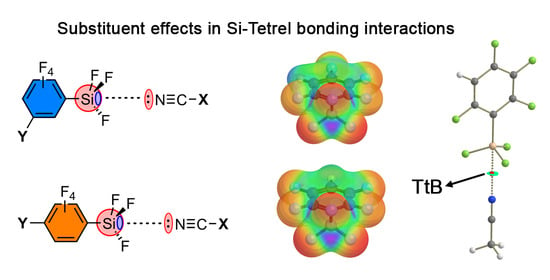
Substituent Effects in Tetrel Bonds Involving Aromatic Silane Derivatives: An ab initio Study
Abstract
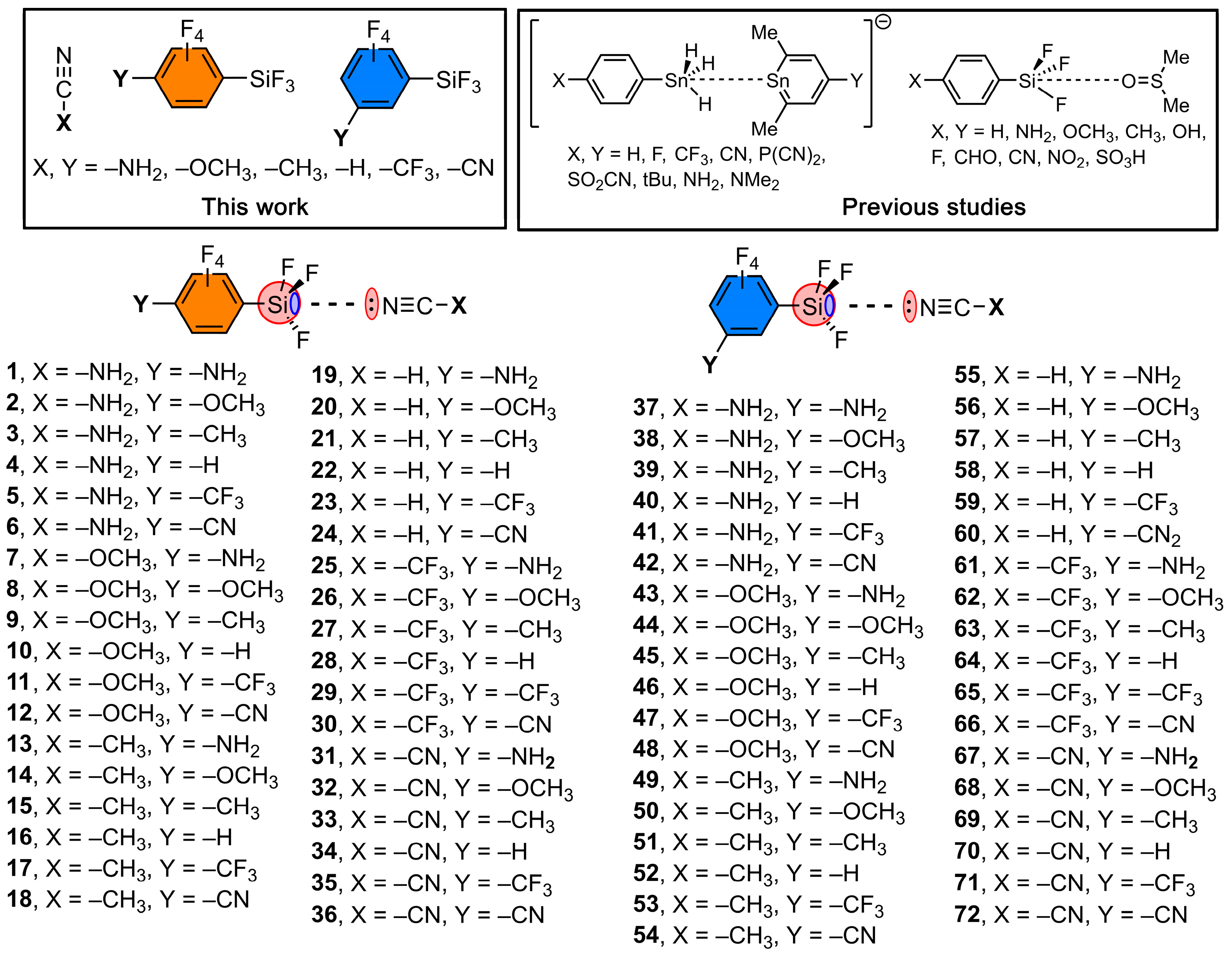
1. Introduction
2. Results and Discussion
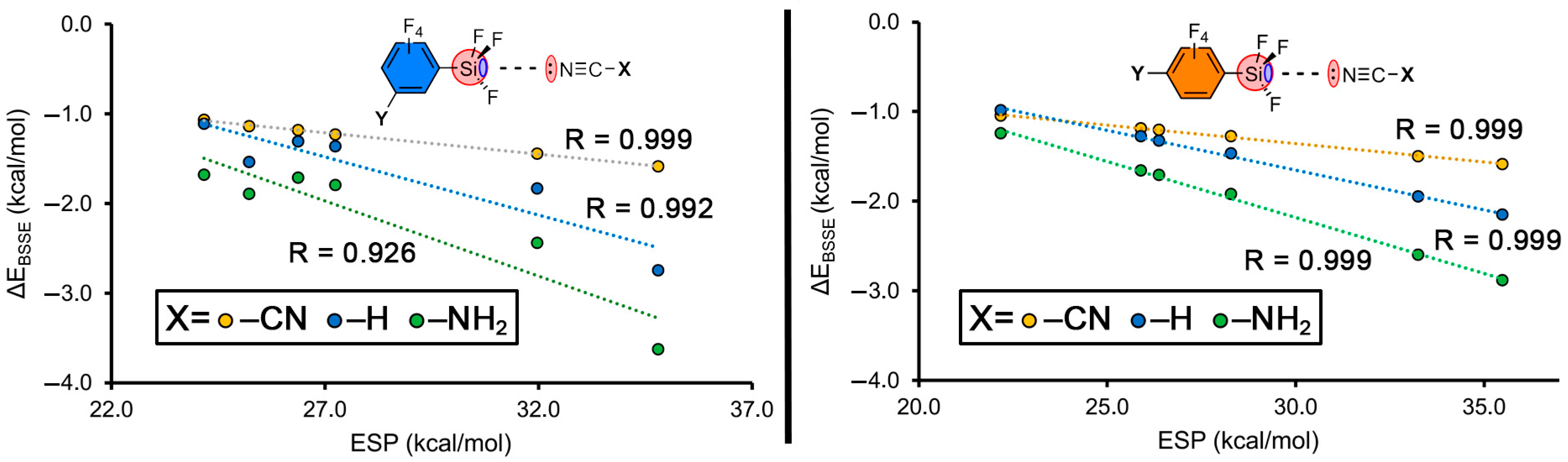
2.1. ESP Analysis
