On the Nature of the Partial Covalent Bond between Noble Gas Elements and Noble Metal Atoms
Abstract
1. Introduction
2. Computational Tools to Analyze the Bonding
3. The General Bonding Picture
4. Types of Noble Gas Compounds
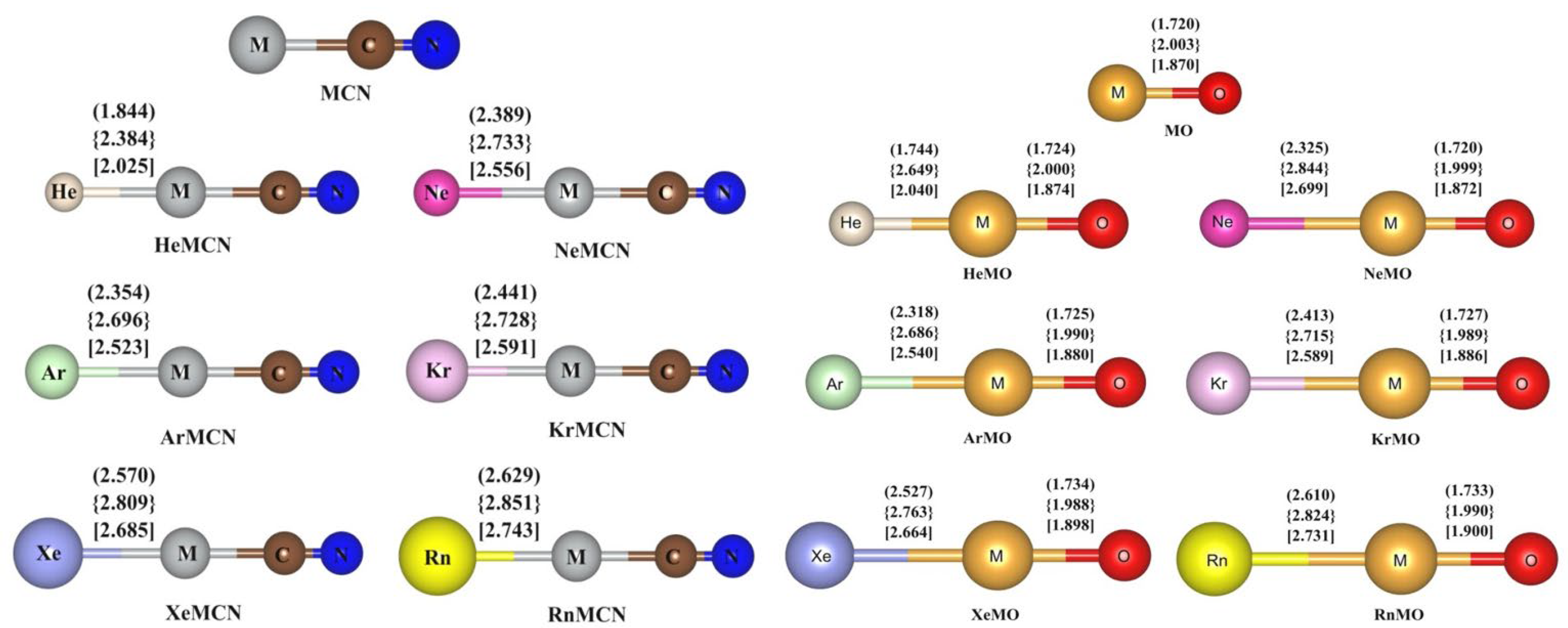
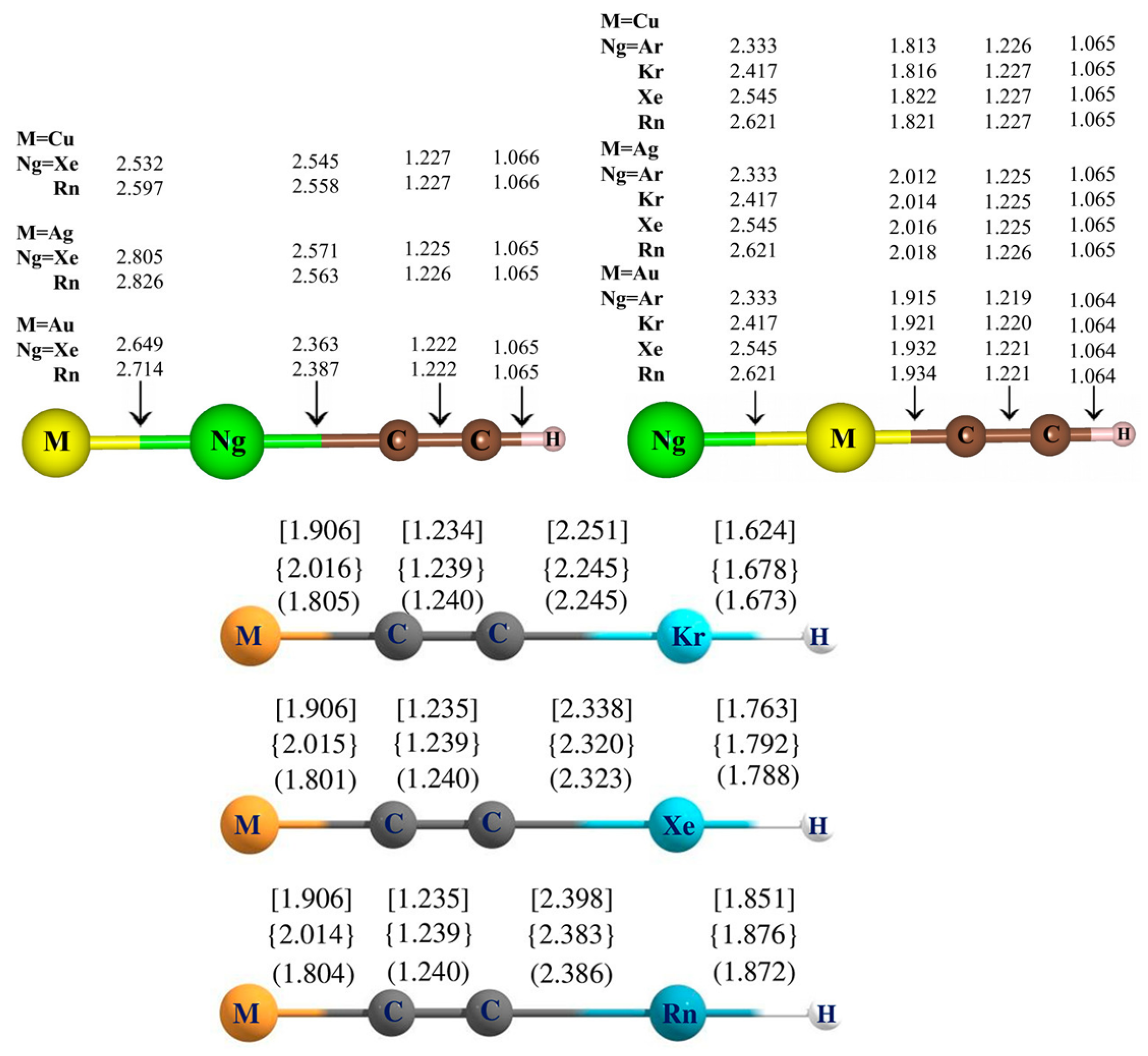
5. Case Studies of Noble Gas−Noble Metal Binding
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kossel, W. Über Molekülbildung als Frage des Atombaus. Ann. Der Phys. 1916, 354, 229–362. [Google Scholar] [CrossRef]
- Von Antropoff, A. Die Wertigkeit der Edelgase und ihre Stellung im periodischen System. II. Angew. Chem. Int. Ed. 1924, 37, 695–696. [Google Scholar] [CrossRef]
- Pauling, L. The formulas of antimonic acid and the antimonates. J. Am. Chem. Soc. 1933, 55, 1895–1900. [Google Scholar] [CrossRef]
- Bartlett, N.; Lohmann, D. 1005. Fluorides of the noble metals. Part II. Dioxygenyl hexafluoroplatinate (V), O2+[PtF6]-. J. Am. Chem. Soc. 1962, 5253–5261. [Google Scholar] [CrossRef]
- Bartlett, N. Xenon hexafluoroplatinate (V) Xe+[PtF6]-. Proc. Chem. Soc. Lond. 1962, 218, 2933. [Google Scholar]
- Hargittai, I. Neil Bartlett and the first noble-gas compound. Struct. Chem. 2009, 20, 953–959. [Google Scholar] [CrossRef]
- Grandinetti, F. 60 years of chemistry of the noble gases. Nature 2022, 606, 659–661. [Google Scholar] [CrossRef]
- Graham, L.; Graudejus, O.; Jha, N.K.; Bartlett, N. Concerning the nature of XePtF6. Coord. Chem. Rev. 2000, 197, 321–334. [Google Scholar] [CrossRef]
- Streng, A.; Kirshenbaum, A.; Streng, L.; Grosse, A. Preparation of Rare-Gas Fluorides and Oxyfluorides by the Electric Discharge Method and their Properties. In Noble Gas Compounds; Hyman, H.H., Ed.; The University of Chicago Press: Chicago, IL, USA, 1963; pp. 73–80. [Google Scholar]
- Lehmann, J.F.; Mercier, H.P.; Schrobilgen, G.J. The chemistry of krypton. Coord. Chem. Rev. 2002, 233, 1–39. [Google Scholar] [CrossRef]
- Claassen, H.H.; Selig, H.; Malm, J.G. Xenon tetrafluoride. J. Am. Chem. Soc. 1962, 84, 3593. [Google Scholar] [CrossRef]
- Slivnik, J.; Brcic, B.; Volavsek, B.; Marsel, J.; Vrscaj, V.; Smalc, A.; Frlec, B.; Zemljic, Z. Über die Synthese von XeF6. Croat. Chem. Acta 1962, 34, 253. [Google Scholar]
- Turner, J.; Pimentel, G.C. Krypton fluoride: Preparation by the matrix isolation technique. Science 1963, 140, 974–975. [Google Scholar] [CrossRef]
- Nelson, L.Y.; Pimentel, G.C. Infrared detection of xenon dichloride. Inorg. Chem. 1967, 6, 1758–1759. [Google Scholar] [CrossRef]
- Bartlett, N.; Wechsberg, M. The Xenon Difluoride Complexes XeF2 · XeOF4; XeF2 · XeF6 · AsF5 and XeF2 · 2XeF6 · 2AsF5 and Their Relevance to Bond Polarity and Fluoride Ion Donor Ability of XeF2 and XeF6. Z. Anorg. Allg. Chem. 1951, 455, 5–17. [Google Scholar]
- Holloway, J.H.; Hope, E.G. Recent advances in noble-gas chemistry. Adv. Inorg. Chem. 1998, 46, 51–100. [Google Scholar]
- Stein, L. Ionic radon solutions. Science 1970, 168, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Khriachtchev, L.; Pettersson, M.; Runeberg, N.; Lundell, J.; Räsänen, M. A stable argon compound. Nature 2000, 406, 874. [Google Scholar] [CrossRef]
