Ultrasound-Assisted Extraction of Protein from Moringa oleifera Seeds and Its Impact on Techno-Functional Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of Ultrasonic-Assisted Extraction (UAE) of Protein
2.1.1. Fitting the Proposed Model
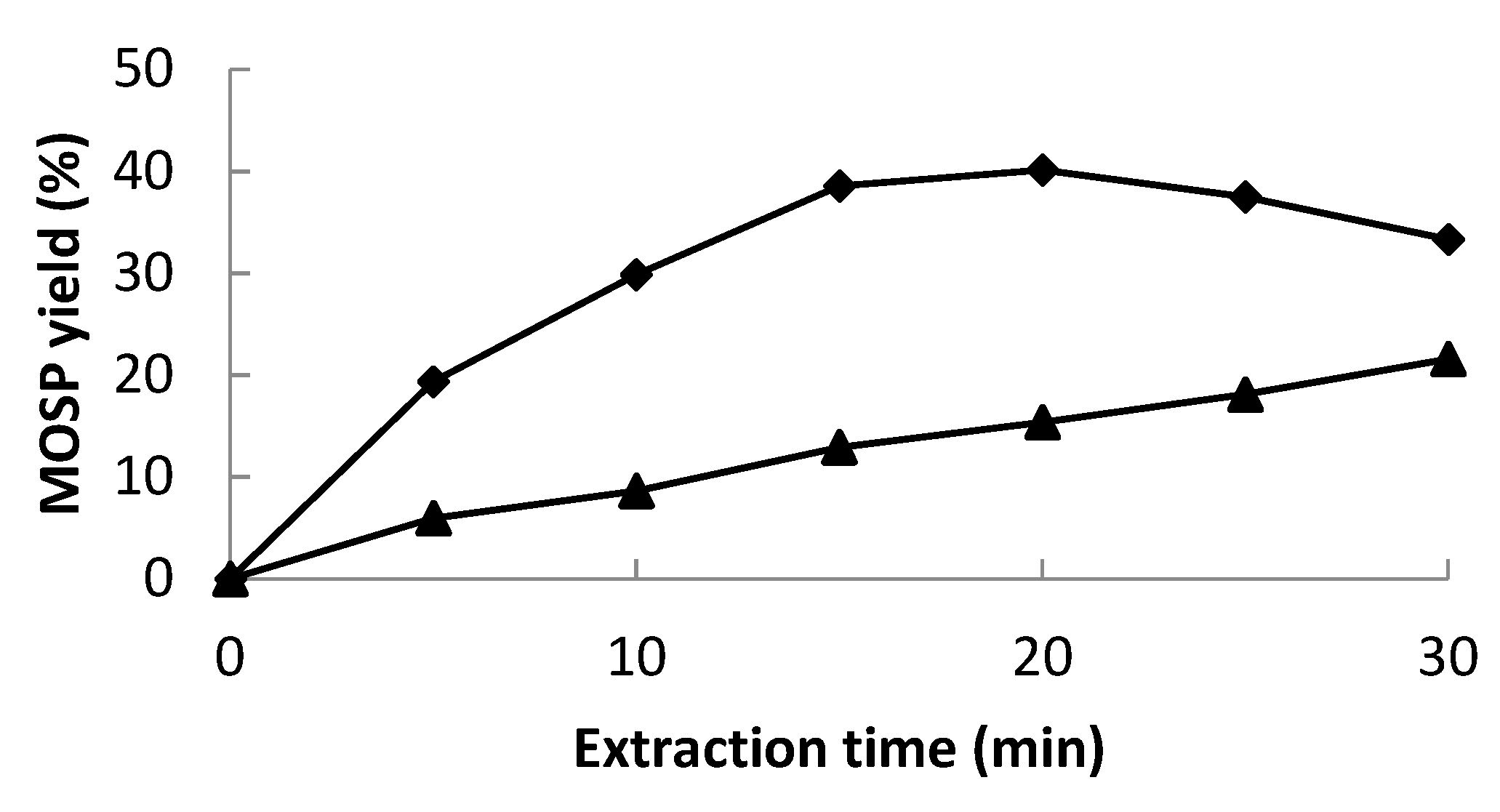
2.1.2. Single-Factor Analysis for Protein Yield
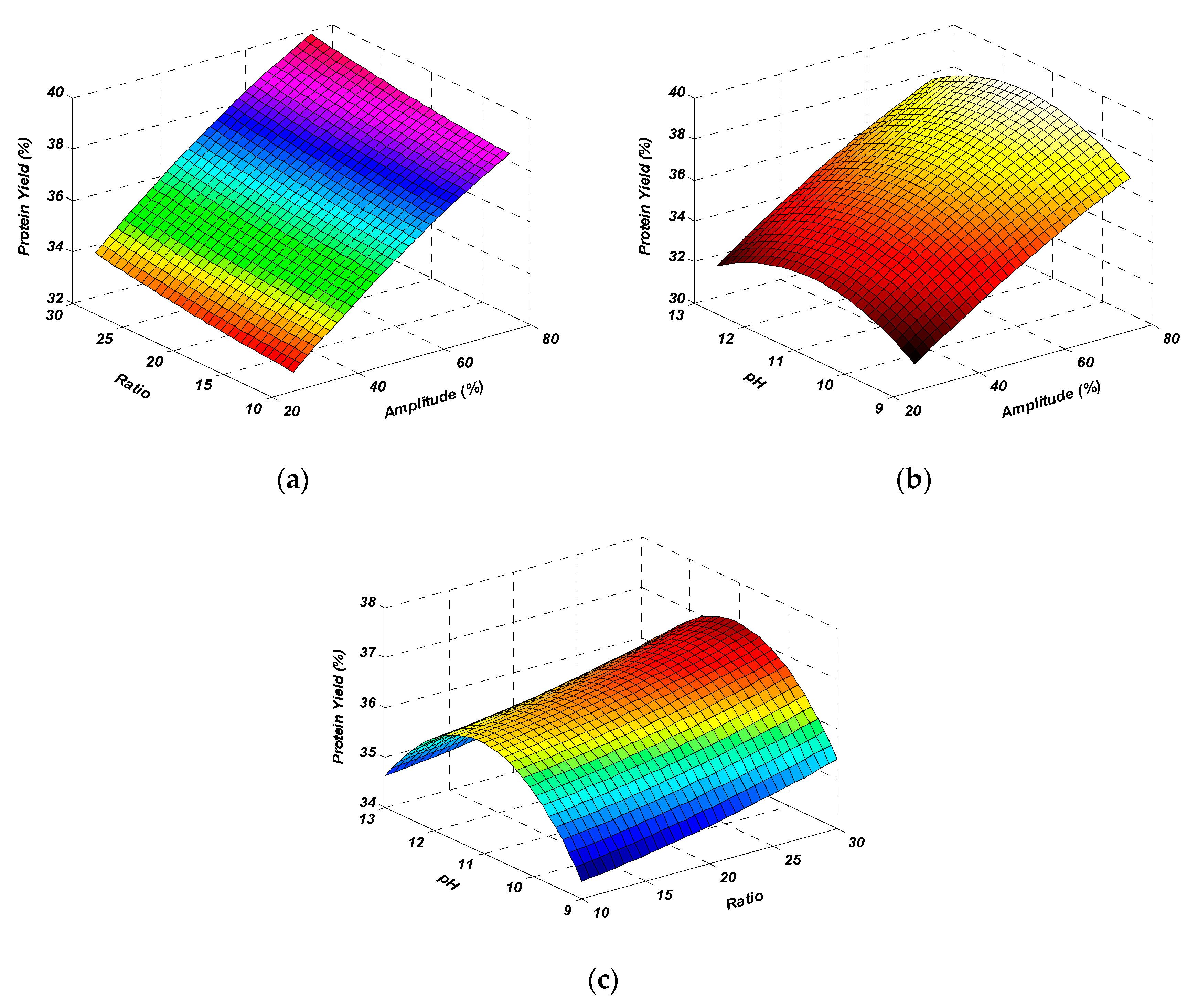
2.1.3. Effect of Mutual Interactions on Protein Yield
