MDACl2-Modified SnO2 Film for Efficient Planar Perovskite Solar Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Device Performance Distribution
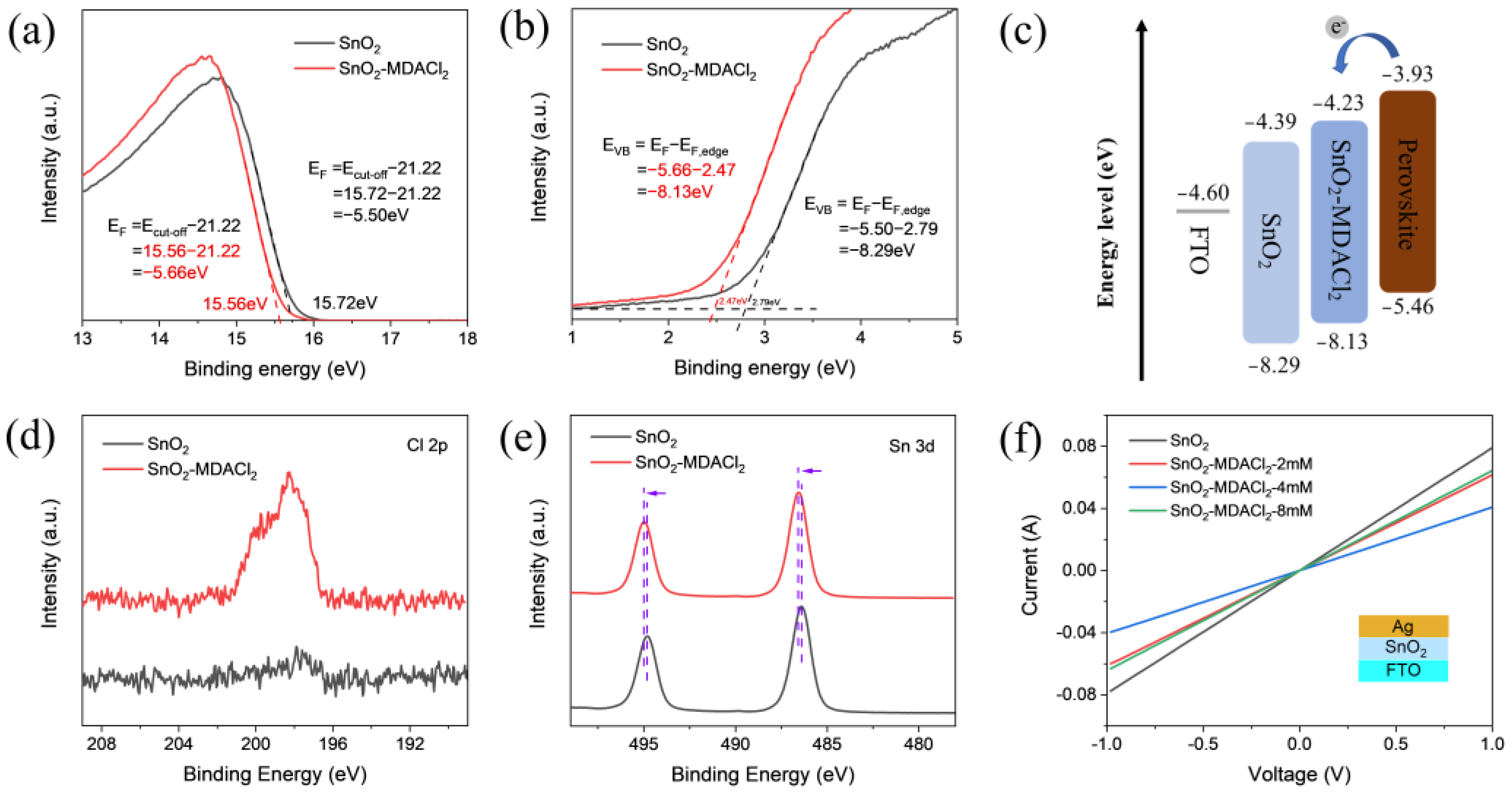
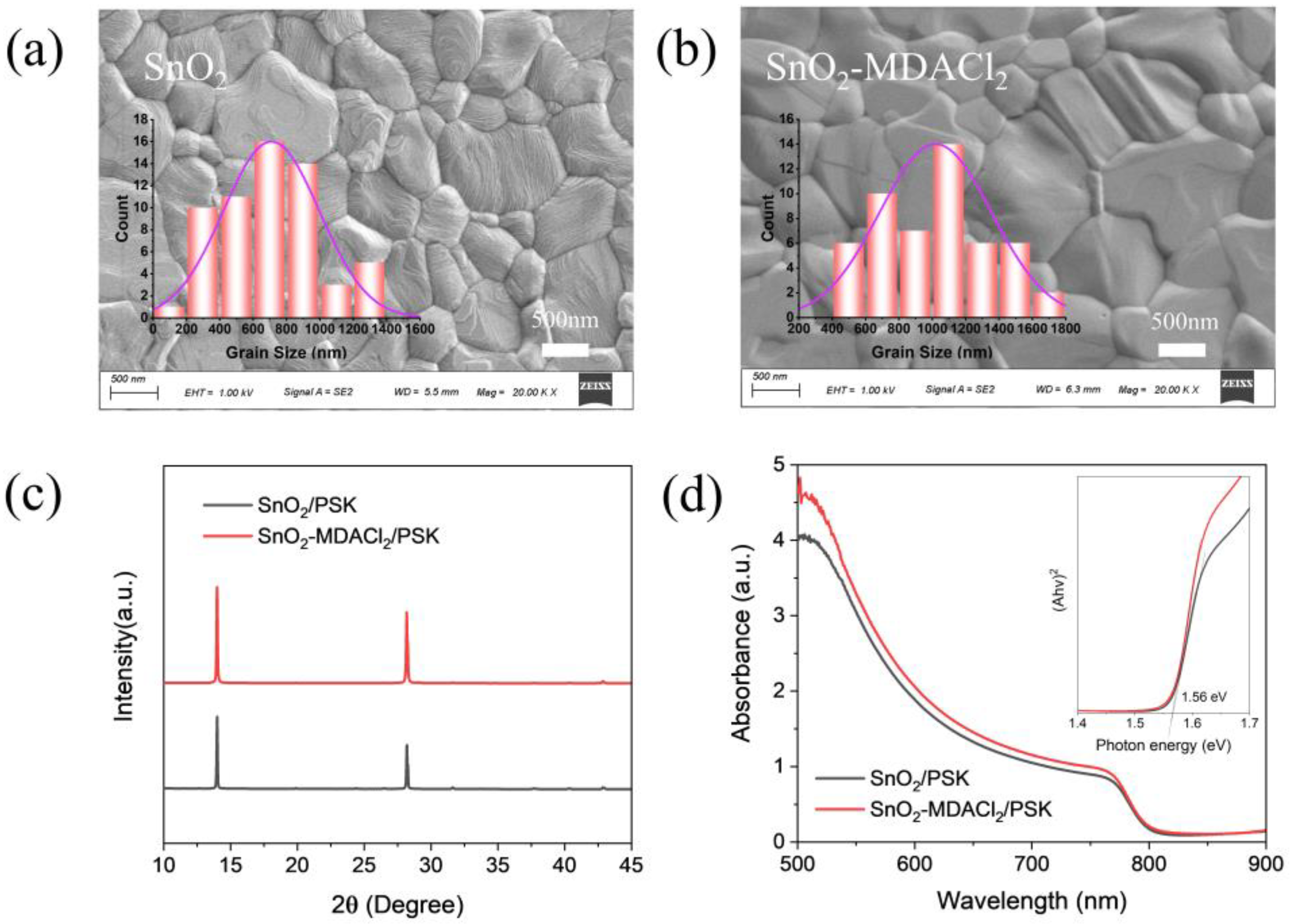
2.2. ETL Characterizations
2.3. Perovskite Film Characterizations
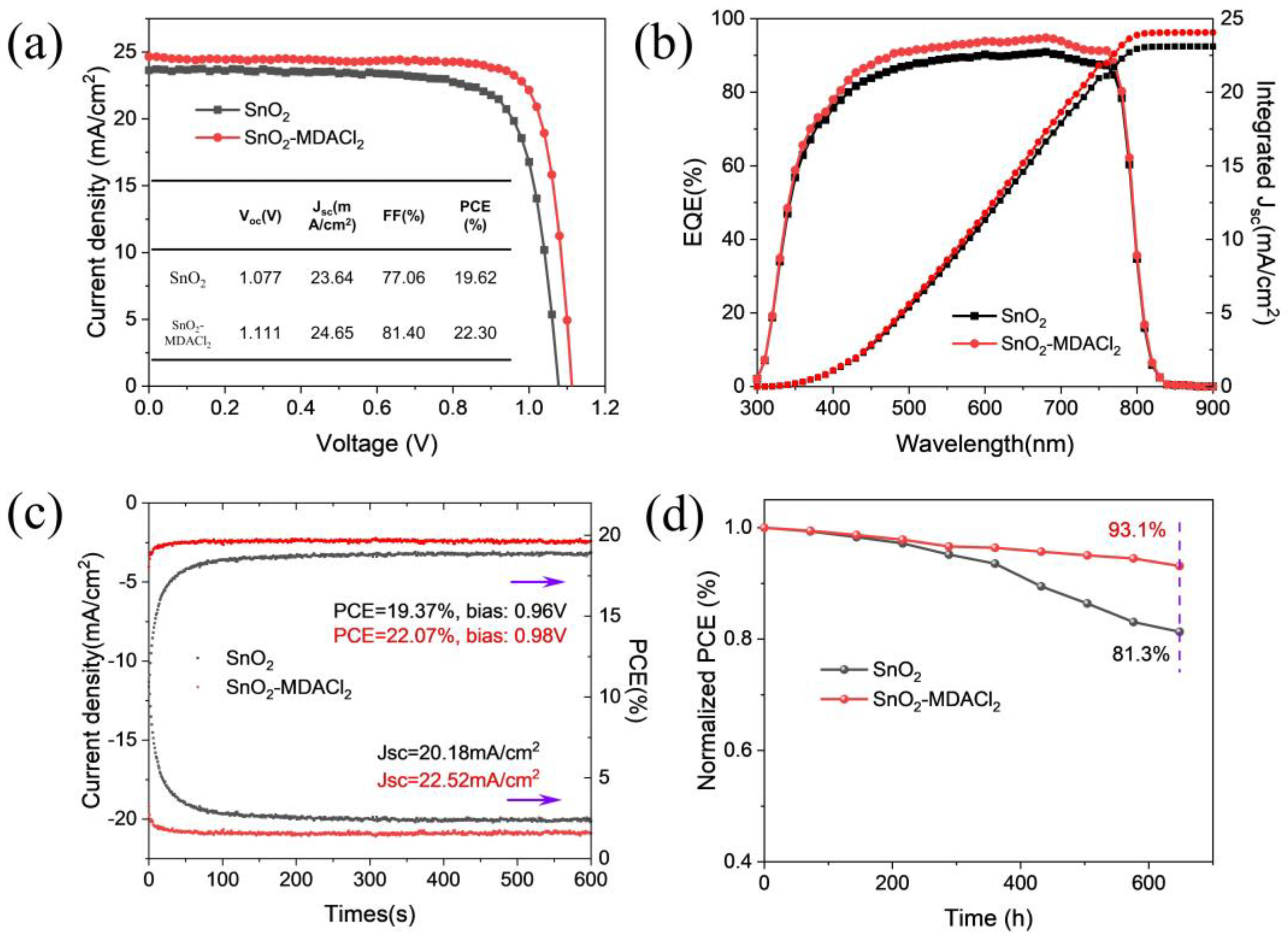
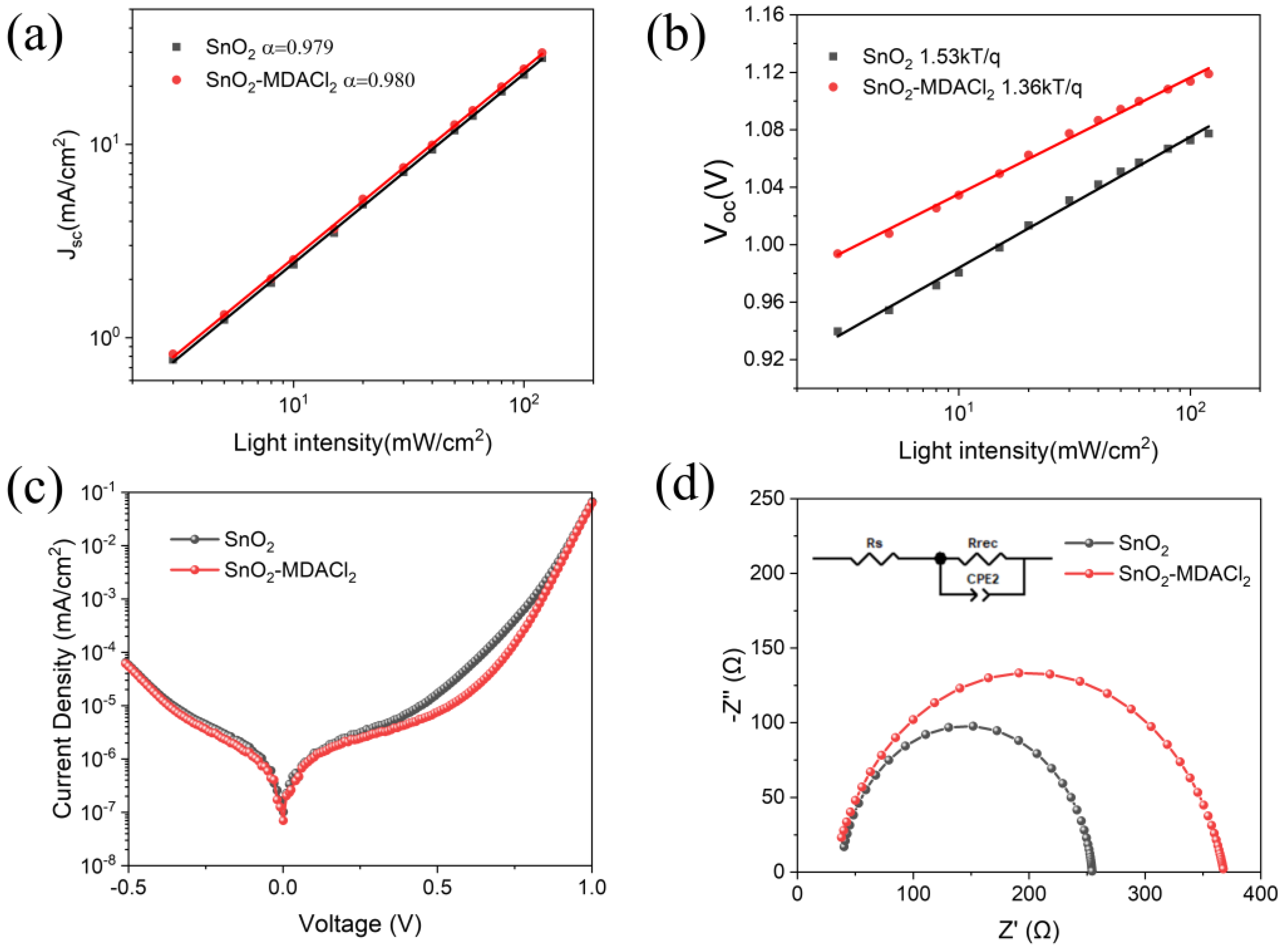
2.4. Device Characterizations
3. Materials and Methods
3.1. Materials
3.2. Device Fabrication
3.3. Characterizations
3.4. Device Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Li, H.; Zhang, W. Perovskite Tandem Solar Cells: From Fundamentals to Commercial Deployment. Chem. Rev. 2020, 120, 9835–9950. [Google Scholar] [CrossRef]
- Schileo, G.; Grancini, G. Lead or no lead? Availability, toxicity, sustainability and environmental impact of lead-free perovskite solar cells. J. Mater. Chem. C 2021, 9, 67–76. [Google Scholar] [CrossRef]
- Wu, G.B.; Liang, R.; Ge, M.Z.; Sun, G.X.; Zhang, Y.; Xing, G.C. Surface Passivation Using 2D Perovskites toward Efficient and Stable Perovskite Solar Cells. Adv. Mater. 2022, 34, 2105635. [Google Scholar] [CrossRef] [PubMed]
- You, P.; Li, G.J.; Tang, G.Q.; Cao, J.P.; Yan, F. Ultrafast laser-annealing of perovskite films for efficient perovskite solar cells. Energy Environ. Sci. 2020, 13, 1187–1196. [Google Scholar] [CrossRef]
- Green, M.A.; Ho-Baillie, A. Perovskite Solar Cells: The Birth of a New Era in Photovoltaics. ACS Energy Lett. 2017, 2, 822–830. [Google Scholar] [CrossRef]
- Tai, Q.; Guo, X.; Tang, G.; You, P.; Ng, T.W.; Shen, D.; Cao, J.; Liu, C.K.; Wang, N.; Zhu, Y. Antioxidant grain passivation for air-stable tin-based perovskite solar cells. Angew. Chem. Int. Ed. 2019, 58, 806–810. [Google Scholar] [CrossRef]
- Al-Dainy, G.A.; Bourdo, S.E.; Saini, V.; Berry, B.C.; Biris, A.S. Hybrid Perovskite Photovoltaic Devices: Properties, Architecture, and Fabrication Methods. Energy Technol. 2017, 5, 373–401. [Google Scholar] [CrossRef]
- Li, J.Y.; Han, Z.Y.; Gu, Y.; Yu, D.J.; Liu, J.X.; Hu, D.W.; Xu, X.B.; Zeng, H.B. Perovskite Single Crystals: Synthesis, Optoelectronic Properties, and Application. Adv. Funct. Mater. 2021, 31, 2008684. [Google Scholar] [CrossRef]
- Oku, T. Crystal structures of perovskite halide compounds used for solar cells. Rev. Adv. Mater. Sci. 2020, 59, 264–305. [Google Scholar] [CrossRef]
- Roghabadi, F.A.; Ahmadi, N.; Ahmadi, V.; Di Carlo, A.; Aghmiuni, K.O.; Tehrani, A.S.; Ghoreishi, F.S.; Payandeh, M.; Fumani, N.M.R. Bulk heterojunction polymer solar cell and perovskite solar cell: Concepts, materials, current status, and opto-electronic properties. Sol. Energy 2018, 173, 407–424. [Google Scholar] [CrossRef]
