Study of Influence of Extraction Method on the Recovery Bioactive Compounds from Peel Avocado
Abstract
1. Introduction
2. Results and Discussion
2.1. Total Phenol Content and Antioxidant Activity
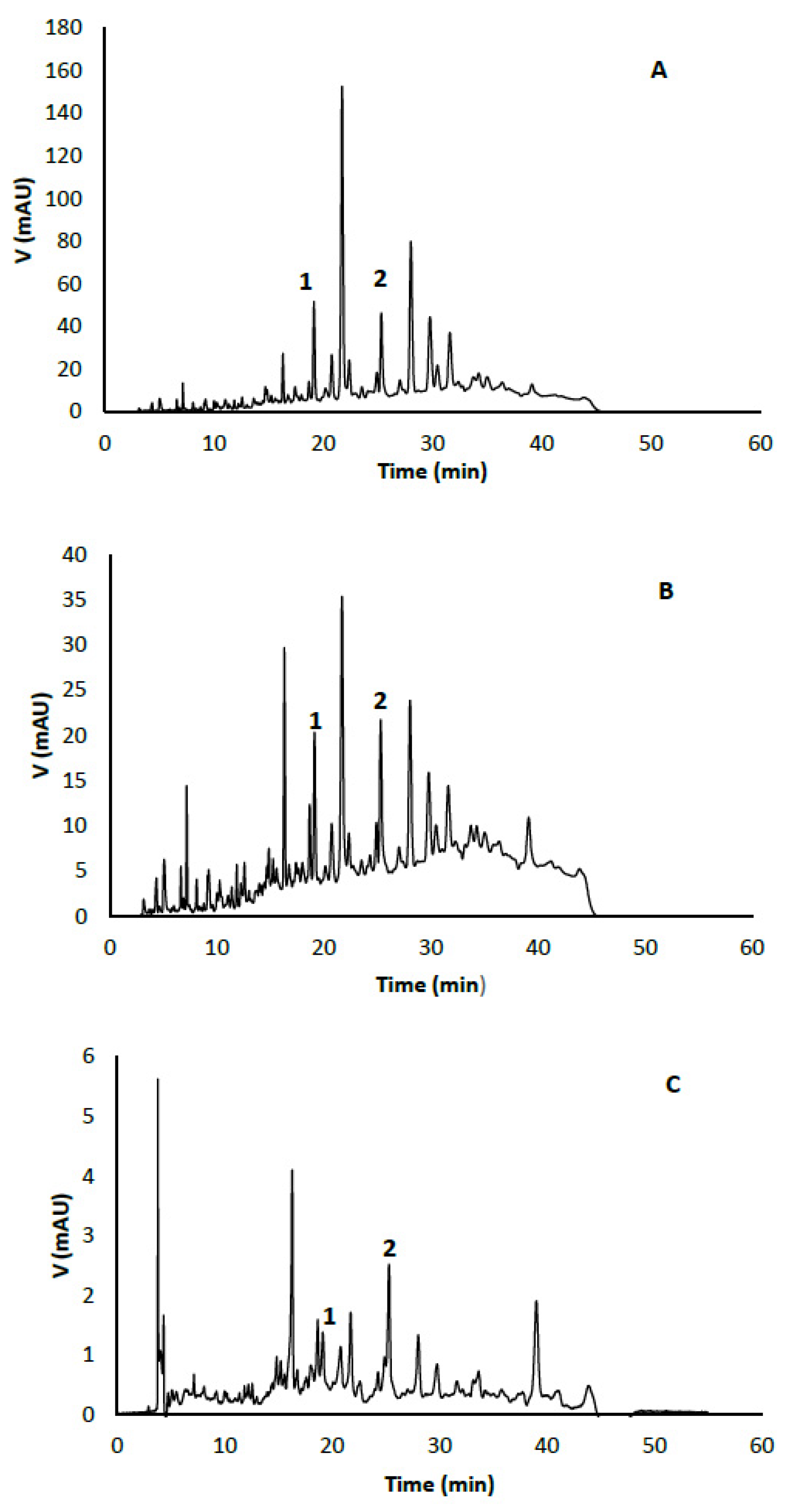
2.2. HPLC Analysis and Compounds’ Identification by UPLC-ESI-MS/MS
2.3. GC-MS Analysis
2.4. Comparison of the Extraction Methods
3. Materials and Methods
3.1. Chemical and Reagents
3.2. Microorganism
3.3. Preparation of Feed Stock and Extraction Methods
Solid-State Fermentation (SSF)
3.4. Total Phenolic Content
3.5. Antioxidant Activity
3.6. HPLC Analysis
3.7. UPLC-ESI-MS/MS Analysis
3.8. GC-MS Analysis
3.9. Statistical Analysis
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- da Silveira, J.S.; Durand, N.; Lacour, S.; Belleville, M.P.; Perez, A.; Loiseau, G.; Dornier, M. Solid-state fermentation as a sustainable method for coffee pulp treatment and production of an extract rich in chlorogenic acids. Food Bioprod. Process. 2019, 115, 175–184. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture of the United Nations. Agriculture Database. Available online: https://www.fao.org/faostat/en/#search/Avocados (accessed on 16 December 2022).
