Ionic Organic Solid 1,3-Bis(sulfomethyl)imidazoliumate as an Effective Metal-Free Catalyst for Sustainable Organic Syntheses
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
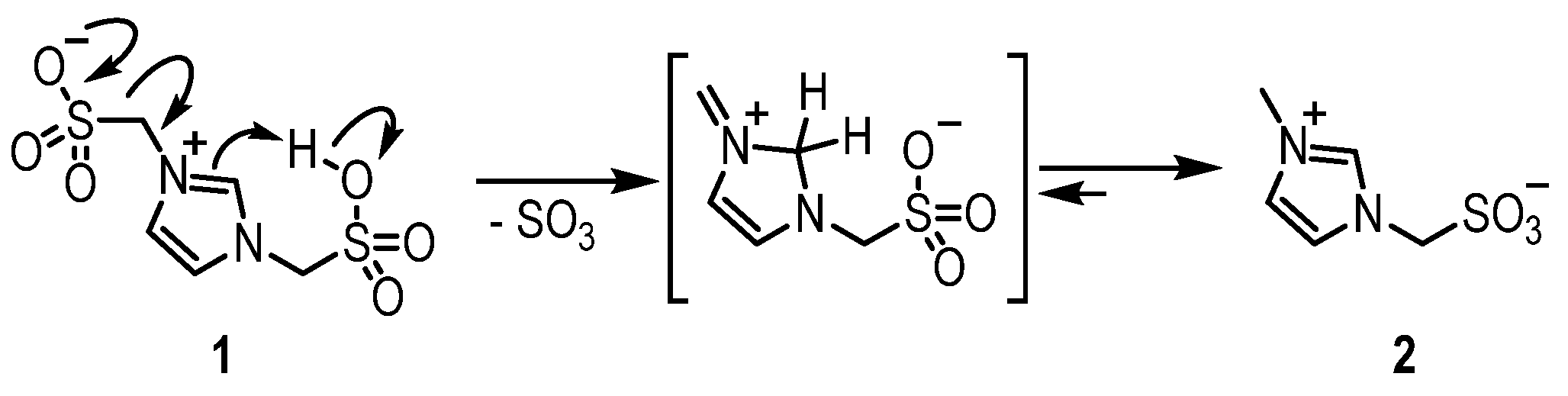
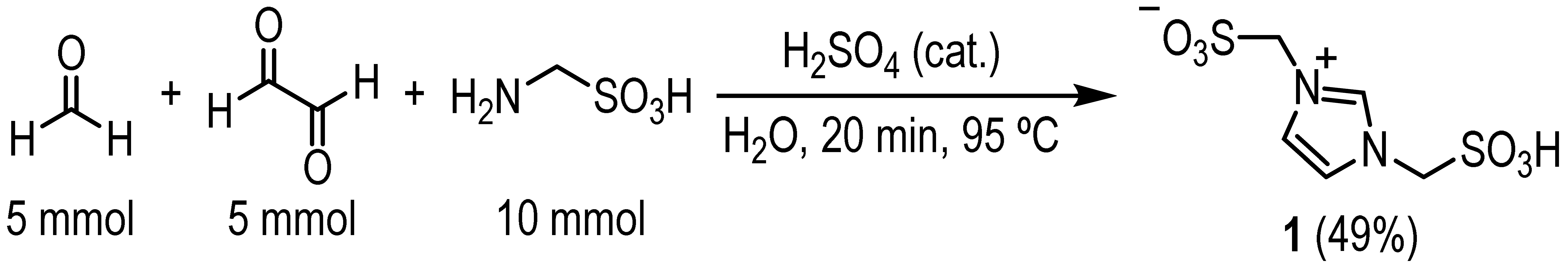
3.1. Procedure for the Preparation of Bsmim (1)
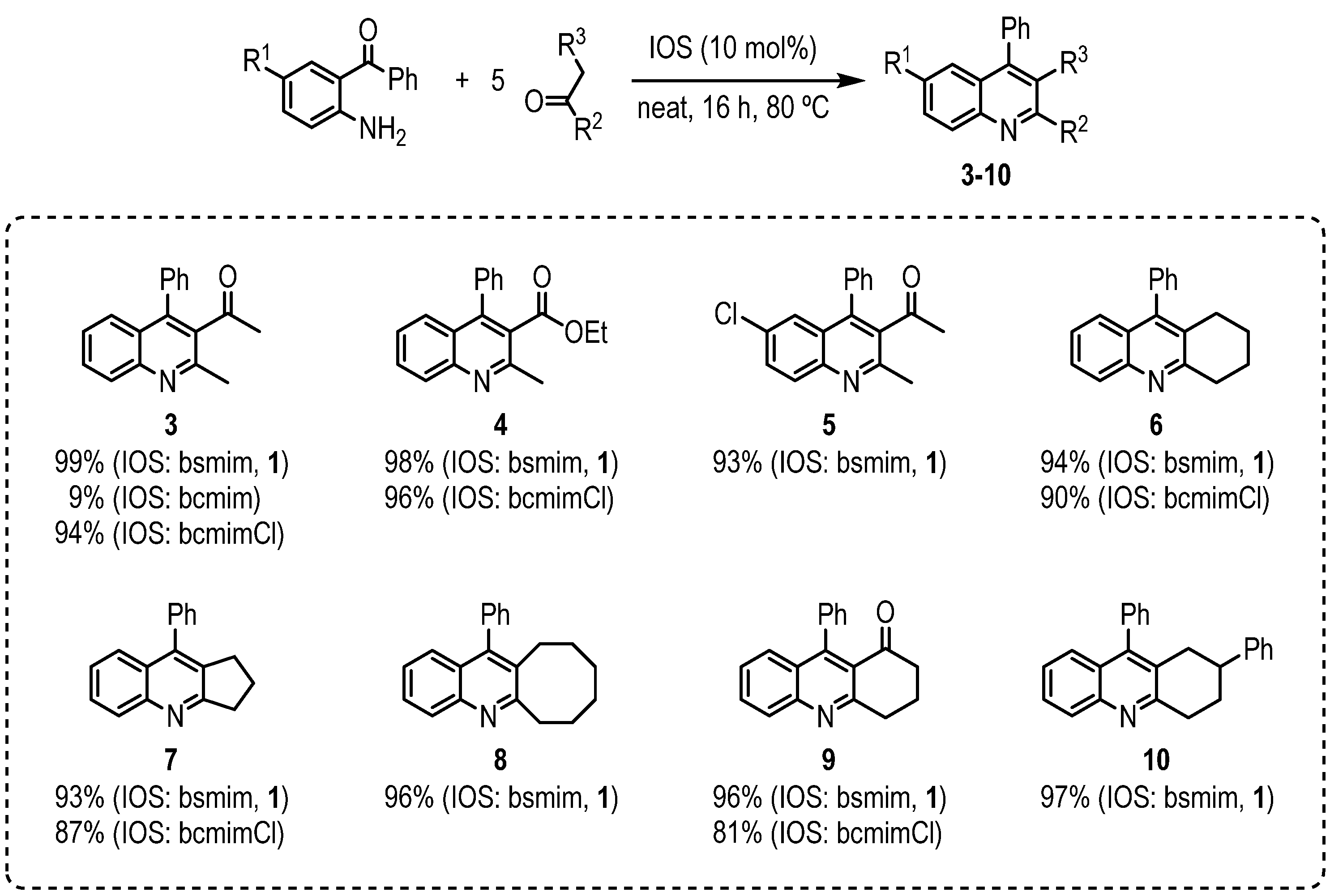
3.2. General Procedure for the Synthesis of Quinolines Promoted by Bsmim
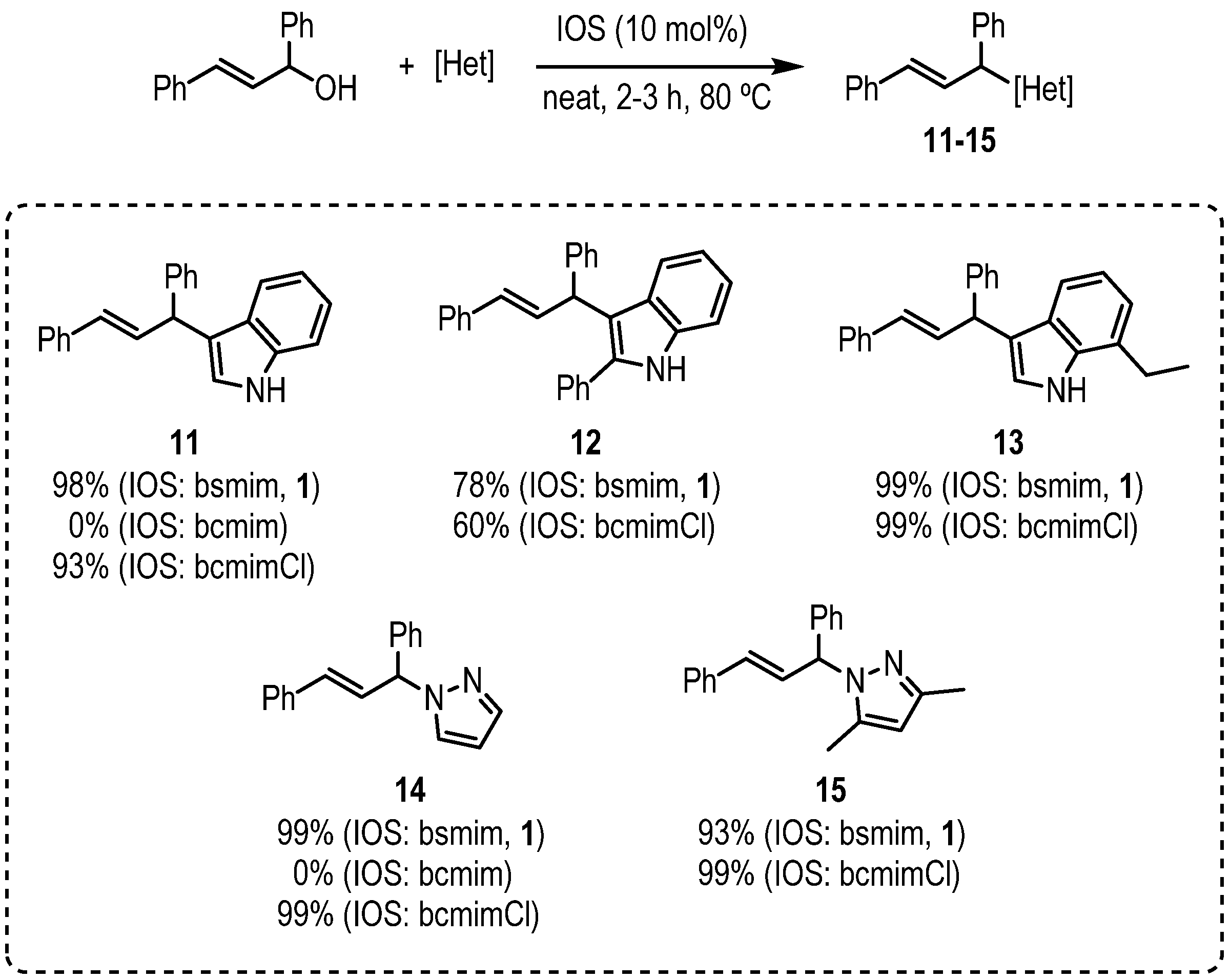
3.3. General Procedure for the Allylation of Heterocycles Promoted by Bsmim
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Liu, X.; Yang, Z.; Wang, D.; Cao, H. Molecular structures and second-order nonlinear optical properties of ionic organic crystal materials. Crystals 2016, 6, 158. [Google Scholar] [CrossRef]
- Torrance, J.B. The relation between conductivity, optical absorption, and lonicity in oxides and organic compounds. J. Solid State Chem. 1992, 96, 59–66. [Google Scholar] [CrossRef]
- Matsunaga, Y. Desorption from metal surfaces by low-energy electrons. J. Chem. Phys. 1964, 41, 1609–1613. [Google Scholar] [CrossRef]
- Soos, Z.G. Theory of π-molecular charge-transfer crystals. Annu. Rev. Phys. Chem. 1974, 25, 121–153. [Google Scholar] [CrossRef]
- Soos, Z.G.; Keller, H.J.; Moroni, W.; Noethe, D. Cation radical salts of phenazine. J. Am. Chem. Soc. 1977, 99, 5040–5044. [Google Scholar] [CrossRef]
- Jérome, F.; Mazaud, A.; Ribault, M.; Bechgaard, K. Superconductivity in a synthetic organic conductor. J. Physique Lett. 1980, 41, 95–98. [Google Scholar] [CrossRef]
- Kim, P.-J.; Jeong, J.-H.; Jazbinsek, M.; Choi, S.-B.; Baek, I.-H.; Kim, J.-T.; Rotermund, F.; Yun, H.; Lee, Y.S.; Günter, P.; et al. Highly efficient organic THz generator pumped at near-infrared: Quinolium single crystals. Adv. Funct. Mater. 2012, 22, 200–209. [Google Scholar] [CrossRef]
