Bioactive Molecules Derived from Plants in Managing Dengue Vector Aedes aegypti (Linn.)
Abstract
1. Introduction
2. Virus Vector
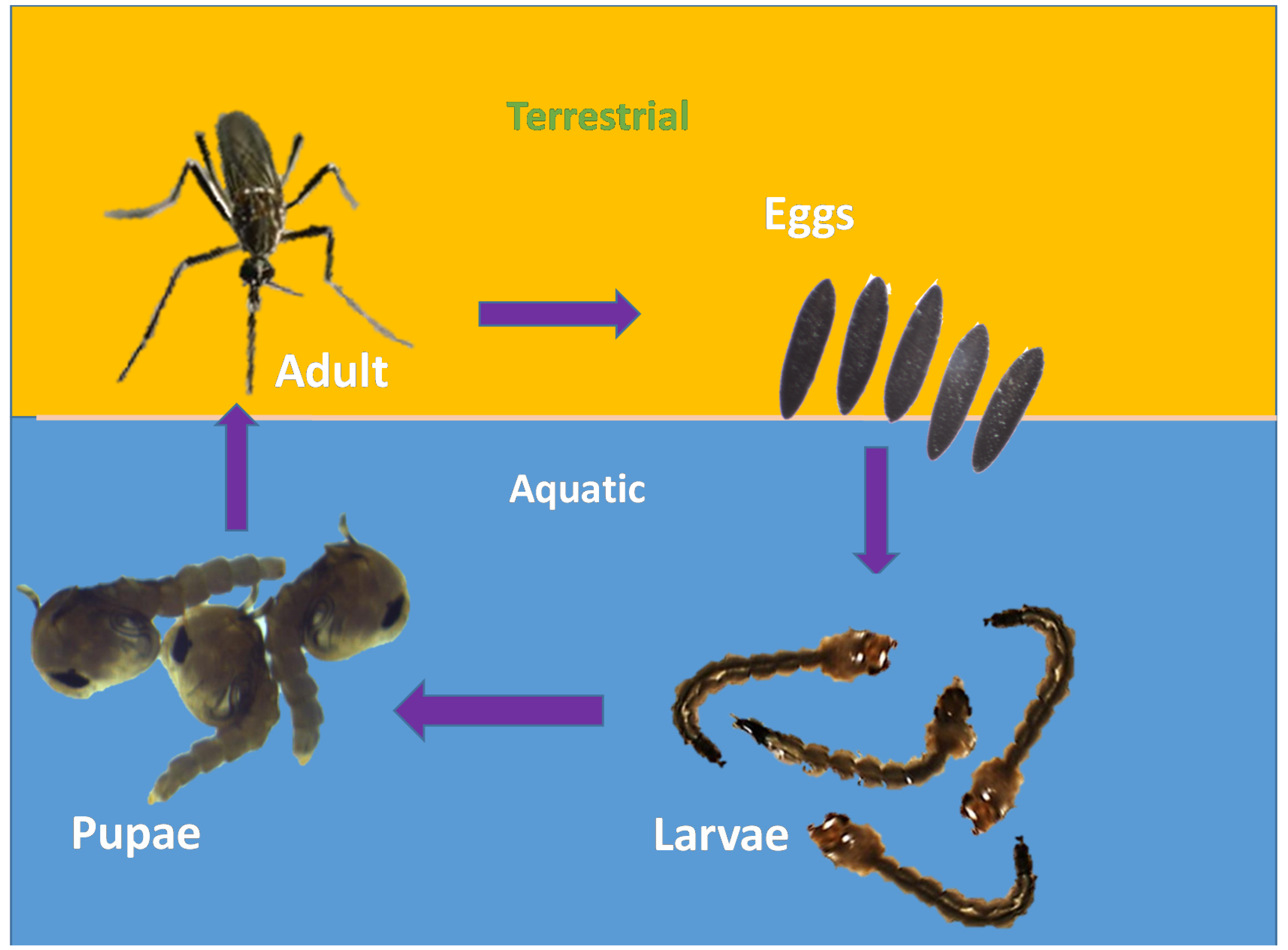
2.1. Life Cycle of Aedes aegypti
- Eggs: About 100 black-coloured eggs are laid by adult female mosquitoes on a wet/moist surface very near the waterline, especially in places such as marshes, plant axils, tree holes and even water containers [29]. Man-made objects such as clay pots, bowls, cups, fountains, barrels, vases and tires are excellent sites for egg laying [30]. The eggs are very hardy and become glued to the wet surface. Due to their ability to endure long periods of drying, they can survive extreme cold and other adverse climatic conditions;
- Larvae: The emergence of larvae from the eggs takes place only after they get fully immersed in water. The process might take days to weeks and some of the eggs require multiple soakings before they hatch. The larvae are aquatic. They hang upside down at an angle from the water surface [29]. They feed on the microorganisms found in water [30]. Siphon is their short respiratory structure, through which they take up oxygen from the air above the water [30]. After undergoing the process of moulting thrice, a larva becomes a pupa.
- Pupa: It takes a larva five days to become a pupa. The pupa continues to develop until the body of the adult mosquito emerges from the pupal skin and exits the water [30];
- Adult: After around 2–3 days, the adult emerges from the pupa [30]. Within two days of emerging, adult mosquitoes mate. Male mosquitoes feed on nectar found in flowers, whereas female mosquitoes consume their blood meal. Though they feed during daytime, their activity peaks at dawn and dusk [29]. After feeding, the mosquitoes look for water surfaces to lay their eggs [30]. They usually prefer to live in close association with humans [29], especially inside homes and buildings where the windows and doors are kept open [30].
2.2. Role in Transmission of Diseases
2.2.1. Dengue Fever
2.2.2. Chikungunya
2.2.3. Zika
3. Protection
4. Chemical Repellents Currently in Use against Aedes aegypti
5. Necessity of Natural Mosquito Repellents
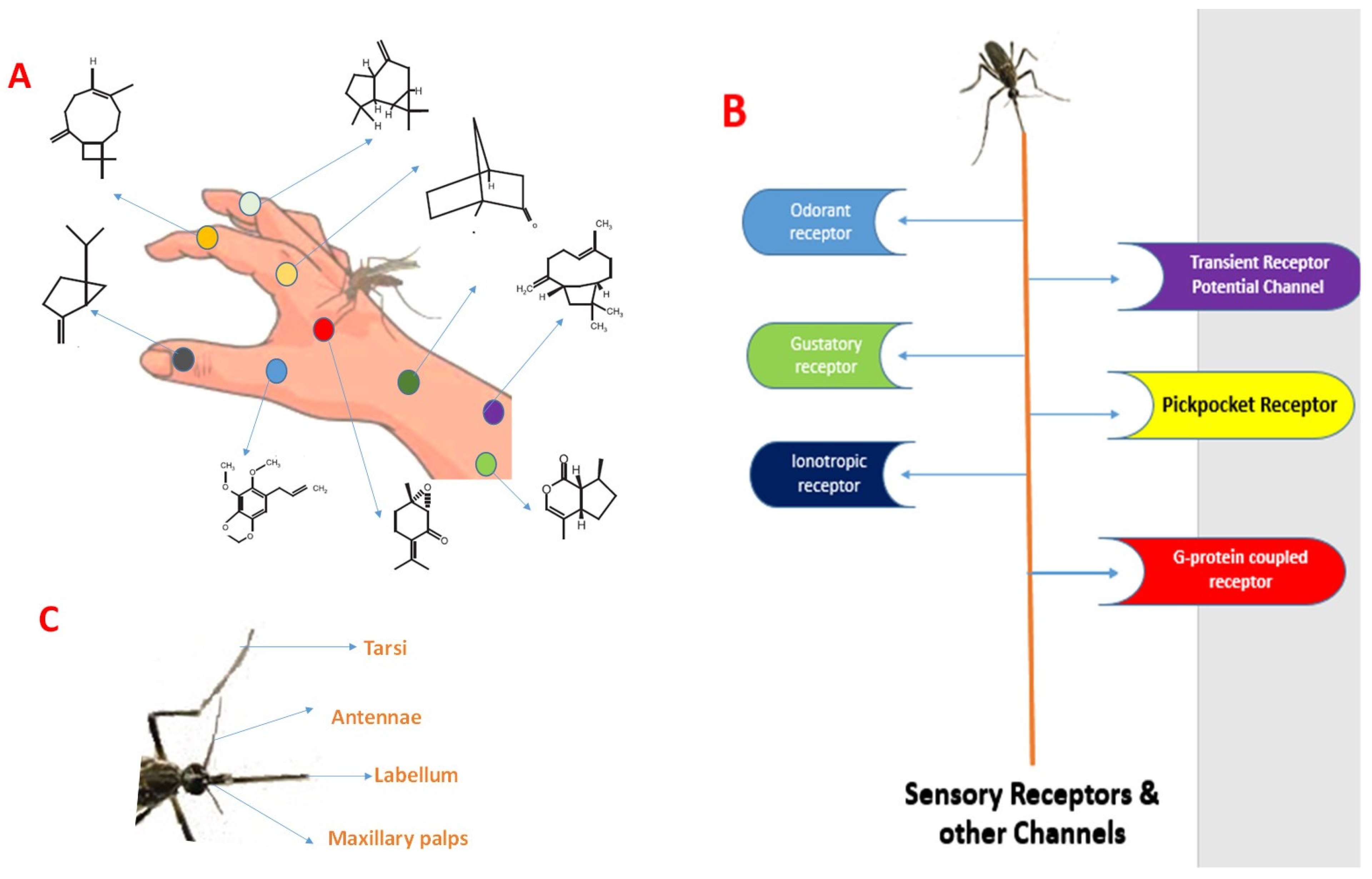
6. Identifying and Screening New Plants for Repellency
7. Plants Tested for Larvicidal Activity against Aedes aegypti
8. Plants Tested for Repellent Activity against Aedes aegypti
9. Plants Tested for Adulticidal Activity against Aedes aegypti
10. Plants Tested for Pupicidal Activity against Aedes aegypti
11. Plants Tested for Ovicidal and Oviposition Deterrent Activities against Aedes aegypti
12. Conclusions and Future Development
- About 40 plant families were involved in these studies against Ae. aegypti and they include Schisandraceae, Rutaceae, Lamiaceae, Annonaceae, Fabaceae, Poaceae, Myrtaceae, Asteraceae, Cupressaceae, Lauraceae, Euphorbiaceae, Cucurbitaceae, Malvaceae, Linaceae, Brassicaceae, Myristicaceae, Canellaceae, Rubiaceae, Amaranthaceae, Zingiberaceae, Apiaceae, Verbenaceae, Apocynaceae, Bignoniaceae, Acanthaceae, Lythraceae, Arecaceae, Amaryllidaceae, Melastomataceae, Rhizophoraceae, Meliaceae, Asparagaceae, Celastraceae, Papaveraceae, Menispermaceae, Acoraceae, Convolvulaceae, Pinaceae, Zygophyllaceae, Anacardiaceae, Ulmaceae and Polygonaceae. All these plant families enriched with essential oils and their bio-active compounds either for its fragrance or other benefits, and the repellents that have been found to repel the dengue vector for a maximum period of 60–180 min.
