Identification of Sildenafil Compound in Selected Drugs Using X-ray Study and Thermal Analysis
Abstract
1. Introduction
2. Results and Discussion
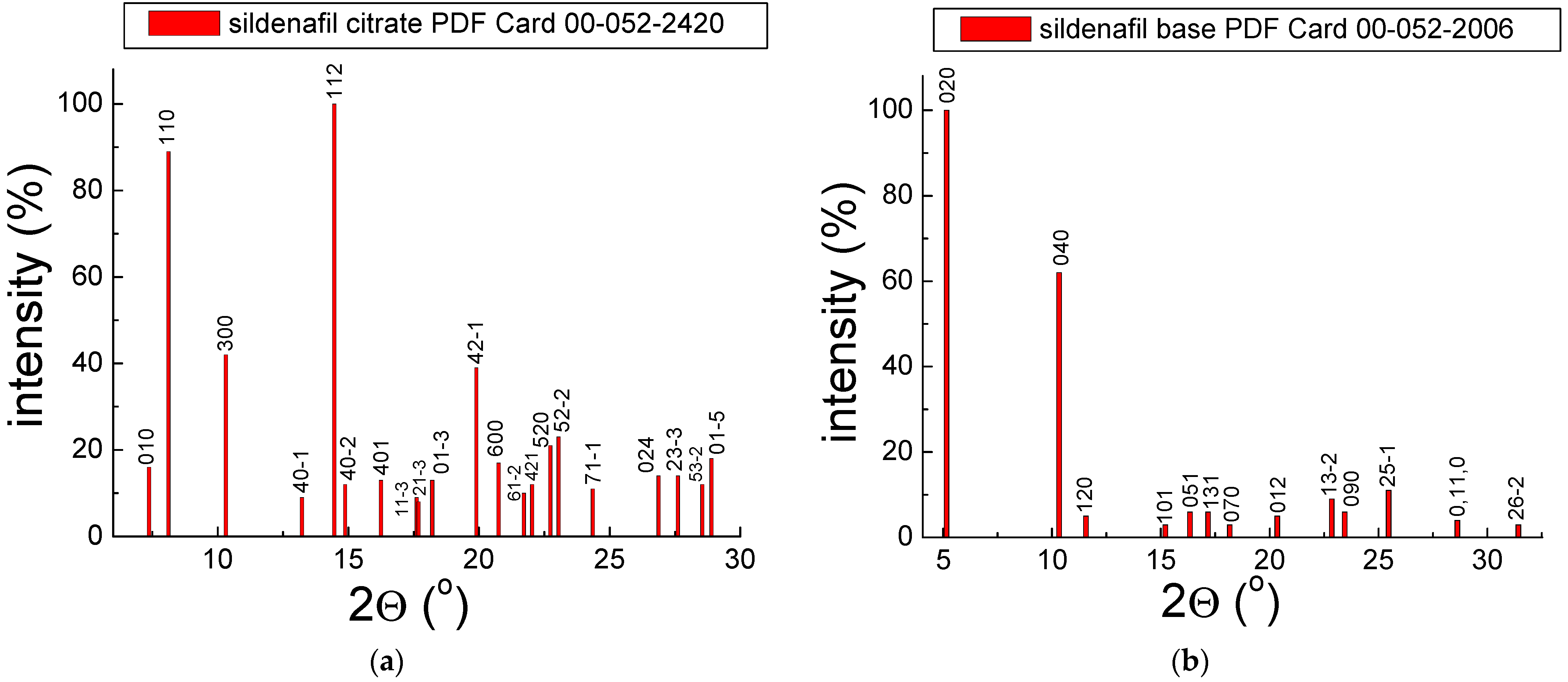
2.1. X-ray Analysis
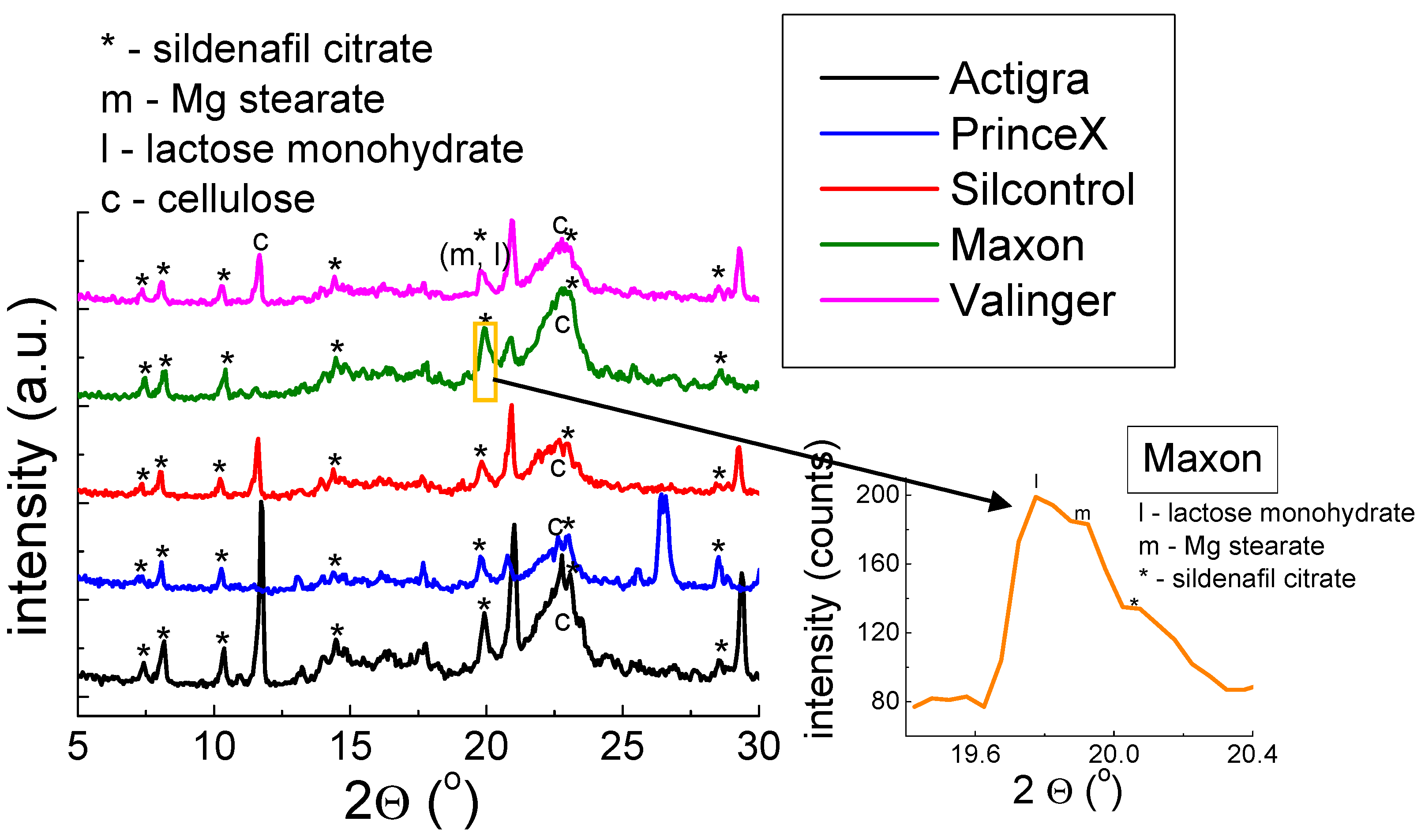
2.1.1. X-ray Study for Drugs with Sildenafil Citrate
