Recent Progress in Modifications, Properties, and Practical Applications of Glass Fiber
Abstract
1. Introduction
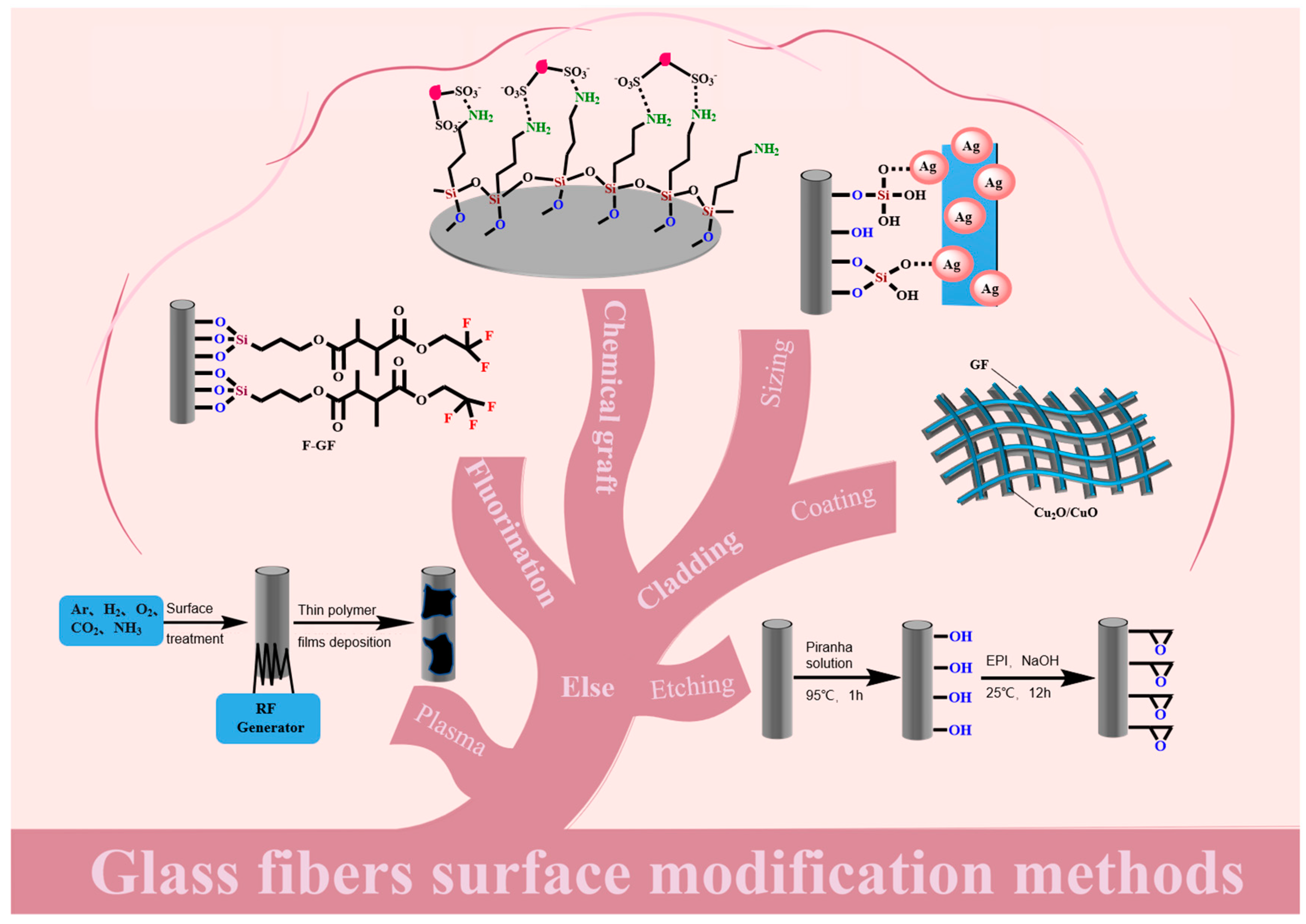
2. Surface Modification Methods
2.1. Coating Modification
2.1.1. Sizing Modification
Film-Forming-Agent Modification
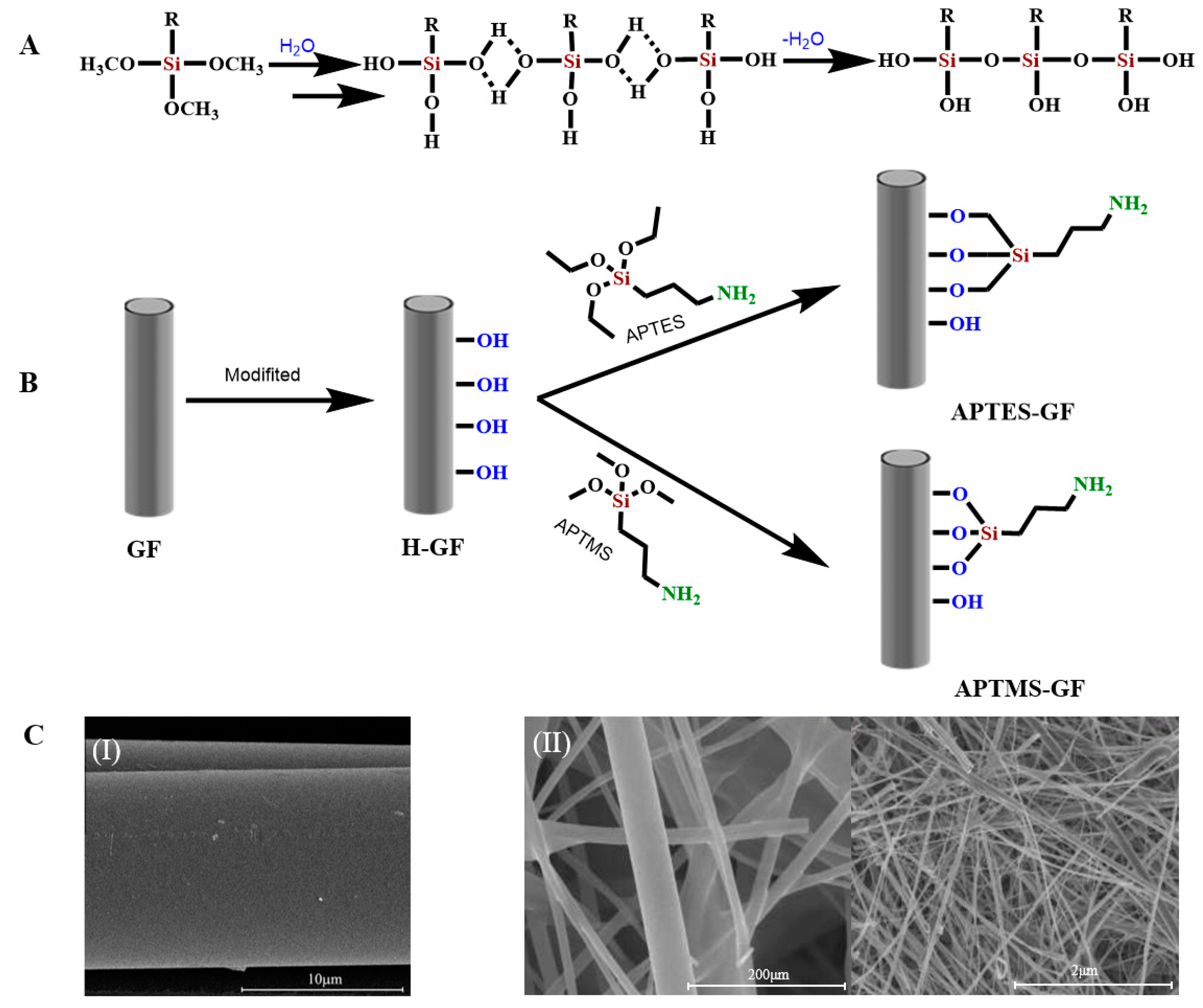
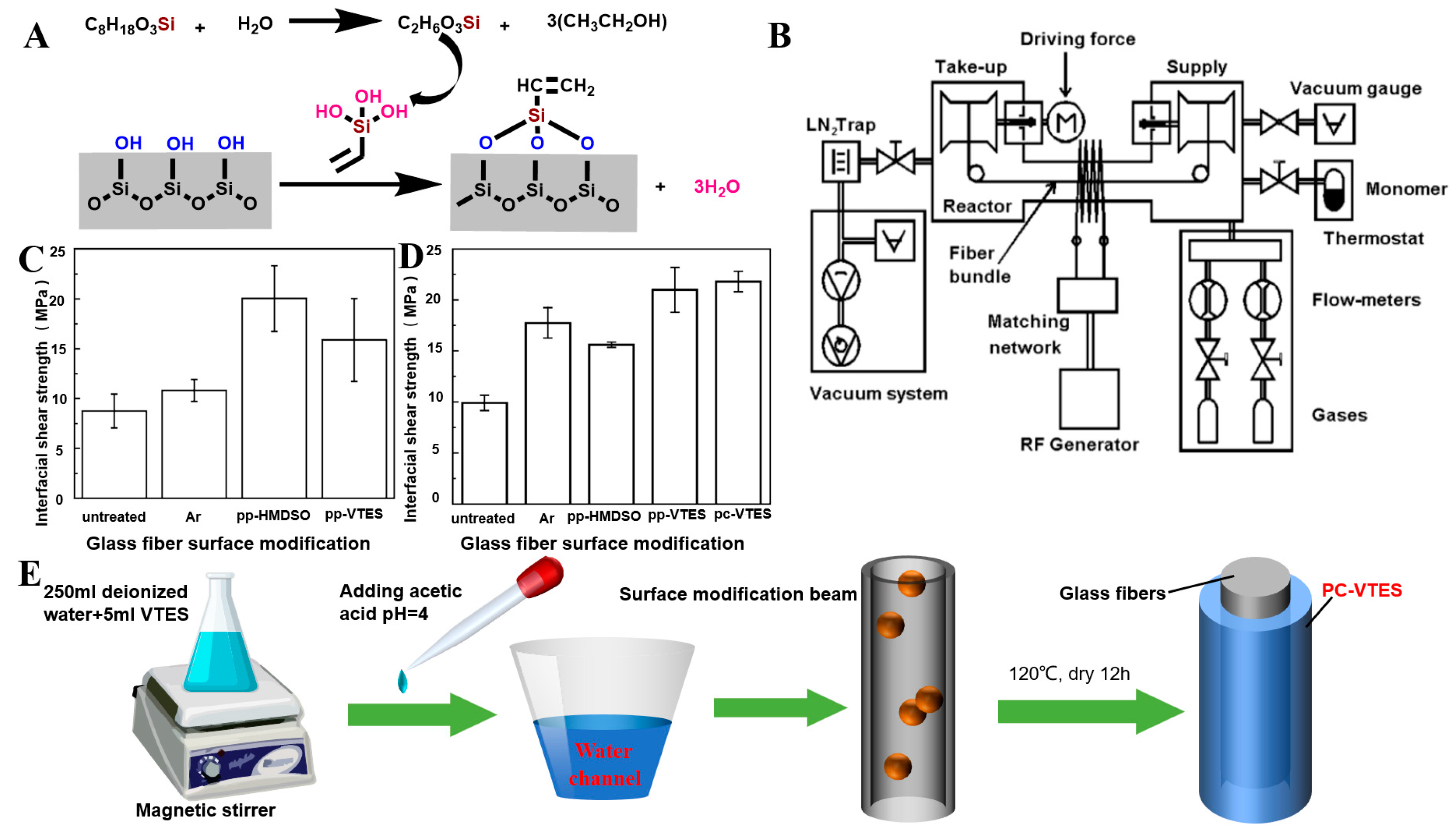
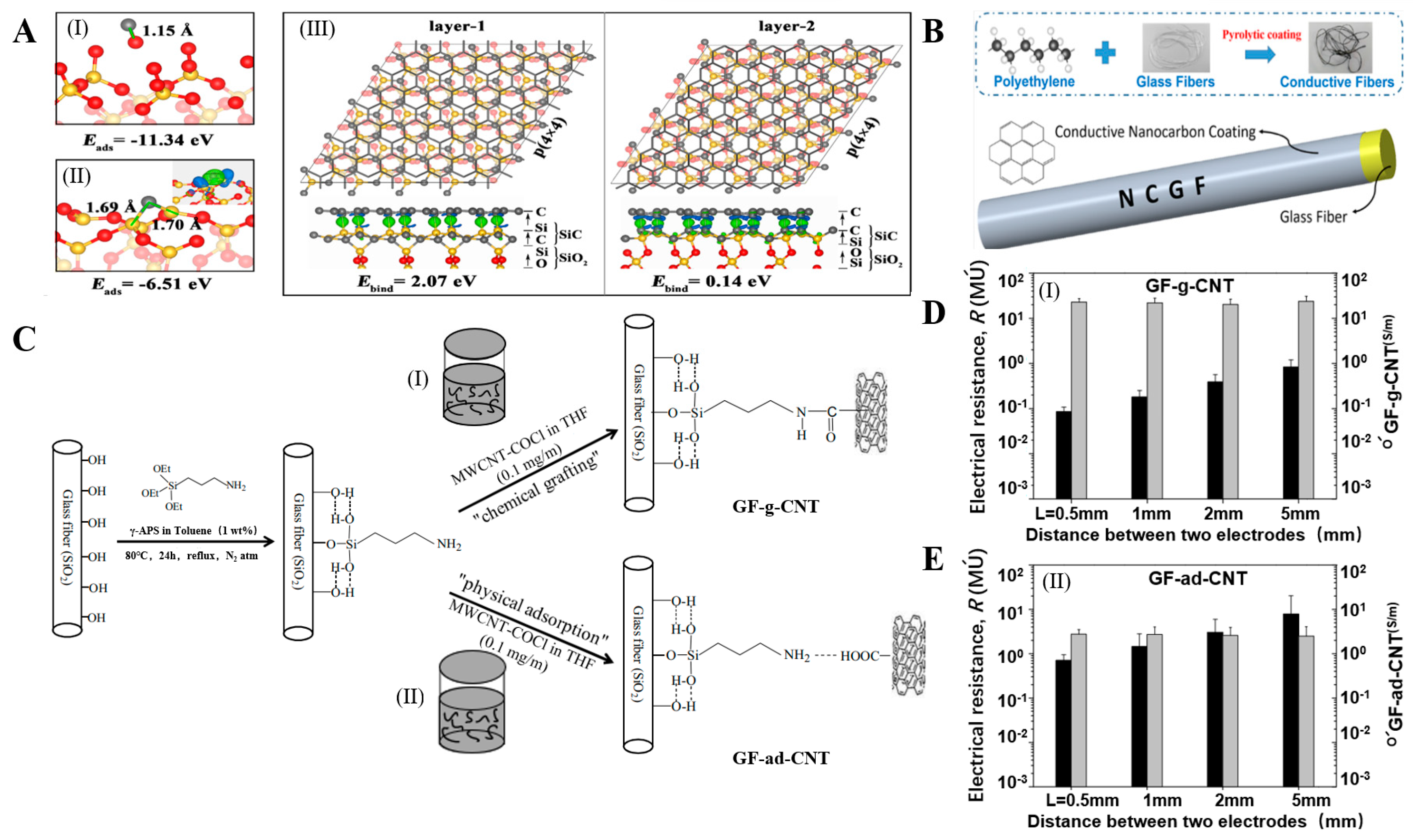
Silane-Coupling-Agent Modification
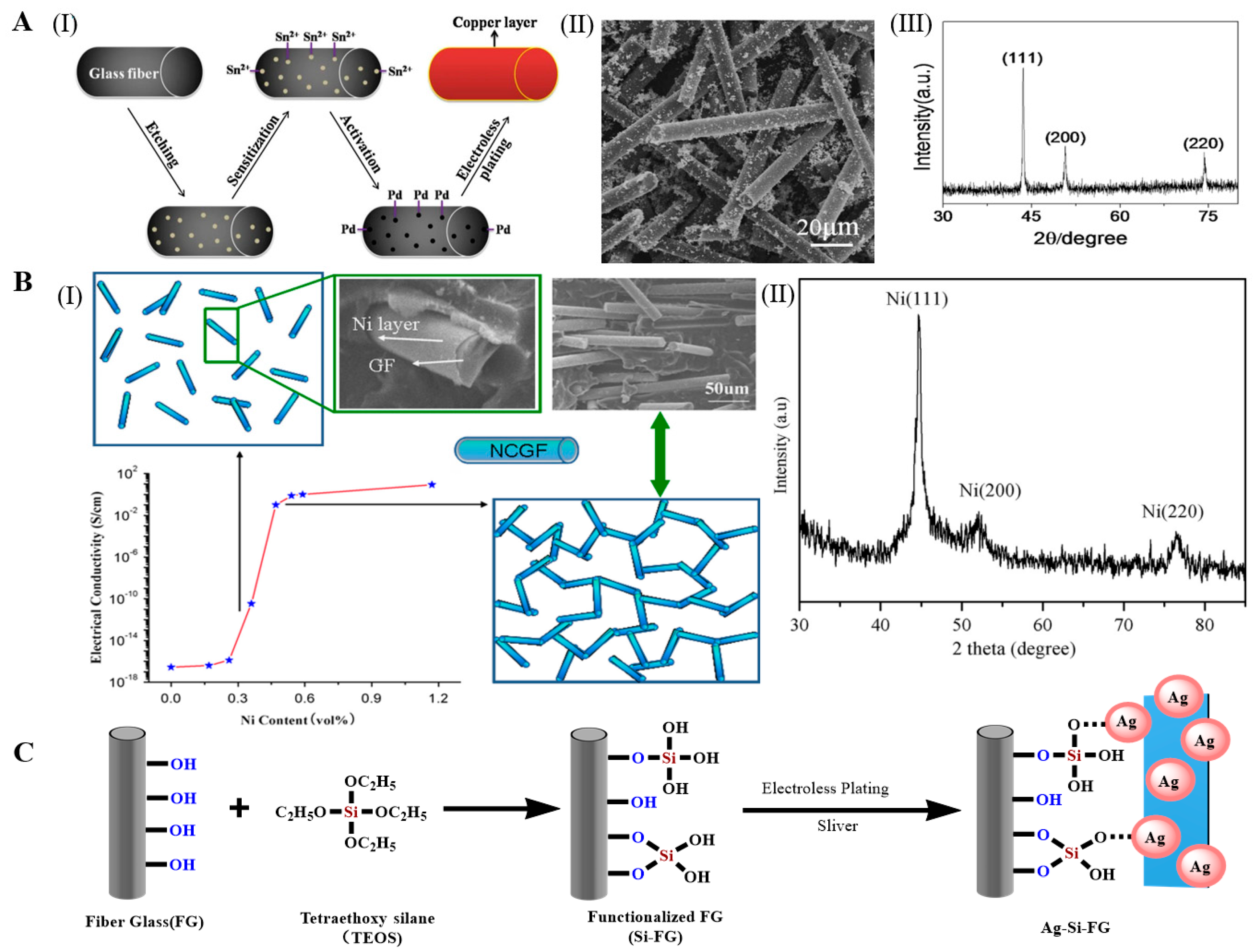
2.1.2. Mental Coating
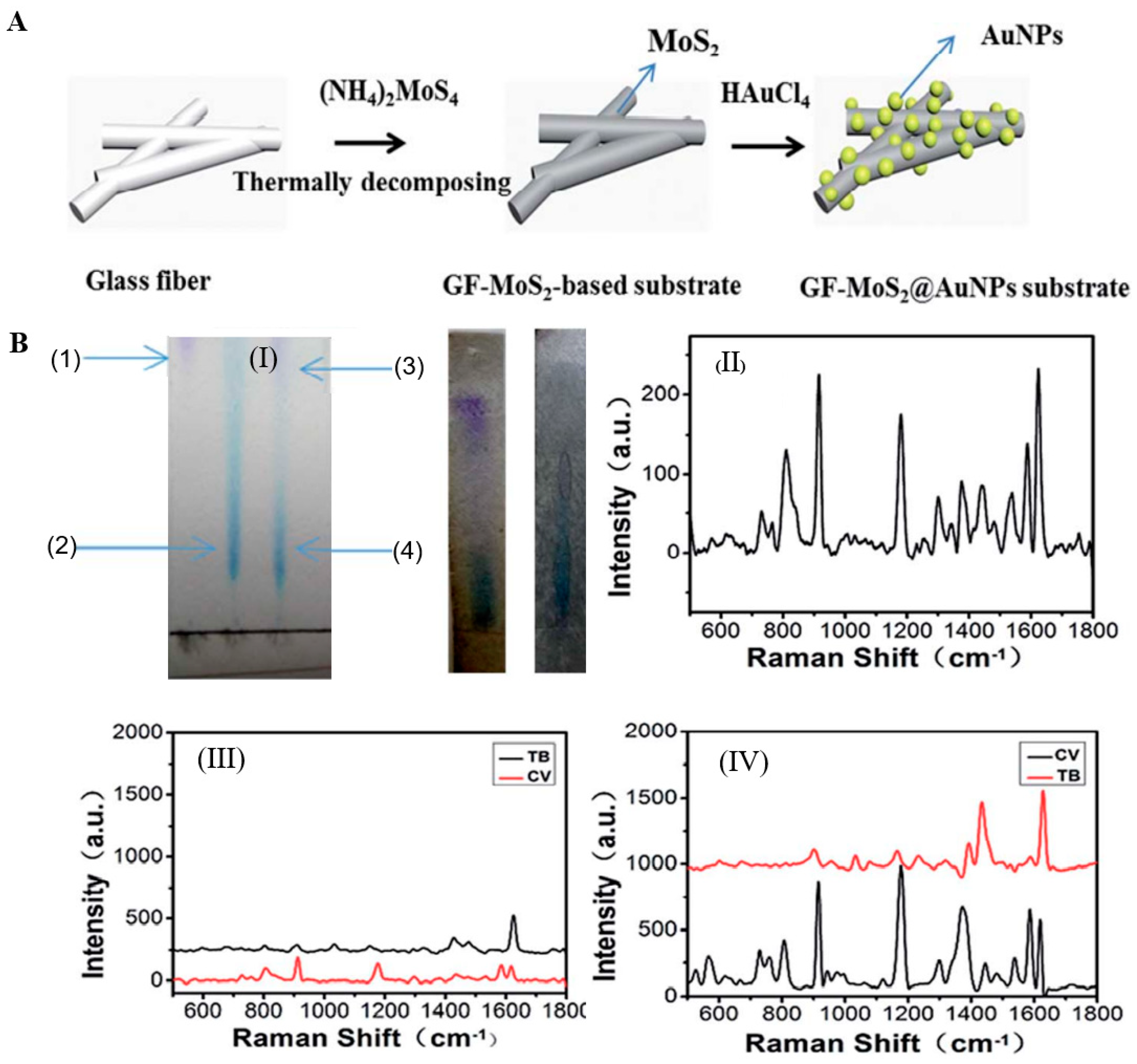
2.1.3. Metal-Oxide Coating
2.1.4. Non-Metallic Coating
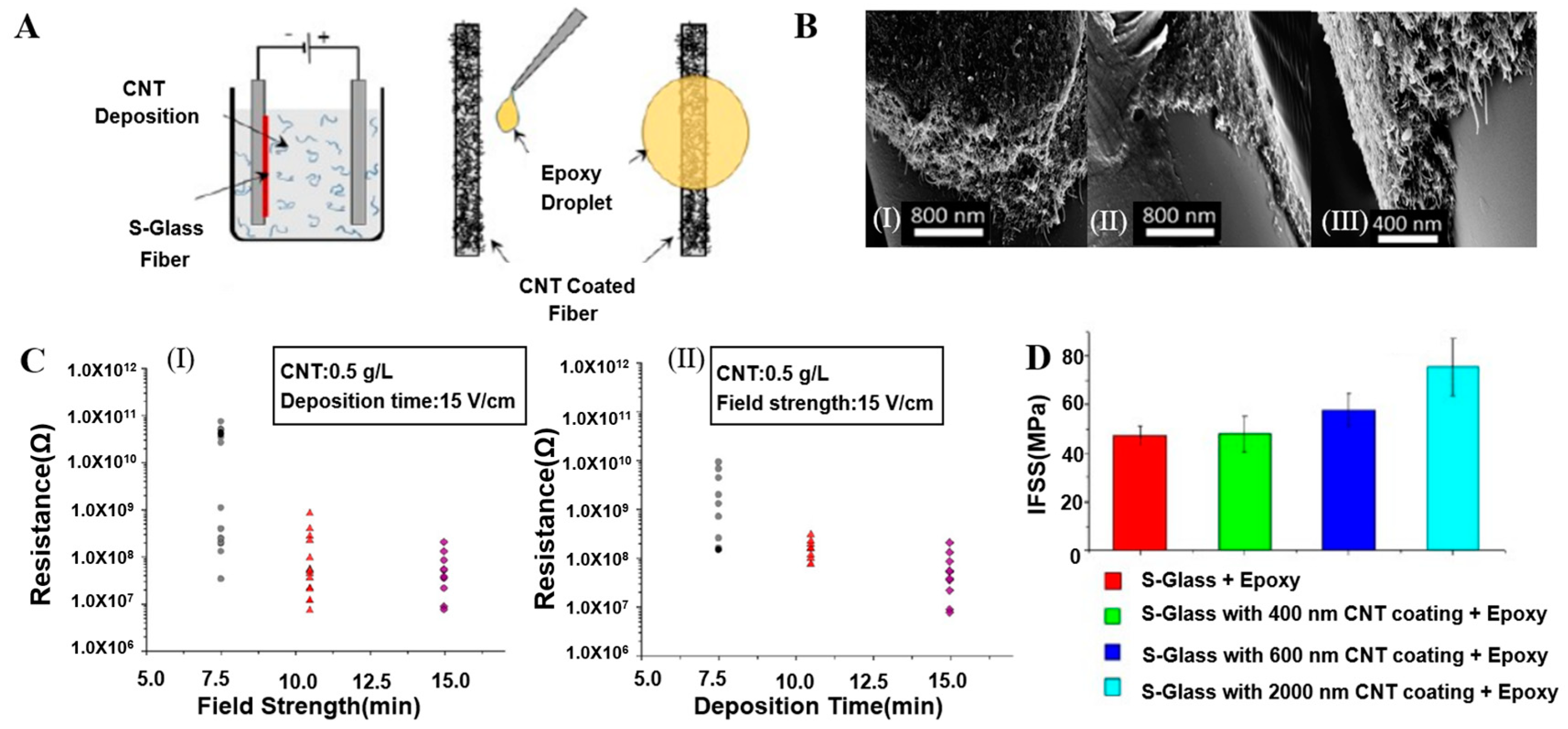
2.1.5. Composite Coating
2.2. Chemical Grafting Modification
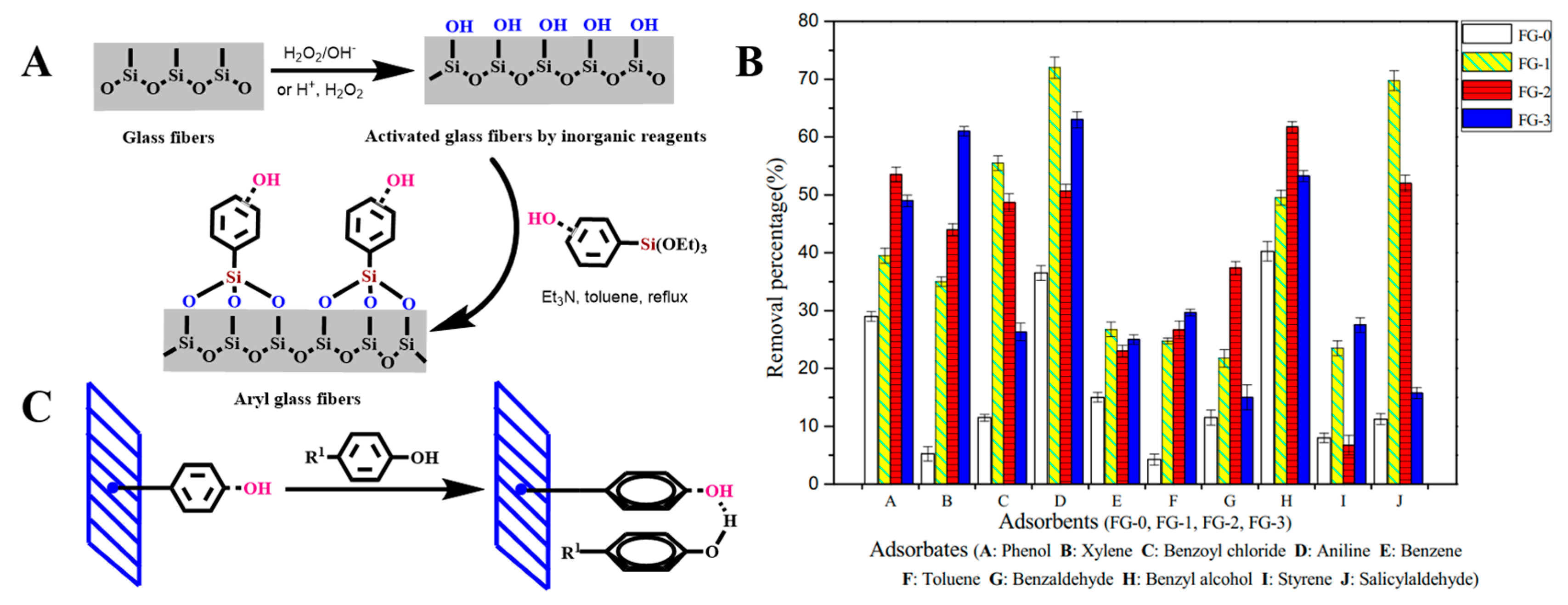
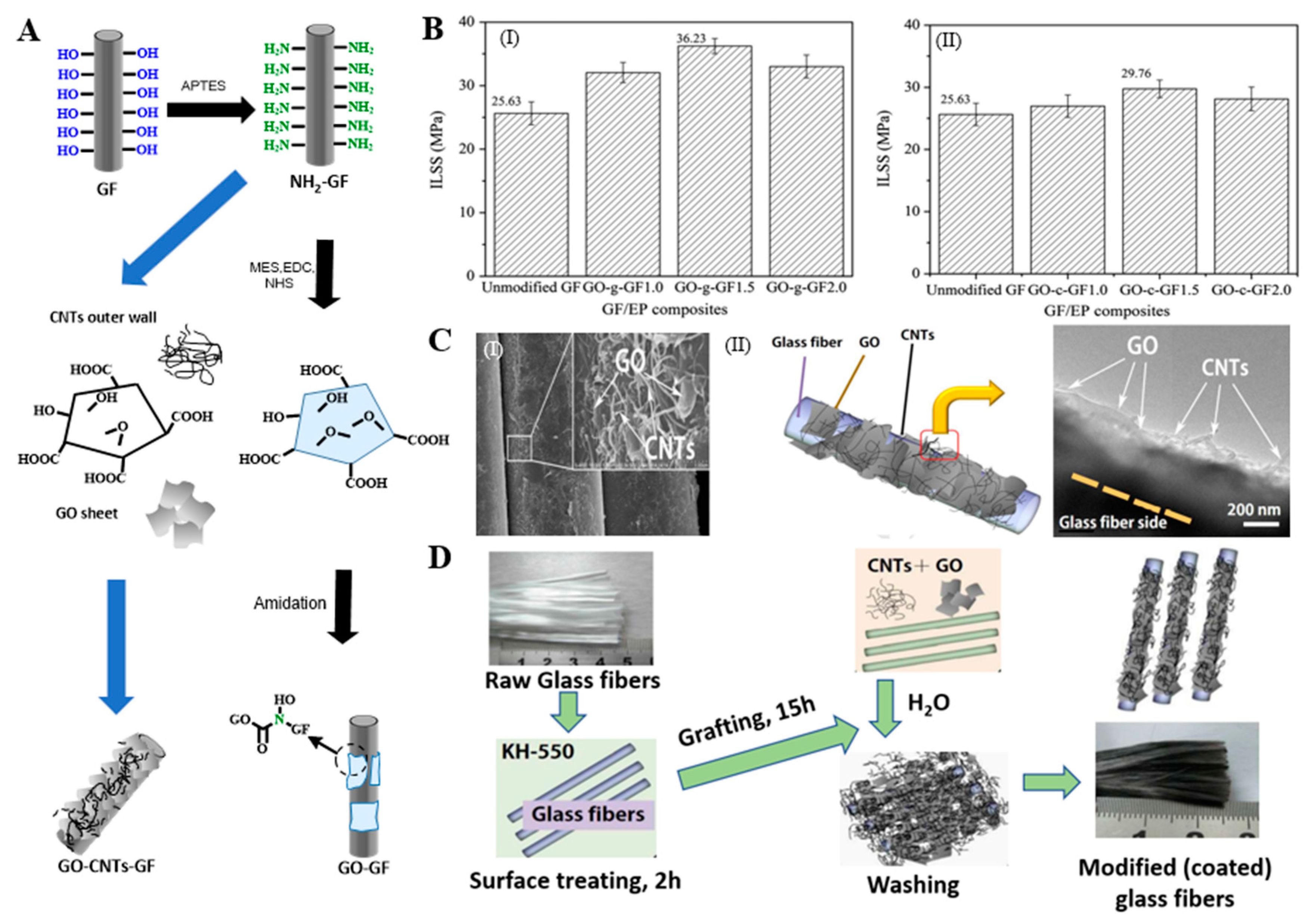
2.2.1. Grafting Organic Functional Groups
2.2.2. Grafting Inorganic Nanomaterials
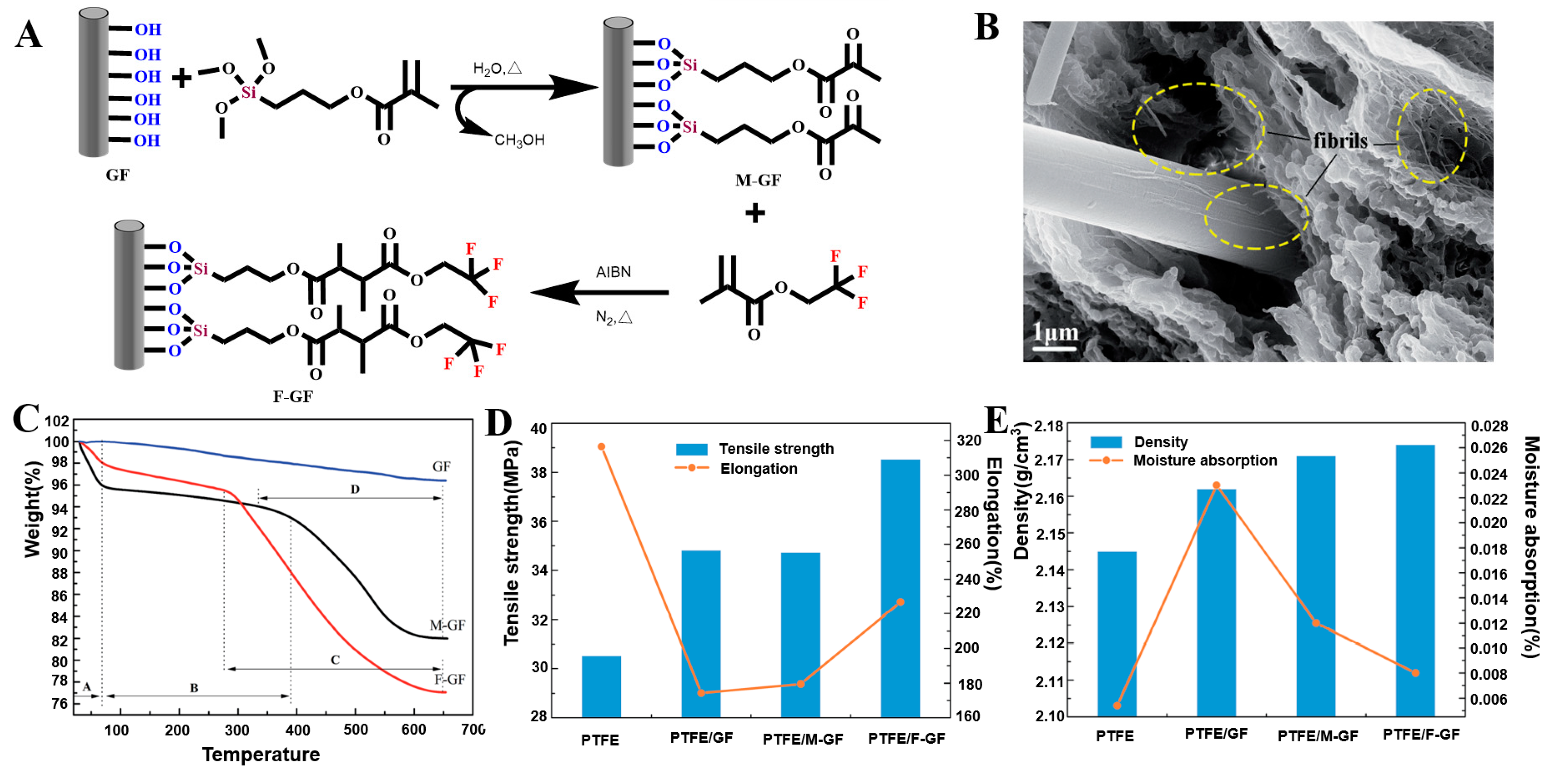
2.3. Fluorination Modification
2.3.1. Direct Gas Fluorination
2.3.2. Solvent Fluorination
2.3.3. Plasma-Assisted Fluorination
