Abstract
We reported herein efficient economic high-pressure synthesis procedures for the synthesis of bis(azoles) and bis(azines) by utilizing the bis(enaminone) intermediate. Bis(enaminone) reacted with hydrazine hydrate, hydroxylamine hydrochloride, guanidine hydrochloride, urea, thiourea, and malononitrile to form the desired bis azines and bis azoles. A combination of elemental analyses and spectral data was used to confirm the structures of the products. Compared with conventional heating, the high-pressure Q-Tube method promotes reactions in a short period of time and provides high yields.
1. Introduction
Temperatures can easily be controlled in the range essential for chemical reactivity, which is probably why organic chemists prefer temperature over high pressures. On the other hand, high-pressure reactors are the workhorses of chemical industries. They can run chemical reactions at pressures of a few thousand atmospheres [1]. Since chemical reactions are influenced by pressure [2], by using pressure, chemists can force chemical reactions to occur and accelerate transitions between solids, liquids, and gases. Initially, we need to focus on the reaction volume and volume of activation in order to understand pressure effects [2]. The activation volume represents a change in volume from the reactant to transition state, and the reaction volume represents a change in volume from the reactant to the product [3]. According to the reported literature that dealt with this issue [3,4], an illustration of bond formations in Figure 1 schematically clarifies the working concept.
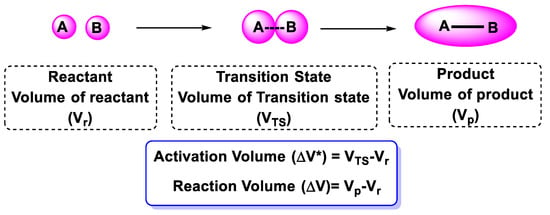
Figure 1.
Activation volume and reaction volume in bond formation reactions.
High-pressure reactions with a positive activation volume or reaction volume are expected to occur more slowly than at low pressure, as the positive activation volume or reaction volume works against compression. Therefore, we should carefully choose the reaction that will occur under pressure [5]. For example, during some bond cleavage phenomena, the transition state has a larger volume than the reactant, because it is less compact, and vice versa for bond formation. An SN1 reaction mechanism (first step), for instance, showing a significantly negative activation volume might seem counterintuitive because a bond breaks and ionizes (or separates) when charges occur. As such, the cleavage of heterolytic bonds does contribute to the positive activation volume, but the contribution of the volume of ions formed with solvent molecules should not be neglected in an overall volume change, which finally produces a negative activation volume. A very important note was reported [6] that led to a useful summary of the activation volume (positive or negative) for the most common reaction, which helped us decide whether to use high-pressure reaction conditions or not.
A positive activation volume was recorded for the following reactions: neutralization, bond cleavage, charge dispersal, and diffusion control. On the other hand, the following reactions showed a negative activation volume: ionization, cyclization, bond formation, displacement, steric hindrance, and charge concentration.
Using high-pressure chemistry, solvents, reagents, and final products can be improved in terms of their physical properties in order to achieve quicker and cleaner transformations. The most modified properties are the physical properties of liquids. A pressure reaction has the advantage of overcoming the solvent’s boiling point [7,8,9]; solvent boiling points are directly proportional to vapor pressure when pressure increases. As defined by the Arrhenius equation, the reaction rate can double relative to every 10 °C increase in temperature [10]. A Q-Tube device for high-pressure chemistry was developed, and it is undoubtedly the most straightforward and cheapest alternative to expensive microwave synthesizers (Figure 2).

Figure 2.
The Q-Tube system.
Q-Tube, unlike other pressure reactors, features a pressure release and resealing system to prevent overpressure explosions [11]. Of note, the conversions and yield values of the Q-Tube high-pressure reactor are higher than that of the MW reactor [12]. Q-Tube influences two factors that enhance the organic reaction. One of them is carried out at an extreme temperature that exceeds the solvent’s boiling point. A temperature increase of 10 °C doubles the reaction rate, according to the Arrhenius equation, as represented in Equation (1). The other factor is an increase in the concentration of substrates due to the high pressure exerted by the Q-Tube system that reduces the reaction volume, including liquids; consequently, the collision frequency increases, leading to faster reactions.
A rate constant is defined as k, activation energy is defined as Ea, and a gas constant is defined as R (8.3145 J/Kmol). In contrast, temperature is defined as T. A frequency factor of A, with units of L∙mol−1∙s−1, is calculated by considering the frequency of reactions and the likelihood of correct molecular orientation [13,14]. On the other hand, pyridine rings attracted particular attention regarding their unique biological activities. It has been claimed that many pyridine derivatives possess engaging biological and pharmacological properties, such as antimicrobial [15], antitubercular [16], anticonvulsant, analgesic, anti-inflammatory [17], and anticancer properties [18,19,20]. In addition, one of the most elementary classes that exhibit potential biological and pharmacological activities is bis-heterocyclic compounds [21,22,23]. Moreover, enaminones are valuable intermediates for synthesizing several heterocyclic systems in addition to their pharmacological potentialities [24,25,26]. Aiming to obtain bis-pyridine enaminone as a precursor for different pyridine-azoles hybrids and pyridine-azine hybrids via green synthesis for biological screening purposes, several studies show that pyridine–azole hybrids and pyridine–azine hybrids have fascinating medicinal properties [27]. These hybrids show the highly potent and selective mGlu5 receptor antagonist with good brain penetration and receptor occupancy [28] as well as calcium channel blocking for the treatment of hypertension [29]. Moreover, they exhibited excellent antifungal activity against Candida albicans [30] and inhibited bacterial biofilms, DNA gyrase, and Mycobacterium tuberculosis [31]. Moreover, they demonstrated antihyperglycemic activity [32], in which their compounds metabolically stabilized the glucokinase activator [33] and exhibited acute oral glucose-lowering efficacy. In addition, some hybrids showed antiviral activities as potent efficacy against human cytomegalovirus (CMV) and the varicella-zoster virus [34]. For cancer treatments, several hybrids showed cytotoxicity against several human cancer cells, including ovarian cancer cells [35] and gastric cancer cells [36], with antiproliferative effects [37]. Green chemistry is based on the design (or redesign) of products and/or manufacturing processes to reduce their impact on human health and the environment [38,39]; therefore, the green organic synthesis trend induces the prevention of the harmful impact on the environment. Catalysis, and any system that reduces reaction time to save energy and lower emissions, is an excellent choice by applying different green chemistry principles [38,39,40]. Our previous results encouraged us to continue synthesizing biologically active heterocyclic compounds [41,42,43,44,45,46] using other green chemistry methods [47,48,49,50,51,52,53,54,55,56,57]. A high-pressure Q-Tube reactor is used in this study to synthesize bis(enaminone), an essential building block for synthesizing bis-heterocyclic compounds.
