Design, Synthesis and Biological Evaluation of Quinoline-8-Sulfonamides as Inhibitors of the Tumor Cell-Specific M2 Isoform of Pyruvate Kinase: Preliminary Study
Abstract
1. Introduction
2. Results and Discussion
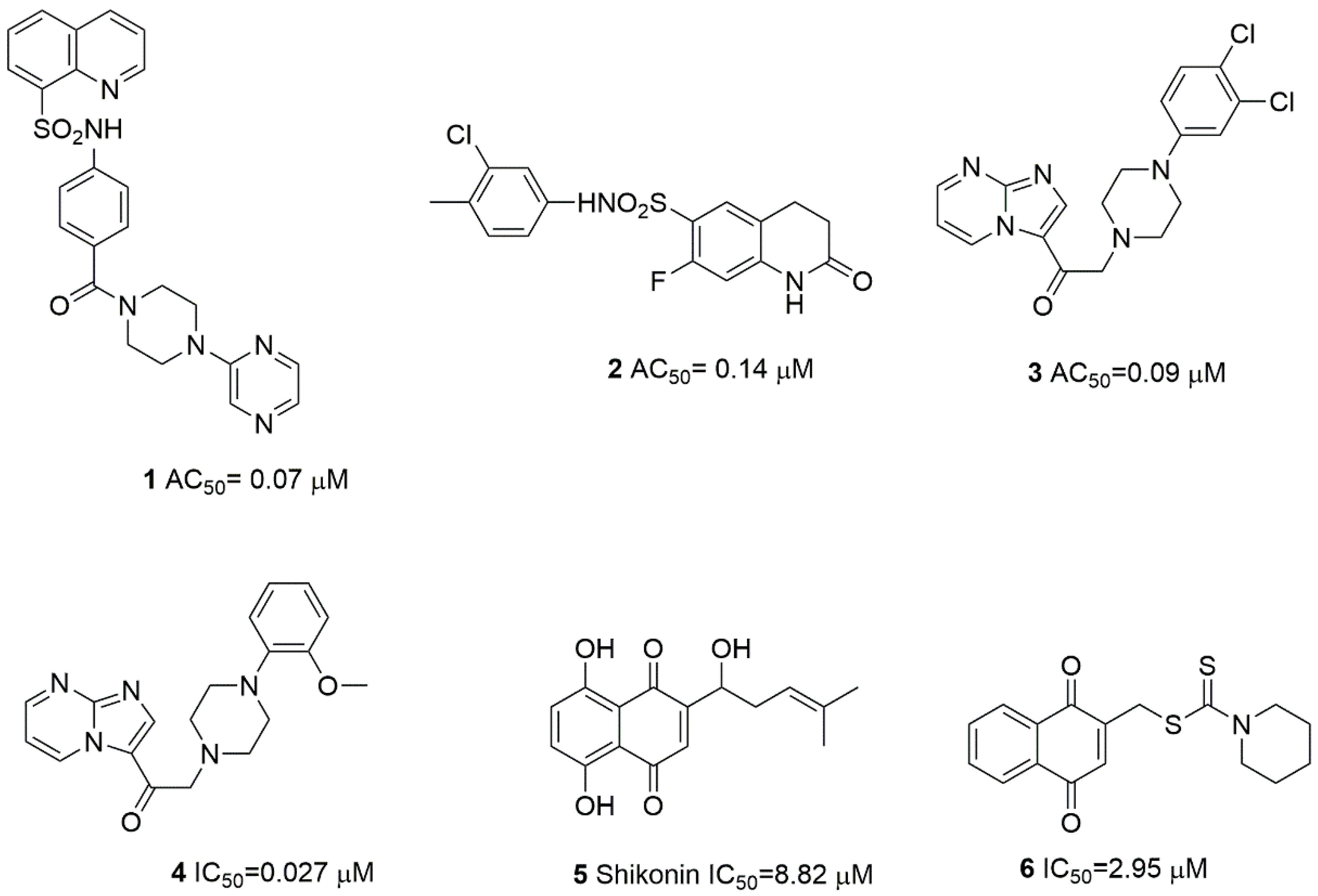
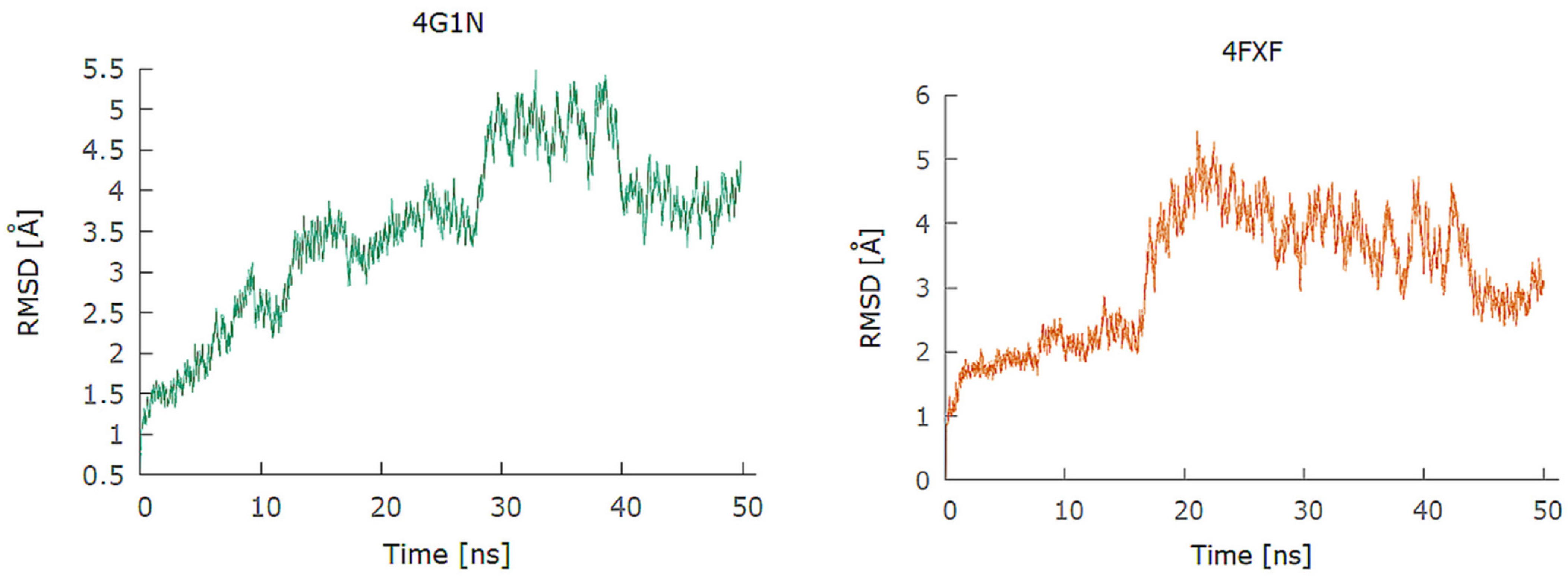
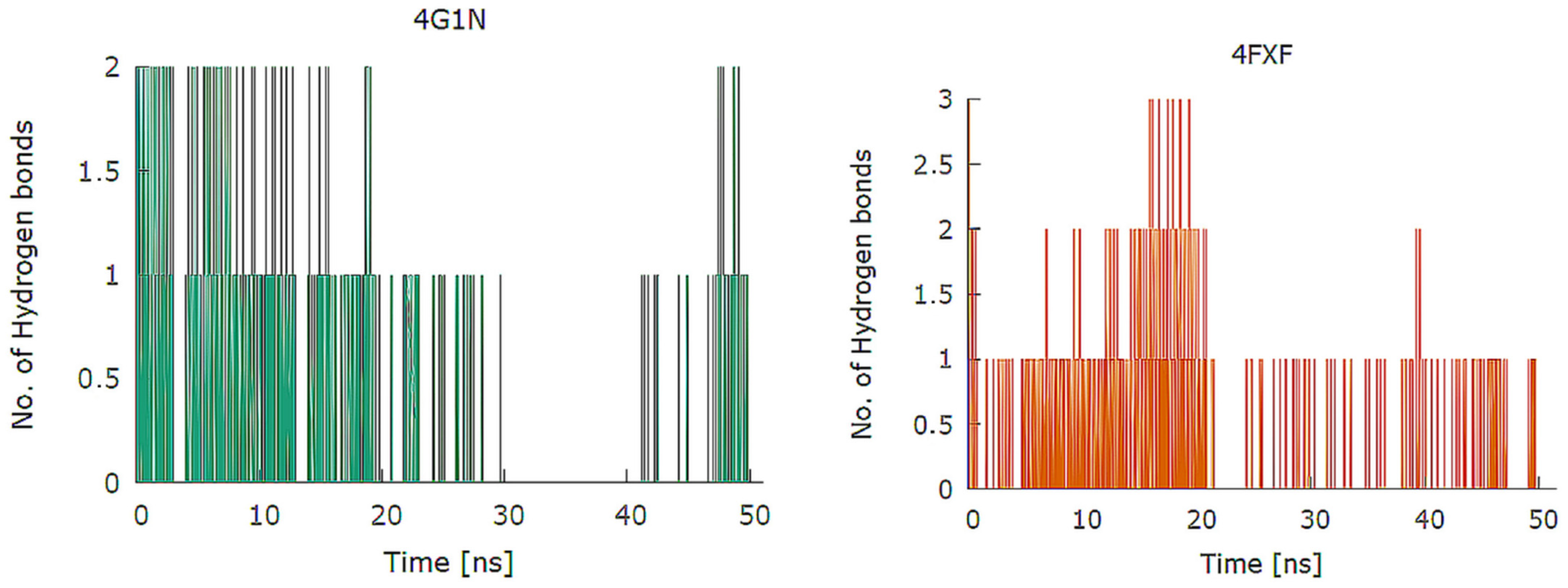
2.1. Design and In Silico Prediction of PKM-2 Modulators
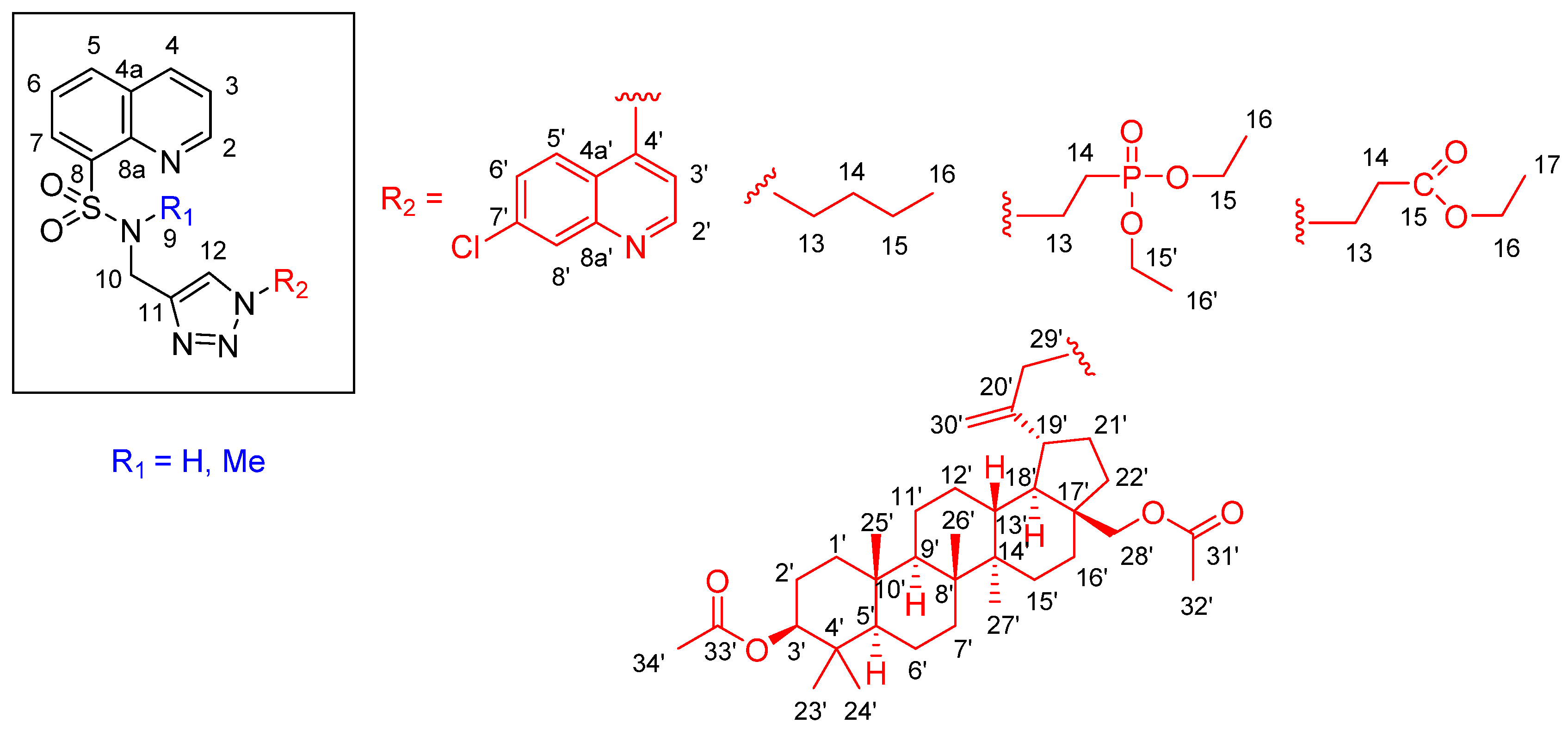
2.2. Chemistry
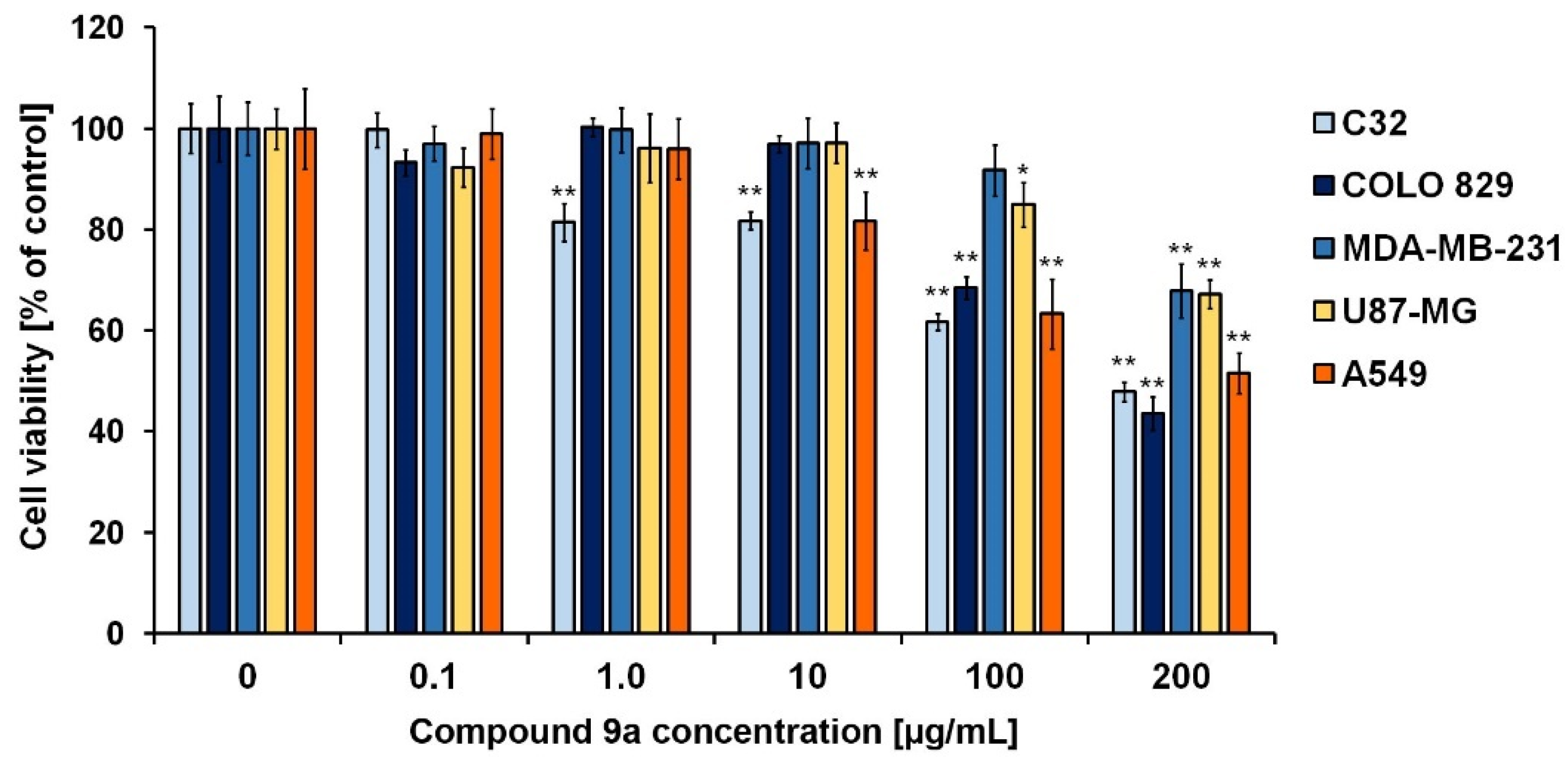
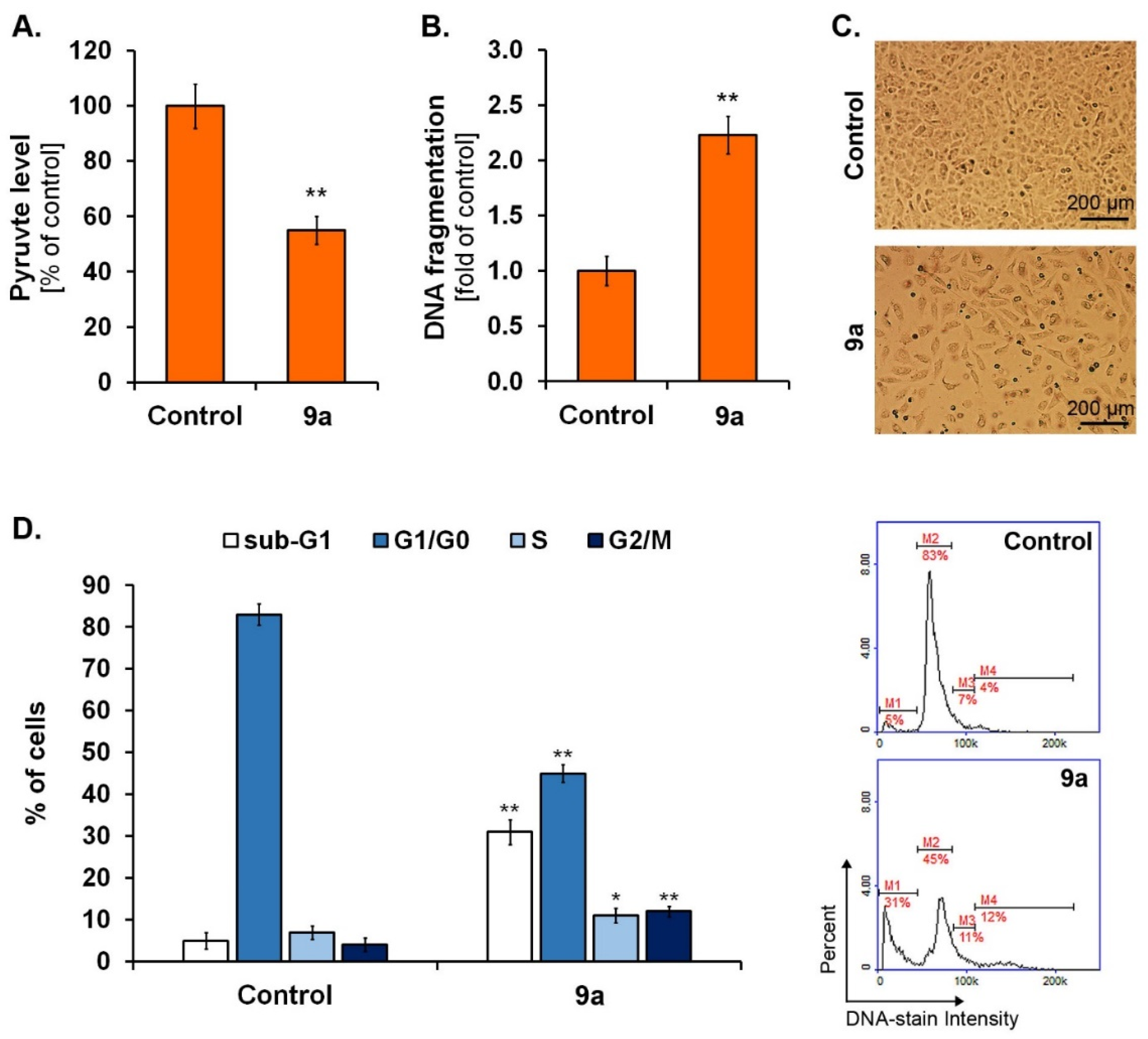
2.3. In Vitro Studies
3. Materials and Methods
3.1. In Silico
3.2. Chemistry
3.2.1. General Chemistry Methods
3.2.2. Synthesis Sulfonamides (8a,b)
3.2.3. Synthesis Triazoles (9a–e)
3.2.4. Procedure B
3.3. In Vitro Study
3.3.1. Chemicals
3.3.2. Cell Culture
3.3.3. WST-1 Assay
3.3.4. Quantitative Analysis of Cellular Pyruvate Level
3.3.5. DNA Fragmentation and Cell Cycle Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Martirosyan, A.R.; Rahim-Bata, R.; Freeman, A.B.; Clarke, C.D.; Howard, R.L.; Strobl, J.S. Differentiation-inducing quinolines as experimental breast cancer agents in the MCF-7 human breast cancer cell model. Biochem. Pharmacol. 2004, 68, 1729–1738. [Google Scholar] [CrossRef]
- Solary, E.; Drenou, B.; Campos, L.; De Crémoux, P.; Mugneret, F.; Moreau, P.; Lioure, B.; Falkenrodt, A.; Witz, B.; Bernard, M.; et al. Quinine as a multidrug resistance inhibitor: A phase 3 multicentric randomized study in adult de novo acute myelogenous leukemia. Blood 2003, 102, 1202–1210. [Google Scholar] [CrossRef]
- Gerrits, C.J.H.; Burris, H.; Schellens, J.H.M.; Planting, A.S.T.; van den Burg, M.E.L.; Rodriguez, G.I.; van Beurden, V.; Loos, W.J.; Hudson, I.; Fields, S.; et al. Five days of oral topotecan (hycamtin®), a phase I and pharmacological study in adult patients with solid tumours. Eur. J. Cancer 1998, 34, 1030–1035. [Google Scholar] [CrossRef]
