“Green” nZVI-Biochar as Fenton Catalyst: Perspective of Closing-the-Loop in Wastewater Treatment
Abstract
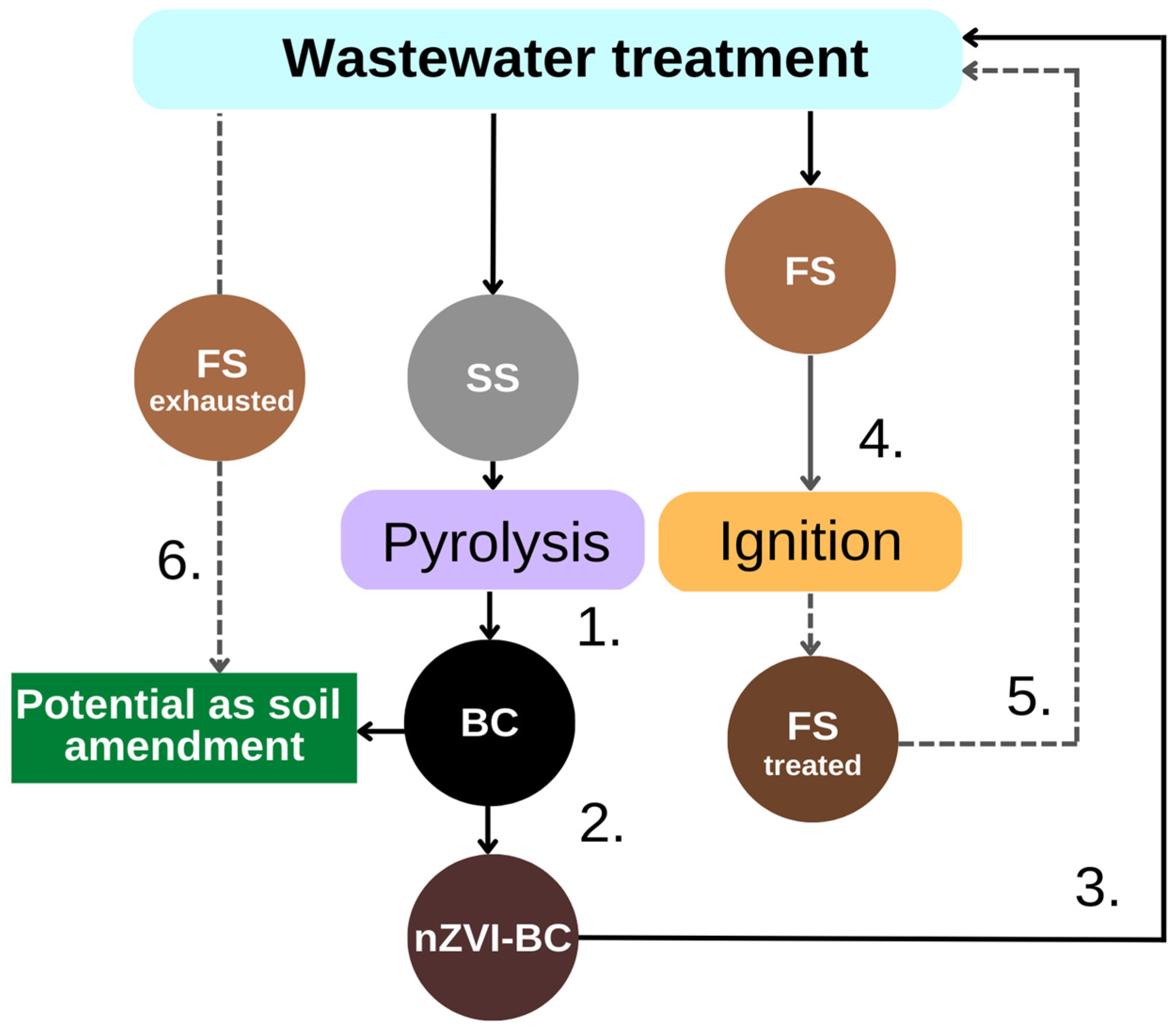
1. Introduction
2. Results and Discussion
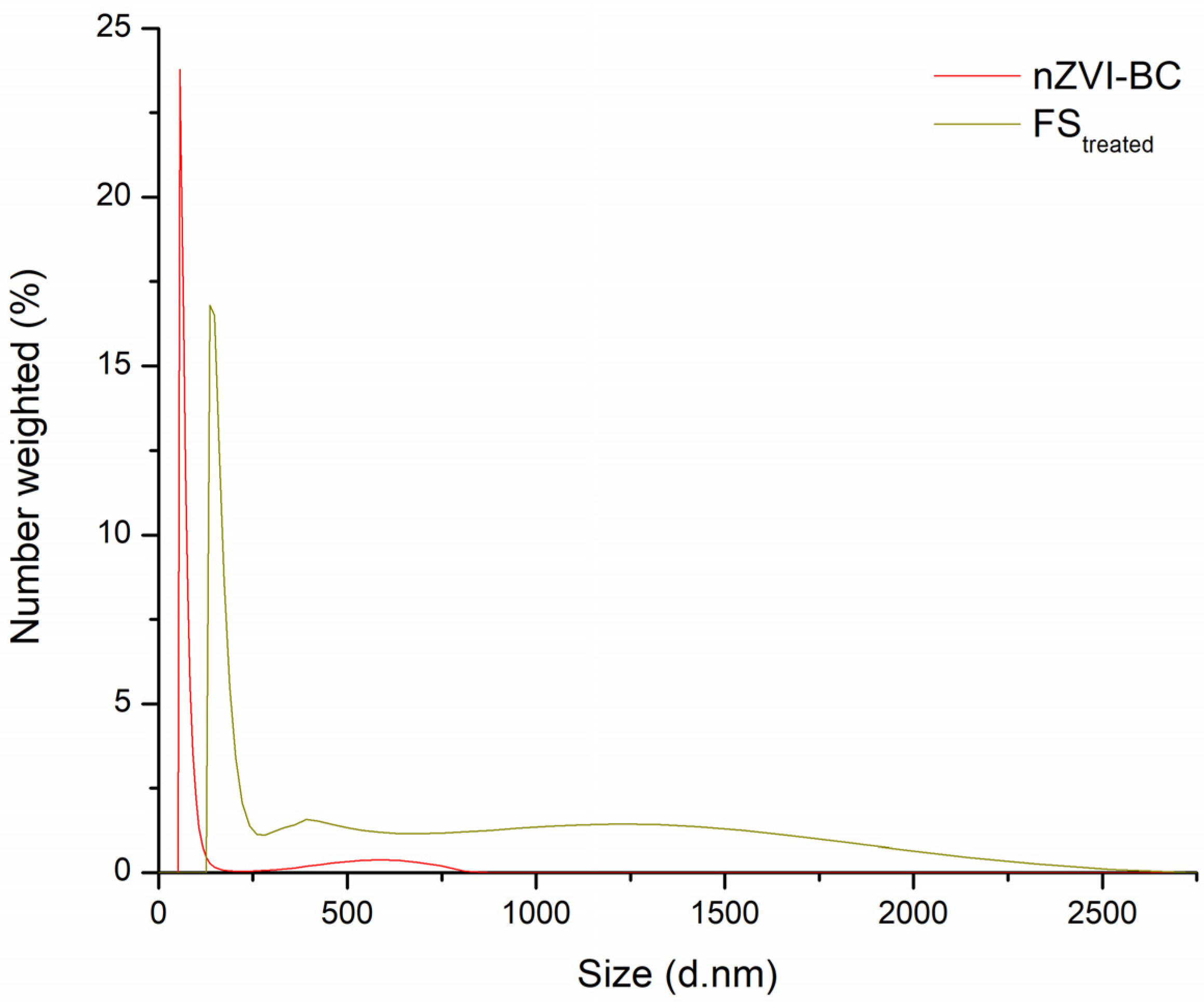
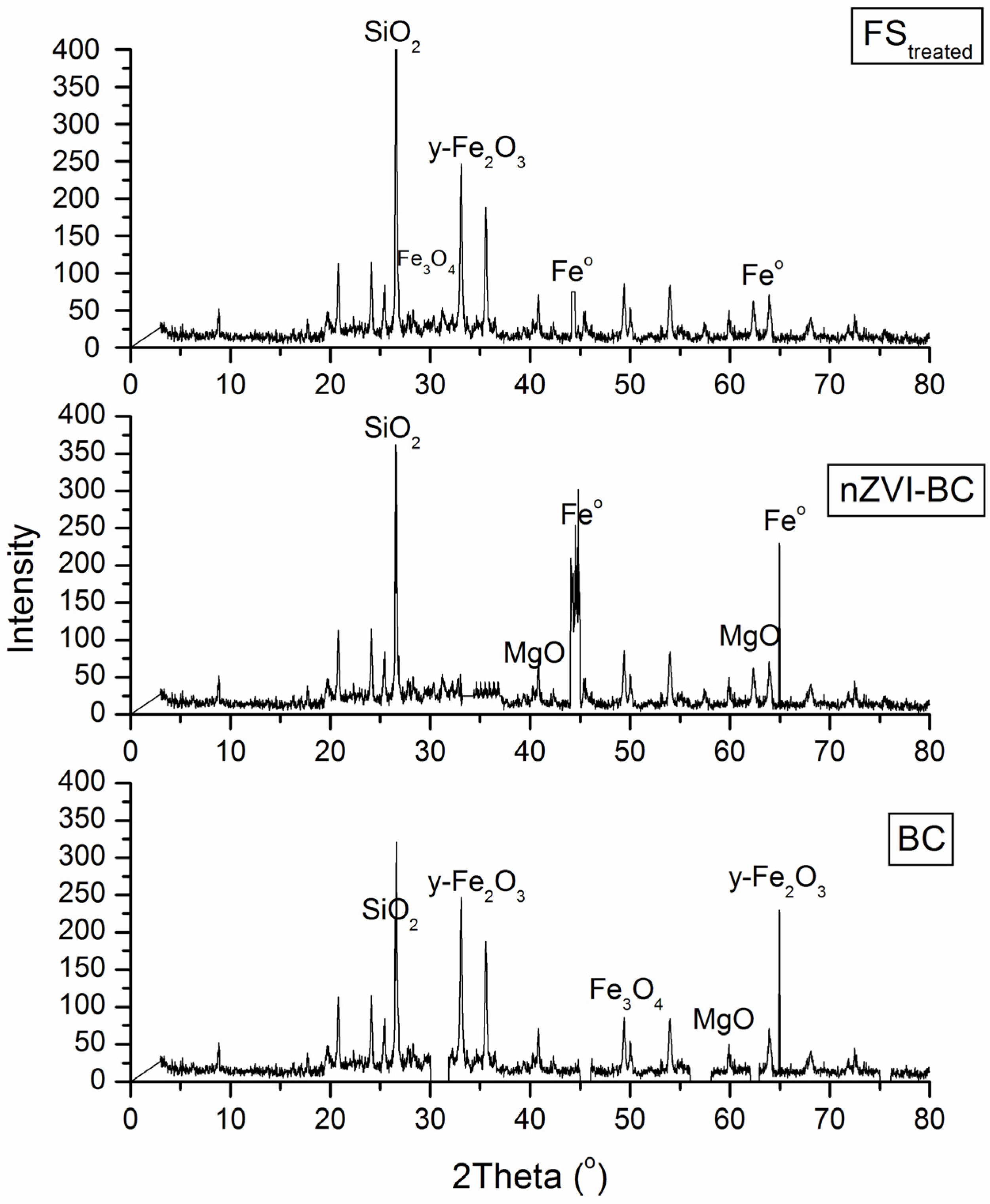
2.1. Characterization of Raw and Modified BC-Based Materials
2.2. DSD Model Evaluation and Fenton Process Optimization
2.3. Treatment and Characterization of Real Effluent of RB4 Dye under Optimal Conditions of Fenton Process Catalyzed with nZVI-BC
2.4. FTIR Analysis of Effluents
2.5. Mechanism of RB4 Removal by nZVI-BC
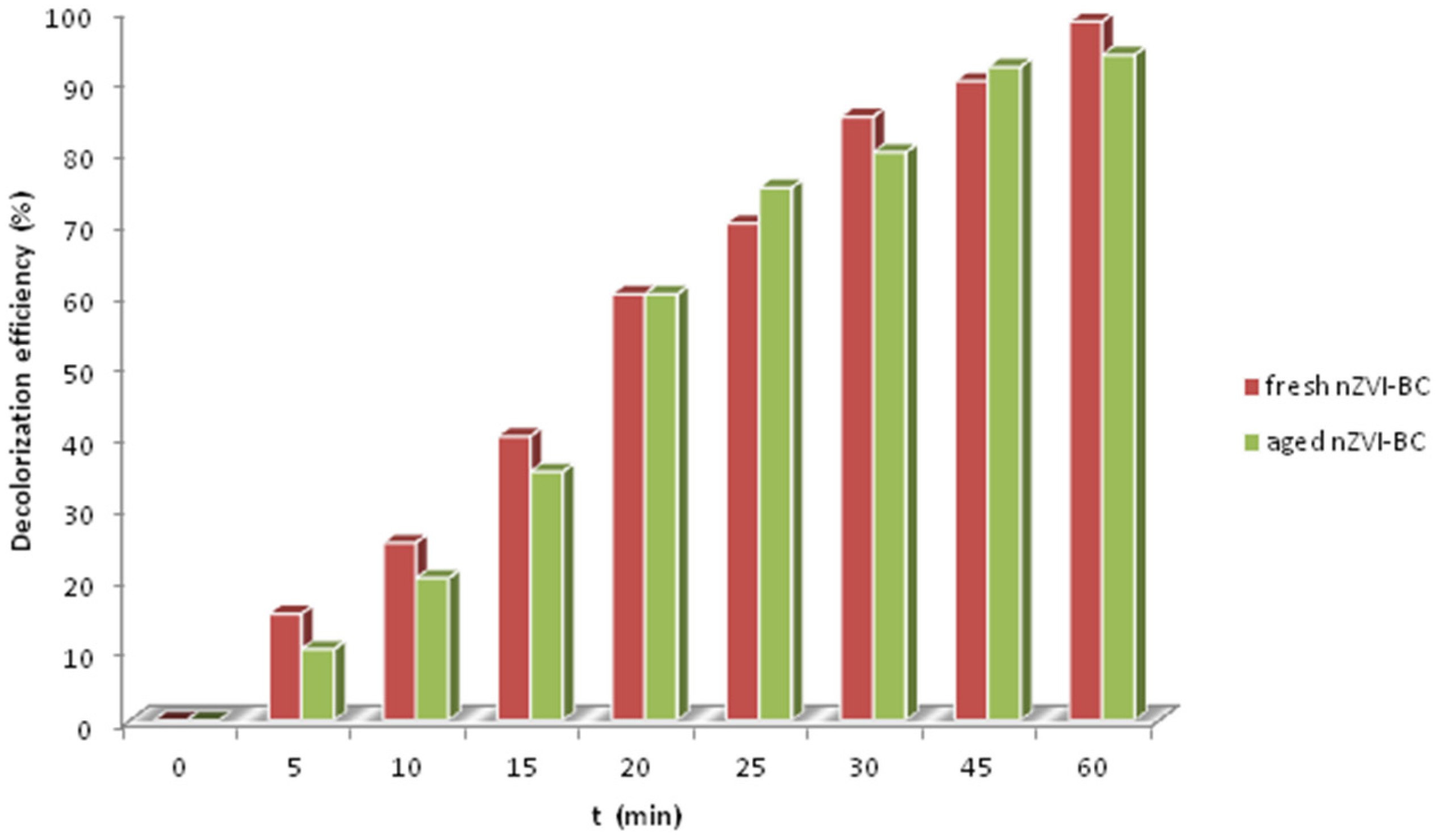
2.6. Stability of nZVI-BC
2.7. Fenton Sludge as a Nutrient Resource
