Photocatalytic Applications of ReS2-Based Heterostructures
Abstract
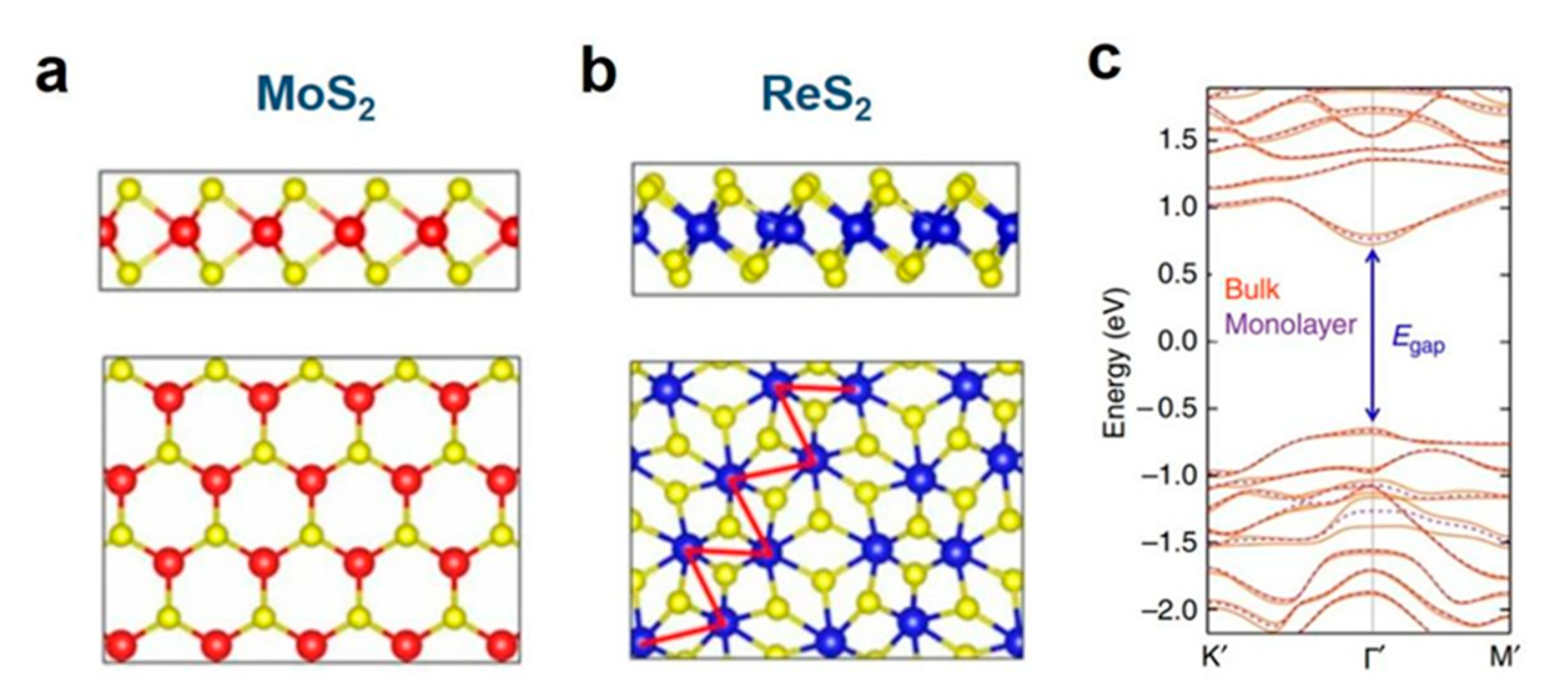
1. Introduction
2. Synthetic Methods for ReS2 and Its Composite Material
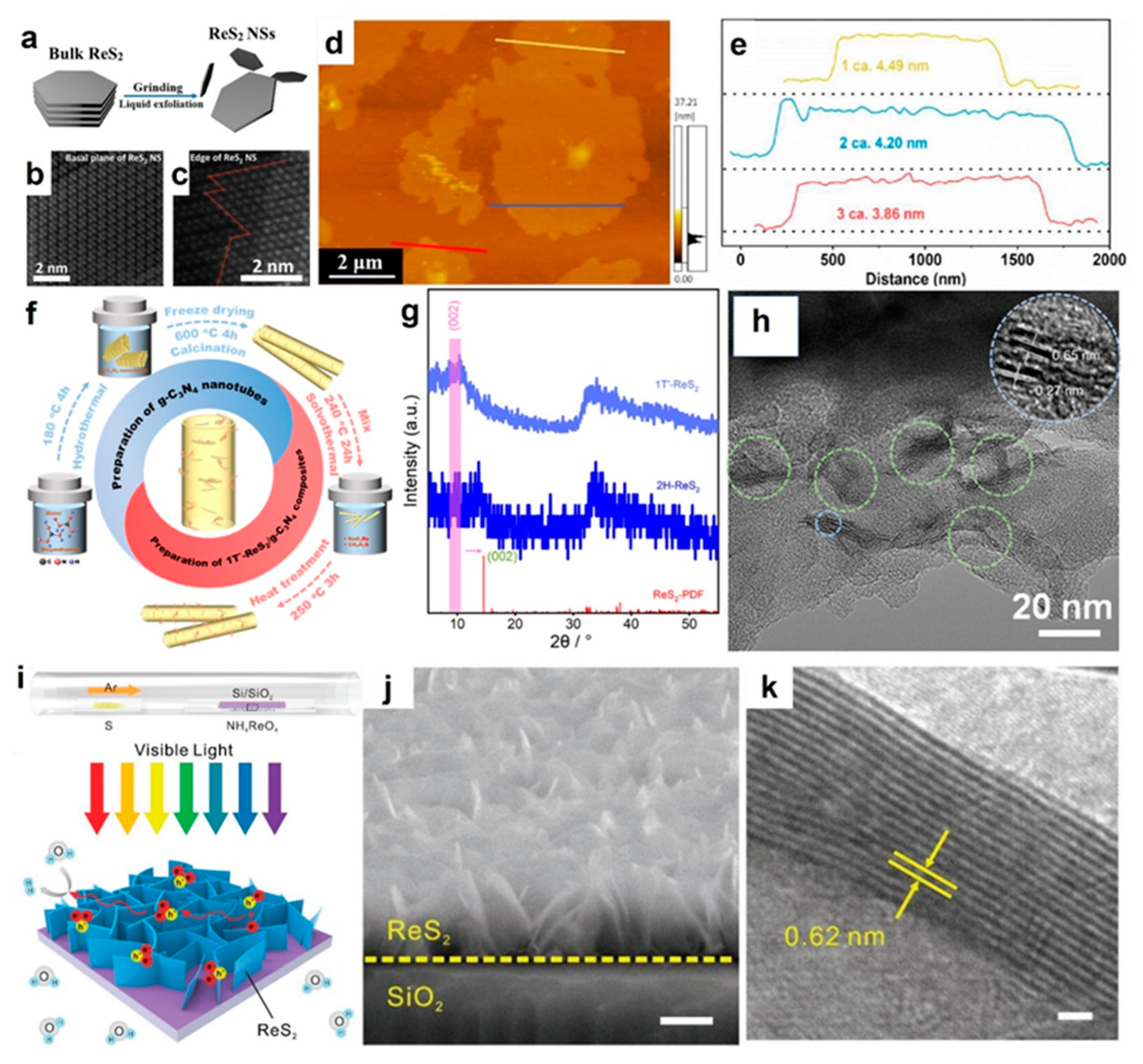
2.1. Exfoliation from the Bulk ReS2
2.2. Hydrothermal Synthesis Reaction
2.3. Chemical Vapor Deposition Technique
3. Heterostructured ReS2 Composites
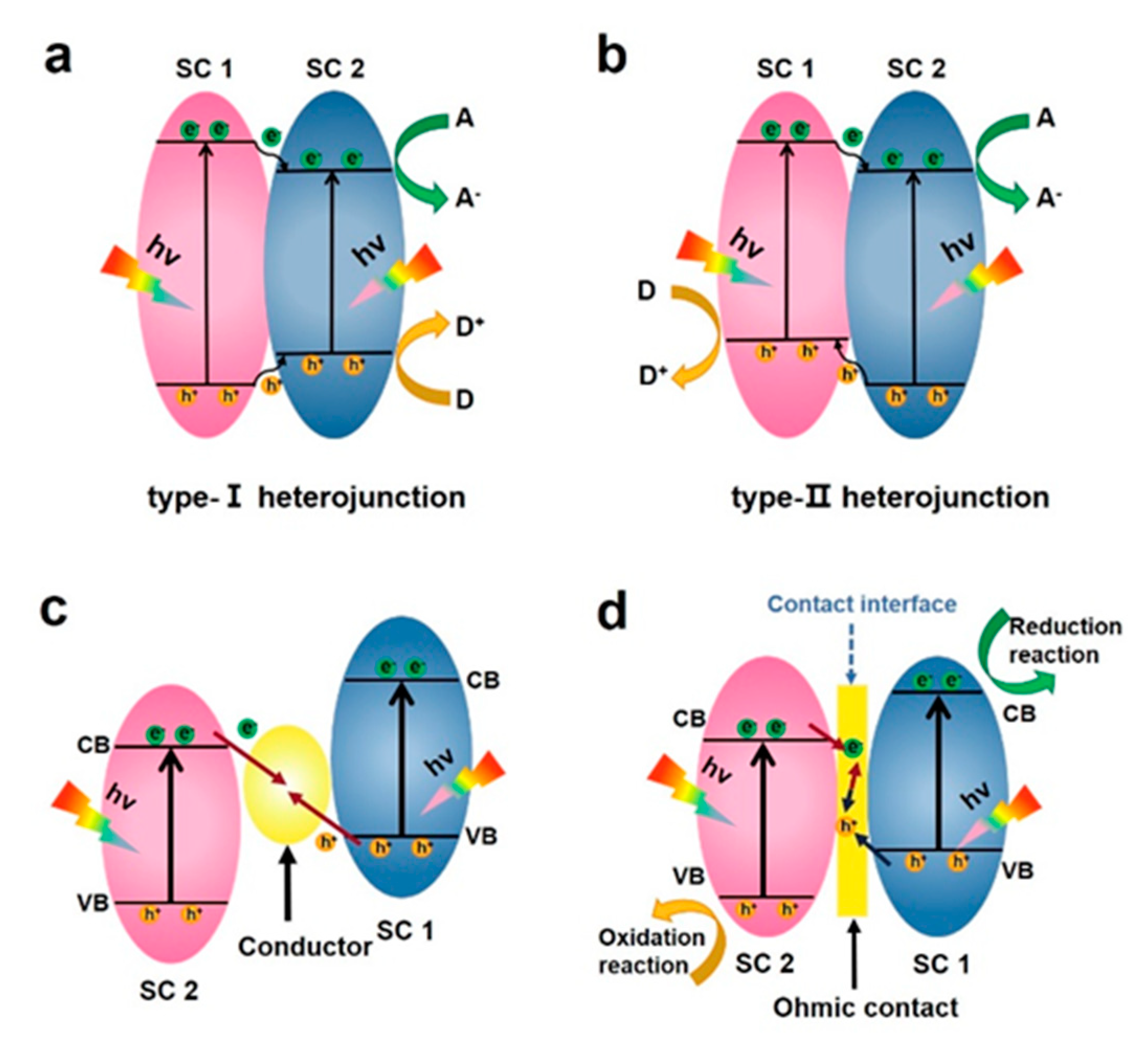
3.1. Type I Heterostructure
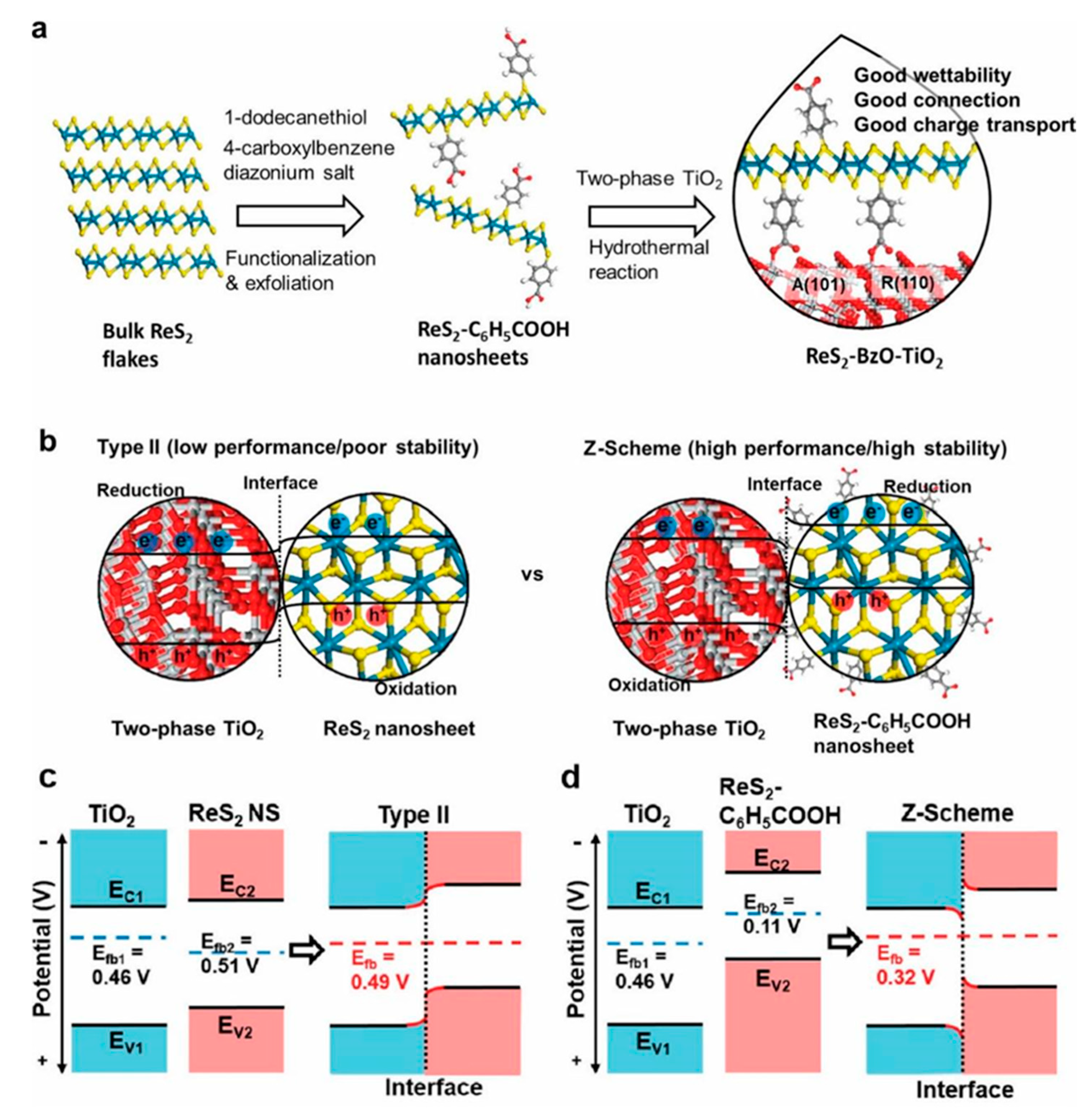
3.2. Type II Heterostructure
3.3. Z-Scheme Heterojunction
4. Photocatalytic Applications of Heterostructure ReS2
4.1. Photocatalytic Hydrogen Production
4.2. Photocatalytic CO2 Reduction
4.3. Degradation of Organic Pollutants
4.4. Photocatalytic Reduction of Metal Ions
4.5. Photocatalytic Water Disinfection
5. Summary and Outlook
- Developing new synthetic methodologies to achieve precise synthesis of ReS2 and its composites. Mechanical mixing is usually used in the construction of heterojunctions for ReS2, but precise design for the interactions between the two or more materials has not yet been realized in the preparation of photocatalysts, resulting in random morphology and exposed crystal faces of the composites. With the growth mechanism of ReS2 as a basis, rational control of the growth conditions is expected to precisely control the size and crystal face orientation. In addition, reducing the size of ReS2 down to sub-10-nanometers is another intriguing direction due to the enhanced quantum confinement effect and derived novel photonic properties; however, structural controllability including particle diameter, edge structure, phase transition, etc. is highly important and is also well deserving of more research attention.
- Surface engineering strategies toward tailorable physicochemical properties of ReS2. Few studies on the design and modification of the surface structure of ReS2 have been reported for photocatalytic research. Surface defects can become centers of the electron−hole complex, but they are not entirely inutile. For example, introducing Re vacancies in a reasonable way can enhance the adsorption ability of H+. In addition, organic molecules can form chemical bonds on the surface S vacancies, which can improve the interface wettability of ReS2 on the one hand and fine-tune its energy band position on the other hand.
