Magnetic Solid-Phase Extraction Based on Silica and Graphene Materials for Sensitive Analysis of Emerging Contaminants in Wastewater with the Aid of UHPLC-Orbitrap-MS
Abstract
1. Introduction
2. Results
2.1. UHPLC-Orbitrap MS
2.2. Characterization of Synthesized Materials
2.2.1. Graphene-Based (GO) Composites
X-ray Diffraction Technique (XRD)
FT-IR Spectroscopy of Magnetic Graphene Based Nanocomposites
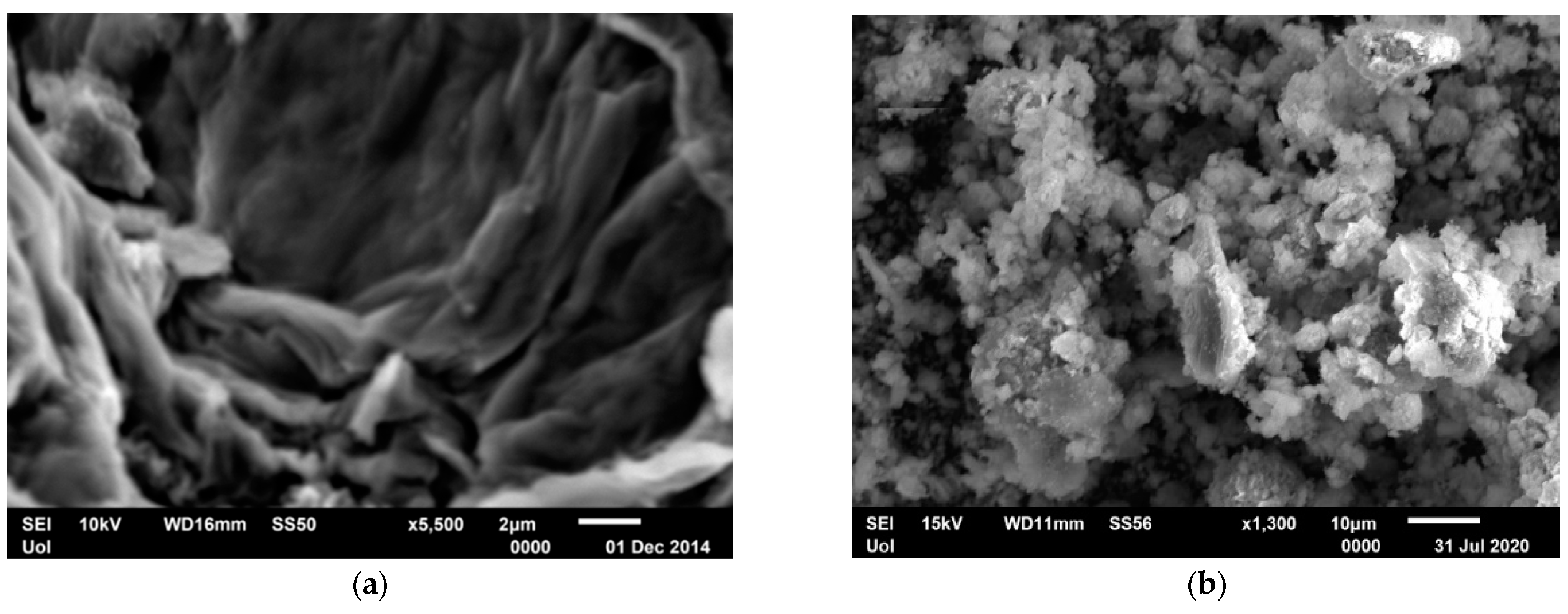
Scanning Electron Microscopy (SEM) of GO and mrGO
2.3. Optimization of Magnetic Solid-Phase Extraction (MSPE)
2.3.1. Optimization of MSPE- Fe3O4@SiO2@C18
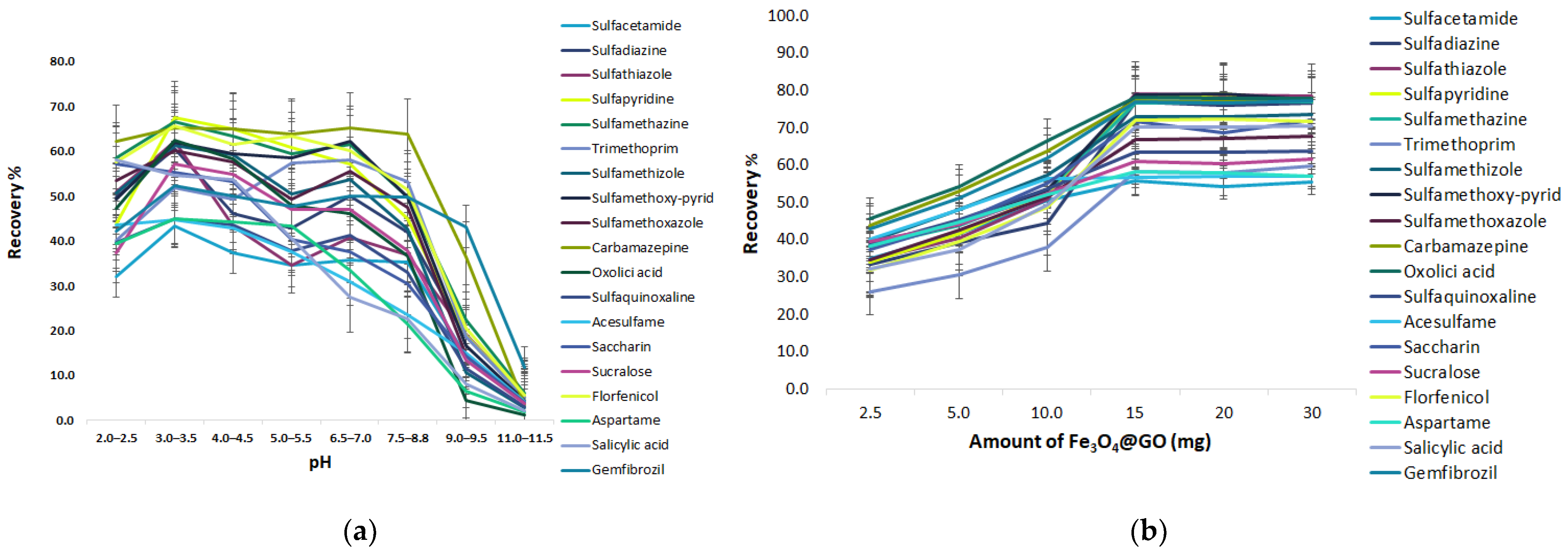
Effect of the pH
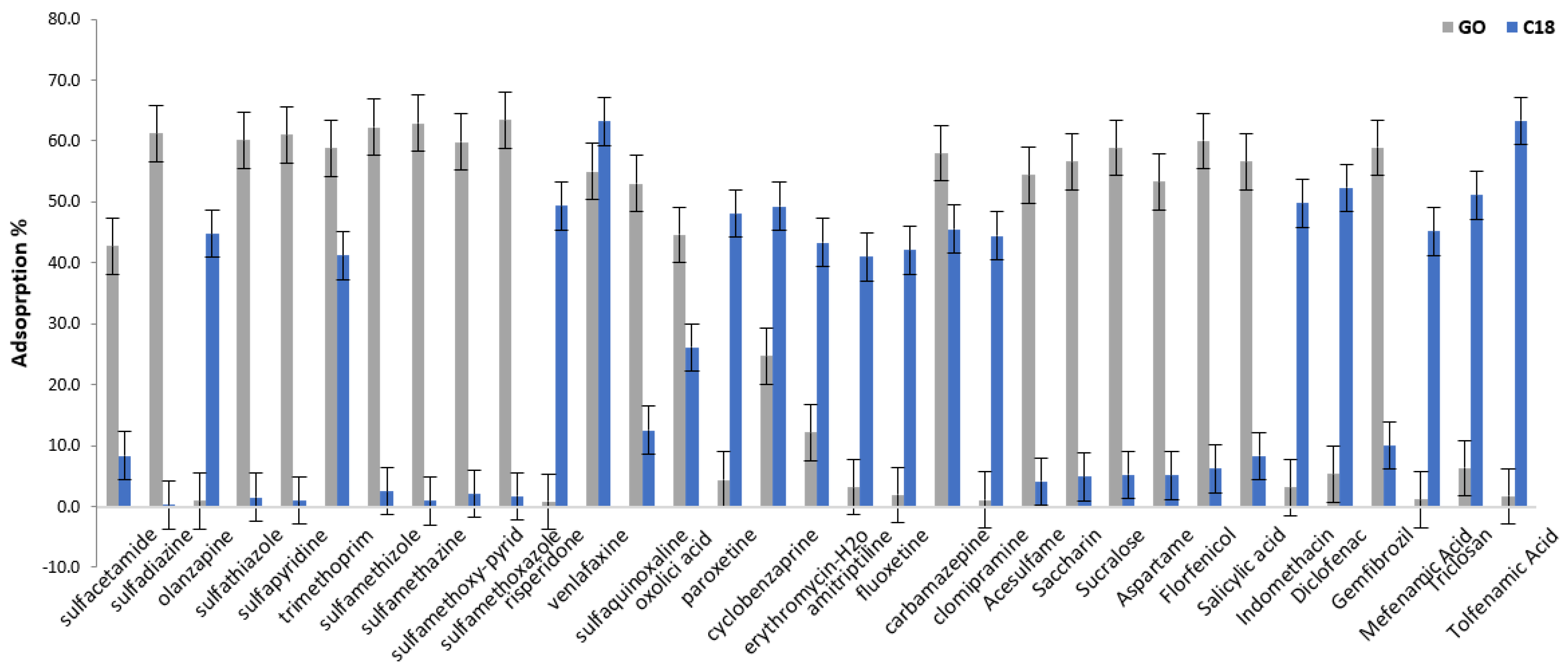
Amount of GO@Fe3O4
Effect of Extraction Time on Fe3O4 @GO-MSPE
Type of Desorption Solvent
Elution Volume and Desorption Time
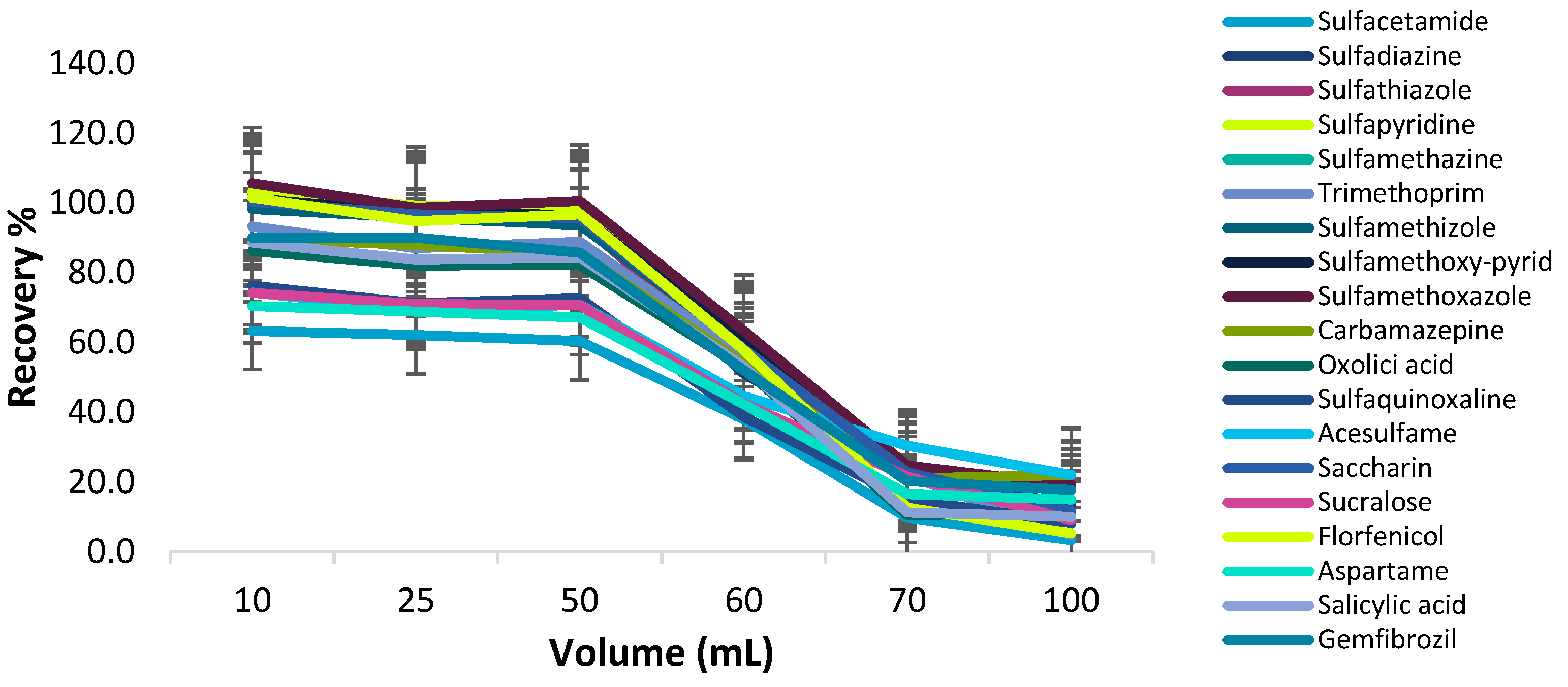
Effect of Sample Volume
2.3.2. Optimization of MSPE- Fe3O4@SiO2@C18
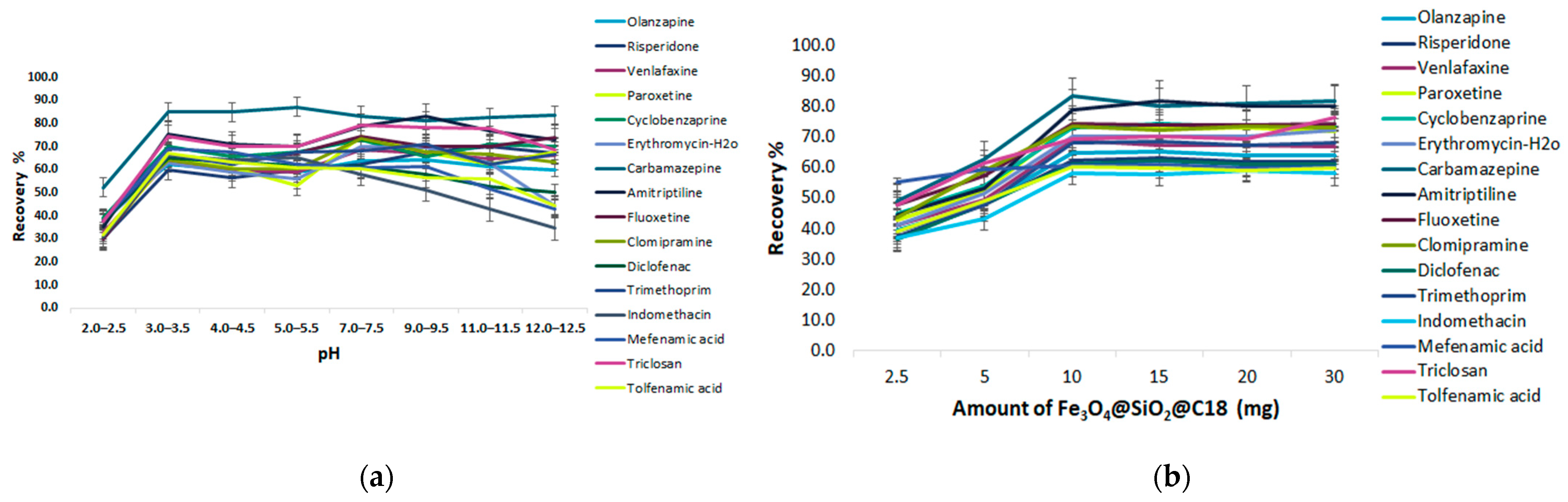
Effect of pH
Amount of Fe3O4@SiO2@C18
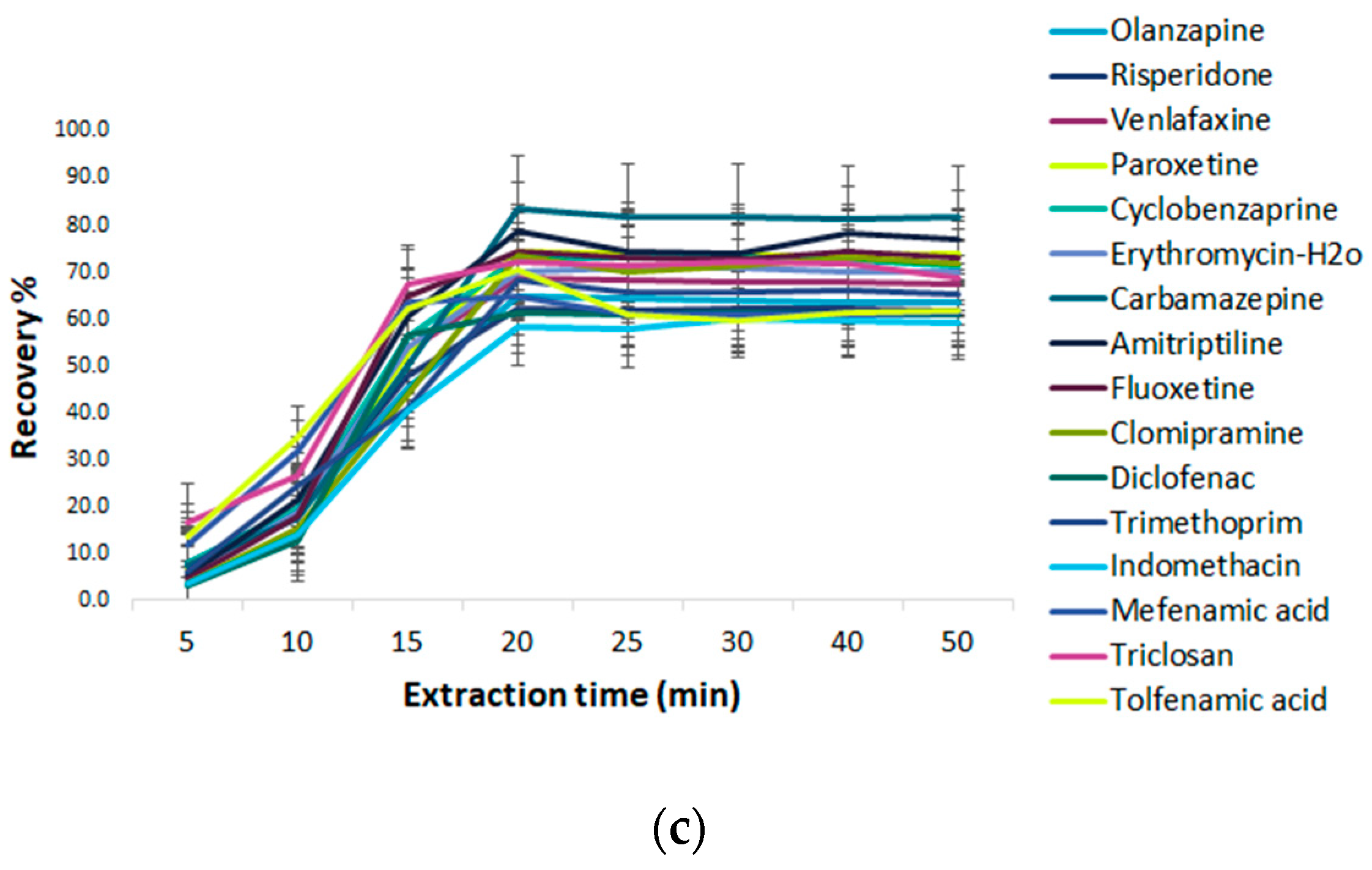
Effect of Extraction Time on Fe3O4@SiO2@C18 -MSPE
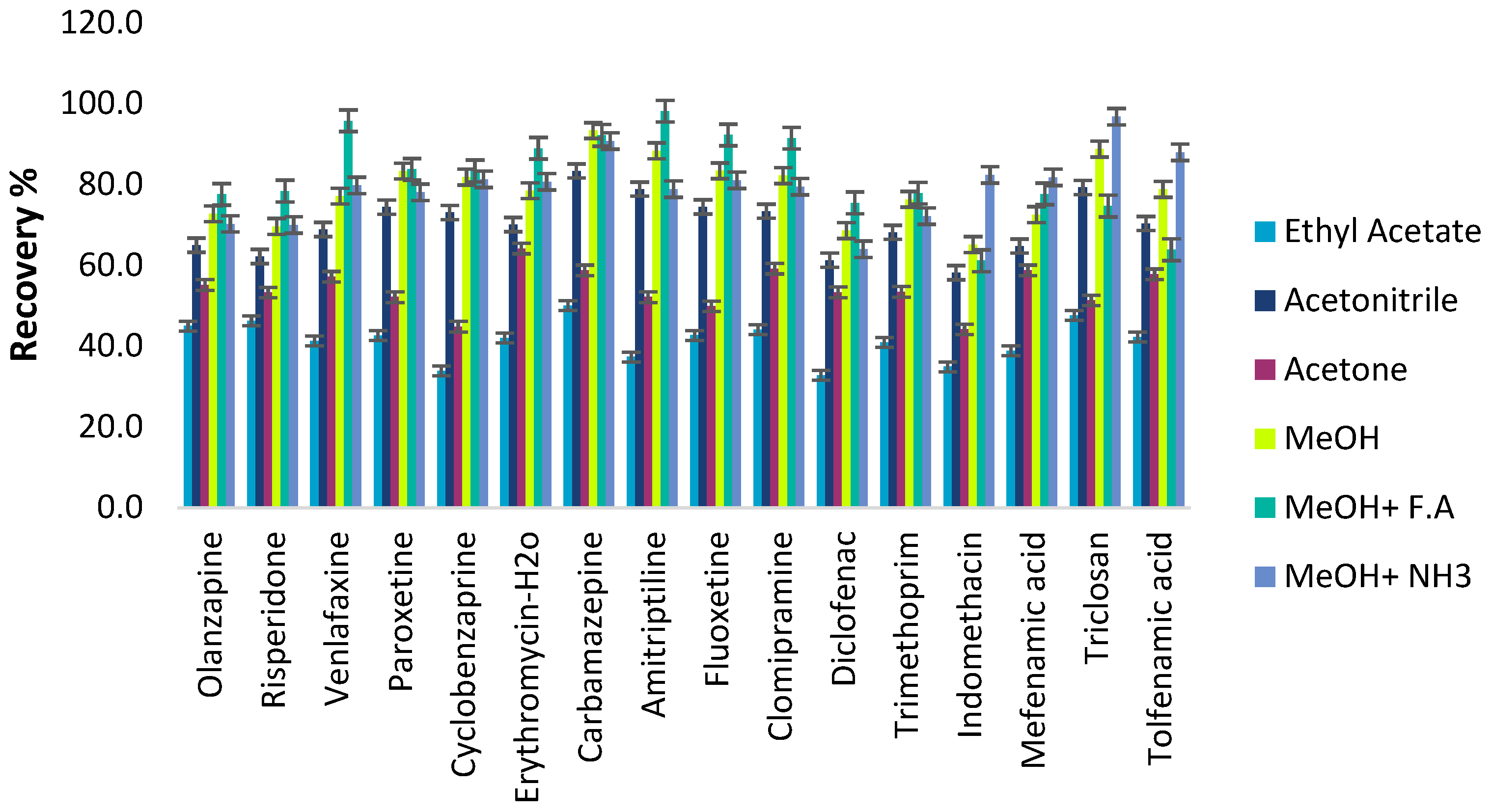
Desorption Conditions
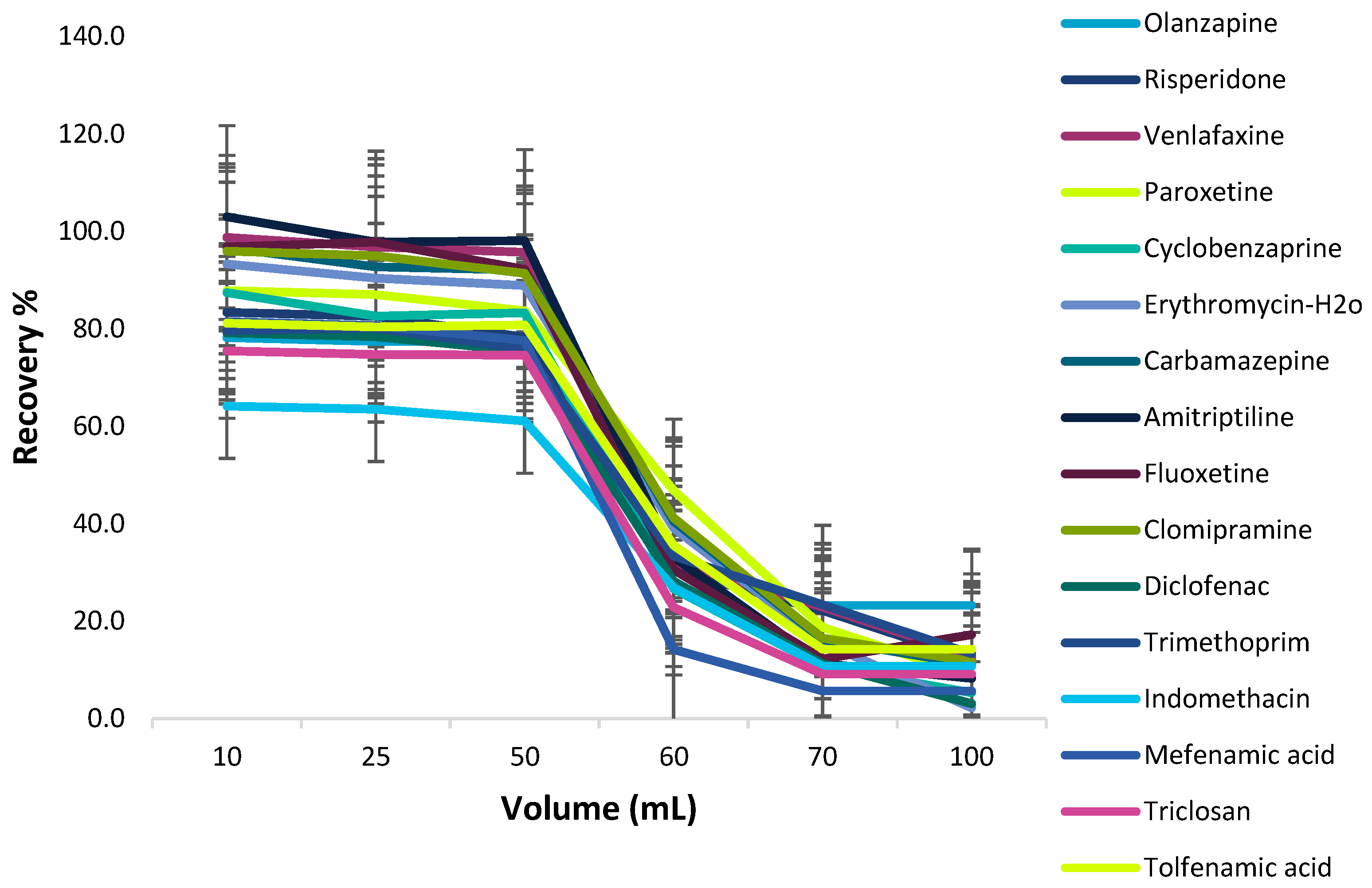
Effect of Sample Volume
2.4. Reuse of Magnetic Sorbents
2.5. Validation of Magnetic Solid-Phase Extraction
2.5.1. Accuracy
2.5.2. Sensitivity and Linearity
2.5.3. Precision
2.5.4. Matrix Effect (ME)
2.6. Analysis of the Real Samples
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Equipment
3.3. UHPLC–LTQ Orbitrap MS Analysis
3.4. Preparation of Magnetic Materials
3.4.1. Preparation of Fe3O4@SiO2@C18
3.4.2. Preparation of Fe3O4@GO
Synthesis of Magnetic Graphene Oxide (mGO)
Synthesis of Magnetic Reduced Graphene Oxide (mrGO)
3.5. Application of MSPE for the Extraction of ECs from Hospital and Urban Wastewater
3.5.1. MSPE@C18
