Abstract
Plant virus diseases seriously affect the yield and quality of agricultural products, and their prevention and control are difficult. It is urgent to develop new and efficient antiviral agents. In this work, a series of flavone derivatives containing carboxamide fragments were designed, synthesized, and systematically evaluated for their antiviral activities against tobacco mosaic virus (TMV) on the basis of a structural–diversity–derivation strategy. All the target compounds were characterized by 1H-NMR, 13C-NMR, and HRMS techniques. Most of these derivatives displayed excellent in vivo antiviral activities against TMV, especially 4m (inactivation inhibitory effect, 58%; curative inhibitory effect, 57%; and protection inhibitory effect, 59%), which displayed similar activity to ningnanmycin (inactivation inhibitory effect, 61%; curative inhibitory effect, 57%; and protection inhibitory effect, 58%) at 500 μg mL−1; thus, it emerged as a new lead compound for antiviral research against TMV. Antiviral mechanism research by molecular docking demonstrated that compounds 4m, 5a, and 6b could interact with TMV CP and disturb virus assembly.
1. Introduction
Plant diseases caused by fungal and viral pathogens are extremely destructive to crops, seriously affecting the growth and maturity of crops, causing huge economic losses and triggering a food crisis [1,2,3]. Tobacco mosaic virus (TMV), known as “plant cancer”, is one of the earliest and most extensively researched model viruses [4]. It has a very wide host range and can infect more than 800 kinds of plants in 65 families, including tobacco, pepper, tomato, eggplant, etc. [5]. Once infected, the virus will transfer from the infected cells to the adjacent healthy cells, step-by-step destroying the host’s defense system. At present, it is still difficult to prevent and treat it [6,7,8]. Ribavirin is widely used as a plant virus inhibitor to prevent plant virus diseases, but its inhibitory effect is less than 50% at 500 μg/mL. To date, no effective agrochemicals can absolutely restrain TMV once it has infected plants [9,10]. Therefore, it is of great significance to develop new antiviral agents. Natural products (NPs) have a long history as a source of compounds and inspiration for pharmaceuticals and crop protection compounds [11,12,13,14,15,16]. However, NPs often lack the necessary physicochemical properties, efficacy, and acceptable biological and environmental profiles that constrain their suitability for use in agricultural settings, so only a few NPs can be directly used [17]. Nevertheless, NPs have had their greatest impact as inspiration or models for crop protection products to develop agrochemicals [17]. For example, Dufulin is a biological antiviral agent inspired by an α-aminophosphonic derivative in sheep [18,19].
Flavonoids are plant secondary metabolites with a C6-C3-C6 skeleton, which widely exist in plants and play an important role in plant breeding and metabolism [20]. According to their chemical structures, flavonoids can be divided into chalcones, flavanones, flavanonols, flavones, flavonols, isoflavones, etc. [21]. Flavonoids have many pharmacological effects including antioxidation, anti-inflammatory, anti-carcinogenic, anti-viral, and fungicidal effects [22,23,24,25,26,27], and have always been used as lead compounds in the development of medicines and pesticides. For instance, the flavone skeleton has been embedded in the marketed medicines flavoxate hydrochloride and efloxate (Figure 1). The anti-TMV activities of vitexin (Figure 1), quercetin, fistulaflavonoids, and flavonoid glycosides have been confirmed [28,29,30]. Chen et al. isolated some isoflavones from tobacco roots and stems, and applied them to control plant viruses. They found that some of the isoflavones had better antiviral properties than that of commercial agents [31]. Two new flavones, 8-hydroxy-7-(3-hydroxypropyl)-2′-methoxyflavone and 8-hydroxy-7-(2-hydroxyethyl)-2′-methoxyflavone, were isolated from the whole plants of Cassia pumila and found to have potential anti-TMV activities with inhibition rates of 30.2% and 28.2%, respectively [32,33]. Encouraged by these results, a series of oxazinyl flavonoids were synthesized and evaluated against TMV in our previous work [34]. The carboxamide group plays an important role in commercialized registered pesticides. There is no “absent” amide bond in the chemical structure of commercial succinate dehydrogenase inhibitor (SDHIs) fungicides, so they are also called carboxamide fungicides, such as the marketed fungicides valifenalate, boscalid, and flubeneteram, etc. (Figure 1). Among these compounds, amide bonds act as an important bridge connecting the carboxylic acid parts and the amine moiety. The hydrophobic tail is mostly consisted of aromatic amines of five or six members, while the structural types of polar moiety are relatively diverse, such as pyrazole, pyridine, pyrazine, benzene ring, etc. [35,36] Many methods for obtaining amide functions have been improved or developed, which makes the synthesis of these compounds more convenient and greener [37,38].
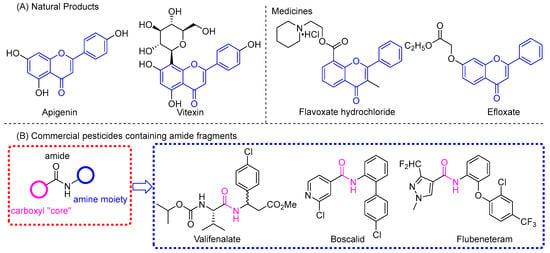
Figure 1.
Chemical structures of flavonoids (A) and carboxamide fragments (B) with biological activities.
In our previous work, we synthesized a series of oxazinyl flavonoids (Figure 2) [34], which displayed higher anti-TMV activities than that of the natural product apigenin (Figure 1). The anti-TMV activity of 7-(benzyloxy)-2-phenyl-4H-chromen-4-one (2c) synthesized in our previous work was significantly higher than that of 7-hydroxy flavone (1) (Table 1). It was found that substituents of hydroxyl oxygen atom had a significant effect on the activity. In this paper, a series of flavone derivatives containing the carboxamide fragments were designed and synthesized based on structural diversity derivation with flavonoid as the mother nucleus structure (Figure 2), and their anti-TMV activities were systematically investigated to find new high-activity compounds for plant protection. The structures of all target compounds were confirmed by 1H NMR, 13C NMR, and HRMS. Using 7-hydroxy flavone as the lead compound, carboxylic acid parts were introduced on the position of 7-OH through a bridge of the amide bond. Further antiviral mechanism research exhibited that flavone derivatives containing an amide fragment could interact with TMV CP (coat protein) and inhibit virus assembly.
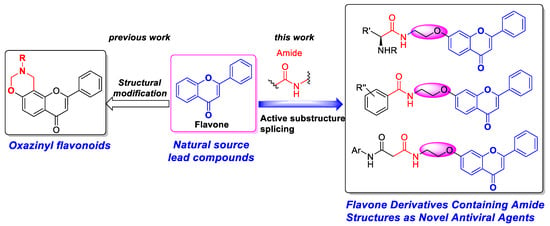
Figure 2.
Design of flavone derivatives containing amide fragments.

Table 1.
In vivo antiviral activity of compounds 2a–2e, 4a–4n, 5a–5e, and 6a–6d against TMV.
2. Results
2.1. Chemistry
7-Hydroxyacetophenone was synthesized according to the method in the literature [34,39]. Compounds 2a–2f were synthesized from 7-hydroxyflavone by reacting with different substituted halogenated hydrocarbons in high yields (Scheme 1).
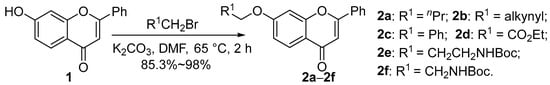
Scheme 1.
Synthesis of 2a–2f.
With compound 2f in hand, deprotection of the tert-butyl carbonyl group of 2f with trifluoroacetic acid resulted in amine 3. Compounds 4–6 were successfully obtained by amidation reaction in the presence of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDCI) and 1-hydroxybenzotriazole (HOBt) (Scheme 2).
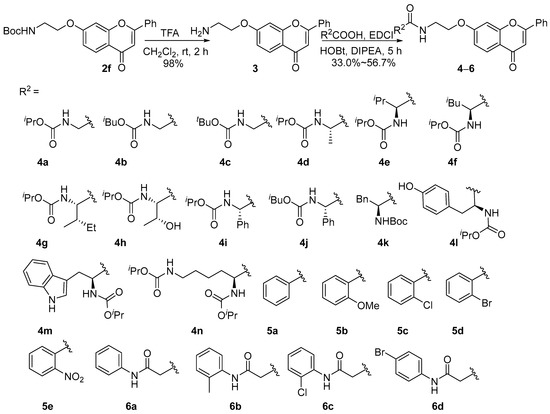
Scheme 2.
Synthesis of 4a–4n, 5a–5e, and 6a–6d.
2.2. Phytotoxic Activity
According to the phytotoxic activity tests, the target compounds were found to be harmless to plants at a concentration of 500 μg/mL. At this concentration, the leaves of tobacco were not found to be rotten or spotted, which could grow healthily and normally. Detailed testing procedures can be seen in our previous report [5,7] and can be found in the Supplementary Materials.