- Lee, S.-H.; Lu, J.; Lee, S.-J.; Han, J.-H.; Jeong, C.-U.; Lee, S.-C.; Li, X.; Jazbinšek, M.; Yoon, W.; Yun, H.; et al. Benzothiazolium single crystals: A new class of nonlinear optical crystals with efficient THz wawe generation. Adv. Mater. 2017, 29, 1701748. [Google Scholar] [CrossRef]
- Palaniyasan, E.; Radhakrishnan, A.; Maidur, S.R.; Patil, P.S.; Muppudathi, A.L.; Jeyaperumal, K.S. Synthesis, growth, and characterization of single-crystal benzo[e]indolium for third-order nonlinear optical properties. J. Electron. Mater. 2022, 51, 3531–3541. [Google Scholar] [CrossRef]
- Zhou, W.; Wu, X.; Ma, P.; Zhou, F.; Li, Z.; Niu, R.; Yang, J.; Wang, Y.; Zhang, X.; Song, Y.; et al. Enhanced ultrafast nonlinear absorption and optical limiting of indolium squaraine for laser protection. Opt. Mater. 2022, 126, 112178. [Google Scholar] [CrossRef]
- Abate, A.; Brischetto, M.; Cavallo, G.; Lahtinen, M.; Metrangolo, P.; Pilati, T.; Radice, S.; Resnati, G.; Rissanen, K.; Terraneo, G. Dimensional encapsulation of I−···I2···I− in an organic salt crystal matrix. Chem. Commun. 2010, 46, 2724–2726. [Google Scholar] [CrossRef]
- Lin, J.; Martí-Rujas, J.; Metrangolo, P.; Pilati, T.; Radice, S.; Resnati, G.; Terraneo, G. Solution and solid state synthesis of the discrete polyiodide I73– under modular cation templation. Cryst. Growth Des. 2012, 12, 5757–5762. [Google Scholar] [CrossRef]
- Pringle, J.M.; Howlett, P.C.; MacFarlane, D.R.; Forsyth, M. Organic ionic plastic crystals: Recent advances. J. Mater. Chem. 2010, 20, 2056–2062. [Google Scholar] [CrossRef]
