Wild Artichoke (Cynara cardunculus subsp. sylvestris, Asteraceae) Leaf Extract: Phenolic Profile and Oxidative Stress Inhibitory Effects on HepG2 Cells
Abstract
1. Introduction
2. Results
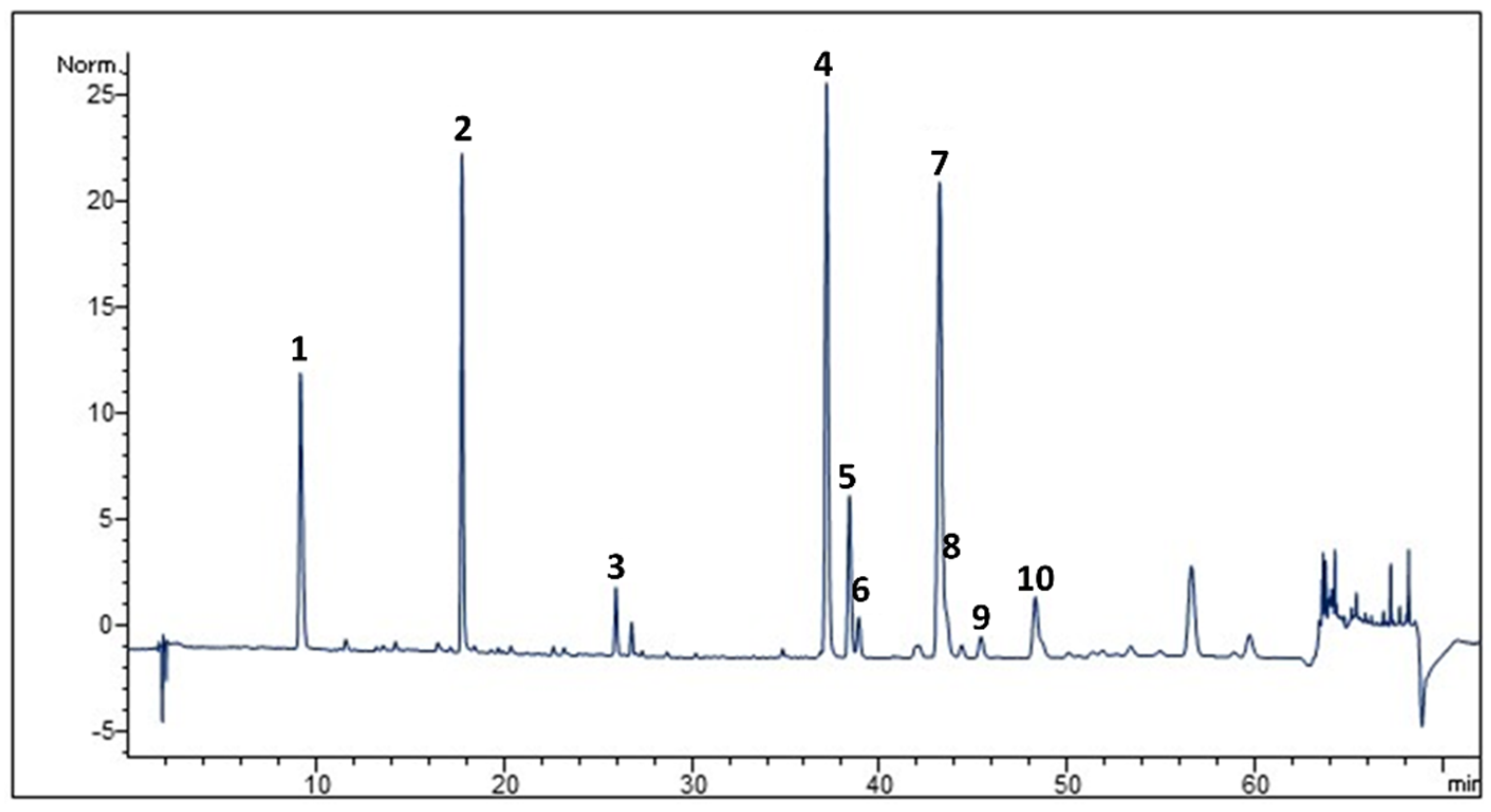
2.1. Phytochemical Analysis
2.2. Cell Viability
2.3. Cell-Free Antioxidant Properties
2.4. Cell-Based Antioxidant Properties
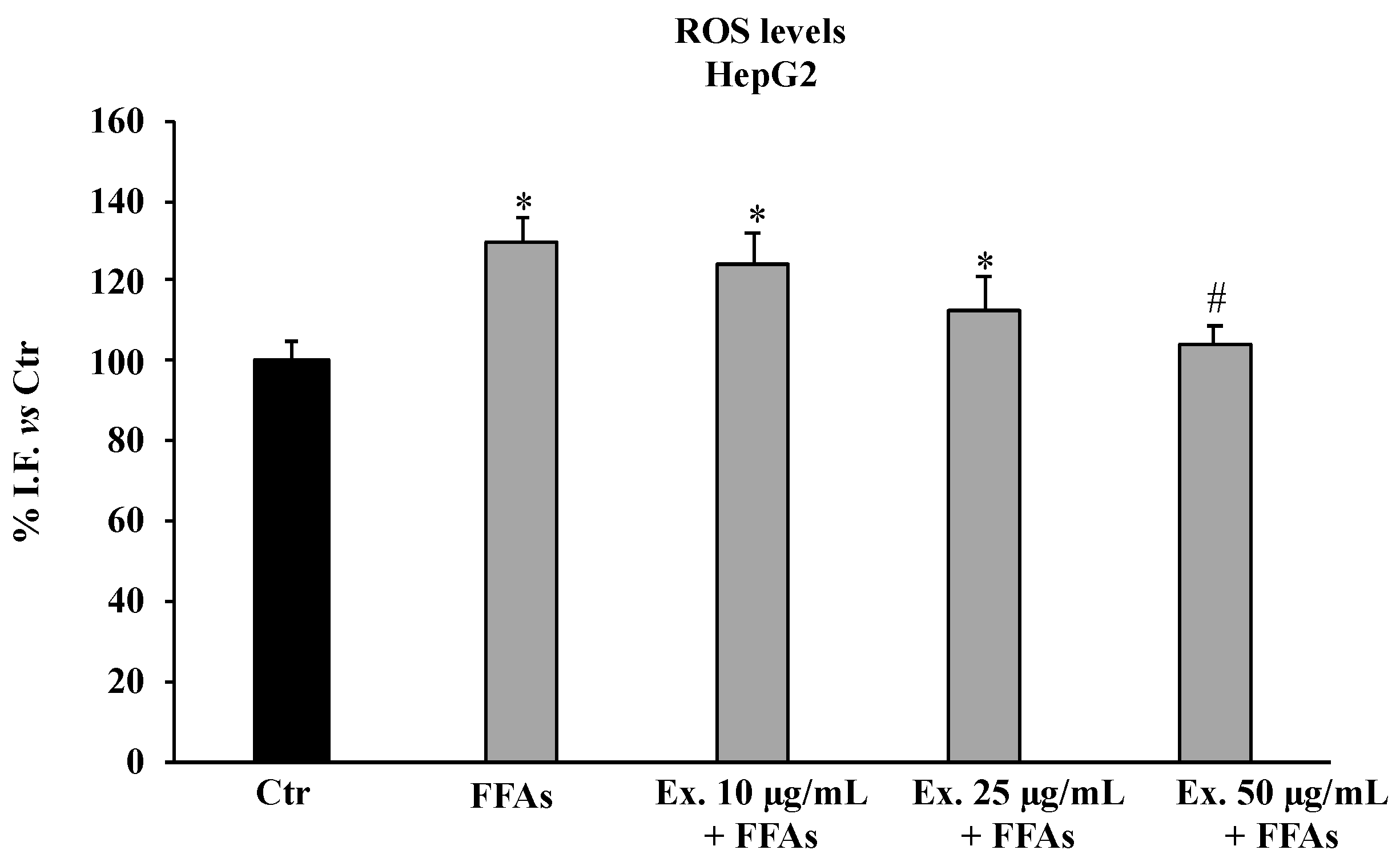
2.4.1. Cynara sylvestris Extract Reduced Reactive Oxygen Species Levels
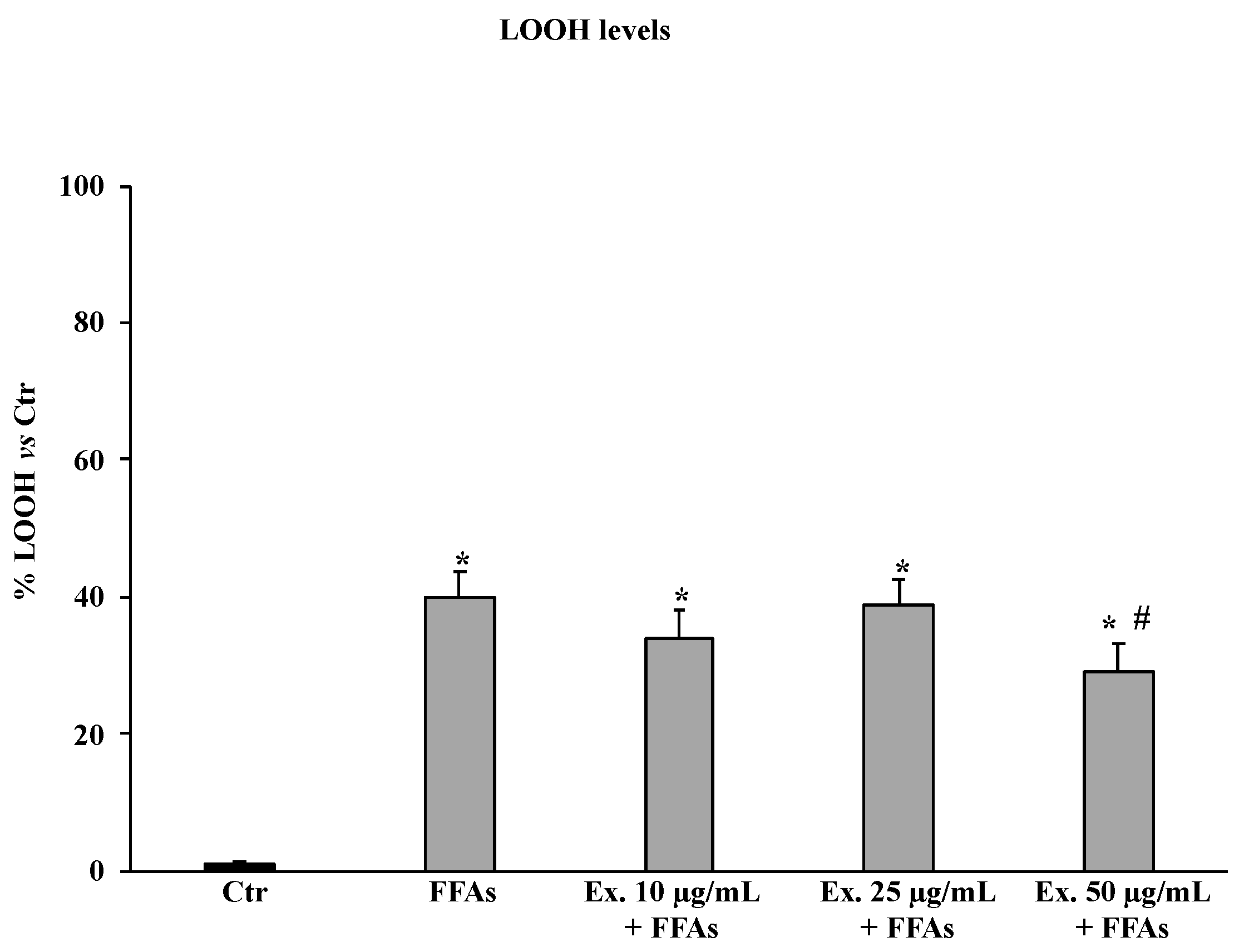
2.4.2. Cynara sylvestris Extract Reduced Lipid Hydroperoxide Levels
2.4.3. Cynara sylvestris Extract Preserved Total Thiol Group Levels
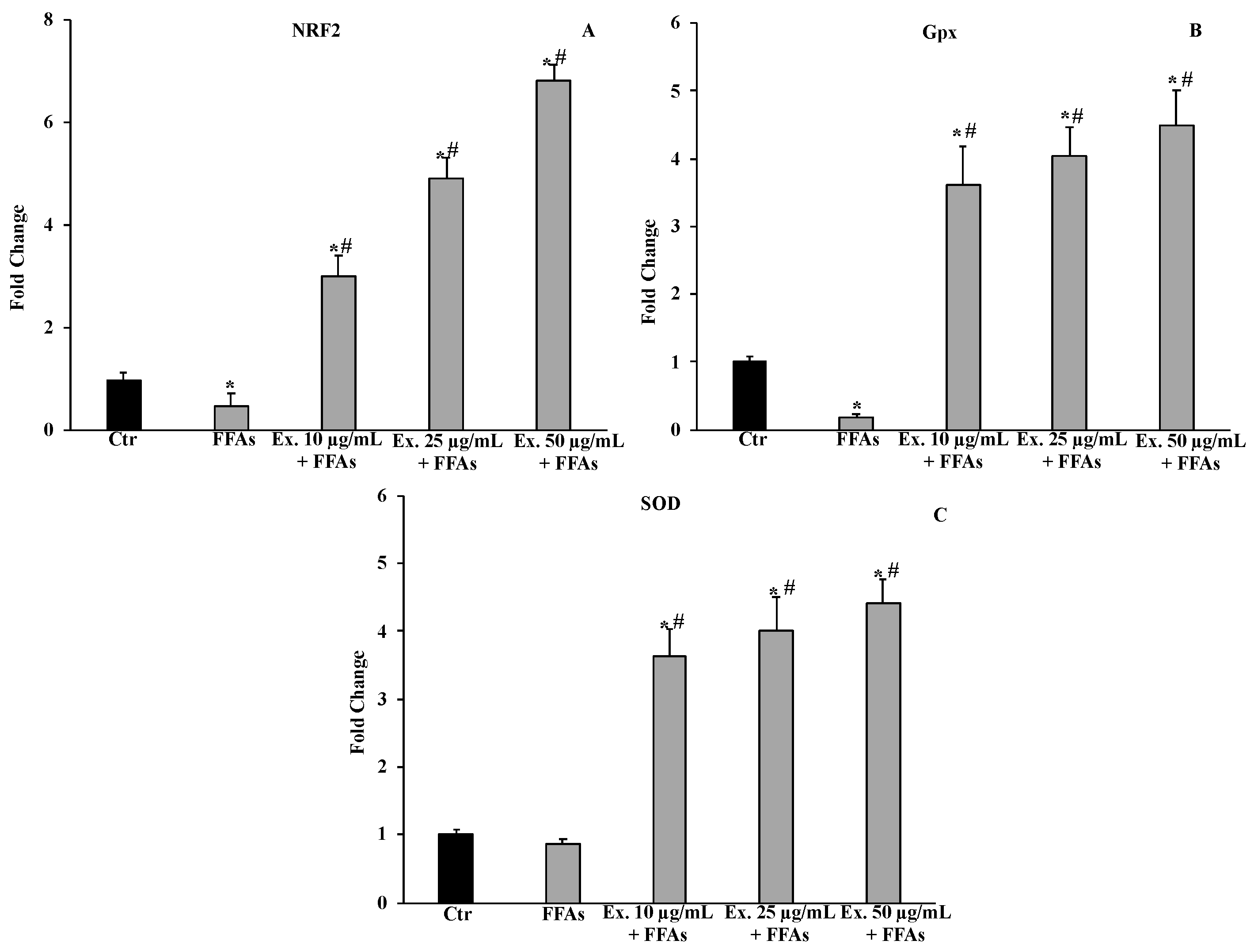
2.4.4. Effect of Cynara sylvestris Extract on Nrf2, Gpx, SOD1 mRNA Expression Levels
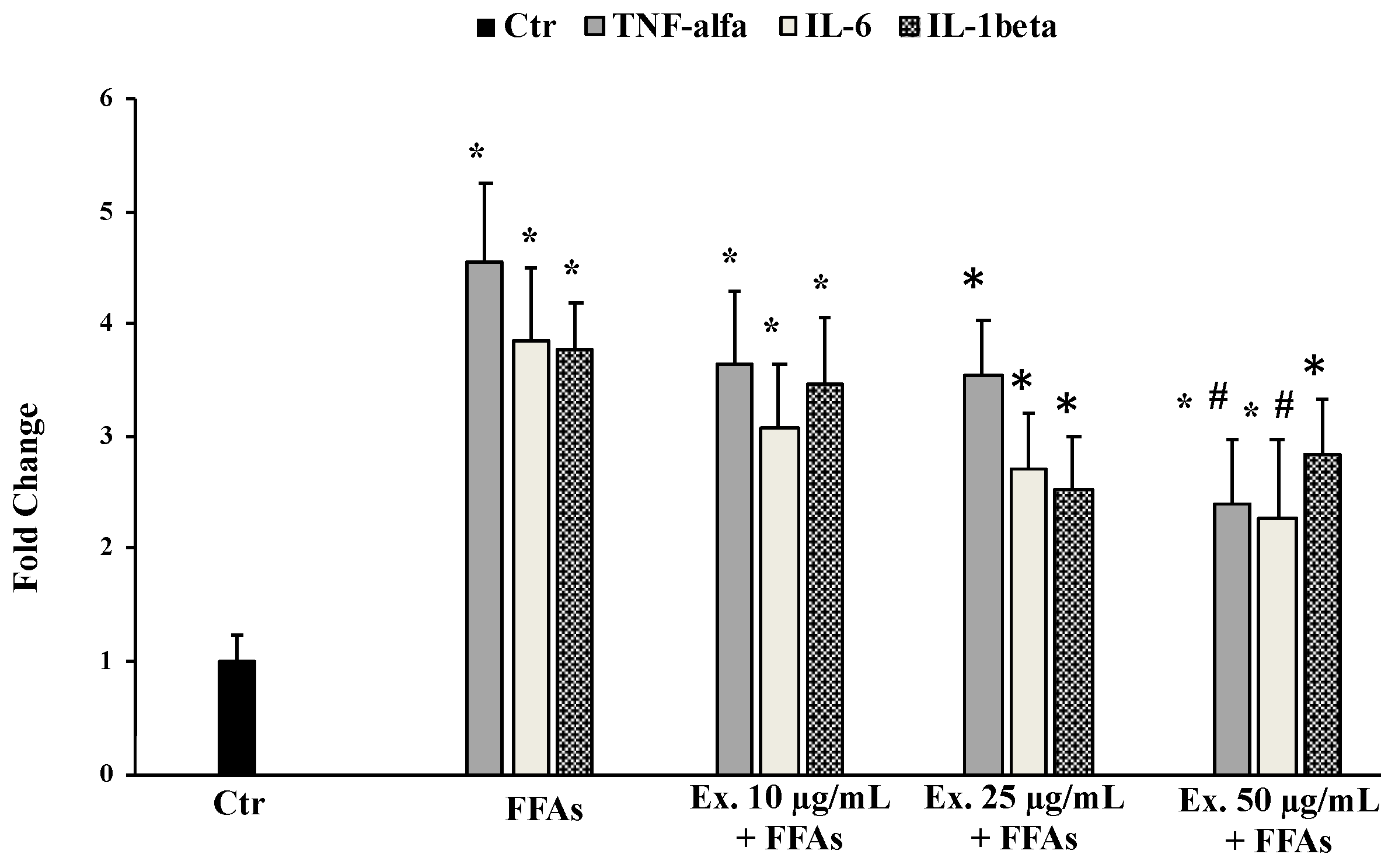
2.4.5. Effect of Cynara sylvestris Extract on TNF-α, IL-6, and IL-1β mRNA Expression
2.4.6. Artemia salina Lethality Bioassay
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Collection and Extraction Procedure
4.3. Determination of Total Flavonoid and Total Phenolic Content
4.4. HPLC-DAD Analysis
4.5. DPPH Test
4.6. Cell Culture and Treatments
4.7. MTT Assay
4.8. Reactive Oxygen Species Assay
4.9. Lipid Peroxidation Determination
4.10. Determination of Total Thiol Groups
4.11. RNA Extraction and Reverse Transcription-Quantitative Polymerase Chain Reaction
4.12. Artemia Salina Lethality Bioassay
4.13. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Velez, Z.; Campinho, M.A.; Guerra, Â.R.; García, L.; Ramos, P.; Guerreiro, O.; Felício, L.; Schmitt, F.; Duarte, M. Biological characterization of Cynara cardunculus L. methanolic extracts: Antioxidant, anti-proliferative, anti-migratory and anti-angiogenic activities. Agriculture 2012, 2, 472–492. [Google Scholar] [CrossRef]
- Pignone, D.; Sonnante, G. Wild artichokes of south Italy: Did the story begin here? Genet. Resour. Crop Evol. 2004, 51, 577–580. [Google Scholar] [CrossRef]