2.3. Antiviral Activity
In Vivo Anti-TMV Activity
The anti-TMV activities of designed compounds are shown in Table 1 with commercial ribavirin and ningnanmycin as the control drugs. According to the results in Table 1, compounds 2a–2f, 4a–4n, 5a–5e, and 6a–6d exhibited moderate to excellent anti-TMV activities, except for 4e and 4f. Almost all the target compounds showed higher anti-TMV activities than ribavirin. Compound 4m exhibited the highest anti-TMV activity at 500 μg/mL (inactivation activity, 58%; curative activity, 57%; protection activity, 59%), which was significantly higher than that of ribavirin (inactivation activity, 39%; curative activity, 40%; protection activity, 39%) and similar to that of ningnamycin (inactivation activity, 61%; curative activity, 57%; protection activity, 58%).
2.4. Mode of Action Studies
Docking Studies
To further study the mechanism of the interaction between flavone derivatives and TMV CP, we use AutoDock Vina 1.1.2 for molecular docking [40]. The docking poses are ranked according to their docking sites, and the lowest binding energy of the macromolecule–ligand complex is considered the best. It can be proven that there are some H-bond interactions and strong binding affinity between flavone derivatives containing amide fragments and TMV CP.
3. Discussion
3.1. Synthesis
According to the reported method [39,41], the 7-position hydroxyl group of 7-hydroxyacetophenone was smoothly reacted with bromo compounds in basic K2CO3 to gain the compounds 2a–2f (Scheme 1). Starting from intermediate 2f, compound 3 can be successfully obtained by treating it with trifluoroacetic acid. The addition of isopropyl chloroformate, isobutyl chloroformate, or di-tert-butyl dicarbonate to a solution of amino acids in aqueous sodium hydroxide produced amino acid derivatives in good yield [42]. Compounds 4–6 can be successfully gained by the amidation reaction of compound 3 with amino acid derivatives [43], benzoic acid and its derivatives, and malonic acid derivatives [44] in the presence of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDCI) and 1-hydroxybenzotriazole (HOBt) (Scheme 2). We mainly introduced structural diversity functional groups into C-7 of 7-hydroxyflavone by the active structures splicing strategy. Amino acids, aromatic carboxylic acids, and malonic acid derivatives fragments were introduced into the flavonoid skeleton to achieve structural diversity derivation of flavonoid compounds.
The structures of obtained compounds were confirmed by 1H, 13C NMR spectroscopy, and HRMS analysis. 7-Hydroxyflavone (1) was obtained by our previously reported method [34], which is consistent with the literature report [41]. Compound 4a was used as an example for chemical structure characterization analysis. The spectrum of 1H NMR (Figure S15) exhibited one methyl signal at δH 1.16, three signals of methylene at δH 3.51, δH 3.60, and δH 4.17, one signal of oxygenated methine at δH 4.63–4.83. Moreover, as described in Figure S16, the data of 13C NMR for 4a indicated 19 signals of carbon, including five saturated carbons (δC 67.7, 67.5, 43.9, 38.5, and 22.5). These spectroscopic features confirmed that the amino acid fragment was successfully introduced into 7-hydroxyflavone.
3.2. Structure–Activity Relationship of the Antiviral Activity
The antiviral activities of compounds 2a–2f, 4a–4n, 5a–5e, and 6a–6d were listed in Table 1, with commercial plant virucides ningnanmycin and ribavirin as controls. Most of these compounds showed higher antiviral activities than ribavirin and were obviously superior to compound 1. As shown in Table 1, compounds 2a–2f with alkyl substitutions on the 7-position of the O atom were higher than that of ribavirin. In contrast, the anti-TMV activity of the substituent containing the ester group (2d), the Boc (t-butyloxycarbonyl) protected amino group (2e and 2f), and the amino group (3) were obviously better than that of compounds containing the simple alkyl substituent (2a, 2b, and 2c).
Amino acids play important roles both as building blocks of peptides and proteins and as important constituents of biologically active compounds, such as benthiavalicarb, iprovalicarb, and valiphenal [45]. As early as 1951, Li et al. reported that some amino acids had certain effects on the reproduction of TMV [46]. Amino acid gossypol Schiff bases were evaluated for their anti-TMV activities by Zhang et al. [47]. To systematically investigate the effect of amino acid fragments with different structures on the anti-TMV activity, 10 kinds of cheap and commercial amino acids, including simple glycine and L-tryptophan containing indole, were used as starting materials to react with different chloroformates to obtain corresponding carboxylic acids and the target compounds 4a–4n. In general, compounds 4a–4n containing amino acid fragments at the 7-position of the O atom displayed higher levels of anti-TMV activity than compounds with smaller groups at the same position. Among compounds 4a, 4d–4e, 4f–4g, 4i, 4l, and 4n, protected by the isopropoxycarbonyl group, glycine derivative 4a had the best antiviral activity. L-valine derivatives (4e), L-leucine derivatives (4f), L-isoleucine derivatives (4g), L-2-phenylglycine derivatives (4i), and L-2-(4-OH-benzyl)glycine derivatives (4l) were not conducive to anti-TMV activity (4a > 4n > 4d > 4i > 4e > 4g ≈ 4l > 4e ≈ 4f). In particular, the inhibition rate of compounds 4e and 4f are lower than 40% (4e: inactivation inhibitory effect, 39%; curative inhibitory effect, 37%; and protection inhibitory effect, 39%; 4f: inactivation inhibitory effect, 38%; curative inhibitory effect, 36%; and protection inhibitory effect, 36%) at 500 μg mL−1. However, compound 4m, starting from (isopropoxycarbonyl)-L-tryptophan, showed excellent anti-TMV activity (inactivation inhibitory effect, 58%; curative inhibitory effect, 57%; and protection inhibitory effect, 59%), which is similar to that of ningnanmycin (inactivation inhibitory effect, 61%; curative inhibitory effect, 57%; and protection inhibitory effect, 58%) at 500 μg mL−1. The different protective groups of amino acids also had certain effects on antiviral activity. Compared with compounds 4a, 4b, and 4c, when the amino protecting group was isopropoxycarbonyl, it was superior to tert-butyloxycarbonyl and iso-butyloxycarbonyl. This rule was consistent with the anti-TMV activity of compounds 4i and 4j.
Compounds 5a–5e, prepared from benzoic acid and its derivatives with compound 3, have higher anti-TMV activity than ribavirin, but the antiviral activity was greatly reduced when the ortho position of the benzene ring of carboxyamide group contained methoxy (5b: inactivation inhibitory effect, 45%; curative inhibitory effect, 44%; and protection inhibitory effect, 46%) at 500 μg mL−1. Compounds 6a–6e, synthesized from 3-oxo-3-(phenylamino)propanoic acid, exhibited higher anti-TMV activity than that of ribavirin. Different substituents on the benzene ring of the amino group were beneficial to the improvement of activity and could increase the anti-TMV activity. In particular, compound 6b (inactivation activity, 54%; curative activity, 57%; protection activity, 54%) exhibited significantly higher activity than ribavirin (inactivation activity, 39%; curative activity, 40%; protection activity, 39%) at 500 μg/mL.
Summarizing the structure–activity relationship, it was found that carboxylic acid parts introduced on the position of 7-OH through a bridge of the amide bond could improve the anti-TMV activity.
3.3. Study on the Mechanism of Anti-TMV Activity
Molecular Docking Study
To further investigate and evaluate the detailed intermolecular interactions between flavone derivatives and potential targets, molecular docking was performed. The detailed calculation procedures for molecular docking research were carried out according to the literature and are described in the Supplementary Materials [48]. Compounds 4m, 5a, and 6b were selected for molecular docking with TMV CP (PDB code 1EI7) [49]. For comparison, compounds 1 and 2a were also chosen for molecular docking with TMV CP, and the results were in the Supplementary Materials (Figure S61). As depicted in Figure 3, compounds 4m, 5a, and 6b folded similar to a hairpin and entered into the hole-shaped active binding pocket created by TMV CP (Figure 3). The results showed that compound 4m was laid into the TMV CP active pocket and formed seven conventional hydrogen bonds with the active sites of ARG 134 (2.8 Å and 2.2 Å), ASP 264 (2.3 Å), SER 255 (2.4 Å), LYS 268 (2.6 Å and 2.3 Å), GLU 131 (2.3 Å) (Figure 3A). Due to the folding of compound 4m, the carbonyl groups and amino groups were fully exposed, which made it easier to interact with amino acid residues. As shown in Figure 3A, not only the flavonoid structure and carboxamide group can interact with TMV CP, but also the indole has a hydrogen bond with TMV CP. The active O atom at position 7 of compounds 5a and 6b can interact with free amine N-H of the adjacent two amino acids ASN 73 and GLY137 to form hydrogen bonds (Figure 3B,C). Compound 5a can also provide an O atom of the carboxyl group to form a hydrogen bond with SER 255, while compound 6b bonds with SER 138 as seen in Figure 3B,C. In particular, the two aromatic rings of compounds 5a and 6b were almost parallel, forming the additional pi–pi interactions effect. Compared with Figure S61A, although compound 1 will also be embedded in the same cavity, its interaction mode is obviously different, and the benzene ring of the flavonoid structure was at the innermost end. Compound 1 provided an O atom to form two hydrogen bonds with LYS 268 and ARG 134, and supplied -OH to form a hydrogen bond with ARG 134. As shown in Figure S61B, after the alkylation of the hydroxyl group, the method of flavonoid embedding into the TMV CP cavity had changed significantly. Compound 2a was laid into the TMV CP active pocket and formed two conventional hydrogen bonds with the active sites of GLN 257 (2.7 Å) and ASN 73 (2.4 Å).