- Frenking, G. Another noble gas conquered. Nature 2000, 406, 836. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X. Infrared Spectra of NgBeS (Ng = Ne, Ar, Kr, Xe) and BeS2 in Noble-Gas Matrices. J. Phys. Chem. A 2013, 117, 1508–1513. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, M.; Zhou, M.; Andrada, D.M.; Frenking, G. Experimental and Theoretical Studies of the Infrared Spectra and Bonding Properties of NgBeCO3 and a Comparison with NgBeO (Ng = He, Ne, Ar, Kr, Xe). J. Phys. Chem. A 2014, 119, 2543–2552. [Google Scholar] [CrossRef]
- Yu, W.; Liu, X.; Xu, B.; Xing, X.; Wang, X. Infrared Spectra of Novel NgBeSO2 Complexes (Ng = Ne, Ar, Kr, Xe) in Low Temperature Matrixes. J. Phys. Chem. A 2016, 120, 8590–8598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, W.L.; Zhao, L.; Chen, M.; Zhou, M.; Li, J.; Frenking, G. A Very Short Be-Be Distance but No Bond: Synthesis and Bonding Analysis of Ng-Be2O2-Ng0 (Ng, Ng = Ne, Ar, Kr, Xe). Chem. Eur. J. 2017, 23, 2035–2039. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Oganov, A.R.; Goncharov, A.F.; Stavrou, E.; Lobanov, S.; Saleh, G.; Qian, G.-R.; Zhu, Q.; Gatti, C.; Deringer, V.L.; et al. A stable compound of helium and sodium at high pressure. Nat. Chem. 2017, 9, 440–445. [Google Scholar] [CrossRef]
- Pyykko, P. Predicted chemical bonds between rare gases and Au. J. Am. Chem. Soc. 1995, 117, 2067–2070. [Google Scholar] [CrossRef]
- Schr€oder, D.; Schwarz, H.; Hrusak, J.; Pyykko, P. Cationic gold (I) complexes of xenon and of ligands containing the donor atoms oxygen, nitrogen, phosphorus, and sulfur. Inorg. Chem. 1998, 37, 624–632. [Google Scholar] [CrossRef]
- Read, J.P.; Buckingham, A.D. Covalency in ArAu+ and Related Species? J. Am. Chem. Soc. 1997, 119, 9010–9013. [Google Scholar] [CrossRef]
- Evans, C.J.; Lesarri, A.; Gerry, M.C.L. Noble Gas—Metal Chemical Bonds. Microwave Spectra, Geometries, and Nuclear Quadrupole Coupling Constants of Ar-AuCl and Kr-AuCl. J. Am. Chem. Soc. 2000, 122, 6100–6105. [Google Scholar] [CrossRef]
- Seidel, S.; Seppelt, K. Xenon as a complex ligand: The tetra Xenono Gold (II) cation in AuXe42+(Sb2F11−)2. Science 2000, 290, 117–118. [Google Scholar] [CrossRef]
- Cooke, S.A.; Gerry, M.C.L. Insights into the Xenon–Silver Halide Interaction from a Rotational Spectroscopic Study of XeAgF and XeAgCl. Phys. Chem. Chem. Phys. 2004, 6, 3248–3256. [Google Scholar] [CrossRef]
- Cooke, S.A.; Gerry, M.C.L. XeAuF. J. Am. Chem. Soc. 2004, 126, 17000–17008. [Google Scholar] [CrossRef]
- Thomas, J.M.; Walker, N.R.; Cooke, S.A.; Gerry, M.C.L. Microwave Spectra and Structures of KrAuF, KrAgF, and KrAgBr; 83Kr Nuclear Quadrupole Coupling and the Nature of Noble Gas−Noble Metal Halide Bonding. J. Am. Chem. Soc. 2004, 126, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Michaud, J.M.; Cooke, S.A.; Gerry, M.C.L. Rotational Spectra, Structures, Hyperfine Constants, and the Nature of the Bonding of KrCuF and KrCuCl. Inorg. Chem. 2004, 43, 3871–3881. [Google Scholar] [CrossRef]
- Michaud, J.M.; Gerry, M.C.L. XeCu Covalent Bonding in XeCuF and XeCuCl, Characterized by Fourier Transform Microwave Spectroscopy Supported by Quantum Chemical Calculations. J. Am. Chem. Soc. 2006, 128, 7613–7621. [Google Scholar] [CrossRef] [PubMed]
- Lantto, P.; Vaara, J. Calculations of nuclear quadrupole coupling in noble gas–noble metal fluorides: Interplay of relativistic and electron correlation effects. J. Chem. Phys. 2006, 125, 174315. [Google Scholar] [CrossRef]
