Retrospective on Exploring MXene-Based Nanomaterials: Photocatalytic Applications
Abstract
1. Introduction
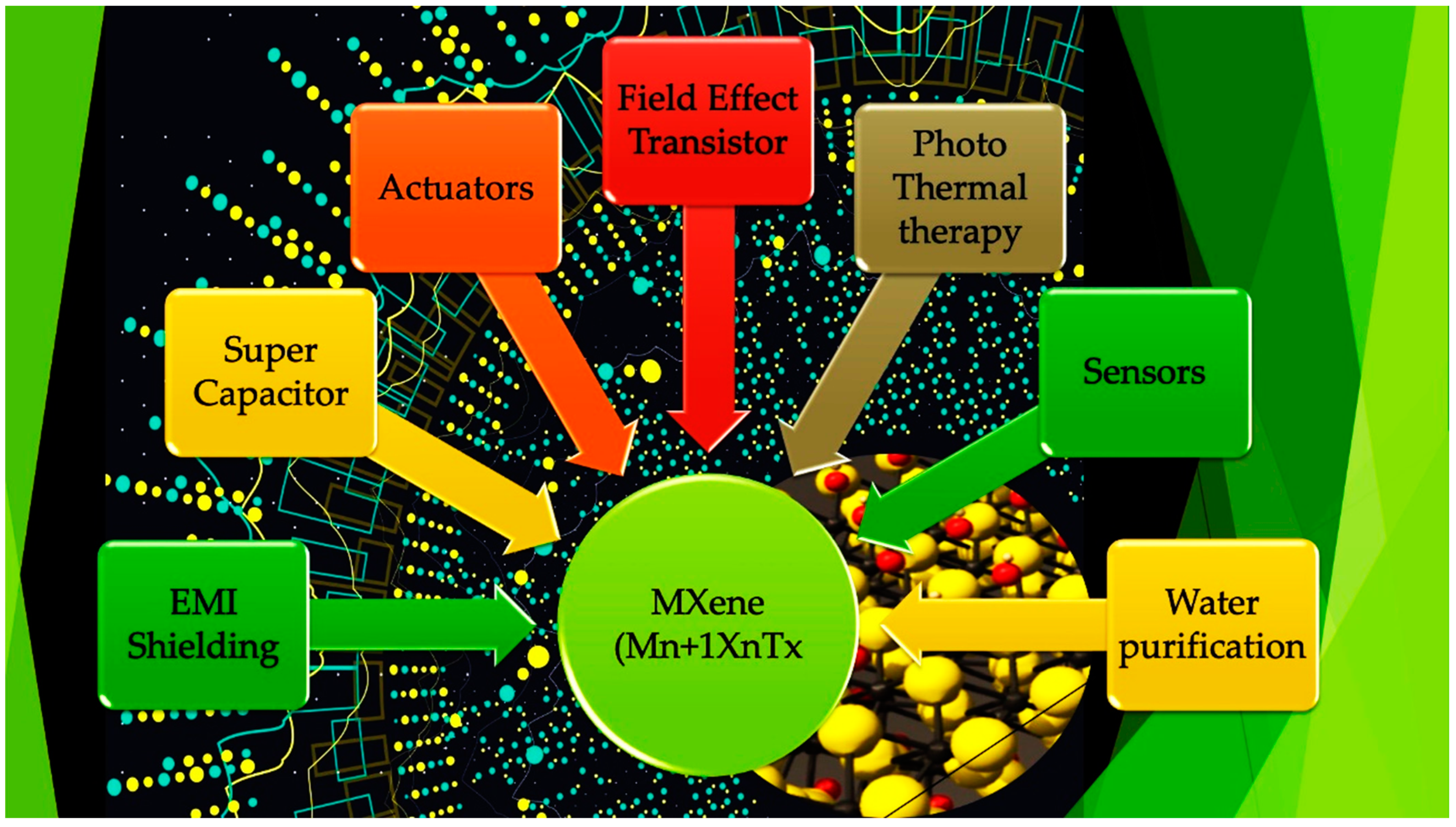
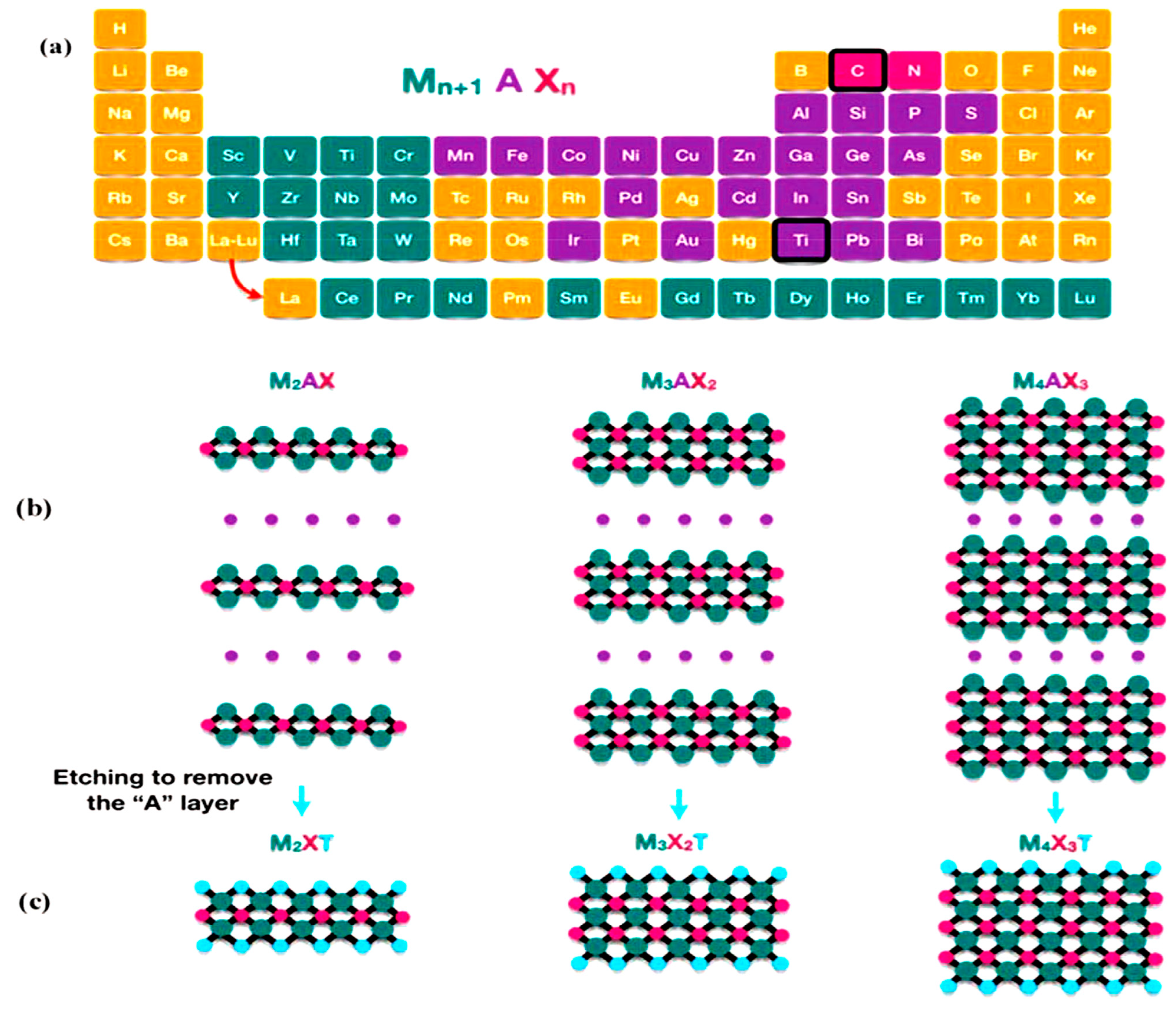
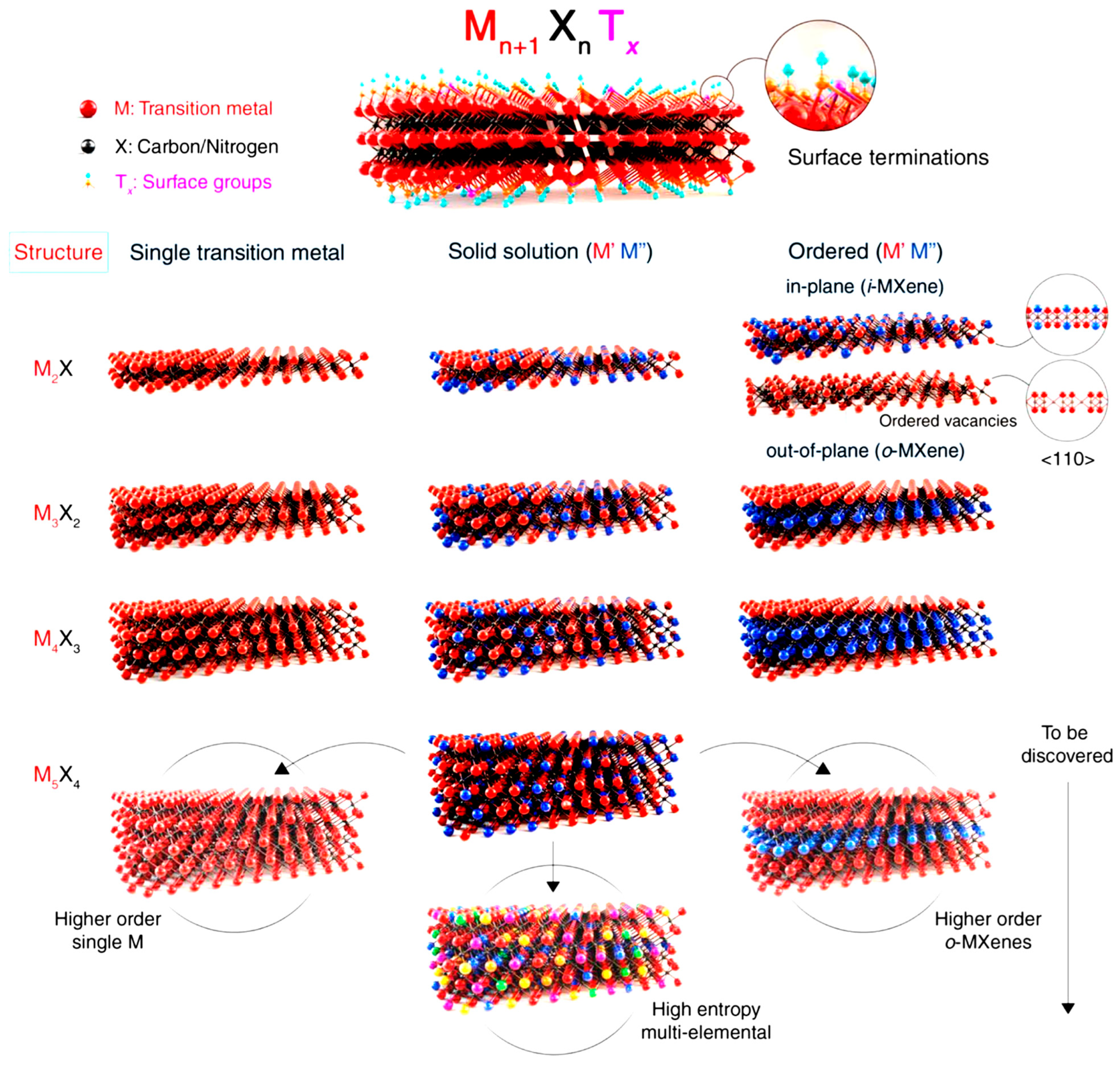
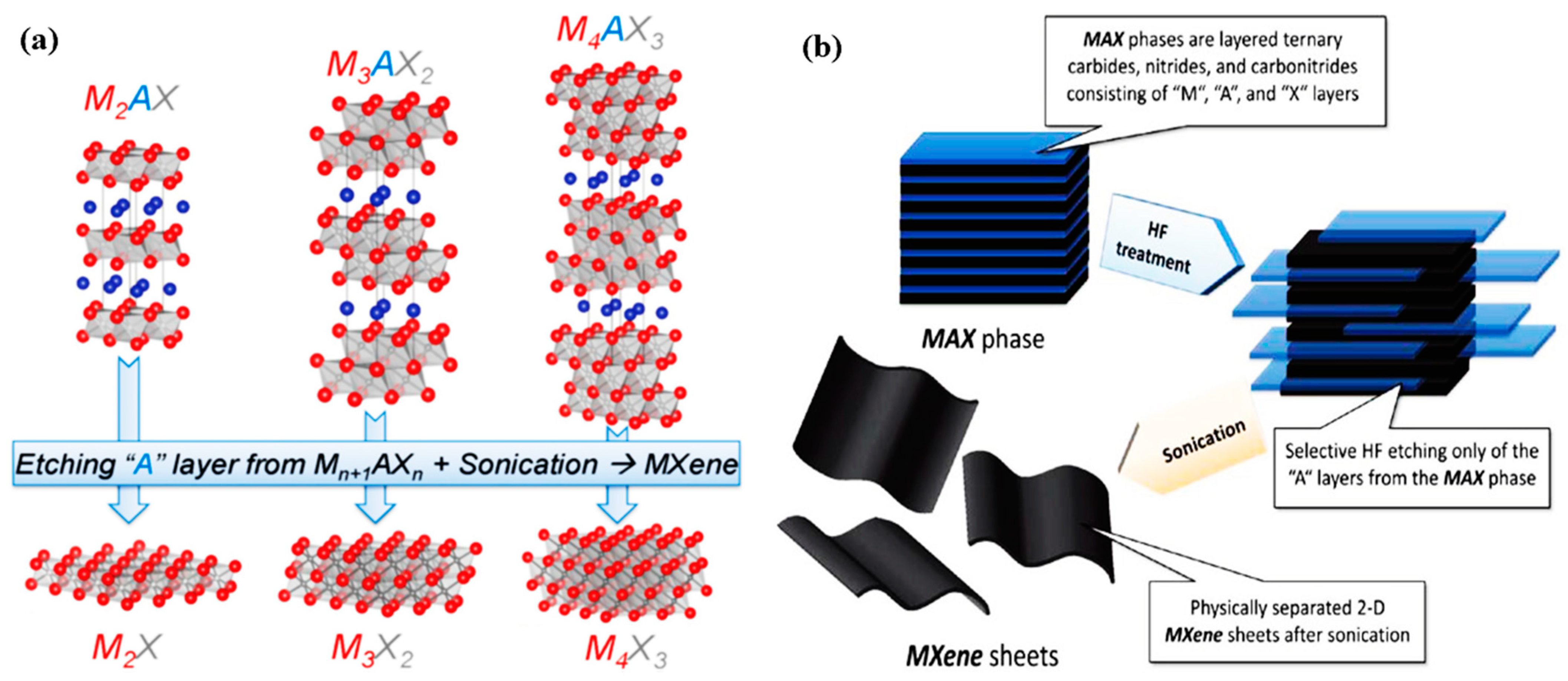
2. Introduction of Mxenes
3. MXenes as Adsorbents
4. Synthesis of MXene-Derived Heterostructure Photocatalyst
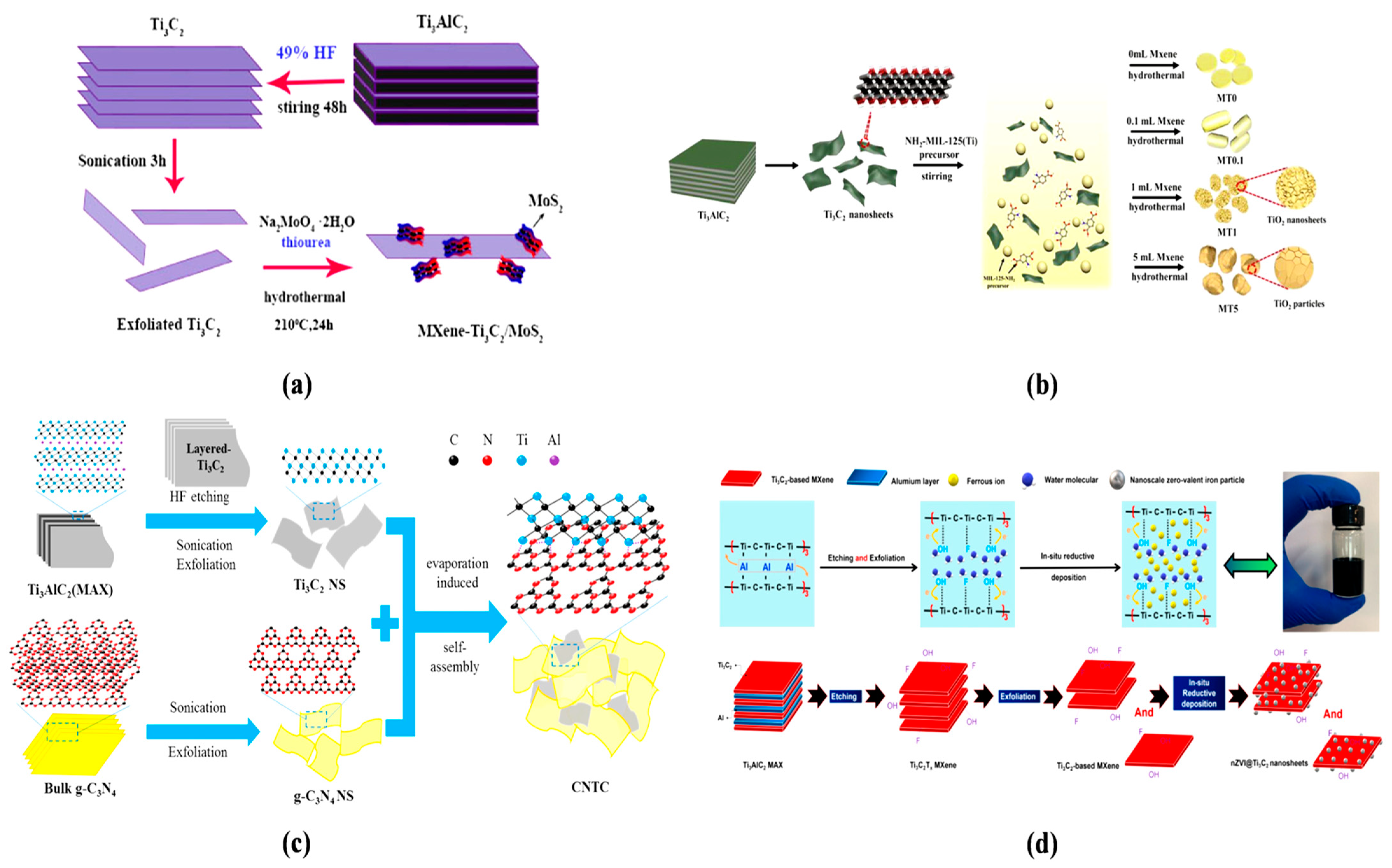
4.1. Evaporation-Induced Synthesis of MXene Composites
4.2. In Situ Reductive Deposition Synthesis
4.3. The Flux Synthesis Method of Nanocomposites
4.4. Solvothermal Method
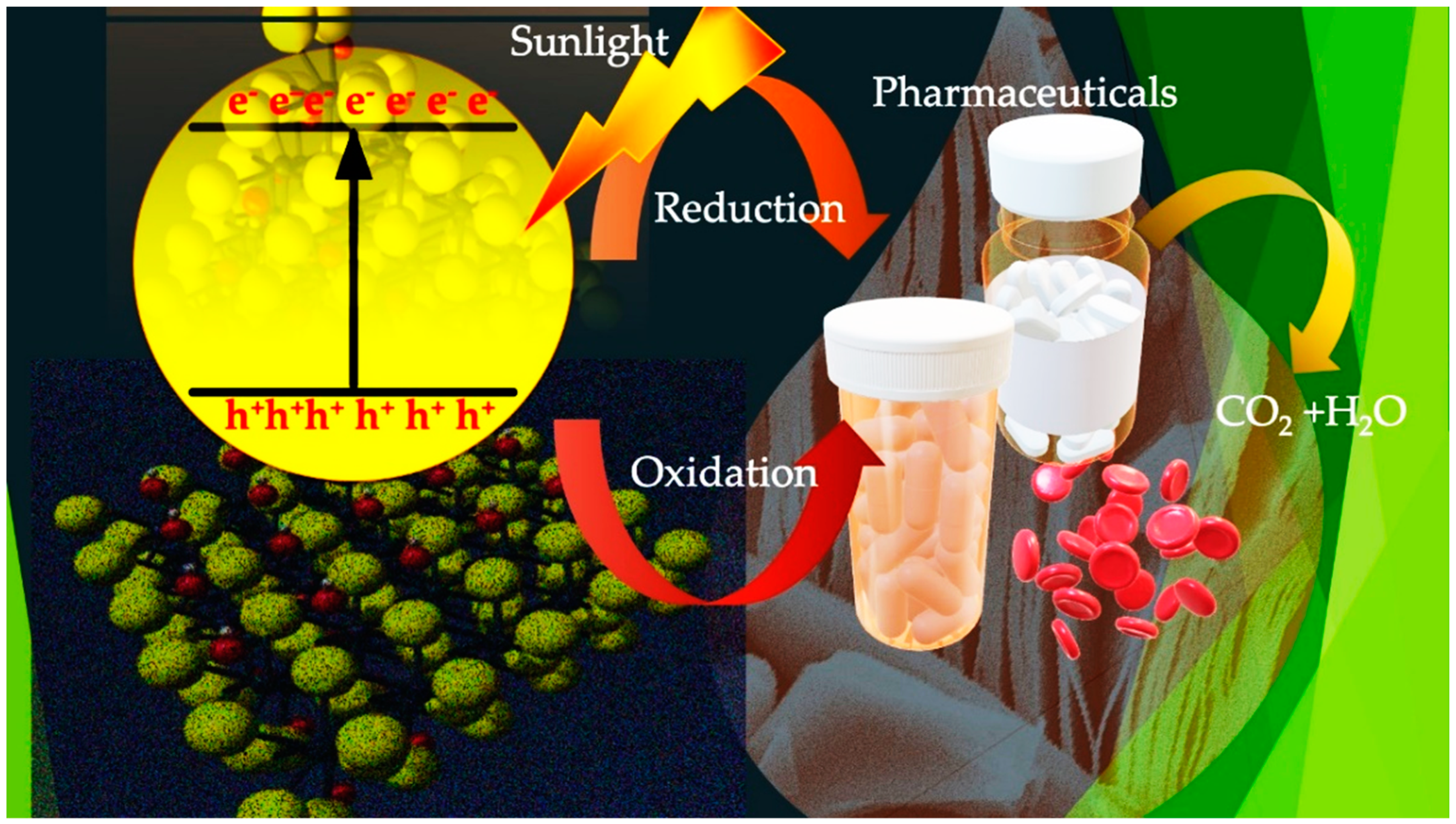
5. Photocatalysis by MXenes
6. MXene-Based Photocatalysis
MXene-Based Photocatalytic Materials
7. Carbon-Based MXene Composites
7.1. Metal-Based MXene Nanocomposites
7.2. MXene–Metal Tungstate Composites
7.3. MXene–Metal-Based Composites
7.4. MXene–Metal and Oxide–Metal Nanocomposites
7.5. MXene–Metal Sulfide Nanocomposites
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, S.; Di Paolo, C.; Wu, X.; Seiler, T.-B.; Hollert, H. Optimization of screening-level risk assessment and priority of emerging pollutants–the case of pharmaceuticals in European surface waters. Environ. Int. 2019, 128, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liao, S.; Qi, F.; Liu, R.; Xiao, T.; Hu, J.; Li, K.; Wang, R.; Min, Y. Direct deposition of two-dimensional MXene nanosheets on commercially available filter for fast and efficient dye removal. J. Hazard. Mater. 2020, 384, 121367. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Wang, R.; Yin, J.; Jiao, T.; Chen, K.; Zou, G.; Zhang, L.; Zhou, J.; Zhang, L.; Peng, Q. Preparation and dye degradation performances of self-assembled MXene-Co3O4 nanocomposites synthesized via solvothermal approach. ACS Omega 2019, 4, 3946. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Cao, Y.; Chen, Q. Synthesis of an Ag2WO4/Ti3C2 Schottky composite by electrostatic traction and its photocatalytic activity. Ceram. Int. 2019, 45, 22298–22307. [Google Scholar]
- Zhao, D.; Cai, C. Preparation of Bi2MoO6/Ti3C2 MXene heterojunction photocatalysts for fast tetracycline degradation and Cr(vi) reduction. Inorg. Chem. Front. 2020, 7, 2799–2808. [Google Scholar]
- Damptey, L.; Bright, N.J.; Camila, S.R.; Varagnolo, S.; Nicholas, P.P.; Vimalnath, S.; David, D.A.; Kumar, R.V.; Sithara, P.S.; Dermot, B.V.K.T.; et al. Surface Functionalized MXenes for Wastewater Treatment—A Comprehensive Review. Glob. Chall. 2022, 6, 2100120. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional transition metal carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. MXenes: A new family of two dimensional materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef]
- Hemanth, N.R.; Kandasubramanian, B. Recent advances in 2D MXenes for enhanced cation intercalation in energy harvesting applications: A review. Chem. Eng. J. 2020, 392, 123678. [Google Scholar] [CrossRef]
- Anasori, B.; Xie, Y.; Beidaghi, M.; Lu, J.; Hosler, B.C.; Hultman, L.; Kent, P.R.C.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional, ordered, double transition metals carbides (MXenes). ACS Nano 2015, 9, 9507–9516. [Google Scholar] [CrossRef] [PubMed]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Sun, W.; Shah, S.A.; Chen, Y.; Tan, Z.; Gao, H.; Habib, T.; Radovic, M.; Green, M.J. Electrochemical etching of Ti2AlC to Ti2CTx (MXene) in low-concentration hydrochloric acid solution. J. Mater. Chem. A. 2017, 5, 21663–21668. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, P.; Wang, F.; Ricciardulli, A.G.; Lohe, M.R.; Blom, P.W.M.; Feng, X. Fluoride-free synthesis of two-dimensional titanium carbide (MXene) using a binary aqueous system. Angew. Chem. 2018, 130, 15717–15721. [Google Scholar] [CrossRef]
- Lei, J.-C.; Zhang, X.; Zhou, Z. Recent advances in MXene: Preparation, properties, and applications. Front. Phys. 2015, 10, 276–286. [Google Scholar] [CrossRef]
- Deysher, G.; Shuck, C.E.; Hantanasirisakul, K.; Frey, N.C.; Foucher, A.C.; Maleski, K.; Sarycheva, A.; Shenoy, V.B.; Stach, E.A.; Anasori, B.; et al. Synthesis of Mo4VAlC4 MAX phase and two-dimensional Mo4VC4 MXene with five atomic layers of transition metals. ACS Nano 2020, 14, 204–217. [Google Scholar] [CrossRef]
- VahidMohammadi, A.; Rosen, J.; Gogotsi, Y. The world of two-dimensional carbides and nitrides (MXenes). Science 2021, 372, 1581. [Google Scholar] [CrossRef]
- Barsoum, M.W.; El-Raghy, T. Synthesis and characterization of a remarkable ceramic: Ti3SiC2. J. Am. Ceram. Soc. 1996, 79, 1953–1956. [Google Scholar] [CrossRef]
- Anasori, B.; Gogotsi, Y. (Eds.) 2D Metal Carbides and Nitrides (MXene); Springer International Publishing: Cham, Switzerland, 2019; pp. 89–109. [Google Scholar]
- Szuplewska, A.; Kulpinska, D.; Dybko, A.; Chudy, M.; Jastrzębska, A.M.; Olszyna, A.; Brzózka, Z. Future applications of MXenes in biotechnology, nanomedicine, and sensors. Trends Biotechnol. 2019, 38, 264–279. [Google Scholar] [CrossRef]