2.1.2. X-ray Study for Drugs with Sildenafil Base
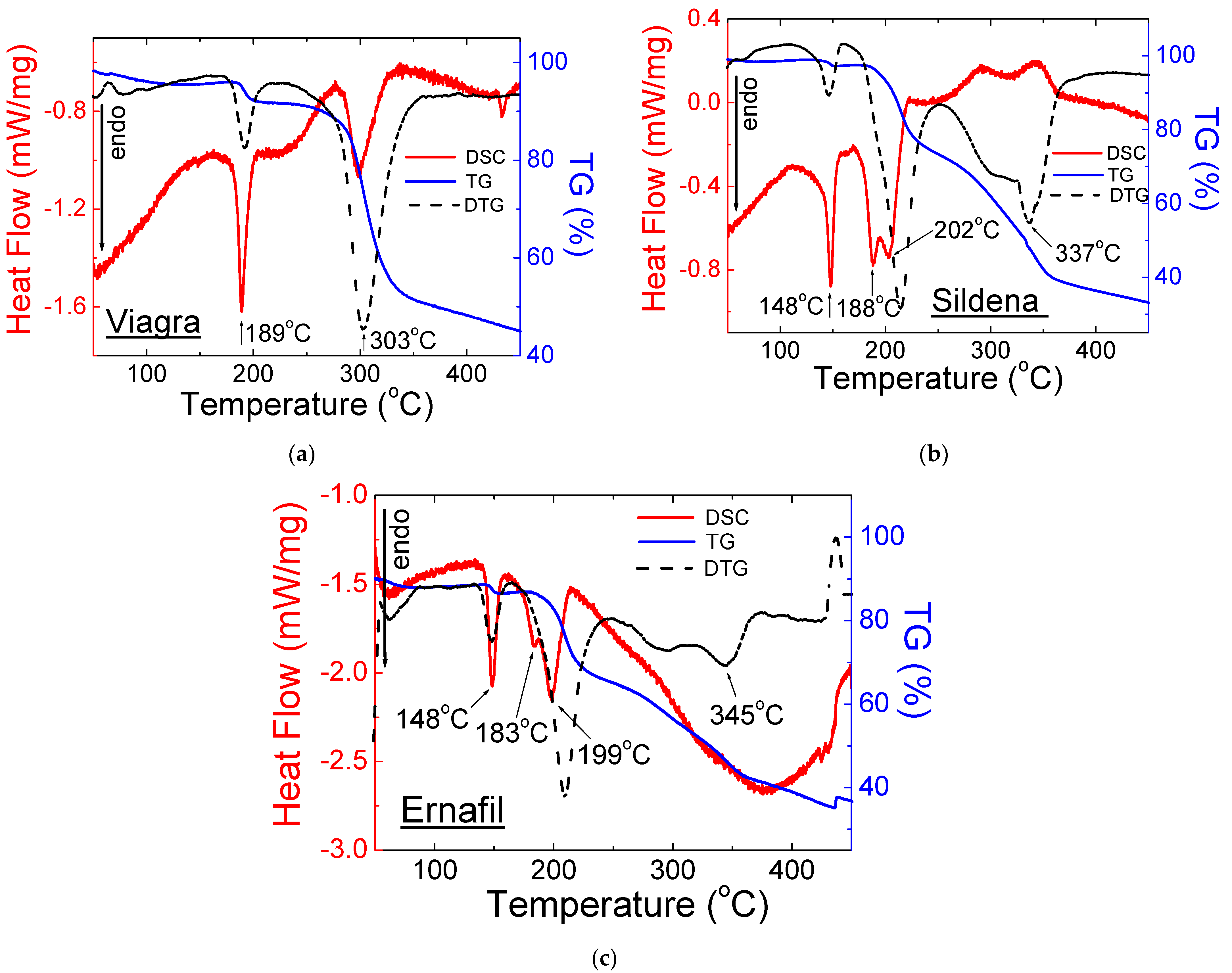
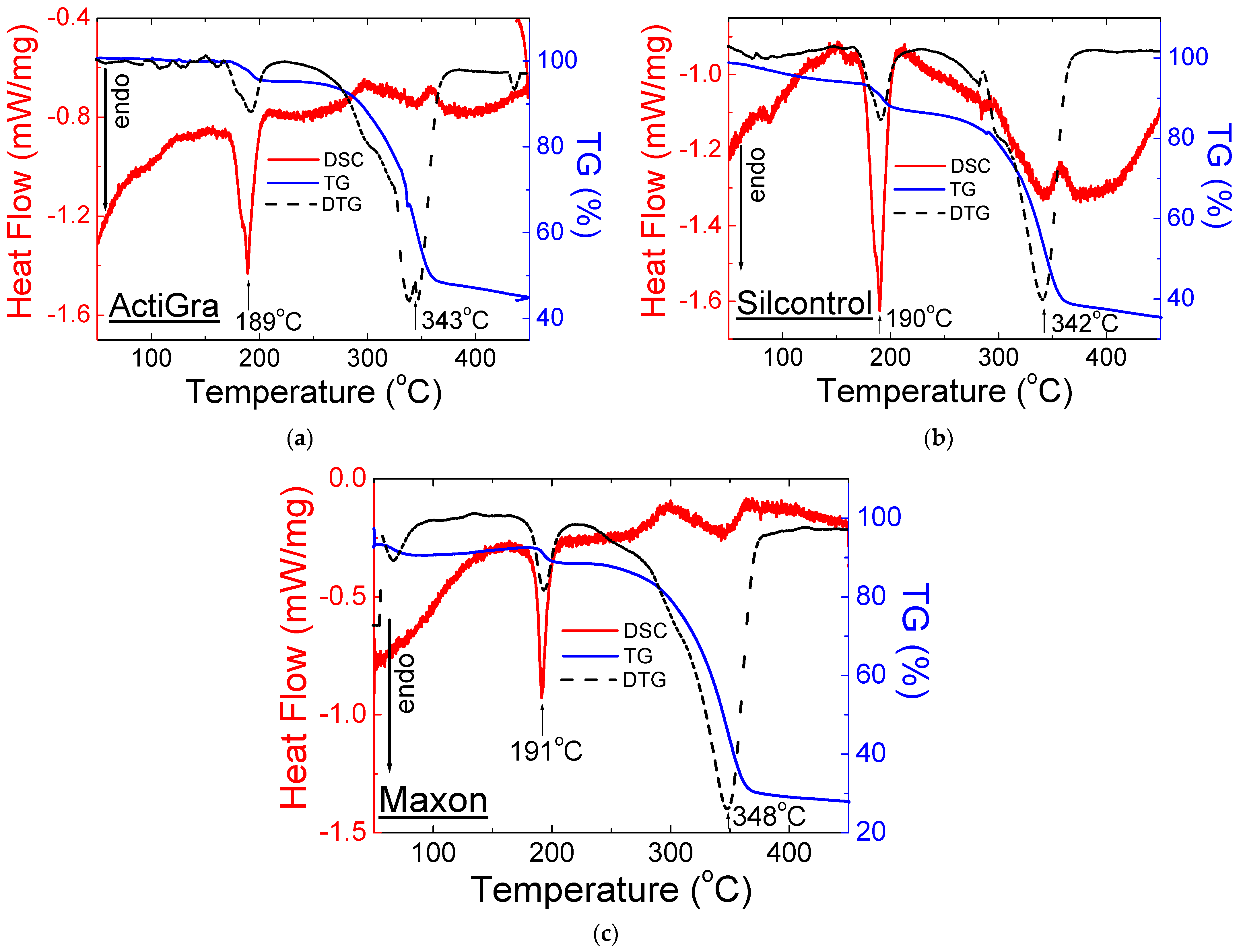
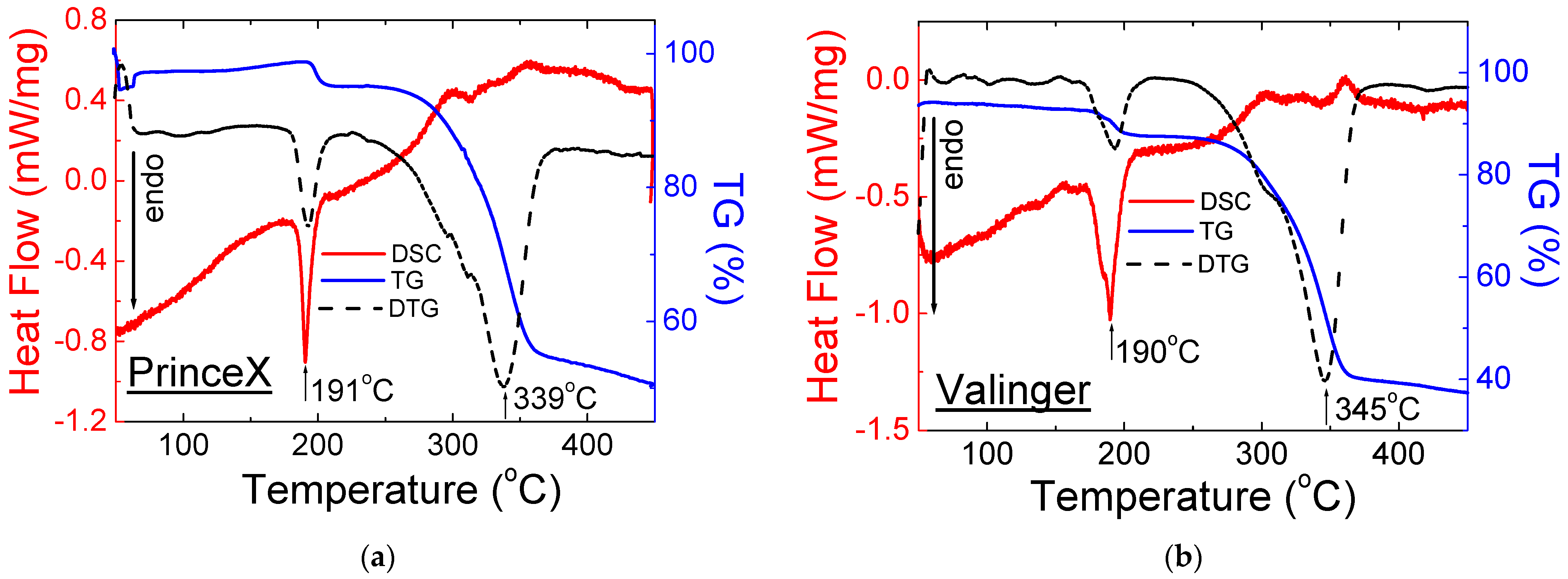
2.2. Thermal Analysis
2.2.1. Drugs with Sildenafil Citrate
2.2.2. Drugs with Sildenafil Base
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. X-ray Analysis of Samples of Selected Drugs Containing Sildenafil
3.2.2. Thermal Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sildenafil DrugBank. Available online: https://go.drugbank.com/drugs/DB00203 (accessed on 30 January 2023).
