Antiulcer Activity of Anthraquinone–Flavonoid Complex of Rumex tianschanicus Losinsk
Abstract
1. Introduction
2. Results
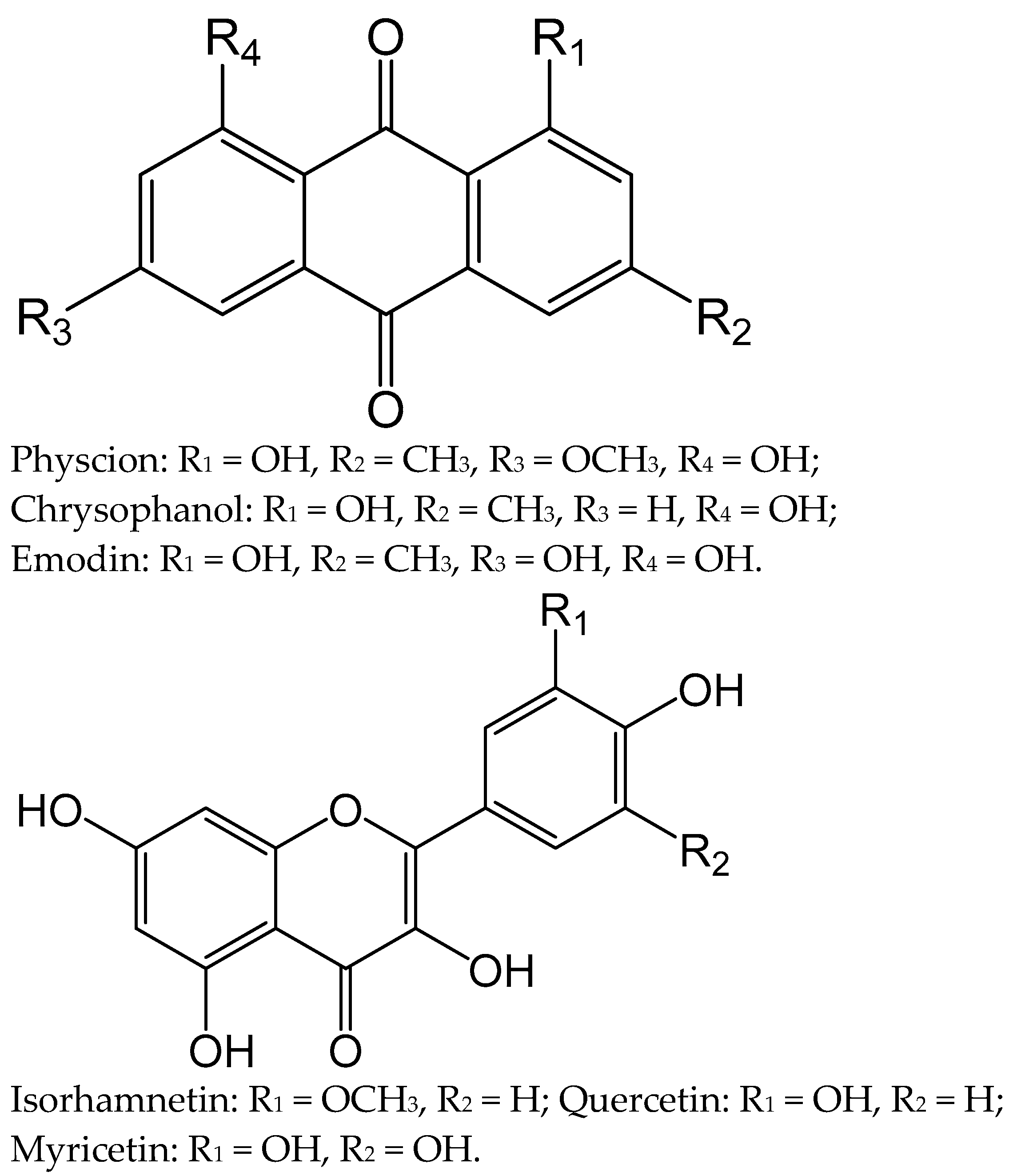
2.1. Identification of Major Components (1–6) of the Polyphenolic Fraction of R. tianschanicus Roots’ Anthraquinone–Flavonoid Complex
2.2. Effect of Anthraquinone–Flavonoid Complex from R. tianschanicus on Body Weight and Organ Mass Ratios in Rats in an Indomethacin Gastric Ulcer Experimental Model
2.3. Effect of Anthraquinone–Flavonoid Complex R. tianschanicus on Models of Indomethacin Damage to the Gastric Mucosa in Rats
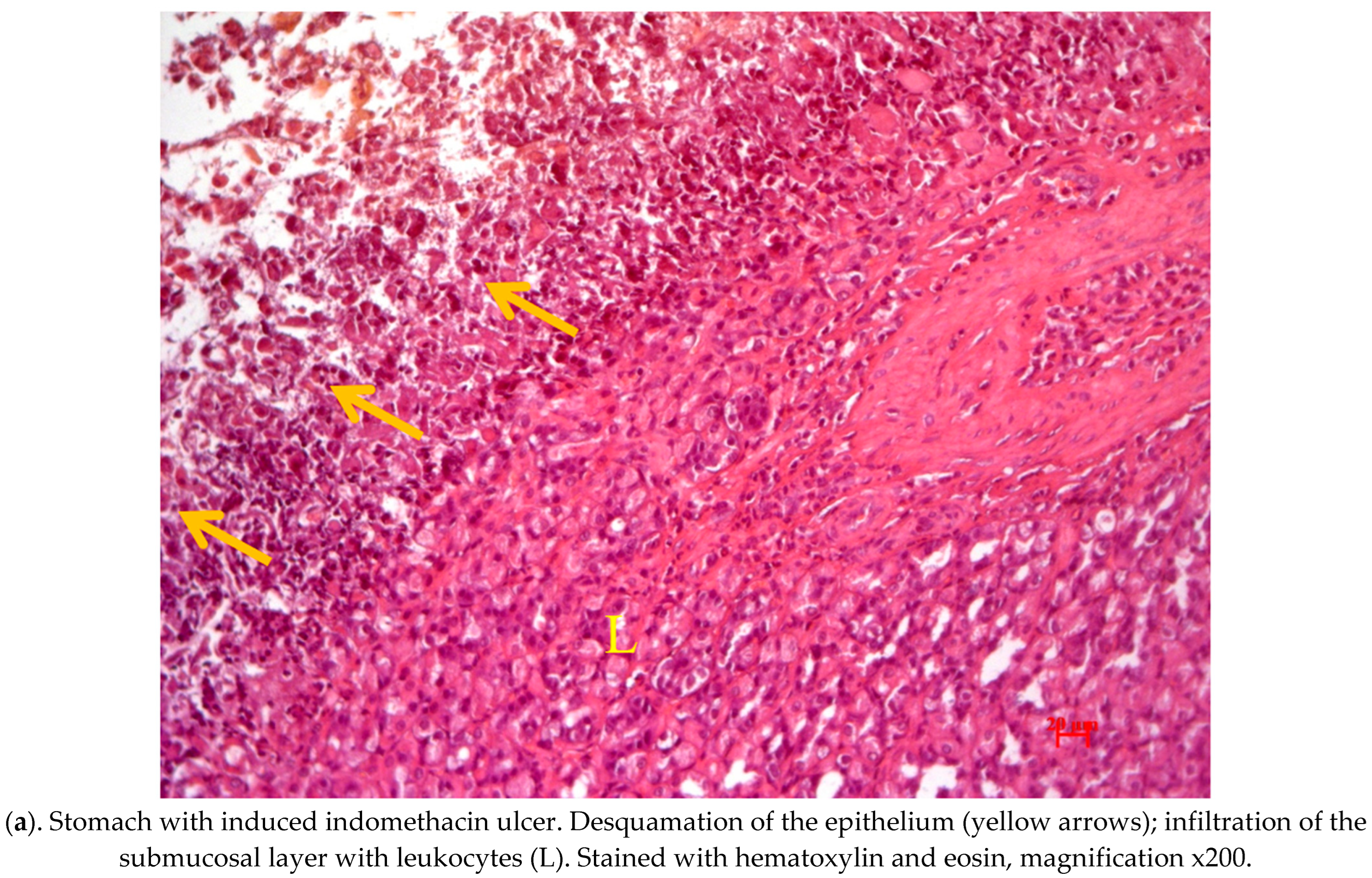
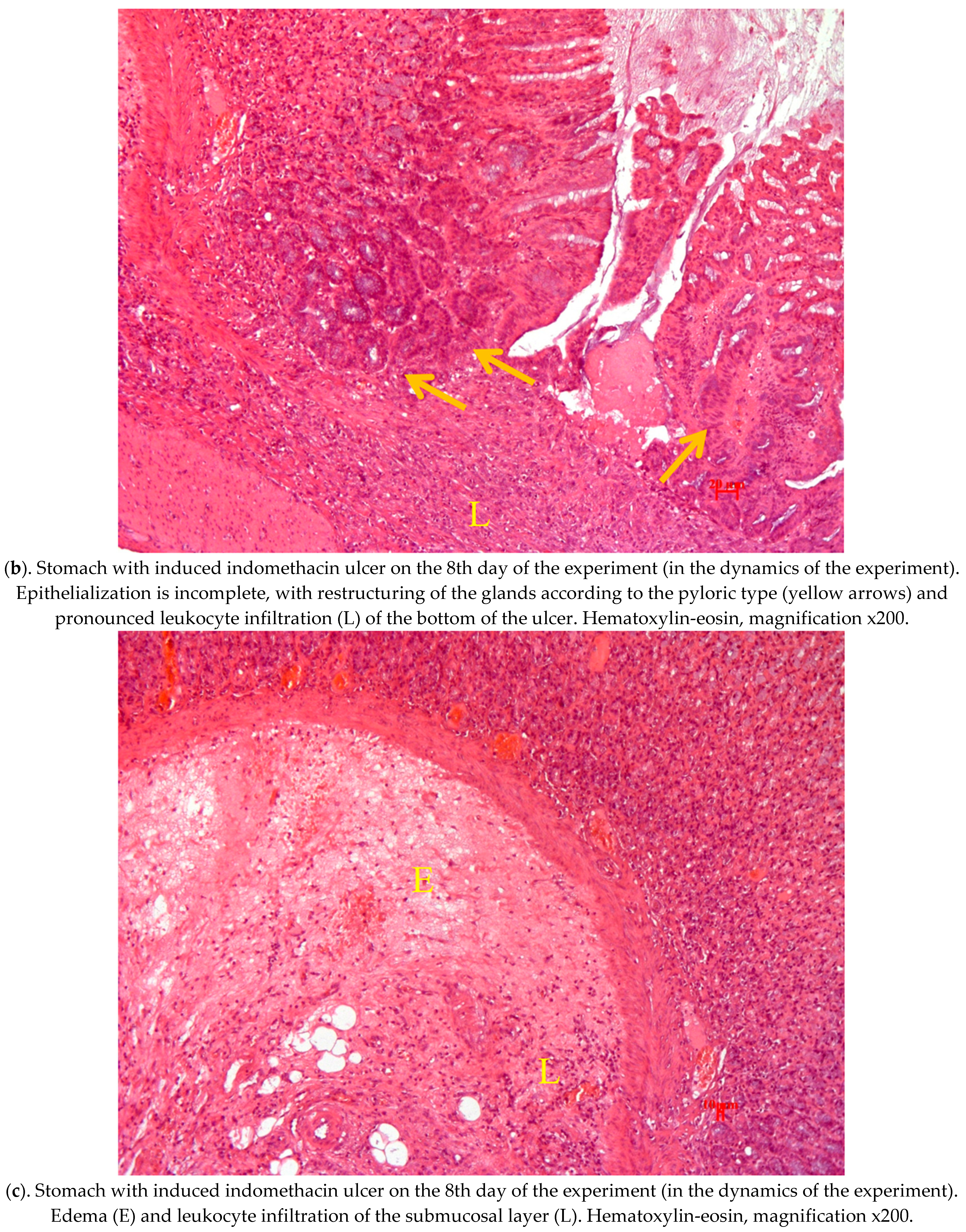
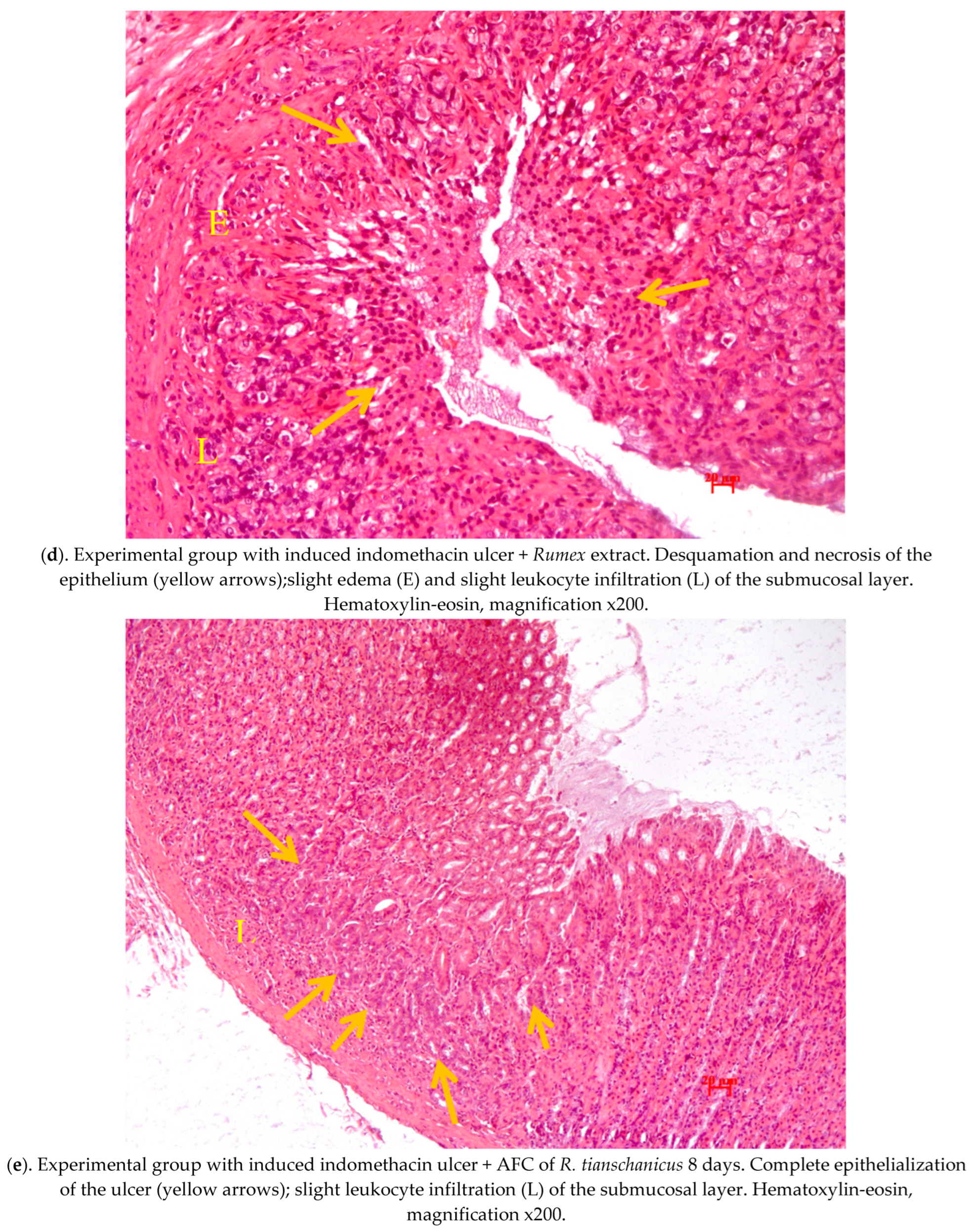
2.4. Pathological Study of Experimental Animals’ Stomachs
3. Discussion
4. Materials and Methods
4.1. Plant Material Collection and Preparation
4.2. General Experimental Procedures
4.3. Extraction and Isolation
4.4. Animals
4.5. Experiment Design
4.6. Histological Examinations
4.7. Statistical Data Processing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hansson, L.E. Risk of stomach cancer in patients with peptic ulcer disease. World J. Surg. 2000, 24, 315–320. [Google Scholar] [CrossRef]
- Lanas, A.; Chan, F.K.L. Peptic ulcer disease. Lancet 2017, 390, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Khedher, A.; Dhibi, S.; Bouzenna, H.; Akermi, S.; El Feki, A.; Teles, P.H.V.; Almeida, R.G.S.; Hfaiedh, N. Atividade antiulcerogênica e antioxidante do extrato etanólico de Plantago ovata em ratos. Braz. J. Biol. 2022, 84, e255120. [Google Scholar] [CrossRef]
- Azmatullah, S.; Khan, A.; Qazi, N.G.; Nadeem, H.; Irshad, N. Pharmacological evaluation of newly synthesized organotin IV complex for antiulcer potential. BMC Pharmacol. Toxicol. 2022, 23, 58. [Google Scholar] [CrossRef] [PubMed]
- Hamauzu, Y.; Irie, M.; Kondo, M.; Fujita, T. Antiulcerative properties of crude poloyphenols and juice of apple, and Chinese quince extracts. Food Chem. 2008, 108, 488–495. [Google Scholar] [CrossRef]