- Torregrosa-Chinillach, A.; Chinchilla, R. Synthesis of xanthones, thioxanthones and acridones by a metal-free photocatlytic oxidation using visible light and molecular oxygen. Molecules 2021, 26, 974. [Google Scholar] [CrossRef]
- Torregrosa-Chinillach, A.; Chinchilla, R. Visible light-induced aerobic oxidative dehydrogenation of C-N/C-O to C=N/C=O bonds using metal-free photocatalysts: Recent developments. Molecules 2022, 27, 497. [Google Scholar] [CrossRef]
- Jen, W.S.; Wiener, J.J.M.; MacMillan, D.W.C. New strategies for organic catalysis: The first enantioselective organocatalytic 1,3-dipolar cycloaddition. J. Am. Chem. Soc. 2000, 122, 9874–9875. [Google Scholar] [CrossRef]
- Itsuno, S.; Yamamoto, S.; Takata, S. Synthesis of cinchonidinium salts containing sulfonamide functionalities and their catalytic activity in asymmetric alkylation reactions. Tetrahedron Lett. 2014, 55, 6117–6120. [Google Scholar] [CrossRef]
- Bacaicoa, S.; Goossens, E.; Sundén, H. Aerobic oxidative N-heterocyclic carbene-catalyzed formal [3+3] cyclization for the synthesis of tetrasubstituted benzene derivatives. Org. Lett. 2022, 24, 9146–9150. [Google Scholar] [CrossRef]
- Allegue, A.; Albert-Soriano, M.; Pastor, I.M. A comparative study of hydroxyl- and carboxylate-functionalized imidazolium and benzimidazolium salts as precursors for N-heterocyclic carbene ligands. Appl. Organomet. Chem. 2015, 29, 624–632. [Google Scholar] [CrossRef]
- Arduengo, A.J.; Harlow, R.L.; Kline, M.A. A stable crystalline carbene. J. Am. Chem. Soc. 1991, 113, 361–363. [Google Scholar] [CrossRef]
- Gisbert, P.; Trillo, P.; Pastor, I.M. Comparative study of catalytic systems formed by palladium and acyl-substituted imidazolium salts. ChemistrySelect 2018, 3, 887–893. [Google Scholar] [CrossRef]