2.2. Energetic Study
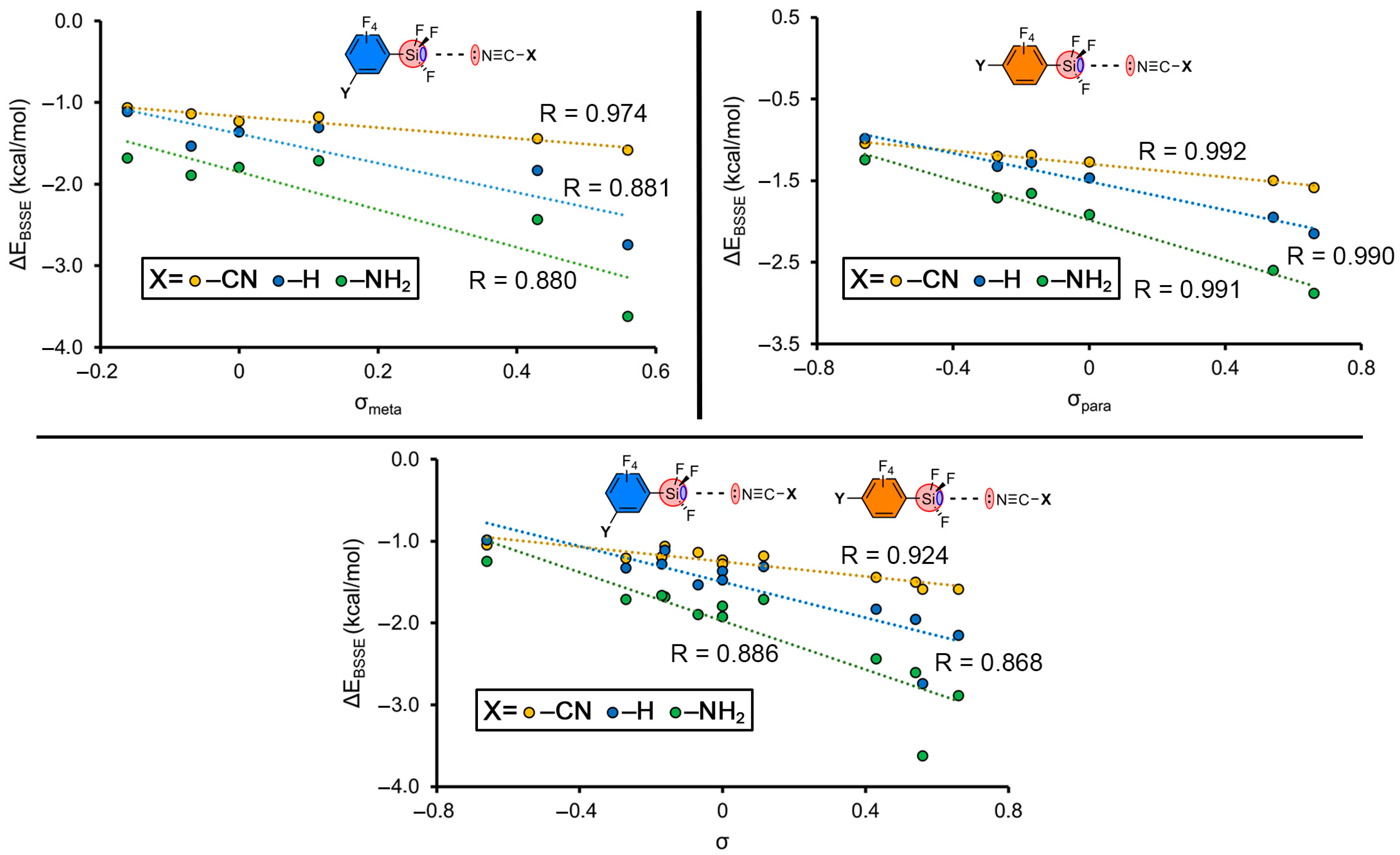
2.3. Hammett’s Representations
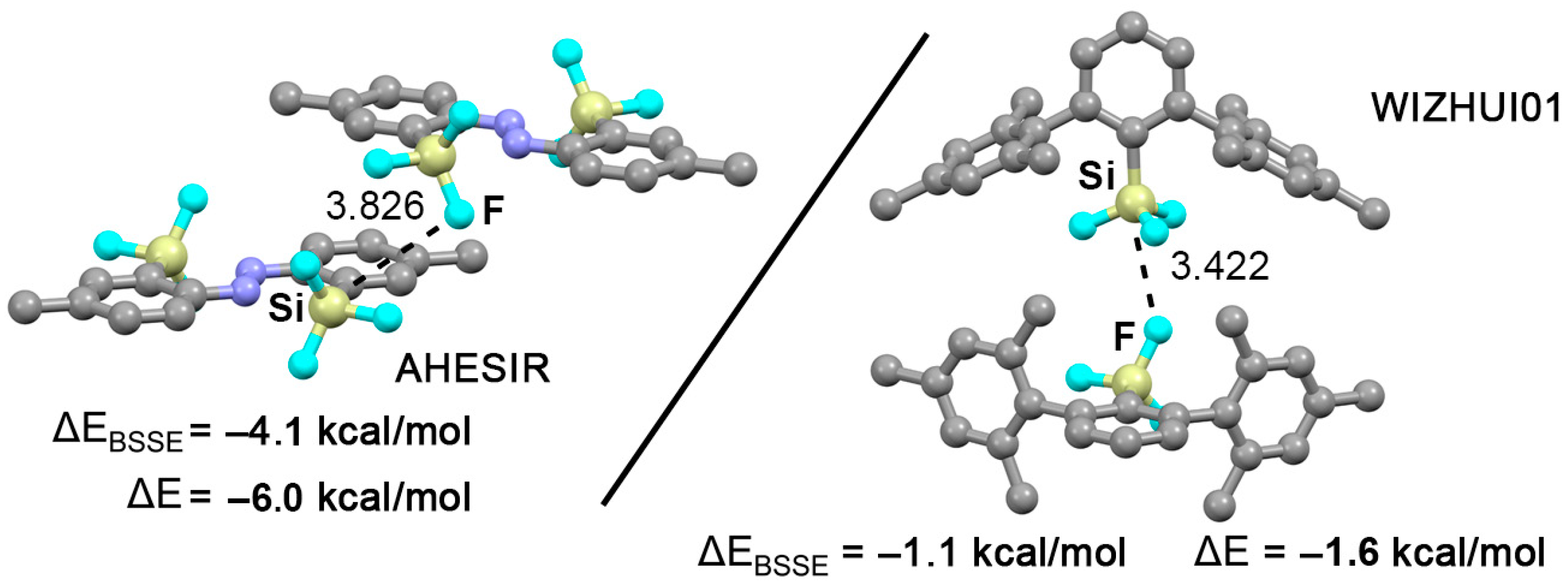
2.4. CSD Search
3. Conclusions
4. Materials and Methods
4.1. General Considerations
4.2. CSD Survey
- dSi···A ≤ sum of vdW radii + 0.5 Å.