2.1.4. Optimization and Validation
2.2. Functional Properties of MOSP
2.2.1. Solubility
2.2.2. Water (WHC)- and Oil-Holding Capacity (OHC)
2.2.3. Emulsion Capacity and Emulsion Stability
2.2.4. Foaming Capacity and Foaming Stability
2.3. Structural Study of MOSP
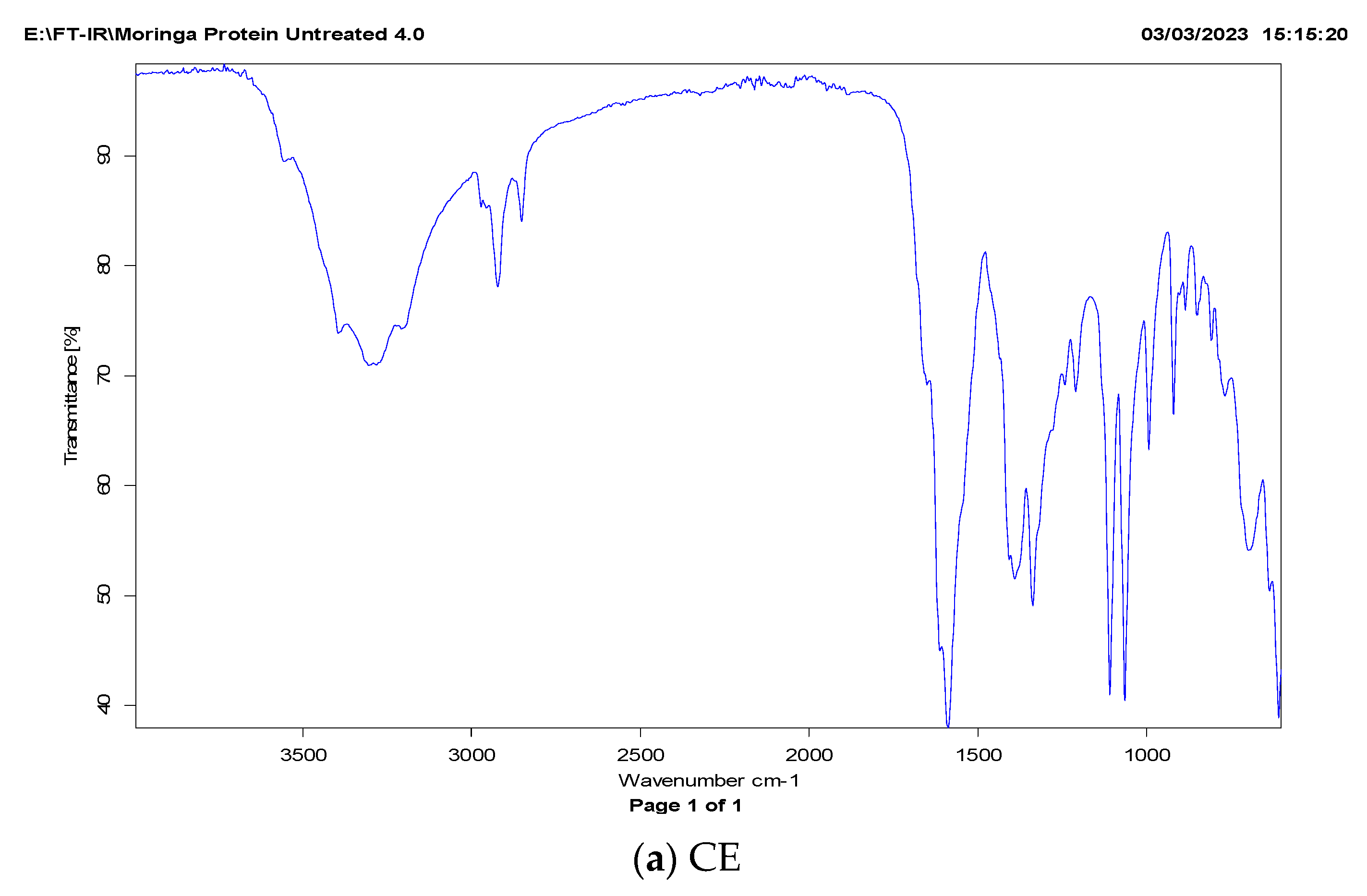
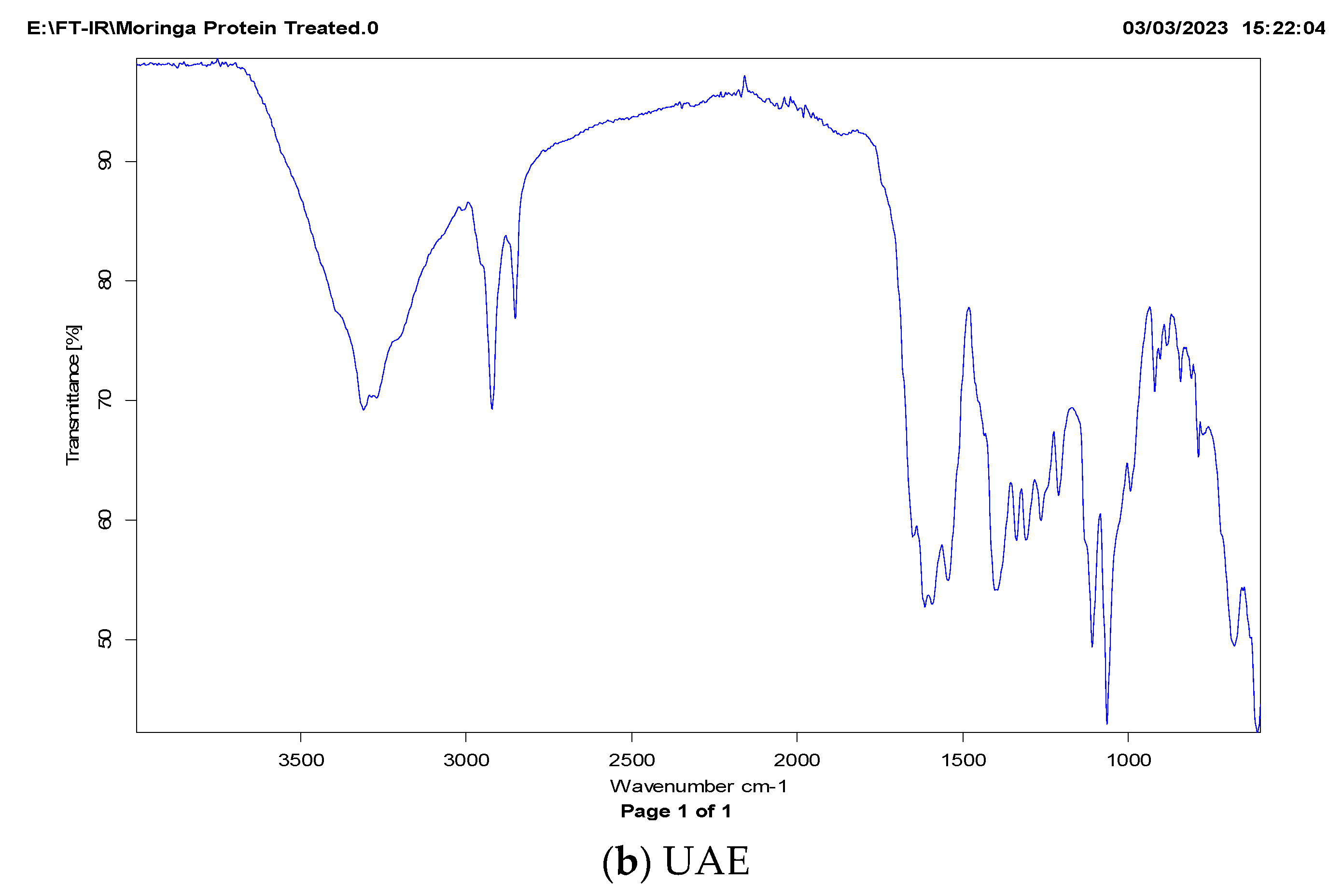
2.3.1. FT-IR Analysis
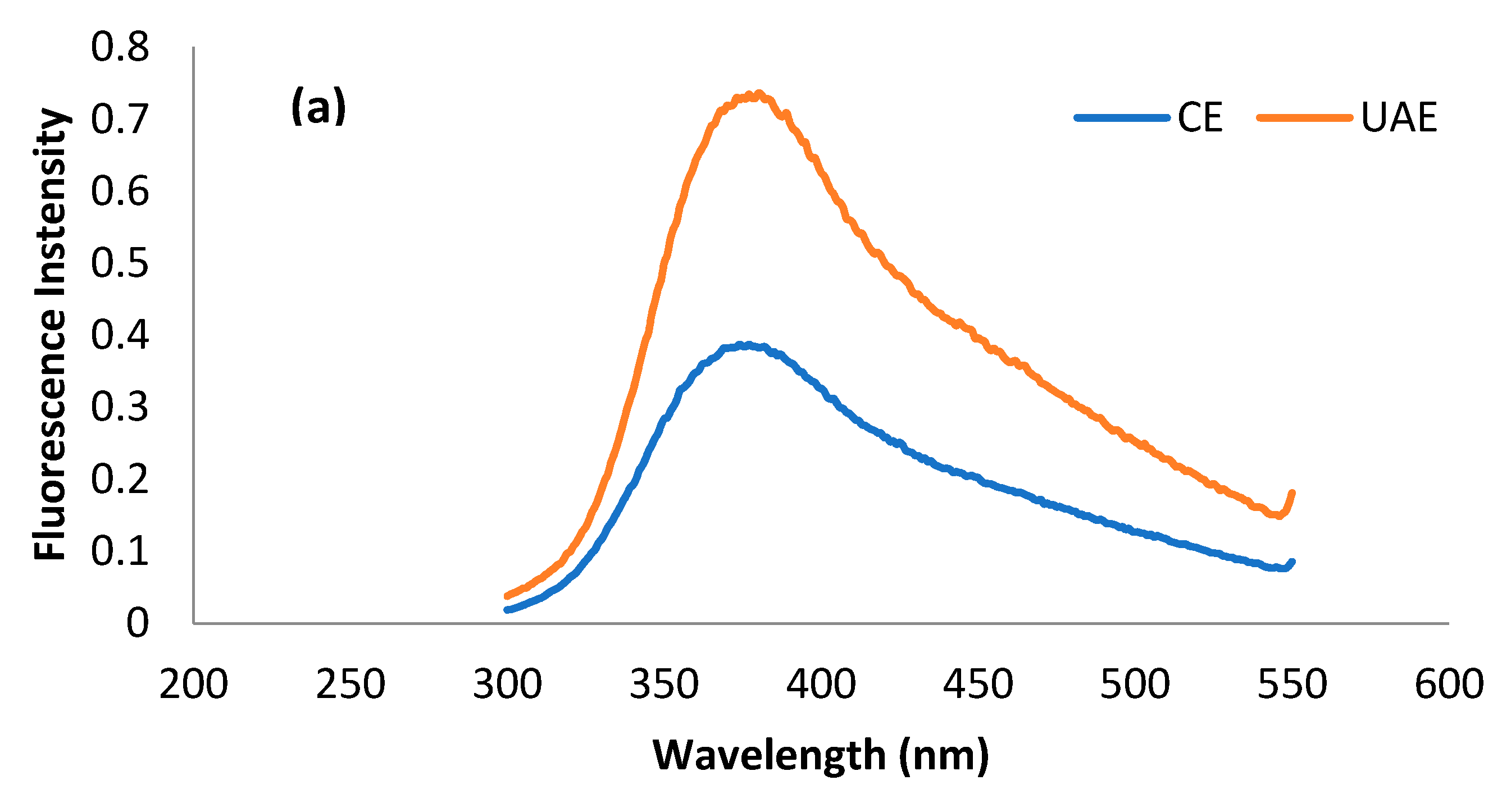
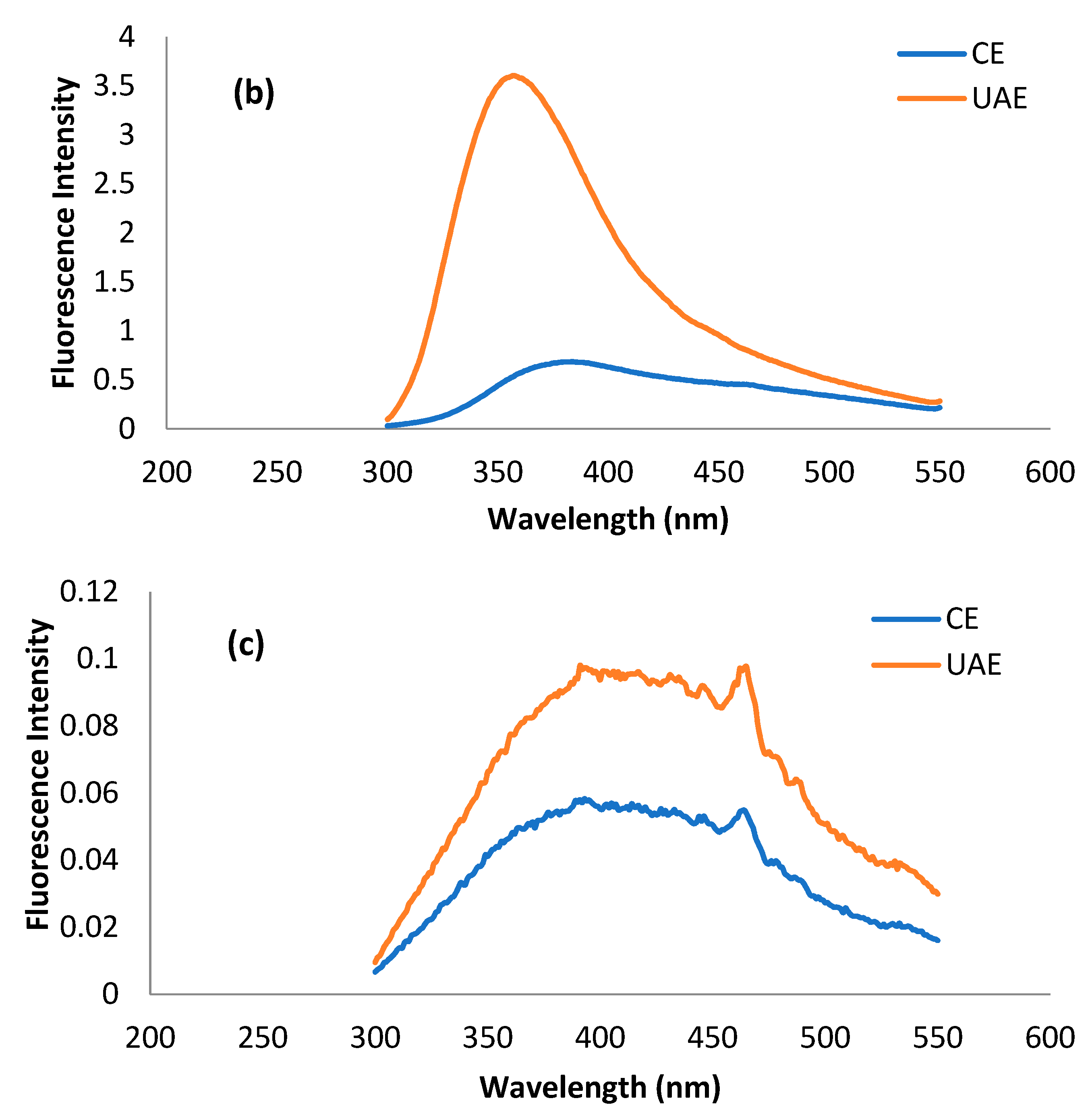
