Abstract
The food production industry is a significant contributor to the generation of millions of tonnes of waste every day. With the increasing public concern about waste production, utilizing the waste generated from popular fruits and vegetables, which are rich in high-added-value compounds, has become a focal point. By efficiently utilizing food waste, such as waste from the fruit and vegetable industries, we can adopt a sustainable consumption and production pattern that aligns with the Sustainable Development Goals (SDGs). This paper provides an overview of the high-added-value compounds derived from fruit and vegetable waste and their sources. The inclusion of bioactive compounds with antioxidant, antimicrobial, and antibrowning properties can enhance the quality of materials due to the high phenolic content present in them. Waste materials such as peels, seeds, kernels, and pomace are also actively employed as adsorbents, natural colorants, indicators, and enzymes in the food industry. Therefore, this article compiles all consumer-applicable uses of fruit and vegetable waste into a single document.
1. Introduction
The generation of food waste and by-products is viewed as a significant issue with adverse environmental, economic, and social effects. Food waste minimization is a widespread goal across the country. Despite global efforts, food insecurity remains prevalent. In 2020, the FAO estimated that approximately 721 to 811 million people would experience hunger, with 98% of them residing in developing countries [1]. Approximately 14% of the world’s food supply, valued at around $400 billion, is lost annually between harvest and commercialization [2]. Contrarily, it is estimated that 17% of food is lost or wasted during the retail and consumer stages [3]. Reducing food loss and waste is a crucial strategy for enhancing the efficiency, safety, quality, and sustainability of our food systems. The Food and Agriculture Organization (FAO) is committed to minimizing food waste throughout the production chain from harvest to sale [4]. Additionally, researchers must actively engage in recovering and revaluing crop waste by-products to develop innovations for use in the food industry. This approach can reduce food waste and create more resilient food systems.
During the processing of fruits and vegetables, a wide range of by-products are generated, including a significant quantity of waste in the juice industry. This waste includes leaves, peels, unwanted pulp, seeds, cull fruits, and stones [5,6], which contain high levels of bioactive substances such as polysaccharides (starch, cellulose, and pectin) and proteins [7,8]. Numerous studies have identified a variety of bioactive substances in fruit and vegetable by-products (FVBP)—including vitamin C and phytochemical compounds, such as flavonoids, anthocyanins, carotenoids, and phenolic acids [9,10,11]. Food waste has significant potential for producing edible films and coatings. Food-related biowaste can come from family cafeterias, industrial biowaste, decomposable plants, and botanical garden waste [12]. Packaging made from fruit and vegetable waste is a practical solution for reducing the manufacturing costs of edible films and coatings while adding value to food by-products. Additionally, the performance of the food packaging system can be improved through the different biological qualities of FVBP, such as dietary fibers, antioxidants, and antimicrobials [13,14,15,16].
Extracts of FVBP that contain antioxidant properties have been utilized to create innovative functional food products that are highly sought-after and well-received by consumers [17,18,19]. Furthermore, due to their bioactive properties that offer health benefits to humans, these natural additives can also be utilized in the food, biotechnology, and pharmaceutical industries [20]. However, their practical application is limited by their instability when exposed to regular food processing and storage conditions, such as changes in pH, temperature, light, oxygen, and ions. To address this challenge, an encapsulation method has recently been developed to enhance the stability and water solubility of these bioactive components while protecting them from external factors [21,22]. This review aims to highlight the value-added products that can be derived from fruits and vegetable waste, such as packaging, edible film, natural pigments, encapsulation, and the removal of toxic substances. Additionally, this review provides a comprehensive overview of the various extraction methods used to extract bioactive components from FVBP, including maceration, distillation, microwave-assisted extraction, supercritical fluid extraction, pressurized liquid extraction, pulsed electric field, and ultrasound-assisted extraction.
2. Bioactive Components in Fruits and Vegetables Waste
The increasing amount of agroindustrial waste has sparked numerous studies aimed at discovering bioactive compounds, especially in FVBP. These organic waste products contain affordable bioactive compounds (as listed in Table 1) that can be used to create innovative functional food ingredients and additives.
2.1. Fruit
The type of waste produced is generally determined by the fruit used. Fruit peels and seed characteristics are used to categorize them, which helps to determine the processing approach and necessary steps. Berry waste contains anthocyanins, which are responsible for their high total phenolic content and antioxidant activity, which can be affected by the solvent and extraction conditions. For example, blackberry pomace [23], blueberry juice [24]; blackberry, blueberry, and jaboticaba skin [25]; apple peels [26]; strawberry fruit peels [27]; raspberry pomace [28]; and red dragon fruit peels [29] can all be used to extract phenolic acid and anthocyanins compounds. Bioactive components like carotenoids, phenolic acids, and flavonoids can commonly be found in tomato skin [30], pumpkin seeds and peel [31], papaya peel [32], and melon peel [33], and their presence in fruit waste provides antioxidant properties. They can serve as natural preservatives and colorants/indicators in food applications.
El-Beltagi et al. [34] employed agroindustrial waste of dried red beetroot peel extract (DRBP) to evaluate its total phenolic compound, flavonoids, and betalains contents as well as its antioxidant capacity. Additionally, the extract was used as a natural preservative in preserving the quality of Nile tilapia fish filet stored at 5 °C for ten days. Researchers discovered that the DRBP aqueous extract contained compounds with potential antioxidant activity, including a high concentration of TPC (832 mg/100 g), flavonoids (234 mg/100 g), and betalains (535 mg/100 g), making it a suitable natural preservative agent. Fernando et al. [35] recovered the waste materials of red beetroot juice by converting it into high-value chemicals containing betalain, phenolic acid, and flavonoid compounds. The data show that the dried pulp of red beetroot produced a high bioactive yield via ultrasound-assisted extraction. The findings also reported that betalains degraded quickly at room temperature compared to −20 °C. In contrast, the temperature affected phenolic acids and antioxidative activity less.
García-Cayuela et al. [36] also extracted the same compounds of phenolic acids, flavonoids, and betalains from the whole fruit, pulp, and peel of six Mexican and Spanish prickly pear (Opuntia ficus-indica L. Mill.) cultivars. The highest concentration of betacyanins (1372–2176 μg/g dry whole fruit) was obtained from the purple cultivars of Spanish Morada and Mexican Pelota, whereas the highest amount of betaxanthin (435–488 μg/g dry whole fruit) was found in red Spanish Sanguinos and yellow Mexican Diamante cultivars. Additionally, the Spanish Morada cultivar has also been reported to have the greatest phenolic content of 49,012 μg/g dry peel.
Recently, Lončarić et al. [37] extracted phenolic, anthocyanins, and flavonol from blueberry pomace using green extraction methods such as high voltage electrical discharges (HVED), pulsed electric field (PEF), and ultrasound-assisted extraction (UAE). Antioxidant activity (0.83 mmol TE/g dw) and total polyphenols content (TPC) (10.52 mg of gallic acid equivalent (GAE) per g of dry weight (dw)) were both maximized through PEF-assisted extraction in the ethanol-based solvent. Meanwhile, PEF-assisted extraction produced the highest yields of anthocyanin (1757.32 g/g of dw) and flavanol (297.86 g/g of dw) in the methanol-based solvent. The highest yields of phenolic acid (625.47 g/g of dw) and flavonol (157.54 g/g of dw) were obtained in the ethanol-based solvent. These outcomes indicated PEF as a promising green extraction method that can increase the polyphenol extraction yield from blueberry pomace. Gulsunoglu et al. [38], on the other hand, extracted phenolic and flavonoid contents and further enhanced the compounds via fermentation with Aspergillus spp. The highest levels of total phenolics (1440 ± 37 and 1202 ± 88 mg GAE/100 g dm) and flavonoid contents (382 ± 47 and 495 ± 19 mg CE/100 g dm) were obtained from A. niger ZDM2 and A. tubingensis ZDM1, respectively. The antioxidant activity of apple peel fermented with these species was 3 and 5 times higher, respectively, than that of the unfermented sample, as measured by the CUPRAC and DPPH methods.
2.2. Vegetable
A considerable amount of waste is generated throughout the cultivation, marketing, and processing of vegetables. This waste is particularly noticeable in the production of various vegetable products such as canned vegetables [39], vegetable juice [40], vegetable concentrate [41], vegetable jam [42], and vegetable-based fermented drinks [43]. As with fruit, the characteristics of vegetable products vary depending on the type of vegetable utilized. Moreover, vegetables can be grouped into categories based on the part of the plant that is used for food preparation. Some plants can even be classified in multiple categories since they have more than one edible part, such as beetroots, which can be consumed as both roots and leaves.
In a study by Lucera et al. [44], vegetable waste from broccoli and artichokes was utilized as a source of phenolic acids and flavonoids. These compounds were extracted and used to fortify cheese samples, resulting in a significant increase in antioxidant capacity. Another study by Salas-Millán et al. [45] explored the potential of broccoli stalk waste as a novel fermented health food using its own microbiota. The natural fermentation process was carried out at 25 °C for 6 days, followed by 6 days of storage at 4 °C. The highest levels of phenolic acids and flavonoids were observed on the third day of fermentation. Chitrakar et al. [46] extracted compounds from asparagus waste and turned them into powder to assess their impact on chip properties. Their findings indicated that the inclusion of asparagus waste led to a significant increase in total chlorophyll (161%) and total phenolic (46%) and total flavonoid (79%) contents. Meanwhile, Zhang et al. [47] isolated compounds from Asparagus Officinalis roots sourced from Chinese and New Zealand cultivars.
In a recent study by Sagar et al. [48], the flavonoid and total phenolic content of onion skin from 15 Indian cultivars were quantified. The results showed that the cv. “NHRDF Red” cultivar had the highest total phenolic and flavonoid contents, while cv. “Bhima Shubhra” had the lowest, demonstrating maximum and minimum antioxidant capacity for cv. “NHRDF Red” and cv. “Bhima Shubhra,” respectively. The study also concluded that the onion skin of cv. “Hissar-2” and cv. “NHRDF Red” were the best sources of flavonoids and natural antioxidants. Similarly, Milea et al. [49] utilized yellow onion waste to create ingredients with multifunctional activities. They obtained the extract through green solvent aqueous extraction of flavonoids, resulting in a total flavonoid content of 50.21 ± 0.09 mg quercetin equivalent (QE)/g dry weight (DW) and antioxidant activity of 250.81 ± 6.76 mM Trolox/g DW. Additionally, Joly et al. [50] extracted phenolic and flavonoid compounds from potato waste using maceration and heating-assisted extraction. The quantitative growth of these compounds was facilitated by heating-assisted extraction. The study also found that under the specified operation conditions, the total phenolic content of unpeeled potato samples was higher than that of peeled samples, indicating that the total phenolic content accumulated in the skin tissue.
In addition, Vaz et al. [51] investigated the waste of artichoke, red pepper, carrot, and cucumber to obtain concentrates of phenolic acids, flavonoids, and other dietary fibers. Their results showed that artichoke waste had the highest concentration of phenolic compounds at 8340.7 mg/kg, while red pepper, carrot, and cucumber had much lower concentrations at 304.4 mg/kg, 217.4 mg/kg, and 195.7 mg/kg, respectively. In another study, phenolic, flavonoid, anthocyanin, and carotenoid components were extracted from potato peel and flesh from four potato varieties. The concentrations of total phenolic, flavonoids, and anthocyanins were 1.58, 2.91, and 1.46 times higher, respectively, in the peel compared to the flesh. Antioxidant activity measurements using FRAP, DPPH, and ABTS assays were also 1.64, 2.19, and 1.66 times higher in the peel than in the flesh, respectively [52]. Moreover, Doulabi et al. [53] extracted phenolic, flavonoid, and anthocyanin compounds from eggplant peel waste using microwave-assisted extraction. They found that a higher microwave power, low ethanol concentrations, and liquid–solid ratios resulted in a greater yield of bioactive compounds and increased the total anthocyanin, phenolic, and flavonoid contents.

Table 1.
Bioactive compounds from fruit and vegetable waste.
Table 1.
Bioactive compounds from fruit and vegetable waste.
| Fruits and Vegetable Waste | Bioactive Compounds | Extraction Method | References |
|---|---|---|---|
| Fruits waste | |||
| Blackberry pomace | Phenolic acids, anthocyanins | Pressurized liquid extraction (PLE), supercritical fluid extraction (SFE) | [23] |
| Blueberry (Vaccinium ashei) juice | Hot water bath and steam pretreatments | [24] | |
| Blackberry, blueberry and jaboticaba skin | - | [25] | |
| Apple peels | - | [26] | |
| Strawberry fruit peel | - | [27] | |
| Raspberry pomace | Solid phase extraction (SPE) | [28] | |
| Red dragon fruit peels | Methanol extraction | [29] | |
| Tomato skin | Carotenoids, phenolic acids, and flavonoids | Supercritical CO2 extraction (SC-CO2) | [30] |
| Pumpkin seeds, peel, flesh | Aqueous phase extraction | [31] | |
| Papaya peel | Ultrasound assisted extraction (UAE) | [32] | |
| Melon peel | - | [33] | |
| Red beetroot peel extract | Phenolic acids, flavonoids, betalains | Maceration technique | [34] |
| Red beetroot juice | Ultrasound assisted extraction (UAE) | [35] | |
| Pulp and peel of prickly pear (Opuntia ficus-indica L. Mill) tissues | Single extraction | [36] | |
| Blueberry pomace extract | Phenolic acids, anthocyanins, flavonol | High voltage electrical discharges (HVED), pulsed electric field (PEF), ultrasound-assisted extraction (UAE) | [37] |
| Apple peels | Phenolic, flavonoids | - | [38] |
| Vegetable by-products | |||
| Broccoli and artichokes waste | Phenolic, flavonoids | Methanolic extraction | [44] |
| Broccoli waste (stalk) | - | [45] | |
| Asparagus leaf waste | - | [46] | |
| Asparagus Officinalis roots | - | [47] | |
| Onion skin | Pressurized hot water extraction | [48] | |
| Yellow onion skin | Hot water extraction | [49] | |
| Potato waste | Maceration, hot water extraction | [50] | |
| Artichoke, red pepper, carrot, and cucumber waste | Ultrasonic processor | [51] | |
| Potato peel | Phenolic, flavonoids, anthocyanins, carotenoids | - | [52] |
| Eggplant peel | Phenolic, flavonoids, anthocyanins | Microwave-assisted extraction (MAE) | [53] |
3. Extraction Methods
Many bioactive compounds can be found in fruit and vegetable waste. To separate, identify, characterize, and extract these compounds in the proper way, it is crucial to know where they come from and what methods can be used for a particular plant matrix. Extraction technique is necessary to extract the desired bioactive component from a complex plant matrix, to enhance the sensitivity and selectivity of the analytical approach, to change the bioactive compound into an identification and separation form, and to have reproducible extraction methods. The bioactive compound can be extracted from fruit and vegetable waste using conventional and nonconventional techniques [54]. Maceration and hydrodistillation, microwave-assisted extraction, supercritical fluid extraction, pressurized liquid extraction, and pulsed electric field are examples of extraction techniques of bioactive compounds.
3.1. Maceration and Hydrodistillation
This method is one of the extraction techniques used for the recovery of bioactive compounds. Alrugaibah et al. [55] observed the efficacy of natural deep eutectic solvents (NDES) in extracting procyanidins and anthocyanins from cranberry pomace with the assistance of ultrasounds. The study demonstrates that the NDES of a certain composition have greater extraction efficiency and more selectivity for procyanidins or anthocyanins from cranberry pomace than 75% ethanol. The NDES 2 with choline chloride:betaine hydrochloride:levulinic acid (1:1:2) and 32 mL water/100 mL NDES extracted the maximum concentration of procyanidins (32.5 mg/g). The most anthocyanins were extracted from NDES 8, which was made up of glucose:lactic acid (1:5) and 20 mL/100 mL water. The amount extracted was 1.58 mg/g, which was 1.79-fold as much as the yield from 75%. The greatest extraction yields predicted by artificial neural networking (ANN) and response surface methodology (RSM) were comparable to the experimental yields, indicating that artificial neural networks are an alternative or superior predictive modeling method to RSM. Wu et al. [56] extracted essential oils from grape and pomelo peel using hydrodistillation combined with electrofluidic pretreatment. Using a variety of approaches, the authors reported a 43% increase in the essential oil recovery yield from grape peel and a 93% increase from pomelo peel. Due to the breakdown of cell contents, fluid impedance decreased during the IEF pretreatment phase, and essential oil yield decreased slightly as frequency increased. Askin [57] compared the aroma profiles of essential oils extracted using hydrodistillation from orange peel waste. Some components—such as 1-nonanol, β-citral, E-2-decanol, and o-hydroxybiphenyl—which were negligible in oils obtained from fresh peels, were found to be significantly elevated in oils obtained from MW400.
Ramli et al. [58] extracted the Citrus hystrix (Kaffir lime) and Ananas comosus (pineapple) peels using maceration techniques. Compounds such as citronellal, linalool, citronellol, terpinen-4-ol, isoPulegol, and -cubebene were found during phytochemical screening of C. hystrix peels. Meanwhile, benzene, 1,3-bis (phenoxyphenoxy), 2-furancarboxaldehyde, 5-methyl-, 2-furancarboxal-dehyde, 5-(hydroxymethyl)-, and 4H-pyran-4-one, 2,3-dihydro-3, 5-dihydroxy-6-methyl were identified in A. comosus peel. Cvetanović et al. [59] also optimized the maceration extraction of tannins from Tamarindus indica L. seed. RSM revealed the optimal extraction parameters to be a methanol concentration of 69.99 percent, an extraction temperature of 23.38 °C, and a solvent-to-sample ratio of 1:20. Using the HPLC-ESI-MS/MS technology, the extract obtained under optimal circumstances was chemically characterized. Coelho et al. [60] extracted bioactive compounds from mango peel liqueurs (Mangifera indica L.) using the maceration technique. Maceration with pectinase produced liqueurs with a greater concentration of quercetin-3-O-glucopyranoside. In regard to mango varieties, the bioactive content of Haden liqueurs was higher than that of Tommy Atkins liqueurs. The liqueurs had a high level of antioxidant activity.