| Element | Concentration, mg/kg | Directive 86/278/EEC [46] | Regulation on Emission Limit Values for Pollutants in Waters and the Deadlines for Their Reaching (“Official Gazette of the RS” No67/11, 48/12 and 01/16) [45] | EBC [48]. | IBI | |||
|---|---|---|---|---|---|---|---|---|
| EBC-Feed Clas I | EBC-AgroBio, Class II | EBC-Agro, Class III | EBC-Material, Class IV | |||||
| Cr | 95 | - | 100–1000 | 70 | 70 | 90 | 250 | 93 |
| Ni | 55.01 | 300–400 | 60–400 | 25 | 25 | 50 | 250 | 47 |
| Cu | 197.49 | 1000–1750 | 700–1750 | 70 | 70 | 100 | 250 | 143 |
| Zn | 1045 | 2500–4000 | 1500–4000 | 200 | 200 | 400 | 750 | 416 |
| As | 4.71 | - | 15–75 | 2 | 13 | 13 | 15 | 13 |
| Cd | 1.89 | 20–40 | 2.5–40 | 0.8 | 0.7 | 1.5 | 5 | 1.4 |
| Pb | 16.90 | 750–1200 | 120–1200 | 10 | 45 | 150 | 250 | 121 |
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Sludge Sampling and BC Synthesis
3.3. Synthesis of nZVI-BC Catalyst
3.4. Characterization of BC, nZVI-BC, and FStreated
3.5. Physico-Chemical Analysis of RB 4 before and after Treatment
3.6. Definitive Screening Design
3.7. Fenton Sludge (FS) Reuse
3.7.1. Application of FStreated in RB4 Decolorization
3.7.2. Nutrient-Release Experiments
4. Conclusions and Outlook for Long-Term Sustainability
- The applied DSD methodology indicated very low concentrations of the obtained nZVI and hydrogen peroxide were required for almost maximum decolorization efficiency. The results are promising from the aspect of overcoming low operating pH value in terms of its adjustment.