2.3.4. Mechanochemical Fluorination
2.4. Other Modification Methods
2.4.1. Redox Modification
2.4.2. Acid–Base Etching Modification
2.4.3. Plasma Treatment Modification
2.4.4. Doping Modification
3. Characteristics of MGF
3.1. Surface Structure
3.1.1. Diameter Evolution
3.1.2. Surface Area
3.1.3. Coating Thickness
3.2. Wettability
3.3. Electrical Performance
3.4. Mechanical Properties
3.5. Stability
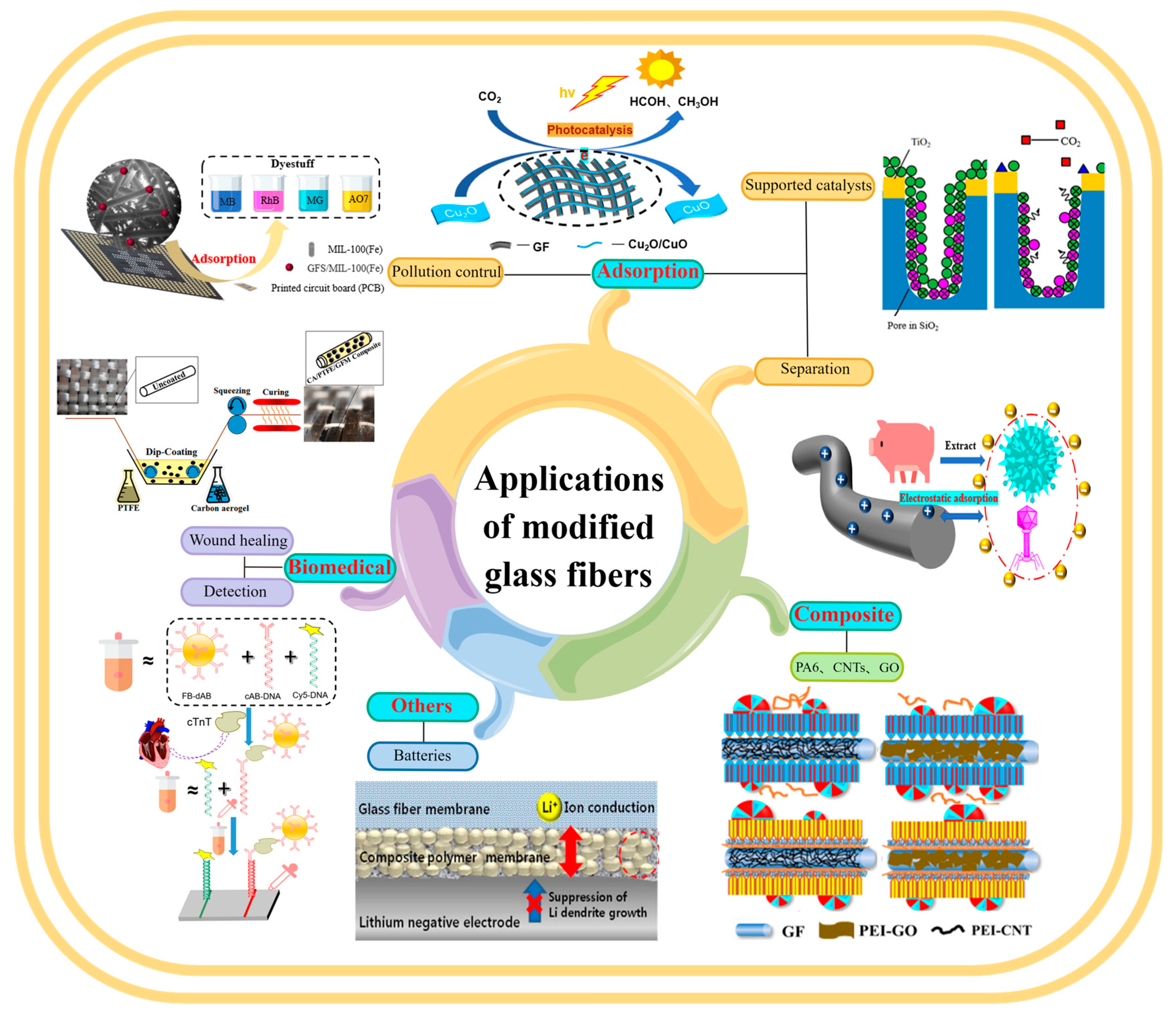
4. Applications of MGF
4.1. Adsorption
4.1.1. Separation
4.1.2. Pollution Control
4.1.3. Supported Catalysts
4.1.4. Gas Storage
4.2. Composite Reinforcement Materials
4.2.1. Resin/Fiber-Reinforced Materials
4.2.2. Thermoplastic/Fiber-Reinforced Materials
4.2.3. Natural Fiber/Fiber-Reinforced Materials
4.3. Biomedical Science
4.4. Other Applications
5. Summary and Outlook
- I.
- Fine structural engineering is the key factor in optimizing the physical and chemical properties of GF. Refined structural engineering distinguishes the specific contributions of GFs with different structures in their performance. This provides guidance for the design of specific functionally graded materials and ultimately achieving customized MGF products to meet the needs of different application fields, as well as providing guidance for targeted, precise modification of other materials.
- II.
- Development of new GF modification methods is required. Using the MGF surface active group, ingenious three-dimensional symmetric configurations can be designed to realize composites with other functional materials and develop more green and environmentally friendly high-performance materials.
- III.
- Application of MGF in the field of adsorption needs to be expanded. In some gaseous media, especially aerosols, under very low detection conditions, combined with the special sites of MGF, new strong adsorption forces, adsorbent enrichment storage, directional transfer, and desorption need to be investigated to achieve reuse and the perfect combination of MGF and environmental media.
- IV.