3.2. Microwave-Assisted Extraction
Microwave-assisted extraction (MAE) is a technology that utilizes microwave energy to extract phytochemicals. Carbone et al. [61] extracted kiwi juice pomace for the recovery of natural antioxidants through microwave-assisted extraction (MAE). On the extraction yield of total polyphenols, temperature (T) was the most impactful factor, followed by solid-to-solvent ratio € and solvent compositi€ (C), although extraction€me (E) did not have a significant linear effect (TPs). The total polyphenols (TPs) yield varied from 1.30–4.87 mg GAE g−1 dw. Doulabi et al. [53] also evaluated the extraction of bioactive compounds from eggplant peel waste by using microwave-assisted extraction methods. The extraction yield, TAC, TPC, and TFC rose significantly (p < 0.01) in parallel with the microwave power increase and the usage of lower ethanol concentrations and lower liquid–solid ratios. The maximum predicted extraction yield (3.27%), TPC (1049.84 µg GAE/mL), TFC (130.40 µg QE/mL), and TAC (6.99 mg/L), as well as the minimum predicted IC50 (0.52 mL/mg), were obtained simultaneously when power, extraction time, liquid–solid ratio, ethanol concentration, and pH of solvent were 269.82 W, 7.98 min, 5.01 mL/g, 73.49%, and 3.06%, respectively. Overall, effective extraction of bioactive components from eggplant peels was possible under optimal microwave settings. Kurtulbaş et al. [62] used the MAE method to extract bioactive components from peach waste. Based on the results obtained, the highest yields were obtained with an 80% ethanol solvent system at 500 W microwave power for 90 s. Under deep freezer (−20 °C) conditions, the total phenols, anthocyanins, and main phenolic components (p-hydroxybenzoic acid and p-coumaric acid) had calculated shelf lives of 111, 107, 88, and 83 days, respectively.
Furthermore, da Rocha and Noreña [63] extracted bioactive compounds from grape pomace using the MAE and UAE methods. The results showed that for both methods of extraction, the amounts of total phenolic compounds and antioxidant activity measured by ABTS and DPPH increased over time, and the best extraction condition was a microwave at 1000 W for 10 min. However the amount of phenolic compounds and antioxidant activity were lower than when exhaustive extraction was done with acidified methanol solution. Lasunon et al. [64] studied the effect of the MAE method on bioactive compounds from tomato waste. For hydrophilic parts, the best extraction conditions were 180 W for 90 s and 450 W for 30 s, which gave the highest total phenolic compound content (280.10 mg GAE/100 g) and total flavonoid content (9832.52 mg CE/100 g DM). The condition of MAE which yielded the high bioactive compound would exhibit lower antioxidant activity. Araújo et al. [65] extracted the bioactive molecules from avocado seeds by using the MAE method. The optimized extracts made with acetone and ethanol had a high polyphenolic content (307.09 ± 14.16 and 254.40 ± 16.36 mg GAE/g extract) and high antioxidant activity as measured by DPPH (266.56 ± 2.76 and 221.69 ± 20.12 mg ET/g extract), ABTS (607.28 ± 4.71 and 516.34 ± 11.81 mg ET/g extract), and ORAC (475.55 ± 47.82). MAE is a green, energy-efficient, and quick way to extract bioactive components from avocado seeds without affecting their antioxidant activity. This makes it a good alternative to traditional protocols in the herbal industry and medicine.
3.3. Supercritical Fluid Extraction
Supercritical fluids are commonly used in business as an alternative to solvent extraction and are considered “environmentally friendly” due to the removal of harmful solvents from operations. In this regard, Pellicano et al. [30] optimized supercritical fluid extraction (SPE) to extract bioactive compounds from tomato skin waste. After 80 min at a pressure of 550 bar, the greatest oil output of 79% was obtained. This oleoresin was determined to be the richest in carotenoids, with lycopene and β-carotene concentrations of 0.86 and 1.5 mg/100 g, respectively. Naringenin was the most abundant flavonoid found, with a maximum level of 84.04 mg/kg DW in oleoresin extracted at 550 bar, followed by caffeic acid (26.60 mg/kg DW). Kupnik et al. [66] extracted bioactive constituents from pomegranate peel waste by using a supercritical fluid extraction method. The LC-MS/MS approach was used to identify and quantify phenolic compounds in selected extracts. The contents of various flavonoids and phenolic acids were determined. At 20 MPa, SFE extract had the highest total polyphenol concentration (11,561.84 µg/g), with ellagic acid (7492.53 µg/g) being the most abundant. Madhumeena et al. [67] investigated the SPE method for the extraction of bioactive components from pineapple waste. The ferulic acid was then quantified using high performance liquid chromatography (HPLC). Ferulic acid is a hydroxycinnamic acid that can be transformed into vanillic acid (flavor compound). Ferulic acid was discovered to be present in 0.76968/100 g of pineapple peel powder.
Soldan et al. [68] extracted oleoresin from Capsicum annuum industrial waste using a supercritical fluid extraction method. Result shows that the total mass yields obtained by SFE varied between 9.38 and 10.08 %. The extracts contained bioactive substances, such as phenolics, flavonoids, fatty acids, and carotenoids, but possessed insufficient antioxidant activity. De Andrade Lima et al. [69] also used SFE to extract carotenoids from vegetable waste. From the results found in the research, the total carotenoid recovery (TCR) was greater than 90% w/w for most samples, and the most highly extracted compound was β-carotene (TCRs 88–100% w/w). According to the findings, the optimized SFE conditions can be utilized as a universal model for carotenoid extraction from various fruit and vegetable matrices, as well as a viable strategy for adding value to these waste streams by producing carotenoid-rich extracts. Romano et al. [70] extracted bioactive compounds from citrus peel using a supercritical CO2 method. When 20% ethanol was utilized as a cosolvent in both liquid (at 20 MPa and 20 °C) and supercritical (at 30 MPa and 60 °C) CO2 extraction, the best yields were obtained. Furthermore, the extracts prepared with liquid CO2 + 20% ethanol contained the most naringin (35.26, 44.05, and 19.86 mg g−1 in orange, tangerine, and lemon peel extracts, respectively) and terpenes, particularly limonene. These results indicate that extraction with a liquid CO2 and ethanol mixture could be a viable alternative to standard solvent extraction, using 80% less organic solvent and yielding extracts with good antiradical capacity and high volatile organic compound content.
3.4. Pressurized Liquid Extraction
Pressurized liquid extraction (PLE) is a method of solid–liquid extraction that uses pressurized liquids as the extractant solvent at elevated temperatures and pressures below their critical point. The PLE applies high pressures to the solvent, causing it to remain liquid past its typical boiling point [71]. Garcia et al. [72] investigated the recovery of bioactive compounds from pomegranate (Punica granatum L.) peel using pressurized liquid extraction (PLE). Based on the result obtained by the researchers, the TPC and punicalagin contents of PPE-PLE were 164.3 ± 10.7 mg GAE/g DW and 17 ± 3.6 mg/g DW, respectively. PPE-PLE demonstrated promising results in terms of AMA and cytotoxicity. Indeed, the AMA was impacted by the polyphenol content and other extracted bioactive components. Santos et al. [23] used feijoa (Acca sellowiana (O. Berg) Burret) peel to extract bioactive compounds via low- and high-pressure techniques. In this study, PLE proved to be the best extraction method for phenolics and antioxidants. Bioactive compounds such as ferulic, gallic and ellagic acid were identified using liquid chromatography–mass spectrometry (LC-ESI-MS/MS) as the major phenolic compounds found in feijoa peel extracts. Lasta et al. [73] applied the PLE technique for the recovery of phenolic compounds from beetroot waste. This study found that some of the abundant phenolic compounds, such as ferulic acid, vitexin, and sinapaldehyde, were obtained from the extracts. These findings suggest that the PLE technique may be a viable option for extracting phenolic compounds from beets that have a high biological potential and might be used in formulations for the nutraceutical, functional food, or pharmaceutical industries.
Andrade et al. [74] used ultrasound-assisted pressurized liquid method to extract anthocyanins from Aronia melanocarpa pomace. The extraction is performed in continuous mode to enhance the yields and the extraction yield was found to be increased by 19%. In a 200 W ultrasonic bath, optimal conditions of 70 °C, 180 bar, and a solvent content of 1.5% wt. citric acid was attained. These settings resulted in anthocyanin extraction of 88.0% by weight in 45 min. Pukalskiene et al. [75] recovered bioactive compounds from strawberry (Fragaria x ananassa) pomace using pressurized liquid extraction. The most prevalent elements in strawberry pomace extracts were quercetin-3-glucuronide; kaempferol-3-glucuronide; and tiliroside, ellagic, malic, succinic, citric, and p-coumaric acids. For the first time, the cytotoxicity, antiproliferative, and cellular antioxidant activities of strawberry pomace extracts obtained by PLE were reported. The PLE-EtOH extract demonstrated the greatest antiproliferative efficacy with no cytotoxicity. Both PLE extracts had a high antioxidant capacity and protected Caco-2 cells from stress. It is reasonable to believe that the biological activities of the extracts are related to the presence of the flavonoids (anthocyanins, catechins) and phenolic acids discovered and quantified in this study (ellagic, coumaric). Guzmán-Lorite et al. [76] compared pressurized liquids with high intensity focused ultrasounds for the extraction of proteins from pomegranate seed waste. Based on the findings, pressurized liquid extraction methods gained higher levels of proteins, peptides, and phenolics. Other interesting chemicals were discovered in addition to phenolic compounds, which were strongly coextracted via PLE using proteins such as azelaic acid.
3.5. Pulsed Electric Field
Pulse electric field (PEF) extraction is a nonthermal, green, and selective extraction technology that does not require a large amount of energy and that has been shown to be effective in short extraction times [77]. PEF has mostly been used in the food sector to reduce microbial growth/pasteurization and to improve the extraction of phytochemicals from fruit and vegetable industry waste [78]. Lakka et al. [79] evaluated pulsed electric field polyphenol extraction from Vitis vinifera, Sideritis scardica, and Crocus sativus. The finding shows that PEF treatment enhanced the recovery in total polyphenols for all the three plants examined. A considerable increase was observed in each plant examined and PEF condition used, albeit lower electric field intensities up to 1.4 kV/cm proved to be more beneficial. Total polyphenol content increased by 49.15%, 35.25%, and 44.36%, respectively, at the optimum electric field strengths of 1.4 kV/cm for V. vinifera and 1.2 kV/cm for S. scardica and C. sativus. Tehrani et al. [80] extracted valuable compounds in onion (Allium cepa L.) waste using a pulsed electric field (PEF). TPC, DPPH, FRAP, quercetin, and extraction yield were 48.912 ± 6 mg/kg, 50.366 ± 1%, 465.414 ± 5 µmFe2/l, 31.761 ± 0.5 mg/100 g, and 88.107 ± 1% respectively. The findings suggested that PEF could be a highly successful approach for continuous extraction of natural chemicals. Hwang et al. [81] also used Citrus unshiu peel waste for the recovery of hesperidin and narirutin using PEF method. Hesperidin concentration peaked at 46.96 ± 3.37 mg/g peel (dry basis) after PEF treatment at 120 s combined with SWE at 150 °C for 15 min, while narirutin concentration peaked at 8.76 ± 0.83 mg/g after PEF treatment at 120 s along with SWE at 190 °C for 5 min. Both hesperidin and narirutin concentrations increased with PEF treatment time. The PEF improved the extraction of hesperidin and narirutin by 22.1% and 33.6%, respectively. This work shows that PEF pretreatment can improve the SWE of flavonoids from C. unshiu peel.
Andreou et al. [82] applied pulsed electric field technique to enhance the product yield and waste valorisation in industrial tomato processing. According to the results obtained, The PEF treatment reduced peel detachment work by 72.3% without affecting ultimate tomato firmness. After PEF processing, the highest amount of tomato juice that could be made was 20.5% more than from an untreated sample. PEF at 5 kV/cm enhanced carotenoid extraction by 56.4% (14.31 mg lycopene/100 g tomato waste). The extraction of phenolic compounds from tomato waste increased (56.16 mg GA/kg) at PEF conditions of 2 kV/cm and 700 pulses. Lakka et al. [83] enhanced the recovery of polyphenols from Rosa canina, Calendula officinalis, and Castanea sativa using PEF method. The PEF approach appeared effective in enhancing polyphenol extraction from all three plant materials examined. In the case of Rosa canina fruits, the application of a moderate to high electric field, up to 1.4 kV/cm, boosted total and individual polyphenol recovery to 63.79% and 84%, respectively. Extraction of high-added-value compounds via PEF from olive leaves has been performed by Pappas et al. [84]. Using a pulse duration of 10 μs, the greatest PEF effect was recorded for aqueous ethanol at a concentration of 25% v/v. The total polyphenols increased by 31.85%, whereas the particular metabolites increased by 265.67%. The recovery of polyphenols was discovered to be dependent on the solvent, the duration of the treatment pulse, and the structure of the extracted metabolites.
3.6. Ultrasound Assisted Extraction
Ultrasound-assisted extraction (UAE) is a useful method of extraction that works better than traditional extraction. Some of the benefits of UAE are that it takes less time, is easier to use, uses less solvent, keeps temperatures down, saves energy, and increases yield. UAE has the chance to improve extraction yields through cavitation and better mass transfer [85]. Pectin is found in the cell wall and middle lamella of many plants, including fruits and vegetables. This has led many researchers to try to get pectin from waste peel, pomace, and rind of plants. Fruit peels, such as passion fruit [86], orange [87], banana [88], grapefruit [89,90,91,92], mango [93], and pomegranate [94]; vegetables, such as eggplant [95] and jackfruit [96]; and other parts, such as grape pomace [97], tomato waste [98], durian rind [99], and sunflower head [100] have been used to extract pectin. Pomegranate peel, grape pomace, tomato waste, grapefruit peel, orange peel, and eggplant peel have all produced pectin yields in excess of 25% when processed using UAE. Ultrasonic factors including frequency, power, duty cycle, power intensity, sonication duration, and extraction variables like temperature, LSR (liquid (solvent) to solid ratio), and pH of solvent all have a role in determining the final pectin yield either alone or via interactive effects. Several results have proven that UAE can boost pectin output and shorten the time required for extraction. Wang et al. [101] compared chemical extraction with UAE extraction of pectin from grapefruit peel and found that UAE extraction produced a greater yield (16.34%) and reduced extraction time by 37.7%.
Guandalini et al. [102] did the UAE of pectin from the residue of phenolic compound extracted mango peel and found a 50% increase in yield (from 5.61 to 8.6). The yield of UAE pectin from rehydrated mango peel increased by 31% (from 6.2 to 8.1) compared to pectin extraction without ultrasound without affecting the quality of pectin. Similarly, Oliveira et al. [86] observed a 1.6-fold increase in the pectin yield via UAE of passion fruit peel over chemical extraction. Grassino et al. [98] found that conventional extraction of pectin at 60 °C gave the highest yield but that 15 min UAE produced higher-quality pectin. For extraction at 80 °C, the yield of pectin was the same after 24 h of traditional extraction and 15 min of UAE. This shows very clearly that UAE speeds up the extraction process. Characterization of pectin extracted through the UAE has been performed to find out about its quality and properties. Eggplant peel, grape pomace, passion fruit peel, sunflower head, and grapefruit peel have all been used to obtain high-methoxyl pectin (degree of esterification >50%). A chosen frequency between 30 and 40 kHz has been used to extract anthocyanin out of grape waste [103], pectin out of tomato waste [98], hemicellulose out of grape pomace [104], and oil out of papaya seed [105]. The choice of a constant low frequency may have to do with the idea that fewer cavitation bubbles with larger diameters favor a large cavitation effect, which decreases as the frequency of the ultrasound keeps increasing [106].
Brahmi et al. [107] optimized the conditions for ultrasound-assisted extraction of phenolic compounds from Opuntia ficus-indica [L.] Mill. flowers. From the study, UAE was statistically more effective than traditional extraction (maceration and Soxhlet extraction) methods at removing bioactive chemicals. UAE extracted 24.4 ± 0.82 mg of total phenolics as mg gallic acid equivalent (GAE)/g of dry weight (DW). The UAE procedure significantly increased the yield and decreased the extraction time compared to conventional methods (maceration and Soxhlet extraction). González-Silva et al. [108] extracted phenolic compounds from Psidium cattleianum leaves using the UAE method, and their results were optimized using response surface methodology (RSM) by assessing the effect of time of extraction (XET = 2, 4, and 6 min), sonication amplitude (XSA = 60, 80, and 100%), and pulse cycle (XPC = 0.4, 0.7, and 1 s). According to the RSM, the optimal parameters for extracting the maximum soluble polyphenol content and yield from UAE (158.18 mg/g dry matter [DM] and 15.81%) comprise a sonication amplitude of 100% for four minutes at a pulse cycle of 0.6 s. Using the response surface approach, the conditions for ultrasonic aided extraction (UAE) of phenolic chemicals from rye bran (RB) were optimized by Iftikhar et al. [109]. The best conditions for UAE were time: 29 min, temperature: 66 °C, and solid-to-solvent ratio: 1:45 (g/mL), yielding a total phenolic content of 245.74 mg GAE/100 g dry weight. UAE resulted in a greater yield of total phenolic and flavonoid components than conventional extraction (CE), but only a minor difference was observed in the total flavonol and proanthocyanidin contents. In every antioxidant test, the UAE extract had more antioxidant activity (DPPH, FRAP, ABTS, reducing power, and H2O2).
4. Valorization of Fruits and Vegetable Waste into Valuable Applications
In parallel with the food waste reduction strategies, recovery and revaluing of crop wastes have been actively conducted. The valorization of crop wastes are discussed in detail in the subsections below.
4.1. Antioxidant
The food sector is becoming increasingly influential in the outcome of novel biomaterials featuring antioxidant characteristics as it has the advantage of enhancing the shelf life of food products [110]. Compounds that are rich in polyphenols, such as phenolic acids and flavonoids, have been reported to have excellent antioxidant capacity [111], as those compounds can scavenge the reactive oxygen species (ROS) present in the food products [112]. Recently, Rangaraj et al. [113] developed an active gelatin film incorporated with date fruit syrup waste extract (DSWE) as an antioxidant additive for food storage studies. The active gelatin/DSWE blend films show a high release profile of the active phenolic compounds and higher antioxidant capacity in an aqueous food medium when compared to lipid-based food stimulants. Venturi et al. [114] used potato peels, which contain bioactive compounds and have higher antioxidant power to enhance the qualitative parameters of fresh-cut apples. During storage, a substantial anti-browning effect and a slowing of the softening of fruits were identified. The observed results indicate that potato extracts are suitable as antioxidant supplements for fresh-cut fruits, hence minimizing the usage of hazardous chemicals. Kurek et al. [112,115] created a chitosan and pectin film mixed with blackcurrant pomace powder to reduce the losses of food production, and this film is focused on food coating and wrappers. Based on the research, the antioxidant activity of the film was increased 30-fold. This film is suitable to be used as active or intelligent packaging films for antioxidant activity of fresh produce.
