Abstract
This study aims to evaluate the toxicity of ZnS nanoparticles (ZnS NP50 = 50 µg/L and ZnS NP100 = 100 µg/L) and diethyl (3-cyano-1-hydroxy-2-methyl-1-phenylpropyl)phosphonate or P (P50 = 50 µg/L and P100 = 100 µg/L) in the clams Ruditapes decussatus using chemical and biochemical approaches. The results demonstrated that clams accumulate ZnS NPs and other metallic elements following exposure. Moreover, ZnS NPs and P separately lead to ROS overproduction, while a mixture of both contaminants has no effect. In addition, data showed that exposure to P100 resulted in increased levels of oxidative stress enzyme activities catalase (CAT) in the gills and digestive glands. A similar trend was also observed in the digestive glands of clams treated with ZnS100. In contrast, CAT activity was decreased in the gills at the same concentration. Exposure to ZnS100 and P100 separately leads to a decrease in acetylcholinesterase (AChE) levels in both gills and digestive glands. Thus, AChE and CAT after co-exposure to an environmental mixture of nanoparticles (ZnS100) and phosphonate (P100) did not show any differences between treated and non-treated clams. The outcome of this work certifies the use of biomarkers and chemical assay when estimating the effects of phosphonate and nanoparticles as part of an ecotoxicological assessment program. An exceptional focus was given to the interaction between ZnS NPs and P. The antioxidant activity of P has been demonstrated to have an additive effect on metal accumulation and antagonistic agents against oxidative stress in clams treated with ZnS NPs.
1. Introduction
Contaminants are unavoidably released into aquatic ecosystems and eventually reach sediment and water as a result of anthropogenic actions [1,2].
Nanoparticles and phosphonates are among the most widely used products in the world [3] and are detected at different concentrations in different compartments of marine ecosystems [4]. These contaminants have harmful effects on aquatic organisms through interference with a range of biological and biochemical pathways [1,2]. Most species can accumulate nanoparticles and phosphonates from contaminated sources, leading to contamination of the entire ecosystem [4] and consequently humans through the food chain [5].
Zinc sulfide nanoparticles (ZnS NPs) have been used for a large number of applications [6]. Because of photochemical processes and dissociation, these types of particles can release toxic metal ions into the aquatic environment [7]. In addition, ZnS NPs have been shown to exhibit some changes in the physicochemical parameters of bodies of water, such as dissolved oxygen level and pH, in turn regulating water quality and hence affecting aquatic fauna [8]. The problem of its toxicity in aquatic species depends on their size, charge, and duration of exposure according to Aye et al. [9], but no data are yet available in marine waters.
Among phosphonates, diethyl (3-cyano-1-hydroxy-2-methyl-1-phenyl propyl) phosphonate (P) is a novel type of hydroxy phosphonate used as a pharmaceutical compound, having received considerable attention for its biological activity as an antibiotic [10] and enzyme inhibitor [11] and for its anticancer and antioxidant activity [12]. Moreover, the toxic effects of this new compound have not yet been investigated. However, the toxic effects of exposure to these two xenobiotics remain distant from clear, and most studies have been performed on each contaminant separately. The need to recognize the eco-toxicological impacts of these xenobiotics on aquatic organisms has become essential, and links between the effects of NPs and phosphonates should be investigated.
Recently, biomarkers and chemical approaches have been used to evaluate the toxicological effects of xenobiotics on bivalves as filter-feeding organisms. It is well documented that bivalves have been utilized as indicators due to their tolerance against pollutants, having a central ecological position [13], and representing the potential transport of NPs through food chains [14]. In particular, the clam Ruditapes decussatus, abundant in the Mediterranean Sea, represents a suitable model organism for determining the impacts of NPs and phosphonates.
NPs and phosphonates can penetrate organisms and generate reactive oxygen species (ROS), causing increases in antioxidant enzyme activities [15] and neurotoxic effects [16]. In addition, NPs and phosphonate could lead to LPO increases and protein and DNA damage [17], as well as decreases in lysosomal membrane stabilities and phagocytic activity [18].
Due to widespread NPs and phosphonate occurrence in aquatic ecosystems and their toxic effects on different organisms, it is necessary to understand the mechanism of their toxicity after individual or combined exposure and to identify potential organ-based biomarkers for assessing biological interactions. From this perspective, the present research is focused on the mechanistic explanation of ZnS and P toxicities (separately and in combination) in the marine bivalve R. decussatus, and it illustrates the potential biomarkers for screening and monitoring the interaction and toxicity of these chemicals in marine organisms. In addition, considering the important role of the gills as a first barrier against the uptake of contaminants and of the digestive glands as key organs in energetic metabolisms [19,20], toxicity and interaction were evaluated in these organs to obtain a more specific ecotoxicological database.
2. Results
2.1. ZnS NPs Characterization
The morphology of pure ZnS nanoparticles was determined by transmission electron microscopy. The TEM images in Figure 1a show that ZnS nanoparticles with a quasi-spherical shape are formed. The size of the prepared ZnS particles is about 5 nm. Lattice fringes can be clearly distinguished for a single ZnS nanocrystal (Figure 1b) as 0.31-nm interplane spacing of the (111) plane of ZnS in the cubic blende structure. Analysis by energy spectroscopy (EDAX) shows that the sample has only peaks of zinc and sulfur, thus confirming the high-purity ZnS nanoparticles (Figure 1c). The presence of peaks (<1 eV) is due to the copper grid used for the TEM/EDAX experiments.
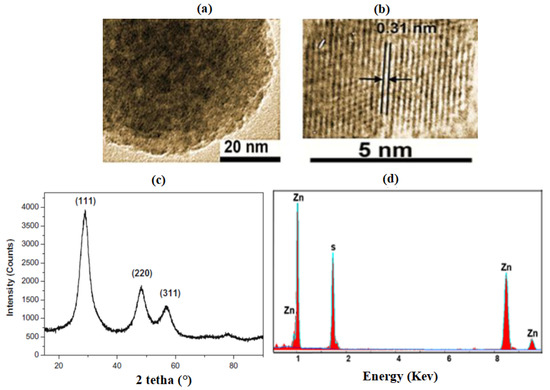
Figure 1.
TEM images (a,b), DRX (c), and EDAX spectra (d) of ZnS nanoparticles.
2.2. Metal Content in the Clams
Table 1 shows that Fe, Cd, Pb, and Zn concentrations decreased after exposure to ZnS50 and P50 compounds separately in the whole soft tissue of R. decussatus. In contrast, metal content increased significantly (p < 0.05) in clams exposed to ZnS100. In addition, Cu concentrations increased significantly in clams with increased P concentrations, while Cd, Fe, Pb, and Zn decreased in P50- and P100-treated clams. Our results also showed an important increase in metal content in the whole soft tissue of clams treated with the mixture (M100).

Table 1.
Metal content in the whole soft tissues of control and treated clams (g/kg dw).