- Barsoum, M.W. The Mn+1AXn phases: A new class of solids: Thermodynamically stable nanolaminates. Prog. Solid State Chem. 2000, 28, 201–281. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Lan, J.-H.; Wang, L.; Wu, Q.-Y.; Wang, C.-Z.; Bo, T.; Chai, Z.-F.; Shi, W.-Q. Adsorption of uranyl species on hydroxylated titanium carbide nanosheet: A first principles study. J. Hazard. Mater. 2016, 308, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Shekhirev, M.; Shuck, C.E.; Sarycheva, A.; Gogotsi, Y. Characterization of MXenes at every step, from their precursors to single flakes and assembled films. Prog. Mater. Sci. 2021, 120, 100757. [Google Scholar] [CrossRef]
- Rasool, K.; Pandey, R.P.; Rasheed, P.A.; Buczek, S.; Gogotsi, Y.; Mahmoud, K.A. Water treatment and environmental remediation applications of two-dimensional metal carbides (MXenes). Mater. Today 2019, 30, 80–102. [Google Scholar] [CrossRef]
- Hantanasirisakul, K.; Alhabeb, M.; Lipatov, A.; Maleski, K.; Anasori, B.; Salles, P.; Ieosakulrat, C.; Pakawatpanurut, P.; Sinitskii, A.; May, S.J.; et al. Effects of synthesis and processing on optoelectronic properties of titanium carbonitride MXene. Chem. Mater. 2019, 31, 2941–2951. [Google Scholar] [CrossRef]
- Khazaei, M.; Arai, M.; Sasaki, T.; Chung, C.-Y.; Venkataramanan, N.S.; Estili, M.; Sakka, Y.; Kawazoe, Y. Novel electronic and magnetic properties of two dimensional transition metal carbides and nitrides. Adv. Funct. Mater. 2013, 23, 2185–2192. [Google Scholar] [CrossRef]
- Khazaei, M.; Arai, M.; Sasaki, T.; Estili, M.; Sakka, Y. Two-dimensional molybdenum carbides: Potential thermoelectric materials of the MXene family. Phys. Chem. Chem. Phys. 2014, 16, 7841–7849. [Google Scholar] [CrossRef]
- Liang, X.; Rangom, Y.; Kwok, C.Y.; Pang, Q.; Nazar, L.F. Interwoven MXene nanosheet/carbon-nanotube composites as Li–S cathode hosts. Adv. Mater. 2017, 29, 1603040. [Google Scholar] [CrossRef]
- Naguib, M.; Adams, R.A.; Zhao, Y.; Zemlyanov, D.; Varma, A.; Nanda, J.; Pol, V.G. Electrochemical performance of MXenes as K-ion battery anodes. Chem. Commun. 2017, 53, 6883–6886. [Google Scholar] [CrossRef]
- Urbankowski, P.; Anasori, B.; Hantanasirisakul, K.; Yang, L.; Zhang, L.; Haines, B.; May, S.J.; Billinge, S.J.L.; Gogotsi, Y. 2D molybdenum and vanadium nitrides synthesized by ammoniation of 2D transition metal carbides (MXenes). Nanoscale. 2017, 9, 17722–17730. [Google Scholar] [CrossRef]
- Huang, K.; Li, Z.; Lin, J.; Han, G.; Huang, P. Two-dimensional transition metal carbides and nitrides (MXenes) for biomedical applications. Chem. Soc. Rev. 2018, 47, 5109–5124. [Google Scholar] [CrossRef]
- Hong Ng, V.M.; Huang, H.; Zhou, K.; Lee, P.S.; Que, W.; Xu, J.Z.; Kong, L.B. Recent progress in layered transition metal carbides and/or nitrides (MXenes) and their composites: Synthesis and applications. J. Mater. Chem. A 2017, 5, 3039–3068. [Google Scholar] [CrossRef]
- Zhu, J.; Ha, E.; Zhao, G.; Zhou, Y.; Huang, D.; Yue, G.; Hu, L.; Sun, N.; Wang, Y.; Lee, L.Y.S.; et al. Recent advance in MXenes: A promising 2D material for catalysis, sensor and chemical adsorption. Coord. Chem. Rev. 2017, 352, 306–327. [Google Scholar] [CrossRef]
- Zhan, X.; Si, C.; Zhou, J.; Sun, Z. MXene and MXene-based composites: Synthesis, properties and environment-related applications. Nanoscale Horiz. 2020, 5, 235–258. [Google Scholar] [CrossRef]
- Sinha, A.; Zhao, H.; Huang, Y.; Lu, X.; Chen, J.; Jain, R. MXene: An emerging material for sensing and biosensing. Trends Anal. Chem. 2018, 105, 424–435. [Google Scholar] [CrossRef]
- Wang, N.N.; Wang, H.; Wang, Y.-Y.; Wei, Y.-H.; Si, J.-Y.; Yuen, A.C.Y.; Xie, J.-S.; Yu, B.; Zhu, S.-E.; Lu, H.-D.; et al. Robust, lightweight, hydrophobic, and fire-retarded polyimide/MXene aerogels for effective oil/water separation. ACS Appl. Mater. Interfaces 2019, 11, 40512–40523. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, A.; Chen, J.; Jia, J.; Zhou, W.; Wang, L.; Hu, Q. Preparation of Ti3C2 and Ti2C MXenes by fluoride salts etching and methane adsorptive properties. Appl. Surf. Sci. 2017, 416, 781–789. [Google Scholar] [CrossRef]
- Guo, Z.; Zhou, J.; Zhu, L.; Sun, Z. MXene: A promising photocatalyst for water splitting. J. Mater. Chem. A 2016, 4, 11446–11452. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Z.; Yang, Q.; Tan, L.; Dong, L.; Dong, M. Ultrathin Ti3C2Tx (MXene) nanosheets wrapped NiSe2 octahedral crystal for enhanced supercapacitor performance and synergetic electrocatalytic water splitting. Nano-Micro Lett. 2019, 11, 31. [Google Scholar] [CrossRef]
- Zhang, Q.; Teng, J.; Zou, G.; Peng, Q.; Du, Q.; Jiao, T.; Xiang, J. Efficient phosphate sequestration for water purification by unique sandwich-like MXene/magnetic iron oxide nanocomposites. Nanoscale 2016, 8, 7085–7093. [Google Scholar] [CrossRef]