- Ebada, S.S.; Al-Jawabri, N.A.; Youssef, F.S.; Albohy, A.; Aldalaien, S.M.; Disi, A.M.; Proksch, P. In vivo antiulcer activity, phytochemical exploration, and molecular modelling of the polyphenolic-rich fraction of Crepis sancta extract. Inflammopharmacology 2020, 28, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Upadhya, V.; Pai, S.R.; Naik, P.M.; Al-Mssallem, M.Q.; Alessa, F.M. Comparative Quantification of the Phenolic Compounds, Piperine Content, and Total Polyphenols along with the Antioxidant Activities in the Piper trichostachyon and P. nigrum. Molecules 2022, 27, 5965. [Google Scholar] [CrossRef]
- Sitpayeva, G.T.; Kudabayeva, L.M.; Dimeyeva, N.A.; Gemejiyeva, P.G.; Vesselova, V. Crop wild relatives of Kazakhstani Tien Shan: Flora, vegetation, resources. Plant Divers. 2020, 42, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Ageeva, N.T.; Baitenov, M.B.; Goloskokov, V.P.; Korzhilova, V.S.; Pavlov, N.V.; Polikov, P.P. Flora of Kazakhstan; Pavlov, N.V., Ed.; KazSSR Academy of Sciences: Alma-Ata, Kazakhstan, 1960; Volume 3, ISBN 978-5-4458-5985-7. (In Russian) [Google Scholar]
- Li, J.J.; Li, Y.X.; Li, N.; Zhu, H.-T.; Wang, D.; Zhang, Y.-J. The genus Rumex (Polygonaceae): An ethnobotanical, phytochemical and pharmacological review. Nat. Prod. Bioprospect. 2022, 12, 21. [Google Scholar] [CrossRef]
- Berillo, D.; Kozhahmetova, M.; Lebedeva, L. Overview of the Biological Activity of Anthraquinons and Flavanoids of the Plant Rumex Species. Molecules 2022, 27, 1204. [Google Scholar] [CrossRef]
- Vasas, A.; Orban-Gyapai, O.; Hohmann, J. The Genus Rumex: Review of traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2015, 175, 198–228. [Google Scholar] [CrossRef] [PubMed]
- Muzychkina, R.A.; Kurbatova, N.V.; Korulkin, D.Y. Component composition and biological activity of polyphenolic metabolites of Rumex tianschanicus Los. KazNU Bull. 2016, 69, 22–31. (In Russian) [Google Scholar]