- Among these, plants belonging to the Rutaceae, Lamiaceae, Asteraceae, Apiaceae, Myrtaceae, Poaceae and Fabaceae families have been frequently researched in recent times for their larvicidal, adulticidal, pupicidal and ovicidal activities against Ae. aegypti and other mosquito vectors.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DSS | Dengue Shock Syndrome |
| DHF | Dengue Haemorrhagic Fever |
| DEN-2 | Dengue Virus Type-2 |
| STI | Sterile Insect Technique |
| DEET | N, N-diethyl-meta-toluamide |
| Lao PDR | Lao People’s Democratic Republic |
| CDIAL | Cinnamodial |
| CHIKV | Chikungunya Virus |
| CML | Cinnamolide |
| CMOS | Cinnamosmolide |
| CPCD | Capsicodendrin |
| DDD | Dichlorodiphenyldichloroethane |
| DDT | Dichlorodiphenyltrichloroethane |
| DENV EO | Dengue Fever Virus Essential Oil |
| GOI | Government of India |
| ICMR | Indian Council of Medical Research |
| LC | Lethal Concentration |
| NCBI | National Center for Biotechnology Information |
| NIV | National Institute of Virology |
| PMD | Para-menthane-3,8-diol |
| POLYG | Polygodial |
| RNA | Ribonucleic Acid |
| UGAN | Ugandensolide |
| USD | United States Dollars |
| VRDL | Virus Research & Diagnostic Labs |
| WARB | Warburganal |
| WHO | World Health Organization |
| YFV | Yellow Fever Virus |
| ZIKV | Zika Virus |
| ZVD | Zika Virus Disease |
References
- Edwin, E.-S.; Vasantha-Srinivasan, P.; Senthil-Nathan, S.; Thanigaivel, A.; Ponsankar, A.; Pradeepa, V.; Selin-Rani, S.; Kalaivani, K.; Hunter, W.B.; Abdel-Megeed, A.; et al. Anti-dengue efficacy of bioactive andrographolide from Andrographis paniculata (Lamiales: Acanthaceae) against the primary dengue vector Aedes aegypti (Diptera: Culicidae). Acta Trop. 2016, 163, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Chandra, G.; Bhattacharjee, I.; Chatterjee, S.N.; Ghosh, A. Mosquito control by larvivorous fish. Indian J. Med. Res. 2008, 127, 13–27. [Google Scholar] [PubMed]
- World Health Organisation. “World Health Day 2014: Preventing Vector-Borne Diseases”. Available online: https://www.who.int/news/item/02-04-2014-world-health-day-2014-preventing-vector-borne-diseases (accessed on 16 May 2022).
- Beerntsen, B.T.; James, A.A.; Christensen, B.M. Genetics of mosquito vector competence. Microbiol. Mol. Biol. Rev. 2000, 64, 115–137. [Google Scholar] [CrossRef] [PubMed]
- Hahn, C.S.; French, O.G.; Foley, P.; Martin, E.N.; Taylor, R.P. Bispecific monoclonal antibodies mediate binding of dengue virus to erythrocytes in a monkey model of passive viremia. J. Immunol. 2001, 166, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Hemingway, J.; Ranson, H. Insecticide resistance in insect vectors of human disease. Annu. Rev. Entomol. 2000, 45, 371–391. [Google Scholar] [CrossRef]
- Amala, K.; Karthi, S.; Ganesan, R.; Radhakrishnan, N.; Srinivasan, K.; Mostafa, A.E.Z.M.; Al-Ghamdi, A.A.; Alkahtani, J.; Elshikh, M.S.; Senthil-Nathan, S.; et al. Bioefficacy of Epaltes divaricata (L.) n-Hexane extracts and their major metabolites against the Lepidopteran pests Spodoptera litura (fab.) and dengue mosquito Aedes aegypti (Linn.). Molecules 2012, 26, 3695. [Google Scholar] [CrossRef]
- Kamaraj, C.; Karthi, S.; Reegan, A.D.; Balasubramani, G.; Ramkumar, G.; Kalaivani, K.; Zahir, A.A.; Deepak, P.; Senthil-Nathan, S.; Rahman, M.; et al. Green synthesis of gold nanoparticles using Gracilaria crassa leaf extract and their ecotoxicological potential: Issues to be considered. Environ. Res. 2022, 213, 113711. [Google Scholar] [CrossRef]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Gibbons, R.V.; Vaughn, D.W. Dengue: An escalating problem. BMJ 2002, 324, 1563–1566. [Google Scholar] [CrossRef]
- WHO. Trade, Foreign Policy, Diplomacy and Health: Public–Private Partnerships for Health. Available online: http://www.who.int/trade/glossary/story077/en/ (accessed on 16 May 2022).
