Nature-Inspired Bioactive Compounds: A Promising Approach for Ferroptosis-Linked Human Diseases?
Abstract
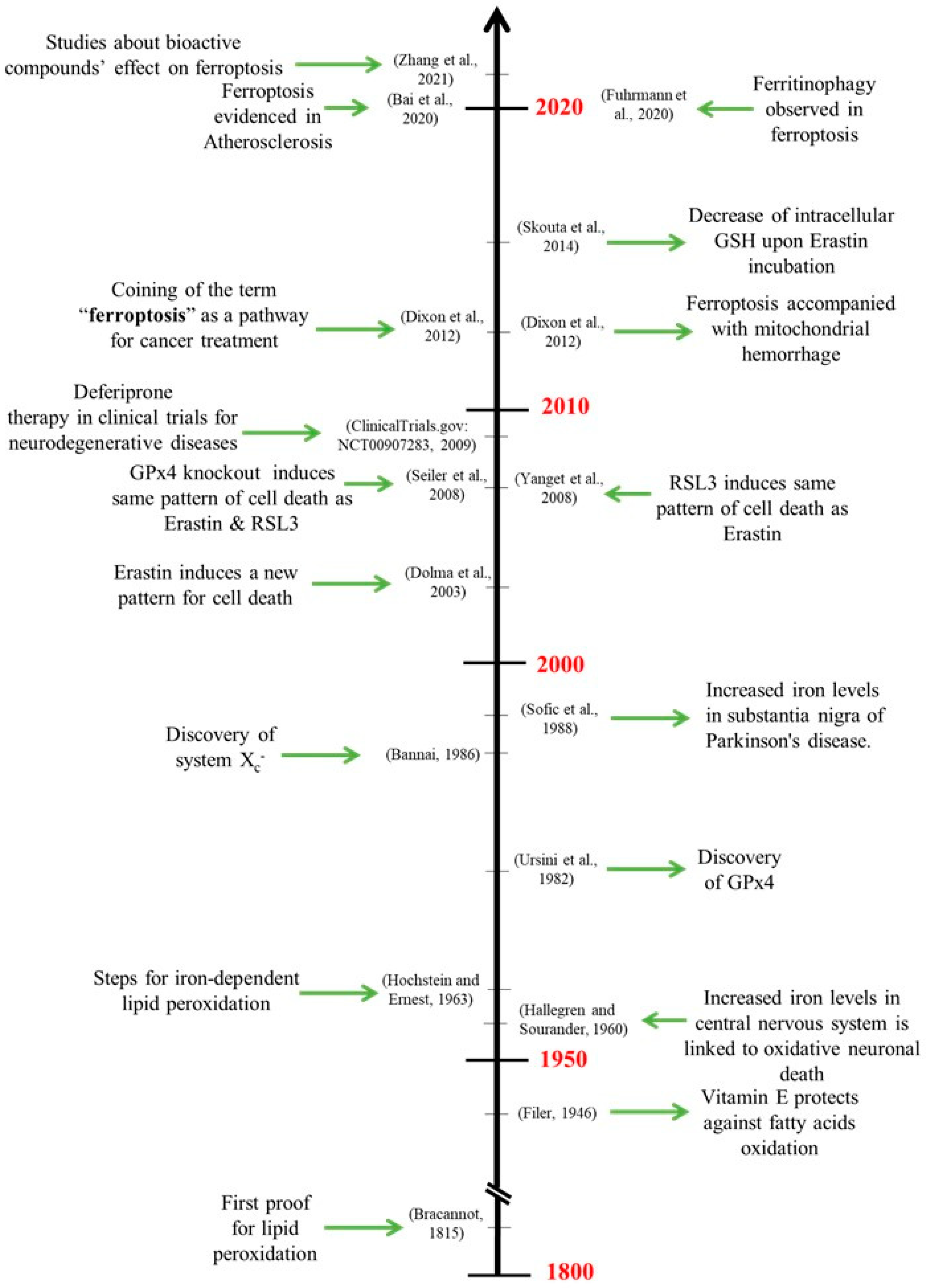
1. Introduction
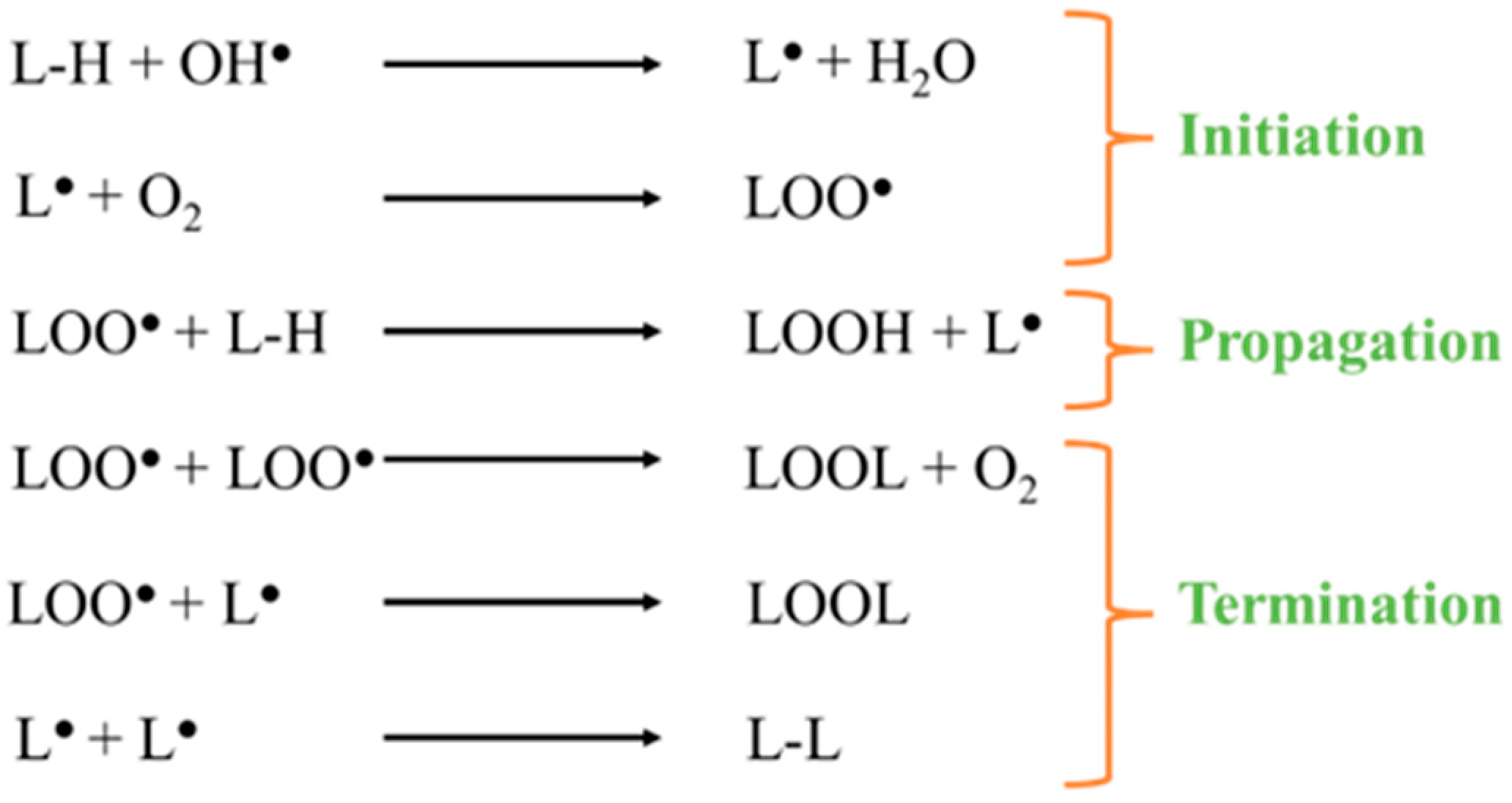
2. Iron-Catalyzed Lipid Peroxidation in Cells
3. Ferroptosis, a Cell Death Type among Others
4. Physiological Regulation of Ferroptosis
5. Pharmacological Modulation of Ferroptosis
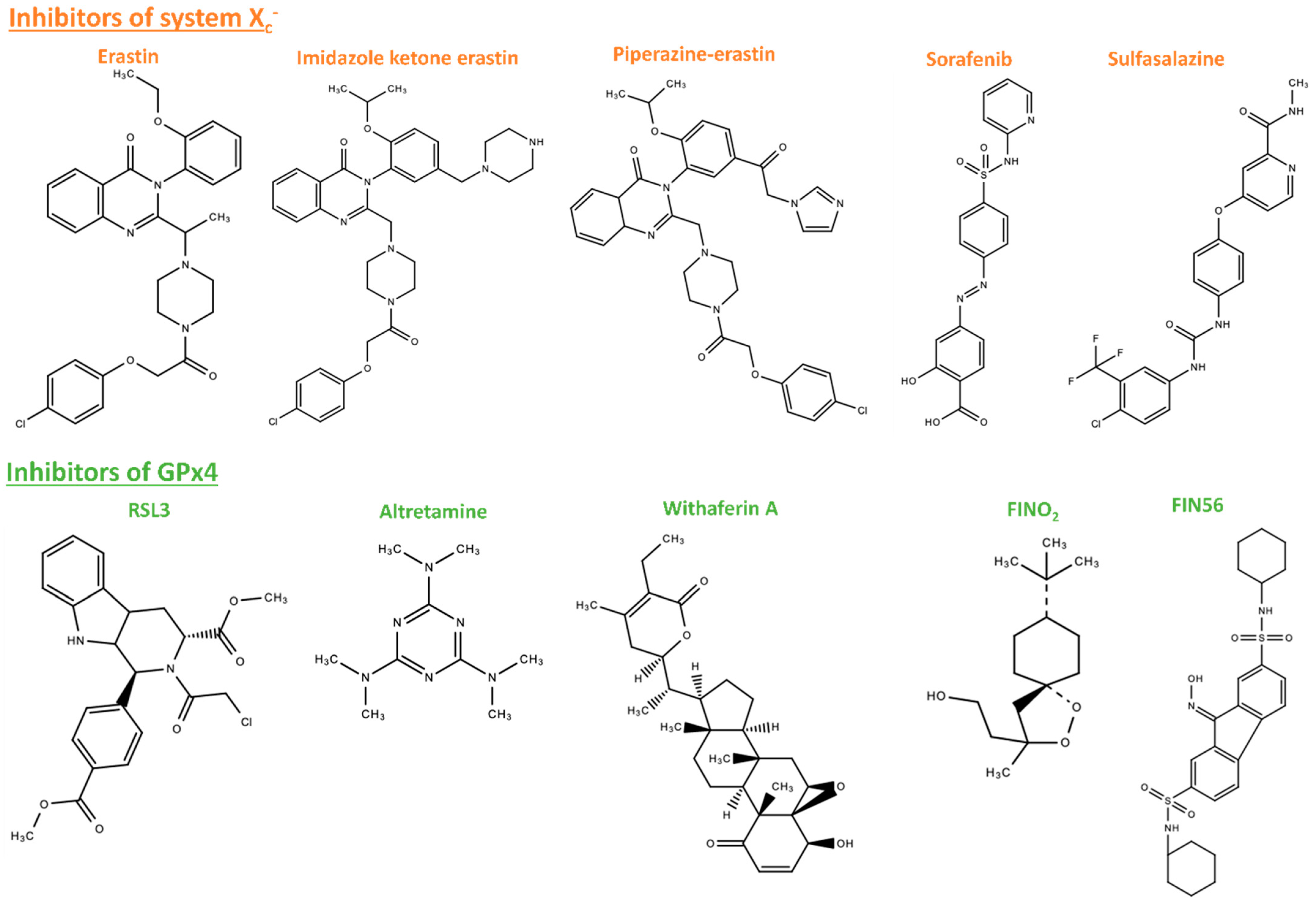
5.1. Ferroptosis Inducers
5.2. Ferroptosis Inhibitors
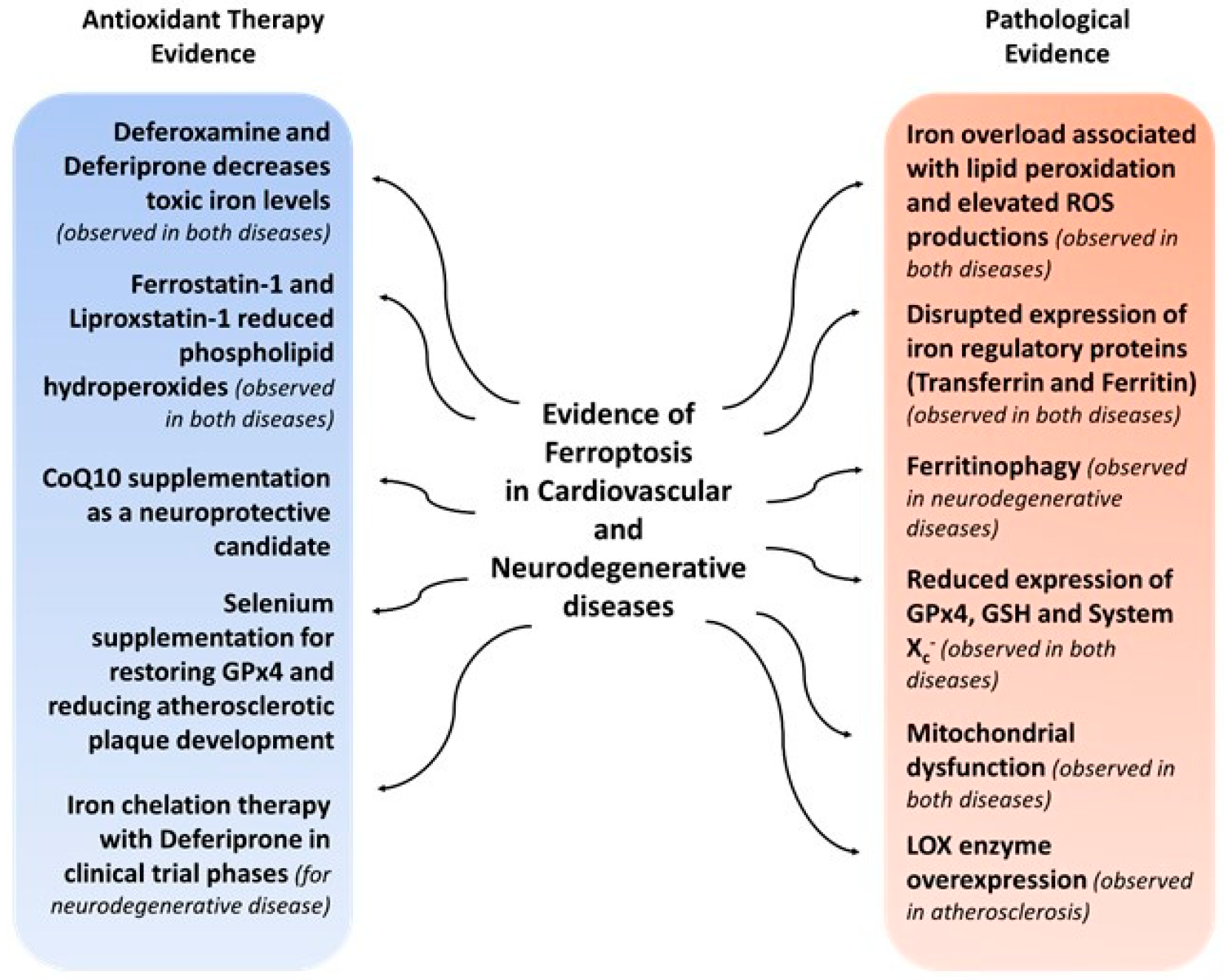
6. Ferroptosis in Cardiovascular and Neurodegenerative Diseases
7. Ferroptosis Modulation Inspired by Nature
7.1. Natural Compounds
7.2. Bioactive Peptides
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blaustein, R. The Great Oxidation Event. BioScience 2016, 66, 189–195. [Google Scholar] [CrossRef]
- Carter, R. Oxygen: The Molecule That Made the World. J. R. Soc. Med. 2003, 96, 46–47. [Google Scholar] [CrossRef]
- Fischer, W.W.; Hemp, J.; Valentine, J.S. How Did Life Survive Earth’s Great Oxygenation? Curr. Opin. Chem. Biol. 2016, 31, 166–178. [Google Scholar] [CrossRef]
- Benzie, I.F.F. Evolution of Antioxidant Defence Mechanisms. Eur. J. Nutr. 2000, 39, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, P.; Zhai, B.; Zhang, M.; Xiang, Y.; Fang, J.; Xu, S.; Gao, Y.; Chen, X.; Sui, X.; et al. The Emerging Role of Ferroptosis in Inflammation. Biomed. Pharmacother. 2020, 127, 110108. [Google Scholar] [CrossRef]