- Fu, L.; Yan, Z.; Zhao, Q.; Yang, H. Novel 2D nanosheets with potential applications in heavy metal purification: A review. Adv. Mater. Interfaces 2018, 5, 1801094. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Zhang, N.; Zhou, Z. Adsorptive environmental applications of MXene nanomaterials: A review. RSC Adv. 2018, 8, 19895–19905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Nicolosi, V. Graphene and MXene-based transparent conductive electrodes and supercapacitors. Energy Storage Mater. 2019, 16, 102–125. [Google Scholar] [CrossRef]
- Shahzad, F.; Alhabeb, M.; Hatter, C.B.; Anasori, B.; Man Hong, S.; Koo, C.M.; Gogotsi, Y. Electromagnetic interference shielding with 2D transition metal carbides (MXenes). Science 2016, 353, 1137–1140. [Google Scholar] [CrossRef]
- Cao, W.T.; Chen, F.F.; Zhu, Y.J.; Zhang, Y.G.; Jiang, Y.Y.; Ma, M.G.; Chen, F. Binary strengthening and toughening of MXene/cellulose nanofiber composite paper with nacre-inspired structure and superior electromagnetic interference shielding properties. ACS Nano 2018, 12, 4583–4593. [Google Scholar] [CrossRef]
- Ihsanullah, I. Potential of MXenes in Water Desalination: Current Status and Perspectives. Nano-Micro Lett. 2020, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowska, A. Colloidal Properties and Stability of 2D Ti3C2 and Ti2C MXenes in Water. Int. J. Electrochem. Sci. 2018, 13, 10837–10847. [Google Scholar]
- Orimolade, B.O.; Idris, A.O.; Feleni, U.; Mamba, B. Recent advances in degradation of pharmaceuticals using Bi2WO6 mediated photocatalysis—A comprehensive review. Environ. Pollut. 2021, 289, 117891. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, D.; Shen, K.; Nie, S.; Liu, M.; Huang, H.; Deng, F.; Zhou, N.; Zhang, X.; Wei, Y. Biomimetic anchoring of Fe3O4 onto Ti3C2 MXene for highly efficient removal of organic dyes by Fenton reaction. J. Environ. Chem. Eng. 2020, 8, 104369. [Google Scholar] [CrossRef]
- Jiang, X.; Kuklin, A.V.; Baev, A.; Ge, Y.; Ågren, H.; Zhang, H.; Prasad, P.N. Two-dimensional MXenes: From morphological to optical, electric, and magnetic properties and applications. Phys. Rep. 2020, 848, 1–58. [Google Scholar] [CrossRef]
- Szuplewska, A.; Kulpińska, D.; Jakubczak, M.; Dybko, A.; Chudy, M.; Olszyna, A.; Brzózka, Z.; Jastrz˛ebska, A.M. The 10th anniversary of MXenes: Challenges and prospects for their surface modification toward future biotechnological applications. Adv. Drug Deliv. Rev. 2022, 182, 114099. [Google Scholar] [CrossRef]
- Jakubczak, M.; Karwowska, E.; Rozmysłowska-Wojciechowska, A.; Petrus, M.; Woźniak, J.; Mitrzak, J.; Jastrz˛ebska, A.M. Filtration Materials Modified with 2D Nanocomposites—A New Perspective for Point-of-Use Water Treatment. Materials 2021, 14, 182. [Google Scholar] [CrossRef] [PubMed]
- Szuplewska, A.; Rozmysłowska-Wojciechowska, A.; Pózniak, S.; Wojciechowski, T.; Birowska, M.; Popielski, M.; Chudy, M.; Ziemkowska, W.; Chlubny, L.; Moszczynska, D.; et al. Multilayered stable 2D nano-sheets of Ti2NTx MXene: Synthesis, characterization, and anticancer activity. J. Nanobiotechnol. 2019, 17, 114. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Mendes, R.G.; Bachmatiuk, A.; Zhao, L.; Ta, H.Q.; Gemming, T.; Liu, H.; Liu, Z.; Rummeli, M.H. Applications of 2D MXenes in energy conversion and storage systems. Chem. Soc. Rev. 2019, 48, 72–133. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, S.; Shi, R.; Waterhouse, G.I.N.; Tang, J.; Zhang, T. Two-dimensional photocatalyst design: A critical review of recent experimental and computational advances. Mater. Today 2020, 34, 78–91. [Google Scholar] [CrossRef]
- Xu, J.; Li, W.; Hou, Y. Two-Dimensional Magnetic Nanostructures. Trends Chem. 2020, 2, 163–173. [Google Scholar] [CrossRef]
- Anichini, C.; Czepa, W.; Pakulski, D.; Aliprandi, A.; Ciesielski, A.; Samorì, P. Chemical sensing with 2D materials. Chem. Soc. Rev. 2018, 47, 4860–4908. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bai, Y.; Shi, X.; Su, N.; Nie, G.; Zhang, R.; Nie, H.; Ye, L. Applications of MXene (Ti3C2Tx) in photocatalysis: A review. Mater. Adv. 2021, 2, 1570–1594. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Sahoo, S.; Wang, N.; Huczko, A. Graphene research and their outputs: Status and prospect. J. Sci. Adv. Mater. Devices 2020, 5, 10–29. [Google Scholar] [CrossRef]
- Katsnelson, M.I. Graphene: Carbon in two dimensions. Mater. Today 2007, 10, 20–27. [Google Scholar] [CrossRef]
- Mas-Ballesté, R.; Navarro, C.; Zamora, F. 2D materials: To graphene and beyond. Nanoscale 2011, 3, 20–30. [Google Scholar] [CrossRef]
- Jakubczak, M.; Szuplewska, A.; Rozmysłowska-Wojciechowska, A.; Rosenkranz, A.; Jastrz˛ebska, A.M. Novel 2D MBenes-Synthesis, Structure, and Biotechnological Potential. Adv. Funct. Mater. 2021, 31, 2103048. [Google Scholar] [CrossRef]
- Zha, X.-J.; Zhao, X.; Pu, J.-H.; Tang, L.-S.; Ke, K.; Bao, R.-Y.; Bai, L.; Liu, Z.-Y.; Yang, M.-B.; Yang, W. Flexible Anti-Biofouling MXene/Cellulose Fibrous Membrane for Sustainable Solar-Driven Water Purification. ACS Appl. Mater. Interfaces 2019, 11, 36589. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-Y.; Li, Y.-H.; Zhang, F.; Tang, Z.-R.; Xu, Y.-J. Visible-light-driven integrated organic synthesis and hydrogen evolution over 1D/2D CdS-Ti3C2Tx MXene composites. Appl. Catal. B Environ. 2020, 269, 118783. [Google Scholar] [CrossRef]