- A recycling route to add value, recycle waste glass as a raw material for GF preparation, and reuse the used MGF needs to be developed to expand production and reduce the cost of GF to realize a sustainable circular supply chain and circular economy.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, Y.; Jiang, L.; Jiang, Z.; Chen, S.; Ma, J. Surface hydrophilizing modification of polytetrafluoroethylene/glass fiber fabric through oxidant-induced polydopamine deposition. J. Ind. Text. 2020, 50, 364–379. [Google Scholar] [CrossRef]
- Aberem, M.B.; Feng, W.; Ait-Kadi, A.; Riedl, B.; Brisson, J. Modification of glass fiber surface by nylon-6,6 grafting. Compos. Interfaces 2012, 12, 425–443. [Google Scholar] [CrossRef]
- Parizi, M.J.G.; Shahverdi, H.; Roa, J.J.; Pipelzadeh, E.; Martinez, M.; Cabot, A.; Guardia, P. Improving Mechanical Properties of Glass Fiber Reinforced Polymers through Silica-Based Surface Nanoengineering. ACS Appl. Polym. Mater. 2020, 2, 2667–2675. [Google Scholar] [CrossRef]
- Wang, F.; Yu, J.; Ge, A.; Liang, X.; Lu, S.; Zhao, C.; Liu, L. Comparison of the physical properties of heat-treated and hydrophobic modified glass fiber felt. J. Ind. Text. 2021, 51, 1422S–1440S. [Google Scholar] [CrossRef]
- Dehrooyeh, S.; Vaseghi, M.; Sohrabian, M.; Sameezadeh, M. Glass fiber/Carbon nanotube/Epoxy hybrid composites: Achieving superior mechanical properties. Mech. Mater. 2021, 161, 104025. [Google Scholar] [CrossRef]
- Xiang, G.; Yin, J.; Qu, G.; Sun, P.; Hou, P.; Huang, J.; Xu, X. Construction of ZnCo2S4@Ni(OH)2 core–shell nanostructures for asymmetric supercapacitors with high energy densities. Inorg. Chem. Front. 2019, 6, 2135–2141. [Google Scholar] [CrossRef]
- Mansour, M.A.; Ismail, M.H.B.; Imran Latif, Q.B.A.; Alshalif, A.F.; Milad, A.; Bargi, W.A.A. A Systematic Review of the Concrete Durability Incorporating Recycled Glass. Sustainability 2023, 15, 3568. [Google Scholar] [CrossRef]
- Li, C.-D.; Chen, Z.-F.; Saeed, M.-U. Characterization of hybrid glass wool suspensions and optimization of microstructure and tensile strength of the associated wet-laid mats by various blendings and numbers of beating revolutions. Mater. Struct. 2016, 49, 1861–1869. [Google Scholar] [CrossRef]
- Ferry, L.; Cuesta, J.L.; Chivas, C.; Hoy, G.M.W.; Dvir, H. Incorporation of a grafted brominated monomer in glass fiber reinforced polypropylene to improve the fire resistance. Polym. Degrad. Stab. 2001, 74, 449–456. [Google Scholar] [CrossRef]
- Li, L.; Li, B.; Tang, F. Influence of maleic anhydride-grafted EPDM and flame retardant on interfacial interaction of glass fiber reinforced PA-66. Eur. Polym. J. 2007, 43, 2604–2611. [Google Scholar] [CrossRef]
- Kallel, H.; Doumouro, J.; Krachmalnicoff, V.; Wilde, Y.D.; Joulain, K. Thermal emission from a single glass fiber. J. Quant. Spectrosc. Radiat. Transf. 2019, 236, 106598. [Google Scholar] [CrossRef]
- Di, X.; Gao, Y.; Bao, C.; Hu, Y.; Xie, Z.G. Optimization of glass fiber based core materials for vacuum insulation panels with laminated aluminum foils as envelopes. Vacuum 2013, 97, 55–59. [Google Scholar] [CrossRef]
- Yang, L.; Sáez, E.R.; Nagel, U.; Thomason, J.L. Can thermally degraded glass fibre be regenerated for closed-loop recycling of thermosetting composites? Compos. Part A Appl. Sci. Manuf. 2015, 72, 167–174. [Google Scholar] [CrossRef]
- Wu, C.; Chen, Z.; Wang, F.; Hu, Y.; Rao, Z.; Wang, E.; Zhang, X. Preparation and characterization of ultralight glass fiber wool/phenolic resin aerogels with a spring-like structure. Compos. Sci. Technol. 2019, 179, 125–133. [Google Scholar] [CrossRef]
- Yang, Y.; Li, B.; Chen, Z.; Saeed, M.U.; Chen, Z.; Li, C.; Wu, C.; Li, Y.; Fu, R. Effect of cross-sectional morphology and composite structure of glass fiber felts on their corresponding acoustic properties. Fibers Polym. 2016, 17, 97–103. [Google Scholar] [CrossRef]
- Wang, F.; Chen, Z.; Wu, C.; Yang, Y.; Zhang, D.; Li, S. Analysis of acoustic performance of glass fiber felts after water absorption and their estimation results by artificial neural network. J. Text. Inst. 2020, 111, 1008–1016. [Google Scholar] [CrossRef]
- Todorova, K.; Kolev, V.; Nacheva, G.; Ivanov, I.G. Isolation of Plasmid DNA by Adsorption on Glass Fibers. Biotechnol. Biotechnol. Equip. 2014, 16, 145–147. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Tan, H.; Du, X. Non-calcined ZrO2 sol-coated hollow glass fibre membrane: Preparation, microstructure, and dye separation. Ceram. Int. 2021, 47, 12906–12915. [Google Scholar] [CrossRef]
- Gao, H.T.; Liu, X.H.; Zhang, S.J.; Qi, J.L. Synergistic effect of glass fibre and Al powder on the mechanical properties of glass-ceramics. Ceram. Int. 2018, 44, 15167–15175. [Google Scholar] [CrossRef]
- Parveen, S.; Pichandi, S.; Goswami, P.; Rana, S. Novel glass fibre reinforced hierarchical composites with improved interfacial, mechanical and dynamic mechanical properties developed using cellulose microcrystals. Mater. Des. 2020, 188, 108448. [Google Scholar] [CrossRef]
- Mazrouei-Sebdani, Z.; Naeimirad, M.; Peterek, S.; Begum, H.; Galmarini, S.; Pursche, F.; Baskin, E.; Zhao, S.; Gries, T.; Malfait, W.J. Multiple assembly strategies for silica aerogel-fiber combinations—A review. Mater. Des. 2022, 223, 111228. [Google Scholar] [CrossRef]
- Guo, R.; Jiang, S.; Hu, M.; Zhan, Y.; Cheng, K.; Duan, G. Adsorption of volatile benzene series compounds by surface-modified glass fibers: Kinetics, thermodynamic adsorption efficiencies, and mechanisms. Environ. Sci. Pollut. Res. Int. 2021, 28, 30898–30907. [Google Scholar] [CrossRef]
- Siddiqui, U.; Khalid, H.; Ghafoor, S.; Javaid, A.; Asif, A.; Khan, A.S. Analyses on mechanical and physical performances of nano-apatite grafted glass fibers based dental composites. Mater. Chem. Phys. 2021, 263, 124188. [Google Scholar] [CrossRef]
- Cova, F.; Benedetto, A.; Chiodini, N.; Lorenzi, R.; Vedda, A.; Ouspenski, V. Influence of the fiber drawing process on mechanical and vibrational properties of sol-gel silica glass. J. Non-Cryst. Solids 2021, 555, 120534. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, Y.-R.; Yuen, A.C.-Y.; Chen, T.B.-Y.; Chan, M.-C.; Peng, L.-Z.; Yang, W.-J.; Zhu, S.-E.; Yang, B.-H.; Hu, K.-H.; et al. Synthesis of phosphorus-containing silane coupling agent for surface modification of glass fibers: Effective reinforcement and flame retardancy in poly(1,4-butylene terephthalate). Chem. Eng. J. 2017, 321, 257–267. [Google Scholar] [CrossRef]
- GAO, S.; MADER, E.; Plonka, R. Nanocomposite coatings for healing surface defects of glass fibers and improving interfacial adhesion. Compos. Sci. Technol. 2008, 68, 2892–2901. [Google Scholar] [CrossRef]
- Mattson, B.; Aksnes, E.; Huse, J.; Tveten, C.; Redford, K.; Stori, A. Silane-modified polymers as interphases in glass-fibre-reinforced composites: 2. Grafting and crosslinking of interphase materials. Compos. Interfaces 2012, 4, 77–94. [Google Scholar] [CrossRef]
- Chen, H.-C. Dyeing of glass fiber fabrics having amino groups with acid dyestuff and after-chroming. J. Appl. Polym. Sci. 1997, 66, 1039–1048. [Google Scholar] [CrossRef]
- Czarnecka, P.; Jani, M.; Sengottuvel, S.; Mrózek, M.; Dąbczyński, P.; Filipkowski, A.; Kujawa, I.; Pysz, D.; Gawlik, W.; Wojciechowski, A.M. Magnetically-sensitive nanodiamond thin-films on glass fibers. Opt. Mater. Express 2022, 12, 444. [Google Scholar] [CrossRef]
- Torza, S. The continuous coating of glass fibers. J. Appl. Phys. 1976, 47, 4017–4020. [Google Scholar] [CrossRef]
- Hashimoto, K.; Fujisawa, T.; Kobayashi, M.; Yosomiya, R. Graft copolymerization of glass fiber and its application. J. Appl. Polym. Sci. 1982, 27, 4529–4539. [Google Scholar] [CrossRef]
- Guo, M.; Meng, F.; Li, G.; Luo, J.; Ma, Y.; Xia, X. Effective Antibacterial Glass Fiber Membrane Prepared by Plasma-Enhanced Chemical Grafting. ACS Omega 2019, 4, 16591–16596. [Google Scholar] [CrossRef]
- Krishnamurthy, A.; Tao, R.; Senses, E.; Doshi, S.M.; Burni, F.A.; Natarajan, B.; Hunston, D.; Thostenson, E.T.; Faraone, A.; Forster, A.L.; et al. Multiscale Polymer Dynamics in Hierarchical Carbon Nanotube Grafted Glass Fiber Reinforced Composites. ACS Appl. Polym. Mater. 2019, 1, 1905–1917. [Google Scholar] [CrossRef] [PubMed]
- Karunagaran, N.; Rajadurai, A. Effect of surface treatment on mechanical properties of glass fiber/stainless steel wire mesh reinforced epoxy hybrid composites. J. Mech. Sci. Technol. 2016, 30, 2475–2482. [Google Scholar] [CrossRef]
- Bashir, S.T.; Yang, L.; Liggat, J.J.; Thomason, J.L. Kinetics of dissolution of glass fibre in hot alkaline solution. J. Mater. Sci. 2018, 53, 1710–1722. [Google Scholar] [CrossRef]
- Tomao, V.; Siouffi, A.M.; Denoyel, R. Influence of time and temperature of hydrothermal treatment on glass fibers surface. J. Chromatogr. A 1998, 829, 367–376. [Google Scholar] [CrossRef]
- Cech, V.; Knob, A.; Hosein, H.-A.; Babik, A.; Lepcio, P.; Ondreas, F.; Drzal, L.T. Enhanced interfacial adhesion of glass fibers by tetravinylsilane plasma modification. Compos. Part A Appl. Sci. Manuf. 2014, 58, 84–89. [Google Scholar] [CrossRef]
- Cech, V.; Knob, A.; Lasota, T.; Lukes, J.; Drzal, L.T. Surface modification of glass fibers by oxidized plasma coatings to improve interfacial shear strength in GF/polyester composites. Polym. Compos. 2019, 40, 199. [Google Scholar] [CrossRef]
- Liverani, L. Copper-doped cotton-like malleable electrospun bioactive glass fibers for wound healing applications. Mater. Lett. X 2022, 14, 100133. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, J.; Wu, J.; Song, L.; Han, Y.; Wang, Z.; Yu, Q. Glass fiber reinforced PET modified by few-layer black phosphorus. Polym. Adv. Technol. 2021, 32, 3515–3522. [Google Scholar] [CrossRef]
- Mahmood, H.; Simonini, L.; Dorigato, A.; Pegoretti, A. Graphene Deposition on Glass Fibers by Triboelectrification. Appl. Sci. 2021, 11, 3123. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, N.; Ge, Y.; Zhu, H.; Feng, X.; Liu, R.; Ma, Y.; Wang, L.; Hou, W. Flexible all-solid-state micro-supercapacitor based on Ni fiber electrode coated with MnO2 and reduced graphene oxide via electrochemical deposition. Sci. China Mater. 2018, 61, 243–253. [Google Scholar] [CrossRef]
- Huang, Y.; Shi, K.; Liao, Z.; Wang, Y.; Wang, L.; Zhu, F. Studies of electroless Ni-Co-P ternary alloy on glass fibers. Mater. Lett. 2007, 61, 1742–1746. [Google Scholar] [CrossRef]