- Calderón-Oliver, M.; Escalona-Buendía, H.B.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Pedroza-Islas, R.; Ponce-Alquicira, E. Optimization of the antioxidant and antimicrobial response of the combined effect of nisin and avocado byproducts. LWT-Food Sci. Technol. 2016, 65, 46–52. [Google Scholar] [CrossRef]
- López-Cobo, A.; Gómez-Caravaca, A.M.; Pasini, F.; Caboni, M.F.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC-DAD-ESI-QTOF-MS and HPLC-FLD-MS as valuable tools for the determination of phenolic and other polar compounds in the edible part and by-products of avocado. LWT 2016, 73, 505–513. [Google Scholar] [CrossRef]
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F.; Arora, A. Bioactives from fruit processing wastes: Green approaches to valuable chemicals. Food Chem. 2017, 225, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Velderrain-Rodríguez, G.R.; Quero, J.; Osada, J.; Martín-Belloso, O.; Rodríguez-Yoldi, M.J. Phenolic-rich extracts from avocado fruit residues as functional food ingredients with antioxidant and antiproliferative properties. Biomolecules 2021, 11, 977. [Google Scholar] [CrossRef] [PubMed]
- García-Vargas, M.C.; Contreras, M.D.M.; Gómez-Cruz, I.; Romero-García, J.M.; Castro, E. Avocado-derived biomass: Chemical composition and antioxidant potential. Proceedings 2021, 70, 100. [Google Scholar] [CrossRef]
- Abdulkhaleq, L.A.; Assi, M.A.; Noor, M.H.M.; Abdullah, R.; Saad, M.Z.; Taufiq-Yap, Y.H. Therapeutic uses of epicatechin in diabetes and cancer. Vet. World 2017, 10, 869. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, D.; Liu, H.; Wang, Z.; Hui, T. Chlorogenic acid and Epicatechin: An efficient inhibitor of heterocyclic amines in charcoal roasted lamb meats. Food Chem. 2022, 368, 130865. [Google Scholar] [CrossRef]
- Dulf, F.V.; Vodnar, D.C.; Socaciu, C. Effects of solid-state fermentation with two filamentous fungi on the total phenolic contents, flavonoids, antioxidant activities and lipid fractions of plum fruit (Prunus domestica L.) by-products. Food Chem. 2016, 209, 27–36. [Google Scholar] [CrossRef]
- Cui, H.Y.; Niranjana Murthy, H.; Moh, S.H.; Cui, Y.Y.; Lee, E.J.; Paek, K.Y. Comparison of conventional and ultrasound-assisted methods for extraction of nutraceutical compounds from Dendrobium candidum. CyTA J. Food 2014, 12, 355–359. [Google Scholar] [CrossRef]
- Jing, C.L.; Dong, X.F.; Tong, J.M. Optimization of ultrasonic-assisted extraction of flavonoid compounds and antioxidants from alfalfa using response surface method. Molecules 2015, 20, 15550–15571. [Google Scholar] [CrossRef] [PubMed]
- Dahmoune, F.; Nayak, B.; Moussi, K.; Remini, H.; Madani, K. Optimization of microwave-assisted extraction of polyphenols from Myrtus communis L. leaves. Food Chem. 2015, 166, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Papoutsis, K.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Bowyer, M.C.; Scarlett, C.J.; Vuong, Q.V. Optimizing a sustainable ultrasound-assisted extraction method for the recovery of polyphenols from lemon by-products: Comparison with hot water and organic solvent extractions. Eur. Food Res. Technol. 2018, 244, 1353–1365. [Google Scholar] [CrossRef]