- Bell, A.S.; Brown, D.; Terrett, N.K. (Pfizer Inc.): Pyrazolopyrimidinone Antianginal Agents. US 5,250,534, 5 October 1993. [Google Scholar]
- Javaroni, V.; Neves, M.F. Erectile dysfunction and hypertension: Impact on cardiovascular risk and treatment. Int. J. Hypertens. 2012, 2012, 627278. [Google Scholar] [CrossRef] [PubMed]
- Yafi, F.A.; Jenkins, L.; Albersen, M.; Corona, G.; Isidori, A.M.; Goldfarb, S.; Maggi, M.; Nelson, C.J.; Parish, S.; Salonia, A.; et al. Erectile dysfunction. Nat. Rev. Dis. Prim. 2016, 2, 16003. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.B. Option and Final Order, Civil No. 2:10cv128; United States District Court Eastern District of Virginia, Norfolk Division: Norfolk, VA, USA, 2015.
- Viagra. Available online: https://www.viagra.com/Taking-viagra (accessed on 30 January 2023).
- Pentakova, M.; Jampilek, J. Patent history of Viagra®. Chem. Listy 2015, 109, 415–417. [Google Scholar]
- Pfizer Inc. Financial Report. 2012. Available online: http://www.pfizer.com/investors/financial_reports/financial_reports (accessed on 30 January 2023).
- Mikulic, M. Pfizer’s Viagra Revenue Worldwide 2003–2019. Last Update: 27 July 2022. Available online: https://www.statista.com/statistics/264827/pfizers-worldwide-viagra-revenue-since-2003/ (accessed on 30 January 2023).
- Sansone, A.; Cuzin, B.; Jannini, E.A. Facing counterfeit medications in sexual medicine. A systematic scoping review on social strategies and technological solutions. Sex Med. 2021, 9, 100437. [Google Scholar] [CrossRef] [PubMed]
- Jendrzejewska, I. Application of X-Ray powder diffraction for analysis of selected dietary supplements containing magnesium and calcium. Front. Chem. 2020, 8, 672. [Google Scholar] [CrossRef] [PubMed]
- Maurin, J.K.; Plucinski, F.; Mazurek, A.P.; Fijalek, Z. The usefulness of simple X-ray powder diffraction analysis for counterfeit control–The Viagra® example. J. Pharm. Biomed. Anal. 2007, 43, 1514. [Google Scholar] [CrossRef] [PubMed]
- Jendrzejewska, I.; Goryczka, T.; Pietrasik, E.; Klimontko, J.; Jampilek, J. X-ray and thermal analysis of selected drugs containing acetaminophen. Molecules 2020, 25, 5909. [Google Scholar] [CrossRef] [PubMed]
- Jendrzejewska, I.; Musiol, R.; Goryczka, T.; Pietrasik, E.; Klimontko, J.; Jampilek, J. The useful of X-ray diffraction and thermal analysis to study dietary supplements containing iron. Molecules 2022, 27, 197. [Google Scholar] [CrossRef] [PubMed]
- Jendrzejewska, I.; Zajdel, P.; Pietrasik, E.; Barsova, Z.; Goryczka, T. Application of X-ray powder diffraction and differential scanning calorimetry for identification of counterfeit drugs. Monatsh. Chem. 2018, 149, 977–985. [Google Scholar] [CrossRef] [PubMed]
- The ICDD PDF-4; The International Centre for Diffraction Data: Newtown Township, PA, USA, 2018.
- Bojarski, Z.; Lągiewka, E. X-ray Structural Analysis; PWN: Warszawa, Poland, 1988. [Google Scholar]
- DeWitt, K.M. X-ray Powder Diffraction Method Development and Validation for the Identification of Counterfeit Pharmaceuticals; U.S. Food and Drug Administration: Cincinnati, OH, USA, 2015.
- USP Pharmacopeial Convention. <941> Characterisation of Crystalline and Partially Crystalline Solids by X-ray Powder Diffraction; General; The United States Pharmacopeial Convention: Rockville, MD, USA, 2011. [Google Scholar]
- Sildenafil Citrate, ChemSrc. Available online: https://www.chemsrc.com/en/cas/171599-83-0_663469.html (accessed on 1 February 2023).