2. Results and Discussions
The design of the Q-Tube™ system allows it to be operated safely and accurately with high reproducibility. The design is clarified in Figure 3, showing the presence of a Qian cap (Figure 3a), which consists of a Teflon septa and needle (Figure 3b) to release pressure via the perforation of the Teflon septa (acts as active ventilation for safety, avoiding a rapid increase in pressure).
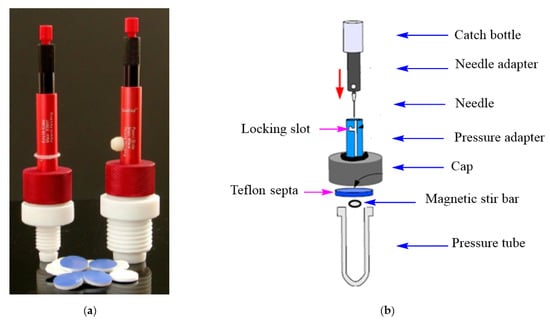
Figure 3.
Detailed schematic diagram for the Q-Tube system and Qian cap photo. (a) Qian cap photo and Teflon septa (b) Schematic diagram for the Q tube System.
The reactivity of enaminone towards different nucleophiles is frequently studied by many reports [58,59]. Scheme 1, Scheme 2 and Scheme 3 show the synthetic procedures used to obtain the target compounds. In a reflux and/or Q-Tube reactor, we used N,N-dimethylformamide dimethyl acetal (DMF-DMA) 2 to react with 2,6-diacetylpyridine 1 in the presence of a little excess of DMF-DMA to produce a single product identified as bis(pyridine)enaminone 3 (Scheme 1). A high percentage of yield was observed when using high-pressure reactors (Q-Tubes) instead of the conventional reflux method [60,61]. With the previously reported microwave radiation method, almost the same % yield was obtained utilizing high-pressure reactors (Q-Tubes) [62]. Scheme 1 and Table 1 show comparative results between the two preparation methods.
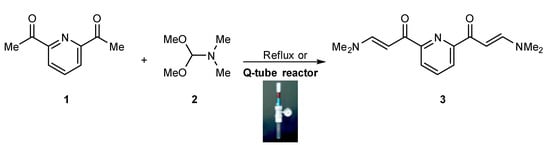
Scheme 1.
Reaction of 2,6-diacetylpyridine 1 with DMF-DMA 2 to produce bis(pyridine)enaminone 3 utilizing Q-Tube.
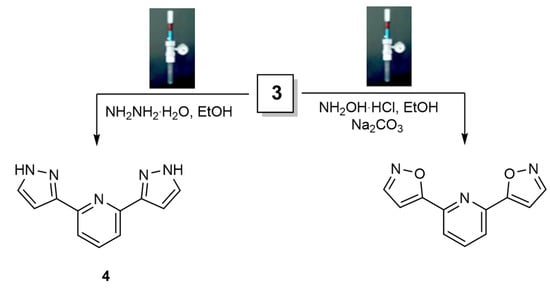
Scheme 2.
Reaction of bis(pyridine)enaminone 3 with different nitrogen nucleophiles to synthesize bis(pyrazole) 4 and bis(isoxazole) 5 utilizing the Q-Tube.
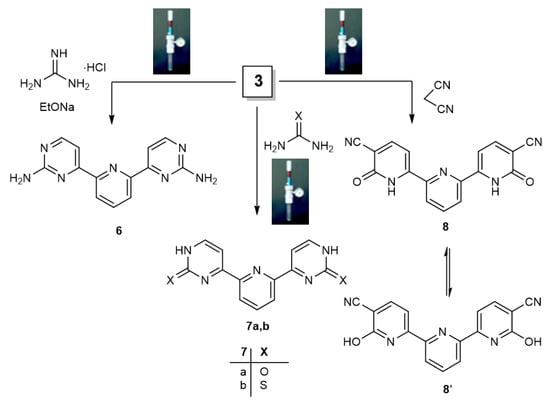
Scheme 3.
Reaction of bis(pyridine)enaminone 3 with guanidine hydrochloride, urea/thiourea, or malononitrile to synthesize bis(aminopyrimidine) 6, bis(pyrimidines) (7a, 7b) or bis(pyridine) 8, respectively, by utilizing the Q-Tube.

Table 1.
Effect of the Q-Tube method versus conventional the heating synthesis of bis(pyridine)enaminone.
The analytical data for both preparation methods agreed with structure 3 (Scheme 1). The 1H spectrum of the isolated compound exhibited two singlet signals at δ 2.90 and 3.20 due to N,N-dimethylamino protons, two doublet signals at δ 6.55 and 7.85 (J = 12.3 Hz) due to olefinic protons, and pyridine ring protons showed two doublet signals at δ 8.074 ppm and a triplet signal at δ 8.012 ppm. Based on the olefinic proton coupling constant value of 12.3 Hz, bis(enaminone) 3 has an E-configuration [63]. By condensing hydrazine hydrate and hydroxylamine with bis(enaminone), we investigated its potential for the synthesis of novel bis(pyrazoles) and bis(isoxazoles). Compound 3 reacted with hydrazine monohydrate in ethanol in the high-pressure reactor Q-Tube. A single product was identified as bis(pyrazole) derivative 4 (Scheme 2). There are two doublets identified as one doublet for CH-4 pyrazole at δ 6.95 ppm and one doublet for CH-5 pyrazole at δ 7.62 ppm in its 1HNMR spectroscopy [64].
