Abstract
Glioblastoma (GBM) is the most aggressive and frequent primary brain tumor, with a poor prognosis and the highest mortality rate. Currently, GBM therapy consists of surgical resection of the tumor, radiotherapy, and adjuvant chemotherapy with temozolomide. Consistently, there are poor treatment options and only modest anticancer efficacy is achieved; therefore, there is still a need for the development of new effective therapies for GBM. Indole is considered one of the most privileged scaffolds in heterocyclic chemistry, so it may serve as an effective probe for the development of new drug candidates against challenging diseases, including GBM. This review analyzes the therapeutic benefit and clinical development of novel indole-based derivatives investigated as promising anti-GBM agents. The existing indole-based compounds which are in the pre-clinical and clinical stages of development against GBM are reported, with particular reference to the most recent advances between 2013 and 2022. The main mechanisms of action underlying their anti-GBM efficacy, such as protein kinase, tubulin and p53 pathway inhibition, are also discussed. The final goal is to pave the way for medicinal chemists in the future design and development of novel effective indole-based anti-GBM agents.
1. Introduction
In recent decades, brain tumors have an increasingly frequent incidence [1]. The average annual incidence rate of all brain tumors in the years 2013–2017 was 23.79 per 100,000, with a higher rate in females than males (26.31 versus 21.09), being 7.08 per 100,000 the average annual incidence rate of primary malignant brain tumors. It has been estimated that 83,830 new cases of primary brain tumors and other central nervous system (CNS) tumors were diagnosed in the United States in 2020 and this includes an expected 24,970 primary malignant tumors [2]. In 2020, worldwide, about 308,102 people were diagnosed with a primary tumor of the brain or spinal cord. Finally, in the United States, in 2022, about 25,050 cases (14,170 men and 10,880 women) of primary brain and spinal cord tumors were diagnosed [3].
Primary brain tumors are mainly termed gliomas and, based on their cellular origin, they can be classified in astrocytic tumors, oligodendrogliomas, mixed gliomas and ependymomas [4]. Glioblastoma (GBM) is a solid tumor of the brain or spinal cord originating from a microglial cell subtype, termed astrocytes. Brain tumors have also been classified, from the mildest to the most aggressive, in four grades [5]: pilocytic astrocytoma (I), diffuse astrocytoma (II), anaplastic astrocytoma (III), and GBM (IV). GBM represents the most aggressive brain tumor, mainly due to its ability to penetrate healthy tissues thanks to the maintenance of sustained angiogenesis. GBM is also the most frequent primary brain tumor, accounting for approximately 15% of all primary brain tumors in adults and with a worldwide incidence of less than 10 per 100,000 people [6,7,8,9]. Furthermore, among the various brain tumors, GBM also has a poor prognosis and higher mortality rate [1,10].
Currently, the GBM therapeutic protocol consists of surgical resection, radiotherapy, and chemotherapy with temozolomide. Complete surgical resection of the tumor improves survival rates, but it is extremely difficult, due to infiltration into the surrounding tissues and, as a result, GBM recurs, often leading to the death of patients [11,12]. Both radiotherapy and temozolomide induce DNA double strand breaks thus activating apoptosis to achieve GBM cell death. In clinical trials, patients with GBM still have an average survival of approximately 15–18 months and a time to recurrence of approximately 7 months, with a 5-year survival of less than 10% or even shorter in older patients [13]. In addition, once GBM has recurred, the mean overall survival was approximately 24–44 weeks [14]. There are poor treatment options for inexorable recurrence, and with chemotherapy only modest anticancer activity is achieved [15]. The standard therapy for recurrent GBM are DNA alkylating drugs, such as lomustine and carmustine, and bevacizumab, an antiangiogenic agent approved by the U.S. Food and Drug Administration (FDA) for the care of relapsing GBM [16]. Moreover, the over-activation or dysregulation of several signaling pathways in GBM can lead to the uncontrolled growth of primary and recurrent tumors [17]. Therefore, there is still a need for the development of new targeted therapeutic treatments that are effective in GBM [18,19].
The research in this field is based on the evidence that the deregulation of three main pathways have been highlighted in GBM, namely the receptor tyrosine kinases (RTKs)/Ras/PI3K pathway (altered in 88% of GBM patients), the p53 pathway (altered in 87% of GBM patients), and the retinoblastoma (RB) protein pathway (altered in 78% of GBM patients) [17].
Heterocyclic compounds, containing at least one heteroatom such as oxygen, nitrogen or sulfur, can be considered both acceptors and donors of hydrogen bonds, so that they can effectively bind to various molecular targets eliciting numerous pharmacological effects [20,21]. Moreover, they are able to alter the liposolubility and therefore the aqueous solubility of drug molecules, thus achieving suitable and remarkable pharmacotherapeutic properties [22]. In this respect, nitrogen-containing heterocycles, as recently highlighted by the FDA, are considered the most relevant heterocyclic nuclei in drug design [23] and, in particular, indole has gained considerable interest in the last decades due to its multiple bioactivities [24].
Indole is a naturally occurring heterocycle, in which the five-membered electron-rich pyrrole ring and the benzene portion confer electronic and steric properties so as to make it a hetero-annulate nucleus widely exploited in drug discovery campaigns. Accordingly, thanks also to its bioavailability and pharmacological activities, indole is considered one of the most privileged scaffolds in medicinal chemistry with potential for different therapeutic applications [25].
In the field of anticancer drugs, the approval and use of alkaloids containing indole, such as vincristine and vinblastine, inspired the researchers to design and synthesize many other indole-based compounds, in order to obtain structurally different leads with distinctive mechanisms of antitumor action [20,26,27].
The following section focuses on the recent advances (2013–2022) made in the design of anti-GMB indole-based compounds, that may act through the modulation of various molecular targets such as kinases, tubulin and p53.
2. Indole Derivatives as Anti-Glioblastoma Agents
Despite several studies highlighted many pharmacological applications of indole-based compounds as anticancer agents [28,29,30,31,32], there are only few reviews describing the recent advances in the discovery of small molecules for GBM treatment [14,33] and none of these are specifically focused on the development of indole-based compounds for the treatment of GBM. This report constitutes a brief review focused on the indole-based derivatives in subclinical and clinical stages of development as anti-GBM agents, with particular reference to the most recent medicinal chemistry advances reported between 2013 and 2022. When possible, structure–activity relationships (SARs) are also discussed.
2.1. Indole-Based Anti-GBM Compounds Acting on Receptor Tyrosine Kinases (RTKs)/Ras/PI3K Pathway
In GBM, the commonly mutated or amplified RTKs are the epidermal growth factor receptor (EGFR) (57.4%), platelet-derived growth factor receptor (PDGFR) (13.1%), mesenchymal epithelial transition (MET) (1.6%), and fibroblast growth factor receptor 2/3 (FGFR2/3) (3.2%). Actually, these altered RTKs affect most of the cellular pathways and processes, including apoptosis, cell growth and survival [34].
2.1.1. Indole-Based PDGFR and VEGFR Inhibitors
It has been reported that GBM stem cell (GSC) growth is supported by abnormal angiogenesis and that these cancer cells can also transdifferentiate directly into endothelial cells or growth factors, such as vascular endothelial growth factor (VEGF), thus regulating the tumor vascular system [35]. Moreover, the platelet-derived growth factor (PDGF) and its receptor (PDGFR) are also overexpressed in approximately 16% of GBM. Therefore, a simultaneous administration of VEGF and PDGF pathway inhibitors could produce a beneficial effect in chemoradiotherapy protocols [36]. Actually, several VEGFR/PDGFR inhibitors are marketed for cancer therapy and have been exploited for the treatment of GBM and, among them, the indole derivative Sunitinib (Sutent, Pfizer) 1 (Chart 1), already approved in 2006 by the FDA for the treatment of renal cell carcinoma and gastrointestinal stromal tumor [14]. In preclinical studies, Sunitinib 1, despite its limited brain penetration in mice, showed a direct antiproliferative effect and prolonged survival in GBM mice [37]. Unfortunately, clinical evaluations showed that 1 did not appear to be particularly useful in patients with GBM [14]. In fact, in a phase II trial, single-agent Sunitinib 1 with continuous high dosing (37.5 mg) did not prolong the six-month progression-free survival in patients with recurrent GBM. This is mainly due to the toxicity of Sunitinib 1 which prevented continued treatment beyond 3 weeks (necessary to achieve optimal pharmacokinetics), thus resulting in suboptimal drug exposure for these GBM patients [37].
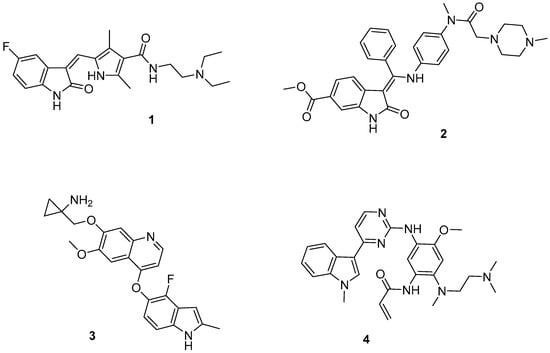
Chart 1.
Indole-based PDGFR/VEGFR inhibitors (1–3) and EGFR inhibitor (4).
Analogously, Nintedanib (Cyendiv, Boehringer-Ingelheim) 2 (Chart 1), a potent triple VEGFR, PDGFR, and FGFR angiokinase inhibitor, does not elicit beneficial effects in GBM, likely due to its limited CNS penetration. Anlotinib 3 (Chart 1) is an oral, selective VEGFR2 inhibitor with significantly fewer side effects than 1 [38], approved in China for the therapeutic treatment of patients with non-small cell lung cancer (NSCLC) [39]. A clinical case of recurrent GBM revealed a reduction in tumor size 26 days after treatment with compound 3, although the tumor continued to progress 2 months after the therapy with 3 [40]. To date, a 50-participant clinical trial evaluating the efficacy of compound 3 in the treatment of recurrent GBM is being recruited (NCT04004975).