- Combining novel characterization methods with theoretical calculations. The surface and interface structure changes in photocatalysis should be thoroughly investigated from both microscopic and transient aspects. Real-time monitoring of intermediates and catalytic products is essential for understanding the photocatalytic mechanism and further optimizing the performance of photocatalysts. For instance, in situ Fourier Transform Infrared spectroscopy and online mass spectrometry can probe the source of hydrogen and the fate of the sacrifice reagents in photocatalytic hydrogen evolution [59]. Ultra-high spatial and temporal resolution technique, such as tip-enhanced Raman spectroscopy, can greatly improve the signal-to-noise ratio and spatial resolution, allowing for the characterization of single molecules and even single chemical bonds. Moreover, the theoretical simulation of the model systems, particularly first-principles, external field simulations, and micro-reaction dynamics simulations, is essential for exploring the fundamental mechanisms of photocatalysis. The binding energy between various intermediates and catalysts can be obtained by calculating the adsorption energy and charge density difference, further reflecting the configuration of the reaction intermediates on the catalyst surface.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tian, J.; Zhao, Z.; Kumar, A.; Boughton, R.I.; Liu, H. Recent progress in design, synthesis, and applications of one-dimensional TiO2 nanostructured surface heterostructures: A review. Chem. Soc. Rev. 2014, 43, 6920–6937. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Lai, X.; Yang, N.; Zhai, J.; Kisailus, D.; Su, F.; Wang, D.; Jiang, L. Hierarchically Ordered Macro−Mesoporous TiO2−Graphene Composite Films: Improved Mass Transfer, Reduced Charge Recombination, and Their Enhanced Photocatalytic Activities. ACS Nano 2011, 5, 590–596. [Google Scholar] [CrossRef]
- Khan, M.M.; Ansari, S.A.; Pradhan, D.; Ansari, M.O.; Han, D.H.; Lee, J.; Cho, M.H. Band gap engineered TiO2 nanoparticles for visible light induced photoelectrochemical and photocatalytic studies. J. Mater. Chem. A 2014, 2, 637–644. [Google Scholar] [CrossRef]
- Katal, R.; Salehi, M.; Davood Abadi Farahani, M.H.; Masudy-Panah, S.; Ong, S.L.; Hu, J. Preparation of a New Type of Black TiO2 under a Vacuum Atmosphere for Sunlight Photocatalysis. ACS Appl. Mater. Interfaces 2018, 10, 35316–35326. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ye, K.; Cao, D.; Gao, P.; Qiu, M.; Liu, L.; Yang, P. Efficient Charge Separation from F– Selective Etching and Doping of Anatase-TiO2{001} for Enhanced Photocatalytic Hydrogen Production. ACS Appl. Mater. Interfaces 2018, 10, 19633–19638. [Google Scholar] [CrossRef]
- Tong, H.; Ouyang, S.; Bi, Y.; Umezawa, N.; Oshikiri, M.; Ye, J. Nano-photocatalytic Materials: Possibilities and Challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, Y.; Bao, J.; Sheng, X.; Fang, J.; Zhao, S.; Zhang, Y.; Chen, W. Hierarchical Honeycomb Br-, N-Codoped TiO2 with Enhanced Visible-Light Photocatalytic H2 Production. ACS Appl. Mater. Interfaces 2018, 10, 18796–18804. [Google Scholar] [CrossRef]
- Bian, Z.; Tachikawa, T.; Zhang, P.; Fujitsuka, M.; Majima, T. Au/TiO2 Superstructure-Based Plasmonic Photocatalysts Exhibiting Efficient Charge Separation and Unprecedented Activity. J. Am. Chem. Soc. 2014, 136, 458–465. [Google Scholar] [CrossRef]
- Yang, R.; Fan, Y.; Zhang, Y.; Mei, L.; Zhu, R.; Qin, J.; Hu, J.; Chen, Z.; Hau Ng, Y.; Voiry, D.; et al. 2D Transition Metal Dichalcogenides for Photocatalysis. Angew. Chem. Int. Ed. 2023, 62, e202218016. [Google Scholar] [CrossRef]
- Faraji, M.; Yousefi, M.; Yousefzadeh, S.; Zirak, M.; Naseri, N.; Jeon, T.H.; Choi, W.; Moshfegh, A.Z. Two-dimensional materials in semiconductor photoelectrocatalytic systems for water splitting. Energy Environ. Sci. 2019, 12, 59–95. [Google Scholar] [CrossRef]
- Tong, R.; Ng, K.W.; Wang, X.; Wang, S.; Wang, X.; Pan, H. Two-dimensional materials as novel co-catalysts for efficient solar-driven hydrogen production. J. Mater. Chem. A 2020, 8, 23202–23230. [Google Scholar] [CrossRef]
- Lotsch, B.V. Vertical 2D Heterostructures. Annu. Rev. Mater. Res. 2015, 45, 85–109. [Google Scholar] [CrossRef]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically Thin MoS2: A New Direct-Gap Semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef]
- Fu, Q.; Han, J.; Wang, X.; Xu, P.; Yao, T.; Zhong, J.; Zhong, W.; Liu, S.; Gao, T.; Zhang, Z.; et al. 2D Transition Metal Dichalcogenides: Design, Modulation, and Challenges in Electrocatalysis. Adv. Mater. 2021, 33, e1907818. [Google Scholar] [CrossRef]
- Pi, L.; Li, L.; Liu, K.; Zhang, Q.; Li, H.; Zhai, T. Recent Progress on 2D Noble-Transition-Metal Dichalcogenides. Adv. Funct. Mater. 2019, 29, 1904932. [Google Scholar] [CrossRef]
- Wu, E.; Xie, Y.; Wang, S.; Zhang, D.; Hu, X.; Liu, J. Multi-level flash memory device based on stacked anisotropic ReS2-boron nitride-graphene heterostructures. Nanoscale 2020, 12, 18800–18806. [Google Scholar] [CrossRef]
- Mukherjee, B.; Hayakawa, R.; Watanabe, K.; Taniguchi, T.; Nakaharai, S.; Wakayama, Y. ReS2/h-BN/Graphene Heterostructure Based Multifunctional Devices: Tunneling Diodes, FETs, Logic Gates, and Memory. Adv. Electron. Mater. 2021, 7, 2000925. [Google Scholar] [CrossRef]
- Qin, J.-K.; Qiu, G.; He, W.; Jian, J.; Si, M.-W.; Duan, Y.-Q.; Charnas, A.; Zemlyanov, D.Y.; Wang, H.-Y.; Shao, W.-Z.; et al. Epitaxial Growth of 1D Atomic Chain Based Se Nanoplates on Monolayer ReS2 for High-Performance Photodetectors. Adv. Funct. Mater. 2018, 28, 1806254. [Google Scholar] [CrossRef]