- Martínez, R.; Pastor, I.M.; Yus, M. Biscarboxy-functionalized imidazole and palladium as highly active catalytic system in protic solvents: Methanol and water. Synthesis 2014, 46, 2965–2975. [Google Scholar] [CrossRef]
- Albert-Soriano, M.; Pastor, I.M. Metal-organic framework based on copper and carboxylate-imidazole as robust and effective catalyst in the oxidative amidation of carboxylic acids and formamides. Eur. J. Org. Chem. 2016, 2016, 5180–5188. [Google Scholar] [CrossRef]
- Albert-Soriano, M.; Trillo, P.; Soler, T.; Pastor, I.M. Versatile barium and calcium imidazolium-dicarboxylate heterogeneous catalysts in quinoline synthesis. Eur. J. Org. Chem. 2017, 2017, 6375–6381. [Google Scholar] [CrossRef]
- Gisbert, P.; Albert-Soriano, M.; Pastor, I.M. Effective and sustainable access to quinolines and acridines: A heterogeneous imidazolium salt mediates C-C and C-N bond formation. Eur. J. Org. Chem. 2019, 2019, 4928–4940. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, H.; Liu, C.-J.; Cao, D.; Wang, B.; Sun, Y.; Abdukader, A. Highly efficient Brønsted acidic ionic liquid promoted direct diazenylation of pyrazolones with aryltriazenes under mild conditions. Asian J. Org. Chem. 2017, 6, 102–107. [Google Scholar] [CrossRef]
- Fei, Z.; Zhao, D.; Geldbach, T.J.; Scopelliti, R.; Dyson, P.J. Brønsted acidic ionic liquids and their zwitterions: Synthesis, characterization and pKa determination. Chem. Eur. J. 2004, 10, 4886–4893. [Google Scholar] [CrossRef]
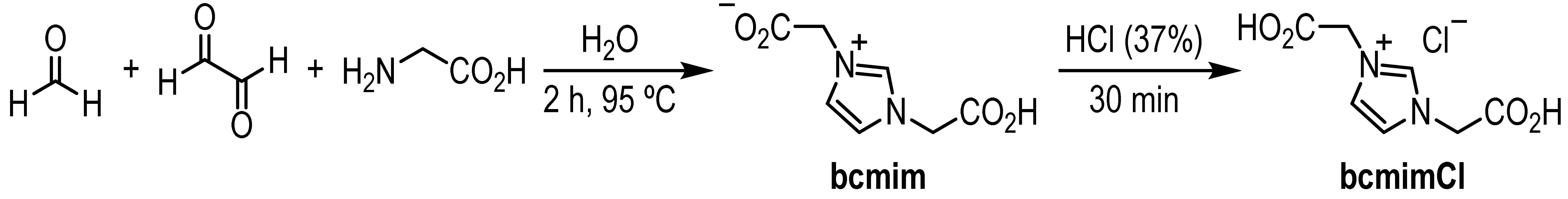
- Albert-Soriano, M.; Hernández-Martínez, L.; Pastor, I.M. 1,3-Bis(carboxymethyl)imidazolium chloride as a metal-free and recyclable catalyst for the synthesis of N-allylanilines by allylic substitution of alcohols. ACS Sustain. Chem. Eng. 2018, 6, 14063–14070. [Google Scholar] [CrossRef]