2.3. Hydrogen Peroxide Levels
The hydrogen peroxide levels were examined in both the digestive glands and gills of R. decussatus clam exposed to ZnS, P, and their combination (Figure 2a). No significant differences were observed in the digestive glands between control and treated clams. Moreover, ZnS50 and P50s showed no significant differences in H2O2 levels in gills compared to the control group. In the same organs, ZnS100 and P100 added separately showed significant increases in H2O2 levels by 76% and 62%, respectively, compared to the control group (p < 0.05). In contrast, no significant changes were observed in the gills and digestive glands of clams treated with M100 (p < 0.05).
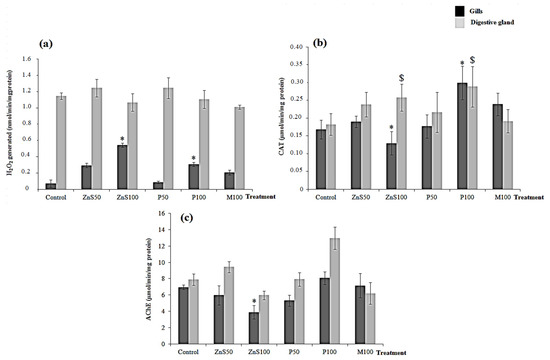
Figure 2.
Antioxidant and neurotoxic effects of ZnS NPs and P: (a) hydrogen peroxide (H2O2) (b) CAT and (c) AChE in the gills and digestive glands of Ruditapes decussatus clams when exposed to increasing concentrations of ZnS NPs and P over 10 days. Values are the mean ± SD. (*) indicates significant differences (p < 0.05) and ($) indicates significant differences in digestive gland (p < 0.05).
2.4. Catalase Activity
No significant differences were observed in the gills and digestive glands of clams exposed to ZnS50 and P50 (Figure 2b). Significant increases in CAT activity were observed in the digestive glands of clams exposed to ZnS100 from 0.182 to 0.257 µmol/min/mg protein, but a significant decrease in this activity occurred in their gills. CAT activity was found to be stimulated after exposure to 100 µg/L per 3 g of compound from 0.168 to 0.2990 µmol/min/mg protein in gills and 0.182 to 0.288 in digestive glands (Figure 2b). In contrast, no significant changes in CAT activities occurred in the gills and digestive glands of clams exposed to the mixture of ZnS NPs and P compound (p < 0.05).
2.5. AChE Activity
No significant differences were observed after 10 days of exposure to ZnS50 and P50 in both gills and digestive glands (Figure 2c). In contrast, significant inhibition (p < 0.05) was detected in gills by 28.2% and digestive glands by 13.77% of specimens exposed to ZnS100. The exposure of clams to M100 did not change AChE activity in the gills and digestive glands compared to the control group (Figure 2c).
3. Discussion
3.1. Metal Content in Clams
The study of human impact on bivalves is of great significance from ecological, economic, and public health points of view. The results of the present study add more data about the toxicity caused by NPs and pharmaceutical compounds, particularly ZnS NPs, and phosphonates. These findings represent the first data on the in vivo effects of ZnS NPs and phosphate P added separately or in combination with metal variations and the enzymatic status of clams. The results demonstrate that exposure to ZnS NP suspensions induces significant changes in metal content in clams. R. decussatus accumulate Fe, Cu, and Zn in the total tissue. In agreement with our results, Vale et al. [21] reported an increase in metal concentrations in Danio rerio fish exposed to increased ZnO NP concentrations. These data suggest that metal accumulations are related to chemical and biochemical processes of detoxification of free radicals generated by NPs. It is also important to note that Cd, Fe, and Cu levels increased with increasing ZnS NPs concentrations in clam tissues. This outcome is probably due to the adsorption of these metals by ZnS NPs [21]. Eventually, ZnS NPs increased the bioavailability of these metals initially present in seawater, and this speciation allows the importation of metals into the cells [21].
Exposure of clams to phosphate P induced a decrease in most metal concentrations in the clams’ tissues. These results could be attributed to the high affinity of this compound against metals, as described by Aouani et al. [22], who suggested that the P=O moiety is responsible for the metal’s ion chelating effect.
Nevertheless, the opposite effect was demonstrated in our study, showing that P induced an additive effect on metal uptake in the presence of ZnS NPs. Comparable results were reported by Gagné et al. [23] in several aquatic species contaminated with a mixture of ZnO and pharmaceuticals. Alsop and Wood [24] observed an additive effect on Cu levels in zebrafish contaminated with Cu and fluoxetine.
3.2. Oxidative Stress and Modulating Effects
Once passed through the membrane barrier, NPs and pharmaceuticals produce reactive oxygen species (ROS), causing oxidative stress [21,25,26]. Our results revealed that the highest concentration of ZnS NPs induces overproduction of hydrogen peroxide in the gills. These results were confirmed by those in the literature [27]. Additionally, Asharani et al. [28] and Hussain et al. [29] showed that Ag NPs contribute to high ROS production by released ions [30]. This overproduction is related to the photocatalytic properties of ZnS NPs [31]. These data suggest that ROS overproduction is related to the photocatalytic aspect of ZnO NPs, and this disturbance could be the result of released Zn2+ in cells [32]. In the present study, P100 increased H2O2 levels only in gills, whereas no excessive production was observed with P50. This increase is related to the chemical nature of this phosphonate. According to Liendro et al. [33], organophosphorus compounds led to ROS production in Oryzias latipes. Similar effects were reported by Bebianno et al. [34] and Maranho et al. [35] in R. philippinarum and R. decussatus after exposure to a mixture of organophosphorus compounds. González et al. [36] and Cruz et al. [37] indicated that pharmaceutical products accumulated in R. decussatus clams, contributing to free radical generation.
Unlike metal uptake, exposure to a mixture of ZnS NPs and P induces an antagonistic effect against ROS production. This decrease is related to the ability of α-hydroxyphosphonate to neutralize excess hydrogen peroxide levels, as reported by Naidu et al. [38]. The addition of P opposes the toxic effects of nanoparticles by preventing ROS overproduction. Thus, both phosphorous and nitrile groups present in P mobilize and remove H2O2 from the organism [22]. In addition, Rao et al. [12] confirmed that phosphorous density has a crucial role in controlling oxygenated species affinity. It is also important to mention that metals react directly with ROS. However, chemical analysis by ICP shows that these two xenobiotics stimulate the non-enzymatic molecular defense system. Similarly, copper, zinc, and iron concentrations increase as a result of exposure to catalyst H2O2 levels, as reported by Fenton [39]. Zinc and copper ions are also catalysts of this radical, according to Mohanty and Samanta [40]. Similarly, iron is likely to induce a reduction in intracellular H2O2, according to [36].