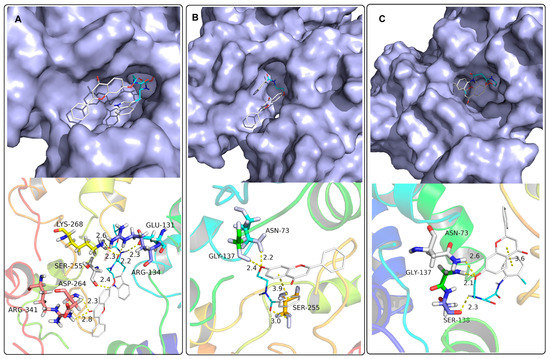
Figure 3.
Molecule docking results of 4m (A), 5a (B), and 6b (C) with TMV CP.
The binding free energies of compounds 4m, 5a, and 6b to TMV CP were −8.6 kcal/mol, −8.1 kcal/mol, and −8.4 kcal/mol, respectively. In contrast, the binding free energies of compounds 1 and 2a to TMV CP were only −7.3 kcal/mol and −7.5 kcal/mol, respectively. The lower the binding free energy, the higher the affinity between the receptor and the ligand [50]. The results of molecular docking showed that the binding energy of amino acid, aromatic formic acid, and malonic acid derivatives introduced at the oxygen atom through the amide bond were lower than that of oxazinyl flavonoids [34], which were more conducive to anti-TMV activity.
The results of molecular docking showed that the introduction of ethyl made the molecular structure more flexible, and it could be embedded into the cavity of TMV CP as stably as a hairpin. Through the bridging of the amide bond, it can not only provide the interaction sites with TMV CP, but also facilitate the introduction of other active groups. Compounds 4–6 bearing different groups could interact with TMV CP to disturb the assembly of TMV virus particles, thus showing good anti-TMV activity. They were also consistent with the activity test.
4. Materials and Methods
4.1. Synthetic Procedures
4.1.1. Reagents and Instruments
All reagents used were analytical reagent (AR) grade or chemically pure (CR), which were purchased from commercial sources (Tianjin Guangda Chemical Reagents Ltd., Tianjin, China). The melting point of the target compounds were measured on an X-4 binocular microscope (Beijing Zhongke Instrument Co., Ltd., Beijing, China). NMR spectra were acquired with a 400 MHz (100 MHz for 13C) instrument (Bruker, Billerica, MA, USA) at room temperature. Chemical shifts were measured relative to residual solvent peaks of CDCl3 (1H: δ = 7.26 ppm; 13C: δ = 77.0 ppm) and DMSO-d6 (1H: δ = 2.5 and 3.3 ppm; 13C: δ = 39.9 ppm) as internal standards. The following abbreviations are used to designate chemical shift multiplicities: s = singlet, d = doublet, dd = doublet of doublets, t = triplet, m = multiplet, and brs = broad singlet. HRMS data were recorded with a QFT-ESI instrument (Varian, Palo Alto, CA, USA).
4.1.2. Synthesis of Compounds 2a–2f
Synthesis of compounds 2a–2e. 7-Hydroxyflavone (0.119 g, 0.5 mmol) and anhydrous K2CO3 (0.414 g, 3 mmol) were added into N,N-dimethylformamide (DMF) (3 mL), then the mixture was stirred at room temperature for 30 min. The mixture continued to stir and control the temperature at 70 °C for 2 h after adding halohydrocarbon compounds (1.5 mmol). After 7-hydroxyflavone was completely consumed (monitored by Thin Layer chromatography, TLC), the mixture was diluted with water (10 mL) and washed with ethyl acetate (3 × 15 mL). The combined organic phase was dried with anhydrous sodium sulfate. The crude product was obtained after removing the solvent in vacuo and purified by column chromatography.
- 7-Butoxy-2-phenyl-4H-chromen-4-one (2a). Light yellow solid, 92.5% yield, m.p. 82–85 °C; 1H NMR (400 MHz, CDCl3) δ 8.13 (d, J = 8.7 Hz, 1H), 8.01–7.83 (m, 2H), 7.59–7.45 (m, 3H), 6.98 (d, J = 11.1 Hz, 2H), 6.77 (s, 1H), 4.10 (s, 2H), 1.84 (d, J = 6.5 Hz, 2H), 1.53 (h, J = 7.3 Hz, 2H), 1.01 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 177.9, 163.8, 163.0, 158.0, 131.9, 131.4, 129.0, 127.0, 126.2, 117.7, 114.8, 107.5, 100.9, 68.5, 31.0, 19.2, 13.8; HR-MS (ESI): calcd for C19H18O3 [M + H]+ 295.1329, found (ESI+) 295.1336.
- 2-Phenyl-7-(prop-2-yn-1-yloxy)-4H-chromen-4-one (2b). Light brown solid, 95.0% yield, m.p. 164–169 °C; 1H NMR (400 MHz, CDCl3) δ 8.20 (d, J = 8.8 Hz, 1H), 7.97–7.91 (m, 2H), 7.59–7.52 (m, 3H), 7.14–7.05 (m, 2H), 6.81 (s, 1H), 4.86 (s, 2H), 2.65 (s, 1H); 13C NMR (100 MHz, CDCl3) δ 177.8, 163.2, 161.9, 157.7, 131.8, 131.5, 129.0, 127.2, 126.2, 118.5, 114.7, 107.6, 101.8, 77.4, 76.6, 56.2; HR-MS (ESI): calcd for C18H12O3 [M + H]+ 277.0859, found (ESI+) 277.0862.
- 7-(Benzyloxy)-2-phenyl-4H-chromen-4-one (2c). White solid, 95% yield, m.p. 173–180 °C; 1H NMR (400 MHz, CDCl3) δ 8.16 (d, J = 8.6 Hz, 1H), 7.96–7.88 (m, 2H), 7.52 (d, J = 5.7 Hz, 3H), 7.43–7.48 (m, 5H), 7.07 (d, J = 9.7 Hz, 2H), 6.78 (s, 1H), 5.20 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 177.9, 163.4, 163.2, 158.0, 135.8, 131.9, 131.5, 129.1, 128.8, 128.5, 127.6, 127.2, 126.3, 118.0, 115.0, 107.6, 101.6, 70.6; HR-MS (ESI): calcd for C22H17O3 [M + H]+ 329.1172, found (ESI+) 329.1176.
- Ethyl 2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)acetate (2d). Light brown solid, 85.3% yield, m.p. 89–93 °C; 1H NMR (400 MHz, CDCl3) δ 8.16 (d, J = 8.8 Hz, 1H), 7.97–7.71 (m, 2H), 7.52 (d, J = 5.5 Hz, 3H), 7.08–6.98 (m, 1H), 6.99–6.89 (m, 1H), 6.77 (s, 1H), 4.75 (s, 2H), 4.31 (q, J = 7.1 Hz, 2H), 1.32 (t, J = 7.1 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 177.8, 168.0, 163.3, 162.2, 157.8, 131.8, 131.6, 129.1, 127.5, 126.2, 118.7, 114.3, 107.7, 101.7, 65.1, 61.8, 14.2; HR-MS (ESI): calcd for C19H16O5 [M + H]+ 325.1071, found (ESI+) 325.1069.
- Tert-butyl(3-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)propyl)carbamate (2e). White solid, 90.2% yield, m.p. 144–150 °C; 1H NMR (400 MHz, CDCl3) δ 8.13 (d, J = 9.0 Hz, 1H), 7.92 (d, J = 6.4 Hz, 2H), 7.53 (s, 3H), 6.99 (d, J = 7.7 Hz, 2H), 6.86 (s, 1H), 4.15 (t, J = 5.6 Hz, 2H), 3.36 (s, 2H), 2.11–2.01 (m, 2H), 1.45 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 177.9, 163.4, 163.0, 157.9, 156.1, 131.8, 131.4, 129.0, 127.0, 126.2, 117.8, 114.7, 107.5, 100.9, 79.3, 66.4, 37.7, 29.5, 28.4; HR-MS (ESI): calcd for C23H25NO5 [M + H]+ 396.1806, found (ESI+) 396.1802.
- Tert-butyl (2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)carbamate (2f). White solid, 98.0% yield, m.p. 129–132 °C; 1H NMR (400 MHz, CDCl3) δ 8.13 (d, J = 8.4 Hz, 1H), 7.93–7.87 (m, 2H), 7.52 (d, J = 4.9 Hz, 3H), 6.98 (d, J = 9.0 Hz, 2H), 6.76 (s, 1H), 5.02 (s, 1H), 4.16 (s, 2H), 3.60 (s, 2H), 1.46 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 177.8, 163.1, 163.1, 157.9, 155.9, 131.7, 131.5, 129.0, 127.1, 126.2, 118.0, 114.7, 107.5, 101.0, 79.8, 67.8, 39.9, 28.4; HR-MS (ESI): calcd for C19H16O5 [M + H]+ 382.1649, found (ESI+) 382.1648.