- The valorization of the obtained Fenton sludge was performed by reusing it as a new heterogeneous Fenton catalyst (FStreated) at the same optimized conditions as for homogenous nZVI-BC catalyst. The almost 100% degradation efficiency of RB4 was obtained, with no pH correction, because the treatment took place at pH = 3.75.
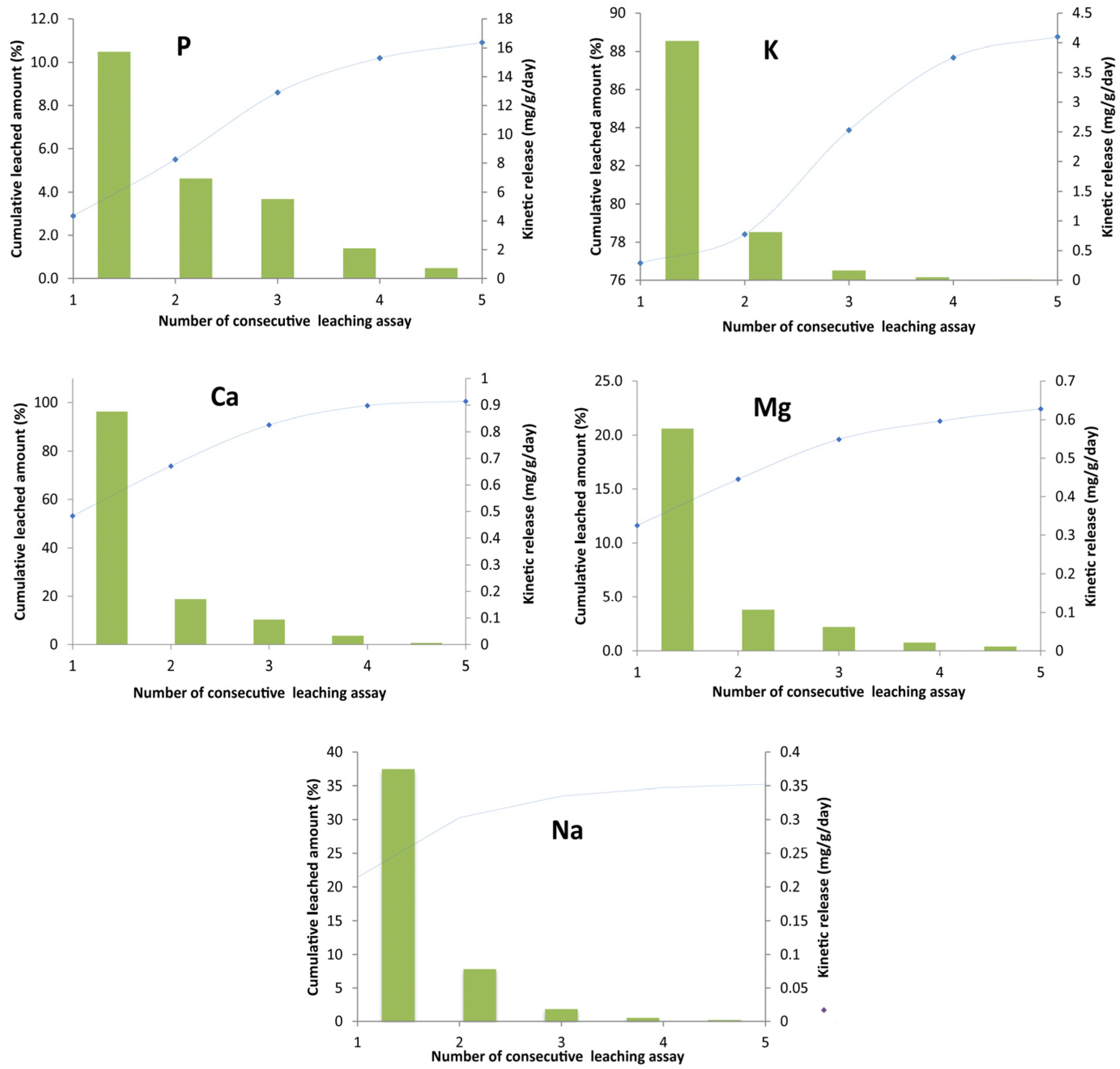
- Nutrient-release experiments demonstrated the nanomaterial’s high capability to supplement agricultural fields with macronutrients (K, Mg, Ca, P, Na) through their release over excessive periods with slow rates. This is the principal key for optimal agricultural plant growth and soil maturing as well as for reduced environmental pollution risks by these elements.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rajasulochana, P.; Preethy, V. Comparison on efficiency of various techniques in treatment of waste and sewage water e a comprehensive review. Resour. Technol. 2016, 2, 175–184. [Google Scholar] [CrossRef]
- Mateo-Sagasta, J.; Raschid-Sally, L.; Thebo, A. Global wastewater and sludge production, treatment and use. In Wastewater: Economic Asset in an Urbanizing World; Springer: Amsterdam, The Netherlands, 2015; pp. 15–38. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Dhanjal, D.S.; Datta, S.; Bhatia, D.; Dhiman, J.; Samuel, J.; Prasad, R.; Singh, J. A sustainable paradigm of sewage sludge biochar: Valorization, opportunities, challenges and future prospects. J. Clean. Prod. 2020, 269, 122259. [Google Scholar] [CrossRef]
- European Environment Agency. Beyond Water Quality—Sewage Treatment in a Circular Economy; European Environment Agency: Copenhagen, Denmark, 2022; Volume 60, p. TH–AL-22-005-EN-N. [CrossRef]
- Domini, M.; Bertanza, G.; Vahidzadeh, R.; Pedrazzani, R. Sewage Sludge Quality and Management for Circular Economy Opportunities in Lombardy. Appl. Sci. 2022, 12, 10391. [Google Scholar] [CrossRef]
- Ghobani, M.; Konvalina, P.; Walkiewicz, A.; Neugschwandtner, R.W.; Kopecky, M.; Zamanian, K.; Chen, W.-H.; Bucur, D. Feasibility of Biochar Derived from Sewage Sludge to Promote Sustainable Agriculture and Mitigate GHG Emissions—A Review. Int. J. Environ. Res. Public Health 2022, 19, 12983. [Google Scholar] [CrossRef] [PubMed]
- Kacprzak, M.; Kupich, I. The specificities of the circular economy (CE) in the municipal wastewater and sewage sludge sector—Local circumstances in Poland. Clean Technol. Environ. Policy 2021. [Google Scholar] [CrossRef]
- Fijalkowski, K.; Rorat, A.; Grobelak, A.; Kacprzak, M.J. The presence of contaminations in sewage sludge e the current situation. J. Environ. Manag. 2017, 203, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Urra, J.; Alkorta, I.; Mijangos, I.; Epelde, L.; Garbisu, C. Application of sewage sludge to agricultural soil increases the abundance of antibiotic resistance genes without altering the composition of prokaryotic communities. Sci. Total Environ. 2019, 647, 1410–1420. [Google Scholar] [CrossRef]
- Goldan, E.; Nedeff, V.; Barsan, N.; Culea, M.; Tomozei, C.; Panainte-Lehadus, M.; Mosnegutu, E. Evaluation of the Use of Sewage Sludge Biochar as a Soil Amendment—A Review. Sustainability 2022, 14, 5309. [Google Scholar] [CrossRef]
- Méndez, A.; Cárdenas-Aguiar, E.; Paz-Ferreiro, J.; Plaza, C.; Gascó, G. The effect of sewage sludge biochar on peat-based growing media. Biol. Agric. Hortic. 2017, 33, 40–51. [Google Scholar] [CrossRef]
- Ye, Y.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Fu, Q.; Wei, W.; Ni, B.; Cheng, D.; Liu, Y. A critical review on utilization of sewage sludge as environmental functional materials. Bioresour. Technol. 2022, 363, 1279814. [Google Scholar] [CrossRef]
- Figueiredo, C.C.D.; Chagas, J.K.M.; da Silva, J.; Paz-Ferreiro, J. Short-term effects of a sewage sludge biochar amendment on total and available heavy metal content of a tropical soil. Geoderma 2019, 344, 31–39. [Google Scholar] [CrossRef]