Antioxidant enzymes, such as CAT, are involved in protecting organisms from the harmful effects of H2O2 generated by xenobiotics [41]. Our results showed normal CAT activity in the gills and digestive glands after exposure to ZnS50. Similar results were also found in Crassostrea gigas oysters exposed to 50 μg/L of ZnO NPs [19] and Scrobicularia plana exposed to 10 μg/L of CuO NPs [26]. In contrast, stimulation of enzymatic defense mechanisms was demonstrated in Scrobicularia plana [20] and E. complanata exposed to a higher concentration of ZnO NPs [23]. It has been shown that CAT activity is much more important in the digestive glands than in other organs in bivalves exposed to a wide variety of nanoparticles [42]. This finding confirms the large difference in response between the gills and the digestive glands of specimens exposed to ZnS100. The same pattern of development has been reported in E. complanata exposed to ZnO NPs (2 mg/L) [23]. In addition, our results are in agreement with the studies of Ali et al. [43], who found that CAT activity in the digestive glands of Lymnaea luteola exposed to ZnO NPs increases with increasing concentration. CAT inhibition in clams treated with ZnS100 could be attributed to hydrogen peroxide overproduction. Gomes et al. [44] showed that CAT inhibition in Mytilus galloprovincialis is due to the interaction of Ag NPs with the thiol group of CAT. Moreover, Mohanty and Samanta [40] showed that CAT inhibition is directly related to the reaction between metal ions and the -SH group of this enzyme and indirectly with the superoxide anion [45].
CAT activity is also known to be strongly influenced by pharmaceuticals [37]. In the present study, the high concentration of P stimulated the antioxidant defense system in a concentration-dependent manner. Aquatic invertebrates are among the most sensitive aquatic species to pharmaceuticals. Martin-Díaz et al. [46] and Parolini et al. [47] showed that the antioxidant defense system’s activation against high levels of H2O2 in the digestive glands of M. galloprovinciallis and Dreissena polymorpha are dependently exposed to concentrations of pharmaceuticals. Our results showed a gradual increase in CAT activity in clams contaminated with two concentrations of P (P50 = 50 μg/L and P100 = μg/L).
CAT activity remains intact after exposure to the mixture (M100) of ZnS NPs and P. This result is related to the phosphonate compound P acting as an antagonist against ZnS NPs by a mechanism that has not yet been developed.
Aouani et al. [22] suggested that P possesses the ability to chelate DPPH radicals by donating an electron. Since the phosphorus group has an affinity for oxygen, it is also possible to have an affinity toward ROS, leading to a decrease in CAT activity. Our results prove that this compound can chelate free radicals, and then it can constitute a defense agent against the accumulation of NPs in the gills and digestive glands. The possible interactions are still unclear, and the mode of action is not well elucidated. In contrast, a study performed on R. decussatus showed that the exposure of this species to a mixture of metal ions and pharmaceuticals led to an increase in CAT activity in the digestive glands [48].
3.3. Neurotoxic Effects of ZnS NPs and P
AChE is the most commonly used biomarker of neurotoxicity in invertebrates [15], and this enzyme is inhibited by several neurotoxic agents [49]. The results of our study showed that ZnS NPs and P inhibit AChE activity in Ruditapes decussates at high concentrations without leading to muscle tetanization since no mortalities were observed during the experiment.
AChE inhibition is a concentration-dependent response since ZnS50 and P50 had no significant changes in clams, and the inhibition is observed in specimens treated with high concentrations (ZnS100 and P100). AChE activity was inhibited in Hedist diversicolor and Nereis diversicolor annelids exposed to increasing concentrations of ZnO [20]. Similarly, CuO NPs have been shown to contribute ta significant inhibition of AChE activity in R. decussatus after 5 days of exposure [34].
This inhibition of AChE is probably due to the adsorption of ZnS NPs to AChE active sites. This observation was confirmed by the hypothesis of [50], who showed AChE inactivation by Zn2+ ions. The denaturation of serine residues at the active site of AChE by Zn2 + ions may also be the cause of AChE inhibition and the accumulation of acetylcholine in the synaptic space [51]. The theory of a modification of the electrostatic field of AChE by ions was also suggested by Radic et al. [52].
AChE inhibition is strongly correlated with a crucial increase in hydrogen peroxide after exposure to ZnS NP100 in the gills and digestive glands and to P100 in the gills. This result is in agreement with Schallreuter et al. [53], who explained that AChE activity becomes inactive due to the high production of H2O2. In addition, dependence was demonstrated by Antonio et al. [54], who discovered a positive correlation between the increase in CAT activity and the inhibition of AChE activity in aquatic species. In addition, Buffet et al. [26] showed that copper, zinc, and silver nano-oxides did not cause any changes in AChE activity in Scrobicularia plana clams treated with 100 μg/L. This finding proves that AChE activity is very variable between species. Pan et al. [9] also showed stimulation of AChE activity in S. plana contaminated with 100 mg/L of AuNPs and that this increase was associated with a compensatory response.
AChE is highly sensitive to pharmaceutical product variation [35]. Twenty percent or more of a decrease in AChE activity following exposure to any contaminant is considered a consequence of neurotoxicity caused by this pollutant [55]. Based on this theory, we can conclude from our results that P at a relatively high concentration has a neurotoxic effect on R. decussatus clams.
Similar neurotoxic effects have been observed in R. decussatus exposed to OMTOS (2-(4-methoxyphenyl)-5,6-trimethylene-4H-1,3,2-oxathiaphosphorin-2-sulfide) [56]. The inhibition of AChE activity was also demonstrated by Mattson et al. [57], who showed that a pharmaceutical (neguvon, an antiparasitic) inhibited this activity in salmonids and fish.
Brewer et al. [58], Sandahl et al. [59], and Sturve et al. [60] also showed inhibition of AChE by organophosphorus compounds. These authors suggested that the decrease in AChE activity in Terapon jarbua fish is strongly correlated with the bioaccumulation of organophosphorus products. In addition, Nunes et al. [61] found that, in the crustacean Artemia parthenogenetica, exposure to pharmaceutical substances, such as diazepam, inhibits AChE activity. Milan et al. [62] reported that inhibition of AChE activity is organo-dependent in R. philippinarum clams exposed to pharmaceuticals (100 μg/L) with greater inhibition in gills than in other organs, while the most recent studies showed that, for the same species contaminated with 50 μg/L of pharmaceutical product (antibiotics and antidepressants), AChE inhibition is more important in the digestive glands [63]. These authors have linked the neurotoxic effects of these pharmaceuticals with their modes of action. These products are known for their ability to inhibit AChE activity [64]. This relationship explains the inhibition of AChE by P, known for its antibacterial activity according to Pokalwar et al. [65] and Reddy et al. [66].
Contrary to the neurotoxic effects of P in the gills, significant stimulation of AChE activity in the digestive glands was observed after the administration of P100. This increase in AChE activity can be explained by an overcompensation of the gene codes for this enzyme and a reduction in the gene code for the acetylcholine vesicle transporter to decrease neuronal hyperexcitation [67]. The increased concentrations of acetylcholine are at the origin of continuous nerve transmission. The regulation of the expression of these genes makes it possible to decrease the availability and/or increase the hydrolysis of the neurotransmitter at the level of the synaptic space. In addition, this increase may be the consequence of the production of new forms of AChE, which trap inhibitors of this enzyme (AChE-R) and which hydrolyzes acetylcholine (AChE -S) [68].
