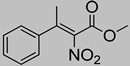
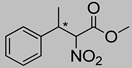
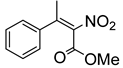
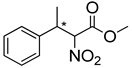
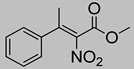
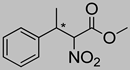
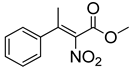
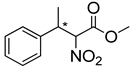
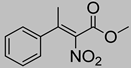
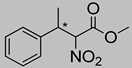
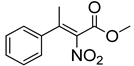
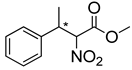
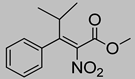
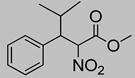
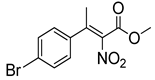
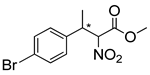
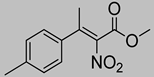
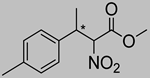
Abstract
Starting from commercially available ketones, a reproducible and reliable strategy for the synthesis of tetrasubstituted nitroalkenes was successfully developed, using a two-step procedure; the HWE olefination of the ketone to form the corresponding α,β-unsaturated esters is followed by a nitration reaction to introduce the nitro group in the α position of the ester group. The enantioselective organocatalytic reduction of these compounds has also been preliminarily studied, to access the functionalized enantioenriched nitroalkanes, which are useful starting materials for further synthetic elaborations. The absolute configuration of the reduction product was established by chemical correlation of the chiral nitroalkane with a known product; preliminary DFT calculations were also conducted to rationalize the stereochemical outcome of the organocatalytic enantioselective reduction.
1. Introduction
Among unnatural α-amino acids, α,α-disubstituted amino acids are key biological scaffolds with many specific roles and properties that have made them increasingly attractive in the fields of organic chemistry, biochemical research and drug discovery [1,2,3]. They have unique structural properties, which make them ideal candidates to be included in the design of new pharmaceutically active compounds, as well as intermediates for the study of pathological pathways [4,5,6].
Synthetic approaches for the synthesis of di- or trisubstituted nitroalkenes, valuable intermediates for the synthesis of α,α-disubstituted amino acids, are abundant in the literature, but there are very scarce data reporting synthetic routes for tetrasubstituted nitroalkenes [7,8]. Herein we describe a reproducible methodology for the synthesis of these compounds (Figure 1) that started from easily available ketones (1), which are converted to tetrasubstituted nitroolefins (3), by reaction of an acrylate intermediate (2), with an appropriate nitration reagent. Nitroalkenes will be organocatalytically reduced to afford enantioenriched nitroalkanes (4), highly functionalized chiral starting materials for further transformations (Figure 1).
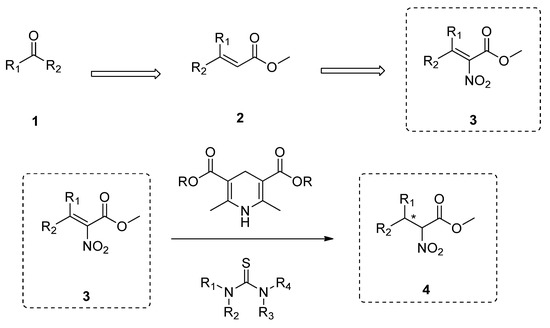
Figure 1.
Synthetic approach for the synthesis of tetrasubstituted nitroalkenes and their enantioselective catalytic reduction.
2. Results and Discussion
2.1. Synthesis of Tetrasubstituted Nitroalkenes
We started the investigations by exploring a two-step synthetic strategy for the synthesis of tetrasubstituted nitroalkenes (Scheme 1). The first reaction involves the formation of acrylates 2a–f, by reaction of different commercially available ketones 1a–f, using Horner–Wadstworth–Emmons reaction conditions [9]. The results are summarized in Table 1.
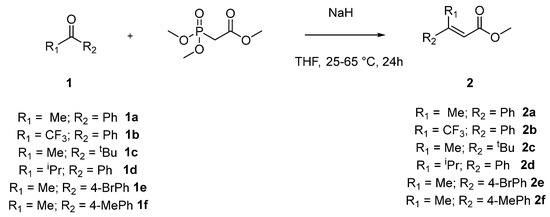
Scheme 1.
