Physico-Chemical Study of Mn(II), Co(II), Cu(II), Cr(III), and Pd(II) Complexes with Schiff-Base and Aminopyrimidyl Derivatives and Anti-Cancer, Antioxidant, Antimicrobial Applications
Abstract
1. Introduction
2. Results and Discussion
2.1. Fourier Transform Infrared Spectra
2.2. Magnetic Moments
2.3. Electronic Spectra
2.4. Theoretical Study
2.5. Thermal Analysis
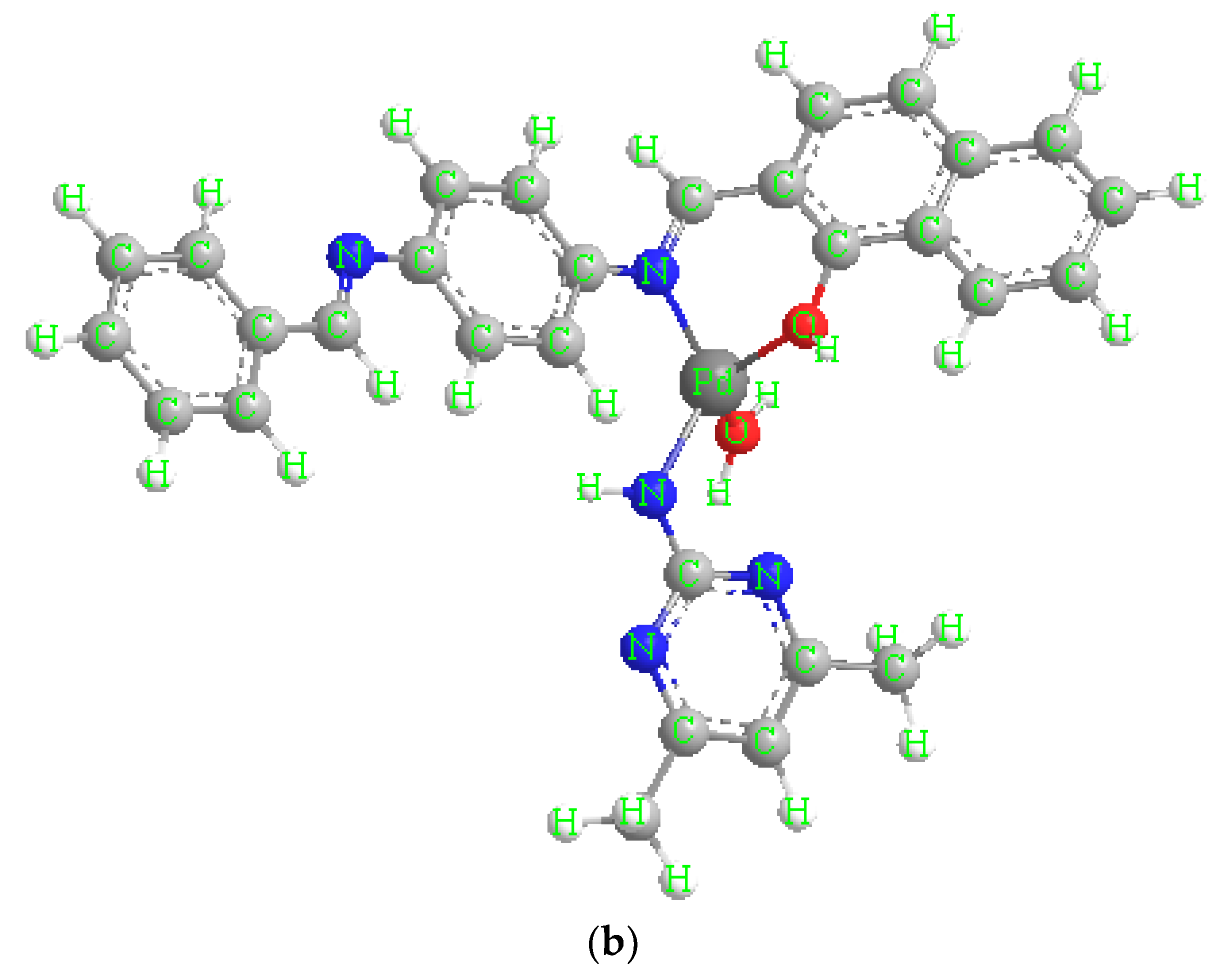
[Pd(HL)(ADMPY)(H2O)]·Cl2
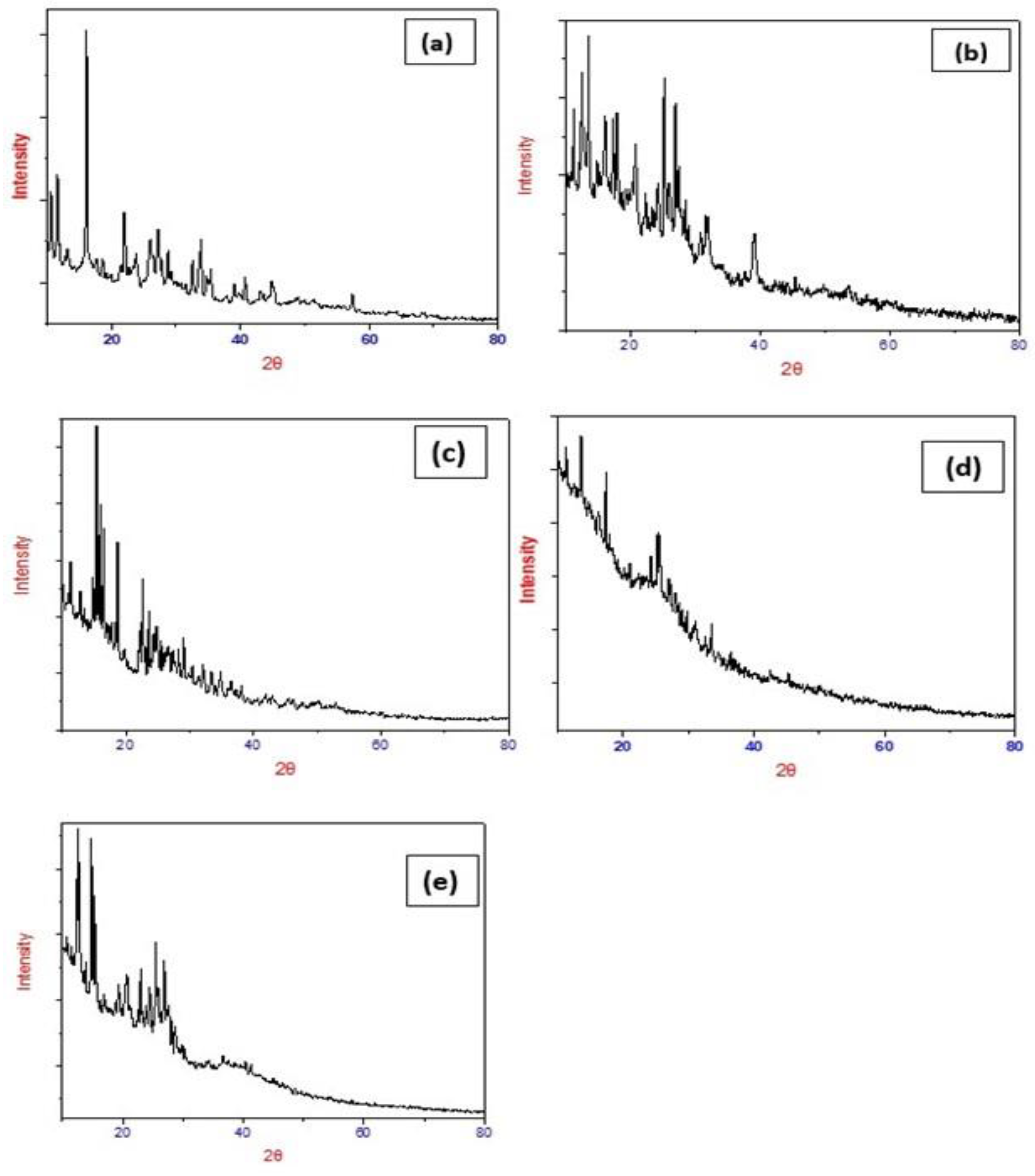
2.6. X-ray Powder Diffraction (XRD)
2.7. Morphological and Structural Properties of Synthesized Metal-Ligand Complexes
2.8. Biological Activity
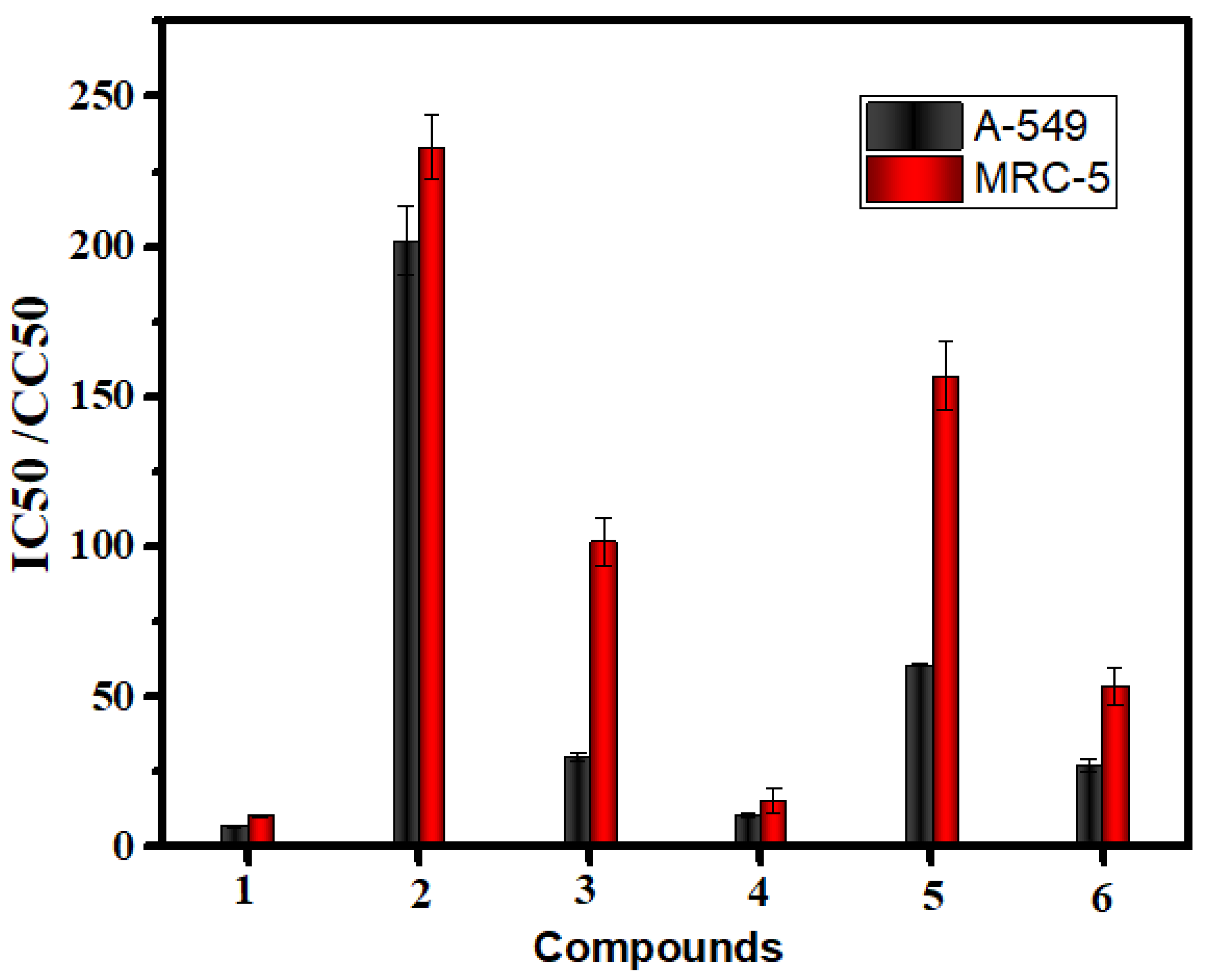
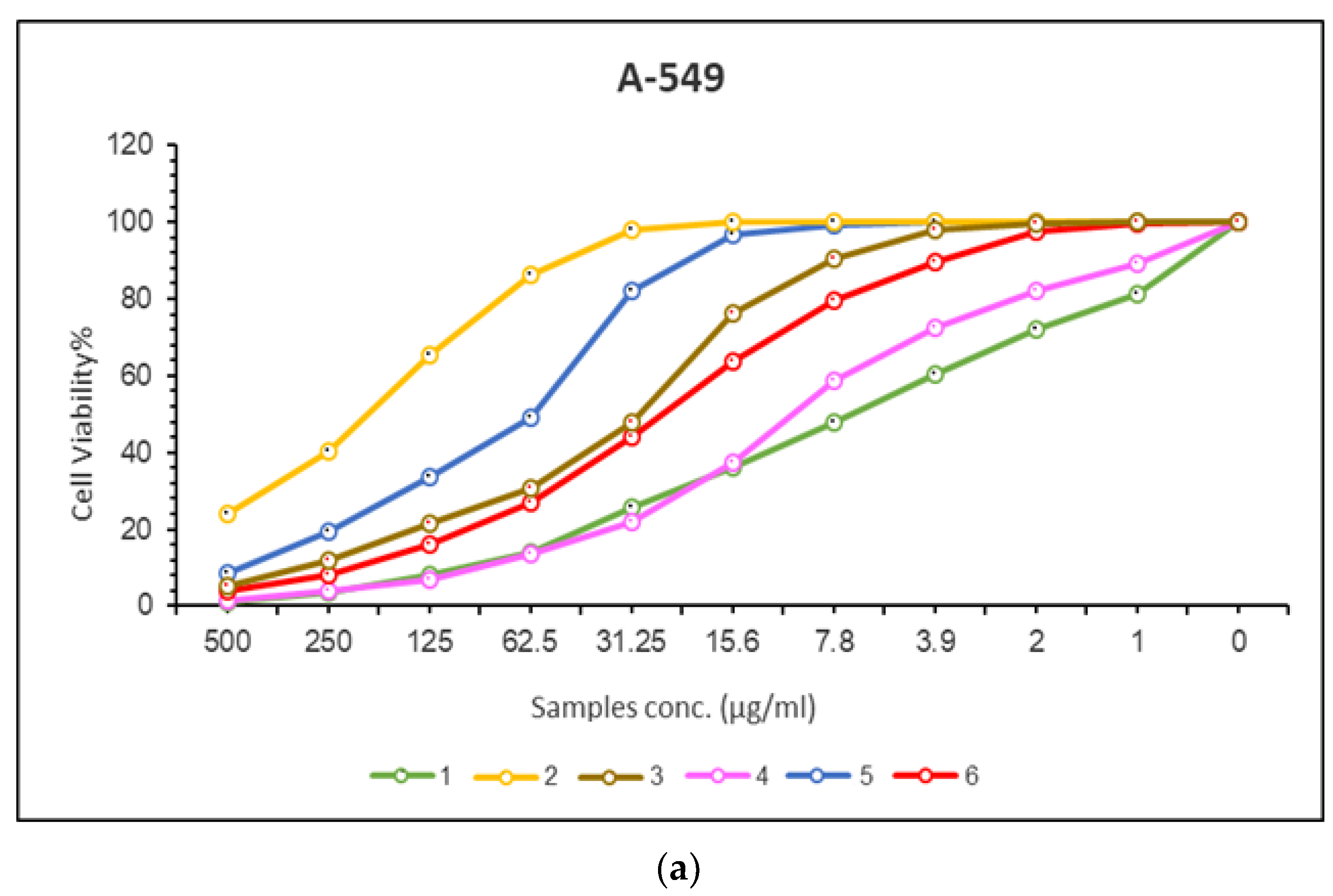
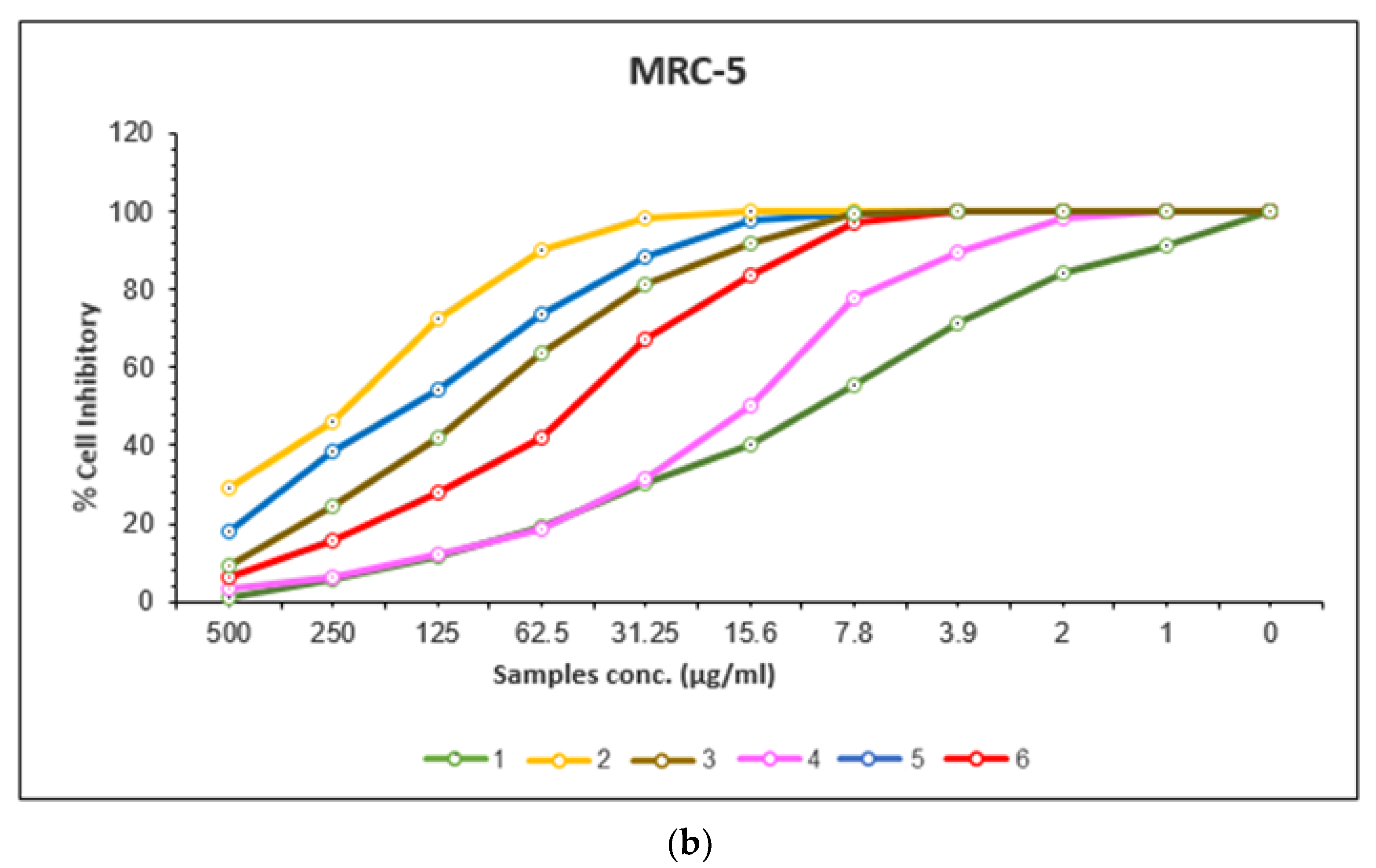
2.8.1. In Vitro Anticancer Activities
2.8.2. Antimicrobial Activity
Antifungal Screening
Antibacterial Screening
2.8.3. Antioxidant Assays
3. Materials and Methods
3.1. Physical Measurements for Complexes
3.2. Microbial Strains and Culture Media
3.3. Computational Studies
3.4. Antioxidant Activity
(DPPH) Radical Scavenging Assay
3.5. Cytotoxicity Testing
3.5.1. Mammalian Cell Lines
3.5.2. Mammalian Cell Lines
3.5.3. Chemicals Used
3.5.4. Crystal Violet Stain (1%)
3.5.5. Cell Line Propagation
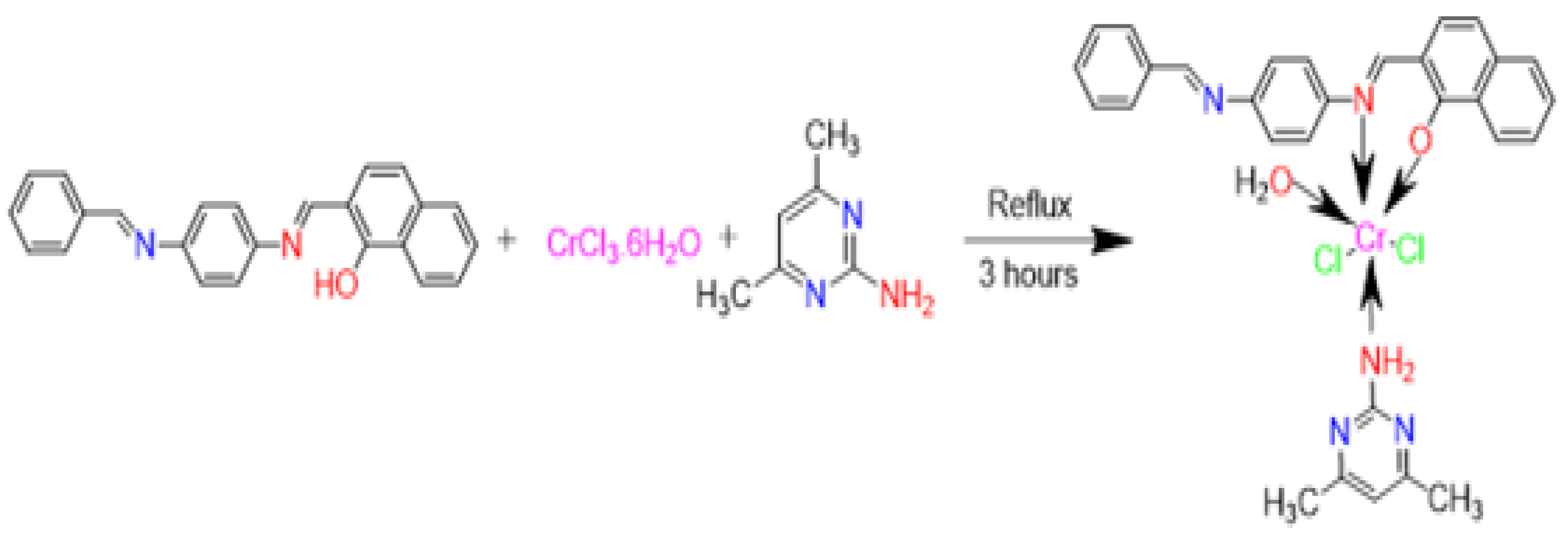
3.6. Synthesis of the Metals Mixed Ligand Complexes
3.6.1. [Mn(HL)(ADMPY)(H2O)Cl2] (1)
3.6.2. [Co(HL)(ADMPY)(H2O)Cl2]·H2O (2)
3.6.3. [Cu(HL)(ADMPY)(H2O)Cl2] (3)
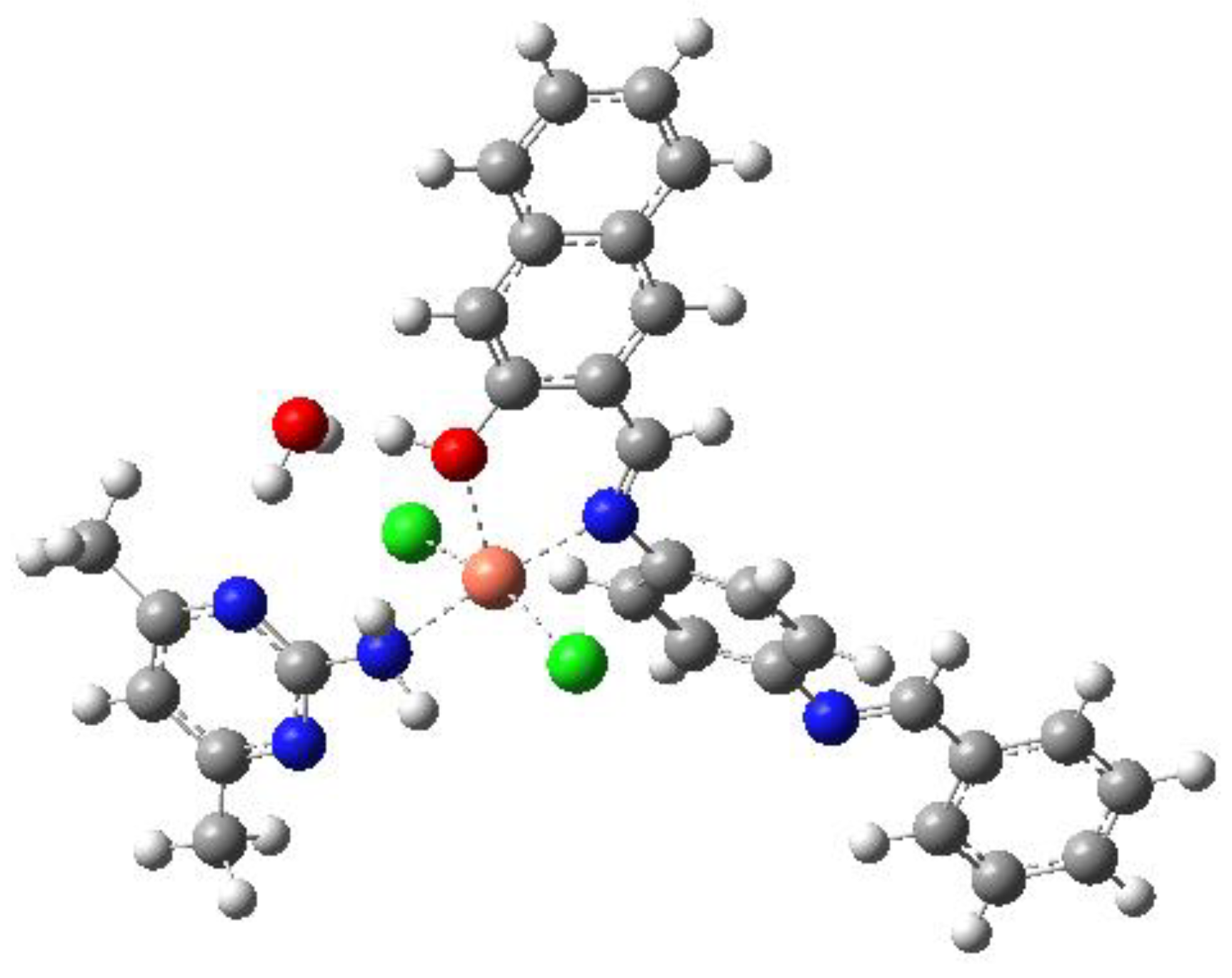
3.6.4. [Cr(HL)(ADMPY)(H2O)Cl2]·H2O (4)
3.6.5. [Pd(HL)(ADMPY)(H2O)]·Cl2 (5)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uddin, M.N.; Ahmed, S.S.; Alam, S.M.R. REVIEW: Biomedical applications of Schiff base metal complexes. J. Coord. Chem. 2020, 73, 3109–3149. [Google Scholar] [CrossRef]
- Omar, M.; El-Halim, H.F.A.; Khalil, E.A. Synthesis, characterization, and biological and anticancer studies of mixed ligand complexes with Schiff base and 2,2′-bipyridine. Appl. Organomet. Chem. 2017, 31, e3724. [Google Scholar] [CrossRef]
- Xavier, A.; Srividhya, N. Synthesis and Study of Schiff base Ligands. IOSR J. Appl. Chem. 2014, 7, 6–15. [Google Scholar] [CrossRef]