- Muzychkina, R.A. Natural Anthraquinones. Biological Properties and Physico-Chemical Characteristics; Phasis: Moscow, Russia, 1998; p. 864. ISBN 5-7036-0041-3. (In Russian) [Google Scholar]
- Litvinenko, Y.A.; Muzychkina, R.A. Phytochemical Investigation of Biologically Active Substances in Certain Kazakhstan Rumex Species. 1. Chem. Nat. Compd. 2003, 39, 446–449. [Google Scholar] [CrossRef]
- Feduraev, P.; Skrypnik, L.; Nebreeva, S.; Dzhobadze, G.; Vatagina, A.; Kalinina, E.; Pungin, A.; Maslennikov, P.; Riabova, A.; Krol, O.; et al. Variability of Phenolic Compound Accumulation and Antioxidant Activity in Wild Plants of Some Rumex Species (Polygonaceae). Antioxidants 2022, 11, 311. [Google Scholar] [CrossRef]
- Harborne, J.B. Phytochemical Methods—A Guide to Modern Techniques of Plant Analysis, 3rd ed.; Chapman and Hall: London, UK, 1998; ISBN 0-412-57260. [Google Scholar]
- Evans, W.C.; Evans, D. Trease and Evans Pharmacognosy, 16th ed.; W.B. Saunders: Philadelphia, PA, USA, 2009; ISBN 9780702029332. [Google Scholar]
- Manojlovic, N.T.; Solujic, S.; Sukdolak, S.; Krstic, L.J. Isolation and antimicrobial activity of anthraquinones from some species of the lichen genus Xanthoria. J. Serb. Chem. Soc. 2000, 65, 555–560. [Google Scholar] [CrossRef]
- Selim, N.M.; Elgazar, A.A.; Abdel-Hamid, N.M.; Abu El-Magd, M.R.; Yasri, A.; El Hefnawy, H.M.; Sobeh, M. Chrysophanol, Physcion, Hesperidin and Curcumin Modulate the Gene Expression of Pro-Inflammatory Mediators Induced by LPS in HepG2: In Silico and Molecular Studies. Antioxidants 2019, 8, 371. [Google Scholar] [CrossRef]
- Sungkeun, C.; Yonghyun, P.; Seuk, C.; Inkyu, K.; Youngwan, S.; Kiwoong, C.; Jongheon, S. Anthraquinones and Sterols from the Korean Marine Echiura Urechis unicintus. J. Korean Chem. Soc. 1998, 42, 64–68. [Google Scholar]