- Shen, J.Y.; Shen, J.; Zhang, W.; Yu, X.; Tang, H.; Zhang, M.; Zulfiwar; Liu, Q. Built-in electric field induced CeO2/Ti3C2-MXene Schottky-junction for coupled photocatalytic tetracycline degradation and CO2 reduction. Ceram. Int. 2019, 45, 24146–24153. [Google Scholar] [CrossRef]
- Daixun, J.; Sun, X.; Wu, X.; Zhang, S.; Qu, X.; Shi, L.; Zhang, Y.; Du, F. MXene-Ti3C2 assisted one-step synthesis of carbon-supported TiO2/Bi4NbO8Cl heterostructures for enhanced photocatalytic water decontamination. Nanophotonics 2020, 9, 2077–2088. [Google Scholar]
- Qin, J.; Hu, X.; Li, X.; Yin, Z.; Liu, B.; Lam, K.-h. 0D/2D AgInS2/MXene Z-scheme heterojunction nanosheets for improved ammonia photosynthesis of N2. Nanomater. Energy 2019, 61, 27–35. [Google Scholar] [CrossRef]
- Zhao, D.; Cai, C. Layered Ti3C2 MXene modified two-dimensional Bi2WO6 composites with enhanced visible light photocatalytic performance. Mater. Chem. Front. 2019, 3, 2521–2528. [Google Scholar] [CrossRef]
- Li, J.; Zhao, L.; Wang, S.; Li, J.; Wang, G.; Wang, J. In situ fabrication of 2D/3D g-C3N4/Ti3C2 (MXene) heterojunction for efficient visible-light photocatalytic hydrogen evolution. Appl. Surf. Sci. 2020, 515, 145922. [Google Scholar] [CrossRef]
- Cai, T.; Wang, L.; Liu, Y.; Zhang, S.; Dong, W.; Chen, H.; Yi, X.; Yuan, J.; Xia, X.; Liu, C.; et al. Ag3PO4/Ti3C2 MXene interface materials as a Schottky catalyst with enhanced photocatalytic activities and anti-photocorrosion performance. Appl. Catal. B Environ. 2018, 239, 545–554. [Google Scholar] [CrossRef]
- He, F.; Zhu, B.; Cheng, B.; Yu, J.; Ho, W.; Macyk, W. 2D/2D/0D TiO2/C3N4/Ti3C2 MXene composite S-scheme photocatalyst with enhanced CO2 reduction activity. Appl. Catal. B Environ. 2020, 272, 119006. [Google Scholar] [CrossRef]
- Li, B.; Song, H.; Han, F.; Wei, L. Photocatalytic oxidative desulfurization and denitrogenation for fuels in ambient air over Ti3C2/g-C3N4 composites under visible light irradiation. Appl. Catal. B Environ. 2020, 269, 118845. [Google Scholar] [CrossRef]
- Xu, T.; Wang, J.; Cong, Y.; Jiang, S.; Zhang, Q.; Zhu, H.; Li, Y.; Li, X. Ternary BiOBr/TiO2/Ti3C2Tx MXene nanocomposites with heterojunction structure and improved photocatalysis performance. Chin. Chem. Lett. 2020, 31, 1022–1025. [Google Scholar] [CrossRef]
- Fang, H.; Pan, Y.; Yan, H.; Qin, X.; Wang, C.; Xu, L.; Pan, C. Facile preparation of Yb3+/Tm3+ co-doped Ti3C2/Ag/Ag3VO4 composite with an efficient charge separation for boosting visible-light photocatalytic activity. Appl. Surf. Sci. 2020, 527, 146909. [Google Scholar] [CrossRef]
- Vigneshwaran, S.; Karthikeyan, P.; Park, C.M.; Meenakshi, S. Boosted insights of novel accordion-like (2D/2D) hybrid photocatalyst for the removal of cationic dyes: Mechanistic and degradation pathways. J. Environ. Manag. 2020, 273, 111125. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Du, Y.; Liao, W. Enhanced photocatalytic performance of TiO2@C nanosheets derived from two-dimensional Ti2CTx. Ceram. Int. 2018, 44, 7042–7046. [Google Scholar] [CrossRef]
- Yao, Z.; Sun, H.; Sui, H.; Liu, X. Construction of BPQDs/Ti3C2@TiO2 composites with favorable charge transfer channels for enhanced photocatalytic activity under visible light irradiation. Nanomaterials 2020, 10, 15. [Google Scholar] [CrossRef]
- Yin, J.; Zhan, F.; Jiao, T.; Wang, W.; Zhang, G.; Jiao, J.; Jiang, G.; Zhang, Q.; Gu, J.; Peng, Q. Facile preparation of self-assembled MXene@Au@CdS nanocomposite with enhanced photocatalytic hydrogen production activity. Sci. China-Mater. 2020, 63, 2228–2238. [Google Scholar] [CrossRef]
- Li, Y.; Ding, L.; Yin, S.; Liang, Z.; Xue, Y.; Wang, X.; Cui, H.; Tian, J. Photocatalytic H2 Evolution on TiO2 Assembled with Ti3C2 MXene and Metallic 1T-WS2 as Co-catalysts. Nano-Micro Lett. 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Li, Y.J.; Yang, S.; Liang, Z.; Xue, Y.; Cui, H.; Tian, J. 1T-MoS2 nanopatch/Ti3C2 MXene/TiO2 nanosheet hybrids for efficient photocatalytic hydrogen evolution. Mater. Chem. Front. 2019, 3, 2673–2680. [Google Scholar] [CrossRef]
- Ding, X.; Li, Y.; Li, C.; Wang, W.; Feng, L.; Han, D. 2D visible-light-driven TiO2@Ti3C2/g-C3N4 ternary heterostructure for high photocatalytic activity. J. Mater. Sci. 2019, 54, 9385–9396. [Google Scholar] [CrossRef]
- Wu, Z.; Liang, Y.; Yuan, X.; Zou, D.; Fang, J.; Jiang, L.; Zhang, J.; Yang, H.; Xiao, Z. MXene Ti3C2 derived Z–scheme photocatalyst of graphene layers anchored TiO2/g–C3N4 for visible light photocatalytic degradation of refractory organic pollutants. Chem. Eng. J. 2020, 394, 124921. [Google Scholar] [CrossRef]
- Wu, S.; Su, Y.; Zhu, Y.; Zhang, Y.; Zhu, M. In-situ growing Bi/BiOCl microspheres on Ti3C2 nanosheets for upgrading visible-light-driven photocatalytic activity. Appl. Surf. Sci. 2020, 520, 146339. [Google Scholar] [CrossRef]
- Mohanty, R.; Mishra, A.; Khatei, J.; Singh, L.; Mahapatra, D.M. Adapting 2D Nanomaterials for Advanced Applications; ACS Publications: Washington, DC, USA, 2020; pp. 1–31. [Google Scholar]