- Al Zoubi, W.; Al-Hamdani, A.; Ahmed, S.D.; Ko, Y.G. Synthesis, characterization, and biological activity of Schiff bases metal complexes. J. Phys. Org. Chem. 2018, 31, e3752. [Google Scholar] [CrossRef]
- Chaudhary, N.K.; Guragain, B.; Chaudhary, S.K.; Mishra, P. Schiff base metal complex as a potential therapeutic drug in medical science: A critical review. Bibechana 2021, 18, 214–230. [Google Scholar] [CrossRef]
- Jaziri, E.; Louis, H.; Gharbi, C.; Unimuke, T.O.; Agwamba, E.C.; Mathias, G.E.; Fugita, W.; Nasr, C.B.; Khedhiri, L. Synthesis, X-ray crystallography, molecular electronic property investigation, and leukopoiesis activity of novel 4,6-dimethyl-1,6-dihydropyridin-2-amino nitrate hybrid material. J. Mol. Struct. 2022, 1268, 133733. [Google Scholar] [CrossRef]
- Al-Fakeh, M.S.; Al-Otaibi, N.F. Nd2O3, Cr2O3, and V2O3 Nanoparticles via Calcination: Synthesis, Characterization, and Antimicrobial and Antioxidant Activities. J. Nanotechnol. 2022, 2022, 7794939. [Google Scholar] [CrossRef]
- Al-Fakeh, M.S.; Allazzam, G.A.; Yarkandi, N.H. Preparation, Properties and Antimicrobial evaluation of a new metal complex with Diphosphine ligand and 2-Aminopyridine. SYLWAN 2021, 9, 165. [Google Scholar]
- Al-Fakeh, M.S.; Allazzam, G.A.; Yarkandi, N.H. Ni(II), Cu(II), Mn(II) and Fe(II) Metal Complexes Containing 1,3-Bis(diphenylphosphino)-propane and Pyridine Derivative: Synthesis, Characterization, and Antimicrobial Activity. Int. J. Biomater. 2021, 2021, 4981367. [Google Scholar] [CrossRef] [PubMed]
- Alorini, T.A.; Al-Hakimi, A.N.; Saeed, S.E.-S.; Alhamzi, E.H.L.; Albadri, A.E. Synthesis, characterization, and anticancer activity of some metal complexes with a new Schiff base ligand. Arab. J. Chem. 2022, 15, 103559. [Google Scholar] [CrossRef]
- Al-Fakeh, M.S.; Messaoudi, S.; Alresheedi, F.I.; Albadri, A.E.; El-Sayed, W.A.; Saleh, E.E. Preparation, Characterization, DFT Calculations, Antibacterial and Molecular Docking Study of Co(II), Cu(II), and Zn(II) Mixed Ligand Complexes. Crystals 2023, 13, 118. [Google Scholar] [CrossRef]
