Development of an HPLC-PDA Method for the Determination of Capsanthin, Zeaxanthin, Lutein, β-Cryptoxanthin and β-Carotene Simultaneously in Chili Peppers and Products
Abstract
1. Introduction
2. Results and Discussion
2.1. HPLC Condition Optimization
2.1.1. Wavelength
2.1.2. Mobile Phase Gradient
2.1.3. Mobile Phase
2.1.4. Flow Rate of Mobile Phase
2.1.5. Column Temperature
2.2. Extraction Condition Optimization
2.3. Methodological Evaluation
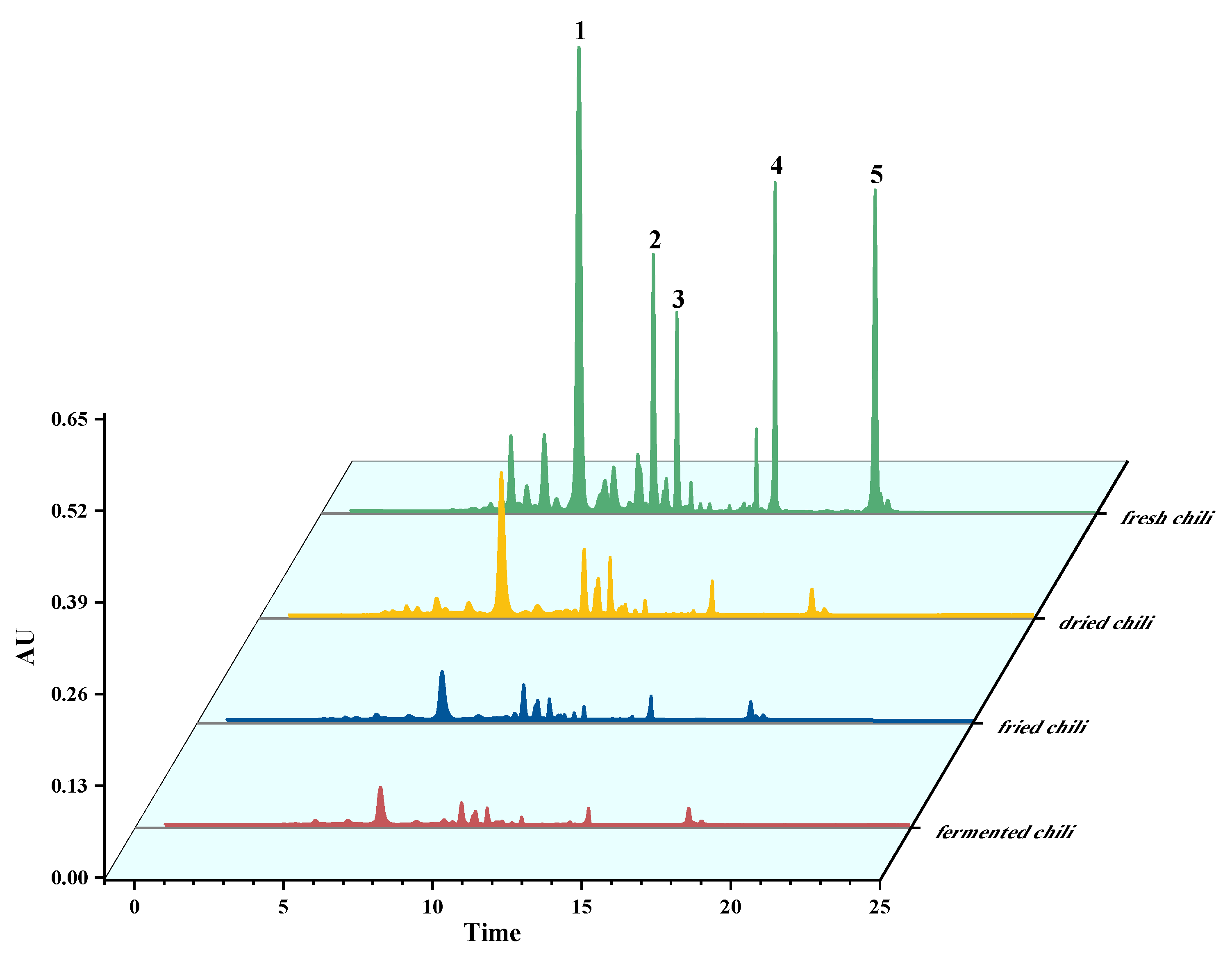