4.1.3. Synthesis of Compounds 4a–4n, 5a–5e, and 6a–6d
- 7-(2-Aminoethoxy)-2-phenyl-4H-chromen-4-one (3). A solution of compound 2f (0.381 g, 1.0 mmol, 1.0 equiv.) in CH2Cl2 (3 mL) was added to CF3COOH (TFA, 3 mL) and stirred at room temperature for 2 h. After the completion of the reaction, the solvent was removed in vacuum, and the crude product was purified by flash chromatography to obtain crude product 3·CF3COOH. The crude product was washed by saturated NaHCO3 solution to obtain compound 3. White solid, 98% yield; 1H NMR (400 MHz, DMSO-d6) δ 8.10 (d, J = 6.7 Hz, 2H), 7.94 (d, J = 8.8 Hz, 1H), 7.59 (d, J = 6.4 Hz, 3H), 7.32 (s, 1H), 7.07 (d, J = 8.4 Hz, 1H), 6.96 (s, 1H), 4.09 (t, J = 5.4 Hz, 2H), 3.17 (s, 2H), 2.94 (s, 2H); 13C NMR (100 MHz, DMSO-d6) δ 176.9, 163.2, 162.7, 157.9, 132.2, 129.6, 126.8, 126.7, 117.9, 115.6, 107.3, 102.1, 68.0.
Compounds 4a–4n: To a stirred solution of 3·CF3COOH (0.395 g, 1 mmol) in CH2Cl2 (3 mL), diisopropylethylamine (0.387 g, 3 mmol), 1-hydroxybenzotriazole (HOBt) (0.203 g, 1.5 mmol), and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI) (0.288 g, 1.5 mmol) were added, and the reaction mixture was stirred for 30 min at 0 °C. Subsequently, carboxylic acid (1.2 mmol) was added, and the resulting solution was stirred at room temperature for 5 h. After the completion of the reaction, the organic phase was washed with water (10 mL) and dried with MgSO4. The crude product was purified by recrystallization to obtain compounds 4a–4n.
- Isopropyl(2-oxo-2-((2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)amino)ethyl)carbamate (4a). White solid, 56.7% yield, m.p. 143–146 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.09 (d, J = 7.8 Hz, 3H), 7.94 (d, J = 8.8 Hz, 1H), 7.58 (d, J = 6.7 Hz, 3H), 7.34 (s, 1H), 7.17 (t, J = 5.4 Hz, 1H), 7.06 (d, J = 8.8 Hz, 1H), 6.97 (s, 1H), 4.83–4.63 (m, 1H), 4.17 (t, J = 4.9 Hz, 2H), 3.60 (d, J = 5.8 Hz, 2H), 3.51 (d, J = 5.3 Hz, 2H), 1.16 (d, J = 6.1 Hz, 6H); 13C NMR (100 MHz, DMSO-d6) δ 176.9, 170.2, 163.5, 162.7, 157.9, 156.7, 132.2, 131.7, 129.6, 126.7, 117.7, 115.5, 107.3, 102.1, 67.7, 67.5, 43.9, 38.5, 22.5; HR-MS (ESI): calcd for C23H24N2O6 [M + H]+ 425.1707, found (ESI+) 425.1704.
- Isobutyl(2-oxo-2-((2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)amino)ethyl)carbamate (4b). White solid, 53.5% yield, m.p. 141–143 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.10 (d, J = 7.3 Hz, 3H), 7.95 (d, J = 8.8 Hz, 1H), 7.59 (d, J = 6.6 Hz, 3H), 7.35 (s, 1H), 7.28 (t, J = 5.8 Hz, 1H), 7.07 (d, J = 8.7 Hz, 1H), 6.98 (s, 1H), 4.17 (t, J = 4.7 Hz, 2H), 3.72 (d, J = 6.5 Hz, 2H), 3.60 (d, J = 5.7 Hz, 2H), 3.51 (d, J = 5.1 Hz, 2H), 1.82 (dt, J = 13.6, 6.7 Hz, 1H), 0.87 (d, J = 6.5 Hz, 6H); 13C NMR (100 MHz, DMSO-d6) δ 177.0, 170.2, 163.5, 162.8, 157.9, 157.3, 129.6, 126.8, 117.7, 115.6, 107.3, 102.1, 70.5, 67.7, 43.9, 38.5, 28.1, 19.4; HR-MS (ESI): calcd for C24H26N2O6 [M + H]+ 439.1864, found (ESI+) 439.1898.
- Tert-butyl(2-oxo-2-((2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)amino)ethyl)carbamate (4c). White solid, 43.5% yield, m.p. 151–153 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.09 (t, J = 8.0 Hz, 3H), 7.95 (d, J = 8.8 Hz, 1H), 7.59 (d, J = 6.5 Hz, 3H), 7.35 (s, 1H), 7.07 (d, J = 8.6 Hz, 1H), 6.97 (d, J = 5.4 Hz, 2H), 4.17 (s, 2H), 3.53 (dd, J = 15.0, 5.5 Hz, 4H), 1.37 (s, 9H); 13C NMR (100 MHz, DMSO-d6) δ 177.0, 170.3, 163.5, 162.7, 158.0, 156.3, 132.2, 131.6, 129.6, 126.7, 117.7, 115.6, 107.2, 102.0, 78.6, 67.7, 43.7, 38.5, 28.7; HR-MS (ESI): calcd for C24H26N2O6 [M + H]+ 439.1864, found (ESI+) 439.1893.
- (S)-Isopropyl(1-oxo-1-((2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)amino)propan-2-yl)carbamate (4d). White solid, 37.5% yield, m.p. 134–136 °C; = +0.6° (c 1.0, CH3OH); 1H NMR (400 MHz, DMSO-d6) δ 8.08 (d, J = 6.8 Hz, 3H), 7.94 (d, J = 8.7 Hz, 1H), 7.58 (d, J = 6.4 Hz, 3H), 7.32 (s, 1H), 7.13 (d, J = 6.9 Hz, 1H), 7.05 (d, J = 8.8 Hz, 1H), 6.96 (s, 1H), 4.87 –4.54 (m, 1H), 4.16 (s, 2H), 4.10–3.94 (m, 1H), 3.61–3.43 (m, 2H), 1.18 (d, J = 7.0 Hz, 3H), 1.14 (d, J = 5.7 Hz, 6H); 13C NMR (100 MHz, DMSO-d6) δ 176.9, 173.6, 163.5, 162.7, 157.9, 155.9, 132.1, 131.6, 129.5, 126.7, 126.6, 117.7, 115.5, 107.2, 102.0, 67.6, 67.4, 50.4, 38.5, 22.5, 18.7; HR-MS (ESI): calcd for C24H26N2O6 [M + H]+ 439.1864, found (ESI+) 439.1891.
- (S)-Isopropyl(3-methyl-1-oxo-1-((2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)amino) butan-2-yl) carbamate (4e). White solid, 41.3% yield, m.p. 164–168 °C; = +3.2° (c 1.0, CH3OH); 1H NMR (400 MHz, DMSO-d6) δ 8.17 (s, 1H), 8.10 (d, J = 6.9 Hz, 2H), 7.95 (d, J = 8.8 Hz, 1H), 7.59 (d, J = 6.4 Hz, 3H), 7.34 (s, 1H), 7.04 (d, J = 8.8 Hz, 1H), 6.97 (s, 1H), 6.92 (d, J = 8.4 Hz, 1H), 4.71 (p, J = 5.9 Hz, 1H), 4.17 (s, 2H), 3.81 (t, J = 7.6 Hz, 1H), 3.52 (ddd, J = 45.7, 14.0, 5.8 Hz, 2H), 2.01–1.81 (m, 1H), 1.14 (d, J = 5.1 Hz, 6H), 0.81 (d, 6H); 13C NMR (100 MHz, DMSO-d6) δ 176.4, 171.7, 163.0, 162.2, 157.4, 155.8, 131.6, 131.1, 129.1, 126.2, 117.2, 115.0, 106.7, 101.5, 66.9, 60.1, 37.9, 30.2, 22.0, 19.1, 18.2; HR-MS (ESI): calcd for C26H30N2O6 [M + H]+ 467.2177, found (ESI+) 467.2179.
- (S)-Isopropyl(4-methyl-1-oxo-1-((2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)amino) pentan-2-yl) carbamate (4f). White solid, 40.5% yield, m.p. 132–136 °C; = −7.6° (c 1.0, CH3OH); 1H NMR (400 MHz, DMSO-d6) δ 8.19–8.04 (m, 3H), 7.95 (d, J = 8.8 Hz, 1H), 7.59 (d, J = 6.3 Hz, 3H), 7.33 (s, 1H), 7.06 (t, J = 9.9 Hz, 2H), 6.97 (s, 1H), 4.70 (dt, J = 11.9, 5.9 Hz, 1H), 4.17 (s, 2H), 3.99 (d, J = 6.0 Hz, 1H), 3.49 (dd, J = 20.4, 3.9 Hz, 2H), 1.61–1.51 (m, 1H), 1.38 (dt, J = 14.0, 7.8 Hz, 2H), 1.13 (d, J = 5.7 Hz, 6H), 0.81 (t, J = 6.5 Hz, 6H); 13C NMR (100 MHz, DMSO-d6) δ 176.4, 172.9, 163.0, 162.2, 157.4, 155.6, 131.6, 131.1, 129.1, 126.2, 117.2, 115.0, 106.7, 101.5, 67.1, 66.9, 53.0, 40.8, 38.0, 24.2, 22.8, 21.5; HR-MS (ESI): calcd for C27H32N2O6 [M + H]+ 481.2333, found (ESI+) 481.2343.