- Al-Fakeh, M.S. Supramolecular metal coordination polymers containing from 1,3-di(4-pyridyl)-propane and 2-amino4-methylthiazole. Biochem. Biotechnol. Res. 2017, 5, 30–38. [Google Scholar]
- Al-Fakeh, M.S. Sonochemical Synthesis and Characterizations of Nanosized metal coordination polymers derived from 5-(3-pyridyl)-1,3,4-oxadiazole-2-thiole and 2-aminothiazole. Eur. Chem. Bull. 2016, 5, 450–455. [Google Scholar] [CrossRef]
- Al-Zaidi, B.H.; Hasson, M.M.; Ismail, A.H. New complexes of chelating Schiff base: Synthesis, spectral investigation, antimicrobial, and thermal behavior studies. J. Appl. Pharm. Sci. 2019, 9, 45–57. [Google Scholar] [CrossRef]
- Rehman, M.; Ali, S.; Munawar, K.S. Synthesis, spectroscopic characterization and biological applications of novel zinc complexes with schiff bases. Key Eng. Mater. 2018, 778, 293–300. [Google Scholar] [CrossRef]
- El-Boraey, H. Structural and thermal studies of some aroylhydrazone Schiff’s bases-transition metal complexes. J. Therm. Anal. Calorim. 2005, 81, 339–346. [Google Scholar] [CrossRef]
- Thilagavathi, G.; Arivazhagan, M. Density functional theory calculation and vibrational spectroscopy study of 2-amino-4,6-dimethyl pyrimidine (ADMP). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 389–395. [Google Scholar] [CrossRef]
- Panchal, P.K.; Patel, D.H.; Patel, M. Preparation and thermal, spectroscopic, and antibacterial studies of some mixed-ligand complexes. Synth. React. Inorg. Met. Org. Chem. 2004, 34, 1223–1235. [Google Scholar] [CrossRef]
- Mohamed, G.G.; Omar, M.; Ibrahim, A.A. Biological activity studies on metal complexes of novel tridentate Schiff base ligand. Spectroscopic and thermal characterization. Eur. J. Med. Chem. 2009, 44, 4801–4812. [Google Scholar] [CrossRef]
- Aly, A.A.; Al-Fakeh, M.S.; Ghandour, M.A.; Abu-Zied, B.M. New nano-sized supramolecular metal coordination polymers derived from 1,2-bis (2-pyridyl)-ethene and benzimidazole. Nano Sci. Nano Technol. Indian J. NSNTAIJ 2013, 7, 197–203. [Google Scholar]
- Al-Fakeh, M.S. Synthesis, thermal stability and kinetic studies of copper(II) and cobalt(II) complexes derived from 4-aminobenzohydrazide and 2-mercaptobenzothiazole. Eur. Chem. Bull. 2020, 9, 403–409. [Google Scholar] [CrossRef]