- C/X–Si···A between 160 and 180 degrees (X = C, F, Cl, Br and I and A = any atom).
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lehn, J.M. Supramolecular Chemistry: Concepts and Perspectives, 1st ed.; Wiley VCH: Weinheim, Germany, 1995. [Google Scholar]
- Schneider, H.J. Supramolecular Systems in Biomedical Fields, 1st ed.; RSC Publishing: Cambridge, UK, 2013. [Google Scholar]
- Steed, A.W.; Atwood, J.L. Supramolecular Chemistry, 1st ed.; John Wily & Sons, Ltd.: Chichester, UK, 2009. [Google Scholar]
- Cragg, P.J. Supramolecular Chemistry: From Biological Inspiration to Biomedical Applications, 1st ed.; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar]
- Politzer, P.; Murray, J. σ-Hole Interactions: Perspectives and Misconceptions. Crystals 2017, 7, 212. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Ho, P.S.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the halogen bond (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- Aakeroy, C.B.; Bryce, D.L.; Desiraju, G.R.; Frontera, A.; Legon, A.C.; Nicotra, F.; Rissanen, K.; Scheiner, S.; Terraneo, G.; Metrangolo, P.; et al. Definition of the chalcogen bond (IUPAC Recommendations 2019). Pure Appl. Chem. 2019, 91, 1889–1892. [Google Scholar] [CrossRef]
- Zahn, S.; Frank, R.; Hey-Hawkins, E.; Kirchner, B. Pnicogen bonds: A new molecular linker? Chem. Eur. J. 2011, 17, 6034–6038. [Google Scholar] [CrossRef]
- Bauzá, A.; Mooibroek, T.J.; Frontera, A. Tetrel Bonding Interaction: Rediscovered Supramolecular Force? Angew. Chem. Int. Ed. 2013, 52, 12317–12321. [Google Scholar] [CrossRef] [PubMed]
- Bauzá, A.; Frontera, A. σ/π-Hole noble gas bonding interactions: Insights from theory and experiment. Coord. Chem. Rev. 2020, 404, 213112. [Google Scholar] [CrossRef]
- Bauzá, A.; Frontera, A.; Mooibroek, T.J. NO3− anions can act as Lewis acid in the solid state. Nat. Commun. 2017, 8, 14522. [Google Scholar] [CrossRef] [PubMed]
- García-Llinás, X.; Bauzá, A.; Seth, S.K.; Frontera, A. Importance of R–CF3···O Tetrel Bonding Interactions in Biological Systems. J. Phys. Chem. A 2017, 28, 5371–5376. [Google Scholar] [CrossRef] [PubMed]
- Bauzá, A.; Sharko, A.V.; Senchyk, G.A.; Rusanov, E.B.; Frontera, A.; Domasevitch, K.V. π–hole interactions at work: Crystal engineering with nitro-derivatives. Cryst. Eng. Comm. 2017, 19, 1933–1937. [Google Scholar] [CrossRef]
- Bauzá, A.; Frontera, A.; Mooibroek, T.J. π-hole interactions involving nitro compounds: Directionality of nitrate esters. Cryst. Growth Des. 2016, 16, 5520–5524. [Google Scholar] [CrossRef]
- Hazari, A.; Das, L.K.; Bauzá, A.; Frontera, A.; Ghosh, A. The influence of H-bonding on the ‘ambidentate’coordination behaviour of the thiocyanate ion to Cd (II): A combined experimental and theoretical study. Dalton Trans. 2014, 43, 8007–8015. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding and other σ-hole interactions: A perspective. Phys. Chem. Chem. Phys. 2013, 15, 11178–11189. [Google Scholar] [CrossRef] [PubMed]
- Bauza, A.; Mooibroek, T.J.; Frontera, A. The Bright Future of Unconventional σ/π-Hole Interactions. Chem. Phys. Chem. 2015, 16, 2496–2517. [Google Scholar] [CrossRef]