- Danielsen, K.; Aksnes, D.W.; Francis, G.W. NMR study of some anthraquinones from rhubarb. Magn. Reson. Chem. 1992, 30, 359–360. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, Z.; Wu, N.; Xu, W.; Han, L.; Li, N.; Han, Y. Two Novel Naphthalene Glucosides and an Anthraquinone Isolated from Rumex dentatus and Their Antiproliferation Activities in Four Cell Lines. Molecules 2012, 17, 843–850. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Lim, M.-Y.; Lee, H.-S. Emodin isolated from cassia obtusifolia (leguminosae) seed shows larvicidal activity against three mosquito species. J. Agric. Food Chem. 2003, 51, 7629–7631. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, F.; Chen, L.-J.; Xiong, H.-R.; Liu, Y.-Y.; Luo, F.; Hou, W.; Xiao, H.; Yang, Z.-Q. In Vitro and in Vivo Studies of the Inhibitory Effects of Emodin Isolated from Polygonum cuspidatum on Coxsakievirus B4. Molecules 2013, 18, 11842–11858. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Núñez, E.J.; Zamilpa, A.; González-Cortazar, M.; Olmedo-Juárez, A.; Cardoso-Taketa, A.; Sánchez-Mendoza, E.; Tapia-Maruri, D.; Salinas-Sánchez, D.O.; Mendoza-de Gives, P. Isorhamnetin: A Nematocidal Flavonoid from Prosopis laevigata Leaves Against Haemonchus contortus Eggs and Larvae. Biomolecules 2020, 10, 773. [Google Scholar] [CrossRef] [PubMed]
- El-Kader, A.M.A.; El-Readi, M.Z.; Ahmed, A.S.; Nafady, A.M.; Wink, M.; Ibraheim, Z.Z. Polyphenols from aerial parts of Polygonum bellardii and their biological activities. Pharm. Biol. 2013, 51, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Gliszczyńska, A.; Nowaczyk, M. Lipid Formulations and Bioconjugation Strategies for Indomethacin Therapeutic Advances. Molecules 2021, 26, 1576. [Google Scholar] [CrossRef]
- Vysochina, G.I. Phenolic Compounds in the Systematics and Phylogeny of the Buckwheat Family; Nauka: Novosibirsk, Russia, 2004; p. 240. (In Russian) [Google Scholar]
- Kukina, T.P. Study of anthracene derivatives from the roots and rhizomes of some Rumex species. In Proceedings of the Materials II International Scientific Conference, Almaty, Kazakhstan, 10–13 October 2007; p. 221. (In Russian). [Google Scholar]