- Al-Fakeh, M.S. Synthesis, characterization and anticancer activity of NiO nanoparticles from a Ni(II) complex derived from chitosan and pyridine derivative. Bulg. Chem. Commun. 2021, 53, 321–326. [Google Scholar] [CrossRef]
- Aly, A.A.M.; Ghandour, M.; Alfakeh, M.S. Synthesis and characterization of transition metal coordination polymers derived from 1,4-Benzenedicarboxylic acid and Azoles. Turk. J. Chem. 2012, 36, 69–79. [Google Scholar]
- Al-Fakeh, M.S. Synthesis and characterization of coordination polymers of 1, 3-di (4-pyridyl)-propane and 2-aminobenzothiazole with Mn(II), Co(II), Cu(II) and Ni(II) ions. J. Chem. Pharm. Res. 2018, 10, 77–83. [Google Scholar]
- Bargujar, S.; Chandra, S.; Chauhan, R.; Rajor, H.K.; Bhardwaj, J. Synthesis, spectroscopic evaluation, molecular modelling, thermal study and biological evaluation of manganese(II) complexes derived from bidentate N,O and N,S donor Schiff base ligands. Appl. Organomet. Chem. 2021, 32, e4149. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, P.K.; Sinha, N.; Chaudhari, S.; Sharma, S. Spectroscopic characterisation of metal complexes with tetradentate ligand. J. Phys. Sci. 2018, 29, 1–11. [Google Scholar] [CrossRef]
- Moustafa, I.M.I.; Magda, H. Synthesis, Spectroscopic Studies and Biological Evaluation of Co(II), Ni(II), Cu(II) and Zr(IV) Complexes of Azo Dyes and Thiamine Hydrochloride as Antimicrobial Agents. Mod. Chem. Appl. 2017, 5, 1–7. [Google Scholar] [CrossRef]
- Al-zaidi, B.H.; Rasheed, A.M.; Al-bayati, S.M.; Hussein, A.M. Preparation and Characterization of Some Chelate Complexe with New bi-dentate (N, O) bis-Schiff base Ligand Among different types of coordination ligands. J. Kufa Chem. Sci. 2016, 2, 49–61. [Google Scholar]
- Al-Nahary, T.T. Synthesis and characterization of metal complexes of Cr(III), Mn(II), Fe(III), Co(II), Ni(II), Cu(II), Ru(III), Rh(III) and Pd(II) with derivatives of 1,3,4-thiadiazole-2,5-dithiol as new ligands. J. Saudi Chem. Soc. 2009, 13, 253–257. [Google Scholar] [CrossRef]