- Valashedi, M.R.; Najafi-Ghalehlou, N.; Nikoo, A.; Bamshad, C.; Tomita, K.; Kuwahara, Y.; Sato, T.; Roushandeh, A.M.; Roudkenar, M.H. Cashing in on Ferroptosis against Tumor Cells: Usher in the next Chapter. Life Sci. 2021, 285, 119958. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Yang, M.; Dong, X. Recent Progress in Ferroptosis Inducers for Cancer Therapy. Adv. Mater. Deerfield Beach Fla. 2019, 31, e1904197. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free Radicals, Natural Antioxidants, and Their Reaction Mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Elias, R.J.; Kellerby, S.S.; Decker, E.A. Antioxidant Activity of Proteins and Peptides. Crit. Rev. Food Sci. Nutr. 2008, 48, 430–441. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, R.; Geng, Y.; Chen, K.; Wang, L.; Imam, M.U. The Regulatory Effects and the Signaling Pathways of Natural Bioactive Compounds on Ferroptosis. Foods 2021, 10, 2952. [Google Scholar] [CrossRef]
- Bai, T.; Li, M.; Liu, Y.; Qiao, Z.; Wang, Z. Inhibition of Ferroptosis Alleviates Atherosclerosis through Attenuating Lipid Peroxidation and Endothelial Dysfunction in Mouse Aortic Endothelial Cell. Free Radic. Biol. Med. 2020, 160, 92–102. [Google Scholar] [CrossRef]
- Fuhrmann, D.C.; Mondorf, A.; Beifuß, J.; Jung, M.; Brüne, B. Hypoxia Inhibits Ferritinophagy, Increases Mitochondrial Ferritin, and Protects from Ferroptosis. Redox Biol. 2020, 36, 101670. [Google Scholar] [CrossRef]
- Skouta, R.; Dixon, S.J.; Wang, J.; Dunn, D.E.; Orman, M.; Shimada, K.; Rosenberg, P.A.; Lo, D.C.; Weinberg, J.M.; Linkermann, A.; et al. Ferrostatins Inhibit Oxidative Lipid Damage and Cell Death in Diverse Disease Models. J. Am. Chem. Soc. 2014, 136, 4551–4556. [Google Scholar] [CrossRef] [PubMed]
- Imperial College London. A Pilot Clinical Trial With the Iron Chelator Deferiprone in Parkinson’s Disease; Clinicaltrials.gov: London, UK, 2020.