- Yogarajalakshmi, P.; Poonguzhali, T.V.; Ganesan, R.; Karthi, S.; Senthil-Nathan, S.; Krutmuang, P.; Radhakrishnan, N.; Mohammad, F.; Kim, T.-J.; Vasantha-Srinivasan, P. Toxicological screening of marine red algae Champia parvula (C. Agardh) against the dengue mosquito vector Aedes aegypti (Linn.) and its non-toxicity against three beneficial aquatic predators. Aquat. Toxicol. 2020, 222, 105474. [Google Scholar] [CrossRef]
- Bouyer, J.; Lefrançois, T. Boosting the sterile insect technique to control mosquitoes. Trends Parasitol. 2014, 30, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, S.D.; Drake, L.L.; Price, D.P.; Hammond, J.I.; Hansen, I.A. The Efficacy of Some Commercially Available Insect Repellents for Aedes aegypti (Diptera: Culicidae) and Aedes albopictus (Diptera: Culicidae). J. Insect Sci. 2015, 15, 140. [Google Scholar] [CrossRef] [PubMed]
- Walshe, D.P.; Garner, P.; Adeel, A.A.; Pyke, G.H.; Burkot, T.R. Larvivorous fish for preventing malaria transmission. Cochrane Database Syst. Rev. 2017, 12, CD008090. [Google Scholar] [CrossRef] [PubMed]
- Kats, L.B.; Ferrer, R.P. Alien predators and amphibian declines: Review of two decades of science and the transition to conservation. Divers. Distrib. 2003, 9, 99–110. [Google Scholar] [CrossRef]
- Montella, I.R.; Martins, A.J.; Viana-Medeiros, P.F.; Lima, J.B.P.; Braga, I.A.; Valle, D. Insecticide Resistance Mechanisms of Brazilian Aedes aegypti Populations from 2001 to 2004. Am. J. Trop. Med. Hyg. 2007, 77, 467–477. [Google Scholar] [CrossRef]
- Diniz, T.C.; Silva, J.C.; de Lima-Saraiva, S.R.G.; de Almeida Ribeiro, F.P.R.; Pacheco, A.G.M.; de Freitas, R.M.; Quintans-Júnior, L.J.; de Souza Siqueira Quintans, J.; Mendes, R.L.; da Silva Almeida, J.R.G. The Role of Flavonoids on Oxidative Stress in Epilepsy. Oxid. Med. Cell. Longev. 2015, 2015, 171756. [Google Scholar] [CrossRef]
- Ranson, H.; N’Guessan, R.; Lines, J.; Moiroux, N.; Nkuni, Z.; Corbel, V. Pyrethroid resistance in African anopheline mosquitoes: What are the implications for malaria control? Trends Parasitol. 2011, 27, 91–98. [Google Scholar] [CrossRef]
- Marcombe, S.; Mathieu, R.B.; Pocquet, N.; Riaz, M.-A.; Poupardin, R.; Sélior, S.; Darriet, F.; Reynaud, S.; Yébakima, A.; Corbel, V.; et al. Insecticide Resistance in the Dengue Vector Aedes aegypti from Martinique: Distribution, Mechanisms and Relations with Environmental Factors. PLoS ONE 2012, 7, e30989. [Google Scholar] [CrossRef]
- Stark, J.D.; Banks, J.E. “Selective” Pesticides: Are They Less Hazardous to the Environment? BioScience 2001, 51, 980–982. [Google Scholar] [CrossRef]
- Osimitz, T.; Murphy, J.; Fell, L.; Page, B. Adverse events associated with the use of insect repellents containing N,N-diethyl-m-toluamide (DEET). Regul. Toxicol. Pharmacol. 2010, 56, 93–99. [Google Scholar] [CrossRef]
- Chen-Hussey, V.; Behrens, R.; Logan, J.G. Assessment of methods used to determine the safety of the topical insect repellent N,N-diethyl-m-toluamide (DEET). Parasites Vectors 2014, 7, 173. [Google Scholar] [CrossRef]
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Rosa, E.R. Neuropharmacological studies of Piper auritum Kunth (Piperaceae): Antinociceptive and anxiolytic-like effects. J. Med. Plant Res. 2013, 7, 1718–1729. [Google Scholar] [CrossRef]
- Soejarto, D.D.; Gyllenhaal, C.; Kadushin, M.R.; Southavong, B.; Sydara, K.; Bouamanivong, S.; Xaiveu, M.; Zhang, H.-J.; Franzblau, S.G.; Tan, G.T.; et al. An ethnobotanical survey of medicinal plants of Laos toward the discovery of bioactive compounds as potential candidates for pharmaceutical development. Pharm. Biol. 2011, 50, 42–60. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Chetia, D. Plant Flavonoids as Potential Source of Future Antimalarial leads. Syst. Rev. Pharm. 2016, 8, 13–18. [Google Scholar] [CrossRef]
- Morrison, A.C.; Zielinski-Gutierrez, E.; Scott, T.W.; Rosenberg, R. Defining Challenges and Proposing Solutions for Control of the Virus Vector Aedes aegypti. PLoS Med. 2008, 5, e68. [Google Scholar] [CrossRef] [PubMed]
- Aedes Mosquito Genus. Available online: https://www.britannica.com/animal/Aedes (accessed on 24 September 2022).
- Mosquito Life Cycle Aedes aegypti. Available online: https://www.cdc.gov/dengue/resources/factsheets/mosquitolifecyclefinal.pdf (accessed on 24 September 2022).
- Shepard, D.S.; Undurraga, E.A.; Halasa, Y.A. Economic and Disease Burden of Dengue in Southeast Asia. PLOS Neglected Trop. Dis. 2013, 7, e2055. [Google Scholar] [CrossRef]
- Gubler, D.J. Dengue and Dengue Hemorrhagic Fever. Clin. Microbiol. Rev. 1998, 11, 480–496. [Google Scholar] [CrossRef]
- Bahl, A.; Bakhshi, S. Dengue Fever in Patients with Pediatric Malignancy on Chemotherapy: A Concern in Tropical Countries. Pediatr. Blood Cancer 2011, 57, 1249–1250. [Google Scholar] [CrossRef]
- Staples, J.E.; Breiman, R.F.; Powers, A.M. Chikungunya Fever: An Epidemiological Review of a Re-Emerging Infectious Disease. Clin. Infect. Dis. 2009, 49, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Chikungunya Transmission Cycles in Lao PDR. Available online: https://www.pasteur.la/project-carried-on-in-the-lab/chikungunya-transmission-cycles-in-lao-pdr/ (accessed on 16 May 2022).
- Cavrini, F.; Gaibani, P.; Pierro, A.M.; Rossini, G.; Landini, M.P.; Sambri, V. Chikungunya: An emerging and spreading arthropod-borne viral disease. J. Infect. Dev. Ctries. 2009, 3, 744–752. [Google Scholar] [CrossRef]
- Zika Virus Infection—India. Available online: http://www.who.int/csr/don/26-may2017-zika-ind/en/ (accessed on 16 June 2017).
- Hamid, P.H.; Ninditya, V.I.; Prastowo, J.; Haryanto, A.; Taubert, A.; Hermosilla, C. Current Status of Aedes aegypti Insecticide Resistance Development from Banjarmasin, Kalimantan, Indonesia. BioMed Res. Int. 2018, 2018, 1735358. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Shi, W.-Q.; Wu, J.-T.; Li, Y.-Y.; Xue, J.-B.; Zhang, Y. Resistance to pyrethroid and organophosphate insecticides, and the geographical distribution and polymorphisms of target-site mutations in voltage-gated sodium channel and acetylcholinesterase 1 genes in Anopheles sinensis populations in Shanghai, China. Parasites Vectors 2019, 12, 96. [Google Scholar] [CrossRef] [PubMed]
- Shukla, D.; Wijayapala, S.; Vankar, P.S. Effective mosquito repellent from plant-based formulation. Int. J. Mosq. Res. 2018, 5, 19–24. [Google Scholar]
- Reichert, W.; Ejercito, J.; Guda, T.; Dong, X.; Wu, Q.; Ray, A.; Simon, J.E. Repellency Assessment of Nepeta cataria Essential Oils and Isolated Nepetalactones on Aedes aegypti. Sci. Rep. 2019, 9, 1524. [Google Scholar] [CrossRef]
- Sritabutra, D.; Soonwera, M. Repellent activity of herbal essential oils against Aedes aegypti (Linn.) and Culex quinquefasciatus (Say.). Asian Pac. J. Trop. Dis. 2013, 3, 271–276. [Google Scholar] [CrossRef]
- Tamta, G.; Mehra, N.; Tandon, S. Traditional Uses, Phytochemical and Pharmacological Properties of Ficus auriculata: A Review. J. Drug Deliv. Ther. 2021, 11, 163–169. [Google Scholar] [CrossRef]
- Maia, M.F.; Moore, S.J. Plant-based insect repellents: A review of their efficacy, development and testing. Malar. J. 2011, 10, S11–S14. [Google Scholar] [CrossRef]
- Soonwera, M.; Moungthipmalai, T.; Aungtikun, J.; Sittichok, S. Combinations of plant essential oils and their major compositions inducing mortality and morphological abnormality of Aedes aegypti and Aedes albopictus. Heliyon 2022, 8, e09346. [Google Scholar] [CrossRef]
