O,S-Acetals in a New Modification of oxo-Friedel–Crafts–Bradsher Cyclization—Synthesis of Fluorescent (Hetero)acenes and Mechanistic Considerations
Abstract
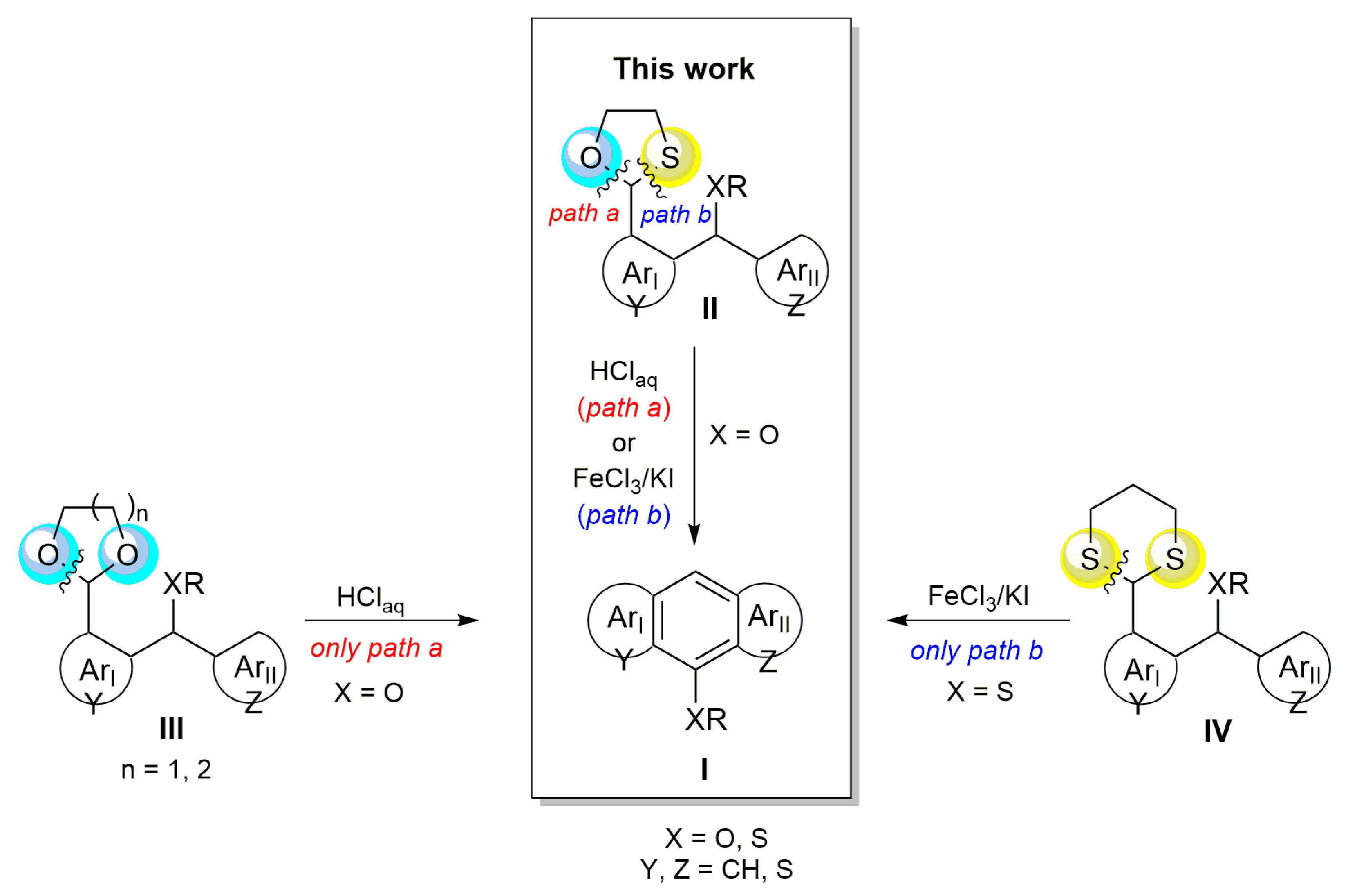
1. Introduction
2. Results and Discussion
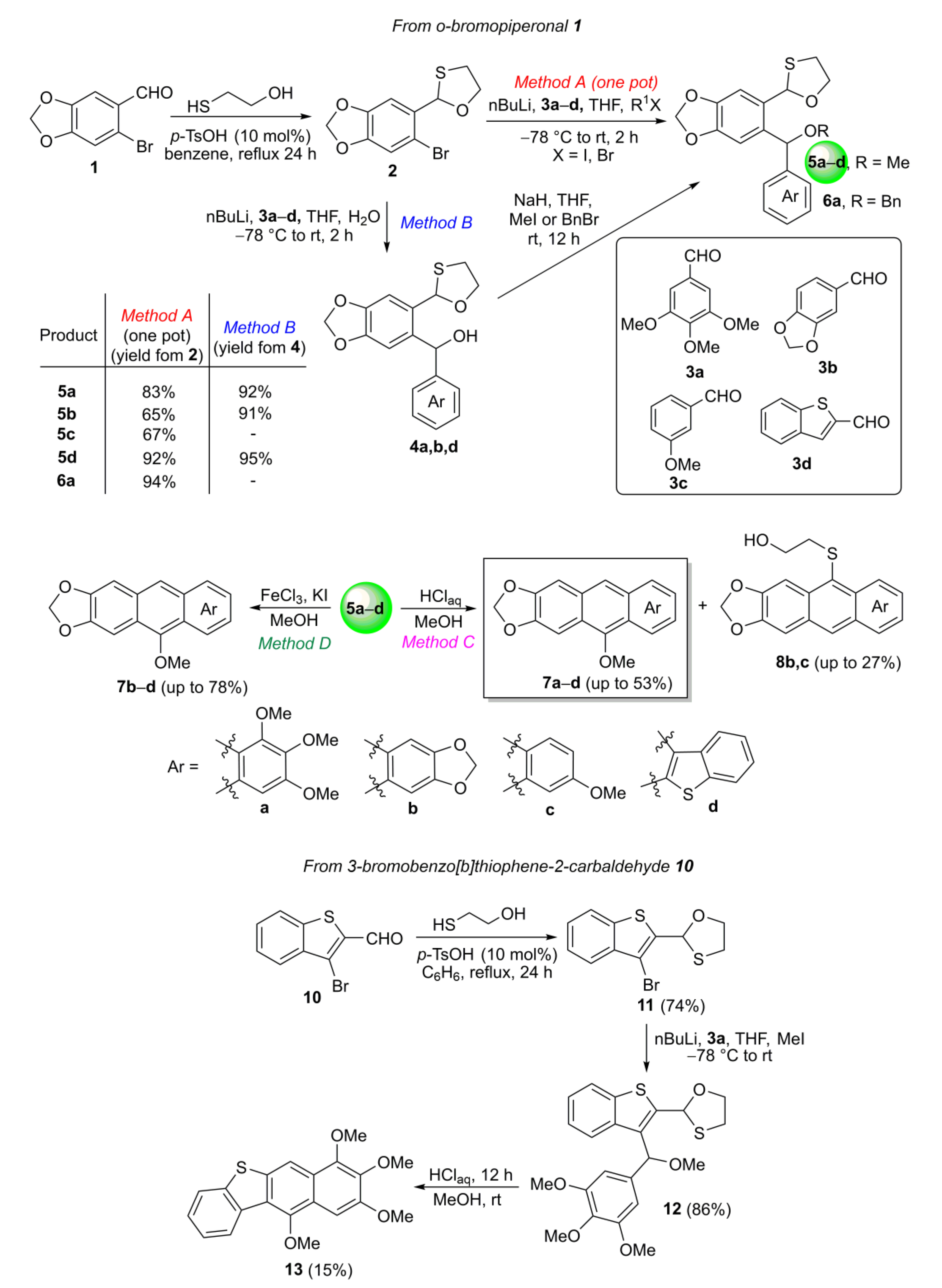
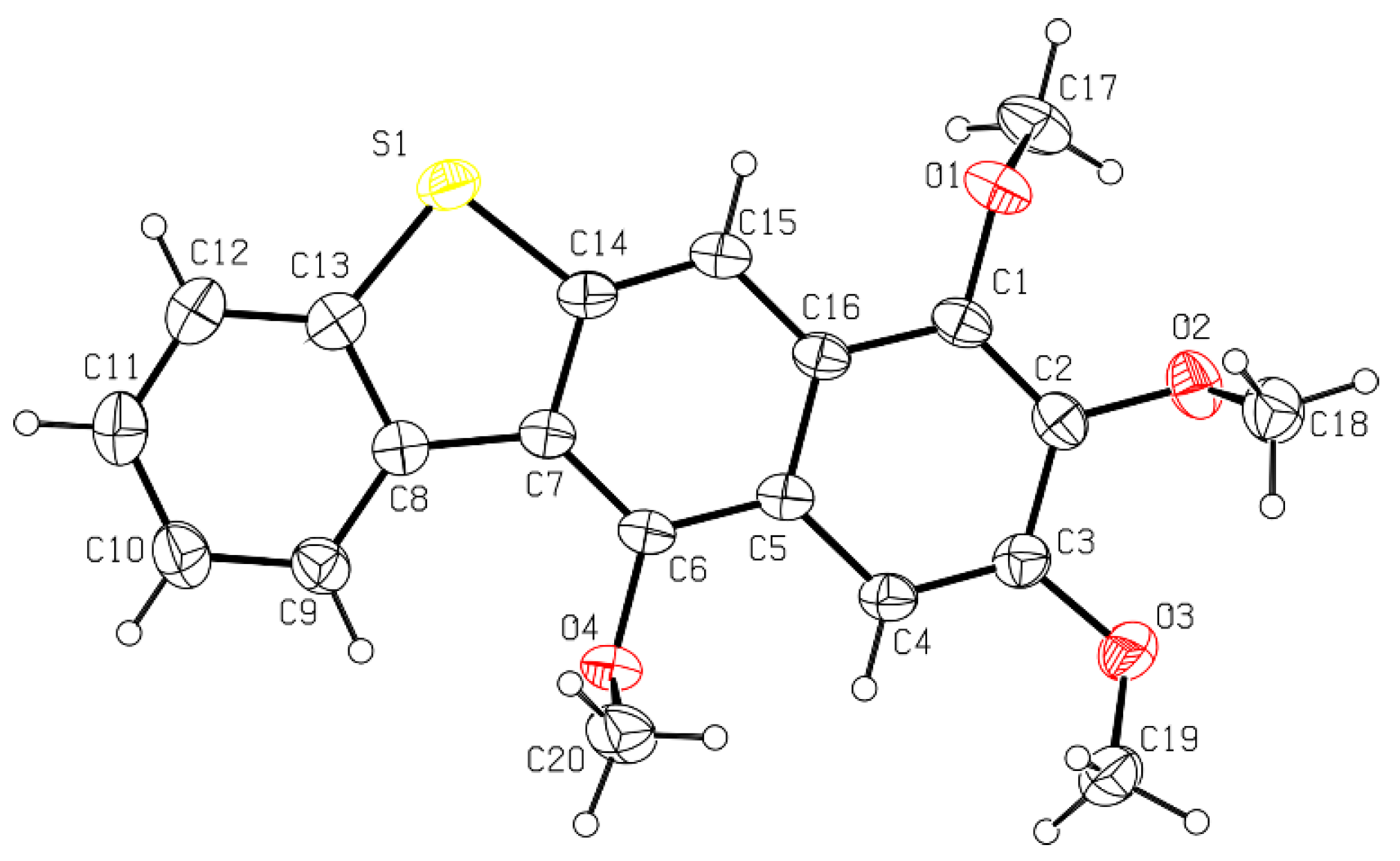
2.1. Synthesis
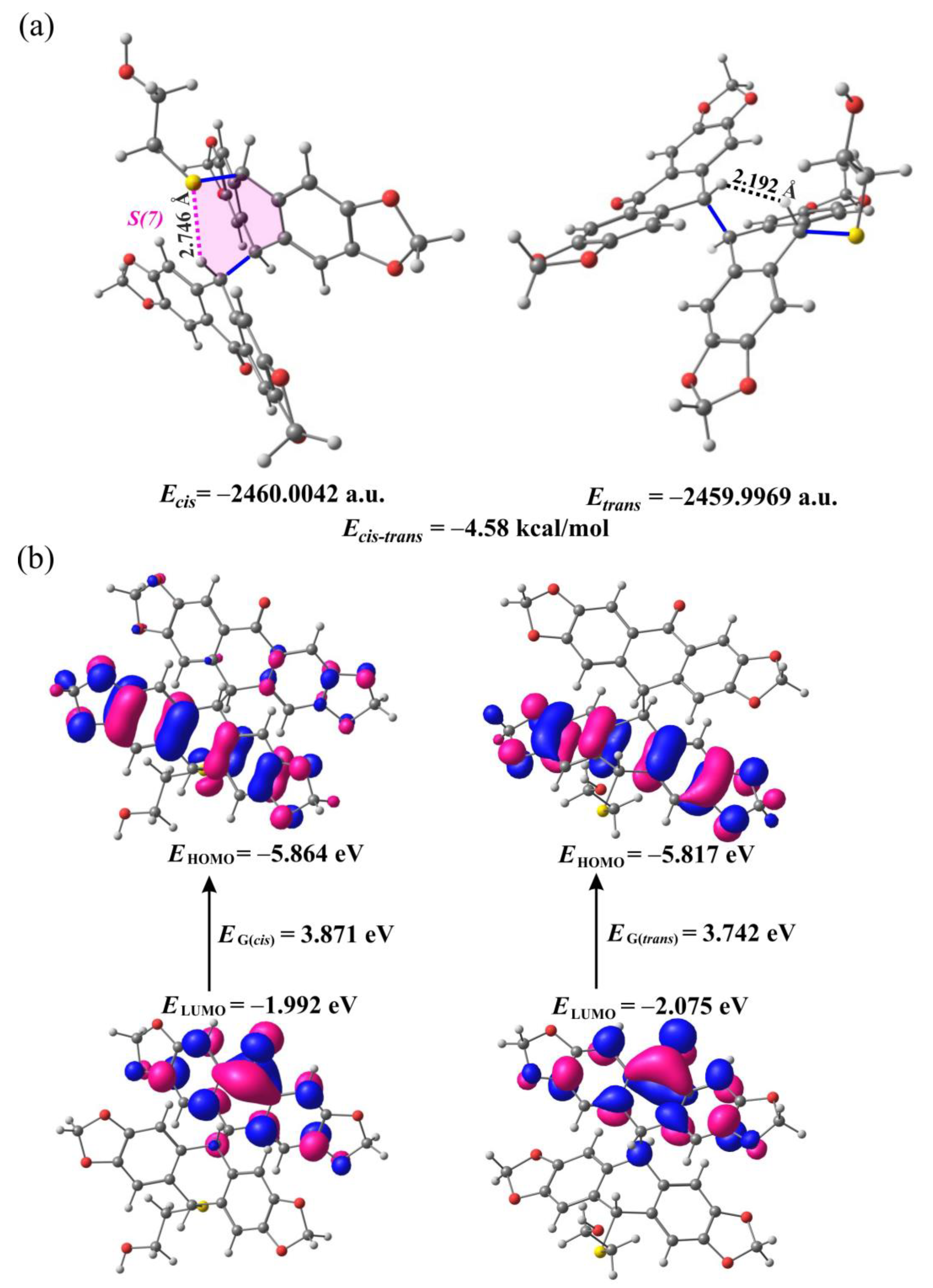
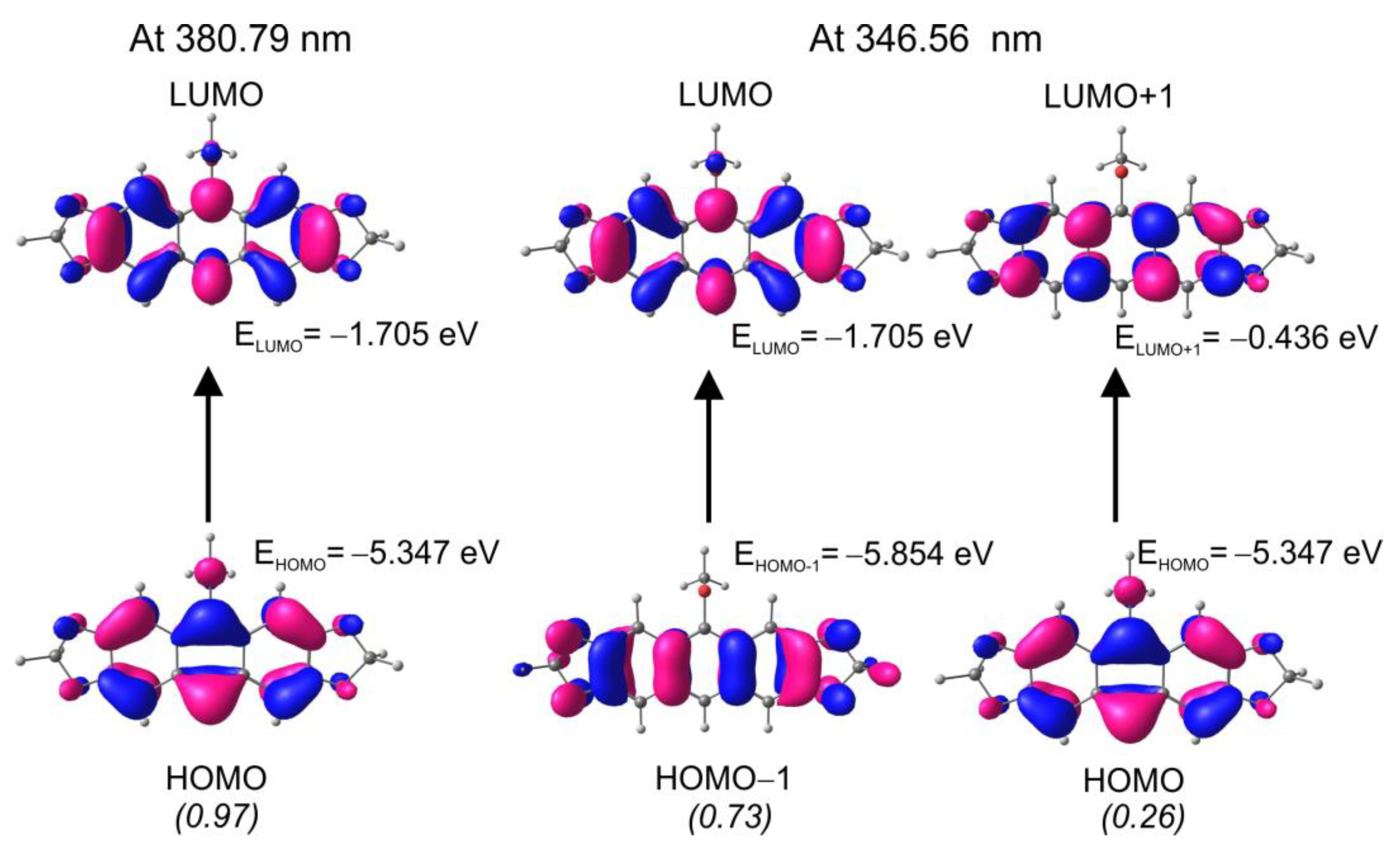
2.2. DFT Calculations for 9a and 9b
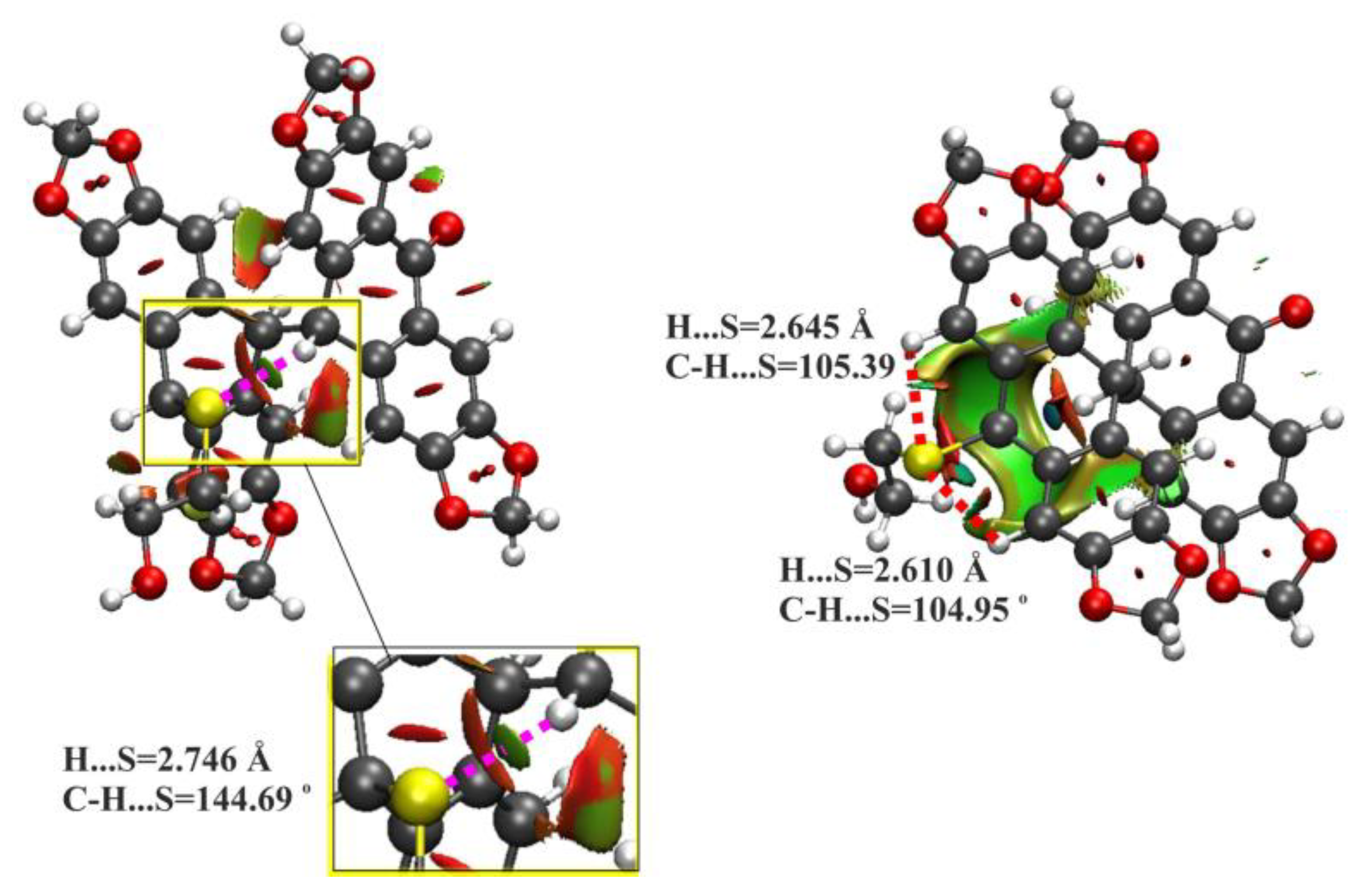
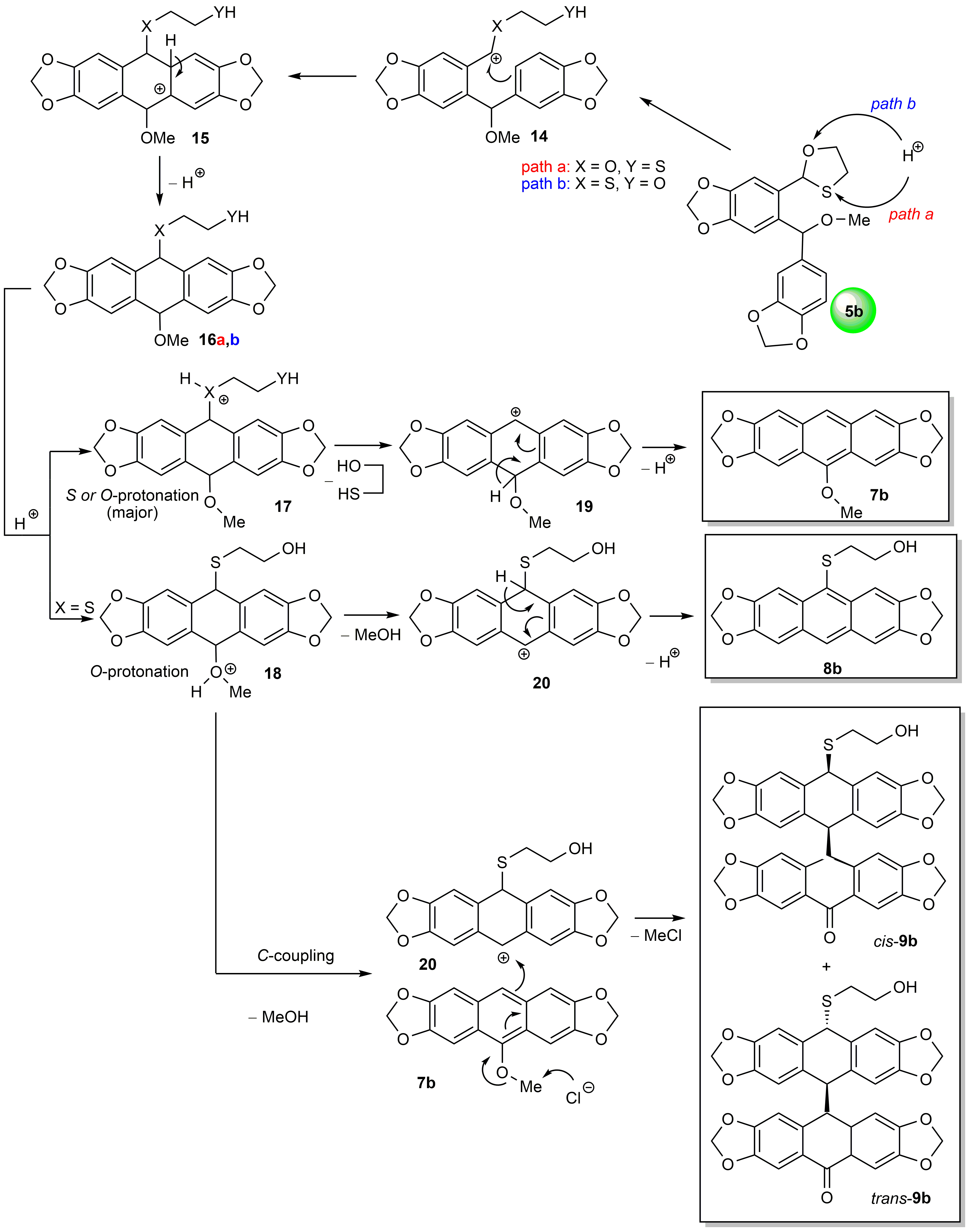
2.3. Mechanistic Considerations and DFT Calculations
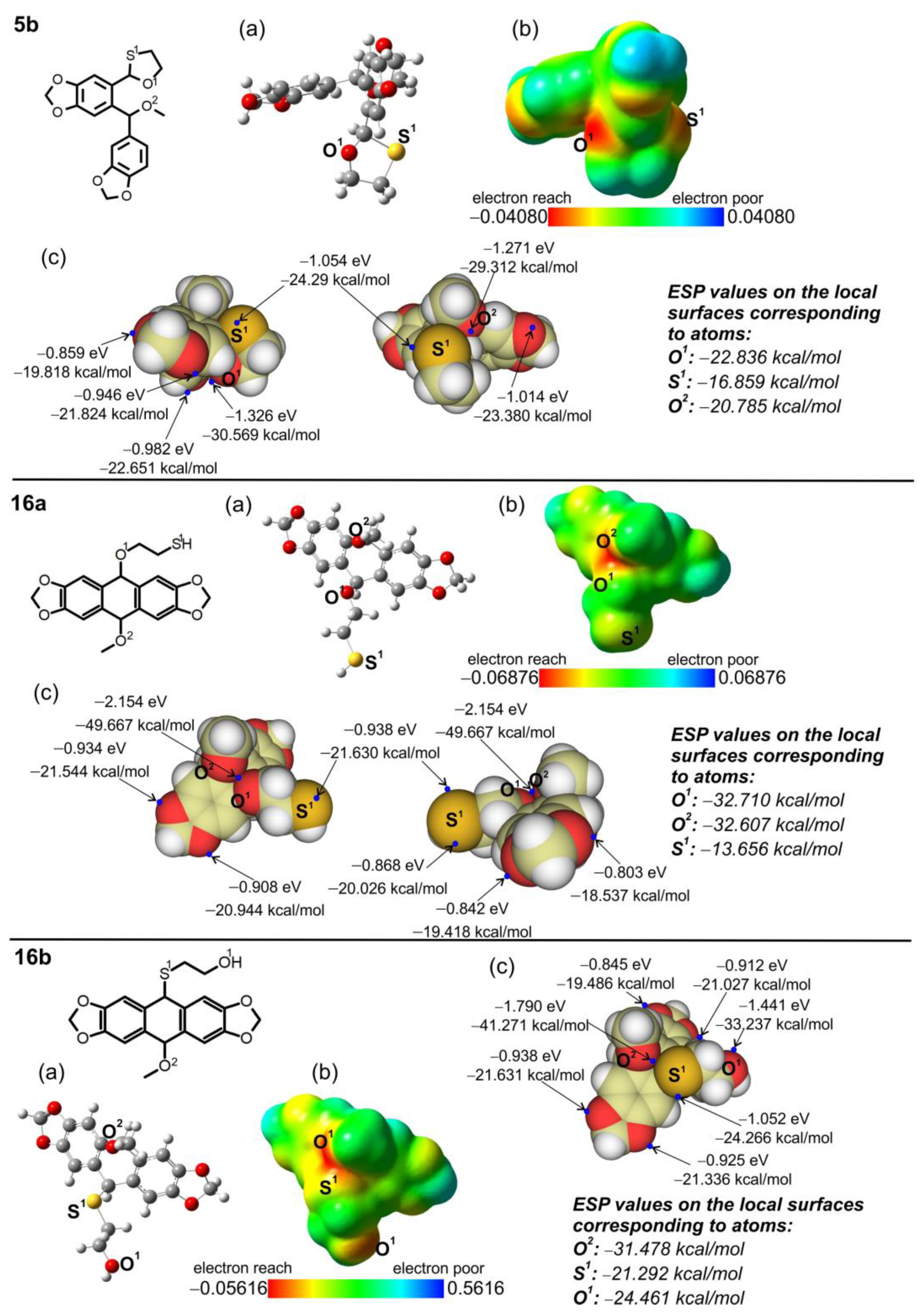
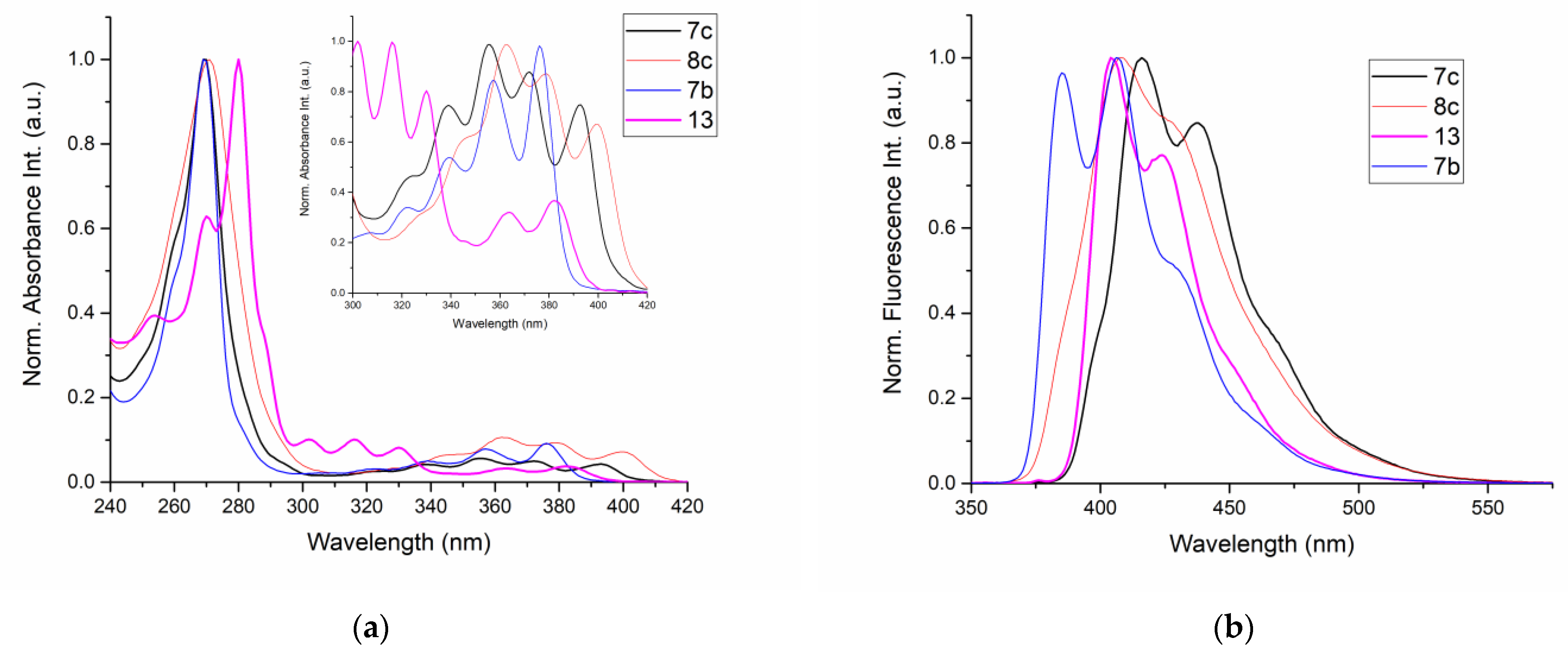
2.4. Electron Character of RO-Acenes 7, 13 and HO(CH2)2S-Acenes 8
2.5. Photopysical Properties
3. Materials and Methods
3.1. Synthesis of o-Bromopiperonal O,S-acetal 2
3.2. General Procedure for the Synthesis of o-(O,S-acetalaryl)arylmethanols 4
3.3. General Procedure for the One-Pot Synthesis of o-(O,S-acetalaryl)arylmethyl Methyl Ethers 5 from o-Bromopiperonal O,S-acetal 2 (Method A)
3.4. Procedure for the Synthesis of o-(O,S-acetalaryl)arylmethyl Benzyl Ether 6a
3.5. General Procedure for the Synthesis of o-(O,S-acetalaryl)arylmethyl Methyl Ethers 5 from o-(O,S-acetalaryl)arylmethanols 4 (Method B)
3.6. General Procedure for the Synthesis of MeO-Substituted Acenes 7, 13 and 8 Using HClaq (Method C)
3.7. General Procedure for the Synthesis of MeO-Substituted Acene 7 Using FeCl3/KI in MeOH (Method D)
3.8. Synthesis of 3-Bromobenzo[b]thiophene-2-carbaldehyde O,S-acetal 11
3.9. Synthesis of o-(O,S-acetalaryl)arylmethyl Methyl Ether 12
3.10. Synthesis of Acene 13 Using HClaq
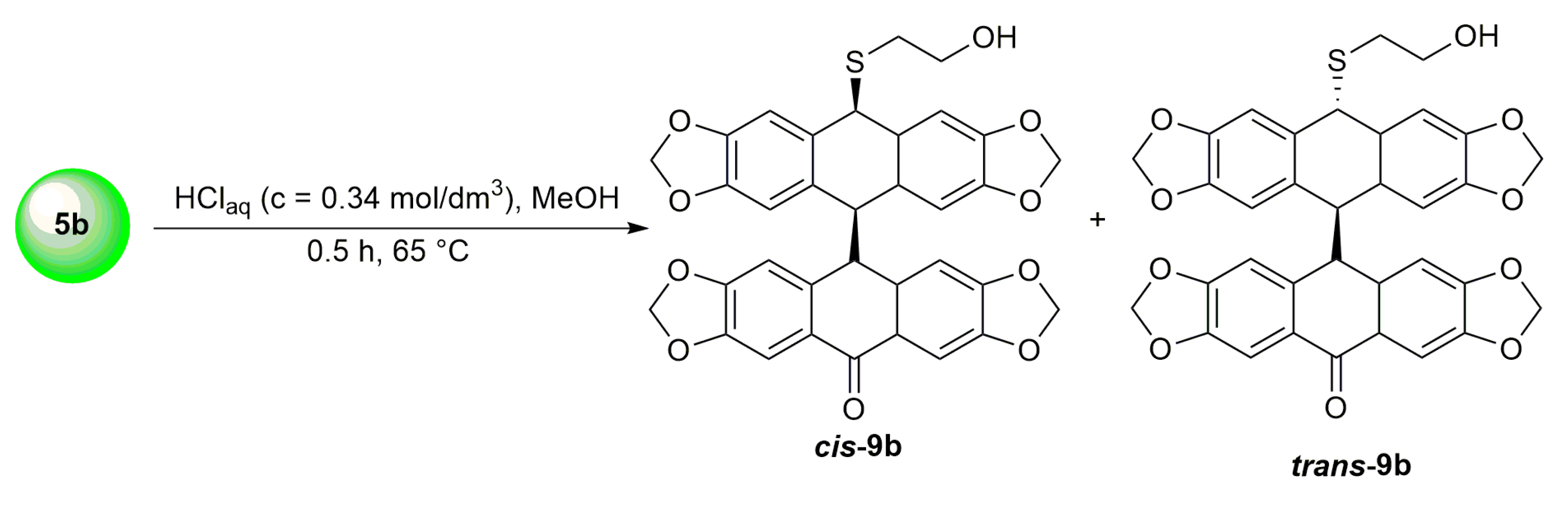
3.11. Synthesis of Dimeric Isomers cis-9b and trans-9b from o-(O,S-acetalaryl)arylmethyl Methyl Ether 5b Using HClaq
