Ratiometric Near-Infrared Fluorescence Liposome Nanoprobe for H2S Detection In Vivo
Abstract
1. Introduction
2. Results and Discussion
2.1. Design of Ratiometric NIR Fluorescence Liposome Nanoprobe for H2S Detection
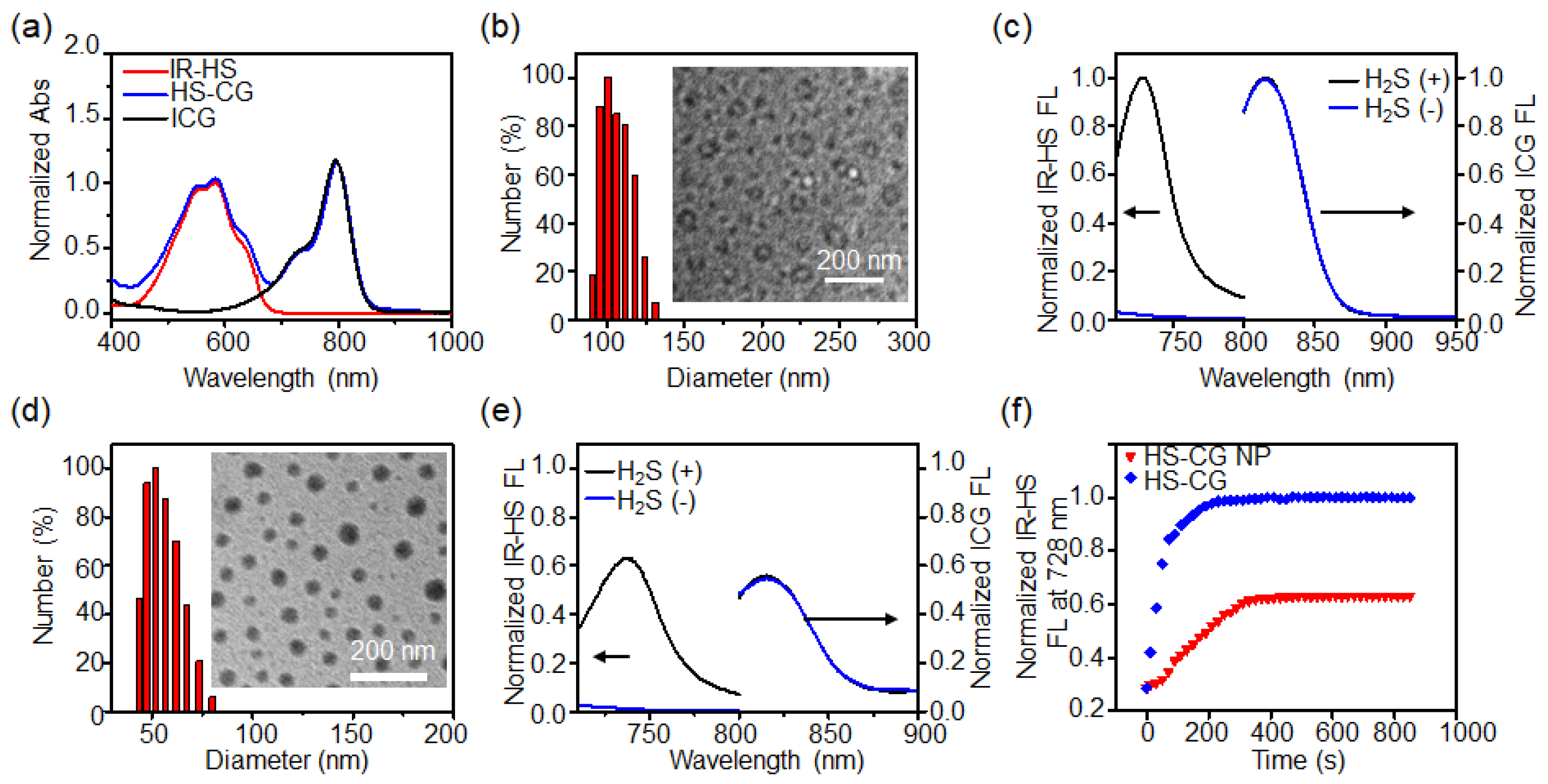
2.2. Synthesis and Characterization of Liposome Nanoprobe HS-CG
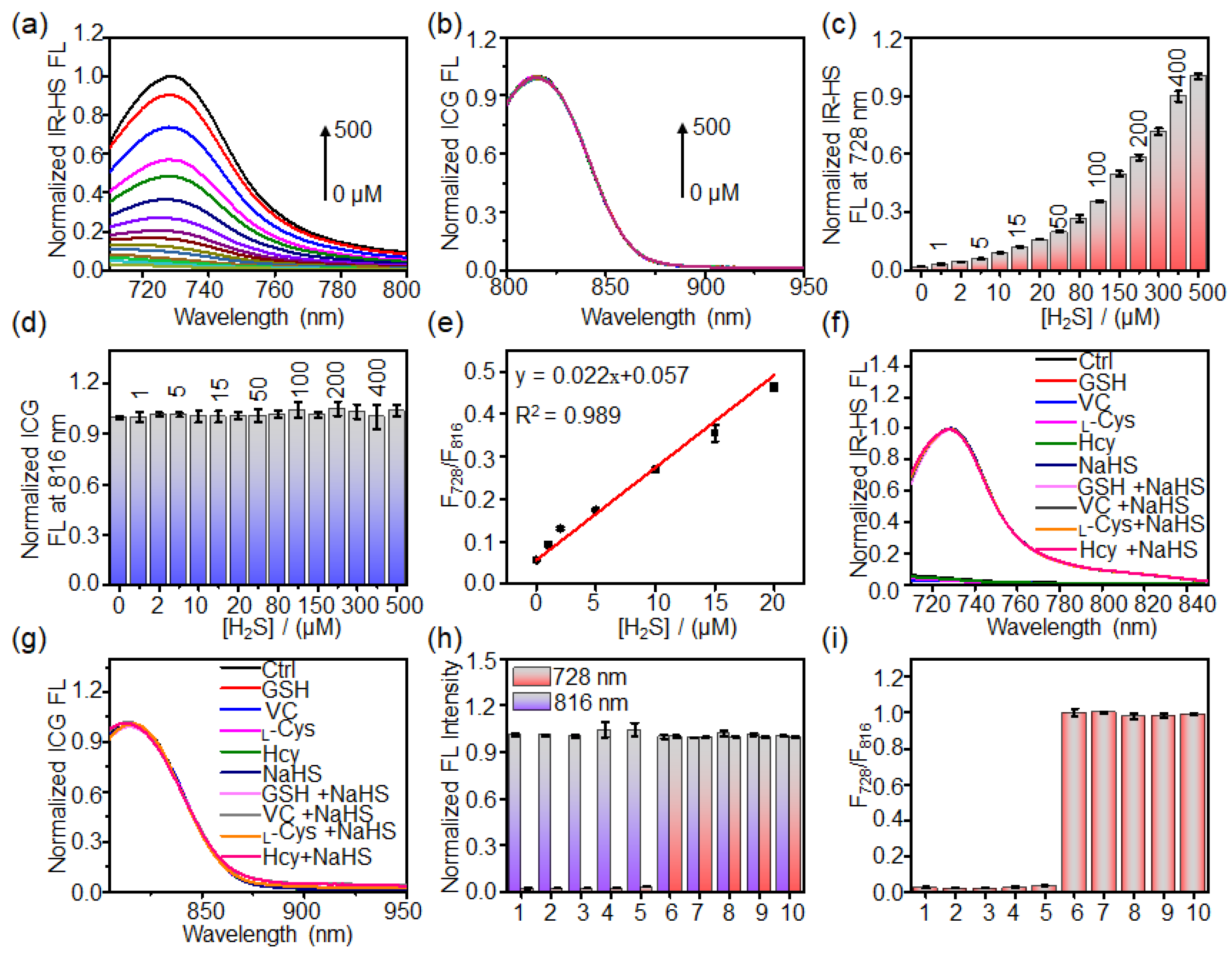
2.3. Response of Liposome Nanoprobe HS-CG toward H2S In Vitro
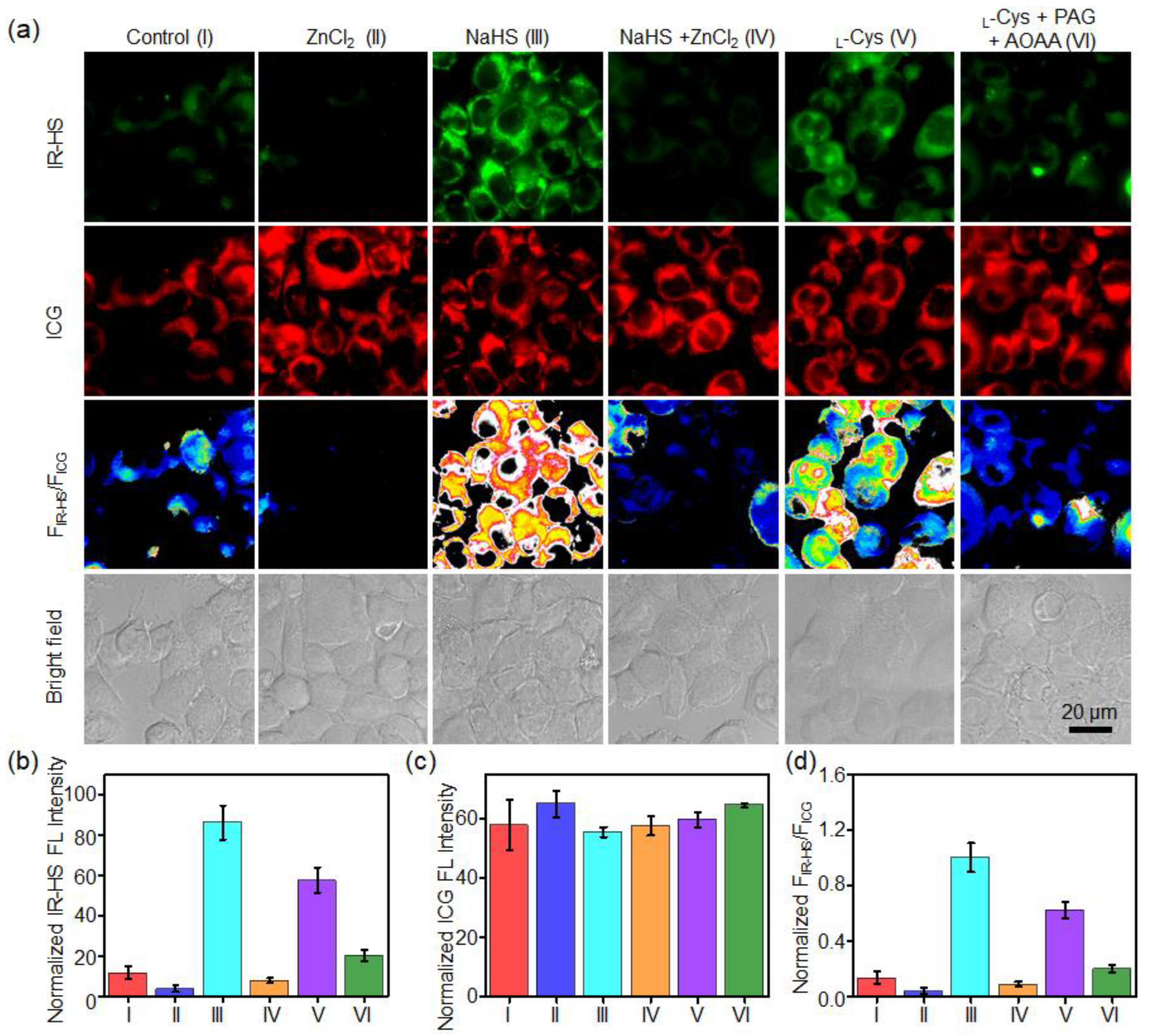
2.4. Imaging of H2S in Tumor Cells
2.5. Imaging of H2S in Tumor Cells
3. Materials and Methods
3.1. Materials and Instrumentation
3.2. Preparation of Liposome Nanoprobe HS-CG
3.3. Investigation of the Sensitivity and Selectivity of HS-CG toward H2S
3.4. Cell Culture
3.5. Ratiometric Imaging of H2S in Living Tumor Cells
3.6. Animals and Tumor Models
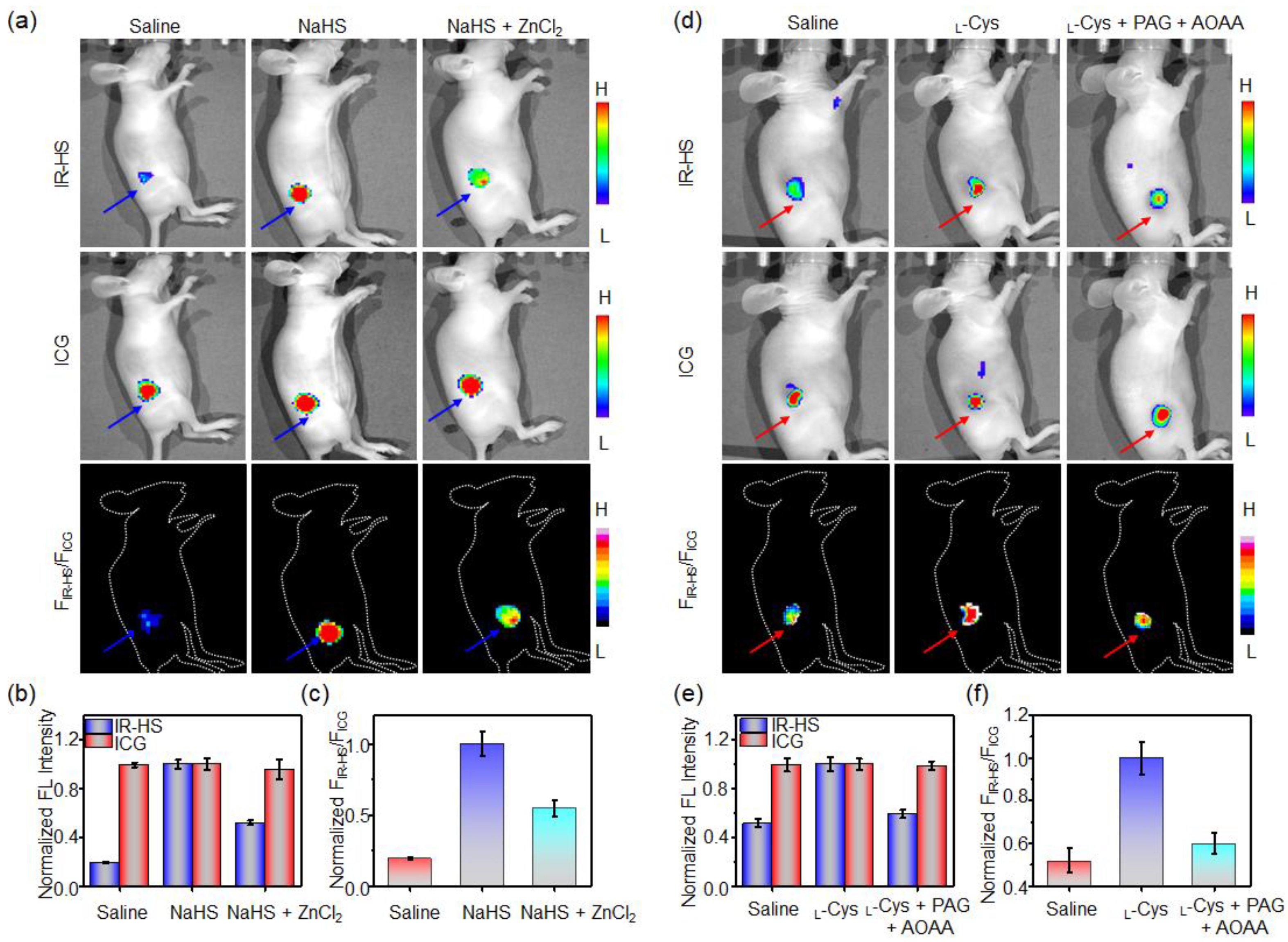
3.7. Ratiometric Fluorescence Imaging of Exogeneous H2S in Mice
3.8. Ratiometric Imaging of Endogenous H2S Tumor of Mice
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hammers, M.D.; Taormina, M.J.; Cerda, M.M.; Montoya, L.A.; Seidenkranz, D.T.; Parthasarathy, R.; Pluth, M.D. A bright fluorescent probe for H2S enables analyte-responsive, 3D imaging in live zebrafish using light sheet fluorescence microscopy. J. Am. Chem. Soc. 2015, 137, 10216–10223. [Google Scholar] [CrossRef] [PubMed]
- Magierowski, M.; Magierowska, K.; Kwiecien, S.; Brzozowski, T. Gaseous mediators nitric oxide and hydrogen sulfide in the mechanism of gastrointestinal integrity, protection and ulcer healing. Molecules 2015, 20, 9099–9123. [Google Scholar] [CrossRef] [PubMed]