Kam et al. [116] utilized durian leaf extract in gelatin-based film for antioxidant activity enhancement of biodegradable film as active packaging. This research revealed that gelatin-based film with added 0.5% crude extract of durian leaf has 17.6 times higher DPPH scavenging activity than the negative control sample. Gelatin film with 0.5% durian leaf extract reduced the oxidation of palm oil at a rate that was three times lower. Deshmukh et al. [117] also employed guar gum/carboxymethyl cellulose incorporated with litchi shell waste extract (GCH/LSE) for antioxidant active packaging. According to the results obtained by the researchers, GCH/LSE 20% shows the highest antioxidant activity, which is 91.52%. Active GCH/LSE films are good at maintaining roasted peanuts’ oxidative stability. The GCH/LSE films were proven to be effective antioxidant packaging for foods with a high lipid content. Orqueda et al. [118] tested an active edible film using red chilto (Solanum betaceum) peel and seed to reduce the oxidation of salmon filets during storage. Based on the experiment conducted, samples collected from chilto peel had the highest concentration of phenolic compounds. Antioxidant pectin-based film can be found in S. betaceum fruit peel, and it successfully shows remarkable antioxidant properties that protect the salmon filets.
Ribeiro et al. [119] created pectin–phenolic antioxidant films from mango peels for food packaging applications. Result shows that film with aqueous or methanolic extracts provides higher antioxidant activity based on the inhibition of the DPPH radical. The extracts, on the other hand, increased elongation and the water vapor barrier and also provided films with a greater antioxidant capacity, making them promising materials as active food packaging/coating, particularly for food products susceptible to lipid oxidation, such as edible nuts, fruits, and breakfast cereals. Merino et al. [120] developed a highly antioxidant bioplastic film from avocado peels and seeds. The findings suggest that combining hydrolysis, plasticization, and pectin blending is critical for obtaining materials with competitive mechanical properties, biodegradability, excellent oxygen barrier properties, high antioxidant activity, optical clarity, and component migration suitable for food contact applications. The created materials, which stand out for their antioxidant activity, natural composition, and ecologically safe technique of creation, thereby constitute an appropriate and sustainable alternative to conventional nonbiodegradable plastic food packaging materials. A new active coating was developed using Cucumis metuliferus (CM) fruit-extract-loaded acetate cellulose coating for antioxidant active packaging [121]. To assess the total phenolic components and antioxidant activity of CM, the extraction procedure was initially optimized. Release studies of CM from the cellulose acetate layer to a fatty food simulant verified the antioxidant efficiency of bilayer packaging.
Gaikwad et al. [13] created a biocomposite film made of polyvinyl alcohol and apple pomace that possesses antioxidant qualities and may be used in active food packaging applications for the storage of soybean oil. They discovered that the inclusion of apple pomace into PVA films increased the overall phenolic content and the antioxidant capabilities of the films in delaying lipid oxidation. Meanwhile, Matta et al. [122] created an active edible film of methylcellulose (MC) containing extracts of green apple (Granny Smith) skin by incorporating ethanolic extracts of freeze-dried apple skin (EEFD) and aqueous extracts of apple skin (AES). The results indicate that including extract of green apple skin into MC films increases the films’ overall phenolic contents and antioxidant capabilities. The potential for developing these films into functional packaging materials for food to ensure quality and safety and broaden the shelf life of packaged foods is therefore significant, and it represents an attractive alternative to the conventional packaging materials in terms of food safety and shelf life extension. Similarly, Han and Song [123] conducted a study on the antioxidant properties of mandarin (Citrus unshiu) peel pectin films comprising sage (Salvia officinalis) leaf extract for environmentally and biodegradable food packaging in which the biopolymer films were made from pectin derived from mandarin peel, a waste of fruit processing. Antioxidant activity of the film increased as the amount of sage leaf extract increased, which indirectly proved the antioxidant properties of sage leaf extract.
Furthermore, Saberi et al. [14] utilized pea starch and guar gum (PSGG) to produce food packaging that was incorporated with natural antioxidants comprising of epigallocatechin gallate (EGCG), blueberry ash (BBA) fruit extract, macadamia (MAC) peel extract, and banana (BAN) peel extract. The findings showed that EGCG-PSGG films had the most antioxidant power, followed by BBA-PSGG, MAC-PSGG, and BAN-PSGG films, at all concentrations (0.75–3 mg mL−1) when measured using DPPH radical scavenging ability assay, cupric reducing antioxidant (CUPRAC), and ferric reducing activity power (FRAP). Inclusion of natural additives has been demonstrated to improve the moisture barrier of the films. Shahbazi [124] combined ethanolic red grape seed extract and Ziziphora clinopodioides essential oil into chitosan and gelatin films to enhance the antibacterial, antioxidant, physical, and mechanical properties of the chitosan (Ch) and gelatin (Ge) films. The two most important chemicals in the ZEO are carvacrol and thymol, which provide antibacterial and antioxidant properties for ZEO. In addition, mango peel extract (MPE) has revealed its robust free-radical-scavenging capabilities and was combined with fish gelatin films to improve the physical, barrier, mechanical, and antioxidant capabilities of the developed film [125].
Interestingly, Melo et al. [126] integrated three chemical fractions derived from mango kernels to create active films: mango kernel starch (MKS) as a matrix, mango kernel fat (MKF), and phenolic extract (MKPE). MKPE was added to give the films active qualities such as primarily antioxidant and UV absorber. The active films are especially appealing for use as bags, pouches, or coatings for oxidizable foods. Additionally, Moghadam et al. [127] created edible antioxidant films using mung bean protein and pomegranate peel. The current study effectively enhanced mung bean protein-based edible films with varying amounts of pomegranate peel as a natural bioactive chemical to develop an antibacterial and antioxidant packaging system. Pomegranate peel, a low-cost by-product of the food industry, has the potential to improve the bio-functional qualities of mung bean protein film. Rodsamran and Sothornvit [128] developed a pectin fraction from pineapple peel to serve as biopolymer films. As expected, TPC and antioxidant activity against DPPH and ABTS radicals in pectin films rose significantly when the PPS to water ratio increased in both stimulation media. The results demonstrated that the whole pineapple peel pectin extract solution (PPS) can be used as a natural plasticizer to produce pectin films with enhanced film properties, particularly in terms of the water vapor barrier and antioxidant properties, and that this research has the potential to become an effective active film or coating for food applications.
Additionally, Vargas-Torrico et al. [129] created gelatin/carboxymethylcellulose active films with Hass avocado peel extract as berry preservation packaging. According to their findings, avocado extract gave active films antifungal and antioxidant properties and altered the physicochemical characteristics of active films. Talón et al. [130] created edible films with a polyphenol from thyme extract (TE) as an antioxidant incorporated into chitosan and starch matrix. Inclusion of TE in the polymer matrix has been shown to improve the mechanical properties of the film as well as providing great antioxidant capacity. The findings also suggested that these antioxidant films may be used for coating applications to extend the shelf life of items susceptible to oxidative processes. Moreover, Urbina et al. [131] employed 100% apple-waste-derived multicomponent films as antioxidants in developing an active packaging. The researcher creates nano papers (NPs) with higher hydrophobicity and antioxidant activity from bacterial cellulose. PHA coatings with varying concentrations of apple extract (e) with antioxidant activity were also applied to the nano papers (NP/PHA-e). The integration of apple extract in the PHA coating supplied free radical scavenging capability to the films since the extracts retained their antioxidant potential after the films were processed. Kurek et al. [11] examined blueberry and red grape skin extract incorporated with chitosan (CS) and carboxymethyl cellulose (CMC) as an antioxidant film. The result showed that blueberry and red grape skin pomace extracts gives a great antioxidant activity. The summary of fruits and vegetables waste used as antioxidant in food shown in Table 2.

Table 2.
Usage of fruits and vegetables waste as antioxidant in food applications.
4.2. Antimicrobial
As a result of recent worldwide food-borne microbial outbreaks, researchers have been looking for novel approaches to preventing microbial growth in food without compromising its flavor, texture, or safety. Use of antimicrobial packaging is one strategy that gives consumers more peace of mind about the quality and security of their food. Therefore, the use of polymers or antimicrobial agents to create barrier-enhanced or active packaging materials is a promising strategy for preventing the growth and dissemination of microorganisms in food as summarize in Table 3. Recently, Ali et al. [16] employed pomegranate peel particles (PGP) mixed with starch as antimicrobial films which can also be used as food grade packaging material. PGP suppressed the development of both gram-positive (S. aureus) and gram-negative (Salmonella) bacteria. The research showed that created films displayed good antibacterial properties against both S. aureus and Salmonella. It was observed that as the concentration of PGP increased, so did the inhibition zone of the films against the targeted bacteria. PGP was significantly more effective against S. aureus than it was against Salmonella. Comparably, Hanani et al. [132] investigated fish gelatin films’ antibacterial characteristics as active packaging using pomegranate (Punica granatum L.) peel powder (PPP). Inclusion of PPP increased the physicochemical properties of the films as well as providing antibacterial capabilities. One of the most sensitive bacteria to the active film was determined to be Staphylococcus aureus (S. aureus), followed by Listeria monocytogenes (L. monocytogenes) as well as Escherichia coli (E. coli). For S. aureus, the most significant inhibitory zone (7.00 mm) was detected surrounding the film containing 5% PPP. These findings indicate that fish gelatin incorporating PPP has tremendous potential as a compelling film with antibacterial capabilities and can help maintain food quality and extend shelf life. Saleem and Saeed [133] studied the orange (Citrus sinensis L.), yellow lemon (Citrus limonia Osbeck), and banana (Musa acuminata) peel extracts as wide-range natural antimicrobial agents for agrowaste minimization. This study discovered that gram-negative bacteria are more sensitive to the extracts, with Klebsiella pneumoniae (gram-negative) bacteria exhibiting the highest sensitivity to the yellow lemon peel extract and the largest zone of inhibition.
Jodhani and Nataraj [134] utilized aloe vera gel and lemon peel extract to maintain the shelf life of banana (Musa spp.). The antifungal properties of lemon peel extract have been successfully tested on Colletotrichum musae, a decay-causing pathogen in bananas. The coating extended the shelf life of bananas up to 9 days without any disease incident. The combination effect on aloe vera and lemon peel essential oil effectively reduced quality losses of bananas without the use of chemical preservatives and hazardous fungicides. Meydanju et al. [135] developed a biodegradable film from lemon peel powder combined with xanthan gum (XG) and TiO2–Ag nanoparticles to inhibit the growth of Escherichia coli and Staphylococcus aureus. The synergistic effect of XG/TiO2-Ag and lemon peel powder has been reported to increase the growth of inhibition zone diameter. Additionally, Alparslan and Baygar [136] utilized orange (Citrus sinensis [L.] Osbeck) peel and combined it with chitosan films to monitor and evaluate the shelf life of deepwater pink shrimp. The antimicrobial properties of the combined film were tested on Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Candida albicans. The combination of chitosan film and OPEO (CH+OPEO) was effective in extending the shelf life of fresh shrimp to 15 days, whereas the only chitosan-coated (CH) group had a shelf life of 10 days, and the uncoated samples (control) only had a shelf life of 7 days.
Furthermore, Agdar et al. [137] evaluated the effectiveness of the salep gum coating containing orange peel essential oil in inhibiting the microbial growth on fish filets stored in refrigerated temperature for 16 days. The combined film prevented the growth of total aerobic mesophilic, coliforms, psychrophilic, and lactic acid bacteria. Additionally, the shelf life of fish filets was extended to 12 days when coated with salep gum containing 0.5% orange peel essential oil. Ramli et al. [138] observed the antibacterial activity of durian (Durio zibethinus) seed and peel extracts for effective shelf life enhancement of preserved meat. In terms of antibacterial characteristics, studies indicate that seeds have promising antimicrobial properties against E. coli, P. vulgaris, S. marcescens, and S. aureus and greater inhibitory zone size than peeling. Mayeli et al. [139] innovated a coating layer enriched with zein and sour orange peel extract to act as an antibacterial coating for refrigerated fish. The developed coating reduced the number of Enterobacteriaceae, Pseudomonas spp., and psychrotrophic bacteria during the storage period. The combined effect of the coating layers extended fish freshness by an average of 12 days. Shukor et al. [140] innovated antimicrobial packaging films using jackfruit peel waste for the shelf life of cherry tomatoes. The samples were tested for 7 days and the antimicrobial activity was done using Escherichia coli and Staphylococcus aureus. Based on the results gained, both Escherichia coli and Staphylococcus aureus were inhibited by films containing 10 wt% thymol. The application of these active films to actual food demonstrated that cherry tomato samples exhibited a considerable reduction in fungus growth.

Table 3.
Usage of peel waste as antimicrobial in food applications.
Table 3.
Usage of peel waste as antimicrobial in food applications.
| Source of Waste | By-Product | Targeted Food Products | Extraction Method | Antimicrobial Effects | References |
|---|---|---|---|---|---|
 Pomegranate | Peel | - | Homogenization |
| [16] |
 Pomegranate | Peel | - | Methanol extraction |
| [132] |
| Orange/Yellow lemon/Banana | Peel | - | Homogenization |
| [133] |
 Lemon | Peel | Banana | Homogenization |
| [133,135] |
 Orange | Peel | Deepwater pink shrimp, fish filets, fish | Methanolic and ethanolic extraction |
| [136,137,139] |
 Durian | Peel | Meat | Rotary evaporation method |
| [138] |
 Jackfruit | Peel | Cherry tomato | Homogenization |
| [140] |
4.3. Antibrowning
The browning reaction, caused by an enzyme, is a serious issue with fresh produce. It alters the food’s quality in an unfavorable way, shortens its shelf life, and decreases its value on the market. Chemicals, especially sulfites, can inhibit enzymes responsible for browning. Reports indicate, however, that it poses health risks to consumers. Recently, Thivya et al. [141] discovered a new alternative to prevent the browning of fresh-cut apples and potatoes by proposing an active packaging composed of sodium alginate (SA) and carboxymethyl (CMC) along with shallot onion waste extract (SOWEs). Shallot onion waste extracts were reported to have an antibrowning effect due to their high content of polyphenols and antioxidant activity. The incorporation of SOWEs in SA/CMC active packaging has successfully inhibited the enzymatic reaction of polyphenol oxidase that is responsible for enzymatic browning in fruits and vegetables. Similarly, Tinello et al. [142] inactivated polyphenol oxidase enzyme using juices and distillates extracted from different onion varieties (white, yellow, and red) and inner layers of Borettana onion wastes. The inhibitory action of the extracted anti-browning agents was tested on commercial mushroom tyrosinase. The studies not only inhibited browning action but also extended the shelf life and improved the sensory properties of the food products. Interestingly, Liang et al. [143] successfully inhibited browning action in fresh-cut asparagus lettuce using extractable condensed tannins (ECTs) extracted from durian shells. The inhibitory action is predominant due to the presence of B-type procyanidins, propelargonidins and prodelphinidins, as well as a low degree of 3-O-galloylation, which are potent compounds that inhibit tyrosinase activity in fruits and vegetables.
Furthermore, Jirasuteeruk and Theerakulkait [144] investigated the anti-browning properties of mango peel extract from different varieties of mangoes. It was reported that mango extract from the Chok-Anan cultivar showed to have greater inhibition action compared to the Nam-Dok-Mai and Kaew cultivars. Chok-Anan mangoes exhibited high phenolics content and therefore inhibited polyphenol oxidase to a greater extent than the other two cultivars. Faiq and Theerakulkait [145] also reported that papaya peel crude extract has high bioactive phenolic compounds and hence has successfully inhibited browning reaction in potatoes, apples, and bananas. Martínez-Hernández et al. [146] produced an antibrowning agent from tomato skin which has a very high lycopene content. Fresh-cut apples were dipped in the lycopene microspheres, which successfully controlled the browning reaction after 9 days at 5 °C. Tinello et al. [147] utilized the juices from unripe grapes to serve as antibrowning and antioxidant agents to dried “Golden Delicious” apple slices. The appearance of the treated apple slices showed good results, demonstrating that the antibrowning agent of unripe grape juices effectively inhibited polyphenol oxidase activity. The usage of fruits and vegetables waste as antibrowning agents was summarized in Table 4.

Table 4.
Usage of fruits and vegetables waste as antibrowning agents in food applications.
4.4. Adsorbents
There is an alarming level of contamination in the aqueous medium, atmosphere, and geosphere due to the large number of toxic and poisonous chemicals released into our environment by various industries. Heavy metal pollution of the aqueous medium is a growing environmental crisis. Particularly problematic in treating industrial wastewater are toxic metals like zinc, mercury, lead, chromium, cadmium, nickel, and copper. Most of them cause cancer and are not biodegradable; they also form aggregates in living organisms. In order to safely remove pollutants and metallic elements from industrial effluents and wastewater, considerable in-depth industrial research has been conducted. As a result, several forms of fruit waste could be utilized to absorb or eliminate hazardous compounds, such as pesticides, heavy metals, and dyes.
Hussain et al. [148] study the flavonoids, total phenolics, and antioxidant properties of agricultural wastes and their capacity to eliminate pesticide waste using banana peels. Banana peels contain chlorophyll, hemicellulose, pectin, cellulose, and other low-molecular-weight compounds with higher antioxidant activity and phenolic content. At a concentration of 9 g, banana peels successfully decreased diazinon by 63.86%, while parathion was lowered by 50.34%. This study shows that agricultural wastes effectively remove diazinon from water, and their usage is environmentally friendly. Maia et al. [149] created activated carbon using banana peel waste using NaOH and pyrolysis to remove methylene blue. The results revealed that the activated carbon derived from banana peel waste has a vast surface area, which is required to remove blue methylene. The maximum dye removal efficiency was 99.8% when the MB starting concentration was 25 mg/L, the sorbent was 0.03 g, and the contact duration was 60 min. Banana-peel-activated carbon coated with Al2O3-Chitosan was developed. Ramutshatsha-Makhwedzha et al. [150] tested this study for the adsorption removal process of cadmium and lead from wastewater and found it to be successful. The BPAC composite was proven reusable until the third cycle of adsorption–desorption (% Re > 80). Based on the data obtained, the produced material could remove Cd2+ and Pb2+ up to 99.9% of the time.