- El-Boraey, H.A. Coordination behavior of tetraaza [N4] ligand towards Co(II), Ni(II), Cu(II), Cu(I) and Pd(II) complexes: Synthesis, spectroscopic characterization and anticancer activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 97, 255–262. [Google Scholar] [CrossRef]
- Ntonga, A.P.; Baldovini, N.; Mouray, E.; Mambu, L.; Belong, P.; Grellier, P. Activity of Ocimum basilicum, Ocimum canum and Cymbopogon citratus essential oils against Plasmodium falciparum and mature-stage larvae of Anopheles funestus ss. Parasite 2014, 21, 33. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
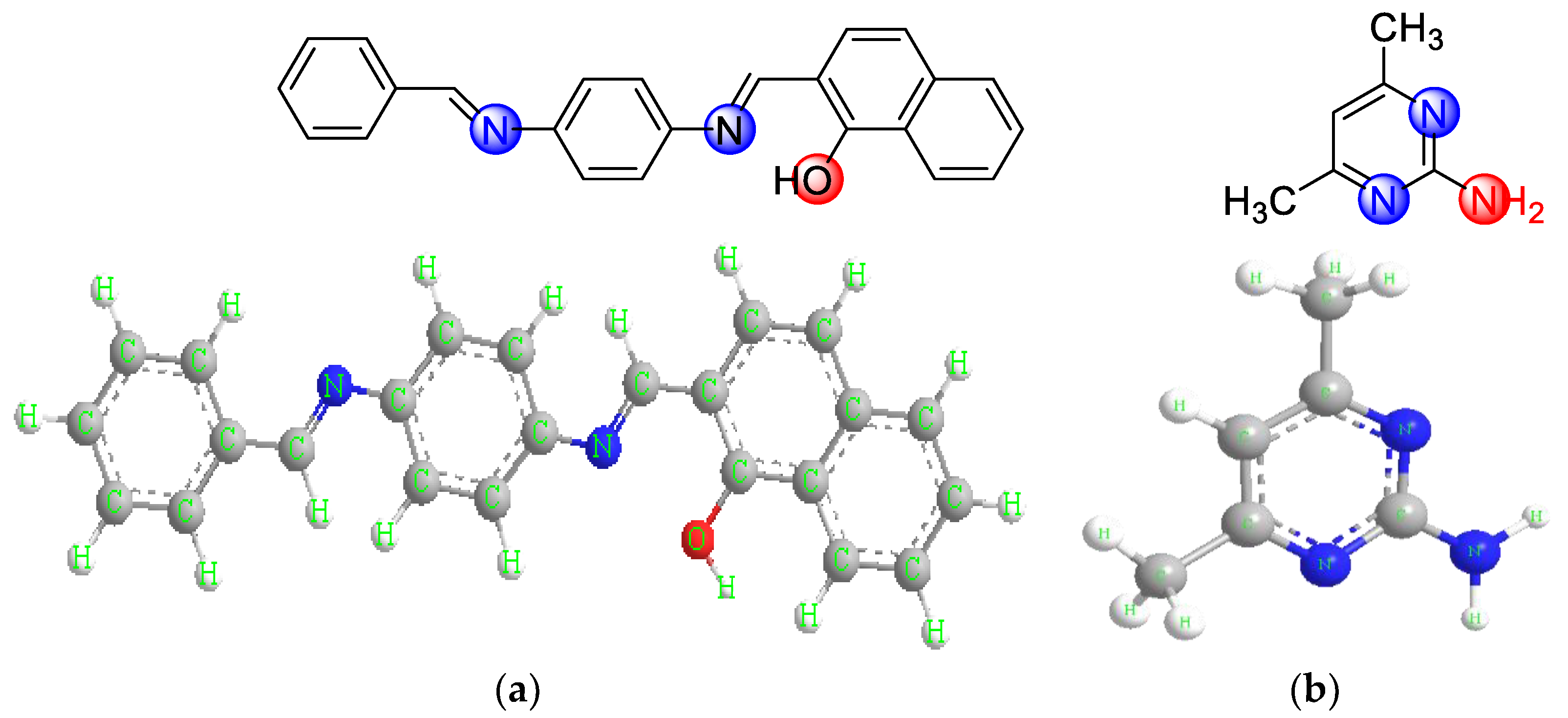







| Group | (HL) | (ADMPY) | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|---|
| υ(OH) | 3350 | - | 3410 | 3345 | 3438 | 3326 | 3354 |
| υ(OH) lattice water | - | - | - | 3547 | - | 3548 | - |
| υ(OH) coordinated water | - | - | 3330 | 3305 | 3314 | 3312 | 3302 |
| υas/s (NH2) | - | 3396, 3310 | Disappear | Disappear | Disappear | Disappear | Disappear |
| δ(NH2) | - | 1633 | 1680 | 1666 | 1680 | 1668 | 1698 |
| υ(C=N) | 1618, 1604 | - | 1616, 1548 | 1625, 1592 | 1617, 1550 | 1613, 1588 | 1610, 1575 |
| δ(OH) in-plane | 850 | - | 834 | 826 | 833 | 821 | 819 |
| υ(C=C) | 1540 | - | 1530 | 1509 | 1526 | 1538 | 1536 |
| υ(C-O) | 1310 | - | 1297 | 1316 | 1297 | 1309 | 1305 |
| υ(M-O) | - | - | 640 | 612 | 634 | 626 | 620 |
| υ(M-N) | - | - | 566 | 547 | 560 | 554 | 544 |
| υ(M-Cl) | - | - | 442 | 419 | 440 | 430 | 424 |
| Ligands and Complexes | Vmax (nm) | vmax (cm−1) | Assignment | μeff. (B.M) | Geometry | dn |
|---|---|---|---|---|---|---|
| (HL) | 259 | 38,610 | π→π∗ | - | - | - |
| (ADMPY) | 314 | 31,847 | n→π∗ | - | - | - |
| Mn(II) complex | 270 | 37,037 | π→π∗ | 5.84 | ||
| 319 | 31,347 | n→π∗ | Octahedral | d5 | ||
| 455 | 21,978 | d-d transition | ||||
| Co (II) complex | 252 | 39,682 | π→π∗ | 4.90 | ||
| 309 | 32,620 | n→π∗ | Octahedral | d7 | ||
| 426 | 23,474 | d-d transition | ||||
| Cu(II) complex | 261 | 38,314 | π→π∗ | 1.72 | ||
| 300 | 33,333 | n→π∗ | Octahedral | d9 | ||
| 415 | 24,096 | d-d transition | ||||