3.5.2. MSPE@GO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richardson, S.D.; Kimura, S.Y. Water Analysis: Emerging Contaminants and Current Issues. Anal. Chem. 2016, 88, 546–582. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.S.; Murphy, M.; Mendola, N.; Wong, V.; Carlson, D.; Waring, L. Characterization of Pharmaceuticals and Personal Care products in hospital effluent and waste water influent/effluent by direct-injection LC-MS-MS. Sci. Total Environ. 2015, 518–519, 459–478. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Wang, L.; Wei, C.; Li, J.; Zhang, J.; Zhou, Z.; Liang, Y. Sucralose and acesulfame as an indicator of domestic wastewater contamination in Wuhan surface water. Ecotoxicol. Environ. Saf. 2020, 189, 109980. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, M.; Zioris, I.; Danis, T.; Bikiaris, D.; Lambropoulou, D. Comprehensive investigation of a wide range of pharmaceuticals and personal care products in urban and hospital wastewaters in Greece. Sci. Total Environ. 2019, 694, 133565. [Google Scholar] [CrossRef] [PubMed]
- Yamini, Y.; Faraji, M. Extraction and determination of trace amounts of chlorpromazine in biological fluids using magnetic solid phase extraction followed by HPLC. J. Pharm. Anal. 2014, 4, 279–285. [Google Scholar] [CrossRef]
- Ansari, S.; Karimi, M. Novel developments and trends of analytical methods for drug analysis in biological and environmental samples by molecularly imprinted polymers. TrAC-Trends Anal. Chem. 2017, 89, 146–162. [Google Scholar] [CrossRef]
- Tiede, K.; Boxall, A.; Tear, S.; Lewis, J.; David, H.; Hassellov, M. Detection and characterization of engineered nanoparticles in food and the environment. Food Addit. Contam. Part A 2008, 25, 795–821. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Xiao, C.; Chen, L.; Yang, P.; Liu, Q.; Wang, J.; Liu, X. Rapid Determination of Trace Sulfonamides in Milk by Graphene Oxide-Based Magnetic Solid Phase Extraction Coupled with HPLC–MS/MS. Food Anal. Methods 2016, 9, 2521–2530. [Google Scholar] [CrossRef]