Wang et al. [151] investigated activated carbon generated using tangerine seed waste for high-performance carbamate pesticide adsorption of plants and water. A novel activated carbon produced from waste tangerine seed was effectively synthesized in this work. Batch adsorption investigations revealed that the pseudo-second-order kinetic model and the Langmuir isotherm model predicted TSAC adsorption behavior well. Bendiocarb, metolcarb, isoprocarb, pirimicarb, carbaryl, and methiocarb have adsorption capacities of 7.97, 9.11, 13.95, 39.37, 44.64, and 93.46 mg/g, respectively. Furthermore, this adsorption mechanism was both spontaneous and exothermic. Gnanasekaran et al. [152] used orange peel extract with 3D ZnO/SnO2 for the removal of chlorophenol effluent. The research found that the existing various (Zn2+, Sn4+, and Sn2+) states aided in postponing the transmission of electron–hole recombination to achieve photocatalytic chlorophenol degradation. In this study, a green 3D composite system is used to absorb the chlorophenol by 77.5% through photocatalysis. Aminu and Oladepo [153] created orange-peel-mediated synthesis of silver nanoparticles for the detection of mercury (II) ions. Their results found that the golden-brown AgNPs colloid solution turned colorless when applied to water. AgNPs show good sensitivity and selectivity for the colorimetric detection of mercury (II) ions with the limit of detection og 1.24 × 10−6 mol/L.
Hassan et al. [154] also used date pits for the removal of organophosphorus pesticide from water. Three types of date pits were used as adsorbents, including roasted date pits, activated date pits, and nanoactivated date pits. The observation shows that nanoactivated date pits have high removal percentage and removal capacity of profenofos from aqueous solution compared to roasted date pits and activated date pits. Mohammad and El-Sayed [155] created activated carbon peach stones for the removal of imidacloprid. The researcher used two types of activated carbon (PSAC 300 and PSAC 500). The result shows that both PSAC 300 and PSAC 500 were able to remove imidacloprid, with about 80% and 99% removal efficiencies, respectively. Overall, PSAC 500 exhibited the maximum adsorption capacity of 39.37 mg/g. Al-Ghouti and Sweleh [156] employed activated carbon (ACOS) from black and green olive stones to remove methylene blue (MB) in water successfully. It was also discovered that black activated carbon olive stones had the most significant N%, H%, and C% prior to adsorption. Furthermore, the highest absorption of methylene blue occurred at the optimal pH value of 10. Methylene blue adsorption capacities were 714 mg g−1 and 769 mg g−1 for black and green activated carbon olive stones, respectively.
Batool et al. [157] removed organochlorine pesticides (OCPs) using a zerovalent iron (Fe0) supported on biochar nanocomposite (Fe0-BRtP) from Nephelium lappaceum (Rambutan) fruit peel waste. The experiment found that Fe0-BRtP combined the advantages of adsorption and dechlorination of OCPs in aqueous solution and up to 96–99% removal was obtained within 120 min. The removal efficiency of regenerated Fe0-BRtP was 89–92% after being reused five times. This Fe0-BRtP nanocomposite could be used as a green and low-cost prospective material for adsorption and reduction of OCPs in aquatic environments. Shakoor and Nasar [158] used citrus limetta peel waste as a methylene blue dye adsorbent. Langmuir adsorption isotherm was found to be the best fit for the data. The maximum adsorption capacity for a monolayer coverage was found to be 227.3 mg/g. The results show that CLP is a very effective, low-cost method of removing dyes from wastewater. Salama [159] investigated cellulose grafted with soy protein isolate (SPI) to remove organic dyes from wastewater. This method exhibited adsorption capacity up to 454 mg/g. The efficiency of MB removal was 95% after four adsorption-desorption cycles. This method is a new sustainable, cost-effective, and reusable hybrid material which can successfully adsorb dyes in wastewater. Goksu and Tanaydin [160] devised a technique for removing crystal violet (CV) color that uses almond shells as an effective adsorbent, and according to the findings of this study, almond shells are effective adsorbents for extracting crystal violet (CV) from aqueous solutions. On almond shells, the excellent adsorption capacity was reported to be 1.075 mg g−1. According to the results of the adsorption experiment, almond shells might be utilized to effectively remove CV in aqueous solutions (Table 5).

Table 5.
Removal or adsorbent capacities of targeted analytes using fruits and vegetables waste.
4.5. Indicator in Packaging
Petrochemical-derived synthetic pigments have been widely employed in a variety of food items. However, these colors negatively impact human health, making it necessary for the scientific community to search for safer, natural, and eco-friendly pigments. Recently, the pigments industry has expanded fast due to its numerous applications in food. As a result, it requires sustainable pigments manufacturing from renewable bio-resources. The valorization of vegetal wastes can fulfill the needs of natural pigment production at the industrial scale for food, medicinal, and cosmeceutical uses (Table 6). Natural colors, such as anthocyanins, betalains, carotenoids, and chlorophylls, are abundant in these wastes [161]. Figure 1 below shows the source of natural pigments.
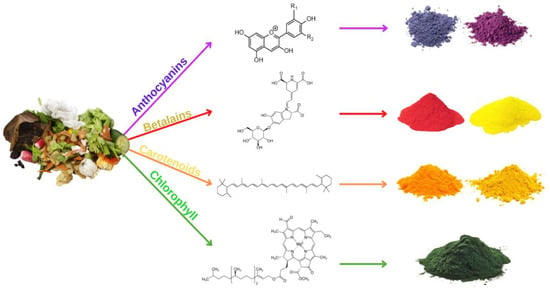
Figure 1.
Natural source of pigments.
4.5.1. Anthocyanins
Anthocyanins are vacuolar polyphenolic pigments that are naturally occurring, water-soluble, and nontoxic [162]. There are roughly 700 different anthocyanin structures recognized, and they have been found in red cabbage [163], black carrot [164], red radish [165], and purple sweet potatoes [166] as well as in blackcurrants [167], cherries [168], and berries [169]. Anthocyanins are flavonoids, and the most trivial anthocyanidins found in the majority of colored plants include pelargonidin, delphinidin, cyanidin, petunidin, peonidin, and malvidin [170]. Anthocyanins differ in hue due to structural changes in hydroxyl groups, their quantity, the location of sugar moieties, cultivar type, and agricultural conditions [171].
Recently, Santos et al. [172] developed an active intelligent packaging based on sodium alginate films incorporated with 40% of Clitoria ternatea extract (CTE) to monitor the quality of milk, pork, and shrimp. The proposed packaging showed a colorimetric potential as CTE is a pH sensitive compound and it changes color at different pH. The blue color of the film changed to purple and green when loaded with 40% CTE and used to evaluate the perishability of milk and meat products, respectively (shrimp and pork). Zhao et al. [173] also utilized waste from purple sweet potato peel extracts (PPE) and combined it with sodium alginate to produce a pH-sensitive intelligent indicator film. The designed film changes color from pink to blue in the presence of total volatile nitrogen, indicating the freshness level of chicken stored at 4 °C for 5 days and 25 °C for 60 h. Similarly, Abdillah et al. [174] developed a halochromic indicator film composed of arrowroot starch/iota-carrageenan incorporated with anthocyanins extracted from Kyoho skin (KSE) to monitor the freshness of shrimp. Indicator films incorporating natural KSE anthocyanin showed pH sensitivity by changing color from purple to red in an acidic environment, from purple to green in an ammonium environment, and from purple to yellow in highly alkaline conditions.
Esfahani et al. [175] innovated an intelligent edible film from cassava starch and pomegranate peel powder (PPP) to monitor the freshness of lamb meat. The intelligent edible film exhibited color changes from red to green when tested on lamb meat stored at 25 °C due to the presence of total volatile basic nitrogen (TVB-N) in meat. Additionally, Capello et al. [176] extracted anthocyanins from agricultural waste of jabuticaba fruit (Plinia cauliflora) and purple sweet potato (Ipomoea Batatas L.) peels to serve as colorimetric indicator films. At the recommended storage temperatures, changes in color from red to blue were clearly evident in both colorimetric indicator films used to track meat freshness. He et al. [177] proposed pH-responsive color absorbent pads from polyvinyl alcohol (PVA), agarose (AG), and purple sweet potato anthocyanins (PSPA) to evaluate the freshness of meat. It was found that the pads’ colors changed noticeably as the pH was adjusted from 3 to 10, with the pad containing 9% PSPA exhibiting the most distinctive color change from pink to green. As a result, the agricultural waste has promising future applications as a colorimetric indicator for fresh meat, which would significantly contribute to the maintenance of food safety and the enhancement of food storage quality.
4.5.2. Betalains
Regarding naturally occurring pigments, betalains are second only to anthocyanins and are primarily classified as betacyanins and betaxanthins [178]. These pigments give fruits and vegetable red-purple (betacyanin) and yellow-orange (betaxanthin) hues, respectively [179]. These chemicals are widely found as pigments in Beta vulgaris (beetroot) [180], Opuntia (prickly pears) [181], Hylocereus [182], and Mammillaria species [180]. Betalains are betalamic acid immonium conjugates containing amino groups and cyclic-dihydroxyphenylalanine (cyclo-DOPA) [183]. Betaxanthins are betalamic acid moieties synthesized with amino acids or amines. Condensation interactions between betalamic acid and cyclo-dopa produce betanidin, a betacyanin precursor. Betanidins have 29 distinct structures in nature due to glycosylation or acylation. Betanins have also been subjected to chemical reactions such as decarboxylation, deglycosylation, hydrolysis, isomerization, and dehydrogenation [184,185]. However, no published study specifies the number of betanin isomers or breakdown products.
Contemporarily, Ai et al. [186] developed a real-time intelligent film from film-forming substrates (FFS), glycerol, and red pitaya peel (RPP) extract to monitor the freshness level of pork. Betacyanins were extracted from red pitaya peel to act as a pH-sensitive indicator. In order to detect deterioration in the freshness of pork, the pH of the film was adjusted to 7.0. This film was more sensitive to ammonia than the original film, which had a pH of 4.3. During the deterioration process of pork, a change in film color was observed which correlated with the built-up total volatile base nitrogen. Kanatt [187] designed an active/intelligent packaging film constructed from polyvinyl alcohol (PVA) and gelatin incorporated with Amaranthus leaf extract (ALE) to evaluate the meat freshness of fish and chicken. Due to the rich bioactive compounds of ALE, the packaging provided antioxidants by minimizing oxidative rancidity, antibacterial by preventing microbial growth and intelligent properties to the film by showing evident color changes from red to yellow on the spoilage of the meat. Interestingly, the shelf life of the meat was extended up to 12 days.
Meanwhile, Ateteallah et al. [188] utilized beetroot juice containing betalains and carrot pulps as natural colorants to improve color and natural flavor and promote healthy constituents in the ice cream. Additionally, the addition of 5% of betalains to ice cream aids in improving total phenol and antioxidant properties of the ice cream. Hernández-Carranza et al. [189] utilized the peel and mucilage of red cactus pear to improve the color, total flavonoids, total phenolic compounds, total betalains, and antioxidant capacity of yogurt. The results reported that the betalains produce a nice magenta color in yogurt. Additionally, Fathordoobady et al. [182] utilized the peel of red dragon fruit (Hylocereus polyrhizus L.) and extracted betacyanin compound from it. Betacyanins were loaded into alginate microspheres and were characterized in terms of their encapsulation efficiency, particle size, uniformity, and morphology. Data demonstrated that alginate-loaded betacyanin extract can be a promising microcapsule that serves as a carrier for the delivery of antioxidants. Alginate-microsphere-loaded betacyanins have proven to be an efficient model for use as food colorant additives and for oral delivery.
4.5.3. Carotenoids
Carotenoids are essential natural pigments which are lipophilic and account for the red, yellow, or orange color spectrum in various fruits and vegetables. Carotenoids are found in all trans and cis isomers [177,190,191]. Carotenes (-carotene, -carotene, and lycopene) and xanthophylls (lutein, zeaxanthin, and -cryptoxanthin) are the primary types of carotenoids [192]. Although both carotenes and xanthophylls include phytochemical chains of hydrogen and carbon, the presence of hydroxyl groups that indicate oxygenated carotenoids in structure distinguishes them [193].
Gungor and Torun [194] extracted β-carotene from peels of pumpkins using the maceration, microemulsion, and ultrasound-assisted methods with sunflower oil as the solvent. Data reported that mayonnaise made with sunflower oil rich in β-carotene was well received sensory-wise, and it retained all of its other distinctive qualities. The results of yellow color and peroxide values demonstrated that β-carotene fortified mayonnaise was more resistant to oxidation during storage than the control mayonnaise. Sharma et al. [195] employed leftovers of the internal fluffy portion along with fibrous strands of ripe pumpkin to extract β-carotene pigment. The extracted carotene pigment can then be used as a natural food colorant in processed food products, increasing both the visual appeal and nutritional value of the product due to the role of β-carotene as a precursor of vitamin A. Lombardelli et al. [196] developed a novel food colorant of carotenoid-containing chromoplasts from the unsold tomatoes. The stability of carotenoid-containing chromoplasts has been studied, and the chromoplasts have been reported to retain the colorimetric parameters of red-orange pigments when stored at 4 °C and 25 °C in the dark.
4.5.4. Chlorophyll
Chlorophyll is a natural oil-soluble, amphiphilic green pigment found widely in plants [197]. Chlorophyll molecules have a lipophilic hydrocarbon tail and a hydrophilic (porphyrin) group head (phytol group) [198]. It is often considered insoluble in polar solutions because of its lipophilic hydrocarbon chains as a phytol tail [199]. Chlorophyll in plant meals is classified into chlorophyll a (blue-green) and chlorophyll b (yellow-green) [200]. Chlorophyll a has a methyl group (-CH3) at position 7-carbon, whereas chlorophyll b has an aldehyde group (-CHO) [201]. The demand for chlorophyll is constantly growing owing to increased knowledge of the usage of natural colorants and the health advantages they provide.
Aznury et al. [202] extracted chlorophyll from moringa leaf and incorporated it in yogurt, providing a green color. The higher the amount of moringa leaf extract, the darker the yogurt due to the high concentration of chlorophyll. Additionally, the addition of moringa leaf extract to yogurt samples has been reported to have a fairly high concentration of vitamin C and was highly preferred. Meanwhile, Rohajatien et al. [203] employed pegagan leaf (Centella asiatica L.) extract to improve the physical properties and antioxidant attributes of mochi ice cream. Addition of pegagan leaf extract produced yellowish-green mochi ice cream with an excellent antioxidant capacity. Molina et al. [204] extracted chlorophyll pigments from the aerial parts of carrot (Daucus carota L.) and tomato (Solanum lycopersicum var. cerasiforme). The findings reported that aerial parts of the tomato have higher chlorophyll concentration than carrot. The evaluated waste products showed promising results for use as sources of natural pigments with potential application in a variety of industrial fields as colorants, including the food, pharmaceutical, and cosmetic industries.

Table 6.
Fruits and vegetables waste as natural colorant/indicators in food applications.
Table 6.
Fruits and vegetables waste as natural colorant/indicators in food applications.
| Sources of Waste | Natural Pigments | Extraction Method | Function | Application | Results | References |
|---|---|---|---|---|---|---|
| Clitoria ternatea extract (CTE) | Anthocyanin | Homogenization | Freshness indicator | Milk, shrimp, and pork |
| [172] |
| Purple sweet potato peel extracts (PPE) | Anthocyanin | Homogenization | Freshness indicator | Chicken |
| [173] |
| Kyoho skin extract | Anthocyanin | Homogenization | Freshness indicator | Shrimp |
| [174] |
| Pomegranate peel powder | Anthocyanin | Homogenization | Freshness indicator | Lamb |
| [175] |
| Jabuticaba fruit (Plinia cauliflora) and purple sweet potato (Ipomoea Batatas L.) peels | Anthocyanin | Homogenization | Freshness indicator | Meat pieces |
| [176] |
| Purple sweet potato peel | Anthocyanin | Homogenization | Freshness indicator | Meat |
| [177] |
| Red pitaya peel | Betacyanins | Homogenization | Freshness indicator | Pork |
| [186] |
| Amaranthus leaf extract | Betalains | Homogenization | Freshness indicator | Fish and chicken |
| [187] |
| Beetroot juice | Betalains | Homogenization | Natural food colorant | Ice cream |
| [188] |
| Peel and mucilage of red cactus pear | Betalains | Hot water extraction | Natural food colorant | Yogurt |
| [189] |
| Peel of red dragon fruit (Hylocereus polyrhizus L.) | Betacyanins | Supercritical fluid extraction | Natural food colorant | - |
| [182] |
| Peels of pumpkin | β-carotene | Maceration, ultrasound-assisted extraction | Natural food colorant and antioxidant | Mayonnaise |
| [194] |
| Leftovers of the internal fluffy portion along with fibrous strands of ripe pumpkin | β-carotene | Homogenization | Potential food colorant | - |
| [195] |
| Unsold tomatoes | Carotenoid | Enzymatic-assisted extraction | Food colorant | - |
| [196] |
| Moringa leaf | Chlorophyll | Homogenization | Food colorant and functional food | Yogurt |
| [202] |
| Pegagan leaf extract | Chlorophyll | Homogenization | Food colorant and antioxidant | Mochi ice cream |
| [203] |
| Aerial parts of carrot (Daucus carota L.) and tomato (Solanum lycopersicum var. cerasiforme) | Chlorophyll | Maceration, ultrasound-assisted extraction | Potential colorant | - |
| [204] |
4.6. Enzymes
In the industrial sector, enzymes play a crucial role in reducing chemical loads, eliminating toxic substances, and reducing pollution [205]. Industrial enzymes currently have a market value of USD 5.9 billion, with a projected market value of USD 8.7 billion in 2026 and a compound annual growth rate of 6.5% [206]. A wide range of enzymes are commonly used in the industry, including lipases, carbohydrases, proteases, and polymerases [207]. Most of the waste produced by the food industry is lignocellulosic, which contains enzymes that are essential raw materials for production and processing [208]. As an example, bromelain, which is extracted from pineapple peel, can improve food digestion [209] and soften beef meat [210] and can be transformed into value-added products (Table 7).