Synthesis of acrylates intermediates (2).

Table 1.
Synthesis of acrylate intermediates 2a–f.
Compounds 2a–f were obtained after reaction of the corresponding commercially available ketones with trimethylphosphonoacetate and sodium hydride in THF for 24 h with good to moderate yields and excellent diastereoselectivities after purification with column chromatography. The reactions were performed at room temperature, but with compounds 2c, 2d and 2f (Table 1, entries 3,4,6) it was necessary to heat the reaction to 66 °C. Their E/Z ratio was checked by 1H-NMR of reaction crude. The low yield observed for the isolation of pure product 2c (Table 1, entry 3) can be explained by its high volatility, and optimization of the product isolation is underway.
Then, our efforts were concentrated on the nitration reaction of these acrylate intermediates using common nitration reagents such as HNO3 [10,11]. However, the use of nitric acid mainly led to the formation of products with the nitro group on the aromatic ring, and the yields after purification were very low (<10%). Furthermore, several problems were also detected during the isolation process. Selective nitration conditions for double bonds were also considered, but no formation of the desired nitroalkane was observed. Thus, alternative strategies involving the condensation between acetophenone and ethyl nitroacetate or the reaction between phenylacetylene with ethyl nitroacetate catalyzed by indium salts were also explored, [12,13,14,15] but without any satisfactory results (see Supplementary Materials).
Finally, Buevich and co-workers reported the first example of a α-nitro addition to a cinammic ester for the synthesis of dehydrophenylalanine derivatives, which are precursors of α-amino acids, by utilizing a CAN-NaNO2 system [16]. Therefore, we decided to investigate the methodology for the synthesis of target tetrasubstituted nitroalkenes 3a–f starting from acrylates 2a–f (Scheme 2).
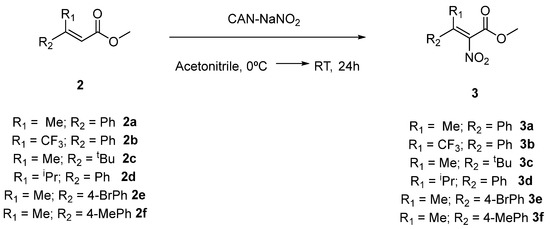
Scheme 2.
Synthesis of tetrasubstituted nitroalkenes 3a–f.
The results are summarized in Table 2.

Table 2.
Synthesis of tetrasubstituted nitroalkenes 3a–f.
The nitration reaction of acrylates led to the formation of the corresponding tetrasubstituted nitroacrylates 3a–f in low to moderate yields after chromatographic purification, and typically in a 6/4 diasteoisomeric ratio, except in the case of 3d, when a single isomer was isolated. In case of nitroacrylates 3a,e (Table 2, entries 1 and 5), two different fractions corresponding to the two separated diastereoisomers were obtained after purification., while for product 3f (Table 2, entry 6) a single fraction containing both, non-separable, isomers, was obtained after chromatography.
The nitration of acrylate 2b (Table 2, entry 2), did not lead to the formation of the corresponding nitroacrylate 3b, but (Z)-methyl 4,4,4-trifluoro-3-(4-nitrophenyl)but-2-enoate was obtained as major compound in this reaction in 76% yield. The nitration of acrylate 2c (Table 2, entry 3) did not afford any product.
As previously mentioned, in the nitration of acrylates 2a,e (Table 2, entries 1 and 5), it was possible to separate the two diastereoisomers. In order to clarify the configuration of the two products, additional NMR experiments were conducted on the isomers of compound 3a (Figure 2).
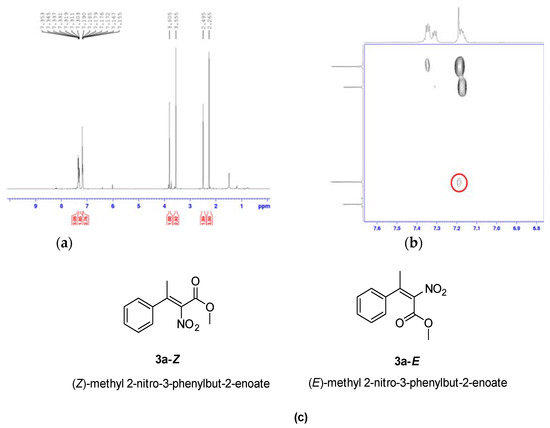
Figure 2.
