Recovery of Polyphenolic Compounds and Vitamins from the Stinging Nettle Leaves: Thermal and Behavior and Biological Activity of Obtained Extracts
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Profile
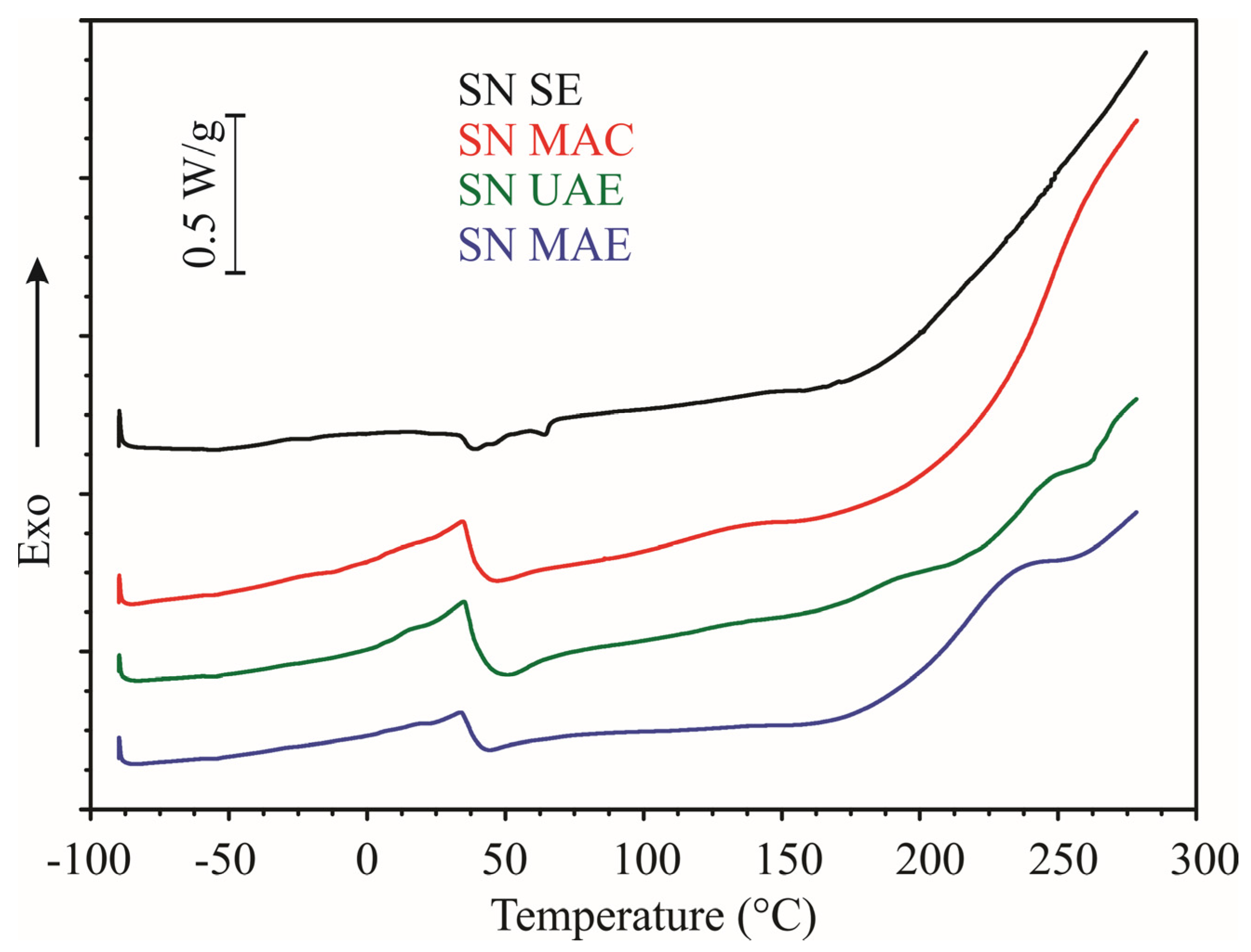
2.2. Thermal Properties
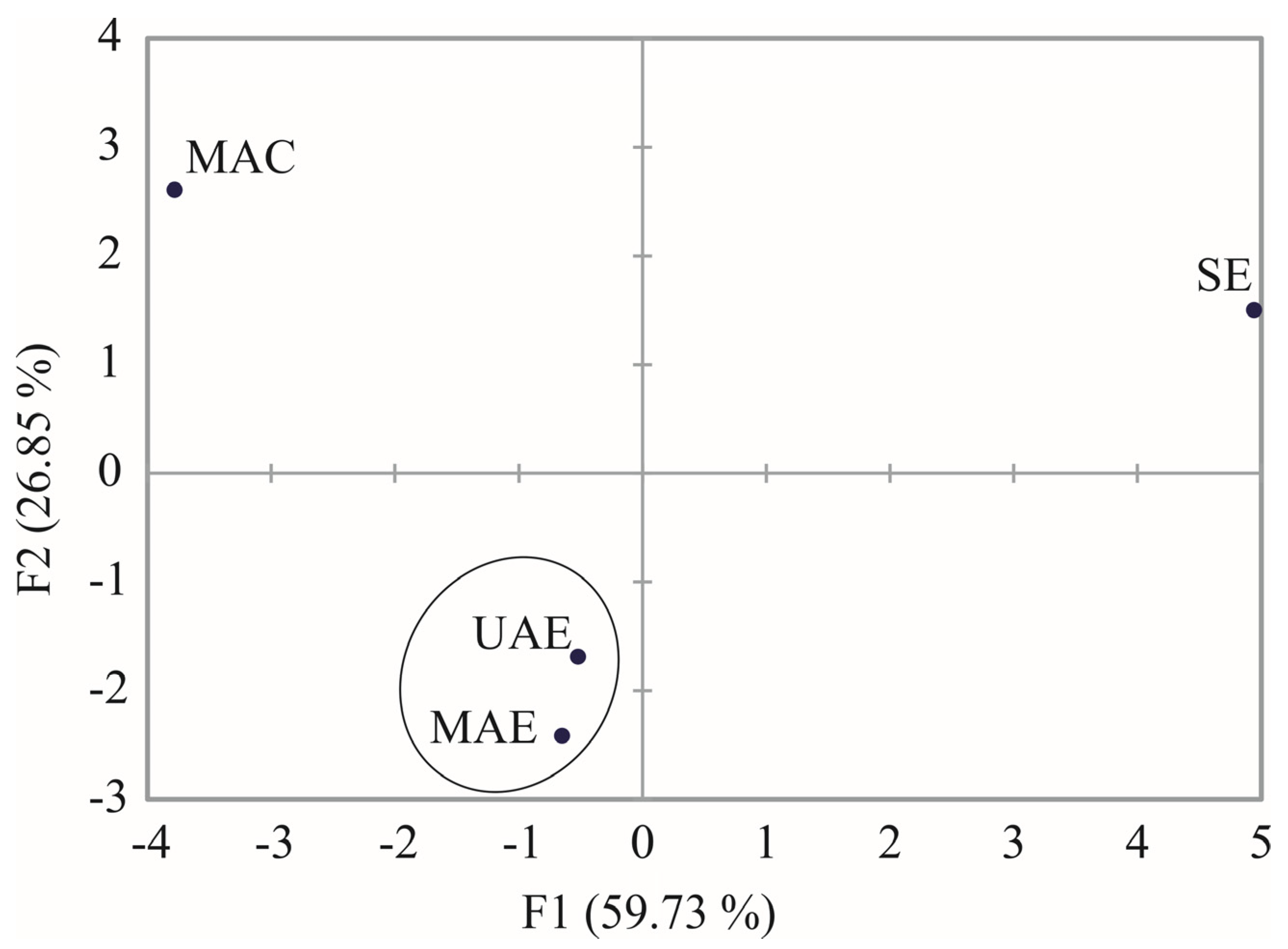
2.3. Principal Component Analysis
3. Materials and Methods
3.1. Plant Material
3.1.1. Extraction Procedures
3.1.2. Polyphenolic Profile
3.1.3. Vitamin Content
3.1.4. Thermal Analysis
3.1.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Orčić, D.; Francišković, M.; Bekvalac, K.; Svirčev, E.; Beara, I.; Lesjak, M.; Mimica-Dukić, N. Quantitative determination of plant phenolics in Urtica dioica extracts by high-performance liquid chromatography coupled with tandem mass spectrometric detection. Food Chem. 2014, 143, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Pinelli, P.; Ieri, F.; Vignolini, P.; Bacci, L.; Baronti, S.; Romani, A. Extraction and HPLC analysis of phenolic compounds in leaves, stalks, and textile fibers of Urtica dioica L. J. Agric. Food Chem. 2008, 56, 9127–9132. [Google Scholar] [CrossRef] [PubMed]
- Leporatti, M.; Corradi, L. Ethnopharmacobotanical remarks on the Province of Chieti town (Abruzzo, Central Italy). J. Ethnopharmacol. 2001, 74, 17–40. [Google Scholar] [CrossRef] [PubMed]
- Di Virgilio, N.; Papazoglou, E.G.; Jankauskiene, Z.; Di Lonardo, S.; Praczyk, M.; Wielgusz, K. The potential of stinging nettle (Urtica dioica L.) as a crop with multiple uses. Ind. Crops Prod. 2015, 68, 42–49. [Google Scholar] [CrossRef]
- Güder, A.; Korkmaz, H. Evaluation of in-vitro antioxidant properties of hydroalcoholic solution extracts Urtica dioica L., Malva neglecta Wallr. and their mixture. Iran. J. Pharm. Res. 2012, 11, 913–923. [Google Scholar] [PubMed]
- Ghaima, K.K.; Hashim, N.M.; Ali, S.A. Antibacterial and antioxidant activities of ethyl acetate extract of nettle (Urtica dioica) and dandelion (Taraxacum officinale). J. Appl. Pharm. Sci. 2013, 3, 96–99. [Google Scholar] [CrossRef]
- Gülçin, İ.; Küfrevioǧlu, Ö.İ.; Oktay, M.; Büyükokuroǧlu, M.E. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J. Ethnopharmacol. 2004, 90, 205–215. [Google Scholar] [CrossRef]