- Sildenafil Citrate-Product Description. ChemicalBook. Available online: https://www.chemicalbook.com/ChemicalProductProperty_US_CB9407725.aspx (accessed on 1 February 2023).
- Canbay, H.S.; Doğantürk, M. Compatibility Studies of Sildenafil with Different Excipients by Using TGA, DSC, XRD and FTIR. Celal Bayar Univ. J. Sci. 2019, 15, 401–407. [Google Scholar]
- Hosny, K.M.; Mosli, H.A.; Hassan, A.H. Soy polysaccharide as a novel superdisintegrant in sildenafil citrate sublingual tablets: Preparation, characterization, and in vivo evaluation. Drug Des. Dev. Ther. 2015, 9, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Julio, T.A.; Zamara, I.F.; Garcia, J.S.; Trevisan, M.G. Compatibility of sildenafil citrate and pharmaceutical excipients by thermal analysis and LC–UV. J. Therm. Anal. Calorim. 2013, 111, 2037–2044. [Google Scholar] [CrossRef]
- Viagra–Sildenafil Citrate, Chemistry Review–Accessdata. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/NDA/98/viagra/chem_rev.pdf (accessed on 1 February 2023).
- Melnikov, P.; Corbi, P.P.; Cuin, A.; Cavicchioli, M.; Guimaraes, W.R. Physicochemical properties of sildenafil citrate (Viagra) and sildenafil base. J. Pharm. Sci. 2003, 92, 2140–2143. [Google Scholar] [CrossRef] [PubMed]
- Magnesium Stearate, Baerlocher GmbH. Available online: https://www.baerlocher.com/products/metal-soaps/magnesium-stearate/ (accessed on 1 February 2023).
- Magnesium Stearate, CENTRO-CHEM. Available online: https://www.centro-chem.pl/en/products/magnesium-stearate (accessed on 1 February 2023).
- Magnesium Stearate, Merck. Available online: https://www.merckmillipore.com/PL/pl/product/PARTECK-LUB-MST,MDA_CHEM-100663 (accessed on 1 February 2023).
- Magnesium Stearate, PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Magnesium-stearate#section=Computed-Properties (accessed on 1 February 2023).
- Magnesium Stearate. ChemicalBook. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB5330900.htm (accessed on 1 February 2023).
- Lactose Monohydrate, Merck. Available online: https://www.merckmillipore.com/PL/pl/product/Lactose-monohydrate,MDA_CHEM-107657?ReferrerURL=https%3A%2F%2Fwww.google.com%2F (accessed on 1 February 2023).
- Lactose Monohydrate, ChemicalBook. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB8685418.htm (accessed on 1 February 2023).
- Ortiz, R.S.; Mariotti, K.C.; Schwab, N.; Sabin, G. Fingerprinting of sildenafil citrate and tadalafil tablets in pharmaceutical formulation via X-ray fluorescence (XRF) spectrometry. J. Pharm. Biomed. Anal. 2012, 58, 7. [Google Scholar] [CrossRef] [PubMed]
- Allabdalla, M.A. Chemical characterisation of counterfeit captagon tablets seized in Jordan. Forensic Sci. Int. 2005, 152, 185. [Google Scholar] [CrossRef] [PubMed]
- Gaudiano, M.C.; Valvo, L.; Bertocchi, P.; Manna, L. RP-HPLC study of the degradation of diclofenac and piroxicam in the presence of hydroxyl radicals. J. Pharm. Biomed. Anal. 2003, 32, 151. [Google Scholar] [CrossRef] [PubMed]

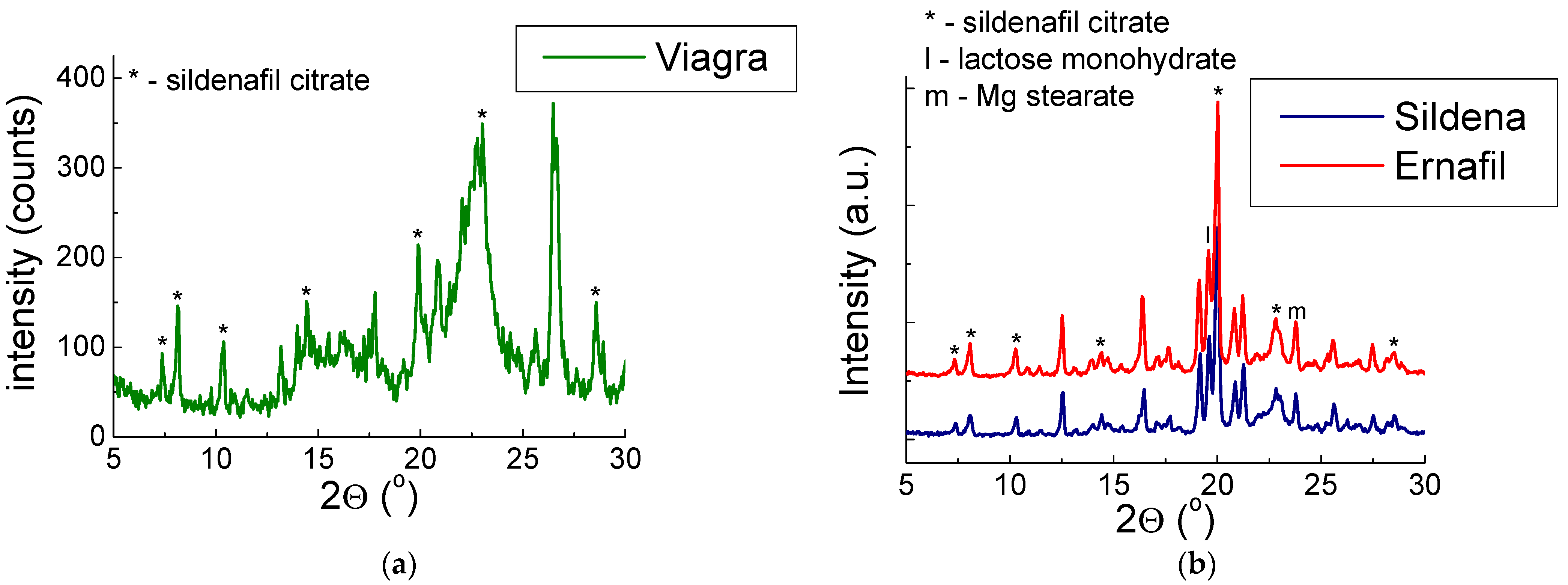