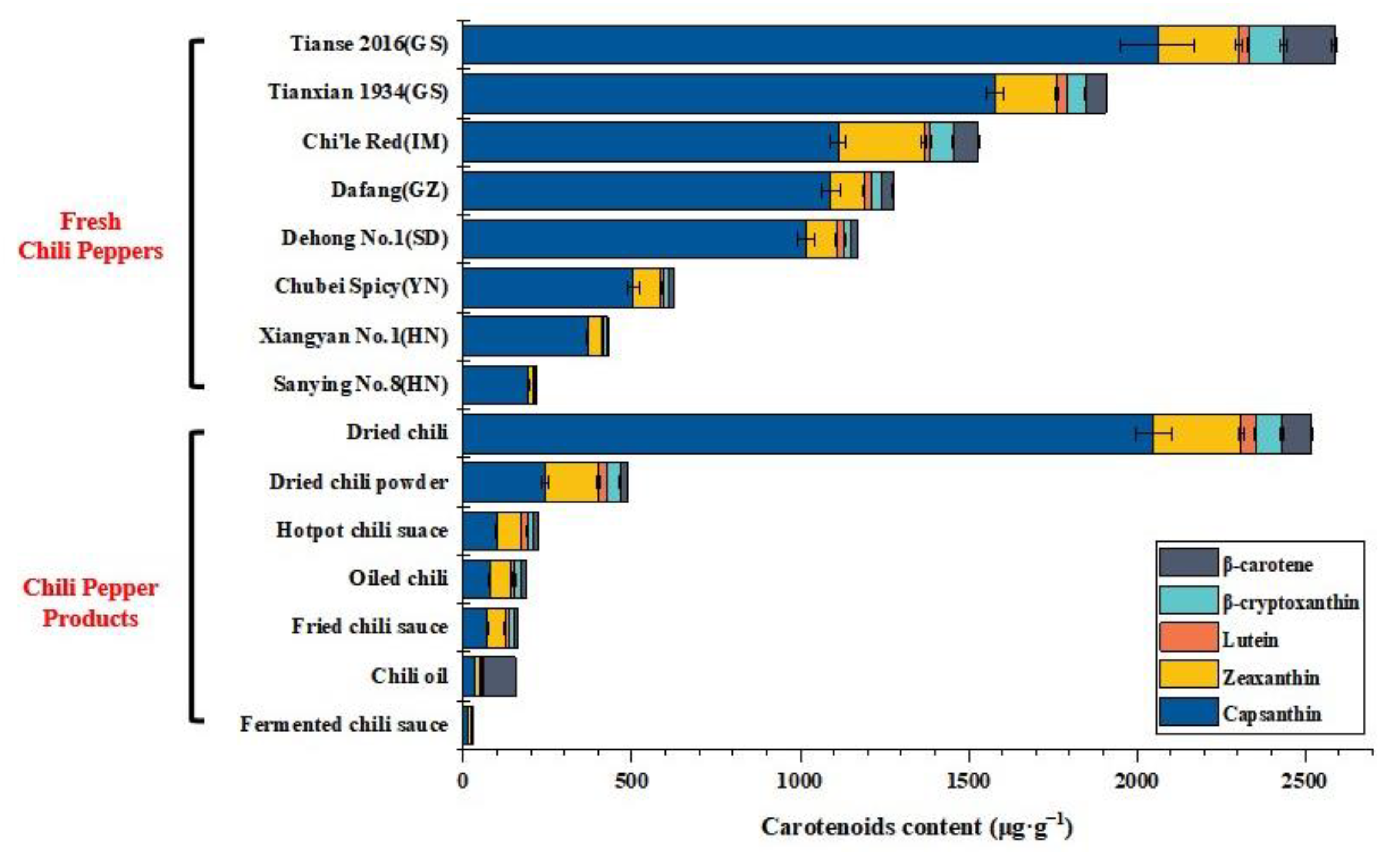
2.4. Application of the Method to Fresh Chili Peppers and Chili Pepper Products Analysis
3. Materials and Methods
3.1. Reagents and Materials
3.2. Apparatus and Software
3.3. Standard Solutions and Samples
3.4. Samples Extraction and Saponification
3.4.1. Optimized Parameters
3.4.2. One Factor Design
3.5. HPLC Conditions
3.5.1. Optimized Parameters
3.5.2. One Factor Design
3.6. HPLC Determination
- Xi—the content of each carotenoid in the sample, μg/g.
