Abstract
The AT1 receptor has mainly been associated with the pathological effects of the renin-angiotensin system (RAS) (e.g., hypertension, heart and kidney diseases), and constitutes a major therapeutic target. In contrast, the AT2 receptor is presented as the protective arm of this RAS, and its targeting via specific agonists is mainly used to counteract the effects of the AT1 receptor. The discovery of a local RAS has highlighted the importance of the balance between AT1/AT2 receptors at the tissue level. Disruption of this balance is suggested to be detrimental. The fine tuning of this balance is not limited to the regulation of the level of expression of these two receptors. Other mechanisms still largely unexplored, such as S-nitrosation of the AT1 receptor, homo- and heterodimerization, and the use of AT1 receptor-biased agonists, may significantly contribute to and/or interfere with the settings of this AT1/AT2 equilibrium. This review will detail, through several examples (the brain, wound healing, and the cellular cycle), the importance of the functional balance between AT1 and AT2 receptors, and how new molecular pharmacological approaches may act on its regulation to open up new therapeutic perspectives.
1. Introduction
Over the past decades, the team of Professor Jeffrey Atkinson, who recently passed away (1943–2023) and who taught Pharmacology at the Faculty of Pharmacy of Nancy for over 25 years, has contributed to demonstrating the major role of the renin angiotensin system (RAS) in the regulation of the cardiovascular system and the cerebral circulation [1,2,3,4].
The RAS is an important hormonal system involved in numerous physiological processes, as shown by the copious literature devoted to the exploration and comprehension of this system since the discovery of renin in the late 19th century by Robert Tigerstedt [5]. The systemic RAS is known for its involvement in vascular homeostasis, blood pressure regulation, and sodium and water retention in the kidney [6]. Angiotensinogen, a glycoprotein produced by the liver and released in the blood is the first element of the systemic RAS. When blood pressure drops, the juxtaglomerular apparatus in the kidney releases renin, an enzyme which cleaves angiotensinogen into angiotensin I (Ang I), an inactive decapeptide. Subsequently, Ang I will be turned into angiotensin II (Ang II) by the angiotensin I-converting enzyme (ACE) [7], mainly expressed at the surface of endothelial cells. Ang II, an octapeptide, is the main endogenous ligand of the RAS, and binds to two main receptors: the angiotensin II type 1 receptor (AT1) and the angiotensin II type 2 receptor (AT2), both belonging to the G protein-coupled receptor (GPCR) family [8,9]. The hydrolysis of Ang II [10] by the angiotensin II-converting enzyme 2 (ACE2) produces the heptapeptide Ang-(1-7). Ang-(1-7) mediates signaling via the Mas receptor (MasR) and the Mas-related G protein-coupled receptor member D (MrgD receptor) (Figure 1). In addition, decarboxylation of Ang-(1-7) transforms it into alamandin, which is also able to bind to the MrGD receptor. These two axes have been the subject of many recent reviews, and will not be discussed in this article [11,12,13]. The AT4 receptor, whose ligand is Ang IV, has been identified as a transmembrane enzyme, insulin-regulated membrane aminopeptidase (IRAP) [14]. In addition to its vasorelaxant effect in cerebral [15] and renal [16] vascular beds, the AT4 receptor seems to be involved in memory and in Alzheimer’s disease [17,18].
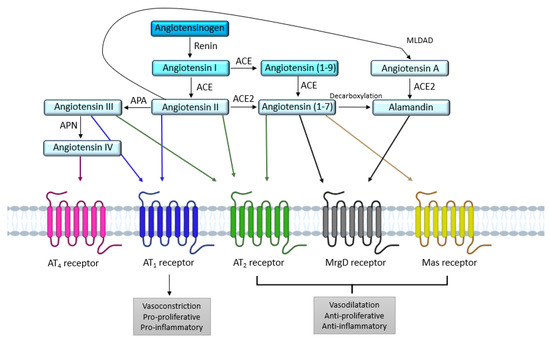
Figure 1.
Overview of the different receptors and enzymes involved in the renin angiotensin system. ACE: angiotensin I-converting enzyme; ACE2: angiotensin II-converting enzyme 2; APA: aminopeptidase A; APN: aminopeptidase N; AT1: angiotensin II type 1 receptor; AT2: angiotensin II type 2 receptor; MLDAD: mononuclear leukocyte-derived aspartate decarboxylase; MrgD: Mas-related G protein-coupled receptor, member D.
The two main receptors for Ang II, the AT1 and AT2 receptors, share a similar affinity for Ang II [9] but exert opposite actions [19,20]. These characteristics suggest that the stimulation of RAS and Ang II production may lead to physiological responses that directly reflect the functional balance between AT1 and AT2 receptors. From a systemic point of view, most of the known effects of RAS activation (elevated blood pressure, water and sodium retention, aldosterone release…) are subsequent to AT1 receptor activation, as the expression and activity of AT2 receptor seem too low to counteract AT1 receptor stimulation. Apart from the systemic RAS, many studies have shown the existence of a localized expression of RAS components in various tissues. For instance, Campbell and Habener in 1986 measured angiotensinogen mRNA levels in 17 different organs in rats; brain, spinal cord, aorta, and mesentery levels were similar to hepatic levels, whereas the levels were lower in the kidney, adrenal, atria, lung, large intestine, spleen, and stomach [21]. Moreover, in humans, the expression of angiotensinogen mRNA is also found in different organs as indicated by the Human Protein Atlas.
These results indicate that many of the tissue-specific actions of angiotensin II may be mediated by local tissue RAS, independently of the circulating RAS. Thus, at this tissue level, a possible balance of these AT1/AT2 receptor appears to be of major importance in the regulation of RAS activity, and the fine tuning of this AT1/AT2 balance seems critical.
The objective of this review is to emphasize the importance of this local functional balance between AT1 and AT2 receptors in physiological and pathophysiological processes. After a brief analysis of the structure and signalization of these two receptors, which have been recently reviewed [22,23], we will highlight the importance of the AT1/AT2 equilibrium. We will not illustrate this point through a systematic review, but through several examples chosen to emphasize the ubiquitous aspect of this major regulation of physiological functions. As we are interested in the vascular and cerebrovascular effects of this system, these will be described first, followed by the cell cycle/cancerization process and wound healing. In the last chapters, we will discuss the mechanisms of this AT1/AT2 balance, as well as different perspectives to be considered in order to modulate it.
2. AT1 and AT2 Angiotensin II Receptors
2.1. AT1 Receptor
The AT1 receptor is responsible for vasoconstriction, cell growth and proliferation, oxidative stress, inflammation, and also hypertrophy and hyperplasia [24,25]. Most of these actions result from the activation of intracellular signaling pathways involving several phospholipases and kinases (see below).
This receptor is expressed in several organs such as artery walls (smooth muscle cells), wherein it is highly expressed [26], but also in the heart (cardiomyocytes) [27], kidney (glomeruli, proximal convoluted tubules) [28] and brain (neurons, microglia cells) [29]. Two isoforms, the AT1A and AT1B receptors [30], have been identified in rodents, showing a sequence homology of more than 96%, and identical functions. In humans, only one isoform has been identified.
The AT1 receptor is a GPCR classically described to activate the phospholipase C (PLC) via Gq protein, although it also interacts with Gi, G12/13, and Gs proteins [20].
2.1.1. Structure
Advances in protein crystallization have led to the elucidation of crystalline structures for GPCRs, providing insight into activation and signaling mechanisms. As GPCRs are often involved in diseases, these crystalline structures also pave the way for structured drug design. The emergence of X-ray crystallography allowed the first crystalline structure of the AT1 receptor to be co-crystallized with an angiotensin II receptor blocker (ARB) [31].
AT1 receptor is a member of the seven transmembrane or GPCR family. Its sequence of 359 amino acids includes three N-glycosylation sites (that enable the proper folding of the receptor and account for its trafficking to the membrane) and four cysteine residues at the extracellular regions [20] (Figure 2). In addition to the two cysteines involved in a disulfide bridge between the first and second extracellular loops as for all GPCRs, the AT1 receptor contains an additional pair of extracellular cysteine residues. These cysteine residues are located on the N-terminal part and the third extracellular loop, and thus form a second disulfide bridge responsible for maintaining the conformation of the AT1 receptor and its binding to Ang II [32]. The cytoplasmic region of the receptor, composed of three intracellular loops and the C-terminal tail, contains sites that can be phosphorylated by several serine/threonine kinases, such as protein kinase C (PKC), and also has four cysteine residues which are involved in disulfide bridges [20].
The AT1 receptor can adopt three different conformations that directly influence its activity [33]. The inactive conformation is stabilized by ARBs and does not lead to any downstream signaling. The “canonical active” conformation is observed after the binding of the endogenous ligand (Ang II), and allows the activation of many different signaling pathways (see below). The binding of Ang II results in a movement of the seventh transmembrane domain on the intracellular side, allowing the recruitment of G proteins or of β-arrestin. Finally, the “active alternative” conformation (incomplete movement of the seventh transmembrane domain on the intracellular side) prevents G protein coupling to the DRY motif of the receptor (Figure 1), and only allows recruitment and stimulation of the β-arrestin signaling pathway [33,34].
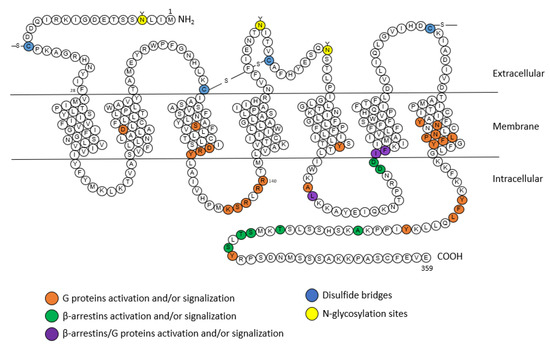
Figure 2.
Snake plot of the rat AT1A receptor (modified from [20,35,36]). Orange: amino acids for the activation of G proteins, green: amino acids for the activation of β-arrestins, purple: amino acids for the activation of G proteins and β-arrestins, blue: disulfide bridges, yellow: N-glycosylation sites. A: alanine; C: cysteine; D: aspartic acid; E: glutamic acid; F: phenylalanine; G: glycine; H: histidine; I: isoleucine; K: lysine; L: leucine; M: methionine; N: asparagine; P: proline; Q: glutamine; R: arginine; S: serine; T: threonine; V: valine; W: tryptophane; Y: tyrosine.
2.1.2. Signaling
G Protein Pathway
As evoked above, the AT1 receptor, once activated by Ang II, adopts the canonical active conformation, allowing its coupling to G proteins (Figure 3).
The activation of phospholipase C (PLC) is subsequent to Gαq protein activation. This activation releases the αq and βγ subunits of the Gαq protein. The αq subunit activates the PLC, leading to the hydrolysis of phosphatidyl-inositol-4,5-diphosphate (PIP2) into inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) [37]. The production of IP3 induces a release of calcium (Ca2+) from the endoplasmic reticulum, and then Ca2+ complexes with calmodulin; this then activates myosin light-chain kinase (MLCK). Phosphorylation of myosin light chains (MLC) leads to muscle cell contraction and thus vasoconstriction. Following the elevation in Ca2+ induced by the PLC, the phospholipase D (PLD) is activated, causing hydrolysis of phospholipids (mainly phosphatidylcholine) to generate phosphatidic acid, which is itself transformed into DAG [38]. Subsequently, DAG activates PKC, which induces the activation of PLC; thus, this positive feedback allows the continuous maintenance of PLC activity [39]. The Gαq subunit can also stimulate cell growth and proliferation through the activation via phosphorylation of several downstream proteins such as mitogen-activated protein kinases (MAPKs), janus kinases (JAKs), and transcription signal transducer and activator (STAT) proteins [40].
AT1 receptor stimulation also induces the activation of phospholipase A2 (PLA2) via the activation of the Gαq protein. Once activated, PLA2 allows the release of arachidonic acid from membrane phospholipids [41]. Arachidonic acid is then transformed into eicosanoids such as prostaglandins or thromboxane [42] by cyclooxygenases or lipooxygenases. Several of these eicosanoids play a role in Ang II-induced contraction, whereas others (PGI2, PGE2) oppose it [42]. The AT1 receptor may also recruit other G proteins, such as the G12/13 protein, involved in the activation of the RhoA/ROCK (Rho-associated protein kinase) signaling. These ROCKs are serine-threonine kinases with targets involved in the regulation of contractility [43,44].
β-Arrestins
After stimulation by Ang II, the AT1 receptor is phosphorylated on its intracellular C-terminal serine and threonine residues by GPCR kinases (GRKs). This phosphorylation increases the affinity of β-arrestin-1 and β-arrestin-2 equally for the AT1 receptor. The β-arrestins’ recruitment is known to inhibit G protein-induced signaling by interfering with the conformation of the receptor; it is also known to initiate, with the help of clathrins and AP-2 adaptor proteins, the internalization and sequestration of the AT1 receptor coupled to its ligand, as well as the membrane recycling of the receptors [34,45].
Originally, arrestins were identified as central players in the desensitization and internalization of GPCRs. In addition to modulating GPCR signaling, in 1999, Robert Lefkowitz’s team showed that β-arrestins can also initiate a second wave of signaling [46]. Other studies, such as that of Tohgo, have shown that overexpression of β-arrestin-1 or β-arrestin-2 leads to a decrease in inositol-phosphate (IP) production following AT1 receptor stimulation [47]. In addition to the modulation of GPCR signaling through desensitization and internalization, β-arrestins also act as signaling scaffolds for various signaling pathways.
For example, β-arrestins are able to recruit at the plasma membrane proteins belonging to the Src family (internalization of the receptor is not necessary) [48]. These Src allow the activation of different kinases such as ERKs, leading to a decrease in the production of IP following the stimulation of AT1 receptor [47].
NADPH
Griendling et al. were the first to demonstrate the implication of nicotinamide adenine dinucleotide phosphate (NADPH) in oxidative stress mediated by the AT1 receptor [49]. Using rat aortic VSMCs, they showed that treatment of VSMCs with Ang II for 4–6 h caused a nearly threefold increase in intracellular O2- consumption. Ang II stimulates the activity of NAD(P)H oxidase (NOX), and thus generates reactive oxygen species (ROS) [49].
NOX comprises five subunits, and in the absence of stimulation, some of its subunits are cytosolic, while others are membrane-bound [50]. Ang II, via processes involving several players such as c-Src, PLD, PKC, PI3K, and transactivation of EGFR (epidermal growth factor receptor), induces phosphorylation of the p47phox subunit, which causes the formation of a complex between cytosolic subunits, followed by transfer to the membrane, wherein the complex associates with membrane subunits to give the active form of the oxidase [50]. This will lead to the production of reactive oxygen species (ROS) such as H2O2 or superoxide. These ROS are able to activate transcription factors such as activator protein-1 (AP-1) and nuclear factor kβ (NF-kβ), which will induce the expression of pro-inflammatory genes [44].
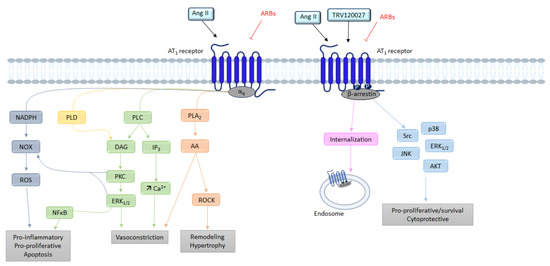
Figure 3.
Overview of the different signaling pathways following AT1 receptor activation. AA: arachidonic acid; Ang II: angiotensin II; ARBs: angiotensin receptor blockers; AT1: angiotensin II type 1 receptor; AT2: angiotensin II type 2 receptor; Ca2+: calcium; DAG: diacylglycerol; ERK1/2: extracellular signal regulated kinase ½; IP3: inositol triphosphate; JNK: c-Jun N-terminal kinase; NFκB: nuclear factor kappa B; NOX: NADPH oxidase; PLA2: phospholipase A2; PLC: phospholipase C; PLD: phospholipase D; PKC: protein kinase; ROCK: Rho-associated protein kinase; ROS: reactive oxygen species; TRV120027: β-arrestin-biased AT1 agonists.
2.2. AT2 Receptor
The AT2 receptor was initially not widely studied because of its low abundance in tissues making its study more difficult. However, it seems to play an important role in the development of the circulatory system, in particular by allowing the differentiation of precursor cells into smooth muscle cells during gestation, thus influencing the structure and function of blood vessels [19]. After birth, AT2 receptors are restricted to certain tissues such as those of the brain, heart, vascular endothelium, kidney, uterus, and ovary [20]. Besides their decrease in locomotion and exploratory behavior associated with a decrease in spontaneous movements and rearing activity, AT2 receptor-KO mice suffer from impaired drinking response to water deprivation [51,52]. Moreover, AT2 receptor expression is upregulated during inflammation, and its stimulation reduces organ damage [53].
2.2.1. Structure
The first crystalline structures of the AT2 receptor were published in 2017 [54] demonstrating commonalities such as an extracellular loop 2 (ECL2) β-hairpin conformation.