- Ng, N.T.; Wan Ibrahim, W.A.; Sutirman, Z.A.; Sanagi, M.M.; Abdul Keyon, A.S. Magnetic nanomaterials for preconcentration and removal of emerging contaminants in the water environment. Nanotechnol. Environ. Eng. 2023, 8, 297–315. [Google Scholar] [CrossRef]
- Abujaber, F.; Zougagh, M.; Jodeh, S.; Ríos, Á.; Guzmán Bernardo, F.J.; Rodríguez Martín-Doimeadios, R.C. Magnetic cellulose nanoparticles coated with ionic liquid as a new material for the simple and fast monitoring of emerging pollutants in waters by magnetic solid phase extraction. Microchem. J. 2018, 137, 490–495. [Google Scholar] [CrossRef]
- Manousi, N.; Rosenberg, E.; Deliyanni, E.; Zachariadis, G.A.; Samanidou, V. Magnetic solid-phase extraction of organic compounds based on graphene oxide nanocomposites. Molecules 2020, 25, 1148. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Szczepańska, N.; de la Guardia, M.; Namieśnik, J. Modern trends in solid phase extraction: New sorbent media. TrAC-Trends Anal. Chem. 2016, 77, 23–43. [Google Scholar] [CrossRef]
- Azzouz, A.; Kailasa, S.K.; Lee, S.S.; Rascón, A.J.; Ballesteros, E.; Zhang, M.; Kim, K.H. Review of nanomaterials as sorbents in solid-phase extraction for environmental samples. TrAC-Trends Anal. Chem. 2018, 108, 347–369. [Google Scholar] [CrossRef]
- Lu, Q.; Guo, H.; Zhang, Y.; Tang, X.; Lei, W.; Qi, R.; Chu, J.; Li, D.; Zhao, Q. Graphene oxide-Fe3O4 nanocomposite magnetic solid phase extraction followed by UHPLC-MS/MS for highly sensitive determination of eight psychoactive drugs in urine samples. Talanta 2020, 206, 120212. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Latorre, C.; Barciela-García, J.; García-Martín, S.; Peña-Crecente, R.M.; Otárola-Jiménez, J. Magnetic solid-phase extraction using carbon nanotubes as sorbents: A review. Anal. Chim. Acta 2015, 892, 10–26. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, R.; Yu, H.; Li, J.; Liu, L.; Wang, S.; Chen, X.; Chan, T.W.D. Magnetic solid-phase extraction of sulfonamide antibiotics in water and animal-derived food samples using core-shell magnetite and molybdenum disulfide nanocomposite adsorbent. J. Chromatogr. A 2020, 1610, 460543. [Google Scholar] [CrossRef] [PubMed]
- Synaridou, M.E.S.; Sakkas, V.A.; Stalikas, C.D.; Albanis, T.A. Evaluation of magnetic nanoparticles to serve as solid-phase extraction sorbents for the determination of endocrine disruptors in milk samples by gas chromatography mass spectrometry. J. Chromatogr. A 2014, 1348, 71–79. [Google Scholar] [CrossRef]
- Guo, L.; Ye, P.; Wang, J.; Fu, F.; Wu, Z. Three-dimensional Fe3O4-graphene macroscopic composites for arsenic and arsenate removal. J. Hazard. Mater. 2015, 298, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Cao, M.; Ma, X.; Zhu, Y.; Hu, C. Superparamagnetic high-surface-area Fe3O4 nanoparticles as adsorbents for arsenic removal. J. Hazard. Mater. 2012, 217–218, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Thi, N.; Hoan, V.; Thu, A.; Van Duc, H.; Cuong, N.D.; Khieu, D.Q.; Vo, V.; Vuong, N.T.; Vn, H. Fe3O4/Reduced Graphene Oxide Nanocomposite: Synthesis and Its Application for Toxic Metal Ion Removal. J. Chem. 2016, 2016, 2418172. [Google Scholar] [CrossRef]
- Rai, A.K.; Gim, J.; Thi, T.V.; Ahn, D.; Cho, S.J.; Kim, J. High Rate capability and long cycle stability of Co3O4/cofe2O4 nanocomposite as an anode material for high-performance secondary lithium ion batteries. J. Phys. Chem. C 2014, 118, 11234–11243. [Google Scholar] [CrossRef]