- Tutunchi, P.; Roufegarinejad, L.; Hamishehkar, H.; Alizadeh, A. Extraction of red beet extract with β-cyclodextrin-enhanced ultrasound assisted extraction: A strategy for enhancing the extraction efficacy of bioactive compounds and their stability in food models. Food Chem. 2019, 297, 124994. [Google Scholar] [CrossRef]
- Thomas, L.; Larroche, C.; Pandey, A. Current developments in solid-state fermentation. Biochem. Eng. J. 2013, 81, 146–161. [Google Scholar] [CrossRef]
- Ramos, B.; Maziero, G.; Leone, A.; Dutra, J.; Pereira, R.; Kitagawa, R.R. Avocado seeds (Persea americana Mill.) prevents indomethacin-induced gastric ulcer in mice. Food Res. Int. 2019, 119, 751–760. [Google Scholar] [CrossRef]
- Morais, D.R.; Rotta, E.M.; Sargi, S.C.; Schmidt, E.M.; Bonafe, E.G.; Eberlin, M.N.; Sawaya, A.C.; Visentainer, J.V. Antioxidant activity, phenolics and UPLC–ESI (–)–MS of extracts from different tropical fruits parts and processed peels. Food Res. Int. 2015, 77, 392–399. [Google Scholar] [CrossRef]
- Shi, D.; Xu, W.; Balan, P.; Wong, M.; Chen, W.; Popovich, D.G. In Vitro Antioxidant properties of New Zealand Hass avocado byproduct (Peel and Seed) fractions. ACS Food Sci. Technol. 2021, 1, 579–587. [Google Scholar] [CrossRef]
- Azwanida, N.N. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med. Aromat. Plants 2015, 4, 2167-0412. [Google Scholar]
- Del Castillo-Llamosas, A.; Rodríguez-Martínez, B.; Pablo, G.; Eibes, G.; Garrote, G.; Gullón, B. Hydrothermal treatment of avocado peel waste for the simultaneous recovery of oligosaccharides and antioxidant phenolics. Biores. Technol. 2021, 342, 125981. [Google Scholar] [CrossRef]
- Kamaraj, M.; Dhana Rangesh Kumar, V.; Nithya, T.G.; Danya, U. Assessment of antioxidant, antibacterial activity and phytoactive compounds of aqueous extracts of avocado fruit peel from Ethiopia. Int. J. Pept. Res. Ther. 2020, 26, 1549–1557. [Google Scholar] [CrossRef]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. J. Funct. Foods 2015, 14, 111–125. [Google Scholar] [CrossRef]
- Yeo, J.; Shahidi, F. Critical re-evaluation of DPPH assay: Presence of pigments affects the results. J. Agric. Food Chem. 2019, 67, 7526–7529. [Google Scholar] [CrossRef]
- Figueroa, J.G.; Borrás-Linares, I.; Del Pino-García, R.; Curiel, J.A.; Lozano-Sánchez, J.; Segura-Carretero, A. Functional ingredient from avocado peel: Microwave-assisted extraction, characterization and potential applications for the food industry. Food Chem. 2021, 352, 129300. [Google Scholar] [CrossRef]
- Figueroa, J.G.; Borrás-Linares, I.; Lozano-Sánchez, J.; Segura-Carretero, A. Comprehensive identification of bioactive compounds of avocado peel by liquid chromatography coupled to ultra-high-definition accurate-mass Q-TOF. Food Chem. 2018, 245, 707–716. [Google Scholar] [CrossRef]
- Rojas-García, A.; Fuentes, E.; Cádiz-Gurrea, M.D.L.L.; Rodriguez, L.; Villegas-Aguilar, M.D.C.; Palomo, I.; Arráez-Román, D.; Segura-Carretero, A. Biological evaluation of avocado residues as a potential source of bioactive compounds. Antioxidants 2022, 11, 1049. [Google Scholar] [CrossRef]