- Seiler, A.; Schneider, M.; Förster, H.; Roth, S.; Wirth, E.K.; Culmsee, C.; Plesnila, N.; Kremmer, E.; Rådmark, O.; Wurst, W.; et al. Glutathione Peroxidase 4 Senses and Translates Oxidative Stress into 12/15-Lipoxygenase Dependent- and AIF-Mediated Cell Death. Cell Metab. 2008, 8, 237–248. [Google Scholar] [CrossRef]
- Yang, A.; Stockwell, B.R. Synthetic Lethal Screening Identifies Compounds Activating Iron-Dependent, Nonapoptotic Cell Death in Oncogenic-RAS-Harboring Cancer Cells. Chem. Biol. 2008, 15, 234–245. [Google Scholar] [CrossRef]
- Dolma, S.; Lessnick, S.L.; Hahn, W.C.; Stockwell, B.R. Identification of Genotype-Selective Antitumor Agents Using Synthetic Lethal Chemical Screening in Engineered Human Tumor Cells. Cancer Cell 2003, 3, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Sofic, E.; Riederer, P.; Heinsen, H.; Beckmann, H.; Reynolds, G.P.; Hebenstreit, G.; Youdim, M.B. Increased Iron (III) and Total Iron Content in Post Mortem Substantia Nigra of Parkinsonian Brain. J. Neural Transm. 1988, 74, 199–205. [Google Scholar] [CrossRef]
- Bannai, S. Exchange of Cystine and Glutamate across Plasma Membrane of Human Fibroblasts. J. Biol. Chem. 1986, 261, 2256–2263. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M. Lipid Peroxidation and Ferroptosis: The Role of GSH and GPx4. Free Radic. Biol. Med. 2020, 152, 175–185. [Google Scholar] [CrossRef]
- Hochstein, P.; Ernster, L. ADP-Activated Lipid Peroxidation Coupled to the TPNH Oxidase System of Microsomes. Biochem. Biophys. Res. Commun. 1963, 12, 388–394. [Google Scholar] [CrossRef]
- Hallgren, B.; Sourander, P. The Non-Haemin Iron in the Cerebral Cortex in Alzheimer’s Disease. J. Neurochem. 1960, 5, 307–310. [Google Scholar] [CrossRef]
- Filer, L.J.; Rumery, R.; Mason, K. Specific Unsaturated Fatty Acids in the Production of Acid Fast Pigment in the Vitamine E Deficient Rat and the Protective Action of Tocopherols. In Proceedings of the Transactions of the First Conference on Biological Antioxidants, New York, NY, USA, 10–11 October 1946. [Google Scholar]
- Braconnot, H. Mémoire Sur La Nature Des Corps Gras. Anna. Chim. 1815, 93, 225–277. [Google Scholar]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on Iron and Its Importance for Human Health. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Yiannikourides, A.; Latunde-Dada, G.O. A Short Review of Iron Metabolism and Pathophysiology of Iron Disorders. Medicines 2019, 6, 85. [Google Scholar] [CrossRef] [PubMed]
- Ems, T.; St Lucia, K.; Huecker, M.R. Biochemistry, Iron Absorption. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Knutson, M.D. Iron Transport Proteins: Gateways of Cellular and Systemic Iron Homeostasis. J. Biol. Chem. 2017, 292, 12735–12743. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.C.; Schmidt, P.J. Iron Homeostasis. Annu. Rev. Physiol. 2007, 69, 69–85. [Google Scholar] [CrossRef]
- Minotti, G.; Aust, S.D. The Role of Iron in the Initiation of Lipid Peroxidation. Chem. Phys. Lipids 1987, 44, 191–208. [Google Scholar] [CrossRef]
- Fenton, H.J.H. LXXIII.—Oxidation of Tartaric Acid in Presence of Iron. J. Chem. Soc. Trans. 1894, 65, 899–910. [Google Scholar] [CrossRef]
- Yaman, S.O.; Ayhanci, A. Lipid Peroxidation; Intech Open: Rijeka, Croatia, 2021; ISBN 978-1-83968-826-3. [Google Scholar]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, e360438. [Google Scholar] [CrossRef]
- Yin, H.; Xu, L.; Porter, N.A. Free Radical Lipid Peroxidation: Mechanisms and Analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef]
- Guéraud, F.; Atalay, M.; Bresgen, N.; Cipak, A.; Eckl, P.M.; Huc, L.; Jouanin, I.; Siems, W.; Uchida, K. Chemistry and Biochemistry of Lipid Peroxidation Products. Free Radic. Res. 2010, 44, 1098–1124. [Google Scholar] [CrossRef]
- Negre-Salvayre, A.; Auge, N.; Ayala, V.; Basaga, H.; Boada, J.; Brenke, R.; Chapple, S.; Cohen, G.; Feher, J.; Grune, T.; et al. Pathological Aspects of Lipid Peroxidation. Free Radic. Res. 2010, 44, 1125–1171. [Google Scholar] [CrossRef]
- Vaca, C.E.; Wilhelm, J.; Harms-Ringdahl, M. Interaction of Lipid Peroxidation Products with DNA. A Review. Mutat. Res. Genet. Toxicol. 1988, 195, 137–149. [Google Scholar] [CrossRef]
- Benzie, I.F.F. Lipid Peroxidation: A Review of Causes, Consequences, Measurement and Dietary Influences. Int. J. Food Sci. Nutr. 1996, 47, 233–261. [Google Scholar] [CrossRef]
- Natto, C.; Kawamura, M.; Yamamoto, Y. Lipid Peroxides as the Initiating Factor of Atherosclerosis. Ann. N. Y. Acad. Sci. 1993, 676, 27–45. [Google Scholar] [CrossRef]
- Nilsson, J.; Regnström, J.; Frostegård, J.; Stiko, A. Lipid Oxidation and Atherosclerosis. Herz 1992, 17, 263–269. [Google Scholar]
- Galluzzi, L.; Vitale, I.; Abrams, J.M.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; Dawson, T.M.; Dawson, V.L.; El-Deiry, W.S.; Fulda, S.; et al. Molecular Definitions of Cell Death Subroutines: Recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012, 19, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.F.R.; Wyllie, A.H.; Currie, A.R. Apoptosis: A Basic Biological Phenomenon with Wide-Ranging Implications in Tissue Kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B. (Ed.) Molecular Biology of the Cell, 5th ed.; Garland Science: New York, NY, USA, 2008; ISBN 978-0-8153-4105-5. [Google Scholar]
- Raychaudhuri, S. A Minimal Model of Signaling Network Elucidates Cell-to-Cell Stochastic Variability in Apoptosis. PLoS ONE 2010, 5, e11930. [Google Scholar] [CrossRef] [PubMed]
- Tixeira, R.; Caruso, S.; Paone, S.; Baxter, A.A.; Atkin-Smith, G.K.; Hulett, M.D.; Poon, I.K.H. Defining the Morphologic Features and Products of Cell Disassembly during Apoptosis. Apoptosis Int. J. Program. Cell Death 2017, 22, 475–477. [Google Scholar] [CrossRef] [PubMed]
- deCathelineau, A.M.; Henson, P.M. The Final Step in Programmed Cell Death: Phagocytes Carry Apoptotic Cells to the Grave. Essays Biochem. 2003, 39, 105–117. [Google Scholar] [CrossRef]
- Raffray, M.; Cohen, G.M. Apoptosis and Necrosis in Toxicology: A Continuum or Distinct Modes of Cell Death? Pharmacol. Ther. 1997, 75, 153–177. [Google Scholar] [CrossRef]
- Proskuryakov, S.Y.; Konoplyannikov, A.G.; Gabai, V.L. Necrosis: A Specific Form of Programmed Cell Death? Exp. Cell Res. 2003, 283, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Vanden Berghe, T.; Linkermann, A.; Jouan-Lanhouet, S.; Walczak, H.; Vandenabeele, P. Regulated Necrosis: The Expanding Network of Non-Apoptotic Cell Death Pathways. Nat. Rev. Mol. Cell Biol. 2014, 15, 135–147. [Google Scholar] [CrossRef]
- Vandenabeele, P.; Galluzzi, L.; Vanden Berghe, T.; Kroemer, G. Molecular Mechanisms of Necroptosis: An Ordered Cellular Explosion. Nat. Rev. Mol. Cell Biol. 2010, 11, 700–714. [Google Scholar] [CrossRef]
- Jorgensen, I.; Miao, E.A. Pyroptotic Cell Death Defends against Intracellular Pathogens. Immunol. Rev. 2015, 265, 130–142. [Google Scholar] [CrossRef]
- Gong, W.; Shi, Y.; Ren, J. Research Progresses of Molecular Mechanism of Pyroptosis and Its Related Diseases. Immunobiology 2020, 225, 151884. [Google Scholar] [CrossRef]
- David, K.K.; Andrabi, S.A.; Dawson, T.M.; Dawson, V.L. Parthanatos, a Messenger of Death. Front. Biosci. Landmark Ed. 2009, 14, 1116–1128. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S.; Chesarone-Cataldo, M.; Todorova, T.; Huang, Y.-H.; Chang, P. A Systematic Analysis of the PARP Protein Family Identifies New Functions Critical for Cell Physiology. Nat. Commun. 2013, 4, 2240. [Google Scholar] [CrossRef]
- Riegman, M.; Sagie, L.; Galed, C.; Levin, T.; Steinberg, N.; Dixon, S.J.; Wiesner, U.; Bradbury, M.S.; Niethammer, P.; Zaritsky, A.; et al. Ferroptosis Occurs through an Osmotic Mechanism and Propagates Independently of Cell Rupture. Nat. Cell Biol. 2020, 22, 1042–1048. [Google Scholar] [CrossRef]
- Yagoda, N.; von Rechenberg, M.; Zaganjor, E.; Bauer, A.J.; Yang, W.S.; Fridman, D.J.; Wolpaw, A.J.; Smukste, I.; Peltier, J.M.; Boniface, J.J.; et al. RAS–RAF–MEK-Dependent Oxidative Cell Death Involving Voltage-Dependent Anion Channels. Nature 2007, 447, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Friedmann Angeli, J.P.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E.; et al. Inactivation of the Ferroptosis Regulator Gpx4 Triggers Acute Renal Failure in Mice. Nat. Cell Biol. 2014, 16, 1180–1191. [Google Scholar] [CrossRef]
- Wei, S.; Qiu, T.; Yao, X.; Wang, N.; Jiang, L.; Jia, X.; Tao, Y.; Wang, Z.; Pei, P.; Zhang, J.; et al. Arsenic Induces Pancreatic Dysfunction and Ferroptosis via Mitochondrial ROS-Autophagy-Lysosomal Pathway. J. Hazard. Mater. 2020, 384, 121390. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, F.; Yin, H.; Huang, Z.; Lin, Z.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, Present and Future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- An, J.-R.; Su, J.-N.; Sun, G.-Y.; Wang, Q.-F.; Fan, Y.-D.; Jiang, N.; Yang, Y.-F.; Shi, Y. Liraglutide Alleviates Cognitive Deficit in Db/Db Mice: Involvement in Oxidative Stress, Iron Overload, and Ferroptosis. Neurochem. Res. 2021, 47, 279–294. [Google Scholar] [CrossRef]
- Karmi, O.; Sohn, Y.-S.; Zandalinas, S.I.; Rowland, L.; King, S.; Nechushtai, R.; Mittler, R. Disrupting CISD2 Function in Cancer Cells Primarily Impacts Mitochondrial Labile Iron Levels and Triggers TXNIP Expression. Free Radic. Biol. Med. 2021, 176, 92–104. [Google Scholar] [CrossRef]
- Alim, I.; Caulfield, J.T.; Chen, Y.; Swarup, V.; Geschwind, D.H.; Ivanova, E.; Seravalli, J.; Ai, Y.; Sansing, L.H.; Ste Marie, E.J.; et al. Selenium Drives a Transcriptional Adaptive Program to Block Ferroptosis and Treat Stroke. Cell 2019, 177, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Homma, T.; Kobayashi, S.; Fujii, J. Cysteine Preservation Confers Resistance to Glutathione-Depleted Cells against Ferroptosis via CDGSH Iron Sulphur Domain-Containing Proteins (CISDs). Free Radic. Res. 2020, 54, 397–407. [Google Scholar] [CrossRef]