- Kong, L.; Liu, J.; Han, Q.; Zhou, Q.; He, J. Integrating metabolomics and physiological analysis to investigate the toxicological mechanisms of sewage sludge-derived biochars to wheat. Ecotoxicol. Environ. Saf. 2019, 185, 109664. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Liu, X.; Tang, J.; Zhang, S. Biochar-supported nanosized zero-valent iron (nZVI-BC) composites for removal of nitro and chlorinated contaminants. Chem. Eng. J. 2022, 431, 133187. [Google Scholar] [CrossRef]
- Deng, J.; Dong, H.; Zhang, C.; Jiang, Z.; Cheng, Y.; Hou, K.; Zhang, L.; Fan, C. Nanoscale zero-valent iron/biochar composite as an activator for Fenton-like removal of sulfamethazine. Sep. Purif. Technol. 2018, 202, 130–137. [Google Scholar] [CrossRef]
- Kecic, V.; Kerkez, Ð.; Prica, M.; Luzanin, O.; Becelic-Tomin, M.; Pilipovic, D.T.; Dalmacija, B. Optimization of azo printing dye removal with oak leaves-nZVI/H2O2system using statistically designed experiment. J. Clean. Prod. 2018, 202, 65–80. [Google Scholar] [CrossRef]
- Wang, R.-Z.; Huang, D.-L.; Liu, Y.-G.; Zhang, C.; Lai, C.; Wang, X.; Zeng, G.-M.; Gong, X.-M.; Duan, A.; Zhang, Q.; et al. Recent advances in biochar-based catalysts: Properties, applications and mechanisms for pollution remediation. Chem. Eng. J. 2019, 371, 380–403. [Google Scholar] [CrossRef]
- Slijepčević, N.; Pilipović, D.T.; Kerkez, D.J.; Krčmar, D.; Bečelić-Tomin, M.; Beljin, J.; Dalmacija, B. A cost effective method for immobilization of Cu and Ni polluted river sediment with nZVI synthesized from leaf extract. Chemosphere 2021, 263, 127816. [Google Scholar] [CrossRef] [PubMed]
- Turabik, M.; Simsek, U.T. Effect of synthesis parameters on the particle size of the zero valent iron particles. Inorg. Nano Met. Chem. 2017, 47, 1033–1043. [Google Scholar] [CrossRef]
- Liu, H.; Hu, G.; Basar, I.A.; Li, J.; Lyczko, N.; Nzihou, A.; Eskicioglu, C. Phosphorus recovery from municipal sludge-derived ash and hydrochar through wet-chemical technology: A review towards sustainable waste management. Chem. Eng. J. 2021, 417, 129300. [Google Scholar] [CrossRef]
- He, Y.; Li, J.; Zhao, Y.; Yang, C.; Xu, C.; Liu, X.; Xing, X.; Tie, J.; Li, R.; Zheng, J. Sewage-sludge derived activated carbon impregnated with polysulfide-sulfidated nZVI: A promising material for Cr(VI) reductive stabilization. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128614. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, M.; Zhou, Y.C.; Li, J.; Wang, B.; Gao, S.; Sato, K.; Feng, W.; Yin, A.D.; Igalavithana, P.; et al. Biochar-supported nZVI (nZVI/ BC) for contaminant removal from soil and water: A critical review. J. Hazard. Mater. 2019, 373, 820–834. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Paik, S.Y.; Ryu, J.; Choi, K.O.; Kang, T.S.; Lee, J.K.; Song, C.W.; Ko, S. Dynamic light scattering-based method to determine primary particle size of iron oxide nanoparticles in simulated gastrointestinal fluid. Food Chem. 2014, 161, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, N.; Kumar, K.M.M.; Balasubramnian, K.S.; Karunamurthy, K.; Varadharajan, R. Facile Synthesis of Iron Oxide, Iron-Cobalt and Zero Valent Iron Nanoparticles and Evaluation of Their Anti Microbial Activity, Free Radicle Scavenginging Activity and Antioxidant Assay. Dig. J. Nanomater. Biostructures 2013, 8, 765–775. [Google Scholar]
- Chen, Z.; Xiao, X.; Chen, B.; Zhu, L. Quantification of chemical states, dissociation constants and contents of oxygen-containing groups on the surface of biochars produced at different temperatures. Environ. Sci. Technol. 2014, 49, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.W.; Feng, Q.Y.; Yang, H.; Alam, E.; Gao, B.; Gu, D.M. Modified biochar supported Ag/Fe nanoparticles used for removal of cephalexin in solution: Characterization, kinetics and mechanisms. Colloids Surf. A Physicochem. Eng. Asp. 2017, 517, 63–71. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B. Removal of arsenic, methylene blue, and phosphate by biochar/alooh nanocomposite. Chem. Eng. J. 2013, 226, 286–292. [Google Scholar] [CrossRef]