- Hartle, M.D.; Pluth, M.D. A practical guide to working with H2S at the interface of chemistry and biology. Chem. Soc. Rev. 2016, 45, 6108–6117. [Google Scholar] [CrossRef] [PubMed]
- Calvert, J.W.; Coetzee, W.A.; Lefer, D.J. Novel insights into hydrogen sulfide–mediated cytoprotection. Antioxid. Redox Signal. 2010, 12, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Predmore, B.L.; Lefer, D.J.; Gojon, G. Hydrogen sulfide in biochemistry and medicine. Antioxid. Redox Signal. 2012, 17, 119–140. [Google Scholar] [CrossRef]
- Takano, Y.; Echizen, H.; Hanaoka, K. Fluorescent probes and selective inhibitors for biological studies of hydrogen sulfide-and polysulfide-mediated signaling. Antioxid. Redox Signal. 2017, 27, 669–683. [Google Scholar] [CrossRef]
- Fernandes, V.S.; Ribeiro, A.S.; Martínez, P.; López-Oliva, M.E.; Barahona, M.V.; Orensanz, L.M.; Martínez-Sáenz, A.; Recio, P.; Benedito, S.; Bustamante, S. Hydrogen sulfide plays a key role in the inhibitory neurotransmission to the pig intravesical ureter. PLoS ONE 2014, 9, e113580. [Google Scholar] [CrossRef]
- Yang, G.; Wu, L.; Jiang, B.; Yang, W.; Qi, J.; Cao, K.; Meng, Q.; Mustafa, A.K.; Mu, W.; Zhang, S. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine γ-lyase. Science 2008, 322, 587–590. [Google Scholar] [CrossRef]
- Lefer, D.J. A new gaseous signaling molecule emerges: Cardioprotective role of hydrogen sulfide. Proc. Natl. Acad. Sci. USA 2007, 104, 17907–17908. [Google Scholar] [CrossRef]