Previously, Hussain et al. [211] extracted bromelain from pineapple core using the maceration extraction method. A maceration of chicken breast meat with 100% core extract shows an 86% reduction in hardness and pH decreases from 5.87 to 4.99. Meanwhile, Singh et al. [212] analyzed the effect of bromelain enzyme extracted from four different pineapple wastes—peel, stem, core, and crown—on tenderizing chicken and beef meat. Rizqiati et al. [213] investigated the effect of drying on cayenne pineapple crown enzyme characteristics—including protein content, activity units, and specific activity—in terms of moisture content, yield, and characteristics. The results obtained showed that the optimal drying temperature was 55 °C because it had the highest moisture content, protein content, and enzyme characteristics as well as the longest immersion duration for the best texture of meat. Lipases have been applied as food additives in the modification of taste in the food industry [214]. Recently, Okino-Delgado et al. [215] examined different varieties and fractions of orange wastes as sources of lipases. Among the fruit varieties studied, bagasse, peel, and frit lipases showed optimal pH values between 6.0 and 8.0 and optimal temperatures between 30 °C and 60 °C. Next, Tambun et al. [216] successfully produced fatty acids directly from avocado seeds by activating the lipase enzyme. The highest fatty acid content found was 11.67%. Furthermore, there is an enzyme called papain present in the bark, leaves, and fruit of the papaya plant [217].
Papain is extracted from fruit and stem latex can be utilized as a primary ingredient in brewing and winemaking [218]. Singla et al. [32] previously investigated the effects of microwave treatment and enzyme addition on ultrasound-assisted extraction (UAE) of bioactive compounds and antioxidant activity from papaya peels. Extracts prepared using UAE extraction were found to have antioxidant activity, indicating that they are useful for preparing plant extracts that contain antioxidants. Phothiwicha et al. [219] evaluated the bioactivity of papain extracted from Chaya and papaya stalks on beef meat. The texture and mastication of beef samples marinated with papain had a softer texture and were easier to chew after the marination. Amylases can therefore be used in a variety of industries, such as food, fermentation, and pharmaceuticals [220]. At present, Abdullah et al. [221] produced a safe sweetener in the form of glucose syrup using alpha amylase extracted from mango seed core, which can be used as an alternative to artificial sweeteners through enzymatic reactions. In a study which tested the amylase production ability of Bacillus subtilis K-18 (KX881940) by hydrolyzing potato peels as carbon sources, Mohtaq et al. [222] found that the bacterium can produce amylase as a result of starch hydrolysis.

Table 7.
Application of enzyme extract from fruit and vegetable waste in the food industry.
Table 7.
Application of enzyme extract from fruit and vegetable waste in the food industry.
| Enzyme | Fruits and Vegetable Waste | Extraction Method | Application | References |
|---|---|---|---|---|
| Bromelain and protease | Core and seed of pineapple and jackfruit | Homogenization | Increasing the tenderness of tough muscle in bovine meat | [210] |
| Bromelain | Pineapple core | Homogenization | Reduction in hardness of chicken meat | [211] |
| Peel, stem, core, and crown of pineapple | Deionized water | Tenderization of chicken and beef meats | [212] | |
| Pineapple crown | Homogenization | Improve meat texture | [213] | |
| Lipase | Bagasse, peel, and frit lipases from the orange | Inline extractor | Juice production | [215] |
| Avocado seed | Extract using n-hexane solvents | Production of fatty acids | [216] | |
| Papain | Papaya peel | Ultrasound assisted extraction | Source of bioactive compounds for food | [32] |
| Chaya and papaya stalks | Enzyme-assisted extraction | Halal beef tenderizer | [219] | |
| α-amylase | Mango seed core | Homogenization | Glucose syrup alternative substitute | [220] |
| Potato peel | Homogenization | Hydrolysis of starch to produce sugars | [222] |
5. Current Status, Opportunities and Prospects for Fruits and Vegetables
Fruits and vegetables are agricultural commodities that generate waste during post-harvest processing, distribution, and consumption. While some by-products are utilized as fuel, construction materials, or animal feed, most of it is discarded. However, many agricultural by-products have been found to possess good nutritional properties and can be used as functional foods and components, thereby reducing production costs and improving food sustainability. Utilizing fruit and vegetable waste to boost the nutritional content of functional foods can also help address the impending food security crisis. Although there are safety concerns surrounding the use of these by-products for human consumption, thorough evaluation of factors such as the source of the by-products, processing methods, potential contaminants, nutritional composition, and health benefits or risks can ensure the safety of valorized by-products [223,224] Adhering to food safety regulations and conducting thorough testing and analysis can unlock the potential of fruit and vegetable by-products while ensuring consumer safety.
However, innovative fruit and vegetable waste is currently unavailable in the market, which is a significant gap in today’s sustainability-focused world. The high-risk investment involved in fruit and vegetable waste recycling is one possible reason for this gap. Additionally, consumers may perceive by-products as unsafe or unacceptable for consumption, leading to reluctance to purchase food containing them. To develop a better ecosystem for valorization of fruit and vegetable waste, a stronger technoeconomic perspective is required with a focus on low-volume, high-value, and better-sustainability alternatives that utilize green methods for extraction. Opportunities for fruits and vegetables waste have expanded in recent years, including nutraceuticals, flavoring agents, bioactive chemicals in packaging, and waste nanoparticles. These waste-derived value-added substances can be ingested in modest quantities, offering additional advantages. Thus, it is crucial to explore promising technologies for effective fruit and vegetable waste valorization in the near future.
6. Conclusions and Future Perspectives
In recent years, there has been a growing interest in utilizing waste and by-products generated from agriculture and food manufacturing. Fruit and vegetable waste, which includes peel, skin, fruits, kernels, seeds, and leaves, offers a plethora of benefits—such as antioxidant, antimicrobial, and antibrowning properties—as well as bioactive chemicals that can be utilized in various applications. This review focuses on the potential utilization of food waste for its antioxidant, antimicrobial, antibrowning, adsorbent, natural pigment, and enzyme properties. The extraction of natural pigments requires innovative and economically feasible resources, suitable extraction techniques, advancements in bioprocessing technologies, new inventions in pigment stabilization, applicability of a technique for a wide range of pigments, the possibility of developing pigment-enriched food ingredients and bioactives, and a trend towards developing pigment-enriched foods and bioactives. While green environmental techniques can absorb toxic compounds, such as heavy metals and pesticides, future research should focus on addressing concerns such as incomplete degradation of hazardous substances, high operational costs, high energy requirements, lower efficiency, and the regeneration of bioadsorbents for future treatments. It is important to note that before employing functional compounds derived from FVBP, additional research, such as toxicological tests, is required to ensure that the constituent is free of pesticides and other harmful chemicals. Bioactivity research is also necessary to determine the bioaccessibility and bioavailability of phytochemicals derived from these wastes.
Author Contributions
N.M.N.‘A. and K.R. drafted the manuscript and collected the data. K.R. developed the framework and finalized and supervised the research. W.X.L.F. and J.M.V. conceived the study and participated in its design and coordination. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Universiti Malaysia Sabah, grant number GUG0559-1/2022.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank all the researchers involved in this project.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
FAO—Food and Agriculture Organization; FVBP—fruit and vegetable by-products; TPC—total polyphenols content; DRBP—dried red beetroot peel; HVED—high-voltage electrical discharges; PEF—pulsed electric field; UAE—ultrasound-assisted extraction; GAE—gallic acid equivalent; CUPRAC—cupric reducing antioxidant capacity; DPPH—2,2-diphenyl-1-picryl-hydrazyl-hydrate; FRAP—ferric reducing antioxidant power; ABTS—2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid); PLE—pressurized liquid extraction; SFE—supercritical fluid extraction; SPE—solid phase extraction; SC-CO2—supercritical CO2 extraction; NDES—natural deep eutectic solvents; ANN—artificial neural networking; RSM—response surface methodology; HPLC ESI-MS/MS—high-performance liquid chromatography/electrospray ionization tandem mass spectrometry; MAE—microwave-assisted extraction; LC-MS/MS—liquid chromatography with tandem mass spectrometry; HPLC—high-performance liquid chromatography; TCR—total carotenoid recovery; DSWE—date fruit syrup waste extract; ROS—reactive oxygen species; GCH/LSE—guar gum/carboxymethyl cellulose incorporated with lychee shell waste extract; EGCG—epigallocatechin gallate; BBA—blueberry ash; CMC—carboxymethyl cellulose; PEE—pulsed electric extraction; PGP—pomegranate peel particles; PPP—pomegranate peel powder; SA—sodium alginate; CMC—carboxymethyl; SOWE—shallot onion waste extract; ECTs—extractable condensed tannins; OCPs—organochlorine pesticides; Fe0—zerovalent iron; TVB-N—total volatile basic nitrogen; cyclo-DOPA—cyclic-dihydroxyphenylalanine.
References
- UNICEF. The State of Food Security and Nutrition in the World 2021; FAO Publisher: Rome, Italy, 2021. [Google Scholar]
- FAO, I. The State of Food and Agriculture 2019. Moving Forward on Food Loss and Waste Reduction; FAO Publisher: Rome, Italy, 2019; pp. 2–13. [Google Scholar]
- UNEP. Food Waste Index Report 2021. Available online: https://www.unep.org/resources/report/unep-food-waste-index-report-2021 (accessed on 15 September 2022).
- FAO. Food Loss and Waste 2022. Available online: https://www.fao.org/nutrition/capacity-development/food-loss-and-waste/en/ (accessed on 15 September 2022).
- de los Ángeles Fernández, M.; Espino, M.; Gomez, F.J.; Silva, M.F. Novel approaches mediated by tailor-made green solvents for the extraction of phenolic compounds from agro-food industrial by-products. Food Chem. 2018, 239, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Amaya-Cruz, D.M.; Rodríguez-González, S.; Pérez-Ramírez, I.F.; Loarca-Piña, G.; Amaya-Llano, S.; Gallegos-Corona, M.A.; Reynoso-Camacho, R. Juice by-products as a source of dietary fibre and antioxidants and their effect on hepatic steatosis. J. Funct. Foods 2015, 17, 93–102. [Google Scholar] [CrossRef]
- Bas-Bellver, C.; Barrera, C.; Betoret, N.; Seguí, L. Turning agri-food cooperative vegetable residues into functional powdered ingredients for the food industry. Sustainability 2020, 12, 1284. [Google Scholar] [CrossRef]
- Ni, N.; Dumont, M.J. Protein-based hydrogels derived from industrial byproducts containing collagen, keratin, zein and soy. Waste Biomass Valorization 2017, 8, 285–300. [Google Scholar] [CrossRef]
- Barros, H.D.; Baseggio, A.M.; Angolini, C.F.; Pastore, G.M.; Cazarin, C.B.; Marostica-Junior, M.R. Influence of different types of acids and pH in the recovery of bioactive compounds in Jabuticaba peel (Plinia cauliflora). Int. Food Res. J. 2019, 124, 16–26. [Google Scholar] [CrossRef]
- Machado, A.P.D.; Rezende, C.A.; Rodrigues, R.A.; Barbero, G.F.; Rosa, P.D.V.E.; Martínez, J. Encapsulation of anthocyanin-rich extract from blackberry residues by spray-drying, freeze-drying and supercritical antisolvent. Powder Technol. 2018, 340, 553–562. [Google Scholar] [CrossRef]
- Kurek, M.; Hlupić, L.; Garofulić, I.E.; Descours, E.; Ščetar, M.; Galić, K. Comparison of protective supports and antioxidative capacity of two bio-based films with revalorised fruit pomaces extracted from blueberry and red grape skin. Food Packag. Shelf Life 2019, 20, 100315. [Google Scholar] [CrossRef]
- Ardolino, F.; Parrillo, F.; Arena, U. Biowaste-to-biomethane or biowaste-to-energy? An LCA study on anaerobic digestion of organic waste. J. Clean. Prod. 2018, 174, 462–476. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Lee, J.Y.; Lee, Y.S. Development of polyvinyl alcohol and apple pomace bio-composite film with antioxidant properties for active food packaging application. J. Food Sci. Technol. 2016, 53, 1608–1619. [Google Scholar] [CrossRef]
- Saberi, B.; Vuong, Q.V.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Physical, barrier, and antioxidant properties of pea starch-guar gum biocomposite edible films by incorporation of natural plant extracts. Food Bioprocess Technol. 2017, 10, 2240–2250. [Google Scholar] [CrossRef]
- Šeregelj, V.; Pezo, L.; Šovljanski, O.; Lević, S.; Nedović, V.; Markov, S.; Tomić, A.; Čanadanović-Brunet, J.; Vulić, J.; Šaponjac, V.T.; et al. New concept of fortified yogurt formulation with encapsulated carrot waste extract. LWT 2021, 138, 110732. [Google Scholar] [CrossRef]
- Ali, A.; Chen, Y.; Liu, H.; Yu, L.; Baloch, Z.; Khalid, S.; Zhu, J.; Chen, L. Starch-based antimicrobial films functionalized by pomegranate peel. Int. J. Biol. Macromol. 2019, 129, 1120–1126. [Google Scholar] [CrossRef]
- Maner, S.; Sharma, A.K.; Banerjee, K. Wheat flour replacement by wine grape pomace powder positively affects physical, functional and sensory properties of cookies. Proc. Natl. Acad. Sci. India Sect. B 2017, 87, 109–113. [Google Scholar] [CrossRef]
- Ayo, J.A.; Kajo, N. Effect of soybean hulls supplementation on the quality of acha based biscuits. Am. J. Agric. Biol. Sci. 2016, 6, 49–56. [Google Scholar] [CrossRef]
- Marchiani, R.; Bertolino, M.; Ghirardello, D.; McSweeney, P.L.; Zeppa, G. Physicochemical and nutritional qualities of grape pomace powder-fortified semi-hard cheeses. J. Food Sci. Technol. 2016, 53, 1585–1596. [Google Scholar] [CrossRef]
- Coman, V.; Teleky, B.E.; Mitrea, L.; Martău, G.A.; Szabo, K.; Călinoiu, L.F.; Vodnar, D.C. Bioactive potential of fruit and vegetable wastes. Adv. Food Nutr. Res. 2020, 91, 157–225. [Google Scholar] [CrossRef]
- Gomes, S.; Finotelli, P.V.; Sardela, V.F.; Pereira, H.M.; Santelli, R.E.; Freire, A.S.; Torres, A.G. Microencapsulated Brazil nut (Bertholletia excelsa) cake extract powder as an added-value functional food ingredient. LWT 2019, 116, 108495. [Google Scholar] [CrossRef]
- Souza, A.C.P.; Gurak, P.D.; Marczak, L.D.F. Maltodextrin, pectin and soy protein isolate as carrier agents in the encapsulation of anthocyanins-rich extract from jaboticaba pomace. Food Bioprod. Process. 2017, 102, 186–194. [Google Scholar] [CrossRef]
- Santos, P.H.; Ribeiro, D.H.B.; Micke, G.A.; Vitali, L.; Hense, H. Extraction of bioactive compounds from feijoa (Acca sellowiana (O. Berg) Burret) peel by low and high-pressure techniques. J. Supercrit. Fluids 2019, 145, 219–227. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, G.; Wang, W.; Yue, J.; Yue, P.; Gao, X. Anthocyanin profile, color and antioxidant activity of blueberry (Vaccinium ashei) juice as affected by thermal pretreatment. Int. J. Food Prop. 2019, 22, 1035–1046. [Google Scholar] [CrossRef]
- Rigolon, T.C.B.; Barros, F.A.R.D.; Vieira, É.N.R.; Stringheta, P.C. Prediction of total phenolics, anthocyanins and antioxidant capacity of blackberry (Rubus sp.), blueberry (Vaccinium sp.) and jaboticaba (Plinia cauliflora (Mart.) Kausel) skin using colorimetric parameters. Food Sci. Technol. 2020, 40, 620–625. [Google Scholar] [CrossRef]
- Riaz, A.; Aadil, R.M.; Amoussa, A.M.O.; Bashari, M.; Abid, M.; Hashim, M.M. Application of chitosan-based apple peel polyphenols edible coating on the preservation of strawberry (Fragaria ananassa cv Hongyan) fruit. J. Food Process. Preserv. 2021, 45, e15018. [Google Scholar] [CrossRef]