- Lien, W.-F.; Huang, P.-C.; Tseng, S.-C.; Cheng, C.-H.; Lai, S.-M.; Liaw, W.-C. Electroless silver plating on tetraethoxy silane-bridged fiber glass. Appl. Surf. Sci. 2012, 258, 2246–2254. [Google Scholar] [CrossRef]
- Xu, C.; Liu, G.; Chen, H.; Zhou, R.; Liu, Y. Fabrication of conductive copper-coated glass fibers through electroless plating process. J. Mater. Sci. Mater. Electron. 2014, 25, 2611–2617. [Google Scholar] [CrossRef]
- Xu, C.; Zhou, R.; Chen, H.; Hou, X.; Liu, G.; Liu, Y. Silver-coated glass fibers prepared by a simple electroless plating technique. J. Mater. Sci. Mater. Electron. 2014, 25, 4638–4642. [Google Scholar] [CrossRef]
- Zhang, Z. Electrical conductive Cu/glass fibre composites prepared by electroless plating. Micro Nano Lett. 2014, 9, 83–86. [Google Scholar] [CrossRef]
- Zuo, J.; Chen, S.; Luo, C.; Chen, D. Preparation of electroless copper coated glass fiber and piezoresistive properties of copper coated glass fiber reinforced plastics. Appl. Surf. Sci. 2015, 349, 319–326. [Google Scholar] [CrossRef]
- Alcañiz-Monge, J.; Lozano-Castello, D.; Hahn, K.; Grobert, N.; Rühle, M. Characterisation of conductive CVD carbon–glass fibres. Carbon 2004, 42, 2349–2351. [Google Scholar] [CrossRef]
- Yang, C.; Xu, Y.; Zhang, C.; Sun, Z.; Chen, C.; Li, X.; Jiang, S.; Man, B. Facile synthesis 3D flexible core-shell graphene/glass fiber via chemical vapor deposition. Nanoscale Res. Lett. 2014, 9, 394. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Cheng, L.; Tan, J.; Yang, W. Conductive Nanocarbon-Coated Glass Fibers. J. Phys. Chem. C 2020, 124, 17806–17810. [Google Scholar] [CrossRef]
- Mahmood, H.; Tripathi, M.; Pugno, N.; Pegoretti, A. Enhancement of interfacial adhesion in glass fiber/epoxy composites by electrophoretic deposition of graphene oxide on glass fibers. Compos. Sci. Technol. 2016, 126, 149–157. [Google Scholar] [CrossRef]
- Hao, B.; Ma, Q.; Yang, S.; Mäder, E.; Ma, P.-C. Comparative study on monitoring structural damage in fiber-reinforced polymers using glass fibers with carbon nanotubes and graphene coating. Compos. Sci. Technol. 2016, 129, 38–45. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, H.; Peng, H. Synthesis and photovoltaic application of platinum-modified conducting aligned nanotube fiber. Sci. China Mater. 2015, 58, 289–293. [Google Scholar] [CrossRef][Green Version]
- Shang, Y.; Shi, B.; Doshi, S.M.; Chu, T.; Qiu, G.; Du, A.; Zhao, Y.; Xu, F.; Thostenson, E.T.; Fu, K.K. Rapid Nanowelding of Carbon Coatings onto Glass Fibers by Electrothermal Shock. ACS Appl. Mater. Interfaces 2020, 12, 37722–37731. [Google Scholar] [CrossRef]
- Feller, J.F.; Grohens, Y. Coupling ability of silane grafted poly(propene) at glass fibers/poly(propene) interface. Compos. Part A Appl. Sci. Manuf. 2004, 35, 1–10. [Google Scholar] [CrossRef]
- Thomason, J. A review of the analysis and characterisation of polymeric glass fibre sizings. Polym. Test. 2020, 85, 106421. [Google Scholar] [CrossRef]
- Gorowara, R.L.; Kosik, W.E.; McKnight, S.H.; McCullough, R.L. Molecular characterization of glass fiber surface coatings for thermosetting polymer matrix/glass fiber composites. Compos. Part A 2001, 32, 323–329. [Google Scholar] [CrossRef]
- Thomason, J.L.; Nagel, U.; Yang, L.; Bryce, D. A study of the thermal degradation of glass fibre sizings at composite processing temperatures. Compos. Part A Appl. Sci. Manuf. 2019, 121, 56–63. [Google Scholar] [CrossRef]
- Thomason, J.L. Glass fibre sizing: A review. Compos. Part A Appl. Sci. Manuf. 2019, 127, 105619. [Google Scholar] [CrossRef]
- Abdul Hamid, Z.M.; Florea, M.; Fliegener, S.; Schober, M.; Hohe, J.; Rühe, J. Chemical Modification of Fiber-Matrix Interfaces of Glass Fiber Reinforced Thermoplastics and Methods for Interface Characterization. Adv. Eng. Mater. 2019, 21, 1800590. [Google Scholar] [CrossRef]
- Cech, V.; Prikryl, R.; Balkova, R.; Vanek, J.; Grycova, A. The influence of surface modifications of glass on glass fiber/polyester interphase properties. J. Adhes. Sci. Technol. 2013, 10, 1299–1320. [Google Scholar] [CrossRef]
- Luo, N.; Zhong, H.; Yang, M.; Yuan, X.; Fan, Y. Modifying glass fiber surface with grafting acrylamide by UV-grafting copolymerization for preparation of glass fiber reinforced PVDF composite membrane. J. Environ. Sci. 2016, 39, 208–217. [Google Scholar] [CrossRef]
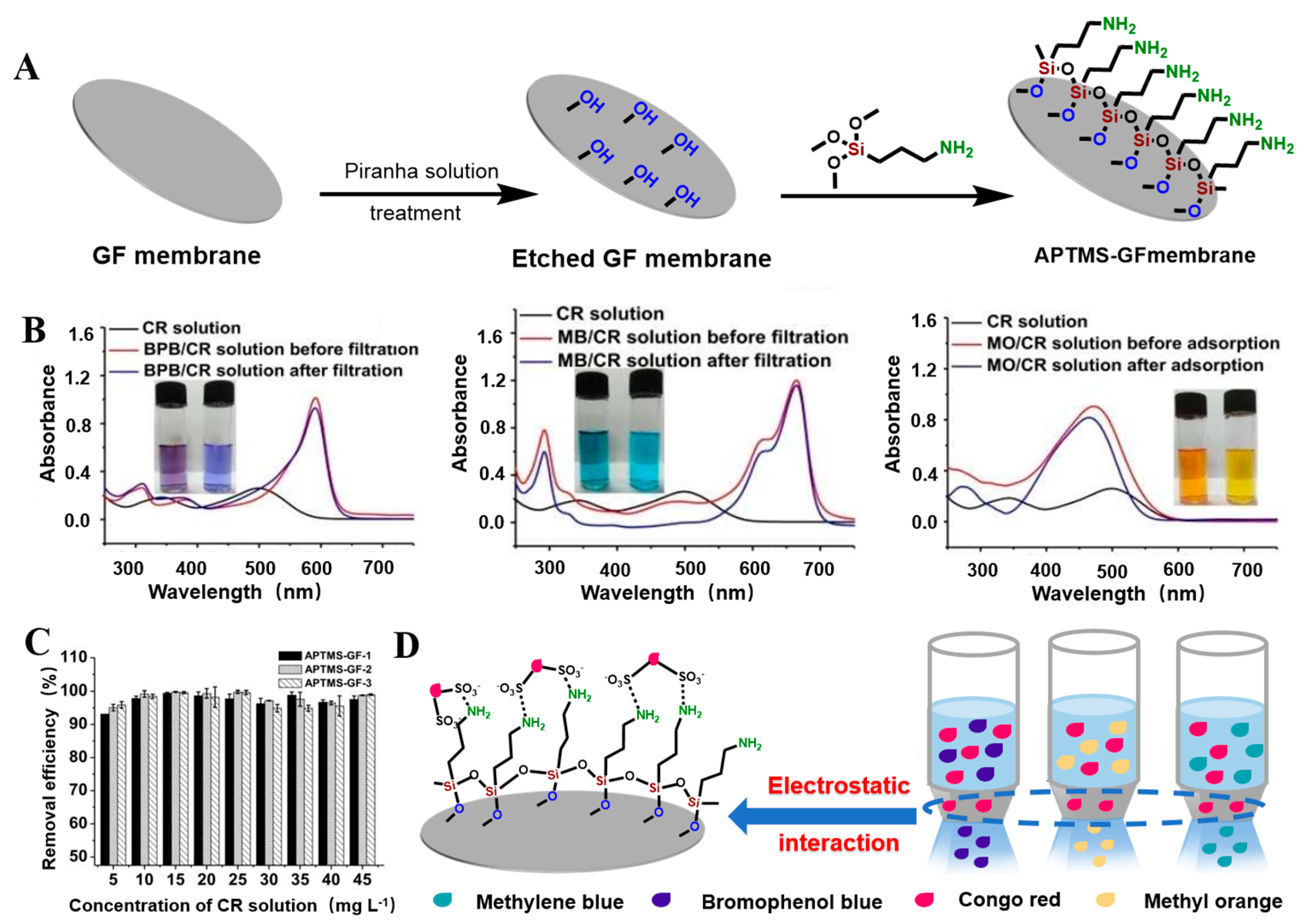
- Li, J.L.; Gao, Y.H.; Jin, C.X.; Wang, Y.; He, M.; Dong, W.W.; Zhao, J.; Li, D.; Shang, H.B. Facile Surface Modification of Glass-Fiber Membrane with Silylating Reagent through Chemical Bonding for the Selective Separation and Recycling of Diverse Dyes from Aqueous Solutions. ChemistrySelect 2018, 3, 12734–12741. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, D.; Jin, X.; Wang, C.; Wang, D.; Ge, H. Modifying glass fibers with graphene oxide: Towards high-performance polymer composites. Compos. Sci. Technol. 2014, 97, 41–45. [Google Scholar] [CrossRef]
- Turrión, S.G.; Olmos, D.; González-Benito, J. Complementary characterization by fluorescence and AFM of polyaminosiloxane glass fibers coatings. Polym. Test. 2005, 24, 301–308. [Google Scholar] [CrossRef]
- Sarr, M.M.; Inoue, H.; Kosaka, T. Study on the improvement of interfacial strength between glass fiber and matrix resin by grafting cellulose nanofibers. Compos. Sci. Technol. 2021, 211, 108853. [Google Scholar] [CrossRef]
- Sorokin, A.E.; Petrova, G.N. Lubricants and Coupling Agents in the Processes of the Liquid-Phase Modification of the Surface of Carbon and Glass Fiber Fillers in the Production of Structural Materials: A Review. Theor. Found. Chem. Eng. 2020, 54, 737–744. [Google Scholar] [CrossRef]
- Song, K.-S.; Nimse, S.B.; Sonawane, M.D.; Lin, Y.; Zhou, Z.; Kim, T. A glass fibre membrane platform for ultra-sensitive detection of cardiac troponin T. Analyst 2017, 142, 3816–3821. [Google Scholar] [CrossRef]
- Tzounis, L.; Kirsten, M.; Simon, F.; Mäder, E.; Stamm, M. The interphase microstructure and electrical properties of glass fibers covalently and non-covalently bonded with multiwall carbon nanotubes. Carbon 2014, 73, 310–324. [Google Scholar] [CrossRef]
- Zhao, Y.; Pan, X.; Zhang, L.; Xu, Y.; Li, C.; Wang, J.; Ou, J.; Xiu, X.; Man, B.; Yang, C. Dense AuNP/MoS 2 hybrid fabrication on fiber membranes for molecule separation and SERS detection. RSC Adv. 2017, 7, 36516–36524. [Google Scholar] [CrossRef]
- Xu, C.; Tian, M.; Liu, L.; Zou, H.; Zhang, L.; Wang, W. Fabrication and Properties of Silverized Glass Fiber by Dopamine Functionalization and Electroless Plating. J. Electrochem. Soc. 2012, 159, D217–D224. [Google Scholar] [CrossRef]
- Eskandari, V.; Kordzadeh, A.; Zeinalizad, L.; Sahbafar, H.; Aghanouri, H.; Hadi, A.; Ghaderi, S. Detection of molecular vibrations of atrazine by accumulation of silver nanoparticles on flexible glass fiber as a surface-enhanced Raman plasmonic nanosensor. Opt. Mater. 2022, 128, 112310. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J. Antibacterial Coating of Glass Fiber Filters with Silver Nanoparticles (AgNPs) and Glycidyltrimethylammonium Chloride (GTAC). Fibers Polym. 2018, 19, 2080–2087. [Google Scholar] [CrossRef]
- Zhou, R.; Chen, H.; Liu, G.; Zhao, G.; Liu, Y. Conductive and magnetic glass microsphere/cobalt composites prepared via an electroless plating route. Mater. Lett. 2013, 112, 97–100. [Google Scholar] [CrossRef]
- Duan, H.; Yang, J.; Yang, Y.; Zhao, G.; Liu, Y. TiO2 hybrid polypropylene/nickel coated glass fiber conductive composites for highly efficient electromagnetic interference shielding. J. Mater. Sci. Mater. Electron. 2017, 28, 5725–5732. [Google Scholar] [CrossRef]
- Guo, C.; Duan, H.; Dong, C.; Zhao, G.; Liu, Y.; Yang, Y. Preparation of the polypropylene/nickel coated glass fibers conductive composites with a low percolation threshold. Mater. Lett. 2015, 143, 124–127. [Google Scholar] [CrossRef]
- Alcázar, J.C.B.; Lemos, R.M.J.; Conde, M.C.M.; Chisini, L.A.; Salas, M.M.S.; Noremberg, B.S.; Motta, F.V.d.; Demarco, F.F.; Tarquinio, S.B.C.; Carreño, N.L.V. Preparation, characterization, and biocompatibility of different metal oxide/PEG-based hybrid coating synthesized by sol–gel dip coating method for surface modification of titanium. Prog. Org. Coat. 2019, 130, 206–213. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Chen, J.; Zhong, F.; Ma, C. Study on treatment of printing and dyeing waste gas in the atmosphere with Ce-Mn/GF catalyst. Arab. J. Geosci. 2021, 14, 493. [Google Scholar] [CrossRef]
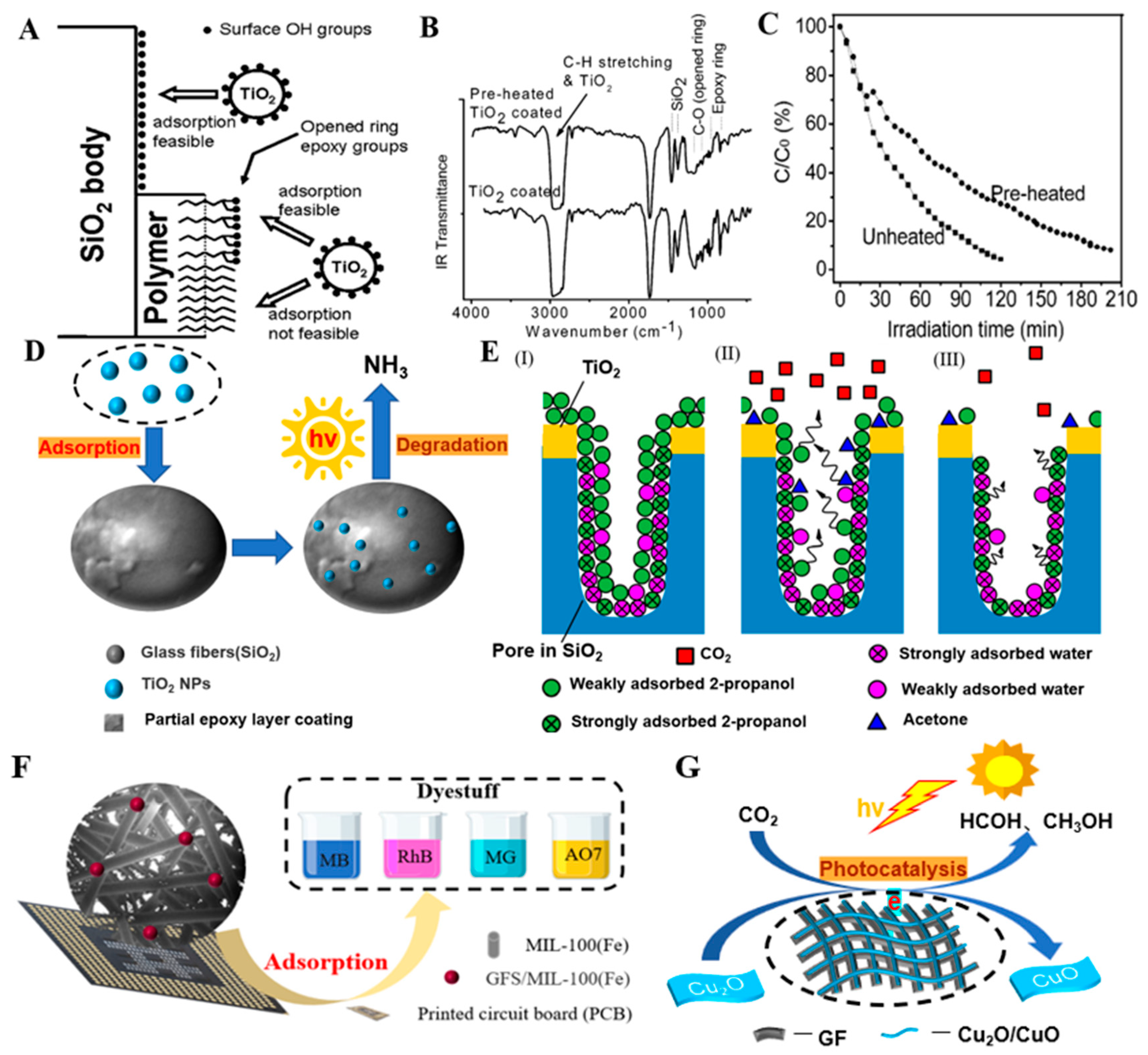
- Ávila-López, M.A.; Luévano-Hipólito, E.; Torres-Martínez, L.M. CuO coatings on glass fibers: A hybrid material for CO2 adsorption and photocatalytic reduction to solar fuels. J. Mater. Sci. Mater. Electron. 2020, 31, 13957–13969. [Google Scholar] [CrossRef]
- Yanagida, S.; Hirayama, K.; Iwasaki, K.; Yasumori, A. Adsorption and Photocatalytic Decomposition of Gaseous 2-Propanol Using TiO2-Coated Porous Glass Fiber Cloth. Catalysts 2019, 9, 82. [Google Scholar] [CrossRef]
- Aminian, M.K.; Taghavinia, N.; Irajizad, A.; Mahdavi, S.M. Adsorption of TiO2 Nanoparticles on Glass Fibers. J. Phys. Chem. C 2007, 111, 9794–9798. [Google Scholar] [CrossRef]
- Fukugaichi, S. Fixation of Titanium Dioxide Nanoparticles on Glass Fiber Cloths for Photocatalytic Degradation of Organic Dyes. ACS Omega 2019, 4, 15175–15180. [Google Scholar] [CrossRef]
- Gutierrez, L.; Li, X.; Wang, J.; Nangmenyi, G.; Economy, J.; Kuhlenschmidt, T.B.; Kuhlenschmidt, M.S.; Nguyen, T.H. Adsorption of rotavirus and bacteriophage MS2 using glass fiber coated with hematite nanoparticles. Water Res. 2009, 43, 5198–5208. [Google Scholar] [CrossRef]
- Reghat, M.; Mirabedini, A.; Tan, A.M.; Weizman, Y.; Middendorf, P.; Bjekovic, R.; Hyde, L.; Antiohos, D.; Hameed, N.; Fuss, F.K.; et al. Graphene as a piezo-resistive coating to enable strain monitoring in glass fiber composites. Compos. Sci. Technol. 2021, 211, 108842. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhou, M.; Zhao, W.; Hu, J.; Fu, H. Anti-corrosion epoxy/modified graphene oxide/glass fiber composite coating with dual physical barrier network. Prog. Org. Coat. 2022, 167, 106823. [Google Scholar] [CrossRef]
- Fang, M.; Xiong, X.; Hao, Y.; Zhang, T.; Wang, H.; Cheng, H.-M.; Zeng, Y. Preparation of highly conductive graphene-coated glass fibers by sol-gel and dip-coating method. J. Mater. Sci. Technol. 2019, 35, 1989–1995. [Google Scholar] [CrossRef]