2.1.2. Indole-Based EGFR Inhibitors
Osimertinib (Tagrisso, Astra Zeneca) 4 (Chart 1), an oral EGFR inhibitor with very high brain penetration properties, was able, in preclinical evaluation, to significantly inhibit the growth of six GBM cell lines [41]. In an in vivo GBM model, compound 4 was shown to significantly inhibit tumor growth and to prolong the survival time of animals. A patient with multifocal GBM characterized by multiple EGFR mutations had a complete response to compound 4 at only one of the tumor sites [42]. This case highlights the heterogeneity of this tumor, which is still a major challenge for EGFR-targeted GBM therapy, together with the limited brain permeability of most TKIs and the low specificity of small molecules inhibiting GBM-related EGFR mutations [14].
2.1.3. Indole-Based PI3K/AKT/mTOR Pathway Inhibitors
Among RTK pathways, PI3K/AKT/mTOR is one of the pivotal triggers of the development of glioma, with a lack of apoptosis and consequent unregulated cell growth [43,44]. Moreover, in clinical analysis of patients with GBM, the PI3K pathway is abnormally activated, resulting in a poor prognosis [45].
In the search for new PDK1/Akt inhibitors as a promising treatment for GBM, the oxindole nucleus present in different PI3K/Akt pathway inhibitors [46,47,48], a small family of 2-oxindole derivatives of general formula 5 (Chart 2, Figure 1) were synthesized and evaluated in vitro [49]. In particular, the substitution pattern at the 5-position was investigated with regard to the activity on PDK1 and Akt kinases; several side chains were introduced at this position, including the 3,4-dimethoxytetrahydroisoquinoline-N-acetamide, 3,4-dimethoxybenzylamino-N-acetamido group (a group with greater flexibility and conformational freedom), substituents with an electron-rich linker like the arylurea- and arylmethyleneamino-sulfonyl moieties, or with a less hindered group, like the methanesulphonylamido one. Human pancreatic adenocarcinoma cell line ASPC1 [50] was used to preliminarily evaluate the cytotoxicity of the sixteen synthesized compounds, as this cell line is a suitable model to study the inhibition of the PI3K-AKT pathway.
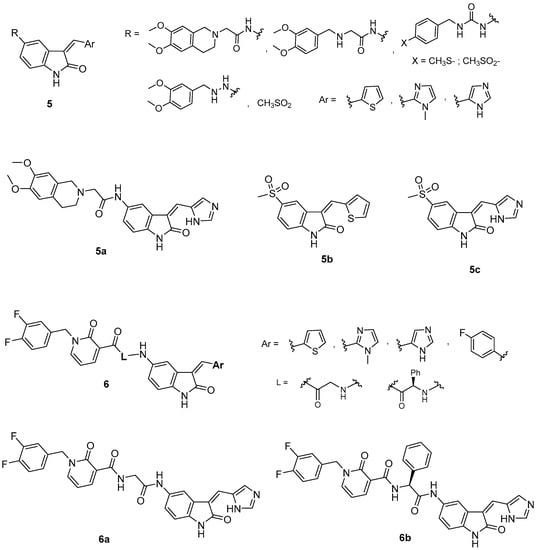
Chart 2.
Indole-based PI3K/AKT/mTOR pathway inhibitors (5–6).
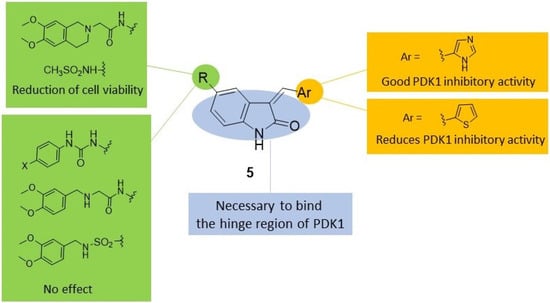
Figure 1.
SAR [49] summary for PDK1 inhibitory activity and effects induced on ASPC1 cell viability by indole-based derivatives 5.
The results obtained showed that, within compounds bearing the 3,4-dimethoxytetrahydroisoquinoline alkylamido side chain, only derivative 5a (Chart 2) diminished the cell viability to about 68%. The substitution of this chain with thiomethylphenylureido or methylsulphonylphenylureido groups resulted in deleterious activity, whereas replacement with a small electron rich group, such as the methanesulphonamido group (compounds 5b–c, Chart 2), maintained a comparable effect on cell viability with respect to compound 5a (68% for 5b and 74% for 5c). The substitution of the 3,4-dimethoxytetrahydroisoquinoline-N-acetamido group with a more flexible and a more conformationally free group, such as the 3,4-dimethoxybenzylamino-N-acetamido and 3,4-dimethoxyaminosulphonyl moieties, respectively, diminished the antiproliferative potency (>85%). Compounds 5a–c were further investigated on the PDK1/Akt pathway, and only 5a (Chart 2) was shown to inhibit PDK1 kinase and also other downstream effectors essential for GBM-derived stem cell survival.
Moreover, compound 5a was able to effectively reduce, in a concentration-dependent manner, the viability of two different human GBM cell lines, ANGMS-CSS and U118MG (GI50 values ranging from 3.98 to 29.93 μM), which are resistant to conventional chemotherapeutic agents, such as temozolomide. Of note, based on its drug likeness and CNS multiparameter optimization (CNS MPO) score, compound 5a should have very good blood–brain barrier (BBB) penetration properties [49].
Later, some new 2-oxo-indole-pyridonyl derivatives, of general formula 6 (Chart 2, Figure 2), with improved activity were reported. The preliminary PDK1 screening of these compounds evidenced that the combination of the 2-oxindole framework with the 2-pyridonyl one is important for the kinase affinity. Indeed, replacement of the 2-oxoindole core with the tetrahydroisoquinoline moiety furnished compounds that were inactive against PDK1. Within the pyridonyl series, the substitution at the 3-position of the 2-oxoindole moiety significantly affects the PDK1 affinity. Notably, the (1H-imidazol-5-yl)methylene group improves the potency against PDK1 in the series of directly fused derivatives, as well as in those with a spacer (L) between the two heterocycles. Even the spacer (glycine or phenylglycine) significantly affects the activity, indicating the glycine is the best linker. In particular, the oxoindolepyridonyl derivative 6a (Chart 2) was found to be the best PDK1 inhibitor with an IC50 value of 0.112 μM in the PDK1 inhibition assay. Moreover, compound 6a was able to inhibit U87MG cells and GSCs proliferation with an IC50 value of 449.7 nM and 3.36 nM, respectively [51].
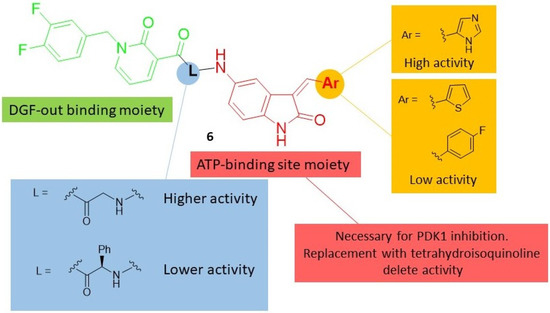
Figure 2.
SAR [51] summary for PDK1 inhibition activity of indole-based derivatives 6.
The simultaneous inhibition of PDK1 and aurora kinase A (AurA), both playing a crucial role in cell survival, may be an innovative strategy to treat GBM, overcoming its resistance and recurrence. In this context, the 1-phenyl derivative of 6a, namely 6b, (Chart 2), thanks to its dual PDK1/Aurora Kinase A inhibitory activity was able to induce apoptosis in U87MG cells with a decrease in cell proliferation and migration. Moreover, 6b inhibited the formation and proliferation of GSCs isolated from U87MG cells with an IC50 value of 8.3 nM. A similar behavior was also observed against GSCs isolated from U343MG and ANGM-CSS cells, as compound 6b reduced their proliferation with IC50 values of 0.05 and 0.04 μM, respectively [52].
2.2. Indole-Based Tubulin Polymerization Inhibitors
Recent studies showed that GBM cells are particularly sensitive to mitotic destruction compared to healthy cells, so the use of therapies that disrupt microtubule (MT) assembly could represent a promising strategy to treat this type of cancer [53]. In support of this, devices that generate electromagnetic fields and work by disrupting MTs have shown significant efficacy and have been approved as new treatments for GBM [54]. In this context, antitubulin agents could have anticancer efficacy in patients with GBM. However, unfortunately, most antitubulin agents do not easily cross the BBB [55].
A library of 2-aroylindole agents that interact with the colchicine-binding site, inhibiting tubulin polymerization, and are insensitive to multidrug resistance efflux pumps that can hinder brain penetration, was developed [56,57,58]. Afterwards, a series of alkylindole compounds able to kill glioma cells in vitro [59] was designed, and, among this class, ST-11 (7, Chart 3), acts directly on MT, disturbing MT dynamics and contributing to aberrant spindle formation. ST-11, 7 showed efficacy in killing GBM cells U251, A172, and U87 and the GSC lines BT74, MGG4, and MGG8 at micromolar concentrations (EC50 from 2.4 to 8.6 μM) [60]. Moreover, in vivo studies showed that, unlike current antitubulin agents, compound 7 was able to readily cross the BBB and, in a mouse model of GBM, it was able to reduce tumor volume without evident toxicity [60].