- de Oliveira, A.A.; França, L.P.; Ramos, A.D.S.; Ferreira, J.L.P.; Maria, A.C.B.; Oliveira, K.M.; Jr, E.S.; da Silva, J.N.; Branches, A.D.; Barros, G.D.A.; et al. Larvicidal, adulticidal and repellent activities against Aedes aegypti L. of two commonly used spices, Origanum vulgare L. and Thymus vulgaris L. South Afr. J. Bot. 2021, 140, 17–24. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; da Silva, A.A.; de Freitas, J.C.C.; Martins, V.E.P.; Ferreira, M.A.P.; Ferreira, A.C.S.; Cabeça, C.L.S.; Rodrigues, A.L.M.; Alves, D.R.; de Morais, S.M. Larvicidal activity of Annona mucosa Jacq. extract and main constituents rolliniastatin 1 and rollinicin against Aedes aegypti and Aedes albopictus. Ind. Crop. Prod. 2021, 169, 113678. [Google Scholar] [CrossRef]
- Manh, H.D.; Hue, D.T.; Hieu, N.T.T.; Tuyen, D.T.T.; Tuyet, O.T. The Mosquito Larvicidal Activity of Essential Oils from Cymbopogon and Eucalyptus Species in Vietnam. Insects 2020, 11, 128. [Google Scholar] [CrossRef] [PubMed]
- Almadiy, A.A. Chemical composition, insecticidal and biochemical effects of two plant oils and their major fractions against Aedes aegypti, the common vector of dengue fever. Heliyon 2020, 6, e04915. [Google Scholar] [CrossRef]
- Amarasinghe, L.; Wickramarachchi, P.; Aberathna, A.; Sithara, W.; De Silva, C. Comparative study on larvicidal activity of green synthesized silver nanoparticles and Annona glabra (Annonaceae) aqueous extract to control Aedes aegypti and Aedes albopictus (Diptera: Culicidae). Heliyon 2020, 6, e04322. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; Sampaio, C.D.G.; De Souza, J.S.N.; Campos, A.R.; Da Silva, A.B.R.; De Morais, S.M.; Martins, V.E.P. Different susceptibilities of Aedes aegypti and Aedes albopictus larvae to plant-derived products. Rev. Da Soc. Bras. De Med. Trop. 2019, 52, e20180197. [Google Scholar] [CrossRef] [PubMed]
- Pratheeba, T.; Taranath, V.; Gopal, D.S.; Natarajan, D. Antidengue potential of leaf extracts of Pavetta tomentosa and Tarenna asiatica (Rubiaceae) against dengue virus and its vector Aedes aegypti (Diptera: Culicidae). Heliyon 2019, 5, e02732. [Google Scholar] [CrossRef] [PubMed]
- Morejón, B.; Pilaquinga, F.; Domenech, F.; Ganchala, D.; Debut, A.; Neira, M. Larvicidal Activity of Silver Nanoparticles Synthesized Using Extracts of Ambrosia arborescens (Asteraceae) to Control Aedes aegypti L. (Diptera: Culicidae). J. Nanotechnol. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Fayemiwo, K.A.; Adeleke, M.A.; Okoro, O.P.; Awojide, S.H.; Awoniyi, I.O. Larvicidal efficacies and chemical composition of essential oils of Pinus sylvestris and Syzygium aromaticum against mosquitoes. Asian Pac. J. Trop. Biomed. 2013, 4, 30–34. [Google Scholar] [CrossRef]
- Lima, T.C.; da Silva, T.K.M.; Silva, F.L.; Barbosa-Filho, J.M.; Marques, M.O.M.; Santos, R.L.C.; Cavalcanti, S.C.D.H.; de Sousa, D.P. Larvicidal activity of Mentha x villosa Hudson essential oil, rotundifolone and derivatives. Chemosphere 2014, 104, 37–43. [Google Scholar] [CrossRef]
- Pitasawat, B.; Champakaew, D.; Choochote, W.; Jitpakdi, A.; Chaithong, U.; Kanjanapothi, D.; Rattanachanpichai, E.; Tippawangkosol, P.; Riyong, D.; Tuetun, B.; et al. Aromatic plant-derived essential oil: An alternative larvicide for mosquito control. Fitoterapia 2007, 78, 205–210. [Google Scholar] [CrossRef]
- Reegan, A.D.; Gandhi, M.R.; Paulraj, M.G.; Balakrishna, K.; Ignacimuthu, S. Effect of niloticin, a protolimonoid isolated from Limonia acidissima L. (Rutaceae) on the immature stages of dengue vector Aedes aegypti L. (Diptera: Culicidae). Acta Trop. 2014, 139, 67–76. [Google Scholar] [CrossRef]
- Manh, H.D.; Tuyet, O.T. Larvicidal and Repellent Activity of Mentha arvensis L. Essential Oil against Aedes aegypti. Insects 2020, 11, 198. [Google Scholar] [CrossRef] [PubMed]
- Alievi, K.; Capoani, G.T.; Buzatto, M.; Miorando, D.; Serpa, P.Z.; Fogolari, O.; Ignácio, Z.M.; Simões, D.A.; Busato, M.A.; Lutinski, J.A.; et al. Ateleia glazioveana and Ocimum basilicum: Plants with potential larvicidal and repellent against Aedes aegypti (Diptera, Culicidae). Res. Soc. Dev. 2021, 10, 17. [Google Scholar] [CrossRef]
- Hari, I.; Mathew, N. Larvicidal activity of selected plant extracts and their combination against the mosquito vectors Culex quinquefasciatus and Aedes aegypti. Environ. Sci. Pollut. Res. 2018, 25, 9176–9185. [Google Scholar] [CrossRef]
- Ishak, A.R.; Dom, N.C.; Hussain, H.; Sabri, N.H. Biolarvacidal Potential of Ipomoea cairica Extracts against Key Dengue Vectors. Procedia-Soc. Behav. Sci. 2014, 153, 180–188. [Google Scholar] [CrossRef]
- Louis, M.M.; Pushpa, V.; Balakrishna, K.; Ganesan, P. Mosquito larvicidal activity of Avocado (Persea americana Mill.) unripe fruit peel methanolic extract against Aedes aegypti, Culex quinquefasciatus and Anopheles stephensi. S. Afr. J. Bot. 2020, 133, 1–4. [Google Scholar] [CrossRef]
- Munusamy, R.G.; Appadurai, D.R.; Kuppusamy, S.; Michael, G.P.; Savarimuthu, I. Ovicidal and larvicidal activities of some plant extracts against Aedes aegypti L. and Culex quinquefasciatus Say (Diptera: Culicidae). Asian Pac. J. Trop. Dis. 2016, 6, 468–471. [Google Scholar] [CrossRef]
- Ruiz-Guerrero, R.; Rodríguez-Pérez, M.A.; Norzagaray-Campos, M. Toxicity of Mexican native plant extracts against larvae of Aedes aegypti (Diptera: Culicidae). Asian Pac. J. Trop. Biomed. 2015, 5, 287–291. [Google Scholar] [CrossRef]
- Govindarajan, M. Larvicidal and repellent activities of Sida acuta Burm. F. (Family: Malvaceae) against three important vector mosquitoes. Asian Pac. J. Trop. Med. 2010, 3, 691–695. [Google Scholar] [CrossRef]
- Kim, M.-K.; Jang, Y.-S.; Ahn, Y.-J.; Lee, D.-K.; Lee, H.-S. Larvicidal Activity of Australian and Mexican Plant Extracts against Aedes aegypti and Culex pipiens pallens (Diptera: Culicidae). J. Asia-Pacific Èntomol. 2002, 5, 227–231. [Google Scholar] [CrossRef]
- Qadir, U. Bioefficacy of Anamirta cocculus Linn. (Menispermaceae) seed extracts against dengue vector, Aedes aegypti Linn. (Diptera: Culicidae). Asian Pac. J. Trop. Dis. 2014, 4, S556–S562. [Google Scholar] [CrossRef]
- Imam, H.; Zarnigar; Sofi, G. Mosquito larvicidal efficay of Acorus calamus extracts against Aedes aegypti L. larvae. Asian Pac. J. Trop. Dis. 2014, 4, S181–S185. [Google Scholar] [CrossRef]
- El-Sheikh, T.M.; Al-Fifi, Z.I.; Alabboud, M.A. Larvicidal and repellent effect of some Tribulus terrestris L., (Zygophyllaceae) extracts against the dengue fever mosquito, Aedes aegypti (Diptera: Culicidae). J. Saudi Chem. Soc. 2012, 20, 13–19. [Google Scholar] [CrossRef]
- Chantawee, A.; Soonwera, M. Efficacies of four plant essential oils as larvicide, pupicide and oviposition deterrent agents against dengue fever mosquito, Aedes aegypti Linn. (Diptera: Culicidae). Asian Pac. J. Trop. Biomed. 2018, 8, 217. [Google Scholar] [CrossRef]
- Inocente, E.A.; Nguyen, B.; Manwill, P.K.; Benatrehina, A.; Kweka, E.; Wu, S.; Cheng, X.; Rakotondraibe, L.H.; Piermarini, P.M. Insecticidal and Antifeedant Activities of Malagasy Medicinal Plant (Cinnamosma sp.) Extracts and Drimane-Type Sesquiterpenes against Aedes aegypti Mosquitoes. Insects 2019, 10, 373. [Google Scholar] [CrossRef]
- Rodrigo, V.B.; Anderson, M.; Jesus, G.; Carlos, G.; Diego, D. Transformation of trans-anethole using the plant pathogenic fungus Colletotrichum acutatum as biocatalyst. Rev. Mex. De Ing. Química 2015, 14, 653–666. [Google Scholar]
- SpectraBase 2,6,10,15,19,23-Hexamethyltetracosane. Available online: https://spectrabase.com/compound/6bTmueR7Ifk (accessed on 7 June 2022).
- Chemical Properties of Cis-Tagetenone. Available online: https://chemeo.com/cid/76-423-8/cis-tagetenone (accessed on 9 June 2022).
- PubChem Explore Chemistry. Available online: https://pubchem.ncbi.nlm.nih.gov/compound (accessed on 13 June 2022).
- (Z)-azarone. Available online: https://greenmolbd.gov.bd/compound/2407 (accessed on 12 June 2022).
- RSC Publishing Home-Chemical Journals, Books and Databases. Available online: https://pubs.rsc.org (accessed on 7 June 2022).
- 1,8-cineole. Available online: https://tcichemicals.com/IN/en/p/C0542 (accessed on 5 June 2022).