- Varghese, A.; Saha, D.; Thakar, K.; Jindal, V.; Ghosh, S.; Medhekar, N.V.; Ghosh, S.; Lodha, S. Near-Direct Bandgap WSe2/ReS2 Type-II pn Heterojunction for Enhanced Ultrafast Photodetection and High-Performance Photovoltaics. Nano Lett. 2020, 20, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Xing, Y.; Han, J.; Cui, B.; Lei, T.; Tu, H.; Guan, B.; Zeng, Z.; Zhang, B.; Lv, W. Ultrasensitive and Broad-Spectrum Photodetectors Based on InSe/ReS2 Heterostructure. Adv. Opt. Mater. 2022, 10, 2101772. [Google Scholar] [CrossRef]
- Su, W.; Zhang, S.; Liu, C.; Tian, Q.; Liu, X.; Li, K.; Lv, Y.; Liao, L.; Zou, X. Interlayer Transition Induced Infrared Response in ReS2/2D Perovskite van der Waals Heterostructure Photodetector. Nano Lett. 2022, 10192–10199. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.U.; Bahng, J.; Dang, X.D.; Oh, S.; Duong, H.P.; Kang, S.S.; Yu, H.M.; Sakong, W.; Kim, M.; Choi, H.-S.; et al. Adaptive photocurrent generation of ReS2-2D Te heterostructure. Nano Energy 2022, 102, 107720. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Song, W.; Zhang, Y.; Hou, Z.; Zhou, G.; Zhang, Z.; Liu, J. In-situ growth of ReS2/NiS heterostructure on Ni foam as an ultra-stable electrocatalyst for alkaline hydrogen generation. Chem. Eng. J. 2023, 451, 138905. [Google Scholar] [CrossRef]
- Zhou, G.; Guo, Z.; Shan, Y.; Wu, S.; Zhang, J.; Yan, K.; Liu, L.; Chu, P.K.; Wu, X. High-efficiency hydrogen evolution from seawater using hetero-structured T/Td phase ReS2 nanosheets with cationic vacancies. Nano Energy 2019, 55, 42–48. [Google Scholar] [CrossRef]
- Ng, S.; Iffelsberger, C.; Sofer, Z.; Pumera, M. Tunable Room-Temperature Synthesis of ReS2 Bicatalyst on 3D- and 2D-Printed Electrodes for Photo- and Electrochemical Energy Applications. Adv. Funct. Mater. 2020, 30, 1910193. [Google Scholar] [CrossRef]
- Cheng, P.; Liu, Y.; Ziegler, M.; Klingenhof, M.; Wang, D.; Zhang, Z.; Strasser, P.; Schaaf, P. Improving Silicon Photocathode Performance for Water Reduction through Dual Interface Engineering and Integrating ReS2 Photocatalyst. ACS Appl. Energy Mater. 2022, 5, 8222–8231. [Google Scholar] [CrossRef]
- Tongay, S.; Sahin, H.; Ko, C.; Luce, A.; Fan, W.; Liu, K.; Zhou, J.; Huang, Y.-S.; Ho, C.-H.; Yan, J.; et al. Monolayer behaviour in bulk ReS2 due to electronic and vibrational decoupling. Nat. Commun. 2014, 5, 3252. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Komsa, H.-P.; Yeh, C.-H.; Björkman, T.; Liang, Z.-Y.; Ho, C.-H.; Huang, Y.-S.; Chiu, P.-W.; Krasheninnikov, A.V.; Suenaga, K. Single-Layer ReS2: Two-Dimensional Semiconductor with Tunable In-Plane Anisotropy. ACS Nano 2015, 9, 11249–11257. [Google Scholar] [CrossRef]
- Feng, Y.; Zhou, W.; Wang, Y.; Zhou, J.; Liu, E.; Fu, Y.; Ni, Z.; Wu, X.; Yuan, H.; Miao, F.; et al. Raman vibrational spectra of bulk to monolayer ReS2 with lower symmetry. Phys. Rev. B 2015, 92, 054110. [Google Scholar] [CrossRef]
- Rahman, M.; Davey, K.; Qiao, S.Z. Advent of 2D Rhenium Disulfide (ReS2): Fundamentals to Applications. Adv. Funct. Mater. 2017, 27, 1606129. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, W.; Zhang, J.; Zhu, X.; Zhang, Q.; Zhang, Y.; Ren, Z.; Song, S.; Wang, J.; Ying, Z.; et al. Highly Efficient Photocatalytic Hydrogen Evolution by ReS2 via a Two-Electron Catalytic Reaction. Adv. Mater. 2018, 30, e1707123. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Zhang, H.; Qu, J.; Shan, J.; Chen, S.; Yang, F.; Zheng, R.; Cairney, J.; Song, L.; Jing, L.; et al. Atomic-Level Insights into the Edge Active ReS2 Ultrathin Nanosheets for High-Efficiency Light-to-Hydrogen Conversion. ACS Mater. Lett. 2020, 2, 1484–1494. [Google Scholar] [CrossRef]
- Zhang, Q.; Fu, L. Novel Insights and Perspectives into Weakly Coupled ReS2 toward Emerging Applications. Chem 2019, 5, 505–525. [Google Scholar] [CrossRef]
- Cao, Y.-D.; Sun, Y.-H.; Shi, S.-F.; Wang, R.-M. Anisotropy of two-dimensional ReS2 and advances in its device application. Rare Met. 2021, 40, 3357–3374. [Google Scholar] [CrossRef]
- Xie, X.; Mao, M.; Qi, S.; Ma, J. ReS2-Based electrode materials for alkali-metal ion batteries. CrystEngComm 2019, 21, 3755–3769. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, H.; Zhang, D.W.; Zhou, P. Electronic and Optoelectronic Applications Based on ReS2. Phys. Status Solidi Rapid Res. Lett. 2019, 13, 1800658. [Google Scholar] [CrossRef]
- Fadhel, M.M.; Ali, N.; Rashid, H.; Sapiee, N.M.; Hamzah, A.E.; Zan, M.S.D.; Aziz, N.A.; Arsad, N. A Review on Rhenium Disulfide: Synthesis Approaches, Optical Properties, and Applications in Pulsed Lasers. Nanomaterials 2021, 11, 2367. [Google Scholar] [CrossRef]
- Ren, H.; Xiang, G. Recent Progress in Research on Ferromagnetic Rhenium Disulfide. Nanomaterials 2022, 12, 3451. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Huang, J.; Li, S.; Meng, A.; Li, Z. Interfacial chemical bond and internal electric field modulated Z-scheme Sv-ZnIn2S4/MoSe2 photocatalyst for efficient hydrogen evolution. Nat. Commun. 2021, 12, 4112. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wang, Y.; Dong, C.-L.; Huang, Y.-C.; Chen, J.; Xue, F.; Shen, S.; Guo, L. Boron-doped nitrogen-deficient carbon nitride-based Z-scheme heterostructures for photocatalytic overall water splitting. Nat. Energy 2021, 6, 388–397. [Google Scholar] [CrossRef]
- Xi, Y.; Wang, F.; Feng, H.; Xiong, Y.; Huang, Q. Cauliflower-like Mn0.2Cd0.8S decorated with ReS2 nanosheets for boosting photocatalytic H2 evolution activity. New J. Chem. 2021, 45, 15949–15955. [Google Scholar] [CrossRef]
- Ma, X.; Cheng, H. ReS2 with unique trion behavior as a co-catalyst for enhanced sunlight hydrogen production. J. Colloid Interface Sci. 2022, 634, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, K.; Pan, G.M.; Luo, Z.J.; Xie, Y.; Li, Y.Y.; Lin, Y.J.; Hao, Z.H.; Zhou, L.; Ding, S.J.; et al. Largely enhanced photocatalytic hydrogen production rate of CdS/(Au-ReS2) nanospheres by the dielectric-plasmon hybrid antenna effect. Nanoscale 2018, 10, 19586–19594. [Google Scholar] [CrossRef]