- Sarı, F.; Koçyiğit, M. Ethnobotanical Usages of the Turkish Rumex Taxa. Turk. J. Biosci. Collect. 2021, 5, 123–140. [Google Scholar] [CrossRef]
- Litvinenko, Y.A.; Muzychkina, R.A. New antioxidant phytopreparation from Rumex thyrsiflorus roots. III. Chem. Nat. Compd. 2008, 44, 239–240. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Sisay, Z.W.; Aragaw, T.J. Evaluation of the Anti-Ulcer Activity of Hydromethanolic Crude Extract and Solvent Fractions of the Root of Rumex nepalensis in Rats. J. Exp. Pharmacol. 2020, 12, 325–337. [Google Scholar] [CrossRef]
- Bae, J.Y.; Lee, Y.S.; Han, S.Y.; Jeong, E.J.; Lee, M.K.; Kong, J.Y.; Lee, D.H.; Cho, K.J.; Lee, H.S.; Ahn, M.J. A Comparison between Water and Ethanol Extracts of Rumex acetosa for Protective Effects on Gastric Ulcers in Mice. Biomol. Ther. 2012, 20, 425–430. [Google Scholar] [CrossRef]
- Amandeep, K.; Sunil, K.; Ramica, S. Assessment of Anti-Ulcer Activity of Rheum emodii Rhizomes Extract. Indo Glob. J. Pharm. Sci. 2012, 2, 333–341. [Google Scholar]
- Prateeksha; Yusuf, M.A.; Singh, B.N.; Sudheer, S.; Kharwar, R.N.; Siddiqui, S.; Abdel-Azeem, A.M.; Fernandes Fraceto, L.; Dashora, K.; Gupta, V.K. Chrysophanol: A Natural Anthraquinone with Multifaceted Biotherapeutic Potential. Biomolecules 2019, 9, 68. [Google Scholar] [CrossRef]
- Diaz-Muñoz, G.; Miranda, I.L.; Sartori, S.K.; Rezende, D.C.; Marisa, A.N. Chapter 11—Anthraquinones: An Overview. Stud. Nat. Prod. Chem. 2018, 58, 313–338. [Google Scholar] [CrossRef]
- Luo, S.; Deng, X.; Liu, Q.; Pan, Z.; Zhao, Z.; Zhou, L.; Luo, X. Emodin ameliorates ulcerative colitis by the flagellin-TLR5 dependent pathway in mice. Int. Immunopharmacol. 2018, 59, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Alexander, V.A.D.; Radhakrishnan, A.; Subramani, P. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef]
- Gudiya, G.; Mohd, A.S.; Mohd Muazzam, K.; Mohd, A.; Rabiya, A.; Md Azizur, R.; Md Afroz, A.; Md Arshad; Mohammad, K. Current Pharmacological Trends on Myricetin. Drug Res. 2020, 70, 448–454. [Google Scholar] [CrossRef]