- de Falco, B.; Incerti, G.; Amato, M.; Lanzotti, V. Artichoke: Botanical, agronomical, phytochemical, and pharmacological overview. Phytochem. Rev. 2015, 14, 993–1018. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Pereira, C.; Tzortzakis, N.; Barros, L.; Ferreira, I.C.F.R. Nutritional value and bioactive compounds characterization of plant parts from Cynara cardunculus L. (Asteraceae) cultivated in Central Greece. Front. Plant Sci. 2018, 9, 459. [Google Scholar] [CrossRef]
- Lattanzio, V.; Kroon, P.A.; Linsalata, V.; Cardinali, A. Globe artichoke: A functional food and source of nutraceutical ingredients. J. Funct. Foods 2009, 1, 131–144. [Google Scholar] [CrossRef]
- Borgognone, D.; Cardarelli, M.; Rea, E.; Lucini, L.; Colla, G. Salinity source-induced changes in yield, mineral composition, phenolic acids and flavonoids in leaves of artichoke and cardoon grown in floating system. J. Sci. Food Agric. 2014, 94, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Speroni, E.; Cervellati, R.; Govoni, P.; Guizzardi, S.; Renzulli, C.; Guerra, M.C. Efficacy of different Cynara scolymus preparations on liver complaints. J. Ethnopharmacol. 2003, 86, 203–211. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. EMA/HMPC/194014/2017. Community Herbal Monograph on Cynara cardunculus L. (syn. Cynara scolymus L.), Folium. Available online: https://www.ema.europa.eu/en/medicines/herbal/cynarae-folium (accessed on 5 October 2022).
- Hernández Bermejo, J.E.; Delucchi, G.; Charra, G.; Pochettino, M.L.; Hurrell, J.A. “Cardos” of two worlds: Transfer and resignification of the uses of thistles from the Iberian Peninsula to Argentina. Ethnobiol. Conserv. 2019, 8, 1–22. [Google Scholar]
- Pandino, G.; Lombardo, S.; Williamson, G.; Mauromicale, G. Polyphenol profile and content in wild and cultivated Cynara cardunculus L. Ital. J. Agron. 2012, 7, e35. [Google Scholar] [CrossRef]
- Pandino, G.; Courts, F.L.; Lombardo, S.; Mauromicale, G.; Williamson, G. Caffeoylquinic acids and flavonoids in the immature inflorescence of globe artichoke, wild cardoon, and cultivated cardoon. J. Agric. Food Chem. 2010, 58, 1026–1031. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Mauromicale, G.; Williamson, G. Phenolic acids and flavonoids in leaf and floral stem of cultivated and wild Cynara cardunculus L. genotypes. Food Chem. 2011, 126, 417–422. [Google Scholar] [CrossRef]