- Isopropyl((2S,3S)-3-methyl-1-oxo-1-((2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl) amino)pentan-2-yl)carbamate (4g). White solid, 37.5% yield, m.p. 180–187 °C; = +1.6° (c 1.0, CH3OH); 1H NMR (400 MHz, DMSO-d6) δ 8.18 (s, 1H), 8.10 (d, J = 6.8 Hz, 2H), 7.95 (d, J = 8.9 Hz, 1H), 7.59 (d, J = 6.4 Hz, 3H), 7.34 (s, 1H), 7.04 (d, J = 8.7 Hz, 1H), 7.00–6.89 (m, 2H), 4.71 (dt, J = 11.7, 5.8 Hz, 1H), 4.17 (s, 2H), 3.84 (t, J = 7.8 Hz, 1H), 3.51 (dd, J = 35.8, 4.9 Hz, 2H), 1.66 (s, 1H), 1.39 (s, 2H), 1.13 (d, J = 5.1 Hz, 6H), 0.82–0.74 (m, 6H); 13C NMR (100 MHz, DMSO-d6) δ 176.9, 172.2, 163.5, 162.7, 157.9, 156.2, 132.2, 131.6, 129.6, 126.7, 117.7, 115.5, 107.2, 102.0, 67.6, 67.4, 59.5, 38.4, 36.8, 24.9, 22.5, 15.7, 11.4; HR-MS (ESI): calcd for C27H32N2O6 [M + H]+ 481.2333, found (ESI+) 481.2338.
- (Isopropyl((2S,3R)-3-hydroxy-1-oxo-1-((2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl) amino)butan-2-yl)carbamate (4h). White solid, 33.0% yield, m.p. 134–137 °C; = +4.5° (c 1.0, CH3OH); 1H NMR (400 MHz, DMSO-d6) δ 8.12 (d, J = 5.8 Hz, 3H), 7.97 (d, J = 8.8 Hz, 1H), 7.62 (s, 3H), 7.36 (s, 1H), 7.08 (d, J = 8.5 Hz, 1H), 6.99 (s, 1H), 6.58 (d, J = 7.4 Hz, 1H), 4.92–4.65 (m, 2H), 4.20 (s, 2H), 3.93 (d, J = 6.2 Hz, 2H), 3.54 (dd, J = 18.9, 5.0 Hz, 2H), 1.28–1.12 (m, 6H), 1.05 (d, J = 4.6 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ 171.3, 163.5, 162.7, 156.3, 132.2, 131.7, 129.6, 126.7, 115.6, 107.3, 67.7, 67.7, 67.2, 61.1, 38.6, 22.5, 20.5; HR-MS (ESI): calcd for C25H28N2O7 [M + H]+ 469.1970, found (ESI+) 469.1977.
- (S)-Isopropyl(2-oxo-2-((2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)amino)-1-phenylethyl)carbamate (4i).White solid, 47.5% yield, m.p. 131–134 °C; = +17.0° (c 1.0, CH3OH); 1H NMR (400 MHz, DMSO-d6) δ 8.48 (s, 1H), 8.09 (s, 2H), 7.94 (d, J = 8.7 Hz, 1H), 7.60 (s, 4H), 7.41 (d, J = 7.0 Hz, 2H), 7.32–7.21 (m, 4H), 7.02 (d, J = 8.7 Hz, 2H), 5.25 (d, J = 8.0 Hz, 1H), 4.92–4.53 (m, 1H), 4.16 (s, 2H), 3.50 (s, 2H), 1.20–1.07 (m, 6H); 13C NMR (100 MHz, DMSO-d6) δ 177.0, 170.9, 163.4, 162.7, 157.9, 155.8, 139.1, 131.6, 129.6, 128.7, 128.0, 127.6, 126.7, 126.6, 117.7, 115.5, 107.2, 101.9, 67.7, 67.5, 58.4, 38.7, 22.4; HR-MS (ESI): calcd for C29H28N2O6 [M + H]+ 501.2020, found (ESI+) 501.2023.
- (S)-Isobutyl(2-oxo-2-((2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)amino)-1-phenylethyl)carbamate (4j). White solid, 49.0% yield, m.p. 130–133 °C; = +26.0° (c 1.0, CH3OH); 1H NMR (400 MHz, DMSO-d6) δ 8.52 (s, 1H), 8.10 (d, J = 5.9 Hz, 2H), 7.94 (d, J = 8.7 Hz, 1H), 7.69 (d, J = 7.7 Hz, 1H), 7.60 (s, 3H), 7.42 (d, J = 7.1 Hz, 2H), 7.33–7.19 (m, 4H), 7.01 (d, J = 8.5 Hz, 1H), 6.97 (s, 1H), 5.25 (d, J = 8.2 Hz, 1H), 4.16 (s, 2H), 3.72 (d, J = 6.1 Hz, 2H), 3.51 (s, 2H), 1.98–1.68 (m, 1H), 0.86 (d, J = 5.5 Hz, 6H); 13C NMR (100MHz, DMSO-d6) δ 176.92, 170.85, 163.41, 162.68, 157.88, 156.35, 139.04, 132.15, 131.61, 129.56, 128.66, 127.62, 126.66, 117.67, 115.48, 107.23, 102.01, 70.56, 67.51, 58.53, 38.69, 28.05, 19.31; HR-MS (ESI): calcd for C30H30N2O6 [M + H]+ 515.2177, found (ESI+) 515.2170.
- (S)-Tert-butyl(1-oxo-1-((2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)amino)-3-phenylpropan-2-yl)carbamate (4k). White solid, 42.5% yield, m.p. 120–125 °C; = −8.8° (c 1.0, CH3OH); 1H NMR (400 MHz, DMSO-d6) δ 8.21 (s, 1H), 8.10 (d, J = 5.3 Hz, 2H), 7.96 (d, J = 8.7 Hz, 1H), 7.60 (s, 3H), 7.34 (s, 1H), 7.23 (s, 4H), 7.15 (s, 1H), 7.07 (d, J = 8.2 Hz, 1H), 6.97 (s, 1H), 6.91 (d, J = 8.1 Hz, 1H), 4.14 (s, 3H), 3.51 (d, J = 22.2 Hz, 2H), 3.04–2.62 (m, 2H), 1.28 (s, 9H); 13C NMR (100 MHz, DMSO-d6) δ 177.1, 172.6, 163.6, 162.8, 158.0, 155.7, 138.5, 132.3, 131.6, 129.6, 128.5, 126.7, 117.7, 115.6, 107.2, 102.0, 78.6, 67.6, 56.2, 38.6, 38.1, 28.6; HR-MS (ESI): calcd for C31H32N2O6 [M + H]+ 529.2333, found (ESI+) 529.2330.
- (S)-Isopropyl(3-(4-hydroxyphenyl)-1-oxo-1-((2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)amino)propan-2-yl)carbamate (4l). White solid, 37.2% yield, m.p. 160–164 °C; = −7.9° (c 1.0, CH3OH); 1H NMR (400 MHz, DMSO-d6) δ 8.30 (s, 1H), 8.20–8.07 (m, 2H), 7.98 (d, J = 8.8 Hz, 1H), 7.61 (d, J = 6.6 Hz, 3H), 7.36 (d, J = 2.1 Hz, 1H), 7.30 (d, J = 8.1 Hz, 2H), 7.22 (d, J = 8.5 Hz, 1H), 7.09 (d, J = 8.0 Hz, 3H), 7.00 (d, J = 1.7 Hz, 1H), 4.83 (p, J = 6.3 Hz, 1H), 4.65 (p, J = 6.4 Hz, 1H), 4.24 (s, 1H), 4.15 (q, J = 5.6 Hz, 2H), 3.35 (d, J = 1.9 Hz, 2H), 3.04–2.70 (m, 2H), 1.28 (d, J = 6.2 Hz, 6H); 13C NMR (100 MHz, DMSO-d6) δ 176.4, 163.0, 162.2, 157.4, 152.5, 149.2, 135.7, 131.7, 131.1, 130.2, 129.1, 126.2, 120.7, 117.2, 115.0, 106.7, 72.6, 66.9, 55.9, 21.3; HR-MS (ESI): calcd for C30H31N2O7 [M + H]+ 531.2126, found (ESI+) 531.2121.