- Mou, C.H.; Witek, H.A. Theoretical study of noble-gas containing metal halides. J. Chem. Phys. 2008, 129, 244310. [Google Scholar] [CrossRef]
- Zou, W.; Liu, Y.; Boggs, J.E. Theoretical study of RgMF (Rg= He, Ne; M= Cu, Ag, Au): Bonded structures of helium. Chem. Phys. Lett. 2009, 482, 207–210. [Google Scholar] [CrossRef]
- Chen, R.; Zhu, H.; Xie, D.Q.; GuoSen, G.S. Theoretical prediction of the noble gas complexes HeAuF and NeAuF. Sci. Chin. Ser. B Chem. 2009, 52, 1987–1990. [Google Scholar] [CrossRef]
- Evans, C.J.; Wright, T.G.; Gardner, A.M. Geometries and Bond Energies of the He− MX, Ne− MX, and Ar− MX (M= Cu, Ag, Au; X= F, Cl) Complexes. J. Phys. Chem. A 2010, 114, 4446–4454. [Google Scholar] [CrossRef]
- Beyhan, S.M.; Gotz, A.W.; Jacob, C.R.; Visscher, L. The weak covalent bond in NgAuF (Ng = Ar, Kr, Xe): A challenge for subsystem density functional theory. J. Chem. Phys. 2010, 132, 044114. [Google Scholar] [CrossRef]
- Wang, X.; Andrews, L.; Brosi, F.; Riedel, S. Matrix Infrared Spectroscopy and Quantum-Chemical Calculations for the Coinage-Metal Fluorides: Comparisons of Ar-AuF, Ne-AuF, and Molecules MF2 and MF3. Chem. Eur. J. 2013, 19, 1397–1409. [Google Scholar] [CrossRef]
- Zhang, P.X.; Zhao, Y.F.; Hao, F.Y.; Zhang, G.H.; Song, X.D.; Li, X.Y. Bonding analysis for NgMOH (Ng= Ar, Kr and Xe; M= Cu and Ag). Mol. Phys. 2008, 106, 1007–1014. [Google Scholar] [CrossRef]
- Zhang, P.X.; Zhao, Y.F.; Hao, F.Y.; Li, X.Y. Bonding analysis for NgAuOH (Ng= Kr, Xe). Int. J. Quantum Chem. 2008, 108, 937–944. [Google Scholar] [CrossRef]
- Ghanty, T.K. Insertion of noble-gas atom (Kr and Xe) into noble-metal molecules (AuF and AuOH): Are they stable? J. Chem. Phys. 2005, 123, 074323. [Google Scholar] [CrossRef]
- Ghanty, T.K. How strong is the interaction between a noble gas atom and a noble metal atom in the insertion compounds MNgF (M=Cu and Ag, and Ng=Ar, Kr, and Xe)? J. Chem. Phys. 2006, 124, 124304. [Google Scholar] [CrossRef]
- Glendening, E.D.; Landis, C.R.; Weinhold, F. NBO6.0: Natural bond orbital analysis program. J. Comput. Chem. 2013, 34, 1429–1437. [Google Scholar] [CrossRef]
- Wiberg, K.B. Application of the Pople-Santry-Segal CNDO Method to the Cyclopropylcarbinyl and Cyclobutyl Cation and to Bicyclobutane. Tetrahedron 1968, 24, 1083–1096. [Google Scholar] [CrossRef]
- Bader, R.F. Atoms in molecules. Acc. Chem. Res. 1985, 18, 9–15. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Bader, R.F.W. Molecular fragments or chemical bonds. Acc. Chem. Res. 1975, 8, 34–40. [Google Scholar] [CrossRef]
- Silvi, B.; Savin, A. Classification of chemical bonds based on topological analysis of electron localization functions. Nature 1994, 371, 683–686. [Google Scholar] [CrossRef]
- Becke, A.D.; Edgecombe, K.E. A simple measure of electron localization in atomic and molecular systems. J. Chem. Phys. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Fonseca, G.C.; Handgraaf, J.W.; Baerends, E.J.; Bickelhaupt, F.M. Voronoi deformation density (VDD) charges: Assessment of the Mulliken, Bader, Hirshfeld, Weinhold, and VDD methods for charge analysis. J. Comput. Chem. 2004, 25, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Haaland, A.; Helgaker, T.U.; Ruud, K.; Shorokhov, D.J. Should Gaseous BF3 and SiF4Be Described as Ionic Compounds? J. Chem. Educ. 2000, 77, 1076. [Google Scholar] [CrossRef]