- You, P.; Liu, Z.; Tai, Q.; Liu, S.; Yan, F. Efficient semitransparent perovskite solar cells with graphene electrodes. Adv. Mater. 2015, 27, 3632–3638. [Google Scholar] [CrossRef] [PubMed]
- Jost, M.; Kegelmann, L.; Korte, L.; Albrecht, S. Monolithic Perovskite Tandem Solar Cells: A Review of the Present Status and Advanced Characterization Methods Toward 30% Efficiency. Adv. Energy Mater. 2020, 10, 1904102. [Google Scholar] [CrossRef]
- Veeramalai, C.P.; Feng, S.; Zhang, X.M.; Pammi, S.V.N.; Pecunia, V.; Li, C.A.B. Lead-halide perovskites for next-generation self-powered photodetectors: A comprehensive review. Photonics Res. 2021, 9, 968–991. [Google Scholar] [CrossRef]
- Mei, F.; Sun, D.; Mei, S.; Feng, J.; Zhou, Y.; Xu, J.; Xiao, X. Recent progress in perovskite-based photodetectors: The design of materials and structures. Adv. Phys. X 2019, 4, 1592709. [Google Scholar] [CrossRef]
- Andreani, L.C.; Bozzola, A.; Kowalczewski, P.; Liscidini, M.; Redorici, L. Silicon solar cells: Toward the efficiency limits. Adv. Phys. X 2019, 4, 1548305. [Google Scholar] [CrossRef]
- You, P.; Tang, G.; Yan, F. Two-dimensional materials in perovskite solar cells. Mater. Today Energy 2019, 11, 128–158. [Google Scholar] [CrossRef]
- Sum, T.C.; Mathews, N. Advancements in perovskite solar cells: Photophysics behind the photovoltaics. Energy Environ. Sci. 2014, 7, 2518–2534. [Google Scholar] [CrossRef]
- Gao, P.; Gratzel, M.; Nazeeruddin, M.K. Organohalide lead perovskites for photovoltaic applications. Energy Environ. Sci. 2014, 7, 2448–2463. [Google Scholar] [CrossRef]
- Rong, Y.G.; Hu, Y.; Mei, A.Y.; Tan, H.R.; Saidaminov, M.I.; Seok, S.I.; McGehee, M.D.; Sargent, E.H.; Han, H.W. Challenges for commercializing perovskite solar cells. Science 2018, 361, eaat8235. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- NERL. Best Research Cell Efficiencies. Available online: www.nrel.gov/pv/cell-efficiency.html (accessed on 23 February 2023).
- Dai, Z.; Yadavalli, S.K.; Chen, M.; Abbaspourtamijani, A.; Qi, Y.; Padture, N.P. Interfacial toughening with self-assembled monolayers enhances perovskite solar cell reliability. Science 2021, 372, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, M.M.; Yadav, P.; Tavakoli, R.; Kong, J. Surface Engineering of TiO2 ETL for Highly Efficient and Hysteresis-Less Planar Perovskite Solar Cell (21.4%) with Enhanced Open-Circuit Voltage and Stability. Adv. Energy Mater. 2018, 8, 1800794. [Google Scholar] [CrossRef]
- Yang, D.; Zhou, X.; Yang, R.; Yang, Z.; Yu, W.; Wang, X.; Li, C.; Liu, S.; Chang, R.P.H. Surface optimization to eliminate hysteresis for record efficiency planar perovskite solar cells. Energy Environ. Sci. 2016, 9, 3071–3078. [Google Scholar] [CrossRef]
- Mahmood, K.; Sarwar, S.; Mehran, M.T. Current status of electron transport layers in perovskite solar cells: Materials and properties. RSC Adv. 2017, 7, 17044–17062. [Google Scholar] [CrossRef]