- Muhaimin; Yusnaidar; Syahri, W.; Madyawati, L.; Utami, A.; Amanda, B.R.; Heriyanti, H.; Chaerunisaa, A. Screening and Potential Analysis of Methanolic Leaf Extract of Mangrove Plants at East Coast Sumatera as Repellent against Aedes aegypti. J. Pharm. Sci. Res. 2018, 10, 2228–2231. [Google Scholar]
- Misni, N.; Nor, Z.M.; Ahmad, R. New Candidates for Plant-Based Repellents against Aedes aegypti. J. Am. Mosq. Control. Assoc. 2016, 32, 117–123. [Google Scholar] [CrossRef]
- Mukandiwa, L.; Eloff, J.N.; Naidoo, V. Repellent and mosquitocidal effects of leaf extracts of Clausena anisata against the Aedes aegypti mosquito (Diptera: Culicidae). Environ. Sci. Pollut. Res. 2016, 23, 11257–11266. [Google Scholar] [CrossRef]
- Soonwera, M. Efficacy of essential oils from Citrus plants against mosquito vectors Aedes aegypti (Linn.) and Culex quinquefasciatus (Say). Int. J. Agric. Technol. 2015, 11, 669–681. [Google Scholar]
- Rajkumar, S.; Jebanesan, A. Prevention of Dengue fever through plant based mosquito repellent Clausena dentata (Willd.) M. Roem (Family: Rutaceae) essential oil against Aedes aegypti l. (Diptera: Culicidae) mosquito. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 231–234. [Google Scholar] [PubMed]
- Lim, V.; Narawi, M.M.; Chiu, H.I.; Tung, W.H.; Tan, J.J.; Lee, C.K. Selected Essential Oils as Repellents against Aedes aegypti: Validation of the Bioconstituents Using Gas Chromatography. J. Essent. Oil Bear. Plants 2019, 22, 1058–1073. [Google Scholar] [CrossRef]
- Azeem, M.; Zaman, T.; Tahir, M.; Haris, A.; Iqbal, Z.; Binyameen, M.; Nazir, A.; Shad, S.A.; Majeed, S.; Mozūraitis, R. Chemical composition and repellent activity of native plants essential oils against dengue mosquito, Aedes aegypti. Ind. Crop. Prod. 2019, 140, 111609. [Google Scholar] [CrossRef]
- Champakaew, D.; Junkum, A.; Chaithong, U.; Jitpakdi, A.; Riyong, D.; Sanghong, R.; Intirach, J.; Muangmoon, R.; Chansang, A.; Tuetun, B.; et al. Angelica sinensis (Umbelliferae) with proven repellent properties against Aedes aegypti, the primary dengue fever vector in Thailand. Parasitol. Res. 2015, 114, 2187–2198. [Google Scholar] [CrossRef] [PubMed]
- Rajeswary, M.; Govindarajan, M. Adulticidal properties of Pithecellobium dulce (Roxb.) Benth. (Family: Fabaceae) against dengue vector, Aedes aegypti (Linn.) (Diptera: Culicidae). Asian Pac. J. Trop. Dis. 2014, 4, S449–S452. [Google Scholar] [CrossRef]
- Castillo, R.M.; Stashenko, E.; Duque, J.E. Insecticidal and Repellent Activity of Several Plant-Derived Essential Oils against Aedes aegypti. J. Am. Mosq. Control. Assoc. 2017, 33, 25–35. [Google Scholar] [CrossRef]
- Torawane, S.; Andhale, R.; Pandit, R.; Mokat, D.; Phuge, S. Screening of some weed extracts for ovicidal and larvicidal activities against dengue vector Aedes aegypti. J. Basic Appl. Zool. 2021, 82, 36. [Google Scholar] [CrossRef]
- Cantrell, C.L.; Jones, A.M.P.; Ali, A. Isolation and Identification of Mosquito (Aedes aegypti) Biting-Deterrent Compounds from the Native American Ethnobotanical Remedy Plant Hierochloë odorata (Sweetgrass). J. Agric. Food Chem. 2016, 64, 8352–8358. [Google Scholar] [CrossRef] [PubMed]
- Autran, E.; Neves, I.; Da Silva, C.; Santos, G.; Da Câmara, C.; Navarro, D. Chemical composition, oviposition deterrent and larvicidal activities against Aedes aegypti of essential oils from Piper marginatum Jacq. (Piperaceae). Bioresour. Technol. 2009, 100, 2284–2288. [Google Scholar] [CrossRef]
- Senthil-Nathan, S. A Review of Resistance Mechanisms of Synthetic Insecticides and Botanicals, Phytochemicals, and Essential Oils as Alternative Larvicidal Agents against Mosquitoes. Front. Physiol. 2020, 10, 1591. [Google Scholar] [CrossRef]
- Radhakrishnan, N.; Karthi, S.; Raghuraman, P.; Ganesan, R.; Srinivasan, K.; Edwin, E.S.; Ganesh-Kumar, S.; Esa, N.M.; Senthil-Nathan, S.; Vasantha-Srinivasan, P.; et al. Chemical screening and mosquitocidal activity of essential oil derived from Mikania scandens (L.) Willd. against Anopheles gambiae Giles and their non-toxicity on mosquito predators. All Life. 2023, 16, 2169959. [Google Scholar] [CrossRef]
- Shyam-Sundar, N.; Karthi, S.; Senthil-Nathan, S.; Narayanan, K.R.; Santoshkumar, B.; Sivanesh, H.; Chanthini, K.M.-P.; Stanley-Raja, V.; Ramasubramanian, R.; Abdel-Megeed, A.; et al. Eco-friendly biosynthesis of TiO2 nanoparticles using Desmostachya bipinnata extract: Larvicidal and pupicidal potential against Aedes aegypti and Spodoptera litura and acute toxicity in non-target organisms. Sci. Total. Environ. 2023, 858, 159512. [Google Scholar] [CrossRef] [PubMed]
- Chellappandian, M.; Senthil-Nathan, S.; Vasantha-Srinivasan, P.; Karthi, S.; Kalaivani, K.; Hunter, W.B.; Ali, H.M.; Salem, M.Z.; Abdel-Megeed, A. Volatile toxin of Limonia acidissima (L.) produced larvicidal, developmental, repellent, and adulticidal toxicity effects on Aedes aegypti (L.). Tox. Rev. 2022, 41, 119–128. [Google Scholar] [CrossRef]
- Chellappandian, M.; Senthil-Nathan, S.; Karthi, S.; Vasantha-Srinivasan, P.; Kalaivani, K.; Hunter, W.B.; Ali, A.M.; Veerabahu, C.; Elshikh, M.S.; Al Farraj, D.A. Larvicidal and repellent activity of N-methyl-1-adamantylamine and oleic acid a major derivative of bael tree ethanol leaf extracts against dengue mosquito vector and their biosafety on natural predator. Environ. Sci. Pollut. Res. 2021, 29, 15654–15663. [Google Scholar] [CrossRef]