- Kataki, M.S.; Murugamani, V.; Rajkumari, A.; Mehra, P.S.; Awasthi, D.; Yadav, R.S. Antioxidant, hepatoprotective, and anthelmintic activities of methanol extract of Urtica dioica L. leaves. Pharm. Crop. 2012, 3, 38–46. [Google Scholar] [CrossRef]
- Kukric, Z.; Topalic-Trivunovic, L.; Kukavica, B.; Matos, S.; Pavicic, S.; Boroja, M.; Savic, A. Characterization of antioxidant and antimicrobial activities of nettle leaves (Urtica dioica L.). Acta Period. Technol. 2012, 43, 257–272. [Google Scholar] [CrossRef]
- Upton, R. Stinging nettles leaf (Urtica dioica L.): Extraordinary vegetable medicine. J. Herb. Med. 2013, 3, 9–38. [Google Scholar] [CrossRef]
- Đurović, S.; Pavlić, B.; Šorgić, S.; Popov, S.; Savić, S.; Petronijević, M.; Radojković, M.; Cvetanović, A.; Zeković, Z. Chemical composition of stinging nettle leaves obtained by different analytical approaches. J. Funct. Foods 2017, 32, 18–26. [Google Scholar] [CrossRef]
- Đurović, S.; Šorgić, S.; Popov, S.; Radojković, M.; Zeković, Z. Isolation and GC Analysis of Fatty Acids: Study Case of Stinging Nettle Leaves. In Carboxylic Acid-Key Role in Life Sciences; InTech: London, UK, 2018. [Google Scholar]
- Đurović, S.; Zeković, Z.; Šorgić, S.; Popov, S.; Vujanović, M.; Radojković, M. Fatty acid profile of stinging nettle leaves: Application of modern analytical procedures for sample preparation and analysis. Anal. Methods 2018, 10, 1080–1087. [Google Scholar] [CrossRef]
- Đurović, S.; Šorgić, S.; Popov, S.; Pezo, L.; Mašković, P.; Blagojević, S.; Zeković, Z. Recovery of biologically active compounds from stinging nettle leaves part I: Supercritical carbon dioxide extraction. Food Chem. 2022, 373, 131724. [Google Scholar] [CrossRef] [PubMed]
- Guil-Guerrero, J.L.; Rebolloso-Fuentes, M.M.; Isasa, M.E.T. Fatty acids and carotenoids from stinging nettle (Urtica dioica L.). J. Food Compos. Anal. 2003, 16, 111–119. [Google Scholar] [CrossRef]
- Otles, S.; Yalcin, B. Phenolic Compounds Analysis of Root, Stalk, and Leaves of Nettle. Sci. World J. 2012, 2012, 564367. [Google Scholar] [CrossRef]
- Zeković, Z.; Cvetanović, A.; Švarc-Gajić, J.; Gorjanović, S.; Sužnjević, D.; Mašković, P.; Savić, S.; Radojković, M.; Đurović, S. Chemical and biological screening of stinging nettle leaves extracts obtained by modern extraction techniques. Ind. Crops Prod. 2017, 108, 423–430. [Google Scholar] [CrossRef]