- Shao, B.; Liu, Z.; Zeng, G.; Wang, H.; Liang, Q.; He, Q.; Cheng, M.; Zhou, C.; Jiang, L.; Song, B. Two-dimensional transition metal carbide and nitride (MXene) derived quantum dots (QDs): Synthesis, properties, applications and prospects. J. Mater. Chem. A 2020, 8, 7508–7535. [Google Scholar] [CrossRef]
- He, P.; Wang, X.X.; Cai, Y.Z.; Shu, J.C.; Zhao, Q.L.; Yuan, J.; Cao, M.S. Tailoring Ti3C2Tx nanosheets to tune local conductive network as an environmentally friendly material for highly efficient electromagnetic interference shielding. Nanoscale 2019, 11, 6080. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-W.; Unnikrishnan, B.; Chen, I.-W.P.; Harroun, S.G.; Chang, H.-T.; Huang, C.-C. Excellent oxidation resistive MXene aqueous ink for micro-supercapacitor application. Energy Storage Mater. 2020, 25, 563–571. [Google Scholar] [CrossRef]
- Wei, Z.; Peigen, Z.; Wubian, T.; Xia, Q.; Yamei, Z.; Zheng Ming, S. Alkali treated Ti3C2Tx MXenes and their dye adsorption performance. Mater. Chem. Phys. 2018, 206, 270. [Google Scholar] [CrossRef]
- Shahzad, A.; Rasool, K.; Miran, W.; Nawaz, M.; Jang, J.; Mahmoud, K.A.; Lee, D.S. Two-Dimensional Ti3C2Tx MXene Nanosheets for Efficient Copper Removal from Water. ACS Sustain. Chem. Eng. 2017, 5, 11481. [Google Scholar]
- Peng, Q.; Guo, J.; Zhang, Q.; Xiang, J.; Liu, B.; Zhou, A.; Liu, R.; Tian, Y. Unique Lead Adsorption Behavior of Activated Hydroxyl Group in Two-Dimensional Titanium Carbide J. Am. Chem. Soc. 2014, 136, 4113. [Google Scholar] [CrossRef]
- Shahzad, A.; Rasool, K.; Miran, W.; Nawaz, M.; Jang, J.; Mahmoud, K.A.; Lee, D.S.J. Mercuric ion capturing by recoverable titanium carbide magnetic nanocomposite. J. Hazard. Mater. 2018, 344, 811. [Google Scholar]
- Qiao, X.Q.; Hu, F.C.; Tian, F.Y.; Hou, D.F.; Li, D.S. Equilibrium and kinetics studies on MB adsorption by ultrathin 2D MoS2 nanosheets. RSC Adv. 2016, 6, 11631. [Google Scholar] [CrossRef]
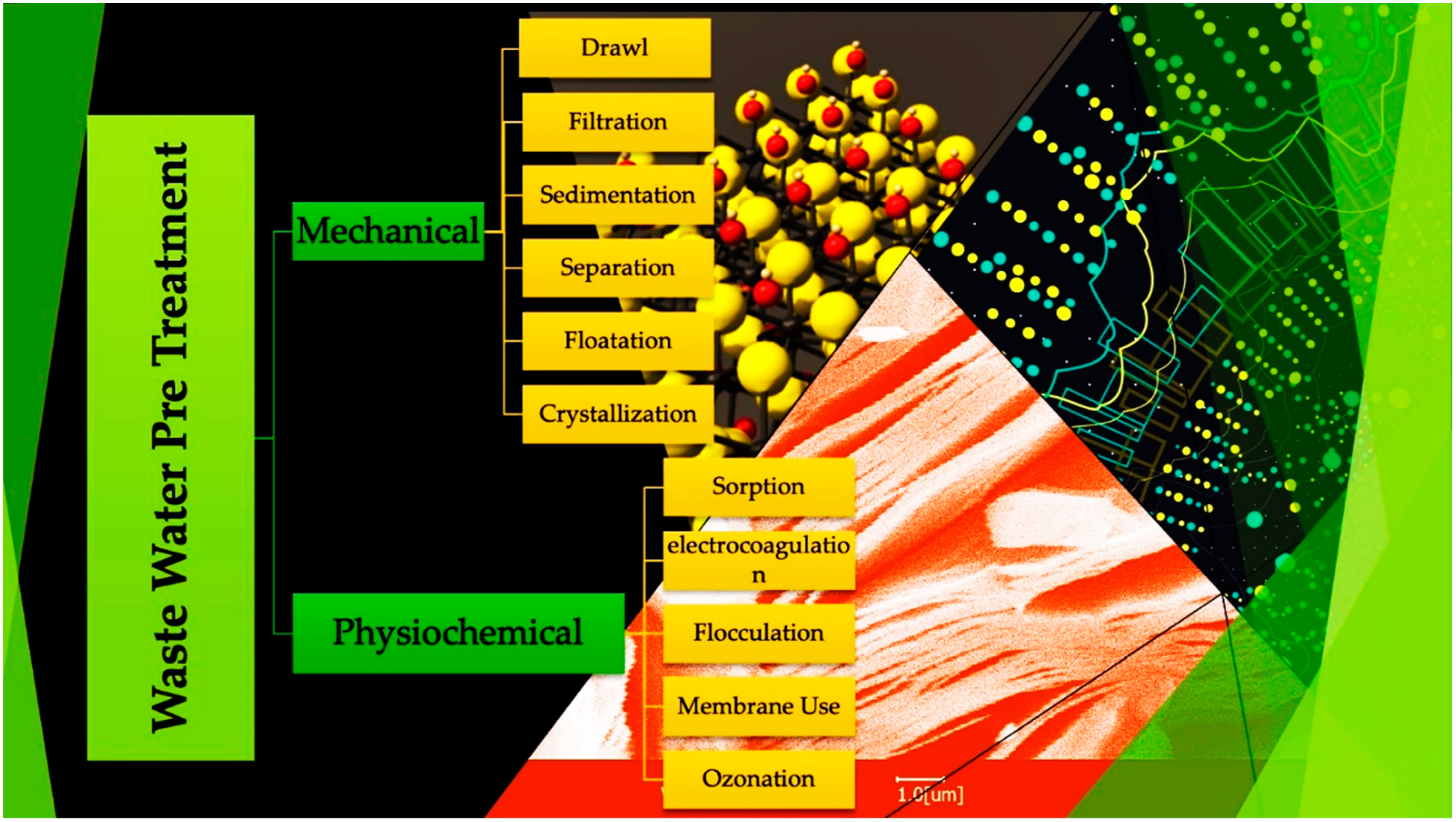
- Gunatilake, S.K. Methods of Removing Heavy Metals from Industrial Wastewater. J. Multidiscip. Eng. Sci. Stud. 2015, 1, 12. [Google Scholar]
- Demirbas, A.J. Heavy metal adsorption onto agro-based waste materials: A review. J. Hazard. Mater. 2008, 157, 220. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazard. Mater. 2012, 211, 317. [Google Scholar] [CrossRef]
- Tofighy, M.A.; Mohammadi, T.J. Adsorption of divalent heavy metal ions from water using carbon nanotube sheets. J. Hazard. Mater. 2011, 185, 140. [Google Scholar] [CrossRef] [PubMed]
- Meng, A.; Zhang, L.; Cheng, B.; Yu, J. Dual cocatalysts in TiO2 photocatalysis. Adv. Mater. 2019, 31, 1807660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, W.; Xi, H.; Li, Q.; Shen, M.; Ying, G.; Zhang, J. Polydopamine functionalized cellulose-MXene composite aerogel with superior adsorption of methylene blue. Cellulose 2021, 28, 4281. [Google Scholar] [CrossRef]
- Mu, W.; Du, S.; Yu, Q.; Li, X.; Wei, H.; Yang, Y. Improving barium ion adsorption on two-dimensional titanium carbide by surface modification. Dalton. Trans. 2018, 47, 8375. [Google Scholar] [CrossRef]
- Liu, T.; Liu, X.; Graham, N.; Yu, W.; Sun, K.J. Two-dimensional MXene incorporated graphene oxide composite membrane with enhanced water purification performance. Membr. Sci. 2020, 593, 117431. [Google Scholar]
- Jin, L.; Chai, L.; Yang, W.; Wang, H.; Zhang, L. Two-Dimensional Titanium Carbides (Ti3C2Tx) Functionalized by Poly(m-phenylenediamine) for Efficient Adsorption and Reduction of Hexavalent Chromium. Int. J. Environ. Res. Public Health 2019, 17, 167. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, H.; Xu, J.; Du, X.; Cheng, X.; Du, Z.; Wang, H. Fabrication of MXene/PEI functionalized sodium alginate aerogel and its excellent adsorption behavior for Cr(VI) and Congo Red from aqueous solution. J. Hazard. Mater. 2021, 416, 125777. [Google Scholar] [CrossRef]
- Yang, G.; Hu, X.; Liang, J.; Huang, Q.; Dou, J.; Tian, J.; Deng, F.; Liu, M.; Zhang, X.; Wei, Y.J. Surface functionalization of MXene with chitosan through in-situ formation of polyimidazoles and its adsorption properties. J. Hazard. Mater. 2021, 419, 126220. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, L.; Sun, D.; Zhang, Y.; Liu, B.; Hu, Q.; Zhou, A. Synthesis and thermal stability of two-dimensional carbide MXene Ti3C2. Mater. Sci. Eng. B 2015, 191, 33. [Google Scholar] [CrossRef]
- Ghosh, A.; Nayak, A.K.; Pal, A. Nano-Particle-Mediated Wastewater Treatment: A Review. Curr. Pollut. 2017, 3, 17–30. [Google Scholar] [CrossRef]
- Zunita, M. Graphene oxide-based nanofilteration for Hg removal from wastewater: A mini Review. Membranes 2021, 11, 269. [Google Scholar] [CrossRef] [PubMed]
- Kommu, A.; Singh, J.K. A review on graphene-based materials for removal of toxic pollutants from wastewater. Soft Mater. 2020, 18, 297–322. [Google Scholar] [CrossRef]