The AT2 receptor is composed of 363 amino acids and shares 34% homology with the AT1 receptor. The AT2 receptor has seven transmembrane domains, an extracellular amino terminus and an intracellular carboxy terminus (Figure 4) [9]. However the AT2 receptor seems to have unique structural and functional differences, unlike other GPCRs (including AT1 receptor), the AT2 receptor is not internalized after stimulation by its endogenous agonist (Ang II) and this stimulation does not lead to the binding of stable GTP analogues [20].
The AT2 receptor also has a nanomolar affinity for Ang II, similar to that of the AT1 receptor (Table 1). At the N-terminal part AT2 receptor has five n-glycosylation sites and 14 cysteine residues [20]. The second intracellular loop consists of a potential PKC phosphorylation site. The cytoplasmic tail contains three consensus PKC phosphorylation sites but also a phosphorylation site for the cyclic AMP-dependent protein kinase [55]. The third intracellular loop of the receptor is involved in coupling to Gi proteins [56], and thus is responsible for inhibiting AT1 receptor-dependent IP3 production. This third loop is also essential for the signal transduction of AT2 receptor via MAPK, and extracellular signal-regulated kinase (ERK) inactivation [55].
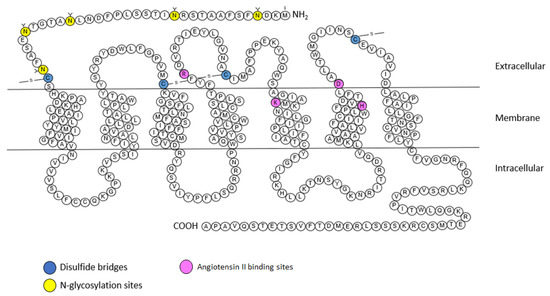
Figure 4.
Snake plot of the rat AT2 receptor (modified from [57,58,59,60]). Blue: disulfide bridges; yellow: N-glycosylation sites. Purple: Angiotensin II binding sites. A: alanine; C: cysteine; D: aspartic acid; E: glutamic acid; F: phenylalanine; G: glycine; H: histidine; I: isoleucine; K: lysine; L: leucine; M: methionine; N: asparagine; P: proline; Q: glutamine; R: arginine; S: serine; T: threonine; V: valine; W: tryptophane; Y: tyrosine.
2.2.2. Signaling
The effects produced by stimulation of the AT2 receptor are the result of the activation of intracellular signaling pathways different from those of the AT1 receptor [61]. It is interesting to note that unlike most other GPCRs, the AT2 receptor does not associate with β-arrestins [62]. Although the AT2 receptor-induced signaling pathways are not yet well understood, they involve in particular the NO, the bradykinin (BK), and the activation of several proteins with tyrosine phosphatase activity [63] (Figure 5).
G Protein Pathway
Before being cloned and identified, this receptor was considered independent of any interaction with G proteins [64]. Nevertheless, several biochemical and functional studies have indicated that the AT2 receptor may recruit Gi [19,56], thereby resulting in activation of the NO-cyclic GMP (cGMP)-protein kinase Gi pathway [65]. Subsequently cGMP activates PKGi, which dephosphorylates myosin light chains via MLCK, thus preventing calcium from leaving the endoplasmic reticulum.
Moreover, Gi recruitment leads to downstream activation of various phosphatases, such as MAPks, SH2-domain-containing phosphatase 1 (SHP-1), and serine/threonine phosphatase 2A (PP2A), resulting in the opening of delayed rectifier K+ channels and inhibition of T-type Ca2+ channels [40]. The activation of Gβγ subunits by the AT2 receptor can induce the release of arachidonic acid via the PLA2. The metabolites produced by arachidonic acid (prostaglandins or thromboxane) appear to contribute to AT2 receptor-mediated vasodilation [66].
We have seen previously that AT1 receptor-mediated vasoconstriction is mediated by the RhoA/ROCK pathway, among others,. Studies by Savoia have shown that the AT2 receptor decreases the activation of the RhoA/ROCK pathway [67], and that this decrease seems to be associated with an increase in the expression of PKGI, which inactivates RhoA by phosphorylating it [68].
Bradykinin
Siragy et al. has suggested that AT2 receptor activity is mediated by stimulation of bradykinin (BK) production [69]. This hypothesis was later confirmed by showing that AT2 receptor inhibits the activity of Na+/H+ exchangers, resulting in acidification of the cell environment, which ultimately results in the release of BK [70]. AT2 receptor-dependent stimulation of BK receptors (B2 receptor) seems to activate protein kinase A (PKA), which phosphorylates eNOS [71]. Furthermore, the proximity of the two receptors allows heterodimerization of AT2 receptors and B2 receptors, increasing the production of cGMP and NO [72]. In addition to these pathways, the AT2 receptor is capable of inducing NO-independent vasodilation. Indeed, AT2 receptor is able to induce hyperpolarization of smooth muscle cells by inducing vasodilation mediated by potassium channels [73]. Inhibition of these channels would abolish this vasodilation.
In conclusion, via activation of the AT2 receptor, Ang II leads to vasodilation, anti-proliferative, and pro-apoptotic effects, meaning the effects of AT1 receptor activation can be counteracted [74].
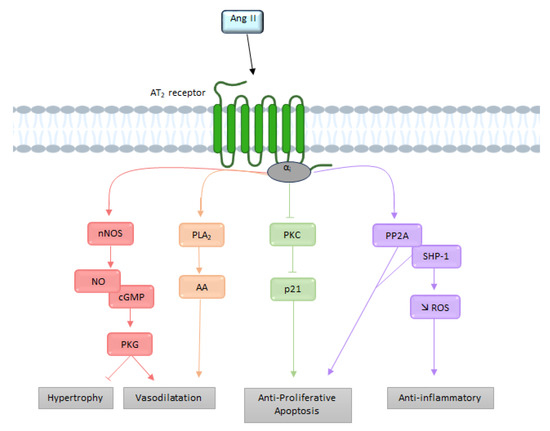
Figure 5.
Overview of the different signaling pathways following AT2 receptor activation. AA: arachidonic acid; Ang II: angiotensin II; AT2: angiotensin II type 2 receptor; cGMP: cyclic guanosine monophosphate; NO: nitric oxide; nNOS: neuronal nitric oxide synthase; PLA2: phospholipase A2; PKC: protein kinase; PP2A: serine/threonine phosphatase 2A; ROS: reactive oxygen species; SHP-1: SH2-domain-containing phosphatase 1.
3. The Functional AT1/AT2 Receptors Balance
We have seen previously that the RAS is expressed in several tissues. We will now discuss, through four different examples, the physiological and pathophysiological implication of the AT1/AT2 functional balance [75]. Several phenomena may interfere with this balance, such as a change in receptors’ expression, or an increase of the production of Ang II.
3.1. Systemic Cardiovascular Impact
Most of the known physiological and pathological actions of Ang II are mediated by AT1 receptors. One of the first systemic effects of the AT1 receptor to be discovered was the regulation of blood pressure. A decrease in blood pressure will induce a decrease in renal perfusion, leading to a release of renin. The renin will then cleave angiotensinogen (in excess) into Ang I, which will in turn be cleaved into Ang II, which will cause vasoconstriction of the vessels, thereby increasing peripheral resistance with the effect of increasing blood pressure [76]. In addition, Ang II can also increase blood pressure via a decrease in renal excretion of water and sodium [6].
In 1999, Horiuchi and his team showed that AT2 receptors have opposite effects to those of AT1 receptors [19]. AT2 receptors also play an important role in the regulation of renal function, particularly with respect to Na+ and water excretion, leading to a reduction in blood pressure. However, studies have shown that stimulation of the AT2 receptors by an agonist does not always lead to a reduction in blood pressure, but that it depends on the model, as shown in this review [77].
In physiological conditions, the AT1 receptor’s effects predominate during Ang II stimulation, due to its higher abundance in tissues.
3.2. AT1/AT2 Balance in the Brain and Cerebral Circulation
3.2.1. Cerebral Circulation
The discovery of the presence of cerebral Ang II in 1971 revealed a cerebral RAS in dogs and rats [78]. Subsequently, AT1 and AT2 receptors were localized in cerebral blood vessels in rats [51], and then in the human brain [79].
In a rat cranial window model, Vincent et al. showed that Ang II-induced vasoconstriction of cerebral arterioles was abolished when an AT1 receptor antagonist was used, resulting in vasodilation, which was itself abolished when AT2 receptor antagonists were used [80]. In physiological conditions, the AT1 receptor will allow vasoconstriction of cerebral arterioles while the AT2 receptor has the opposite effect. Moreover, in the same cranial window model, our team showed that the AT1 receptor was involved in the structural remodeling of cerebral arterioles in hypertensive rats [81]. This remodeling of cerebral arterioles induces a decrease in the internal diameter of vessels that is reversed when AT1 receptor antagonists are used [82].
The AT1 and AT2 receptors and changes in the AT1/AT2 equilibrium are particularly important contributors to the regulation of cerebral circulation. Indeed, the vasoconstriction of cerebral arterioles observed during Ang II stimulation is the sum of AT1 receptor-dependent vasoconstriction and AT2 receptor-dependent vasodilation in physiological conditions.
Importantly, under pathological conditions with deregulation of the cerebral circulation, such as cerebral ischemia, altered function and expression of RAS components in the cerebral vasculature is observed. During ischemic stroke, increased AT1 receptor-dependent vasoconstriction of cerebral vessels has been shown in the presence of Ang II, despite decreased AT1 receptor gene expression [83]. To counteract this, it has been shown that AT2 receptor gene expression is increased following stroke, but AT2 receptor protein expression remains unchanged in the middle cerebral arteries, which limits the beneficial impact that AT2 receptor agonists may have [84].
3.2.2. Cardiovascular Regulation
The two receptors are expressed inside or near the medulla oblongata, the brain region which regulates cardiac rhythm and blood pressure. They are exclusively found in the neurons rather than the glia.
The localization of AT1 receptors in the brain has been determined primarily by receptor autoradiography [32,85,86]. These studies demonstrated a wide distribution of AT1 receptors’ expression in the brain, including several regions involved in cardiovascular regulation. This distribution of AT1 receptors in the brain was confirmed by the development of transgenic mice expressing the AT1 receptor fused with eGFP [87]. High densities of AT1 receptors are found in the subfornical organ (SFO), the paraventricular nucleus (PVN), the area postrema (AP), the nucleus of the solitary tract (NTS), and the rostral ventrolateral medulla (RVLM) [87]. AT1 receptors are almost exclusively localized in neurons rather than in microglia, or astrocytes in these cardiovascular control centers [88]. Furthermore, the AT1 receptor appears to be predominantly expressed on glutamatergic neurons [89].
Similarly, the results obtained with AT2 receptor-eGFP mice demonstrate the localization of AT2 receptor-positive cells in or near brain areas that directly influence sympathetic output and blood pressure control. For example, the use of this mouse not only confirmed the presence of AT2 receptor-containing neurons in the intermediate NTS, but also demonstrated that these neurons are predominantly GABAergic [90]. The AT2 receptor–eGFP mouse also revealed a high concentration of AT2 receptor-positive neurons in the AP, as well as AT2 receptor neuronal fibers in the RVLM and PVN [90]. Furthermore, a number of AT2 receptor-positive neuronal fibers and terminals in the PVN were found to be derived from the AT2 receptor-containing GABAergic neurons that surround this nucleus [89].
It has been shown that AT1 receptors can directly influence the neurons of the cardiovascular centers to induce sympatho-excitation and increase blood pressure, indeed; the mRNA expression levels of the AT1 receptor in the RVLM and the NTS were higher in hypertensive rats in comparison to normotensive rats [91]. Furthermore, the administration of the AT1 receptor antagonist losartan into the brain reduces blood pressure in hypertensive rats [91]. The first evidence that stimulation of the brain of AT2 receptors lowers blood pressure was demonstrated by the fact that AT1 receptor-mediated pressor responses to Ang II were amplified in the presence of the AT2 receptor antagonist PD123319 [92]. Furthermore, the stimulation of RVLM AT2 receptor by a specific agonist (CGP42112A) results in a drop in blood pressure [93].
3.2.3. Neuroinflammation
Inflammation is another example of the dysregulation of the AT1/AT2 receptors’ balance in the brain. Cells present in the brain such as astrocytes or microglial cells are the main sources of inflammation. In general, the activation of the AT1 receptor in macrophages leads to the activation of the pro-inflammatory axis of the RAS, while activation of the AT2 receptor promotes the activation of an anti-inflammatory axis [94,95].
In contrast, in pathological conditions such as inflammation, these receptors (and in particular, AT1/NOX signaling) are upregulated. NOX-derived superoxides are amplified by the activation of NF-kβ and the RhoA/ROCK pathway, leading to the production of ROS. In addition, through a feedback mechanism, activation of the RhoA/ROCK pathway enables increased expression of the AT1 receptor via NF-kβ [96]. To compensate for this mechanism, it was shown by Rompe et al. that the AT2 receptor can induce the dephosphorylation of I-κB (NF-κβ inhibitor), thus allowing it to bind to NF-κβ to prevent its nuclear translocation (and transcription of the AT1 receptor) [53].
However, it has been shown that although AT2 receptor expression is generally upregulated, as the AT1 receptor as a compensatory mechanism, this phenomenon attenuates during aging. For example, in young and healthy brains of rats, and as previously described, there is a balance between the pro-oxidative and pro-inflammatory axis induced by AT1 receptor, and between the protective axis mediated by the AT2 receptor [97]. Aging causes an overactivation of the pro-inflammatory axis of the RAS, while the protective axis is unchanged. Indeed, a significant increase in NOX activity and levels of the pro-inflammatory cytokines was observed in aged rats, which revealed a pro-oxidative and pro-inflammatory state in the aged substantia nigra [97]. Moreover, aged rats also showed upregulation of AT1 receptor expression, which was inhibited by administration of candesartan (an AT1 receptor blocker), and downregulation of AT2 receptor expression [98].
Through these examples, we have seen different mechanisms of deregulation of the AT1/AT2 balance in the brain. Although the upregulation of the two receptors can partly explain this deregulation, it is certainly not the only element involved in the appearance of pathologies, as we will see in the following example.
3.3. Cellular Cycle
The proliferation of normal cells is regulated by kinases called cyclin-dependent kinases (CDKs). The main actors in this cell cycle are cyclins, which regulate CDKs, enabling cells to progress through the cell cycle [99]. We have seen previously that AT1 receptors promote cell proliferation pathways, in particular via the MAPK pathway, which allows the expression of cyclin and CDKs, itself allowing the advancement of cells in the cell cycle (Figure 6) [100]. Diep et al. studied the expression of cell cycle proteins in Ang II-infused rats. A significant increase in 3H-thymidine incorporation in the mesenteric arteries was observed, reflecting the entry of cells into DNA replication phase (S phase). Furthermore, this incorporation was associated with high expression of cyclin D1 and CDK4 [101]. The use of losartan in these animals completely abolished 3H-thymidine incorporation and restored the expression level of cyclin D1 and CDK4.
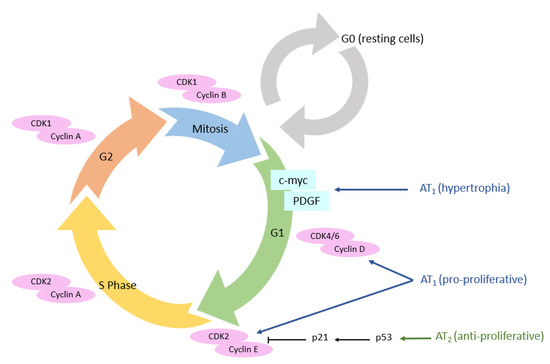
Figure 6.
AT1 and AT2 receptors’ expression and their impact on cell cycle. AT1 receptor: angiotensin II type 1 receptor; AT2 receptor: angiotensin II type 2 receptor; CDK: cyclin-dependent kinases.
Conversely, AT2 receptors are involved in the activation of anti-proliferative pathways [102]. In human aortic endothelial cells and after stimulation with C21 (an AT2 receptor agonist), an activation of p53 protein was observed [103]. This protein is a key tumor suppressor which can lead either to a transient arrest of cell proliferation (to repair DNA damages, for example) or to an irreversible arrest of cell proliferation called senescence, leading to cell death [104]. When p53 is not activated, histone deacetylase-1 (HDAC1) deacetylates p53, resulting in its degradation; in contrast, HDAC1 inhibitors (such as the vorinostat) sensitize cells to apoptosis by increasing p53 acetylation. This AT2 receptor-coupled HDAC1/p53 signaling pathway appears to have a role in physiological apoptosis and cell turnover involving p53 [103].
The AT1 receptor directly induces cell hypertrophy, notably through the MAPk pathway, but also through the β-catenin pathway [105,106]. Cyclin D1 will enable the transition from the G0 to the G1 phase of the cell cycle. The next expected step for cells in G1, is the S phase, leading to cell proliferation. However, Geisterfer et al. reported that Ang II induces hypertrophy, but not proliferation, in confluently cultured rat aortic smooth muscle cells [107]. Subsequently, several studies revealed that Ang II stimulates the expression of immediate early genes exclusively expressed in the G1 phase (c-myc and PDGF (platelet-derived growth factor)) [108]. Thus, these genes, once expressed after Ang II stimulation, will allow the return of resting cells to the G1 phase, and not necessarily progression to S phase, which consists of G1 phase arrest and hypertrophy [109].