- de Sánchez-Mata, M.C.; Tardío, J. (Eds.) Mediterranean Wild Edible Plants; Springer: New York, NY, USA, 2016; ISBN 978-1-4939-3327-3. [Google Scholar]
- Fiol, C.; Prado, D.; Mora, M.; Alava, J.I. Nettle cheese: Using nettle leaves (Urtica dioica) to coagulate milk in the fresh cheese making process. Int. J. Gastron. Food Sci. 2016, 4, 19–24. [Google Scholar] [CrossRef]
- Krawęcka, A.; Sobota, A.; Pankiewicz, U.; Zielińska, E.; Zarzycki, P. Stinging Nettle (Urtica dioica L.) as a Functional Component in Durum Wheat Pasta Production: Impact on Chemical Composition, In Vitro Glycemic Index, and Quality Properties. Molecules 2021, 26, 6909. [Google Scholar] [CrossRef]
- Đurović, S.; Vujanović, M.; Radojković, M.; Filipović, J.; Filipović, V.; Gašić, U.; Tešić, Ž.; Mašković, P.; Zeković, Z. The functional food production: Application of stinging nettle leaves and its extracts in the baking of a bread. Food Chem. 2020, 312, 126091. [Google Scholar] [CrossRef]
- Mašković, P.; Veličković, V.; Mitić, M.; Đurović, S.; Zeković, Z.; Radojković, M.; Cvetanović, A.; Švarc-Gajić, J.; Vujić, J. Summer savory extracts prepared by novel extraction methods resulted in enhanced biological activity. Ind. Crops Prod. 2017, 109, 875–881. [Google Scholar] [CrossRef]
- Bucar, F.; Britzmann, B.; Streit, B.; Weigend, M. LC-PDA-MS-profiles of phenolic compounds in extracts of aerial parts of Urtica species. Planta Med. 2006, 72, 152. [Google Scholar] [CrossRef]
- Roleira, F.M.F.; Tavares-da-Silva, E.J.; Varela, C.L.; Costa, S.C.; Silva, T.; Garrido, J.; Borges, F. Plant derived and dietary phenolic antioxidants: Anticancer properties. Food Chem. 2015, 183, 235–258. [Google Scholar] [CrossRef]
- Ćetković, G.S.; Čanadanović-Brunet, J.M.; Djilas, S.M.; Tumbas, V.T.; Markov, S.L.; Cvetković, D.D. Antioxidant potential, lipid peroxidation Inhibition and antimicrobial activities of Satureja montana L. subsp. kitaibelii extracts. Int. J. Mol. Sci. 2007, 8, 1013–1027. [Google Scholar] [CrossRef]