- Wang, P.; Li, R.; Chen, B.; Hou, F.; Zhang, J.; Zhao, Y.; Zhang, X. Gradient Energy Alignment Engineering for Planar Perovskite Solar Cells with Efficiency Over 23%. Adv. Mater. 2020, 32, 1905766. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, H.; Feng, S.; Yang, L.; Dong, H.; Wang, J.; Tian, C.; Li, L.; Lu, H.; Jeong, J.; et al. Modulation of perovskite crystallization processes towards highly efficient and stable perovskite solar cells with MXene quantum dot-modified SnO2. Energy Environ. Sci. 2021, 14, 3447–3454. [Google Scholar] [CrossRef]
- Zhu, P.; Gu, S.; Luo, X.; Gao, Y.; Li, S.; Zhu, J.; Tan, H. Simultaneous Contact and Grain-Boundary Passivation in Planar Perovskite Solar Cells Using SnO2-KCl Composite Electron Transport Layer. Adv. Energy Mater. 2020, 10, 1903083. [Google Scholar] [CrossRef]
- Masood, M.T.; Qudsia, S.; Hadadian, M.; Weinberger, C.; Nyman, M.; Ahläng, C.; Dahlström, S.; Liu, M.; Vivo, P.; Österbacka, R. Investigation of well-defined pinholes in TiO2 electron selective layers used in planar heterojunction perovskite solar cells. Nanomaterials 2020, 10, 181. [Google Scholar] [CrossRef]
- Culu, A.; Kaya, I.C.; Sonmezoglu, S. Spray-Pyrolyzed Tantalium-Doped TiO2 Compact Electron Transport Layer for UV-Photostable Planar Perovskite Solar Cells Exceeding 20% Efficiency. ACS Appl. Energy Mater. 2022, 5, 3454–3462. [Google Scholar] [CrossRef]
- Huang, H.; Cui, P.; Chen, Y.; Yan, L.; Yue, X.; Qu, S.; Wang, X.; Du, S.; Liu, B.; Zhang, Q. 24.8%-efficient planar perovskite solar cells via ligand-engineered TiO2 deposition. Joule 2022, 6, 2186–2202. [Google Scholar] [CrossRef]
- Wu, M.; Duan, Y.; Yang, L.; You, P.; Li, Z.; Wang, J.; Zhou, H.; Yang, S.; Xu, D.; Zou, H. Multifunctional Small Molecule as Buried Interface Passivator for Efficient Planar Perovskite Solar Cells. Adv. Funct. Mater. 2023, 2300128. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, X.; You, J. SnO2: A Wonderful Electron Transport Layer for Perovskite Solar Cells. Small 2018, 14, 1801154. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Guo, Y.; Wen, J.; Liu, H.; Yang, G.; Qin, P.; Fang, G. Review on the Application of SnO2 in Perovskite Solar Cells. Adv. Funct. Mater. 2018, 28, 1802757. [Google Scholar] [CrossRef]
- Ke, W.; Fang, G.; Liu, Q.; Xiong, L.; Qin, P.; Tao, H.; Wang, J.; Lei, H.; Li, B.; Wan, J.; et al. Low-Temperature Solution-Processed Tin Oxide as an Alternative Electron Transporting Layer for Efficient Perovskite Solar Cells. J. Am. Chem. Soc. 2015, 137, 6730–6733. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Feng, J.; Liu, Z.; Duan, Y.; Zhan, S.; Yang, S.; He, K.; Li, Y.; Zhou, Y.; Yuan, N.; et al. Record-Efficiency Flexible Perovskite Solar Cells Enabled by Multifunctional Organic Ions Interface Passivation. Adv. Mater. 2022, 34, 2201681. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yuan, J.; Xi, J.; Du, J.; Tian, J. Multiple-Function Surface Engineering of SnO2 Nanoparticles to Achieve Efficient Perovskite Solar Cells. J. Phys. Chem. Lett. 2021, 12, 9142–9148. [Google Scholar] [CrossRef]