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yang, Y.; Börjesson, K. Electroactive covalent organic frameworks: A new choice for organic electronics. Trends Chem. 2022, 4, 60–75. [Google Scholar] [CrossRef]
- Koprowski, M.; Owsianik, K.; Knopik, Ł.; Vivek, V.; Romaniuk, A.; Różycka-Sokołowska, E.; Bałczewski, P. Comprehensive Review on Synthesis, Properties, and Applications of Phosphorus (PIII, PIV, PV) Substituted Acenes with More Than Two Fused Benzene Rings. Molecules 2022, 27, 6611. [Google Scholar] [CrossRef]
- Bałczewski, P.; Kowalska, E.; Różycka-Sokołowska, E.; Skalik, J.; Owsianik, K.; Koprowski, M.; Marciniak, B.; Guziejewski, D.; Ciesielski, W. Mono-Aryl/Alkylthio-Substituted (Hetero)acenes of Exceptional Thermal and Photochemical Stability by the Thio-Friedel–Crafts/Bradsher Cyclization Reaction. Chem.—A Eur. J. 2019, 25, 14148–14161. [Google Scholar] [CrossRef]
- Im, Y.; Byun, S.Y.; Kim, J.H.; Lee, D.R.; Oh, C.S.; Yook, K.S.; Lee, J.Y. Recent Progress in High-Efficiency Blue-Light-Emitting Materials for Organic Light-Emitting Diodes. Adv. Funct. Mater. 2017, 27, 1603007. [Google Scholar] [CrossRef]
- Yuan, Q.; Wang, T.; Yu, P.; Zhang, H.; Zhang, H.; Ji, W. A review on the electroluminescence properties of quantum-dot light-emitting diodes. Org. Electron. 2021, 90, 106086. [Google Scholar] [CrossRef]
- Lim, H.; Woo, S.-J.; Ha, Y.H.; Kim, Y.-H.; Kim, J.-J. Breaking the Efficiency Limit of Deep-Blue Fluorescent OLEDs Based on Anthracene Derivatives. Adv. Mater. 2022, 34, 2100161. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, T.; Dong, S.; Wen, Z.; Xu, H.; Miao, Y.; Wang, H.; Yu, J. Anthracene and carbazole based asymmetric fluorescent materials for high-efficiency deep-blue non-doped organic light emitting devices with CIEy=0.06. Dye. Pigment. 2022, 199, 110047. [Google Scholar] [CrossRef]