- A—peak area of each carotenoid in the sample solution.
- A0—peak area of each carotenoid in the sample dilution solution.
- As—peak area of each carotenoid in the standard solution.
- Cs—concentration of each carotenoid in the standard solution, mg/L.
- C—concentration of each carotenoid in the sample solution, mg/L.
- V—extract liquid in mL.
- f—dilution ratio of sample solution.
- m—mass of sample in g.
3.7. Validation Procedures
- W—detection of ion concentration, mg/L.
- S/N—instrument signal to noise ratio.
- 100—conversion factor.
- 10−6—conversion factor.
- V—constant volume of sample, mL.
- f—sample dilution ratio.
- m—sample weight, g.
- m—content of added standard products, mg/L.
- X1—detection of sample extracts added standard products, mg/L.
- X0—detection of sample extracts, mg/L.
3.8. Application of the Method to Fresh Chili Peppers and Chili Pepper Products Analysis
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- FAO. 2019. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 1 January 2023).
- Baenas, N.; Belović, M.; Ilic, N.; Moreno, D.; García-Viguera, C. Industrial use of pepper (Capsicum annum L.) derived products: Technological benefits and biological advantages. Food Chem. 2019, 274, 872–885. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Gontarek-Castro, E.; Jafari, S.M. Up-to-date strategies and future trends towards the extraction and purification of Capsaicin: A comprehensive review. Trends Food Sci. Technol. 2022, 123, 161–171. [Google Scholar] [CrossRef]
- Villa-Rivera, M.G.; Ochoa-Alejo, N. Chili Pepper Carotenoids: Nutraceutical Properties and Mechanisms of Action. Molecules 2020, 25, 5573. [Google Scholar] [CrossRef] [PubMed]
- Azlan, A.; Sultana, S.; Huei, C.S.; Razman, M.R. Antioxidant, Anti-Obesity, Nutritional and Other Beneficial Effects of Different Chili Pepper: A Review. Molecules 2022, 27, 898. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.E.; Abraham, A.; Kulkarni, G.; Shettigar, N.; Dave, T.; Kulkarni, M. Capsanthin, a Plant-Derived Xanthophyll: A Review of Pharmacology and Delivery Strategies. AAPS PharmSciTech 2021, 22, 203. [Google Scholar] [CrossRef]
- Donato, P.; Giuffrida, D.; Oteri, M.; Inferrera, V.; Dugo, P.; Mondello, L. Supercritical Fluid Chromatography × Ultra-High Pressure Liquid Chromatography for Red Chilli Pepper Fingerprinting by Photodiode Array, Quadrupole-Time-of-Flight and Ion Mobility Mass Spectrometry (SFC × RP-UHPLC-PDA-Q-ToF MS-IMS). Food Anal. Methods 2018, 11, 3331–3341. [Google Scholar] [CrossRef]
- Ponder, A.; Kulik, K.; Hallmann, E. Occurrence and Determination of Carotenoids and Polyphenols in Different Paprika Powders from Organic and Conventional Production. Molecules 2021, 26, 2980. [Google Scholar] [CrossRef]
- Tan, J.; Li, M.-F.; Li, R.; Jiang, Z.-T.; Tang, S.-H.; Wang, Y. Front-face synchronous fluorescence spectroscopy for rapid and non-destructive determination of free capsanthin, the predominant carotenoid in chili (Capsicum annuum L.) powders based on aggregation-induced emission. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 255, 119696. [Google Scholar] [CrossRef]
- Morales-Soriano, E.; Panozzo, A.; Ugás, R.; Grauwet, T.; Van Loey, A.; Hendrickx, M. Carotenoid profile and basic structural indicators of native Peruvian chili peppers. Eur. Food Res. Technol. 2019, 245, 717–732. [Google Scholar] [CrossRef]