In esophageal adenocarcinomal cells (EACs), Fujihara et al. showed that telmisartan induces antitumoral effects in EAC, both in vitro and in vivo. Following inhibition of the AT1 receptor by telmisartan, cell cycle arrest in the G0/G1 phase is induced via the Akt/mTOR pathway in EAC cells [110]. Similar results were obtained in breast cancer and cholangiocarcinoma cells [111]. Moreover, this stop in the cell cycle was accompanied by a high decrease in cell cycle-related proteins, such as cyclin E, cyclin D1, and their catalytic sub-units, Cdk4 and Cdk6. These experiments show that increased cell proliferation in cancer is at least partially related to actions mediated by the AT1 receptor. In contrast, overexpression of AT2 receptors in SMMC7721 cells was shown to reduce S-phase cells and increase G1-phase cells via suppression of CDK4 and cyclin D1 expression, thereby reducing proliferation [112].
Many studies have reported that an increase in the presence of AT1 and AT2 receptors is found in different types of cancer, and is directly linked to a worse prognosis in terms of tumor aggressiveness [113,114,115]. The overexpression of the AT1 receptor has been demonstrated in several in vitro models, such as mammary carcinoma cells in culture, pancreatic adenocarcinoma cells, and hepatocarcinoma cells, but also in vivo in various tumors including estrogen receptor positive breast cancers, glioblastoma, and ovarian cancers [116,117]. Despite its anti-proliferative effect, the AT2 receptor has also been shown to be overexpressed by several cancers such as astrocytomas [113] and lung tumors [118] in vivo. In an astrocytoma model, it was shown that out of 133 tumors, 10% of low-grade tumors were positive for the AT1 receptor, versus 67% for high-grade tumors; 17% of low-grade tumors were positive for the AT2 receptor, versus 53% for high-grade tumors [113].
It has been shown in several models of cancer cells overexpressing the AT2 receptor that this overexpression promotes apoptosis of these cells [112,119,120]. In addition, the effects of C21 were studied in prostate cancer (using human LNCaP prostate cancer cells and prostate adenocarcinoma transgenic rats) and leiomyosarcoma cells. In both studies, the authors were able to find antiproliferative and pro-apoptotic effects of the AT2 receptor [121,122].
3.4. Wound Healing
Several studies reported dynamic changes in angiotensin receptor expression during the different phases of wound healing [123,124].
The expression of AT1 and AT2 receptors in the skin of young rats was first shown in 1992 by Viswanathan and Saavedra [125]. AT1 and AT2 receptors are expressed in human fibroblasts, keratinocytes, and vascular endothelial cells. Both AT1 and AT2 receptors are found in myofibroblasts and keratinocytes in rodents [126]. RAS components are present in the epidermal and dermal layers, but also in subcutaneous fat tissues, in microvessels, and in appendages such as hair follicles [126,127]. However, expression of RAS components in skin has also been demonstrated at the protein level, with results confirmed at the mRNA level [128].
Regarding the functional role of the RAS in skin physiology, a recent study by Jiang et al. reported that Ang II promotes differentiation of keratinocytes from bone marrow-derived mesenchymal stem cells (BMdSC) under physiological conditions [129]. Moreover, Ang II has been shown to increase vascular permeability to recruit inflammatory cells and to induce angiogenesis [130]; the AT1 receptor promotes migration, while the AT2 receptor inhibits it.
During wound healing, an organism will regulate the expression levels of AT1 and AT2 receptors, enabling a response to Ang II that is adapted to the situation. Immediately after wounding, an increase in both AT1 and AT2 receptor expression is observed, which seems slightly delayed and weaker for AT2 receptors [131]. In cultured keratinocytes, this regulation is detectable at the mRNA level 1 h after wounding, but the protein expression of AT1 receptor peaks at 3 h, and that of AT2 receptor peaks at 12 h after wounding [126]. This specific early increase in AT1 receptors could play a role in promoting blood clotting, initiating the inflammatory phase and inducing re-epithelialization by stimulating keratinocyte proliferation and migration [132,133].
In vivo, the wound healing process leads to an increase in receptor expression; this is higher for the AT1 receptor than for the AT2 receptor during the early phases of wound closure. Subsequently, there is a decrease in the expression of both receptors during the inflammation process, followed by an increase during re-epithelization.
Finally, during the last phase (remodeling), an increase in AT1 and AT2 receptors has been demonstrated, but this time with a dominance of the AT2 receptor over the AT1 receptor (Figure 7) [123,133].
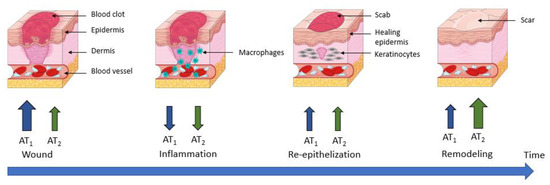
Figure 7.
AT1 and AT2 receptors’ expression during wound healing. AT1 receptor: angiotensin II type 1 receptor; AT2 receptor: angiotensin II type 2 receptor.
This is consistent with what is known about both receptors, since in the early phases of wound healing and re-epithelization, a pro-proliferative action of AT1 receptors is required to allow wound closure. Therefore, it has been shown that in mice KO for AT1 receptors and rats treated with an AT1 receptor antagonist, wound closure was delayed [134,135]. In contrast, in AT2 receptor KO mice, re-epithelization was accelerated. These results support that during the wound healing process, the antiproliferative effect of AT2 receptors is complementary to the pro-proliferative effects of AT1 receptors under physiological conditions.
During the remodeling phase of wound healing, AT2 receptor expression is stronger than AT1 receptor expression. The antifibrotic properties seem essential for the formation of a resistant scar tissue. Indeed, in AT2 receptor-KO mice, the skin breaks under lower tension than in wild-type mice [135]. This increased expression of the two receptors contributes to the localized Ang II action in the wounded area rather than in the unaffected skin. Thus, the changes in the AT1/AT2 receptor ratio observed in tissue repair may cause a switch in the dominating subtype, and consequently a change in the response to Ang II [136].
The formation of hypertrophic scars or keloids is a recurrent problem resulting from an insufficient control of proliferative and fibrotic processes in wound healing [137]. Indeed, it has been shown that it is the overactivated cutaneous RAS that is involved in this process via the AT1 receptor [20,138]. Indeed, these receptors, once stimulated by Ang II, are known to act in a pro-fibrotic manner. Studies have also shown that the level of Ang II and AT1 receptor expression were increased in hypertrophic scars and keloids in both rodents and humans. As a result, several experiments were conducted to inhibit the profibrotic effect of AT1 receptor by using ARBs to prevent or treat hypertrophic scars and keloids in preclinical models. All these studies, regardless of the species, resulted in a reduction in scar size [139,140].
As evoked above, AT1 and AT2 receptors have the same affinity for Ang II, but induce opposite physiological responses. Thus, the physiological response to Ang II production will reflect the functional balance between these two receptors. In the next section, we will discuss the different mechanisms that may contribute to the fine tuning of this functional AT1/AT2 receptor balance.
4. Mechanisms Regulating the AT1/AT2 Functional Balance
4.1. Functional Opposition vs. Expression Level
The response to Ang II results from the balance between the effects of each of these receptors. As we have seen previously, the effects of the AT1 receptor predominate because it is the most abundant.
This functional balance was confirmed in vivo using knockout mice for either the AT1 receptor or AT2 receptor. In AT1A receptor KO mice, there was an absence of hypertensive response following Ang II injection, which is normally observed in wild-type mice. In addition, systemic blood pressure was markedly decreased in these mice [141]. In the same AT1A receptor KO mice and after treatment with an AT1 receptor antagonist, a decrease in blood pressure was observed in mice pretreated with ACE, thus showing that AT1B receptors also seem to play a role in the regulation of blood pressure. In contrast, in AT2 receptor KO mice, vasoconstriction to Ang II is more important than in wild-type mice [142]. Thus, in cerebral arteries, the effects observed are the sum of constrictor effects mediated by the AT1 receptor and dilator effects mediated by the AT2 receptor.
Furthermore, as we have seen in Section 3.4, during the wound healing phenomenon, there is a modification of the expression of the receptors according to the phase of the process: a stronger expression of the AT1 receptor at the time of re-epithelization, and a stronger expression of the AT2 receptor at the time of the remodeling phase.
The expression level of the receptors is also related to the mechanisms that regulate them. At the AT1 receptor level, overexpression of ATRAP (AT1 receptor-associated protein) inhibits AT1 receptor-dependent PLC activation [143], inositol phosphate production, and cell proliferation [144], indicating that ATRAP acts as a negative regulator of AT1 receptor signaling [145]. In addition, overexpression of ATRAP decreases membrane expression of the AT1 receptor due to its increased internalization [146]. In contrast, expression of ARAP-1 (ankyrin repeat and pleckstrin homology domain-containing protein 1) restores receptor membrane expression [147]. Moreover, at the renal level, overexpression of ARAP1 in mice causes hypertension and renal hypertrophy, effects that are suppressed by an ARB, suggesting that ARAP1 potentiates AT1 receptor signaling [148].
The AT2 receptor interacts with ATIP (AT2 receptor interaction protein) [149]. Decreased expression of this protein results in retention of the AT2 receptor in cellular compartments, reducing their expression on the cell surface, and reducing AT2 receptor-related effects. Expression of the AT2 receptor at the membrane induces an increase in ATIP expression, creating a positive feedback loop [149]. PARP-1 (poly(ADP-ribose) polymerase-1) plays an important role in the regulation of AT2 receptor expression. Indeed, in addition to repressing the transcription of the gene coding for the AT2 receptor, it activates the transcription of the ATIP gene [150]. The AT2 receptor also interacts with another protein: the transcription factor PLZF (promyelocytic zinc finger protein). Once bound to the AT2 receptor, PLZF will cause its internalization, thus decreasing its membrane expression; then, PLZF will migrate to the nucleus, allowing the transcription of PI3K [151], which is involved in the activation of eNOS (endothelial nitric oxide synthase).
Although the level of expression may partly explain the predominance of the effects of one receptor over the other, it turns out that in some cases, this is more complex. For example, we have seen that after stroke, there is an increase in AT1 receptor-dependent vasoconstriction, despite a decrease in AT1 receptor gene expression [83]. Furthermore, Foulquier et al. showed that high salt intake for 4 days was associated with abolition of AT2 receptor-mediated vasodilation because of decreased aldosterone levels, but also with decreased cerebrovascular AT2 receptor protein levels without mRNA changes [152]. If this high salt intake is maintained for 30 days, in addition to the abolition of AT2 receptor-mediated vasodilation, we observe AT2 receptor-mediated vasoconstriction, although neither the mRNA nor protein levels of the AT2 receptor are altered by high salt intake.
4.2. Direct AT1/AT2 Receptors Interactions
GPCRs have the ability to form homodimers and heterodimers that can change receptor properties. In this section, we will discuss the implication of these homodimers and heterodimers on AT1 and AT2 receptor signaling (Figure 8).
4.2.1. AT1 Receptor Dimerization
Abdallah et al. showed that increased levels of AT1 receptor homodimers were present on monocytes from patients with hypertension, which is an atherogenic risk factor, and that they were related to increased Ang II-dependent monocyte activity and adhesiveness [153]. This increase leads to the formation of atherosclerotic lesions. In this study, in addition to showing that inhibition of Ang II release prevents the formation of AT1 receptor homodimers, they observed that dimerized receptors increase Gq/11-mediated inositol phosphate signaling [153]. In addition, the constitutive formation of homodimers of the AT1 receptor are formed during biosynthesis, as the receptors are trafficked through the endoplasmic reticulum. Furthermore, the constitutive nature of receptor dimerization was not affected by treatment with agonists or antagonists [154]. These data show that AT1 receptor homodimerization can enhance Ang II-mediated signaling, which may have a pathological effect (e.g., arthrosclerosis), but that these homodimers are not affected by receptor agonists and antagonists.
The AT1 receptor and B2 receptor can form a heterodimeric complex. This heterodimerization between the AT1 receptor and B2 receptor in HEK-293 cells increased the efficacy and potency of Ang II, but decreased the potency and the efficacy of BK. To confirm this, the authors compared the Ang II- or BK-stimulated increase in inositol phosphates in HEK-293 cells expressing the indicated receptors. Furthermore, AT1/B2 heteromerization is also involved in the increase in Ang II hypersensitivity in preeclampsia [155]. Preeclampsia is a pregnancy complication characterized by high blood pressure, and it may cause serious complications for the mother and fetus [156]. The presence of AT1/B2 heterodimers has been reported in human placental biopsies from pregnancies with preeclampsia [157].
In 2005, Kostenis et al. showed that the AT1 receptor could form a heterodimer with the MasR [158]. In their study, they transfected the human forms of Mas and AT1 receptors, individually and in combination, into CHO-K1 cells, and then assessed intracellular Ca2+ mobilization after stimulation with different doses of Ang II. The MasR alone did not respond to Ang II stimulation, while cells expressing the AT1 receptor increased their intracellular Ca2+ level. Upon coexpression of MasR with the AT1 receptor, a reduction in the potency and maximal efficiency of Ang II in increasing Ca2+ mobilization was observed. In the same model, increasing concentrations of losartan induced significant rightward shifts in Ang II concentration–response curves in cells expressing both the AT1 receptor alone and the AT1/MasR heterodimer. In contrast, the Ang II-induced elevation of intracellular Ca2+ and its decrease in the presence of MasR were not affected by the presence of Ang-(1-7). Furthermore, in vivo data corroborate the results obtained in cell lines, as MasR-KO animals demonstrated a significant increase in the vasoactive properties of Ang II [158].
4.2.2. AT2 Receptor Dimerization
AT2 receptor homodimerization was first described by Miura et al. in PC12W cells and CHO cells transfected with AT2R [159]. They also showed that these AT2 receptor homodimers allow constitutive signaling that leads to apoptosis. Furthermore, dimerization and pro-apoptotic signaling were not altered following AT2 receptor stimulation, suggesting that this homodimerization is ligand-independent, which has also been reported in transfected HEK-293 cells [160]. Zha et al. showed in NRK-52E rat kidney epithelial cells that AT2 receptor homodimerization is observed under high-glucose conditions, which may be a direct effect of receptor dimerization susceptibility or an indirect effect due to increased AT2 receptor expression under high-glucose conditions [161].
Like AT1 receptors, AT2 receptors can form heterodimers with B2 receptors. We have already seen that AT2 receptors mediate a vasodilatory cascade that includes BK, NO, and cGMP. Using a KO mouse model for B2R, they showed that when AT2 and B2 receptors are simultaneously activated in vivo, NO and cGMP production increases [162]. In a PC12W cell model, heterodimerization of these receptors was shown without any stimulation, suggesting the presence of constitutive heterodimers [72]. Furthermore, the use of an AT2 receptor agonist (CGP42112A) combined with a B2 receptor agonist (BK) or antagonist (icatibant) allows for an increase in receptor expression alone, but also for the formation of heterodimers [72]. Thus, the maximal increase in cGMP and NO production is observed when AT2 receptor is stimulated and the B2 receptor is blocked; this result is in agreement with another study showing that B2 receptor blockade increases the effect of AT2 receptors on cGMP and NO production [163]. Since the rate of heterodimer formation depends on the expression level of AT2 and B2 receptors, controlling the expression level of these receptors would influence the formation of dimers, thus increasing (or not) the AT2 receptors’ effects.
The AT2 receptors is also able to form a heterodimeric complex with MasR [164]. This dimerization influences the RAS-protective Ang II/AT2 axis, resulting in increased NO production and promoting diuretic–natriuretic response in obese Zucker rats [164]. Several studies suggest that these receptors may be functionally interdependent; indeed, the AT2 receptor antagonist (PD123319) reduced the vasodepressor effects of the MasR agonist Ang-(1-7) [165]. Similarly, Ang-(1-7) mediated endothelium-dependent vasodilation in the cerebral arteries [166] and aortic rings of salt-fed animals [167] was inhibited by PD123319 as well as the MasR antagonist (A-779). In mouse astrocytes isolated from AT2 receptor KO, MasR was not responsive to Ang-(1–7), and in astrocytes isolated from MasR KO, AT2 receptors were not responsive to C21, suggesting that in murine astrocytes in primary culture, AT2 and MasR are functionally dependent on each other [168]. In contrast, another study showed that the vasoconstriction effect induced by Ang II was slightly increased, and the vasodilation induced by the AT2 receptor agonist CGP42112A was not altered in mice KO for MasR [169].