- Zayed, A.; Serag, A.; Farag, M.A. Cynara cardunculus L.: Outgoing and potential trends of phytochemical, industrial, nutritive and medicinal merits. J. Funct. Foods 2020, 69, 103937. [Google Scholar] [CrossRef]
- Silva, L.R.; Jacinto, T.A.; Coutinho, P. Bioactive compounds from cardoon as health promoters in metabolic disorders. Foods 2022, 11, 336. [Google Scholar] [CrossRef]
- Mandim, F.; Petropoulos, S.A.; Dias, M.I.; Pinela, J.; Kostić, M.; Soković, M.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Barros, L. Phenolic composition and biological properties of Cynara cardunculus L. var. altilis petioles: Influence of the maturity stage. Antioxidants 2021, 10, 1907. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.B.; Affes, H.; Ksouda, K.; Dhouibi, R.; Sahnoun, Z.; Hammami, S.; Zeghal, K.M. Pharmacological studies of artichoke leaf extract and their health benefits. Plant Foods Hum. Nutr. 2015, 70, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Ferro, Y.; Pujia, R.; Mazza, E.; Lascala, L.; Lodari, O.; Maurotti, S.; Pujia, A.; Montalcini, T. A new nutraceutical (Livogen Plus®) improves liver steatosis in adults with non-alcoholic fatty liver disease. J. Transl. Med. 2022, 20, 377. [Google Scholar] [CrossRef] [PubMed]
- Ferro, Y.; Montalcini, T.; Mazza, E.; Foti, D.; Angotti, E.; Gliozzi, M.; Nucera, S.; Paone, S.; Bombardelli, E.; Aversa, I.; et al. Randomized clinical trial: Bergamot citrus and wild cardoon reduce liver steatosis and body weight in non-diabetic individuals aged over 50 years. Front. Endocrinol. 2020, 11, 494. [Google Scholar] [CrossRef]
- Liao, K.C.; Jhuang, J.H.; Yao, H.T. Artichoke leaf extract supplementation lowers hepatic oxidative stress and inflammation and increases multidrug resistance-associated protein 2 in mice fed a high-fat and high-cholesterol diet. Food Funct. 2021, 12, 7239–7249. [Google Scholar] [CrossRef]
- Oppedisano, F.; Muscoli, C.; Musolino, V.; Carresi, C.; Macrì, R.; Giancotta, C.; Bosco, F.; Maiuolo, J.; Scarano, F.; Paone, S.; et al. The protective effect of Cynara cardunculus extract in diet-induced NAFLD: Involvement of OCTN1 and OCTN2 transporter subfamily. Nutrients 2020, 12, 1435. [Google Scholar] [CrossRef]