- Xing, C.; Zhao, H.; Yu, G.; Guo, L.; Hu, Y.; Chen, T.; Jiang, L.; Li, X. Modification of g-C3N4 Photocatalyst with Flower-like ReS2 for Highly Efficient Photocatalytic Hydrogen Evolution. ChemCatChem 2020, 12, 6385–6392. [Google Scholar] [CrossRef]
- Jing, Q.; Zhang, H.; Huang, H.; Fan, X.; Zhang, Y.; Hou, X.; Xu, Q.; Ni, Z.; Qiu, T. Ultrasonic exfoliated ReS2 nanosheets: Fabrication and use as co-catalyst for enhancing photocatalytic efficiency of TiO2 nanoparticles under sunlight. Nanotechnology 2019, 30, 184001. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Ito, Y.; Tan, Y.; Yamaguchi, H.; Hojo, D.; Hirata, A.; Voiry, D.; Chhowalla, M.; Chen, M. Chemically exfoliated ReS2 nanosheets. Nanoscale 2014, 6, 12458–12462. [Google Scholar] [CrossRef]
- Yu, Z.G.; Cai, Y.; Zhang, Y.-W. Robust Direct Bandgap Characteristics of One- and Two-Dimensional ReS2. Sci. Rep. 2015, 5, 13783. [Google Scholar] [CrossRef]
- Ran, J.; Zhang, H.; Qu, J.; Shan, J.; Davey, K.; Cairney, J.M.; Jing, L.; Qiao, S.Z. Significantly Raised Visible-Light Photocatalytic H2 Evolution on a 2D/2D ReS2 /In2ZnS4 van der Waals Heterostructure. Small 2021, 17, 2100296. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, D.; Xia, B.; Xu, H.; Tang, Y.; Davey, K.; Ran, J.; Qiao, S.-Z. ReS2 Nanosheets with In Situ Formed Sulfur Vacancies for Efficient and Highly Selective Photocatalytic CO2 Reduction. Small Sci. 2021, 1, 2000052. [Google Scholar] [CrossRef]
- Liu, N.; Dai, W.; Fei, F.; Xu, H.; Lei, J.; Quan, G.; Zheng, Y.; Zhang, X.; Tang, L. Insights into the photocatalytic activation persulfate by visible light over ReS2/MIL-88B(Fe) for highly efficient degradation of ibuprofen: Combination of experimental and theoretical study. Sep. Purif. Technol. 2022, 297, 121545. [Google Scholar] [CrossRef]
- Zhan, X.; Ou, D.; Zheng, Y.; Li, B.; Xu, L.; Yang, H.; Yang, W.; Zhang, H.; Hou, H.; Yang, W. Boosted photocatalytic hydrogen production over two-dimensional/two-dimensional Ta3N5/ReS2 van der Waals heterojunctions. J. Colloid Interface Sci. 2023, 629, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Shao, Y.; Hu, J.; Qu, J.; Yang, X.; Yang, F.; Ming Li, C. Ultrathin layered 2D/2D heterojunction of ReS2/high-crystalline g-C3N4 for significantly improved photocatalytic hydrogen evolution. Chem. Eng. J. 2022, 448, 137613. [Google Scholar] [CrossRef]
- Knirsch, K.C.; Berner, N.C.; Nerl, H.C.; Cucinotta, C.S.; Gholamvand, Z.; McEvoy, N.; Wang, Z.; Abramovic, I.; Vecera, P.; Halik, M.; et al. Basal-Plane Functionalization of Chemically Exfoliated Molybdenum Disulfide by Diazonium Salts. ACS Nano 2015, 9, 6018–6030. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Seo, S.; Luo, Y.; Sun, Y.; Oh, S.; Nguyen, C.T.K.; Seo, C.; Kim, J.H.; Kim, J.; Lee, H. Efficient and Stable Solar Hydrogen Generation of Hydrophilic Rhenium-Disulfide-Based Photocatalysts via Chemically Controlled Charge Transfer Paths. ACS Nano 2020, 14, 1715–1726. [Google Scholar] [CrossRef]
- Wang, X.; Xue, Y.; Liang, Z.; Tian, J.; Zhang, X.; Chen, X. Insights into the function of semi-metallic 1T’ phase ReS2 as cocatalyst decorated g-C3N4 nanotubes for enhanced photocatalytic hydrogen production activity. Mater. Today Adv. 2022, 15, 100257. [Google Scholar] [CrossRef]
- Wang, X.; Chen, B.; Yan, D.; Zhao, X.; Wang, C.; Liu, E.; Zhao, N.; He, F. Distorted 1T-ReS2 Nanosheets Anchored on Porous TiO2 Nanofibers for Highly Enhanced Photocatalytic Hydrogen Production. ACS Appl. Mater. Interfaces 2019, 11, 23144–23151. [Google Scholar] [CrossRef]
- Ye, L.; Ma, Z.; Deng, Y.; Ye, Y.; Li, W.; Kou, M.; Xie, H.; Zhikun, X.; Zhou, Y.; Xia, D.; et al. Robust and efficient photocatalytic hydrogen generation of ReS2/CdS and mechanistic study by on-line mass spectrometry and in situ infrared spectroscopy. Appl. Catal. B 2019, 257, 117897. [Google Scholar] [CrossRef]
- Zhan, X.; Fang, Z.; Li, B.; Zhang, H.; Xu, L.; Hou, H.; Yang, W. Rationally designed Ta3N5@ReS2 heterojunctions for promoted photocatalytic hydrogen production. J. Mater. Chem. A 2021, 9, 27084–27094. [Google Scholar] [CrossRef]
- Xiong, X.; Yan, A.; Zhang, X.; Huang, F.; Li, Z.; Zhang, Z.; Weng, H. ReS2/ZnIn2S4 heterojunctions with enhanced visible-light-driven hydrogen evolution performance for water splitting. J. Alloys Compd. 2021, 873, 159850. [Google Scholar] [CrossRef]
- Chen, R.; Ma, M.; Luo, Y.; Qian, L.; Xu, S. Enhanced interfacial effect between CdS and ReS2 on boosted hydrogen evolution performance via phase structure engineering. J. Solid State Chem. 2022, 312, 123238. [Google Scholar] [CrossRef]
- Song, T.; Wang, J.; Su, L.; Xu, H.; Bai, X.; Zhou, L.; Tu, W. Promotion effect of rhenium on MoS2/ReS2@CdS nanostructures for photocatalytic hydrogen production. Mol. Catal. 2021, 516, 111939. [Google Scholar] [CrossRef]
- Liu, J.; Qi, F.; Zhang, N.; Yang, J.; Liang, Z.; Tian, C.; Zhang, W.; Tang, X.; Wu, D.; Huang, Q. Three-dimensional structural ReS2@Cu2O/Cu heterojunction photocatalysts for visible-light-driven CO2 reduction. J. Mater. Sci. 2022, 57, 15474–15487. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, Q.; Xie, Y.; Lan, Z.; Prezhdo, O.V.; Saidi, W.A.; Zhao, J. Delocalized Impurity Phonon Induced Electron-Hole Recombination in Doped Semiconductors. Nano Lett. 2018, 18, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Zhang, Y.; Zhang, Y.; Liu, Z. Chemical vapour deposition of group-VIB metal dichalcogenide monolayers: Engineered substrates from amorphous to single crystalline. Chem. Soc. Rev. 2015, 44, 2587–2602. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, H.; Li, L.-J. Recent advances in controlled synthesis of two-dimensional transition metal dichalcogenides via vapour deposition techniques. Chem. Soc. Rev. 2015, 44, 2744–2756. [Google Scholar] [CrossRef]