2.3.2. Intrinsic Fluorescence Patterns
3. Material and Methods
3.1. Raw Materials and Chemicals
3.2. Ultrasonic-Assisted Extraction (UAE) of Seed Protein
3.3. Protein Quantification by Bradford Method
3.4. Isolation of Seed Protein
3.5. Functional Properties of M. oleifera Seed Protein (MOSP)
3.5.1. Solubility
3.5.2. Water- and Oil-holding Capacity
3.5.3. Emulsion Capacity and Emulsion Stability
3.5.4. Foaming Capacity and Stability
3.6. Fourier-Transform Infrared (FT-IR) Spectroscopy
3.7. Fluorescence Spectroscopy
3.8. Experimental Design and Statistical Analysis
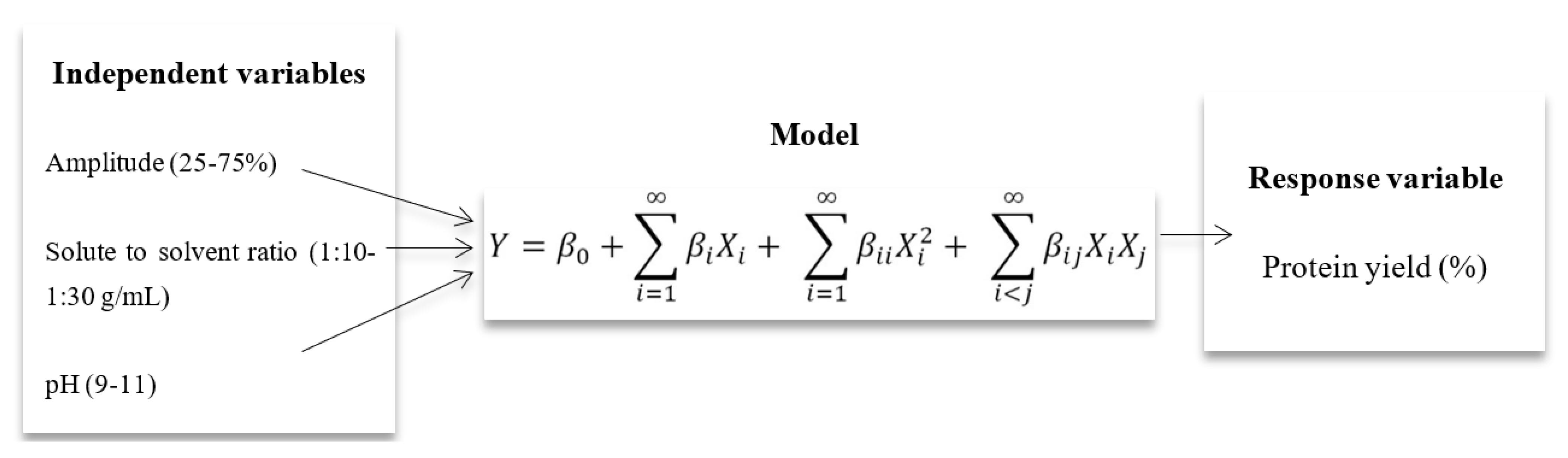 where Y denotes the predicted value of the response variable; β0 denotes the intercepts; and βi, βii, and βij are the linear, second order, and interaction regression coefficients predictable by the model, respectively. Xi and Xj are the values of studied or independent variables.