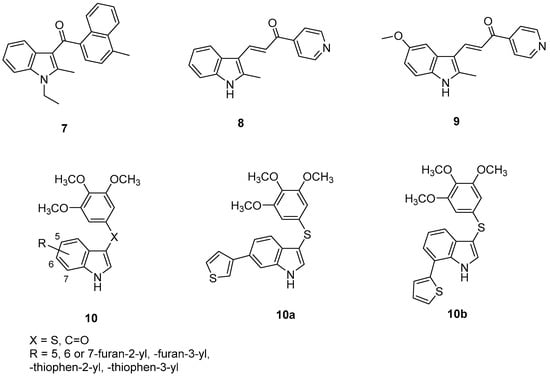
Chart 3.
Indole-based tubulin polymerization inhibitors (7–10) in GBM.
Another alkylindole derivative, compound 8 (Chart 3), was a promising anti-GBM agent [61] with a mechanism of induction of methuosis (a nonapoptotic cell death associated with vacuolization in both temozolomide-resistant and nonresistant GBM cells) [62]. Compound 8, at 1 μM concentration, was able to induce transient effects in U251 GBM cell morphology within 4 h, but most of the vacuoles were dispelled in 24 h, while they remained persistent for 24 h and beyond at higher concentration (10 μM). Moreover, despite its therapeutic potential, compound 8 presented a limited solubility in aqueous solutions [61]. Previous SAR studies on this class of compounds evidenced the availability of some flexibility at the 5-position of the indole ring, so that modifications were made at this position with groups that would increase polarity at physiological pH, while maintaining a methyl at the 2-position. This study led to the development of a more potent derivative, compound 9 (Chart 3) [63], able to inhibit U251 GBM cells’ proliferation with a lower IC50 value (1.9 μM) than compound 8 (4.8 μM). Further studies on alkylindole derivatives indicated that some particular substitutions at the indole 2-position could cause microtubule disruption, instead of methuosis, resulting in less potent compounds [63].
In 2018, twenty new 3-arylthio- and 3-aroyl-1H-indole derivatives (general formula 10, Chart 3, Figure 3) substituted at the 5-, 6- or 7-position of the indole scaffold with a heterocyclic ring were designed [64]. Among them, some 6- and 7-heterocyclyl-1H-indole compounds were able to host tubulin polymerization, by inhibiting the binding of colchicine to tubulin at submicromolar concentrations, and the growth of MCF-7 cancer cells with IC50 values in the range of 150–350 nM. Two representative very potent derivatives of the 6- and 7-heterocyclyl-1H-indoles, 10a (R = -thiophen-3-yl) and 10b (R = -thiophen-2-yl), respectively, resulted in the most interesting compounds of all the series. Compound 10a efficaciously inhibited tubulin polymerization at 50 nM, its activity comparable to that of vinblastine (a breast cancer drug, inhibitor of tubulin polymerization), while compound 10b resulted in affecting the mitotic spindle structural organization, without fully inhibiting tubulin polymerization, at a concentration of 100 nM. Compounds 10a and 10b potently inhibited proliferation of a panel of cancer cells and the NCI/ADR-RES multidrug resistant cell line at low nanomolar concentrations. Moreover, in cell cycle analysis, compound 10a became effective at 20 nM and induced 77% G2/M arrest at 50 nM, and resulted in being a potent inhibitor of the U87MG cell line at 16 nM concentration, being more active than the yet reported arylthioindoles featuring the imidazole-1-yl or pyridine-4-yl moiety at 2-position of the indole [65].
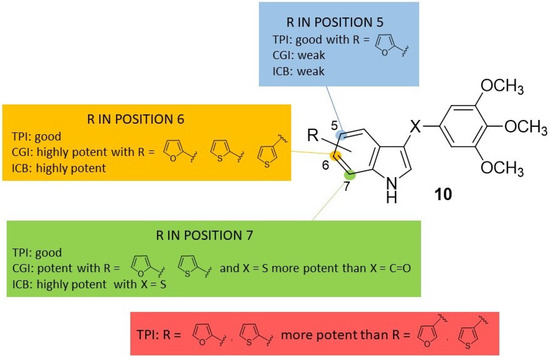
Figure 3.
SAR [64] summary for tubulin polymerization inhibition (TPI), MCF-7 cell growth inhibition (CGI) and inhibition of colchicine binding (ICB) of indole based derivatives 10.
In 2019, a series of bis-heterocyclic analogues of isocombretastatin were reported [66]. In previous SAR studies, the 3′,4′,5′-trimethoxyphenyl pattern, present in combrestatin and isocombretastatin as A-ring, resulted in an important requirement for optimal tubulin polymerization inhibition, whereas structural modifications of the B-ring were well tolerated [67]. Replacement of the isocombrestatin B-ring with heterocycles such as pyridine [68] and carbazole [69] (11, Chart 4) resulted in affording comparable activities rather than a methoxyphenol nucleus. Moreover, it was evidenced that the use of indole on the B-ring gave good results [70]. The more challenging replacement of the trimethoxyphenyl group of isocombrestatin with heterocycles such as quinoline [71] and quinazoline [72] were invaluable. In this context, 17 isocombreastatin analogues with general formula 12 (Chart 4, Figure 4), were investigated [66]. In these compounds, the trimethoxy A-ring was replaced by a pyrimidinedionyl, a quinolinyl, a quinazolinyl, or a pyridinyl moiety, while the B-ring was replaced with a carbazolyl or an indolyl moiety. Among all the new derivatives, compounds 12a–c (Chart 4) showed very high antiproliferative activity against five different cancer cell lines including the GBM cell line, U87MG, in the range 0.81–61.9 nM. These results confirmed that the replacement of both the aromatic A- and B-rings of isocombrestatin by quinaldine and 2,6-dimethylpyridine (ring A) and by carbazole and indole (ring B) was successful. In particular, the most interesting compound, 12a, was found to efficiently inhibit tubulin polymerization, with an IC50 of 2.0 μM (value comparable to that of combrestatin and isocombrestatin), by targeting the colchicine-binding site of tubulin. Moreover, 12a was able to arrest the cell cycle in the G2/M phase at a 10 nM concentration and, interestingly, showed high antiproliferative activity in P-glycoprotein (PGP) overexpressing multidrug-resistant cells lines (K562-R and HT-29). Thus, 12a results are of high clinical significance because PGP expression is mostly associated with chemotherapy clinical resistance. Finally, thanks to its favorable physicochemical properties, compound 12a was permeable to the BBB, and it also showed good antiproliferative activity against the human GBM-cancer cell line U87MG. All these properties make compound 12a a promising potential candidate for the treatment of GBM [66].
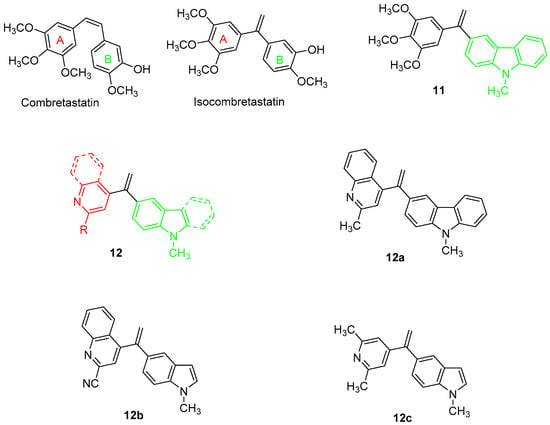
Chart 4.
Indole-based tubulin polymerization inhibitors (11–12) in GBM.
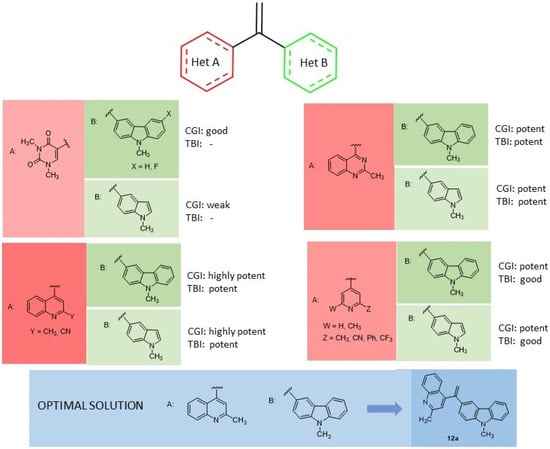
Figure 4.
SAR [66] summary for HCT 116 cell growth inhibition (CGI) and tubulin polymerization inhibition (TBI) of indole based derivatives 12.
2.3. Indole-Based Inhibitors of the p53 Pathway
The insurgence/progression of GBM, as well as the related chemoresistance, have often been attributed to the altered functioning of the p53 tumor suppressor derived by modifications within the p53 signaling pathway, including the overexpression of Murine Double Minute-2 (MDM2) [73,74]. MDM2 is an oncoprotein and it is the main physiological negative regulator of p53. It can bind to the p53 transactivation domain and interfere with p53 transcriptional regulatory mechanisms. Moreover, MDM2 is an E3 ubiquitin ligase promoting p53 proteasomal degradation. For all these reasons, inhibition of p53-MDM2 interaction and the consequent reactivation of endogenous p53 activity could represent a useful therapeutic strategy for the treatment of GBM [73,74].
MDM2 accumulates at high concentrations in cancer cells, including GBM cells, so that p53 availability is often reduced due to p53-MDM2 binding. MDM2 inhibitors are an attractive anticancer strategy for blocking p53-MDM2 protein–protein interaction, thus inhibiting the cell growth in various cancers. Anyhow, the efficacy of these inhibitors in GBM still deserves a further in depth investigation [75,76].