- Chatzimarkou, A.; Stalikas, C. Adsorptive Removal of Estriol from Water Using Graphene-Based Materials and Their Magnetite Composites: Heterogeneous Fenton-Like Non-toxic Degradation on Magnetite/Graphene Oxide. Int. J. Environ. Res. 2020, 14, 269–287. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Travlou, N.A.; Kalogirou, O.; Deliyanni, E.A. Magnetic graphene oxide: Effect of preparation route on reactive black 5 adsorption. Materials 2013, 6, 1360–1376. [Google Scholar] [CrossRef] [PubMed]
- Qi, T.; Huang, C.; Yan, S.; Li, X.-J.; Pan, S.-Y. Synthesis, characterization and adsorption properties of magnetite/reduced graphene oxide nanocomposites. Talanta 2015, 144, 1116–1124. [Google Scholar] [CrossRef]
- Sheshmani, S.; Falahat, B.; Nikmaram, F.R. Preparation of magnetic graphene oxide-ferrite nanocomposites for oxidative decomposition of Remazol Black B. Int. J. Biol. Macromol. 2017, 97, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Chatzimitakos, T.G.; Karali, K.K.; Stalikas, C.D. Magnetic graphene oxide as a convenient nanosorbent to streamline matrix solid-phase dispersion towards the extraction of pesticides from vegetables and their determination by GC–MS. Microchem. J. 2019, 151, 104247. [Google Scholar] [CrossRef]
- Chen, H.; Gao, B.; Li, H. Removal of sulfamethoxazole and ciprofloxacin from aqueous solutions by graphene oxide. J. Hazard. Mater. 2015, 282, 201–207. [Google Scholar] [CrossRef]
- Liu, F.F.; Zhao, J.; Wang, S.; Xing, B. Adsorption of sulfonamides on reduced graphene oxides as affected by pH and dissolved organic matter. Environ. Pollut. 2016, 210, 85–93. [Google Scholar] [CrossRef]
- 2002/657/EC: Commission Decision of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results (Text with EEA relevance) (notified under document number C(2002) 3044)-Publica.