- O’Reilly, S.; Rowinsky, E.K. The clinical status of irinotecan (CPT-11), a novel water soluble camptothecin analogue. Crit. Rev. Oncol. Hematol. 1996, 24, 47–70. [Google Scholar] [CrossRef]
- Vultur, A.; Buettner, R.; Kowolik, C.; Liang, W.; Smith, D.; Boschelli, F.; Jove, R. SKI-606 (bosutinib), a novel Src kinase inhibitor, suppresses migration and invasion of human breast cancer cells. Mol. Cancer Ther. 2008, 7, 1185–1194. [Google Scholar] [CrossRef]
- Matsui, J.; Funahashi, Y.; Uenaka, T.; Watanabe, T.; Tsuruoka, A.; Asada, M. Multikinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 by inhibiting vascular endothelial growth factor receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin. Cancer Res. 2008, 14, 5459–5465. [Google Scholar] [CrossRef]
- You, W.K.; Sennino, B.; Williamson, C.W.; Falcón, B.; Hashizume, H.; Yao, L.C.; Aftab, D.T.; Mc Donald, D.M. VEGF and c-Met blockade amplify angiogenesis inhibition in pancreatic islet cancer. Cancer Res. 2011, 71, 4758–4768. [Google Scholar] [CrossRef]
- Sparano, J.A.; Moulder, S.; Kazi, A.; Coppola, D.; Negassa, A.; Vahdat, L.; Li, T.; Pellegrino, C.; Fineberg, S.; Munster, P.; et al. Phase II trial of tipifarnib plus neoadjuvant doxorubicin-cyclophosphamide in patients with clinical stage IIB-IIIC breast cancer. Clin. Cancer Res. 2009, 15, 2942–2948. [Google Scholar] [CrossRef]
- Fenical, W.; Jensen, P.; MacMillan, J.; Hughes, C.C.; LaClair, J.J. Ammosamides as Anticancer Agents. WO. Patent 2009/006319, 8 January 2009. [Google Scholar]
- Zajdel, P.; Partyka, A.; Marciniec, K.; Bojarski, A.J.; Pawłowski, M.; Wesołowska, A. Quinoline- and isoquinoline-sulfonamide analogs of aripiprazole: Novel antipsychotic agents? Future Med. Chem. 2014, 6, 57–75. [Google Scholar] [CrossRef]
- Zajdel, P.; Kos, T.; Marciniec, K.; Satała, G.; Canale, V.; Kamiński, K.; Hołuj, M.; Lenda, T.; Koralewski, R.; Bednarski, M.; et al. Novel multi-target azinesulfonamides of cyclic amine derivatives as potential antipsychotics with pro-social and pro-cognitive effects. Eur. J. Med. Chem. 2018, 145, 790–804. [Google Scholar] [CrossRef]