Further NMR experiments performed for tetrasubstituted nitroacrylate 3a. (a): NMR spectra of a mixture fraction of compound 3a. (b): NOE contact observed between OMe and Ph groups in the more abundant form of the tetrasubstituted nitroacrylate 3a. (c): structures of nitroacrylates 3a–Z and 3a–E.
A chemical shift study on the methoxy group signal of both Z/E forms of the tetrasubstituted nitroalkene 3a has been performed, using a NOESY experiment. As illustrated in Figure 2a, the OMe group of the molecule is more shielded (3.55 ppm) in the more abundant form, suggesting that it can be near to the shielding cone of the aromatic ring (corresponding to the (E) isomer), while it resonates at 3.8 ppm in the less abundant isomer of Figure 2a.
NOESY experimental results, showing through-space correlation within the molecule, were also acquired to predict if the more abundant isomer of the synthetized tetrasubstituted nitroalkene corresponds to (E) or (Z), considering that the NOE contact between the methoxy group and the phenyl ring can only be observed in the (E) isomer. The analysis of NOE contacts suggested that the significant cross peak between the OMe group and phenyl ring (Figure 2b) is present only in the major isomer that can be determined to have the (E) configuration (product 3a–E).
2.2. Enantioselective Reduction of Tetrasubstituted Nitroalkenes (3)
The use of Hantzsch esters as biomimetic reducing agents [17] has been reported in different organocatalytic reductions of nitroalkenes, but also ketoimines and ketoesters [18,19,20]. In addition, Hantzsch esters are easily synthetized, and their structure readily tuned in order to maximize the efficiency of enantioselective reactions [21].
The enantioselective reduction of the synthetized tetrasubstituted nitroalkenes (3) using Hantzsch esters as a reductive agent and a thiourea based chiral catalyst was investigated to obtain the corresponding functionalized nitroalkanes (4) (Scheme 3), in the presence of a few chiral bifunctional catalysts A–D, representative of different classes of the most popular organocatalysts for this transformation. The results are reported in Table 3.
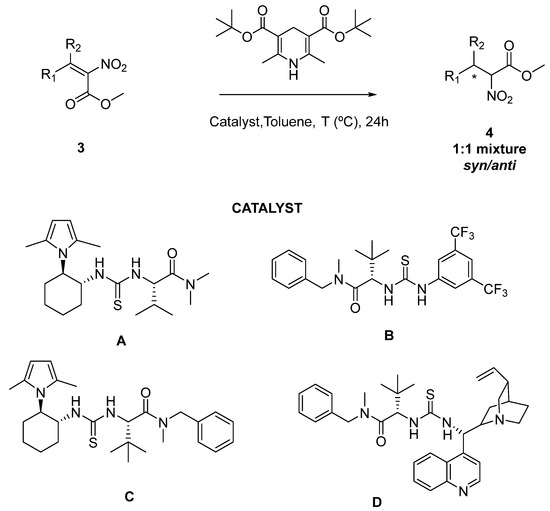
Scheme 3.
Organocatalytic reduction of tetrasubstituted nitroalkenes.

Table 3.
Enantioselective reduction of tetrasubstituted nitroalkenes.
In general, compounds 4 were obtained in a 1:1 mixture of syn/anti products after 24 h of reaction time. Initial experiments were conducted using nitroalkane 3a (Table 3, entries 1–8) as model substrate. The reactions performed in the presence of catalyst A starting from different mixtures of nitroalkene 3a (Table 3, entries 1–3), demonstrate a different reactivity of the E–Z isomers of this compound, showing that one isomer reacted more quickly than the other one. This interesting discovery was confirmed when the reaction was performed with a pure fraction of the less reactive isomer (which was previously assigned as having a Z configuration) and no reaction was observed (Table 3, entry 1). Starting from differently enriched mixtures in the E isomer led to similar results (entries 2–3), leading to the chiral alkanes in up to 67% enantiomeric excess (entries 2–3). Attempts to increase the yield by operating at a higher temperature or to improve the enantioselectivity by running the reaction at a lower temperature did not lead to any significant results. The enantioselectivity of the reaction was measured by HPLC analysis of the pure samples on the chiral stationary phase, and two pairs of enantiomers, corresponding to syn/anti products, were found (see experimental section).