- Musolino, V.; Gliozzi, M.; Bombardelli, E.; Nucera, S.; Carresi, C.; Maiuolo, J.; Mollace, R.; Paone, S.; Bosco, F.; Scarano, F.; et al. The synergistic effect of Citrus bergamia and Cynara cardunculus extracts on vascular inflammation and oxidative stress in non-alcoholic fatty liver disease. J. Tradit. Complement. Med. 2020, 10, 268–274. [Google Scholar] [CrossRef]
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxid. Med. Cell. Longev. 2018, 2018, 9547613, Erratum in Oxid. Med. Cell. Longev. 2021, 2021, 9757921. [Google Scholar] [CrossRef] [PubMed]
- Delli Bovi, A.P.; Marciano, F.; Mandato, C.; Siano, M.A.; Savoia, M.; Vajro, P. Oxidative Stress in Non-alcoholic Fatty Liver Disease. An Updated Mini Review. Front Med. 2021, 26, 595371. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.R.; Jovanova, B.; Panovska, T.K. Toxicological evaluation of the plant products using brine shrimp (Artemia salina L.) model. Maced. Pharm. Bull. 2014, 60, 9–18. [Google Scholar] [CrossRef]
- Libralato, G.; Prato, E.; Migliore, L.; Cicero, A.M.; Manfra, L. A review of toxicity testing protocols and endpoints with Artemia spp. Ecol. Indic. 2016, 69, 35–49. [Google Scholar] [CrossRef]
- Peña-Oyarzun, D.; Bravo-Sagua, R.; Diaz-Vega, A.; Aleman, L.; Chiong, M.; Garcia, L.; Bambs, C.; Troncoso, R.; Cifuentes, M.; Morselli, E.; et al. Autophagy and oxidative stress in non-communicable diseases: A matter of the inflammatory state? Free Radic. Biol. Med. 2018, 124, 61–78. [Google Scholar] [CrossRef]
- Seyedsadjadi, N.; Grant, R. The Potential Benefit of Monitoring Oxidative Stress and Inflammation in the Prevention of Non-Communicable Diseases (NCDs). Antioxidants 2020, 10, 15. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef]
- Forni, C.; Facchiano, F.; Bartoli, M.; Pieretti, S.; Facchiano, A.; D’Arcangelo, D.; Norelli, S.; Valle, G.; Nisini, R.; Beninati, S.; et al. Beneficial Role of Phytochemicals on Oxidative Stress and Age-Related Diseases. BioMed Res. Int. 2019, 2019, 8748253. [Google Scholar] [CrossRef]
- Gantenbein, K.V.; Kanaka-Gantenbein, C. (Mediterranean Diet as an Antioxidant: The Impact on Metabolic Health and Overall Wellbeing. Nutrients 2021, 13, 1951. [Google Scholar] [CrossRef]