- Lužanin, O.; Gudurić, V.; Ristić, I.; Muhič, S. Investigating impact of five build parameters on the maximum flexural force in FDM specimens—A definitive screening design approach. Rapid Prototyp. J. 2017, 23, 1088–1098. [Google Scholar] [CrossRef]
- Mohammed, E.; Naugler, C.; Far, B. Chapter 32—Emerging business intelligence framework for a clinical laboratory through big data analytics. In Emerging Trends in Computational Biology, Bioinformatics, and Systems Biology; Elsevier: New York, NY, USA, 2015; pp. 577–602. [Google Scholar]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Matavos-Aramyan, S.; Moussavi, M. Advances in Fenton and Fenton based oxidation processes for industrial effluent contaminants control-A review. Int. J. Environ. Sci. Nat. Resour. 2017, 2, 1–18. [Google Scholar] [CrossRef]
- Zhang, M.; Dong, H.; Zhao, L.; Wang, D.; Meng, D. A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef]
- Becelic-Tomin, M.; Dalmacija, B.; Rajic, L.J.; Tomasevic, D.; Kerkez, D.J.; Watson, M.; Prica, M. Degradation of anthraquinone dye Reactive Blue 4 in pyrite ash catalyzed Fenton reaction. Sci. World J. 2014, 2014, 234654. [Google Scholar] [CrossRef] [PubMed]
- Demarchis, L.; Minella, M.; Nisticò, R.; Maurino, V.; Minero, C.; Vione, D. Photo-Fenton reaction in the presence of morphologically controlled hematite as iron source. J. Photochem. Photobiol. A Chem. 2015, 307, 99–107. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Ou, C.; Faheem, J.S.; Yu, H.; Jiao, Z.; Han, W.; Sun, X.; Li, J.; Wang, L. Reuse of Fenton sludge as an iron source for NiFe2O4 synthesis and its application in the Fenton-based process. J. Environ. Sci. China 2017, 53, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Belete, Y.Z.; Ziemann, E.; Gross, A.; Bernstein, R. Facile activation of sludge-based hydrochar by Fenton oxidation for ammonium adsorption in aqueous media. Chemosphere 2021, 273, 128526. [Google Scholar] [CrossRef]
- Gao, L.; Cao, Y.; Wang, L.; Li, S. A review on sustainable reuse applications of Fenton sludge during wastewater treatment. Front. Environ. Sci. Eng. 2022, 16, 77. [Google Scholar] [CrossRef]
- Pachhade, K.; Sandhya, S.; Swaminathan, K. Ozonation of reactive dye, Procion red MX-5B catalyzed by metal ions. J. Hazard. Mater. 2009, 167, 313–318. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Zimmerman, A.R.; Li, Y.; Ma, L.; Harris, W.G.; Migliaccio, K.W. Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite. Bioresour. Technol. 2014, 175, 391–395. [Google Scholar] [CrossRef]
- Mandic, A.K.; Becelic-Tomin, M.; Milidrag, G.P.; Raseta, M.; Kerkez, D.J. Application of impregnated biocarbon produced from soybean hulls in dye decolorization. Hem. Ind. 2021, 75, 307–320. [Google Scholar] [CrossRef]
- Xia, J.; Shen, Y.; Zhang, H.; Hu, X.; Mian, M.M.; Zhang, W.-H. Synthesis of magnetic nZVI@biochar catalyst from acid precipitated black liquor and Fenton sludge and its application for Fenton-like removal of rhodamine B dye. Ind. Crops Prod. 2022, 187, 11544. [Google Scholar] [CrossRef]
- Wang, T.; Su, J.; Jin, X.Y.; Chen, Z.L.; Megharaj, M.; Naidu, R. Functional clay supported bimetallic nZVI/Pd nanoparticles used for removal of methyl orange from aqueous solution. J. Hazard. Mater. 2013, 262, 819–825. [Google Scholar] [CrossRef]
- Han, L.; Xue, S.; Zhao, S.; Yan, J.; Qian, L.; Chen, M. Biochar Supported Nanoscale Iron Particles for the Efficient Removal of Methyl Orange Dye in Aqueous Solutions. PLoS ONE 2015, 10, e0132067. [Google Scholar] [CrossRef] [PubMed]
- Regulation on Emission Limit Values for Pollutants in Waters and the Deadlines for Their Reaching. Official Gazette of the Republic of Serbia No 67 (13.09.2011), 48(10.05.2012) and 01(06.01.2016.). Available online: https://leap.unep.org/countries/rs/national-legislation/regulation-emission-limit-values-pollutants-water-and-deadlines (accessed on 13 December 2022).
- European Commission. Protection of the Environment, and in particular of the soil, when sewage sludge is used in agriculture. Official J. Eur. Communities 1986, 4, 6–12. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31986L0278&from=EN (accessed on 11 November 2022).