- Chen, Y.; Zuo, X.; He, Y.; Qian, F.; Zuo, S.; Zhang, Y.; Liang, L.; Chen, Z.; Zhao, K.; Liu, Z.; et al. Dual Passivation of Perovskite and SnO2 for High-Efficiency MAPbI3 Perovskite Solar Cells. Adv. Sci. 2021, 8, 2001466. [Google Scholar] [CrossRef]
- Choi, K.; Lee, J.; Kim, H.I.; Park, C.W.; Kim, G.-W.; Choi, H.; Park, S.; Park, S.A.; Park, T. Thermally stable, planar hybrid perovskite solar cells with high efficiency. Energy Environ. Sci. 2018, 11, 3238–3247. [Google Scholar] [CrossRef]
- Huang, S.; Li, P.; Wang, J.; Huang, C.-C.; Xue, Q.; Fu, N. Modification of SnO2 electron transport Layer: Brilliant strategies to make perovskite solar cells stronger. Chem. Eng. J. 2022, 439, 135687. [Google Scholar] [CrossRef]
- Tao, J.; Liu, X.; Shen, J.; Wang, H.; Xue, J.; Su, C.; Guo, H.; Fu, G.; Kong, W.; Yang, S. Functionalized SnO2 films by using EDTA-2 M for high efficiency perovskite solar cells with efficiency over 23%. Chem. Eng. J. 2022, 430, 132683. [Google Scholar] [CrossRef]
- Sonmezoglu, S.; Akin, S. Suppression of the interface-dependent nonradiative recombination by using 2-methylbenzimidazole as interlayer for highly efficient and stable perovskite solar cells. Nano Energy 2020, 76, 105127. [Google Scholar] [CrossRef]
- Huang, Y.; Li, S.; Wu, C.; Wang, S.; Wang, C.; Ma, R. Introduction of LiCl into SnO2 electron transport layer for efficient planar perovskite solar cells. Chem. Phys. Lett. 2020, 745, 137220. [Google Scholar] [CrossRef]
- Xie, G.; Lu, X.; Duan, J.; Dong, Y.; Jiang, X.; Tu, F.; Duan, Y.; Tang, Q. Alkali chloride doped SnO2 electron-transporting layers for boosting charge transfer and passivating defects in all-inorganic CsPbBr3 perovskite solar cells. J. Mater. Chem. A 2021, 9, 15003–15011. [Google Scholar] [CrossRef]
- Ponzoni, A. Morphological effects in SnO2 chemiresistors for ethanol detection: A review in terms of central performances and outliers. Sensors 2020, 21, 29. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Guo, H.; Zhang, H.; Yang, J.; Chen, H.; Wang, L.; Hao, F.; Niu, X. Chlorine-doped SnO2 hydrophobic surfaces for large grain perovskite solar cells. J. Mater. Chem. C 2020, 8, 11638–11646. [Google Scholar] [CrossRef]