- Isnain, F.S.; Liao, N.-C.; Tsai, H.-Y.; Hsu, J.-L.; Tsai, P.-J.; Wardani, A.K.; Chen, Y.-K. Protective Effect of Ethanolic Extract of Djulis Hull on Indomethacin-Induced Gastric Injury. Appl. Sci. 2023, 13, 594. [Google Scholar] [CrossRef]
- Abd-Eldayem, A.M.; Alnasser, S.M.; Abd-Elhafeez, H.H.; Soliman, S.A.; Abdel-Emam, R.A. Therapeutic Versus Preventative Use of Ginkgo biloba Extract (EGb 761) against Indomethacin-Induced Gastric Ulcer in Mice. Molecules 2022, 27, 5598. [Google Scholar] [CrossRef]
- Elsanhoty, R.M.; Soliman, M.S.M.; Khidr, Y.A.; Hassan, G.O.O.; Hassan, A.R.A.; Aladhadh, M.; Abdella, A. Pharmacological Activities and Characterization of Phenolic and Flavonoid Compounds in Solenostemma argel Extract. Molecules 2022, 27, 8118. [Google Scholar] [CrossRef]
- Derelanko, M.J.; Long, J.F. Effect of corticosteroids on indomethacin-induced intestinal ulceration in the rat. Dig. Dis. Sci. 1980, 25, 823–829. [Google Scholar] [CrossRef]
- Qazi, N.G.; Khan, A.-U.; Abbasi, S.W.; Shah, F.A.; Rasheed, F.; Ali, F.; Hassan, S.S.U.; Bungau, S. Pharmacological Basis of Rumex hastatus D. Don in Gastrointestinal Diseases with Focusing Effects on H+/K+-ATPase, Calcium Channels Inhibition and PDE Mediated Signaling: Toxicological Evaluation on Vital Organs. Molecules 2022, 27, 5919. [Google Scholar] [CrossRef]
- Ajaib, M.; Ishtiaq, S.; Ishtiaq, M.; Maqbool, M.; Bhatti, K.H.; Khan, A.; Afreen, A.; Hussain, T.; Sardar, T.; Gul, A.; et al. Analysis of antidiabetic, antiulcer and analgesic potential of traditional ethnomedicinal plant Emex spinosa (L.) Campd. from Azad Jammu and Kashmir. PLoS ONE 2022, 17, e0274706. [Google Scholar] [CrossRef] [PubMed]
- Aleid, I.S.; Alfheeaid, H.A.; Aljutaily, T.; Alhomaid, R.M.; Alharbi, H.F.; Althwab, S.A.; Abdel-Rahman, H.A.; AlGeffari, M.A.; Barakat, H. Gastroprotective Effects of Spirulina platensis, Golden Kiwifruit Flesh, and Golden Kiwifruit Peel Extracts Individually or in Combination against Indomethacin-Induced Gastric Ulcer in Rats. Nutrients 2021, 13, 3499. [Google Scholar] [CrossRef] [PubMed]