Aguirre-Martinez et al. [69] showed an activation of the cholinergic system in clam R. philippinarum exposed to several families of pharmaceutical products. These authors have shown that exposure to an antibacterial drug (Novobiocin = 0.1 to 50 μg/L) and another anti-inflammatory product (Ibuprofen = 5 to 50 μg/L) is likely to induce a progressive increase in AChE activity in the digestive gland. Similarly, AChE stimulation in clam was also detected following contamination with a low concentration of caffeine (0.1 μg/L). Aguirre-Martinez et al. [69] pointed out that this stimulation is closely linked to the level of contamination and is probably contaminant-dependent. This was confirmed by Aguirre-Martinez et al. [69], which corroborated the hypothesis of a significant increase in AChE as a transient effect of the drug, suggesting that clams attenuate neurotoxic effects by increasing AChE activity in the digestive gland. Zhang and Greenberg, [70] and Oliveira et al. [63] also indicated that the cholinergic system activation offers the advantage of being a powerful indicator of neurotoxicity in clams exposed to a pharmaceutical product. The authors suggest that this increase may be a coping mechanism but not a defense against stress [71].
The mixture of ZnS NPs and P at similar concentrations showed an antagonist AChE response. This outcome would suggest that P possesses the ability to activate the cholinergic system in the presence of NPs.
4. Materials and Methods
4.1. ZnS NPs and Diethyl (3-cyano-1-hydroxy-2-methyl-1-phenyl propyl) phosphonate Synthesis
To synthesize the ZnS NPs, zinc acetate dihydrates [Zn (OAc) 2. 2 H2O) Aldrich, AR grade] (1.15 g) and thiourea (0.47 g) were dissolved in 25 mL of 1,3-propanediol, and the mixture was heated to 190 °C and kept at this temperature for 2 h under continuous magnetic stirring. At the end of the reaction, the precipitate was centrifuged, washed several times with ethanol, and then dried in a vacuum at 60 °C for 12 h to yield a white, dry ZnS powder. The crystalline structure of the obtained powder was characterized by X-ray diffraction (XRD) (an INEL diffractometer with a copper anticathode (= 1.54060 Å)). High-resolution TEM (Philips Tecnai F-20 SACTEM operating at 200 Kv) images provided further insight into the structural information of the ZnS NPs.
Diethyl (3-cyano-1-hydroxy-2-methyl-1-phenyl propyl)phosphonate (P) was synthesized in 77% yield, according to the procedure reported by Aouani et al. [22], which involves the reaction of 3-methyl-4-oxo-4-phenyl-butane nitrile with diethylphosphite at room temperature for 5 h in the presence of magnesium oxide as solid support (Scheme 1).
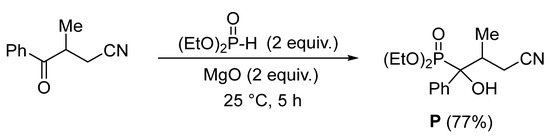
Scheme 1.
Synthesis of phosphonate P.
Experimental procedure: Diethylphosphite (0.02 mol) was added to the ketonitrile (0.01 mol), and the mixture was heated at 60 °C and stirred until the ketonitrile was dissolved. Then, magnesium oxide (0.02 mol) was added, and the mixture was stirred at 25 °C for 5 h. The magnesium oxide was filtered and washed with dichloromethane (3 × 10 mL). The filtrate was extracted with water (2 × 30 mL). The organic phase was dried over Na2SO4 and concentrated under a vacuum. The obtained residue was chromatographed on a silica gel column using a mixture of ether and hexane (8:2) as an eluent.
The physical and spectral data for compound P were: brown solid; mp 182-184 °C; 31P NMR (121.5 MHz, CDCl3): δ(ppm) = 21.6 (min), 22.0 (maj); maj/min: 61/39; 1H NMR (300.1 MHz, CDCl3): δ (ppm) = 0.94–1.46 (m, 6H, CH3-CH2-O); 2.16 (d, 3H, 3JHH = 9.0 Hz, CH3-CH, min); 2.20 (d, 3H, 3JHH = 9.0 Hz, CH3-CH, maj); 2.49–2.78 (m, 2H, CH-CH2-CN); 3.50–3.82 (m, 1H, CH-CH2-CN); 3.98–4.22 (m, 4H, CH3-CH2-O); 5.22 (br s, 1H, OH, min); 5.52 (br s, 1H, OH, maj); 7.12–7.62 (m, 5H, arom-H); 13C NMR (75.5 MHz, CDCl3): δ (ppm) = 13.7 (d, 3JCP = 8.2 Hz, CH-CH2-CN); 16.2 (d, 3JCP = 6.0 Hz, CH3-CH2-O); 16.4 (d, 3JCP = 6.0 Hz, CH3-CH2-O); 19.8 (s, CH3-CH, min); 20.2 (s, CH3-CH, maj); 37.7 (d, 2JCP = 7.0 Hz, CH-CH2-CN); 61.1 (d, 2JCP = 6.0 Hz, CH3-CH2-O); 61.4 (d, 2JCP = 6.0 Hz, CH3-CH2-O); 75,8 (d, 1JCP = 160.5 Hz, P-C-OH, maj); 79.1 (d, 1JCP = 161.8 Hz, P-C-OH, min); 118.5 (s, CN, maj); 119.5 (s, CN, min); phenyl carbons: δ (ppm) = 125.5, 125.7, 125.9, 127.4, 127.7, 127.9, 128.1, 128.2, 128.3, 128.4, 128.6, 128.9, 132.9, 133.7; IR (neat): νP=O = 1256 cm−1, νCN = 2246 cm−1, νOH = 3483 cm−1; EI-HRMS: calculated for C15H22NO4P: 311.1286 (M+), found: 311.1282.
4.2. In Vivo Exposure
Clams (R. decussatus) of between 4.22 and 3.25 cm in shell length were collected in the Bizerte lagoon, Northern Tunisia. Acclimation occurred in 2-L glass tanks for 1 week before starting the exposure. The following experimental treatments were established in triplicate tanks. Clams were divided into groups of 5 per tank and exposed at the same time to ZnS NPs, P, or a combination of the 2 compounds (M100) for 10 days. Two concentrations of 50 and 100 µg L−1 were considered for ZnS NPs and diethyl (3-cyano-1-hydroxy-1-phenyl-2-methyl propyl) phosphate or P. The exposure treatments were labeled as follows: ZnS50 and ZnS100 for dissolved 50 and 100 µg L−1 of ZnS, P50; and P100 for dissolved 50 and 100 µg L−1 of phosphonate and M100 for dissolved ZnS100 and P100 mixture.