| No. of Diffraction Line | 2θ (o) Exp. | 2θ (o) ICDD | J/Jmax (%) Exp. | J/Jmax (%) ICDD | |Δ2θ| * | dhkl (Å) Exp. | dhkl (Å) CDD | hkl ** |
|---|---|---|---|---|---|---|---|---|
| Viagra® | ||||||||
| 1. | 7.3981 | 7.3730 | 27 | 16 | 0.0251 | 11.94 | 11.98 | 010 |
| 2. | 8.1870 | 8.1122 | 41 | 89 | 0.0748 | 10.79 | 10.89 | 110 |
| 3. | 10.2926 | 10.3135 | 33 | 42 | 0.0209 | 8.59 | 8.57 | 300 |
| 4. | 14.4514 | 14.4612 | 42 | 100 | 0.0098 | 6.12 | 6.12 | 112 |
| 5. | 19.9320 | 19.8997 | 57 | 39 | 0.0323 | 4.45 | 4.46 | 42-1 |
| 6. | 23.0929 | 23.0460 | 93 | 23 | 0.0469 | 3.85 | 3.86 | 52-2 |
| Ernafil® | ||||||||
| 1. | 7.3615 | 7.3730 | 10 | 16 | 0.0115 | 12.00 | 11.98 | 010 |
| 2. | 8.0668 | 8.1122 | 15 | 89 | 0.0454 | 10.95 | 10.89 | 110 |
| 3. | 10.3448 | 10.3135 | 13 | 42 | 0.0313 | 8.54 | 8.57 | 300 |
| 4. | 14.3992 | 14.4612 | 11 | 100 | 0.0620 | 6.15 | 6.12 | 112 |
| 5. | 20.0366 | 19.8997 | 100 | 39 | 0.1369 | 4.43 | 4.46 | 42-1 |
| 6. | 22.9624 | 23.0460 | 25 | 23 | 0.0836 | 3.87 | 3.86 | 52-2 |
| Sildena® | ||||||||
| 1. | 7.3824 | 7.3730 | 11 | 16 | 0.0094 | 11.96 | 11.98 | 010 |
| 2. | 8.0564 | 8.1122 | 14 | 89 | 0.0558 | 10.97 | 10.89 | 110 |
| 3. | 10.2508 | 10.3135 | 13 | 42 | 0.0313 | 8.62 | 8.57 | 300 |
| 4. | 14.4671 | 14.4612 | 14 | 100 | 0.0059 | 6.12 | 6.12 | 112 |
| 5. | 19.9739 | 19.8997 | 100 | 39 | 0.0742 | 4.44 | 4.46 | 42-1 |
| 6. | 23.0407 | 23.0460 | 63 | 23 | 0.0053 | 3.86 | 3.86 | 52-2 |
| Valinger® | ||||||||
| 1. | 7.3981 | 7.3730 | 25 | 16 | 0.0251 | 11.94 | 11.98 | 010 |
| 2. | 8.0825 | 8.1122 | 32 | 89 | 0.0297 | 10.93 | 10.89 | 110 |
| 3. | 10.2926 | 10.3135 | 28 | 42 | 0.0209 | 8.59 | 8.57 | 300 |
| 4. | 14.4514 | 14.4612 | 38 | 100 | 0.0098 | 6.12 | 6.12 | 112 |
| 5. | 19.8276 | 19.8997 | 43 | 39 | 0.0721 | 4.48 | 4.46 | 42-1 |
| 6. | 23.2341 | 23.0460 | 53 | 23 | 0.1881 | 3.83 | 3.86 | 52-2 |
| ActiGra® | ||||||||
| 1. | 7.3992 | 7.3730 | 20 | 16 | 0.0262 | 11.94 | 11.98 | 010 |
| 2. | 8.2111 | 8.1122 | 29 | 89 | 0.0989 | 10.76 | 10.89 | 110 |
| 3. | 10.3469 | 10.3135 | 27 | 42 | 0.0361 | 8.54 | 8.57 | 300 |
| 4. | 14.4723 | 14.4612 | 32 | 100 | 0.0111 | 6.11 | 6.12 | 112 |
| 5. | 19.9289 | 19.8997 | 45 | 39 | 0.0292 | 4.45 | 4.46 | 42-1 |
| 6. | 23.0930 | 23.0460 | 64 | 23 | 0.0470 | 3.85 | 3.86 | 52-2 |
| Silcontrol® | ||||||||
| 1. | 7.3824 | 7.3730 | 23 | 16 | 0.0094 | 11.96 | 11.98 | 010 |
| 2. | 8.0564 | 8.1122 | 31 | 89 | 0.0558 | 10.96 | 10.89 | 110 |
| 3. | 10.3083 | 10.3135 | 25 | 42 | 0.0052 | 8.57 | 8.57 | 300 |
| 4. | 14.4096 | 14.4612 | 33 | 100 | 0.0516 | 6.14 | 6.12 | 112 |
| 5. | 19.8641 | 19.8997 | 42 | 39 | 0.0356 | 4.47 | 4.46 | 42-1 |
| 6. | 23.0407 | 23.0460 | 63 | 23 | 0.0053 | 3.86 | 3.86 | 52-2 |
| Maxon® | ||||||||
| 1. | 7.4922 | 7.3730 | 23 | 16 | 0.1192 | 11.79 | 11.98 | 010 |
| 2. | 8.1714 | 8.1122 | 31 | 89 | 0.0592 | 10.81 | 10.89 | 110 |
| 3. | 10.3605 | 10.3135 | 31 | 42 | 0.0470 | 8.53 | 8.57 | 300 |
| 4. | 14.5246 | 14.4612 | 43 | 100 | 0.0634 | 6.09 | 6.12 | 112 |
| 5. | 19.9774 | 19.8997 | 66 | 39 | 0.0777 | 4.44 | 4.46 | 42-1 |
| 6. | 23.0930 | 23.0460 | 96 | 23 | 0.0470 | 3.85 | 3.86 | 52-2 |
| PrinceX® | ||||||||