- Vasantha-Srinivasan, P.; Karthi, S.; Ganesan, R.; Senthil-Nathan, S.; Krutmuang, P.; Chellappandian, M.; Radhakrishnan, N.; Ponsankar, A.; Karthick, K.; Nelofer, A.-R. The efficacy of methanolic extract of Swietenia mahagoni Jacq. (Meliaceae) and a commercial insecticide against laboratory and field strains of Aedes aegypti (Linn.) and their impact on its predator Toxorhnchites splendens. Biocatal. Agric. Biotechnol. 2021, 31, 101915. [Google Scholar] [CrossRef]


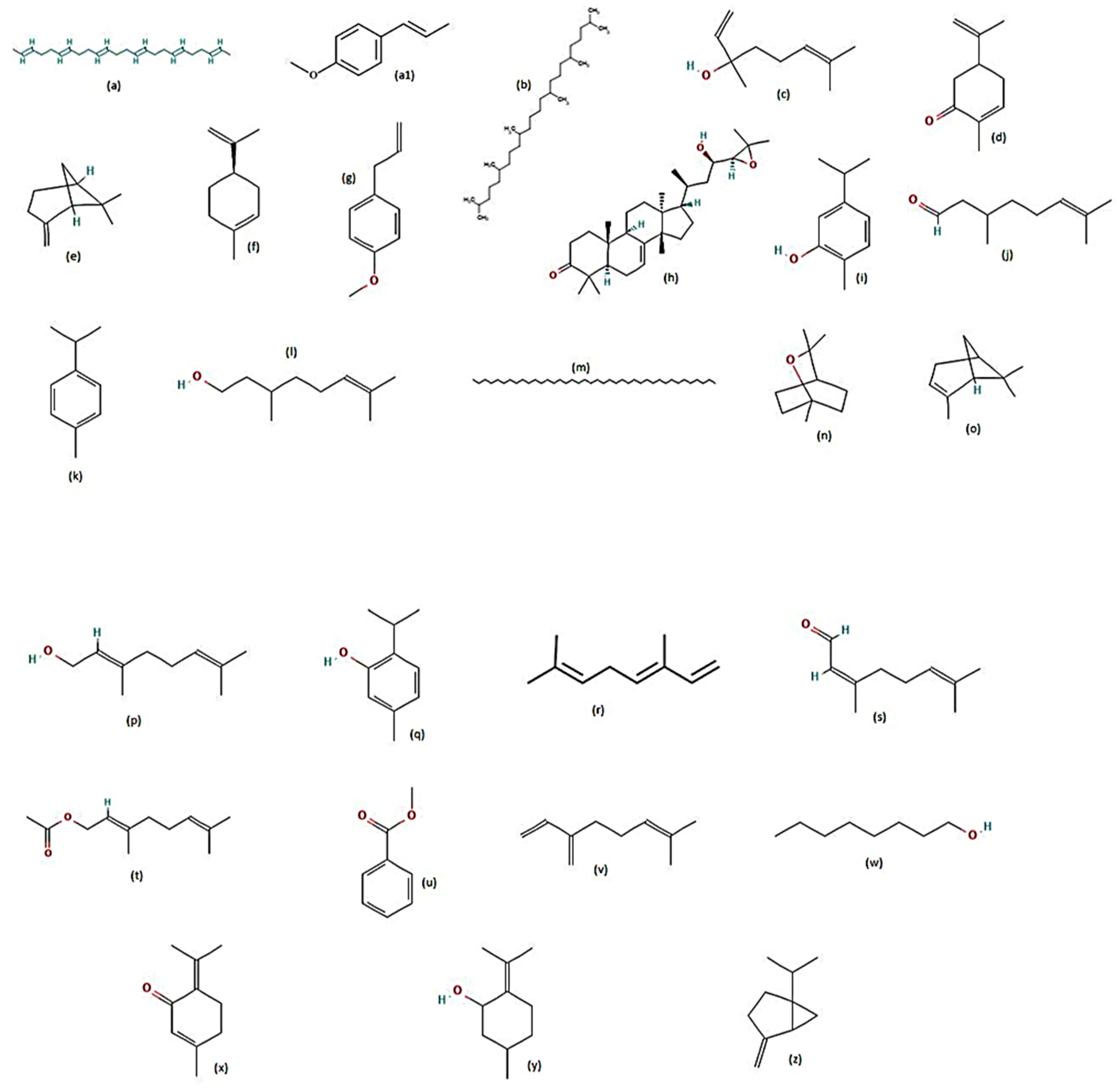
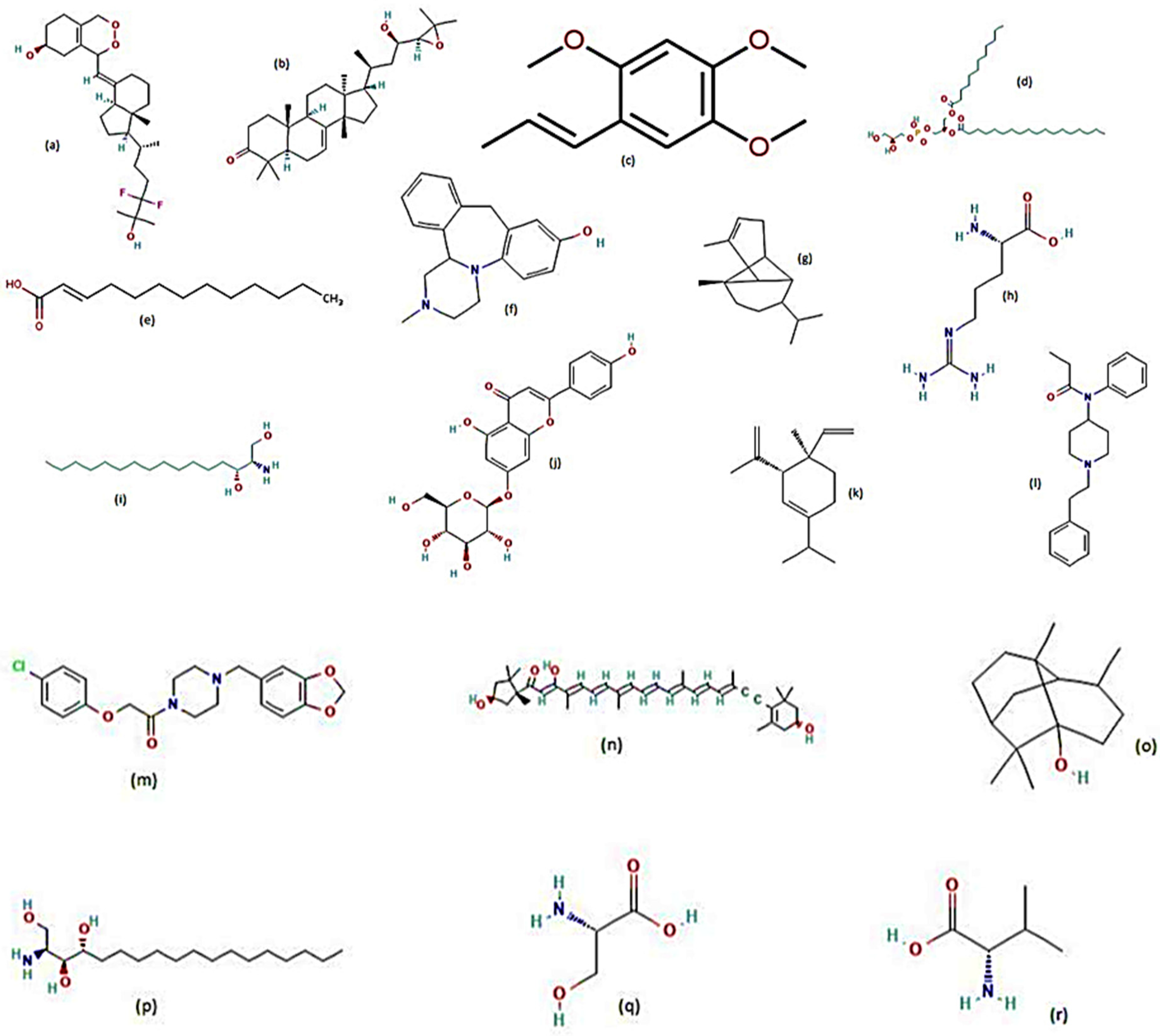
| Species/Plant Family | Part of Plant | Extract | Metabolites Detected | LC50 | LC90 | Time of Exposure to Larvae (hours) | Reference |
|---|---|---|---|---|---|---|---|
| Illicium verum (Schisandraceae) | Fruit | Essential oil | Trans-anethole | 2.4 to 3.4 % | - | 6 | [45] |
| Zanthoxylum limonella (Rutaceae) | Fruit | Essential oil | Limonene | 2.5 to 2.7% | - | 6 | [45] |
| Origanum vulgare (Lamiaceae) | Aerial parts | Essential oil | EO containing terpinen-4-ol, carvacrol, thymol | 37.5 μg/mL | - | 48 | [46] |
| Thymus vulgaris (Lamiaceae) | Aerial parts | Essential oil | EO containing thymol, p-cymene, γ-terpinene | 38.9 μg/mL | - | 48 | [46] |
| Annona mucosa (Annonaceae) | Seed | Ethanolic Extract | Rolliniatstatin 1, rollinicin | Rolliniastatin 1 = 0.43 μg/mL Rollinicin = 0.78 μg/mL | - | 24 | [47] |
| Cymbopogon citratus (Poaceae) | Leaves | Essential oil | EO containing citral, geranial, geraniol, β-myrcene | 120.6 ppm | - | 24 | [48] |
| Cymbopogon winteratus (Poaceae) | Leaves | Essential oil | EO containing citronellal, citronellol, geraniol, elemol | 38.8 ppm | - | 24 | [48] |
| Eucalyptus citriodora (Myrtaceae) | Leaves | Essential oil | EO containing citronellal, citronellol, isopulegol | 104.4 ppm | - | 24 | [48] |
| Eucalyptus camaldulensis (Myrtaceae) | Leaves | Essential oil | EO containing 1,8-cineole, α-pinene, citronellyl acetate | 33.7 ppm | - | 24 | [48] |
| Achillea bieberstenii (Asteraceae) | Aerial parts | Essential oil | EO containing α-terpinene, p-cymene | EO = 23.6 μL/L α-terpinene = 70.1 μL/L | - | 24 | [49] |
| Juniperus procera (Cupressaceae) | Aerial parts | Essential oil | EO containing eugenol, β-caryophyllene | EO = 12.2 μL/L Eugenol = 38.3 μL/L | - | 24 | [49] |
| Annona glabra (Annonaceae) | Leaves | Extract | Silver nanoparticles (An-AgNPs) | 5.945 mg/L for 24 h 3.5485 mg/L for 48 h | - | 24, 48 | [50] |
| Brassica napus (Brassicaceae) | Leaves | Essential oil | Lipids and fatty acid methyl esters | Fatty acid methyl esters = 342.8 ppm | - | 24 | [51] |
| Pavetta tomentosa (Rubiaceae) | Fresh Leaves | Extract | 2,6,10,14,18,22-tetracosane hexane; 2,6,10,15,19,23-hexamethyltetracosane | Crude extract = 5.96 μg/mL | Crude extract = 7.49 μg/mL | 24 | [52] |
| Tarenna asiatica (Rubiaceae) | Fresh Leaves | Extract | Tetracontane | Crude extract = 1.28 μg/mL | Crude extract = 1.99 μg/ml | 24 | [52] |
| Ambrosia arborescens (Asteraceae) | Leaves | Extract | Silver nanoparticles | 0.28 ppm | 0.43 ppm | 24 | [53] |
| Pinus sylvestris (Pinaceae) | Needles | Essential oil | 3-cyclohexane-1-methanol, alpha, alpha.4-trimethyl | EO = 100.39mg/L | - | 24 | [54] |
| Syzygium aromaticum (Myrtaceae) | Buds | Essential oil | Eugenol, eugenyl acetate | EO = 92.56 mg/L | - | 24 | [54] |
| Mentha villosa (Lamiaceae) | Leaves | Essential oil | EO containing rotundifolone | EO = 45 ppm Rotundifolone = 62.5 ppm | - | 24 | [55] |
| Carum carvi (Apiaceae) | Voucher specimen | Essential oil | Carvone, limonene, γ-terpenene | EO = 54.62 ppm | - | 24 | [56] |
| Apium graveolens (Apiaceae) | Voucher specimen | Essential oil | D-limonene, phthalides | EO = 42.07 ppm | - | 24 | [56] |
| Foeniculum vulgare (Apiaceae) | Voucher specimen | Essential oil | Trans-anethole, D-limonene, estragole | EO = 49.32 ppm | - | 24 | [56] |
| Zanthoxylum limonella (Rutaceae) | Voucher specimen | Essential oil | D-limonene, terpinen-4-ol, sabinene | EO = 24.61 ppm | - | 24 | [56] |
| Curcuma zedoaria (Zingiberaceae) | Voucher specimen | Essential oil | 1,8-cineole, p-cymene, α-phellandrene | EO = 31.87 ppm | - | 24 | [56] |
| Limonia acidissima (Rutaceae) | Leaves | Extract | Niloticin | 0.44 ppm | 1.17 ppm | 24 | [57] |
| Mentha arvensis (Lamiaceae) | Fresh Leaves | Essential oil | (Corn mint oil) containing menthol, methyl acetate, menthone and limonene | 78.1 ppm | 125.7 ppm | 24 | [58] |
| Plant Species/Family | Part of the Plant | Metabolites Detected | % Repellency | % Protection/Duration of Protection | Complete Protection Time (CPT) | Time of Exposure to theMosquito | Ref. |
|---|---|---|---|---|---|---|---|
| Origanum vulgare (Lamiaceae) | Aerial parts | Terpinen-4-ol, carvacrol, thymol | 8.9% to 37.8% (Essential oil) | - | - | 24 h | [46] |
| Thymus vulgaris (Lamiaceae) | Aerial parts | Thymol, p-cymene, γ-terpenes | 4.4% to 68.9% (Essential oil) | - | - | 24 h | [46] |
| Ateleia glazioviana (Fabaceae) | Leaves | Flavonoids | 84.5% (Dichloroethane extract) | - | - | 24 h | [59] |
| Ocimum basilicum (Lamiaceae) | Leaves | Eucalyptol, linalool, eugenol | 70.5% (Alcoholic spray derived from essential oil) | - | - | 24 h | [59] |
| Myristica fragrans (Myristicaceae) | Nutmeg oil | α-pinene, terpinen-4-ol, safrole | - | 100% protection for first 2 h, 90 to 23.32% for the next 4 h | - | 6 h | [84] |
| Mentha piperita (Lamiaceae) | Peppermint oil | Menthol | - | 96.67% to 27.78% for the first 4 h | - | 6 h | [84] |