- Paíga, P.; Santos, L.H.M.L.M.; Delerue-Matos, C. Development of a multi-residue method for the determination of human and veterinary pharmaceuticals and some of their metabolites in aqueous environmental matrices by SPE-UHPLC–MS/MS. J. Pharm. Biomed. Anal. 2017, 135, 75–86. [Google Scholar] [CrossRef]
- Gros, M.; Rodríguez-Mozaz, S.; Barceló, D. Fast and comprehensive multi-residue analysis of a broad range of human and veterinary pharmaceuticals and some of their metabolites in surface and treated waters by ultra-high-performance liquid chromatography coupled to quadrupole-linear ion trap tandem. J. Chromatogr. A 2012, 1248, 104–121. [Google Scholar] [CrossRef]
- Huerta, B.; Rodriguez-Mozaz, S.; Nannou, C.; Nakis, L.; Ruhí, A.; Acuña, V.; Sabater, S.; Barcelo, D. Determination of a broad spectrum of pharmaceuticals and endocrine disruptors in biofilm from a waste water treatment plant-impacted river. Sci. Total Environ. 2016, 540, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Kokotou, M.G.; Thomaidis, N.S. Determination of eight artificial sweeteners in wastewater by hydrophilic interaction liquid chromatography-tandem mass spectrometry. Anal. Methods 2013, 5, 3825–3833. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raychaudhuri, U.; Chakraborty, R. Artificial sweeteners—A review. J. Food Sci. Technol. 2014, 51, 611–621. [Google Scholar] [CrossRef]
- Soh, L.; Connors, K.A.; Brooks, B.W.; Zimmerman, J. Fate of sucralose through environmental and water treatment processes and impact on plant indicator species. Environ. Sci. Technol. 2011, 45, 1363–1369. [Google Scholar] [CrossRef]
- Oppenheimer, J.; Eaton, A.; Badruzzaman, M.; Haghani, A.W.; Jacangelo, J.G. Occurrence and suitability of sucralose as an indicator compound of wastewater loading to surface waters in urbanized regions. Water Res. 2011, 45, 4019–4027. [Google Scholar] [CrossRef]
- Henderson, A.; Ng, B.; Landeweer, S.; Quinete, N.; Gardinali, P. Assessment of Sucralose, Caffeine and Acetaminophen as Anthropogenic Tracers in Aquatic Systems Across Florida. Bull. Environ. Contam. Toxicol. 2020, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Jmaiff Blackstock, L.K.; Wawryk, N.J.P.; Jiang, P.; Hrudey, S.E.; Li, X.F. Recent applications and critical evaluation of using artificial sweeteners to assess wastewater impact. Curr. Opin. Environ. Sci. Health 2019, 7, 26–33. [Google Scholar] [CrossRef]
- Quoc Tuc, D.; Elodie, M.G.; Pierre, L.; Fabrice, A.; Marie-Jeanne, T.; Martine, B.; Joelle, E.; Marc, C. Fate of antibiotics from hospital and domestic sources in a sewage network. Sci. Total Environ. 2017, 575, 758–766. [Google Scholar] [CrossRef]
- Yilmaz, G.; Kaya, Y.; Vergili, I.; Beril Gönder, Z.; Özhan, G.; Ozbek Celik, B.; Altinkum, S.M.; Bagdatli, Y.; Boergers, A.; Tuerk, J. Characterization and toxicity of hospital wastewaters in Turkey. Environ. Monit. Assess. 2017, 189, 55. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Silva-Agredo, J.; Botero-Coy, A.M.; Moncayo-Lasso, A.; Hernández, F.; Torres-Palma, R.A. Effective elimination of fifteen relevant pharmaceuticals in hospital wastewater from Colombia by combination of a biological system with a sonochemical process. Sci. Total Environ. 2019, 670, 623–632. [Google Scholar] [CrossRef]
- Thai, P.K.; Ky, L.X.; Binh, V.N.; Nhung, P.H.; Nhan, P.T.; Hieu, N.Q.; Dang, N.T.T.; Tam, N.K.B.; Anh, N.T.K. Occurrence of antibiotic residues and antibiotic-resistant bacteria in effluents of pharmaceutical manufacturers and other sources around Hanoi, Vietnam. Sci. Total Environ. 2018, 645, 393–400. [Google Scholar] [CrossRef]
- Dasenaki, M.E.; Thomaidis, N.S. Multianalyte method for the determination of pharmaceuticals in wastewater samples using solid-phase extraction and liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 4229–4245. [Google Scholar] [CrossRef] [PubMed]
- Kosma, C.I.; Lambropoulou, D.A.; Albanis, T.A. Investigation of PPCPs in wastewater treatment plants in Greece: Occurrence, removal and environmental risk assessment. Sci. Total Environ. 2014, 466–467, 421–438. [Google Scholar] [CrossRef]
- Nannou, C.I.; Kosma, C.I.; Albanis, T.A. Occurrence of pharmaceuticals in surface waters: Analytical method development and environmental risk assessment. Int. J. Environ. Anal. Chem. 2015, 95, 1242–1262. [Google Scholar] [CrossRef]
- Kaklamanos, G.; Vincent, U.; Von Holst, C. Multi-residue method for the detection of veterinary drugs in distillers grains by liquid chromatography–Orbitrap high resolution mass spectrometry. J. Chromatogr. A 2013, 1322, 38–48. [Google Scholar] [CrossRef]
- Commission Decision of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off. J. Eur. Communities 2002, L221, 8–36.