| 1. | 7.3458 | 7.3730 | 25 | 16 | 0.0270 | 12.02 | 11.98 | 010 |
| 2. | 8.0825 | 8.1122 | 38 | 89 | 0.0297 | 10.93 | 10.89 | 110 |
| 3. | 10.3448 | 10.3135 | 33 | 42 | 0.0313 | 8.54 | 8.57 | 300 |
| 4. | 14.3992 | 14.4612 | 28 | 100 | 0.0620 | 6.15 | 6.12 | 112 |
| 5. | 19.8798 | 19.8997 | 40 | 39 | 0.0199 | 4.46 | 4.46 | 42-1 |
| 6. | 23.0930 | 23.0460 | 62 | 23 | 0.0470 | 3.85 | 3.86 | 52-2 |
| No. of Diffraction Line | 2θ (o) Exp. | 2θ (o) ICDD | J/Jmax (%) Exp. | J/Jmax (%) ICDD | |Δ2θ| * | dhkl (Å) Exp. | dhkl (Å) ICDD | hkl ** |
|---|---|---|---|---|---|---|---|---|
| DoppelSil® | ||||||||
| 1. | 5.2089 | 5.1545 | 13 | 100 | 0.0544 | 16.95 | 17.13 | 020 |
| 2. | 10.3866 | 10.3256 | 11 | 62 | 0.0610 | 8.51 | 8.56 | 040 |
| 3. | 16.4159 | 16.3409 | 27 | 6 | 0.0750 | 5.40 | 5.42 | 051 |
| 4. | 17.1525 | 17.1703 | 13 | 6 | 0.0178 | 5.17 | 5.16 | 131 |
| 5. | 22.8631 | 22.8538 | 16 | 9 | 0.0093 | 3.89 | 3.88 | 090 |
| 6. | 25.5695 | 25.4645 | 22 | 11 | 0.1050 | 3.48 | 3.49 | 25-1 |
| Maxigra® | ||||||||
| 1. | 5.0236 | 5.1545 | 16 | 100 | 0.1309 | 17.58 | 17.13 | 020 |
| 2. | 10.2202 | 10.3256 | 15 | 62 | 0.1054 | 8.65 | 8.56 | 040 |
| 3. | 16.2660 | 16.3409 | 43 | 6 | 0.0749 | 5.44 | 5.42 | 051 |
| 4. | 16.9891 | 17.1703 | 17 | 6 | 0.1812 | 5.21 | 5.16 | 131 |
| 5. | 22.6831 | 22.8538 | 19 | 9 | 0.1707 | 3.92 | 3.88 | 090 |
| 6. | 25.4501 | 25.4645 | 22 | 11 | 0.0144 | 3.50 | 3.49 | 25-1 |
| Mensil® | ||||||||
| 1. | 5.0107 | 5.1545 | 17 | 100 | 0.1438 | 17.62 | 17.13 | 020 |
| 2. | 10.1835 | 10.3256 | 16 | 62 | 0.1421 | 8.68 | 8.56 | 040 |
| 3. | 16.2746 | 16.3409 | 44 | 6 | 0.0663 | 5.44 | 5.42 | 051 |
| 4. | 16.9755 | 17.1703 | 15 | 6 | 0.1948 | 5.22 | 5.16 | 131 |
| 5. | 22.6646 | 22.8538 | 18 | 9 | 0.1892 | 3.92 | 3.88 | 090 |
| 6. | 25.4194 | 25.4645 | 20 | 11 | 0.0451 | 3.50 | 3.49 | 25-1 |
| Inventum™ | ||||||||
| 1. | 5.0236 | 5.1545 | 15 | 100 | 0.1309 | 17.58 | 17.13 | 020 |
| 2. | 10.1865 | 10.3256 | 14 | 62 | 0.1391 | 8.68 | 8.56 | 040 |
| 3. | 16.2379 | 16.3409 | 37 | 6 | 0.1030 | 5.45 | 5.42 | 051 |
| 4. | 16.9797 | 17.1703 | 16 | 6 | 0.1906 | 5.22 | 5.16 | 131 |
| 5. | 22.6694 | 22.8538 | 17 | 9 | 0.1844 | 3.92 | 3.88 | 090 |
| 6. | 25.4164 | 25.4645 | 19 | 11 | 0.0481 | 3.50 | 3.49 | 25-1 |
| No. | Name of Drug | Weight Loss (%) | Onset (°C) | Offset (°C) | Peak Maximum (°C) | Peak Height (mW) | Peak Area (J) | Enthalpy (J/g) |
|---|---|---|---|---|---|---|---|---|
| 1 | Viagra® | 55 | 185 | 194 | 189 | 3.75 | 0.52 | 72.6 |
| 290 | 303 | 298 | 2.29 | 0.74 | 103 | |||
| 2 | Ernafil® | 65 | 144 | 153 | 148 | 3.10 | 0.25 | 52.3 |
| 189 | 210 | 183, 199 | 3.00 | 0.67 | 140 | |||
| 3 | Sildena® | 68 | 144 | 151 | 148 | 4.48 | 0.54 | 75.1 |
| 181 | 214 | 188, 202 | 4.47 | 1.50 | 209 | |||
| 4 | Valinger® | 63 | 177 | 197 | 190 | 3.87 | 0.60 | 97.5 |
| 343 | 357 | 348 | 0.48 | 0.08 | 13.4 | |||
| 5 | ActiGra® | 55 | 185 | 196 | 189 | 3.36 | 0.51 | 93.3 |
| 345 | 356 | 354 | 0.43 | 0.07 | 13.4 | |||
| 6 | Silcontrol® | 65 | 180 | 195 | 190 | 4.23 | 0.66 | 107 |
| 328 | 334 | 334 | 0.77 | 0.28 | 44.4 | |||