When thiourea catalyst B was used (Table 3, entry 6), the corresponding nitroalkane 3a was obtained with good yield, but the enantioselectivity observed was lower than with catalyst A (Table 3, entry 2), which was the best catalyst for this transformation. When the thiourea catalysts C and D were tested (Table 3, entries 7,8), the yields were drastically reduced, with a very low quantity of product obtained after purification. With the optimized conditions in hand, the enantioselective reduction of nitroalkenes 3d–f (Table 3, entries 9–11) was carried out using Hanztsch ester and thiourea catalyst A. Compounds 4d–f were obtained with good yields after purification, but generally low or modest enantioselectivities.
2.3. Determination of the Absolute Configuration of Nitroalkanes (4)
The absolute configuration of the reduction product, the nitroalkane (4), was experimentally established by converting 4a into a known compound. To achieve this goal, two approaches were attempted (Scheme 4).
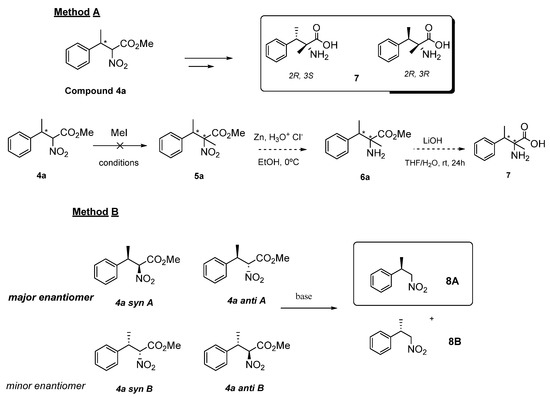
Scheme 4.
Strategies for the experimental determination of the absolute configuration of nitroal-kane 4a.
The first strategy (Scheme 4, method A) explored the transformation of 4a into the known compound 7, by a three-step procedure involving the alkylation of nitroalkane 4a, followed by the reduction of the nitro group and hydrolysis of the ester moiety as depicted in Scheme 4 [22,23,24]. However, despite several conditions being attempted, the alkylation did not lead to the desired product, but mixtures of unreacted starting material and O-alkylated products were obtained (In an additional experimental effort, we have observed that nitroalkane 4a reacted with “softer” electrophiles, such as methyl vinyl ketone in a Michael reaction, additional studies are underway to further explore these transformations).
Thus, our efforts were focused on an alternative approach (Scheme 4, Method B). The decarboxylation of the ester moiety of 4a (as 50:50 mixture of syn/anti isomers, enantioenriched) to afford the corresponding trisubstituted nitroalkane 8 was then explored [25]. This simple approach was found to be effective and the desired decarboxylated nitroalkane 8 was finally synthetized. The experimental optical rotation was measured using a polarimeter and by comparison with the literature data, the compound 8, derived from the major enantiomer of product 4a (4a syn A and 4a anti A), was established to have the R-configuration [26].
DFT computational studies on the enantioselective reduction of tetrasubstituted nitroalkanes were preliminarily performed to rationalize the stereochemical outcome of the reaction, using the Gaussian g16 package using Catalyst A as the model catalyst. All geometries of reactants and products (ground states and transition states) were located at a B3LYP/6-31G (d,p) level of theory and finer electronic energies were successively obtained, increasing the basis set up to 6/311 + (2df,2pd) with B3LYP functional [27]. In Figure 3 four possible complexes of nitroalkene 3a with catalyst A and the geometries of the TS leading to the formation of the four stereoisomers of nitroalkane 4a are represented.
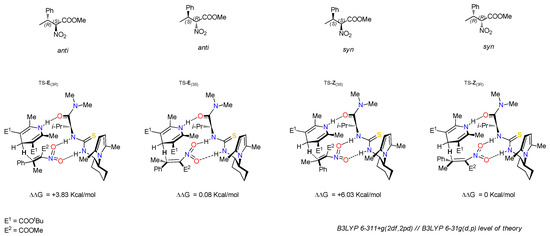
Figure 3.