- De Proft, F.; Van Alsenoy, C.; Peeters, A.; Langenaeker, W.; Geerlings, P. Atomic charges, dipole moments, and Fukui functions using the Hirshfeld partitioning of the electron density. J. Comput. Chem. 2002, 23, 1198–1209. [Google Scholar] [CrossRef] [PubMed]
- Jensen, F. Introduction To Computational Chemistry; John Wiley and Sons: New York, NY, USA, 1999. [Google Scholar]
- Brom, J.M.; Schmitz, B.J.; Thompson, J.D.; Cramer, C.J.; Truhlar, D.G. A Class IV Charge Model for Boron Based on Hybrid Density Functional Theory. J. Phys. Chem. A 2003, 107, 6483–6488. [Google Scholar] [CrossRef]
- Bader, R.F.W.; Matta, C.F. Atoms in molecules as non-overlapping, bounded, space-filling open quantum systems. Found. Chem. 2013, 15, 253–276. [Google Scholar] [CrossRef]
- Bader, R.F.W.; Zou, P.F. An atomic population as the expectation value of a quantum observable. Chem. Phys. Lett. 1992, 191, 54–58. [Google Scholar]
- Borocci, S.; Grandinetti, F.; Sanna, N. Noble-gas compounds: A general procedure of bonding analysis. J. Chem. Phys. 2022, 156, 014104. [Google Scholar] [CrossRef]
- Makarewicz, E.; Gordon, A.J.; Berski, S. Nature of the Bonding in the AuNgX (Ng = Ar, Kr, Xe; X = F, Cl, Br, I) Molecules. Topological Study on Electron Density and the Electron Localization Function (ELF). J. Phys. Chem. A 2015, 119, 2401–2412. [Google Scholar] [CrossRef]
- Borocci, S.; Giordani, M.; Grandinetti, F. Bonding Motifs of Noble-Gas Compounds as Described by the Local Electron Energy Density. J. Phys. Chem. A 2015, 119, 6528–6541. [Google Scholar] [CrossRef]
- Borocci, S.; Grandinetti, F.; Nunzi, F.; Sanna, N. Classifying the chemical bonds involving the noble-gas atoms. New J. Chem. 2020, 44, 14536–14550. [Google Scholar] [CrossRef]
- Michalak, A.; Mitoraj, M.; Ziegler, T. Bond orbitals from chemical valence theory. J. Phys. Chem. A 2008, 112, 1933–1939. [Google Scholar] [CrossRef]
- Mitoraj, M.P.; Michalak, A.; Ziegler, T. A combined charge and energy decomposition scheme for bond analysis. J. Chem. Theory Comput. 2009, 5, 962–975. [Google Scholar]
- Pan, S.; Saha, R.; Chattaraj, P.K. Exploring the nature of silicon-noble gas bonds in H3SiNgNSi and HSiNgNSi compounds (Ng = Xe, Rn). Int. J. Mol. Sci. 2015, 16, 6402–6418. [Google Scholar] [CrossRef]
- Tonner, R.; Frenking, G. Divalent carbon (0) chemistry, part 1: Parent compounds. Chem. Eur. J. 2008, 14, 3260–3272. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Baerends, E.J.; Ziegler, T.; Autschbach, J.; Bashford, D.; B_erces, A.; Bickelhaupt, F.M.; Bo, C.; Boerrigter, P.M.; Cavallo, L.; Chong, D.P.; et al. ADF2013.01; SCM, Theoretical Chemistry; Vrije Universiteit: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Pal, R.; Patra, S.G.; Chattaraj, P.K. Can a chemical bond be exclusively covalent or ionic? J. Chem. Sci. 2022, 134, 108. [Google Scholar] [CrossRef]
- Li, T.H.; Liu, Y.L.; Lin, R.J.; Yeh, T.Y.; Hu, W.P. On the stability of noble gas molecules. Chem. Phys. Lett. 2007, 434, 38–41. [Google Scholar] [CrossRef]
- Krapp, A.; Frenking, G. Is this a chemical bond? a theoretical study of Ng2@C60 (Ng= He, Ne, Ar, Kr, Xe). Chem. Eur. J. 2007, 13, 8256–8270. [Google Scholar] [CrossRef]