- Davanagere, M.P.; Maiti, B. 1,3-Bis(carboxymethyl)imidazolium chloride as a sustainable, recyclable, and metal-free ionic catalyst for the Biginelli multicomponent reaction in neat conditions. ACS Omega 2021, 6, 26035–26047. [Google Scholar] [CrossRef]
- Gisbert, P.; Pastor, I.M. Efficient thiophene synthesis mediated by 1,3-bis(carboxymethyl)imidazolium chloride: C-C and C-S bond formation. Eur. J. Org. Chem. 2020, 2020, 4319–4325. [Google Scholar] [CrossRef]
- Albert-Soriano, M.; Pastor, I.M. Anion-dependent imidazolium-based catalysts for allylation of aniline with tunable regioselectivity. Adv. Synth. Catal. 2020, 362, 2494–2502. [Google Scholar] [CrossRef]
- Trummal, A.; Lipping, L.; Kaljurand, I.; Koppel, I.A.; Leito, I. Acidity of strong acids in water and dimethyl sulfoxide. J. Phys. Chem. A 2016, 120, 3663–3669. [Google Scholar] [CrossRef]
- Zhang, S.; Baker, J.; Pulay, P. A reliable and efficient first principles-based method for predicting pKa values. 2. Organic acids. J. Phys. Chem. A 2010, 114, 432–442. [Google Scholar] [CrossRef]
- Khazaei, A.; Zolfigol, M.A.; Moosavi-Zare, A.R.; Zare, A.; Ghaemi, E.; Khakyzadeh, V.; Asgari, Z.; Hasaninejad, A. Sulfonic acid functionalized imidazolium salts/FeCl3 as novel and highly efficient catalytic systems for the synthesis of benzimidazoles at room temperature. Sci. Iran. 2011, 18, 1365–1371. [Google Scholar] [CrossRef]
- Khaligh, N.G.; Mihankhah, T.; Johan, M.R. One-pot synthesis of coumarins using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride as an efficient task-specific ionic liquid. Polycycl. Aromat. Compd. 2021, 41, 1712–1721. [Google Scholar] [CrossRef]