- Riwar, L.J.; Trapp, N.; Root, K.; Zenobi, R.; Diederich, F. Supramolecular Capsules: Strong versus Weak Chalcogen Bonding. Angew. Chem. Int. Ed. 2018, 57, 17259–17264. [Google Scholar] [CrossRef]
- Wonner, P.; Dreger, A.; Vogel, L.; Engelage, E.; Huber, S.M. Chalcogen Bonding Catalysis of a Nitro-Michael Reaction. Angew. Chem. Int. Ed. 2019, 58, 16923–16927. [Google Scholar] [CrossRef] [PubMed]
- Borissov, A.; Marques, I.; Lim, J.Y.C.; Félix, V.; Smith, M.D.; Beer, P.D. Anion Recognition in Water by Charge-Neutral Halogen and Chalcogen Bonding Foldamer Receptors. J. Am. Chem. Soc. 2019, 141, 4119–4129. [Google Scholar] [CrossRef] [PubMed]
- Mallada, B.; Gallardo, A.; Lamanec, M.; de la Torre, B.; Špirko, V.; Hobza, P.; Jelinek, P. Real-space imaging of anisotropic charge of σ-hole by means of Kelvin probe force microscopy. Science 2021, 374, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, J.; Ling, K.B.; Cockroft, S.L. The Origin of Chalcogen-Bonding Interactions. J. Am. Chem. Soc. 2017, 139, 15160–15167. [Google Scholar] [CrossRef]
- Macchione, M.; Goujon, A.; Strakova, K.; Humeniuk, H.V.; Licari, G.; Tajkhorshid, E.; Sakai, N.; Matile, S. A Chalcogen-Bonding Cascade Switch for Planarizable Push-Pull Probes. Angew. Chem. Int. Ed. 2019, 58, 15752–15756. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. An overview of strengths and directionalities of noncovalent interactions: σ-holes and π-holes. Crystals 2019, 9, 165. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Clark, T.; Resnati, G. The σ-hole revisited. Phys. Chem. Chem. Phys. 2017, 19, 32166–32178. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Pilati, T.; Resnati, G.; Terraneo, G. Naming interactions from the electrophilic site. Cryst. Growth Des. 2014, 14, 2697–2702. [Google Scholar] [CrossRef]
- Terraneo, G.; Resnati, G. Bonding Matters. Cryst. Growth Des. 2017, 17, 1439–1440. [Google Scholar] [CrossRef]
- Scheiner, S. Steric crowding in tetrel bonds. J. Phys. Chem. A 2018, 122, 2550–2562. [Google Scholar] [CrossRef] [PubMed]
- Bauzá, A.; Mooibroek, T.J.; Frontera, A. Tetrel Bonding Interactions. Chem. Rec. 2016, 16, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Mani, D.; Arunan, E. The X–C⋯Y (X = O/F, Y = O/S/F/Cl/Br/N/P) ‘carbon bond’ and hydrophobic interactions. Phys. Chem. Chem. Phys. 2013, 15, 14377–14383. [Google Scholar] [CrossRef]
- Sethio, D.; Oliveira, V.; Kraka, E. Quantitative Assessment of Tetrel Bonding Utilizing Vibrational Spectroscopy. Molecules 2018, 23, 2763. [Google Scholar] [CrossRef]
- Heywood, V.L.; Alford, T.P.J.; Roeleveld, J.J.; Deprez, S.J.L.; Verhoofstad, A.; van der Vlugt, J.I.; Domingos, S.R.; Schnell, M.; Davis, A.P.; Mooibroek, T.J. Observations of tetrel bonding between sp3-carbon and THF. Chem. Sci. 2020, 11, 5289–5293. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.; Wang, Y.-B. Tetrel bonding on Graphene. Comp. Theor. Chem. 2019, 1147, 8–12. [Google Scholar] [CrossRef]
- Taylor, M.S. Anion recognition based on halogen, chalcogen, pnictogen and tetrel bonding. Coord. Chem. Rev. 2020, 413, 213720. [Google Scholar] [CrossRef]