- Wu, L.; Zeng, W.; Ishigaki, Y.; Zhang, J.; Bai, H.; Harimoto, T.; Suzuki, T.; Ye, D. A Ratiometric Photoacoustic Probe with a Reversible Response to Hydrogen Sulfide and Hydroxyl Radicals for Dynamic Imaging of Liver Inflammation. Angew. Chem. Int. Ed. 2022, 61, e202209248. [Google Scholar]
- Li, J.; Xie, L.; Li, B.; Yin, C.; Wang, G.; Sang, W.; Li, W.; Tian, H.; Zhang, Z.; Zhang, X. Engineering a Hydrogen-Sulfide-Based Nanomodulator to Normalize Hyperactive Photothermal Immunogenicity for Combination Cancer Therapy. Adv. Mater. 2021, 33, 2008481. [Google Scholar] [CrossRef] [PubMed]
- Panagaki, T.; Randi, E.B.; Augsburger, F.; Szabo, C. Overproduction of H2S, generated by CBS, inhibits mitochondrial Complex IV and suppresses oxidative phosphorylation in Down syndrome. Proc. Natl. Acad. Sci. USA 2019, 116, 18769–18771. [Google Scholar] [CrossRef] [PubMed]
- Panagaki, T.; Lozano-Montes, L.; Janickova, L.; Zuhra, K.; Szabo, M.P.; Majtan, T.; Rainer, G.; Maréchal, D.; Herault, Y.; Szabo, C. Overproduction of hydrogen sulfide, generated by cystathionine β-synthase, disrupts brain wave patterns and contributes to neurobehavioral dysfunction in a rat model of down syndrome. Redox Biol. 2022, 51, 102233. [Google Scholar] [CrossRef] [PubMed]
- Giovinazzo, D.; Bursac, B.; Sbodio, J.I.; Nalluru, S.; Vignane, T.; Snowman, A.M.; Albacarys, L.M.; Sedlak, T.W.; Torregrossa, R.; Whiteman, M. Hydrogen sulfide is neuroprotective in Alzheimer’s disease by sulfhydrating GSK3β and inhibiting Tau hyperphosphorylation. Proc. Natl. Acad. Sci. USA 2021, 118, e2017225118. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Zhang, Y.; Zhou, Y.; Liu, Q.; Chen, X.; Liu, X.; Grune, T.; Shi, L.; Hou, M.; Liu, Z. Effects of methionine intake on cognitive function in mild cognitive impairment patients and APP/PS1 Alzheimer’s Disease model mice: Role of the cystathionine-β-synthase/H2S pathway. Redox Biol. 2022, 59, 102595. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Yu, Y.; Zhu, L.; Lai, N.; Zhang, L.; Guo, Y.; Lin, X.; Yang, D.; Ren, N.; Zhu, Z. Implications of hydrogen sulfide in colorectal cancer: Mechanistic insights and diagnostic and therapeutic strategies. Redox Biol. 2023, 59, 102601. [Google Scholar] [CrossRef]
- Gong, W.; Jiang, L.; Zhu, Y.; Jiang, M.; Chen, D.; Jin, Z.; Qin, S.; Yu, Z.; He, Q. An Activity-Based Ratiometric Fluorescent Probe for In Vivo Real-Time Imaging of Hydrogen Molecules. Angew. Chem. Int. Ed. 2022, 61, e202114594. [Google Scholar] [CrossRef]
- Lan, Q.; Yu, P.; Yan, K.; Li, X.; Zhang, F.; Lei, Z. Polymethine molecular platform for ratiometric fluorescent probes in the second near-infrared window. J. Am. Chem. Soc. 2022, 144, 21010–21015. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, H.; Wang, L.; Qin, X.; Jiang, B.P.; Ji, S.C.; Shen, X.C.; Liang, H. A general approach to design dual ratiometric fluorescent and photoacoustic probes for quantitatively visualizing tumor hypoxia levels In Vivo. Angew. Chem. Int. Ed. 2022, 61, e202107076. [Google Scholar]
- Srikun, D.; Miller, E.W.; Domaille, D.W.; Chang, C.J. An ICT-based approach to ratiometric fluorescence imaging of hydrogen peroxide produced in living cells. J. Am. Chem. Soc. 2008, 130, 4596–4597. [Google Scholar] [CrossRef]