- Sharghi, H.; Shiri, P.; Aberi, M. An overview on recent advances in the synthesis of sulfonated organic materials, sulfonated silica materials, and sulfonated carbon materials and their catalytic applications in chemical processes. Beilstein J. Org. Chem. 2018, 14, 2745–2770. [Google Scholar] [CrossRef]
- Xu, D.-Q.; Wu, J.; Luo, S.-P.; Zhang, J.-X.; Wu, J.-Y.; Du, X.-H.; Xu, Z.-Y. Fischer indole synthesis catalyzed by novel SO3H-functionalized ionic liquids in water. Green Chem. 2009, 11, 1239–1246. [Google Scholar] [CrossRef]
- Khaligh, N.G.; Teng, L.S.; Ling, O.C.; Johan, M.R.; Ching, J.J. 4-Imidazol-1-yl-butane-1-sulfonic acid or a novel liquid salt? The NMR analysis and dual solvent-catalytic efficiency for one-pot synthesis of xanthenes. J. Mol. Liq. 2019, 278, 19–32. [Google Scholar] [CrossRef]
- Sim, S.E.; Kwon, S.; Koo, S. Bis-sulfonic acid ionic liquids for the conversion of Fructose to 5-Hydroxymethyl-2-furfural. Molecules 2012, 17, 12804–12811. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Ohno, H. Triple ion-type imidazolium salts: A new class of single-ion conductive matrix. Ionics 2002, 8, 267–271. [Google Scholar] [CrossRef]
- Girard, A.-L.; Simon, N.; Zanatta, M.; Marmitt, S.; Gonçalves, P.; Dupont, J. Insights on recyclable catalytic system composed of task-specific ionic liquids for the chemical fixation of carbon dioxide. Green Chem. 2014, 16, 2815–2825. [Google Scholar] [CrossRef]
- Jónsson, E.; Armand, M.B.; Johansson, J.P. Anions and Derived Salts with High Dissociation in Non-Protogenic Solvents. U.S. Patent 9269987, 23 February 2016. Available online: https://image-ppubs.uspto.gov/dirsearch-public/print/downloadPdf/9269987 (accessed on 23 February 2023).