- Takizawa, Y.; Chung, D.D.L. Through-thickness thermal conduction in glass fiber polymer-matrix composites and its enhancement by composite modification. J. Mater. Sci. 2016, 51, 3463–3480. [Google Scholar] [CrossRef]
- Jing, M.; Che, J.; Xu, S.; Liu, Z.; Fu, Q. The effect of surface modification of glass fiber on the performance of poly(lactic acid) composites: Graphene oxide vs. silane coupling agents. Appl. Surf. Sci. 2018, 435, 1046–1056. [Google Scholar] [CrossRef]
- Ning, N.; Zhang, W.; Yan, J.; Xu, F.; Wang, T.; Su, H.; Tang, C.; Fu, Q. Largely enhanced crystallization of semi-crystalline polymer on the surface of glass fiber by using graphene oxide as a modifier. Polymer 2013, 54, 303–309. [Google Scholar] [CrossRef]
- Periyasamy, A.P.; Venkataraman, M.; Kremenakova, D.; Militky, J.; Zhou, Y. Progress in Sol-Gel Technology for the Coatings of Fabrics. Materials 2020, 13, 1838. [Google Scholar] [CrossRef] [PubMed]
- Siyal, M.I.; Khan, A.A.; Lee, C.-K.; Kim, J.-O. Surface modification of glass fiber membranes by fluorographite coating for desalination of concentrated saline water with humic acid in direct-contact membrane distillation. Sep. Purif. Technol. 2018, 205, 284–292. [Google Scholar] [CrossRef]
- Moriche, R.; Jiménez-Suárez, A.; Sánchez, M.; Prolongo, S.G.; Ureña, A. Graphene nanoplatelets coated glass fibre fabrics as strain sensors. Compos. Sci. Technol. 2017, 146, 59–64. [Google Scholar] [CrossRef]
- Ye, J.; Fang, J.; Zhang, L.; Li, C. Transcrystalline induced by MWCNTs and organic nucleating agents at the interface of glass fiber/polypropylene. Polym. Compos. 2018, 39, 3424–3433. [Google Scholar] [CrossRef]
- Tamrakar, S.; An, Q.; Thostenson, E.T.; Rider, A.N.; Haque, B.Z.G.; Gillespie, J.W. Tailoring Interfacial Properties by Controlling Carbon Nanotube Coating Thickness on Glass Fibers Using Electrophoretic Deposition. ACS Appl. Mater. Interfaces 2016, 8, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Rider, A.N.; Thostenson, E.T. Hierarchical composite structures prepared by electrophoretic deposition of carbon nanotubes onto glass fibers. ACS Appl. Mater. Interfaces 2013, 5, 2022–2032. [Google Scholar] [CrossRef]
- Zhang, Y.; Mi, C. Improved hydrothermal aging performance of glass fiber-reinforced polymer composites via silica nanoparticle coating. J. Appl. Polym. Sci. 2019, 137, 48652. [Google Scholar] [CrossRef]
- Abe, I.; Sato, K.; Abe, H.; Naito, M. Formation of Porous Fumed Silica Coating on the Surface of Glass Fibers by a Dry Mechanical Processing Technique. Adv. Powder Technol. 2008, 19, 311–320. [Google Scholar] [CrossRef]
- Pogula, S.D.; Patwardhan, S.V.; Perry, C.C.; Gillespie, J.W.; Yarlagadda, S.; Kiick, K.L. Continuous silica coatings on glass fibers via bioinspired approaches. Langmuir ACS J. Surf. Colloids 2007, 23, 6677–6683. [Google Scholar] [CrossRef]
- Li, M.; Chen, Z.; Yang, L.; Li, J.; Xu, J.; Chen, C.; Wu, Q.; Yang, M.; Liu, T. Antibacterial Activity and Mechanism of GO/Cu2O/ZnO Coating on Ultrafine Glass Fiber. Nanomaterials 2022, 12, 1857. [Google Scholar] [CrossRef]
- Wei, Y.; Tang, P.; Huang, M.; Pan, Y. Fixation of BiOI/BiOBr/MoS2 Powders on Fiber Cloths for Photocatalytic Degradation of Ammonia Nitrogen from Aqueous Solution. Environ. Pollut. 2020, 9, 14. [Google Scholar] [CrossRef]
- Zhang, H.-p.; Han, W.; Tavakoli, J.; Zhang, Y.-p.; Lin, X.; Lu, X.; Ma, Y.; Tang, Y. Understanding interfacial interactions of polydopamine and glass fiber and their enhancement mechanisms in epoxy-based laminates. Compos. Part A Appl. Sci. Manuf. 2019, 116, 62–71. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, B.; Su, J.; Han, J. Preparation, Characterization and Properties of Montmorillonite Modified PTFE/Glass Fiber Composites. Fibers Polym. 2020, 21, 1126–1133. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Chang, C.-S.; Suen, S.-Y. Protein adsorption separation using glass fiber membranes modified with short-chain organosilicon derivatives. J. Membr. Sci. 2007, 305, 125–135. [Google Scholar] [CrossRef]
- Su, I.-F.; Chen, L.-J.; Suen, S.-Y. Adsorption separation of terpene lactones from Ginkgo biloba L. extract using glass fiber membranes modified with octadecyltrichlorosilane. J. Sep. Sci. 2005, 28, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, S.M.; Sheikhzadeh, M.; Abtahi, S.M.; Zadhoush, A. Shear behavior of soft-matrix composites reinforced with polyethylene loop-formed fibers. Iran. Polym. J. 2013, 22, 15–24. [Google Scholar] [CrossRef]
- Nimse, S.B.; Song, K.-S.; Kim, J.; Ta, V.-T.; Nguyen, V.-T.; Kim, T. A generalized probe selection method for DNA chips. Chem. Commun. 2011, 47, 12444–12446. [Google Scholar] [CrossRef]
- Ta, V.-T.; Nimse, S.B.; Song, K.-S.; Kim, J.; Sayyed, D.R.; Nguyen, V.-T.; Kim, T. Characterization of the mixed self-assembled monolayer at the molecular scale. Chem. Commun. 2011, 47, 11261–11263. [Google Scholar] [CrossRef]
- Cao, Z.; Hao, T.; Wang, P.; Zhang, Y.; Cheng, B.; Yuan, T.; Meng, J. Surface modified glass fiber membranes with superior chemical and thermal resistance for O/W separation. Chem. Eng. J. 2017, 309, 30–40. [Google Scholar] [CrossRef]
- Li, Z.F.; Ruckenstein, E. Strong adhesion and smooth conductive surface via graft polymerization of aniline on a modified glass fiber surface. J. Colloid Interface Sci. 2002, 251, 343–349. [Google Scholar] [CrossRef]
- Hou, X.; Wang, Y.; Hou, J.; Sun, G.; Zhang, C. Effect of polyaniline-modified glass fibers on the anticorrosion performance of epoxy coatings. J. Coat. Technol. Res. 2017, 14, 407–415. [Google Scholar] [CrossRef]
- González-Benito, J.; Cabanelas, J.C.; Aznar, A.; Vigil, M.R.; Bravo, J.; Serrano, B.; Baselga, J. Photophysics of a pyrene probe grafted onto silanized glass fiber surfaces. J. Lumin. 1997, 72–74, 451–453. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Takeuchi, T.; Ito, M.; Kokubo, T. CaO-B2O3-SiO2 glass fibers for wound healing. J. Mater. Sci. Mater. Med. 2022, 33, 15. [Google Scholar] [CrossRef]
- Ren, J.; Chen, L.; Liu, Z.; Song, Q.; Liu, C. Study on the heat transfer reinforcement of glass fiber/epoxy resin composites by grafting and dispersing graphene oxide. Compos. Sci. Technol. 2021, 216, 109039. [Google Scholar] [CrossRef]
- Hua, Y.; Li, F.; Liu, Y.; Huang, G.-W.; Xiao, H.-M.; Li, Y.-Q.; Hu, N.; Fu, S.-Y. Positive synergistic effect of graphene oxide/carbon nanotube hybrid coating on glass fiber/epoxy interfacial normal bond strength. Compos. Sci. Technol. 2017, 149, 294–304. [Google Scholar] [CrossRef]
- Ku-Herrera, J.J.; Avilés, F.; Nistal, A.; Cauich-Rodríguez, J.V.; Rubio, F.; Rubio, J.; Bartolo-Pérez, P. Interactions between the glass fiber coating and oxidized carbon nanotubes. Appl. Surf. Sci. 2015, 330, 383–392. [Google Scholar] [CrossRef]
- Jin, J.; Zhang, L.; Chen, W.; Li, C.-Z. Synthesis of glass fiber-multiwall carbon nanotube hybrid structures for high-performance conductive composites. Polym. Compos. 2013, 34, 1313–1320. [Google Scholar] [CrossRef]
- Zhang, L.; Su, D.; Jin, L.; Li, C. Polyamide 6 composites reinforced with glass fibers modified with electrostatically assembled multiwall carbon nanotubes. J. Mater. Sci. 2012, 47, 5446–5454. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, L.; Li, C. Polyamide 6 composite with highly improved mechanical properties by PEI-CNT grafted glass fibers through interface wetting, infiltration and crystallization. Polymer 2019, 172, 253–264. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, L.; Li, C. Largely enhanced transcrystalline formation and properties of polypropylene on the surface of glass fiber as induced by PEI-CNT and PEI-GO modification. Polymer 2020, 186, 122025. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, L.; Li, C. The combined effect of impregnated rollers configuration and glass fibers surface modification on the properties of continuous glass fibers reinforced polypropylene prepreg composites. Compos. Sci. Technol. 2020, 197, 108259. [Google Scholar] [CrossRef]
- Chen, X.; Fan, K.; Liu, Y.; Li, Y.; Liu, X.; Feng, W.; Wang, X. Recent Advances in Fluorinated Graphene from Synthesis to Applications: Critical Review on Functional Chemistry and Structure Engineering. Adv. Mater. 2022, 34, e2101665. [Google Scholar] [CrossRef] [PubMed]
- Agopian, J.-C.; Téraube, O.; Charlet, K.; Dubois, M. A review about the fluorination and oxyfluorination of carbon fibres. J. Fluor. Chem. 2021, 251, 109887. [Google Scholar] [CrossRef]
- Jung, M.-J.; Kim, J.W.; Im, J.S.; Park, S.-J.; Lee, Y.-S. Nitrogen and hydrogen adsorption of activated carbon fibers modified by fluorination. J. Ind. Eng. Chem. 2009, 15, 410–414. [Google Scholar] [CrossRef]
- Sugiyama, H.; Hattori, Y. Selective and enhanced CO2 adsorption on fluorinated activated carbon fibers. Chem. Phys. Lett. 2020, 758, 137909. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Yuan, Y.; Li, E.; Wang, F. Effects of surface fluoride-functionalizing of glass fiber on the properties of PTFE/glass fiber microwave composites. RSC Adv. 2017, 7, 22810–22817. [Google Scholar] [CrossRef]
- Clark*, J.H.; Williamson, C.J. Mild Surface Fluorination of Glass Fibres. J. Mater. Chem. 1993, 3, 579–581. [Google Scholar] [CrossRef]
- Yin, X.; Sun, C.; Zhang, B.; Song, Y.; Wang, N.; Zhu, L.; Zhu, B. A facile approach to fabricate superhydrophobic coatings on porous surfaces using cross-linkable fluorinated emulsions. Chem. Eng. J. 2017, 330, 202–212. [Google Scholar] [CrossRef]
- Hottentot, D.J.; Kusano, Y.; Fæster, S. Fluorination of sized glass fibres for decreased wetting by atmospheric pressure plasma treatment in He/CF4. J. Adhes. 2020, 96, 2–12. [Google Scholar]
- Kusano, Y.; Cederløf, D.J.H.; Fæster, S. Plasma Surface Modification of Glass Fibre Sizing for Manufacturing Polymer Composites. Key Eng. Mater. 2020, 843, 159–164. [Google Scholar] [CrossRef]
- Mahmoud, M.; Kraxner, J.; Kaňková, H.; Hujová, M.; Chen, S.; Galusek, D.; Bernardo, E. Porous Glass Microspheres from Alkali-Activated Fiber Glass Waste. Materials 2022, 15, 1043. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, X.; Fan, W.; Wang, S.; Xiao, C.; Jantzen, C. Preparation of Porous Glass-Based Thick Film Using Diffusion-Induced Phase Separation from Scrap Glass. J. Am. Ceram. Soc. 2014, 97, 3128–3134. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, P.; Cao, Y.; Yu, P.; Chen, M.; Zhou, X. Evaluation of fiber surface modification via air plasma on the interfacial behavior of glass fiber reinforced laminated veneer lumber composites. Constr. Build. Mater. 2020, 233, 117315. [Google Scholar] [CrossRef]