- Aron, A.T.; Loehr, M.O.; Bogena, J.; Chang, C.J. An endoperoxide reactivity-based FRET probe for ratiometric fluorescence imaging of labile iron pools in living cells. J. Am. Chem. Soc. 2016, 138, 14338–14346. [Google Scholar] [CrossRef]
- Wang, H.; He, Z.; Yang, Y.; Zhang, J.; Zhang, W.; Zhang, W.; Li, P.; Tang, B. Ratiometric fluorescence imaging of Golgi H2O2 reveals a correlation between Golgi oxidative stress and hypertension. Chem. Sci. 2019, 10, 10876–10880. [Google Scholar] [CrossRef]
- Wang, Z.; Detomasi, T.C.; Chang, C.J. A dual-fluorophore sensor approach for ratiometric fluorescence imaging of potassium in living cells. Chem. Sci. 2021, 12, 1720–1729. [Google Scholar] [CrossRef] [PubMed]
- Abeywickrama, C.S.; Pang, Y. Synthesis of a bis [2-(2′-hydroxyphenyl) benzoxazole] pyridinium derivative: The fluoride-induced large spectral shift for ratiometric response. New J. Chem. 2021, 45, 9102–9108. [Google Scholar] [CrossRef]
- Ge, X.; Lou, Y.; Su, L.; Chen, B.; Guo, Z.; Gao, S.; Zhang, W.; Chen, T.; Song, J.; Yang, H. Single wavelength laser excitation ratiometric NIR-II fluorescent probe for molecule imaging In Vivo. Anal. Chem. 2020, 92, 6111–6120. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Ye, M.; Wang, Z.; Liu, P.; Li, H.; Lu, C.; Wang, Y.; Liang, T.; Li, H.; Li, C. A ratiometric near-infrared fluorescent probe with a large emission peak shift for sensing and imaging hypochlorous acid. Sens. Actuators B Chem. 2021, 343, 130063. [Google Scholar] [CrossRef]