| Ocimum basilicum (Lamiaceae) | Basil oil | Methyl chavicol, geraniol, methyl eugenol | - | 98.9% to 2.22% for 5 h | - | 6 h | [84] |
| Chenopodium ambrosioides (Amaranthaceae) | Aerial parts | Trans-pinocarveol, cis-carveol, trans-pinocarvyl acetate | 39.7% (Essential oil) | - | - | 5 min | [85] |
| Conyza sumatrensis (Asteraceae) | Aerial parts | Cis-lachnophyllum ester, limonene, trans-β-ocimene | 51.4% (Essential oil) | - | - | 5 min | [85] |
| Erigeron canadensis (Asteraceae) | Aerial parts | Limonene, matricaria ester | 80% (Essential oil) | - | - | 5 min | [85] |
| Eucalyptus camaldulensis (Myrtaceae) | Fresh leaves | Pinocarveol, myrtenol, β-phellandrene | 13.7% (Essential oil) | - | - | 5 min | [85] |
| Mentha spicata (Lamiaceae) | Aerial parts | Piperitenone oxide, eucalyptol | 100% (Essential oil) | - | - | 5 min | [85] |
| Parthenium hysterophorus (Asteraceae) | Aerial parts | Germacrene-D, β-myrcene, trans-β-ocimene | 63.9% (Essential oil) | - | - | 5 min | [85] |
| Targetes minuta (Asteraceae) | Aerial parts | Cis-β-ocimene, cis-tagetenone, limonene | 50.2% (Essential oil) | - | - | 5 min | [85] |
| Nepeta cataria (Lamiaceae) | CR9, CR3 crude essential oils | Z, E-nepetalactone and E, Z nepetalactone isomers | 10% CR9 crude oil showed >95% repellency for the first 2 h | - | - | 24 h | [41] |
| Cymbopogon citratus (Poaceae) | Leaves | Citral, limonene, α-terpineol, citronellol | - | - | The blend of all extracts was used For 1% w/v-1 h 2% w/v-2 to 3 h 5% w/v-5 to 6 h | - | [40] |
| Lantana Camara (Verbenaceae) | Leaves | Oleanonic acid, lantadene A&B, lantanilic acid | |||||
| Calotropis gigantea (Apocynaceae) | Leaves | Acetate, citrates, chloride | |||||
| Ocimum sanctum (Lamiaceae) | Leaves, flowers, branches | Eugenol, ursolic acid, rosmarinic acid | |||||
| Azadirachta indica (Meliaceae) | Leaves | Nimbolinin, nimbin, quercetin, nimbidiol | |||||
| Calotropis Procera (Apocynaceae) | Leaves | Uscharin, calotoxin, calotropeol acetate | |||||
| Angelica sinensis (Apiaceae / Umbelliferae) | Rhizome and root | 3-N-butylphthalide, butylidenephthalide, di-iso-octyl phthalate, ligustilide | - | - | 2.5 h (EO) 2.5 h (Ethanolic extract) 7.5 h (Hexane extract) 1.75 h (Acetone extract) 0.5 h (Methanolic extract) | Every 3 min in a 30 min interval | [86] |
| Mentha arvensis (Lamiaceae) | Fresh leaves | Essential oil (corn mint oil) containing menthol, methyl acetate, menthone, and limonene | - | - | 25% EO–45 min 90 min (50% EO) 165 min (100% EO) | - | [58] |
| Species/Plant Family | Part of Plant | Metabolites Detected | LC50 | LC90 | % Mortality | Time of Exposure to the Mosquito | Reference |
|---|---|---|---|---|---|---|---|
| Origanum vulgare (Lamiaceae) | Aerial parts | EO containing terpinen-4-ol, carvacrol, thymol | 14.3 μg/mL | - | - | 90 min | [46] |
| Thymus vulgaris (Lamiaceae) | Aerial parts | EO containing thymol, p-cymene, γ-terpinene | 11.7 μg/mL | - | - | 90 min | [46] |
| Achillea bieberstenii (Asteraceae) | Aerial parts | EO containing α-terpinene, p-cymene | 30.2 μL/L (EO) 66.8 μL/L (α-terpinene) 54.1 μL/L (p-cymene) | - | - | 24 h | [49] |
| Juniperus procera (Cupressaceae) | Aerial parts | EO containing eugenol, β-caryophyllene | 10.1 μL/L (EO) 18.3 μL/L (Eugenol) 46.4 μL/L (β-caryophyllene) | - | - | 24 h | [49] |
| Pavetta tomentosa (Rubiaceae) | Fresh leaves | 2,6,10,14,18,22-tetracosane hexane; 2,6,10,15,19,23-hexamethyltetracosane | 32.105 μg/mL (Crude extract) | 41.001 μg/mL (Crude extract) | - | 60 min | [52] |
| Tarenna asiatica (Rubiaceae) | Fresh leaves | Tetracontane | 9.012 μg/mL (Crude extract) | 11.854 μg/mL (Crude extract) | - | 60 min | [52] |
| Lippia alba (Verbenaceae) | Dry whole plant (voucher specimen) | Limonene, carvone, piperitenone | - | - | 24% (at 390 ppm of EO after 24 h) | 24 h | [88] |
| Lippia origanoides (Verbenaceae) | Dry whole plant (voucher specimen) | Carvacrol, p-cymene, thymol | - | - | 68% (at 300 ppm of EO within 2 min) | 24 h | [88] |
| Eucalyptus citriodora (Myrtaceae) | Dry whole plant (voucher specimen) | Citronellol, pulegol, citronellal | - | - | 13% (at 390 ppm of EO after 24 h) | 24 h | [88] |
| Cymbopogon flexuosus (Poaceae) | Dry whole plant (voucher specimen) | Neral, geraniol, geranyl acetate | - | - | >92% (at 1000 ppm of EO after 60 min) | 24 h | [88] |
| Citrus sinensis (Rutaceae) | Dry whole plant (voucher specimen) | Limonene, myrcene, n-octanol | - | - | 76% (at 390 ppm of EO after 24 h) | 24 h | [88] |
| Cananga odorata (Annonaceae) | Dry whole plant (voucher specimen) | Methyl benzoate, linalool | - | - | >92% (at 1000 ppm of EO after 60 min) | 24 h | [88] |
| Swinglea glutinosa (Rutaceae) | Dry whole plant (voucher specimen) | α-pinene, β-pinene, sabinene,1,8- cineole | - | - | >92% (at 1000 ppm of EO after 120 min) | 24 h | [88] |
| Tagetes lucida (Asteraceae) | Dry whole plant (voucher specimen) | Myrcene, estragole, trans-β-ocimene | - | - | >92% (at 1000 ppm of EO after 24 h) | 24 h | [88] |
| Plant Species/Family | Part of Plant | Metabolites Detected | LC50 | LC90 | % Mortality | LT50 | Time of Exposure to the Mosquito | Reference |
|---|---|---|---|---|---|---|---|---|
| Illicium verum (Schisandraceae) | Fruit | Trans-anethole | - | - | 86.4 to 100% (5% trans-anethole) | 6.9 to 28.8 h (5% trans-anethole) 1.5 to 5.2 h (2.5% EO + 2.5% trans-anethole) | 72 h | [45] |