- Haykir, G.; Aydemir, M.; Han, S.H.; Gumus, S.; Hizal, G.; Lee, J.Y.; Turksoy, F. The investigation of sky-blue emitting anthracene-carbazole derivatives: Synthesis, photophysics and OLED applications. Org. Electron. 2018, 59, 319–329. [Google Scholar] [CrossRef]
- Lim, H.; Cheon, H.J.; Lee, G.S.; Kim, M.; Kim, Y.-H.; Kim, J.-J. Enhanced Triplet–Triplet Annihilation of Blue Fluorescent Organic Light-Emitting Diodes by Generating Excitons in Trapped Charge-Free Regions. ACS Appl. Mater. Interfaces 2019, 11, 48121–48127. [Google Scholar] [CrossRef]
- Song, D.; Yu, Y.; Yue, L.; Zhong, D.; Zhang, Y.; Yang, X.; Sun, Y.; Zhou, G.; Wu, Z. Asymmetric thermally activated delayed fluorescence (TADF) emitters with 5,9-dioxa-13b-boranaphtho[3,2,1-de]anthracene (OBA) as the acceptor and highly efficient blue-emitting OLEDs. J. Mater. Chem. C 2019, 7, 11953–11963. [Google Scholar] [CrossRef]
- Park, J.; Jang, B.; Moon, Y.J.; Lee, H.; Kim, Y.K.; Yoon, S.S. Efficient deep blue organic light emitting diodes based on [2,7,7,13,13-pentamethyl-9-(10-phenylanthracen-9-yl)-7,13-dihydrobenzo[5,6]-s-indaceno[1,2-g]quinoline] derivatives. Mol. Cryst. Liq. Cryst. 2020, 705, 120–126. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, W.; Ye, S.; Zhang, Q.; Duan, Y.; Guo, R.; Wang, L. Molecular engineering of anthracene-based emitters for highly efficient nondoped deep-blue fluorescent OLEDs. J. Mater. Chem. C 2020, 8, 9678–9687. [Google Scholar] [CrossRef]
- Desai, N.K.; Kolekar, G.B.; Patil, S.R. Fabrication and Characterization of Anthracene Doped P-terphenyl Thin Films By Spin Coating Technique; Investigation of Fluorescence Properties. J. Fluoresc. 