- US Food & Drug Administration Office of Foods and Veterinary Medicine. Momerandum of Public Health Service Food and Drug AdministrationAcceptance Criteria for Confirmation of Identity of Chemical Residues using Exact Mass Data within the Office of Foods and Veterinary Medicine. September 2015; pp. 1–16. Available online: https://www.fda.gov/media/96499/download (accessed on 14 April 2022).
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef] [PubMed]
- Kalaboka, M.; Chrimatopoulos, C.; Jim, C.; Boti, V. Exploring the Efficiency of UHPLC-Orbitrap MS for the Determination of 20 Pharmaceuticals and Acesulfame K in Hospital and Urban Wastewaters with the Aid of FPSE. Separations 2020, 7, 46. [Google Scholar] [CrossRef]
- Fan, W.; Lai, Q.; Zhang, Q.; Wang, Y. Nanocomposites of TiO2 and reduced graphene oxide as efficient photocatalysts for hydrogen evolution. J. Phys. Chem. C 2011, 115, 10694–10701. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Calza, P.; Hadjicostas, C.; Sakkas, V.A.; Sarro, M.; Minero, C.; Medana, C.; Albanis, T.A. Photocatalytic transformation of the antipsychotic drug risperidone in aqueous media on reduced graphene oxide-TiO2 composites. Appl. Catal. B Environ. 2016, 183, 96–106. [Google Scholar] [CrossRef]
- Maidatsi, K.V.; Chatzimitakos, T.G.; Sakkas, V.A.; Stalikas, C.D. Octyl-modified magnetic graphene as a sorbent for the extraction and simultaneous determination of fragrance allergens, musks, and phthalates in aqueous samples by gas chromatography with mass spectrometry. J. Sep. Sci. 2015, 38, 3758–3765. [Google Scholar] [CrossRef] [PubMed]





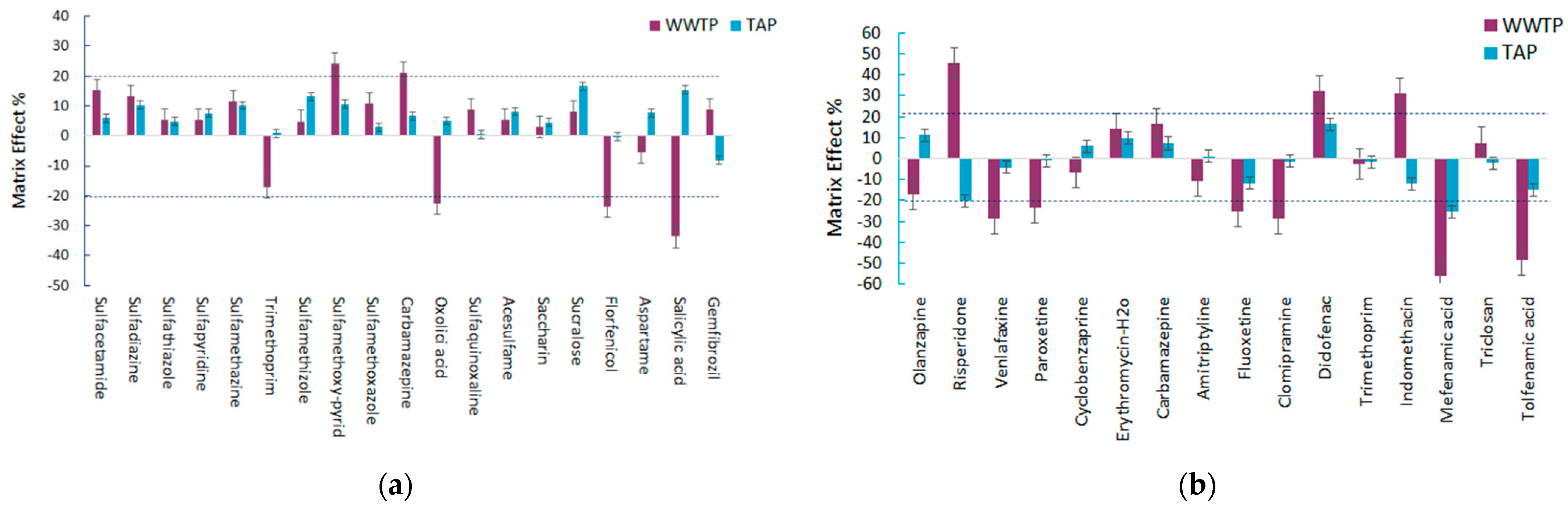

| Optimized Extraction Conditions | MSPE–Fe3O4@GO | MSPE –Fe3O4@SiO2@C18 |
|---|---|---|
| pH | 3.0 | 7.0 |
| Sorbent Amount Extraction Time Elution Solvent Elution volume–Desorption Time | 15 mg | 10 mg |
| 15 min | 20 min | |
| MeOH + 1%NH3 | MeOH + 1% f.a | |
| 2 × 2 mL–2 × 60 s | 2 × 1 mL–2 × 30 s |
| MSPE@Fe3O4@GO | MSPE@Fe3O4@SiO2@C18 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TAP WATER | EFFLUENT | TAP WATER | EFFLUENT | ||||||||||||||
| Compound | R2 | MDL ng/L | MQL ng/L | ME % | R2 | MDL ng/L | MQL ng/L | ME % | R2 | MDL ng/L | MQL ng/L | ME % | R2 | MDL ng/L | MQL ng/L | ME % | |
| Sulfacetamide | 0.9991 | 3.8 | 12.9 | 5.9 | 0.9973 | 9.8 | 32.8 | 15.4 | Olanzapine | 0.9991 | 1.5 | 4.4 | 11.1 | 0.9948 | 1.80 | 5.8 | −17.0 |
| Sulfadiazine | 0.9987 | 3.2 | 10.4 | 10.3 | 0.9967 | 5.1 | 12.9 | 13.1 | Risperidone | 0.9951 | 0.6 | 1.8 | −20.3 | 0.9912 | 1.45 | 4.9 | 45.9 |
| Sulfathiazole | 0.9990 | 3.9 | 13.9 | 4.8 | 0.9970 | 4.3 | 14.6 | 5.3 | Venlafaxine | 0.9990 | 0.3 | 0.9 | −4.2 | 0.9988 | 0.65 | 1.8 | −28.7 |
| Sulfapyridine | 0.9994 | 4.1 | 11.3 | 7.6 | 0.9975 | 3.9 | 13.5 | 5.3 | Paroxetine | 0.9968 | 1.9 | 5.8 | −0.9 | 0.9933 | 3.84 | 11.2 | −23.3 |
| Sulfamethazine | 0.9997 | 1.1 | 3.4 | 10.1 | 0.9949 | 1.3 | 3.5 | 11.6 | Cyclobenzaprine | 0.9992 | 0.5 | 1.5 | 5.9 | 0.9987 | 0.49 | 1.6 | −6.7 |
| Trimethoprim | 0.9998 | 0.4 | 1.2 | 0.8 | 0.9978 | 1.1 | 2.1 | −17.2 | Ery-H2O | 0.9998 | 2.3 | 7.0 | 9.7 | 0.9991 | 2.35 | 7.2 | 14.4 |
| Sulfamethizole | 0.9989 | 1.9 | 6.4 | 13.1 | 0.9959 | 2.0 | 6.8 | 4.9 | Carbamazepine | 1.0000 | 0.3 | 0.9 | 7.3 | 0.9994 | 0.38 | 1.1 | 16.6 |
| Sulfamethoxy-pyrid | 0.9995 | 0.8 | 2.5 | 10.5 | 0.9928 | 1.2 | 3.3 | 24.3 | Amitriptyline | 0.9958 | 1.3 | 3.8 | 1.1 | 0.9948 | 1.39 | 4.1 | −10.6 |
| Sulfamethoxazole | 0.9998 | 0.9 | 2.7 | 2.9 | 0.9978 | 0.9 | 2.9 | 10.8 | Fluoxetine | 0.9981 | 1.3 | 3.8 | −11.8 | 0.9969 | 1.68 | 5.1 | −25.1 |