A control series without ZnS NPs and P was run in parallel. Aeration at the bottom of the tank was used to minimize agglomeration and subsequent sedimentation of the contaminants, and the seawater was changed every 48 h. For the duration of the experiment (10 days), the clams were fed, with the seawater changed every 48 h. Test animals were checked daily. No mortality was observed, and all animals were seen to be feeding normally.
4.3. Metal Analysis
Heavy metals and electrolytes present in the clams were determined using an Optima 7300 DV optical emission spectrometer. After 10 days of exposure, the clams were dissected, and the whole tissue was weighed. The whole tissue was then placed in the oven at 105 °C for 30 min to be dried. The samples are mineralized separately by a mixture of HNO3 5% (3 mL), H2O2 (3 mL), and H2O (1 mL). The digestion was continued in a microwave for 20 min at 720 W. The samples were diluted with 50 mL of ultrapure water [72]. The solution was filtered to eliminate the organic particles that remained in the suspension and entrainer interferences during measurement. Finally, the samples were analyzed by ICP-AES using reference material of mussel tissue (NIST 2976) [73]. The detection limits for Cd, Zn, Fe, Cu, and Pb were in the range of 0.006–25 mg/L. The results are expressed in g kg-1 wet weight. Values reported were corrected for background levels determined in blank sterile filtered seawater.
4.4. Biochemical Analysis
After 10 days of exposure, the digestive glands and gills of each individual were dissected on ice. Protein extractions were performed and homogenized by a polytron homogenizer in 10 mMTris/HCl with a pH of 7.2, containing 500 mM sucrose, 1 mM EDTA and 1 mM PMSF; supernatants were collected by centrifugation at 9000× g (4 °C for 30 min). Protein concentrations were quantified according to Bradford’s [74] method based on a colorimetric reaction using bovine serum albumin (BSA). Hydrogen peroxide (H2O2) levels were measured following the method of Wolff [75]: a volume of 0.1 mL of the supernatant was added to 900 mL of FOX1 reagent (100 mxylenol orange, 100 mM sorbitol, 250 mM ammonium ferrous sulfate, and 25 mM H2SO4), vortexed, and incubated at room temperature for 30 min. The sample was then centrifuged at low speed for 3 min, and the absorbance of the supernatant was read at 560 nm. CAT activity was measured by the decrease in absorbance at 240 nm due to H2O2 consumption according to the method of Aebi [76]. The reaction volume and reaction time were 1 mL and 1 min, respectively. The reaction solution contained 80 mM phosphate buffer with a pH of 6.5 and 50 mM H2O2 [77]. Specific CAT activities are reported as µmol/min/mg protein.
AChE activity was determined using the method of Ellman et al. [78]. Measurements of AChE activity were initiated by the addition of acetylthiocholine, and then absorbance was read at 412 nm. Results were expressed in µmol/min/mg of the hydrolyzed substrate relative to the total protein content.
4.5. Data Analysis
Metal content, H2O2 levels, and enzymatic activities were compared among samples using Statistica software, version 8.0. After testing ANOVA assumptions, statistical significance was evaluated through one-way ANOVA. Tukey’s HSD test allowed pairwise comparisons between experimental conditions, and a significant difference was considered at p < 0.05.
5. Conclusions
The toxicity of phosphonates, specifically diethyl (3-cyano-1-hydroxy-2-methyl-1-phenyl propyl) phosphonate (P) and ZnS NPs, in marine clams is highlighted in the present study. The study revealed that phosphonate had both additive and antagonistic toxic effects when present with ZnS NPs. Consequently, exposure to these pollutants disrupted the metal content and oxidant/antioxidant balance in the clams’ gills and digestive glands, leading to an increase in CAT activity, H2O2 overproduction, and neurotoxic effects. The results suggest that phosphonate could be utilized as a new product to control NP toxicity in aquatic environments. To verify the findings of this initial study, additional tests examining the toxicity of ZnS NPs and P at various concentrations are necessary.
Author Contributions
Biomarker assessment and Writing—original draft, W.S. and I.B.; Validation and writing—original draft, A.K. and S.G.; Literature investigation, I.A. and A.F.; Review and editing, S.T.; Funding acquisition and review and editing, M.I.A.; Literature investigation and formal analysis, S.A.A. and A.A.Q.; Statistical analyses, H.B.; Review and editing, F.B.; Conceptualization, supervision, review, and editing, B.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors express their appreciation to the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University for funding this work through Research Group no. RG-21-09-91.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data in the article are available from the corresponding author upon reasonable request.
Acknowledgments
The authors express their appreciation to the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University for funding this work through Research Group no. RG-21-09-91.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Sample Availability
Samples of all the compounds are available from the authors.
References
- Moreno-González, R.; Rodríguez-Mozaz, S.; Huerta, B.; Barceló, D.; León, M.V. Do pharmaceuticals bioaccumulate in marine molluscs and fish from a coastal lagoon? Environ. Res. 2016, 146, 282–298. [Google Scholar] [CrossRef] [PubMed]
- Alygizakis, N.A.; Gago-ferrero, P.; Borova, V.L.; Pavlidou, A.; Hatzianestis, I.; Thomaidis, N.S. Occurrence and spatial distribution of pharmaceuticals, drugs of abuse and related metabolites in offshore seawater. Sci. Total. Environ. 2016, 541, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.; Araujo, A.N.; Fachini, A.; Pena, A.; Delerue-Matos, C.; Montenegro, M. Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. J. Hazard. Mater. 2010, 175, 45–95. [Google Scholar] [CrossRef]
- Melvin, S.D. 2015. Chemosphere Oxidative stress, energy storage, and swimming performance of Limnodyna stesperonii tadpoles exposed to a sub-lethal pharmaceutical mixture throughout development. Chemosphere 2015, 1, 8. [Google Scholar]
- Tauxe-Wuersch, A.; De Alencastro, L.F.; Grandjean, D.; Tarradellas, J. Occurrence of several acidic drugs in sewage treatment plants in Switzerland and risk assessment. Water Res. 2005, 39, 1761–1772. [Google Scholar] [CrossRef]