- Sirijan, M.; Pipattanawong, N.; Saeng-on, B.; Chaiprasart, P. Anthocyanin content, bioactive compounds and physico-chemical characteristics of potential new strawberry cultivars rich in-anthocyanins. J. Berry Res. 2020, 10, 397–410. [Google Scholar] [CrossRef]
- Szymanowska, U.; Baraniak, B. Antioxidant and potentially anti-inflammatory activity of anthocyanin fractions from pomace obtained from enzymatically treated raspberries. Antioxidants 2019, 8, 299. [Google Scholar] [CrossRef] [PubMed]
- Prabowo, I.; Utomo, E.P.; Nurfaizy, A.; Widodo, A.; Widjajanto, E.; Rahadju, P. Characteristics and antioxidant activities of anthocyanin fraction in red dragon fruit peels (Hylocereus polyrhizus) extract. Drug Invent. Today 2019, 12, 670–678. [Google Scholar]
- Pellicanò, T.M.; Sicari, V.; Loizzo, M.R.; Leporini, M.; Falco, T.; Poiana, M. Optimizing the supercritical fluid extraction process of bioactive compounds from processed tomato skin by-products. Food Sci. Technol. 2019, 40, 692–697. [Google Scholar] [CrossRef]
- Hussain, A.; Kausar, T.; Din, A.; Murtaza, M.A.; Jamil, M.A.; Noreen, S.; Rehman, H.U.; Shabbir, H.; Ramzan, M.A. Determination of total phenolic, flavonoid, carotenoid, and mineral contents in peel, flesh, and seeds of pumpkin (Cucurbita maxima). J. Food Process. Preserv. 2021, 45, e15542. [Google Scholar] [CrossRef]
- Singla, M.; Singh, A.; Sit, N. Effect of microwave and enzymatic pretreatment and type of solvent on kinetics of ultrasound assisted extraction of bioactive compounds from ripe papaya peel. J. Food Process Eng. 2022, 42, e14119. [Google Scholar] [CrossRef]
- Gómez-García, R.; Campos, D.A.; Oliveira, A.; Aguilar, C.N.; Madureira, A.R.; Pintado, M. A chemical valorisation of melon peels towards functional food ingredients: Bioactives profile and antioxidant properties. Food Chem. 2021, 335, 127579. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; El-Mogy, M.M.; Parmar, A.; Mansour, A.T.; Shalaby, T.A.; Ali, M.R. Phytochemical characterization and utilization of dried red beetroot (Beta vulgaris) peel extract in maintaining the quality of Nile Tilapia fish fillet. Antioxidants 2022, 11, 906. [Google Scholar] [CrossRef]
- Fernando, G.S.N.; Wood, K.; Papaioannou, E.H.; Marshall, L.J.; Sergeeva, N.N.; Boesch, C. Application of an ultrasound-assisted extraction method to recover betalains and polyphenols from red beetroot waste. ACS Sustain. Chem. Eng. 2021, 9, 8736–8747. [Google Scholar] [CrossRef]
- García-Cayuela, T.; Gómez-Maqueo, A.; Guajardo-Flores, D.; Welti-Chanes, J.; Cano, M.P. Characterization and quantification of individual betalain and phenolic compounds in Mexican and Spanish prickly pear (Opuntia ficus-indica L. Mill) tissues: A comparative study. J. Food Compos. Anal. 2019, 76, 1–13. [Google Scholar] [CrossRef]
- Lončarić, A.; Celeiro, M.; Jozinović, A.; Jelinić, J.; Kovač, T.; Jokić, S.; Lores, M. Green extraction methods for extraction of polyphenolic compounds from blueberry pomace. Foods 2020, 9, 1521. [Google Scholar] [CrossRef]
- Gulsunoglu, Z.; Purves, R.; Karbancioglu-Guler, F.; Kilic-Akyilmaz, M. Enhancement of phenolic antioxidants in industrial apple waste by fermentation with Aspergillus spp. Biocatal. Agric. Biotechnol. 2020, 25, 101562. [Google Scholar] [CrossRef]
- Vilarinho, F.; Lestido-Cardama, A.; Sendón, R.; Rodríguez Bernaldo de Quirós, A.; Vaz, M.D.F.; Sanches-Silva, A. HPLC with fluorescence detection for determination of bisphenol A in canned vegetables: Optimization, validation and application to samples from Portuguese and Spanish markets. Coatings 2020, 10, 624. [Google Scholar] [CrossRef]
- Yang, F.; Wang, Y.P.; Zhao, H. Quality enhancement of fermented vegetable juice by probiotic through fermented yam juice using Saccharomyces cerevisiae. Food Sci. Technol. 2019, 40, 26–35. [Google Scholar] [CrossRef]
- Sobota, A.; Wirkijowska, A.; Zarzycki, P. Application of vegetable concentrates and powders in coloured pasta production. Int. J. Food Sci. 2020, 55, 2677–2687. [Google Scholar] [CrossRef]
- Perumpuli, P.A.B.N.; Fernando, G.; Kaumal, M.; Arandara, M.; Silva, S. Development of low sugar vegetable jam from beetroot (Beta vulgaris L.): Studies on Physicochemical Sensory and Nutritional Properties. J. Theor. Appl. Sci. 2018, 10, 22–27. [Google Scholar]
- Siroli, L.; Camprini, L.; Pisano, M.B.; Patrignani, F.; Lanciotti, R. Volatile molecule profiles and anti-Listeria monocytogenes activity of nisin producers Lactococcus lactis strains in vegetable drinks. Front. Microbiol. 2019, 10, 563. [Google Scholar] [CrossRef]
- Lucera, A.; Costa, C.; Marinelli, V.; Saccotelli, M.A.; Del Nobile, M.A.; Conte, A. Fruit and vegetable by-products to fortify spreadable cheese. Antioxidants 2018, 7, 61. [Google Scholar] [CrossRef]
- Salas-Millán, J.Á.; Aznar, A.; Conesa, E.; Conesa-Bueno, A.; Aguayo, E. Functional food obtained from fermentation of broccoli by-products (stalk): Metagenomics profile and glucosinolate and phenolic compounds characterization by LC-ESI-QqQ-MS/MS. LWT 2022, 169, 113915. [Google Scholar] [CrossRef]
- Chitrakar, B.; Zhang, M.; Zhang, X.; Bhandari, B. Valorization of asparagus-leaf by-product through nutritionally enriched chips to evaluate the effect of powder particle size on functional properties and rutin contents. Dry. Technol. 2022, 41, 34–45. [Google Scholar] [CrossRef]
- Zhang, H.; Birch, J.; Yang, H.; Xie, C.; Kong, L.; Dias, G.; Bekhit, A.E.D. Effect of solvents on polyphenol recovery and antioxidant activity of isolates of Asparagus Officinalis roots from Chinese and New Zealand cultivars. Int. J. Food Sci. 2018, 53, 2369–2377. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Gonzalez-Aguilar, G.A. Quantification of flavonoids, total phenols and antioxidant properties of onion skin: A comparative study of fifteen Indian cultivars. J. Food Sci. Technol. 2020, 57, 2423–2432. [Google Scholar] [CrossRef]
- Milea, Ș.A.; Crăciunescu, O.; Râpeanu, G.; Oancea, A.; Enachi, E.; Bahrim, G.E.; Stănciuc, N. Multifunctional ingredient from aqueous flavonoidic extract of yellow onion skins with cytocompatibility and cell proliferation properties. Appl. Sci. 2021, 11, 7243. [Google Scholar] [CrossRef]
- Joly, N.; Souidi, K.; Depraetere, D.; Wils, D.; Martin, P. Potato by-products as a source of natural Chlorogenic acids and phenolic compounds: Extraction, characterization, and antioxidant capacity. Molecules 2020, 26, 177. [Google Scholar] [CrossRef] [PubMed]
- Vaz, A.A.; Odriozola-Serrano, I.; Oms-Oliu, G.; Martín-Belloso, O. Physicochemical properties and bioaccessibility of phenolic compounds of dietary fibre concentrates from vegetable by-products. Foods 2022, 11, 2578. [Google Scholar] [CrossRef] [PubMed]
- Makori, S.I.; Mu, T.H.; Sun, H.N. Profiling of polyphenols, flavonoids and anthocyanins in potato peel and flesh from four potato varieties. Potato Res. 2022, 65, 193–208. [Google Scholar] [CrossRef]
- Doulabi, M.; Golmakani, M.T.; Ansari, S. Evaluation and optimization of microwave-assisted extraction of bioactive compounds from eggplant peel by-product. J. Food Process. Preserv. 2020, 44, e14853. [Google Scholar] [CrossRef]
- Rodriguez Garcia, S.L.; Raghavan, V. Green extraction techniques from fruit and vegetable waste to obtain bioactive compounds—A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6446–6466. [Google Scholar] [CrossRef]
- Alrugaibah, M.; Yagiz, Y.; Gu, L. Use natural deep eutectic solvents as efficient green reagents to extract procyanidins and anthocyanins from cranberry pomace and predictive modeling by RSM and artificial neural networking. Sep. Purif. Technol. 2021, 255, 117720. [Google Scholar] [CrossRef]
- Wu, F.; Jin, Y.; Xu, X.; Yang, N. Electrofluidic pretreatment for enhancing essential oil extraction from citrus fruit peel waste. J. Clean. Prod. 2017, 159, 85–94. [Google Scholar] [CrossRef]
- Aşkin, B. Comparison of aroma profiles of essential oils extracted by hydro-distillation from orange peel waste dried by various methods. J. Food Nutr. Res. 2021, 60, 271–278. [Google Scholar]
- Ramli, R.; Ahmad Zaghlul, N.S.; Ahmad Nasir, N.A.H. The potential of antioxidants and phytochemicals components in fruit waste (peel) of Citrus hystrix and Ananas comosus. In Charting the Sustainable Future of ASEAN in Science and Technology, Proceedings from the 3rd International Conference on the Future of ASEAN (ICoFA) 2019, Shah Alam, Malaysia, 5 October 2019; Springer: Berlin/Heidelberg, Germany, 2020; pp. 123–135. [Google Scholar] [CrossRef]
- Cvetanović, A.; Uysal, S.; Pavlić, B.; Sinan, K.I.; Llorent-Martínez, E.J.; Zengin, G. Tamarindus indica L. seed: Optimization of maceration extraction recovery of tannins. Food Anal. Methods 2020, 13, 579–590. [Google Scholar] [CrossRef]
- Coelho, E.M.; de Souza, M.E.A.O.; Corrêa, L.C.; Viana, A.C.; de Azevêdo, L.C.; dos Santos Lima, M. Bioactive compounds and antioxidant activity of mango peel liqueurs (Mangifera indica L.) produced by different methods of maceration. Antioxidants 2019, 8, 102. [Google Scholar] [CrossRef]
- Carbone, K.; Amoriello, T.; Iadecola, R. Exploitation of kiwi juice pomace for the recovery of natural antioxidants through microwave-assisted extraction. Agriculture 2020, 10, 435. [Google Scholar] [CrossRef]
- Kurtulbaş, E.; Sevgen, S.; Samli, R.; Şahin, S. Microwave-assisted extraction of bioactive components from peach waste: Describing the bioactivity degradation by polynomial regression. Biomass Convers. 2022, 1–11. [Google Scholar] [CrossRef]
- da Rocha, C.B.; Noreña, C.P.Z. Microwave-assisted extraction and ultrasound-assisted extraction of bioactive compounds from grape pomace. Int. J. Food Eng. 2020, 16. [Google Scholar] [CrossRef]
- Lasunon, P.; Phonkerd, N.; Tettawong, P.; Sengkhamparn, N. Effect of microwave-assisted extraction on bioactive compounds from industrial tomato waste and its antioxidant activity. Food Res. 2021, 5, 468–474. [Google Scholar] [CrossRef]
- Araújo, R.G.; Rodriguez-Jasso, R.M.; Ruiz, H.A.; Govea-Salas, M.; Pintado, M.E.; Aguilar, C.N. Process optimization of microwave-assisted extraction of bioactive molecules from avocado seeds. Ind. Crops Prod. 2020, 154, 112623. [Google Scholar] [CrossRef]
- Kupnik, K.; Leitgeb, M.; Primožič, M.; Postružnik, V.; Kotnik, P.; Kučuk, N.; Marevci, M.K. Supercritical fluid and conventional extractions of high value-added compounds from pomegranate peels waste: Production, quantification and antimicrobial activity of bioactive constituents. Plants 2022, 11, 928. [Google Scholar] [CrossRef] [PubMed]
- Madhumeena, S.; Preetha, R.; Prasad, S. Effective utilization of pineapple waste. J. Phys. Conf. Ser. 2021, 1979, 012001. [Google Scholar] [CrossRef]
- Soldan, A.C.F.; Arvelos, S.; Watanabe, E.O.; Hori, C.E. Supercritical fluid extraction of oleoresin from Capsicum annuum industrial waste. J. Clean. Prod. 2021, 297, 126593. [Google Scholar] [CrossRef]
- de Andrade Lima, M.; Kestekoglou, I.; Charalampopoulos, D.; Chatzifragkou, A. Supercritical fluid extraction of carotenoids from vegetable waste matrices. Molecules 2019, 24, 466. [Google Scholar] [CrossRef] [PubMed]
- Romano, R.; De Luca, L.; Aiello, A.; Rossi, D.; Pizzolongo, F.; Masi, P. Bioactive compounds extracted by liquid and supercritical carbon dioxide from citrus peels. Int. J. Food Sci. 2022, 57, 3826–3837. [Google Scholar] [CrossRef]
- Lizárraga-Velázquez, C.E.; Leyva-López, N.; Hernández, C.; Gutiérrez-Grijalva, E.P.; Salazar-Leyva, J.A.; Osuna-Ruíz, I.; Ávalos-Soriano, A. Antioxidant molecules from plant waste: Extraction techniques and biological properties. Processes 2020, 8, 1566. [Google Scholar] [CrossRef]
- García, P.; Fredes, C.; Cea, I.; Lozano-Sánchez, J.; Leyva-Jiménez, F.J.; Robert, P.; Vergara, C.; Jimenez, P. Recovery of bioactive compounds from pomegranate (Punica granatum L.) peel using pressurized liquid extraction. Foods 2021, 10, 203. [Google Scholar] [CrossRef]
- Lasta, H.F.B.; Lentz, L.; Rodrigues, L.G.G.; Mezzomo, N.; Vitali, L.; Ferreira, S.R.S. Pressurized liquid extraction applied for the recovery of phenolic compounds from beetroot waste. Biocatal. Agric. Biotechnol. 2019, 21, 101353. [Google Scholar] [CrossRef]
- Andrade, T.A.; Hamerski, F.; Fetzer, D.E.L.; Roda-Serrat, M.C.; Corazza, M.L.; Norddahl, B.; Errico, M. Ultrasound-assisted pressurized liquid extraction of anthocyanins from Aronia melanocarpa pomace. Sep. Purif. Technol. 2021, 276, 119290. [Google Scholar] [CrossRef]
- Pukalskienė, M.; Pukalskas, A.; Dienaitė, L.; Revinytė, S.; Pereira, C.V.; Matias, A.A.; Venskutonis, P.R. Recovery of bioactive compounds from strawberry (Fragaria × ananassa) pomace by conventional and pressurized liquid extraction and assessment their bioactivity in human cell cultures. Foods 2021, 10, 1780. [Google Scholar] [CrossRef]
- Guzmán-Lorite, M.; Marina, M.L.; García, M.C. Pressurized liquids vs. high intensity focused ultrasounds for the extraction of proteins from a pomegranate seed waste. Innov. Food Sci. Emerg. Technol. 2022, 77, 102958. [Google Scholar] [CrossRef]
- Maza, M.A.; Pereira, C.; Martínez, J.M.; Camargo, A.; Álvarez, I.; Raso, J. PEF treatments of high specific energy permit the reduction of maceration time during vinification of Caladoc and Grenache grapes. IFSET 2020, 63, 102375. [Google Scholar] [CrossRef]
- Pataro, G.; Carullo, D.; Falcone, M.; Ferrari, G. Recovery of lycopene from industrially derived tomato processing by-products by pulsed electric fields-assisted extraction. Innov. Food Sci. Emerg. Technol. 2020, 63, 102369. [Google Scholar] [CrossRef]
- Lakka, A.; Bozinou, E.; Makris, D.P.; Lalas, S.I. Evaluation of pulsed electric field polyphenol extraction from Vitis vinifera, Sideritis scardica and Crocus sativus. Chem. Eng. 2021, 5, 25. [Google Scholar] [CrossRef]
- Tehrani, M.G.; Elhamirad, A.H.; Azarpazhooh, E.; Pedramnia, A.; Sharayei, P. Natural valuable compound extraction from onion by-products using a pulsed electric field. IJBCS 2019, 12, 171–180. [Google Scholar]
- Hwang, H.J.; Kim, H.J.; Ko, M.J.; Chung, M.S. Recovery of hesperidin and narirutin from waste Citrus unshiu peel using subcritical water extraction aided by pulsed electric field treatment. Food Sci. Biotechnol. 2021, 30, 217–226. [Google Scholar] [CrossRef]
- Andreou, V.; Dimopoulos, G.; Dermesonlouoglou, E.; Taoukis, P. Application of pulsed electric fields to improve product yield and waste valorization in industrial tomato processing. J. Food Eng. 2020, 270, 109778. [Google Scholar] [CrossRef]
- Lakka, A.; Bozinou, E.; Stavropoulos, G.; Samanidis, I.; Athanasiadis, V.; Dourtoglou, V.G.; Makris, D.P.; Lalas, S.I. Enhancement of polyphenols recovery from Rosa canina, Calendula officinalis and Castanea sativa using Pulsed Electric Field. Beverages 2021, 7, 63. [Google Scholar] [CrossRef]
- Pappas, V.M.; Lakka, A.; Palaiogiannis, D.; Bozinou, E.; Ntourtoglou, G.; Batra, G.; Lalas, S.I. Use of Pulsed Electric Field as a low-temperature and high-performance “green” extraction technique for the recovery of high added value compounds from olive leaves. Beverages 2021, 7, 45. [Google Scholar] [CrossRef]
- Sharayei, P.; Azarpazhooh, E.; Zomorodi, S.; Ramaswamy, H.S. Ultrasound assisted extraction of bioactive compounds from pomegranate (Punica granatum L.) peel. LWT 2019, 101, 342–350. [Google Scholar] [CrossRef]
- de Oliveira, C.F.; Giordani, D.; Lutckemier, R.; Gurak, P.D.; Cladera-Olivera, F.; Marczak, L.D.F. Extraction of pectin from passion fruit peel assisted by ultrasound. LWT 2016, 71, 110–115. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Khodaiyan, F.; Kazemi, M.; Najari, Z. Optimization and characterization of pectin extracted from sour orange peel by ultrasound assisted method. Int. J. Biol. Macromol. 2019, 125, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Maran, J.P.; Priya, B.; Al-Dhabi, N.A.; Ponmurugan, K.; Moorthy, I.G.; Sivarajasekar, N. Ultrasound assisted citric acid mediated pectin extraction from industrial waste of Musa balbisiana. Ultrason. Sonochem. 2017, 35, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Bagherian, H.; Ashtiani, F.Z.; Fouladitajar, A.; Mohtashamy, M. Comparisons between conventional, microwave-and ultrasound-assisted methods for extraction of pectin from grapefruit. Chem. Eng. Process. 2011, 50, 1237–1243. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Xu, Y.; Cao, Y.; Jiang, Z.; Ding, T.; Liu, D. Ultrasound-assisted heating extraction of pectin from grapefruit peel: Optimization and comparison with the conventional method. Food Chem. 2015, 178, 106–114. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, L.; Bailina, Y.; Ge, Z.; Ding, T.; Ye, X.; Liu, D. Effects of ultrasound and/or heating on the extraction of pectin from grapefruit peel. J. Food Eng. 2014, 126, 72–81. [Google Scholar] [CrossRef]