- Fernández-López, J.; Pérez-Alvarez, A.J.; Viuda-Martos, M. Beneficial Health Effects of Bioactive Compounds Present in Spices and Aromatic Herbs. In Studies in Natural Products Chemistry: Bioactive Natural Products Volume 37; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Nederlands, 2012; pp. 115–134. [Google Scholar]
- Di Carlo, G.; Mascolo, N.; Izzo, A.A.; Capasso, F. Flavonoids: Old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999, 65, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.G. Antioxidant activity of medicinal and aromatic plants. A review. Flavour Fragr. J. 2010, 25, 291–312. [Google Scholar] [CrossRef]
- Tsao, R.; Deng, Z. Separation procedures for naturally occurring antioxidant phytochemicals. J. Chromatogr. B 2004, 812, 85–99. [Google Scholar] [CrossRef]
- Rembe, J.-D.; Fromm-Dornieden, C.; Stuermer, E.K. Effects of Vitamin B Complex and Vitamin C on Human Skin Cells: Is the Perceived Effect Measurable? Adv. Skin Wound Care 2018, 31, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Sahib, A.S.; Al-Jawad, F.H.; Alkaisy, A.A. Effect of antioxidants on the incidence of wound infection in burn patients. Ann. Burns Fire Disasters 2010, 23, 199–205. [Google Scholar] [PubMed]
- Lima, C.; Pereira, A.; Silva, J.; Oliveira, L.; Resck, M.; Grechi, C.; Bernardes, M.; Olímpio, F.; Santos, A.; Incerpi, E.; et al. Ascorbic acid for the healing of skin wounds in rats. Brazilian J. Biol. 2009, 69, 1195–1201. [Google Scholar] [CrossRef]
- Boyce, S.T.; Supp, A.P.; Swope, V.B.; Warden, G.D. Vitamin C Regulates Keratinocyte Viability, Epidermal Barrier, and Basement Membrane In Vitro, and Reduces Wound Contraction After Grafting of Cultured Skin Substitutes. J. Investig. Dermatol. 2002, 118, 565–572. [Google Scholar] [CrossRef]
- Gisondi, P.; Fantuzzi, F.; Malerba, M.; Girolomoni, G. Folic acid in general medicine and dermatology. J. Dermatol. Treat. 2007, 18, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S. State diagram of foods: Its potential use in food processing and product stability. Trends Food Sci. Technol. 2006, 17, 129–141. [Google Scholar] [CrossRef]
- Zlatanović, S.; Ostojić, S.; Micić, D.; Rankov, S.; Dodevska, M.; Vukosavljević, P.; Gorjanović, S. Thermal behaviour and degradation kinetics of apple pomace flours. Thermochim. Acta 2019, 673, 17–25. [Google Scholar] [CrossRef]