- Fratianni, A.; Niro, S.; Alam, M.; Cinquanta, L.; Di Matteo, M.; Adiletta, G.; Panfili, G. Effect of a physical pre-treatment and drying on carotenoids of goji berries (Lycium barbarum L.). LWT 2018, 92, 318–323. [Google Scholar] [CrossRef]
- Kamal, M.; Ali, R.; Rahman, M.; Shishir, M.R.I.; Yasmin, S.; Sarker, S.H. Effects of processing techniques on drying characteristics, physicochemical properties and functional compounds of green and red chilli (Capsicum annum L.) powder. J. Food Sci. Technol. 2019, 56, 3185–3194. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Kumar, V.; Kumar, S.; Pinakin, D.J.; Babbar, N.; Kaur, J.; Sharma, B.R. Process optimization for drying of Bauhinia variegata flowers: Effect of different pre-treatments on quality attributes. J. Food Process. Preserv. 2022, 46, e16229. [Google Scholar] [CrossRef]
- Saengrayap, R.; Tansakul, A.; Mittal, G.S. Effect of far-infrared radiation assisted microwave-vacuum drying on drying characteristics and quality of red chilli. J. Food Sci. Technol. 2015, 52, 2610–2621. [Google Scholar] [CrossRef]
- Nagy, Z.; Daood, H.; Koncsek, A.; Molnár, H.; Helyes, L. The simultaneous determination of capsaicinoids, tocopherols, and carotenoids in pungent pepper powder. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 199–209. [Google Scholar] [CrossRef]
- Perrut, M.; Perrut, V. Supercritical fluid applications in the food industry. In Gases in Agro-Food Processes; Elsevier: Amsterdam, The Netherlands, 2019; pp. 483–509. [Google Scholar]
- Mendoza, N.N.G.; Rodríguez, S.A.V. Improvement of the extraction of carotenoids and capsaicinoids of chili pepper native (Capsicum baccatum), assisted with cellulolytic enzymes. Rev. Peru. Biol. 2020, 27, 55–60. [Google Scholar] [CrossRef]
- Li, N.; Li, J.; Ding, D.; Xie, J.; Zhang, J.; Li, W.; Ma, Y.; Gao, F.; Niu, T.; Wang, C.; et al. Optimum Parameters for Extracting Three Kinds of Carotenoids from Pepper Leaves by Response Surface Methodology. Separations 2021, 8, 134. [Google Scholar] [CrossRef]
- Giuffrida, D.; Donato, P.; Dugo, P.; Mondello, L. Recent Analytical Techniques Advances in the Carotenoids and Their Derivatives Determination in Various Matrixes. J. Agric. Food Chem. 2018, 66, 3302–3307. [Google Scholar] [CrossRef]
- Uragami, C.; Saito, K.; Yoshizawa, M.; Molnár, P.; Hashimoto, H. Unified analysis of optical absorption spectra of carotenoids based on a stochastic model. Arch. Biochem. Biophys. 2018, 650, 49–58. [Google Scholar] [CrossRef]
- Patsilinakos, A.; Ragno, R.; Carradori, S.; Petralito, S.; Cesa, S. Carotenoid content of Goji berries: CIELAB, HPLC-DAD analyses and quantitative correlation. Food Chem. 2018, 268, 49–56. [Google Scholar] [CrossRef]
- Rodríguez, E.; Sánchez-Prieto, M.; Olmedilla-Alonso, B. Assessment of carotenoid concentrations in red peppers (Capsicum annuum) under domestic refrigeration for three weeks as determined by HPLC-DAD. Food Chem. X 2020, 6, 100092. [Google Scholar] [CrossRef]
- Czyrski, A.; Sznura, J. The application of Box-Behnken-Design in the optimization of HPLC separation of fluoroquinolones. Sci. Rep. 2019, 9, 19458. [Google Scholar] [CrossRef] [PubMed]