- Wang, W.; Wu, X.; Chantapakul, T.; Wang, D.; Zhang, S.; Ma, X.; Liu, D. Acoustic cavitation assisted extraction of pectin from waste grapefruit peels: A green two-stage approach and its general mechanism. Int. Food Res. J. 2017, 102, 101–110. [Google Scholar] [CrossRef]
- Hosseini, S.; Parastouei, K.; Khodaiyan, F. Simultaneous extraction optimization and characterization of pectin and phenolics from sour cherry pomace. Int. J. Biol. Macromol. 2020, 158, 911–921. [Google Scholar] [CrossRef]
- Moorthy, I.G.; Maran, J.P.; Muneeswari, S.; Naganyashree, S.; Shivamathi, C.S. Response surface optimization of ultrasound assisted extraction of pectin from pomegranate peel. Int. J. Biol. Macromol. 2015, 72, 1323–1328. [Google Scholar] [CrossRef]
- Kazemi, M.; Khodaiyan, F.; Hosseini, S.S. Eggplant peel as a high potential source of high methylated pectin: Ultrasonic extraction optimization and characterization. LWT 2019, 105, 182–189. [Google Scholar] [CrossRef]
- Moorthy, I.G.; Maran, J.P.; Ilakya, S.; Anitha, S.L.; Sabarima, S.P.; Priya, B. Ultrasound assisted extraction of pectin from waste Artocarpus heterophyllus fruit peel. Ultrason. Sonochem. 2017, 34, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Minjares-Fuentes, R.; Femenia, A.; Garau, M.C.; Meza-Velázquez, J.A.; Simal, S.; Rosselló, C. Ultrasound-assisted extraction of pectins from grape pomace using citric acid: A response surface methodology approach. Carbohydr. Polym. 2014, 106, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Grassino, A.N.; Brnčić, M.; Vikić-Topić, D.; Roca, S.; Dent, M.; Brnčić, S.R. Ultrasound assisted extraction and characterization of pectin from tomato waste. Food Chem. 2016, 198, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Wai, W.W.; Alkarkhi, A.F.; Easa, A.M. Optimization of pectin extraction from durian rind (Durio zibethinus) using response surface methodology. J. Food Sci. 2009, 74, C637–C641. [Google Scholar] [CrossRef]
- Ponmurugan, K.; Al-Dhabi, N.A.; Maran, J.P.; Karthikeyan, K.; Moothy, I.G.; Sivarajasekar, N.; Manoj, J.J.B. Ultrasound assisted pectic polysaccharide extraction and its characterization from waste heads of Helianthus annus. Carbohydr. Polym. 2017, 173, 707–713. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Jiang, P.; Hu, L.; Zhi, Z.; Chen, J.; Liu, D. Characterization of pectin from grapefruit peel: A comparison of ultrasound-assisted and conventional heating extractions. Food Hydrocoll. 2016, 61, 730–739. [Google Scholar] [CrossRef]
- Guandalini, B.B.V.; Rodrigues, N.P.; Marczak, L.D.F. Sequential extraction of phenolics and pectin from mango peel assisted by ultrasound. Int. Food Res. J. 2019, 119, 455–461. [Google Scholar] [CrossRef]
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: A comparison. IFSET 2008, 9, 85–91. [Google Scholar] [CrossRef]
- Minjares-Fuentes, R.; Femenia, A.; Garau, M.C.; Candelas-Cadillo, M.G.; Simal, S.; Rosselló, C. Ultrasound-assisted extraction of hemicelluloses from grape pomace using response surface methodology. Carbohydr. Polym. 2016, 138, 180–191. [Google Scholar] [CrossRef]
- Samaram, S.; Mirhosseini, H.; Tan, C.P.; Ghazali, H.M.; Bordbar, S.; Serjouie, A. Optimisation of ultrasound-assisted extraction of oil from papaya seed by response surface methodology: Oil recovery, radical scavenging antioxidant activity, and oxidation stability. Food Chem. 2015, 172, 7–17. [Google Scholar] [CrossRef]
- Leong, T.; Ashokkumar, M.; Kentish, S. The fundamentals of power ultrasound—A review. Acoust. Aust. 2011, 39, 54–63. [Google Scholar]
- Brahmi, F.; Blando, F.; Sellami, R.; Mehdi, S.; De Bellis, L.; Negro, C.; Makhlouf-Boulekbache, L. Optimization of the conditions for ultrasound-assisted extraction of phenolic compounds from Opuntia ficus-indica [L.] Mill. flowers and comparison with conventional procedures. Ind. Crops Prod. 2022, 184, 114977. [Google Scholar] [CrossRef]
- González-Silva, N.; Nolasco-González, Y.; Aguilar-Hernández, G.; Sáyago-Ayerdi, S.G.; Villagrán, Z.; Acosta, J.L.; Anaya-Esparza, L.M. Ultrasound-assisted extraction of phenolic compounds from Psidium cattleianum leaves: Optimization using the response surface methodology. Molecules 2022, 27, 3557. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, M.; Zhang, H.; Iftikhar, A.; Raza, A.; Begum, N.; Tahamina, A.; Wang, J. Study on optimization of ultrasonic assisted extraction of phenolic compounds from rye bran. LWT 2020, 134, 110243. [Google Scholar] [CrossRef]
- Goulas, V.; Hadjivasileiou, L.; Primikyri, A.; Michael, C.; Botsaris, G.; Tzakos, A.G.; Gerothanassis, I.P. Valorization of carob fruit residues for the preparation of novel bi-functional polyphenolic coating for food packaging applications. Molecules 2019, 24, 3162. [Google Scholar] [CrossRef]
- Piechowiak, T.; Skóra, B.; Grzelak-Błaszczyk, K.; Sójka, M. Extraction of antioxidant compounds from blueberry fruit waste and evaluation of their in vitro biological activity in human keratinocytes (HaCaT). Food Anal. Methods 2021, 14, 2317–2327. [Google Scholar] [CrossRef]
- Cadiz-Gurrea, M.D.L.L.; Pinto, D.; Delerue-Matos, C.; Rodrigues, F. Olive fruit and leaf wastes as bioactive ingredients for cosmetics—A preliminary study. Antioxidants 2021, 10, 245. [Google Scholar] [CrossRef]
- Rangaraj, V.M.; Rambabu, K.; Banat, F.; Mittal, V. Effect of date fruit waste extract as an antioxidant additive on the properties of active gelatin films. Food Chem. 2021, 355, 129631. [Google Scholar] [CrossRef]
- Venturi, F.; Bartolini, S.; Sanmartin, C.; Orlando, M.; Taglieri, I.; Macaluso, M.; Lucchesini, M.; Trivellini, A.; Zinnia, A.; Mensuali, A. Potato peels as a source of novel green extracts suitable as antioxidant additives for fresh-cut fruits. Appl. Sci. 2019, 9, 2431. [Google Scholar] [CrossRef]
- Kurek, M.; Benbettaieb, N.; Ščetar, M.; Chaudy, E.; Repajić, M.; Klepac, D.; Valić, S.; Debeaufort, F. Characterization of food packaging films with blackcurrant fruit waste as a source of antioxidant and color sensing intelligent material. Molecules 2021, 26, 2569. [Google Scholar] [CrossRef]
- Kam, W.Y.J.; Mirhosseini, H.; Abas, F.; Hussain, N.; Hedayatnia, S.; Chong, H.L.F. Antioxidant activity enhancement of biodegradable film as active packaging utilizing crude extract from durian leaf waste. Food Control 2018, 90, 66–72. [Google Scholar] [CrossRef]
- Deshmukh, R.K.; Akhila, K.; Ramakanth, D.; Gaikwad, K.K. Guar gum/carboxymethyl cellulose based antioxidant film incorporated with halloysite nanotubes and litchi shell waste extract for active packaging. Int. J. Biol. Macromol. 2022, 201, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Orqueda, M.E.; Méndez, D.A.; Martínez-Abad, A.; Zampini, C.; Torres, S.; Isla, M.I.; López-Rubio, A.; Fabra, M.J. Feasibility of active biobased films produced using red chilto wastes to improve the protection of fresh salmon fillets via a circular economy approach. Food Hydrocoll. 2022, 133, 107888. [Google Scholar] [CrossRef]
- Ribeiro, A.C.B.; Cunha, A.P.; da Silva, L.M.R.; Mattos, A.L.A.; de Brito, E.S.; de Souza, M.D.S.M.; de Azeredo, H.M.C.; Ricardo, N.M.P.S. From mango by-product to food packaging: Pectin-phenolic antioxidant films from mango peels. Int. J. Biol. Macromol. 2021, 193, 1138–1150. [Google Scholar] [CrossRef] [PubMed]
- Merino, D.; Bertolacci, L.; Paul, U.C.; Simonutti, R.; Athanassiou, A. Avocado peels and seeds: Processing strategies for the development of highly antioxidant bioplastic films. ACS Appl. Mater. Interfaces 2021, 13, 38688–38699. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; Garrido, L.; Faba, S.; Guarda, A.; Galotto, M.J.; López de Dicastillo, C. Cucumis metuliferus fruit extract loaded acetate cellulose coatings for antioxidant active packaging. Polymers 2020, 12, 1248. [Google Scholar] [CrossRef]
- Matta, E.; Tavera-Quiroz, M.J.; Bertola, N. Active edible films of methylcellulose with extracts of green apple (Granny Smith) skin. Int. J. Biol. Macromol. 2019, 124, 1292–1298. [Google Scholar] [CrossRef]
- Han, H.S.; Song, K.B. Antioxidant activities of mandarin (Citrus unshiu) peel pectin films containing sage (Salvia officinalis) leaf extract. Int. J. Food Sci. 2020, 55, 3173–3181. [Google Scholar] [CrossRef]
- Shahbazi, Y. The properties of chitosan and gelatin films incorporated with ethanolic red grape seed extract and Ziziphora clinopodioides essential oil as biodegradable materials for active food packaging. Int. J. Biol. Macromol. 2017, 99, 746–753. [Google Scholar] [CrossRef]
- Adilah, A.N.; Jamilah, B.; Noranizan, M.A.; Hanani, Z.N. Utilization of mango peel extracts on the biodegradable films for active packaging. Food Packag. Shelf Life 2018, 16, 1–7. [Google Scholar] [CrossRef]
- Melo, P.E.; Silva, A.P.M.; Marques, F.P.; Ribeiro, P.R.; Brito, E.S.; Lima, J.R.; Azeredo, H.M. Antioxidant films from mango kernel components. Food Hydrocoll. 2019, 95, 487–495. [Google Scholar] [CrossRef]
- Moghadam, M.; Salami, M.; Mohammadian, M.; Khodadadi, M.; Emam-Djomeh, Z. Development of antioxidant edible films based on mung bean protein enriched with pomegranate peel. Food Hydrocoll. 2020, 104, 105735. [Google Scholar] [CrossRef]
- Rodsamran, P.; Sothornvit, R. Preparation and characterization of pectin fraction from pineapple peel as a natural plasticizer and material for biopolymer film. Food Bioprod. Process. 2019, 118, 198–206. [Google Scholar] [CrossRef]
- Vargas-Torrico, M.F.; Aguilar-Méndez, M.A.; von Borries-Medrano, E. Development of gelatin/carboxymethylcellulose active films containing Hass avocado peel extract and their application as a packaging for the preservation of berries. Int. J. Biol. Macromol. 2022, 206, 1012–1025. [Google Scholar] [CrossRef]
- Talón, E.; Trifkovic, K.T.; Nedovic, V.A.; Bugarski, B.M.; Vargas, M.; Chiralt, A.; González-Martínez, C. Antioxidant edible films based on chitosan and starch containing polyphenols from thyme extracts. Carbohydr. Polym. 2017, 157, 1153–1161. [Google Scholar] [CrossRef]
- Urbina, L.; Eceiza, A.; Gabilondo, N.; Corcuera, M.Á.; Retegi, A. Valorization of apple waste for active packaging: Multicomponent polyhydroxyalkanoate coated nanopapers with improved hydrophobicity and antioxidant capacity. Food Packag. Shelf Life 2019, 21, 100356. [Google Scholar] [CrossRef]
- Hanani, Z.N.; Yee, F.C.; Nor-Khaizura, M.A.R. Effect of pomegranate (Punica granatum L.) peel powder on the antioxidant and antimicrobial properties of fish gelatin films as active packaging. Food Hydrocoll. 2019, 89, 253–259. [Google Scholar] [CrossRef]
- Saleem, M.; Saeed, M.T. Potential application of waste fruit peels (orange, yellow lemon and banana) as wide range natural antimicrobial agent. J. King Saud Univ. Sci. 2020, 32, 805–810. [Google Scholar] [CrossRef]
- Jodhani, K.A.; Nataraj, M. Synergistic effect of Aloe gel (Aloe vera L.) and Lemon (Citrus Limon L.) peel extract edible coating on shelf life and quality of banana (Musa spp.). J. Food Meas. Charact. 2021, 15, 2318–2328. [Google Scholar] [CrossRef]
- Meydanju, N.; Pirsa, S.; Farzi, J. Biodegradable film based on lemon peel powder containing xanthan gum and TiO2–Ag nanoparticles: Investigation of physicochemical and antibacterial properties. Polym. Test. 2022, 106, 107445. [Google Scholar] [CrossRef]
- Alparslan, Y.; Baygar, T. Effect of chitosan film coating combined with orange peel essential oil on the shelf life of deepwater pink shrimp. Food Bioprocess Technol. 2017, 10, 842–853. [Google Scholar] [CrossRef]
- Agdar GhareAghaji, M.; Zomordi, S.; Gharekhani, M.; Hanifian, S. Effect of edible coating based on salep containing orange (Citrus sinensis) peel essential oil on shelf life of rainbow trout (Oncorhynchus mykiss) fillets. J. Food Process. Preserv. 2021, 45, e15737. [Google Scholar] [CrossRef]
- Ramli, A.N.M.; Sukri, N.A.M.; Azelee, N.I.W.; Bhuyar, P. Exploration of antibacterial and antioxidative activity of seed/peel extracts of Southeast Asian fruit Durian (Durio zibethinus) for effective shelf-life enhancement of preserved meat. J. Food Process. Preserv. 2021, 45, e15662. [Google Scholar] [CrossRef]
- Mayeli, M.; Mehdizadeh, T.; Tajik, H.; Esmaeli, F.; Langroodi, A.M. Combined impacts of zein coating enriched with methanolic and ethanolic extracts of sour orange peel and vacuum packing on the shelf life of refrigerated rainbow trout. Flavour Fragr. J. 2019, 34, 460–470. [Google Scholar] [CrossRef]
- Shukor, U.A.A.; Nordin, N.; Tawakkal, I.S.M.A.; Talib, R.A.; Othman, S.H. Utilization of jackfruit peel waste for the production of biodegradable and active antimicrobial packaging films. In Biopolymers and Biocomposites from Agro-Waste for Packaging Applications; Woodhead Publishing: Sawston, UK, 2021; pp. 171–192. [Google Scholar] [CrossRef]
- Thivya, P.; Bhosale, Y.K.; Anandakumar, S.; Hema, V.; Sinija, V.R. Development of active packaging film from sodium alginate/carboxymethyl cellulose containing shallot waste extracts for anti-browning of fresh-cut produce. Int. J. Biol. Macromol. 2021, 188, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Tinello, F.; Mihaylova, D.; Lante, A. Valorization of onion extracts as anti-browning agents. Food Sci. Biotechnol. 2020, 3, 16–21. [Google Scholar] [CrossRef]
- Liang, D.; Liu, L.; Qin, Z.; Li, G.; Ding, B.; Chen, H.; Wang, Z. Antioxidant and antityrosinase activity of extractable condensed tannins from durian shells with antibrowning effect in fresh-cut asparagus lettuce model. LWT 2022, 162, 113423. [Google Scholar] [CrossRef]
- Jirasuteeruk, C.; Theerakulkait, C. Inhibitory effect of different varieties of mango peel extract on enzymatic browning in potato purée. Agric. Nat. Resour. 2020, 54, 217–222. [Google Scholar]
- Faiq, M.; Theerakulkait, C. Effect of papaya peel extract on browning inhibition in vegetable and fruit slices. Ital. J. Food Sci. 2018, 30, 57–61. [Google Scholar]
- Martínez-Hernández, G.B.; Castillejo, N.; Artés-Hernández, F. Effect of fresh–cut apples fortification with lycopene microspheres, revalorized from tomato by-products, during shelf life. Postharvest Biol. Technol. 2019, 156, 110925. [Google Scholar] [CrossRef]
- Tinello, F.; Mihaylova, D.; Lante, A. Effect of dipping pre-treatment with unripe grape juice on dried “Golden Delicious” apple slices. Food Bioprocess Technol. 2018, 11, 2275–2285. [Google Scholar] [CrossRef]
- Maia, L.S.; Duizit, L.D.; Pinhatio, F.R.; Mulinari, D.R. Valuation of banana peel waste for producing activated carbon via NaOH and pyrolysis for methylene blue removal. Carbon Lett. 2021, 31, 749–762. [Google Scholar] [CrossRef]
- Zaini, H.M.; Roslan, J.; Saallah, S.; Munsu, E.; Sulaiman, N.S.; Pindi, W. Banana peels as a bioactive ingredient and its potential application in the food industry. J. Funct. Foods 2022, 92, 105054. [Google Scholar] [CrossRef]
- Ramutshatsha-Makhwedzha, D.; Mbaya, R.; Mavhungu, M.L. Application of activated carbon banana peel coated with al2o3-chitosan for the adsorptive removal of lead and cadmium from wastewater. Materials 2022, 15, 860. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.L.; Xie, T.; Cao, J. Activated carbon derived from waste tangerine seed for the high-performance adsorption of carbamate pesticides from water and plant. Bioresour. Technol. 2020, 316, 123929. [Google Scholar] [CrossRef] [PubMed]
- Gnanasekaran, L.; Priya, A.K.; Gracia, F. Orange peel extract influenced partial transformation of SnO2 to SnO in green 3D-ZnO/SnO2 system for chlorophenol degradation. J. Hazard. Mater. 2022, 424, 127464. [Google Scholar] [CrossRef]
- Aminu, A.; Oladepo, S.A. Fast orange peel-mediated synthesis of silver nanoparticles and use as visual colorimetric sensor in the selective detection of mercury (II) ions. Arab. J. Sci. Eng. 2021, 46, 5477–5487. [Google Scholar] [CrossRef]
- Hassan, S.S.; Al-Ghouti, M.A.; Abu-Dieyeh, M.; McKay, G. Novel bioadsorbents based on date pits for organophosphorus pesticide remediation from water. J. Environ. Chem. Eng. 2020, 8, 103593. [Google Scholar] [CrossRef]
- Mohammad, S.G.; El-Sayed, M.M. Removal of imidacloprid pesticide using nanoporous activated carbons produced via pyrolysis of peach stone agricultural wastes. Chem. Eng. Commun. 2021, 208, 1069–1080. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Sweleh, A.O. Optimizing textile dye removal by activated carbon prepared from olive stones. Environ. Technol. Innov. 2019, 16, 100488. [Google Scholar] [CrossRef]