- Kusano, Y. Atmospheric Pressure Plasma Processing for Polymer Adhesion: A Review. J. Adhes. 2014, 90, 755–777. [Google Scholar] [CrossRef]
- Yukihiro Kusano, T.L.A.; Toftegaard, H.L. Plasma treatment of carbon fibres and glass-fibre-reinforced polyesters at atmospheric pressure for adhesion improvement. Mater. Eng. Innov. 2014, 5, 122–137. [Google Scholar] [CrossRef]
- Cech, V.; Marek, A.; Knob, A.; Valter, J.; Branecky, M.; Plihal, P.; Vyskocil, J. Continuous surface modification of glass fibers in a roll-to-roll plasma-enhanced CVD reactor for glass fiber/polyester composites. Compos. Part A Appl. Sci. Manuf. 2019, 121, 244–253. [Google Scholar] [CrossRef]
- Cech, V.; Prikryl, R.; Balkova, R.; Grycova, A.; Vanek, J. Plasma surface treatment and modification of glass fibers. Compos. Part A 2002, 33, 1367–1372. [Google Scholar] [CrossRef]
- Kwon, D.-J.; Shin, P.-S.; Kim, J.-H.; Park, H.-S.; Beak, Y.-M.; DeVries, K.L.; Park, J.-M. Reinforcing effects of glass fiber/p-DCPD with fiber concentrations, types, lengths and surface treatment. Compos. Part B Eng. 2017, 123, 74–80. [Google Scholar] [CrossRef]
- Lee, J.-M.; Moon, J.-S.; Shim, D.; Choi, B.-H. Effect of glass fiber distributions on the mechanical and fracture behaviors of injection-molded glass fiber-filled polypropylene with 2-Hole Tension specimens. Compos. Sci. Technol. 2019, 170, 190–199. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kwon, D.-J.; Shin, P.-S.; Park, H.-S.; Baek, Y.-M.; DeVries, K.L.; Park, J.-M. Evaluation of interfacial and mechanical properties of glass fiber and p-DCPD composites with surface treatment of glass fiber. Compos. Part B Eng. 2018, 153, 420–428. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, J.; Zhou, L.; Wang, Z.; Liang, W. Effect of fiber surface modification on the lifetime of glass fiber reinforced polymerized cyclic butylene terephthalate composites in hygrothermal conditions. Mater. Des. 2015, 85, 14–23. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, M.; Kumar, S.; Mohapatra, S.K. Properties of Glass Fiber Hybrid Composites: A Review. Polym.-Plast. Technol. Eng. 2016, 56, 455–469. [Google Scholar] [CrossRef]
- Norström, A.E.E.; Fagerholm, H.M.; Rosenholm, J.B. Surface characterization of silane-treated industrial glass fibers. J. Adhes. Sci. Technol. 2001, 15, 665–679. [Google Scholar] [CrossRef]
- Bakaev, V.A.; Rivera, L.O.; Pantano, C.G. A Dynamic Volumetric Method for Measuring Adsorption of Water on Glass Fibers. J. Phys. Chem. C 2015, 119, 22504–22513. [Google Scholar] [CrossRef]
- Chowdhury, S.C.; Prosser, R.; Sirk, T.W.; Elder, R.M.; Gillespie, J.W. Glass fiber-epoxy interactions in the presence of silane: A molecular dynamics study. Appl. Surf. Sci. 2021, 542, 148738. [Google Scholar] [CrossRef]
- Mohamed, O.A.; Kewalramani, M.; Khattab, R. Fiber Reinforced Polymer Laminates for Strengthening of RC Slabs against Punching Shear: A Review. Polymers 2020, 12, 685. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Z.; Li, K.; Wang, X.; Wei, L. Elastic and Stretchable Functional Fibers: A Review of Materials, Fabrication Methods, and Applications. Adv. Fiber Mater. 2021, 3, 1–13. [Google Scholar] [CrossRef]
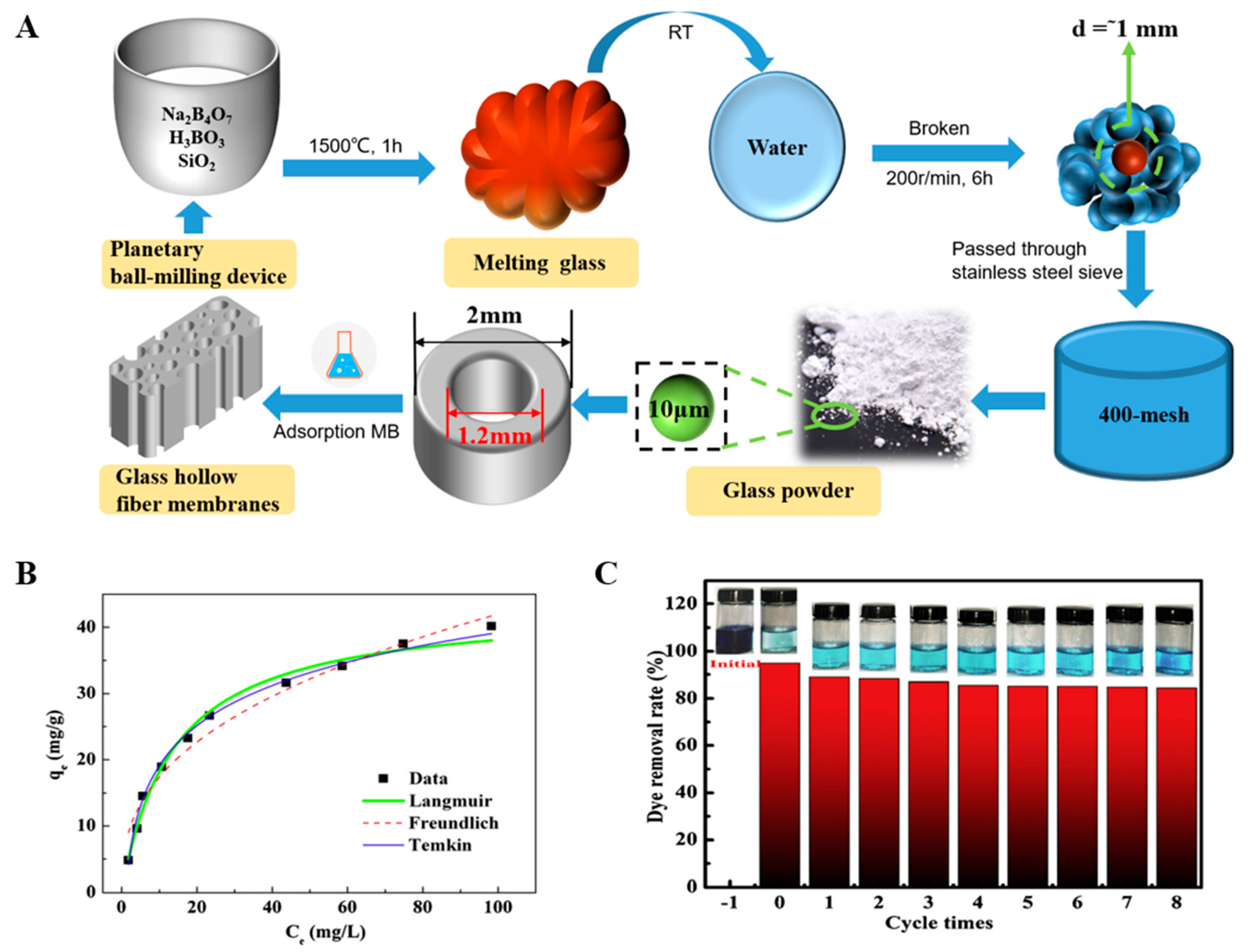
- Zhang, Y.; Liu, J.; Du, X.; Shao, W. Preparation of reusable glass hollow fiber membranes and methylene blue adsorption. J. Eur. Ceram. Soc. 2019, 39, 4891–4900. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Dutta, B.K. On the adsorption and diffusion of Methylene Blue in glass fibers. J. Colloid Interface Sci. 2005, 286, 807–811. [Google Scholar] [CrossRef]
- Guan, C.; Tao, Y.; Chen, S.; Zhu, J.; Gao, X. Synthesis of glass fiber sphere using waste printed circuit board to support MIL-100(Fe) for dye adsorption in water. Environ. Sci. Pollut. Res. 2021, 28, 46281–46290. [Google Scholar] [CrossRef]
- Letenkova, I.V. Investigation of Thermodynamics of Adsorption of Cadmium Ions Cd (II) from Model Solutions by Glass Fiber Materials. IOP Conf. Ser. Earth Environ. Sci. 2021, 852, 012059. [Google Scholar] [CrossRef]
- Fuhrmann, M.; Fitts, J.P. Adsorption of Trace Metals on Glass Fiber Filters. J. Environ. Qual. 2004, 33, 1943. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, A.; Selmert, V.; Weinrich, H.; Kungl, H.; Tempel, H.; Eichel, R.A. Tailored Gas Adsorption Properties of Electrospun Carbon Nanofibers for Gas Separation and Storage. ChemSusChem 2020, 13, 3180–3191. [Google Scholar] [CrossRef]
- Im, J.S.; Jung, M.J.; Lee, Y.-S. Effects of fluorination modification on pore size controlled electrospun activated carbon fibers for high capacity methane storage. J. Colloid Interface Sci. 2009, 339, 31–35. [Google Scholar] [CrossRef]
- Larasati, Z.S.; Widiastuti, N. Adsorption-desorption of CO2 and H2 gases on zeolite-X supported on glass fiber. AIP Conf. Proc. 2018, 2049, 020083. [Google Scholar]
- Hao, X.; Zhou, H.; Sun, L.; Lin, D.; Ou, R.; Wang, Q. Research progress and application of co-extruded wood plastic composites. J. For. Eng. 2021, 6, 27–38. [Google Scholar]
- Ding, L.; Han, X.; Cao, L.; Chen, Y.; Ling, Z.; Han, J.; He, S.; Jiang, S. Characterization of natural fiber from manau rattan (Calamus manan) as a potential reinforcement for polymer-based composites. J. Bioresour. Bioprod. 2021, 7, 190–200. [Google Scholar] [CrossRef]
- Tian, F.; Xu, X. Dynamical mechanical behaviors of rubber-filled wood fiber composites with urea formaldehyde resin. J. Bioresour. Bioprod. 2022, 7, 320–327. [Google Scholar] [CrossRef]
- Ren, D.; Li, K.; Chen, L.; Chen, S.; Han, M.; Xu, M.; Liu, X. Modification on glass fiber surface and their improved properties of fiber-reinforced composites via enhanced interfacial properties. Compos. Part B Eng. 2019, 177, 107419. [Google Scholar] [CrossRef]
- Nguyen, N.T.T.; Kiyotaka, O.; Kazuya, O.; Toru, F.; Ou, S.; Hidehiko, T.; Yukiko, F. Effect of Submicron Glass Fiber Modification on Mechanical Properties of Short Carbon Fiber Reinforced Polymer Composite with Different Fiber Length. J. Compos. Sci. 2020, 4, 5. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Wang, X.; Lei, H.; Huo, J. Chemical modification of starch with epoxy resin to enhance the interfacial adhesion of epoxy-based glass fiber composites. RSC Adv. 2016, 6, 84187–84193. [Google Scholar] [CrossRef]
- Garg, M.; Sharma, S.; Mehta, R. Role of curing conditions and silanization of glass fibers on carbon nanotubes (CNTs) reinforced glass fiber epoxy composites. Compos. Interfaces 2016, 24, 233–253. [Google Scholar] [CrossRef]
- Kusano, Y.; Norrman, K.; Singh, S.V.; Leipold, F.; Morgen, P.; Bardenshtein, A.; Krebs, N. Ultrasound enhanced 50 Hz plasma treatment of glass-fiber-reinforced polyester at atmospheric pressure. J. Adhes. Sci. Technol. 2013, 27, 825–833. [Google Scholar] [CrossRef]
- Chen, M.; Wan, C.; Zhang, Y.; Zhang, A.Y. Fibre Orientation and Mechanical Properties of Short Glass Fibre Reinforced PP Composites. Polym. Polym. Compos. 2005, 13, 253–262. [Google Scholar] [CrossRef]
- Lin, J.; Guo, Z.; Hong, B.; Xu, J.; Fan, Z.; Lu, G.; Wang, D.; Oeser, M. Using recycled waste glass fiber reinforced polymer (GFRP) as filler to improve the performance of asphalt mastics. J. Clean. Prod. 2022, 336, 130357. [Google Scholar] [CrossRef]
- Della Gatta, R.; Viscusi, A.; Perna, A.S.; Caraviello, A.; Astarita, A. Cold spray process for the production of AlSi10Mg coatings on glass fibers reinforced polymers. Mater. Manuf. Process. 2021, 36, 106–121. [Google Scholar] [CrossRef]
- Gonçalves, R.M.; Martinho, A.; Oliveira, J.P. Recycling of Reinforced Glass Fibers Waste: Current Status. Materials 2022, 15, 1596. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, H.-M.; Feng, Q.-P.; Fu, S.-Y. Synergistic effect of carbon nanotubes and n-butyl glycidyl ether on matrix modification for improvement of tensile performance of glass fiber/epoxy composites. Compos. Part A Appl. Sci. Manuf. 2014, 62, 39–44. [Google Scholar] [CrossRef]
- Jamshaid, F.; Khan, R.U.; Islam, A. Performance tuning of glass fiber/epoxy composites through interfacial modification upon integrating with dendrimer functionalized graphene oxide. J. Appl. Polym. Sci. 2021, 138, 50876. [Google Scholar] [CrossRef]
- Liao, M.; Yang, Y.; Hamada, H. Mechanical performance of glass woven fabric composite: Effect of different surface treatment agents. Compos. Part B Eng. 2016, 86, 17–26. [Google Scholar] [CrossRef]
- Yıldız, S.; Karaağaç, B.; Güzeliş, S.G. Utilization of glass fiber reinforced polymer wastes. Polym. Compos. 2021, 42, 412–423. [Google Scholar] [CrossRef]
- Luo, G.; Li, W.; Liang, W.; Liu, G.; Ma, Y.; Niu, Y.; Li, G. Coupling effects of glass fiber treatment and matrix modification on the interfacial microstructures and the enhanced mechanical properties of glass fiber/polypropylene composites. Compos. Part B Eng. 2017, 111, 190–199. [Google Scholar] [CrossRef]
- Safri, S.N.A.; Sultan, M.T.H.; Shah, A.U.M. Characterization of benzoyl treated sugar palm/glass fibre hybrid composites. J. Mater. Res. Technol. 2020, 9, 11563–11573. [Google Scholar] [CrossRef]
- Nayak, S.K.; Mohanty, S.; Samal, A.S.K. Hybridization Effect of Glass Fibre on Mechanical, Morphological and Thermal Properties of Polypropylene-Bamboo/Glass Fibre Hybrid Composites. Polym. Polym. Compos. 2010, 18, 205–218. [Google Scholar] [CrossRef]
- Guo, R.; Xian, G.; Li, F.; Li, C.; Hong, B. Hygrothermal resistance of pultruded carbon, glass and carbon/glass hybrid fiber reinforced epoxy composites. Constr. Build. Mater. 2022, 315, 125710. [Google Scholar] [CrossRef]
- Raj, F.M.; Nagarajan, V.A.; Elsi, S.S. Mechanical, physical and dynamical properties of glass fiber and waste fishnet hybrid composites. Polym. Bull. 2017, 74, 1441–1460. [Google Scholar] [CrossRef]
- Bishetti, P. Glass Fiber Reinforced Concrete. Int. J. Civ. Eng. 2019, 6, 23–26. [Google Scholar] [CrossRef]
- Garoushi, S.; Vallittu, P.; Lassila, L. Hollow glass fibers in reinforcing glass ionomer cements. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2017, 33, e86–e93. [Google Scholar] [CrossRef]
- Gao, S.L.; Mäder, E.; Plonka, R. Coatings for glass fibers in a cementitious matrix. Acta Mater. 2004, 52, 4745–4755. [Google Scholar] [CrossRef]