In this context, in 2013 a novel spirooxoindolepyrrolidine MDM2 inhibitor, 13 (Chart 5) [77], was investigated to assess its activity on human GBM cell lines [75]. Compound 13 was able to block p53–MDM2 interaction inducing a time-dependent increment of p53 expression due to the release of p53. Compound 13 was also reported to induce cell cycle arrest and apoptosis, thus effectively inhibiting human GBM cell growth in vitro. In vivo, the administration of 13 in the human xenograft GBM mouse model brought p53 activation, arrest of cell proliferation and induction of apoptosis in tumor tissue. Interestingly, 13 was non-toxic both in an in vitro healthy human cell model and also in an in vivo mouse model. Moreover, the co-administration of 13 with temozolomide produced a synergistic inhibitory effect on GBM cell viability in vitro, thus indicating a possibility of reducing the dose of temozolomide in the treatment of GBM [75].
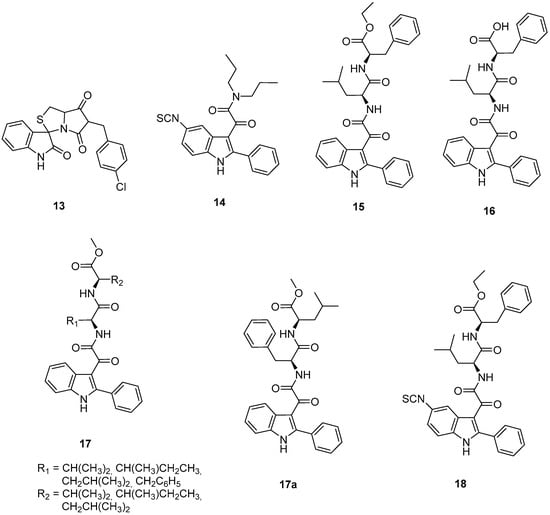
Chart 5.
Indole-based murine double minute-2 (MDM2) inhibitors (13–18).
The mitochondrial translocator protein (TSPO) has been found to play a key role in several cellular processes, such as cell life/death, and TSPO ligands have been demonstrated to be valid candidates for the treatment of several diseases, including cancer [78,79]. It is well known that there is no correlation between TSPO ligands’ binding affinity and their in vitro efficacy. This not only makes it difficult to assess structure–activity relationships for the discovery of new ligands, but also questions the specificity of the observed effects. In this context, the concept of residence time (RT), i.e., the time that a ligand spends on its target, has to be considered, as it is a parameter capable of influencing the pharmacological activity of the ligand itself. Taking this concept into account, the effects on the life/death processes of GBM cells produced by a reversibly binding TSPO ligand and a covalently binding one (compound 14, Chart 5), both belonging to the class of phenylindolylglyoxylamides (PIGA), were studied in parallel [80]. The results showed that nanomolar concentrations of the irreversible compound 14 were requested to observe the same effects elicited by micromolar concentrations of the reversible PIGA ligand, highlighting that a stable ligand–receptor interaction is crucial to increase the ligands’ efficacy.
In multifactorial diseases such as cancer, therapies targeting multiple cell pathways have emerged. In this context, the same authors have exploited the idea to design dual target compounds, able to bind to TSPO and inhibit the p53–MDM2 interaction. To this purpose the PIGA class was investigated [81], taking into consideration the available SARs for binding to TSPO [82,83], and also the crystallographic p53–MDM2 interaction data [84]. This study led to the rational design of the 2-phenylindolglyoxylyldipeptide 15 and its acid analogue 16 (Chart 5), both able to efficaciously dissociate the p53–MDM2 complex, with IC50 values in the nanomolar range (IC50 values of 11.65 ± 0.49 nM and 202.0 ± 21.2 nM for 15 and 16, respectively).
In particular, compound 15 was shown to be about ten times more potent than the reference MDM2 inhibitors, nutlin-3 [85] or 13 [75], dissociating the MDM2–p53 complex with IC50 values of 108.0 ± 4.5 nM and 121.7 ± 14.5 nM, respectively. GBM cell incubation with compounds 15 and 16 resulted in a dose-dependent increase in p53 protein levels, ascribed to a decreased interaction between p53 and MDM2, and a reactivation of p53 functions. Moreover, in GBM cells, these compounds caused mitochondrial membrane potential (Δψm) dissipation, emphasizing that the dual targeting of MDM2 and TSPO amplifies the mitochondrial permeability transition pore (MPTP) opening, and a dose dependent cell viability inhibition. These effects are exerted by compounds 15 and 16 with higher potency with respect to the single target reference standards (PK11195 for TSPO and nutlin-3 for p53-MDM2) singularly applied, confirming the synergistic effect due to the concomitant modulation of the two targets [75,81,86].
Subsequently, a lead optimization process was carried out with the development of some further PIGA derivatives (general formula 17, Chart 5, Figure 5) with different dipeptides linked to the glyoxylyl moiety [87]. As observed in the three-dimensional complex of MDM2–15 in the MDM2 N-terminus region, in the Leu26 as well as Phe55 pockets, either aromatic or aliphatic residues were present; thus, in series 17 the terminal benzyl group of the lead compound 15 was converted into aliphatic side chains of different length and the Leu side chain into other aliphatic chains or into a benzyl group. Among the compounds of the new series, 17a (Chart 5) emerged as the most potent dual inhibitor of p53–MDM2 interaction (IC50 4.3 ± 0.6 nM)—TSPO ligand (Ki 87.2 ± 6.8 nM). Compound 17a was able to reactivate p53 function and inhibit cell growth of GBM cells through cell cycle arrest and apoptosis. It was ineffective on a GBM cell line expressing mutant p53, whereas it was able to affect the viability of GSCs resistant to therapies and responsible for GBM recurrence. Finally, 17a was preferentially active toward tumor cells compared to healthy ones [87].
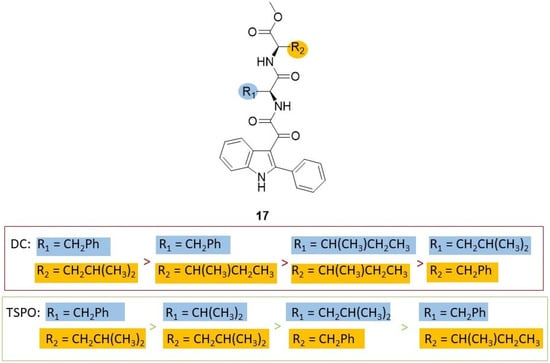
Figure 5.
SAR [87] summary for dissociation of human p53/MDM2 complex (DC) and binding affinity to TSPO (TSPO) of indole based derivatives 17.
As highlighted above, reversible drugs may not be able to maintain their therapeutic effect over time by favoring the activation of alternative signaling pathways that escape their action causing resistance, a dual-targeted compound 18 (Chart 5) has been developed, based on the structure of derivative 15, able to produce a long-lasting binding profile to TSPO and MDM2 [88,89]. Specifically, compound 18 features a 5-isothiocyanate group able to covalently bind SH or NH groups of the target proteins, thus binding to TSPO and MDM2 in a covalent manner, with Ki values of 108 ± 10 nM, and IC50 of 6.81 ± 0.79 nM, respectively. Moreover, compound 18 inhibited GBM cell growth to a greater extent and with long-lasting effects with respect to the reversible analogue 15, evidencing that the irreversible TSPO/MDM2 dual-targeting may represent an interesting alternative to overtake the time-limited effects of traditional drugs for GBM [89].
Some metals different from platinum, exploited in complexes with drugs in clinical use worldwide [90,91,92], were attractive as their various oxidation states, stability and coordination geometries, as well as thermodynamic and kinetic characteristics, may contribute to selectively bind biological targets, triggering specific cellular responses and, consequently, pharmacological effects [93,94,95]. In particular, ruthenium-based complexes with a direct metal–metal bond and a mixed valence (II,III) [96,97] have been exploited in cancer therapy because, by anchoring ligands with antiproliferative activity, it is possible to obtain the multiple release of the active molecules in the tumor site, but also the exploitation of the combined pharmacological effects of active ligands and ruthenium [98,99,100,101].
In this context, two Ru2 (II,III) complexes, 19a–b (Chart 6) obtained from compounds 16 and 17a, respectively, were synthesized and thoroughly characterized [102,103]. Compound 19a was completely inactive in GBM models due to its high chemical stability: the steric protection of the dipeptidyl chain prevents the release of the active ligand by the metal and, consequently, the expected antitumor effects [102]. Instead, complex 19b [103], characterized by the presence of four molecules of compound 17a as ligands coordinating the Ru2 core, when assayed on U87MG cells, showed a cytotoxic potency lying in the µM range (IC50 25.5 ± 5.7 µM). Complex 19b thus resulted in being more reactive, and thus more active as an anticancer agent compared to its isomer 19a, reasonably thanks to the increased accessibility of the Ru2 core to the attacking nucleophiles. In fact, the substantial difference between the two isomers 19a and 19b resides in the position of the phenyl substituent which in 19a is in the α-position with respect to the carboxylic group, resulting in a greater steric obstacle on Ru2 with consequent shielding from nucleophilic attacks by the target proteins.
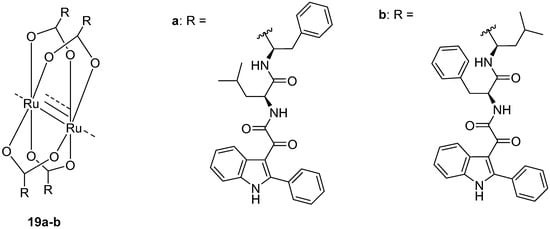
Chart 6.
Indole based murine double minute-2 (MDM2) inhibitors (19) in GBM.