- Jang, S.; Chapa-Dubocq, X.R.; Tyurina, Y.Y.; St Croix, C.M.; Kapralov, A.A.; Tyurin, V.A.; Bayır, H.; Kagan, V.E.; Javadov, S. Elucidating the Contribution of Mitochondrial Glutathione to Ferroptosis in Cardiomyocytes. Redox Biol. 2021, 45, 102021. [Google Scholar] [CrossRef]
- Dixon, S.J.; Patel, D.N.; Welsch, M.; Skouta, R.; Lee, E.D.; Hayano, M.; Thomas, A.G.; Gleason, C.E.; Tatonetti, N.P.; Slusher, B.S.; et al. Pharmacological Inhibition of Cystine-Glutamate Exchange Induces Endoplasmic Reticulum Stress and Ferroptosis. eLife 2014, 3, e02523. [Google Scholar] [CrossRef]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Organelle-Specific Regulation of Ferroptosis. Cell Death Differ. 2021, 28, 2843–2856. [Google Scholar] [CrossRef]
- Guo, J.; Duan, L.; He, X.; Li, S.; Wu, Y.; Xiang, G.; Bao, F.; Yang, L.; Shi, H.; Gao, M.; et al. A Combined Model of Human IPSC-Derived Liver Organoids and Hepatocytes Reveals Ferroptosis in DGUOK Mutant MtDNA Depletion Syndrome. Adv. Sci. 2021, 8, 2004680. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Angeli, J.P.F.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.; Fulda, S.; Gascon, S.; Hatzios, S.K.; Kagan, V.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Yang, H.; Rivera, Z.; Jube, S.; Nasu, M.; Bertino, P.; Goparaju, C.; Franzoso, G.; Lotze, M.T.; Krausz, T.; Pass, H.I.; et al. Programmed Necrosis Induced by Asbestos in Human Mesothelial Cells Causes High-Mobility Group Box 1 Protein Release and Resultant Inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 12611–12616. [Google Scholar] [CrossRef]
- Mou, Y.; Wang, J.; Wu, J.; He, D.; Zhang, C.; Duan, C.; Li, B. Ferroptosis, a New Form of Cell Death: Opportunities and Challenges in Cancer. J. Hematol. Oncol. 2019, 12, 34. [Google Scholar] [CrossRef]
- Shimada, K.; Skouta, R.; Kaplan, A.; Yang, W.S.; Hayano, M.; Dixon, S.J.; Brown, L.M.; Valenzuela, C.A.; Wolpaw, A.J.; Stockwell, B.R. Global Survey of Cell Death Mechanisms Reveals Metabolic Regulation of Ferroptosis. Nat. Chem. Biol. 2016, 12, 497–503. [Google Scholar] [CrossRef]
- Hou, W.; Xie, Y.; Song, X.; Sun, X.; Lotze, M.T.; Zeh, H.J.; Kang, R.; Tang, D. Autophagy Promotes Ferroptosis by Degradation of Ferritin. Autophagy 2016, 12, 1425–1428. [Google Scholar] [CrossRef]
- Feng, H.; Schorpp, K.; Jin, J.; Yozwiak, C.E.; Hoffstrom, B.G.; Decker, A.M.; Rajbhandari, P.; Stokes, M.E.; Bender, H.G.; Csuka, J.M.; et al. Transferrin Receptor Is a Specific Ferroptosis Marker. Cell Rep. 2020, 30, 3411–3423. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. Acsl4 Dictates Ferroptosis Sensitivity by Shaping Cellular Lipid Composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Winter, G.E.; Musavi, L.S.; Lee, E.D.; Snijder, B.; Rebsamen, M.; Superti-Furga, G.; Stockwell, B.R. Human Haploid Cell Genetics Reveals Roles for Lipid Metabolism Genes in Nonapoptotic Cell Death. ACS Chem. Biol. 2015, 10, 1604–1609. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Kim, K.J.; Gaschler, M.M.; Patel, M.; Shchepinov, M.S.; Stockwell, B.R. Peroxidation of Polyunsaturated Fatty Acids by Lipoxygenases Drives Ferroptosis. Proc. Natl. Acad. Sci. USA 2016, 113, E4966–E4975. [Google Scholar] [CrossRef]
- Park, T.-J.; Park, J.H.; Lee, G.S.; Lee, J.-Y.; Shin, J.H.; Kim, M.W.; Kim, Y.S.; Kim, J.-Y.; Oh, K.-J.; Han, B.-S.; et al. Quantitative Proteomic Analyses Reveal That GPX4 Downregulation during Myocardial Infarction Contributes to Ferroptosis in Cardiomyocytes. Cell Death Dis. 2019, 10, 835. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Conrad, M. Iron and Ferroptosis: A Still Ill-defined Liaison. IUBMB Life 2017, 69, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Fradejas, N.; Carlson, B.A.; Rijntjes, E.; Becker, N.-P.; Tobe, R.; Schweizer, U. Mammalian Trit1 Is a TRNA([Ser]Sec)-Isopentenyl Transferase Required for Full Selenoprotein Expression. Biochem. J. 2013, 450, 427–432. [Google Scholar] [CrossRef]
- Cardoso, B.R.; Hare, D.J.; Bush, A.I.; Roberts, B.R. Glutathione Peroxidase 4: A New Player in Neurodegeneration? Mol. Psychiatry 2017, 22, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Gaucher, C.; Boudier, A.; Bonetti, J.; Clarot, I.; Leroy, P.; Parent, M. Glutathione: Antioxidant Properties Dedicated to Nanotechnologies. Antioxidants 2018, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Hassannia, B.; Vandenabeele, P.; Berghe, T.V. Targeting Ferroptosis to Iron Out Cancer. Cancer Cell 2019, 35, 830–849. [Google Scholar] [CrossRef]
- Cao, J.Y.; Dixon, S.J. Mechanisms of Ferroptosis. Cell. Mol. Life Sci. 2016, 73, 2195–2209. [Google Scholar] [CrossRef]
- Gao, M.; Monian, P.; Quadri, N.; Ramasamy, R.; Jiang, X. Glutaminolysis and Transferrin Regulate Ferroptosis. Mol. Cell 2015, 59, 298–308. [Google Scholar] [CrossRef]
- Guo, J.; Xu, B.; Han, Q.; Zhou, H.; Xia, Y.; Gong, C.; Dai, X.; Li, Z.; Wu, G. Ferroptosis: A Novel Anti-Tumor Action for Cisplatin. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2018, 50, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, X.; Liu, L.; Yu, B.; Xue, Y.; Liu, Y. Erastin Sensitizes Glioblastoma Cells to Temozolomide by Restraining XCT and Cystathionine-γ-Lyase Function. Oncol. Rep. 2015, 33, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Hsu, J.L.; Chen, C.-T.; Wang, Y.-N.; Hsu, M.-C.; Chang, S.-S.; Du, Y.; Ko, H.-W.; Herbst, R.; Hung, M.-C. Caspase-Independent Cell Death Is Involved in the Negative Effect of EGFR Inhibitors on Cisplatin in Non-Small Cell Lung Cancer Cells. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 845–854. [Google Scholar] [CrossRef]
- Sato, M.; Kusumi, R.; Hamashima, S.; Kobayashi, S.; Sasaki, S.; Komiyama, Y.; Izumikawa, T.; Conrad, M.; Bannai, S.; Sato, H. The Ferroptosis Inducer Erastin Irreversibly Inhibits System xc− and Synergizes with Cisplatin to Increase Cisplatin’s Cytotoxicity in Cancer Cells. Sci. Rep. 2018, 8, 968. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.-L.; Kim, E.H.; Jang, H.J.; Park, J.Y.; Shin, D. Induction of Ferroptotic Cell Death for Overcoming Cisplatin Resistance of Head and Neck Cancer. Cancer Lett. 2016, 381, 96–103. [Google Scholar] [CrossRef]
- Pan, X.; Lin, Z.; Jiang, D.; Yu, Y.; Yang, D.; Zhou, H.; Zhan, D.; Liu, S.; Peng, G.; Chen, Z.; et al. Erastin Decreases Radioresistance of NSCLC Cells Partially by Inducing GPX4-mediated Ferroptosis. Oncol. Lett. 2019, 17, 3001–3008. [Google Scholar] [CrossRef]
- Shibata, Y.; Yasui, H.; Higashikawa, K.; Miyamoto, N.; Kuge, Y. Erastin, a Ferroptosis-Inducing Agent, Sensitized Cancer Cells to X-Ray Irradiation via Glutathione Starvation In Vitro and In Vivo. PLoS ONE 2019, 14, e0225931. [Google Scholar] [CrossRef]
- Cobler, L.; Zhang, H.; Suri, P.; Park, C.; Timmerman, L.A. XCT Inhibition Sensitizes Tumors to γ-Radiation via Glutathione Reduction. Oncotarget 2018, 9, 32280–32297. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, H.; Daniels, J.D.; Zandkarimi, F.; Liu, H.; Brown, L.M.; Uchida, K.; O’Connor, O.A.; Stockwell, B.R. Imidazole Ketone Erastin Induces Ferroptosis and Slows Tumor Growth in a Mouse Lymphoma Model. Cell Chem. Biol. 2019, 26, 623–633. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef]
- Takayama, T.; Kubo, T.; Morikawa, A.; Morita, T.; Nagano, O.; Saya, H. Potential of Sulfasalazine as a Therapeutic Sensitizer for CD44 Splice Variant 9-Positive Urogenital Cancer. Med. Oncol. 2016, 33, 45. [Google Scholar] [CrossRef]
- Ma, M.-Z.; Chen, G.; Wang, P.; Lu, W.-H.; Zhu, C.-F.; Song, M.; Yang, J.; Wen, S.; Xu, R.-H.; Hu, Y.; et al. Xc− Inhibitor Sulfasalazine Sensitizes Colorectal Cancer to Cisplatin by a GSH-Dependent Mechanism. Cancer Lett. 2015, 368, 88–96. [Google Scholar] [CrossRef]
- Lachaier, E.; Louandre, C.; Godin, C.; Saidak, Z.; Baert, M.; Diouf, M.; Chauffert, B.; Galmiche, A. Sorafenib Induces Ferroptosis in Human Cancer Cell Lines Originating from Different Solid Tumors. Anticancer Res. 2014, 34, 6417–6422. [Google Scholar] [PubMed]
- Louandre, C.; Ezzoukhry, Z.; Godin, C.; Barbare, J.-C.; Mazière, J.-C.; Chauffert, B.; Galmiche, A. Iron-Dependent Cell Death of Hepatocellular Carcinoma Cells Exposed to Sorafenib. Int. J. Cancer 2013, 133, 1732–1742. [Google Scholar] [CrossRef]
- Guo, W.; Zhao, Y.; Zhang, Z.; Tan, N.; Zhao, F.; Ge, C.; Liang, L.; Jia, D.; Chen, T.; Yao, M.; et al. Disruption of XCT Inhibits Cell Growth via the ROS/Autophagy Pathway in Hepatocellular Carcinoma. Cancer Lett. 2011, 312, 55–61. [Google Scholar] [CrossRef]
- Gout, P.W.; Buckley, A.R.; Simms, C.R.; Bruchovsky, N. Sulfasalazine, a Potent Suppressor of Lymphoma Growth by Inhibition of the xc− Cystine Transporter: A New Action for an Old Drug. Leukemia 2001, 15, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.L.; Saha, A.; Liu, J.; Tadi, S.; Tiziani, S.; Yan, W.; Triplett, K.; Lamb, C.; Alters, S.E.; Rowlinson, S.; et al. Systemic Depletion of L-Cyst(e)Ine with Cyst(e)Inase Increases Reactive Oxygen Species and Suppresses Tumor Growth. Nat. Med. 2017, 23, 120–127. [Google Scholar] [CrossRef]
- Shin, D.; Kim, E.H.; Lee, J.; Roh, J.-L. Nrf2 Inhibition Reverses Resistance to GPX4 Inhibitor-Induced Ferroptosis in Head and Neck Cancer. Free Radic. Biol. Med. 2018, 129, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Zhang, R.; Liu, S.; Duan, T.; Zhai, L.; Zhang, M.; Han, X.; Xiang, Y.; Huang, X.; Lin, H.; et al. RSL3 Drives Ferroptosis Through GPX4 Inactivation and ROS Production in Colorectal Cancer. Front. Pharmacol. 2018, 9, 1371. [Google Scholar] [CrossRef]
- Hassannia, B.; Wiernicki, B.; Ingold, I.; Qu, F.; Van Herck, S.; Tyurina, Y.Y.; Bayır, H.; Abhari, B.A.; Angeli, J.P.F.; Choi, S.M.; et al. Nano-Targeted Induction of Dual Ferroptotic Mechanisms Eradicates High-Risk Neuroblastoma. J. Clin. Investig. 2018, 128, 3341–3355. [Google Scholar] [CrossRef]