where Y denotes the predicted value of the response variable; β0 denotes the intercepts; and βi, βii, and βij are the linear, second order, and interaction regression coefficients predictable by the model, respectively. Xi and Xj are the values of studied or independent variables.4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kristo, E.; Corredig, M. Functional properties of food proteins. In Applied Food Protein Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 47–73. [Google Scholar]
- Mune, M.A.M.; Bassogog, C.B.B.; Nyobe, E.C.; Minka, S.R.R. Physicochemical and functional properties of moringa oleifera seed and leaf flour. Cogent Food Agric. 2016, 2, 1220352. [Google Scholar] [CrossRef]
- Sujatha, B.; Patel, P. Moringa oleifera–nature’s gold. Imp. J. Interdiscip. Res. 2017, 3, 1175–1179. [Google Scholar]
- Abbas, R.; Elsharbasy, F.; Fadlelmula, A. Nutritional values of moringa oleifera, total protein, Amino Acid Vitamins Minerals, Carbohydrates, Total Fat and Crude Fiber, under the Semi-Arid Conditions of Sudan. J. Microb. Biochem. Technol. 2018, 10, 56–58. [Google Scholar]
- Chumark, P.; Khunawat, P.; Sanvarinda, Y.; Phornchirasilp, S.; Morales, N.P.; Phivthong-ngam, L.; Ratanachamnong, P.; Srisawat, S.; Pongrapeeporn, K.-u.S. The in vitro and ex vivo antioxidant properties, hypolipidaemic and antiatherosclerotic activities of water extract of moringa oleifera lam. Leaves. J. Ethnopharmacol. 2008, 116, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Jahan, S.; Shahjahan, M.; Rasna, S.S.; Aktar, M.; Sultana, S.; Ahmed, S.M.; Sabrin, F.; Nahar, S. Antibacterial effect of moringa (moringa oleifera) leaf ethanolic extract against staphylococcus aureus and escherichia coli. Mymensingh Med. J. 2022, 31, 976–982. [Google Scholar] [PubMed]
- Saleem, A.; Saleem, M.; Akhtar, M.F. Antioxidant, anti-inflammatory and antiarthritic potential of moringa oleifera lam: An ethnomedicinal plant of moringaceae family. S. Afr. J. Bot. 2020, 128, 246–256. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; Albalawi, S.M.; Athar, M.T.; Khan, A.Q.; Al-Shahrani, H.; Islam, M. Moringa oleifera as an anti-cancer agent against breast and colorectal cancer cell lines. PLoS ONE 2015, 10, e0135814. [Google Scholar] [CrossRef]
- Buddin, M.; Rithuan, M.A.; Surni, M.; Jamal, N.M.; Faiznur, M. Ultrasonic assisted extraction (uae) of moringa oleifera seed oil: Kinetic study. ASM Sci. J. 2018, 11, 158–166. [Google Scholar]
- El-Hack, A.; Mohamed, E.; Alagawany, M.; Elrys, A.S.; Desoky, E.-S.M.; Tolba, H.; Elnahal, A.S.; Elnesr, S.S.; Swelum, A.A. Effect of forage moringa oleifera l.(moringa) on animal health and nutrition and its beneficial applications in soil, plants and water purification. Agriculture 2018, 8, 145. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, X.-Y.; Liu, L.-N.; Xie, Y.-P.; Ke, Y.-J.; Cai, Z.-J.; Wu, G.-J. Ultrasonic-assisted extraction and functional properties of wampee seed protein. Food Sci. Technol. 2019, 39, 324–331. [Google Scholar] [CrossRef]
- Phongthai, S.; Lim, S.T.; Rawdkuen, S. Ultrasonic-assisted extraction of rice bran protein using response surface methodology. J. Food Biochem. 2017, 41, e12314. [Google Scholar] [CrossRef]
- Chemat, F.; Abert Vian, M.; Fabiano-Tixier, A.-S.; Nutrizio, M.; Režek Jambrak, A.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chemistry 2020, 22, 2325–2353. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochemistry 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Yolmeh, M.; Jafari, S.M. Applications of response surface methodology in the food industry processes. Food Bioprocess Technol. 2017, 10, 413–433. [Google Scholar] [CrossRef]
- Liu, Z.D.; Guo, B.H.; Su, M.Y.; Wang, Y.Y. Effect of ultrasonic treatment on the functional properties of whey protein isolates. Adv. Mater. Res. 2012, 443, 660–665. [Google Scholar] [CrossRef]
- Aguilar-Acosta, L.A.; Serna-Saldivar, S.O.; Rodríguez-Rodríguez, J.; Escalante-Aburto, A.; Chuck-Hernández, C. Effect of ultrasound application on protein yield and fate of alkaloids during lupin alkaline extraction process. Biomolecules 2020, 10, 292. [Google Scholar] [CrossRef]
- Elhag, H.E.E.A.; Naila, A.; Nour, A.H.; Ajit, A.; Sulaiman, A.Z.; Abd Aziz, B. Optimization of protein yields by ultrasound assisted extraction from eurycoma longifolia roots and effect of agitation speed. J. King Saud Univ. -Sci. 2019, 31, 913–930. [Google Scholar] [CrossRef]
- Zhao, Y.; Wen, C.; Feng, Y.; Zhang, J.; He, Y.; Duan, Y.; Zhang, H.; Ma, H. Effects of ultrasound-assisted extraction on the structural, functional and antioxidant properties of dolichos lablab l. Protein. Process Biochem. 2021, 101, 274–284. [Google Scholar] [CrossRef]