- Hu, Q.; Jung, J.; Chen, D.; Leong, K.; Song, S.; Li, F.; Mohan, B.C.; Yao, Z.; Prabhakar, A.K.; Lin, X.H.; et al. Biochar industry to the circular economy. Sci. Total Environ. 2021, 757, 143820. [Google Scholar] [CrossRef] [PubMed]
- European Biochar Certificate (EBC). Guidelines for a Sustainable Production of Biochar; Version 6.1 of 19th June; European Biochar Foundation (EBC): Arbaz, Switzerland, 2021; Available online: https://www.european-biochar.org/media/doc/2/version_en_9_2e.pdf (accessed on 11 November 2022).
- McFarland, M.; Provin, T. Essential Nutrients for Plants—How Do Nutrients Affect Plant Growth? 2014. Available online: https://agrilife.org/texaslocalproduce-2/files/2018/07/Essential-Nutrients-for-Plants.pdf (accessed on 15 November 2022).
- Ferjani, A.I.; Jellali, S.; Akrout, H.; Limousy, L.; Hamdi, H.; Thevenin, N.; Jeguirim, M. Nutrient retention and release from raw exhausted grape marc biochars and an amended agricultural soil: Static and dynamic investigation. Environ. Technol. Innov. 2020, 19, 100885. [Google Scholar] [CrossRef]
- Mukherjee, A.; Zimmerman, A.R. Organic carbon and nutrient release from a range of laboratory-produced biochars and biochar-soil mixtures. Geoderma 2013, 193, 122–130. [Google Scholar] [CrossRef]
- Hadroug, S.; Jellali, S.; Leahy, J.J.; Kwapinska, M.; Jeguirim, M.; Hamdi, H.; Kwapinski, W. Pyrolysis process as a sustainable management option of poultry manure: Characterization of the derived biochars and assessment of their nutrient release capacities. Water 2019, 11, 2271. [Google Scholar] [CrossRef]
- Li, W.; Feng, X.; Song, W.; Guo, M. Transformation of Phosphorus in Speciation and Bioavailability During Converting Poultry Litter to Biochar. Front. Sustain. Food Syst. 2018, 2, 20. [Google Scholar] [CrossRef]
- Sun, H.; Lu, H.; Chu, L.; Shao, H.; Shi, W. Biochar applied with appropriate rates can reduce N leaching, keep N retention and not increase NH3 volatilization in a coastal saline soil. Sci. Total Environ. 2017, 575, 820–825. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Y.; Chiu, P.C.; Imhoff, P.T.; Guo, M. Phosphorus release behaviors of poultry litter biochar as a soil amendment. Sci. Total Environ. 2015, 512-513, 454–463. [Google Scholar] [CrossRef]
- Wojas, N.A.; Swerin, A.; Wallqvist, V.; Järn, M.; Schoelkopf, J.; Gane, P.A.C.; Claesson, P.M. Surface-Modified and Unmodified Calcite: Effects of Water and Saturated Aqueous Octanoic Acid Droplets on Stability and Saturated Fatty Acid Layer Organization. Langmuir 2021, 37, 14135–14146. [Google Scholar] [CrossRef]
- Khan, M.B.; Cui, X.; Jilani, G.; Tang, L.; Lu, M.; Cao, X.; Sahito, Z.A.; Hamid, Y.; Hussain, B.; Yang, X.; et al. New insight into the impact of biochar during vermi-stabilization of divergent biowastes: Literature synthesis and research pursuits. Chemosphere 2020, 238, 124679. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Duan, X.; Zhang, C.; Wang, S.; Ren, N.; Ho, S.H. Graphitic biochar catalysts from anaerobic digestion sludge for nonradical degradation of micropollutants and disinfection. Chem. Eng. J. 2020, 384, 123244. [Google Scholar] [CrossRef]
- Machado, S.; Pinto, S.L.; Grosso, J.P.; Nouws, H.P.A.; Albergaria, J.T.; Delerue-Matos, C. Green production of zero-valent iron nanoparticles using tree leaf extracts. Sci. Total Environ. 2013, 445, 1–8. [Google Scholar] [CrossRef]
- Mortazavian, S.; Jones-Lepp, T.; Bae, J.-H.; Chun, D.; Bandala, E.R.; Moon, J. Heat-treated biochar impregnated with zero-valent iron nanoparticles for organic contaminants removal from aqueous phase: Material characterizations and kinetic studies. J. Ind. Eng. Chem. 2019, 76, 197–214. [Google Scholar] [CrossRef]
- SRPS ISO 6060; Water Quality—Determination of the Chemical Oxygen Demand. International Organization for Standardization: Geneve, Switzerland, 1994.
- SRPS ISO 8245; Water Quality—Guidelines for the Determination of Total Organic Carbon (TOC). International Organization for Standardization: Geneve, Switzerland, 2007.