- Suleyman, H.; Demirezer, L.O.; Kuruuzum-Uz, A. Effects of Rumex patientia root extract on indomethacine and ethanol induced gastric damage in rats. DiePharm 2004, 59, 147–149. [Google Scholar]
- Mashkovskii, M.D.; Babayan, E.A.; Oboimakova, A.N.; Bulayev, V.M.; Severtsev, V.A.; Lyubimov, B.I.; Sokolov, S.D.; Tentsova, A.I. USSR State Pharmacopoeia, 11th ed.; Part 2, General Analytical Methods, Plant Materials; USSR Ministry of Health, Medicine: Moscow, Russia, 1990. (In Russian) [Google Scholar]
- Tulegenova, A.U.; Berdimuratova, G.D.; Dauletbakova, F.D.; Adekenov, S.M.; Arystanova, S.N.; Baimukanov, S.A.; Dernovoi, A.G.; Kuzdenbayeva, R.S.; Lokshin, V.N.; Muminov, T.A. State Pharmacopoeia of the Republic of Kazakhstan, 1st ed.; Zhibek Zholy: Almaty, Kazakhstan, 2008; Volume 1, p. 592. (In Russian) [Google Scholar]
- Tulegenova, A.U.; Berdimuratova, G.D.; Puchkina, L.D.; Arystanova, S.N.; Baimukanov, S.A.; Doskaliyev, Z.A.; Kuzdenbayeva, R.S.; Lokshin, V.N.; Pak, L.Y.-B.; Sabdenalyev, D.M. State Pharmacopoeia of the Republic of Kazakhstan, 1st ed.; Zhibek Zholy: Almaty, Kazakhstan, 2009; Volume 2, p. 802. (In Russian) [Google Scholar]
- Lillie, R.D. Pathohistological Technique and Practical Histochemistry; Mir: Moscow, Russia, 1969; p. 648. (In Russian) [Google Scholar]

| Animal Groups | Total Body Weight, g | Heart, g | Kidneys, g | Liver, g | Stomach, g | Spleen, g |
|---|---|---|---|---|---|---|
| Control | 225.5 ± 7.2 | 1.8 ± 0.4 | 1.7 ± 0.5 | 8.9 ± 0.6 | 9.6 ± 0.6 | 3.6 ± 0.4 |
| Stomach ulcer | 210.2 ± 8.2 * | 1.6 ± 0.3 | 1.8 ± 0.4 | 7.8 ± 0.5 * | 8.3 ± 0.5 * | 3.3 ± 0.4 |
| Prophylactic injection | 238.4 ± 6.7* | 1.9 ± 0.6 | 1.9 ± 0.4 | 9.2 ± 0.6 | 10.2 ± 0.9 * | 3.9 ± 0.6 |
| Single injection | 228.4 ± 5.9 | 2.1 ± 0.6 | 1.8 ± 0.5 | 9.1 ± 0.7 | 9.8 ± 0.8 | 3.7 ± 0.7 |