- Huang, H.; Pang, H.; Huang, J.; Zhao, H.; Liao, B. Synthesis and characterization of ground glass fiber reinforced polyurethane-based polymer concrete as a cementitious runway repair material. Constr. Build. Mater. 2020, 242, 117221. [Google Scholar] [CrossRef]
- Haddaji, Y.; Majdoubi, H.; Mansouri, S.; Alomayri, T.S.; Allaoui, D.; Manoun, B.; Oumam, M.; Hannache, H. Microstructure and flexural performances of glass fibers reinforced phosphate sludge based geopolymers at elevated temperatures. Case Stud. Constr. Mater. 2022, 16, e00928. [Google Scholar] [CrossRef]
- Fang, Y.; Cui, P.; Ding, Z.; Zhu, J.-X. Properties of a magnesium phosphate cement-based fire-retardant coating containing glass fiber or glass fiber powder. Constr. Build. Mater. 2018, 162, 553–560. [Google Scholar] [CrossRef]
- Manalo, A.; Jackson, M. Behaviour of pultruded glass fibre reinforced composites guardrail system. Adv. Struct. Eng. 2018, 21, 545–556. [Google Scholar] [CrossRef]
- Michael Raj, F.; Nagarajan, V.A.; SahayaElsi, S. Waste to poles: Discarded fishnet/glass fiber and polyester for building electrical poles. Polym. Compos. 2018, 39, 2997–3005. [Google Scholar] [CrossRef]
- Ebadzadsahraei, S.; Hassan Vakili, M. Comparison of Corrosion Resistance of Fiberglass/Epoxy and Fiberglass/Polyester Composite Pipes for Application in Natural Gas Transportation. J. Pipeline Syst. Eng. Pract. 2019, 10, 41. [Google Scholar] [CrossRef]
- Skovron, J.D.; Zhuge, J.; Gou, J.; Gordon, A.P. Effect of nanopaper coating on flexural properties of a fire-treated glass fiber reinforced polyester composite. J. Compos. Mater. 2016, 50, 3995–4013. [Google Scholar] [CrossRef]
- Naidu, G.G.; Ramakrishna, R.; Ezri, B.G. Strengthening of Soil by adding Lime and Glass Fiber as Stabilizing Materials for the Construction of High Rise Buildings. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1126, 012070. [Google Scholar] [CrossRef]
- Trejbal, J. Mechanical properties of lime-based mortars reinforced with plasma treated glass fibers. Constr. Build. Mater. 2018, 190, 929–938. [Google Scholar] [CrossRef]
- Gu, A.; Liang, G.; Yuan, L. Novel preparation of glass fiber reinforced polytetrafluoroethylene composites for application as structural materials. Polym. Adv. Technol. 2009, 20, 39–42. [Google Scholar] [CrossRef]
- Tjong, S.C.; Xu, S.-A.; Li, R.K.Y.; Mai, Y.W. Fracture characteristics of short glass fibre/maleated styrene-ethylene-butylene-styrene/polypropylene hybrid composite. Polym. Int. 2002, 51, 1248–1255. [Google Scholar] [CrossRef]
- Seraji, A.A.; Aghvami-Panah, M.; Shams-Ghahfarokhi, F. Evaluation of ultimate engineering properties of polytetrafluoroethylene/carbon-aerogel/glass fiber porous composite. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 128975. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.B.; Lee, J.S.; Kim, J.W. Improvement in mechanical properties of glass fiber fabric/PVC composites with chopped glass fibers and coupling agent. Mater. Res. Express 2017, 4, 075303. [Google Scholar] [CrossRef]
- Gowda, S.; Basavarajaiah, S. Mechanical and tribological performance evaluation of maleic anhydride grafted ethylene octene copolymer toughened acrylonitrile butadiene styrene/polyamide 6 composites strengthened with glass fibres. J. Text. Inst. 2021, 112, 1240–1248. [Google Scholar] [CrossRef]
- Sim, J.-H.; Yu, S.-H.; Yoon, H.-S.; Kwon, D.-J.; Lee, D.-H.; Bae, J.-S. Characteristic evaluation and finite element analysis of glass fiber/recycled polyester thermoplastic composites by cross-sectional shape of glass fiber. Compos. Part B Eng. 2016, 223, 109095. [Google Scholar] [CrossRef]
- Yang, Y.; Hou, T.; Dong, C.; Duan, H.; Zhao, G.; Liu, Y. Crystallization induced enhancement on electrical conductivity and strength of highly conductive PP composites. J. Polym. Res. 2016, 23, 12252. [Google Scholar] [CrossRef]
- Jiang, J.; Mei, C.; Pan, M.; Lu, F. Effects of hybridization and interface modification on mechanical properties of wood flour/polymer composites reinforced by glass fibers. Polym. Compos. 2019, 40, 3601–3610. [Google Scholar] [CrossRef]
- Bi, X.; Huang, R. 3D printing of natural fiber and composites: A state-of-the-art review. Mater. Des. 2022, 222, 111065. [Google Scholar] [CrossRef]
- Zhao, H.; Miao, Q.; Huang, L.; Zhou, X.; Chen, L. Preparation of long bamboo fiber and its reinforced polypropylene membrane composites. J. For. Eng. 2021, 6, 96–103. [Google Scholar]
- Fatima, A.; Yasir, S.; Khan, M.S.; Manan, S.; Ullah, M.W.; Ul-Islam, M. Plant extract-loaded bacterial cellulose composite membrane for potential biomedical applications. J. Bioresour. Bioprod. 2021, 6, 26–32. [Google Scholar] [CrossRef]
- Tian, F.; Guan, M.; Du, K.; Yong, C.; Zhao, C. Fiber properties of straw pretreated by fermentation and its degradable composites. J. For. Eng. 2022, 7, 128–133. [Google Scholar]
- Peng, L.; Yi, J.; Yang, X.; Xie, J.; Chen, C. Development and characterization of mycelium bio-composites by utilization of different agricultural residual byproducts. J. Bioresour. Bioprod. 2023, 8, 78–89. [Google Scholar] [CrossRef]
- Wambua, P.; Ivens, J.; Verpoest, I. Natural fibres: Can they replace glass in fibre reinforced plastics? Compos. Sci. Technol. 2003, 63, 1259–1264. [Google Scholar] [CrossRef]
- Ramesh, M.; Palanikumar, K.; Reddy, K.H. Mechanical property evaluation of sisal-jute-glass fiber reinforced polyester composites. Compos. Part B: Eng. 2013, 48, 1–9. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, C.; Yang, M.; Wang, D.; Peng, S.; Tian, Z.; Shuai, C. Copper-doped mesoporous bioactive glass endows magnesium-based scaffold with antibacterial activity and corrosion resistance. Mater. Chem. Front. 2021, 5, 7228–7240. [Google Scholar] [CrossRef]
- Liu, L.; Sun, H.; Zhang, J.; Xu, B.; Gao, Y.; Qi, D.; Mao, Z.; Wu, J. Trilayered Fibrous Dressing with Wettability Gradient for Spontaneous and Directional Transport of Massive Exudate and Wound Healing Promotion. Adv. Fiber Mater. 2022. [Google Scholar] [CrossRef]
- Chen, F.; Xiao, H.; Peng, Z.Q.; Zhang, Z.P.; Rong, M.Z.; Zhang, M.Q. Thermally conductive glass fiber reinforced epoxy composites with intrinsic self-healing capability. Adv. Compos. Hybrid Mater. 2021, 4, 1048–1058. [Google Scholar] [CrossRef]
- Fang, X.; Wei, S.; Kong, J. Paper-based microfluidics with high resolution, cut on a glass fiber membrane for bioassays. Lab A Chip 2014, 14, 911–915. [Google Scholar] [CrossRef]
- Vallittu, P.K. High-aspect ratio fillers: Fiber-reinforced composites and their anisotropic properties. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2015, 31, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rantala, L.I.; Lastumäki, T.M.; Peltomäki, T.; Vallittu, P.K. Fatigue resistance of removable orthodontic appliance reinforced with glass fibre weave. J. Oral Rehabil. 2003, 30, 501–506. [Google Scholar] [CrossRef]
- Huang, X.; Cheng, H.; Luo, W.; Zhang, W.; Jiang, M.; Yang, C.; Yu, T.; Cai, Z.; Xu, Z.; Shu, X.; et al. Er-Activated Hybridized Glass Fiber for Laser and Sensor in the Extended Wavebands. Adv. Opt. Mater. 2021, 9, 2101394. [Google Scholar] [CrossRef]
- Saito, M.; Takizawa, M. Teflon-clad As-S glass infrared fiber with low-absorption loss. J. Appl. Phys. 1986, 59, 1450–1452. [Google Scholar] [CrossRef]
- Pureza, P.C.; Brower, D.T.; Aggarwal, I.D. Fluoride Glass Fibers with Improved Mechanical Strength. J. Am. Ceram. Soc. 1989, 72, 1980–1981. [Google Scholar] [CrossRef]
- Hirao, K.; Higuchi, M.; Soga, N. Upconversion mechanism of Pr3+ -doped fluoride fiber glass. J. Lumin. 1994, 60–61, 115–118. [Google Scholar] [CrossRef]
- Toru Mizunami, H.I.; Takagi, K. Gain saturation characteristics of Raman amplification in silica and fluoride glass optical fibers. Opt. Commun. 1993, 97, 74–78. [Google Scholar] [CrossRef]
- Zhan, H.; Huang, Z.; Wen, J.; Jiang, L.; Wang, J.; Jing, F.; Lin, A. Single-mode fluorotellurite glass fiber. Opt. Mater. 2016, 53, 142–145. [Google Scholar] [CrossRef]
- Woo, H.-S.; Kim, J.-H.; Moon, Y.-B.; Kim, W.K.; Ryu, K.H.; Kim, D.-W. A dual membrane composed of composite polymer membrane and glass fiber membrane for rechargeable lithium-oxygen batteries. J. Membr. Sci. 2018, 550, 340–347. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, J.; Zeng, J.; Huang, J.; Zhao, J. VGCF 3D conducting host coating on glass fiber filters for lithium metal anodes. Chem. Commun. 2018, 54, 1178–1181. [Google Scholar] [CrossRef]
- Sebastian, J.; Schehl, N.; Bouchard, M.; Boehle, M.; Li, L.; Lagounov, A.; Lafdi, K. Health monitoring of structural composites with embedded carbon nanotube coated glass fiber sensors. Carbon 2014, 66, 191–200. [Google Scholar] [CrossRef]
- Matsubara, Y.O.H. Carbon-matrix composites with continuous glass fiber and carbon black for maximum strain sensing. Carbon 2007, 45, 1152–1159. [Google Scholar]
- Jiao, T.; Pu, C.; Xing, W.; Lv, T.; Li, Y.; Wang, H.; He, J. Characterization of Engineering-Suitable Optical Fiber Sensors Packaged with Glass Fiber-Reinforced Polymers. Symmetry 2022, 14, 973. [Google Scholar] [CrossRef]
- Zhao, C.; Xia, Z.; Wang, X.; Nie, J.; Huang, P.; Zhao, S. 3D-printed highly stable flexible strain sensor based on silver-coated-glass fiber-filled conductive silicon rubber. Mater. Des. 2020, 193, 108788. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Song, Y.; Wu, D.; Mao, X.; Yang, X.; Jiang, S.; Zhang, C.; Guo, R. Recent Progress in Modifications, Properties, and Practical Applications of Glass Fiber. Molecules 2023, 28, 2466. https://doi.org/10.3390/molecules28062466
Wu Y, Song Y, Wu D, Mao X, Yang X, Jiang S, Zhang C, Guo R. Recent Progress in Modifications, Properties, and Practical Applications of Glass Fiber. Molecules. 2023; 28(6):2466. https://doi.org/10.3390/molecules28062466
Chicago/Turabian StyleWu, Yawen, Yangyang Song, Di Wu, Xiaowei Mao, Xiuling Yang, Shaohua Jiang, Chunmei Zhang, and Rui Guo. 2023. "Recent Progress in Modifications, Properties, and Practical Applications of Glass Fiber" Molecules 28, no. 6: 2466. https://doi.org/10.3390/molecules28062466
APA StyleWu, Y., Song, Y., Wu, D., Mao, X., Yang, X., Jiang, S., Zhang, C., & Guo, R. (2023). Recent Progress in Modifications, Properties, and Practical Applications of Glass Fiber. Molecules, 28(6), 2466. https://doi.org/10.3390/molecules28062466