By using the high-pressure reactor Q-Tube, compound 3 also condensed with hydroxylamine hydrochloride in the presence of a base such as sodium carbonate in ethanol as a solvent. Bis(isoxazole) 5 was detected exclusively in TLC. 1HNMR indicated that two characteristic doublets were obtained for the isolated compound, with one of them at δ 8.78 (d, J = 1.86 Hz CH-5 isoxazole) downfield while the other was at δ 7.25 (d, J = 1.86 Hz CH-4 isoxazole) upfield [65], which is completely consistent with the proposed structure depicted in Scheme 2. It is noteworthy that to find an advantage of the high-pressure reactor on the reactions shown in Scheme 2, these reactions had been carried out with conventional heating; for both compounds 4 and 5, almost the same yield but shorter reaction times was found with the high-pressure Q-Tube reactor. Scheme 2 and Table 2 represent the time of reactions and % yield.

Table 2.
Effect of the Q-Tube method versus the conventional heating synthesis of bis(azoles).
The Q-Tube reactor demonstrated that bis(enaminone) 3 is an important precursor in synthesizing bis(azines) 6–8 from its reactions with guanidine, urea, thiourea, and malononitrile; only one product was obtained in each case (Scheme 3). By utilizing the high-pressure Q-Tube in comparison to conventional heating (Scheme 3), bis(enaminone) 3 gives excellent yields and the fastest reaction times for pyrimidines resulting from reactions with guanidine hydrochloride, urea, and/or thiourea. The IR spectrum of compound 6 shows symmetric and asymmetric bands at 3250–3450 cm−1 due to the amino group [64]. Its 1HNMR shows a D2O exchangeable singlet peak at δ 6.79, representing the amino group [65].
The IR spectrum of compound 7a exhibits an OH absorbance band at 3284 cm−1 [64], which confirms the presence of tautomeric isomer “enol form is predominant”, as shown in Figure 4. Moreover, 1HNMR confirms the presence of the D2O exchangeable signal for OH due to two Hydroxy groups at 5.89 ppm (cf. Materials and Methods).
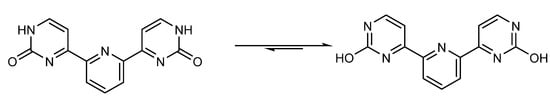
Figure 4.
Tautomeric isomers of compound 7a.
Compound 7b shows a distinct absorbance band due to C=S in IR at 1407 cm−1 and a signal in 13CNMR above 185 ppm for (C=S). As tested by TLC, only one compound formed from the reaction of compound 3 with malononitrile in ethanol and drops of piperidine at 120 °C/25 psi. A band at 2174 cm−1 is evident in the IR spectrum of the formed compound 8 due to the cyano group, and the 1HNMR spectrum is consistent with the formed mixture (cf. Materials and Method). The obtained spectroscopic data confirm that compound 8 was identified as bis(pyridine) via the pyridine bridge (Scheme 3). According to Table 3, the compounds formed using the high-pressure Q-Tube have a higher yield and a shorter reaction time than the conventional heating method.

Table 3.
Effect of the Q-Tube method versus the conventional heating synthesis of bis(azines).
Of note, the formation of compound 8 is in line with the reported literature [66], which suggested that malononitrile substitutes the dimethylamino group first to give intermediate A, followed by cyclization to bisaminopyran (intermediate B) and then the ring opening of the formed aminopyran to afford intermediate C, which readily eliminates dimethylamine to afford the final isolable pyridinone derivative 8 (in a Dimroth-type rearrangement) (Scheme 4).
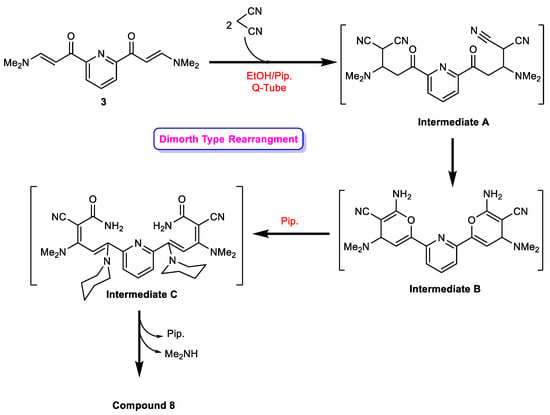
Scheme 4.
Suggested mechanism for the formation of compound 8.
Finally, we succeeded in introducing a technique considered as state of the art in modern green organic chemistry techniques. Green chemistry tends to perform organic reactions under atmospheric pressures, avoiding the high amount of energy that is consumed to obtain highly pressurized reactions, or develops a new catalyst to reduce the operating temperature and pressure for the process; consequently, less energy is consumed, which is good for the industry. However, the Q-Tube system works in a manner that does not consume more energy to attain high pressure as the pressure released in our reactions described above is considered autogenic pressure. Another important advantage is the high reproducibility of the obtained results. On the other hand, we succeeded in applying the use of this economic Q-Tube system in comparison to the microwave reactor in order to synthesize the bis(azoles)-pyridine hybrids and bis(azines)-pyridine hybrids of expected high biological and pharmacological activities.
3. Materials and Methods
3.1. General
All melting points were measured on a Gallenkamp Electrothermal melting point apparatus (Gallenkamp, Cambridge, UK) and were uncorrected. The infrared spectra (KBr disks) in 4000 to 400 cm−1 were recorded on a Perkin-Elmer Frontier spectrometer (Perkin-Elmer, San Diego, CA, USA). The N.M.R. spectra were recorded on a 850 and 600 MHz NMR spectrometer (Bruker, Fällanden, Switzerland) that is deuterated in dimethylsulphoxide (DMSO-d6) and chloroform (CDCl3). Chemical shifts were quoted in δ and were related to that of the solvent. Mass spectrometry was carried out using a direct probe controller inlet part in a single quadrupole mass analyzer (Thermo Fisher Scientific GCMS Model I.S.Q. LT, Carlsbad, CA, USA) using Thermo X-Calibur Software at the regional center for mycology and biotechnology (R.C.M.B.), Al-Azhar University, Cairo. The reaction temperature was manually input depending on the boiling point of the solvent used and stabilized for more than an hour. Q-Tube-assisted reactions were performed in a Q-Tube-safe pressure reactor from Q Labtech (Washington, DC, USA) equipped with a cap/sleeve, pressure adapter (120 psi), needle, borosilicate glass tube, Teflon septum, and catch bottle. Elemental analyses were performed using a Perkin-Elmer 2400 Analyzer (Perkin-Elmer, San Diego, CA, USA). T.L.C. Sigma-Aldrich (St Louis, MA, USA) silica gel was used on TLC Al foils and a silica gel matrix, with a fluorescent indicator at 254 nm. The 1HNMR spectra of the synthesized compounds can be found in Supplementary Materials (Figures S1–S6)
3.2. General Procedure for Preparation of Dienaminone 3
Method I: A mixture of compound 1 (10 mmol) and DMF-DMA (25 mmol) was refluxed at 90–100 °C overnight. The mixture was left at room temperature, and the solid was collected by hexane and petroleum ether 40–60 and then washed by ethanol.