| 7 | Maxon® | 72 | 187 | 196 | 191 | 3.02 | 0.31 | 66.4 |
| 343 | 367 | 353 | 0.73 | 0.25 | 52.7 | |||
| 8 | PrinceX® | 49 | 187 | 197 | 191 | 5.05 | 0.69 | 103.1 |
| 314 | 324 | 319 | 0.46 | 0.05 | 8.10 |
| No. | Name of Drug | Weight Loss (%) | Onset (°C) | Offset (°C) | Peak Maximum (°C) | Peak Height (mW) | Peak Area (J) | Enthalpy (J/g) |
|---|---|---|---|---|---|---|---|---|
| 1 | DoppelSil® | 57 | 142 | 150 | 147 | 2.50 | 0.28 | 68.0 |
| 174 | 188 | 183 | 0.71 | 0.02 | 4.22 | |||
| 195 | 207 | 202 | 2.96 | 0.23 | 55.0 | |||
| 2 | Maxigra® | 31 | 135 | 148 | 140 | 6.68 | 0.77 | 72.6 |
| 179 | 183 | 181 | 1.35 | 0.08 | 7.70 | |||
| 194 | 206 | 201 | 9.78 | 1.25 | 118 | |||
| 3 | Mensil® | 27 | 140 | 148 | 143 | 5.50 | 0.53 | 65.0 |
| 180 | 186 | 182 | 0.88 | 0.05 | 5.73 | |||
| 192 | 202 | 198 | 6.24 | 0.70 | 86.7 | |||
| 4 | Inventum™ | 24 | 142 | 150 | 146 | 4.70 | 0.45 | 73.2 |
| 180 | 186 | 182 | 0.88 | 0.04 | 5.73 | |||
| 192 | 202 | 198 | 6.24 | 0.54 | 86.7 |
| No. | Product Name (Manufacturer) | Sildenafil Compound Content in 1 Tablet (mg) | Mass (%) of Sildenafil Compound in 1 Tablet |
|---|---|---|---|
| 1 | Viagra® (Pfizer) | 100 | 15.7 |
| 2 | Ernafil® (Teva) | 100 | 18.8 |
| 3 | Sildena® (Hemofarm) | 100 | 16.2 |
| 4 | Valinger® (Orion Pharma) | 25 | 16.1 |
| 5 | Maxigra® (Polpharma) | 25 | 18.5 |
| 6 | Mensil® (Hasco-Lek S.A.) | 25 | 18.8 |
| 7 | DoppelSil® (Doppelherz Pharma) | 25 | 18.1 |
| 8 | Inventum™ (Aflofarm Farmacja Polska) | 25 | 18.9 |
| 9 | Maxon® (Adamed Pharma) | 25 | 16.1 |
| 10 | ActiGra® (Biofarm) | 25 | 16.6 |
| 11 | Silcontrol® (Zentiva) | 25 | 16.1 |
| 12 | PrinceX® (Accord Healthcare) | 25 | 16.6 |
| No. | Chemical Compound | Chemical Formula | No. PDF Card |
|---|---|---|---|
| 1 | sildenafil citrate | C28H38N6O11S | 00–052–2420 |
| 2 | sildenafil base | C22H30N6O4S | 00–052–2006 |
| 3 | lactose monohydrate | C12H22O11·H2O | 00–001–0333 |
| 4 | magnesium stearate | C36H70MgO4 | 00–005–0292 |
| 5 | cellulose | C6H10O5 | 00–003–0223 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jendrzejewska, I.; Goryczka, T.; Pietrasik, E.; Klimontko, J.; Jampilek, J. Identification of Sildenafil Compound in Selected Drugs Using X-ray Study and Thermal Analysis. Molecules 2023, 28, 2632. https://doi.org/10.3390/molecules28062632
Jendrzejewska I, Goryczka T, Pietrasik E, Klimontko J, Jampilek J. Identification of Sildenafil Compound in Selected Drugs Using X-ray Study and Thermal Analysis. Molecules. 2023; 28(6):2632. https://doi.org/10.3390/molecules28062632
Chicago/Turabian StyleJendrzejewska, Izabela, Tomasz Goryczka, Ewa Pietrasik, Joanna Klimontko, and Josef Jampilek. 2023. "Identification of Sildenafil Compound in Selected Drugs Using X-ray Study and Thermal Analysis" Molecules 28, no. 6: 2632. https://doi.org/10.3390/molecules28062632
APA StyleJendrzejewska, I., Goryczka, T., Pietrasik, E., Klimontko, J., & Jampilek, J. (2023). Identification of Sildenafil Compound in Selected Drugs Using X-ray Study and Thermal Analysis. Molecules, 28(6), 2632. https://doi.org/10.3390/molecules28062632