- Kishimoto, S.; Kato, A.; Naito, A.; Sakamoto, Y.; Iida, S. Attempts of homo p–n junction formation in ZnS by impurity co-doping with vapor phase epitaxy. Phys. Stat. Sol. 2002, 1, 391. [Google Scholar] [CrossRef]
- Bedia, J.; Muelas-Ramos, V.; Peñas-Garzón, M.; Gómez-Avilés, A.; Rodríguez, J.J.; Belver, C. A review on the synthesis and characterization of metal organic frameworks for photocatalytic water purification. Catalysts 2019, 9, 52. [Google Scholar] [CrossRef]
- Chatterjee, N.; Bhattacharjee, B. An analytic contemplation of the conspicuous vicissitudes in the histomorphology of corpuscles of Stannius of a freshwater catfish Mystustengara (Hamilton, 1822) due to the Exposure of ZnS Nanoparticles. Scientifica 2015, 2015, 697053. [Google Scholar] [CrossRef]
- Aye, M.; Di Giorgio, C.; Mekaouche, M.; Steinberg, J.G.; Brerro-Saby, C.; Barthélémy, P.; De Méo, M.; Jammes, Y. Genotoxicity of intraperitoneal injection of lipoamphiphile CdSe/ZnS quantum dots in rats. Mut. Res. Genet. Toxicol. Environ. Mutagen. 2013, 758, 48–55. [Google Scholar] [CrossRef]
- Sonar, S.S.; Kategaonkar, A.H.; Ware, M.N.; Gill, C.H.; Shingate, B.B.; Shingare, M.S. Ammonium metavanadate: An effective catalyst for synthesis of α-hydroxyphosphonates. Arkivoc 2009, 2, 138–148. [Google Scholar] [CrossRef]
- Patel, D.V.; Rielly-Gauvin, K.; Ryono, D.E. Preparation of peptidic α-hydroxy phosphonates a new class of transition state analog renin inhibitors. Tetrahedron Lett. 1990, 31, 55. [Google Scholar] [CrossRef]
- Rao, K.U.M.; Sundar, C.S.; Prasad, S.S.; Rani, C.R.; Reddy, C.S. Neat Synthesis and Anti-oxidant Activity of α-Hydroxyphosphonates. Bull. Korean Chem. Soc. 2011, 32, 33–43. [Google Scholar] [CrossRef]
- Dellali, M.; Gnassia Barelli, M.; Roméo, M.; Aϊssa, P. The use of acetylcholinesterase activity in Ruditapes decussatus and Mytilusgalloprovincialis in the biomonitoring of Bizerta lagoon. Comp. Biochem. Physiol. C 2001, 130, 227–235. [Google Scholar]
- Canesi, L.; Ciacci, C.; Fabbri, R.; Marcomini, A.; Pojana, G.; Gallo, G. 2012. Bivalve molluscs as a unique target group for nanoparticle toxicity. Mar. Environ. Res. 2012, 76, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.F.; Buffet, P.E.; Poirier, L.; Amiard-Triquet, C.; Gilliland, D.; Joubert, Y.; Pilet, P.; Guibbolini, M.; Risso de Faverney, C.; Romeo, M.; et al. Size dependent bioaccumulation and ecotoxicity of gold nanoparticles in an endobenthic invertebrate: The Tellinid clam Scrobicularia plana. Environ. Pollut. 2012, 168, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, J.; Li, F.; Gao, D.; Xing, B. Adsorption and inhibition of acetylcholinesterase by different nanoparticles. Chemosphere 2009, 77, 67–73. [Google Scholar] [CrossRef]
- Chen, Z.; Meng, H.; Xing, G.; Chen, C.; Zhao, Y.; Jia, G.; Wang, T.; Yuan, H.; Ye, C.; Zhao, F.; et al. Acute toxicological effects of copper nanoparticles in vivo. Toxicol. Lett. 2006, 163, 109–120. [Google Scholar] [CrossRef]
- Barmo, C.; Ciacci, C.; Canonico, B.; Fabbri, R.; Cortese, K.; Balbi, T.; Marcomini, A.; Pojana, G.; Gallo, G.; Canesi, L. In vivo effects of n-TiO2 on digestive gland and immune function of the marine bivalve Mytilus galloprovincialis. Aqua. Toxicol. 2013, 132–133, 9–18. [Google Scholar] [CrossRef]
- Trevisan, R.; Delapedra, G.; Mello, D.F.; Arl, M.; Schmidt, E.C.; Meder, F.; Monopoli, M.; Cargnin-Ferreira, E.; Bouzon, Z.L.; Fisher, A.S.; et al. Gills are an initial target of zinc oxide nanoparticles in oysters Crassostrea gigas, leading to mitochondrial disruption and oxidative stress. Aqua. Toxicol. 2014, 153, 27–38. [Google Scholar] [CrossRef]
- Buffet, P.E.; Amiard-Triquet, C.; Dybowska, A.; Risso-de Faverney, C.; Guibbolini, M.; Valsami-Jones, E.; Mouneyrac, C. Fate of isotopically labeled zinc oxide nanoparticles in sediment and effects on two endobenthic species, the clam Scrobicularia plana and the ragworm Hedistediversicolor. Ecotoxicol. Environ. Saf. 2012, 84, 191–198. [Google Scholar] [CrossRef]
- Vale, G.; Mehennaoui, K.; Cambier, S.; Libralato, G.; Jomini, S.; Domingos, R.F. Manufactured nanoparticles in the aquatic environment-biochemical responses on freshwater organisms: A critical overview. Aqua. Toxicol. 2016, 170, 162–174. [Google Scholar] [CrossRef]
- Aouani, I.; Lahbib, K.; Touil, S. Green Synthesis and Antioxidant Activity of Novel γ-cyano-α-hydroxyphosphonate Derivatives. Med. Chem. 2015, 11, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Gagné, F.; Turcotte, P.; Auclair, J.; Gagnon, C. The effects of zinc oxide nanoparticles on the metallome in freshwater mussels. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2013, 158, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Alsop, D.; Wood, C.M. Metal and pharmaceutical mixtures: Is ion loss the mechanism underlying acute toxicity and widespread additive toxicity in zebrafish? Aquat. Toxicol. 2013, 140–141, 257–267. [Google Scholar] [CrossRef]
- Fouqueray, M.; Dufils, B.; Vollat, B.; Chaurand, P.; Botta, C.; Abacci, K.; Labille, J.; Rose, J.; Garric, J. Effects of aged TiO2 nanomaterial from sunscreen on Daphnia magna exposed by dietary route. Environ. Pollut. 2012, 163, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Buffet, P.E.; Pan, J.F.; Poirier, L.; Amiard-Triquet, C.; Amiard, J.C.; Gaudin, P. Biochemical and behavioural responses of the endobenthic bivalve Scrobicularia plana to silver nanoparticles in seawater and microalgal food. Ecotoxicol. Environ. Saf. 2013, 89, 117–124. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Moller, L. Copper oxide nanoparticles are highly toxic: A comparison between metal oxide nanoparticles and carbon nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Asharani, P.V.; Mun, G.L.K.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009, 3, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.M.; Hess, K.L.; Gearhart, J.M.; Geiss, K.T.; Schlager, J.J. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol. Vitr. 2005, 19, 975–983. [Google Scholar] [CrossRef]
- Navarro, E.; Baun, A.; Behra, R.; Hartmann, N.B.; Filser, J.; Miao, A.; Quigg, A.; Santschi, P.H.; Sigg, L. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants and fungi. Ecotoxicology 2008, 17, 372–386. [Google Scholar] [CrossRef]