- Khatua, M.; Pan, S.; Chattaraj, P.K. Movement of Ng2 molecules confined in a C60 cage: An ab initio molecular dynamics study. Chem. Phys. Lett. 2014, 610, 351–356. [Google Scholar] [CrossRef]
- Pan, S.; Gupta, A.; Saha, R.; Merino, G.; Chattaraj, P.K. A coupled-cluster study on the noble gas binding ability of metal cyanides versus metal halides (metal = Cu, Ag, Au). J. Comput. Chem. 2015, 36, 2168–2176. [Google Scholar] [CrossRef]
- Pan, S.; Saha, R.; Kumar, A.; Gupta, A.; Merino, G.; Chattaraj, P.K. A noble interaction: An assessment of noble gas binding ability of metal oxides (metal = Cu, Ag, Au). Int. J. Quantum Chem. 2016, 116, 1016–1024. [Google Scholar] [CrossRef]
- Ghara, M.; Pan, S.; Deb, J.; Kumar, A.; Sarkar, U.; Chattaraj, P.K. A computational study on structure, stability and bonding in Noble Gas bound metal Nitrates, Sulfates and Carbonates (metal = Cu, Ag, Au). J. Chem. Sci. 2016, 128, 1537–1548. [Google Scholar] [CrossRef]
- Pople, J.A.; Head-Gordon, M.; Raghavachari, K. Quadratic configuration interaction. A general technique for determining electron correlation energies. J. Chem. Phys. 1987, 87, 5968–5975. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Dunning Jr, T.H. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H., Jr.; Harrison, R.J. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar]
- Woon, D.E.; Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations. III. The atoms aluminum through argon. J. Chem. Phys. 1993, 98, 1358–1371. [Google Scholar] [CrossRef]
- Peterson, K.A.; Woon, D.E.; Dunning, T.H., Jr. Benchmark calculations with correlated molecular wave functions. IV. The classical barrier height of the H+ H2→ H2+ H reaction. J. Chem. Phys. 1994, 100, 7410–7415. [Google Scholar]
- Jana, G.; Saha, R.; Pan, S.; Kumar, A.; Merino, G.; Chattaraj, P.K. Noble gas binding ability of metal-bipyridine monocationic complexes (metal = Cu, Ag, Au): A computational study. ChemistrySelect 2016, 1, 5842–5849. [Google Scholar] [CrossRef]
- Jana, G.; Pan, S.; Merino, G.; Chattaraj, P.K. MNgCCH (M = Cu, Ag, Au; Ng = Xe, Rn): The First Set of Compounds with M–Ng–C Bonding Motif. J. Phys. Chem. A 2017, 121, 6491–6499. [Google Scholar] [CrossRef]
- Jana, G.; Pan, S.; Merino, G.; Chattaraj, P.K. Noble Gas Inserted Metal Acetylides (Metal = Cu, Ag, Au). J. Phys. Chem. A 2018, 122, 7391–7401. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Hybrid Meta Density Functional Theory Methods for Thermochemistry, Thermochemical Kinetics, and Noncovalent Interactions: The MPW1B95 and MPWB1K Models and Comparative Assessments for Hydrogen Bonding and van der Waals Interactions. J. Phys. Chem. A 2004, 108, 6908–6918. [Google Scholar] [CrossRef]
- Moller, C.; Plesset, M.S. Note on an Approximation Treatment for Many-Electron Systems. Phys. Rev. 1934, 46, 618. [Google Scholar] [CrossRef]
- Woon, D.E.; Dunning Jr, T.H. Gaussian basis sets for use in correlated molecular calculations. V. Core-valence basis sets for boron through neon. J. Chem. Phys. 1995, 103, 4572–4585. [Google Scholar] [CrossRef]
- Pearson, R.G. The principle of maximum hardness. Acc. Chem. Res. 1993, 26, 250–255. [Google Scholar] [CrossRef]
- Pearson, R.G. Maximum Chemical and Physical Hardness. J. Chem. Educ. 1999, 76, 267. [Google Scholar] [CrossRef]