- Zhang, S.; Gu, H.; Chen, S.-C.; Zheng, Q. KF-Doped SnO2 as an electron transport layer for efficient inorganic CsPbI2 Br perovskite solar cells with enhanced open-circuit voltages. J. Mater. Chem. C 2021, 9, 4240–4247. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, K.; Hu, J.; Li, L. Coagulated SnO2 Colloids for High-Performance Planar Perovskite Solar Cells with Negligible Hysteresis and Improved Stability. Angew. Chem. 2019, 58, 11497–11504. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhang, W.; Cai, Q.; Xu, X.; Dong, H.; Mu, C.; Zhang, J.-P. Precursor Engineering of the Electron Transport Layer for Application in High-Performance Perovskite Solar Cells. Adv. Sci. 2021, 8, 2102845. [Google Scholar] [CrossRef] [PubMed]
- Min, H.; Kim, M.; Lee, S.-U.; Kim, H.; Kim, G.; Choi, K.; Lee, J.H.; Seok, S.I. Efficient, stable solar cells by using inherent bandgap of α-phase formamidinium lead iodide. Science 2019, 366, 749–753. [Google Scholar] [CrossRef]
- Min, H.; Lee, D.Y.; Kim, J.; Kim, G.; Lee, K.S.; Kim, J.; Paik, M.J.; Kim, Y.K.; Kim, K.S.; Kim, M.G. Perovskite solar cells with atomically coherent interlayers on SnO2 electrodes. Nature 2021, 598, 444–450. [Google Scholar] [CrossRef]
- You, P.; Tang, G.; Cao, J.; Shen, D.; Ng, T.-W.; Hawash, Z.; Wang, N.; Liu, C.-K.; Lu, W.; Tai, Q. 2D materials for conducting holes from grain boundaries in perovskite solar cells. Light Sci. Appl. 2021, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Schlipf, J.; Wussler, M.; Petrus, M.L.; Jaegermann, W.; Bein, T.; Müller-Buschbaum, P.; Docampo, P. Hybrid perovskite/perovskite heterojunction solar cells. ACS Nano 2016, 10, 5999–6007. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Shin, D.; Rhee, R.; Yun, S.; Yeom, K.M.; Chun, D.H.; Lee, S.; Kim, D.; Yi, Y.; Noh, J.H. Band alignment engineering between planar SnO2 and halide perovskites via two-step annealing. J. Phys. Chem. Lett. 2019, 10, 6545–6550. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Lin, Z.; Cai, Q.; Wen, X.; Mu, C. Choline chloride-modified SnO2 achieving high output voltage in MAPbI3 perovskite solar cells. ACS Appl. Energy Mater. 2020, 3, 3504–3511. [Google Scholar] [CrossRef]
- Biesinger, M.C. Accessing the robustness of adventitious carbon for charge referencing (correction) purposes in XPS analysis: Insights from a multi-user facility data review. Appl. Surf. Sci. 2022, 597, 153681. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. The same chemical state of carbon gives rise to two peaks in X-ray photoelectron spectroscopy. Sci. Rep. 2021, 11, 11195. [Google Scholar] [CrossRef]
- Xi, J.; Yuan, J.; Du, J.; Yan, X.; Tian, J. Efficient Perovskite Solar Cells Based on Tin Oxide Nanocrystals with Difunctional Modification. Small 2022, 18, 2203519. [Google Scholar] [CrossRef]
- Gaggiotti, G.; Galdikas, A.; Kačiulis, S.; Mattogno, G.; Setkus, A. Surface chemistry of tin oxide based gas sensors. J. Appl. Phys. 1994, 76, 4467–4471. [Google Scholar] [CrossRef]