- Anania, C.; Perla, F.M.; Olivero, F.; Pacifico, L.; Chiesa, C. Mediterranean diet and nonalcoholic fatty liver disease. World J. Gastroenterol. 2018, 24, 2083–2094. [Google Scholar] [CrossRef]
- Pinelli, P.; Agostini, F.; Comino, C.; Lanteri, S.; Portis, E.; Romani, A. Simultaneous quantification of caffeoyl esters and flavonoids in wild and cultivated cardoon leaves. Food Chem. 2007, 105, 1695–1701. [Google Scholar] [CrossRef]
- Carpentieri, S.; Augimeri, G.M.; Ceramella, J.; Vivacqua, A.; Sinicropi, M.S.; Pataro, G.; Bonofiglio, D.; Ferrari, G. Antioxidant and Anti-Inflammatory Effects of Extracts from Pulsed Electric Field-Treated Artichoke By-Products in Lipopolysaccharide-Stimulated Human THP-1 Macrophages. Foods 2022, 11, 2250. [Google Scholar] [CrossRef] [PubMed]
- Kollia, E.; Markaki, P.; Zoumpoulakis, P.; Proestos, C. Comparison of Different Extraction Methods for the Determination of the Antioxidant and Antifungal Activity of Cynara scolymus and C. cardunculus Extracts and Infusions. Nat. Prod. Res. 2017, 12, 423–426. [Google Scholar] [CrossRef]
- Cui, W.; Chen, S.L.; Hu, K.Q. Quantification and mechanisms of oleic acid-induced steatosis in HepG2 cells. Am. J. Transl. Res. 2010, 2, 95–104. [Google Scholar] [PubMed]
- Müller, F.A.; Shana, J.; Sturla, S.S. Human in vitro models of nonalcoholic fatty liver Disease. Curr. Opin. Toxicol. 2019, 16, 9–16. [Google Scholar] [CrossRef]
- Miccadei, S.; Di Venere, D.; Cardinali, A.; Romano, F.; Durazzo, A.; Foddai, M.S.; Fraioli, R.; Mobarhan, S.; Maiani, G. Antioxidative and apoptotic properties of polyphenolic extracts from edible part of artichoke (Cynara scolymus L.) on cultured rat hepatocytes and on human hepatoma cells. Nutr. Cancer 2008, 60, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, D.; Park, S.J.; Kim, K.S.; Park, G.D.; Kim, O.K.; Lee, J. Artichoke Extract Directly Suppresses Inflammation and Apoptosis in Hepatocytes During the Development of Non-Alcoholic Fatty Liver Disease. J. Med. Food 2021, 24, 1058–1067. [Google Scholar] [CrossRef]
- Lü, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Bio. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef]