- Micić, D.M.; Ostojić, S.B.; Simonović, M.B.; Pezo, L.L.; Simonović, B.R. Thermal behavior of raspberry and blackberry seed flours and oils. Thermochim. Acta 2015, 617, 21–27. [Google Scholar] [CrossRef]
- Mašković, P.Z.; Veličković, V.; Đurović, S.; Zeković, Z.; Radojković, M.; Cvetanović, A.; Švarc-Gajić, J.; Mitić, M.; Vujić, J. Biological activity and chemical profile of Lavatera thuringiaca L. extracts obtained by different extraction approaches. Phytomedicine 2018, 38, 118–124. [Google Scholar] [CrossRef]
- Cvetanović, A.; Švarc-Gajić, J.; Gašić, U.; Tešić, Ž.; Zengin, G.; Zeković, Z.; Đurović, S. Isolation of apigenin from subcritical water extracts: Optimization of the process. J. Supercrit. Fluids 2017, 120, 32–42. [Google Scholar] [CrossRef]

| Compound | Content (mg/L) | |||
|---|---|---|---|---|
| SN SE | SN MAC | SN UAE | SN MAE | |
| Protocatechuic acid | 3.41 ± 0.10 b | ND * | 4.65 ± 0.20 a | ND |
| p-Hydroxybenzoic acid | 3.34 ± 0.12 a | ND | 1.09 ± 0.06 c | 1.42 ± 0.03 b |
| Caffeic acid | 31.65 ± 0.52 b | 0.73 ± 0.02 d | 20.98 ± 0.52 c | 53.78 ± 0.53 a |
| Vanillic acid | 2.18 ± 0.09 a | 2.09 ± 0.12 a | 1.09 ± 0.01 c | 1.42 ± 0.01 b |
| Aesculin | 3.50 ± 0.15 a | 0.15 ± 0.00 c | 1.93 ± 0.06 b | 3.57 ± 0.03 a |
| 5-O-Caffeoylquinic acid | 152.38 ± 2.25 a | 0.29 ± 0.01 d | 31.08 ± 0.36 c | 70.99 ± 0.46 b |
| p-Coumaric acid | 3.15 ± 0.09 d | 5.74 ± 0.21 b | 4.64 ± 0.05 c | 10.00 ± 0.11 a |
| Ferulic acid | 2.86 ± 0.08 b | ND | 1.81 ± 0.03 c | 6.61 ± 0.09 a |
| p-Hydroxyphenylacetic acid | 1.49 ± 0.05 a | 1.55 ± 0.06 a | 0.30 ± 0.00 c | 0.91 ± 0.03 b |
| Quercetin-3-O-galactoside | 11.70 ± 0.15 a | 0.01 ± 0.00 b | 0.05 ± 0.00 b | 0.14 ± 0.01 b |
| Rutin | 43.26 ± 0.36 a | 0.04 ± 0.00 d | 1.03 ± 0.03 c | 2.57 ± 0.08 b |
| Apigenin-7-O-glucoside | 0.06 ± 0.00 a | ND | ND | 0.03 ± 0.00 b |
| Quercetin | 4.32 ± 0.16 | ND | ND | ND |
| Luteolin | 0.10 ± 0.00 | ND | ND | ND |
| Naringin | 7.38 ± 0.11 a | ND | 2.23 ± 0.02 c | 3.68 ± 0.06 b |
| Kaempferol | 0.40 ± 0.03 | ND | ND | ND |
| Apigenin | 0.06 ± 0.00 | ND | ND | ND |
| Isorhamnetin-3-O-rutinoside | 7.65 ± 0.09 a | ND | 0.13 ± 0.00 b | 0.17 ± 0.00 b |
| Taxifolin | 0.12 ± 0.01 d | 0.45 ± 0.01 b | 0.25 ± 0.00 c | 0.61 ± 0.00 a |