The four possible TS geometries for nitroalkene 3a complexes with catalyst A.
Transition states responsible for the hydride transfer were located assuming the coordination of the nitro group of the nitroalkane 3a to the thiourea moiety and of the Hantzsch ester NH group with the catalyst carboxyamide group, according to the so-called Takemoto model. The energy profile is depicted in Figure 4.
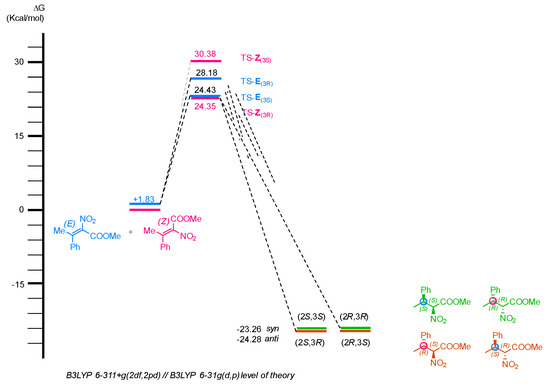
Figure 4.
DFT calculations performed for the enantioselective reduction of tetrasubstituted nitroal-kene 3a.
In Figure 5 the geometries of the transition states originated by the E-isomer of compound 3a are illustrated.
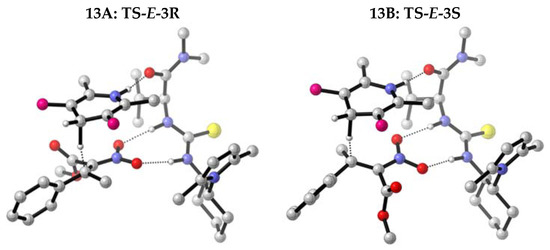
Figure 5.
Transition states formed by the E- isomer of nitroacrylate 3a.
The blue spheres represent the nitrogen atom of the nitro group of nitroalkane 3a and of the thiourea catalyst A, the yellow one represents the sulfur atom of the thiourea moiety, and the red spheres represent the oxygen atoms of nitro and ester groups of compound 3a, and of the carboxyamide group of the catalyst. The pink sphere represents the tert-butyl groups of the Hantzsch ester, carbon atoms are grey and hydrogen atoms are white. The broken lines showed the H-bonding interaction between the nitro group of 3a and the thiourea moiety of the catalyst, and the transfer hydride between Hantzsch ester and carbon C3 of the nitroolefin.
According to the calculations, among the two transition states originating from Z-olefin, TS-Z-(3R) is the lowest in energy and would lead to the formation of the final product with a R-configuration at the C3 carbon, in agreement with the experimental data. However, the (Z) isomer was found to be very poorly reactive, while, as established in NMR analysis, the more reactive isomer is the (E)-nitroacrylate, that, according to the calculations should preferably afford the (S) enantiomer at C3 carbon of compound 4a. These findings are in contrast with the experimentally established absolute configuration (R) for the major enantiomer derived from the reaction of the E isomer of 3a. Therefore, we can conclude that, at the moment, the proposed TS according to the Takemoto model, is not able to explain why the E isomer should be more reactive than the Z isomer, and, furthermore, cannot predict the correct configuration at the C3 of the nitroalkane. Those results are probably an indication that other coordination modes are active in the TS of the reactions, and other models need to be taken into consideration to rationalize the stereochemical outcome of the reaction.
3. Materials and Methods
Reactions were monitored by analytical thin-layer chromatography (TLC) using silica gel 60 F254 pre-coated glass plates (0.25 mm thickness) and visualized using UV light. Flash chromatography was carried out on silica gel (230–400 mesh). Proton NMR spectra were recorded on spectrometers operating at 300 MHz (Bruker Fourier 300); proton chemical shifts are reported in ppm (δ) with the solvent reference relative to tetramethylsilane (TMS) employed as the internal standard (CDCl3: δ = 7.26 ppm). 13C-NMR spectra were recorded on 300 MHz spectrometers (Bruker Fourier 300) operating at 75 MHz, with complete proton decoupling; carbon chemical shifts are reported in ppm (δ) relative to TMS with the respective solvent resonance as the internal standard (CDCl3: δ = 77.0 ppm). Mass spectra and accurate mass analysis were carried out on a VG AUTOSPEC- M246 spectrometer (double-focusing magnetic sector instrument with EBE geometry) equipped with EI source or with LCQ Fleet ion trap mass spectrometer, ESI source, with acquisition in positive ionization mode in the mass range of 50–2000 m/z. Dry solvents were purchased and stored under nitrogen over molecular sieves (bottles with crown caps). All chemicals were purchased from commercial suppliers and used without further purification unless otherwise specified.