- Suresh, P.; Vijaya, J.J.; Kennedy, L.J. Synergy effect in the photocatalytic degradation of textile dyeing waste water by using microwave combustion synthesized zinc oxide supported activated carbon. React. Kinet. Mech. Catal. 2015, 114, 767–780. [Google Scholar] [CrossRef]
- Zhang, H.; Ji, Z.; Xia, T.; Meng, H.; Low-Kam, C.; Liu, R.; Pokhrel, S.; Lin, S.; Wang, X.; Liao, Y.P. Use of metal oxide nanoparticle band gap to develop a predictive paradigm for oxidative stress and acute pulmonary inflammation. ACS Nano 2012, 6, 4349–4368. [Google Scholar] [CrossRef] [PubMed]
- Liendro, N.; Ferrari, A.; Mardirosian, M.; Lascano, C.I.; Venturino, A. Toxicity of the insecticide chlorpyrifos to the South American toad Rhinella arenarum at larval developmental stage. Environ. Toxicol. Pharmacol. 2015, 39, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Bebianno, M.J.; Geret, F.; Hoarau, P.; Serafim, M.A.; Coelho, M.R.; Gnassia-Barelli, M.; Romeo, M. Biomarkers in Ruditapes decussatus: A potential bioindicator species. Biomarkers 2004, 9, 305–330. [Google Scholar] [CrossRef]
- Maranho, L.A.; André, C.; Del Valls, T.A.; Gagné, F.; Martín-Díaz, M.L. In situ evaluation of wastewater discharges and the bioavailability of contaminants to marine biota. Sci. Tot. Environ. 2015, 538, 876–887. [Google Scholar] [CrossRef] [PubMed]
- González, P.M.; Puntarulo, S. Fe, oxidative and nitrosative metabolism in the Antarctic limpet Nacellaconcinna. Comp. Biochem. Physio. Part A 2016, 200, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Cruz, D.; Almeida, A.; Calisto, A.; Esteves, V.I.; Schneider, R.J.; Wrona, F.J.; Soares, A.M.V.M.; Figueira, A.; Freitas, R. Caffeine impacts in the clam Ruditapes philippinarum: Alterations on energy reserves, metabolic activity and oxidative stress biomarkers. Chemosphere 2016, 160, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Naidu, K.R.; Kumar, K.S.; Arulselvan, P.; Reddy, C.B.; Lasekan, O. Synthesis of α Hydroxyphosphonates and Their Antioxidant Properties. Arch. Pharm. Chem. Life Sci. 2012, 345, 957–963. [Google Scholar] [CrossRef]
- Fenton, H.J.H. Oxidation of tartaric acid in presence of iron. J. Chem. Soc. Trans. 1894, 65, 899. [Google Scholar] [CrossRef]
- Mohanty, D.; Samanta, L. Multivariate analysis of potential biomarkers of oxidative stress in Notopterusnotopterus tissues from Mahanadi River as a function of concentration of heavy metals. Chemosphere 2016, 155, 28–38. [Google Scholar] [CrossRef]
- Hermes-Lima, M. Oxygen in biology and biochemistry: Role of free radicals. In Functional Metabolism: Regulation and Adaptation; Storey, K.B., Ed.; Wiley-Liss: Hoboken, NJ, USA, 2005; pp. 319–368. [Google Scholar]
- Canesi, L.; Ciacci, C.; Vallotto, D.; Gallo, G.; Marcomini, A.; Pojana, G. In vitro effects of suspensions of selected nanoparticles (C60 fullerene, TiO2, SiO2) on Mytilus hemocytes. Aquat. Toxicol. 2010, 96, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Ali, D.; Alarifi, S.; Kumar, S.; Ahamed, M.; Siddiqui, M.A. Oxidative stress and genotoxic effect of zinc oxide nanoparticles in freshwater snail Lymnaealuteola L. Aqua. Toxicol. 2012, 124–125, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.; Pereira, C.G.; Cardoso, C.; Sousa, V.S.; Teixeira, M.R.; Pinheiro, J.P.; Bebianno, M.J. Effects of silver nanoparticles exposure in the mussel Mytilus galloprovincialis. Mar. Environ. Res. 2014, 101, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Atli, G.; Alptekin, O.; Tukel, S.; Canli, M. Response of catalase activity to Ag2þ, Cd2þ, Cr2þ, Cu2þ and Zn2þ in five tissues of freshwater fish Oreochromis niloticus. Comp. Biochem. Physiol. C 2006, 143, 218–224. [Google Scholar]
- Martin-Diaz, L.; Franzellitti, S.; Buratti, S.; Valbonesi, P.; Capuzzo, A.; Fabbri, E. Effects of environmental concentrations of the antiepilectic drug carbamazepine on biomarkers and cAMP-mediated cell signaling in the mussel Mytilus galloprovincialis. Aquat. Toxicol. 2009, 94, 177–185. [Google Scholar] [CrossRef]
- Parolini, M.; Magni, S.; Castiglioni, S.; Binelli, A. Amphetamine exposure imbalanced antioxidant activity in the bivalve Dreissena polymorpha causing oxidative and genetic damage. Chemosphere 2016, 144, 207–213. [Google Scholar] [CrossRef]
- Kamel, N.; Jebali, J.; Banni, M.; Ben Khedher, S.; Chouba, L.; Boussetta, H. Biochemical responses and metals levels in Ruditapes decussatus after exposure to treated municipal effluents. Ecotoxicol. Environ. Saf. 2012, 82, 40–46. [Google Scholar] [CrossRef]
- Matozzo, V.; Tomei, A.; Marin, M.G. Acetylcholinesterase as a biomarker of exposure to neurotoxic compounds in the clam Tapes philippinarum from the Lagoon of Venice. Mar. Pollut. Bull. 2005, 50, 1686–1693. [Google Scholar] [CrossRef]
- Hamza-Chaffai, A.; Roméo, M.; Gnassia-Barelli, M.; El Abed, A. Effect of copper and lindane on some biomarkers measured in the clam Ruditapes decussates. Bull. Environ. Conta. Toxicol. 1998, 61, 397–404. [Google Scholar] [CrossRef]
- Gaitonde, D.; Sarkar, A.; Kaisary, S.; Silva, C.D.; Dias, C.; Rao, D.P.; Ray, D.; Nagarajan, R.; Sousa, S.N.D.; Sarker, S.; et al. Acetylcholinesterase activities in marine snail (Cronia contracta) as a biomarker of neurotoxic contaminants along the Goa coast, west coast of India. Ecotoxicology 2006, 15, 353–358. [Google Scholar] [CrossRef]
- Radic, Z.; Kirchhoff, P.D.; Quinn, D.M.; McCammon, J.A.; Taylor, P. Electrostatic influence on the kinetics of ligand binding to acetylcholinesterase: Distinctions between active center ligands and fasciculin. J. Bio. Chem. 1997, 272, 23265–23277. [Google Scholar] [CrossRef] [PubMed]
- Schallreuter, K.U.; Elwary, S.M.; Gibbons, N.C. Activation/deactivation of acetylcholinesterase by H2O2: More evidence for oxidative stress in vitiligo. Biochem. Biophys. Res. Commun. 2004, 315, 502–508. [Google Scholar] [CrossRef]
- Antonio, M.T.; Corredor, L.; Leret, M.L. Study of the activity of several brain enzymes like markers of the neurotoxicity induced by perinatal exposure to lead and/orcadmium. Toxicol. Lett. 2003, 143, 331–340. [Google Scholar] [CrossRef] [PubMed]
- US Environmental Protection Agency. SCE Policy Issues Related to the Food Quality Protection Act. Office of Pesticide Programs Science Policy on the Use of Cholinesterase Inhibition for Risk Assessment of Organophosphate and Carbamate Pesticides; OOP Docket # 00560; Federal register; US Environmental Protection Agency: Washington, DC, USA, 1998; Volume 63, p. 214.