- Blaser, H.; Dostert, C.; Mak, T.W.; Brenner, D. TNF and ROS Crosstalk in Inflammation. Trends Cell Biol. 2016, 26, 249–261. [Google Scholar] [CrossRef]
- Karkucinska-Wieckowska, A.; Simoes, I.C.M.; Kalinowski, P.; Lebiedzinska-Arciszewska, M.; Zieniewicz, K.; Milkiewicz, P.; Górska-Ponikowska, M.; Pinton, P.; Malik, A.N.; Krawczyk, M.; et al. Mitochondria, oxidative stress and nonalcoholic fatty liver disease: A complex relationship. Eur. J. Clin. Investig. 2022, 52, e13622. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Gao, Y.Q.; Zhang, Y.; Wang, H.; Liu, G.S.; Lei, J.Y. Chlorogenic acid alleviates autophagy and insulin resistance by suppressing JNK pathway in a rat model of nonalcoholic fatty liver disease. J. Biosci. 2018, 43, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Kamel, A.M.; Farag, M.A. Therapeutic Potential of Artichoke in the Treatment of Fatty Liver: A Systematic Review and Meta-Analysis. J. Med. Food. 2022, 25, 931–942. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Z.; Chen, L.; Sun, G. Hypolipidemic Effects and Preliminary Mechanism of Chrysanthemum Flavonoids, Its Main Components Luteolin and Luteoloside in Hyperlipidemia Rats. Antioxidants 2021, 10, 1309. [Google Scholar] [CrossRef] [PubMed]
- Costea, L.; Chițescu, C.L.; Boscencu, R.; Ghica, M.; Lupuliasa, D.; Mihai, D.P.; Deculescu-Ioniță, T.; Duțu, L.E.; Popescu, M.L.; Luță, E.A.; et al. The Polyphenolic Profile and Antioxidant Activity of Five Vegetal Extracts with Hepatoprotective Potential. Plants 2022, 11, 1680. [Google Scholar] [CrossRef]
- Zheng, Q.S.; Sun, X.L.; Xu, B.; Li, G.; Song, M. Mechanisms of apigenin-7-glucoside as a hepatoprotective agent. Biomed. Environ. Sci. 2005, 18, 65–70. [Google Scholar]
- Genovese, C.; Garozzo, A.; D’Angeli, F.; Malfa, G.A.; Bellia, F.; Tomasello, B.; Nicolosi, D.; Malaguarnera, R.; Ronsisvalle, S.; Guadagni, F.; et al. Orobanche crenata Forssk. Extract Affects Human Breast Cancer Cell MCF-7 Survival and Viral Replication. Cells 2022, 11, 1696. [Google Scholar] [CrossRef]