- Sires, I.; Brillas, E.; Oturan, M.A.; Panizza, M. Electrochemical advanced oxidation processes: Today and tomorrow. A review. Environ. Sci. Pollut. Res. 2014, 21, 8336–8367. [Google Scholar] [CrossRef]
- Ibrahim, Y.; Kassab, A.; Eid, K.; Abdullah, A.M.; Ozoemena, K.I.; Elzatahry, A. Unveiling fabrication and environmental remediation of mxene-based nanoarchitectures in toxic metals removal from wastewater: Strategy and mechanism. Nanomaterials 2020, 10, 885. [Google Scholar] [CrossRef]
- Lindsey, M.E.; Meyer, M.; Thurman, E.M. Analysis of trace level of Sulfonamide and Tetracycline Antimicrobials in Ground water and Surface water using Solid-Phase Extraction and Liquid Chromatography/Mass spectrometery. Anal. Chem. 2001, 73, 4640–4646. [Google Scholar] [CrossRef]
- Santos, J.L.; Aparicio, I.; Callejon, M.; Alonso, E. Occurrence of pharmaceutically active compounds during 1-year in wastewater from four wastewater treatment plants in Seville (Spain). J. Hazard. Mater. 2009, 164, 1509–1516. [Google Scholar] [CrossRef]
- Kosma, C.I.; Nannou, C.I.; Boti, V.I.; Albanis, T.A. Psychiatrics and selected metabolites in hospital and urban wastewater: Occurrence, removal, mass loading, seasonal influence and risk assessment. Sci. Total Environ. 2019, 659, 1473–1483. [Google Scholar] [CrossRef]
- Vickers, N.J. Animal communication: When I am calling you, will you answer too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef] [PubMed]
- Monteil, H.; Pechaud, Y.; Oturan, M.A. A review on efficiency and cost effectiveness of electro- and bio-electro-Fenton processes: Applications to the treatment of pharmaceutical pollutants in water. Chem. Eng. J. 2019, 376, 119577. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.-H.; Nghiiem, L.-D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Kharel, S.; Stapf, M.; Miehe, U.; Ekbald, M.; Cimbritz, M.; Falas, P.; Nilsson, J.; Sehlen, R.; Bester, K. Ozone dose dependent formation and removal of ozonation products of pharmaceuticals in pilot and full-scale municipal wastewater treatment plants. Sci. Total Environ. 2020, 731, 139064. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.; Harris, S.; Morris, C.; Commind, E.; Cormican, M. Hospital Effluent: Impact on the Microbial Environment and Risk to the Human Health; Report No. 162; Environmental Protection Agency: Wexford, Ireland, 2016. [Google Scholar]
- Dominika, S.; Dominika, B.; Marcinowski, P.; Bogacki, J.; Michał, J.; Agnieszka, J.-E. Two-Dimensional Nanostructures in the World of Advanced Oxidation Processes. Catalysts 2022, 12, 358. [Google Scholar]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction photocatalysis. Adv. Mater. 2017, 29, 1601694. [Google Scholar]
- Chen, Z.; Yang, S.; Tian, Z.; Zhu, B. Nis and graphene as dual cocatalysts for the enhanced photocatalytic H2 production activity of g-C3N4. Appl. Surf. Sci. 2019, 469, 657. [Google Scholar] [CrossRef]
- Gao, M.; Zhu, L.; Peh, C.K.; Ho, W. Solar absorber material and system designs for photothermal water vaporization towards clean water and energy production. Energy Environ. Sci. 2019, 12, 841. [Google Scholar]
- Yi, X.; Yuan, J.; Tang, H.; Du, Y.; Hassan, B.; Yin, K.; Chen, Y.; Liu, X. Embedding few layer Ti3C2Tx into alkalized g-C3N4 nanosheets for efficient photocatalytic degradation. J. Colloid Interface Sci. 2020, 571, 297–306. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, M.; Liang, H.; Chen, J.; Xu, L.; Niu, J. Novel dual-effective Z-scheme heterojunction with g-C3N4, Ti3C2 MXene and black phosphorus for improving visible light-induced degradation of ciprofloxacin. Appl. Catal. B Environ. 2021, 291, 120105. [Google Scholar] [CrossRef]
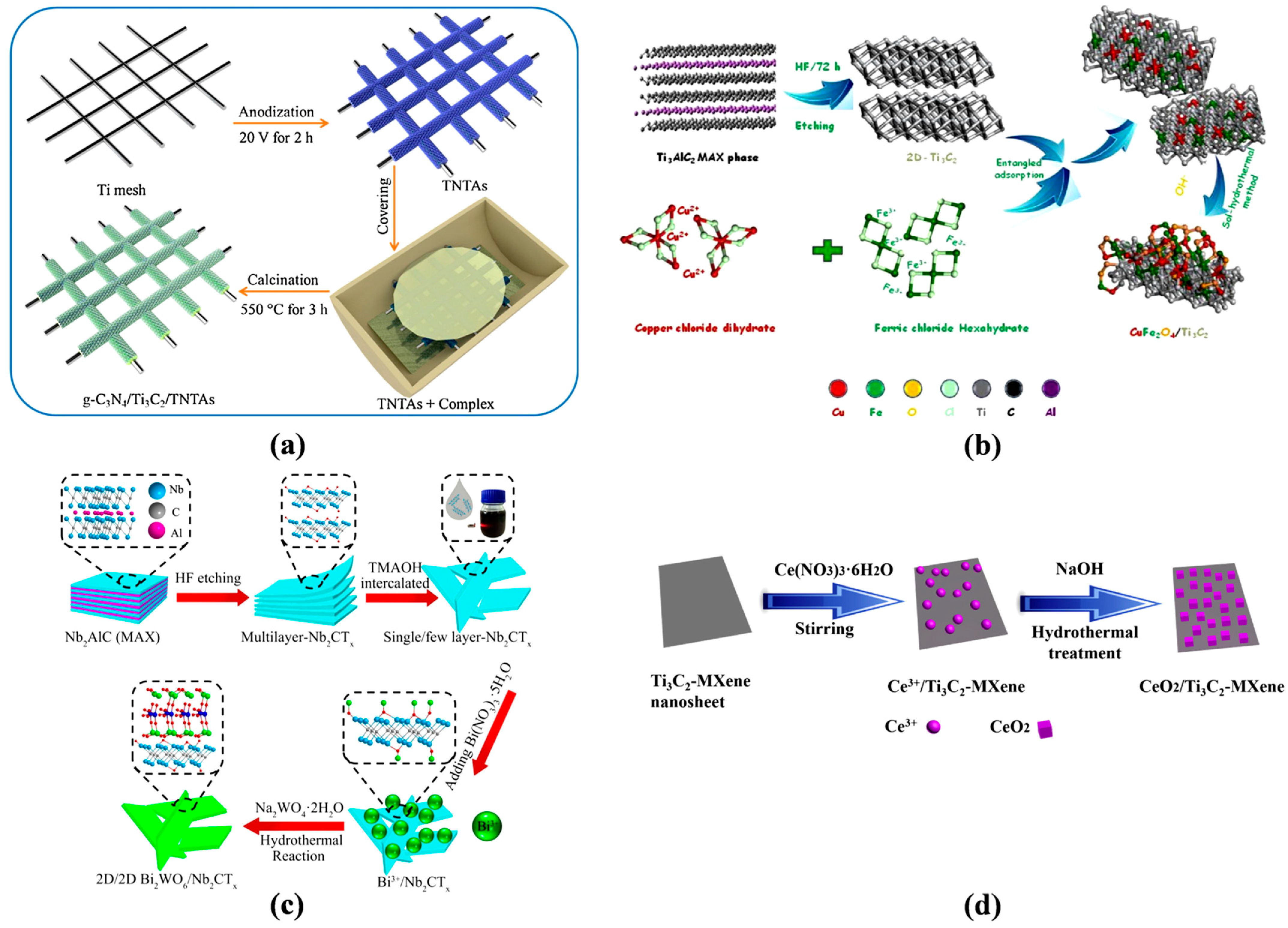
- Diao, Y.; Yan, M.; Li, X.; Zhou, C.; Peng, B.; Chen, H.; Zhang, H. In-situ grown of g-C3N4/Ti3C2/TiO2 nanotube arrays on Ti meshes for efficient degradation of organic pollutants under visible light irradiation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 594, 124511. [Google Scholar] [CrossRef]
- Cao, Y.; Fang, Y.; Lei, X.; Tan, B.; Hu, X.; Liu, B.; Chen, Q. Fabrication of novel CuFe2O4/MXene hierarchical heterostructures for enhanced photocatalytic degradation of sulfonamides under visible light. J. Hazard. Mater. 2020, 387, 122021. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Guo, R.; Xiao, H.; Ren, E.; Song, Q.; Xiang, C.; Lai, X.; Lan, J.; Jiang, S. Bi2WO6/Nb2CTx MXene hybrid nanosheets with enhanced visible-light-driven photocatalytic activity for organic pollutants degradation. Appl. Surf. Sci. 2020, 505, 144595. [Google Scholar] [CrossRef]
- Zou, X.; Zhao, X.; Zhang, J.; Lv, W.; Qiu, L.; Zhang, Z. Photocatalytic degradation of ranitidine and reduction of nitrosamine dimethylamine formation potential over MXene–Ti3C2/MoS2 under visible light irradiation. J. Hazard. Mater. 2021, 413, 125424. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Yang, Q.; Wang, D.; Yao, F.; Cao, J.; Chen, Z.; Huang, X.; Yang, Y.; Li, X. Mxene-modulated dual-heterojunction generation on a metal-organic framework (MOF) via surface constitution reconstruction for enhanced photocatalytic activity. Chem. Eng. J. 2020, 390, 124519. [Google Scholar] [CrossRef]