- Bauzá, A.; Seth, S.K.; Frontera, A. Tetrel bonding interactions at work: Impact on tin and lead coordination compounds. Coord. Chem. Rev. 2019, 384, 107–125. [Google Scholar] [CrossRef]
- Mundlapati, V.R.; Sahoo, D.K.; Bhaumik, S.; Jena, S.; Chandrakar, A.; Biswal, H.S. Noncovalent Carbon-Bonding Interactions in Proteins. Angew. Chem. Int. Ed. 2018, 57, 16496. [Google Scholar] [CrossRef]
- Dutta, J.; Sahoo, D.K.; Jena, S.; Tulsiyan, K.D.; Biswal, H.S. Non-covalent interactions with inverted carbon: A carbo-hydrogen bond or a new type of hydrogen bond? Phys. Chem. Chem. Phys. 2020, 22, 8988–8997. [Google Scholar] [CrossRef]
- Murray, J.S.; Lane, P.; Clark, T.; Riley, K.E.; Politzer, P. σ-Holes, π-holes and electrostatically-driven interactions. J. Mol. Mod. 2012, 18, 541–548. [Google Scholar] [CrossRef]
- Burgi, H.B.; Dunitz, J.D.; Shefter, E. Geometrical reaction coordinates. II. Nucleophilic addition to a carbonyl group. J. Am. Chem. Soc. 1973, 95, 5065–5067. [Google Scholar]
- Jönsson, B.; Karlström, G.; Wennerström, H. Ab initio molecular orbital calculations on the water-carbon dioxide system: Molecular complexes. Chem. Phys. Lett. 1975, 30, 58–59. [Google Scholar] [CrossRef]
- Peterson, K.I.; Klemperer, W. Structure and internal rotation of H2O−CO2, HDO−CO2 and D2O−CO2 van der Waals complexes. J. Chem. Phys. 1984, 80, 2439–2445. [Google Scholar] [CrossRef]
- Peng, Y.P.; Sharpe, S.W.; Shin, S.K.; Wittig, C.; Beaudet, R.A. Infrared spectroscopy of CO2–D(H)Br: Molecular structure and its reliability. J. Chem. Phys. 1992, 97, 5392. [Google Scholar]
- Leopold, K.R.; Fraser, G.T.; Klemperer, W. Rotational spectrum and structure of the complex HCNCO2. J. Chem. Phys. 1984, 80, 1039. [Google Scholar] [CrossRef]
- Alkorta, I.; Rozas, I.; Elguero, J. Molecular Complexes between Silicon Derivatives and Electron-Rich Groups. J. Phys. Chem. A 2001, 105, 743–749. [Google Scholar] [CrossRef]
- Alkorta, I.; Elguero, J.; Fruchier, A.; Macquarrie, D.J.; Virgili, A. Aminopropylsilanes versus silatranes: An experimental and theoretical study. J. Organomet. Chem. 2001, 625, 148–153. [Google Scholar] [CrossRef]
- Liu, M.; Li, Q.; Scheiner, S. Comparison of tetrel bonds in neutral and protonated complexes of pyridineTF3 and furanTF3 (T= C, Si, and Ge) with NH3. Phys. Chem. Chem. Phys. 2017, 19, 5550–5559. [Google Scholar] [CrossRef]
- Murray, J.S.; Lane, P.; Politzer, P. Expansion of the sigma-hole concept. J. Mol. Model. 2009, 15, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.S.; Concha, M.C.; Politzer, P. Molecular surface electrostatic potentials as guides to Si-O-N angle contraction: Tunable σ-holes. J. Mol. Model. 2011, 7, 2151–2157. [Google Scholar] [CrossRef] [PubMed]
- Patia, S.; Rappoport, Z. (Eds.) The Chemistry of Functional Groups. In The Chemistry of Organic Germanium, Tin and Lead Compounds; Wiley: Hoboken, NJ, USA, 1995; Volume 19. [Google Scholar]
- Parr, J. Comprehensive Coordination Chem II; McCleverty, J.A., Meye, T., Jr., Eds.; Elsevier Pergamon: Oxford, UK, 2004; Volume 3, p. 545. [Google Scholar]