- Zereshki, P.; Yao, P.; He, D.; Wang, Y.; Zhao, H. Interlayer charge transfer in ReS2/WS2 van der Waals heterostructures. Phys. Rev. B 2019, 99, 195438. [Google Scholar] [CrossRef]
- Varghese, A.; Joseph, D.; Ghosh, S.; Thakar, K.; Mcdhckar, N.; Lodha, S. WSe2/ReS2 vdW Heterostructure for Versatile Optoelectronic Applications. In Proceedings of the 2018 76th Device Research Conference (DRC), Santa Barbara, CA, USA, 24–27 June 2018. [Google Scholar]
- Wei, N.; Cai, J.; Wang, R.; Wang, M.; Lv, W.; Ci, H.; Sun, J.; Liu, Z. Elevated polysulfide regulation by an ultralight all-CVD-built ReS2@N-Doped graphene heterostructure interlayer for lithium-sulfur batteries. Nano Energy. 2019, 66, 104190. [Google Scholar]
- Li, J.; Song, P.; Zhao, J.; Vaklinova, K.; Zhao, X.; Li, Z.; Qiu, Z.; Wang, Z.; Lin, L.; Zhao, M.; et al. Printable two-dimensional superconducting monolayers. Nat. Mater. 2021, 20, 181–187. [Google Scholar]
- Liu, D.; Hong, J.; Li, X.; Zhou, X.; Jin, B.; Cui, Q.; Chen, J.; Feng, Q.; Xu, C.; Zhai, T.; et al. Synthesis of 2H-1T′ WS2-ReS2 Heterophase Structures with Atomically Sharp Interface via Hydrogen-Triggered One-Pot Growth. Adv. Funct. Mater. 2020, 30, 1910169. [Google Scholar] [CrossRef]
- Chen, B.; Wu, K.; Suslu, A.; Yang, S.; Cai, H.; Yano, A.; Soignard, E.; Aoki, T.; March, K.; Shen, Y.; et al. Controlling Structural Anisotropy of Anisotropic 2D Layers in Pseudo-1D/2D Material Heterojunctions. Adv. Mater. 2017, 29, 1701201. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Hong, J.; Wang, X.; Li, X.; Feng, Q.; Tan, C.; Zhai, T.; Ding, F.; Peng, H.; Xu, H. Diverse Atomically Sharp Interfaces and Linear Dichroism of 1T’ ReS2-ReSe2 Lateral p–n Heterojunctions. Adv. Funct. Mater. 2018, 28, 1804696. [Google Scholar] [CrossRef]
- Zhang, T.; Jiang, B.; Xu, Z.; Mendes, R.G.; Xiao, Y.; Chen, L.; Fang, L.; Gemming, T.; Chen, S.; Rummeli, M.H.; et al. Twinned growth behaviour of two-dimensional materials. Nat. Commun. 2016, 7, 13911. [Google Scholar] [CrossRef]
- Lim, J.; Kadyrov, A.; Jeon, D.; Choi, Y.; Bae, J.; Lee, S. Contact Engineering of Vertically Grown ReS2 with Schottky Barrier Modulation. ACS Appl. Mater. Interfaces 2021, 13, 7529–7538. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, J.; Huang, X.; Zhou, Y.; Chen, Y.; Xia, J.; Wang, H.; Xie, Y.; Yu, H.; Lei, J.; et al. A library of atomically thin metal chalcogenides. Nature 2018, 556, 355–359. [Google Scholar] [CrossRef]
- Ghoshal, D.; Yoshimura, A.; Gupta, T.; House, A.; Basu, S.; Chen, Y.; Wang, T.; Yang, Y.; Shou, W.; Hachtel, J.A.; et al. Theoretical and Experimental Insight into the Mechanism for Spontaneous Vertical Growth of ReS2 Nanosheets. Adv. Funct. Mater. 2018, 28, 1801286. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Yu, J. Low-Symmetry Two-Dimensional ReS2 and its Heterostructures: Chemical Vapor Deposition Synthesis and Properties. Prog. Chem. 2022, 34, 1440–1452. [Google Scholar]
- Gao, J.; Li, L.; Tan, J.; Sun, H.; Li, B.; Idrobo, J.C.; Singh, C.V.; Lu, T.M.; Koratkar, N. Vertically Oriented Arrays of ReS2 Nanosheets for Electrochemical Energy Storage and Electrocatalysis. Nano Lett. 2016, 16, 3780–3787. [Google Scholar] [CrossRef]
- Kang, Y.B.; Han, X.; Kim, S.; Yuan, H.; Ling, N.; Ham, H.C.; Dai, L.; Park, H.S. Structural Engineering of Ultrathin ReS2 on Hierarchically Architectured Graphene for Enhanced Oxygen Reduction. ACS Nano 2021, 15, 5560–5566. [Google Scholar] [CrossRef]
- He, X.; Liu, F.; Hu, P.; Fu, W.; Wang, X.; Zeng, Q.; Zhao, W.; Liu, Z. Chemical Vapor Deposition of High-Quality and Atomically Layered ReS2. Small 2015, 11, 5423–5429. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chu, H.; Zhang, X.; Pan, H.; Zhao, S.; Li, D. Heterostructure ReS2/GaAs Saturable Absorber Passively Q-Switched Nd:YVO4 Laser. Nanoscale Res. Lett. 2019, 14, 112. [Google Scholar] [CrossRef] [PubMed]
- Butanovs, E.; Kuzmin, A.; Piskunov, S.; Smits, K.; Kalinko, A.; Polyakov, B. Synthesis and characterization of GaN/ReS2, ZnS/ReS2 and ZnO/ReS2 core/shell nanowire heterostructures. Appl. Surf. Sci. 2021, 536, 147841. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Cheng, P.; Song, W.; Zhang, X.; Rong, S.; Gao, X.; Zhou, G.; Zhang, Z.; Liu, J. Universal substrate growth of Ag-modified ReS2 as visible-light-driven photocatalyst for highly efficient water disinfection. Chem. Eng. J. 2022, 430, 132918. [Google Scholar] [CrossRef]
- Zhou, G.; Wu, Q.; Wu, L.; Liu, L.; Wang, D.; Wang, P. Reaction kinetic acceleration induced by atomic-hybridized channels in carbon quantum dot/ReS2 composites for efficient Cr(VI) reduction. Appl. Catal. B 2022, 300, 119807. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, W.; Li, Z.; Zhang, Z.; Zhou, G. A new strategy: Fermi level control to realize 3D pyramidal NiCo-LDH/ReS2/n-PSi as a high-performance photoanode for the oxygen evolution reaction. J. Mater. Chem. C 2022, 10, 3848–3855. [Google Scholar] [CrossRef]
- Ha, E.; Liu, W.; Wang, L.; Man, H.W.; Hu, L.; Tsang, S.C.; Chan, C.T.; Kwok, W.M.; Lee, L.Y.; Wong, K.Y. Cu2ZnSnS4/MoS2-Reduced Graphene Oxide Heterostructure: Nanoscale Interfacial Contact and Enhanced Photocatalytic Hydrogen Generation. Sci. Rep. 2017, 7, 39411. [Google Scholar] [CrossRef]