3. Conclusions
Glioblastoma (GBM) is still one of the most recurrent and aggressive CNS tumors with a poor prognosis due its unique molecular characteristics; these are the presence of a population of stem-like cells (glioma stem cells, GSCs) with ability of self-renewal and tumorigenicity that makes GBM resistant to chemotherapy and radiotherapy. Moreover, the propensity of GBM cells to infiltrate/invade the adjacent normal brain tissues and along blood vessels makes it difficult to fully resect the tumor and limits the effect of local radiotherapy. A poor prognosis for GBM is also due to the existence of the BBB making it difficult for drugs to enter the CNS and achieve sufficient effective concentration, and to a still poor knowledge of tumor-intrinsic dominant signaling pathways as well as tumor-specific antigenic profiles.
In the last decades, improved genomic, epigenetic, transcriptomic, and proteomic characterization of GBM and of the brain microenvironment and immune system interaction have helped in developing innovative clinical trials. In this context, the efficacy of biomacromolecules is increasingly recognized in the search for new therapeutic strategies for GBM such as immunotherapy, including immune checkpoint blockade, chimeric antigen receptor T (CART) cell therapy, oncolytic virotherapy and vaccine therapy.
To date, many researchers are trying to use synergistic immunotherapies as a good therapeutic prospect for GBM treatment. Oncolytic viruses were also shown to promote tumor regression by inducing immunogenic cell death mainly in tumor cells: some of them are approved for the treatment of certain malignant gliomas, even if further studies have to be carried out to assess which GBM patients would benefit the most. The personalized vaccination trials were quite feasible and with a certain therapeutic potential, with autologous dendritic cell vaccines resulting in superior therapeutic efficacy compared to peptide vaccines; surely, this approach to GBM treatment warrants further in-depth studies.
In the treatment of GBM, small molecules, due to their rather simple structure, have a greater advantage in entering the CNS. In addition, the cost of the small molecules is much lower than that of biological macromolecules, making them more attractive to patients. Moreover, the discovery of more targeted small molecules with excellent BBB permeability necessary for innovative treatment of GBM may aid in silico methods such as virtual screening (VS), that can rapidly provide a set of biologically active compounds in an economically efficient manner. Very recently, the employment of artificial intelligence methods such as deep and machine learning has added value in small-molecule drug discovery. These methods give access to better success rates by overcoming the intrinsic hurdles connected with standard ligand- and receptor-based methods. Thus, although there is still much to understand on the mechanisms and biomarkers of GBM, and intense research and clinical development are required to optimize the available molecules and to overcome their potential side effects, with the help of these innovative in silico discovery technologies small molecules may represent the main direction for the development of drugs for an efficient and effective treatment of GBM.
The indole nucleus is considered a privileged structure in medicinal chemistry and, overall, indole compounds have emerged as a valuable tool in the ongoing battle against life-threatening diseases, such as cancer. In fact, the vast amount of indole compounds entering the pre-clinical and clinical study phases highlights the significant progress achieved in this area.
In this context, the present review summarized the state of the art on indole-based compounds in the pre-clinical and clinical stages of development against GBM, mainly focusing on the most recent advances in medicinal chemistry of indole derivatives with anti-GBM activity (in vitro and/or in vivo), reported between 2013 and 2022.
In the treatment of GBM, most of the molecular targets studied in recent years involve kinases, and in particular PI3K, PDK1, CK2, c-Src, Akt/PKB, FAK, VEFRG, and EGFR. Other enzymes, signaling pathways and cellular processes are also involved, including G-quadruplex, HDAC, HSP90, microtubule, p53, among others. The indole-based derivatives as anti-GBM agents, herein reported, act mainly on three targets which are essentially kinases, tubulin and p53. In most of the studies performed, in vitro studies in GBM cell lines, mainly U87MG and U118MG, were involved, and some of the indole-based compounds reported were found to have promising activity.
With this review we aimed to provide an outlook on existing data of the indole-based derivatives that might help the medicinal chemists in the future design and development of more efficient and selective novel indole-based leads for in vitro and in vivo studies, so that new more effective GBM drug candidates can be unveiled and introduced to the market and into clinical usage.
Author Contributions
Conceptualization, S.S., S.T. and F.D.S.; writing—original draft preparation, S.S.; writing—review and editing, S.S., S.T. and F.D.S.; visualization, E.B. (Elisabetta Barresi), E.B. (Emma Baglini), V.P.; supervision, S.T. and F.D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The author would like to thank the University of Pisa (Fondi di Ateneo) for supporting this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, J.; McKay, R.M.; Parada, L.F. Malignant Glioma: Lessons from Genomics, Mouse Models, and Stem Cells. Cell 2012, 149, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro Oncol. 2020, 22, IV1–IV96. [Google Scholar] [CrossRef] [PubMed]
- Cancer.Net Editorial Board Brain Tumor: Statistics | Cancer.Net. Available online: https://www.cancer.net/cancer-types/brain-tumor/statistics (accessed on 17 January 2023).
- Carvalho, B.; Lopes, J.M.; Silva, R.; Peixoto, J.; Leitão, D.; Soares, P.; Fernandes, A.C.; Linhares, P.; Vaz, R.; Lima, J. The Role of C-Met and VEGFR2 in Glioblastoma Resistance to Bevacizumab. Sci. Rep. 2021, 11, 6067. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of Its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar] [CrossRef]
- Tamimi, A.F.; Juweid, M. Epidemiology and Outcome of Glioblastoma. In Glioblastoma; De Vleeschouwer, S., Ed.; Codon Publications: Brisbane, Australia, 2017; pp. 143–153. ISBN 9780994438126. [Google Scholar]
- Ostrom, Q.T.; Liao, P.; Stetson, L.C.; Barnholtz-Sloan, J.S. Epidemiology of Glioblastoma and Trends in Glioblastoma Survivorship. In Glioblastoma; Elsevier: Amsterdam, The Netherlands, 2016; pp. 11–19. ISBN 9780323479745. [Google Scholar]
- Li, K.; Lu, D.; Guo, Y.; Wang, C.; Liu, X.; Liu, Y.; Liu, D. Trends and Patterns of Incidence of Diffuse Glioma in Adults in the United States, 1973–2014. Cancer Med. 2018, 7, 5281–5290. [Google Scholar] [CrossRef]
- Behl, T.; Sharma, A.; Sharma, L.; Sehgal, A.; Singh, S.; Sharma, N.; Zengin, G.; Bungau, S.; Toma, M.M.; Gitea, D.; et al. Current Perspective on the Natural Compounds and Drug Delivery Techniques in Glioblastoma Multiforme. Cancers 2021, 13, 2765. [Google Scholar] [CrossRef]
- Seker-Polat, F.; Degirmenci, N.P.; Solaroglu, I.; Bagci-Onder, T. Tumor Cell Infiltration into the Brain in Glioblastoma: From Mechanisms to Clinical Perspectives. Cancers 2022, 14, 443. [Google Scholar] [CrossRef]