- Shu, W.; Zang, S.; Wang, C.; Gao, M.; Jing, J.; Zhang, X. An endoplasmic reticulum-targeted ratiometric fluorescent probe for the sensing of hydrogen sulfide in living cells and zebrafish. Anal. Chem. 2020, 92, 9982–9988. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, C.; Yang, Z.; Chen, J.; He, Y.; Jiao, Y.; He, W.; Qiu, L.; Cen, J.; Guo, Z. A ratiometric fluorescent probe for rapid detection of hydrogen sulfide in mitochondria. Angew. Chem. Int. Ed. 2013, 52, 1688–1691. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, X.; Li, K.; Zhu, S.; Guo, Z.; Zhang, L.; Wang, F.; Fei, Q.; Luo, S.; Shi, P. Forster resonance energy transfer switchable self-assembled micellar nanoprobe: Ratiometric fluorescent trapping of endogenous H2S generation via fluvastatin-stimulated upregulation. J. Am. Chem. Soc. 2015, 137, 8490–8498. [Google Scholar] [CrossRef]
- Wang, X.; Sun, J.; Zhang, W.; Ma, X.; Lv, J.; Tang, B. A near-infrared ratiometric fluorescent probe for rapid and highly sensitive imaging of endogenous hydrogen sulfide in living cells. Chem. Sci. 2013, 4, 2551–2556. [Google Scholar] [CrossRef]
- Bae, S.K.; Heo, C.H.; Choi, D.J.; Sen, D.; Joe, E.-H.; Cho, B.R.; Kim, H.M. A ratiometric two-photon fluorescent probe reveals reduction in mitochondrial H2S production in Parkinson’s disease gene knockout astrocytes. J. Am. Chem. Soc. 2013, 135, 9915–9923. [Google Scholar] [CrossRef]
- He, L.; Lin, W.; Xu, Q.; Wei, H. A new strategy to construct a FRET platform for ratiometric sensing of hydrogen sulfide. Chem. Commun. 2015, 51, 1510–1513. [Google Scholar] [CrossRef]