Furthermore, using bioluminescence resonance energy transfer (BRET), the AT2 receptor was found to form a heterodimer with the relaxin family peptide receptor 1 (RXFP1). Relaxin, by binding to RXFP1, is known to be antifibrotic by interfering with transforming growth factor β1 (TGF-β1). Ang II is also known to in-hibit TGF-β1 via the AT2 receptor. Chow et al. investigated the potential interactions of relaxin with the AT2 receptor. The antifibrotic action of relaxin in primary rat kidney myofibroblasts was reduced when combined with an AT2 receptor antagonist (PD123319). This heterodimerization results in the activation of the NO-cGMP-dependent pathway. Although relaxin does not interact directly with the AT2 receptor, AT2/RXFP1 heterodimerization results in the activation of the NO-cGMP-dependent pathway, leading to the disruption of TGF-β1 signaling and thus the latter’s pro-fibrotic effect.
Last but not least, AT2/AT1 heterodimerization was first described by AbdAlla et al. in PC-12 cells, rat fetal fibroblasts, and human myometrial tissue samples [170]. They showed that AT2/AT1 dimerization was constitutive and led to inhibition of the AT1 receptor-mediated G protein pathway. This inhibition of AT1 receptor signaling does not require AT2 receptor activation, as shown by the fact that AT2/AT1 heterodimerization is not affected by AT2 receptor antagonists such as PD123319, and by the persistence of the effect in cells with dimers containing an AT2 receptor mutant that is unable to bind agonists or to initiate AT2 signaling. The authors concluded that the AT2 receptor acts as a kind of reverse agonist of the AT1 receptor by constitutively preventing the conformational changes necessary to initiate AT1 receptor signaling [170].
Attenuation of AT1 receptor signaling by the AT2 receptor (via calcium signaling, ERK1/2 MAPK activation) as well as constitutive AT2/AT1 dimerization has been confirmed in studies by other groups in HeLa cells [171] or HEK-293 transfected with AT2/AT1 [172]. AT2/AT1 dimerization also appears to impact AT2 intracellular trafficking, as AT2/AT1 dimers internalize upon Ang II stimulation (whereas AT2 alone is unable to do so) [171].
More recently, a study demonstrated that AT1 and AT2 receptors form heterodimers that are expressed in the cells of the central nervous system (striatal neurons and microglia). These dimers are new functional units with specific signaling properties, because on the one hand, coactivation of the two receptors reduces Ang II signaling, and on the other hand, they exhibit cross-potentiation: i.e., candesartan (AT1 receptor antagonist), increases the effect of AT2 receptor agonists [172].
In a model of Parkinson’s disease (6-OH-dopamine hemi-lesioned rat), the authors wanted to quantify the quantity of AT2/AT1 dimers in striatal sections of naïve and 6-OH-dopamine hemi-lesioned rats, treated or not with L-DOPA and divided into two groups: those that are dyskinetic and those that are resistant to L-DOPA-induced dyskinesia. First, they demonstrated that the quantity of AT2/AT1 dimers found in the non-lesioned striatum was negligible compared to the lesioned striatum. Furthermore, dyskinetic animals on L-DOPA showed an approximately 2-fold increase in AT2/AT1 dimers (compared to the lesioned rat hemisphere), and dyskinesia-resistant animals showed an approximately 10-fold increase (compared to the non-lesioned control hemisphere) [172]. In this context of Parkinson’s disease, the use of AT1 receptor antagonists coupled to AT2 receptor agonists could potentiate the neuroprotective effects via the AT2 receptor.
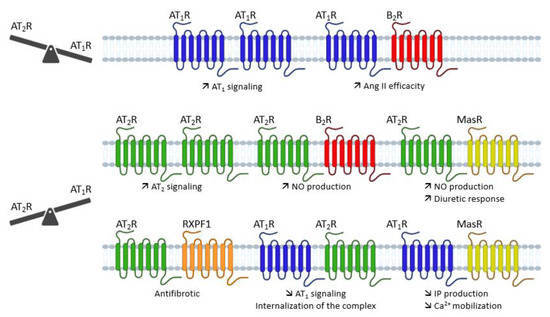
Figure 8.
Overview of the different dimerizations of AT1 and AT2 receptors and their functional impact. Ang II: angiotensin II; AT1R: angiotensin type 1 receptor; AT2R: angiotensin type 2 receptor; B2R: bradikynin receptor B2; Ca2+: calcium; IP: inositol phosphate; MasR: Mas receptor; NO: nitric oxide; RXPF1: relaxin family peptide receptor 1.
In conclusion, the different dimerizations of AT1 or AT2 receptors, depending on the tissue and the co-expressed receptor (AT1, AT2, B2R, MasR), cause a functional change in the activity of the receptors, modifying the AT1/AT2 balance without changing the expression of these receptors.
4.3. Post-Translational Modifications
Post-translational modifications are also mechanisms to modulate GPCR functions, AT1 and AT2 receptors being no exception.
4.3.1. N-Glycosylation
AT1 receptor function is regulated by various post-translational modifications such as N-glycosylation [173]. To study the effects of N-glycosylation on extracellular loops (ECL), artificial N-glycosylation sequences were incorporated into ECL1, ECL2 and ECL3 [174]. In ECL1, N-glycosylation causes a very significant decrease in the ligand affinity and surface expression of the receptor; in ECL2, it leads to the synthesis of a misfolded receptor, and in ECL3 N-glycosylation produces mutant receptors with normal affinity and low surface expression. These results show that N-glycosylation sites alter many properties of the AT1 receptor, such as targeting, folding, affinity, and surface expression [174].
Like the AT1 receptor, the AT2 receptor contains multiple glycosylation sites. The variability in AT2 receptor molecular weight has been shown to be due to different degrees of N-glycosylation [175]. However, this glycosylation does not appear to be involved in AT2 receptor binding and membrane addressing [176]. On the other hand, glycosylation of AT2 receptor could nevertheless play a role in the stability of the receptor and its coupling with its intracellular effectors [176].
4.3.2. Phosphorylation
Phosphorylation is another post-translational modification that can occur on the AT1 receptor [177]. Phosphorylation of AT1 receptor serine/threonine residues is required for β-arrestin recruitment and receptor internalization [178]. In contrast, in the kidneys and arteries, GRK4 has been shown to exacerbate urinary sodium retention and vasoconstriction by increasing AT1 receptor expression [179].
The AT2 receptor is rapidly phosphorylated on a serine by PKC after activation by Ang II. The functional role of AT2 receptor phosphorylation is not known. When triggered by AT1 receptor activation, it appears to modulate the AT2 receptor’s effects, as opposed to AT1’s effects [180].
A more recent study showed that GRK4, by increasing AT2 receptor phosphorylation, impairs AT2 receptor-mediated diuresis and natriuresis without decreasing mRNA or protein levels [181]
4.3.3. S-Nitrosation
S-nitrosation is a mode of post-translational modification that allows the addition of a NO group to the sulfur atom of specific cysteine residues.
The AT1 receptor contains ten cysteine residues. Four of these cysteines are involved in disulfide bridges at the extracellular loops, one is located on the cytoplasmic tail, and the other five are distributed in the transmembrane domains of the receptor (see Section 2.1.1). The affinity of the AT1 receptor for Ang II is decreased in the presence of sodium nitroprusside (SNP), an NO donor. An assessment of the affinity of different mutated AT1 receptors for each of five cysteines revealed the cysteine involved in sensitivity to sodium nitroprusside, cysteine residue 289 [182].
The AT2 receptor has 14 cysteine residues. We have seen that the four cysteines in the extracellular loops are engaged in disulfide bridges. The remaining ten cysteines are distributed on the transmembrane domains and the cytoplasmic tail.
In 2015, Jang showed that the AT1 receptor, AT2 receptor, and ROS appear to be involved in increasing nNOS (neuronal nitric oxide synthase) activity [183]. After stimulation with Ang II, membrane expression of the AT1 receptor decreases, while that of the AT2 receptor increases. In addition, losartan and L-NAME (eNOS inhibitor) inhibit translocation to the membrane of AT2 receptor, suggesting that AT2 receptor translocation may be NO-dependent. The SNP allows for the S-nitrosation of the AT2 receptor, as it does for the AT1 receptor. In addition, a mutation in cysteine 349 induces increased surface expression of the AT2 receptor, suggesting that it plays a role in the translocation of the receptor to the membrane [183].
5. Possible Ways to Tune the AT1/AT2 Functional Balance
5.1. Pushing the Balance Using Agonist/Antagonist Ligands
One of the most obvious means to act on the AT1/AT2 functional balance is to use agonists or antagonists towards one or the other receptor.
In order to rebalance the AT1/AT2 balance, the use of specific molecules targeting our receptors seems to be an interesting avenue. Indeed, this would make it possible to block or activate one specific receptor, which cannot be achieved with ACE inhibitors, for example, as they prevent both receptors’ activation by inhibiting Ang I cleavage.
AT1 receptor antagonists were the first molecules discovered in this sense (Table 1). In the case of pathologies associated with AT1 receptor, the ideal is to be able to abolish the overexpression of the receptor responsible for the imbalance in order to orient it in favor of the AT2 receptor. AT1 receptor antagonists are widely used to treat hypertension as well as cardiac diseases (heart failure or myocardial infarction). In vitro and in vivo, losartan is a reversible competitive AT1 receptor antagonist that inhibits the Ang II-induced vasoconstriction of blood [184]. However, complete blockade of the receptor then amounts to promoting activation of the AT2 receptor, which will tip the balance in the other direction instead of bringing it back to equilibrium.
Another method would be to act on the AT2 receptor itself by using an agonist of the latter. For this, molecules capable of specifically activating the receptor have been developed, such as CGP42112A, which is a peptide, or C21, which is a synthetic compound. A study on the effects of CGP42112A in the same SHR model was performed, and the authors tested CGP42112A in the presence or absence of candesartan. The results showed that the use of candesartan alone at a high concentration lowered blood pressure in SHR rats, and that CGP42112A only provided a depressant effect in the presence of candesartan [185]. Another study on CGP42112A showed that intravenous infusion of the molecule increased NO production in pigs [186]. Wan et al. showed that the use of C21 in hypertensive rats reduced blood pressure [187]; however Foulquier et al. showed that this reduction in blood pressure was dependent on the experimental model used [77].
These studies show that the use of an AT2 receptor agonist alone would not be sufficient to regulate the balance given the predominance of the AT1 receptor in the pathological context. However, it would appear that combining the effects of an AT1 receptor antagonist and an AT2 receptor agonist would be more effective in certain pathologies such as hypertension [188,189].
We have seen that the AT2 receptor has a rather protective role in case of pathology, but a study has shown that the use of PD123319 can reduce the inflammatory response and oxidative stress in rats with induced colitis [190]. In this same study and another one, it was demonstrated that PD123319 may have partial agonistic properties in relation to the AT2 receptor, thus biasing the interpretation of functional data [190,191]. Another study conducted by our group showed that in an SHR model, the combination of the effects of an ARB with an ACE inhibitor induced an exacerbated decrease in middle cerebral artery contraction in SHR [192]. In this specific case, by blocking the AT1 receptor, the AT2 receptor will become dominant, thereby reversing the balance. This phenomenon has been described by Budzyn et al. [193].

Table 1.
Angiotensin receptor ligands.
Table 1.
Angiotensin receptor ligands.
| Agonists | |||
| Compounds | AT1 affinity | AT2 affinity | References |
| Ang II | pIC50 = 8.1 | pIC50 = 9.2 | [194] |
| Ang III | pIC50 = 7.6 | pIC50 = 9.2 | [194] |
| Ang IV | N.A. | pIC50 = 7.3 | [194] |
| Ang-(1-7) | pKi = 6.66 | pIC50 = 6.6 | [194,195] |
| SII | pKd = 6.5 | N.A. | [196] |
| TRV120023 | pEC50 = 7.4 | N.A. | [197] |
| TRV120026 | pEC50 = 7.6 | N.A. | [197] |
| TRV120027 | pEC50 = 7.7 | Ki = 7 nM | [197] |
| CGP42112A | N.A. | pIC50 = 9.6 | [194] |
| C21 | N.A. | pIC50 = 8.6 | [194] |
| Antagonists | |||
| Compounds | AT1 affinity | AT2 affinity | References |
| Losartan | pIC50 = 7.4–8.7 | N.A. | [198] |
| Candesartan | pIC50 = 9.5–9.7 | N.A. | [199] |
| Valsartan | pIC50 = 8.6 | N.A. | [200] |
| Telmisartan | pIC50 = 8.4 | N.A. | [201] |
| PD123177 | N.A. | pIC50 = 8.5–9.5 | [202] |
| PD123319 | N.A. | pIC50 = 8.25 | [194] |
N.A.: non-applicable. EC50: half maximum effective concentration; IC50: half maximum inhibitory concentration; Kd: dissociation constant. Ki: inhibition constant.
5.2. Selective Activation of the β-Arrestin Pathway
The use of biased agonists is another strategy to regulate the AT1/AT2 receptors’ balance. A biased agonist is a receptor-specific ligand capable of selectively activating a single signaling pathway by preferentially stabilizing one of the receptor conformations. This phenomenon is also called “functional selectivity”. In the case of the AT1 receptor, several biased agonists have been developed that allow AT1 receptor to adopt an alternative active conformation [45]. For example SII, TRV120027, and TRV120023 selectively activate the β-arrestin pathway while inhibiting the G protein pathway [197].
SII is a modified Ang II peptide, which can trigger the phosphorylation of the AT1 receptor, and thus β-arrestin recruitment [203]. SII elicits GRK6 and β-arrestin 2-dependent ERK activation, and promotes β-arrestin-regulated Akt activity and mTOR phosphorylation to stimulate protein synthesis [204]. TRV120023 has been reported to only recruit β-arrestin while blocking G protein activation, enhancing myocyte contractility but without promoting hypertrophy, as seen with Ang II [205]. For example, an acute infusion of Ang II increased mean arterial pressure in male SHR, accompanied by a reduction in glomerular filtration rate, whereas TRV120023 blocked the acute infusion of an Ang II-induced hypertensive state in a dose-dependent manner [205].
TRV120027 has been studied in several preclinical studies in a heart failure model, and has shown promising results [206]. A recent study just demonstrated that β-arrestin signaling, mediated by the PAR-1 receptor, produces prolonged activation of MAPK 42/44, which increases PDGF-β secretion. It has been shown that after ischemic stroke, PDGF-β secretion can provide increased protection of endothelial function and barrier integrity [207]. Similarly, there are biased agonists that selectively activate the G protein pathway, such as TRV120055 or TRV120056 [33].
The use of biased agonists could be interesting in a pathological setting. Indeed, unlike ARBs, which completely inhibit the effects of the AT1 receptor, β-arrestin-biased agonists will allow the activation of the β-arrestin pathway, with potentially protective effects. Activation of the β-arrestin pathway to mediate internalization could furthermore reduce the number of AT1 receptors present at the surface. On the other hand, if AT1 receptors are already occupied by a biased agonist, Ang II could then be able to bind to the AT2 receptor. These combined effects could restore the functional balance without blocking the AT1 receptor. In addition to this internalization, β-arrestin may activate beneficial secondary signaling pathways [207].
Although TRV120027 is able to bind to the AT2 receptor with an affinity comparable to that of the AT1 receptor [197], no functional studies have been carried out. Furthermore, as the receptor is unable to interact with β-arrestin and be internalized, most signaling pathways function through the stimulation of the G protein pathway.
5.3. Post-Translational Regulation
We have previously shown that AT1 and AT2 receptors can undergo different post-translation changes (see Section 4.3).
Regarding phosphorylation, we have already established that the AT2 receptor was not able to recruit β-arrestins as would classically be the case for GPCRs, including the AT1 receptor. On the other hand, intervention on certain GRKs, such as GRK4, could have an effect on the AT1/AT2 balance because it would allow, on the one hand, the inhibition of the overexpression of AT1 receptors at the membrane, and on the other hand, a decrease in the phosphorylation of the AT2 receptor and thus the restoration of AT2 receptor signaling.
Although the glycosylations of the AT1 receptor are very important for its membrane addressing and ligand binding, for the AT2 receptor, the role of these glycosylations remains unknown.
AT1 and AT2 receptors both have four cysteines involved in disulfide bridges. It has been shown that reduction of these disulfide bridges by dithiothreitol (DTT) strongly decreases Ang II binding for the AT1 receptor, but not for the AT2 receptor [32].
AT1 and AT2 receptors can undergo S-nitrosation. In a study of our group, rat middle cerebral arteries were pretreated with an NO donor, S-nitrosoglutathione (GSNO), and this led to abolition of Ang II-induced vasoconstriction [208]. The following results were found in an ex vivo rat aortic ring model. A decrease in Ang II-induced vascular contraction was observed when arteries were pretreated with GSNO. In vivo, a decrease in blood pressure response to Ang II was also detected after oral administration of GSNO [209]. In vitro experiments showed that pretreatment of HEK293 cells with GSNO seems not to alter the internalization of activated AT1 receptors [208]. Similar to biased agonists, S-nitrosation disrupts AT1 receptor G protein signaling, the advantage here being that NO is an endogenous product.
This post-translational modification seems to be an interesting tool for directing a rather protective response by not altering the AT1/AT2 balance as antagonists and/or agonists can. Indeed, we have seen that NO is one of the main second messengers of AT2 receptors. We can envisage that the NO produced by the AT2 receptor, which is even more important when the AT2 receptor and B2 receptor form a dimer, could S-nitrosate the AT1 receptor.