- Persano, F.; Gigli, G.; Leporatti, S. Natural Compounds as Promising Adjuvant Agents in The Treatment of Gliomas. Int. J. Mol. Sci. 2022, 23, 3360. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of Radiotherapy with Concomitant and Adjuvant Temozolomide versus Radiotherapy Alone on Survival in Glioblastoma in a Randomised Phase III Study: 5-Year Analysis of the EORTC-NCIC Trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Liu, H.; Qiu, W.; Sun, T.; Wang, L.; Du, C.; Hu, Y.; Liu, W.; Feng, F.; Chen, Y.; Sun, H. Therapeutic Strategies of Glioblastoma (GBM): The Current Advances in the Molecular Targets and Bioactive Small Molecule Compounds. Acta Pharm. Sin. B 2022, 12, 1781–1804. [Google Scholar] [CrossRef]
- Taal, W.; Oosterkamp, H.M.; Walenkamp, A.M.E.; Dubbink, H.J.; Beerepoot, L.V.; Hanse, M.C.J.; Buter, J.; Honkoop, A.H.; Boerman, D.; de Vos, F.Y.F.; et al. Single-Agent Bevacizumab or Lomustine versus a Combination of Bevacizumab plus Lomustine in Patients with Recurrent Glioblastoma (BELOB Trial): A Randomised Controlled Phase 2 Trial. Lancet Oncol. 2014, 15, 943–953. [Google Scholar] [CrossRef]
- Weller, M.; Cloughesy, T.; Perry, J.R.; Wick, W. Standards of Care for Treatment of Recurrent Glioblastoma-Are We There Yet? Neuro Oncol. 2013, 15, 4–27. [Google Scholar] [CrossRef]
- McLendon, R.; Friedman, A.; Bigner, D.; Van Meir, E.G.; Brat, D.J.; Mastrogianakis, G.M.; Olson, J.J.; Mikkelsen, T.; Lehman, N.; Aldape, K.; et al. Comprehensive Genomic Characterization Defines Human Glioblastoma Genes and Core Pathways. Nature 2008, 455, 1061–1068. [Google Scholar] [CrossRef]
- Attia, N.; Mashal, M.; Pemminati, S.; Omole, A.; Edmondson, C.; Jones, W.; Priyadarshini, P.; Mughal, T.; Aziz, P.; Zenick, B.; et al. Cell-Based Therapy for the Treatment of Glioblastoma: An Update from Preclinical to Clinical Studies. Cells 2022, 11, 116. [Google Scholar] [CrossRef]
- Zhai, K.; Mazurakova, A.; Koklesova, L.; Kubatka, P.; Büsselberg, D. Flavonoids Synergistically Enhance the Anti-Glioblastoma Effects of Chemotherapeutic Drugs. Biomolecules 2021, 11, 1841. [Google Scholar] [CrossRef]
- Dhiman, A.; Sharma, R.; Singh, R.K. Target-Based Anticancer Indole Derivatives and Insight into Structure-activity Relationship: A Mechanistic Review Update (2018–2021). Acta Pharm. Sin. B 2022, 12, 3006–3027. [Google Scholar] [CrossRef]
- Gevorgyan, V. Chemistry of Heterocyclic Compounds: A Renaissance. Chem. Heterocycl. Compd. 2012, 48, 1. [Google Scholar] [CrossRef]
- Dua, R.; Shrivastava, S.; Sonwane, S.K.; Srivastava, S.K. Pharmacological Significance of Synthetic Heterocycles Scaffold: A Review. Adv. Biol. Res. 2011, 5, 120–144. [Google Scholar]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef]
- Sharma, V.; Kumar, P.; Pathaka, D. Biological Importance of the Indole Nucleus in Recent Years: A Comprehensive Review. J. Heterocycl. Chem. 2010, 47, 491–502. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Kaushik, N.; Attri, P.; Kumar, N.; Kim, C.H.; Verma, A.K.; Choi, E.H. Biomedical Importance of Indoles. Molecules 2013, 18, 6620–6662. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Li, Y.; Yan, C.; Yan, M.; Tang, Z. Indole: A Privileged Scaffold for the Design of Anti-Cancer Agents. Eur. J. Med. Chem. 2019, 183, 111691. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Dong, W.; Guo, Q.; Li, X.; Huang, L. The Importance of Indole and Azaindole Scaffold in the Development of Antitumor Agents. Eur. J. Med. Chem. 2020, 203, 112506. [Google Scholar] [CrossRef]
- Dadashpour, S.; Emami, S. Indole in the Target-Based Design of Anticancer Agents: A Versatile Scaffold with Diverse Mechanisms. Eur. J. Med. Chem. 2018, 150, 9–29. [Google Scholar] [CrossRef]
- Leoni, A.; Locatelli, A.; Morigi, R.; Rambaldi, M. 2-Indolinone a Versatile Scaffold for Treatment of Cancer: A Patent Review (2008–2014). Expert Opin. Ther. Pat. 2016, 26, 149–173. [Google Scholar] [CrossRef]
- Kumar, N.M.; Kumar, D. Recent Developments on Synthetic Indoles as Potent Anticancer Agents. Chem. Biol. Interface Off. J. ISCB 2013, 5, 276–303. [Google Scholar]
- de Sa Alves, F.; Barreiro, E.; Manssour Fraga, C. From Nature to Drug Discovery: The Indole Scaffold as a Privileged Structure. Mini-Rev. Med. Chem. 2009, 9, 782–793. [Google Scholar] [CrossRef]
- Sravanthi, T.V.; Manju, S.L. Indoles—A Promising Scaffold for Drug Development. Eur. J. Pharm. Sci. 2016, 91, 1–10. [Google Scholar] [CrossRef]
- Fernandes, G.F.d.S.; Fernandes, B.C.; Valente, V.; dos Santos, J.L. Recent Advances in the Discovery of Small Molecules Targeting Glioblastoma. Eur. J. Med. Chem. 2019, 164, 8–26. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the Phosphoinositide 3-Kinase Pathway in Cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef]
- Jhaveri, N.; Chen, T.C.; Hofman, F.M. Tumor Vasculature and Glioma Stem Cells: Contributions to Glioma Progression. Cancer Lett. 2016, 380, 545–551. [Google Scholar] [CrossRef]
- Ahir, B.K.; Engelhard, H.H.; Lakka, S.S. Tumor Development and Angiogenesis in Adult Brain Tumor: Glioblastoma. Mol. Neurobiol. 2020, 57, 2461–2478. [Google Scholar] [CrossRef]
- De Boüard, S.; Herlin, P.; Christensen, J.G.; Lemoisson, E.; Gauduchon, P.; Raymond, E.; Guillamo, J.S. Antiangiogenic and Anti-Invasive Effects of Sunitinib on Experimental Human Glioblastoma. Neuro Oncol. 2007, 9, 412–423. [Google Scholar] [CrossRef]
- Xie, C.; Wan, X.; Quan, H.; Zheng, M.; Fu, L.; Li, Y.; Lou, L. Preclinical Characterization of Anlotinib, a Highly Potent and Selective Vascular Endothelial Growth Factor Receptor-2 Inhibitor. Cancer Sci. 2018, 109, 1207–1219. [Google Scholar] [CrossRef]
- Syed, Y.Y. Anlotinib: First Global Approval. Drugs 2018, 78, 1057–1062. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, J.; Liu, F.; Song, M.; Hou, Y.; Liang, N. Targeted Therapy with Anlotinib for Patient with Recurrent Glioblastoma: A Case Report and Literature Review. Medicine 2019, 98, e15749. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Shi, L.; Shan, Q.; Cao, Q.; Yue, C.; Li, H.; Li, S.; Wang, J.; Gao, S.; et al. The Third-Generation EGFR Inhibitor AZD9291 Overcomes Primary Resistance by Continuously Blocking ERK Signaling in Glioblastoma. J. Exp. Clin. Cancer Res. 2019, 38, 219. [Google Scholar] [CrossRef]
- Makhlin, I.; Salinas, R.D.; Zhang, D.; Jacob, F.; Ming, G.; Song, H.; Saxena, D.; Dorsey, J.F.; Nasrallah, M.P.; Morrissette, J.J.; et al. Clinical Activity of the EGFR Tyrosine Kinase Inhibitor Osimertinib in EGFR -Mutant Glioblastoma. CNS Oncol. 2019, 8, CNS43. [Google Scholar] [CrossRef]
- Brennan, C.W.; Verhaak, R.G.W.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. The Somatic Genomic Landscape of Glioblastoma. Cell 2013, 155, 462. [Google Scholar] [CrossRef]
- Akhavan, D.; Cloughesy, T.F.; Mischel, P.S. MTOR Signaling in Glioblastoma: Lessons Learned from Bench to Bedside. Neuro Oncol. 2010, 12, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Lassen, U.; Sorensen, M.; Gaziel, T.B.; Hasselbalch, B.; Poulsen, H.S. Phase II Study of Bevacizumab and Temsirolimus Combination Therapy for Recurrent Glioblastoma Multiforme. Anticancer Res. 2013, 33, 1657–1660. [Google Scholar] [PubMed]
- Abdulhameed, M.D.M.; Hamza, A.; Liu, J.; Zhan, C.G. Combined 3D-QSAR Modeling and Molecular Docking Study on Indolinone Derivatives as Inhibitors of 3-Phosphoinositide-Dependent Protein Kinase-1. J. Chem. Inf. Model. 2008, 48, 1760–1772. [Google Scholar] [CrossRef] [PubMed]
- Islam, I.; Bryant, J.; Chou, Y.L.; Kochanny, M.J.; Lee, W.; Phillips, G.B.; Yu, H.; Adler, M.; Whitlow, M.; Ho, E.; et al. Indolinone Based Phosphoinositide-Dependent Kinase-1 (PDK1) Inhibitors. Part 1: Design, Synthesis and Biological Activity. Bioorg. Med. Chem. Lett. 2007, 17, 3814–3818. [Google Scholar] [CrossRef] [PubMed]
- Islam, I.; Brown, G.; Bryant, J.; Hrvatin, P.; Kochanny, M.J.; Phillips, G.B.; Yuan, S.; Adler, M.; Whitlow, M.; Lentz, D.; et al. Indolinone Based Phosphoinositide-Dependent Kinase-1 (PDK1) Inhibitors. Part 2: Optimization of BX-517. Bioorg. Med. Chem. Lett. 2007, 17, 3819–3825. [Google Scholar] [CrossRef] [PubMed]