- Acquaviva, R.; D’Angeli, F.; Malfa, G.A.; Ronsisvalle, S.; Garozzo, A.; Stivala, A.; Ragusa, S.; Nicolosi, D.; Salmeri, M.; Genovese, C. Antibacterial and anti-biofilm activities of walnut pellicle extract (Juglans regia L.) against coagulase-negative staphylococci. Nat. Prod. Res. 2021, 35, 2076–2081. [Google Scholar] [CrossRef]
- Malfa, G.A.; Tomasello, B.; Acquaviva, R.; La Mantia, A.; Pappalardo, F.; Ragusa, M.; Renis, M.; Di Giacomo, C. The antioxidant activities of Betula etnensis Rafin. ethanolic extract exert protective and anti-diabetic effects on streptozotocin-induced diabetes in rats. Antioxidants 2020, 9, 847. [Google Scholar] [CrossRef]
- Salerno, L.; Pittalà, V.; Romeo, G.; Modica, M.N.; Siracusa, M.A.; Di Giacomo, C.; Acquaviva, R.; Barbagallo, I.; Tibullo, D.; Sorrenti, V. Evaluation of novel aryloxyalkyl derivatives of imidazole and 1,2,4-triazole as heme oxygenase-1 (HO-1) inhibitors and their antitumor properties. Bioorgan. Med. Chem. 2013, 21, 5145–5153. [Google Scholar] [CrossRef]
- Tomasello, B.; Malfa, G.A.; La Mantia, A.; Miceli, N.; Sferrazzo, G.; Taviano, M.F.; Di Giacomo, C.; Renis, M.; Acquaviva, R. Anti-adipogenic and anti-oxidant effects of a standardised extract of Moro blood oranges (Citrus sinensis (L.) Osbeck) during adipocyte differentiation of 3T3-L1 preadipocytes. Nat. Prod. Res. 2021, 35, 2660–2667. [Google Scholar] [CrossRef] [PubMed]
- Tomasello, B.; Di Mauro, M.D.; Malfa, G.A.; Acquaviva, R.; Sinatra, F.; Spampinato, G.; Laudani, S.; Villaggio, G.; Bielak-Zmijewska, A.; Grabowska, W.; et al. Rapha Myr®, a Blend of Sulforaphane and Myrosinase, Exerts Antitumor and Anoikis-Sensitizing Effects on Human Astrocytoma Cells Modulating Sirtuins and DNA Methylation. Int. J. Mol. Sci. 2020, 21, 5328. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, C.; Acquaviva, R.; Piva, A.; Sorrenti, V.; Vanella, L.; Piva, G.; Casadei, G.; La Fauci, L.; Ritieni, A.; Bognanno, M.; et al. Protective effect of cyanidin 3-O-beta-D-glucoside on ochratoxin A-mediated damage in the rat. Brit. J. Nutr. 2007, 98, 937–943. [Google Scholar] [CrossRef]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobson, L.B.; Nichols, D.E.; McLaughlin, J.L. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef] [PubMed]



| Total Flavonoids (mg CE/g Extract) | Total Polyphenols (mg GAE/g Extract) | DPPH Test IC50 (μg/mL) | |