- Pan, S.; Solà, M.; Chattaraj, P.K. On the validity of the maximum hardness principle and the minimum electrophilicity principle during chemical reactions. J. Phys. Chem. A 2013, 117, 1843–1852. [Google Scholar] [CrossRef]
- Chamorro, E.; Chattaraj, P.K.; Fuentealba, P. Variation of the electrophilicity index along the reaction path. J. Phys. Chem. A 2003, 107, 7068–7072. [Google Scholar] [CrossRef]
- Parthasarathi, R.; Elango, M.; Subramanian, V.; Chattaraj, P.K. Variation of electrophilicity during molecular vibrations and internal rotations. Theor. Chem. Acc. 2005, 113, 257–266. [Google Scholar] [CrossRef]
- Chattaraj, P.K.; Giri, S. A minimum electrophilicity perspective of the HSAB principle. Indian J. Phys. 2007, 81, 871–879. [Google Scholar]
- Miranda-Quintana, R.A.; Chattaraj, P.K.; Ayers, P.W. Finite temperature grand canonical ensemble study of the minimum electrophilicity principle. J. Chem. Phys. 2017, 147, 124103. [Google Scholar] [CrossRef] [PubMed]

| System | BCP | ρ(rc) | ∇2ρ(rc) | G(rc) | V(rc) | H(rc) | G(rc)/ρ(rc) | ELF |
|---|---|---|---|---|---|---|---|---|
| HF | H-F | 0.412 | −0.339 | 0.999 | −0.105 | −0.947 | 2.425 | 0.977 |
| HCl | H-Cl | 0.310 | −0.157 | 0.143 | −0.394 | −0.394 | 0.461 | 0.999 |
| HBr | H-Br | 0.193 | −0.420 | 0.530 | −0.211 | −0.158 | 2.747 | 0.924 |
| LiF | Li-F | 0.079 | 0.705 | 0.163 | −0.149 | 0.014 | 2.056 | 0.062 |
| LiCl | Li-Cl | 0.047 | 0.274 | 0.065 | −0.062 | 0.003 | 1.391 | 0.067 |
| LiBr | Li-Br | 0.040 | 0.198 | 0.048 | −0.046 | 0.002 | 1.210 | 0.070 |
| NaF | Na-F | 0.054 | 0.440 | 0.101 | −0.091 | 0.009 | 1.865 | 0.046 |
| NaCl | Na-Cl | 0.035 | 0.200 | 0.045 | −0.040 | 0.005 | 1.279 | 0.054 |
| NaBr | Na-Br | 0.030 | 0.150 | 0.034 | −0.030 | 0.004 | 1.109 | 0.059 |
| KF | K-F | 0.052 | 0.291 | 0.071 | −0.068 | 0.002 | 1.353 | 0.081 |
| KCl | K-Cl | 0.031 | 0.131 | 0.031 | −0.285 | 0.002 | 0.984 | 0.077 |
| KBr | K-Br | 0.027 | 0.099 | 0.023 | −0.022 | 0.002 | 0.868 | 0.080 |
| MgF2 | Mg-F | 0.082 | 0.759 | 0.179 | −0.168 | 0.011 | 2.177 | 0.058 |
| MgCl2 | Mg-Cl | 0.055 | 0.311 | 0.077 | −0.076 | 0.001 | 1.392 | 0.082 |
| MgBr2 | Mg-Br | 0.048 | 0.222 | 0.056 | −0.057 | −0.001 | 1.171 | 0.095 |
| CaF2 | Ca-F | 0.045 | 0.150 | 0.041 | −0.044 | −0.003 | 0.909 | 0.109 |
| CaCl2 | Ca-Cl | 0.052 | 0.203 | 0.054 | −0.058 | −0.004 | 1.042 | 0.129 |
| CaBr2 | Ca-Br | 0.045 | 0.150 | 0.041 | −0.044 | −0.003 | 0.909 | 0.138 |
| H2 | H-H | 0.250 | −0.982 | 0.548 | −0.246 | −0.246 | 2.195 | 0.999 |
| N2 | N-N | 0.720 | −0.287 | 0.629 | −0.198 | −0.135 | 0.875 | 0.874 |
| O2 | O-O | 0.546 | −0.960 | 0.492 | −0.122 | −0.732 | 0.900 | 0.820 |
| F2 | F-F | 0.294 | 0.564 | 0.287 | −0.433 | −0.146 | 0.977 | 0.628 |
| Cl2 | Cl-Cl | 0.154 | −0.205 | 0.711 | −0.147 | −0.763 | 4.634 | 0.759 |
| CO | C-O | 0.508 | 0.724 | 0.114 | −0.209 | −0.955 | 0.223 | 0.401 |
| H2O | O-H | 0.362 | −0.241 | 0.729 | −0.749 | −0.676 | 2.015 | 0.981 |
| NH3 | N-H | 0.337 | −0.157 | 0.603 | −0.514 | −0.454 | 1.790 | 0.984 |
| CH4 | C-H | 0.278 | −0.947 | 0.428 | −0.322 | −0.279 | 1.542 | 0.984 |
| C2H4 | C-C | 0.359 | −0.112 | 0.147 | −0.574 | −0.428 | 0.409 | 0.926 |
| C2H2 | C-C | 0.426 | −0.125 | 0.314 | −0.940 | −0.626 | 0.737 | 0.830 |
| ArCuNO3 | Ar-Cu | 0.042 | 0.209 | 0.056 | −0.059 | −0.003 | 1.312 | 0.066 |
| Cu-N | 0.069 | 0.322 | 0.096 | −0.111 | −0.015 | 1.397 | 0.106 | |