- Jones, B.; Nachtsheim, C.J. Definitive screening designs with added two-level categorical factors. J. Qual. Technol. 2013, 45, 121–129. [Google Scholar] [CrossRef]
- Milidrag, G.P.; Nikić, J.; Gvoić, V.; Mandić, A.K.; Agbaba, J.; Bečelić-Tomin, M.; Kerkez, D. Photocatalytic Degradation of Magenta Effluent Using Magnetite Doped TiO2 in Solar Parabolic Trough Concentrator. Catalysts 2022, 12, 986. [Google Scholar] [CrossRef]






| Element, % | BC | nZVI-BC | FStreated |
|---|---|---|---|
| C | 37.43 | 27.61 | 19.84 |
| O | 28.83 | 22.17 | 31.40 |
| Si | 9.05 | 8.27 | 7.25 |
| Ca | 6.83 | 2.88 | 2.63 |
| P | 5.79 | 2.76 | 3.85 |
| Fe | 3.83 | 24.97 | 22.69 |
| Al | 3.33 | 3.17 | 2.94 |
| K | 2.89 | 3.75 | 3.62 |
| Mg | 1.27 | 1.37 | 1.34 |
| Na | 0.75 | 1.14 | 1.15 |
| Cl | - | 1.90 | 3.25 |
| Sample | c(H2O2) (mM) | c(nZVI-BC) (mg/L) | c(RB4) (mg/L) | Removal Efficiency (%) |
|---|---|---|---|---|
| 1 | 5.5 | 200 | 150 | 75.77 |
| 2 | 5.5 | 5 | 50 | 18.28 |
| 3 | 10 | 102.5 | 50 | 64.64 |
| 4 | 1 | 102.5 | 150 | 39.37 |
| 5 | 10 | 5 | 100 | 21.62 |
| 6 | 1 | 200 | 100 | 73.97 |
| 7 | 10 | 200 | 50 | 90.05 |
| 8 | 1 | 5 | 150 | 14.77 |
| 9 | 10 | 200 | 150 | 85.35 |
| 10 | 1 | 5 | 50 | 15.78 |
| 11 | 10 | 5 | 150 | 20.47 |
| 12 | 1 | 200 | 50 | 77.81 |
| 13 | 5.5 | 102.5 | 100 | 52.58 |
| 14 | 5.5 | 200 | 150 | 77.9 |
| 15 | 5.5 | 5 | 50 | 14.62 |
| 16 | 10 | 102.5 | 50 | 66.12 |
| 17 | 1 | 102.5 | 150 | 39.74 |
| 18 | 10 | 5 | 100 | 17.05 |
| 19 | 1 | 200 | 100 | 72.01 |
| 20 | 10 | 200 | 50 | 92.96 |
| 21 | 1 | 5 | 150 | 6.00 |
| 22 | 10 | 200 | 150 | 88.06 |
| 23 | 1 | 5 | 50 | 5.03 |
| 24 | 10 | 5 | 150 | 19.35 |
| 25 | 1 | 200 | 50 | 69.86 |
| 26 | 5.5 | 102.5 | 100 | 50.97 |
| 27 | 5.5 | 102.5 | 100 | 47.91 |
| 28 | 5.5 | 102.5 | 100 | 53.45 |
| Descriptive Factor | Value |
|---|---|
| R2 | 0.986522 |
| R2 adj | 0.982671 |
| AIC | 170.1597 |
| BIC | 173.2384 |
| RMSE (Root Mean Square Error) | 3.828282 |
| Parameter | Before Treatment | After Treatment | Mineralization, % |
|---|---|---|---|
| pH | 6.4 | 3.4 | - |
| Conductivity (μS/cm) | 72 | 280.6 | - |
| BOD (mgO2/L) | 0 | 16 | - |
| COD (mgO2/L) | 280 | 105 | 62.5% |
| TOC (mgC/L) | 16 | 10.15 | 36.6% |
| Compounds | Retention Time | Structure |
|---|---|---|
| Dibutyl phtalate | 18.0703 |  |
| Phthalic acid isobutyl octyl ester | 18.066 | 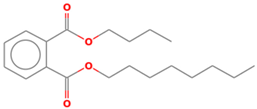 |
| 1,2-Benzenedicarboxylic acid bis (2-methyl propyl) ester | 18.066 | 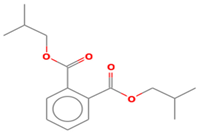 |
| Element | Main Function of Nutrient for Plant Growth [49] | Released Amount at the First Leaching Assay (mg/g) | Percentage from the Contained Fraction in the Biochar (%) | Cumulative Released Amount at the Fifth Leaching Assay (mg/g) | Percentage from the Contained Fraction in the Biochar (%) |
|---|---|---|---|---|---|
| K | Primary—Resistance to diseases, fruit ripening, overall development, water regulation | 4.03 | 76.9 | 6.40 | 89 |
| P | Primary—Healthy vegetation growth, chlorophyll, formation of cells, basis for amino acids and proteins. | 15.7 | 2.9 | 58.2 | 10.9 |
| Ca | Secondary—Plant structure, growth and strength, resistance to diseases | 0.87 | 53.1 | 1.66 | 100 |
| Mg | Secondary—Vegetation development and growth, sugar formation, chlorophyll, fat formation, metabolism | 0.58 | 11.6 | 1.12 | 22.4 |
| Na | Micro- or trace nutrient—Plant growth, leaf color, formation of starch, enzyme development, and activity | 0.38 | 21.4 | 0.62 | 35 |
| Variable | Unit | Encoded Value | Level | ||
|---|---|---|---|---|---|
| −1 | 0 | +1 | |||
| c (H2O2) | mM | X1 | 1 | 5.5 | 10 |
| c (nZVI-BC) | mg/L | X3 | 5 | 102.5 | 200 |
| c (RB4) | mg/L | X2 | 50 | 100 | 150 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leovac Maćerak, A.; Kulić Mandić, A.; Pešić, V.; Tomašević Pilipović, D.; Bečelić-Tomin, M.; Kerkez, D. “Green” nZVI-Biochar as Fenton Catalyst: Perspective of Closing-the-Loop in Wastewater Treatment. Molecules 2023, 28, 1425. https://doi.org/10.3390/molecules28031425
Leovac Maćerak A, Kulić Mandić A, Pešić V, Tomašević Pilipović D, Bečelić-Tomin M, Kerkez D. “Green” nZVI-Biochar as Fenton Catalyst: Perspective of Closing-the-Loop in Wastewater Treatment. Molecules. 2023; 28(3):1425. https://doi.org/10.3390/molecules28031425
Chicago/Turabian StyleLeovac Maćerak, Anita, Aleksandra Kulić Mandić, Vesna Pešić, Dragana Tomašević Pilipović, Milena Bečelić-Tomin, and Djurdja Kerkez. 2023. "“Green” nZVI-Biochar as Fenton Catalyst: Perspective of Closing-the-Loop in Wastewater Treatment" Molecules 28, no. 3: 1425. https://doi.org/10.3390/molecules28031425
APA StyleLeovac Maćerak, A., Kulić Mandić, A., Pešić, V., Tomašević Pilipović, D., Bečelić-Tomin, M., & Kerkez, D. (2023). “Green” nZVI-Biochar as Fenton Catalyst: Perspective of Closing-the-Loop in Wastewater Treatment. Molecules, 28(3), 1425. https://doi.org/10.3390/molecules28031425