2020, 30, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Sun, M.; Xu, L.; Wang, R.; Zhou, H.; Pan, Y.; Zhang, S.; Sun, Q.; Xue, S.; Yang, W. Highly efficient non-doped blue fluorescent OLEDs with low efficiency roll-off based on hybridized local and charge transfer excited state emitters. Chem. Sci. 2020, 11, 5058–5065. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.-S.; Ha, Y.H.; Kwon, S.-K.; Kim, Y.-H.; Kim, J.-J. Design Strategy of Anthracene-Based Fluorophores toward High-Efficiency Deep Blue Organic Light-Emitting Diodes Utilizing Triplet–Triplet Fusion. ACS Appl. Mater. Interfaces 2020, 12, 15422–15429. [Google Scholar] [CrossRef]
- Wu, Z.; Zhu, X.; Li, Y.; Chen, H.; Zhuang, Z.; Shen, P.; Zeng, J.; Chi, J.; Ma, D.; Zhao, Z.; et al. High-Performance Hybrid White OLEDs with Ultra-Stable Emission Color and Small Efficiency Roll-Off Achieved by Incorporating a Deep-Blue Fluorescent Neat Film. Adv. Opt. Mater. 2021, 9, 2100298. [Google Scholar] [CrossRef]
- Kang, S.; Kwon, H.; Jeong, J.; Kim, Y.-C.; Park, J. Synthesis and Electroluminescence Properties of New Blue Emitting Polymer Based on Dual-Core Type for Solution Process OLEDs. Macromol. Res. 2022, 30, 454–459. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, G.; Luo, X.; Tian, X.; Zhang, D.; Guo, S.; Zhou, H.; Miao, Y.; Huang, J.; Wang, H. Anthracene-based blue fluorescence materials utilized in non-doped OLEDs with high luminance and a low efficiency roll-off. Dye. Pigment. 2022, 204, 110391. [Google Scholar] [CrossRef]
- Lee, J.H.; Lin, H.-Y.; Chen, C.-H.; Lee, Y.-T.; Chiu, T.-L.; Lee, J.-H.; Chen, C.-T.; Adachi, C. Deep Blue Fluorescent Material with an Extremely High Ratio of Horizontal Orientation to Enhance Light Outcoupling Efficiency (44%) and External Quantum Efficiency in Doped and Non-Doped Organic Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2021, 13, 34605–34615. [Google Scholar] [CrossRef]