- Xu, D.; Mai, R.; Jiang, Y.; Chen, C.; Wang, R.; Xu, Z.; Kempa, K.; Zhou, G.; Liu, J.-M.; Gao, J. An internal encapsulating layer for efficient, stable, repairable and low-lead-leakage perovskite solar cells. Energy Environ. Sci. 2022, 15, 3891–3900. [Google Scholar] [CrossRef]
- Bi, C.; Wang, Q.; Shao, Y.; Yuan, Y.; Xiao, Z.; Huang, J. Non-wetting surface-driven high-aspect-ratio crystalline grain growth for efficient hybrid perovskite solar cells. Nat. Commun. 2015, 6, 7747. [Google Scholar] [CrossRef]
- Bao, J.; Wang, P.; Zhang, W.; Li, B.; Wu, X.; Xu, L.; Lin, P.; He, H.; Yu, X.; Cui, C. Multifunctional Thiophene-Based Interfacial Passivating Layer for High-Performance Perovskite Solar Cells. ACS Appl. Energy Mater. 2022, 5, 6823–6832. [Google Scholar] [CrossRef]
- Xu, P.; He, H.; Ding, J.; Wang, P.; Piao, H.; Bao, J.; Zhang, W.; Wu, X.; Xu, L.; Lin, P.; et al. Simultaneous Passivation of the SnO2/Perovskite Interface and Perovskite Absorber Layer in Perovskite Solar Cells Using KF Surface Treatment. ACS Appl. Energy Mater. 2021, 4, 10921–10930. [Google Scholar] [CrossRef]
- Tzoganakis, N.; Feng, B.; Loizos, M.; Krassas, M.; Tsikritzis, D.; Zhuang, X.; Kymakis, E. Ultrathin PTAA interlayer in conjunction with azulene derivatives for the fabrication of inverted perovskite solar cells. J. Mater. Chem. C 2021, 9, 14709–14719. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, J.; Arivazhagan, V.; Xiao, K.; Qiang, Y.; Huang, K.; Hu, M.; Cui, C.; Yu, X.; Yang, D. Efficient and highly light stable planar perovskite solar cells with graphene quantum dots doped PCBM electron transport layer. Nano Energy 2017, 40, 345–351. [Google Scholar] [CrossRef]
- Lin, C.C.; Murakami, T.N.; Chikamatsu, M.; Bessho, T.; Furue, M.; Segawa, H. A sodium chloride modification of SnO2 electron transport layers to enhance the performance of perovskite solar cells. ACS Omega 2021, 6, 17880–17889. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Y.; Fu, R.; Zhou, W.; Zhao, Y.; Liu, X.; Yu, D.; Zhao, Q. Efficient Perovskite Solar Cells Fabricated Through CsCl-Enhanced PbI2 Precursor via Sequential Deposition. Adv. Mater. 2018, 30, 1803095. [Google Scholar] [CrossRef]
- Chen, H.; Liu, T.; Zhou, P.; Li, S.; Ren, J.; He, H.; Wang, J.; Wang, N.; Guo, S. Efficient bifacial passivation with crosslinked thioctic acid for high-performance methylammonium lead iodide perovskite solar cells. Adv. Mater. 2020, 32, 1905661. [Google Scholar] [CrossRef]
- Wang, P.; Wang, J.; Zhang, X.; Wang, H.; Cui, X.; Yuan, S.; Lu, H.; Tu, L.; Zhan, Y.; Zheng, L. Boosting the performance of perovskite solar cells through a novel active passivation method. J. Mater. Chem. A 2018, 6, 15853–15858. [Google Scholar] [CrossRef]