- Li, L.; Salvador, P.A.; Rohrer, G.S. Photocatalysts with internal electric fields. Nanoscale 2014, 6, 24–42. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Sun, Y.; Xiao, T.; Tu, W.; Yuan, X.; Zeng, G.; Li, S.; Chew, J.W. Photogenerated charge transfer via interfacial internal electric field for significantly improved photocatalysis in direct Z-scheme oxygen-doped carbon nitrogen/CoAl-layered double hydroxide heterojunction. Appl. Catal. B 2018, 227, 530–540. [Google Scholar] [CrossRef]
- Bellus, M.Z.; Li, M.; Lane, S.D.; Ceballos, F.; Cui, Q.; Zeng, X.C.; Zhao, H. Type-I van der Waals heterostructure formed by MoS2 and ReS2 monolayers. Nanoscale Horiz. 2017, 2, 31–36. [Google Scholar] [CrossRef]
- Wang, F.; Hu, J.; Liang, R.; Lei, W.; Lou, Z.; Pan, X.; Lu, B.; Ye, Z. Novel ReS2/g-C3N4 heterojunction photocatalyst formed by electrostatic self-assembly with increased H2 production. Int. J. Hydrogen Energy 2022, 47, 29284–29294. [Google Scholar] [CrossRef]
- Hu, J.; Li, X.; Qu, J.; Yang, X.; Cai, Y.; Yang, T.; Yang, F.; Li, C.M. Bifunctional honeycomb hierarchical structured 3D/3D ReS2/ZnIn2S4-Sv heterojunction for efficient photocatalytic H2-evolution integrated with biomass oxidation. Chem. Eng. J. 2023, 453, 139957. [Google Scholar] [CrossRef]
- Reza Gholipour, M.; Dinh, C.T.; Beland, F.; Do, T.O. Nanocomposite heterojunctions as sunlight-driven photocatalysts for hydrogen production from water splitting. Nanoscale 2015, 7, 8187–8208. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Uddin, W.; Saleemi, A.S.; Hafeez, M.; Kamil, M.; Mir, I.A.; Sunila; Ullah, R.; Rehman, S.U.; Ling, Z. Optoelectronic properties of MoS2-ReS2 and ReS2-MoS2 heterostructures. Phys. B 2020, 577, 411809. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, Y.; Shen, G.; Hong, K. Synthesis of type-II heterojunction films between ReS2 and XS2 (X = Mo, W) with high electrocatalystic activities in dye-sensitized solar cells. Catal. Commun. 2022, 170, 106497. [Google Scholar] [CrossRef]
- Zhang, W.; Mohamed, A.R.; Ong, W.-J. Z-Scheme Photocatalytic Systems for Carbon Dioxide Reduction: Where Are We Now? Angew. Chem. Int. Ed. 2020, 59, 22894–22915. [Google Scholar] [CrossRef]
- Abdul Nasir, J.; Munir, A.; Ahmad, N.; ul Haq, T.; Khan, Z.; Rehman, Z. Photocatalytic Z-Scheme Overall Water Splitting: Recent Advances in Theory and Experiments. Adv. Mater. 2021, 33, 2105195. [Google Scholar] [CrossRef]
- Wang, L.; Bie, C.; Yu, J. Challenges of Z-scheme photocatalytic mechanisms. Trends Chem. 2022, 4, 973–983. [Google Scholar] [CrossRef]
- Di, T.; Xu, Q.; Ho, W.; Tang, H.; Xiang, Q.; Yu, J. Review on Metal Sulphide-based Z-scheme Photocatalysts. ChemCatChem 2019, 11, 1394–1411. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Z.; Wu, J. Construction of a Z-scheme heterojunction for high-efficiency visible-light-driven photocatalytic CO2 reduction. Nanoscale 2021, 13, 4359–4389. [Google Scholar] [CrossRef]
- Liu, H.; Xu, B.; Liu, J.M.; Yin, J.; Miao, F.; Duan, C.G.; Wan, X.G. Highly efficient and ultrastable visible-light photocatalytic water splitting over ReS2. Phys. Chem. Chem. Phys. 2016, 18, 14222–14227. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, R.; Song, K.; Liu, C.; He, F. Constructing a 2D/2D interfacial contact in ReS2/TiO2 via Ti–S bond for efficient charge transfer in photocatalytic hydrogen production. J. Mater. Chem. A 2021, 9, 23687–23696. [Google Scholar] [CrossRef]
- Lin, B.; Ma, B.; Chen, J.; Zhou, Y.; Zhou, J.; Yan, X.; Xue, C.; Luo, X.; Liu, Q.; Wang, J.; et al. Sea-urchin-like ReS2 nanosheets with charge edge-collection effect as a novel cocatalyst for high-efficiency photocatalytic H2 evolution. Chin. Chem. Lett. 2022, 33, 943–947. [Google Scholar] [CrossRef]
- Guo, L.; Yu, G.; Zhao, H.; Xing, C.; Hu, Y.; Chen, T.; Li, X. Construction of heterojunctions between ReS2 and twin crystal ZnxCd1−xS for boosting solar hydrogen evolution. New J. Chem. 2021, 45, 5137–5145. [Google Scholar] [CrossRef]
- Shi, S.; Sun, Z.; Hu, Y.H. Synthesis, stabilization and applications of 2-dimensional 1T metallic MoS2. J. Mater. Chem. A 2018, 6, 23932–23977. [Google Scholar] [CrossRef]
- Kwak, I.H.; Kwon, I.S.; Debela, T.T.; Seo, J.; Ahn, J.-P.; Yoo, S.J.; Kim, J.-G.; Park, J.; Kang, H.S. Two-dimensional MoS2–melamine hybrid nanostructures for enhanced catalytic hydrogen evolution reaction. J. Mater. Chem. A 2019, 7, 22571–22578. [Google Scholar] [CrossRef]
- Liu, L.; Wu, J.; Wu, L.; Ye, M.; Liu, X.; Wang, Q.; Hou, S.; Lu, P.; Sun, L.; Zheng, J.; et al. Phase-selective synthesis of 1T’ MoS2 monolayers and heterophase bilayers. Nat. Mater. 2018, 17, 1108–1114. [Google Scholar] [CrossRef]
- Yu, Y.; Nam, G.H.; He, Q.; Wu, X.J.; Zhang, K.; Yang, Z.; Chen, J.; Ma, Q.; Zhao, M.; Liu, Z.; et al. High phase-purity 1T’-MoS2- and 1T’-MoSe2-layered crystals. Nat. Chem. 2018, 10, 638–643. [Google Scholar] [CrossRef]
- Ran, J.; Chen, L.; Wang, D.; Talebian-Kiakalaieh, A.; Jiao, Y.; Adel Hamza, M.; Qu, Y.; Jing, L.; Davey, K.; Qiao, S.-Z. Atomic-Level Regulated Two-Dimensional ReSe2: A Universal Platform Boosting Photocatalysis. Adv. Mater. 2023, e2210164. [Google Scholar] [CrossRef]
- Huang, Q.; Yang, J.; Qi, F.; Zhang, W.; Zhang, N.; Liang, Z.; Liu, J.; Tian, C.; Tang, X.; Wu, D.; et al. Visible light driven photocatalytic reduction of CO2 on Au-Pt/Cu2O/ReS2 with high efficiency and controllable selectivity. Chem. Eng. J. 2022, 437, 135299. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Hong, J.; Liu, D.; Feng, Q.; Lei, Z.; Liu, K.; Ding, F.; Xu, H. Nanoassembly Growth Model for Subdomain and Grain Boundary Formation in 1T′ Layered ReS2. Adv. Funct. Mater. 2019, 29, 1906385. [Google Scholar] [CrossRef]
- Ho, C.H.; Huang, Y.S.; Tiong, K.K. In-plane anisotropy of the optical and electrical properties of ReS2 and ReSe2 layered crystals. J. Alloys Compd. 2001, 317–318, 222–226. [Google Scholar] [CrossRef]