- Rahman, M.M.; Lamsal, B.P. Ultrasound-assisted extraction and modification of plant-based proteins: Impact on physicochemical, functional, and nutritional properties. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1457–1480. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Xu, L.; Ma, H. An efficient ultrasound-assisted extraction method of pea protein and its effect on protein functional properties and biological activities. LWT 2020, 127, 109348. [Google Scholar] [CrossRef]
- Gadalkar, S.M.; Rathod, V.K. Extraction of watermelon seed proteins with enhanced functional properties using ultrasound. Prep. Biochem. Biotechnol. 2020, 50, 133–140. [Google Scholar] [CrossRef]
- Dabbour, M.; He, R.; Ma, H.; Musa, A. Optimization of ultrasound assisted extraction of protein from sunflower meal and its physicochemical and functional properties. J. Food Process Eng. 2018, 41, e12799. [Google Scholar] [CrossRef]
- Ly, H.L.; Tran, T.M.C.; Tran, T.T.T.; Ton, N.M.N.; Le, V.V.M. Application of ultrasound to protein extraction from defatted rice bran. Int. Food Res. J. 2018, 25, 695–701. [Google Scholar]
- Yucetepe, A.; Saroglu, O.; Bildik, F.; Ozcelik, B.; Daskaya-Dikmen, C. Optimisation of ultrasound-assisted extraction of protein from spirulina platensis using rsm. Czech J. Food Sci. 2018, 36, 98–108. [Google Scholar] [CrossRef]
- Kramer, R.M.; Shende, V.R.; Motl, N.; Pace, C.N.; Scholtz, J.M. Toward a molecular understanding of protein solubility: Increased negative surface charge correlates with increased solubility. BpJ 2012, 102, 1907–1915. [Google Scholar] [CrossRef]
- Tang, S.-Q.; Du, Q.-H.; Fu, Z. Ultrasonic treatment on physicochemical properties of water-soluble protein from moringa oleifera seed. Ultrason. Sonochemistry 2021, 71, 105357. [Google Scholar] [CrossRef]
- Jambrak, A.R.; Mason, T.J.; Lelas, V.; Herceg, Z.; Herceg, I.L. Effect of ultrasound treatment on solubility and foaming properties of whey protein suspensions. J. Food Eng. 2008, 86, 281–287. [Google Scholar] [CrossRef]
- Jiang, S.; Ding, J.; Andrade, J.; Rababah, T.M.; Almajwal, A.; Abulmeaty, M.M.; Feng, H. Modifying the physicochemical properties of pea protein by ph-shifting and ultrasound combined treatments. Ultrason. Sonochemistry 2017, 38, 835–842. [Google Scholar] [CrossRef]
- Ding, Q.; Zhang, T.; Niu, S.; Cao, F.; Wu-Chen, R.A.; Luo, L.; Ma, H. Impact of ultrasound pretreatment on hydrolysate and digestion products of grape seed protein. Ultrason. Sonochemistry 2018, 42, 704–713. [Google Scholar] [CrossRef]
- Malik, M.A.; Sharma, H.K.; Saini, C.S. High intensity ultrasound treatment of protein isolate extracted from dephenolized sunflower meal: Effect on physicochemical and functional properties. Ultrason. Sonochemistry 2017, 39, 511–519. [Google Scholar] [CrossRef]
- Zhao, C.-B.; Zhou, L.-Y.; Liu, J.-Y.; Zhang, Y.; Chen, Y.; Wu, F. Effect of ultrasonic pretreatment on physicochemical characteristics and rheological properties of soy protein/sugar maillard reaction products. J. Food Sci. Technol. 2016, 53, 2342–2351. [Google Scholar] [CrossRef]
- Zou, Y.; Li, P.; Zhang, K.; Wang, L.; Zhang, M.; Sun, Z.; Sun, C.; Geng, Z.; Xu, W.; Wang, D. Effects of ultrasound-assisted alkaline extraction on the physiochemical and functional characteristics of chicken liver protein isolate. Poult. Sci. 2017, 96, 2975–2985. [Google Scholar] [CrossRef]
- Zayas, J.F. Water holding capacity of proteins. In Functionality of Proteins in Food; Springer: Berlin/Heidelberg, Germany, 1997; pp. 76–133. [Google Scholar]
- Knorr, D.; Zenker, M.; Heinz, V.; Lee, D.-U. Applications and potential of ultrasonics in food processing. Trends Food Sci. Technol. 2004, 15, 261–266. [Google Scholar] [CrossRef]
- Barekat, S.; Soltanizadeh, N. Application of high-intensity ultrasonic radiation coupled with papain treatment to modify functional properties of beef longissimus lumborum. J. Food Sci. Technol. 2019, 56, 224–232. [Google Scholar] [CrossRef]
- Biswas, B.; Sit, N. Effect of ultrasonication on functional properties of tamarind seed protein isolates. J. Food Sci. Technol. 2020, 57, 2070–2078. [Google Scholar] [CrossRef]
- Haque, M.A.; Timilsena, Y.P.; Adhikari, B. Food proteins, structure, and function. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–8. [Google Scholar]
- Pearce, K.N.; Kinsella, J.E. Emulsifying properties of proteins: Evaluation of a turbidimetric technique. J. Agric. Food Chem. 1978, 26, 716–723. [Google Scholar] [CrossRef]
- Omura, M.H.; de Oliveira, A.P.H.; de Souza Soares, L.; dos Reis Coimbra, J.S.; de Barros, F.A.R.; Vidigal, M.C.T.R.; Baracat-Pereira, M.C.; de Oliveira, E.B. Effects of protein concentration during ultrasonic processing on physicochemical properties and techno-functionality of plant food proteins. Food Hydrocoll. 2021, 113, 106457. [Google Scholar] [CrossRef]