3.1. General Procedure for the Synthesis of Tetrasubstituted Nitroalkenes (3)
Tetrasubstituted nitroalkenes (3) were synthetized using a two-step procedure: Firstly the formation of an acrylate intermediate (2) by a Horner–Wasdforth–Emmons reaction of an appropriate ketone (1) with trimethylphosphonoacetate and sodium hydride, following by a nitration reaction of this intermediate with a mixture of CAN-NaNO2 as an effective nitration reagent.
Compounds 2a–f were synthetized using conditions reported in the literature [9]. First, a solution of trimethyl phosphonoacetate (5.21 mmol) in 20 mL of THF was cooled to 0 °C. Then, sodium hydride (5.21 mmol) was added portion-wise and the mixture was stirred for 30 min. After this time, the appropriate ketone (4.17 mmol) was added at the same temperature and the reaction mixture was allowed to warm to room temperature and stirred for 24 h at the right temperature. Then, 20 mL of saturated solution of ammonium chloride was added dropwise and the mixture was extracted with Et2O.
The combined organic phases were dried using MgSO4, filtered and concentrated in vacuo. The solvent was eliminated under reduced pressure and the crude product was purified using column chromatography and hexanes/EtOAc as eluent. The 1H-NMR of compounds 2a–f were in agreement with the published ones. Compounds 2a–f were directly used in the next step after purification.
Acrylates 2a, 2b, 2c, 2d, 2e and 2f (5.68 mmol) were dissolved in 50 mL of acetonitrile and cooled to 0 °C. Then, sodium nitrite (17 mmol) and cerium ammonium nitrate (17 mmol) were added at the same temperature, and the reaction mixture was allowed to warm to room temperature and stirred for 24 h. After this time, the reaction was filtered through a pad of celite, and the filtrate was concentrated under reduced pressure. The residue was poured into cold water and extracted with DCM (3 × 50 mL). The combined organic layers were dried using MgSO4, filtered and concentrated in vacuo. The crude product was purified by column chromatography using an appropriate mixture of solvents to afford nitroacrylates. For further details see the Supporting Information.
3.2. General Procedure for the Enantioselective Synthesis of Nitroalkanes (4)
To a stirred solution of nitroalkenes (3) in toluene (0.3 mmol 0.3M), catalyst A (10 mol%) and Hanztsch ester (1.2 eq, 0.36 mmol) were added. The reaction mixture was heated at 60 °C for 24 h. Then, the mixture was allowed to warm to room temperature and the solvent was eliminated under reduced pressure, and the crude product was purified using column chromatography and an appropriate mixture of eluents.
4. Conclusions
Although the preparation of tetrasubstituted nitroacrylates proved to be very challenging, in this work a reproducible strategy for the synthesis of tetrasubstituted nitroalkenes was successfully developed using a two-step procedure; the HWE olefination of the ketone followed by the reaction of nitration affords the desired tetrasubstituted nitroalkenes (3).
The enantioselective reduction of these synthetized tetrasubstituted nitroalkenes (3) to access the functionalized nitroalkanes (4) was also performed, using a Hantzsch ester as the reductive agent and a thiourea based chiral catalyst, to afford the products with good to moderate yields, in a 1:1 mixture of syn/anti isomers, and up to 67% e.e. Although the level of enantioselectivity could not be considered satisfactory yet, it should be noted that the enantioselective organocatalytic reduction of tetrasubsituted alkenes was almost completely unknown. Even if the poor reactivity of the substrates represents a major problem, the present work demonstrates that the asymmetric catalytic reduction of functionalized nitroacrylates may offer a viable strategy for the synthesis of chiral amino ester derivatives.