- Woo, J.H.; Shimoni, Y.; Yang, W.S.; Subramaniam, P.; Iyer, A.; Nicoletti, P.; Rodríguez Martínez, M.; López, G.; Mattioli, M.; Realubit, R.; et al. Elucidating Compound Mechanism of Action by Network Perturbation Analysis. Cell 2015, 162, 441–451. [Google Scholar] [CrossRef]
- Eaton, J.K.; Furst, L.; Ruberto, R.A.; Moosmayer, D.; Hillig, R.C.; Hilpmann, A.; Zimmermann, K.; Ryan, M.J.; Niehues, M.; Badock, V.; et al. Targeting a Therapy-Resistant Cancer Cell State Using Masked Electrophiles as GPX4 Inhibitors; Cold Spring Harbor Laboratory: New York, NY, USA, 2018; p. 376764. [Google Scholar]
- Weïwer, M.; Bittker, J.A.; Lewis, T.A.; Shimada, K.; Yang, W.S.; MacPherson, L.; Dandapani, S.; Palmer, M.; Stockwell, B.R.; Schreiber, S.L.; et al. Development of Small-Molecule Probes That Selectively Kill Cells Induced to Express Mutant RAS. Bioorg. Med. Chem. Lett. 2012, 22, 1822–1826. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Berleth, N.; Wu, W.; Schlütermann, D.; Deitersen, J.; Stuhldreier, F.; Berning, L.; Friedrich, A.; Akgün, S.; Mendiburo, M.J.; et al. Fin56-Induced Ferroptosis Is Supported by Autophagy-Mediated GPX4 Degradation and Functions Synergistically with MTOR Inhibition to Kill Bladder Cancer Cells. Cell Death Dis. 2021, 12, 1028. [Google Scholar] [CrossRef] [PubMed]
- Gaschler, M.M.; Andia, A.A.; Liu, H.; Csuka, J.M.; Hurlocker, B.; Vaiana, C.A.; Heindel, D.W.; Zuckerman, D.S.; Bos, P.H.; Reznik, E.; et al. FINO2 Initiates Ferroptosis through GPX4 Inactivation and Iron Oxidation. Nat. Chem. Biol. 2018, 14, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.-C.; Chiang, S.-K.; Chen, S.-E.; Yu, Y.-L.; Chou, R.-H.; Chang, W.-C. Heme Oxygenase-1 Mediates BAY 11-7085 Induced Ferroptosis. Cancer Lett. 2018, 416, 124–137. [Google Scholar] [CrossRef]
- Ma, S.; Henson, E.S.; Chen, Y.; Gibson, S.B. Ferroptosis Is Induced Following Siramesine and Lapatinib Treatment of Breast Cancer Cells. Cell Death Dis. 2016, 7, e2307. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, Y.; Hao, J.; Duan, H.-Q.; Zhao, C.-X.; Sun, C.; Li, B.; Fan, B.-Y.; Wang, X.; Li, W.-X.; et al. Deferoxamine Promotes Recovery of Traumatic Spinal Cord Injury by Inhibiting Ferroptosis. Neural Regen. Res. 2019, 14, 532–541. [Google Scholar] [CrossRef]
- Do Van, B.; Gouel, F.; Jonneaux, A.; Timmerman, K.; Gelé, P.; Pétrault, M.; Bastide, M.; Laloux, C.; Moreau, C.; Bordet, R.; et al. Ferroptosis, a Newly Characterized Form of Cell Death in Parkinson’s Disease That Is Regulated by PKC. Neurobiol. Dis. 2016, 94, 169–178. [Google Scholar] [CrossRef]
- Otsu, W.; Ishida, K.; Chinen, N.; Nakamura, S.; Shimazawa, M.; Tsusaki, H.; Hara, H. Cigarette Smoke Extract and Heated Tobacco Products Promote Ferritin Cleavage and Iron Accumulation in Human Corneal Epithelial Cells. Sci. Rep. 2021, 11, 18555. [Google Scholar] [CrossRef]
- Chen, M.-S.; Wang, S.-F.; Hsu, C.-Y.; Yin, P.-H.; Yeh, T.-S.; Lee, H.-C.; Tseng, L.-M. CHAC1 Degradation of Glutathione Enhances Cystine-Starvation-Induced Necroptosis and Ferroptosis in Human Triple Negative Breast Cancer Cells via the GCN2-EIF2α-ATF4 Pathway. Oncotarget 2017, 8, 114588–114602. [Google Scholar] [CrossRef]
- Barnabé, N.; Zastre, J.A.; Venkataram, S.; Hasinoff, B.B. Deferiprone Protects against Doxorubicin-Induced Myocyte Cytotoxicity. Free Radic. Biol. Med. 2002, 33, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Miotto, G.; Rossetto, M.; Di Paolo, M.L.; Orian, L.; Venerando, R.; Roveri, A.; Vučković, A.-M.; Bosello Travain, V.; Zaccarin, M.; Zennaro, L.; et al. Insight into the Mechanism of Ferroptosis Inhibition by Ferrostatin-1. Redox Biol. 2020, 28, 101328. [Google Scholar] [CrossRef]
- Wu, C.; Zhao, W.; Yu, J.; Li, S.; Lin, L.; Chen, X. Induction of Ferroptosis and Mitochondrial Dysfunction by Oxidative Stress in PC12 Cells. Sci. Rep. 2018, 8, 574. [Google Scholar] [CrossRef]
- Li, D.; Zhang, M.; Chao, H. Significance of Glutathione Peroxidase 4 and Intracellular Iron Level in Ovarian Cancer Cells-”utilization” of Ferroptosis Mechanism. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. Al 2021, 70, 1177–1189. [Google Scholar] [CrossRef]
- Hinman, A.; Holst, C.R.; Latham, J.C.; Bruegger, J.J.; Ulas, G.; McCusker, K.P.; Amagata, A.; Davis, D.; Hoff, K.G.; Kahn-Kirby, A.H.; et al. Vitamin E Hydroquinone Is an Endogenous Regulator of Ferroptosis via Redox Control of 15-Lipoxygenase. PLoS ONE 2018, 13, e0201369. [Google Scholar] [CrossRef]
- Princen, H.M.G.; van Duyvenvoorde, W.; Buytenhek, R.; van der Laarse, A.; van Poppel, G.; Leuven, J.A.G.; van Hinsbergh, V.W.M. Supplementation With Low Doses of Vitamin E Protects LDL From Lipid Peroxidation in Men and Women. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 325–333. [Google Scholar] [CrossRef]
- Yamada, N.; Karasawa, T.; Kimura, H.; Watanabe, S.; Komada, T.; Kamata, R.; Sampilvanjil, A.; Ito, J.; Nakagawa, K.; Kuwata, H.; et al. Ferroptosis Driven by Radical Oxidation of N-6 Polyunsaturated Fatty Acids Mediates Acetaminophen-Induced Acute Liver Failure. Cell Death Dis. 2020, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, J.W.; Liu, Z.H.; Shen, Y.; Ding, F.H.; Gu, G.; Liu, J.; Qiu, J.P.; Gao, J.; Zhang, R.Y.; et al. CTRP5 Promotes Transcytosis and Oxidative Modification of Low-Density Lipoprotein and the Development of Atherosclerosis. Atherosclerosis 2018, 278, 197–209. [Google Scholar] [CrossRef]
- Hörkkö, S.; Bird, D.A.; Miller, E.; Itabe, H.; Leitinger, N.; Subbanagounder, G.; Berliner, J.A.; Friedman, P.; Dennis, E.A.; Curtiss, L.K.; et al. Monoclonal Autoantibodies Specific for Oxidized Phospholipids or Oxidized Phospholipid–Protein Adducts Inhibit Macrophage Uptake of Oxidized Low-Density Lipoproteins. J. Clin. Investig. 1999, 103, 117–128. [Google Scholar] [CrossRef]
- Marques, V.B.; Leal, M.A.S.; Mageski, J.G.A.; Fidelis, H.G.; Nogueira, B.V.; Vasquez, E.C.; Meyrelles, S.D.S.; Simões, M.R.; Dos Santos, L. Chronic Iron Overload Intensifies Atherosclerosis in Apolipoprotein E Deficient Mice: Role of Oxidative Stress and Endothelial Dysfunction. Life Sci. 2019, 233, 116702. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xu, L.-H.; Forssell, C.; Sullivan, J.L.; Yuan, X.-M. Overexpression of Transferrin Receptor and Ferritin Related to Clinical Symptoms and Destabilization of Human Carotid Plaques. Exp. Biol. Med. 2008, 233, 818–826. [Google Scholar] [CrossRef]
- Vinchi, F.; Porto, G.; Simmelbauer, A.; Altamura, S.; Passos, S.T.; Garbowski, M.; Silva, A.M.N.; Spaich, S.; Seide, S.E.; Sparla, R.; et al. Atherosclerosis Is Aggravated by Iron Overload and Ameliorated by Dietary and Pharmacological Iron Restriction. Eur. Heart J. 2020, 41, 2681–2695. [Google Scholar] [CrossRef]
- Kapralov, A.A.; Yang, Q.; Dar, H.H.; Tyurina, Y.Y.; Anthonymuthu, T.S.; Kim, R.; St Croix, C.M.; Mikulska-Ruminska, K.; Liu, B.; Shrivastava, I.H.; et al. Redox Lipid Reprogramming Commands Susceptibility of Macrophages and Microglia to Ferroptotic Death. Nat. Chem. Biol. 2020, 16, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Sindrilaru, A.; Peters, T.; Wieschalka, S.; Baican, C.; Baican, A.; Peter, H.; Hainzl, A.; Schatz, S.; Qi, Y.; Schlecht, A.; et al. An Unrestrained Proinflammatory M1 Macrophage Population Induced by Iron Impairs Wound Healing in Humans and Mice. J. Clin. Investig. 2011, 121, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Marques, L.; Negre-Salvayre, A.; Costa, L.; Canonne-Hergaux, F. Iron Gene Expression Profile in Atherogenic Mox Macrophages. Biochim. Biophys. Acta 2016, 1862, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Marcil, V.; Lavoie, J.C.; Emonnot, L.; Seidman, E.; Levy, E. Analysis of the Effects of Iron and Vitamin C Co-Supplementation on Oxidative Damage, Antioxidant Response and Inflammation in THP-1 Macrophages. Clin. Biochem. 2011, 44, 873–883. [Google Scholar] [CrossRef]
- Sampilvanjil, A.; Karasawa, T.; Yamada, N.; Komada, T.; Higashi, T.; Baatarjav, C.; Watanabe, S.; Kamata, R.; Ohno, N.; Takahashi, M. Cigarette Smoke Extract Induces Ferroptosis in Vascular Smooth Muscle Cells. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H508–H518. [Google Scholar] [CrossRef]
- Howard, G.; Wagenknecht, L.E.; Burke, G.L.; Diez-Roux, A.; Evans, G.W.; McGovern, P.; Nieto, F.J.; Tell, G.S. Cigarette Smoking and Progression of Atherosclerosis: The Atherosclerosis Risk in Communities (ARIC) Study. JAMA 1998, 279, 119–124. [Google Scholar] [CrossRef]
- Guo, Z.; Ran, Q.; Roberts, L.J.; Zhou, L.; Richardson, A.; Sharan, C.; Wu, D.; Yang, H. Suppression of Atherogenesis by Overexpression of Glutathione Peroxidase-4 in Apolipoprotein E-Deficient Mice. Free Radic. Biol. Med. 2008, 44, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Wójcicki, J.; Rózewicka, L.; Barcew-Wiszniewska, B.; Samochowiec, L.; Juźwiak, S.; Kadłubowska, D.; Tustanowski, S.; Juzyszyn, Z. Effect of Selenium and Vitamin E on the Development of Experimental Atherosclerosis in Rabbits. Atherosclerosis 1991, 87, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Schwenke, D.C.; Behr, S.R. Vitamin E Combined with Selenium Inhibits Atherosclerosis in Hypercholesterolemic Rabbits Independently of Effects on Plasma Cholesterol Concentrations. Circ. Res. 1998, 83, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Mehta, U.; Kang, B.P.S.; Kukreja, R.S.; Bansal, M.P. Ultrastructural Examination of Rabbit Aortic Wall Following High-Fat Diet Feeding and Selenium Supplementation: A Transmission Electron Microscopy Study. J. Appl. Toxicol. JAT 2002, 22, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, H.; Huang, K. Selenium in the Prevention of Atherosclerosis and Its Underlying Mechanisms. Met. Integr. Biometal Sci. 2017, 9, 21–37. [Google Scholar] [CrossRef]