- Sestito, S.; Nesi, G.; Daniele, S.; Martelli, A.; Digiacomo, M.; Borghini, A.; Pietra, D.; Calderone, V.; Lapucci, A.; Falasca, M.; et al. Design and Synthesis of 2-Oxindole Based Multi-Targeted Inhibitors of PDK1/Akt Signaling Pathway for the Treatment of Glioblastoma Multiforme. Eur. J. Med. Chem. 2015, 105, 274–288. [Google Scholar] [CrossRef]
- Asano, T.; Yao, Y.; Zhu, J.; Li, D.; Abbruzzese, J.L.; Reddy, S.A.G. The PI 3-Kinase/Akt Signaling Pathway Is Activated Due to Aberrant Pten Expression and Targets Transcription Factors NF-ΚB and c-Myc in Pancreatic Cancer Cells. Oncogene 2004, 23, 8571–8580. [Google Scholar] [CrossRef]
- Sestito, S.; Daniele, S.; Nesi, G.; Zappelli, E.; Di Maio, D.; Marinelli, L.; Digiacomo, M.; Lapucci, A.; Martini, C.; Novellino, E.; et al. Locking PDK1 in DFG-out Conformation through 2-Oxo-Indole Containing Molecules: Another Tools to Fight Glioblastoma. Eur. J. Med. Chem. 2016, 118, 47–63. [Google Scholar] [CrossRef]
- Daniele, S.; Sestito, S.; Pietrobono, D.; Giacomelli, C.; Chiellini, G.; Di Maio, D.; Marinelli, L.; Novellino, E.; Martini, C.; Rapposelli, S. Dual Inhibition of PDK1 and Aurora Kinase A: An Effective Strategy to Induce Differentiation and Apoptosis of Human Glioblastoma Multiforme Stem Cells. ACS Chem. Neurosci. 2017, 8, 100–114. [Google Scholar] [CrossRef]
- Ding, Y.; Hubert, C.G.; Herman, J.; Corrin, P.; Toledo, C.M.; Skutt-Kakaria, K.; Vazquez, J.; Basom, R.; Zhang, B.; Risler, J.K.; et al. Cancer-Specific Requirement for BUB1B/BUBR1 in Human Brain Tumor Isolates and Genetically Transformed Cells. Cancer Discov. 2013, 3, 198–211. [Google Scholar] [CrossRef]
- Stupp, R.; Wong, E.T.; Kanner, A.A.; Steinberg, D.; Engelhard, H.; Heidecke, V.; Kirson, E.D.; Taillibert, S.; Liebermann, F.; Dbalý, V.; et al. NovoTTF-100A versus Physician’s Choice Chemotherapy in Recurrent Glioblastoma: A Randomised Phase III Trial of a Novel Treatment Modality. Eur. J. Cancer 2012, 48, 2192–2202. [Google Scholar] [CrossRef]
- Dumontet, C.; Jordan, M.A. Microtubule-Binding Agents: A Dynamic Field of Cancer Therapeutics. Nat. Rev. Drug Discov. 2010, 9, 790–803. [Google Scholar] [CrossRef]
- Beckers, T.; Reissmann, T.; Schmidt, M.; Fiebig, H.H.; Burger, A.M.; Vanhoefer, U.; Mahboobi, S.; Pongratz, H.; Hockemeyer, J.; Frieser, M.; et al. 2-Aroylindoles, a Novel Class of Potent, Orally Active Small Molecule Tubulin Inhibitors. Cancer Res. 2002, 62, 3113–3119. [Google Scholar]
- Kuo, C.C.; Hsieh, H.P.; Pan, W.Y.; Chen, C.P.; Liou, J.P.; Lee, S.J.; Chang, Y.L.; Chen, L.T.; Chen, C.T.; Chang, J.Y. BPR0L075, a Novel Synthetic Indole Compound with Antimitotic Activity in Human Cancer Cells, Exerts Effective Antitumoral Activity in Vivo. Cancer Res. 2004, 64, 4621–4628. [Google Scholar] [CrossRef]
- Löscher, W.; Potschka, H. Blood-Brain Barrier Active Efflux Transporters: ATP-Binding Cassette Gene Family. NeuroRX 2005, 2, 86–98. [Google Scholar] [CrossRef]
- Stella, N.; Kline, T. Composition and Methods for Treating Glioblastoma. 2012. [Google Scholar]
- Cherry, A.E.; Haas, B.R.; Naydenov, A.V.; Fung, S.; Xu, C.; Swinney, K.; Wagenbach, M.; Freeling, J.; Canton, D.A.; Coy, J.; et al. ST-11: A New Brain-Penetrant Microtubule-Destabilizing Agent with Therapeutic Potential for Glioblastoma Multiforme. Mol. Cancer Ther. 2016, 15, 2018–2029. [Google Scholar] [CrossRef]
- Overmeyer, J.H.; Young, A.M.; Bhanot, H.; Maltese, W.A. A Chalcone-Related Small Molecule That Induces Methuosis, a Novel Form of Non-Apoptotic Cell Death, in Glioblastoma Cells. Mol. Cancer 2011, 10, 1–17. [Google Scholar] [CrossRef]
- Maltese, W.A.; Overmeyer, J.H. Methuosis: Nonapoptotic Cell Death Associated with Vacuolization of Macropinosome and Endosome Compartments. Am. J. Pathol. 2014, 184, 1630–1642. [Google Scholar] [CrossRef]
- Robinson, M.W.; Overmeyer, J.H.; Young, A.M.; Erhardt, P.W.; Maltese, W.A. Synthesis and Evaluation of Indole-Based Chalcones as Inducers of Methuosis, a Novel Type of Nonapoptotic Cell Death. J. Med. Chem. 2012, 55, 1940–1956. [Google Scholar] [CrossRef]
- La Regina, G.; Bai, R.; Coluccia, A.; Naccarato, V.; Famiglini, V.; Nalli, M.; Masci, D.; Verrico, A.; Rovella, P.; Mazzoccoli, C.; et al. New 6- and 7-Heterocyclyl-1H-Indole Derivatives as Potent Tubulin Assembly and Cancer Cell Growth Inhibitors. Eur. J. Med. Chem. 2018, 152, 283–297. [Google Scholar] [CrossRef]
- La Regina, G.; Bai, R.; Rensen, W.; Coluccia, A.; Piscitelli, F.; Gatti, V.; Bolognesi, A.; Lavecchia, A.; Granata, I.; Porta, A.; et al. Design and Synthesis of 2-Heterocyclyl-3-Arylthio-1H-Indoles as Potent Tubulin Polymerization and Cell Growth Inhibitors with Improved Metabolic Stability. J. Med. Chem. 2011, 54, 8394–8406. [Google Scholar] [CrossRef] [PubMed]
- Naret, T.; Khelifi, I.; Provot, O.; Bignon, J.; Levaique, H.; Dubois, J.; Souce, M.; Kasselouri, A.; Deroussent, A.; Paci, A.; et al. 1,1-Diheterocyclic Ethylenes Derived from Quinaldine and Carbazole as New Tubulin-Polymerization Inhibitors: Synthesis, Metabolism, and Biological Evaluation. J. Med. Chem. 2019, 62, 1902–1916. [Google Scholar] [CrossRef] [PubMed]
- Tron, G.C.; Pirali, T.; Sorba, G.; Pagliai, F.; Busacca, S.; Genazzani, A.A. Medicinal Chemistry of Combretastatin A4: Present and Future Directions. J. Med. Chem. 2006, 49, 3033–3044. [Google Scholar] [CrossRef] [PubMed]
- Lawson, M.; Hamze, A.; Peyrat, J.F.; Bignon, J.; Dubois, J.; Brion, J.D.; Alami, M. An Efficient Coupling of N-Tosylhydrazones with 2-Halopyridines: Synthesis of 2-α-Styrylpyridines Endowed with Antitumor Activity. Org. Biomol. Chem. 2013, 11, 3664–3673. [Google Scholar] [CrossRef] [PubMed]
- Bzeih, T.; Naret, T.; Hachem, A.; Jaber, N.; Khalaf, A.; Bignon, J.; Brion, J.D.; Alami, M.; Hamze, A. A General Synthesis of Arylindoles and (1-Arylvinyl)Carbazoles: Via a One-Pot Reaction from N -Tosylhydrazones and 2-Nitro-Haloarenes and Their Potential Application to Colon Cancer. Chem. Commun. 2016, 52, 13027–13030. [Google Scholar] [CrossRef]
- Álvarez, R.; Puebla, P.; Díaz, J.F.; Bento, A.C.; García-Navas, R.; De La Iglesia-Vicente, J.; Mollinedo, F.; Andreu, J.M.; Medarde, M.; Peláez, R. Endowing Indole-Based Tubulin Inhibitors with an Anchor for Derivatization: Highly Potent 3-Substituted Indolephenstatins and Indoleisocombretastatins. J. Med. Chem. 2013, 56, 2813–2827. [Google Scholar] [CrossRef]
- Khelifi, I.; Naret, T.; Renko, D.; Hamze, A.; Bernadat, G.; Bignon, J.; Lenoir, C.; Dubois, J.; Brion, J.D.; Provot, O.; et al. Design, Synthesis and Anticancer Properties of IsoCombretaQuinolines as Potent Tubulin Assembly Inhibitors. Eur. J. Med. Chem. 2017, 127, 1025–1034. [Google Scholar] [CrossRef]
- Soussi, M.A.; Provot, O.; Bernadat, G.; Bignon, J.; Desravines, D.; Dubois, J.; Brion, J.D.; Messaoudi, S.; Alami, M. IsoCombretaQuinazolines: Potent Cytotoxic Agents with Antitubulin Activity. ChemMedChem 2015, 10, 1392–1402. [Google Scholar] [CrossRef]
- Ohgaki, H.; Kleihues, P. Genetic Alterations and Signaling Pathways in the Evolution of Gliomas. Cancer Sci. 2009, 100, 2235–2241. [Google Scholar] [CrossRef]
- Cerami, E.; Demir, E.; Schultz, N.; Taylor, B.S.; Sander, C. Automated Network Analysis Identifies Core Pathways in Glioblastoma. PLoS ONE 2010, 5, e8918. [Google Scholar] [CrossRef]
- Costa, B.; Bendinelli, S.; Gabelloni, P.; Da Pozzo, E.; Daniele, S.; Scatena, F.; Vanacore, R.; Campiglia, P.; Bertamino, A.; Gomez-Monterrey, I.; et al. Human Glioblastoma Multiforme: P53 Reactivation by a Novel MDM2 Inhibitor. PLoS ONE 2013, 8, e72281. [Google Scholar] [CrossRef]
- Fang, Y.; Liao, G.; Yu, B. Small-Molecule MDM2/X Inhibitors and PROTAC Degraders for Cancer Therapy: Advances and Perspectives. Acta Pharm. Sin. B 2020, 10, 1253–1278. [Google Scholar] [CrossRef]