Method II: The same above mixture scale was placed in a Q-Tube at 120 °C/under the autogenic pressure (15 psi) for 23–24 min. The products were collected and washed with ethanol: one spot on TLC; eluent (CHCl3:MeOH 85:15).
2,6-Bis(3-dimethylamino-1-oxoprop-2-en-yl)pyridine (3) [62]
Orange shine grain; (Q-Tube) yield 94%; m.p. 236–237 °C, FT-IR cm−1: 3026, 2911 (CH aromatic), 2807(CH aliphatic), 1643(C=O ketone); fingerprint area matched precisely with the product obtained from conventional heating. 1HNMR (850 MHz, DMSO-d6): δ, ppm = 2.96 (s, 6H), 3.20 (s, 6H), 6.55 (s, 2H broad alkene), 7.84–7.85(d, 2H alkene), 8.01–8.07 (dt, 3H, pyridine ring).
3.3. General Procedure for Preparation of Bis(azoles) 4 and 5
Method I: For bis(pyrazoles), A mixture of compound 3 (10 mmol) and hydrazine monohydrate (20 mmol) in ethanol was refluxed at 50–60 °C for 120 min. The mixture was left to cool and then added to iced water. The solid was collected by filtration and washed by EtOH. For bis(isoxazoles), a mixture of compound 3 (10 mmol), hydroxylamine hydrochloride (25 mmol) and sodium carbonate (25 mmol) in EtOH was refluxed at 50–60 °C for 240 min. The mixture was left to cool and then added to iced water. The solid was collected by filtration and washed by EtOH.
Method II: The same above mixture scale was placed in a Q-Tube at 120 °C/under an autogenic pressure of 30 psi for an appropriate period time as examined by TLC. Then, the products were obtained by following the same workup mentioned above.
2,6-Bis(1H-pyrazol-3-yl)pyridine (4) [62]
White cotton; (Q-Tube) yield 75%; m.p.c 257–259 °C, FT-IR cm−1: 3190; 3049; 2947 (CH aromatic), 1591 (C=N), 1596–1564 (C=C aromatic). 1HNMR (850 MHz, DMSO-d6): δ ppm = 6.95 (d, 2H, CH-4 pyrazole), 7.62−7.86 (m, 2H, CH-5 pyrazole and 3H pyridine ring), 13.02 (s, 1H, NH), 13.51 (s, 1H, NH).
2,6-Di(isoxazol-5-yl)pyridine (5) [62]
Beige powder; (Q-Tube) yield 29%; 143–147 °C, FT-IR cm−1: 3126, 3097 (CH aromatic), 1601 (C=N). 1HNMR (600 MHz, DMSO-d6): δ, ppm = 7.25 (d, 2H, CH-4 isoxazole), 8.76 (d, 2H, CH-5 isoxazole), 8.08 (d, 2H, pyridine), 8.19 (t, 1H, pyridine).
3.4. General Procedure for Preparation of Bis(pyrimidines) 6, 7a and 7b
Method I: A mixture of compound 3 (10 mmol), guanidine hydrochloride, urea or thiourea (20 mmol), and sodium ethoxide (30 mmol) in EtOH was refluxed at 60–70 °C for the appropriate time, as examined by TLC. The mixture was left to cool. The solid was collected by filtration and washed by EtOH.
Method II: The same above mixture scale was placed in a Q-Tube at 120 °C under an autogenic pressure of 30 psi for an appropriate period of time as examined by TLC. The products were collected and washed by ethanol. Recrystallization by DMF.
4,4′-(Pyridine-2,6-diyl)bis(pyrimidin-2-amine) (6) [67]
Pink powder; (Q-Tube) yield 97%; m.p. > 300 °C, FT-IR cm−1: 3442; 3290 (NH2), 3145 (CH aromatic), 1596 (C=N), 1549 (C=C aromatic), 1HNMR (850 MHz, DMSO-d6): δ, ppm = 6.79 (4H, NH2), 7.65 (d, 2H, CH-5 pyrimidine), 8.40 (d, 2H, CH-6 pyrimidine), 8.46 (d, 2H, pyridine), 8.16 (t, 1H, pyridine).
4,4′-(Pyridine-2,6-diyl)bis(pyrimidin-2-ol) (7a) [67]
Dark violet powder; (Q-Tube) yield 96% m.p. > 300 °C, FT-IR cm−1: 3284 (OH), 1575 (C=C aromatic). 1HNMR (850 MHz, DMSO-d6): δ, ppm = 5.89 (d, OH), 7.69–8.51 (m, 7H, pyridine and pyrimidine) 9.49 (d, OH). GC-MS: m/z [M]+ 267.84
4,4′-(Pyridine-2,6-diyl)bis(pyrimidine-2(1H)-thione) (7b) [67]
Orange powder; (Q-Tube) yield 41%, m.p. > 300 °C. FT-IR cm−1: 3306 (NH), 1532; 1556 (C=C aromatic), 1605 (C=N); 1HNMR (600 MHz, DMSO-d6): δ, ppm 7.52 (d, 2H, pyrimidine ring) 8.05 (t, 1H, pyridine ring) 8.16 (d, 2H, pyridine ring) 8.38 (d, 2H, pyrimidine ring); 13CNMR (213 MHz, DMSO-d6): δ, ppm = 127.3, 135.4, 141.0, 158.7, 179.2, 194.8. GC-MS: m/z [M]+ 299.27.