- Parasuraman, P.S.; Ho, J.-H.; Lin, M.-H.; Ho, C.-H. In-Plane Axially Enhanced Photocatalysis by Re4 Diamond Chains in Layered ReS2. J. Phys. Chem. C 2018, 122, 18776–18784. [Google Scholar] [CrossRef]
- Liu, W.; Wang, P.; Ao, Y.; Chen, J.; Gao, X.; Jia, B.; Ma, T. Directing Charge Transfer in a Chemical-Bonded BaTiO3 @ReS2 Schottky Heterojunction for Piezoelectric Enhanced Photocatalysis. Adv. Mater. 2022, 34, e2202508. [Google Scholar] [CrossRef]
- Li, H.; Liang, Z.; Deng, Q.; Hu, M.T.; Du, N.; Hou, W. Facile Construction of Defect-rich Rhenium Disulfide/Graphite Carbon Nitride Heterojunction via Electrostatic Assembly for Fast Charge Separation and Photoactivity Enhancement. ChemCatChem 2019, 11, 1633–1642. [Google Scholar] [CrossRef]
- Tu, S.; Guo, Y.; Zhang, Y.; Hu, C.; Zhang, T.; Ma, T.; Huang, H. Piezocatalysis and Piezo-Photocatalysis: Catalysts Classification and Modification Strategy, Reaction Mechanism, and Practical Application. Adv. Funct. Mater. 2020, 30, 2005158. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Wang, W.; Huang, G.; Yu, J.C.; Wong, P.K. Advances in photocatalytic disinfection of bacteria: Development of photocatalysts and mechanisms. J. Environ. Sci. 2015, 34, 232–247. [Google Scholar] [CrossRef]

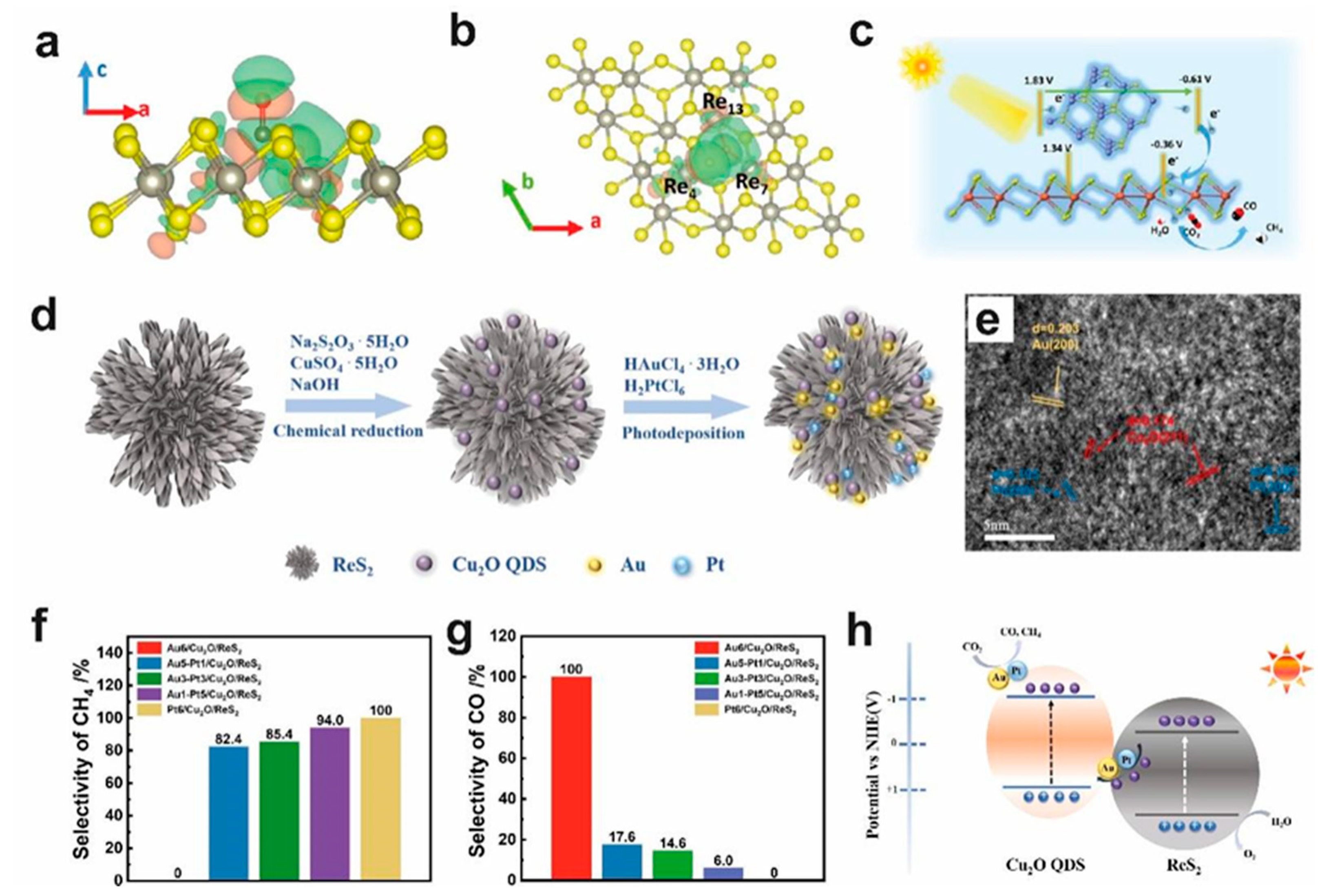
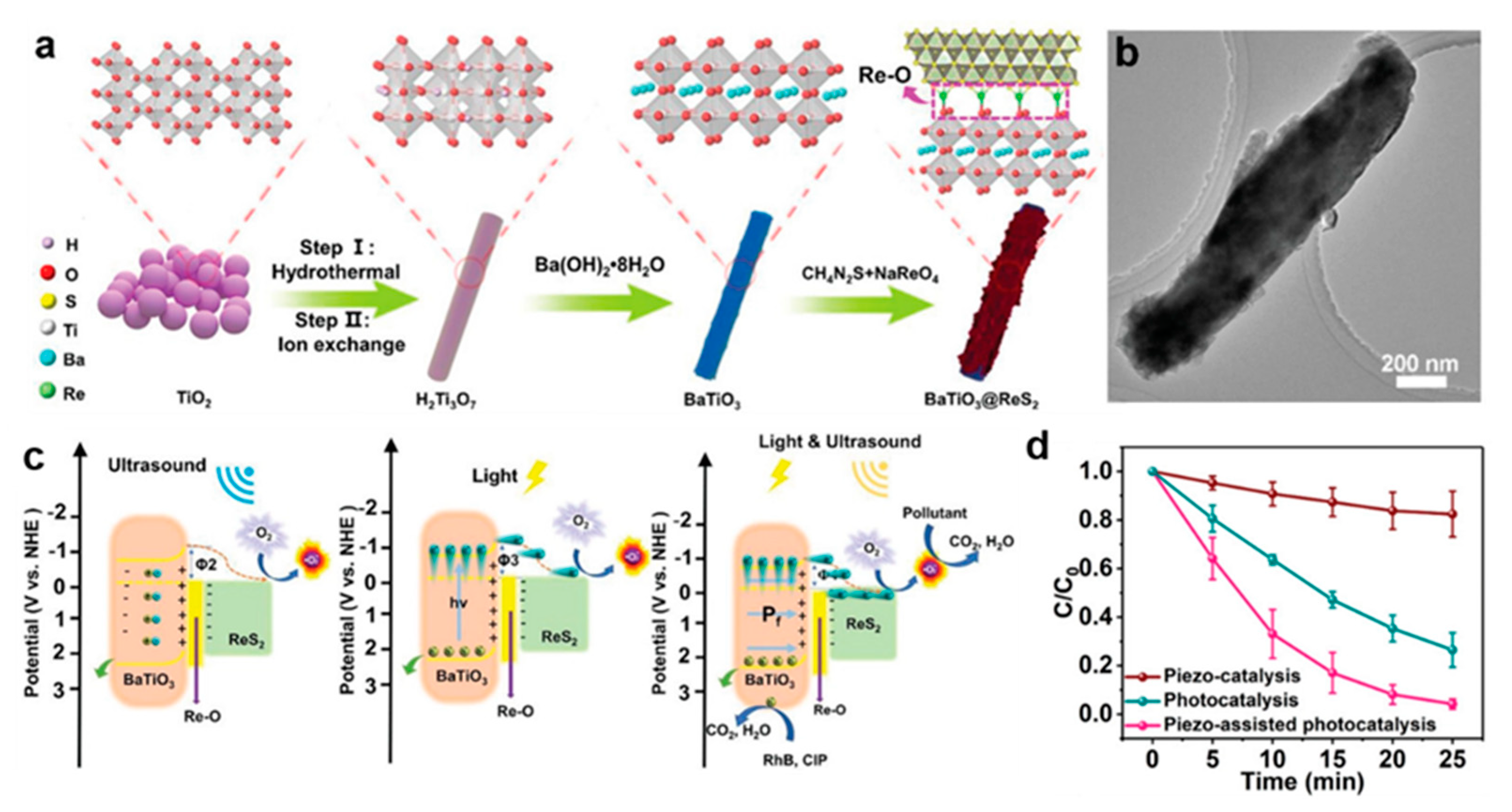


| Photocatalyst | Source of Light | Morphology | Performance | Ref |
|---|---|---|---|---|
| ReS2/ZnIn2S4 | 300 W Xenon arc lamp (λ ≥ 420 nm cutoff filter) | cobblestone structure with particles | 1858.6 μmol h−1 g−1 | [61] |
| ReS2 nanowalls | 300 W Xenon arc lamp (λ ≥ 420 nm cutoff filter) | ReS2 nanowalls | 13 mmol h−1 g−1 | [33] |
| TiO2/ReS2 | 300 W Xenon arc lamp | ReS2 nanosheets and TiO2 nanofibers | 1404 μmol h−1 g−1 | [58] |
| ReS2/TiO2 | Solar simulator (λ ≥ 300 nm) | sea-urchin-like structured | 3.71 mmol h−1 g−1 | [104] |
| ReS2-BzO-TiO2 | Solar simulator | nanosheets | 9.5 mmol h−1 g−1 | [56] |
| CdS/ReS2 | 300 W Xennon lamp (λ ≥ 420 nm UV-cutoff filter) | nanorod | 137.5 mmol h−1 g−1 | [59] |
| ReS2/Zn0.5Cd0.5S | visible light irradiation (λ ≥ 420 nm) | nanospheres on ReS2 nanosheets | 112.10 mmol h−1 g−1 | [105] |
| ReS2/Mn0.2Cd0.8S | 300 W Xenon arc lamp (λ ≥ 420 nm cutoff filter) | cauliflower-like morphology | 17.31 mmol h−1 g−1 | [43] |
| CdS/(Au-ReS2) | 300 W Xenon arc lamp (λ ≥ 420 nm cutoff filter) | ReS2 nanosheets | 3060 μmol h−1 g−1 | [45] |
| g-C3N4/CdS/ReS2 | 300 W Xenon arc lamp (AM 1.5 G filter) | a hollow spherical nano-shell structure | 7141.2 ± 85.7 μmol h−1 g−1 | [44] |
| ReS2/ZnIn2S4-Sv | 300 W Xenon arc lamp | nanoflower | 1.08 mmol h−1 g−1 | [93] |
| ReS2/TiO2 | 300 W Xenon arc lamp | ReS2 ultrathin nanosheets | 1037 μmol h−1 g−1 | [34] |
| ReS2/g-C3N4 | Solar simulator (AM 1.5G) | nanospheres | 1823 mmol h−1 g−1 | [92] |
| ReS2/ZnIn2S4 | Xenon arc lamp (400 nm cutoff light filter) | ReS2 nanosheets | 2515 µmol h−1 g−1 | [50] |
| ReS2/g-C3N4 | 300 W Xenon arc lamp | ultrathin layered 2D/2D structure | 3.46 mmol h−1 g−1 | [54] |
| MoS2/ReS2@CdS | 300 W Xenon arc lamp (λ ≥ 420 nm) | CdS@ReS2 nano-spheres and MoS2 nanoflakes | 171.9 mmol h−1 g−1 | [63] |
| ReS2/TiO2 | 300 W Xenon arc lamp | circle-shaped sheet-like structures 2D TiO2 | 762.3 mmol h−1 g−1 | [103] |
| ReS2/g-C3N4 | visible light irradiation (λ ≥ 420 nm) | ReS2 nanoflowers on the surface of g-C3N4 | 249 μmol h−1 g−1 | [46] |
| ReS2-CdS/P-0.2 | 300 W Xenon arc lamp (λ ≥ 420 nm cutoff filter) | CdS nanorods and ReS2 nanosheet | 14.68 mmol h−1 g−1 | [62] |
| Ta3N5/ReS2 | 300 W Xenon arc lamp | CdS nanorods and ReS2 nanosheet | 615 μmol h−1 g−1 | [53] |
| Photocatalyst | Source of Light | Morphology | Performance | Ref |
|---|---|---|---|---|
| ReS2@Cu2O/Cu | 300 W Xennon lamp (λ ≥ 420 nm UV-cutoff filter) | ReS2 particles on the surface of Cu2O/Cu frameworks | CO (14.3 μmol h−1 g−1) | [64] |
| Au-Pt/Cu2O/ReS2 | 300 W Xennon lamp | flower-like microsphere | CH4 (60.76 μmol h−1 g−1) | [111] |
| ReS2/CdS | visible-light irradiation (λ ≥ 420 nm) | nanosheets | CO (7.1 μmol h−1 g−1) | [51] |
| Photocatalyst | Type | Synthesis Methods | Morphology | Light Source | Application | Efficiency | Cycle | Ref |
|---|---|---|---|---|---|---|---|---|
| ReS2/MIL-88B(Fe) | Type-II | solvothermal method | shuttle structure | Both PS and visible light irradiation | Degradation of Ibuprofen (IBP) | 100% (3 h) | 3 | [52] |
| TiO2@ReS2 | Z-scheme | ultrasonic liquid exfoliation method | ReS2 nanosheets | Solar simulator | Degradation of Rhodamine B (RhB) | 94% (120 min) | 25 | [47] |
| BaTiO3@ReS2 | Type-I | multi-step hydrothermal method | ReS2 nanosheets on BaTiO3 nanorods | UV-vis light | Degradation of RhB | 96% (25 min) | 3 | [115] |
| carbon quantum dots (CQDs)/ReS2 | Type-I | two-step hydrothermal method | rCQDs/ReS2 nanosheets | 300 W Xenon lamp | Cr (VI) | 96% (50 min) | 6 | [86] |
| ReS2/Graphite Carbon Nitride (CN) | Type-II | electrostatic assembly process | ReS2 microspheres and CN nanosheets | 300 W Xenon arc lamp (λ ≥ 420 nm cutoff filter) | RhB | 94% (30 min) | 3 | [116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Li, Y.; Wang, L.; Yu, X. Photocatalytic Applications of ReS2-Based Heterostructures. Molecules 2023, 28, 2627. https://doi.org/10.3390/molecules28062627
Wang N, Li Y, Wang L, Yu X. Photocatalytic Applications of ReS2-Based Heterostructures. Molecules. 2023; 28(6):2627. https://doi.org/10.3390/molecules28062627
Chicago/Turabian StyleWang, Nan, Yashu Li, Lin Wang, and Xuelian Yu. 2023. "Photocatalytic Applications of ReS2-Based Heterostructures" Molecules 28, no. 6: 2627. https://doi.org/10.3390/molecules28062627
APA StyleWang, N., Li, Y., Wang, L., & Yu, X. (2023). Photocatalytic Applications of ReS2-Based Heterostructures. Molecules, 28(6), 2627. https://doi.org/10.3390/molecules28062627