| Cr(III) complex | 295 | 33,898 | π→π∗ | 3.70 | ||
| 345 | 28,985 | n→π∗ | Octahedral | d3 | ||
| 419 | 23,866 | d-d transition | ||||
| Pd(II) complex | 308 | 32,468 | π→π∗ | Diamagnetic | ||
| 355 | 28,169 | n→π∗ | square planar | d8 | ||
| 454 | 22,026 | d-d transition |
| Compound | Step | Temp. Range °C | Assignment | TGA (Wt. loss %) Found (calcd.) |
|---|---|---|---|---|
| Mn(II) complex | 1st 2nd 3rd 4th | 80–140 142–200 200–302 304–550 | Loss of coordinated water molecules (H2O) Loss of two chloride atoms Loss of ligand (ADMPY) Decomposition the rest of the organic ligand (HL) final product (manganese oxide) | 2.59 (2.91) 11.30 (11.48) 19.72 (19.94) 55.86 (56.75) 10.53 (11.48) |
| Co(II) complex | 1st 2nd 3rd 4th | 78–150 152–248 250–396 398–550 | Loss two water molecules, one crystalline and one coordinated. Loss of two chloride atoms Loss of ligand (ADMPY) Decomposition with the formation of final product (cobalt oxide). | 5.40 (5.62) 10.98 (11.08) 19.10 (19.26) 53.11 (54.80) 15.70 (15.93) |
| Cu(II) complex | 1st 2nd 3rd 4th 5th | 83–154 156–270 272–362 364–420 422–550 | Loss of coordinated water molecules (H2O) Loss of two chloride atoms Loss of ligand (ADMPY) Loss of ligand (HL) formation of copper oxide. | 2.67 (2.87) 10.96 (11.33) 19.47 (19.68) 55.80 (56.01) 12.02 (12.71) |
| Cr(II) complex | 1st 2nd 3rd 4th | 81–158 160–221 223–353 355–550 | Loss two water molecules, one crystalline and one coordinated. Loss of two chloride atoms Loss of ligand (ADMPY) Decomposition with the formation of final product chromium oxide. | 5.13 (5.68) 10.98 (11.21) 19.14 (19.48) 54.87 (55.38) 10.56 (10.75) |
| Pd(II) complex | 1st 2nd 3rd | 82–160 162–254 256–550 | Loss of coordinated water molecules (H2O) Loss of two chloride atoms Thermal decomposition of the rest of the complex and forming palladium oxide. | 2.51 (2.69) 10.34 (10.60) 69.56 (70.83) 17.98 (18.30) |
| Parameters | Mn(II) Complex | Co(II) Complex | Cu(II) Complex | Cr(III) Complex | Pd(II) Complex |
|---|---|---|---|---|---|
| Empirical formula | C30H29N5O2MnCl2 | C30H31N5O3CoCl2 | C30H29N5O2CuCl2 | C30H31N5O3CrCl2 | C30H29N5O2PdCl2 |