- Moore, B.P.; Chung, D.H.; Matharu, D.S.; Golden, J.E.; Maddox, C.; Rasmussen, L.; Noah, J.W.; Sosa, M.I.; Ananthan, S.; Tower, N.A.; et al. (S)-N-(2,5-Dimethylphenyl)-1-(quinoline-8-ylsulfonyl)pyrrolidine-2-carboxamide as a small molecule inhibitor probe for the study of respiratory syncytial virus infection. J. Med. Chem. 2012, 55, 8582–8587. [Google Scholar] [CrossRef]
- Baraldi, P.G.; Romagnoli, R.; Tabrizi, M.A.; Falzoni, S.; Di Virgilio, F. Synthesis of conformationally constrained analogues of KN62 a potent antagonist of P2X7 receptor. Biorg. Med. Chem. Lett. 2000, 10, 681–684. [Google Scholar] [CrossRef]
- Kim, Y.H.; Shin, K.J.; Lee, T.G.; Kim, E.; Lee, M.S.; Ryu, S.H.; Suh, P.G. G2 arrest and apoptosis by 2-amino-N-quinoline-8-yl-benzenesulfonamide (QBS), a novel cytotoxic compound. Biochem. Pharmacol. 2005, 69, 1333–1341. [Google Scholar] [CrossRef]
- Bury, M.J.; Casillas, L.; Charnley, A.K.; Demartino, M.P.; Dong, X.; Haile, P.A.; Harris, P.A.; Lakdawala, S.A.; King, B.W.; Marquis, R.W.; et al. Amino-quinolines as Kinase Inhibitors. WO Patent 2011/140442, 10 November 2011. [Google Scholar]
- Lindsley, C.W.; Zhao, Z. Inhibitors of Akt Activity. WO Patent 2003/086403, 23 October 2003. [Google Scholar]
- Billiard, J.; Dennison, J.B.; Briand, J.; Annan, R.S.; Chai, D.; Colón, M.; Dodson, C.S.; Gilbert, S.A.; Greshock, J.; Jing, J.; et al. Quinoline 3-sulfonamides inhibit lactatedehydrogenase A and reverse aerobic glycolysisin cancer cell. Cancer Metab. 2013, 1, 19–36. [Google Scholar] [CrossRef]
- Walsh, M.J.; Brimacombe, K.R.; Veith, H.; Bougie, J.M.; Daniel, T.; Leister, W.; Cantley, L.C.; Israelsen, W.J.; Vander Heiden, M.G.; Shen, M.; et al. 2-Oxo-N-aryl-1,2,3,4-tetrahydroquinoline-6-sulfonamides as activators of the tumor cell specific M2 isoform of pyruvate kinase. Bioorg. Med. Chem. Lett. 2011, 21, 6322–6327. [Google Scholar] [CrossRef]