| Zanthoxylum limonella (Rutaceae) | Fruit | Limonene | - | - | 89.6 to 94.4% (5% d-limonene)) | 23.8 to 28.5 h (5% d-limonene) 7.9 to 15.3 h (2.5% EO + 2.5% d-limonene) | 72 h | [45] |
| Pavetta tomentosa (Rubiaceae) | Fresh leaves | 2,6,10,14,18,22-tetracosane hexane; 2,6,10,15,19,23 -hexamethyltetracosane | For 24 h, Acetone extract = 1.361 μg/mL; Hexane extract = 2.044 μg/mL; Chloroform extract = 2.512 μg/mL For 48 h, Acetone extract = 3.273 μg/mL; Hexane extract = 1.682 μg/mL; Chloroform extract = 2.298 μg/mL | - | - | - | 24 and 48 h | [52] |
| Tarenna asiatica (Rubiaceae) | Fresh leaves | Tetracontane | For 24 h, Acetone extract = 1.682 μg/mL; Hexane extract = 1.990 g/mL; Chloroform extract = 2.429 μg/mL For 48 h, Acetone extract = 4.555 μg/mL; Hexane extract = 3.008 μg/mL; Chloroform extract = 3.975 μg/mL | - | - | - | 24 and 48 h | [52] |
| Lippia alba (Verbenaceae) | Dry whole plant (voucher specimen) | Limonene, carvone, piperitenone | - | - | 24% and 29% after 24 and 48 h (at 390 ppm of EO) | - | 24 and 48 h | [88] |
| Lippia origanoides (Verbenaceae) | Dry whole plant (voucher specimen) | Carvacrol, p-cymene, thymol | - | - | 67% and 73% after 24 and 48 h (at 250 ppm of EO) | - | 24 and 48 h | [88] |
| Eucalyptus citriodora (Myrtaceae) | Dry whole plant (voucher specimen) | Citronellol, pulegol, citronellal | - | - | 13% and 47% after 24 and 48 h (at 390 ppm of EO) | - | 24 and 48 h | [88] |
| Cymbopogon flexuosus (Poaceae) | Dry whole plant (voucher specimen) | Neral, geraniol, geranyl acetate | - | - | 13% and 47% after 24 and 48 h (at 390 ppm of EO) | - | 24 and 48 h | [88] |
| Citrus sinensis (Rutaceae) | Dry whole plant (voucher specimen) | Limonene, myrcene, n-octanol | - | - | 27% and 42% after 24 and 48 h (at 390 ppm of EO) | - | 24 and 48 h | [88] |
| Cananga odorata (Annonaceae) | Dry whole plant (voucher specimen) | Methyl benzoate, linalool | - | - | 27% and 56% after 24 and 48 h (at 390 ppm of EO) | - | 24 and 48 h | [88] |
| Swinglea glutinosa (Rutaceae) | Dry whole plant (voucher specimen) | α-pinene, β-pinene, sabinene,1,8- cineole | - | - | 38% and 73% after 24 and 48 h (at 390 ppm of EO) | - | 24 and 48 h | [88] |
| Tagetes lucida (Asteraceae) | Dry whole plant (voucher specimen) | Myrcene, estragole, trans-β-ocimene | - | - | 56% and 67% after 24 and 48 h (at 390 ppm of EO) | - | 24 and 48 h | [88] |
| Limonia acidissima (Rutaceae) | Leaves | Niloticin | 0.62 ppm (Niloticin) 4.19 ppm (Hexane extract) | 1.45 ppm (Niloticin) 8.10 ppm (Hexane Extract) | - | - | 24 h | [57] |
| Plant Species/Family | Part of the Plant | Metabolite Detected | Ovicidal Activity (% Mortality) | Oviposition Deterrent Activity (Number of Eggs Laid) | Time of Exposure to the Eggs/Adult Aedes aegypti | Ref. |
|---|---|---|---|---|---|---|
| Limonia acidissima (Rutaceae) | Leaves | Niloticin | 83.2% (at 2 ppm of niloticin) | - | 120 h | [57] |
| Cyanthocline purpurea (Asteraceae) | Leaves | 3-n-decyl acrylic acid, C16 sphinganine, mytiloxanthin | >70% (ethanolic extract at 0.2 mg/mL) | - | 48 h | [89] |
| Blumea lacera (Asteraceae) | Leaves | Phytosphingosine, cosmosiin, valine, serine, arginine | ≈75% (ethanolic extract at 0.1 mg/mL) | - | 48 h | [89] |
| Neanotis lancifolia (Rubiaceae) | Leaves | Fentanyl, 8-hydroxy mianserin, 1-dodecanoyl-2-octadecanoyl-glycero-3-phospho-(1′-sn-glycerol) | 90% (ethanolic extract at 0.1 mg/mL) | - | 48 h | [89] |
| Neanotis montholonii (Rubiaceae) | Leaves | (6RS)-6,19-epidioxy-24,24 difluoro-25-hydroxy-6,19-dihydrovitamin D3/(6RS)-6,19-epidioxy-24,24-dif, 1-dodecanoyl-2-octadecanoyl-glycero-3-phospho-(1′-sn-glycerol), fipexide | ≈90% (ethanolic extract at 0.1 mg/mL) | - | 48 h | [89] |
| Piper marginatum (Piperaceae) | Leaves and stem | D-elemene, α-Copaene, patchouli alcohol, (Z)-asarone | - | <50% eggs laid (at 50 and 100 ppm of leaf and stem extracts) <40% eggs laid (essential oil) | 14 h | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Priya, S.S.; Vasantha-Srinivasan, P.; Altemimi, A.B.; Keerthana, R.; Radhakrishnan, N.; Senthil-Nathan, S.; Kalaivani, K.; Chandrasekar, N.; Karthi, S.; Ganesan, R.; et al. Bioactive Molecules Derived from Plants in Managing Dengue Vector Aedes aegypti (Linn.). Molecules 2023, 28, 2386. https://doi.org/10.3390/molecules28052386
Priya SS, Vasantha-Srinivasan P, Altemimi AB, Keerthana R, Radhakrishnan N, Senthil-Nathan S, Kalaivani K, Chandrasekar N, Karthi S, Ganesan R, et al. Bioactive Molecules Derived from Plants in Managing Dengue Vector Aedes aegypti (Linn.). Molecules. 2023; 28(5):2386. https://doi.org/10.3390/molecules28052386
Chicago/Turabian StylePriya, Sridhar Shanmuga, Prabhakaran Vasantha-Srinivasan, Ammar B. Altemimi, Ramji Keerthana, Narayanaswamy Radhakrishnan, Sengottayan Senthil-Nathan, Kandasamy Kalaivani, Nainarpandian Chandrasekar, Sengodan Karthi, Raja Ganesan, and et al. 2023. "Bioactive Molecules Derived from Plants in Managing Dengue Vector Aedes aegypti (Linn.)" Molecules 28, no. 5: 2386. https://doi.org/10.3390/molecules28052386
APA StylePriya, S. S., Vasantha-Srinivasan, P., Altemimi, A. B., Keerthana, R., Radhakrishnan, N., Senthil-Nathan, S., Kalaivani, K., Chandrasekar, N., Karthi, S., Ganesan, R., Alkanan, Z. T., Pal, T., Verma, O. P., & Proćków, J. (2023). Bioactive Molecules Derived from Plants in Managing Dengue Vector Aedes aegypti (Linn.). Molecules, 28(5), 2386. https://doi.org/10.3390/molecules28052386