- Hou, M.; Wang, Y.; Han, M.; Ren, H.; Wang, R.; Zhao, J.; Huang, Q.; Ding, Y.; Zhang, X.; Hou, G. Potassium chloride templated α-FAPbI3 perovskite crystal growth for efficient planar perovskite solar cells. Org. Electron. 2022, 106, 106527. [Google Scholar] [CrossRef]
- Cao, J.; Tang, G.; You, P.; Wang, T.; Zheng, F.; Zhao, J.; Yan, F. Enhanced performance of planar perovskite solar cells induced by van der Waals epitaxial growth of mixed perovskite films on WS2 flakes. Adv. Funct. Mater. 2020, 30, 2002358. [Google Scholar] [CrossRef]
- Li, Y.; Meng, L.; Yang, Y.M.; Xu, G.; Hong, Z.; Chen, Q.; You, J.; Li, G.; Yang, Y.; Li, Y. High-efficiency robust perovskite solar cells on ultrathin flexible substrates. Nat. Commun. 2016, 7, 10214. [Google Scholar] [CrossRef] [PubMed]
- Stolterfoht, M.; Le Corre, V.M.; Feuerstein, M.; Caprioglio, P.; Koster, L.J.A.; Neher, D. Voltage-dependent photoluminescence and how it correlates with the fill factor and open-circuit voltage in perovskite solar cells. ACS Energy Lett. 2019, 4, 2887–2892. [Google Scholar] [CrossRef]
- Kanemitsu, Y. Luminescence spectroscopy of lead-halide perovskites: Materials properties and application as photovoltaic devices. J. Mater. Chem. C 2017, 5, 3427–3437. [Google Scholar] [CrossRef]
- Wolff, C.M.; Caprioglio, P.; Stolterfoht, M.; Neher, D. Nonradiative recombination in perovskite solar cells: The role of interfaces. Adv. Mater. 2019, 31, 1902762. [Google Scholar] [CrossRef]
- Kirchartz, T.; Márquez, J.A.; Stolterfoht, M.; Unold, T. Photoluminescence-based characterization of halide perovskites for photovoltaics. Adv. Energy Mater. 2020, 10, 1904134. [Google Scholar] [CrossRef]
- Tang, G.; You, P.; Tai, Q.; Yang, A.; Cao, J.; Zheng, F.; Zhou, Z.; Zhao, J.; Chan, P.K.L.; Yan, F. Solution-phase epitaxial growth of perovskite films on 2D material flakes for high-performance solar cells. Adv. Mater. 2019, 31, 1807689. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; You, P.; Tai, Q.; Wu, R.; Yan, F. Performance enhancement of perovskite solar cells induced by lead acetate as an additive. Sol. RRL 2018, 2, 1800066. [Google Scholar] [CrossRef]
- Luo, D.; Su, R.; Zhang, W.; Gong, Q.; Zhu, R. Minimizing non-radiative recombination losses in perovskite solar cells. Nat. Rev. Mater. 2020, 5, 44–60. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, X.; Kim, S.G.; Park, N.G. Multifunctional chemical linker imidazoleacetic acid hydrochloride for 21% efficient and stable planar perovskite solar cells. Adv. Mater. 2019, 31, 1902902. [Google Scholar] [CrossRef]
- Liu, Z.; You, P.; Xie, C.; Tang, G.; Yan, F. Ultrathin and flexible perovskite solar cells with graphene transparent electrodes. Nano Energy 2016, 28, 151–157. [Google Scholar] [CrossRef]