- Sato, T. Comprehensive Organometallic Chem II—8—Tin; Abel, E.W., Stone, F.G.A., Wilkinson, G., Eds.; Pergamon Press: Oxford, UK, 1995; Volume 11, p. 355. [Google Scholar]
- Pinhey, J.T. 11—Lead. In Comprehensive Organometallic Chem II; Abel, E.W., Stone, F.G.A., Wilkinson, G., Eds.; Pergamon Press: Oxford, UK, 1995; Volume 11, p. 461. [Google Scholar]
- Greenberg, A.; Wu, G. Structural relationships in silatrane molecules. Struct. Chem. 1990, 1, 79–85. [Google Scholar] [CrossRef]
- Hencsei, P. Evaluation of silatrane structures by correlation relationships. Struct. Chem. 1991, 2, 21–26. [Google Scholar] [CrossRef]
- Voronkov, M.G.; Barishok, V.P.; Petukhov, L.P.; Rahklin, R.G.; Pestunovich, V.A. 1-Halosilatranes. J. Organomet. Chem. 1988, 358, 39–55. [Google Scholar] [CrossRef]
- Lukevics, E.; Dimens, V.; Pokrovska, N.; Zicmane, I.; Popelis, J.; Kemme, A. Addition of nitrile oxides to 2,3-dihydrofurylsilanes. Crystal and molecular structure of tetrahydrofuro-[2,3-d]-isoxazolylsilanes. J. Organomet. Chem. 1999, 586, 200–207. [Google Scholar] [CrossRef]
- Franconetti, A.; Frontera, A. “Like–like” tetrel bonding interactions between Sn centres: A combined ab initio and CSD study. Dalton Trans. 2019, 48, 11208–11216. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Yang, X.; Li, Q. Tetrel Bonds between Phenyltrifluorosilane and Dimethyl Sulfoxide: Influence of Basis Sets, Substitution and Competition. Molecules 2021, 26, 7231. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Bauzá, A.; Quiñonero, D.; Frontera, A.; Deyà, P.M. Substituent effects in halogen bonding complexes between aromatic donors and acceptors: A comprehensive ab initio study. Phys. Chem. Chem. Phys. 2011, 13, 20371–20379. [Google Scholar] [CrossRef]
- Kano, N.; Yamamura, M.; Kawashima, T. 2,2′-Disilylazobenzenes featuring double intramolecular nitrogen⋯silicon coordination: A photoisomerizable fluorophore. Dalton Trans. 2015, 44, 16256–16265. [Google Scholar] [CrossRef] [PubMed]
- Schröder, A.; Lork, E.; Beckmann, J. A monoclinic polymorph of 2,6-Mes2 C6 H3 SiF3. Main Group Met. Chem. 2014, 37, 153. [Google Scholar] [CrossRef]
- Weigend, F.; Häser, M. RI-MP2: First derivatives and global consistency. Theor. Chem. Acc. 1997, 97, 331–340. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Bauzá, A.; Alkorta, I.; Frontera, A.; Elguero, J. On the Reliability of Pure and Hybrid DFT Methods for the Evaluation of Halogen, Chalcogen, and Pnicogen Bonds Involving Anionic and Neutral Electron Donors. J. Chem. Theory Comput. 2013, 9, 5201–5210. [Google Scholar] [CrossRef]
- Ahlrichs, R.; Bar, M.; Haser, M.; Horn, H.; Kolmel, C. Electronic Structure Calculations on Workstation Computers—The Program System turbomole. Chem. Phys. Lett. 1989, 162, 165–169. [Google Scholar] [CrossRef]
- Boys, S.B.; Bernardy, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; Gaussian, Inc.: Wallingford, UK, 2016. [Google Scholar]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Todd, A.; Keith, T.K. AIMAll, version 13.05.06; Gristmill Software: Overland Park, KS, USA, 2013. [Google Scholar]