- Tome, T.; Žigart, N.; Časar, Z.; Obreza, A. Development and Optimization of Liquid Chromatography Analytical Methods by Using AQbD Principles: Overview and Recent Advances. Org. Process Res. Dev. 2019, 23, 1784–1802. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, G.; Yang, B.; Wu, Y.; Du, M.; Kan, J. Insights into the stability of carotenoids and capsaicinoids in water-based or oil-based chili systems at different processing treatments. Food Chem. 2021, 342, 128308. [Google Scholar] [CrossRef] [PubMed]
- Hornero-Méndez, D. A Routine Method for the Extraction and HPLC-DAD Profiling of Major Plant and Food Carotenoids. In Plant and Food Carotenoids; Springer: Berlin/Heidelberg, Germany, 2020; pp. 117–134. [Google Scholar] [CrossRef]
- Ordóñez-Santos, L.E.; Martínez-Girón, J. Thermal degradation kinetics of carotenoids, vitamin C and provitamin A in tree tomato juice. Int. J. Food Sci. Technol. 2020, 55, 201–210. [Google Scholar] [CrossRef]
- Pola, W.; Sugaya, S.; Photchanachai, S. Influence of Postharvest Temperatures on Carotenoid Biosynthesis and Phytochemicals in Mature Green Chili (Capsicum annuum L.). Antioxidants 2020, 9, 203. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, J.; Yu, J.; Lv, J.; Zhang, J.; Wang, X.; Wang, C.; Tang, C.; Zhang, Y.; Dawuda, M.M.; et al. Reversed-Phase High-Performance Liquid Chromatography for the Quantification and Optimization for Extracting 10 Kinds of Carotenoids in Pepper (Capsicum annuum L.) Leaves. J. Agric. Food Chem. 2017, 65, 8475–8488. [Google Scholar] [CrossRef]
- Sarkar, S.; Manna, M.S.; Bhowmick, T.K.; Gayen, K. Extraction of chlorophylls and carotenoids from dry and wet biomass of isolated Chlorella thermophila: Optimization of process parameters and modelling by artificial neural network. Process Biochem. 2020, 96, 58–72. [Google Scholar] [CrossRef]
- Civan, M.; Kumcuoglu, S. Green ultrasound-assisted extraction of carotenoid and capsaicinoid from the pulp of hot pepper paste based on the bio-refinery concept. LWT 2019, 113, 108320. [Google Scholar] [CrossRef]
- Ayob, O.; Hussain, P.R.; Suradkar, P.; Naqash, F.; Rather, S.A.; Joshi, S.; Azad, Z.A.A. Evaluation of chemical composition and antioxidant activity of Himalayan Red chilli varieties. LWT 2021, 146, 111413. [Google Scholar] [CrossRef]
- Gebregziabher, B.; Zhang, S.; Qi, J.; Azam, M.; Ghosh, S.; Feng, Y.; Huai, Y.; Li, J.; Li, B.; Sun, J. Simultaneous Determination of Carotenoids and Chlorophylls by the HPLC-UV-VIS Method in Soybean Seeds. Agronomy 2021, 11, 758. [Google Scholar] [CrossRef]
- Tomlekova, N.; Spasova-Apostolova, V.; Pantchev, I.; Sarsu, F. Mutation Associated with Orange Fruit Color Increases Concentrations of β-Carotene in a Sweet Pepper Variety (Capsicum annuum L.). Foods 2021, 10, 1225. [Google Scholar] [CrossRef]
- Pinheiro-Sant’Ana, H.M.; Anunciação, P.C.; e Souza, C.S.; Filho, G.X.D.P.; Salvo, A.; Dugo, G.; Giuffrida, D. Quali-Quantitative Profile of Native Carotenoids in Kumquat from Brazil by HPLC-DAD-APCI/MS. Foods 2019, 8, 166. [Google Scholar] [CrossRef] [PubMed]
- Hrvolová, B.; Martínez-Huélamo, M.; Colmán-Martínez, M.; Hurtado-Barroso, S.; Lamuela-Raventós, R.M.; Kalina, J. Development of an Advanced HPLC–MS/MS Method for the Determination of Carotenoids and Fat-Soluble Vitamins in Human Plasma. Int. J. Mol. Sci. 2016, 17, 1719. [Google Scholar] [CrossRef] [PubMed]
- GB/T 21266-2007; Determination of Total Capsaicinoid Content and Representation of Pungency Degree in Capsicum and Its Products. Standardization Administration of China: Beijing, China, 2007.