In general, the use of biased agonists of the β-arrestin pathway and S-nitrosation of the AT1 receptor allow for the interruption of the G-protein pathway. In addition, biased agonists allow the activation of signaling pathways with protective effects similar to those of the AT2 receptor.
6. Conclusions
In summary, we have seen through several examples that the AT1/AT2 balance is very important from a physiological point of view, and that the disturbance of this balance leads to the appearance of pathologies, most often as a result of the dominance of the AT1 receptor over the AT2 receptor. However, emerging studies have shown that secondary signaling of β-arrestins could have beneficial effects. This is why a finer regulation of this balance via S-nitrosation of the receptors or the use of biased agonists seems to be interesting, and could open new therapeutic perspectives.
Author Contributions
Conceptualization, F.D., M.C. and C.D.; writing—original draft preparation, M.C.; writing—review and editing, F.D., S.F. and C.D.; supervision, F.D. and S.F.; project administration, F.D. and S.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the French Ministry of Education, Research and Technology (Paris, France, EA3452).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
| ACE | Angiotensin-converting enzyme |
| Ang II | angiotensin II |
| ARB | AT1 receptor blocker |
| AT1 | angiotensin II type 1 receptor |
| AT2 | angiotensin II type 2 receptor |
| B2 | bradykinin receptor |
| BK | bradykinin |
| C21 | compound 21 |
| GPCR | G protein-coupled receptor |
| GRKs | G protein-coupled receptor kinases |
| KO | knock-out |
| MAPK | mitogen-activated protein kinases |
| MasR | Mas receptor |
| NF-kβ | nuclear factor kβ |
| PKC | protein kinase C |
| PLA2 | phospholipase A2 |
| PLC | phospholipase C |
| RAS | renin-angiotensin system |
| ROS | reactive oxygen species |
| VSMC | vascular smooth muscle cells |
References
- Henrion, D.; Chillon, J.-M.; Capdeville-Atkinson, C.; Vinceneux-Feugier, M.; Atkinson, J. Chronic treatment with the angiotensin I converting enzyme inhibitor, perindopril, protects in vitro carbachol-induced vasorelaxation in a rat model of vascular calcium overload. Br. J. Pharmacol. 1991, 104, 966–972. [Google Scholar] [CrossRef]
- Lartaud, I.; Bray-des-Boscs, L.; Chillon, J.M.; Atkinson, J.; Capdeville-Atkinson, C. In vivo cerebrovascular reactivity in Wistar and Fischer 344 rat strains during aging. Am. J. Physiol. Heart Circ. Physiol. 1993, 264, H851–H858. [Google Scholar] [CrossRef]
- Régrigny, O.; Atkinson, J.; Capdeville-Atkinson, C.; Limiñana, P.; Chillon, J.-M. Effect of Lovastatin on Cerebral Circulation in Spontaneously Hypertensive Rats. Hypertension 2000, 35, 1105–1110. [Google Scholar] [CrossRef]
- Atkinson, J. Stroke, high blood pressure and the renin–angiotensin–aldosterone system—New developments. Front. Pharm. 2011, 2, 22. [Google Scholar] [CrossRef]
- Tigerstedt, R.; Bergman, P.Q. Niere und Kreislauf 1. Skand. Arch. Für Physiol. 1898, 8, 223–271. [Google Scholar] [CrossRef]
- Guyton, A.C. Blood Pressure Control—Special Role of the Kidneys and Body Fluids. Science 1991, 252, 1813–1816. [Google Scholar] [CrossRef] [PubMed]
- Skeggs, L.T.; Lentz, K.E.; Gould, A.B.; Hochstrasser, H.; Kahn, J.R. Biochemistry and kinetics of the renin-angiotensin system. Fed. Proc. 1967, 26, 42–47. [Google Scholar]
- Murphy, T.J.; Alexander, R.W.; Griendling, K.K.; Runge, M.S.; Bernstein, K.E. Isolation of a cDNA encoding the vascular type-1 angiotensin II receptor. Nature 1991, 351, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Mukoyama, M.; Nakajima, M.; Horiuchi, M.; Sasamura, H.; Pratt, R.E.; Dzau, V.J. Expression cloning of type 2 angiotensin II receptor reveals a unique class of seven-transmembrane receptors. J. Biol. Chem. 1993, 268, 24539–24542. [Google Scholar] [CrossRef]
- Sevá Pessôa, B.; van der Lubbe, N.; Verdonk, K.; Roks, A.J.M.; Hoorn, E.J.; Danser, A.H.J. Key developments in renin–angiotensin–aldosterone system inhibition. Nat. Rev. Nephrol. 2013, 9, 26–36. [Google Scholar] [CrossRef]
- Simões e Silva, A.; Silveira, K.; Ferreira, A.; Teixeira, M. ACE2, angiotensin-(1-7) and Mas receptor axis in inflammation and fibrosis: Angiotensin-(1-7) in inflammation and fibrosis. Br. J. Pharmacol. 2013, 169, 477–492. [Google Scholar] [CrossRef] [PubMed]
- Povlsen, A.; Grimm, D.; Wehland, M.; Infanger, M.; Krüger, M. The Vasoactive Mas Receptor in Essential Hypertension. JCM 2020, 9, 267. [Google Scholar] [CrossRef]
- Schleifenbaum, J. Alamandine and Its Receptor MrgD Pair Up to Join the Protective Arm of the Renin-Angiotensin System. Front. Med. 2019, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Chai, S.Y.; Fernando, R.; Peck, G.; Ye, S.-Y.; Mendelsohn, F.A.O.; Jenkins, T.A.; Albiston, A.L. What’s new in the renin-angiotensin system?: The angiotensin IV/AT4 receptor. CMLS Cell. Mol. Life Sci. 2004, 61, 2728–2737. [Google Scholar] [CrossRef]
- Kramár, E.A.; Krishnan, R.; Harding, J.W.; Wright, J.W. Role of nitric oxide in angiotensin IV-induced increases in cerebral blood flow. Regul. Pept. 1998, 74, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, T.A.; Handa, R.K.; Harding, J.W.; Wright, J.W. A role for the angiotensin IV/AT4 system in mediating natriuresis in the rat. Peptides 2001, 22, 935–944. [Google Scholar] [CrossRef]
- Wilson, W.L.; Munn, C.; Ross, R.C.; Harding, J.W.; Wright, J.W. The role of the AT4 and cholinergic systems in the Nucleus Basalis Magnocellularis (NBM): Effects on spatial memory. Brain Res. 2009, 1272, 25–31. [Google Scholar] [CrossRef]
- Royea, J.; Hamel, E. Brain angiotensin II and angiotensin IV receptors as potential Alzheimer’s disease therapeutic targets. GeroScience 2020, 42, 1237–1256. [Google Scholar] [CrossRef]
- Horiuchi, M.; Akishita, M.; Dzau, V.J. Recent Progress in Angiotensin II Type 2 Receptor Research in the Cardiovascular System. Hypertension 1999, 33, 613–621. [Google Scholar] [CrossRef]
- Gasparo, M.D.; Catt, K.J.; Inagami, T.; Wright, J.W.; Unger, T. International Union of Pharmacology. XXIII. The Angiotensin II Receptors. Pharmacol. Rev. 2000, 52, 415–472. [Google Scholar]
- Campbell, D.J.; Habener, J.F. Angiotensinogen gene is expressed and differentially regulated in multiple tissues of the rat. J. Clin. Investig. 1986, 78, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Delaitre, C.; Boisbrun, M.; Lecat, S.; Dupuis, F. Targeting the Angiotensin II Type 1 Receptor in Cerebrovascular Diseases: Biased Signaling Raises New Hopes. Int. J. Mol. Sci. 2021, 22, 6738. [Google Scholar] [CrossRef] [PubMed]
- Steckelings, U.M.; Widdop, R.E.; Sturrock, E.D.; Lubbe, L.; Hussain, T.; Kaschina, E.; Unger, T.; Hallberg, A.; Carey, R.M.; Sumners, C. The Angiotensin AT2 Receptor: From a Binding Site to a Novel Therapeutic Target. Pharmacol. Rev. 2022, 74, 1051–1135. [Google Scholar] [CrossRef] [PubMed]
- Hunyady, L.; Catt, K.J. Pleiotropic AT1 Receptor Signaling Pathways Mediating Physiological and Pathogenic Actions of Angiotensin II. Mol. Endocrinol. 2006, 20, 953–970. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiol. Rev. 2018, 98, 1627–1738. [Google Scholar] [CrossRef]
- Pueyo, M.E.; N’Diaye, N.; Michel, J.-B. Angiotensin Il-elicited signal transduction via AT1 receptors in endothelial cells. Br. J. Pharmacol. 1996, 118, 79–84. [Google Scholar] [CrossRef]
- Busche, S.; Gallinat, S.; Bohle, R.-M.; Reinecke, A.; Seebeck, J.; Franke, F.; Fink, L.; Zhu, M.; Sumners, C.; Unger, T. Expression of Angiotensin AT1 and AT2 Receptors in Adult Rat Cardiomyocytes after Myocardial Infarction. Am. J. Pathol. 2000, 157, 605–611. [Google Scholar] [CrossRef]
- Siragy, H.M. AT1 and AT2 receptor in the kidney: Role in health and disease. Semin. Nephrol. 2004, 24, 93–100. [Google Scholar] [CrossRef]
- Lenkei, Z.; Palkovits, M.; Corvol, P.; Llorens-Cortès, C. Expression of Angiotensin Type-1 (AT1) and Type-2 (AT2) Receptor mRNAs in the Adult Rat Brain: A Functional Neuroanatomical Review. Front. Neuroendocrinol. 1997, 18, 383–439. [Google Scholar] [CrossRef]
- Johren, O.; Saavedra, J.M. Expression of AT1A and AT1B angiotensin II receptor messenger RNA in forebrain of 2-wk-old rats. Am. J. Physiol. Endocrinol. Metab. 1996, 271, E104–E112. [Google Scholar] [CrossRef]
- Zhang, H.; Unal, H.; Gati, C.; Han, G.W.; Liu, W.; Zatsepin, N.A.; James, D.; Wang, D.; Nelson, G.; Weierstall, U.; et al. Structure of the Angiotensin Receptor Revealed by Serial Femtosecond Crystallography. Cell 2015, 161, 833–844. [Google Scholar] [CrossRef]
- Ohyama, K.; Yamano, Y.; Sano, T.; Nakagomi, Y.; Hamakubo, T.; Morishima, I.; Inagami, T. Disulfide bridges in extracellular domains of angiotensin II receptor type IA. Regul. Pept. 1995, 57, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Wingler, L.M.; Skiba, M.A.; McMahon, C.; Staus, D.P.; Kleinhenz, A.L.W.; Suomivuori, C.-M.; Latorraca, N.R.; Dror, R.O.; Lefkowitz, R.J.; Kruse, A.C. Angiotensin and biased analogs induce structurally distinct active conformations within a GPCR. Science 2020, 367, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Suomivuori, C.-M.; Latorraca, N.R.; Wingler, L.M.; Eismann, S.; King, M.C.; Kleinhenz, A.L.W.; Skiba, M.A.; Staus, D.P.; Kruse, A.C.; Lefkowitz, R.J.; et al. Molecular mechanism of biased signaling in a prototypical G protein–coupled receptor. Science. 2020, 367, 881–887. [Google Scholar] [CrossRef]
- Aplin, M.; Bonde, M.M.; Hansen, J.L. Molecular determinants of angiotensin II type 1 receptor functional selectivity. J. Mol. Cell. Cardiol. 2009, 46, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, P.; Jagadeesh, G. Structural determinants for binding, activation, and functional selectivity of the angiotensin AT1 receptor. J. Mol. Endocrinol. 2014, 53, R71–R92. [Google Scholar] [CrossRef]
- Kaschina, E.; Unger, T. Angiotensin AT1/AT2 Receptors: Regulation, Signalling and Function. Blood Press. 2003, 12, 70–88. [Google Scholar] [CrossRef]
- Freeman, E.J.; Chisolm, G.M.; Tallant, E.A. Role of calcium and protein kinase C in the activation of phospholipase D by angiotensin II in vascular smooth muscle cells. Arch. Biochem. Biophys. 1995, 319, 84–92. [Google Scholar] [CrossRef]
- Lassegue, B.; Alexander, R.W.; Clark, M.; Akers, M.; Griendling, K.K. Phosphatidylcholine is a major source of phosphatidic acid and diacylglycerol in angiotensin Il-stimulated vascular smooth-muscle cells. Biochem. J. 1993, 292, 509–517. [Google Scholar] [CrossRef]
- Vinturache, A.E.; Smith, F.G. Angiotensin type 1 and type 2 receptors during ontogeny: Cardiovascular and renal effects. Vasc. Pharmacol. 2014, 63, 145–154. [Google Scholar] [CrossRef]
- Rao, G.N.; Lassegue, B.; Alexander, R.W.; Griendling, K.K. Angiotensin 11 stimulates phosphorylation of high-molecular-mass cytosolic phospholipase A2 in vascular smooth-muscle cells. Biochem. J. 1994, 299, 197–201. [Google Scholar] [CrossRef]
- Touyz, R.M.; Schiffrin, E.L. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol. Rev. 2000, 52, 639–672. [Google Scholar] [PubMed]
- Ohtsu, H.; Suzuki, H.; Nakashima, H.; Dhobale, S.; Frank, G.D.; Motley, E.D.; Eguchi, S. Angiotensin II Signal Transduction Through Small GTP-Binding Proteins: Mechanism and Significance in Vascular Smooth Muscle Cells. Hypertension 2006, 48, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Forrester, S.J.; O’Brien, S.; Baggett, A.; Rizzo, V.; Eguchi, S. AT1 receptor signaling pathways in the cardiovascular system. Pharmacol. Res. 2017, 125, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Violin, J.D.; Lefkowitz, R.J. β-Arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol. Sci. 2007, 28, 416–422. [Google Scholar] [CrossRef]
- Luttrell, L.M.; Lefkowitz, R.J. The role of β-arrestins in the termination and transduction of G-protein-coupled receptor signals. J. Cell Sci. 2002, 115, 455–465. [Google Scholar] [CrossRef]
- Tohgo, A.; Pierce, K.L.; Choy, E.W.; Lefkowitz, R.J.; Luttrell, L.M. β-Arrestin Scaffolding of the ERK Cascade Enhances Cytosolic ERK Activity but Inhibits ERK-mediated Transcription following Angiotensin AT1a Receptor Stimulation. J. Biol. Chem. 2002, 277, 9429–9436. [Google Scholar] [CrossRef]
- Peterson, Y.K.; Luttrell, L.M. The Diverse Roles of Arrestin Scaffolds in G Protein–Coupled Receptor Signaling. Pharmacol. Rev. 2017, 69, 256–297. [Google Scholar] [CrossRef]
- Griendling, K.K.; Minieri, C.A.; Ollerenshaw, J.D.; Alexander, R.W. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ. Res. 1994, 74, 1141–1148. [Google Scholar] [CrossRef]
- Touyz, R.M.; Briones, A.M. Reactive oxygen species and vascular biology: Implications in human hypertension. Hypertens. Res. 2011, 34, 5–14. [Google Scholar] [CrossRef]
- Tsutsumi, K.; Saavedra, J.M. Characterization and development of angiotensin II receptor subtypes (AT1 and AT2) in rat brain. Am. J. Physiol. 1991, 261, R209–R216. [Google Scholar] [CrossRef] [PubMed]
- Ichiki, T.; Herold, C.L.; Kambayashi, Y.; Bardhan, S.; Inagami, T. Cloning of the cDNA and the genomic DNA of the mouse angiotensin II type 2 receptor. Biochim. Biophys. Acta (BBA) Biomembr. 1994, 1189, 247–250. [Google Scholar] [CrossRef]
- Rompe, F.; Artuc, M.; Hallberg, A.; Alterman, M.; Ströder, K.; Thöne-Reineke, C.; Reichenbach, A.; Schacherl, J.; Dahlöf, B.; Bader, M.; et al. Direct Angiotensin II Type 2 Receptor Stimulation Acts Anti-Inflammatory Through Epoxyeicosatrienoic Acid and Inhibition of Nuclear Factor κB. Hypertension 2010, 55, 924–931. [Google Scholar] [CrossRef]
- Zhang, H.; Han, G.W.; Batyuk, A.; Ishchenko, A.; White, K.L.; Patel, N.; Sadybekov, A.; Zamlynny, B.; Rudd, M.T.; Hollenstein, K.; et al. Structural basis for selectivity and diversity in angiotensin II receptors. Nature 2017, 544, 327–332. [Google Scholar] [CrossRef]