- (S)-Isopropyl(3-(1H-indol-3-yl)-1-oxo-1-((2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl) amino)propan-2-yl)carbamate (4m). White solid, 54.3% yield, m.p. 105–115 °C; = +2.5° (c 1.0, CH3OH); 1H NMR (400 MHz, DMSO-d6) δ 10.79 (s, 1H), 8.23 (s, 1H), 8.11 (d, J = 6.8 Hz, 2H), 7.95 (d, J = 8.7 Hz, 1H), 7.59 (d, J = 6.0 Hz, 4H), 7.30 (d, J = 6.7 Hz, 2H), 7.13 (s, 1H), 7.05 (d, J = 10.0 Hz, 3H), 6.96 (d, J = 10.6 Hz, 2H), 4.55–4.76 (m, 1H), 4.24 (d, J = 2.9 Hz, 1H), 4.10 (s, 2H), 3.47 (s, 2H), 3.11–2.84 (m, 2H), 1.08 (dd, J = 21.3, 5.8 Hz, 6H); 13C NMR (100 MHz, DMSO-d6) δ 176.5, 172.3, 163.0, 162.2, 157.4, 155.5, 136.0, 131.7, 131.1, 129.1, 127.2, 126.2, 123.7, 120.8, 118.4, 118.1, 117.2, 116.2, 115.0, 111.2, 110.0, 106.7, 101.5, 67.0, 66.9, 55.4, 38.0, 27.8, 21.9; HR-MS (ESI): calcd for C32H31N3O6 [M + H]+ 554.2286, found (ESI+) 554.2285.
- (S)-Diisopropyl(6-oxo-6-((2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)amino)hexane-1,5-diyl)dicarbamate (4n). White solid, 43.6% yield, m.p. 169–173 °C; = −15.3° (c 1.0, CH3OH); 1H NMR (400 MHz, DMSO-d6) δ 8.10 (d, J = 6.8 Hz, 3H), 7.95 (d, J = 8.8 Hz, 1H), 7.59 (d, J = 6.5 Hz, 3H), 7.34 (s, 1H), 7.05 (t, J = 8.0 Hz, 2H), 6.97 (s, 1H), 6.93 (s, 1H), 4.67–4.73 (m, 2H), 4.17 (s, 2H), 3.88–3.93 (m, 1H), 3.43–3.56 (m, 2H), 2.87–2.88 (m, 2H), 1.46–1.54 (m, 2H), 1.32 (d, J = 5.9 Hz, 2H), 1.28–1.17 (m, 2H), 1.12 (d, J = 6.2 Hz, 12H); 13C NMR (100 MHz, DMSO-d6) δ 177.0, 173.0, 163.5, 162.7, 157.9, 156.3, 156.2, 132.2, 131.6, 129.6, 126.7, 117.7, 115.5, 107.2, 102.0, 67.4, 66.9, 55.0, 38.4, 32.1, 29.6, 23.2, 22.5, 22.5; HR-MS (ESI): calcd for C31H39N3O8 [M + H]+ 582.2810, found (ESI+) 582.2807.
- N-(2-((4-Oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)benzamide (5a). White solid, 42.2% yield, m.p. 132–144 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.76 (s, 1H), 8.09 (d, J = 6.2 Hz, 2H), 7.94 (d, J = 8.7 Hz, 1H), 7.87 (d, J = 7.4 Hz, 2H), 7.59 (d, J = 5.7 Hz, 3H), 7.52 (d, J = 7.1 Hz, 1H), 7.46 (t, J = 7.2 Hz, 2H), 7.39 (s, 1H), 7.09 (d, J = 8.8 Hz, 1H), 6.97 (s, 1H), 4.31 (d, J = 5.1 Hz, 2H), 3.71 (d, J = 5.1 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 177.0, 167.2, 163.6, 162.7, 156.0, 134.6, 132.2, 131.8, 131.6, 129.6, 128.8, 127.7, 126.7, 117.7, 115.6, 107.2, 102.1, 67.4, 39.2; HR-MS (ESI): calcd for C24H19NO4 [M + H]+ 386.1387, found (ESI+) 386.1395.
- 2-Methoxy-N-(2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)benzamide (5b). White solid, 43.5% yield, m.p. 95–100 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.46 (s, 1H), 8.09 (d, J = 6.1 Hz, 2H), 7.95 (d, J = 8.7 Hz, 1H), 7.80 (d, J = 6.9 Hz, 1H), 7.59 (s, 3H), 7.47 (t, J = 7.2 Hz, 1H), 7.40 (s, 1H), 7.12 (t, J = 8.7 Hz, 2H), 7.03 (t, J = 7.3 Hz, 1H), 6.97 (s, 1H), 4.31 (s, 2H), 3.88 (s, 3H), 3.79–3.68 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 177.1, 165.9, 163.6, 162.8, 158.0, 157.5, 133.1, 132.3, 131.6, 131.0, 129.6, 126.8, 126.7, 122.8, 121.1, 117.7, 115.6, 112.6, 107.2, 102.0, 67.6, 56.4, 38.9; HR-MS (ESI): calcd for C25H21NO5 [M + H]+ 416.1493, found (ESI+) 416.1485.
- 2-Chloro-N-(2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)benzamide (5c). White solid, 39.8% yield, m.p. 148–154 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.74 (s, 1H), 8.10 (d, J = 6.0 Hz, 2H), 7.96 (d, J = 8.8 Hz, 1H), 7.58 (d, J = 6.1 Hz, 3H), 7.49 (d, J = 7.6 Hz, 1H), 7.45–7.41 (m, 2H), 7.37 (d, J = 8.1 Hz, 2H), 7.09 (d, J = 8.7 Hz, 1H), 6.97 (s, 1H), 4.30 (s, 2H), 3.68 (d, J = 5.1 Hz, 2H); 13C NMR (101 MHz, DMSO-d6) δ 177.0, 167.23, 163.5, 162.7, 158.0, 137.3, 132.2, 131.6, 131.3, 130.4, 130.1, 129.6, 129.3, 127.6, 126.7, 117.7, 115.6, 107.3, 102.0, 67.4, 39.0; HR-MS (ESI): calcd for C24H18ClNO4 [M + H]+ 420.0997, found (ESI+) 420.0995.
- 2-Bromo-N-(2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)benzamide (5d). White solid, 37.5% yield, m.p. 138–148 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.72 (s, 1H), 8.10 (d, J = 6.0 Hz, 2H), 7.96 (d, J = 8.8 Hz, 1H), 7.66–7.53 (m, 4H), 7.39 (q, J = 12.7, 10.6 Hz, 4H), 7.09 (d, J = 8.8 Hz, 1H), 6.97 (s, 1H), 4.30 (s, 2H), 3.81–3.57 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 177.0, 168.1, 163.5, 162.7, 156.0, 139.4, 133.2, 132.2, 131.6, 131.4, 129.6, 129.3, 128.0, 126.7, 119.4, 117.7, 115.6, 107.3, 102.0, 67.4, 39.0; HR-MS (ESI): calcd for C24H18BrNO4 [M + H]+ 464.0492, found (ESI+) 464.0496.
- 2-Nitro-N-(2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)benzamide (5e). White solid, 40.1% yield, m.p. 166–172 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.01 (s, 1H), 8.10 (d, J = 6.4 Hz, 2H), 8.04 (d, J = 7.9 Hz, 1H), 7.96 (d, J = 8.6 Hz, 1H), 7.78 (t, J = 7.2 Hz, 1H), 7.70 (d, J = 7.4 Hz, 1H), 7.61 (d, J = 7.6 Hz, 4H), 7.40 (d, J = 8.1 Hz, 1H), 7.10 (d, J = 8.6 Hz, 1H), 6.98 (s, 1H), 4.30 (s, 2H), 3.77–3.60 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 177.0, 166.5, 163.6, 162.8, 158.0, 147.5, 134.2, 132.8, 132.2, 131.6, 131.3, 129.6, 129.5, 126.7, 124.6, 117.7, 115.6, 107.2, 102.0, 67.4, 39.2; HR-MS (ESI): calcd for C24H18N2O6 [M + H]+ 431.1238, found (ESI+) 431.1236.
- N1-(2-((4-Oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)-N3-phenylmalonamide (6a). White solid, 44.5% yield, m.p. 173–178 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.10 (s, 1H), 8.41 (s, 1H), 8.10 (d, J = 6.9 Hz, 2H), 7.96 (d, J = 8.8 Hz, 1H), 7.62–7.54 (m, 5H), 7.37 (s, 1H), 7.29 (t, J = 7.7 Hz, 2H), 7.06 (dt, J = 14.8, 8.0 Hz, 2H), 6.98 (s, 1H), 4.21 (s, 2H), 3.55 (d, J = 5.0 Hz, 2H), 3.30 (s, 2H); 13C NMR (100 MHz, DMSO-d6) δ 177.0, 167.6, 166.1, 163.5, 162.8, 158.0, 139.4, 132.2, 131.6, 129.6, 129.2, 126.7, 123.9, 119.6, 117.7, 115.6, 107.2, 102.1, 67.7, 45.0, 38.8; HR-MS (ESI): calcd for C26H22N2O5 [M + H]+ 443.1602, found (ESI+) 443.1605.
- N1-(2-((4-Oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)-N3-(o-tolyl)malonamide (6b). White solid, 46.3% yield, m.p. 166–169 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.64 (s, 1H), 8.50 (s, 1H), 8.10 (d, J = 7.1 Hz, 2H), 7.95 (d, J = 8.8 Hz, 1H), 7.57 (dd, J = 14.6, 7.5 Hz, 4H), 7.36 (s, 1H), 7.19 (d, J = 7.3 Hz, 1H), 7.14 (t, J = 7.5 Hz, 1H), 7.06 (q, J = 8.4, 7.3 Hz, 2H), 6.97 (s, 1H), 4.22 (s, 2H), 3.57 (d, J = 5.0 Hz, 2H), 3.36 (s, 2H), 2.21 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 176.9, 168.2, 165.9, 163.4, 162.7, 158.0, 136.7, 132.2, 131.7, 130.9, 130.8, 129.6, 126.7, 126.5, 125.3, 124.2, 117.8, 115.5, 107.3, 102.1, 67.7, 44.0, 38.8, 18.2; HR-MS (ESI): calcd for C27H24N2O5 [M + H]+ 457.1758, found (ESI+) 457.1755.