- Sellami, B.; Aouani, I.; Maalaoui, A.; Dellali, M.; Aïssa, P.; Touil, S.; Sheehan, D.; Mahmoudi, E.; Hamouda, B. Ecotoxicology and Environmental Safety 2- oxathiaphosphorine-2-sul fi de on biomarkers of Mediterranean clams Ruditapesdecussatus. Ecotox. Environ. Saf. 2015, 120, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Mattson, N.S.; Egidius, E.; Solbakken, J.E. Uptake and elimination of (methyl-14C) trichlorfon in blue mussel (Mytilus edulis) and European oyster (Ostrea edulis)—Impact of Neguvon disposal on mollusc farming. Aquaculture 1988, 71, 9–14. [Google Scholar] [CrossRef]
- Brewer, S.K.; Little, E.E.; DeLonay, A.J.; Beauvais, S.L.; Jones, S.B.; Ellersieck, M.R. Behavioral dysfunctions correlate to altered physiology in Rainbow Trout (Oncorynchus mykiss) exposed to cholinesterase-inhibiting chemicals. Arch. Environ. Con. Tox. 2001, 40, 70–76. [Google Scholar]
- Sandahl, J.F.; Baldwin, D.H.; Jenkins, J.J.; Scholz, N.L. Comparative thresholds for acetylcholinesterase inhibition and behavioral impairment in coho salmon exposed to chlorpyrifos. Environ. Toxicol. Chem. 2005, 24, 136–145. [Google Scholar] [CrossRef]
- Sturve, J.; Scarlet, P.; Halling, M.; Kreuger, J.; Macia, A. Environmental monitoring of pesticide exposure and effects on mangrove aquatic organisms of Mozambique. Mar. Environ. Res. 2016, 121, 9–19. [Google Scholar] [CrossRef]
- Nunes, B.; Carvalho, F.; Guilhermino, L. Effects of widely used pharmaceuticals and a detergent on oxidative stress biomarkers of the crustacean Artemia parthenogenetica. Chemosphere 2000, 62, 581–594. [Google Scholar] [CrossRef]
- Milan, M.; Pauletto, M.; Patarnello, T.; Bargelloni, L.; Marin, M.G.; Matozzo, V. Gene transcription and biomarker responses in the clam Ruditapes philippinarum after exposure to ibuprofen. Aquat. Toxicol. 2013, 126, 17–29. [Google Scholar] [CrossRef]
- Oliveira, H.H.P.; Liebel, S.; Rossi, S.C.; Azevedo, A.C.B.; Barrera, E.A.L.; Garcia, J.R.E.; Grötzner, S.R.; Neto, F.F.; Randi, M.A.F.; Ribeiro, C.A.O. Mixtures of benzo(a)pyrene, dichlorodiphenyltrichloroethane and tributyltin are more toxic to Neotropical fish Rhamdiaquelen than isolated exposures. Ecotoxicol. Environ. Saf. 2015, 122, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M.; Dobes, P. Caffeine inhibits acetylcholinesterase, but not butyrylcholinesterase. Int. J. Mol. Sci. 2013, 14, 9873–9882. [Google Scholar] [CrossRef] [PubMed]
- Pokalwar, R.U.; Hangarge, R.V.; Maske, P.V.; Shingare, M.S. Synthesis and antibacterial activities of α -hydroxyphosphonates and α -acetyloxyphosphonates derived from 2-chloroquinoline-3-carbaldehyde. Arkivoc 2006, 6, 196–204. [Google Scholar] [CrossRef]
- Reddy, G.S.; Syamasundar, C.; Prasad, S.S.; Dadapeer, E.; Raju, C.N.; Reddy, C.S. Synthesis, Spectral characterization and antimicrobial activity of α hydroxyphosphonates. Der. Pharma. Chemica. 2012, 4, 2208–2213. [Google Scholar]
- Kaufer, D.; Friedman, A.; Seidman, S. Acute stress facilitates long-lasting changes in cholinergic gene expression. Nature 1998, 393, 373–377. [Google Scholar] [CrossRef]
- Meshorer, E.; Soreq, H. Virtues and woes of AChE alternative splicing in stress related neuropathologies. Trends Neurosci. 2006, 29, 216–224. [Google Scholar] [CrossRef]
- Aguirre-Martinez, G.V.; DelValls, T.A.; Martín-Díaz, M.L. General stress, detoxification pathways, neurotoxicity and genotoxicity evaluated in Ruditapes philippinarum exposed to human pharmaceuticals. Ecotoxicol. Environ. Saf. 2016, 124, 18–31. [Google Scholar] [CrossRef]
- Zhang, X.J.; Greenberg, D.S. Acetylcholinesterase involvement in apoptosis. Front. Mol. Neurosci. 2012, 5, 40. [Google Scholar] [CrossRef]
- Williamson, S.M.; Moffat, C.; Gomersall, M.A.E.; Saranzewa, N.; Connolly, C.N.; Wright, G.A. Exposure to acetylcholinesterase inhibitors alters the physiology and motor function of honeybees. Front. Physiol. 2013, 4, 13. [Google Scholar] [CrossRef]
- Jobansson, C.G. Digestion methods for the determination of the total content of heavy metals, in: Manual of methods in aquatic environment research. Part 1: Methods for detection, measurement and monitoring of water pollution. FAO Fish. Tech. Pap. 1975, 137, 200. [Google Scholar]
- Lavilla, I.; Costas, M.; Gil, S.; Corderí, S.; Sánchez, G.; Bendicho, C. Simplified and miniaturized procedure based on ultrasound-assisted cytosol preparation for the determination of Cd and Cu bound to metallothioneins in mussel tissue by ICP-MS. Talanta 2012, 93, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram of protein utilizing the principal of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Wolff, S.P. Ferrous ion oxidation in presence of ferric iron indicator xylenol orange for measurement of hydroperoxide. Methods Enzymol. 1994, 233, 182–189. [Google Scholar]
- Aebi, H. Catalase. In Methods of Enzymatic Analysis; Bergmayer, H.U., Ed.; Academic Press: London, UK, 1974; pp. 671–684. [Google Scholar]
- Ni, W.; Trelease, R.N.; Eising, R. Two temporally synthesized charge subunits interact to form the five isoformes of cottonseed (Gossypium hirsutum) catalase. Biochemistry 1990, 269, 233–238. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).