- Veiner, H.-L.; Gorbatov, R.; Vardi, M.; Doros, G.; Miller-Lotan, R.; Zohar, Y.; Sabo, E.; Asleh, R.; Levy, N.S.; Goldfarb, L.J.; et al. Pharmacogenomic Interaction between the Haptoglobin Genotype and Vitamin E on Atherosclerotic Plaque Progression and Stability. Atherosclerosis 2015, 239, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-J.; Wei, H.; Frei, B. The Iron Chelator, Desferrioxamine, Reduces Inflammation and Atherosclerotic Lesion Development in Experimental Mice. Exp. Biol. Med. 2010, 235, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, M. PM2.5 Induces Ferroptosis in Human Endothelial Cells through Iron Overload and Redox Imbalance. Environ. Pollut. 2019, 254, 112937. [Google Scholar] [CrossRef] [PubMed]
- Gujja, P.; Rosing, D.R.; Tripodi, D.J.; Shizukuda, Y. Iron Overload Cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 1001–1012. [Google Scholar] [CrossRef]
- Ravingerová, T.; Kindernay, L.; Barteková, M.; Ferko, M.; Adameová, A.; Zohdi, V.; Bernátová, I.; Ferenczyová, K.; Lazou, A. The Molecular Mechanisms of Iron Metabolism and Its Role in Cardiac Dysfunction and Cardioprotection. Int. J. Mol. Sci. 2020, 21, 7889. [Google Scholar] [CrossRef] [PubMed]
- Koleini, N.; Nickel, B.E.; Edel, A.L.; Fandrich, R.R.; Ravandi, A.; Kardami, E. Oxidized Phospholipids in Doxorubicin-Induced Cardiotoxicity. Chem. Biol. Interact. 2019, 303, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Feng, G.; Gauthier, J.M.; Lokshina, I.; Higashikubo, R.; Evans, S.; Liu, X.; Hassan, A.; Tanaka, S.; Cicka, M.; et al. Ferroptotic Cell Death and TLR4/Trif Signaling Initiate Neutrophil Recruitment after Heart Transplantation. J. Clin. Investig. 2019, 129, 2293–2304. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wang, H.; Han, D.; Xie, E.; Yang, X.; Wei, J.; Gu, S.; Gao, F.; Zhu, N.; Yin, X.; et al. Ferroptosis as a Target for Protection against Cardiomyopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 2672–2680. [Google Scholar] [CrossRef]
- Feng, Y.; Madungwe, N.B.; Imam Aliagan, A.D.; Tombo, N.; Bopassa, J.C. Liproxstatin-1 Protects the Mouse Myocardium against Ischemia/Reperfusion Injury by Decreasing VDAC1 Levels and Restoring GPX4 Levels. Biochem. Biophys. Res. Commun. 2019, 520, 606–611. [Google Scholar] [CrossRef]
- Sun, C.; Peng, F.; Li, J.; Cui, X.; Qiao, X.; Zhu, W. Ferroptosis-Specific Inhibitor Ferrostatin-1 Relieves H2O2-Induced Redox Imbalance in Primary Cardiomyocytes through the Nrf2/ARE Pathway. Dis. Markers 2022, 2022, 4539932. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Jiang, W.; Wang, W.; Xiong, R.; Wu, X.; Geng, Q. Ferroptosis and Its Emerging Roles in Cardiovascular Diseases. Pharmacol. Res. 2021, 166, 105466. [Google Scholar] [CrossRef]
- Johnson, W.M.; Wilson-Delfosse, A.L.; Mieyal, J.J. Dysregulation of Glutathione Homeostasis in Neurodegenerative Diseases. Nutrients 2012, 4, 1399–1440. [Google Scholar] [CrossRef]
- Ross, C.A.; Tabrizi, S.J. Huntington’s Disease: From Molecular Pathogenesis to Clinical Treatment. Lancet Neurol. 2011, 10, 83–98. [Google Scholar] [CrossRef]
- Sadagurski, M.; Cheng, Z.; Rozzo, A.; Palazzolo, I.; Kelley, G.R.; Dong, X.; Krainc, D.; White, M.F. IRS2 Increases Mitochondrial Dysfunction and Oxidative Stress in a Mouse Model of Huntington Disease. J. Clin. Investig. 2011, 121, 4070–4081. [Google Scholar] [CrossRef]
- Chen, J.; Marks, E.; Lai, B.; Zhang, Z.; Duce, J.A.; Lam, L.Q.; Volitakis, I.; Bush, A.I.; Hersch, S.; Fox, J.H. Iron Accumulates in Huntington’s Disease Neurons: Protection by Deferoxamine. PLoS ONE 2013, 8, e77023. [Google Scholar] [CrossRef]
- Youdim, M.B.; Riederer, P. The Role of Iron in Senescence of Dopaminergic Neurons in Parkinson’s Disease. J. Neural Transm. Suppl. 1993, 40, 57–67. [Google Scholar] [PubMed]
- Morris, C.M.; Edwardson, J.A. Iron Histochemistry of the Substantia Nigra in Parkinson’s Disease. Neurodegener. J. Neurodegener. Disord. Neuroprot. Neuroregener. 1994, 3, 277–282. [Google Scholar]
- Mann, V.M.; Cooper, J.M.; Daniel, S.E.; Srai, K.; Jenner, P.; Marsden, C.D.; Schapira, A.H. Complex I, Iron, and Ferritin in Parkinson’s Disease Substantia Nigra. Ann. Neurol. 1994, 36, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.A.; Jiménez-Jiménez, F.J.; Fernandez-Calle, P.; Lalinde, L.; Tenias, J.M.; Pondal, M.; Vazquez, A.; Codoceo, R. Serum Lipid Peroxides in Patients with Parkinson’s Disease. Neurosci. Lett. 1992, 136, 137–140. [Google Scholar] [CrossRef]
- Dexter, D.T.; Carter, C.J.; Wells, F.R.; Javoy-Agid, F.; Agid, Y.; Lees, A.; Jenner, P.; Marsden, C.D. Basal Lipid Peroxidation in Substantia Nigra Is Increased in Parkinson’s Disease. J. Neurochem. 1989, 52, 381–389. [Google Scholar] [CrossRef]
- Przedborski, S.; Ischiropoulos, H. Reactive Oxygen and Nitrogen Species: Weapons of Neuronal Destruction in Models of Parkinson’s Disease. Antioxid. Redox Signal. 2005, 7, 685–693. [Google Scholar] [CrossRef]
- Tieu, K.; Ischiropoulos, H.; Przedborski, S. Nitric Oxide and Reactive Oxygen Species in Parkinson’s Disease. IUBMB Life 2003, 55, 329–335. [Google Scholar] [CrossRef]
- Hirsch, E.C. Does Oxidative Stress Participate in Nerve Cell Death in Parkinson’s Disease? Eur. Neurol. 1993, 33 (Suppl. S1), 52–59. [Google Scholar] [CrossRef]
- Magaki, S.; Raghavan, R.; Mueller, C.; Oberg, K.C.; Vinters, H.V.; Kirsch, W.M. Iron, Copper, and Iron Regulatory Protein 2 in Alzheimer’s Disease and Related Dementias. Neurosci. Lett. 2007, 418, 72–76. [Google Scholar] [CrossRef]
- Morris, C.M.; Kerwin, J.M.; Edwardson, J.A. Non-Haem Iron Histochemistry of the Normal and Alzheimer’s Disease Hippocampus. Neurodegener. J. Neurodegener. Disord. Neuroprot. Neuroregener. 1994, 3, 267–275. [Google Scholar]
- Piñero, D.J.; Hu, J.; Connor, J.R. Alterations in the Interaction between Iron Regulatory Proteins and Their Iron Responsive Element in Normal and Alzheimer’s Diseased Brains. Cell. Mol. Biol. 2000, 46, 761–776. [Google Scholar]
- Lovell, M.A.; Markesbery, W.R. Oxidative DNA Damage in Mild Cognitive Impairment and Late-Stage Alzheimer’s Disease. Nucleic Acids Res. 2007, 35, 7497–7504. [Google Scholar] [CrossRef]
- Dei, R.; Takeda, A.; Niwa, H.; Li, M.; Nakagomi, Y.; Watanabe, M.; Inagaki, T.; Washimi, Y.; Yasuda, Y.; Horie, K.; et al. Lipid Peroxidation and Advanced Glycation End Products in the Brain in Normal Aging and in Alzheimer’s Disease. Acta Neuropathol. 2002, 104, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Dang, T.N.; Arseneault, M.; Ramassamy, C. Role of By-Products of Lipid Oxidation in Alzheimer’s Disease Brain: A Focus on Acrolein. J. Alzheimer’s Dis. JAD 2010, 21, 741–756. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhao, J.; Zhao, Y.; Zhou, L.; Qiao, H.; Xu, Q.; Liu, Y. The Role of Ferroptosis in Neurodegenerative Diseases. Mol. Biol. Rep. 2022, 52, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Weiland, A.; Wang, Y.; Wu, W.; Lan, X.; Han, X.; Li, Q.; Wang, J. Ferroptosis and Its Role in Diverse Brain Diseases. Mol. Neurobiol. 2019, 56, 4880–4893. [Google Scholar] [CrossRef] [PubMed]
- Reichert, C.O.; de Freitas, F.A.; Sampaio-Silva, J.; Rokita-Rosa, L.; de Lima Barros, P.; Levy, D.; Bydlowski, S.P. Ferroptosis Mechanisms Involved in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 8765. [Google Scholar] [CrossRef]
- Hambright, W.S.; Fonseca, R.S.; Chen, L.; Na, R.; Ran, Q. Ablation of Ferroptosis Regulator Glutathione Peroxidase 4 in Forebrain Neurons Promotes Cognitive Impairment and Neurodegeneration. Redox Biol. 2017, 12, 8–17. [Google Scholar] [CrossRef]
- Ingold, I.; Berndt, C.; Schmitt, S.; Doll, S.; Poschmann, G.; Buday, K.; Roveri, A.; Peng, X.; Porto Freitas, F.; Seibt, T.; et al. Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell 2018, 172, 409–422. [Google Scholar] [CrossRef]
- Aoyama, K. Glutathione in the Brain. Int. J. Mol. Sci. 2021, 22, 5010. [Google Scholar] [CrossRef]
- Robert, S.M.; Ogunrinu-Babarinde, T.; Holt, K.T.; Sontheimer, H. Role of Glutamate Transporters in Redox Homeostasis of the Brain. Neurochem. Int. 2014, 73, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Manzar, H.; Abdulhussein, D.; Yap, T.E.; Cordeiro, M.F. Cellular Consequences of Coenzyme Q10 Deficiency in Neurodegeneration of the Retina and Brain. Int. J. Mol. Sci. 2020, 21, 9299. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Mercado-Ayon, E.; Mercado-Ayon, Y.; Dong, Y.N.; Halawani, S.; Ngaba, L.; Lynch, D.R. Mitochondrial Dysfunction in the Development and Progression of Neurodegenerative Diseases. Arch. Biochem. Biophys. 2021, 702, 108698. [Google Scholar] [CrossRef]
- Zuo, Y.; Xie, J.; Li, X.; Li, Y.; Thirupathi, A.; Zhang, J.; Yu, P.; Gao, G.; Chang, Y.; Shi, Z. Ferritinophagy-Mediated Ferroptosis Involved in Paraquat-Induced Neurotoxicity of Dopaminergic Neurons: Implication for Neurotoxicity in PD. Oxid. Med. Cell. Longev. 2021, 2021, 9961628. [Google Scholar] [CrossRef]
- Mahoney-Sánchez, L.; Bouchaoui, H.; Ayton, S.; Devos, D.; Duce, J.A.; Devedjian, J.-C. Ferroptosis and Its Potential Role in the Physiopathology of Parkinson’s Disease. Prog. Neurobiol. 2021, 196, 101890. [Google Scholar] [CrossRef]
- Moreau, C.; Duce, J.A.; Rascol, O.; Devedjian, J.-C.; Berg, D.; Dexter, D.; Cabantchik, Z.I.; Bush, A.I.; Devos, D.; FAIRPARK-II study group. Iron as a Therapeutic Target for Parkinson’s Disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2018, 33, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Arenas-Jal, M.; Suñé-Negre, J.M.; García-Montoya, E. Coenzyme Q10 Supplementation: Efficacy, Safety, and Formulation Challenges. Compr. Rev. Food Sci. Food Saf. 2020, 19, 574–594. [Google Scholar] [CrossRef]
- Martin-Bastida, A.; Ward, R.J.; Newbould, R.; Piccini, P.; Sharp, D.; Kabba, C.; Patel, M.C.; Spino, M.; Connelly, J.; Tricta, F.; et al. Brain Iron Chelation by Deferiprone in a Phase 2 Randomised Double-Blinded Placebo Controlled Clinical Trial in Parkinson’s Disease. Sci. Rep. 2017, 7, 1398. [Google Scholar] [CrossRef]
- University Hospital. Conservative Iron Chelation as a Disease-Modifying Strategy in Parkinson’s Disease. European Multicentre, Parallel-Group, Placebo-Controlled, Randomized Clinical Trial of Deferiprone; Clinicaltrials.gov: Lille, France, 2022.