- N1-(2-Chlorophenyl)-N3-(2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)malonamide (6c). White solid, 39.0% yield, m.p. 184–189 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.18 (s, 1H), 8.60 (s, 1H), 8.10 (d, J = 6.8 Hz, 2H), 8.02 (d, J = 8.0 Hz, 1H), 7.94 (d, J = 8.8 Hz, 1H), 7.59 (d, J = 6.9 Hz, 3H), 7.48 (d, J = 8.0 Hz, 1H), 7.36 (s, 1H), 7.31 (t, J = 7.7 Hz, 1H), 7.13 (t, J = 7.6 Hz, 1H), 7.08 (d, J = 8.8 Hz, 1H), 6.97 (s, 1H), 4.30–4.15 (m, 2H), 3.58 (d, J = 4.9 Hz, 2H), 3.45 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 177.0, 168.3, 166.1, 163.5, 162.8, 158.0, 135.2, 132.2, 131.6, 129.9, 129.6, 128.1, 126.7, 126.1, 124.7, 124.1, 117.7, 115.6, 107.2, 102.1, 67.7, 43.6, 38.8; HR-MS (ESI): calcd for C26H21ClN2O5 [M + H]+ 477.1212, found (ESI+) 477.1205.
- N1-(4-Bromophenyl)-N3-(2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)malonamide (6d). White solid, 42.2% yield, m.p. 181–186 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.30 (d, J = 5.0 Hz, 1H), 8.50–8.40 (m, 1H), 8.10 (d, J = 6.2 Hz, 2H), 7.95 (d, J = 8.8 Hz, 1H), 7.64–7.53 (m, 5H), 7.46 (d, J = 8.5 Hz, 2H), 7.36 (s, 1H), 7.08 (d, J = 8.7 Hz, 1H), 6.97 (s, 1H), 4.20 (s, 2H), 3.54 (d, J = 4.8 Hz, 2H), 3.33 (s, 2H); 13C NMR (100 MHz, DMSO-d6) δ 176.9, 167.3, 166.3, 163.5, 162.7, 158.0, 138.8, 132.2, 132.0, 131.7, 129.6, 126.7, 121.5, 117.7, 115.5, 115.4, 107.3, 102.1, 67.7, 45.1, 38.8; HR-MS (ESI): calcd for C26H21BrN2O5 [M + H]+ 521.0707, found (ESI+) 521.0713.
4.2. Biological Assays
Each test was repeated three times at 25 ± 1 °C. The active effect was expressed in percentage scale of 0–100 (0: no activity; 100: total inhibited). The specific test method for the anti-TMV activity was carried out by the literature method [34,51], and detailed bioassay procedures for the anti-TMV activity were described as follows. The in vivo inhibition rates of the compound were then calculated according to the following formula (1) (“av” means average, and controls were not treated with compound).
Inhibition rate (%) = [(av local lesion no. of control − av local lesion no. of drug-treated)/av local lesion no. of control] × 100%
4.2.1. Extraction of TMV
Using Gooding’s method [52] and our previous reports [7,34,51], the TMV viruses were propagated in Nicotiana tabacum L. The infected tobacco leaves were selected and soaked in phosphate buffer, and then filtered through double-layer pledget. The filtrate was centrifuged at 10,000× g for 5 min, treated with PEG twice, and centrifuged again, and the extract was processed at 4 °C. The absorbance value was estimated at 260 nm by an ultraviolet spectrophotometer. The concentration of TMV viruses was obtained using Equation (2).
4.2.2. Inactivation Effect of Compounds against TMV In Vivo
After the compound and TMV viruses were mixed for 30 min, the tobacco leaves were sprinkled with diamonds. The mixture was then inoculated on the left side of the leaves of N. tabacum L., whereas the right side of the leaves were inoculated with the mixture of solvent and the virus for control. After incubating for half an hour, the leaves were rinsed with water and sequentially incubated at 25 °C in a greenhouse at 25 ± 2 °C. The local lesion numbers were counted after 3–4 days. There were three replicates for each compound.
4.2.3. Cultivation Effect of Compounds against TMV In Vivo
Growing leaves of N. tabacum L. of the same ages were selected and sprinkled with diamonds. TMV (concentration of 6.0 × 10−3 mg/mL) was dipped and inoculated on the whole leaves. After inoculation with the TMV viruses for half an hour at 25 °C, the leaves were washed under running water, then allowed to air dry. The compound solution was smeared on the left side, and the solvent was smeared on the right side for control. The local lesion numbers were then counted and recorded 3–4 days after inoculation.
4.2.4. Protective Effect of Compounds against TMV In Vivo
The compound solution was smeared on the left side and the solvent serving as the control was smeared on the right side of growing N. tabacum L. leaves of the same ages. The leaves were then inoculated with the virus after 12 h. A brush was dipped in TMV of 6 × 10−3 mg/mL to inoculate the leaves, which were previously scattered with silicon carbide. The leaves were rubbed softly along the nervature once or twice and then washed with water. The local lesion numbers appearing 3–4 days after inoculation were counted. There were three replicates for each compound.
4.3. Calculation Procedures for Molecular Docking Research
The calculation procedures for molecular docking research consisted of four steps according to the literature method [48,53] and our previously described method [7,34].
Receptor Preparation. The 3D crystal structure of TMV-CP (PDB code:1EI7) was downloaded from the protein data bank, and this was used as the receptor for molecular docking. Water molecules were removed from the target protein and hydrogen atoms were added using AutoDock Tools prior to molecular docking.
Ligand preparation. Target compounds are drawn using ChemOffice 2015 as ligands followed by management of its conformer and the minimization process.
Molecular Docking Using AutoDock Vina 1.1.2. The input files for AutoDock Vina were prepared using AutoDock Tools. The protein was placed in a grid box (grid parameters: center x = 5, center y = −20, center z = 0.8, size x = 60, size y = 60, size z = 56), using AutoDock Vina 1.1.2 at 1.00 Å to define the binding site. The docking procedure was performed using the instructed command prompts.
Analysing and Output Visualization using PyMOL 1.7.2.1. The docking poses were ranked according to their docking scores. The scoring function in Auto Dock was used to predict the binding affinity of one ligand to the receptor molecule. The conformation with the lowest binding affinity was selected for further analysis after the docking process. The docking results included the locations of hydrogen bonds, and closely interacting residues were performed by PyMOL software 1.7.2.1.
5. Conclusions
In this paper, a series of flavone derivatives containing carboxamide fragments were synthesized based on the natural product and evaluated for their antiviral activities against TMV. Most of these compounds displayed good to excellent anti-TMV activities in vivo. The structure–activity relationship revealed that the introduction of amino acids, aromatic carboxylic acids, and malonic acid derivative fragments to the mother nucleus structure on the 7-positions were favorable for their activities. In particular, compound 4m (inactivation activity, 58%; curative activity, 57%; protection activity, 59%) even exhibited similar anti-TMV activity with ningnanmycin (inactivation activity, 61%; curative activity, 57%; protection activity, 58%) at 500 μg/mL, which was significantly higher than that of ribavirin (inactivation activity, 39%; curative activity, 40%; protection activity, 39%). Antiviral mechanism research by molecular docking demonstrated that these flavone derivatives containing carboxamide fragments could interact with TMV CP at multiple sites and inhibit virus assembly. In this study, it was found that the introduction of carboxamide fragments at the 7-position of the flavonoids greatly improved the antiviral activity, which has the potential to become a new type of anti-plant virus agent.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules28052179/s1, Section S1: Phytotoxic activity; Section S2: Copies of NMR spectra (Figures S1–S60); Section S3: Molecule docking results of 1 and 2a with TMV CP (Figure S61). References [7,51] were cited in Supplementary Materials.
Author Contributions
Project administration, supervision, A.L.; writing—original draft, Z.W.; chemical methodology, B.Z.; biological methodology, L.W. and J.W.; docking studies, J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the S&T Program of Hebei (21326504D) and the Hebei Natural Science Foundation (B2020202028).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data used to support the findings of this study are included within the article and Supplementary Materials.
Acknowledgments
The authors also acknowledge the State Key Laboratory of Elemento-Organic Chemistry (Nankai University) for the biological activity test.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 2a–2e, 4a–4n, 5a–5e, and 6a–6d are available from the authors.