- NY/T 1651-2008; Determination of Lycopene in Vegetables and Derived Products-HPLC. Standardization Administration of China: Beijing, China, 2008.
- GB 5009.83-2016; National Food Safety Standard Determination of Carotenoids in Foods. Standardization Administration of China: Beijing, China, 2016.
- Implementing Council Directive. 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off. J. Eur. Communities 2002, 221, 8–36. [Google Scholar]


| Parameters | Capsanthin | Zeaxanthin | Lutein | β-Cryptoxanthin | β-Carotene |
|---|---|---|---|---|---|
| Retention time (min) | 7.541 | 10.187 | 10.476 | 14.345 | 17.873 |
| Standard Curve Equation | Y = 22761X − 24502 | Y = 40516X − 2562.6 | Y = 124707X − 2148 | Y = 74985X − 6336.5 | Y = 85211X − 373.54 |
| R | 0.9985 | 0.9996 | 0.9997 | 0.9999 | 0.9998 |
| LODs (mg/L) | 0.026 | 0.063 | 0.020 | 0.026 | 0.035 |
| LOQs (mg/L) | 0.088 | 0.209 | 0.067 | 0.088 | 0.118 |
| LODsample (mg/kg in the fresh chili) | 0.646 | 1.565 | 0.497 | 0.646 | 0.869 |
| LOQsample (mg/kg in the fresh chili) | 2.185 | 5.191 | 1.664 | 2.185 | 2.931 |
| LODsample (mg/kg in the dried chili) | 0.650 | 1.575 | 0.500 | 0.650 | 0.875 |
| LOQsample (mg/kg in the dried chili) | 2.200 | 5.225 | 1.675 | 2.200 | 2.950 |
| LODsample (mg/kg in the fried chili sauce) | 0.131 | 0.317 | 0.101 | 0.131 | 0.176 |
| LOQsample (mg/kg in fried chili suace) | 0.443 | 1.052 | 0.337 | 0.443 | 0.594 |
| LODsample (mg/kg in the fermented chili sauce) | 0.130 | 0.314 | 0.100 | 0.130 | 0.174 |
| LOQsample (mg/kg in the fermented chili sauce) | 0.439 | 1.042 | 0.334 | 0.439 | 0.588 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Lin, J.; Peng, S.; Zhao, H.; Wang, Y.; Rao, L.; Liao, X.; Zhao, L. Development of an HPLC-PDA Method for the Determination of Capsanthin, Zeaxanthin, Lutein, β-Cryptoxanthin and β-Carotene Simultaneously in Chili Peppers and Products. Molecules 2023, 28, 2362. https://doi.org/10.3390/molecules28052362
Xu J, Lin J, Peng S, Zhao H, Wang Y, Rao L, Liao X, Zhao L. Development of an HPLC-PDA Method for the Determination of Capsanthin, Zeaxanthin, Lutein, β-Cryptoxanthin and β-Carotene Simultaneously in Chili Peppers and Products. Molecules. 2023; 28(5):2362. https://doi.org/10.3390/molecules28052362
Chicago/Turabian StyleXu, Jiayue, Jialu Lin, Sijia Peng, Haoda Zhao, Yongtao Wang, Lei Rao, Xiaojun Liao, and Liang Zhao. 2023. "Development of an HPLC-PDA Method for the Determination of Capsanthin, Zeaxanthin, Lutein, β-Cryptoxanthin and β-Carotene Simultaneously in Chili Peppers and Products" Molecules 28, no. 5: 2362. https://doi.org/10.3390/molecules28052362
APA StyleXu, J., Lin, J., Peng, S., Zhao, H., Wang, Y., Rao, L., Liao, X., & Zhao, L. (2023). Development of an HPLC-PDA Method for the Determination of Capsanthin, Zeaxanthin, Lutein, β-Cryptoxanthin and β-Carotene Simultaneously in Chili Peppers and Products. Molecules, 28(5), 2362. https://doi.org/10.3390/molecules28052362