- Kung, C.; Hixon, J.; Choe, S.; Marks, K.; Gross, S.; Murphy, E.; DeLaBarre, B.; Cianchetta, G.; Sethumadhavan, S.; Wang, X.; et al. Small Molecule Activation of PKM2 in Cancer Cells Induces Serine Auxotrophy. Chem. Biol. 2012, 19, 1187–1198. [Google Scholar] [CrossRef]
- Dong, G.; Mao, Q.; Xia, W.; Xu, Y.; Wang, J.; Xu, L.; Jiang, F. PKM2 and cancer: The function of PKM2 beyond glycolysis. Oncol. Lett. 2016, 11, 1980–1986. [Google Scholar] [CrossRef]
- Vaupel, P.; Schmidberger, H.; Mayer, A. The Warburg effect: Essential part of metabolic reprogramming and central contributor to cancer progression. Int. J. Radiat. Biol. 2019, 95, 912–919. [Google Scholar] [CrossRef]
- Shi, H.S.; Li, D.; Zhang, J.; Wang, Y.S.; Yang, L.; Zhang, H.L.; Wang, X.H.; Mu, B.; Wang, W.; Ma, Y.; et al. Silencing of pkm2 increases the efficacy of docetaxel in human lung cancer xenografts in mice. Cancer Sci. 2010, 101, 1447–1453. [Google Scholar] [CrossRef]
- Zhou, C.F.; Li, X.B.; Sun, H.; Zhang, B.; Han, Y.S.; Jiang, Y.; Zhuang, Q.L.; Fang, J.; Wu, G.H. Pyruvate kinase type M2 is upregulated in colorectal cancer and promotes proliferation and migration of colon cancer cells. IUBMB Life 2012, 64, 775–782. [Google Scholar] [CrossRef]
- Dayton, T.L.; Jacks, T.; Vander Heiden, M.G. PKM2, cancer metabolism, and the road ahead. EMBO Rep. 2016, 17, 1721–1730. [Google Scholar] [CrossRef]
- Ning, X.; Qi, H.; Li, R.; Li, Y.; Jin, Y.; McNutt, M.A.; Li, J.; Yin, Y. Discovery of novel naphthoquinone derivatives as inhibitors of the tumor cell specific M2 isoform of pyruvate kinase. Eur. J. Med. Chem. 2017, 138, 343–352. [Google Scholar] [CrossRef]
- Li, Y.H.; Li, X.F.; Liu, J.T.; Wang, H.; Fan, L.L.; Li, J.; Sun, G.P. PKM2, a potential target for regulating cancer. Gene 2018, 668, 48–53. [Google Scholar] [CrossRef]