- Batool, S.; Shah, A.A.; Bakar, A.F.A.; Maah, M.J.; Bakar, N.K.A. Removal of organochlorine pesticides using zerovalent iron supported on biochar nanocomposite from Nephelium lappaceum (Rambutan) fruit peel waste. Chemosphere 2022, 289, 133011. [Google Scholar] [CrossRef]
- Shakoor, S.; Nasar, A. Removal of methylene blue dye from artificially contaminated water using citrus limetta peel waste as a very low cost adsorbent. J. Taiwan Inst. Chem. Eng. 2016, 66, 154–163. [Google Scholar] [CrossRef]
- Salama, A. New sustainable hybrid material as adsorbent for dye removal from aqueous solutions. J. Colloid Interface Sci. 2017, 487, 348–353. [Google Scholar] [CrossRef]
- Goksu, A.; Tanaydin, M.K. Adsorption of hazardous crystal violet dye by almond shells and determination of optimum process conditions by Taguchi method. Desalin. Water Treat. 2017, 88, 189–199. [Google Scholar] [CrossRef]
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting citrus wastes into value-added products: Economic and environmentally friendly approaches. Nutrition 2017, 34, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Nassour, R.; Ayash, A.; Al-Tameemi, K. Anthocyanin pigments: Structure and biological importance. J. Chem. Pharm. Sci. 2020, 13, 45–57. [Google Scholar]
- Valencia-Arredondo, J.A.; Hernández-Bolio, G.I.; Cerón-Montes, G.I.; Castro-Muñoz, R.; Yáñez-Fernández, J. Enhanced process integration for the extraction, concentration and purification of di-acylated cyanidin from red cabbage. Sep. Purif. Technol. 2020, 238, 116492. [Google Scholar] [CrossRef]
- Türker, D.A.; Doğan, M. Application of deep eutectic solvents as a green and biodegradable media for extraction of anthocyanin from black carrots. LWT 2021, 138, 110775. [Google Scholar] [CrossRef]
- Chayavanich, K.; Thiraphibundet, P.; Imyim, A. Biocompatible film sensors containing red radish extract for meat spoilage observation. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 226, 117601. [Google Scholar] [CrossRef]
- Sohany, M.; Tawakkal, I.S.M.A.; Ariffin, S.H.; Shah, N.N.A.K.; Yusof, Y.A. Characterization of anthocyanin associated purple sweet potato starch and peel-based pH indicator films. Foods 2021, 10, 2005. [Google Scholar] [CrossRef]
- Kowalski, R.; de Mejia, E.G. Phenolic composition, antioxidant capacity and physical characterization of ten blackcurrant (Ribes nigrum) cultivars, their juices, and the inhibition of type 2 diabetes and inflammation biochemical markers. Food Chem. 2021, 359, 129889. [Google Scholar] [CrossRef] [PubMed]
- Molaeafard, S.; Jamei, R.; Marjani, A.P. Co-pigmentation of anthocyanins extracted from sour cherry (Prunus cerasus L.) with some organic acids: Color intensity, thermal stability, and thermodynamic parameters. Food Chem. 2021, 339, 128070. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhang, J.; Jia, Z.; Yang, X.; Zhou, Z. Intelligent pH indicator films containing anthocyanins extracted from blueberry peel for monitoring tilapia fillet freshness. J. Sci. Food Agric. 2021, 101, 1800–1811. [Google Scholar] [CrossRef] [PubMed]
- Velickovic, J.M.; Mitic, M.N.; Arsic, B.B.; Paunovic, D.Đ.; Stojanovic, B.T.; Veljkovic, J.N.; Kostic, D.A. HPLC analysis of extracts of fresh petals of Papaver rhoeas L. Stud. Univ. Babes-Bolyai Chem. 2019, 64, 154. [Google Scholar] [CrossRef]
- Farooque, S.; Rose, P.M.; Benohoud, M.; Blackburn, R.S.; Rayner, C.M. Enhancing the potential exploitation of food waste: Extraction, purification, and characterization of renewable specialty chemicals from blackcurrants (Ribes nigrum L.). J. Agric. Food Chem. 2018, 66, 12265–12273. [Google Scholar] [CrossRef]
- Santos, L.G.; Alves-Silva, G.F.; Martins, V.G. Active-intelligent and biodegradable sodium alginate films loaded with Clitoria ternatea anthocyanin-rich extract to preserve and monitor food freshness. Int. J. Biol. Macromol. 2022, 220, 866–877. [Google Scholar] [CrossRef]
- Zhao, M.; Nuerjiang, M.; Bai, X.; Feng, J.; Kong, B.; Sun, F.; Xia, X. Monitoring dynamic changes in chicken freshness at 4 °C and 25° C using pH-sensitive intelligent films based on sodium alginate and purple sweet potato peel extracts. Int. J. Biol. Macromol. 2022, 216, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Abdillah, A.A.; Lin, H.H.; Charles, A.L. Development of halochromic indicator film based on arrowroot starch/iota-carrageenan using Kyoho skin extract to monitor shrimp freshness. Int. J. Biol. Macromol. 2022, 211, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, A.; Mohammadi Nafchi, A.; Baghaei, H.; Nouri, L. Fabrication and characterization of a smart film based on cassava starch and pomegranate peel powder for monitoring lamb meat freshness. Food Sci. Nutr. 2022, 10, 3293–3301. [Google Scholar] [CrossRef] [PubMed]
- Capello, C.; Trevisol, T.C.; Pelicioli, J.; Terrazas, M.B.; Monteiro, A.R.; Valencia, G.A. Preparation and characterization of colorimetric indicator films based on chitosan/polyvinyl alcohol and anthocyanins from agri-food wastes. J. Polym. Environ. 2021, 29, 1616–1629. [Google Scholar] [CrossRef]
- He, Y.; Li, B.; Du, J.; Cao, S.; Liu, M.; Li, X.; Xu, D. Development of pH-responsive absorbent pad based on polyvinyl alcohol/agarose/anthocyanins for meat packaging and freshness indication. Int. J. Biol. Macromol. 2022, 201, 203–215. [Google Scholar] [CrossRef]
- Fernández-López, J.A.; Fernández-Lledó, V.; Angosto, J.M. New insights into red plant pigments: More than just natural colorants. RSC Adv. 2020, 10, 24669–24682. [Google Scholar] [CrossRef]
- Akbar Hussain, E.; Sadiq, Z.; Zia-Ul-Haq, M. Bioavailability of betalains. In Betalains: Biomolecular Aspects; Springer: Berlin/Heidelberg, Germany, 2018; pp. 165–183. [Google Scholar] [CrossRef]
- Šeremet, D.; Durgo, K.; Jokić, S.; Huđek, A.; Vojvodić Cebin, A.; Mandura, A.; Komes, D. Valorization of banana and red beetroot peels: Determination of basic macrocomponent composition, application of novel extraction methodology and assessment of biological activity in vitro. Sustainability 2020, 12, 4539. [Google Scholar] [CrossRef]
- Yao, X.; Hu, H.; Qin, Y.; Liu, J. Development of antioxidant, antimicrobial and ammonia-sensitive films based on quaternary ammonium chitosan, polyvinyl alcohol and betalains-rich cactus pears (Opuntia ficus-indica) extract. Food Hydrocoll. 2020, 106, 105896. [Google Scholar] [CrossRef]
- Fathordoobady, F.; Jarzębski, M.; Pratap-Singh, A.; Guo, Y.; Abd-Manap, Y. Encapsulation of betacyanins from the peel of red dragon fruit (Hylocereus polyrhizus L.) in alginate microbeads. Food Hydrocoll. 2021, 113, 106535. [Google Scholar] [CrossRef]
- Reyes-Martínez, A.; Antunes-Ricardo, M.; Gutiérrez-Uribe, J.; Santos-Díaz, M.D.S. Enhanced production and identification of antioxidants in in vitro cultures of the cacti Mammillaria candida and Turbinicarpus laui. Appl. Microbiol. Biotechnol. 2019, 103, 2583–2595. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Biological properties and applications of betalains. Molecules 2021, 26, 2520. [Google Scholar] [CrossRef]
- Hou, Y.; Liu, X.; Li, S.; Zhang, X.; Yu, S.; Zhao, G.R. Metabolic engineering of Escherichia coli for de novo production of betaxanthins. J. Agric. Food Chem. 2020, 68, 8370–8380. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Wang, G.; Fang, F.; Zhang, F.; Liao, H. Development of real-time intelligent films from red pitaya peel and its application in monitoring the freshness of pork. J. Sci. Food Agric. 2022, 102, 5512–5522. [Google Scholar] [CrossRef]
- Kanatt, S.R. Development of active/intelligent food packaging film containing Amaranthus leaf extract for shelf life extension of chicken/fish during chilled storage. Food Packag. 2020, 24, 100506. [Google Scholar] [CrossRef]
- Ateteallah, H.; Abd-Elkarim, N.; Hassan, N.A. Effect of adding beetroot juice and carrot pulps on rheological, chemical, nutritional and organoleptic properties of ice cream. JFDS 2019, 10, 175–179. [Google Scholar] [CrossRef]
- Hernández-Carranza, P.; Jattar-Santiago, K.Y.; Avila-Sosa, R.; Pérez-Xochipa, I.; Guerrero-Beltrán, J.A.; Ochoa-Velasco, C.E.; Ruiz-López, I.I. Antioxidant fortification of yogurt with red cactus pear peel and its mucilage. CYTA J. Food 2019, 17, 824–833. [Google Scholar] [CrossRef]
- Umair, M.; Jabbar, S.; Nasiru, M.M.; Lu, Z.; Zhang, J.; Abid, M.; Zhao, L. Ultrasound-assisted extraction of carotenoids from carrot pomace and their optimization through response surface methodology. Molecules 2021, 26, 6763. [Google Scholar] [CrossRef] [PubMed]
- Portillo-López, R.; Morales-Contreras, B.E.; Lozano-Guzmán, E.; Basilio-Heredia, J.; Muy-Rangel, M.D.; Ochoa-Martínez, L.A.; Rosas-Flores, W.; Morales-Castro, J. Vegetable oils as green solvents for carotenoid extraction from pumpkin (Cucurbita argyrosperma Huber) byproducts: Optimization of extraction parameters. J. Food Sci. 2021, 86, 3122–3136. [Google Scholar] [CrossRef]
- Fernandes, A.S.; do Nascimento, T.C.; Jacob-Lopes, E.; De Rosso, V.V.; Zepka, L.Q. Carotenoids: A brief overview on its structure, biosynthesis, synthesis, and applications. Prog. Carotenoid Res. 2018, 1, 1–17. [Google Scholar]
- Miękus, N.; Iqbal, A.; Marszałek, K.; Puchalski, C.; Świergiel, A. Green chemistry extractions of carotenoids from Daucus carota L.—Supercritical carbon dioxide and enzyme-assisted methods. Molecules 2019, 24, 4339. [Google Scholar] [CrossRef]
- Gungor, K.K.; Torun, M. Pumpkin peel valorization using green extraction technology to obtain β-carotene fortified mayonnaise. Waste Biomass Valorization 2022, 13, 4375–4388. [Google Scholar] [CrossRef]
- Sharma, A.; Dhiman, A.K.; Attri, S. Utilization of internal waste fluffy portion of Cucurbita maxima for extraction of ß-carotene pigment. Pigment. Resin Technol. 2020, 49, 81–88. [Google Scholar] [CrossRef]
- Lombardelli, C.; Benucci, I.; Esti, M. Novel food colorants from tomatoes: Stability of carotenoid-containing chromoplasts under different storage conditions. LWT 2021, 140, 110725. [Google Scholar] [CrossRef]
- Derrien, M.; Badr, A.; Gosselin, A.; Desjardins, Y.; Angers, P. Optimization of a green process for the extraction of lutein and chlorophyll from spinach by-products using response surface methodology (RSM). LWT 2017, 79, 170–177. [Google Scholar] [CrossRef]
- Qiu, N.W.; Jiang, D.C.; Wang, X.S.; Wang, B.S.; Zhou, F. Advances in the members and biosynthesis of chlorophyll family. Photosynthetic 2019, 57, 974–984. [Google Scholar] [CrossRef]
- Kuncham, V.S. The Impact of Light Irradiance, Chlorophyll Concentration and Solvent on Chlorophyll Absorbance Spectrum. Master’s Thesis, McGill University, Montreal, QC, Canada, 2021. [Google Scholar]
- Purkiewicz, A.; Pietrzak-Fiećko, R. Antioxidant properties of fruit and vegetable whey beverages and fruit and vegetable mousses. Molecules 2021, 26, 3126. [Google Scholar] [CrossRef]
- Carrillo, C.; Nieto, G.; Martinez-Zamora, L.; Ros, G.; Kamiloglu, S.; Munekata, P.E.; Barba, F.J. Novel approaches for the recovery of natural pigments with potential health effects. J. Agric. Food Chem. 2022, 70, 6864–6883. [Google Scholar] [CrossRef] [PubMed]
- Aznury, M.; Margerty, E.; Awwaliyah, S. Effect of fermentation time and percentage of moringa (Moringa oleifera) flour variations on vitamin c of yogurt. In 4th Forum in Research, Science, and Technology (FIRST-T1-T2-2020); Atlantis Press: Amsterdam, The Netherlands, 2021; pp. 376–383. [Google Scholar] [CrossRef]
- Rohajatien, U.; Hidayati, L.; Safitri, Y.A. The effect of using pegagan leaves (Centella Asiatica L. Urban) extract on the physical properties and antioxidant capacity of mochi ice cream. Bull. Culin. Art Hosp. 2022, 2, 20–24. [Google Scholar] [CrossRef]
- Molina, A.K.; Gomes, L.C.; Prieto, M.A.; Ferreira, I.C.; Pereira, C.; Dias, M.I.; Barros, L. Extraction of chlorophylls from Daucus carota L. and Solanum lycopersicum var. cerasiforme crop by-products. Food Chem. Adv. 2022, 1, 100048. [Google Scholar] [CrossRef]
- Chapman, J.; Ismail, A.E.; Dinu, C.Z. Industrial applications of enzymes: Recent advances, techniques, and outlooks. Catalysts 2018, 8, 238. [Google Scholar] [CrossRef]
- Lugani, Y.; Vemuluri, V.R. Extremophiles Diversity, Biotechnological Applications and Current Trends. In Extremophiles; CRC Press: Boca Raton, FL, USA, 2022; pp. 1–30. [Google Scholar]
- Liu, X.; Kokare, C. Microbial enzymes of use in industry. In Biotechnology of Microbial Enzymes; Academic Press: Cambridge, MA, USA, 2017; pp. 267–298. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. Microbial enzyme production using lignocellulosic food industry wastes as feedstock: A review. Bioengineering 2016, 3, 30. [Google Scholar] [CrossRef]
- Gómez-García, R.; Vilas-Boas, A.A.; Oliveira, A.; Amorim, M.; Teixeira, J.A.; Pastrana, L.; Pintado, M.M.; Campos, D.A. Impact of simulated human gastrointestinal digestion on the bioactive fraction of upcycled pineapple by-products. Foods 2022, 11, 126. [Google Scholar] [CrossRef]
- Ramli, A.N.M.; Hamid, H.A.; Zulkifli, F.H.; Zamri, N.; Bhuyar, P.; Manas, N.H.A. Physicochemical properties and tenderness analysis of bovine meat using proteolytic enzymes extracted from pineapple (Ananas comosus) and jackfruit (Artocarpus heterophyllus) by-products. J. Food Process. Preserv. 2021, 45, e15939. [Google Scholar] [CrossRef]
- Hussain, N.; Weng, C.H.; Munawar, N. Effects of different concentrations of pineapple core extract and maceration process on free-range chicken meat quality. Ital. J. Food Sci. 2022, 34, 124–131. [Google Scholar] [CrossRef]
- Singh, T.A.; Sarangi, P.K.; Singh, N.J. Tenderisation of meat by bromelain enzyme extracted from pineapple wastes. Int. J. Curr. Microbiol. 2018, 7, 3256–3264. [Google Scholar] [CrossRef]
- Rizqiati, H.; Nugraheni, A.; Susanti, S.; Fatmawati, L.; Nuryanto, N.; Arifan, F. Characteristic of isolated crude bromelain extract from cayenne pineapple crown in various drying temperature and its effect on meat texture. Food Res. 2021, 5, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, N.; Revathi, D.; Sheelu, G.; Yamuna Rani, K.; Sridhar, S.; Mehtab, V.; Sumana, C. Recent advances on sources and industrial applications of lipases. Biotechnol. Prog. 2018, 34, 5–28. [Google Scholar] [CrossRef]
- Okino-Delgado, C.H.; Pereira, M.S.; da Silva, J.V.I.; Kharfan, D.; do Prado, D.Z.; Fleuri, L.F. Lipases obtained from orange wastes: Commercialization potential and biochemical properties of different varieties and fractions. Biotechnol. Prog. 2019, 35, e2734. [Google Scholar] [CrossRef] [PubMed]
- Tambun, R.; Tambun, J.A.; Tarigan, I.A.A. Fatty acid production from avocado seed by activating Lipase Enzyme in the seed. IOP Conf. Ser. Mater. Sci. Eng. 2020, 725, 12075. [Google Scholar] [CrossRef]
- Shinde, A.; Kolhe, D.; Hase, D.P.; Chavan, M.J. A brief introduction about carica papaya linn. World J. Pharm. Res. 2020, 9. [Google Scholar] [CrossRef]
- Adiaha, M.S. Effect of nutritional, medicinal and pharmacological properties of papaya (Carica papaya Linn.) to human development: A review. World Sci. News 2017, 2, 238–249. [Google Scholar]
- Phothiwicha, A.; Thamsaad, K.; Boontranurak, K.; Sintuya, P.; Panyoyai, N. Utilization of papain extracted from Chaya and papaya stalks as halal beef tenderiser. IFSTJ 2021, 5, 1–5. [Google Scholar] [CrossRef]
- Kumar, D. A review of the use of microbial amylase in industry. Asian J. Res. Soc. Sci. Humanit. 2021, 11, 57–61. [Google Scholar] [CrossRef]
- Abdullah, T.; Nurhidayatullah, N.; Widiati, B. Seed waste of mango (Mangifera indica) as raw material glucose syrup alternative substitute for synthetic sweetener. J. Pijar Mipa 2022, 17, 271–275. [Google Scholar] [CrossRef]
- Mushtaq, Q.; Irfan, M.; Tabssum, F.; Iqbal Qazi, J. Potato peels: A potential food waste for amylase production. J. Food Process Eng. 2017, 40, e12512. [Google Scholar] [CrossRef]
- Rao, M.; Bast, A.; De Boer, A. Valorized food processing by-products in the EU: Finding the balance between safety, nutrition, and sustainability. Sustainability 2021, 13, 4428. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Álvarez-Rivera, G.; Valdés, A.; Ibáñez, E.; Cifuentes, A. Food by-products and food wastes: Are they safe enough for their valorization? Trends Food Sci. Technol. 2021, 114, 133–147. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).