| Isorhamnetin-3-O-glucoside | 16.54 ± 0.22 a | ND | 0.15 ± 0.00 b | 0.09 ± 0.00 b |
| Daidzein | ND | 0.04 ± 0.00 b | 0.03 ± 0.00 c | 0.08 ± 0.00 a |
| Eriodictyol | 0.12 ± 0.01 | ND | ND | ND |
| Chrysoeriol | 0.11 ± 0.01 | ND | ND | ND |
| Chrysin | 0.04 ± 0.00 | ND | ND | ND |
| Acacetin | 0.02 ± 0.00 | ND | ND | ND |
| Genkwanin | 0.03 ± 0.00 | ND | ND | ND |
| Galangin | 0.09 ± 0.00 | ND | ND | ND |
| Kaempferide | 0.03 ± 0.00 | ND | ND | ND |
| Total | 295.99 | 11.09 | 71.44 | 156.07 |
| Extraction Technique | Vitamin/Content (mg/L) | ||||
|---|---|---|---|---|---|
| C | B1 | B2 | B3 | B6 | |
| SN SE | ND * | ND | 89.63 ± 0.22 a | ND | 1.22 ± 0.03 c |
| SN MAC | 6.69 ± 0.09 c | 4.52 ± 0.05 b | 16.38 ± 0.19 d | ND | ND |
| SN UAE | 74.89 ± 0.12 a | 3.46 ± 0.08 c | 40.27 ± 0.11 c | 28.37 ± 0.09 b | 104.15 ± 1.22 a |
| SN MAE | 60.17 ± 0.13 b | 6.70 ± 0.09 a | 51.16 ± 0.10 b | 197.35 ± 1.05 a | 7.14 ± 0.06 b |
| Parameter | SN SE | SN MAC | SN UAE | SN MAE |
|---|---|---|---|---|
| I weight loss (%) | 1.3 ± 0.6 ab | 2.3 ± 0.5 a | 1.1 ± 0.5 ab | 0.9 ± 0.6 b |
| Ts1 (°C) | 29.3 ± 1.7 a | 25.0 ± 2.5 a | 25.8 ± 2.3 a | 25.1 ± 2.1 a |
| Te1 (°C) | 144.4 ± 2.1 ab | 147.8 ± 1.3 a | 143.3 ± 1.6 ab | 142.9 ± 2.1 b |
| II weight loss (%) | 59.2 ± 2.1 a | 18.3 ± 1.9 c | 32.7 ± 1.7 b | 31.1 ± 2.0 b |
| Ts2 (°C) | 144.4 ± 2.1 ab | 147.8 ± 1.3 a | 143.3 ± 1.6 ab | 142.9 ± 2.1 b |
| Te2 (°C) | 380.3 ± 2.2 a | 308.5 ± 1.4 d | 334.7 ± 2.4 c | 339.7 ± 1.3 b |
| Tp1 (°C) | 194.9 ± 2.4 b | 234.9 ± 1.8 a | 189.2 ± 1.1 c | 233.9 ± 1.4 a |
| Tp2 (°C) | 259.2 ± 2.5 b | / | 241.6 ± 1.4 c | 266.9 ± 1.4 a |
| Tp3 (°C) | 333.5 ± 1.6 a | / | 316.6 ± 2.1 b | 318.2 ± 2.3 b |
| III weight loss (%) | 14.1 ± 1.9 b | 21.1 ± 1.7 a | 13.0 ± 1.2 b | 19.2 ± 1.7 a |
| Ts3 (°C) | 380.3 ± 2.2 a | 308.5 ± 1.4 d | 334.7 ± 2.4 c | 339.7 ± 1.3 b |
| Te3 (°C) | 697.7 ± 1.3 a | 507.3 ± 1.5 c | 565.0 ± 2.0 b | 563.4 ± 2.2 b |
| IV wieght loss (%) | / | 20.1 ± 0.6 a | 10.6 ± 1.1 b | 7.9 ± 1.0 c |
| Ts4 (°C) | / | 507.3 ± 1.5 b | 565.0 ± 2.0 a | 563.4 ± 2.2 a |
| Te4 (°C) | / | 697.8 ± 2.3 a | 697.8 ± 2.2 a | 697.8 ± 1.2 a |
| Residue at 700 °C (%) | 25.4 ± 1.5 b | 38.3 ± 1.3 a | 42.6 ± 2.1 a | 40.9 ± 2.6 a |
| F1 | F2 | |
|---|---|---|