- Di Stefano, V.; Avellone, G.; Bongiorno, D.; Indelicato, S.; Massenti, R.; Lo Bianco, R. Quantitative evaluation of the phenolic profile in fruits of six avocado (Persea americana) cultivars by ultra-high-performance liquid chromatography-heated electrospray-mass spectrometry. Int. J. Food Prop. 2017, 20, 1302–1312. [Google Scholar] [CrossRef]
- Suleria, H.A.; Barrow, C.J.; Dunshea, F.R. Screening and characterization of phenolic compounds and their antioxidant capacity in different fruit peels. Foods 2020, 9, 1206. [Google Scholar] [CrossRef]
- Melgar, B.; Dias, M.I.; Ciric, A.; Sokovic, M.; Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Barros, L.; Ferreira, I.C.R.F. Bioactive characterization of Persea americana Mill. by-products: A rich source of inherent antioxidants. Ind. Crops Prod. 2018, 111, 212–218. [Google Scholar] [CrossRef]
- Rosero, J.C.; Cruz, S.; Osorio, C.; Hurtado, N. Analysis of phenolic composition of byproducts (seeds and peels) of avocado (Persea americana Mill.) cultivated in Colombia. Molecules 2019, 24, 3209. [Google Scholar] [CrossRef]
- Juurlink, B.H.; Azouz, H.J.; Aldalati, A.M.; AlTinawi, B.M.; Ganguly, P. Hydroxybenzoic acid isomers and the cardiovascular system. Nutr. J. 2014, 13, 63. [Google Scholar] [CrossRef]
- Benali, T.; Bakrim, S.; Ghchime, R.; Benkhaira, N.; El Omari, N.; Balahbib, A.; Bouyahya, A.; Taha, D.; Zengin, G.; Hasan, M.M.; et al. Pharmacological insights into the multifaceted biological properties of quinic acid. Biotechnol. Genet. Eng. Rev. 2022, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; XiaoHui, Z. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Liu, A.; Li, P.; Liu, C.; Xiao, W.; Huang, J.; Liu, Z.; Zhang, S. Advances in physiological functions and mechanisms of (−)-epicatechin. Crit. Rev. Food Sci. Nutr. 2021, 61, 211–233. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Qi, Q.L.; Wang, M.T.; Li, Q.Y. Therapeutic potential of naringin: An overview. Pharm. Biol. 2016, 54, 3203–3210. [Google Scholar] [CrossRef] [PubMed]
- Talja, R.A.; Roos, Y.H. Phase and state transition effects on dielectric, mechanical, and thermal properties of polyols. Thermochim. Acta 2001, 380, 109–121. [Google Scholar] [CrossRef]
- Kövilein, A.; Kubisch, C.; Cai, L.; Ochsenreither, K. Malic acid production from renewables: A review. J. Chem. Technol. Biotechnol. 2020, 95, 513–526. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.; Irfan, S.; Lorenzo, J.M.; Shafique, B.; Kanwal, R.; Pateiro, M.; Aadil, R.M.; Arshad, R.N.; Wang, L.; Nayik, G.A.; et al. Sonication, a potential technique for extraction of phytoconstituents: A systematic review. Processes 2021, 9, 1406. [Google Scholar] [CrossRef]