- Li, C.; Yang, F.; Huang, Y.; Huang, C.; Zhang, K.; Yan, L. Comparison of hydrodynamic and ultrasonic cavitation effects on soy protein isolate functionality. J. Food Eng. 2020, 265, 109697. [Google Scholar]
- Khatkar, A.B.; Kaur, A.; Khatkar, S.K.; Mehta, N. Optimization of processing time, amplitude and concentration for ultrasound-assisted modification of whey protein using response surface methodology. J. Food Sci. Technol. 2018, 55, 2298–2309. [Google Scholar] [CrossRef]
- O’sullivan, J.; Murray, B.; Flynn, C.; Norton, I. The effect of ultrasound treatment on the structural, physical and emulsifying properties of animal and vegetable proteins. Food Hydrocoll. 2016, 53, 141–154. [Google Scholar] [CrossRef]
- Mauer, L. Protein | heat treatment for food proteins. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 4868–4872. [Google Scholar]
- Alavi, F.; Chen, L.; Emam-Djomeh, Z. Effect of ultrasound-assisted alkaline treatment on functional property modifications of faba bean protein. Food Chem. 2021, 354, 129494. [Google Scholar] [CrossRef]
- Tan, M.C.; Chin, N.L.; Yusof, Y.A.; Abdullah, J. Effect of high power ultrasonic treatment on whey protein foaming quality. Int. J. Food Sci. Technol. 2016, 51, 617–624. [Google Scholar] [CrossRef]
- Arzeni, C.; Martínez, K.; Zema, P.; Arias, A.; Pérez, O.; Pilosof, A. Comparative study of high intensity ultrasound effects on food proteins functionality. J. Food Eng. 2012, 108, 463–472. [Google Scholar] [CrossRef]
- Tabtabaei, S.; Hijar, B.; Chen, B.K.; Diosady, L.L. Functional properties of protein isolates produced by aqueous extraction of de-hulled yellow mustard. J. Am. Oil Chem. Soc. 2017, 94, 149–160. [Google Scholar] [CrossRef]
- Xiong, T.; Xiong, W.; Ge, M.; Xia, J.; Li, B.; Chen, Y. Effect of high intensity ultrasound on structure and foaming properties of pea protein isolate. Food Res. Int. 2018, 109, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Nham Tran, T.L.; Miranda, A.F.; Mouradov, A.; Adhikari, B. Physicochemical characteristics of protein isolated from thraustochytrid oilcake. Foods 2020, 9, 779. [Google Scholar] [CrossRef]
- Mir, N.A.; Riar, C.S.; Singh, S. Structural modification of quinoa seed protein isolates (qpis) by variable time sonification for improving its physicochemical and functional characteristics. Ultrason. Sonochemistry 2019, 58, 104700. [Google Scholar] [CrossRef]
- Du, Q.-H.; Wu, Y.-H.; Tang, S.-Q.; Ren, M.-H.; Fu, Z. Influences of ultrasonic treatment on structure and functional properties of salt-soluble protein from moringa oleifera seeds. Int. J. Food Sci. Technol. 2021, 56, 5871–5880. [Google Scholar] [CrossRef]
- Aderinola, T.A.; Fagbemi, T.N.; Enujiugha, V.N.; Alashi, A.M.; Aluko, R.E. Amino acid composition and antioxidant properties of moringa oleifera seed protein isolate and enzymatic hydrolysates. Heliyon 2018, 4, e00877. [Google Scholar] [CrossRef]
- Cattan, Y.a.; Patil, D.; Vaknin, Y.; Rytwo, G.; Lakemond, C.; Benjamin, O. Characterization of moringa oleifera leaf and seed protein extract functionality in emulsion model system. Innov. Food Sci. Emerg. Technol. 2022, 75, 102903. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, W.; Yi, J.; Liu, N.; Cao, Y.; Lu, J.; Decker, E.A.; McClements, D.J. Effects of sonication on the physicochemical and functional properties of walnut protein isolate. Food Res. Int. 2018, 106, 853–861. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Z.; Han, D.; Li, Y.; Sun, X.; Wang, Z.; Jin, H. Structural and functional properties changes of β-conglycinin exposed to hydroxyl radical-generating systems. Molecules 2017, 22, 1893. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, L.; Bailina, Y.; Ge, Z.; Ding, T.; Ye, X.; Liu, D. Effects of ultrasound and/or heating on the extraction of pectin from grapefruit peel. J. Food Eng. 2014, 126, 72–81. [Google Scholar] [CrossRef]
- Kruger, N.J. The bradford method for protein quantitation. In The Protein Protocols Handbook; Humana Press: Totowa, NJ, USA, 2009; pp. 17–24. [Google Scholar]
- Kusumah, S.; Andoyo, R.; Rialita, T. Protein isolation techniques of beans using different methods: A review. IOP Conf. Ser. Earth Environ. Sci. 2020, 443, 012053. [Google Scholar] [CrossRef]
- Yılmaz, E.; Hüriyet, Z. Physico-chemical and functional properties of extracted capia pepperseed (capsicum annuum l.) proteins. Waste Biomass Valorization 2017, 8, 871–881. [Google Scholar] [CrossRef]
- Saha, J.; Deka, S.C. Functional properties of sonicated and non-sonicated extracted leaf protein concentrate from diplazium esculentum. Int. J. Food Prop. 2017, 20, 1051–1061. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, J.; Xiong, Y.L. Structural and emulsifying properties of soy protein isolate subjected to acid and alkaline ph-shifting processes. J. Agric. Food Chem. 2009, 57, 7576–7583. [Google Scholar] [CrossRef]