- Hsu, M.C.; Hung, W.C. Pyruvate kinase M2 fuels multiple aspects of cancer cells: From cellular metabolism, transcriptional regulation to extracellular signalling. Mol. Cancer 2018, 17, 35. [Google Scholar] [CrossRef]
- Thonsri, U.; Seubwai, W.; Waraasawapati, S.; Wongkham, S.; Boonmars, T.; Cha’on, B.; Wongkham, C. Antitumor Effect of Shikonin, a PKM2 Inhibitor, in Cholangiocarcinoma Cell Lines. Anticancer Res. 2020, 40, 5115–5124. [Google Scholar] [CrossRef]
- Yuan, M.; McNae, I.W.; Chen, Y.; Blackburn, E.A.; Wear, M.A.; Michels, P.A.M.; Fothergill-Gilmore, L.A.; Hupp, T.; Walkinshaw, M.D. An allostatic mechanism for M2 pyruvate kinase as an amino-acid sensor. Biochem. J. 2018, 475, 1821–1837. [Google Scholar] [CrossRef]
- Patel, S.; Globisch, C.; Pulugu, P.; Kumar, P.; Jain, A.; Shard, A. Novel imidazopyrimidines-based molecules induce tetramerization of tumor pyruvate kinase M2 and exhibit potent antiproliferative profile. Eur. J. Pharm. Sci. 2022, 170, 106112. [Google Scholar] [CrossRef]
- Doiron, J.E.; Le, C.A.; Bacsa, J.; Breton, G.W.; Martin, K.L.; Aller, S.G.; Turlington, M. Structural Consequences of the 1,2,3-triazole as an amide bioisostere in analogues of the cystic fibrosis drugs VX-809 and VX-770. ChemMedChem 2020, 15, 1720–1730. [Google Scholar] [CrossRef]
- Arora, S.; Joshi, G.; Chaturvedi, A.; Heuser, M.; Patil, S.; Kumar, R. A Perspective on Medicinal Chemistry Approaches for Targeting Pyruvate Kinase M2. J. Med. Chem. 2022, 65, 1171–1205. [Google Scholar] [CrossRef]
- Verdonk, M.L.; Cole, J.C.; Hartshorn, M.J.; Murray, C.W.; Taylor, R.D. Improved Protein–Ligand Docking Using GOLD. Proteins Struct. Funct. Genet. 2003, 52, 609–623. [Google Scholar] [CrossRef]
- Respondek, M.; Beberok, A.; Rzepka, Z.; Rok, J.; Wrześniok, D. Mcl-1 Inhibitor Induces Cells Death in BRAF-Mutant Amelanotic Melanoma Trough GSH Depletion, DNA Damage and Cell Cycle Changes. Pathol. Oncol. Res. 2020, 26, 1465–1474. [Google Scholar] [CrossRef]