| Formula weight | 617.42 | 639.42 | 625.72 | 632.16 | 668.60 |
| Crystal system | Monoclinic | Tetragonal | Triclinic | Triclinic | Tetragonal |
| a (Å) | 17.160 | 12.550 | 7.044 | 5.382 | 13.281 |
| b (Å) | 5.861 | 12.550 | 10.298 | 3.345 | 13.281 |
| c (Å) | 16.840 | 13.090 | 13.419 | 14.532 | 6.228 |
| Alfa (°) | 90.000 | 90.000 | 55.914 | 91.749 | 90.000 |
| Beta (°) | 101.310 | 90.000 | 71.226 | 26.757 | 90.000 |
| gamma (°) | 90.000 | 90.000 | 50.082 | 89.817 | 90.000 |
| Volume of unit cell (Å3) Particle Size (nm) | 1661 72 | 2064 67 | 617.34 38 | 117.58 32 | 1098.6 88 |
| No. | Compounds | a A-549 | b MRC-5 | ||
|---|---|---|---|---|---|
| c IC50 Value (µg/mL) | ±S.D | d CC50 Value (µg/mL) | ±S.D | ||
| 1 | cisplatin | 7.11 | 0.39 | 10.51 | 0.37 |
| 2 | Mn(II) complex | 201.86 | 11.48 | 233.01 | 10.74 |
| 3 | Co(II) complex | 29.94 | 1.14 | 101.72 | 8.26 |
| 4 | Cu(II) complex | 10.97 | 0.67 | 15.41 | 4 |
| 5 | Cr(III) complex | 61.46 | 0.21 | 157.65 | 11.28 |
| 6 | Pd(II) complex | 26.54 | 2.08 | 53 | 6.09 |
| Mean Zone of Inhibition in mm for Three Replicates | ||||||||
|---|---|---|---|---|---|---|---|---|
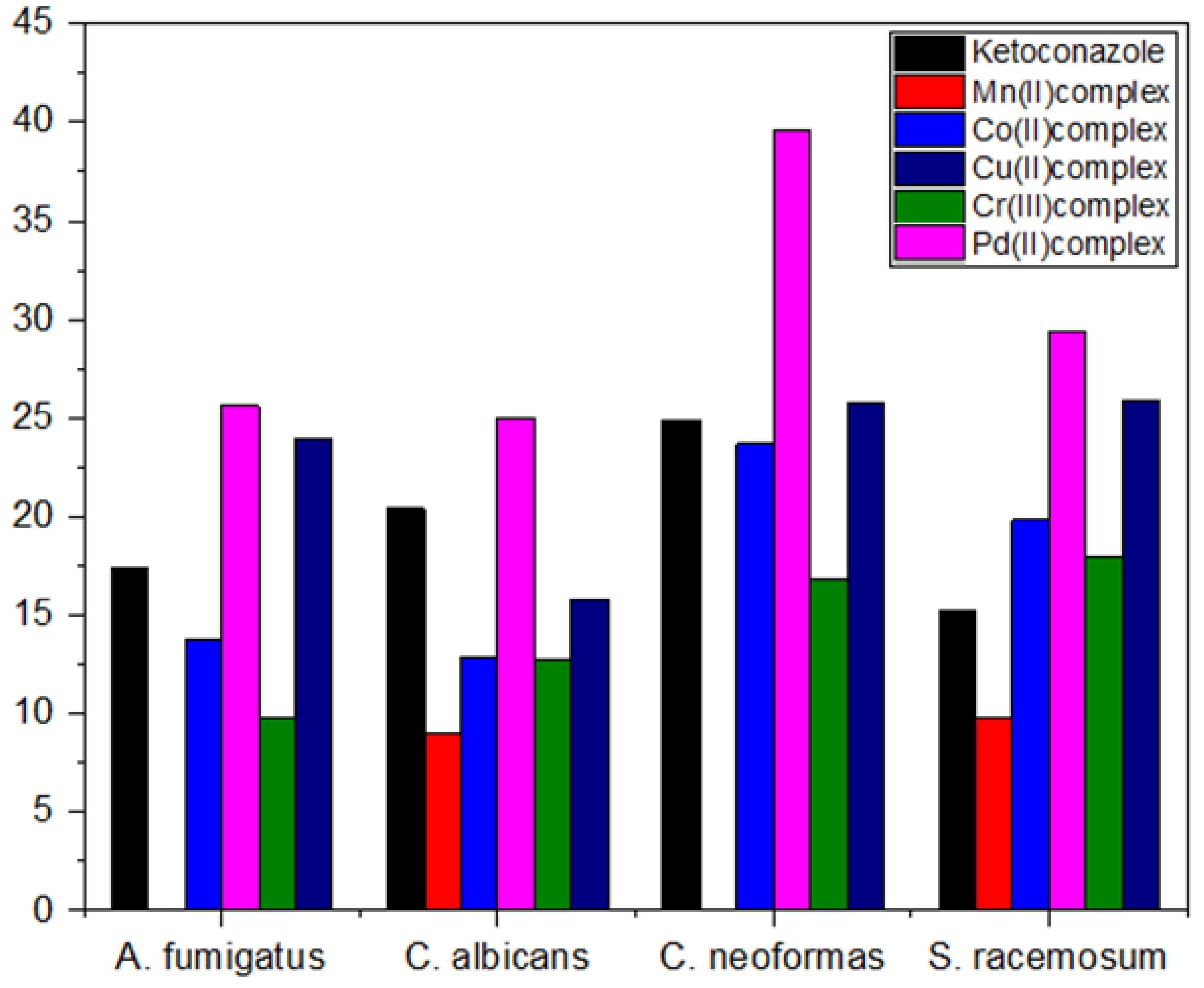
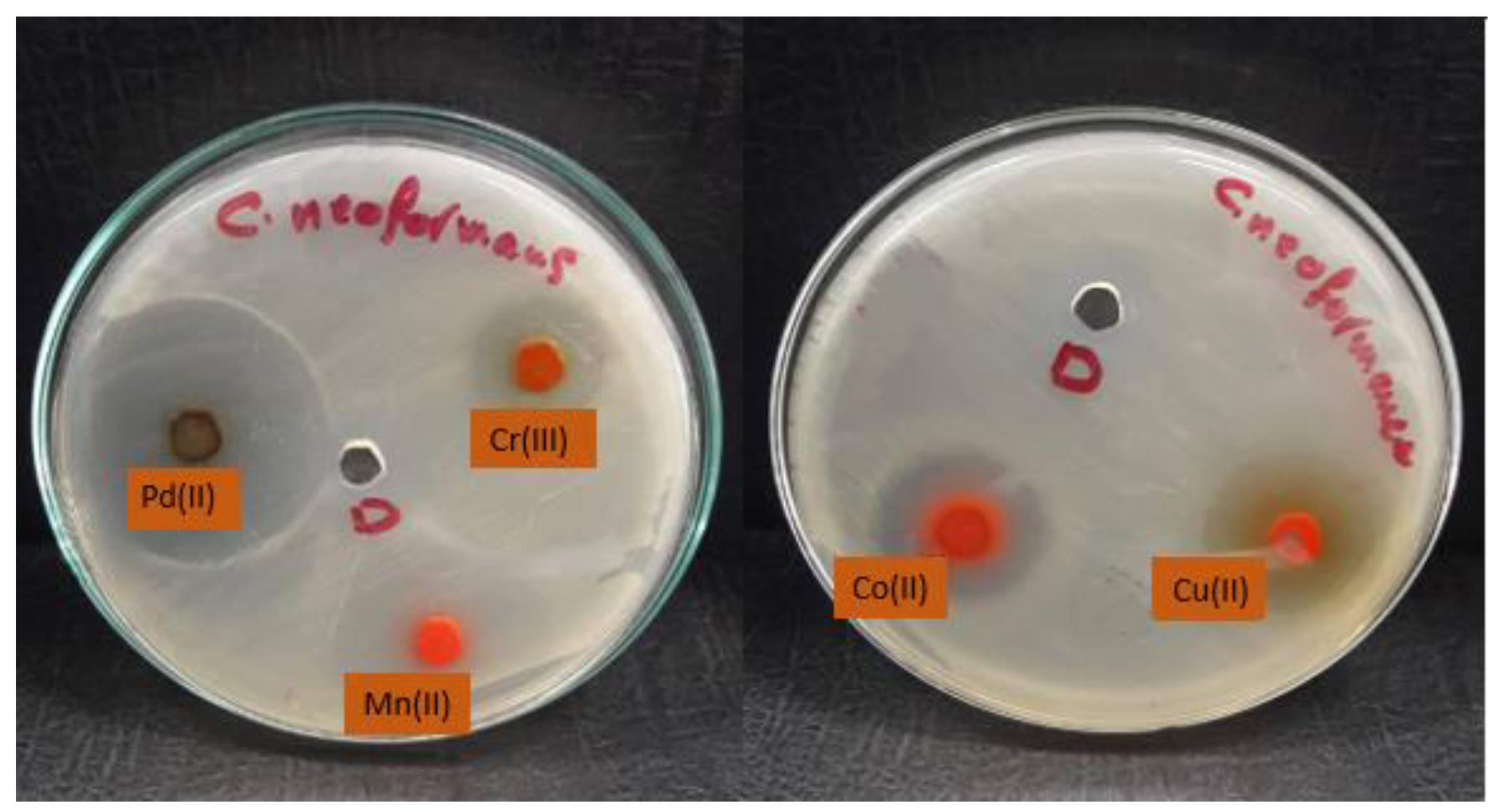
| Compounds | Aspergillus fumigatus | Candida albicans | Cryptococcus neoformas | Syncephalastrum racemosum | ||||
| 1 Ketoconazole | 17.37 | ±0.55 | 20.40 | ±0.82 | 24.87 | ±0.31 | 15.27 | ±0.57 |
| Mn(II) complex | 2 NA | - | 8.97 | ± 0.21 | NA2 | - | 9.80 | ±0.36 |
| Co(II) complex | 13.73 | ±0.32 | 12.90 | ±0.26 | 23.67 | ±0.47 | 19.87 | ±0.38 |
| Cu(II) complex | 23.97 | ±0.47 | 15.80 | ±0.36 | 25.73 | ±0.40 | 25.87 | ±0.21 |
| Cr(III) complex | 9.83 | ±0.32 | 12.77 | ±0.40 | 16.83 | ±0.31 | 17.90 | ±0.30 |
| Pd(II) complex | 25.63 | ±0.49 | 25 | ±0.46 | 39.57 | ±0.57 | 29.40 | ±0.44 |
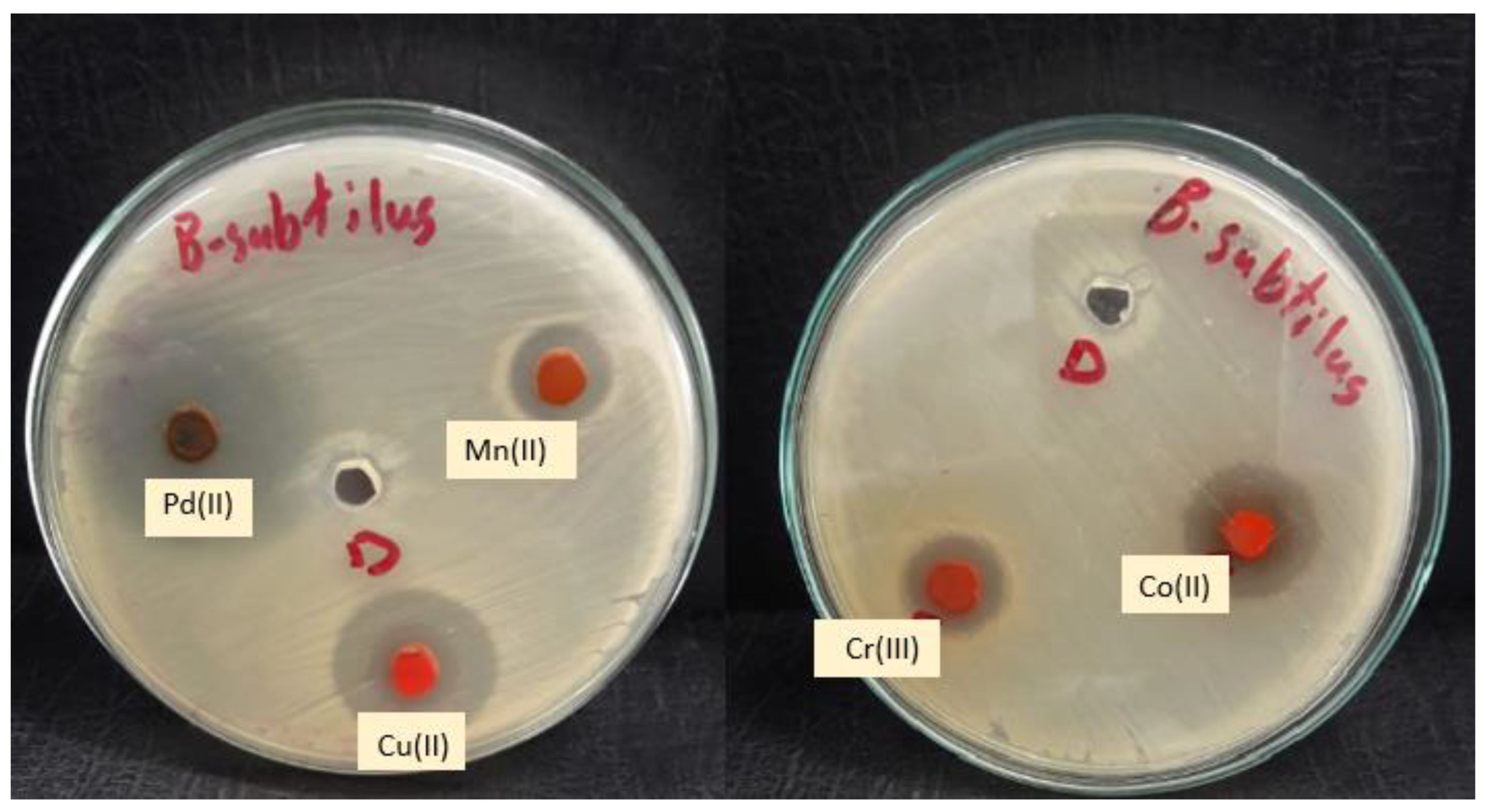
| Mean Zone of Inhibition in mm for Three Replicates | ||||||||
|---|---|---|---|---|---|---|---|---|
| (G+) Gram-Positive Bacteria (G−) Gram-Negative Bacteria | ||||||||
| Compounds | Staphylococcus aureus | Bacillus subtilis | Escherichia coli | Proteus vulgaris | ||||