| Carbamazepine | 1.0000 | 0.9 | 2.6 | 6.7 | 0.9993 | 1.0 | 3.1 | 21.1 | Clomipramine | 0.9992 | 1.4 | 4.1 | −1.2 | 0.998 | 2.01 | 6.1 | −28.9 |
| Oxolici acid | 0.9993 | 1.2 | 3.0 | 5.0 | 0.9981 | 0.6 | 1.8 | −22.6 | Diclofenac | 0.9993 | 6.6 | 19.8 | 16.4 | 0.9928 | 10.9 | 33.6 | 32.0 |
| Sulfaquinoxaline | 0.9987 | 0.7 | 2.0 | 0.6 | 0.9931 | 0.9 | 2.9 | 8.8 | Trimethoprim | 0.9998 | 0.7 | 2.1 | −1.5 | 0.999 | 0.71 | 1.9 | −2.7 |
| Acesulfame | 0.9992 | 10.8 | 32.4 | 8.1 | 0.9941 | 10.4 | 34.8 | 5.4 | Indomethacin | 0.9987 | 5.1 | 15.4 | −12.2 | 0.9954 | 7.0 | 21.8 | 30.9 |
| Saccharin | 0.9993 | 11.2 | 30.7 | 4.5 | 0.9964 | 10.0 | 35.1 | 3.1 | Mefenamic acid | 0.9994 | 5.6 | 16.9 | −25.3 | 0.9977 | 5.9 | 19.8 | −55.8 |
| Sucralose | 0.9990 | 29.4 | 90.5 | 16.5 | 0.9973 | 31.2 | 98.7 | 8.0 | Triclosan | 0.9987 | 2.3 | 6.8 | −2.3 | 0.9952 | 2.5 | 7.1 | 7.6 |
| Florfenicol | 0.9996 | 0.7 | 1.9 | −0.1 | 0.9944 | 0.6 | 2.0 | −23.5 | Tolfenamic acid | 0.9999 | 3.7 | 11.0 | −14.8 | 0.9991 | 4.9 | 15.4 | −48.6 |
| Aspartame | 0.9981 | 22.4 | 69.9 | 7.7 | 0.9964 | 30.2 | 93.9 | −5.6 | |||||||||
| Salicylic acid | 0.9933 | 10.3 | 29.4 | 15.3 | 0.9913 | 11.8 | 37.8 | −33.7 | |||||||||
| Gemfibrozil | 0.9900 | 5.9 | 18.8 | −8.1 | 0.9922 | 9.4 | 29.4 | 8.9 | |||||||||
| Concentration (ngL−1) | ||
|---|---|---|
| Analyte | WWTP-u | WWTP-h |
| Acesulfame | 10,324.6 | 157.6 |
| Amitriptyline | 15.1 | 25.8 |
| Aspartame | <MDL | <MDL |
| Carbamazepine | 133.2 | 478.0 |
| Clomipramine | <MDL | <MQL |
| Cyclobenzaprine | <MDL | <MDL |
| Diclofenac | 152.9 | 156.9 |
| Erythromycin-H2O | <MQL | 14.9 |
| Florfenicol | <MDL | <MDL |
| Fluoxetine | 32.8 | 23.1 |
| Gemfibrozil | <MDL | <MDL |
| Indomethacin | <MDL | <MDL |
| Mefenamic acid | <MDL | <MQL |
| Olanzapine | <MDL | <MDL |
| Oxolinic acid | <MDL | <MDL |
| Paroxetine | <MQL | 40.4 |
| Risperidone | <MDL | <MDL |
| Saccharin | <MQL | <MQL |
| Salicylic acid | 723.6 | 451.8 |
| Sucralose | 1246.1 | <MQL |
| Sulfacetamide | <MDL | 189.5 |
| Sulfadiazine | <MDL | <MQL |
| Sulfamethazine | <MDL | <MDL |
| Sulfamethizole | <MDL | <MDL |
| Sulfamethoxazole | 189.6 | 487.5 |
| Sulfamethoxy-pyridazine | <MDL | <MDL |
| Sulfapyridine | <MDL | 109.3 |
| Sulfaquinoxaline | 12.4 | <MDL |
| Sulfathiazole | <MDL | <MDL |
| Tolfenamic acid | <MDL | <MDL |
| Triclosan | 115.4 | 110.9 |
| Trimethoprim | <MDL | 12.9 |
| Venlafaxine | 145.2 | 165.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalaboka, M.; Sakkas, V. Magnetic Solid-Phase Extraction Based on Silica and Graphene Materials for Sensitive Analysis of Emerging Contaminants in Wastewater with the Aid of UHPLC-Orbitrap-MS. Molecules 2023, 28, 2277. https://doi.org/10.3390/molecules28052277
Kalaboka M, Sakkas V. Magnetic Solid-Phase Extraction Based on Silica and Graphene Materials for Sensitive Analysis of Emerging Contaminants in Wastewater with the Aid of UHPLC-Orbitrap-MS. Molecules. 2023; 28(5):2277. https://doi.org/10.3390/molecules28052277
Chicago/Turabian StyleKalaboka, Maria, and Vasilios Sakkas. 2023. "Magnetic Solid-Phase Extraction Based on Silica and Graphene Materials for Sensitive Analysis of Emerging Contaminants in Wastewater with the Aid of UHPLC-Orbitrap-MS" Molecules 28, no. 5: 2277. https://doi.org/10.3390/molecules28052277
APA StyleKalaboka, M., & Sakkas, V. (2023). Magnetic Solid-Phase Extraction Based on Silica and Graphene Materials for Sensitive Analysis of Emerging Contaminants in Wastewater with the Aid of UHPLC-Orbitrap-MS. Molecules, 28(5), 2277. https://doi.org/10.3390/molecules28052277