| Caffeic acid | 0.4492 | −0.7205 |
| Vanillic acid | 0.2440 | 0.8956 |
| Aesculin | 0.7649 | −0.5315 |
| 5-O-Caffeoylquinic acid | 0.9642 | 0.0101 |
| p-Coumaric acid | −0.4689 | −0.5740 |
| p-Hydroxyphenylacetic acid | 0.1206 | 0.8365 |
| Quercetin-3-O-galactoside | 0.9123 | 0.4036 |
| Rutin | 0.9244 | 0.3708 |
| Taxifolin | −0.6942 | −0.3446 |
| Total | 0.9558 | −0.0286 |
| B2 | 0.9860 | −0.0661 |
| I weight loss | −0.5049 | 0.8625 |
| Ts1 | 0.9409 | 0.3271 |
| Te1 | −0.4669 | 0.8839 |
| II weight loss | 0.9987 | 0.0479 |
| Ts2 | −0.4669 | 0.8839 |
| Te2 | 0.9954 | −0.0560 |
| Tp1 | −0.6495 | 0.1095 |
| III weight loss | −0.6857 | 0.2724 |
| Ts3 | 0.9954 | −0.0560 |
| Te3 | 0.9959 | 0.0884 |
| Residue at 700 °C | −0.7980 | −0.5773 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Đurović, S.; Micić, D.; Šorgić, S.; Popov, S.; Gašić, U.; Tosti, T.; Kostić, M.; Smyatskaya, Y.A.; Blagojević, S.; Zeković, Z. Recovery of Polyphenolic Compounds and Vitamins from the Stinging Nettle Leaves: Thermal and Behavior and Biological Activity of Obtained Extracts. Molecules 2023, 28, 2278. https://doi.org/10.3390/molecules28052278
Đurović S, Micić D, Šorgić S, Popov S, Gašić U, Tosti T, Kostić M, Smyatskaya YA, Blagojević S, Zeković Z. Recovery of Polyphenolic Compounds and Vitamins from the Stinging Nettle Leaves: Thermal and Behavior and Biological Activity of Obtained Extracts. Molecules. 2023; 28(5):2278. https://doi.org/10.3390/molecules28052278
Chicago/Turabian StyleĐurović, Saša, Darko Micić, Saša Šorgić, Saša Popov, Uroš Gašić, Tomislav Tosti, Marija Kostić, Yulia A. Smyatskaya, Stevan Blagojević, and Zoran Zeković. 2023. "Recovery of Polyphenolic Compounds and Vitamins from the Stinging Nettle Leaves: Thermal and Behavior and Biological Activity of Obtained Extracts" Molecules 28, no. 5: 2278. https://doi.org/10.3390/molecules28052278
APA StyleĐurović, S., Micić, D., Šorgić, S., Popov, S., Gašić, U., Tosti, T., Kostić, M., Smyatskaya, Y. A., Blagojević, S., & Zeković, Z. (2023). Recovery of Polyphenolic Compounds and Vitamins from the Stinging Nettle Leaves: Thermal and Behavior and Biological Activity of Obtained Extracts. Molecules, 28(5), 2278. https://doi.org/10.3390/molecules28052278