| Long-lasting effects | 255.5 ± 9.2 * | 2.2 ± 0.2 | 2.1 ± 0.5 | 10.1 ± 0.5 * | 10.7 ± 0.7 * | 4.1 ± 0.6 |
| Animal Groups | Rat Ulcer Prevalence, % | Number of Punctate Ulcers | Number of Large Ulcers | PI | AA |
|---|---|---|---|---|---|
| Control | 100 | 4.8 ± 0.9 | 4.6 ± 0.7 | 12.3 | - |
| Stomach ulcer | 92 | 3.5 ± 0.4 | 4.1 ± 0.6 | 7.4 | 1.5 |
| Prophylactic injection + Stomach ulcer | 60 | 2.1 ± 0.4 * | 1.2 ± 0.5 * | 6.2 | 2.3 |
| Single injection + Stomach ulcer | 35 | 2.7 ± 0.6 * | 2.8 ± 0.8 * | 5.4 | 1.9 |
| Prolonged use + Stomach ulcer | 30 | 1.3 ± 0.5 * | 1.8 ± 0.3 * | 6.1 | 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seitimova, G.A.; Shokan, A.K.; Tolstikova, T.G.; Zhukova, N.A.; Korulkin, D.Y.; Kudrina, N.O.; Litvinenko, Y.A.; Meduntseva, N.D.; Terletskaya, N.V.; Kulmanov, T.E. Antiulcer Activity of Anthraquinone–Flavonoid Complex of Rumex tianschanicus Losinsk. Molecules 2023, 28, 2347. https://doi.org/10.3390/molecules28052347
Seitimova GA, Shokan AK, Tolstikova TG, Zhukova NA, Korulkin DY, Kudrina NO, Litvinenko YA, Meduntseva ND, Terletskaya NV, Kulmanov TE. Antiulcer Activity of Anthraquinone–Flavonoid Complex of Rumex tianschanicus Losinsk. Molecules. 2023; 28(5):2347. https://doi.org/10.3390/molecules28052347
Chicago/Turabian StyleSeitimova, Gulnaz A., Aksholpan K. Shokan, Tatyana G. Tolstikova, Nataliya A. Zhukova, Dmitriy Yu. Korulkin, Nataliya O. Kudrina, Yuliya A. Litvinenko, Nataliya D. Meduntseva, Nina V. Terletskaya, and Timur E. Kulmanov. 2023. "Antiulcer Activity of Anthraquinone–Flavonoid Complex of Rumex tianschanicus Losinsk" Molecules 28, no. 5: 2347. https://doi.org/10.3390/molecules28052347
APA StyleSeitimova, G. A., Shokan, A. K., Tolstikova, T. G., Zhukova, N. A., Korulkin, D. Y., Kudrina, N. O., Litvinenko, Y. A., Meduntseva, N. D., Terletskaya, N. V., & Kulmanov, T. E. (2023). Antiulcer Activity of Anthraquinone–Flavonoid Complex of Rumex tianschanicus Losinsk. Molecules, 28(5), 2347. https://doi.org/10.3390/molecules28052347