- Moreira, S.A.; Alexandre, E.M.; Pintado, M.; Saraiva, J.A. Effect of emergent non-thermal extraction technologies on bioactive individual compounds profile from different plant materials. Food Res. Int. 2019, 115, 177–190. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Carrillo-Hormaza, L.; Ramírez, A.M.; Osorio, E.; Gil, A. Optimization of ultrasound-assisted extraction and rapid resolution analysis of flavanols and methylxanthines for the quality control of cocoa-derived products. Food Anal. Methods 2017, 10, 497–507. [Google Scholar] [CrossRef]
- Saavedra, J.; Córdova, A.; Navarro, R.; Díaz-Calderón, P.; Fuentealba, C.; Astudillo-Castro, C.; Toledo, L.; Enrione, J.; Galvez, L. Industrial avocado waste: Functional compounds preservation by convective drying process. J. Food Eng. 2017, 198, 81–90. [Google Scholar] [CrossRef]
| Extraction Method | mg GAE 1/100 g Dry Matter | % DPPH Inhibition |
|---|---|---|
| M | 972.9 ± 118.6 b | 43.8 ± 3.1 c |
| MβC | 845.4 ± 113.2 a,b | 29.2 ± 4.0 b |
| WS | 635.9 ± 19.7 a | 25.7 ± 1.0 b |
| ES | 953.8 ± 85.6 b | 26.1 ± 0.4 b |
| SSF (14 d) | 800.2 ± 72.7 a,b | 23.7 ± 1.2 a,b |
| SSF (control) | 649.4 ± 53.5 a | 17.1 ± 2.3 a |
| WG | 1562.2 ± 137.9 c | 39.4 ± 1.5 c |
| WGM | 2143.1 ± 85.9 d | 28.5 ± 4.6 b |
| Title 1 | Chlorogenic Acid (mg/100 g Dry Matter) | Epicatechin (mg/100 g Dry Matter) |
|---|---|---|
| M | 73.48 ± 19.14 c | 43.83 ± 6.10 a |
| MβC | 53.60 ± 4.95 b,c | 45.84 ± 13.81 a |
| WS | 29.61 ± 5.25 a,b | 22.85 ± 1.52 a |
| ES | 7.22 ± 0.30 a | 24.84 ± 0.66 a |
| WG | 241.88 ± 7.42 d | 133.64 ± 8.35 b |
| WGM | 244.33 ± 24.44 d | 181.71 ± 31.79 c |
| SFF | N.D. * | N.D. a* |
| No. | Proposed Compound | RT (min) | m/z | Fragmentations | Molecular Formula | Reference |
|---|---|---|---|---|---|---|
| 1 | Quinic acid * | 0.61 | 191.2 | 85.06 | C7H12O6 | [26] |
| 2 | shikimic acid * | 0.66 | 173.18 | 111.07 | C7H10O5 | [27] |
| 3 | Gentisic acid * | 3.44 | 153.15 | 108.2 | C7H6O4 | [26] |
| 4 | 4-hydroxybenzoic acid * | 3.51 | 137.04 | 93.05 | C7H6O3 | [26] |
| 5 | Chlorogenic acid * | 3.80 | 353.1 | 191.2 | C16H18O9 | [28] |
| 6 | 4-O-caffeoylquinic acid | 4.65 | 353.3 | 179.06 | C16H18O9 | [26] |
| 7 | Vanillic acid * | 4.13 | 167.18 | 152.02 | [26] | |
| 8 | Caffeic acid * | 4.27 | 179.19 | 135.08 | C9H8O4 | [26] |
| 9 | Syringic acid * | 4.45 | 197.21 | 182.5 | C9H10O5 | [26] |
| 10 | Coumaric acid * | 5.45 | 163.24 | 119.09 | C9H8O3 | [26] |
| 11 | Ellagic acid * | 6.31 | 301 | 229 | C14H6O8 | |
| 12 | Ferulic acid | 5.99 | 193.24 | 134.04 | C10H10O4 | [23] |
| 13 | Sinapic acid * | 6.04 | 223.24 | 164.06 | C11H12O5 | [28] |
| 14 | Benzoic acid * | 6.79 | 121.1 | 77.1 | C7H6O2 | [26] |
| 15 | trans-cinnamic acid * | 8.83 | 147.17 | 103.08 | C9H8O2 | |
| 16 | 2-hydrobenzoic acid * | 6.78 | 137.04 | 93.05 | C7H6O3 | [29] |
| 17 | Diccaffeoylquinic acid | 7.19 | 515.45 | 353.2 | C25H24O12 | [7] |
| 18 | Coumaric acid (isomer) | 4.0 | 163.24 | 119.08 | C9H8O3 | |
| 19 | Procyanidin B1 * | 3.45 | 577.44 | 289.18 | C30H26O12 | [26] |
| 20 | Catechin * | 3.88 | 289.164 | 245.2 | C15H14O6 | [26] |
| 21 | Procyanidin B2 * | 4.39 | 577.44 | 289.18 | C30H26O12 | [26] |
| 22 | Epicatechin * | 4.79 | 289.164 | 245.2 | C15H14O6 | [30] |
| 23 | Quercetin glucoronide | 6.27 | 477.26 | 301.1 | C21H20O12 | [30] |