| KrCuNO3 | Kr-Cu | 0.053 | 0.188 | 0.053 | −0.058 | −0.006 | 1.000 | 0.096 |
| Cu-N | 0.068 | 0.320 | 0.095 | −0.110 | −0.015 | 1.394 | 0.106 | |
| XeCuNO3 | Xe-Cu | 0.049 | 0.157 | 0.047 | −0.055 | −0.008 | 0.957 | 0.139 |
| Cu-N | 0.068 | 0.317 | 0.094 | −0.109 | −0.148 | 1.391 | 0.105 | |
| RnCuNO3 | Rn-Cu | 0.047 | 0.136 | 0.042 | −0.049 | −0.007 | 0.889 | 0.149 |
| Cu-O | 0.067 | 0.315 | 0.093 | −0.108 | −0.015 | 1.389 | 0.104 | |
| ArAgNO3 | Ar-Ag | 0.026 | 0.110 | 0.028 | −0.028 | 0.000 | 1.053 | 0.055 |
| Ag-N | 0.057 | 0.247 | 0.068 | −0.075 | −0.007 | 1.202 | 0.111 | |
| KrAgNO3 | Kr-Ag | 0.033 | 0.116 | 0.031 | −0.339 | −0.002 | 0.943 | 0.090 |
| Ag-N | 0.057 | 0.245 | 0.068 | −0.074 | −0.007 | 1.202 | 0.110 | |
| XeAgNO3 | Xe-Ag | 0.039 | 0.111 | 0.033 | −0.037 | −0.005 | 0.833 | 0.137 |
| Ag-N | 0.056 | 0.244 | 0.068 | −0.074 | −0.007 | 1.201 | 0.110 | |
| RnAgNO3 | Rn-Ag | 0.039 | 0.100 | 0.030 | −0.036 | −0.005 | 0.776 | 0.153 |
| Ag-N | 0.056 | 0.243 | 0.067 | −0.074 | −0.006 | 1.200 | 0.109 | |
| Ar2Ag2SO4 | Ar-Ag | 0.024 | 0.102 | 0.025 | −0.025 | 0.002 | 1.037 | 0.051 |
| Ag-S | 0.055 | 0.245 | 0.067 | −0.073 | −0.006 | 1.221 | 0.104 | |
| Kr2Ag2SO4 | Kr-Ag | 0.032 | 0.110 | 0.030 | −0.318 | −0.214 | 0.937 | 0.085 |
| Ag-S | 0.055 | 0.245 | 0.067 | −0.073 | −0.006 | 1.220 | 0.104 | |
| Xe2Ag2SO4 | Xe-Ag | 0.038 | 0.109 | 0.032 | −0.036 | −0.004 | 0.834 | 0.132 |
| Ag-S | 0.055 | 0.243 | 0.067 | −0.073 | −0.006 | 1.218 | 0.104 | |
| Rn2Ag2SO4 | Rn-Ag | 0.038 | 0.098 | 0.030 | −0.035 | −0.005 | 0.778 | 0.148 |
| Ag-S | 0.055 | 0.242 | 0.066 | −0.072 | −0.006 | 1.217 | 0.103 |
| System | Bonds | ΔEpauli | ΔEelstat | ΔEorb | ΔEdisp | ΔEint | %Covalent | %Ionic |
|---|---|---|---|---|---|---|---|---|
| HF | H-F | 0.0 | −232.76 | −145.92 | −0.11 | −378.79 | 38.53 | 61.47 |
| HCl | H-Cl | 0.0 | −159.91 | −178.63 | −0.35 | −338.89 | 52.76 | 47.24 |
| HBr | H-Br | −0.01 | −141.69 | −184.5 | −0.43 | −326.61 | 56.56 | 43.44 |
| LiF | Li-F | 41.38 | −210.53 | −24.01 | −0.28 | −193.44 | 10.24 | 89.76 |
| LiCl | Li-Cl | 29.16 | −160.53 | −26.33 | −0.91 | −158.62 | 14.09 | 85.91 |
| LiBr | Li-Br | 27.72 | −147.42 | −26.89 | −1.07 | −147.66 | 15.43 | 84.57 |
| NaF | Na-F | 31.09 | −179.91 | −12.91 | −0.34 | −162.06 | 6.70 | 93.30 |
| NaCl | Na-Cl | 25.45 | −146.21 | −14.95 | −1.03 | −136.74 | 9.28 | 90.72 |
| NaBr | Na-Br | 24.6 | −137.15 | −16.36 | −1.19 | −130.09 | 10.66 | 89.34 |
| KF | K-F | 39.61 | −163.92 | −21.37 | −0.43 | −146.11 | 11.53 | 88.47 |
| KCl | K-Cl | 29.18 | −132.33 | −15.93 | −1.21 | −120.29 | 10.74 | 89.26 |
| KBr | K-Br | 28.28 | −125.09 | −15.59 | −1.36 | −113.75 | 11.08 | 88.92 |
| MgF2 | Mg-F | 95.84 | −734.88 | −82.7 | −0.81 | −722.56 | 10.12 | 89.88 |
| MgCl2 | Mg-Cl | 77.01 | −582.58 | −120.12 | −2.52 | −628.21 | 17.09 | 82.91 |
| MgBr2 | Mg-Br | 69.11 | −528.17 | −141.21 | −2.93 | −603.2 | 21.10 | 78.90 |
| CaF2 | Ca-F | 121.9 | −666.31 | −79.32 | −0.98 | −624.71 | 10.64 | 89.36 |
| CaCl2 | Ca-Cl | 101.73 | −542.27 | −92.56 | −2.82 | −535.92 | 14.58 | 85.42 |
| CaBr2 | Ca-Br | 95.85 | −501.53 | −103.75 | −0.08 | −509.51 | 17.14 | 82.86 |
| H2 | H-H | 226.24 | 6.19 | −410.2 | −0.09 | −177.86 | 101.53 | −1.53 |
| N2 | N-N | 1683.65 | −320.32 | −1830.98 | −0.47 | −468.11 | 85.11 | 14.89 |