- FAIRPARK II|EU Funded Research Project|Parkinson’s Disease—Fair Park II (Fairpark2.Eu). Available online: http://fairpark2.eu/ (accessed on 6 January 2023).
- Forni, D.G.L. Ferrochelating Treatment in Patients Affected by “Neurodegeneration With Brain Iron Accumulation” (NBIA); Clinicaltrials.gov: Cagliari and Genoa, Italy, 2022.
- Abbruzzese, G.; Cossu, G.; Balocco, M.; Marchese, R.; Murgia, D.; Melis, M.; Galanello, R.; Barella, S.; Matta, G.; Ruffinengo, U.; et al. A Pilot Trial of Deferiprone for Neurodegeneration with Brain Iron Accumulation. Haematologica 2011, 96, 1708–1711. [Google Scholar] [CrossRef] [PubMed]
- University Hospital. Conservative Iron Chelation as a Disease-Modifying Strategy in Amyotrophic Lateral Sclerosis: Multicentre, Parallel-Group, Placebo-Controlled, Randomized Clinical Trial of Deferiprone; Clinicaltrials.gov: Lille, France, 2022.
- Llabani, E.; Hicklin, R.W.; Lee, H.Y.; Motika, S.E.; Crawford, L.A.; Weerapana, E.; Hergenrother, P.J. Diverse Compounds from Pleuromutilin Lead to a Thioredoxin Inhibitor and Inducer of Ferroptosis. Nat. Chem. 2019, 11, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wu, Q.; Feng, J.; Yan, L.; Sun, Y.; Liu, S.; Xiang, Y.; Zhang, M.; Pan, T.; Chen, X.; et al. Erianin, a Novel Dibenzyl Compound in Dendrobium Extract, Inhibits Lung Cancer Cell Growth and Migration via Calcium/Calmodulin-Dependent Ferroptosis. Signal Transduct. Target. Ther. 2020, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Xiang, X.; Li, J.; Deng, J.; Fang, Z.; Zhang, L.; Xiong, J. Ruscogenin Induces Ferroptosis in Pancreatic Cancer Cells. Oncol. Rep. 2020, 43, 516–524. [Google Scholar] [CrossRef]
- Fu, F.; Lai, Q.; Hu, J.; Zhang, L.; Zhu, X.; Kou, J.; Yu, B.; Li, F. Ruscogenin Alleviates Myocardial Ischemia-Induced Ferroptosis through the Activation of BCAT1/BCAT2. Antioxidants 2022, 11, 583. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; An, S. Ruscogenin Prevents Folic Acid-Induced Acute Kidney Damage by Inhibiting Rev-Erbα/β-Mediated Ferroptosis. Comput. Intell. Neurosci. 2022, 2022, 8066126. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-C.; Chuang, J.-Y.; Ko, C.-Y.; Kao, T.-J.; Yang, P.-Y.; Yu, C.-H.; Liu, M.-S.; Hu, S.-L.; Tsai, Y.-T.; Chan, H.; et al. AR Ubiquitination Induced by the Curcumin Analog Suppresses Growth of Temozolomide-Resistant Glioblastoma through Disrupting GPX4-Mediated Redox Homeostasis. Redox Biol. 2019, 30, 101413. [Google Scholar] [CrossRef]
- Li, R.; Zhang, J.; Zhou, Y.; Gao, Q.; Wang, R.; Fu, Y.; Zheng, L.; Yu, H. Transcriptome Investigation and In Vitro Verification of Curcumin-Induced HO-1 as a Feature of Ferroptosis in Breast Cancer Cells. Oxid. Med. Cell. Longev. 2020, 2020, 3469840. [Google Scholar] [CrossRef]
- Guerrero-Hue, M.; García-Caballero, C.; Palomino-Antolín, A.; Rubio-Navarro, A.; Vázquez-Carballo, C.; Herencia, C.; Martín-Sanchez, D.; Farré-Alins, V.; Egea, J.; Cannata, P.; et al. Curcumin Reduces Renal Damage Associated with Rhabdomyolysis by Decreasing Ferroptosis-Mediated Cell Death. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 8961–8975. [Google Scholar] [CrossRef]
- de Souza Farias, S.A.; da Costa, K.S.; Martins, J.B.L. Analysis of Conformational, Structural, Magnetic, and Electronic Properties Related to Antioxidant Activity: Revisiting Flavan, Anthocyanidin, Flavanone, Flavonol, Isoflavone, Flavone, and Flavan-3-Ol. ACS Omega 2021, 6, 8908–8918. [Google Scholar] [CrossRef]
- Perez, C.A.; Wei, Y.; Guo, M. Iron-Binding and Anti-Fenton Properties of Baicalein and Baicalin. J. Inorg. Biochem. 2009, 103, 326–332. [Google Scholar] [CrossRef]
- Li, Q.; Li, Q.-Q.; Jia, J.-N.; Sun, Q.-Y.; Zhou, H.-H.; Jin, W.-L.; Mao, X.-Y. Baicalein Exerts Neuroprotective Effects in FeCl3-Induced Posttraumatic Epileptic Seizures via Suppressing Ferroptosis. Front. Pharmacol. 2019, 10, 638. [Google Scholar] [CrossRef] [PubMed]
- Holland, T.M.; Agarwal, P.; Wang, Y.; Leurgans, S.E.; Bennett, D.A.; Booth, S.L.; Morris, M.C. Dietary Flavonols and Risk of Alzheimer Dementia. Neurology 2020, 94, e1749–e1756. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhai, Y.; Chen, J.; Xu, X.; Wang, H. Kaempferol Ameliorates Oxygen-Glucose Deprivation/Reoxygenation-Induced Neuronal Ferroptosis by Activating Nrf2/SLC7A11/GPX4 Axis. Biomolecules 2021, 11, 923. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zeng, J.; Liu, Y.; Liang, M.; Liu, Q.; Li, Z.; Zhao, X.; Chen, D. Inhibitory Effect and Mechanism of Action of Quercetin and Quercetin Diels-Alder Anti-Dimer on Erastin-Induced Ferroptosis in Bone Marrow-Derived Mesenchymal Stem Cells. Antioxidants 2020, 9, 205. [Google Scholar] [CrossRef]
- Zheng, K.; Dong, Y.; Yang, R.; Liang, Y.; Wu, H.; He, Z. Regulation of Ferroptosis by Bioactive Phytochemicals: Implications for Medical Nutritional Therapy. Pharmacol. Res. 2021, 168, 105580. [Google Scholar] [CrossRef]
- Quinn, P.J.; Boldyrev, A.A.; Formazuyk, V.E. Carnosine: Its Properties, Functions and Potential Therapeutic Applications. Mol. Asp. Med. 1992, 13, 379–444. [Google Scholar] [CrossRef]
- Pan, M.; Liu, K.; Yang, J.; Liu, S.; Wang, S.; Wang, S. Advances on Food-Derived Peptidic Antioxidants—A Review. Antioxidants 2020, 9, 799. [Google Scholar] [CrossRef]
- Sarmadi, B.H.; Ismail, A. Antioxidative Peptides from Food Proteins: A Review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef]
- Coda, R.; Rizzello, C.G.; Pinto, D.; Gobbetti, M. Selected Lactic Acid Bacteria Synthesize Antioxidant Peptides during Sourdough Fermentation of Cereal Flours. Appl. Environ. Microbiol. 2012, 78, 1087–1096. [Google Scholar] [CrossRef]
- Csire, G.; Canabady-Rochelle, L.; Averlant-Petit, M.-C.; Selmeczi, K.; Stefan, L. Both Metal-Chelating and Free Radical-Scavenging Synthetic Pentapeptides as Efficient Inhibitors of Reactive Oxygen Species Generation. Metallomics 2020, 12, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.-J.; Huang, L.-H.; Sun, Q.; Jiang, Z.-Q.; Wu, X. Isolation, Identification and Synthesis of Four Novel Antioxidant Peptides from Rice Residue Protein Hydrolyzed by Multiple Proteases. Food Chem. 2015, 179, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, M.; Lin, S.; Cheng, S. Contribution of Specific Amino Acid and Secondary Structure to the Antioxidant Property of Corn Gluten Proteins. Food Res. Int. Ott. Ont. 2018, 105, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, H.; Joshi, R.; Gupta, M. Isolation, Purification and Characterization of Antioxidative Peptide of Pearl Millet (Pennisetum glaucum) Protein Hydrolysate. Food Chem. 2016, 204, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Bamdad, F.; Gänzle, M.; Chen, L. Fractionation and Characterization of Antioxidant Peptides Derived from Barley Glutelin by Enzymatic Hydrolysis. Food Chem. 2012, 134, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.B.; Wang, Z.; Xu, S.Y.; Gao, X.F. Purification and Characterization of a Radical Scavenging Peptide from Rapeseed Protein Hydrolysates. J. Am. Oil Chem. Soc. 2009, 86, 959–966. [Google Scholar] [CrossRef]
- Chen, N.; Yang, H.; Sun, Y.; Niu, J.; Liu, S. Purification and Identification of Antioxidant Peptides from Walnut (Juglans regia L.) Protein Hydrolysates. Peptides 2012, 38, 344–349. [Google Scholar] [CrossRef]
- Yang, R.; Li, X.; Lin, S.; Zhang, Z.; Chen, F. Identification of Novel Peptides from 3 to 10kDa Pine Nut (Pinus koraiensis) Meal Protein, with an Exploration of the Relationship between Their Antioxidant Activities and Secondary Structure. Food Chem. 2017, 219, 311–320. [Google Scholar] [CrossRef]