|---|---|---|---|
| C. sylvestris | 50.32 ± 1.62 | 185.21 ± 1.97 | 20.04 ± 2.52 µg/mL |
| Trolox | 15 µM ± 0.62 |
| Peak | Compound | Wavelength (nm) | Ret. Time (min.) |
|---|---|---|---|
| 1 | Neochlorogenic acid | 325 | 9.11 |
| 2 | Chlorogenic acid | 325 | 17.79 |
| 3 | Cynarine | 325 | 25.92 |
| 4 | Luteolin 7-Glucoside | 346 | 37.21 |
| 5 | Luteolin 7-Glucuronide | 346 | 38.44 |
| 6 | 3,4-Dicaffeoylquinic acid | 325 | 38.93 |
| 7 | 1,5-Dicaffeoylquinic acid | 325 | 43.18 |
| 8 | Apigenin 7-O-Rutinoside | 346 | 43.45 |
| 9 | Apigenin 7-O-Glucoside | 346 | 45.38 |
| 10 | Apigenin 7-O-Glucuronide | 346 | 48.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acquaviva, R.; Malfa, G.A.; Santangelo, R.; Bianchi, S.; Pappalardo, F.; Taviano, M.F.; Miceli, N.; Di Giacomo, C.; Tomasello, B. Wild Artichoke (Cynara cardunculus subsp. sylvestris, Asteraceae) Leaf Extract: Phenolic Profile and Oxidative Stress Inhibitory Effects on HepG2 Cells. Molecules 2023, 28, 2475. https://doi.org/10.3390/molecules28062475
Acquaviva R, Malfa GA, Santangelo R, Bianchi S, Pappalardo F, Taviano MF, Miceli N, Di Giacomo C, Tomasello B. Wild Artichoke (Cynara cardunculus subsp. sylvestris, Asteraceae) Leaf Extract: Phenolic Profile and Oxidative Stress Inhibitory Effects on HepG2 Cells. Molecules. 2023; 28(6):2475. https://doi.org/10.3390/molecules28062475
Chicago/Turabian StyleAcquaviva, Rosaria, Giuseppe Antonio Malfa, Rosa Santangelo, Simone Bianchi, Francesco Pappalardo, Maria Fernanda Taviano, Natalizia Miceli, Claudia Di Giacomo, and Barbara Tomasello. 2023. "Wild Artichoke (Cynara cardunculus subsp. sylvestris, Asteraceae) Leaf Extract: Phenolic Profile and Oxidative Stress Inhibitory Effects on HepG2 Cells" Molecules 28, no. 6: 2475. https://doi.org/10.3390/molecules28062475
APA StyleAcquaviva, R., Malfa, G. A., Santangelo, R., Bianchi, S., Pappalardo, F., Taviano, M. F., Miceli, N., Di Giacomo, C., & Tomasello, B. (2023). Wild Artichoke (Cynara cardunculus subsp. sylvestris, Asteraceae) Leaf Extract: Phenolic Profile and Oxidative Stress Inhibitory Effects on HepG2 Cells. Molecules, 28(6), 2475. https://doi.org/10.3390/molecules28062475