| O2 | O-O | 956.56 | −239.07 | −849.78 | −0.29 | −132.58 | 78.04 | 21.96 |
| F2 | F-F | 335.12 | −98.82 | −381.46 | −0.17 | −145.34 | 79.42 | 20.58 |
| Cl2 | Cl-Cl | 298.94 | −109.56 | −303.5 | −1.29 | −115.41 | 73.48 | 26.52 |
| CO | C-O | 1362.11 | −277.75 | −1502.04 | −0.46 | −418.14 | 84.39 | 15.61 |
| H2O | O-H | 409.15 | −69.44 | −534.56 | −0.21 | −195.04 | 88.50 | 11.50 |
| NH3 | N-H | 418.92 | −84.04 | −514.21 | −0.3 | −179.63 | 85.95 | 14.05 |
| CH4 | C-H | 387.57 | −55.77 | −511.01 | −0.33 | −179.53 | 90.16 | 9.84 |
| C2H4 | C-C | 1154.17 | −453.35 | −908.61 | −1.42 | −209.21 | 66.71 | 33.29 |
| C2H2 | C-C | 1373.8 | −463.1 | −1210.62 | −0.89 | −300.81 | 72.33 | 27.67 |
| ArCuNO3 | Ar-Cu | 18 | −13.27 | −11.36 | −1.38 | −8.01 | 46.12 | 53.88 |
| Cu-N | 67.77 | −186.74 | −47.74 | −2.15 | −168.87 | 20.36 | 79.64 | |
| KrCuNO3 | Kr-Cu | 22.86 | −17.78 | −15.01 | −1.7 | −11.63 | 45.78 | 54.22 |
| Cu-N | 67.03 | −182.95 | −47.83 | −2.24 | −166 | 20.73 | 79.27 | |
| XeCuNO3 | Xe-Cu | 28.91 | −23.59 | −18.59 | −2.19 | −15.45 | 44.07 | 55.93 |
| Cu-N | 66.82 | −178.15 | −48.15 | −2.36 | −161.84 | 21.28 | 78.72 | |
| RnCuNO3 | Rn-Cu | 29.16 | −24.27 | −18.69 | −2.31 | −16.12 | 43.51 | 56.49 |
| Cu-O | 66.98 | −175.24 | −48.22 | −2.41 | −158.89 | 21.58 | 78.42 | |
| ArAgNO3 | Ar-Ag | 81.38 | −6.34 | −5.43 | −1.46 | 68.15 | 46.13 | 53.87 |
| Ag-N | 60.16 | −167.49 | −38.87 | −2.38 | −148.59 | 18.84 | 81.16 | |
| KrAgNO3 | Kr-Ag | 14.98 | −11.05 | −9.14 | −1.8 | −7.01 | 45.27 | 54.73 |
| Ag-N | 59.13 | −164.45 | −38.93 | −2.5 | −146.74 | 19.14 | 80.86 | |
| XeAgNO3 | Xe-Ag | 23.11 | −17.96 | −13.1 | −2.32 | −10.26 | 42.18 | 57.82 |
| Ag-N | 58.81 | −160.71 | −39.21 | −2.57 | −143.68 | 19.61 | 80.39 | |
| RnAgNO3 | Rn-Ag | 25.04 | −19.98 | −13.96 | −2.43 | −11.33 | 41.13 | 58.87 |
| Ag-N | 58.55 | −158.24 | −39.25 | −2.61 | −141.55 | 19.87 | 80.13 | |
| Ar2Ag2SO4 | Ar-Ag | 8.27 | −5.82 | −4.55 | −1.5 | −3.6 | 43.88 | 56.12 |
| Ag-S | 135.09 | −522.47 | −73.49 | −6.9 | −467.78 | 12.33 | 87.67 | |
| Kr2Ag2SO4 | Kr-Ag | 14.25 | −10.55 | −8.04 | −1.88 | −6.22 | 43.25 | 56.75 |
| Ag-S | 134.31 | −511.84 | −76.42 | −7.27 | −461.22 | 12.99 | 87.01 | |
| Xe2Ag2SO4 | Xe-Ag | 22.95 | −17.88 | −11.83 | −2.45 | −9.2 | 39.82 | 60.18 |
| Ag-S | 133.17 | −496.82 | −80.74 | −7.52 | −451.9 | 13.98 | 86.02 | |
| Rn2Ag2SO4 | Rn-Ag | 24.84 | −19.85 | −12.44 | −2.59 | −10.05 | 38.53 | 61.47 |
| Ag-S | 132.96 | −488.06 | −82.97 | −7.65 | −445.72 | 14.53 | 85.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pal, R.; Chattaraj, P.K. On the Nature of the Partial Covalent Bond between Noble Gas Elements and Noble Metal Atoms. Molecules 2023, 28, 3253. https://doi.org/10.3390/molecules28073253
Pal R, Chattaraj PK. On the Nature of the Partial Covalent Bond between Noble Gas Elements and Noble Metal Atoms. Molecules. 2023; 28(7):3253. https://doi.org/10.3390/molecules28073253
Chicago/Turabian StylePal, Ranita, and Pratim Kumar Chattaraj. 2023. "On the Nature of the Partial Covalent Bond between Noble Gas Elements and Noble Metal Atoms" Molecules 28, no. 7: 3253. https://doi.org/10.3390/molecules28073253
APA StylePal, R., & Chattaraj, P. K. (2023). On the Nature of the Partial Covalent Bond between Noble Gas Elements and Noble Metal Atoms. Molecules, 28(7), 3253. https://doi.org/10.3390/molecules28073253