- Contreras-García, J.; Johnson, E.R.; Keinan, S.; Chaudret, R.; Piquemal, J.-P.; Beratan, D.N.; Yang, W. NCIPLOT: A Program for Plotting Noncovalent Interaction Regions J. Chem. Theory Comput. 2011, 7, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Bruno, I.J.; Cole, J.C.; Edgington, P.R.; Kessler, M.; Macrae, C.F.; McCabe, P.; Pearson, J.; Taylor, R. New software for searching the Cambridge Structural Database and visualising crystal structures. Acta Cryst. 2002, 58, 389–397. [Google Scholar] [CrossRef] [PubMed]
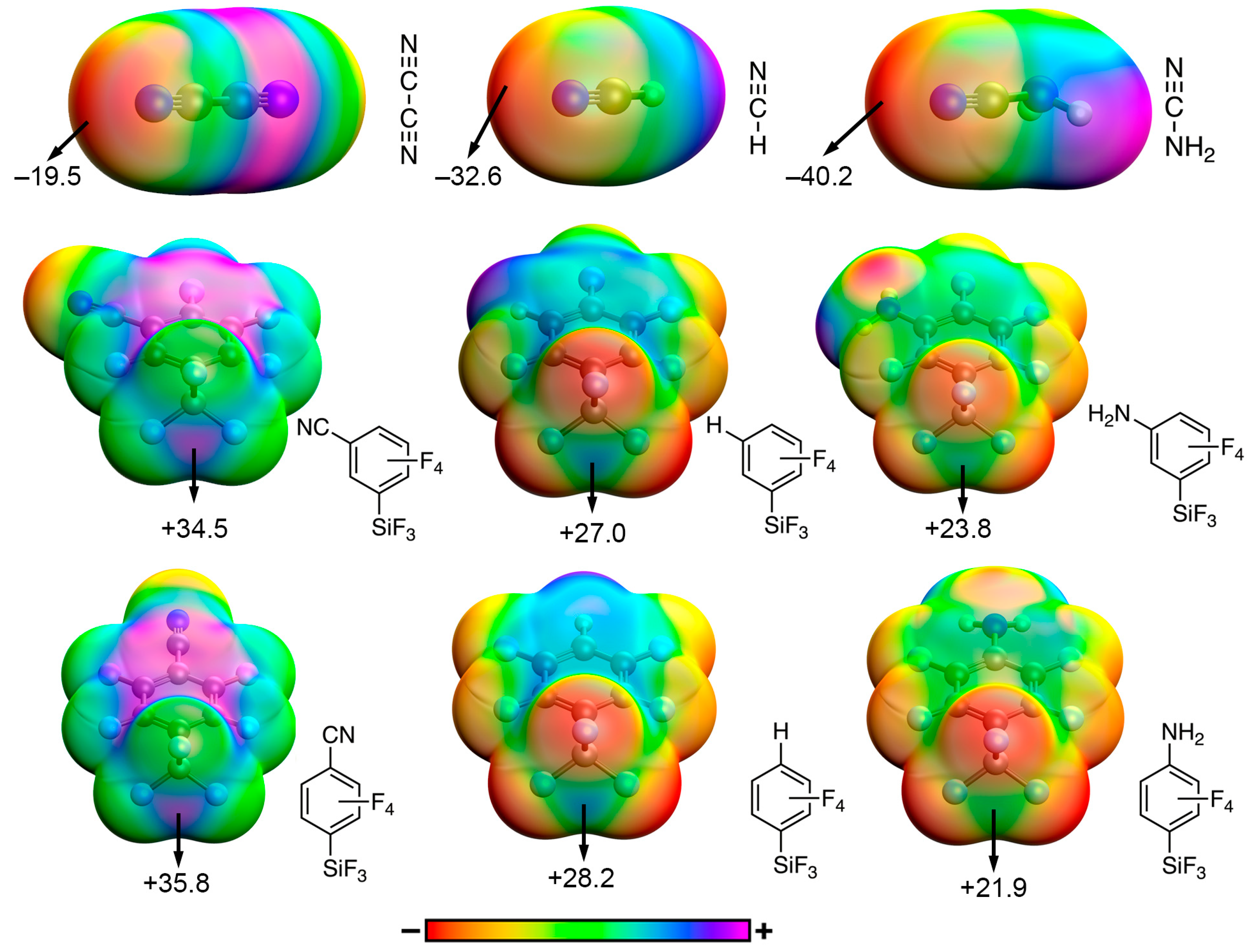
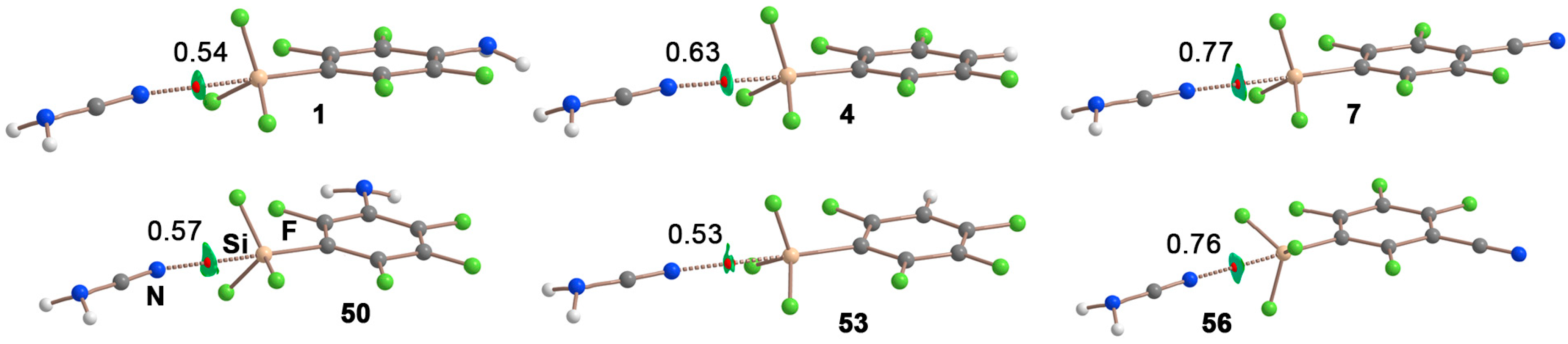
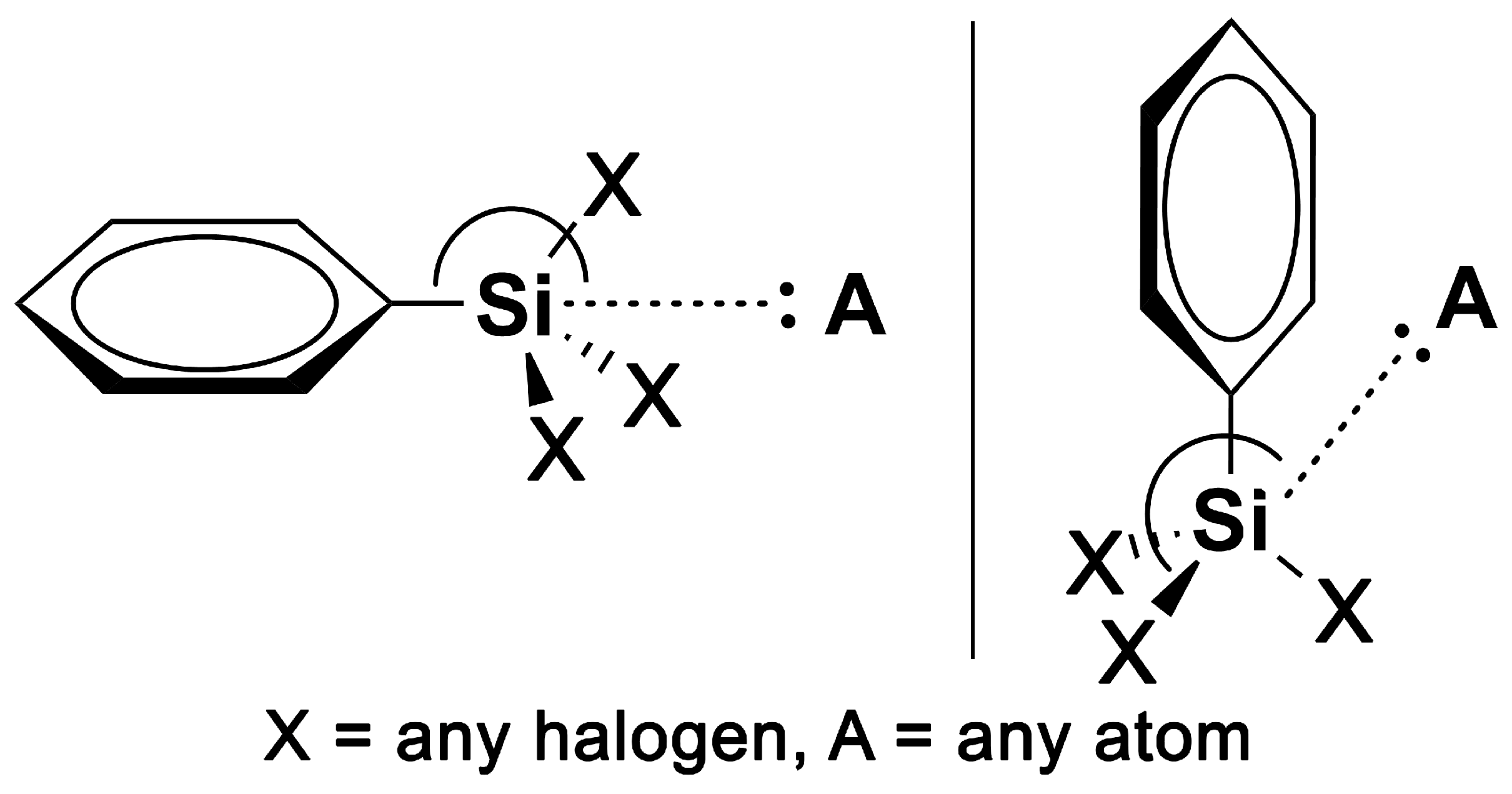
| TtB Donor (para substitution) | ESP (kcal/mol) |
|---|---|
| –CF3 | +33.3 |
| –CN | +35.8 |
| –H | +28.2 |
| –CH3 | +25.9 |
| –NH2 | +21.9 |
| –OCH3 | +26.4 |
| TtB donor (meta substitution) | ESP (kcal/mol) |
| –CF3 | +32.0 |
| –CN | +34.5 |
| –H | +27.0 |
| –CH3 | +25.2 |
| –NH2 | +23.8 |
| –OCH3 | +26.4 |
| TtB acceptor | ESP (kcal/mol) |
| –CF3 | −23.3 |
| –CN | −19.5 |
| –H | −32.6 |
| –CH3 | −38.3 |
| –NH2 | −40.2 |
| –OCH3 | −39.2 |
| Complex | ΔE | ΔEBSSE | d | ρ × 100 |
|---|---|---|---|---|
| 1 (X = –NH2, Y = –NH2) | −1.9 | −1.2 | 3.159 | 0.72 |
| 2 (X = –NH2, Y = –OCH3) | −2.4 | −1.7 | 3.114 | 0.77 |
| 3 (X = –NH2, Y = –CH3) | −2.4 | −1.7 | 3.119 | 0.77 |
| 4 (X =–NH2, Y = –H) | −2.7 | −1.9 | 3.095 | 0.79 |
| 5 (X = –NH2, Y = –CF3) | −3.4 | −2.6 | 3.030 | 0.89 |
| 6 (X = –NH2, Y = –CN) | −3.7 | −2.9 | 3.024 | 0.93 |
| 7 (X = –OCH3, Y = –NH2) | −1.9 | −1.2 | 3.173 | 0.69 |
| 8 (X = –OCH3, Y = –OCH3) | −2.4 | −1.7 | 3.142 | 0.72 |
| 9 (X = –OCH3, Y = –CH3) | −2.3 | −1.6 | 3.132 | 0.74 |
| 10 (X = –OCH3, Y = –H) | −2.6 | −1.9 | 3.108 | 0.77 |
| 11 (X = –OCH3, Y = –CF3) | −3.3 | −2.5 | 3.046 | 0.86 |
| 12 (X = –OCH3, Y = –CN) | −3.6 | −2.8 | 3.024 | 0.89 |
| 13 (X = –CH3, Y = –NH2) | −1.9 | −1.2 | 3.182 | 0.69 |
| 14 (X = –CH3, Y = –OCH3) | −2.3 | −1.6 | 3.139 | 0.74 |
| 15 (X = –CH3, Y = –CH3) | −2.3 | −1.6 | 3.143 | 0.74 |
| 16 (X = –CH3, Y = –H) | −2.5 | −1.8 | 3.121 | 0.77 |
| 17 (X = –CH3, Y = –CF3) | −3.2 | −2.4 | 3.060 | 0.85 |
| 18 (X = –CH3, Y = –CN) | −3.5 | −2.7 | 3.039 | 0.88 |
| 19 (X = –H, Y = –NH2) | −1.5 | −1.0 | 3.259 | 0.42 |
| 20 (X = –H, Y = –OCH3) | −1.9 | −1.3 | 3.221 | 0.64 |
| 21 (X = –H, Y = –CH3) | −1.9 | −1.3 | 3.218 | 0.64 |
| 22 (X = –H, Y = –H) | −2.1 | −1.5 | 3.206 | 0.66 |
| 23 (X = –H, Y = –CF3) | −2.6 | −2.0 | 3.155 | 0.71 |
| 24 (X = –H, Y = –CN) | −2.8 | −2.1 | 3.138 | 0.74 |
| 25 (X = –CF3, Y = –NH2) | −1.5 | −1.0 | 3.310 | 0.37 |
| 26 (X = –CF3, Y = –OCH3) | −1.8 | −1.2 | 3.268 | 0.58 |
| 27 (X = –CF3, Y = –CH3) | −1.7 | −1.1 | 3.283 | 0.57 |
| 28 (X = –CF3, Y = –H) | −1.8 | −1.3 | 3.267 | 0.58 |
| 29 (X = –CF3, Y = –CF3) | −2.2 | −1.6 | 3.228 | 0.62 |
| 30 (X = –CF3, Y = –CN) | −2.3 | −1.7 | 3.214 | 0.47 |
| 31 (X = –CN, Y = –NH2) | −1.6 | −1.0 | 3.324 | 0.69 |
| 32 (X = –CN, Y = –OCH3) | −1.8 | −1.2 | 3.297 | 0.74 |
| 33 (X = –CN, Y = –CH3) | −1.8 | −1.2 | 3.299 | 0.74 |
| 34 (X = –CN, Y = –H) | −1.8 | −1.3 | 3.286 | 0.77 |