- Gomez-Monterrey, I.; Bertamino, A.; Porta, A.; Carotenuto, A.; Musella, S.; Aquino, C.; Granata, I.; Sala, M.; Brancaccio, D.; Picone, D.; et al. Identification of the Spiro(Oxindole-3,3′-Thiazolidine)-Based Derivatives as Potential P53 Activity Modulators. J. Med. Chem. 2010, 53, 8319–8329. [Google Scholar] [CrossRef]
- Chelli, B.; Lena, A.; Vanacore, R.; Da Pozzo, E.; Costa, B.; Rossi, L.; Salvetti, A.; Scatena, F.; Ceruti, S.; Abbracchio, M.P.; et al. Peripheral Benzodiazepine Receptor Ligands: Mitochondrial Transmembrane Potential Depolarization and Apoptosis Induction in Rat C6 Glioma Cells. Biochem. Pharmacol. 2004, 68, 125–134. [Google Scholar] [CrossRef]
- Chelli, B.; Rossi, L.; Da Pozzo, E.; Costa, B.; Spinetti, F.; Rechichi, M.; Salvetti, A.; Lena, A.; Simorini, F.; Vanacore, R.; et al. PIGA (N,N-Di-n-Butyl-5-Chloro-2-(4-Chlorophenyl)Indol-3-Ylglyoxylamide), a New Mitochondrial Benzodiazepine-Receptor Ligand, Induces Apoptosis in C6 Glioma Cells. ChemBioChem 2005, 6, 1082–1088. [Google Scholar] [CrossRef]
- Costa, B.; Da Pozzo, E.; Giacomelli, C.; Taliani, S.; Bendinelli, S.; Barresi, E.; Da Settimo, F.; Martini, C. TSPO Ligand Residence Time Influences Human Glioblastoma Multiforme Cell Death/Life Balance. Apoptosis 2015, 20, 383–398. [Google Scholar] [CrossRef]
- Daniele, S.; Taliani, S.; Da Pozzo, E.; Giacomelli, C.; Costa, B.; Trincavelli, M.L.; Rossi, L.; La Pietra, V.; Barresi, E.; Carotenuto, A.; et al. Apoptosis Therapy in Cancer: The First Single-Molecule Co-Activating P53 and the Translocator Protein in Glioblastoma. Sci. Rep. 2014, 4, 4749. [Google Scholar] [CrossRef]
- Primofiore, G.; Da Settimo, F.; Taliani, S.; Simorini, F.; Patrizi, M.P.; Novellino, E.; Greco, G.; Abignente, E.; Costa, B.; Chelli, B.; et al. N,N-Dialkyl-2-Phenylindol-3-Ylglyoxylamides. A New Class of Potent and Selective Ligands at the Peripheral Renzodiazepine Receptor. J. Med. Chem. 2004, 47, 1852–1855. [Google Scholar] [CrossRef]
- Da Settimo, F.; Simorini, F.; Taliani, S.; La Motta, C.; Marini, A.M.; Salerno, S.; Bellandi, M.; Novellino, E.; Greco, G.; Cosimelli, B.; et al. Anxiolytic-like Effects of N,N-Dialkyl-2-Phenylindol-3-Ylglyoxylamides by Modulation of Translocator Protein Promoting Neurosteroid Biosynthesis. J. Med. Chem. 2008, 51, 5798–5806. [Google Scholar] [CrossRef]
- Shangary, S.; Wang, S. Small-Molecule Inhibitors of the MDM2-P53 Protein-Protein Interaction to Reactivate P53 Function: A Novel Approach for Cancer Therapy. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 223–241. [Google Scholar] [CrossRef]
- Vassilev, L.T.; Vu, B.T.; Graves, B.; Carvajal, D.; Podlaski, F.; Filipovic, Z.; Kong, N.; Kammlott, U.; Lukacs, C.; Klein, C.; et al. In Vivo Activation of the P53 Pathway by Small-Molecule Antagonists of MDM2. Science 2004, 303, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Villalonga-Planells, R.; Coll-Mulet, L.; Martínez-Soler, F.; Castaño, E.; Acebes, J.J.; Giménez-Bonafé, P.; Gil, J.; Tortosa, A. Activation of P53 by Nutlin-3a Induces Apoptosis and Cellular Senescence in Human Glioblastoma Multiforme. PLoS ONE 2011, 6, e18588. [Google Scholar] [CrossRef] [PubMed]
- Daniele, S.; La Pietra, V.; Barresi, E.; Di Maro, S.; Da Pozzo, E.; Robello, M.; La Motta, C.; Cosconati, S.; Taliani, S.; Marinelli, L.; et al. Lead Optimization of 2-Phenylindolylglyoxylyldipeptide Murine Double Minute (MDM)2/Translocator Protein (TSPO) Dual Inhibitors for the Treatment of Gliomas. J. Med. Chem. 2016, 59, 4526–4538. [Google Scholar] [CrossRef] [PubMed]
- Daniele, S.; Giacomelli, C.; Pietrobono, D.; Barresi, E.; Piccarducci, R.; La Pietra, V.; Taliani, S.; Da Settimo, F.; Marinelli, L.; Novellino, E.; et al. Long Lasting Inhibition of Mdm2-P53 Interaction Potentiates Mesenchymal Stem Cell Differentiation into Osteoblasts. Biochim. Biophys. Acta—Mol. Cell Res. 2019, 1866, 737–749. [Google Scholar] [CrossRef]
- Daniele, S.; Barresi, E.; Zappelli, E.; Marinelli, L.; Novellino, E.; Da Settimo, F.; Taliani, S.; Trincavelli, M.L.; Martini, C.; Daniele, S.; et al. Long Lasting MDM2/Translocator Protein Modulator: A New Strategy for Irreversible Apoptosis of Human Glioblastoma Cells. Oncotarget 2016, 7, 7866–7884. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef]
- Cirri, D.; Pillozzi, S.; Gabbiani, C.; Tricomi, J.; Bartoli, G.; Stefanini, M.; Michelucci, E.; Arcangeli, A.; Messori, L.; Marzo, T. PtI2(DACH), the Iodido Analogue of Oxaliplatin as a Candidate for Colorectal Cancer Treatment: Chemical and Biological Features. Dalt. Trans. 2017, 46, 3311–3317. [Google Scholar] [CrossRef]
- Marzo, T.; Ferraro, G.; Merlino, A.; Messori, L. Protein Metalation by Inorganic Anticancer Drugs. Encycl. Inorg. Bioinorg. Chem. 2020, 1–17. [Google Scholar] [CrossRef]
- Benjamin Garbutcheon-Singh, K.; Grant, M.P.; Harper, B.W.; Krause-Heuer, A.M.; Manohar, M.; Orkey, N.; Aldrich-Wright, J.R. Transition Metal Based Anticancer Drugs. Curr. Top. Med. Chem. 2011, 11, 521–542. [Google Scholar] [CrossRef]
- Fricker, S.P. Metal Based Drugs: From Serendipity to Design. Dalt. Trans. 2007, 43, 4903–4917. [Google Scholar] [CrossRef]
- Jungwirth, U.; Kowol, C.R.; Keppler, B.K.; Hartinger, C.G.; Berger, W.; Heffeter, P. Anticancer Activity of Metal Complexes: Involvement of Redox Processes. Antioxid. Redox Signal. 2011, 15, 1085–1127. [Google Scholar] [CrossRef]
- Santos, R.L.S.R.; Bergamo, A.; Sava, G.; De Oliveira Silva, D. Synthesis and Characterization of a Diruthenium(II,III)-Ketoprofen Compound and Study of the in Vitro Effects on CRC Cells in Comparison to the Naproxen and Ibuprofen Derivatives. Polyhedron 2012, 42, 175–181. [Google Scholar] [CrossRef]
- Alves Rico, S.R.; Abbasi, A.Z.; Ribeiro, G.; Ahmed, T.; Wu, X.Y.; De Oliveira Silva, D. Diruthenium(II,III) Metallodrugs of Ibuprofen and Naproxen Encapsulated in Intravenously Injectable Polymer-Lipid Nanoparticles Exhibit Enhanced Activity against Breast and Prostate Cancer Cells. Nanoscale 2017, 9, 10701–10714. [Google Scholar] [CrossRef]
- Alessio, E. Thirty Years of the Drug Candidate NAMI-A and the Myths in the Field of Ruthenium Anticancer Compounds: A Personal Perspective. Eur. J. Inorg. Chem. 2017, 2017, 1549–1560. [Google Scholar] [CrossRef]
- Lin, K.; Zhao, Z.Z.; Bo, H.B.; Hao, X.J.; Wang, J.Q. Applications of Ruthenium Complex in Tumor Diagnosis and Therapy. Front. Pharmacol. 2018, 9, 1323. [Google Scholar] [CrossRef]
- Ribeiro, G.; Benadiba, M.; Colquhoun, A.; de Oliveira Silva, D. Diruthenium(II, III) Complexes of Ibuprofen, Aspirin, Naproxen and Indomethacin Non-Steroidal Anti-Inflammatory Drugs: Synthesis, Characterization and Their Effects on Tumor-Cell Proliferation. Polyhedron 2008, 27, 1131–1137. [Google Scholar] [CrossRef]
- Musumeci, D.; Rozza, L.; Merlino, A.; Paduano, L.; Marzo, T.; Massai, L.; Messori, L.; Montesarchio, D. Interaction of Anticancer Ru(III) Complexes with Single Stranded and Duplex DNA Model Systems. Dalt. Trans. 2015, 44, 13914–13925. [Google Scholar] [CrossRef]
- Barresi, E.; Tolbatov, I.; Pratesi, A.; Notarstefano, V.; Baglini, E.; Daniele, S.; Taliani, S.; Re, N.; Giorgini, E.; Martini, C.; et al. A Mixed-Valence Diruthenium(Ii,Iii) Complex Endowed with High Stability: From Experimental Evidence to Theoretical Interpretation. Dalt. Trans. 2020, 49, 14520–14527. [Google Scholar] [CrossRef]
- Barresi, E.; Tolbatov, I.; Marzo, T.; Zappelli, E.; Marrone, A.; Re, N.; Pratesi, A.; Martini, C.; Taliani, S.; Da Settimo, F.; et al. Two Mixed Valence Diruthenium(Ii,Iii) Isomeric Complexes Show Different Anticancer Properties. Dalt. Trans. 2021, 50, 9643–9647. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).