| Silica−Titania hollow nanoparticles | Size: 25, 50, 75, 100, and 125 nm | In vitro | J774A.1 cells and murine alveolar macrophage cell line | IL-1, IL-6, and TNF-α. These increase TNF-α, IL-1, and IL-6. | [126] |
| Graphene oxide | Size:(10–40 μm) and (50–300 μm) | In vitro | Human monocyte-derived macrophages | This increases IL-1β and decreases IL-10. | [127] |
| Inorganic nanoparticles, particularly iron oxide (IO) and gold (Au) | Size: 4 nm | In vitro | RAW264.7 cells and murine macrophage cell line | These increase M1 polarization and decrease M2 transformation. | [128] |
| Silver particles | Size: 4 nm | In vitro | U937 cells, human lymphoma cell line | These increase IL-8 and ROS. | [129] |
| Carbon nanomaterials | Size: 15, 50, 140 nm | In vitro | THP-1 cells, human monocyte cell line | These increase 5 nm: M2 macrophages, 50 nm: M1/M2 macrophage and 140 nm: M1 macrophages. | [130] |
| Types of Nanocarriers | Drawbacks | Benefits |
|---|---|---|
| Metallic nanoparticles | Particles’ instability, impurity, biologically harmful, explosion, difficulty in synthesis, toxicity | Biocompatible; strong plasma uptake; and uniformity in size, shape, and branch length. Tuned pharmacokinetics and biodistribution |
| Dendrimers | Low hydro solubility and high non-specific toxicity, poly(amidoamine) (PAMAM) dendrimers, and PPI dendrimers attributed toward toxic manifestations | Water soluble and biocompatible, good PK behavior, flexibility in conjugation chemistry, and ability to encapsulate and deliver various bioactive agents [169,170] |
| Polymeric micelles | The low payload of drugs and less stability in an aqueous medium | Biodegradable, self-assembling, and biocompatible. Potential targeting of functional modification, efficient carrier system for hydrophilic drugs, biodegradable |
| Carbon nanotubes | Poorly soluble in water, not biodegradable, toxicity concerns, poor PK (pharmacokinetics) | Ease of synthesis and conjugation of multiple bioactive agents, large surface area, ability to encapsulate and deliver various types of bioactive agents, protects entrapped drug and provides sustained release [171] |
| Liposomes | Fewer stables, leakage, and fusion of encapsulated drug/molecules; high production cost; some may be allergic | Targeted to specific cells or tissues, biocompatible, longer duration of circulation, high stability via encapsulation, high efficacy and therapeutic index of drug |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wahab, S.; Ghazwani, M.; Hani, U.; Hakami, A.R.; Almehizia, A.A.; Ahmad, W.; Ahmad, M.Z.; Alam, P.; Annadurai, S. Nanomaterials-Based Novel Immune Strategies in Clinical Translation for Cancer Therapy. Molecules 2023, 28, 1216. https://doi.org/10.3390/molecules28031216
Wahab S, Ghazwani M, Hani U, Hakami AR, Almehizia AA, Ahmad W, Ahmad MZ, Alam P, Annadurai S. Nanomaterials-Based Novel Immune Strategies in Clinical Translation for Cancer Therapy. Molecules. 2023; 28(3):1216. https://doi.org/10.3390/molecules28031216
Chicago/Turabian StyleWahab, Shadma, Mohammed Ghazwani, Umme Hani, Abdulrahim R. Hakami, Abdulrahman A. Almehizia, Wasim Ahmad, Mohammad Zaki Ahmad, Prawez Alam, and Sivakumar Annadurai. 2023. "Nanomaterials-Based Novel Immune Strategies in Clinical Translation for Cancer Therapy" Molecules 28, no. 3: 1216. https://doi.org/10.3390/molecules28031216
APA StyleWahab, S., Ghazwani, M., Hani, U., Hakami, A. R., Almehizia, A. A., Ahmad, W., Ahmad, M. Z., Alam, P., & Annadurai, S. (2023). Nanomaterials-Based Novel Immune Strategies in Clinical Translation for Cancer Therapy. Molecules, 28(3), 1216. https://doi.org/10.3390/molecules28031216