| 35 (X = –CN, Y = –CF3) | −2.1 | −1.5 | 3.250 | 0.85 |
| 36 (X = –CN, Y = –CN) | −2.2 | −1.6 | 3.238 | 0.89 |
| Complex | ΔE | ΔEBSSE | d | ρ × 100 |
|---|---|---|---|---|
| 37 (X = –NH2, Y = –NH2) | −2.4 | −1.7 | 3.135 | 0.74 |
| 38 (X = –NH2, Y = –OCH3) | −2.4 | −1.7 | 3.112 | 0.77 |
| 39 (X = –NH2, Y = –CH3) | −2.6 | −1.9 | 3.131 | 0.75 |
| 40 (X = –NH2, Y = –H) | −2.5 | −1.8 | 3.162 | 0.71 |
| 41 (X = –NH2, Y = –CF3) | −3.2 | −2.4 | 3.048 | 0.87 |
| 42 (X = –NH2, Y = –CN) | −3.6 | −3.6 | 3.014 | 0.92 |
| 43 (X = –OCH3, Y = –NH2) | −2.3 | −1.6 | 3.160 | 0.71 |
| 44 (X = –OCH3, Y = –OCH3) | −2.7 | −2.0 | 3.128 | 0.75 |
| 45 (X = –OCH3, Y = –CH3) | −2.6 | −1.8 | 3.141 | 0.73 |
| 46 (X = –OCH3, Y = –H) | −2.4 | −1.7 | 3.142 | 0.73 |
| 47 (X = –OCH3, Y = –CF3) | −3.1 | −2.4 | 3.073 | 0.82 |
| 48 (X = –OCH3, Y = –CN) | −3.5 | −2.7 | 3.056 | 0.84 |
| 49 (X = –CH3, Y = –NH2) | −2.0 | −1.3 | 3.176 | 0.70 |
| 50 (X = –CH3, Y = –OCH3) | −2.3 | −1.6 | 3.164 | 0.71 |
| 51 (X = –CH3, Y = –CH3) | −2.1 | −1.5 | 3.157 | 0.72 |
| 52 (X = –CH3, Y = –H) | −2.4 | −1.7 | 3.121 | 0.77 |
| 53 (X = –CH3, Y = –CF3) | −3.0 | −2.3 | 3.070 | 0.84 |
| 54 (X = –CH3, Y = –CN) | −3.4 | −2.6 | 3.045 | 0.88 |
| 55 (X = –H, Y = –NH2) | −1.7 | −1.1 | 3.258 | 0.60 |
| 56 (X = –H, Y = –OCH3) | −1.9 | −1.3 | 3.232 | 0.63 |
| 57 (X = –H, Y = –CH3) | −2.1 | −1.5 | 3.238 | 0.62 |
| 58 (X = –H, Y = –H) | −1.9 | −1.4 | 3.223 | 0.64 |
| 59 (X = –H, Y = –CF3) | −2.4 | −1.8 | 3.168 | 0.70 |
| 60 (X = –H, Y = –CN) | −2.7 | −2.7 | 3.138 | 0.74 |
| 61 (X = –CF3, Y = –NH2) | −1.9 | −1.3 | 3.307 | 0.54 |
| 62 (X = –CF3, Y = –OCH3) | −1.7 | −1.2 | 3.287 | 0.56 |
| 63 (X = –CF3, Y = –CH3) | −2.0 | −1.4 | 3.290 | 0.56 |
| 64 (X = –CF3, Y = –H) | −1.8 | −1.2 | 3.280 | 0.57 |
| 65 (X = –CF3, Y = –CF3) | −2.1 | −1.5 | 3.229 | 0.62 |
| 66 (X = –CF3, Y = –CN) | −2.3 | −1.7 | 3.214 | 0.64 |
| 67 (X = –CN, Y = –NH2) | −1.6 | −1.1 | 3.341 | 0.51 |
| 68 (X = –CN, Y = –OCH3) | −1.7 | −1.2 | 3.302 | 0.55 |
| 69 (X = –CN, Y = –CH3) | −1.7 | −1.1 | 3.318 | 0.53 |
| 70 (X = –CN, Y = –H) | −1.8 | −1.2 | 3.308 | 0.54 |
| 71 (X = –CN, Y = –CF3) | −2.0 | −1.4 | 3.277 | 0.57 |
| 72 (X = –CN, Y = –CN) | −2.2 | −1.6 | 3.245 | 0.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burguera, S.; Frontera, A.; Bauzá, A. Substituent Effects in Tetrel Bonds Involving Aromatic Silane Derivatives: An ab initio Study. Molecules 2023, 28, 2385. https://doi.org/10.3390/molecules28052385
Burguera S, Frontera A, Bauzá A. Substituent Effects in Tetrel Bonds Involving Aromatic Silane Derivatives: An ab initio Study. Molecules. 2023; 28(5):2385. https://doi.org/10.3390/molecules28052385
Chicago/Turabian StyleBurguera, Sergi, Antonio Frontera, and Antonio Bauzá. 2023. "Substituent Effects in Tetrel Bonds Involving Aromatic Silane Derivatives: An ab initio Study" Molecules 28, no. 5: 2385. https://doi.org/10.3390/molecules28052385
APA StyleBurguera, S., Frontera, A., & Bauzá, A. (2023). Substituent Effects in Tetrel Bonds Involving Aromatic Silane Derivatives: An ab initio Study. Molecules, 28(5), 2385. https://doi.org/10.3390/molecules28052385