| 24 | Quercetin 3-O-glucoside | 6.24 | 463.36 | 300.42 | C21H20O12 | [31] |
| 25 | Naringin * | 6.94 | 579.32 | 151.02 | C27H32O14 | [28] |
| 26 | Eriodictyol | 8.33 | 287.28 | 151.04 | C15H12O6 | |
| 27 | Unknown 2 | 6.61 | 609.28 | 300.24 | C27H30O16 |
| No. | TR | Molecular Formula | Molecular Weight (g/mol) | Proposed Compound |
|---|---|---|---|---|
| 1 | 6.448 | C3H6O3 | 90.08 | Lactic acid |
| 2 | 6.634 | C2H4O3 | 75.06 | Glycolic acid |
| 3 | 7.019 | C3H7NO2 | 89.09 | Alanine |
| 4 | 8.424 | C3H4O4 | 104.0615 | Malonic acid |
| 5 | 8.615 | C5H11NO2 | 117.15 | Valine |
| 6 | 8.992 | C7H6O2 | 122.12 | Benzoic acid |
| 7 | 9.186 | C8H16O2 | 144.21 | Octanoic acid |
| 8 | 9.434 | H3PO4 | 98.0 | Phosphoric acid |
| 9 | 9.437 | C3H8O3 | 92.09 | Glycerol |
| 10 | 9.723 | C4H9NO2 | 103.12 | 4-Aminobutanoic acid |
| 11 | 9.903 | C4H6O4 | 118.09 | Succinic acid |
| 12 | 10.217 | C3H6O4 | 106.08 | Glyceric acid |
| 13 | 10.331 | C4H4O4 | 116.1 | Maleic acid |
| 14 | 10.434 | C5H6O4 | 130.09 | Methylmaleic acid |
| 15 | 10.611 | C3H7NO3 | 105.09 | Serine |
| 16 | 10.974 | C4H9NO3 | 119.1192 | Threonine |
| 17 | 12.262 | C4H6O5 | 134.0874 | Malic acid |
| 18 | 12.645 | C4H7NO4 | 133.11 | Aspartic acid |
| 19 | 12.731 | C4H9NO2 | 103.12 | 4-Aminobutanoic acid |
| 20 | 12.977 | C5H12O5 | 152.15 | Pentitol |
| 21 | 13.22 | C7H6O5 | 170.12 | 2,3,4-Trihydroxybutiric acid |
| 22 | 13.294 | C5H6O5 | 146.11 | 2-Oxoglutaric acid |
| 23 | 13.815 | C5H9NO4 | 147.13 | Glutamic acid |
| 24 | 13.895 | C9H11NO2 | 165.19 | Phenylalanine |
| 25 | 15.533 | C5H10N2O3 | 146.14 | Glutamine |
| 26 | 15.96 | C7H10O5 | 174.15 | Shikimic acid |
| 27 | 16.115 | C6H8O7 | 192.024 | Citric acid |
| 28 | 16.772 | C6H12O6 | 180.16 | Fructose |
| 29 | 17.043 | C6H12O6 | 180.16 | Glucose |
| 30 | 17.224 | C6H12O6 | 180.16 | Glucose isomer |
| 31 | 17.27 | C9H11NO3 | 181.19 | Tyrosine |
| 32 | 17.442 | C6H14O6 | 180.17 | Glucitol |
| 33 | 18.031 | C6H12O7 | 196.16 | Gluconic acid |
| 34 | 18.146 | C16H32O2 | 256.4 | Palmitic acid |
| 35 | 18.89 | C6H12O6 | 180.16 | Myo-inositol |
| 36 | 23.572 | C12H22O11 | 342.3 | Sucrose |
| 37 | 24.953 | C15H14O6 | 290.26 | Epicatechin 1 |
| 38 | 25.091 | C15H14O6 | 290.26 | Catechin isomer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Gutiérrez, E. Study of Influence of Extraction Method on the Recovery Bioactive Compounds from Peel Avocado. Molecules 2023, 28, 2557. https://doi.org/10.3390/molecules28062557
Martínez-Gutiérrez E. Study of Influence of Extraction Method on the Recovery Bioactive Compounds from Peel Avocado. Molecules. 2023; 28(6):2557. https://doi.org/10.3390/molecules28062557
Chicago/Turabian StyleMartínez-Gutiérrez, Emir. 2023. "Study of Influence of Extraction Method on the Recovery Bioactive Compounds from Peel Avocado" Molecules 28, no. 6: 2557. https://doi.org/10.3390/molecules28062557
APA StyleMartínez-Gutiérrez, E. (2023). Study of Influence of Extraction Method on the Recovery Bioactive Compounds from Peel Avocado. Molecules, 28(6), 2557. https://doi.org/10.3390/molecules28062557