- Zeng, W.; Wu, L.; Sun, Y.; Wang, Y.; Wang, J.; Ye, D. Ratiometric imaging of MMP-2 activity facilitates tumor detection using activatable near-infrared fluorescent semiconducting polymer nanoparticles. Small 2021, 17, 2101924. [Google Scholar] [CrossRef] [PubMed]
- Maity, D.; Raj, A.; Samanta, P.K.; Karthigeyan, D.; Kundu, T.K.; Pati, S.K.; Govindaraju, T. A probe for ratiometric near-infrared fluorescence and colorimetric hydrogen sulfide detection and imaging in live cells. RSC Adv. 2014, 4, 11147–11151. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Y.Q.; Zhang, J.; Yang, T.; Cao, J.; Zhang, L.; Guo, W. A ratiometric fluorescent probe for biological signaling molecule H2S: Fast response and high selectivity. Chem. Eur. J. 2013, 19, 4717–4722. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.W.; Yang, X.F.; Zhong, Y.; Guo, Y.; Li, Z.; Li, H. A ratiometric fluorescent probe for gasotransmitter hydrogen sulfide based on a coumarin-benzopyrylium platform. Anal. Chim. Acta 2015, 859, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tan, H.; Yan, Y.; Hang, Y.; Yu, F.; Qu, X.; Hua, J. Targetable N-annulated perylene-based colorimetric and ratiometric near-infrared fluorescent probes for the selective detection of hydrogen sulfide in mitochondria, lysosomes, and serum. J. Mater. Chem. B 2017, 5, 2172–2180. [Google Scholar] [CrossRef]
- Wu, L.; Zeng, W.; Feng, L.; Hu, Y.; Sun, Y.; Yan, Y.; Chen, H.-Y.; Ye, D. An activatable ratiometric near-infrared fluorescent probe for hydrogen sulfide imaging In Vivo. Sci. China Chem. 2020, 63, 741–750. [Google Scholar] [CrossRef]