- Respondek, M.; Beberok, A.; Rzepka, Z.; Rok, J.; Wrześniok, D. MIM1 induces COLO829 melanoma cell death through mitochondrial membrane breakdown, GSH depletion, and DNA damage. Fundam. Clin. Pharmacol. 2020, 34, 20–31. [Google Scholar] [CrossRef]
- Respondek, M.; Beberok, A.; Rok, J.; Rzepka, Z.; Wrześniok, D.; Buszman, E. MIM1, the Mcl-1—Specific BH3 mimetic induces apoptosis in human U87MG glioblastoma cells. Toxicol. Vitr. 2018, 53, 126–135. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, S.; Liu, J.; Tian, Y.; Ma, B.; Xu, S.; Fu, Y.; Luo, Y. Secreted Pyruvate Kinase M2 Promotes Lung Cancer Metastasis through Activating the Integrin Beta1/FAK Signaling Pathway. Cell Rep. 2020, 30, 1780–1797. [Google Scholar] [CrossRef]
- Ding, Z.; Xi, J.; Zhong, M.; Chen, F.; Zhao, H.; Zhang, B.; Fang, J. Cynaropicrin induces cell cycle arrest and apoptosis by inhibiting PKM2 to cause DNA damage and mitochondrial fission in A549. J. Agric. Food Chem. 2021, 69, 13557–13567. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.; Gaussian Inc.: Wallingford, UK, 2016. [Google Scholar]
- Cole, J.C.; Nissink, J.W.M.; Taylor, R. Protein-Ligand Docking and Virtual Screening with GOLD in Virtual Screening in Drug Discovery. In Virtual Screening in Drug Discovery, Shoichet, B., Alvarez, J., Eds; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Systèmes, D. Biovia, Discovery Studio Modeling Environment; Dessault Systemes: San Diego, CA, USA, 2016. [Google Scholar]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kale, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD—Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Vanommeslaeghe, K.; Raman, E.P.; MacKerell, A.D.J., Jr. Automation of the CHARMM General Force Field (CGenFF) II: Assignment of bonded parameters and partial atomic charges. Chem. Inf. Model. 2012, 52, 3155–3168. [Google Scholar] [CrossRef]
- Ribeiro, J.V.; Bernardi, R.C.; Rudack, T.; Stone, J.E.; Phillips, J.C.; Freddolino, P.L.; Schulten, K. QwikMD—Integrative molecular dynamics toolkit for novices and experts. Sci. Rep. 2016, 6, 26536. [Google Scholar] [CrossRef]