- Martos, M.; Albert-Soriano, M.; Pastor, I.M. 1,3-Bis(3-carboxypropyl)-1H-imidazole. Molbank 2022, 2022, M1480. [Google Scholar] [CrossRef]
- Sheldon, R.A. Fundamentals of green chemistry: Efficiency in reaction design. Chem. Soc. Rev. 2012, 41, 1437–1451. [Google Scholar] [CrossRef] [PubMed]
- Van Aken, K.; Strekowski, L.; Patiny, L. EcoScale, a semi-quantitative tool to select an organic preparation based on economical and ecological parameters. Beilstein J. Org. Chem. 2006, 2, 3. [Google Scholar] [CrossRef]
- Andraos, J. Reaction Green Metrics: Problems, Exercises and Solutions; CRC Press (Taylor & Francis Group): Boca Raton, FL, USA, 2019. [Google Scholar]
- Martos, M.; Pérez-Almarcha, Y.; Pastor, I.M. DES-type interactions to promote solvent-free and metal-free reactions between nitrogen-containing heterocycles and allylic alcohols. Eur. J. Org. Chem. 2022, 2022, e202201221. [Google Scholar] [CrossRef]
- Tanwar, B.; Kumar, A.; Yogeeswari, P.; Sriram, D.; Chakraborti, A.K. Design, development of new synthetic methodology, and biological evaluation of substituted quinolines as new anti-tubercular leads. Bioorg. Med. Chem. Lett. 2016, 26, 5960–5966. [Google Scholar] [CrossRef]
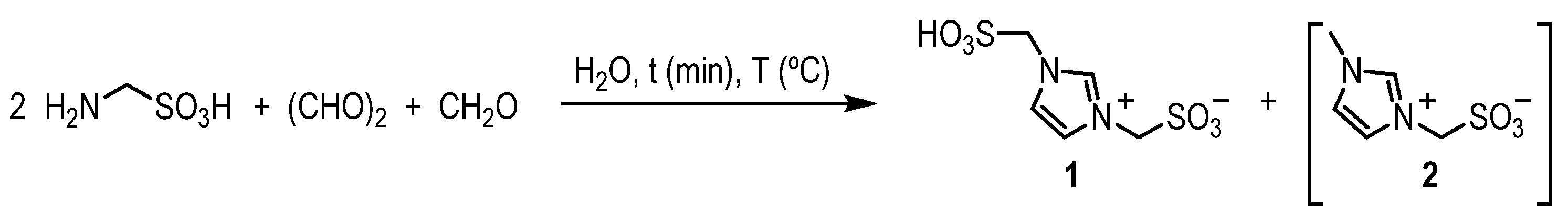
| Entry | Additive | T (°C) | t (min) | Yield (%) | [1:2:AMSA] 2 |
|---|---|---|---|---|---|
| 1 | -- | 95 | 120 | n.d. 4 | -- |
| 2 | H2O (2 mL) | 95 | 120 | 45 | 76:9:15 |
| 3 | -- 3 | 95 | 120 | n.d. 4 | -- |
| 4 | H2O (2 mL) | 95 | 20 | 47 | 86:9:5 |
| 5 | H2O (2 mL) | 120 | 20 | 41 | 75:12:13 |
| 6 | H2O (2 mL) | 120 | 40 | 45 | 68:20:12 |
| 7 | H2SO4 (c., 0.1 mL) | 95 | 20 | 30 | 100:0:0 |
| 8 | H2O (2 mL) + H2SO4 (c., 0.1 mL) | 95 | 20 | 49 | 100:0:0 |
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| IOS | Yield (%) | AE (%) | SF | RME (%) | MRP | VMR | E-Factor | EcoScale |
| bsmim (1) | 49 | 83 | 1 | 5.1 | 0.127 | 0.623 | 18.5 | 64 |
 | ||||||||
| Bcmim 1 | 89 | 77 | 1 | 18.7 | 0.271 | 0.707 | 4.3 | 83 |
| bcmimCl 1,2 | 83 | 80 | 1.15 | 4.4 | 0.076 | 0.647 | 21.7 | 67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martos, M.; Guapacha, A.M.; Pastor, I.M. Ionic Organic Solid 1,3-Bis(sulfomethyl)imidazoliumate as an Effective Metal-Free Catalyst for Sustainable Organic Syntheses. Molecules 2023, 28, 2695. https://doi.org/10.3390/molecules28062695
Martos M, Guapacha AM, Pastor IM. Ionic Organic Solid 1,3-Bis(sulfomethyl)imidazoliumate as an Effective Metal-Free Catalyst for Sustainable Organic Syntheses. Molecules. 2023; 28(6):2695. https://doi.org/10.3390/molecules28062695
Chicago/Turabian StyleMartos, Mario, Angélica M. Guapacha, and Isidro M. Pastor. 2023. "Ionic Organic Solid 1,3-Bis(sulfomethyl)imidazoliumate as an Effective Metal-Free Catalyst for Sustainable Organic Syntheses" Molecules 28, no. 6: 2695. https://doi.org/10.3390/molecules28062695
APA StyleMartos, M., Guapacha, A. M., & Pastor, I. M. (2023). Ionic Organic Solid 1,3-Bis(sulfomethyl)imidazoliumate as an Effective Metal-Free Catalyst for Sustainable Organic Syntheses. Molecules, 28(6), 2695. https://doi.org/10.3390/molecules28062695