- Liu, N.; Lu, N.; Su, Y.; Wang, P.; Quan, X. Fabrication of g-C3N4/Ti3C2 composite and its visible-light photocatalytic capability for ciprofloxacin degradation. Sep. Purif. Technol. 2019, 211, 782–789. [Google Scholar] [CrossRef]
- Ma, Y.; Lv, X.; Xiong, D.; Zhao, X.; Zhang, Z. Catalytic degradation of ranitidine using novel magnetic Ti3C2-based MXene nanosheets modified with nanoscale zero-valent iron particles. Appl. Catal. B Environ. 2021, 284, 119720. [Google Scholar] [CrossRef]
- Fayyaz, A.; Saravanakumar, K.; Talukdar, K.; Kim, Y.; Yoon, Y.; Park, C.M. Catalytic oxidation of naproxen in cobalt spinel ferrite decorated Ti3C2Tx MXene activated persulfate system: Mechanisms and pathways. Chem. Eng. J. 2021, 407, 27842. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Y. 2D Early Transition Metal Carbides (MXenes) for Catalysis. Small 2019, 15, 1804736. [Google Scholar] [CrossRef]
- Kuang, P.; Low, J.; Cheng, B.; Yu, J.; Fan, J.J. MXene-based photocatalysts. Mater. Sci. Technol. 2020, 56, 18. [Google Scholar] [CrossRef]
- Yang, C.; Tan, Q.; Li, Q.; Zhou, J.; Fan, J.; Li, B.; Sun, J.; Lv, K. 2D/2D Ti3C2 MXene/g-C3N4 nanosheets heterojunction for high efficient CO2 reduction photocatalyst: Dual effects of urea. Appl. Catal. B 2020, 268, 118738. [Google Scholar] [CrossRef]
- Xu, C.; Yang, F.; Deng, B.; Che, S.; Yang, W.; Zhang, G.; Sun, Y.; Li, Y.J. RGO-wrapped Ti3C2/TiO2 nanowires as a highly efficient photocatalyst for simultaneous reduction of Cr(VI) and degradation of RhB under visible light irradiation. J. Alloys Compd. 2021, 874, 159865. [Google Scholar] [CrossRef]
- Yang, J.-X.; Yu, W.-B.; Li, C.-F.; Dong, W.-D.; Jiang, L.-Q.; Zhou, N.; Zhuang, Z.-P.; Liu, J.; Hu, Z.-Y.; Zhao, H.; et al. PtO nanodots promoting Ti3C2 MXene in-situ converted Ti3C2/TiO2 composites for photocatalytic hydrogen production. Chem. Eng. J. 2021, 420, 129695. [Google Scholar]
- Zhang, L.; Ma, P.; Dai, L.; Li, S.; Yu, W.; Guan, J. In situ crystallization and growth of TiO2 nanospheres between MXene layers for improved adsorption and visible light photocatalysis. Catal. Sci. Technol. 2021, 11, 3834. [Google Scholar]
- Sha, X.; Huang, H.H.; Sun, S.; Huang, H.H.; Huang, Q.; He, Z.; Liu, M.; Zhou, N.; Zhang, X.; Wei, Y.J. Mussel-inspired preparation of MXene-PDA-Bi6O7 composites for efficient adsorptive removal of iodide ions. Environ. Chem. Eng. 2020, 8, 104261. [Google Scholar]
- Zhao, Q.; Wang, J.; Li, Z.; Guo, Y.; Tang, B.; Abudula, A.; Guan, G. Two-dimensional Ti3C2TX-nanosheets/Cu2O composite as a high-performance photocatalyst for decomposition of tetracycline. Carbon Resour. Convers. 2021, 4, 197. [Google Scholar]
- Nosaka, Y.; Nosaka, A.Y. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Deng, L.; Luo, J.; Crittenden, J.; Liu, C.; Wei, Y.; Wang, L. Destruction of phenicol antibiotics using the UV/H2O2 process: Kinetics, byproducts, toxicity evaluation and trichloromethane formation potential. Chem. Eng. J. 2018, 351, 867–877. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Tariq, A.; Zaheer, A.; Gul, S.; Ali, S.I.; Iqbal, M.Z.; Akinwande, D.; Rizwan, S. Ti3C2-MXene/bismuth ferrite nanohybrids for efficient degradation of organic dyes and colorless pollutants. ACS Omega 2019, 4, 20530–20539. [Google Scholar] [CrossRef]
- Ayesha, J.; Shoomaila, L.; Muhammad, I.; Nazim, H.; Muhammad, B.; Hafiz, M.N.I. MXene-based hybrid composites as photocatalyst for the mitigation of pharmaceuticals. Chemosphere 2022, 291, 133062. [Google Scholar]
- Narayanam, J.M.; Stephenson, C.R. Visible light photoredox catalysis: Applications in organic synthesis. Chem. Soc. Rev. 2011, 40, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Iocozzia, J.; Wang, Y.; Cui, X.; Chen, Y.; Zhao, S.; Li, Z.; Lin, Z. Noble metal–metal oxide nanohybrids with tailored nanostructures for efficient solar energy conversion, photocatalysis and environmental remediation. Energy Environ. Sci. 2017, 10, 402–434. [Google Scholar] [CrossRef]
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Müller, J.-O.; Schlögl, R.; Carlsson, J.M. Graphitic carbon nitride materials: Variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893–4908. [Google Scholar] [CrossRef]
- Masih, D.; Ma, Y.; Rohani, S. Graphitic C3N4 based noble-metal-free photocatalyst systems: A review. Appl. Catal. B Environ. 2017, 206, 556–588. [Google Scholar] [CrossRef]
- Sun, S.; Liang, S. Recent advances in functional mesoporous graphitic carbon nitride (mpg-C3N4) polymers. Nanoscale 2017, 9, 10544–10578. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tan, X.; Wang, S.; Ma, F.; Znad, H.; Shen, Z.; Liu, L.; Liu, S. MXene as a non-metal charge mediator in 2D layered CdS@Ti3C2@TiO2 composites with superior Z-scheme visible light-driven photocatalytic activity. Environ. Sci. Nano 2019, 6, 3158–3169. [Google Scholar] [CrossRef]