- Griendling, K.K.; Lassègue, B.; Alexander, R.W. Angiotensin receptors and their therapeutic implications. Annu. Rev. Pharmacol. Toxicol. 1996, 36, 281–306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Pratt, R.E. The AT2 Receptor Selectively Associates with Giα2 and Giα3 in the Rat Fetus. J. Biol. Chem. 1996, 271, 15026–15033. [Google Scholar] [CrossRef]
- Feng, Y.-H.; Saad, Y.; Karnik, S.S. Reversible inactivation of AT2 angiotensin II receptor from cysteine-disulfide bond exchange. FEBS Lett. 2000, 484, 133–138. [Google Scholar] [CrossRef]
- Heerding, J.N.; Yee, D.K.; Jacobs, S.L.; Fluharty, S.J. Mutational analysis of the angiotensin II type 2 receptor: Contribution of conserved extracellular amino acids. Regul. Pept. 1997, 72, 97–103. [Google Scholar] [CrossRef]
- Yee, D.K.; Kisley, L.R.; Heerding, J.N.; Fluharty, S.J. Mutation of a conserved fifth transmembrane domain lysine residue (Lys215) attenuates ligand binding in the angiotensin II type 2 receptor. Brain Res. Mol. Brain Res. 1997, 51, 238–241. [Google Scholar] [CrossRef]
- Turner, C.A.; Cooper, S.; Pulakat, L. Role of the His273 located in the sixth transmembrane domain of the Angiotensin II receptor subtype AT2 in ligand–receptor interaction. Biochem. Biophys. Res. Commun. 1999, 257, 704–707. [Google Scholar] [CrossRef]
- Steckelings, U.M.; Kaschina, E.; Unger, T. The AT2 receptor—A matter of love and hate. Peptides 2005, 26, 1401–1409. [Google Scholar] [CrossRef]
- Turu, G.; Szidonya, L.; Gáborik, Z.; Buday, L.; Spät, A.; Clark, A.J.L.; Hunyady, L. Differential beta-arrestin binding of AT1 and AT2 angiotensin receptors. FEBS Lett. 2006, 580, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Padia, S.H.; Carey, R.M. AT2 receptors: Beneficial counter-regulatory role in cardiovascular and renal function. Pflug. Arch. Eur. J. Physiol. 2013, 465, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Bottari, S.P.; Taylor, V.; King, I.N.; Bogdal, Y.; Whitebread, S.; de Gasparo, M. Angiotensin II AT2 receptors do not interact with guanine nucleotide binding proteins. Eur. J. Pharmacol. Mol. Pharmacol. 1991, 207, 157–163. [Google Scholar] [CrossRef]
- Stennett, A.K.; Qiao, X.; Falone, A.E.; Koledova, V.V.; Khalil, R.A. Increased vascular angiotensin type 2 receptor expression and NOS-mediated mechanisms of vascular relaxation in pregnant rats. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H745–H755. [Google Scholar] [CrossRef] [PubMed]
- Alexander, L.D.; Ding, Y.; Alagarsamy, S.; Cui, X. Angiotensin II stimulates fibronectin protein synthesis via a Gβγ/arachidonic acid-dependent pathway. Am. J. Physiol. Ren. Physiol. 2014, 307, F287–F302. [Google Scholar] [CrossRef]
- Savoia, C.; Tabet, F.; Yao, G.; Schiffrin, E.L.; Touyz, R.M. Negative regulation of RhoA/Rho kinase by angiotensin II type 2 receptor in vascular smooth muscle cells: Role in angiotensin II-induced vasodilation in stroke-prone spontaneously hypertensive rats. J. Hypertens. 2005, 23, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Savoia, C.; Ebrahimian, T.; He, Y.; Gratton, J.-P.; Schiffrin, E.L.; Touyz, R.M. Angiotensin II/AT2 receptor-induced vasodilation in stroke-prone spontaneously hypertensive rats involves nitric oxide and cGMP-dependent protein kinase. J. Hypertens. 2006, 24, 2417–2422. [Google Scholar] [CrossRef] [PubMed]
- Siragy, H.M.; Carey, R.M. The subtype 2 (AT2) angiotensin receptor mediates renal production of nitric oxide in conscious rats. J. Clin. Investig. 1997, 100, 264–269. [Google Scholar] [CrossRef]
- Tsutsumi, Y.; Matsubara, H.; Masaki, H.; Kurihara, H.; Murasawa, S.; Takai, S.; Miyazaki, M.; Nozawa, Y.; Ozono, R.; Nakagawa, K.; et al. Angiotensin II type 2 receptor overexpression activates the vascular kinin system and causes vasodilation. J. Clin. Investig. 1999, 104, 925–935. [Google Scholar] [CrossRef]
- Yayama, K.; Hiyoshi, H.; Imazu, D.; Okamoto, H. Angiotensin II Stimulates Endothelial NO Synthase Phosphorylation in Thoracic Aorta of Mice With Abdominal Aortic Banding Via Type 2 Receptor. Hypertension 2006, 48, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Abadir, P.M.; Periasamy, A.; Carey, R.M.; Siragy, H.M. Angiotensin II Type 2 Receptor–Bradykinin B2 Receptor Functional Heterodimerization. Hypertension 2006, 48, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Dimitropoulou, C.; White, R.E.; Fuchs, L.; Zhang, H.; Catravas, J.D.; Carrier, G.O. Angiotensin II Relaxes Microvessels Via the AT2 Receptor and Ca2+-Activated K+ (BK Ca) Channels. Hypertension 2001, 37, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, J.M.; Armando, I. Angiotensin II AT2 Receptors Contribute to Regulate the Sympathoadrenal and Hormonal Reaction to Stress Stimuli. Cell. Mol. Neurobiol. 2018, 38, 85–108. [Google Scholar] [CrossRef] [PubMed]
- Foulquier, S.; Steckelings, U.M.; Unger, T. Perspective: A tale of two receptors. Nature 2013, 493, S9. [Google Scholar] [CrossRef]
- Anderson, W.P.; Selig, S.E.; Korner, P.I. Role of Angiotensin II in the Hypertension Induced by Renal Artery Stenosis. Clin. Exp. Hypertens. Part A Theory Pract. 1984, 6, 299–314. [Google Scholar] [CrossRef]
- Foulquier, S.; Steckelings, U.M.; Unger, T. Impact of the AT2 Receptor Agonist C21 on Blood Pressure and Beyond. Curr. Hypertens. Rep. 2012, 14, 403–409. [Google Scholar] [CrossRef]
- Fischer-Ferraro, C.; Nahmod, V.E.; Goldstein, D.J.; Finkielman, S. Angiotensin and renin in rat and dog brain. J. Exp. Med. 1971, 133, 353–361. [Google Scholar] [CrossRef]
- Barnes, J.M.; Steward, L.J.; Barber, P.C.; Barnes, N.M. Identification and characterisation of angiotensin II receptor subtypes in human brain. Eur. J. Pharmacol. 1993, 230, 251–258. [Google Scholar] [CrossRef]
- Vincent, J.-M.; Kwan, Y.W.; Lung Chan, S.; Perrin-Sarrado, C.; Atkinson, J.; Chillon, J.-M. Constrictor and Dilator Effects of Angiotensin II on Cerebral Arterioles. Stroke 2005, 36, 2691–2695. [Google Scholar] [CrossRef]
- Dupuis, F.; Atkinson, J.; Limiñana, P.; Chillon, J.-M. Comparative effects of the angiotensin II receptor blocker, telmisartan, and the angiotensin-converting enzyme inhibitor, ramipril, on cerebrovascular structure in spontaneously hypertensive rats. J. Hypertens. 2005, 23, 1061–1066. [Google Scholar] [CrossRef]
- Foulquier, S.; Dupuis, F.; Perrin-Sarrado, C.; Maguin Gatè, K.; Leroy, P.; Liminana, P.; Atkinson, J.; Capdeville-Atkinson, C.; Lartaud, I. Differential Effects of Short-Term Treatment with Two AT1 Receptor Blockers on Diameter of Pial Arterioles in SHR. PLoS ONE 2012, 7, e42469. [Google Scholar] [CrossRef]
- Stenman, E.; Edvinsson, L. Cerebral Ischemia Enhances Vascular Angiotensin AT1 Receptor–Mediated Contraction in Rats. Stroke 2004, 35, 970–974. [Google Scholar] [CrossRef]
- Vikman, P.; Edvinsson, L. Gene expression profiling in the human middle cerebral artery after cerebral ischemia. Eur. J. Neurol. 2006, 13, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Jancovski, N.; Bassi, J.K.; Nguyen-Huu, T.-P.; Choong, Y.-T.; Palma-Rigo, K.; Davern, P.J.; Gurley, S.B.; Thomas, W.G.; Head, G.A.; et al. Angiotensin Type 1A Receptors in C1 Neurons of the Rostral Ventrolateral Medulla Modulate the Pressor Response to Aversive Stress. J. Neurosci. 2012, 32, 2051–2061. [Google Scholar] [CrossRef] [PubMed]
- De Kloet, A.D.; Wang, L.; Ludin, J.A.; Smith, J.A.; Pioquinto, D.J.; Hiller, H.; Steckelings, U.M.; Scheuer, D.A.; Sumners, C.; Krause, E.G. Reporter mouse strain provides a novel look at angiotensin type-2 receptor distribution in the central nervous system. Brain Struct. Funct. 2016, 221, 891–912. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.D.; Wang, G.; Waters, E.M.; Gonzales, K.L.; Speth, R.C.; Van Kempen, T.A.; Marques-Lopes, J.; Young, C.N.; Butler, S.D.; Davisson, R.L.; et al. Distribution of angiotensin type 1a receptor-containing cells in the brains of bacterial artificial chromosome transgenic mice. Neuroscience 2012, 226, 489–509. [Google Scholar] [CrossRef] [PubMed]
- Elsaafien, K.; de Kloet, A.D.; Krause, E.G.; Sumners, C. Brain Angiotensin Type-1 and Type-2 Receptors in Physiological and Hypertensive Conditions: Focus on Neuroinflammation. Curr. Hypertens. Rep. 2020, 22, 48. [Google Scholar] [CrossRef] [PubMed]
- De Kloet, A.D.; Wang, L.; Pitra, S.; Hiller, H.; Smith, J.A.; Tan, Y.; Nguyen, D.; Cahill, K.M.; Sumners, C.; Stern, J.E.; et al. A Unique “Angiotensin-Sensitive” Neuronal Population Coordinates Neuroendocrine, Cardiovascular, and Behavioral Responses to Stress. J. Neurosci. 2017, 37, 3478–3490. [Google Scholar] [CrossRef]
- De Kloet, A.D.; Pitra, S.; Wang, L.; Hiller, H.; Pioquinto, D.J.; Smith, J.A.; Sumners, C.; Stern, J.E.; Krause, E.G. Angiotensin Type-2 Receptors Influence the Activity of Vasopressin Neurons in the Paraventricular Nucleus of the Hypothalamus in Male Mice. Endocrinology 2016, 157, 3167–3180. [Google Scholar] [CrossRef]
- de Oliveira-Sales, E.B.; Nishi, E.E.; Boim, M.A.; Dolnikoff, M.S.; Bergamaschi, C.T.; Campos, R.R. Upregulation of AT1R and iNOS in the Rostral Ventrolateral Medulla (RVLM) Is Essential for the Sympathetic Hyperactivity and Hypertension in the 2K-1C Wistar Rat Model. Am. J. Hypertens. 2010, 23, 708–715. [Google Scholar] [CrossRef]
- Li, Z.; Iwai, M.; Wu, L.; Shiuchi, T.; Jinno, T.; Cui, T.-X.; Horiuchi, M. Role of AT2 receptor in the brain in regulation of blood pressure and water intake. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H116–H121. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wang, W.-Z.; Wang, W.; Zucker, I.H. Imbalance of Angiotensin Type 1 Receptor and Angiotensin II Type 2 Receptor in the Rostral Ventrolateral Medulla: Potential Mechanism for Sympathetic Overactivity in Heart Failure. Hypertension 2008, 52, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Valero-Esquitino, V.; Lucht, K.; Namsolleck, P.; Monnet-Tschudi, F.; Stubbe, T.; Lucht, F.; Liu, M.; Ebner, F.; Brandt, C.; Danyel, L.A.; et al. Direct angiotensin type 2 receptor (AT2R) stimulation attenuates T-cell and microglia activation and prevents demyelination in experimental autoimmune encephalomyelitis in mice. Clin. Sci. 2015, 128, 95–109. [Google Scholar] [CrossRef]
- Kim, J.-H.; Afridi, R.; Cho, E.; Yoon, J.H.; Lim, Y.-H.; Lee, H.-W.; Ryu, H.; Suk, K. Soluble ANPEP Released From Human Astrocytes as a Positive Regulator of Microglial Activation and Neuroinflammation: Brain Renin–Angiotensin System in Astrocyte–Microglia Crosstalk. Mol. Cell. Proteom. 2022, 21, 100424. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Perez, A.I.; Borrajo, A.; Rodriguez-Pallares, J.; Guerra, M.J.; Labandeira-Garcia, J.L. Interaction between NADPH-oxidase and Rho-kinase in angiotensin II-induced microglial activation: NADPH-Oxidase and Rho-Kinase Interaction. Glia 2015, 63, 466–482. [Google Scholar] [CrossRef]
- Villar-Cheda, B.; Valenzuela, R.; Rodriguez-Perez, A.I.; Guerra, M.J.; Labandeira-Garcia, J.L. Aging-related changes in the nigral angiotensin system enhances proinflammatory and pro-oxidative markers and 6-OHDA-induced dopaminergic degeneration. Neurobiol. Aging 2012, 33, 204.e1–204.e11. [Google Scholar] [CrossRef]
- Labandeira-Garcia, J.L.; Rodríguez-Perez, A.I.; Garrido-Gil, P.; Rodriguez-Pallares, J.; Lanciego, J.L.; Guerra, M.J. Brain Renin-Angiotensin System and Microglial Polarization: Implications for Aging and Neurodegeneration. Front. Aging Neurosci. 2017, 9, 129. [Google Scholar] [CrossRef]
- Campbell, G.J.; Hands, E.L.; Van de Pette, M. The Role of CDKs and CDKIs in Murine Development. Int. J. Mol. Sci. 2020, 21, 5343. [Google Scholar] [CrossRef]
- Han, H.J.; Han, J.Y.; Heo, J.S.; Lee, S.H.; Lee, M.Y.; Kim, Y.H. ANG II-stimulated DNA synthesis is mediated by ANG II receptor-dependent Ca2+/PKC as well as EGF receptor-dependent PI3K/Akt/mTOR/p70S6K1 signal pathways in mouse embryonic stem cells. J. Cell. Physiol. 2007, 211, 618–629. [Google Scholar] [CrossRef]
- Diep, Q.N.; El Mabrouk, M.; Touyz, R.M.; Schiffrin, E.L. Expression of Cell Cycle Proteins in Blood Vessels of Angiotensin II–Infused Rats: Role of AT1 Receptors. Hypertension 2001, 37, 604–608. [Google Scholar] [CrossRef]
- Gingras, B.; Rodier, G.; Giasson, E.; Coulombe, P.; Chassagne, C.; Meloche, S. Expression of angiotensin type II receptor downregulates Cdk4 synthesis and inhibits cell-cycle progression. Oncogene 2003, 22, 2633–2642. [Google Scholar] [CrossRef] [PubMed]
- Peluso, A.A.; Kempf, S.J.; Verano-Braga, T.; Rodrigues-Ribeiro, L.; Johansen, L.E.; Hansen, M.R.; Kitlen, G.; Haugaard, A.H.; Sumners, C.; Ditzel, H.J.; et al. Quantitative Phosphoproteomics of the Angiotensin AT2-Receptor Signaling Network Identifies HDAC1 (Histone-Deacetylase-1) and p53 as Mediators of Antiproliferation and Apoptosis. Hypertension 2022, 79, 2530–2541. [Google Scholar] [CrossRef] [PubMed]
- Cubillos-Rojas, M.; Schneider, T.; Bartrons, R.; Ventura, F.; Rosa, J.L. NEURL4 regulates the transcriptional activity of tumor suppressor protein p53 by modulating its oligomerization. Oncotarget 2017, 8, 61824–61836. [Google Scholar] [CrossRef]
- Yu, L.; Meng, W.; Ding, J.; Cheng, M. Klotho inhibits angiotensin II-induced cardiomyocyte hypertrophy through suppression of the AT1R/beta catenin pathway. Biochem. Biophys. Res. Commun. 2016, 473, 455–461. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Y. Wnt/β-catenin signaling and renin–angiotensin system in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2016, 25, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Geisterfer, A.A.; Peach, M.J.; Owens, G.K. Angiotensin II induces hypertrophy, not hyperplasia, of cultured rat aortic smooth muscle cells. Circ. Res. 1988, 62, 749–756. [Google Scholar] [CrossRef]