| 3 Gentamycin | 24.33 | ±0.15 | 25.93 | ±0.32 | 29.83 | ±0.32 | 25.20 | ±0.26 |
| Mn(II) complex | 8.87 | ±0.42 | 10.73 | ±0.42 | 4 NA | - | 10.85 | ±0.35 |
| Co(II) complex | 12.83 | ±0.42 | 15.77 | ±0.42 | 11.93 | ±0.35 | 17.60 | ±0.62 |
| Cu(II) complex | 13 | ±0.46 | 18.70 | ±0.44 | 13.90 | ±0.26 | 21.20 | ±0.40 |
| Cr(III) complex | 10.87 | ±0.31 | 12 | ±0.40 | 10.80 | ±0.52 | 15.93 | ±0.55 |
| Pd(II) complex | 14.10 | ±0.26 | 29.60 | ±0.52 | 17.67 | ±0.60 | 21.83 | ±0.42 |
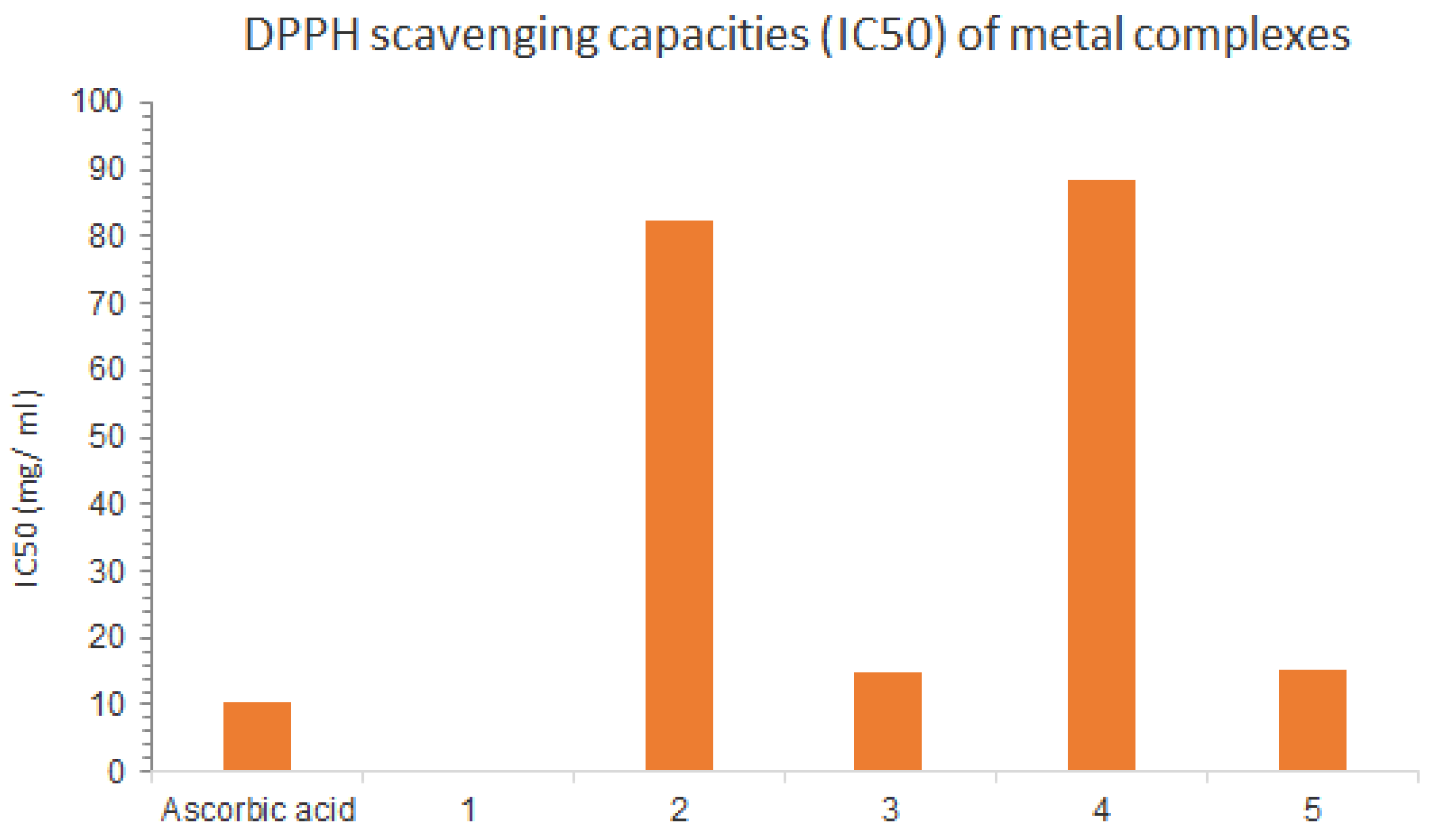
| Compounds | IC50 (mg/mL) |
|---|---|
| Ascorbic acid | 10.21 ± 0.77 |
| Mn(II) complex | weak antioxidant activity |
| Co(II) complex | 82.43 ± 1.85 |
| Cu(II) complex | 13.98 ± 0.52 |
| Cr(III) complex | 87.76 ± 5.11 |
| Pd(II) complex | 15.03 ± 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Fakeh, M.S.; Alsikhan, M.A.; Alnawmasi, J.S. Physico-Chemical Study of Mn(II), Co(II), Cu(II), Cr(III), and Pd(II) Complexes with Schiff-Base and Aminopyrimidyl Derivatives and Anti-Cancer, Antioxidant, Antimicrobial Applications. Molecules 2023, 28, 2555. https://doi.org/10.3390/molecules28062555
Al-Fakeh MS, Alsikhan MA, Alnawmasi JS. Physico-Chemical Study of Mn(II), Co(II), Cu(II), Cr(III), and Pd(II) Complexes with Schiff-Base and Aminopyrimidyl Derivatives and Anti-Cancer, Antioxidant, Antimicrobial Applications. Molecules. 2023; 28(6):2555. https://doi.org/10.3390/molecules28062555
Chicago/Turabian StyleAl-Fakeh, Maged S., Maha A. Alsikhan, and Jawza Sh Alnawmasi. 2023. "Physico-Chemical Study of Mn(II), Co(II), Cu(II), Cr(III), and Pd(II) Complexes with Schiff-Base and Aminopyrimidyl Derivatives and Anti-Cancer, Antioxidant, Antimicrobial Applications" Molecules 28, no. 6: 2555. https://doi.org/10.3390/molecules28062555
APA StyleAl-Fakeh, M. S., Alsikhan, M. A., & Alnawmasi, J. S. (2023). Physico-Chemical Study of Mn(II), Co(II), Cu(II), Cr(III), and Pd(II) Complexes with Schiff-Base and Aminopyrimidyl Derivatives and Anti-Cancer, Antioxidant, Antimicrobial Applications. Molecules, 28(6), 2555. https://doi.org/10.3390/molecules28062555