- Shen, S.; Chahal, B.; Majumder, K.; You, S.-J.; Wu, J. Identification of Novel Antioxidative Peptides Derived from a Thermolytic Hydrolysate of Ovotransferrin by LC-MS/MS. J. Agric. Food Chem. 2010, 58, 7664–7672. [Google Scholar] [CrossRef]
- Yousr, M.; Howell, N. Antioxidant and ACE Inhibitory Bioactive Peptides Purified from Egg Yolk Proteins. Int. J. Mol. Sci. 2015, 16, 29161–29178. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-Y.; Majumder, K.; Wu, J. Oxygen Radical Absorbance Capacity of Peptides from Egg White Protein Ovotransferrin and Their Interaction with Phytochemicals. Food Chem. 2010, 123, 635–641. [Google Scholar] [CrossRef]
- Najafian, L.; Babji, A.S. Isolation, Purification and Identification of Three Novel Antioxidative Peptides from Patin (Pangasius sutchi) Myofibrillar Protein Hydrolysates. LWT-Food Sci. Technol. 2015, 60, 452–461. [Google Scholar] [CrossRef]
- Guo, L.; Hou, H.; Li, B.; Zhang, Z.; Wang, S.; Zhao, X. Preparation, Isolation and Identification of Iron-Chelating Peptides Derived from Alaska Pollock Skin. Process Biochem. 2013, 48, 988–993. [Google Scholar] [CrossRef]
- Bordbar, S.; Ebrahimpour, A.; Zarei, M.; Abdul Hamid, A.; Saari, N. Alcalase-Generated Proteolysates of Stone Fish (Actinopyga lecanora) Flesh as a New Source of Antioxidant Peptides. Int. J. Food Prop. 2018, 21, 1541–1559. [Google Scholar] [CrossRef]
- Kim, S.S.; Ahn, C.-B.; Moon, S.W.; Je, J.-Y. Purification and Antioxidant Activities of Peptides from Sea Squirt (Halocynthia roretzi) Protein Hydrolysates Using Pepsin Hydrolysis. Food Biosci. 2018, 25, 128–133. [Google Scholar] [CrossRef]
- Gallego, M.; Mora, L.; Reig, M.; Toldrá, F. Stability of the Potent Antioxidant Peptide SNAAC Identified from Spanish Dry-Cured Ham. Food Res. Int. 2018, 105, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Escudero, E.; Mora, L.; Fraser, P.D.; Aristoy, M.-C.; Toldrá, F. Identification of Novel Antioxidant Peptides Generated in Spanish Dry-Cured Ham. Food Chem. 2013, 138, 1282–1288. [Google Scholar] [CrossRef]
- Xing, L.-J.; Hu, Y.-Y.; Hu, H.-Y.; Ge, Q.-F.; Zhou, G.-H.; Zhang, W.-G. Purification and Identification of Antioxidative Peptides from Dry-Cured Xuanwei Ham. Food Chem. 2016, 194, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.-Z.; Zhang, W.-G.; Zhou, G.-H.; Xu, X.-L.; Kang, Z.-L.; Yin, Y. Isolation and Identification of Antioxidant Peptides from Jinhua Ham. J. Agric. Food Chem. 2013, 61, 1265–1271. [Google Scholar] [CrossRef]
- Zhang, Q.; Tong, X.; Li, Y.; Wang, H.; Wang, Z.; Qi, B.; Sui, X.; Jiang, L. Purification and Characterization of Antioxidant Peptides from Alcalase-Hydrolyzed Soybean (Glycine max L.) Hydrolysate and Their Cytoprotective Effects in Human Intestinal Caco-2 Cells. J. Agric. Food Chem. 2019, 67, 5772–5781. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Zhang, J.; Feng, Y.; Duan, Y.; Ma, H.; Zhang, H. Purification and Identification of Novel Antioxidant Peptides from Watermelon Seed Protein Hydrolysates and Their Cytoprotective Effects on H2O2-Induced Oxidative Stress. Food Chem. 2020, 327, 127059. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-M.; Wang, Y.-M.; Zhao, Y.-Q.; Chi, C.-F.; Wang, B. Antioxidant Peptides from the Protein Hydrolysate of Monkfish (Lophius litulon) Muscle: Purification, Identification, and Cytoprotective Function on HepG2 Cells Damage by H2O2. Mar. Drugs 2020, 18, 153. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liao, W.; Nimalaratne, C.; Chakrabarti, S.; Wu, J. Purification and Characterization of Antioxidant Peptides from Cooked Eggs Using a Dynamic in Vitro Gastrointestinal Model in Vascular Smooth Muscle A7r5 Cells. NPJ Sci. Food 2018, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Sun, B.; Luo, X.; Zhao, M.; Zheng, F.; Sun, J.; Li, H.; Sun, X.; Huang, M. Cytoprotective Effects of a Tripeptide from Chinese Baijiu against AAPH-Induced Oxidative Stress in HepG2 Cells via Nrf2 Signaling. RSC Adv. 2018, 8, 10898–10906. [Google Scholar] [CrossRef]
- Jiang, N.; Zhang, S.; Zhu, J.; Shang, J.; Gao, X. Hypoglycemic, Hypolipidemic and Antioxidant Effects of Peptides from Red Deer Antlers in Streptozotocin-Induced Diabetic Mice. Tohoku J. Exp. Med. 2015, 236, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Ben Khaled, H.; Ghlissi, Z.; Chtourou, Y.; Hakim, A.; Ktari, N.; Fatma, M.A.; Barkia, A.; Sahnoun, Z.; Nasri, M. Effect of Protein Hydrolysates from Sardinelle (Sardinella aurita) on the Oxidative Status and Blood Lipid Profile of Cholesterol-Fed Rats. Food Res. Int. 2012, 45, 60–68. [Google Scholar] [CrossRef]
- Zou, Z.; Wang, M.; Wang, Z.; Aluko, R.E.; He, R. Antihypertensive and Antioxidant Activities of Enzymatic Wheat Bran Protein Hydrolysates. J. Food Biochem. 2020, 44, e13090. [Google Scholar] [CrossRef]
- Ding, J.-F.; Li, Y.-Y.; Xu, J.-J.; Su, X.-R.; Gao, X.; Yue, F.-P. Study on Effect of Jellyfish Collagen Hydrolysate on Anti-Fatigue and Anti-Oxidation. Food Hydrocoll. 2011, 25, 1350–1353. [Google Scholar] [CrossRef]



| Origin | Peptide Sequences | Antioxidant Activities | References |
|---|---|---|---|
| Soy proteins | VNPESQQGSPR; LLPHH; LLPLPVLK; WLR; MLPVMR; SWLRL; SGDAL; SHECN; LPFAM | ABTS, DPPH, and Hydroxyl radical scavenging activities; Ferric reducing power; Inhibition of Fenton reaction | [208,209,210,211,212] |
| Rice proteins | FRDEHKK; RPNYTDA; TSQLLSDQ; TRTGDPFF; NFHPQ | ABTS, DPPH, and Hydroxyl radical scavenging activities; Lipid peroxidation inhibition in liposome model; Ferric reducing power | [141,208] |
| Cereal proteins (wheat, corn, pearl millet, barley) | KVALMSAGSMH; AGLPM; HALGA; SDRDLLGPNNQYLP; SVNVPL | Oxygen radical absorbance capacity; DPPH and Hydroxyl radical scavenging activities, Lipid peroxidation inhibition in liposome model; Iron (II) chelating activity | [206,209,210,211] |
| Seed proteins (rapeseed, walnuts, pine nut) | PAGPF; ADAF; KWFCT; QWFCT | DPPH radical scavenging activities; Lipid peroxidation inhibition in liposome model; Iron (II) chelating activity, Ferric reducing power | [212,213,214] |
| Egg proteins | WNIP; GWNI; WYGPD; KLSDW; KGLWE; IRW | Oxygen radical absorbance capacity; DPPH radical scavenging activity; Lipid peroxidation inhibition in liposome model | [215,216,217] |
| Fish proteins | VPKNYFHDIV; LVMFLDNQHRVIRH; FVNQPYLLYSVHMK; SCH; GVSGLHID; MTTL; LEW; YYPYQL; LEW; MTTL; YYPYQL | Oxygen radical absorbance capacity; ABTS and DDPH radical scavenging activities; Iron (II) chelating activity | [218,219,220,221] |
| Beef proteins | SNAAC; SAGNPN; DLEE; FWIIE; APYMM; GKFNV | Oxygen radical absorbance capacity; ABTS, DDPH, Hydroxyl, and Superoxide anion radical scavenging activities; Lipid peroxidation inhibition in liposome model; inhibition of Fenton’s reagent-induced protein oxidation; Iron (II) chelating activity | [222,223,224,225] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Hajj, S.; Canabady-Rochelle, L.; Gaucher, C. Nature-Inspired Bioactive Compounds: A Promising Approach for Ferroptosis-Linked Human Diseases? Molecules 2023, 28, 2636. https://doi.org/10.3390/molecules28062636
El Hajj S, Canabady-Rochelle L, Gaucher C. Nature-Inspired Bioactive Compounds: A Promising Approach for Ferroptosis-Linked Human Diseases? Molecules. 2023; 28(6):2636. https://doi.org/10.3390/molecules28062636
Chicago/Turabian StyleEl Hajj, Sarah, Laetitia Canabady-Rochelle, and Caroline Gaucher. 2023. "Nature-Inspired Bioactive Compounds: A Promising Approach for Ferroptosis-Linked Human Diseases?" Molecules 28, no. 6: 2636. https://doi.org/10.3390/molecules28062636
APA StyleEl Hajj, S., Canabady-Rochelle, L., & Gaucher, C. (2023). Nature-Inspired Bioactive Compounds: A Promising Approach for Ferroptosis-Linked Human Diseases? Molecules, 28(6), 2636. https://doi.org/10.3390/molecules28062636