- Naftilan, A.J.; Pratt, R.E.; Dzau, V.J. Induction of Platelet-derived Growth Factor A-chain and c-myc Gene Expressions by Angiotensin 11 in Cultured Rat Vascular Smooth Muscle Cells. J. Clin. Investig. 1989, 83, 1419–1424. [Google Scholar] [CrossRef]
- Wolf, G.; Wenzel, U.O. Angiotensin II and Cell Cycle Regulation. Hypertension 2004, 43, 693–698. [Google Scholar] [CrossRef]
- Fujihara, S.; Morishita, A.; Ogawa, K.; Tadokoro, T.; Chiyo, T.; Kato, K.; Kobara, H.; Mori, H.; Iwama, H.; Masaki, T. The angiotensin II type 1 receptor antagonist telmisartan inhibits cell proliferation and tumor growth of esophageal adenocarcinoma via the AMPKα/mTOR pathway in vitro and in vivo. Oncotarget 2017, 8, 8536–8549. [Google Scholar] [CrossRef]
- Samukawa, E.; Fujihara, S.; Oura, K.; Iwama, H.; Yamana, Y.; Tadokoro, T.; Chiyo, T.; Kobayashi, K.; Morishita, A.; Nakahara, M.; et al. Angiotensin receptor blocker telmisartan inhibits cell proliferation and tumor growth of cholangiocarcinoma through cell cycle arrest. Int. J. Oncol. 2017, 51, 1674–1684. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Liang, Z.; Zhang, Y.; Jie, F.; Li, J.; Fei, Y.; Huang, Z.; Pei, N.; Wang, S.; Li, A.; et al. Effects of Angiotensin II Type 2 Receptor Overexpression on the Growth of Hepatocellular Carcinoma Cells In Vitro and In Vivo. PLoS ONE 2013, 8, e83754. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, O.; Pineda-Olvera, B.; Guevara-Salazar, P.; Hernández-Pedro, N.; Morales-Espinosa, D.; Cerón-Lizarraga, T.L.; González-De la Rosa, C.H.; Rembao, D.; Segura-Pacheco, B.; Sotelo, J. Expression of AT1 and AT2 angiotensin receptors in astrocytomas is associated with poor prognosis. Br. J. Cancer 2008, 99, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Dolley-Hitze, T.; Jouan, F.; Martin, B.; Mottier, S.; Edeline, J.; Moranne, O.; Le Pogamp, P.; Belaud-Rotureau, M.-A.; Patard, J.-J.; Rioux-Leclercq, N.; et al. Angiotensin-2 receptors (AT1-R and AT2-R), new prognostic factors for renal clear-cell carcinoma? Br. J. Cancer 2010, 103, 1698–1705. [Google Scholar] [CrossRef]
- Perini, M.V.; Dmello, R.S.; Nero, T.L.; Chand, A.L. Evaluating the benefits of renin-angiotensin system inhibitors as cancer treatments. Pharmacol. Ther. 2020, 211, 107527. [Google Scholar] [CrossRef]
- Suganuma, T.; Ino, K.; Shibata, K.; Kajiyama, H.; Nagasaka, T.; Mizutani, S.; Kikkawa, F. Functional Expression of the Angiotensin II Type1 Receptor in Human Ovarian Carcinoma Cells and Its Blockade Therapy Resulting in Suppression of Tumor Invasion, Angiogenesis, and Peritoneal Dissemination. Clin. Cancer Res. 2005, 11, 2686–2694. [Google Scholar] [CrossRef]
- Rhodes, D.R.; Ateeq, B.; Cao, Q.; Tomlins, S.A.; Mehra, R.; Laxman, B.; Kalyana-Sundaram, S.; Lonigro, R.J.; Helgeson, B.E.; Bhojani, M.S.; et al. AGTR1 overexpression defines a subset of breast cancer and confers sensitivity to losartan, an AGTR1 antagonist. Proc. Natl. Acad. Sci. USA 2009, 106, 10284–10289. [Google Scholar] [CrossRef]
- Tamura, M.; Yan, H.; Zegarra-Moro, O.; Edl, J.; Oursler, S.; Chard-Bergstrom, C.; Andrews, G.; Kanehira, T.; Takekoshi, S.; Mernaugh, R. Specific single chain variable fragment (ScFv) antibodies to angiotensin II AT2 receptor: Evaluation of the angiotensin II receptor expression in normal and tumor-bearing mouse lung. J. Mol. Hist. 2008, 39, 351–358. [Google Scholar] [CrossRef]
- Pickel, L.; Matsuzuka, T.; Doi, C.; Ayuzawa, R.; Maurya, D.K.; Xie, S.-X.; Berkland, C.; Tamura, M. Over-expression of angiotensin II type 2 receptor gene induces cell death in lung adenocarcinoma cells. Cancer Biol. Ther. 2010, 9, 277–285. [Google Scholar] [CrossRef]
- Pei, N.; Mao, Y.; Wan, P.; Chen, X.; Li, A.; Chen, H.; Li, J.; Wan, R.; Zhang, Y.; Du, H.; et al. Angiotensin II type 2 receptor promotes apoptosis and inhibits angiogenesis in bladder cancer. J. Exp. Clin. Cancer Res. 2017, 36, 77. [Google Scholar] [CrossRef]
- Ito, Y.; Naiki-Ito, A.; Kato, H.; Suzuki, S.; Kuno, T.; Ishiguro, Y.; Takahashi, S.; Uemura, H. Chemopreventive effects of angiotensin II receptor type 2 agonist on prostate carcinogenesis by the down-regulation of the androgen receptor. Oncotarget 2018, 9, 13859–13869. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lützen, U.; Fritsch, J.; Zuhayra, M.; Schütze, S.; Steckelings, U.M.; Recanti, C.; Namsoleck, P.; Unger, T.; Culman, J. Activation of intracellular angiotensin AT₂ receptors induces rapid cell death in human uterine leiomyosarcoma cells. Clin. Sci. 2015, 128, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Aleksiejczuk, M.; Gromotowicz-Poplawska, A.; Marcinczyk, N.; Przylipiak, A.; Chabielska, E. The expression of the renin-angiotensin-aldosterone system in the skin and its effects on skin physiology and pathophysiology. J. Physiol. Pharmacol. 2019, 70. [Google Scholar] [CrossRef]
- Silva, I.M.S.; Assersen, K.B.; Willadsen, N.N.; Jepsen, J.; Artuc, M.; Steckelings, U.M. The role of the renin-angiotensin system in skin physiology and pathophysiology. Exp. Dermatol. 2020, 29, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, M.; Saavedra, J.M. Expression of angiotensin II AT2 receptors in the rat skin during experimental wound healing. Peptides 1992, 13, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Steckelings, U.M.; Henz, B.M.; Wiehstutz, S.; Unger, T.; Artuc, M. Differential expression of angiotensin receptors in human cutaneous wound healing. Br. J. Dermatol. 2005, 153, 887–893. [Google Scholar] [CrossRef]
- Takeda, H.; Katagata, Y.; Kondo, S. Immunohistochemical study of angiotensin receptors in human anagen hair follicles and basal cell carcinoma. Br. J. Dermatol. 2002, 147, 276–280. [Google Scholar] [CrossRef]
- Nehme, A.; Zouein, F.A.; Zayeri, Z.D.; Zibara, K. An Update on the Tissue Renin Angiotensin System and Its Role in Physiology and Pathology. J. Cardiovasc. Dev. Dis. 2019, 6, 14. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, F.; Xu, Y.; Yan, J.-X.; Wu, Y.-D.; Li, S.-H.; Liao, X.; Liang, J.-X.; Li, Z.-H.; Liu, H.-W. A novel role of angiotensin II in epidermal cell lineage determination: Angiotensin II promotes the differentiation of mesenchymal stem cells into keratinocytes through the p38 MAPK, JNK and JAK2 signalling pathways. Exp. Dermatol. 2019, 28, 59–65. [Google Scholar] [CrossRef]
- Rha, E.Y.; Kim, J.W.; Kim, J.H.; Yoo, G. Angiotensin-Converting Enzyme Inhibitor, Captopril, Improves Scar Healing in Hypertensive Rats. Int. J. Med. Sci. 2021, 18, 975–983. [Google Scholar] [CrossRef]
- Jadhav, S.S.; Sharma, N.; Meeks, C.J.; Mordwinkin, N.M.; Espinoza, T.B.; Roda, N.R.; DiZerega, G.S.; Hill, C.K.; Louie, S.G.; Rodgers, K.E. Effects of combined radiation and burn injury on the renin-angiotensin system: CRBI and renin-angiotensin system. Wound Repair. Regen. 2013, 21, 131–140. [Google Scholar] [CrossRef]
- Kamiñska, M.; Mogielnicki, A.; Stankiewicz, A.; Kramkowski, K.; Domaniewski, T.; Buczko, W.; Chabielska, E. Angiotensin Ii Via At1 Receptor Accelerates Arterial Thrombosis In Renovascular Hypertensive Rats. J. Physiol. Pharmacol. 2005, 56, 571–585. [Google Scholar] [PubMed]
- Bernasconi, R.; Nyström, A. Balance and circumstance: The renin angiotensin system in wound healing and fibrosis. Cell. Signal. 2018, 51, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Yahata, Y.; Shirakata, Y.; Tokumaru, S.; Yang, L.; Dai, X.; Tohyama, M.; Tsuda, T.; Sayama, K.; Iwai, M.; Horiuchi, M.; et al. A Novel Function of Angiotensin II in Skin Wound Healing. J. Biol. Chem. 2006, 281, 13209–13216. [Google Scholar] [CrossRef] [PubMed]
- Faghih, M.; Hosseini, S.M.; Smith, B.; Ansari, A.M.; Lay, F.; Ahmed, A.K.; Inagami, T.; Marti, G.P.; Harmon, J.W.; Walston, J.D.; et al. Knockout of Angiotensin AT2 receptors accelerates healing but impairs quality. Aging 2015, 7, 1185–1197. [Google Scholar] [CrossRef]
- Hedayatyanfard, K.; Haddadi, N.; Ziai, S.A.; Karim, H.; Niazi, F.; Steckelings, U.M.; Habibi, B.; Modarressi, A.; Dehpour, A. The renin-angiotensin system in cutaneous hypertrophic scar and keloid formation. Exp. Dermatol. 2020, 29, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Karppinen, S.-M.; Heljasvaara, R.; Gullberg, D.; Tasanen, K.; Pihlajaniemi, T. Toward understanding scarless skin wound healing and pathological scarring. F1000Res 2019, 8, 787. [Google Scholar] [CrossRef]
- Murphy, A.M.; Wong, A.L.; Bezuhly, M. Modulation of angiotensin II signaling in the prevention of fibrosis. Fibrogenes. Tissue Repair. 2015, 8, 7. [Google Scholar] [CrossRef]
- Murphy, A.; LeVatte, T.; Boudreau, C.; Midgen, C.; Gratzer, P.; Marshall, J.; Bezuhly, M. Angiotensin II Type I Receptor Blockade Is Associated with Decreased Cutaneous Scar Formation in a Rat Model. Plast. Reconstr. Surg. 2019, 144, 803e–813e. [Google Scholar] [CrossRef]
- Huang, Y.; Li, J.; Wang, Y.; Chen, D.; Huang, J.; Dai, W.; Peng, P.; Guo, L.; Lei, Y. Intradermal delivery of an angiotensin II receptor blocker using a personalized microneedle patch for treatment of hypertrophic scars. Biomater. Sci. 2023, 11, 583–595. [Google Scholar] [CrossRef]
- Ito, M.; Oliverio, M.I.; Mannon, P.J.; Best, C.F.; Maeda, N.; Smithies, O.; Coffman, T.M. Regulation of blood pressure by the type 1A angiotensin II receptor gene. Proc. Natl. Acad. Sci. USA 1995, 92, 3521–3525. [Google Scholar] [CrossRef]
- Siragy, H.M.; Inagami, T.; Ichiki, T.; Carey, R.M. Sustained hypersensitivity to angiotensin II and its mechanism in mice lacking the subtype-2 (AT2) angiotensin receptor. Proc. Natl. Acad. Sci. USA 1999, 96, 6506–6510. [Google Scholar] [CrossRef] [PubMed]
- Daviet, L.; Lehtonen, J.Y.; Tamura, K.; Griese, D.P.; Horiuchi, M.; Dzau, V.J. Cloning and characterization of ATRAP, a novel protein that interacts with the angiotensin II type 1 receptor. J. Biol. Chem. 1999, 274, 17058–17062. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Ilasaca, M.; Liu, X.; Tamura, K.; Dzau, V.J. The angiotensin II type I receptor-associated protein, ATRAP, is a transmembrane protein and a modulator of angiotensin II signaling. Mol. Biol. Cell. 2003, 14, 5038–5050. [Google Scholar] [CrossRef] [PubMed]
- Mogi, M.; Iwai, M.; Horiuchi, M. Emerging Concepts of Regulation of Angiotensin II Receptors: New Players and Targets for Traditional Receptors. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2532–2539. [Google Scholar] [CrossRef]
- Tanaka, Y.; Tamura, K.; Koide, Y.; Sakai, M.; Tsurumi, Y.; Noda, Y.; Umemura, M.; Ishigami, T.; Uchino, K.; Kimura, K.; et al. The novel angiotensin II type 1 receptor (AT1R)-associated protein ATRAP downregulates AT1R and ameliorates cardiomyocyte hypertrophy. FEBS Lett. 2005, 579, 1579–1586. [Google Scholar] [CrossRef]
- Guo, D.-F.; Chenier, I.; Tardif, V.; Orlov, S.N.; Inagami, T. Type 1 angiotensin II receptor-associated protein ARAP1 binds and recycles the receptor to the plasma membrane. Biochem. Biophys. Res. Commun. 2003, 310, 1254–1265. [Google Scholar] [CrossRef]
- Guo, D.-F.; Chenier, I.; Lavoie, J.L.; Chan, J.S.D.; Hamet, P.; Tremblay, J.; Chen, X.M.; Wang, D.H.; Inagami, T. Development of hypertension and kidney hypertrophy in transgenic mice overexpressing ARAP1 gene in the kidney. Hypertension 2006, 48, 453–459. [Google Scholar] [CrossRef]
- Wruck, C.J.; Funke-Kaiser, H.; Pufe, T.; Kusserow, H.; Menk, M.; Schefe, J.H.; Kruse, M.L.; Stoll, M.; Unger, T. Regulation of transport of the angiotensin AT2 receptor by a novel membrane-associated Golgi protein. Arterioscl. Thromb. Vasc. Biol. 2005, 25, 57–64. [Google Scholar] [CrossRef]
- Reinemund, J.; Seidel, K.; Steckelings, U.M.; Zaade, D.; Klare, S.; Rompe, F.; Katerbaum, M.; Schacherl, J.; Li, Y.; Menk, M.; et al. Poly(ADP-ribose) polymerase-1 (PARP-1) transcriptionally regulates angiotensin AT2 receptor (AT2R) and AT2R binding protein (ATBP) genes. Biochem. Pharmacol. 2009, 77, 1795–1805. [Google Scholar] [CrossRef]
- Senbonmatsu, T.; Saito, T.; Landon, E.J.; Watanabe, O.; Price, E.J.; Roberts, R.L.; Imboden, H.; Fitzgerald, T.G.; Gaffney, F.A.; Inagami, T. A novel angiotensin II type 2 receptor signaling pathway: Possible role in cardiac hypertrophy. EMBO J. 2003, 22, 6471–6482. [Google Scholar] [CrossRef]
- Foulquier, S.; Dupuis, F.; Perrin-Sarrado, C.; Maguin Gaté, K.; Merhi-Soussi, F.; Liminana, P.; Kwan, Y.-W.; Capdeville-Atkinson, C.; Lartaud, I.; Atkinson, J. High salt intake abolishes AT2-mediated vasodilation of pial arterioles in rats. J. Hypertens. 2011, 29, 1392–1399. [Google Scholar] [CrossRef]
- AbdAlla, S.; Lother, H.; Langer, A.; el Faramawy, Y.; Quitterer, U. Factor XIIIA Transglutaminase Crosslinks AT1 Receptor Dimers of Monocytes at the Onset of Atherosclerosis. Cell 2004, 119, 343–354. [Google Scholar] [CrossRef]
- Oro, C.; Qian, H.; Thomas, W.G. Type 1 angiotensin receptor pharmacology: Signaling beyond G proteins. Pharmacol. Ther. 2007, 113, 210–226. [Google Scholar] [CrossRef]
- AbdAlla, S.; Lother, H.; Quitterer, U. AT1-receptor heterodimers show enhanced G-protein activation and altered receptor sequestration. Nature 2000, 407, 94–98. [Google Scholar] [CrossRef]
- Bokslag, A.; van Weissenbruch, M.; Mol, B.W.; de Groot, C.J.M. Preeclampsia; short and long-term consequences for mother and neonate. Early Hum. Dev. 2016, 102, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Quitterer, U.; AbdAlla, S. Pathological AT1R-B2R Protein Aggregation and Preeclampsia. Cells 2021, 10, 2609. [Google Scholar] [CrossRef] [PubMed]
- Kostenis, E.; Milligan, G.; Christopoulos, A.; Sanchez-Ferrer, C.F.; Heringer-Walther, S.; Sexton, P.M.; Gembardt, F.; Kellett, E.; Martini, L.; Vanderheyden, P.; et al. G-Protein–Coupled Receptor Mas Is a Physiological Antagonist of the Angiotensin II Type 1 Receptor. Circulation 2005, 111, 1806–1813. [Google Scholar] [CrossRef]