3.5. General Procedure for Preparation of Bis(pyridine)derivative 8
Method I: A mixture of compound 3 (10 mmol) and malononitrile (20 mmol) and piperidine drops in EtOH was refluxed at 75 °C for 1440 min. The solid was collected by filtration and washed by EtOH.
Method II: The same above mixture scale was placed in a Q-Tube at 120 °C under the autogenic pressure of 30 psi for an appropriate period of time, as examined by TLC. The product was collected and washed by ethanol, Recrystallization by EtOH.
6,6″-Dioxo-1,1″,6,6″-tetrahydro-[2,2′:6′,2″-terpyridine]-5,5″-dicarbonitrile (8) [68]
Orange powder; (Q-Tube) yield 99%, m.p. 202–204 °C; FT-IR cm−1: 3412; 3326 (OH and NH keto-enol form), 2184 (C≡N), 1645 (C=O) and 1534 (C=C aromatic); 1HNMR (850 MHz, DMSO-d6): δ, ppm = 5.62–5.64 (dd, 1H), 6.09–6.11 (dd, 1H), 6.99–7.04 (dd, 2H), 7.48–7.53 (dd, 2H), 8.11 (t, 1H, pyridine), 6.85 (broad s, OH), 6.69 (s, OH).
4. Conclusions
This study developed an efficient green organic synthesis protocol that saves energy by using a high-pressure Q-Tube reactor as an economic and safe alternative to a microwave reactor. A bis(enaminone) precursor was used in this protocol for preparing bis azoles and bis azines of expected biological and pharmacological efficacy. A mechanism for the reaction of bis(enaminone) with malononitrile suggested that the reaction proceeded via a Dimorth-type rearrangement. Modern Q-Tubes with a Qian cap have the fastest reaction time compared to conventional heating methods and almost identical results to microwave reactors. The technique opens the door to working on new substrates for biological screening in the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28052355/s1. Figures S1–S6: The 1HNMR spectra of the synthesized compounds.
Author Contributions
Conceptualization, K.M.A.-Z. and T.S.S.; methodology, K.M.A.-Z., M.S.B. and N.F.A.; formal analysis, M.S.B.; investigation, T.S.S.; resources, K.M.A.-Z.; data curation, M.S.B.; writing—original draft preparation, T.S.S.; writing—review and editing, T.S.S.; visualization, K.M.A.-Z.; supervision, K.M.A.-Z. and N.F.A.; project administration, K.M.A.-Z. and T.S.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deputyship for Research and Innovation, Ministry of Education in Saudi Arabia, for funding this research work via project number MoE-IF-G-20-04.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in Supplementary Materials and upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 3–8 are available from the authors.
References
- Isaacs, N.S. Liquid Phase High Pressure Chemistry; John Wiley & Sons, Inc.: Chichester, UK, 1981. [Google Scholar]
- Chen, B.; Hoffmann, R.; Cammi, R. The Effect of Pressure on Organic Reactions in Fluids—A New Theoretical Perspective. Angew. Chem. Int. Ed. 2017, 56, 11126–11142. [Google Scholar] [CrossRef] [PubMed]
- Jenner, G. High Pressure Molecular Science; Springer: Dordrecht, The Netherlands, 1999; pp. 313–330. [Google Scholar]
- Jenner, G. High Pressure Chemistry, Biochemistry and Materials Science; Springer: Dordrecht, The Netherlands, 1993; pp. 367–392. [Google Scholar]
- Muller, P. Glossary of terms used in physical organic chemistry (IUPAC Recommendations 1994). Pure Appl. Chem. 1994, 66, 1077–1184. [Google Scholar] [CrossRef]
- Le Noble, W.J. High Pressure Chemistry and Biochemistry; Springer: Dordrecht, The Netherlands, 1987; pp. 295–310. [Google Scholar]
- Zhang, Y.; Mosey, N.J. High pressure chemistry of thioaldehydes: A first-principles molecular dynamics study. J. Chem. Phys. 2016, 145, 194506. [Google Scholar] [CrossRef] [PubMed]
- Im, J.; Yim, N.; Kim, J.; Vogt, T.; Lee, Y. High-pressure chemistry of a zeolitic imidazolate framework compound in the presence of different fluids. J. Am. Chem. Soc. 2016, 138, 11477–11480. [Google Scholar] [CrossRef] [PubMed]
- Horvath-Bordon, E.; Riedel, R.; Zerr, A.; McMillan, P.F.; Auffermann, G.; Prots, Y.; Bronger, W.; Kniep, R.; Kroll, P. Highpressure chemistry of nitride-based materials. Chem. Soc. Rev. 2006, 35, 987–1014. [Google Scholar] [CrossRef] [PubMed]
- IUPAC. Compendium of Chemical Terminology, 2nd ed.; McNaught, A.D., Wilkinson, A., Eds.; Oxford University Press: Oxford, UK, 1997; ISBN 0-9678550-9-8. [Google Scholar] [CrossRef]
- Illustrations Together with Exhaustive Technical. Available online: http://www.qlabtech.com (accessed on 3 January 2023).
- Nacca, F.G.; Merlino, O.; Mangiavacchi, F.; Krasowska, D.; Santi, C.; Sancineto, L. The Q-Tube system, a nonconventional technology for green chemistry practitioners. Curr. Green Chem. 2017, 4, 58–66. [Google Scholar] [CrossRef]
- Wurche, F.; Klärner, F.-G. The Effect of Pressure on Organic Reactions: Basic Principles and Mechanistic Applications in High Pressure Chemistry: Synthetic, Mechanistic, and Supercritical Applications; Wiley-VCH Verlag GmbH: Winheim, Germany, 2002; pp. 41–96. [Google Scholar]
- Ball, D.; Key, J. Introductory Chemistry, 1st ed.; BCcampus: Victoria, BC, Canada, 2014; Available online: https://opentextbc.ca/introductorychemistry/ (accessed on 3 January 2023).