- Bałczewski, P.; Kowalska, E.; Różycka-Sokołowska, E.; Uznański, P.; Wilk, J.; Koprowski, M.; Owsianik, K.; Marciniak, B. Organosulfur Materials with High Photo- and Photo-Oxidation Stability: 10-Anthryl Sulfoxides and Sulfones and Their Photophysical Properties Dependent on the Sulfur Oxidation State. Materials 2021, 14, 3506. [Google Scholar] [CrossRef]
- Newman, M.S.; Hussain, N.S. Synthesis of nuclear monobromobenz[a]anthracenes. J. Org. Chem. 1982, 47, 2837–2840. [Google Scholar] [CrossRef]
- Andrus, M.B.; Ye, Z.; Zhang, J. Highly selective glycine phase-transfer catalysis using fluoroanthracenylmethyl cinchonidine catalysts. Tetrahedron Lett. 2005, 46, 3839–3842. [Google Scholar] [CrossRef]
- Harvey, R.G.; Cortez, C.; Sugiyama, T.; Ito, Y.; Sawyer, T.W.; DiGiovanni, J. Biologically active dihydrodiol metabolites of polycyclic aromatic hydrocarbons structurally related to the potent carcinogenic hydrocarbon 7,12-dimethylbenz[a]anthracene. J. Med. Chem. 1988, 31, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Hallman, J.L.; Bartsch, R.A. Synthesis of naphtho[f]ninhydrin. J. Org. Chem. 1991, 56, 6243–6245. [Google Scholar] [CrossRef]
- Platt, K.L.; Oesch, F. Reductive cyclization of keto acids to polycyclic aromatic hydrocarbons by hydroiodic acid-red phosphorus. J. Org. Chem. 1981, 46, 2601–2603. [Google Scholar] [CrossRef]
- Ihmels, H.; Meiswinkel, A.; Mohrschladt, C.J.; Otto, D.; Waidelich, M.; Towler, M.; White, R.; Albrecht, M.; Schnurpfeil, A. Anthryl-Substituted Heterocycles as Acid-Sensitive Fluorescence Probes. J. Org. Chem. 2005, 70, 3929–3938. [Google Scholar] [CrossRef]
- Sangaiah, R.; Gold, A.; Toney, G.E. Synthesis of a series of novel polycyclic aromatic systems: Isomers of benz[a]anthracene containing a cyclopenta-fused ring. J. Org. Chem. 1983, 48, 1632–1638. [Google Scholar] [CrossRef]