- Miura, S.; Karnik, S.S.; Saku, K. Constitutively Active Homo-oligomeric Angiotensin II Type 2 Receptor Induces Cell Signaling Independent of Receptor Conformation and Ligand Stimulation. J. Biol. Chem. 2005, 280, 18237–18244. [Google Scholar] [CrossRef] [PubMed]
- Porrello, E.R.; Pfleger, K.D.G.; Seeber, R.M.; Qian, H.; Oro, C.; Abogadie, F.; Delbridge, L.M.D.; Thomas, W.G. Heteromerization of angiotensin receptors changes trafficking and arrestin recruitment profiles. Cell. Signal. 2011, 23, 1767–1776. [Google Scholar] [CrossRef]
- Zha, D.; Cheng, H.; Li, W.; Wu, Y.; Li, X.; Zhang, L.; Feng, Y.-H.; Wu, X. High glucose instigates tubulointerstitial injury by stimulating hetero-dimerization of adiponectin and angiotensin II receptors. Biochem. Biophys. Res. Commun. 2017, 493, 840–846. [Google Scholar] [CrossRef]
- Abadir, P.M.; Carey, R.M.; Siragy, H.M. Angiotensin AT2 Receptors Directly Stimulate Renal Nitric Oxide in Bradykinin B2-Receptor–Null Mice. Hypertension 2003, 42, 600–604. [Google Scholar] [CrossRef]
- Zhao, Y.; Biermann, T.; Luther, C.; Unger, T.; Culman, J.; Gohlke, P. Contribution of bradykinin and nitric oxide to AT2 receptor-mediated differentiation in PC12 W cells: Angiotensin II and PC12 W cell differentiation. J. Neurochem. 2003, 85, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.N.; Ali, Q.; Samuel, P.; Steckelings, U.M.; Hussain, T. Angiotensin II Type 2 Receptor and Receptor Mas Are Colocalized and Functionally Interdependent in Obese Zucker Rat Kidney. Hypertension 2017, 70, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Walters, P.E.; Gaspari, T.A.; Widdop, R.E. Angiotensin-(1–7) Acts as a Vasodepressor Agent Via Angiotensin II Type 2 Receptors in Conscious Rats. Hypertension 2005, 45, 960–966. [Google Scholar] [CrossRef]
- Durand, M.J.; Raffai, G.; Weinberg, B.D.; Lombard, J.H. Angiotensin-(1-7) and low-dose angiotensin II infusion reverse salt-induced endothelial dysfunction via different mechanisms in rat middle cerebral arteries. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1024–H1033. [Google Scholar] [CrossRef]
- Roks, A.J.; Nijholt, J.; van Buiten, A.; van Gilst, W.H.; de Zeeuw, D.; Henning, R.H. Low sodium diet inhibits the local counter-regulator effect of angiotensin-(1-7) on angiotensin II. J. Hypertens. 2004, 22, 2355–2361. [Google Scholar] [CrossRef]
- Leonhardt, J.; Villela, D.C.; Teichmann, A.; Münter, L.-M.; Mayer, M.C.; Mardahl, M.; Kirsch, S.; Namsolleck, P.; Lucht, K.; Benz, V.; et al. Evidence for Heterodimerization and Functional Interaction of the Angiotensin Type 2 Receptor and the Receptor MAS. Hypertension 2017, 69, 1128–1135. [Google Scholar] [CrossRef]
- Lemos, V.S.; Silva, D.M.R.; Walther, T.; Alenina, N.; Bader, M.; Santos, R.A.S. The Endothelium-Dependent Vasodilator Effect of the Nonpeptide Ang(1-7) Mimic AVE 0991 Is Abolished in the Aorta of Mas-Knockout Mice. J. Cardiovasc. Pharmacol. 2005, 46, 274–279. [Google Scholar] [CrossRef]
- AbdAlla, S.; Lother, H.; Abdel-tawab, A.M.; Quitterer, U. The Angiotensin II AT2 Receptor Is an AT1Receptor Antagonist. J. Biol. Chem. 2001, 276, 39721–39726. [Google Scholar] [CrossRef] [PubMed]
- Inuzuka, T.; Fujioka, Y.; Tsuda, M.; Fujioka, M.; Satoh, A.O.; Horiuchi, K.; Nishide, S.; Nanbo, A.; Tanaka, S.; Ohba, Y. Attenuation of ligand-induced activation of angiotensin II type 1 receptor signaling by the type 2 receptor via protein kinase C. Sci Rep 2016, 6, 21613. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Santisteban, R.; Rodriguez-Perez, A.I.; Muñoz, A.; Reyes-Resina, I.; Labandeira-García, J.L.; Navarro, G.; Franco, R. Angiotensin AT1 and AT2 receptor heteromer expression in the hemilesioned rat model of Parkinson’s disease that increases with levodopa-induced dyskinesia. J. Neuroinflamm. 2020, 17, 243. [Google Scholar] [CrossRef] [PubMed]
- Lanctôt, P.M.; Leclerc, P.C.; Escher, E.; Leduc, R.; Guillemette, G. Role of N-glycosylation in the expression and functional properties of human AT1 receptor. Biochemistry 1999, 38, 8621–8627. [Google Scholar] [CrossRef]
- Lanctot, P.M.; Leclerc, P.C.; Clément, M.; Auger-Messier, M.; Escher, E.; Leduc, R.; Guillemette, G. Importance of N-glycosylation positioning for cell-surface expression, targeting, affinity and quality control of the human AT1 receptor. Biochem. J. 2005, 390, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Servant, G.; Dudley, D.T.; Escher, E.; Guillemette, G. The marked disparity between the sizes of angiotensin type 2 receptors from different tissues is related to different degrees of N-glycosylation. Mol. Pharmacol. 1994, 45, 1112–1118. [Google Scholar]
- Servant, G.; Dudley, D.T.; Escher, E.; Guillemette, G. Analysis of the role of N-glycosylation in cell-surface expression and binding properties of angiotensin II type-2 receptor of rat pheochromocytoma cells. Biochem. J. 1996, 313, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Qian, H. Association of -Arrestin 1 with the Type 1A Angiotensin II Receptor Involves Phosphorylation of the Receptor Carboxyl Terminus and Correlates with Receptor Internalization. Mol. Endocrinol. 2001, 15, 1706–1719. [Google Scholar] [CrossRef]
- Kule, C.E.; Karoor, V.; Day, J.N.E.; Thomas, W.G.; Baker, K.M.; Dinh, D.; Acker, K.A.; Booz, G.W. Agonist-dependent internalization of the angiotensin II type one receptor (AT1): Role of C-terminus phosphorylation in recruitment of β-arrestins. Regul. Pept. 2004, 120, 141–148. [Google Scholar] [CrossRef]
- Chen, K.; Fu, C.; Chen, C.; Jose, P.A.; Zeng, C. Role of GRK4 in the Regulation of Arterial AT1 Receptor in Hypertension. J. Am. Soc. Hypertens. 2016, 10, e3. [Google Scholar] [CrossRef]
- Olivares-Reyes, J.A.; Smith, R.D.; Hunyady, L.; Shah, B.H.; Catt, K.J. Agonist-induced Signaling, Desensitization, and Internalization of a Phosphorylation-deficient AT1A Angiotensin Receptor. J. Biol. Chem. 2001, 276, 37761–37768. [Google Scholar] [CrossRef]
- Zhang, F.; Lei, L.; Huang, J.; Wang, W.; Su, Q.; Yan, H.; Chen, C.; Zheng, S.; Ren, H.; Li, Z.; et al. G-protein-coupled receptor kinase 4 causes renal angiotensin II type 2 receptor dysfunction by increasing its phosphorylation. Clin. Sci. 2022, 136, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, P.C.; Lanctot, P.M.; Auger-Messier, M.; Escher, E.; Leduc, R.; Guillemette, G. S-nitrosylation of cysteine 289 of the AT1 receptor decreases its binding affinity for angiotensin II: S -nitrosylation of the AT1 receptor. Br. J. Pharmacol. 2006, 148, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Chun, J.N.; Godo, S.; Wu, G.; Shimokawa, H.; Jin, C.Z.; Jeon, J.H.; Kim, S.J.; Jin, Z.H.; Zhang, Y.H. ROS and endothelial nitric oxide synthase (eNOS)-dependent trafficking of angiotensin II type 2 receptor begets neuronal NOS in cardiac myocytes. Basic Res. Cardiol. 2015, 110, 21. [Google Scholar] [CrossRef]
- Sica, D.A.; Gehr, T.W.B.; Ghosh, S. Clinical Pharmacokinetics of Losartan. Clin. Pharmacokinet. 2005, 44, 797–814. [Google Scholar] [CrossRef]
- Barber, M.N.; Sampey, D.B.; Widdop, R.E. AT2 Receptor Stimulation Enhances Antihypertensive Effect of AT1 Receptor Antagonist in Hypertensive Rats. Hypertension 1999, 34, 1112–1116. [Google Scholar] [CrossRef]
- Ewert, S.; Laesser, M.; Johansson, B.; Holm, M.; Aneman, A.; Fandriks, L. The angiotensin II receptor type 2 agonist CGP 42112A stimulates NO production in the porcine jejunal mucosa. BMC Pharmacol. 2003, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Wallinder, C.; Plouffe, B.; Beaudry, H.; Mahalingam, A.K.; Wu, X.; Johansson, B.; Holm, M.; Botoros, M.; Karlén, A.; et al. Design, Synthesis, and Biological Evaluation of the First Selective Nonpeptide AT2 Receptor Agonist. J. Med. Chem. 2004, 47, 5995–6008. [Google Scholar] [CrossRef]
- Carey, R. Role of the angiotensin AT2 receptor in blood pressure regulation and therapeutic implications. Am. J. Hypertens. 2001, 14, S98–S102. [Google Scholar] [CrossRef]
- Sumners, C.; de Kloet, A.D.; Krause, E.G.; Unger, T.; Steckelings, U.M. Angiotensin type 2 receptors: Blood pressure regulation and end organ damage. Curr. Opin. Pharmacol. 2015, 21, 115–121. [Google Scholar] [CrossRef]
- Zizzo, M.G.; Caldara, G.; Bellanca, A.; Nuzzo, D.; Di Carlo, M.; Serio, R. PD123319, angiotensin II type II receptor antagonist, inhibits oxidative stress and inflammation in 2, 4-dinitrobenzene sulfonic acid-induced colitis in rat and ameliorates colonic contractility. Inflammopharmacology 2020, 28, 187–199. [Google Scholar] [CrossRef]
- Wagenaar, G.T.M.; Sengers, R.M.A.; Laghmani, E.H.; Chen, X.; Lindeboom, M.P.H.A.; Roks, A.J.M.; Folkerts, G.; Walther, F.J. Angiotensin II type 2 receptor ligand PD123319 attenuates hyperoxia-induced lung and heart injury at a low dose in newborn rats. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, L261–L272. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, F.; Vincent, J.-M.; Limiñana, P.; Chillon, J.-M.; Capdeville-Atkinson, C.; Atkinson, J. Effects of suboptimal doses of the AT1 receptor blocker, telmisartan, with the angiotensin-converting enzyme inhibitor, ramipril, on cerebral arterioles in spontaneously hypertensive rat. J. Hypertens. 2010, 28, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- Budzyn, K.; Sobey, C.G.; Drummond, G.R. Reduced cerebrovascular remodeling and functional impairment in spontaneously hypertensive rats following combined treatment with suboptimal doses of telmisartan and ramipril: Is less really more? J. Hypertens. 2010, 28, 1384–1389. [Google Scholar] [CrossRef] [PubMed]
- Bosnyak, S.; Jones, E.S.; Christopoulos, A.; Aguilar, M.-I.; Thomas, W.G.; Widdop, R.E. Relative affinity of angiotensin peptides and novel ligands at AT1 and AT2 receptors. Clin. Sci. 2011, 121, 297–303. [Google Scholar] [CrossRef]
- Teixeira, L.B.; Parreiras-e-Silva, L.T.; Bruder-Nascimento, T.; Duarte, D.A.; Simões, S.C.; Costa, R.M.; Rodríguez, D.Y.; Ferreira, P.A.B.; Silva, C.A.A.; Abrao, E.P.; et al. Ang-(1-7) is an endogenous β-arrestin-biased agonist of the AT1 receptor with protective action in cardiac hypertrophy. Sci. Rep. 2017, 7, 11903. [Google Scholar] [CrossRef]
- Wei, H.; Ahn, S.; Shenoy, S.K.; Karnik, S.S.; Hunyady, L.; Luttrell, L.M.; Lefkowitz, R.J. Independent β-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc. Natl. Acad. Sci. USA 2003, 100, 10782–10787. [Google Scholar] [CrossRef]
- Violin, J.D.; DeWire, S.M.; Yamashita, D.; Rominger, D.H.; Nguyen, L.; Schiller, K.; Whalen, E.J.; Gowen, M.; Lark, M.W. Selectively engaging β-arrestins at the angiotensin II type 1 receptor reduces blood pressure and increases cardiac performance. J. Pharmacol. Exp. Ther. 2010, 335, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, P.B.; Carini, D.J.; Chiu, A.T.; Duncia, J.V.; Price, W.A.J.; Wells, G.J.; Wong, P.C.; Johnson, A.L.; Wexler, R.R. The discovery of a new class of highly specific nonpeptide angiotensin II receptor antagonists. Am. J. Hypertens. 1991, 4, 275S–281S. [Google Scholar] [CrossRef]
- Vanderheyden, P.M.L.; Fierens, F.L.P.; De Backer, J.P.; Fraeyman, N.; Vauquelin, G. Distinction between surmountable and insurmountable selective AT1 receptor antagonists by use of CHO-K1 cells expressing human angiotensin II AT1 receptors: Insurmountable antagonism of candesartan. Br. J. Pharmacol. 1999, 126, 1057–1065. [Google Scholar] [CrossRef]
- De Gasparo, M.; Whitebread, S. Binding of valsartan to mammalian angiotensin AT1 receptors. Regul. Pept. 1995, 59, 303–311. [Google Scholar] [CrossRef]
- McClellan, K.J.; Markham, A. Telmisartan. Drugs 1998, 56, 1039–1044; discussion 1045–1046. [Google Scholar] [CrossRef]
- Dudley, D.T.; Summerfelt, R.M. Regulated expression of angiotensin II (AT2) binding sites in R3T3 cells. Regul. Pept. 1993, 44, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Kassmann, M.; Nickel, S.; Zhang, C.; Alenina, N.; Anistan, Y.M.; Schleifenbaum, J.; Bader, M.; Welsh, D.G.; Huang, Y.; et al. Myogenic Vasoconstriction Requires Canonical Gq/11 Signaling of the Angiotensin II Type 1 Receptor. J. Am. Heart Assoc. 2022, 11, e022070. [Google Scholar] [CrossRef]
- Jean-Charles, P.-Y.; Kaur, S.; Shenoy, S.K. G Protein–Coupled Receptor Signaling Through β-Arrestin–Dependent Mechanisms. J. Cardiovasc. Pharmacol. 2017, 70, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Viswanathan, G.; Sellig, M.; Jassal, C.; Choi, I.; Garikipati, A.; Xiong, X.; Nazo, N.; Rajagopal, S. β-Arrestin–Mediated Angiotensin II Type 1 Receptor Activation Promotes Pulmonary Vascular Remodeling in Pulmonary Hypertension. JACC Basic Transl. Sci. 2021, 6, 854–869. [Google Scholar] [CrossRef] [PubMed]
- Boerrigter, G.; Soergel, D.G.; Lark, M.W.; Burnett, J.C. TRV120027, a Novel Beta-Arrestin Biased Ligand at the Angiotensin II Type I Receptor, Unloads the Heart and Maintains Renal Function When Added to Furosemide in Experimental Heart Failure. J. Card. Fail. 2011, 17, S63–S64. [Google Scholar] [CrossRef]
- Kanki, H.; Sasaki, T.; Matsumura, S.; Yokawa, S.; Yukami, T.; Shimamura, M.; Sakaguchi, M.; Furuno, T.; Suzuki, T.; Mochizuki, H. β-arrestin-2 in PAR-1-biased signaling has a crucial role in endothelial function via PDGF-β in stroke. Cell Death Dis. 2019, 10, 100. [Google Scholar] [CrossRef]
- Bouressam, M.; Lecat, S.; Raoul, A.; Gaucher, C.; Perrin-Sarrado, C.; Lartaud, I.; Dupuis, F. S-nitrosoglutathione inhibits cerebrovascular angiotensin II-dependent and -independent AT1 receptor responses: A possible role of S-nitrosation. Br. J. Pharmacol. 2019, 176, 2049–2062. [Google Scholar] [CrossRef]
- Pinheiro, L.C.; Oliveira-Paula, G.H.; Ferreira, G.C.; Dal-Cin de Paula, T.; Duarte, D.A.; Costa-Neto, C.M.; Tanus-Santos, J.E. Oral nitrite treatment increases S-nitrosylation of vascular protein kinase C and attenuates the responses to angiotensin II. Redox Biol. 2021, 38, 101769. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).