- Klimesová, V.; Svoboda, M.; Waisser, K.; Pour, M.; Kaustová, J. New pyridine derivatives as potential antimicrobial agents. Farmaco 1999, 54, 666–672. [Google Scholar] [CrossRef]
- Patel, H.; Chaudhari, K.; Jain, P.; Surana, S. Synthesis and in vitro antitubercular activity of pyridine analouges against the resistant Mycobacterium tuberculosis. Bioorganic Chem. 2020, 102, 104099. [Google Scholar] [CrossRef]
- Al-Omar, M.A.; Amr, A.E.-G.E.; Al-Salahi, R.A. Anti-inflammatory, analgesic, anticonvulsant and antiparkinsonian activities of some pyridine derivatives using 2,6-disubstituted isonicotinic acid hydrazides. Arch. Pharm. 2010, 343, 648–656. [Google Scholar] [CrossRef]
- El-Naggar, M.; Almahli, H.; Ibrahim, H.S.; Eldehna, W.M.; Abdel-Aziz, H.A. Pyridine-Ureas as Potential Anticancer Agents: Synthesis and In Vitro Biological Evaluation. Molecules 2018, 23, 1459. [Google Scholar] [CrossRef]
- Davari, A.S.; Abnous, K.; Mehri, S.; Ghandadi, M.; Hadizadeh, F. Synthesis and biological evaluation of novel pyridine derivatives as potential anticancer agents and phosphodiesterase-3 inhibitors. Bioorganic Chem. 2014, 57, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Goh, A.W.; Yu, M.; Adams, J.; Lam, F.; Teo, T.; Li, P.; Noll, B.; Zhong, L.; Diab, S. Discovery of (E)-3-((Styrylsulfonyl)methyl)pyridine and (E)-2-((Styrylsulfonyl)methyl)pyridine Derivatives as Anticancer Agents: Synthesis, Structure–Activity Relationships, and Biological Activities. J. Med. Chem. 2014, 57, 2275–2291. [Google Scholar] [CrossRef]
- Raasch, A.; Scharfenstein, O.; Tränkle, C.; Holzgrabe, U.; Mohr, K. Elevation of Ligand Binding to Muscarinic M2 Acetylcholine Receptors by Bis(ammonio)alkane-Type Allosteric Modulators. J. Med. Chem. 2002, 45, 3809–3812. [Google Scholar] [CrossRef]
- Yang, G.Y.; Oh, K.-A.; Park, N.-J.; Jung, Y.-S. New oxime reactivators connected with CH2O(CH2)nOCH2 linker and their reactivation potency for organophosphorus agents-inhibited acetylcholinesterase. Bioorganic Med. Chem. 2007, 15, 7704–7710. [Google Scholar] [CrossRef] [PubMed]
- Giacomo, B.D.; Bedini, A.; Spadoni, G.; Tarzia, G.; Fraschini, F.; Pannaccib, M.; Lucinib, V. Synthesis and biological activity of new melatonin dimeric derivatives. Bioorganic Med. Chem. 2007, 15, 4643–4650. [Google Scholar] [CrossRef] [PubMed]
- Eddington, N.D.; Cox, D.S.; Roberts, R.R.; Stables, J.P.; Powell, C.B.; Scott, K.R. Enaminones-versatile therapeutic pharmacophores. Further advances. Curr. Med. Chem. 2000, 7, 417–436. [Google Scholar] [CrossRef] [PubMed]
- Elassar, A.-Z.A.; El-Khair, A.A. Recent developments in the chemistry of enaminones. Tetrahedron 2003, 59, 8463–8480. [Google Scholar] [CrossRef]
- Stanovnik, B.; Svete, J. Synthesis of Heterocycles from Alkyl 3-(Dimethylamino)propenoates and Related Enaminones. Chem. Rev. 2004, 104, 2433–2480. [Google Scholar] [CrossRef]
- Falcó, J.L.; Loveras, M.; Buira, I.; Teixidó, J.; Borrell, J.I.; Méndez, E.; Terencio, J.; Palomer, A.; Guglietta, A. Design synthesis and biological activity of acyl substituted 3-amino-5-methyl-1,4,5,7-tetrahydropyrazolo[3,4-b]pyridin-6-ones as potential hypnoticdrugs. Eur. J. Med. Chem. 2005, 40, 1179–1187. [Google Scholar] [CrossRef]
- Anderson, J.J.; Bradbury, M.J.; Giracello, D.R.; Chapman, D.F.; Holtz, G.; Roppe, J.; King, C.; Cosford, N.D.P.; Varney, M.A. In vivo receptor occupancy of mGlu5 receptor antagonists using the novel radioligand [3H]3-methoxy-5-(pyridin-2-ylethynyl)pyridine). Eur. J. Pharmacol. 2003, 473, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Navidpour, L.; Shafaroodi, H.; Miri, R.; Dehpour, A.R.; Shafiee, A. Lipophilic 4-imidazoly-1,4-dihydropyridines: Synthesis, calcium channel antagonist activity and protection against pentylenetetrazole-induced seizure. Farmaco 2004, 59, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, A.; Turan-Zitouni, G.; Kaplancıklı, Z.A.; Iscan, G.; Khan, S.; Demirci, F. Synthesis and the selective antifungal activity of 5,6,7,8-tetrahydroimidazo[1,2-a]pyridine derivatives. Eur. J. Med. Chem. 2010, 45, 2080–2084. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.S.; Perumal, S.; Shetty, K.A.; Yogeeswari, P.; Sriram, D. 1,3-Dipolar cycloaddition of C-aryl-N-phenylnitrones to (R)-1-(1-phenylethyl)-3-[(E)-arylmethylidene]tetrahydro-4(1H)-pyridinones: Synthesis and antimycobacterial evaluation of enantiomerically pure spiroisoxazolidines. Eur. J. Med. Chem. 2010, 45, 124–133. [Google Scholar] [CrossRef]
- Humphries, P.S.; Almaden, J.V.; Barnum, S.J.; Carlson, T.J.; Do, Q.T.; Fraser, J.D.; Hess, M.; Kim, Y.H.; Ogilviec, K.M.; Sun, S. Pyridine-2-propanoic acids: Discovery of dual PPARa/c agonists as antidiabetic agents. Bioorganic Med. Chem. Lett. 2006, 16, 6116–6119. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Nonoshita, K.; Ogino, Y.; Nagae, Y.; Tsukahara, D.; Hosaka, H.; Maruki, H.; Ohyama, S.; Yoshimoto, R.; Sasaki, K.; et al. Discovery of novel 2-(pyridine-2-yl)-1H-benzimidazole derivatives as potent glucokinase activators. Bioorganic Med. Chem. Lett. 2009, 19, 4450–4454. [Google Scholar] [CrossRef]