- Newman, M.S.; Prabhu, V.S.; Veeraraghavan, S. Synthesis of nuclear monobromobenz[a]anthracenes. J. Org. Chem. 1983, 48, 2926–2928. [Google Scholar] [CrossRef]
- Bradsher, C.; Sinclair, E. Notes—Cyclodehydrations in Liquid Sulfur Dioxide. J. Org. Chem. 1957, 22, 79–81. [Google Scholar] [CrossRef]
- Yamato, T.; Sakaue, N.; Shinoda, N.; Matsuo, K. Selective preparation of polycyclic aromatic hydrocarbons. Part 4.1 New synthetic route to anthracenes from diphenylmethanes using Friedel–Crafts intramolecular cyclization. J. Chem. Soc. Perkin Trans. 1 1997, 8, 1193–1200. [Google Scholar] [CrossRef]
- Bałczewski, P.; Skalik, J.; Uznański, P.; Guziejewski, D.; Ciesielski, W. Use of isomeric, aromatic dialdehydes in the synthesis of photoactive, positional isomers of higher analogs of o-bromo(hetero)acenaldehydes. RSC Adv. 2015, 5, 24700–24704. [Google Scholar] [CrossRef]
- Bodzioch, A.; Marciniak, B.; Różycka-Sokołowska, E.; Jeszka, J.K.; Uznański, P.; Kania, S.; Kuliński, J.; Bałczewski, P. Synthesis and Optoelectronic Properties of Hexahydroxylated 10-O-R-Substituted Anthracenes via a New Modification of the Friedel–Crafts Reaction Using O-Protected ortho-Acetal Diarylmethanols. Chem.—A Eur. J. 2012, 18, 4866–4876. [Google Scholar] [CrossRef] [PubMed]
- Bałczewski, P.; Bodzioch, A.; Różycka-Sokołowska, E.; Marciniak, B.; Uznański, P. First Approach to Nitrogen-Containing Fused Aromatic Hydrocarbons as Targets for Organoelectronics Utilizing a New Transformation of O-Protected Diaryl Methanols. Chem.—A Eur. J. 2010, 16, 2392–2400. [Google Scholar] [CrossRef] [PubMed]
- Bałczewski, P.; Kowalska, E.; Skalik, J.; Koprowski, M.; Owsianik, K.; Różycka-Sokołowska, E. Ultrasound-assisted synthesis of RO- and RS-substituted (hetero)acenes via oxo- and thio-Friedel-Crafts/Bradsher reactions. Ultrason. Sonochem. 2019, 58, 104640. [Google Scholar] [CrossRef]