- Wojciechowski, T.; Rozmysłowska-Wojciechowska, A.; Matyszczak, G.; Wrzecionek, M.; Olszyna, A.; Peter, A.; Mihaly-Cozmuta, A.; Nicula, C.; Mihaly-Cozmuta, L.; Podsiadło, S. Ti2C MXene modified with ceramic oxide and noble metal nanoparticles: Synthesis, morphostructural properties, and high photocatalytic activity. Inorg. Chem. 2019, 58, 7602–7614. [Google Scholar] [CrossRef]
- Jang, J.; Shahzad, A.; Woo, S.H.; Lee, D.S. Magnetic Ti3C2Tx (Mxene) for diclofenac degradation via the ultraviolet/chlorine advanced oxidation process. Environ. Res. 2020, 182, 108990. [Google Scholar] [CrossRef]
- Grzegórska, A.; Głuchowski, P.; Karczewski, J.; Ryl, J.; Wysocka, I.; Siuzdak, K.; Trykowski, G.; Grochowska, K.; Zielińska-Jurek, A. Enhanced photocatalytic activity of accordion-like layered Ti3C2 (MXene) coupled with Fe-modified decahedral anatase particles exposing {1 0 1} and {0 0 1} facets. Chem. Eng. J. 2021, 426, 130801. [Google Scholar] [CrossRef]
- Xu, D.; Ma, Y.; Wang, J.; Chen, W.; Tang, Y.; Li, X.; Li, L. Interfacial engineering of 2D/2D MXene heterostructures: Face-to-face contact for augmented photodegradation of amoxicillin. Chem. Eng. J. 2021, 426, 131246. [Google Scholar] [CrossRef]
- Tang, Y.; Hu, X.; Zhang, H.; Cao, J.; Liu, J.; Zhang, L.; Chen, X.; Chen, T. Metal-based MXene nanocomposites for catalytic applications. Nanoscale 2018, 10, 3233–3240. [Google Scholar]
- Wu, Y.; Jiang, H.; Wang, X.; Zhang, Y.; Yu, Y. Recent progress in synthesis, properties and applications of MXene-based nanocomposites. J. Mater. Sci. Technol. 2019, 35, 1987–2000. [Google Scholar]
- Zeng, X.; Yin, L.; Zhang, Y.; Chen, T.; Yu, Y. Metal-based MXene nanocomposites for antibacterial applications. J. Colloid Interface Sci. 2021, 583, 98–110. [Google Scholar]
- Shao, B.; Wang, J.; Liu, Z.; Zeng, G.; Tang, L.; Liang, Q.; He, Q.; Wu, T.; Liu, Y.; Yuan, X. Ti3C2Tx MXene decorated black phosphorus nanosheets with improved visible-light photocatalytic activity: Experimental and theoretical studies. J. Mater. Chem. 2020, 8, 5171–5185. [Google Scholar] [CrossRef]
- Li, S.; Shao, L.; Yang, Z.; Cheng, S.; Yang, C.; Liu, Y.; Xia, X. Constructing Ti3C2 MXene/ZnIn2S4 heterostructure as a Schottky catalyst for photocatalytic environmental remediation. Green Energy Environ. 2020, 7, 246–256. [Google Scholar] [CrossRef]
- Feng, X.; Yu, Z.; Sun, Y.; Shan, M.; Long, R.; Li, X. 3D MXene/Ag2S material as Schottky junction catalyst with stable and enhanced photocatalytic activity and photocorrosion resistance. Separ. Purif. Technol. 2021, 266, 118606. [Google Scholar] [CrossRef]
- Zhuge, Z.; Liu, X.; Chen, T.; Gong, Y.; Li, C.; Niu, L.; Xu, S.; Xu, X.; Alothman, Z.A.; Sun, C.Q. Highly efficient photocatalytic degradation of different hazardous contaminants by CaIn2S4-Ti3C2Tx Schottky heterojunction: An experimental and mechanism study. Chem. Eng. J. 2021, 421, 127838. [Google Scholar] [CrossRef]
| Photocatalysts | Application | Optimal Degradation Efficiency | Reference |
|---|---|---|---|
| CdS–Ti3C2Tx | HER | 15.4 mmol g−1·h−1 | [64] |
| CeO2/Ti3C2 | Removal of C22H24N2O8 + reduction of CO2 | 80.2% in 1h | [65] |
| C–TiO2/Bi4NbO8Cl | MO, CIP, RhB, and 2&4-DCP | >95% | [66] |
| AgInS2/MXene | Reduction of N2 | 38.8 μ⋅mol g−1·h−1 | [67] |
| Bi2WO6/Ti3C2 | C22H25ClN2O8 and RhB | 99.9% and 97% in 20 min and 60 min | [68] |
| g-C3N4/Ti3C2 | HER | 116.2 μ mol/h/g | [69] |
| Ag3PO4/Ti3C2 | C6H4N2O5 and C22H25ClN2O8 | >80% | [70] |
| TiO2/C3N4/Ti3C2 | CO2 reduction | 4.39 μ·mol·g−1·h−1 | [71] |
| Ti3C2/g-C3N4 | Degradation of C5H5N and C4H4S | 80% in 3 h | [72] |
| BiOBr/TiO2/Ti3C2Tx | RhB | 99.8% | [73] |
| Ti3C2/Ag/Ag3VO4 | TC, RhB, and MB | 97%, 96%, and 99% | [74] |
| CS@g-C3N4/MX | RhB and MB | 99% and 98.5% | [75] |
| TiO2@C/MXene | MB | 85.7% | [76] |
| BPQDS/Ti3C2@TiO2 | MO | 93% | [77] |
| MXene@Au@CdS | HER | 17,070.43 μ mol g−1⋅h−1 | [78] |
| 1T-WS2@TiO2@Ti3C2 | HER | 3409.8 μ mol g−1⋅h−1 | [79] |
| Ti3C2/TiO2/1T-MoS2 | HER | 9738 μ mol g−1⋅h−1 | [80] |
| TiO2@Ti3C2/g-C3N4 | C6H5NH2 and RhB | 99.9% and 98.2% | [81] |
| MXene/TiO2/g-C3N4 | BPA, CIP, TC, and RhB | 66.3%, 41.8%, 63.6%, and 92.1% | [82] |
| Ti3C2–Bi/BiOCl | C17H18FN3O3 | 89% | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irfan, S.; Khan, S.B.; Din, M.A.U.; Dong, F.; Chen, D. Retrospective on Exploring MXene-Based Nanomaterials: Photocatalytic Applications. Molecules 2023, 28, 2495. https://doi.org/10.3390/molecules28062495
Irfan S, Khan SB, Din MAU, Dong F, Chen D. Retrospective on Exploring MXene-Based Nanomaterials: Photocatalytic Applications. Molecules. 2023; 28(6):2495. https://doi.org/10.3390/molecules28062495
Chicago/Turabian StyleIrfan, Syed, Sadaf Bashir Khan, Muhammad Aizaz Ud Din, Fan Dong, and Deliang Chen. 2023. "Retrospective on Exploring MXene-Based Nanomaterials: Photocatalytic Applications" Molecules 28, no. 6: 2495. https://doi.org/10.3390/molecules28062495
APA StyleIrfan, S., Khan, S. B., Din, M. A. U., Dong, F., & Chen, D. (2023). Retrospective on Exploring MXene-Based Nanomaterials: Photocatalytic Applications. Molecules, 28(6), 2495. https://doi.org/10.3390/molecules28062495