- Veron, J.-B.; Enguehard-Gueiffier, C.; Snoeck, R.; Andrei, G.; De Clercqb, E.; Gueiffier, A. Influence of 6 or 8-substitution on the antiviral activity of 3-phenethylthiomethylimidazo[1,2-a]pyridine against human cytomegalovirus (HCMV) and varicella-zoster virus (VZV). Bioorganic Med. Chem. 2007, 15, 7209–7219. [Google Scholar] [CrossRef]
- Liu, X.-H.; Liu, H.-F.; Shen, X.; Song, B.-A.; Bhadury, P.S.; Zhu, H.-L.; Liu, J.-X.; Qi, X.-B. Synthesis and molecular docking studies of novel 2-chloropyridine derivatives containing flavone moieties as potential antitumor agents. Bioorganic Med. Chem. Lett. 2010, 20, 4163–4167. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, S.Y.; Kim, J.S.; Song, H.M.; Suh, M.E.; Lee, C.O. Synthesis and cytotoxicity evaluation of pyridin[2,3-f]indole-2,4,9-trione and benz[f]indole-2,4,9-trione derivatives. Bioorganic Med. Chem. 2003, 11, 4791–4796. [Google Scholar] [CrossRef]
- Liu, J.; Cui, G.; Zhao, M.; Cui, C.; Ju, J.; Peng, S. Dual-acting agents that possess reversing resistance and anticancer activities: Design, synthesis, MES-SA/Dx5 cell assay, and SAR of Benzyl 1,2,3,5,11,11a-hexahydro-3,3-dimethyl-1-oxo-6Himidazo[3,4:1,2]pyridin[3,4-b]indol-2-substitutedacetates. Bioorganic Med. Chem. 2007, 15, 7773–7788. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry Theory and Practice; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Nasir Baig, R.B.; Varma, R.S. Solvent-free synthesis. In An Introduction to Green Chemistry Methods; Luque, R., Colmenares, J.C., Eds.; Future Science: London, UK, 2013. [Google Scholar]
- Sheldon, R.A. Green chemistry and resource efficiency: Towards a green economy. Green Chem. 2016, 18, 3180–3183. [Google Scholar] [CrossRef]
- Mokhtar, M.; Alzhrani, G.; Aazam, E.S.; Saleh, T.S.; Panja, S.; Maiti, D. Synergistic Effect of NiLDH@YZ Hybrid and Mechanochemical Agitation on Glaser Homocoupling Reaction. Chem. A Eur. J. 2021, 27, 8875–8885. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, M.; Saleh, T.S.; Narasimharao, K.; Al-Mutairi, E. New green perspective to dihydropyridines synthesis utilizing modified heteropoly acid catalysts. Catal. Today, 2021; in press. [Google Scholar]
- El-bendary, M.M.; Saleh, T.S.; Al-Bogami, A.S. Synthesis and structural characterization of a palladium complex as an anticancer agent, and a highly efficient and reusable catalyst for the Heck coupling reaction under ultrasound irradiation: A convenient sustainable green protocol. Polyhedron 2021, 194, 114924. [Google Scholar] [CrossRef]
- AL-Johani, M.; Al-Zaydi, K.; Mousally, S.; Alqahtani, N.; Elnagdi, N.; Elnagdi, M. Multi Component Reactions under Increased Pressure: On the Mechanism of Formation of Pyridazino [5, 4,3-de][1,6] naphthyridine Derivatives by the Reaction of Malononitrile, Aldehydes and 2-Oxoglyoxalarylhydrazones in Q-Tubes. Molecules 2017, 22, 2114. [Google Scholar] [CrossRef] [PubMed]
- Al-Zaydi, K.M. A simplified green chemistry approaches to synthesis of 2-substituted 1,2,3-triazoles and 4-amino-5-cyanopyrazole derivatives conventional heating versus microwave and ultrasound as ecofriendly energy sources. Ultrason. Sonochem. 2009, 16, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Al-Zaydi, K.M.; Borik, R.M.; Elnagdi, M.H. Arylhydrazononitriles as Precursors to 2-Substituted 1,2,3-triazoles and 4-amino-5-cyano-pyrazole Derivatives Utilizing Microwave and Ultrasound Irradiation. Green Chem. Lett. Rev. 2012, 5, 241–250. [Google Scholar] [CrossRef]
- Alzaydi, K.M.; Saleh, T.S. 2-Aryl hydrazonopropanal pharmacophores as potent cytotoxic agents against human hepatocellular carcinoma cell line. Med. Chem. Res. 2020, 29, 199–205. [Google Scholar] [CrossRef]
- Ahmed, N.S.; Saleh, T.S.; El-Mossalamy, E.-S.H. An efficiently sonochemical synthesis of novel pyrazoles, bipyrazoles and pyrazol-3-ylpyrazolo[3,4-d]pyrimidines incorporating 1H-benzoimidazole moiety. Curr. Org. Chem. 2013, 17, 194–202. [Google Scholar] [CrossRef]
- Shahid, A.; Ahmed, N.S.; Saleh, T.S.; Al-Thabaiti, S.A.; Basahel, S.N.; Schwieger, W.; Mokhtar, M. Solvent-free biginelli reactions catalyzed by hierarchical zeolite utilizing a ball mill technique: A green sustainable process. Catalysts 2017, 7, 84. [Google Scholar] [CrossRef]
- Narasimharao, K.; Al-Sabban, E.; Saleh, T.S.; Gallastegui, A.G.; Sanfiz, A.C.; Basahel, S.; Al-Thabaiti, S.; Alyoubi, A.; Obaid, A.; Mokhtar, M. Microwave assisted efficient protocol for the classic Ullmann homocoupling reaction using Cu–Mg–Al hydrotalcite catalysts. J. Mol. Catal. A Chem. 2013, 379, 152–162. [Google Scholar] [CrossRef]