- Sheng, Z.; Hu, D.; Zheng, M.; Zhao, P.; Liu, H.; Gao, D.; Gong, P.; Gao, G.; Zhang, P.; Ma, Y. Smart human serum albumin-indocyanine green nanoparticles generated by programmed assembly for dual-modal imaging-guided cancer synergistic phototherapy. ACS Nano 2014, 8, 12310–12322. [Google Scholar] [CrossRef]
- Song, S.; Shen, H.; Yang, T.; Wang, L.; Fu, H.; Chen, H.; Zhang, Z. Indocyanine green loaded magnetic carbon nanoparticles for near infrared fluorescence/magnetic resonance dual-modal imaging and photothermal therapy of tumor. ACS Appl. Mater. Int. 2017, 9, 9484–9495. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Liu, Y.; Zhang, J.; Miao, Y.; An, R. Ratiometric Near-Infrared Fluorescence Liposome Nanoprobe for H2S Detection In Vivo. Molecules 2023, 28, 1898. https://doi.org/10.3390/molecules28041898
Wu L, Liu Y, Zhang J, Miao Y, An R. Ratiometric Near-Infrared Fluorescence Liposome Nanoprobe for H2S Detection In Vivo. Molecules. 2023; 28(4):1898. https://doi.org/10.3390/molecules28041898
Chicago/Turabian StyleWu, Luyan, Yili Liu, Junya Zhang, Yinxing Miao, and Ruibing An. 2023. "Ratiometric Near-Infrared Fluorescence Liposome Nanoprobe for H2S Detection In Vivo" Molecules 28, no. 4: 1898. https://doi.org/10.3390/molecules28041898
APA StyleWu, L., Liu, Y., Zhang, J., Miao, Y., & An, R. (2023). Ratiometric Near-Infrared Fluorescence Liposome Nanoprobe for H2S Detection In Vivo. Molecules, 28(4), 1898. https://doi.org/10.3390/molecules28041898