- Caprioglio, P.; Wolff, C.M.; Sandberg, O.J.; Armin, A.; Rech, B.; Albrecht, S.; Neher, D.; Stolterfoht, M. On the origin of the ideality factor in perovskite solar cells. Adv. Energy Mater. 2020, 10, 2000502. [Google Scholar] [CrossRef]
- Almora, O.; Cho, K.T.; Aghazada, S.; Zimmermann, I.; Matt, G.J.; Brabec, C.J.; Nazeeruddin, M.K.; Garcia-Belmonte, G. Discerning recombination mechanisms and ideality factors through impedance analysis of high-efficiency perovskite solar cells. Nano Energy 2018, 48, 63–72. [Google Scholar] [CrossRef]
- Calado, P.; Burkitt, D.; Yao, J.; Troughton, J.; Watson, T.M.; Carnie, M.J.; Telford, A.M.; O’Regan, B.C.; Nelson, J.; Barnes, P.R. Identifying dominant recombination mechanisms in perovskite solar cells by measuring the transient ideality factor. Phys. Rev. Appl. 2019, 11, 044005. [Google Scholar] [CrossRef]
- Almora, O.; Zarazua, I.; Mas-Marza, E.; Mora-Sero, I.; Bisquert, J.; Garcia-Belmonte, G. Capacitive dark currents, hysteresis, and electrode polarization in lead halide perovskite solar cells. J. Phys. Chem. Lett. 2015, 6, 1645–1652. [Google Scholar] [CrossRef]
- Tai, Q.; You, P.; Sang, H.; Liu, Z.; Hu, C.; Chan, H.L.; Yan, F. Efficient and stable perovskite solar cells prepared in ambient air irrespective of the humidity. Nat. Commun. 2016, 7, 11105. [Google Scholar] [CrossRef] [PubMed]
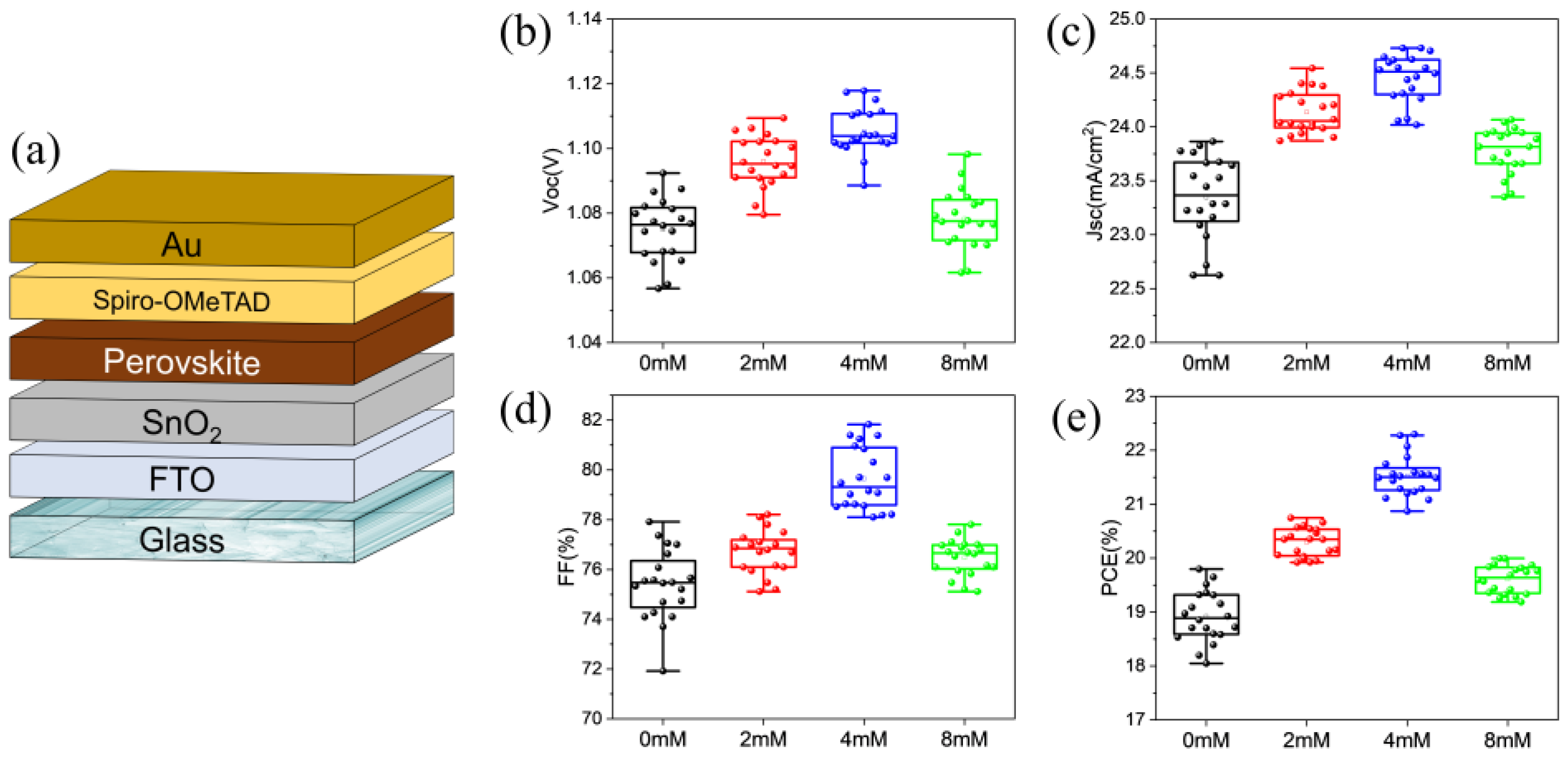

| ETL | VOC (V) | JSC (mA/cm2) | FF (%) | PCE (%) |
|---|---|---|---|---|
| SnO2 | 1.075 ± 0.010 | 23.35 ± 0.39 | 75.39 ± 1.42 | 18.92 ± 0.47 |
| SnO2-MDACl2-2 mM | 1.096 ± 0.008 | 24.14 ± 0.20 | 76.71 ± 0.88 | 20.29 ± 0.27 |
| SnO2-MDACl2-4 mM | 1.105 ± 0.007 | 24.45 ± 0.22 | 79.65 ± 1.24 | 21.53 ± 0.38 |
| SnO2-MDACl2-8 mM | 1.078 ± 0.009 | 23.78 ± 0.21 | 76.48 ± 0.72 | 19.61 ± 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Cui, X.; Xiang, B.; Chen, Y.; Zhao, C.; Wang, L.; Yang, C.; Zhang, G.; Xie, C.; Han, Y.; et al. MDACl2-Modified SnO2 Film for Efficient Planar Perovskite Solar Cells. Molecules 2023, 28, 2668. https://doi.org/10.3390/molecules28062668
Xiao Y, Cui X, Xiang B, Chen Y, Zhao C, Wang L, Yang C, Zhang G, Xie C, Han Y, et al. MDACl2-Modified SnO2 Film for Efficient Planar Perovskite Solar Cells. Molecules. 2023; 28(6):2668. https://doi.org/10.3390/molecules28062668
Chicago/Turabian StyleXiao, Yaodong, Xiangqian Cui, Boyuan Xiang, Yanping Chen, Chaoyue Zhao, Lihong Wang, Chuqun Yang, Guangye Zhang, Chen Xie, Yulai Han, and et al. 2023. "MDACl2-Modified SnO2 Film for Efficient Planar Perovskite Solar Cells" Molecules 28, no. 6: 2668. https://doi.org/10.3390/molecules28062668
APA StyleXiao, Y., Cui, X., Xiang, B., Chen, Y., Zhao, C., Wang, L., Yang, C., Zhang, G., Xie, C., Han, Y., Qiu, M., Li, S., & You, P. (2023). MDACl2-Modified SnO2 Film for Efficient Planar Perovskite Solar Cells. Molecules, 28(6), 2668. https://doi.org/10.3390/molecules28062668