The absolute configuration of the major enantiomer obtained in the enantioselective reduction was established by converting the nitroalkane 4a into a known product. DFT calculations, performed in order to rationalize the stereochemical outcome of the reaction did not lead to satisfactory results. Further studies, considering other alternative coordination modes between the catalyst and the substrate, will be necessary in order to understand the origins of the stereocontrol of the reaction.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28073156/s1.
Author Contributions
Conceptualization, M.B.; methodology, M.S., P.C.G. and S.R.; validation, M.S., P.C.G. and S.R.; formal analysis, F.V.; investigation, P.C.G.; data curation, S.R., F.V. and P.C.G.; writing—original draft preparation, M.B. All authors have read and agreed to the published version of the manuscript.
Funding
M.B. and P.C.G. thank ITN-EID project Marie Sklodowska-Curie Actions Innovative Training Network—TECHNOTRAIN H2020-MSCA-ITN-2018 Grant Agreement 812944. www.technotrain-ITN.eu. M.B. and M.S. thank Taros Chemicals.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request from the authors.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Narancic, T.; Almahboub, S.A.; O’Connor, K.E. Unnatural amino acids: Production and biotechnological potential. World J. Microbiol. Biotechnol. 2019, 35, 67. [Google Scholar] [CrossRef]
- Osberger, T.J.; Rogness, D.C.; Kohrt, J.T.; Stepan, A.F.; White, M.C. Oxidative diversification of amino acids and peptides by small-molecule iron catalysis. Nature 2016, 537, 214–219. [Google Scholar] [CrossRef]
- Bisht, A.; Juyal, D. Unnatural amino acids (UAA’S): A trendy scaffold for pharmaceutical research. J. Drug Deliv. Ther. 2019, 9, 601–609. [Google Scholar]
- Ohfune, Y.; Shinada, T. Enantio- and Diastereoselective Construction of α,α-Disubstituted α-Amino Acids for the Synthesis of Biologically Active Compounds. Eur. J. Org. Chem. 2005, 2005, 5127–5143. [Google Scholar] [CrossRef]
- Li, B.; Zhang, J.; Xu, Y.; Yang, X.; Li, L. Improved synthesis of unnatural amino acids for peptide stapling. Tetrahedron Lett. 2017, 58, 24–28. [Google Scholar] [CrossRef]
- Vogt, H.; Bräse, S. Recent approaches towards the asymmetric synthesis of α,α-disubstituted α-amino acids. Org. Biomol. Chem. 2007, 5, 406–430. [Google Scholar] [CrossRef]
- Noboru, O. Chapter 2: Preparation of Nitro Compounds. In The Nitro Group in Organic Synthesis; Willey-VCH: Hoboken, NJ, USA, 2001; pp. 3–29. [Google Scholar]
- Gabrielli, S.; Chiurchiù, E.; Palmieri, A. β-Nitroacrylates: A Versatile and Growing Class of Functionalized Nitroalkenes. Adv. Synth. Catal. 2019, 361, 630–651. [Google Scholar] [CrossRef]
- Wen, J.; Jiang, J.; Zhang, X. Rhodium-Catalyzed Asymmetric Hydrogenation of α,β-Unsaturated Carbonyl Compounds via Thiourea Hydrogen Bonding. Org. Lett. 2016, 18, 4451–4453. [Google Scholar] [CrossRef]
- Sharma, V.; Kelly, G.T.; Watanabe, C.M.H. Exploration of the Molecular Origin of the Azinomycin Epoxide: Timing of the Biosynthesis Revealed. Org. Lett. 2008, 10, 4815–4818. [Google Scholar] [CrossRef]
- Maity, S.; Manna, S.; Rana, S.; Naveen, T.; Mallick, A.; Maiti, D. Efficient and Stereoselective Nitration of Mono- and Disubstituted Olefins with AgNO2 and TEMPO. J. Am. Chem. Soc. 2013, 135, 3355–3358. [Google Scholar] [CrossRef]