- Jamshidi, H.; Naimi-Jamal, M.R.; Safavi, M.; RayatSanati, K.; Azerang, P.; Tahghighi, A. Synthesis and biological activity profile of novel triazole/quinoline hybrids. Chem. Biol. Drug Des. 2022, 100, 1–12. [Google Scholar] [CrossRef]
- Antimonova, A.N.; Petrenko, N.I.; Shakirov, M.M.; Rybalova, T.V.; Frolova, T.S.; Shul’ts, E.E.; Kukina, T.P.; Sinitsyna, O.I.; Tolstikov, G.A. Synthesis and study of mutagenic properties of lupane triterpenoids containing 1,2,3-triazole fragments in the C-30 position. Chem. Nat. Compd. 2013, 49, 657–664. [Google Scholar] [CrossRef]
- Beberok, A.; Wrześniok, D.; Szlachta, M.; Rok, J.; Rzepka, Z.; Respondek, M.; Buszman, E. Lomefloxacin induces oxidative stress and apoptosis in COLO829 melanoma Cells. Int. J. Mol. Sci. 2017, 18, 2194. [Google Scholar] [CrossRef]

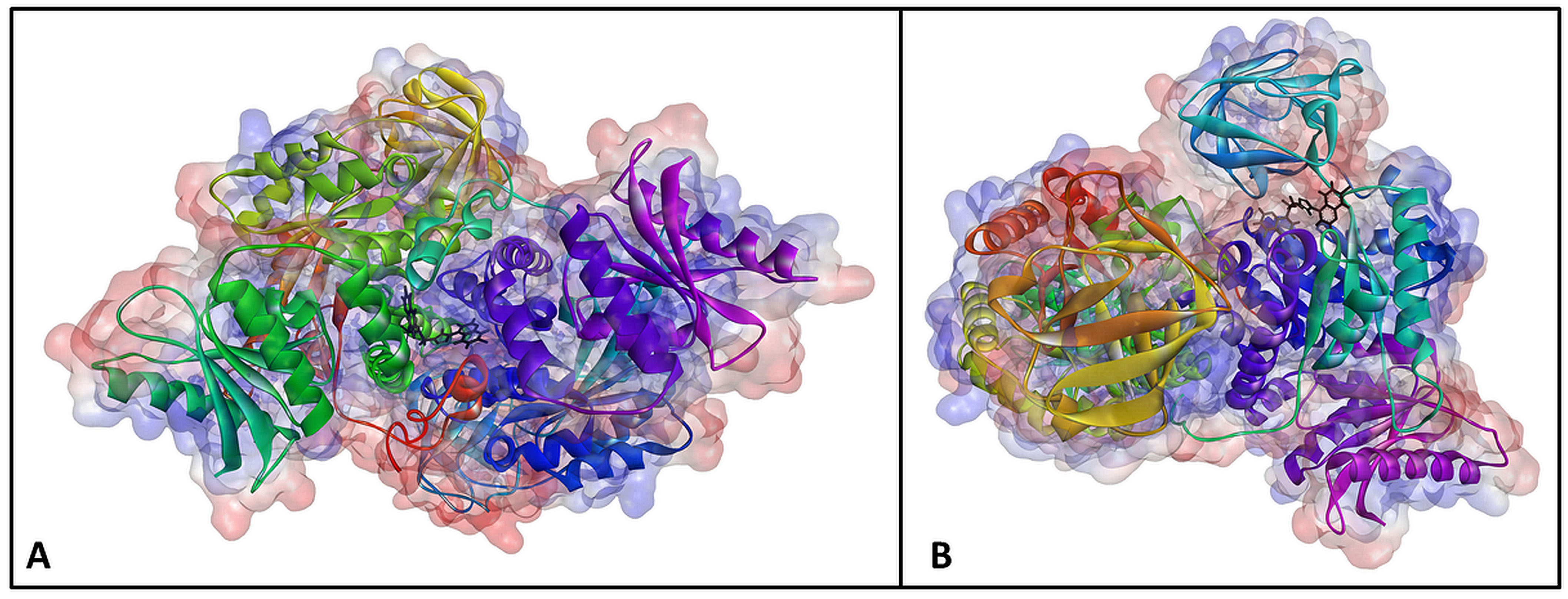
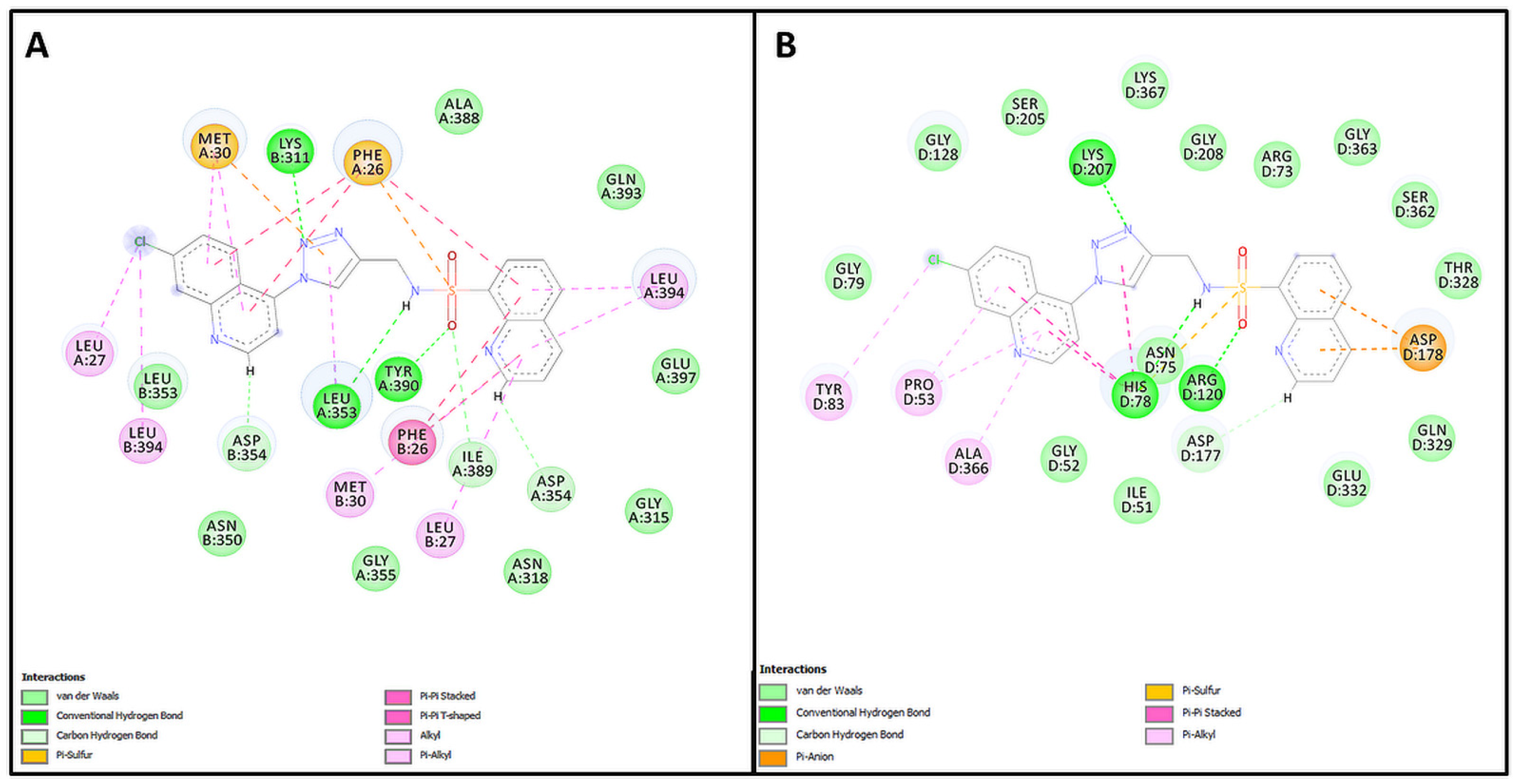
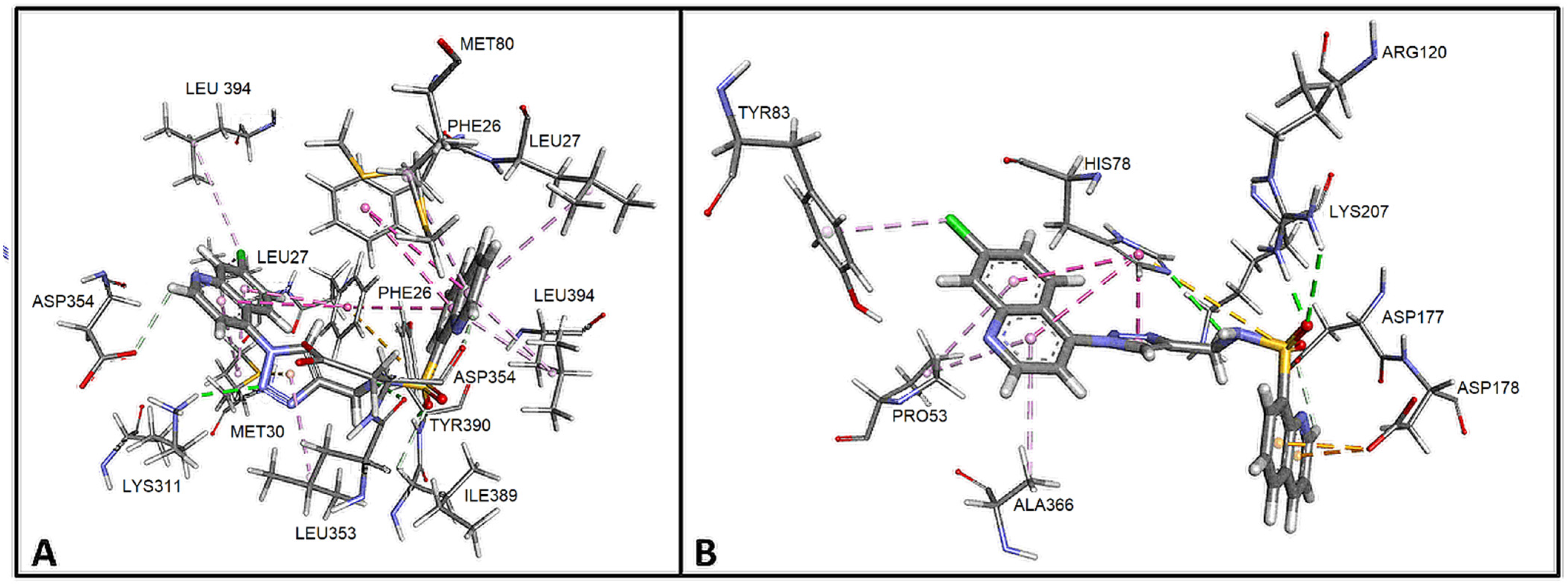

| Compound | PKM2 Structure | |||
|---|---|---|---|---|
| PDB ID: 4G1N | PDB ID: 4XFX | |||
| GOLD Score [a.u.] | ΔG [kcal/mol] | GOLD Score [a.u.] | ΔG [kcal/mol] | |
| 1 | 75.03 | −10.34 | 65.82 | −9.35 |
| 2 | 62.95 | −9.04 | 54.05 | −8.08 |
| 3 | 63.77 | −9.13 | 56.78 | −8.38 |
| 4 | 62.99 | −9.04 | 62.30 | −8.97 |
| 5 | 58.05 | −8.51 | 53.64 | −8.04 |
| 6 | 60.65 | −8.79 | 52.92 | −7.96 |
| 9a | 78.56 | −10.72 | 76.33 | −10.48 |
| 9b | 72.64 | −10.08 | 75.57 | −10.40 |
| 9c | 73.17 | −10.14 | 64.99 | −9.26 |
| 9d | 70.82 | −9.89 | 61.35 | −8.87 |
| 9e | 71.35 | −9.94 | 60.36 | −8.76 |
| Compound | Analyzed Cell Lines—GI50 | |||||
|---|---|---|---|---|---|---|
| C32 | COLO 829 | MDA-MB-231 | U87-MG | A549 | NHDF | |
| 9a | 234 µg/mL (0.520 mM) | 169 µg/mL (0.376 mM) | 274 µg/mL (0.609 mM) | 340 µg/mL (0.756 mM) | 223 µg/mL (0.496 mM) | Negative |
| 9b | 200 µg/mL (0.441 mM) | 266 µg/mL (0.587 mM) | Negative | 347 µg/mL (0.765 mM) | Negative | Negative |