- Saleh, T.S.; Narasimharao, K.; Ahmed, N.S.; Basahel, S.N.; Al-Thabaiti, S.A.; Mokhtar, M. Mg-Al hydrotalcite as an efficient catalyst for microwave assisted regioselective 1,3-dipolar cycloaddition of nitrilimines with the enaminone derivatives: A green protocol. J. Mol. Catal. A Chem. 2013, 367, 12–22. [Google Scholar] [CrossRef]
- Bassyouni, F.A.; Saleh, T.S.; Elhefnawi, M.M.; El-Moez, S.I.A.; El-Senousy, W.M.; Abdel-Rehim, M.E. Synthesis, pharmacological activity evaluation and molecular modeling of new polynuclear heterocyclic compounds containing benzimidazole derivatives. Arch. Pharmacal Res. 2012, 35, 2063–2075. [Google Scholar] [CrossRef]
- Mady, M.F.; Saleh, T.S.; El-Kateb, A.A.; El-Rahman, N.M.A.; El-Moez, S.I.A. Microwave-assisted synthesis of novel pyrazole and pyrazolo[3,4-d]pyridazine derivatives incorporating diaryl sulfone moiety as potential antimicrobial agents. Res. Chem. Intermed. 2016, 42, 753–769. [Google Scholar] [CrossRef]
- Maksod, I.H.A.E.; Saleh, T.S. The use of nano supported nickel catalyst in reduction of p-nitrophenol using hydrazine as hydrogen donor. Green Chem. Lett. Rev. 2010, 3, 127–134. [Google Scholar] [CrossRef]
- Saleh, T.S.; El-Rahman, N.M.A.; Assaker, R.S.A. Microwave promoted a green protocol for solvent free synthesis of 1,5-benzothiazepine and [1,3,4]-thiadiazepine derivatives incorporating thiophene moiety. Green Chem. Lett. Rev. 2012, 5, 315–320. [Google Scholar] [CrossRef]
- Al-Bogami, A.S.; Saleh, T.S.; Mekky, A.E.M.; Shaaban, M.R. Microwave assisted regioselective synthesis and 2D-NMR studies of novel azoles and azoloazines utilizing fluorine-containing building blocks. J. Mol. Struct. 2016, 1121, 167–179. [Google Scholar] [CrossRef]
- Mekky, A.E.M.; Saleh, T.S.; Al-Bogami, A.S. Synthesis of novel pyrazoles incorporating a phenothiazine moiety: Unambiguous structural characterization of the regioselectivity in the 1,3-dipolar cycloaddition reaction using 2D HMBC NMR spectroscopy. Tetrahedron 2013, 69, 6787–6798. [Google Scholar] [CrossRef]
- Kudyakova, Y.S.; Bazhin, D.N.; Goryaeva, M.V.; Burgart, Y.V.; Saloutin, V.I. The use of 2-(1-alkoxyalkylidene)-1,3-dicarbonyl compounds in organic synthesis. Russ. Chem. Rev. 2014, 83, 120–142. [Google Scholar] [CrossRef]
- Mittersteiner, M.; Andrade, V.P.; Bonacorso, H.G.; Martins, M.A.P.; Zanatta, N. The Wonderful World of β-Enamino Diketones Chemistry. Eur. J. Org. Chem. 2020, 2020, 6405–6417. [Google Scholar] [CrossRef]
- Gamez, P.; Steensma, R.H.; Driessen, W.L.; Reedijk, J. Copper(II) compounds of the planar-tridentate ligand 2,6- bis(pyrazol-3-yl)pyridine. Inorganica Chim. Acta 2002, 333, 51–56. [Google Scholar] [CrossRef]
- Lin, Y.-I.; Lang, S.A., Jr. Novel two step synthesis of pyrazoles and isoxazoles from aryl methyl ketones. J. Heterocycl. Chem. 1977, 14, 345–347. [Google Scholar] [CrossRef]
- Pleier, A.K.; Glas, H.; Grosche, M.; Sirsch, P.; Thiel, W.R. Microwave assisted synthesis of 1-aryl-3-dimethylaminoprop-2-enones: A simple and rapid access to 3 (5)-arylpyrazoles. Synthesis 2001, 1, 55–62. [Google Scholar] [CrossRef]
- Shaaban, M.R.; Farag, A.M.; Saleh, T.S.; Osman, F.H. Regioselective synthesis of some novel pyrazoles, isoxazoles, pyrazolo[3,4-d]pyridazines and isoxazolo[3,4-d]pyridazines pendant to benzimidazole. J. Heterocycl. Chem. 2007, 44, 177–181. [Google Scholar] [CrossRef]
- Sumrra, S.H.; Hassan, A.U.; Zafar, M.N.; Shafqat, S.S.; Mustafa, G.; Zafar, M.N.; Zubair, M.; Imran, M. Metal incorporated sulfonamides as promising multidrug targets: Combined enzyme inhibitory, antimicrobial, antioxidant and theoretical exploration. J. Mol. Struct. 2022, 1250, 131710. [Google Scholar] [CrossRef]
- Hassan, A.U.; Sumrra, S.H.; Raza, M.A.; Zubair, M.; Zafar, M.N.; Mughal, E.U.; Nazar, M.F.; Irfan, A.; Imran, M.; Assiri, M.A. Design, facile synthesis, spectroscopic characterization, and medicinal probing of metal-based new sulfonamide drugs: A theoretical and spectral study. Appl. Organomet. Chem. 2021, 35, e6054. [Google Scholar] [CrossRef]
- Abdelrazek, F.M.; Elsayed, A.N. About the reaction of β-dimethyl- amino-α,β-enones with active methylene nitriles. J. Heterocycl. Chem. 2009, 46, 949–953. [Google Scholar] [CrossRef]
- Bejan, E.; Haddou, H.A.; Daran, J.C.; Balavoine, G.G.A. The reaction of enaminones with carboxamidines: A convenient route for the synthesis of polyaza heterocycles. Synthesis 1996, 8, 1012–1018. [Google Scholar] [CrossRef]
- Hassanien, A.Z.A.E.B. 2, 6-Bis [3-N, N-Dimethylamino-1-Oxopropen-1-Yl] Pyridine as a Building Block in Heterocyclic Synthesis: Synthesis of 2, 2′: 6′, 2″-Terpyridines and 2, 6-Bis [Pyrazolyl, Isoxazolyl, Diazepinyl, Pyrazolo [5, 1-a] Pyrimidinyl and Pyrazolo-[4, 3-D] Pyridazinyl] Pyridines. J. Chem. Res. 2004, 8, 536–540. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).