- Rodríguez, J.M.; Pujol, M.D. Straightforward synthesis of nitroolefins by microwave- or ultrasound-assisted Henry reaction. Tetrahedron Lett. 2011, 52, 2629–2632. [Google Scholar] [CrossRef]
- Zhu, L.; Yan, P.; Zhang, L.; Chen, Z.; Zeng, X.; Zhong, G. TiCl4/DMAP mediated Z-selective knovenagel condensation of isatins with nitroacetates and related compounds. RSC Adv. 2017, 7, 51352–51358. [Google Scholar] [CrossRef]
- Zhang, J.; Blazecka, P.G.; Mark, P.A.; Lovdahl, T.; Curran, T. Indium (III) mediated Markovnikov addition of malonates and β-ketoesters to terminal alkynes and the formation of Knoevenagel condensation products. Tetrahedron 2005, 61, 7807–7813. [Google Scholar] [CrossRef]
- Nakamura, M.; Fujimoto, T.; Endo, K.; Nakamura, E. Stereoselective Synthesis of Tetra-Substituted Olefins via Addition of Zinc Enolates to Unactivated Alkynes. Org. Lett. 2004, 6, 4837–4840. [Google Scholar] [CrossRef]
- Buevich, A.V.; Wu, Y.; Chan, T.M.; Stamford, A. An unusual contra-Michael addition of NaNO2–ceric ammonium nitrate to acrylic esters. Tetrahedron Lett. 2008, 49, 2132–2135. [Google Scholar] [CrossRef]
- Zheng, C.; You, S.-L. Transfer hydrogenation with Hantzsch esters and related organic hydride donors. Chem. Soc. Rev. 2012, 41, 2498–2518. [Google Scholar] [CrossRef]
- Nolwenn, J.A.; Ozores, M.L.; List, B. Organocatalytic Asymmetric Transfer Hydrogenation of Nitroolefins. J. Am. Chem. Soc. 2007, 129, 8976–8977. [Google Scholar]
- Bernardi, L.; Fochi, M. A General Catalytic Enantioselective Transfer Hydrogenation Reaction of β,β-Disubstituted Nitroalkenes Promoted by a Simple Organocatalyst. Molecules 2016, 21, 1000. [Google Scholar] [CrossRef]
- Massolo, E.; Benaglia, M.; Orlandi, M.; Rossi, S.; Celentano, G. Enantioselective Organocatalytic Reduction of β-Trifluoromethyl Nitroalkenes: An Efficient Strategy for the Synthesis of Chiral β-Trifluoromethyl Amines. Chem. Eur. J. 2015, 21, 3589–3595. [Google Scholar] [CrossRef]
- Barrios-Rivera, J.; Xu, Y.; Willis, M.; Vyas, V.K. A diversity of recently reported methodology for asymmetric imine reduction. Org. Chem. Front. 2020, 7, 3312–3342. [Google Scholar] [CrossRef]
- Davis, F.A.; Liang, C.H.; Liu, H. Asymmetric Synthesis of β-Substituted α-Amino Acids Using 2H-Azirine-2-carboxylate Esters. Synthesis of 3,3-Disubstituted Aziridine-2-carboxylate Esters. J. Org. Chem. 1997, 62, 3796–3797. [Google Scholar] [CrossRef]
- Gagnot, G.; Hervin, V.; Coutant, E.P.; Desmons, S.; Baatallah, R.; Monnot, V.; Janin, Y.L. Synthesis of unnatural α-amino esters using ethyl nitroacetate and condensation or cycloaddition reactions. Beilstein J. Org. Chem. 2018, 14, 2846–2852. [Google Scholar] [CrossRef] [PubMed]
- Harel, T.; Rozen, S. Transforming Natural Amino Acids into α-Alkyl-Substituted Amino Acids with the Help of the HOF·CH3CN Complex. J. Org. Chem. 2007, 72, 6500–6503. [Google Scholar] [CrossRef]
- Metz, A.E.; Kozlowski, M.C. 2-Aryl-2-nitroacetates as Central Precursors to Aryl Nitromethanes, α-Ketoesters, and α-Amino Acids. J. Org. Chem. 2013, 78, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Hostmann, T.; Molloy, J.J.; Bussmann, K.; Gilmour, R. Light-Enabled Enantiodivergence: Stereospecific Reduction of Activated Alkenes Using a Single Organocatalyst Enantiomer. Org. Lett. 2019, 21, 10164–10168. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016; Available online: https://gaussian.com/citation/ (accessed on 13 December 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).