| 9c | 269 µg/mL (0.691 mM) | 179 µg/mL (0.460 mM) | Negative | Negative | Negative | 87 µg/mL (0.223 mM) |
| 9d | 209 µg/mL (0.605 mM) | 130 µg/mL (0.376 mM) | Negative | 328 µg/mL (0.949 mM) | Negative | Negative |
| 9e | 303 µg/mL (0.373 mM) | Negative | 314 µg/mL (0.386 mM) | Negative | Negative | Negative |
| Reference compound | Dacarbazine 9 µg/mL (0.049 mM) [34] | Dacarbazine negative [35] | Cisplatin 4 µg/mL (0.013 mM) | Temozolomide negative [36] | Cisplatin 196 µg/mL (0.653 mM) | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marciniec, K.; Rzepka, Z.; Chrobak, E.; Boryczka, S.; Latocha, M.; Wrześniok, D.; Beberok, A. Design, Synthesis and Biological Evaluation of Quinoline-8-Sulfonamides as Inhibitors of the Tumor Cell-Specific M2 Isoform of Pyruvate Kinase: Preliminary Study. Molecules 2023, 28, 2509. https://doi.org/10.3390/molecules28062509
Marciniec K, Rzepka Z, Chrobak E, Boryczka S, Latocha M, Wrześniok D, Beberok A. Design, Synthesis and Biological Evaluation of Quinoline-8-Sulfonamides as Inhibitors of the Tumor Cell-Specific M2 Isoform of Pyruvate Kinase: Preliminary Study. Molecules. 2023; 28(6):2509. https://doi.org/10.3390/molecules28062509
Chicago/Turabian StyleMarciniec, Krzysztof, Zuzanna Rzepka, Elwira Chrobak, Stanisław Boryczka, Małgorzata Latocha, Dorota Wrześniok, and Artur Beberok. 2023. "Design, Synthesis and Biological Evaluation of Quinoline-8-Sulfonamides as Inhibitors of the Tumor Cell-Specific M2 Isoform of Pyruvate Kinase: Preliminary Study" Molecules 28, no. 6: 2509. https://doi.org/10.3390/molecules28062509
APA StyleMarciniec, K., Rzepka, Z., Chrobak, E., Boryczka, S., Latocha, M., Wrześniok, D., & Beberok, A. (2023). Design, Synthesis and Biological Evaluation of Quinoline-8-Sulfonamides as Inhibitors of the Tumor Cell-Specific M2 Isoform of Pyruvate Kinase: Preliminary Study. Molecules, 28(6), 2509. https://doi.org/10.3390/molecules28062509