- Mondal, E.; Sahu, P.R.; Khan, A.T. A Useful and Catalytic Method for Protection of Carbonyl Compounds into the Corresponding 1,3-Oxathiolanes and Deprotection to the Parent Carbonyl Compounds. Synlett 2002, 2002, 0463–0467. [Google Scholar] [CrossRef]
- Djerassi, C.; Gorman, M. Studies in Organic Sulfur Compounds. VI.1 Cyclic Ethylene and Trimethylene Hemithioketals. J. Am. Chem. Soc. 1953, 75, 3704–3708. [Google Scholar] [CrossRef]
- Liang, X.; Gao, S.; Yang, J.; He, M. Synthesis of a Novel Strong Brønsted Acidic Ionic Liquid and its Catalytic Activities for the Oxathioacetalization. Catal. Lett. 2008, 125, 396–400. [Google Scholar] [CrossRef]
- Yus, M.; Nájera, C.; Foubelo, F. The role of 1,3-dithianes in natural product synthesis. Tetrahedron 2003, 59, 6147–6212. [Google Scholar] [CrossRef]
- Morton, D.R.; Hobbs, S.J. Facile preparation of cyclic ethylene thioketals and thioacetals with 2-phenyl- and 2-chloro-1,3,2-dithiaborolanes. J. Org. Chem. 1979, 44, 656–658. [Google Scholar] [CrossRef]
- Fuji, K.; Ueda, M.; Sumi, K.; Kajiwara, K.; Fujita, E.; Iwashita, T.; Miura, I. Chemistry of carbanions stabilized by sulfur. 1. Chemistry of 1,3-oxathianes. Synthesis and conformation of 2-substituted 1,3-oxathianes. J. Org. Chem. 1985, 50, 657–661. [Google Scholar] [CrossRef]
- Satchell, D.P.N.; Satchell, R.S. Mechanisms of hydrolysis of thioacetals. Chem. Soc. Rev. 1990, 19, 55–81. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Quantitative analysis of molecular surface based on improved Marching Tetrahedra algorithm. J. Mol. Graph. Model. 2012, 38, 314–323. [Google Scholar] [CrossRef]
- ACD/Percepta, Version 14.0.0; Advanced Chemistry Development, Inc.: Toronto, ON, Canada, 2015.