- Phongthai, S.; Lim, S.-T.; Rawdkuen, S. Optimization of microwave-assisted extraction of rice bran protein and its hydrolysates properties. J. Cereal Sci. 2016, 70, 146–154. [Google Scholar] [CrossRef]


| Run | Independent Variables (Coded Values) | Independent Variables (Actual Values) | Response (MOSP Yield (%)) | |||||
|---|---|---|---|---|---|---|---|---|
| A: Amplitude (%) | B: Solute-to-Solvent Ratio (g/mL) | C: pH | A: Amplitude (%) | B: Solute-to-Solvent Ratio (g/mL) | C: pH | Measured | Predicted | |
| 1 | 0 | 0 | −1 | 50 | 1:20 | 9 | 34.76 | 34.77 |
| 2 | 1 | −1 | 1 | 75 | 1:10 | 13 | 37.34 | 37.32 |
| 3 | −1 | 0 | 0 | 25 | 1:20 | 11 | 33.15 | 33.13 |
| 4 | −1 | 1 | 1 | 25 | 1:30 | 13 | 32.10 | 32.11 |
| 5 (c.p.) | 0 | 0 | 0 | 50 | 1:20 | 11 | 36.62 | 36.59 |
| 6 (c.p.) | 0 | 0 | 0 | 50 | 1:20 | 11 | 36.52 | 36.59 |
| 7 | 1 | −1 | −1 | 75 | 1:10 | 9 | 37.06 | 37.07 |
| 8 | −1 | −1 | 1 | 25 | 1:10 | 13 | 31.17 | 31.18 |
| 9 | 0 | −1 | 0 | 50 | 1:10 | 11 | 36.22 | 36.20 |
| 10 | 1 | 0 | 0 | 75 | 1:20 | 11 | 39.30 | 39.29 |
| 11 | 1 | 1 | 1 | 75 | 1:30 | 13 | 38.28 | 38.29 |
| 12 (c.p.) | 0 | 0 | 0 | 50 | 1:20 | 11 | 36.57 | 36.59 |
| 13 | 0 | 0 | 1 | 50 | 1:20 | 13 | 35.02 | 34.99 |
| 14 | −1 | 1 | −1 | 25 | 1:30 | 9 | 31.94 | 31.93 |
| 15 | 0 | 1 | 0 | 50 | 1:30 | 11 | 37.20 | 37.19 |
| 16 | −1 | −1 | −1 | 25 | 1:10 | 9 | 30.92 | 30.91 |
| 17 | 1 | 1 | −1 | 75 | 1:30 | 9 | 38.11 | 38.10 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 111.81 | 9 | 12.42 | 11,418.21 | <0.0001 ** |
| A: Amplitude | 94.93 | 1 | 94.93 | 87,243.93 | <0.0001 ** |
| B: Solute-to-solvent ratio | 2.42 | 1 | 2.42 | 2224.75 | <0.0001 ** |
| C: pH | 0.1254 | 1 | 0.1254 | 115.29 | <0.0001 ** |
| AB | 0.0002 | 1 | 0.0002 | 0.1838 | 0.6810 ns |
| AC | 0.0002 | 1 | 0.0002 | 0.1838 | 0.6810 ns |
| BC | 0.0050 | 1 | 0.0050 | 4.60 | 0.0693 ns |
| A2 | 0.3720 | 1 | 0.3720 | 341.87 | <0.0001 ** |
| B2 | 0.0338 | 1 | 0.0338 | 31.11 | 0.0008 ** |
| C2 | 7.81 | 1 | 7.81 | 7180.25 | <0.0001 ** |
| Residual | 0.0076 | 7 | 0.0011 | ||
| Lack of Fit | 0.0026 | 5 | 0.0005 | 0.2093 | 0.9308 ns |
| Pure Error | 0.0050 | 2 | 0.0025 | ||
| Cor Total | 111.82 | 16 | |||
| R2 | 0.9999 | ||||
| R2 adjusted | 0.9998 | ||||
| Factor | Coefficient Estimate | df | Standard Error | 95% CI Low | 95% CI High | VIF |
|---|---|---|---|---|---|---|
| Intercept | 36.59 | 1 | 0.0141 | 36.55 | 36.62 | |
| A: Amplitude | 3.08 | 1 | 0.0104 | 3.06 | 3.11 | 1.0000 |
| B: Solute to solvent ratio | 0.4920 | 1 | 0.0104 | 0.4673 | 0.5167 | 1.0000 |
| C: pH | 0.1120 | 1 | 0.0104 | 0.0873 | 0.1367 | 1.0000 |
| AB | 0.0050 | 1 | 0.0117 | −0.0226 | 0.0326 | 1.0000 |
| AC | 0.0050 | 1 | 0.0117 | −0.0226 | 0.0326 | 1.0000 |
| BC | −0.0250 | 1 | 0.0117 | −0.0526 | 0.0026 | 1.0000 |
| A2 | −0.3726 | 1 | 0.0202 | −0.4203 | −0.3250 | 1.54 |
| B2 | 0.1124 | 1 | 0.0202 | 0.0647 | 0.1600 | 1.54 |
| C2 | −1.71 | 1 | 0.0202 | −1.76 | −1.66 | 1.54 |
| Functional Properties | MOSP (CE) | MOSP (UAE) |
|---|---|---|
| Solubility (%) | 5.56 ± 0.13 | 29.82 ± 0.21 |
| WHC (g/g) | 0.86 ± 0.009 | 1.02 ± 0.006 |
| OHC (g/g) | 0.91 ± 0.015 | 1.91 ± 0.013 |
| Emulsion capacity (mg/mL) | 58.39 ± 1.68 | 75.93 ± 1.19 |
| Foaming capacity (%) | 13.21 ± 0.27 | 24.23 ± 0.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatima, K.; Imran, M.; Ahmad, M.H.; Khan, M.K.; Khalid, W.; AL-Farga, A.; Alansari, W.S.; Shamlan, G.; Eskandrani, A.A. Ultrasound-Assisted Extraction of Protein from Moringa oleifera Seeds and Its Impact on Techno-Functional Properties. Molecules 2023, 28, 2554. https://doi.org/10.3390/molecules28062554
Fatima K, Imran M, Ahmad MH, Khan MK, Khalid W, AL-Farga A, Alansari WS, Shamlan G, Eskandrani AA. Ultrasound-Assisted Extraction of Protein from Moringa oleifera Seeds and Its Impact on Techno-Functional Properties. Molecules. 2023; 28(6):2554. https://doi.org/10.3390/molecules28062554
Chicago/Turabian StyleFatima, Khushar, Muhammad Imran, Muhammad Haseeb Ahmad, Muhammad Kamran Khan, Waseem Khalid, Ammar AL-Farga, Wafa S. Alansari, Ghalia Shamlan, and Areej A. Eskandrani. 2023. "Ultrasound-Assisted Extraction of Protein from Moringa oleifera Seeds and Its Impact on Techno-Functional Properties" Molecules 28, no. 6: 2554. https://doi.org/10.3390/molecules28062554
APA StyleFatima, K., Imran, M., Ahmad, M. H., Khan, M. K., Khalid, W., AL-Farga, A., Alansari, W. S., Shamlan, G., & Eskandrani, A. A. (2023). Ultrasound-Assisted Extraction of Protein from Moringa oleifera Seeds and Its Impact on Techno-Functional Properties. Molecules, 28(6), 2554. https://doi.org/10.3390/molecules28062554