- Míšek, J.; Vargas Jentzsch, A.; Sakurai, S.; Emery, D.; Mareda, J.; Matile, S. A Chiral and Colorful Redox Switch: Enhanced π Acidity in Action. Angew. Chem. Int. Ed. 2010, 49, 7680–7683. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Xing, Z.; Fang, B.; Xie, X.; She, X. Visible light photoredox catalyzed deprotection of 1,3-oxathiolanes. Org. Biom. Chem. 2020, 18, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Björk, M.; Grivas, S. Synthesis of novel 2-aminoimidazo[4,5-b]pyridines, including the thieno analogue of the cooked-food mutagen IFP. J. Heterocycl. Chem. 2006, 43, 101–109. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sanchez, P.; Contreras-Garcia, J.; Cohen, A.J.; Yang, W. Revealing noncovalent interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
- Chemcraft—Graphical Software for Visualization of Quantum Chemistry Computations. Available online: https://www.chemcraftprog.com (accessed on 1 March 2023).
- Humphrey, W.; Dalke, A.; Schulten, K. VMD—Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
| Substrate | Reaction Conditions | RO-Acene (Yield) 1 | HO(CH2)2S-Acene (Yield) 1 |
|---|---|---|---|
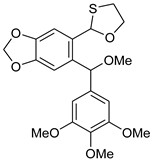 5a | HCl (0.17 mol/dm3, 10 equiv.), MeOH, 72 h, rt | 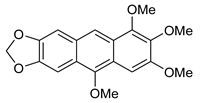 7a (53%) | not observed |
 5b | HCl (0.17 mol/dm3, 10 equiv.), MeOH, 72 h, rt | 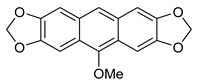 7b (37%) | 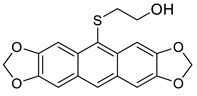 8b (12%) |
| HCl (0.17 mol/dm3, 10 equiv.), MeOH, 72 h, rt then 8 h, 65 °C | 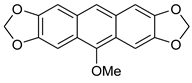 7b (8%) | 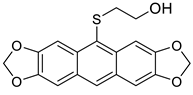 8b (25%) | |
| HCl (0.34 mol/dm3, 10 equiv.), MeOH, 0.5 h, 65 °C | not observed | 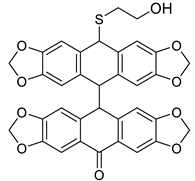 9b (55%) | |
| FeCl3/KI, MeOH, 3 h, 65 °C | 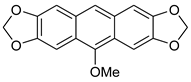 7b (36%) | not observed | |
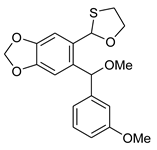 5c | HCl (0.17 mol/dm3, 10 equiv.), 2 h, 65 °C then 12 h, rt |  7c (35%) | 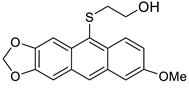 8c (9%) |
| HCl, (0.34 mol/dm3, 5 equiv.), 4 d, rt |  7c (25%) | 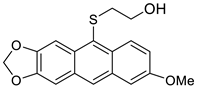 8c (27%) | |
| FeCl3/KI, MeOH, 12 h, 65 °C | 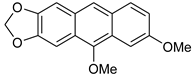 7c (78%) | not observed | |
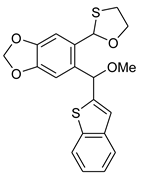 5d | HCl (0.17 mol/dm3, 10 equiv.), MeOH, 72 h, rt |  7d (62%) | not observed |
| HCl (0.17 mol/dm3, 10 equiv.), MeOH, 8 h, 65 °C | 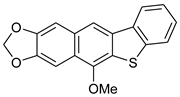 7d (33%) | not observed | |
| HCl (1.78 mol/dm3, 100 equiv.), 3 h, rt | not observed | 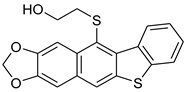 8d (14%) 2 | |
| FeCl3/KI, MeOH, 12 h, 65 °C | 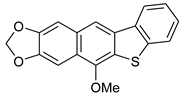 7d (62%) | not observed | |
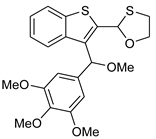 12 | HCl (0.34 mol/dm3, 10 equiv.), MeOH, 12 h, rt | 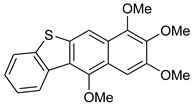 13 (15%) | not observed |
| Compound | Absorption λmax (nm) | Emission 1 λmax (nm) | Stokes Shift (cm−1) |
|---|---|---|---|
| 7b | 269, 322, 339, 357, 376 | 385, 406 | 621 |
| 7c | 270, 323, 339, 356, 372, 393 | 416, 438 | 1472 |
| 8c | 269, 363, 379, 400 | 408, 428 | 553 |
| 13 | 280, 302, 317, 330, 363, 383 | 404, 424 | 1420 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owsianik, K.; Różycka-Sokołowska, E.; Bałczewski, P. O,S-Acetals in a New Modification of oxo-Friedel–Crafts–Bradsher Cyclization—Synthesis of Fluorescent (Hetero)acenes and Mechanistic Considerations. Molecules 2023, 28, 2474. https://doi.org/10.3390/molecules28062474
Owsianik K, Różycka-Sokołowska E, Bałczewski P. O,S-Acetals in a New Modification of oxo-Friedel–Crafts–Bradsher Cyclization—Synthesis of Fluorescent (Hetero)acenes and Mechanistic Considerations. Molecules. 2023; 28(6):2474. https://doi.org/10.3390/molecules28062474
Chicago/Turabian StyleOwsianik, Krzysztof, Ewa Różycka-Sokołowska, and Piotr Bałczewski. 2023. "O,S-Acetals in a New Modification of oxo-Friedel–Crafts–Bradsher Cyclization—Synthesis of Fluorescent (Hetero)acenes and Mechanistic Considerations" Molecules 28, no. 6: 2474. https://doi.org/10.3390/molecules28062474
APA StyleOwsianik, K., Różycka-Sokołowska, E., & Bałczewski, P. (2023). O,S-Acetals in a New Modification of oxo-Friedel–Crafts–Bradsher Cyclization—Synthesis of Fluorescent (Hetero)acenes and Mechanistic Considerations. Molecules, 28(6), 2474. https://doi.org/10.3390/molecules28062474