References
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; Mcroberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.; Philip, S.; Jacob, M.K.; Narayanan, S.P.; Jacob, C.K.; Kochupurackal, J. Phenazine-1-carboxylic acid mediated anti-oomycete activity of the endophytic Alcaligenes sp. EIL-2 against Phytophthora meadii. Microbiol. Res. 2015, 170, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Wang, S.; Song, D.; Cao, X.; Huang, W.; Ke, S. Discovery of γ-Lactam alkaloid derivatives as potential fungicidal agents targeting steroid biosynthesis. J. Agric. Food Chem. 2020, 68, 14438–14451. [Google Scholar] [CrossRef]
- Guo, J.; Hao, Y.; Ji, X.; Wang, Z.; Liu, Y.; Ma, D.; Li, Y.; Pang, H.; Ni, J.; Wang, Q. Optimization, structure–activity relationship, and mode of action of nortopsentin analogues containing thiazole and oxazole moieties. J. Agric. Food Chem. 2019, 67, 10018–10031. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, T.; Zhou, Y.; Shi, L.; Lu, A.; Wang, Z. Discovery of cysteine and its derivatives as novel antiviral and antifungal agents. Molecules 2021, 26, 383. [Google Scholar] [CrossRef]
- Guo, W.; Yan, H.; Ren, X.; Tang, R.; Sun, Y.; Wang, Y.; Feng, J. Berberine induces resistance against tobacco mosaic virus in tobacco. Pest Manag. Sci. 2019, 76, 1804–1813. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yang, S.; Li, H.; Lu, A.; Wang, Z.; Yao, Y.; Wang, Q. Discovery, structural optimization, and mode of action of essramycin alkaloid and its derivatives as anti-tobacco mosaic virus and anti-phytopathogenic fungus agents. J. Agric. Food Chem. 2019, 68, 471–484. [Google Scholar] [CrossRef]
- Scholthof, K.-B.G. Tobacco mosaic virus: A model system for plant biology. Annu. Rev. Phytopathol. 2004, 42, 13–34. [Google Scholar] [CrossRef]
- Reichman, M.; Devash, Y.; Suhadolnik, R.J.; Sela, I. Human leukocyte interferon and the antiviral factor (AVF) from virus-infected plants stimulate plant tissues to produce nucleotides with antiviral activity. Virology 1983, 128, 240–244. [Google Scholar] [CrossRef]
- Gan, X.; Wang, Y.; Hu, D.; Song, B. Design, synthesis, and antiviral activity of novel chalcone derivatives containing a purine moiety. Chin. J. Chem. 2017, 35, 665–672. [Google Scholar] [CrossRef]
- Cai, L.; Zhang, W.; Jia, H.; Feng, H.; Wei, X.; Chen, H.; Wang, D.; Xue, Y.; Sun, X. Plant-derived compounds: A potential source of drugs against Tobacco mosaic virus. Pestic. Biochem. Physiol. 2020, 169, 104589. [Google Scholar] [CrossRef]
- Cantrell, C.L.; Dayan, F.E.; Duke, S.O. Natural products as sources for new pesticides. J. Nat. Prod. 2012, 75, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.; Reker, D.; Schneider, P.; Schneider, G. Counting on natural products for drug design. Nat. Chem. 2016, 8, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Liu, J.; Xu, S.; Zhu, Z.; Xu, J. The structural modification of natural products for novel drug discovery. Expert Opin. Drug Discov. 2016, 12, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Mihorianu, M.; Franz, M.H.; Jones, P.G.; Freytag, M.; Kelter, G.; Fiebig, H.-H.; Tamm, M.; Neda, I. N-Heterocyclic carbenes derived from imidazo-[1,5-a]pyridines related to natural products: Synthesis, structure and potential biological activity of some corresponding gold(I) and silver(I) complexes. Appl. Organomet. Chem. 2016, 30, 581–589. [Google Scholar] [CrossRef]
- Sparks, T.C.; Duke, S.O. Structure simplification of natural products as a lead generation approach in agrochemical discovery. J. Agric. Food Chem. 2021, 69, 8324–8346. [Google Scholar] [CrossRef] [PubMed]
- Song, B.A.; Zhang, G.P.; Hu, D.Y.; Pang, L.; Yang, S.; Liu, G.; Wang, H. N-Substituted Benzothiazolyl-1-substitutedphenyl-O,O-dialkyl-α-aminophosphonate Ester Derivatives Preparation and Application. China Patent CN 1687088, 2005. [Google Scholar]
- Chen, Z.; Li, G.J.; Fan, H.T.; Liu, J.J.; Bi, L.; Yang, S.; Song, B.A.; Hu, D.Y. The study of efficiency of dufulin against southern rice black-streaked dwarf virus. Chin. Agr. Sci. Bull. 2011, 27, 250–254. [Google Scholar]
- Martens, S.; Mithöfer, A. Flavones and flavone synthases. Phytochemistry 2005, 66, 2399–2407. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Shah, S.A.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial effects of flavonoids and their structure-activity relationship study: A comparative interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.-S. Recent advances in natural antifungal flavonoids and their derivatives. Bioorganic Med. Chem. Lett. 2019, 29, 126589. [Google Scholar] [CrossRef]
- Orhan, D.D.; Özçelik, B.; Özgen, S.; Ergun, F. Antibacterial, antifungal, and antiviral activities of some flavonoids. Microbiol. Res. 2010, 165, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as potential anti-inflammatory molecules: A review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef] [PubMed]
- Benavente-García, O.; Castillo, J. Update on uses and properties of Citrus flavonolds: New findings in anticancer, cardiovascular, and anti-inflammatory activity. J. Agric. Food Chem. 2008, 56, 6185–6205. [Google Scholar] [CrossRef]
- Jia, L.-G.; Sheng, Z.-W.; Xu, W.-F.; Li, Y.-X.; Liu, Y.-G.; Xia, Y.-J.; Zhang, J.-H. Modulation of anti-oxidation ability by proanthocyanidins during germination of Arabidopsis thaliana seeds. Mol. Plant 2012, 5, 472–481. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Aoshima, A.; Ikeshiro, Y.; Chen, Y.-P.; Furukawa, H.; Itoigawa, M.; Fujioka, T.; Mihashi, K.; Cosentino, L.M.; Morris-Natschke, S.L.; et al. Anti-HIV benzylisoquinoline alkaloids and flavonoids from the leaves of Nelumbo nucifera, and structure–activity correlations with related alkaloids. Bioorganic Med. Chem. 2005, 13, 443–448. [Google Scholar] [CrossRef]
- Krcatović, E.; Rusak, G.; Bezić, N.; Krajacić, M. Inhibition of tobacco mosaic virus infection by quercetin and vitexin. Acta Virol. 2008, 52, 119–124. [Google Scholar]
- Zhao, W.; Zeng, X.; Zhang, T.; Wang, L.; Yang, G.; Chen, Y.-K.; Hu, Q.; Miao, M. Flavonoids from the bark and stems of Cassia fistula and their anti-tobacco mosaic virus activities. Phytochem. Lett. 2013, 6, 179–182. [Google Scholar] [CrossRef]
- Li, Y.; Ye, S.; Hu, Z.; Hao, N.; Bo, X.; Liang, H.; Tian, X. Identification of anti- TMV active flavonoid glycosides and their mode of action on virus particles from Clematis lasiandra Maxim. Pest Manag. Sci. 2021, 77, 5268–5277. [Google Scholar] [CrossRef]
- Chen, Z.; Tan, J.; Yang, G.; Miao, M.; Chen, Y.; Li, T. Isoflavones from the roots and stems of Nicotiana Tabacum and their anti-tobacco mosaic virus activities. Phytochem. Lett. 2012, 5, 233–235. [Google Scholar] [CrossRef]
- Kong, W.-S.; Xing, H.-H.; Li, J.; Ye, L.; Liu, X.; Li, Y.-P.; Rao, G.-X.; Zhou, M.; Yang, G.; Hu, Q.-F.; et al. Two new flavones from Cassia pumila and their anti-tobacco mosaic virus activity. Chem. Nat. Compd. 2018, 54, 1048–1051. [Google Scholar] [CrossRef]
- Yao, J.M.; Pu, L.L.; Rao, J.R.; Wang, M.; Guo, C.; Lei, Z.W. Research progress of anti-plant virus agents based on structural diversification. Agrochemicals 2021, 60, 859–865. [Google Scholar] [CrossRef]
- Ma, Y.C.; Wang, L.; Lu, A.D.; Xue, W. Synthesis and biological activity of novel oxazinyl flavonoids as antiviral and anti-phytopathogenic fungus agents. Molecules 2022, 27, 6875. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Gao, M.Q.; Zhu, X.L.; Yang, G.F. Research progress on carboxamide fungicides targeting succinate dehydrogenase. Chin. J. Pestic. Sci. 2019, 21, 673–680. [Google Scholar]
- Luo, B.; Ning, Y. Comprehensive overview of carboxamide derivatives as succinate dehydrogenase inhibitors. J. Agric. Food Chem. 2022, 70, 957–975. [Google Scholar] [CrossRef]
- Chaparro, S.; Rojas, H.A.; Castillo, J.C.; Portilla, J.; Romanelli, G.P.; Pineda, A.; Elsharif, A.M.; Martinez, J.J.; Luque, R. Solventless amide synthesis catalyzed by biogenic CaCO3 materials. ACS Sustain. Chem. Eng. 2020, 8, 13139–13146. [Google Scholar] [CrossRef]
- Zeng, S.; Liu, J.; Anankanbil, S.; Chen, M.; Guo, Z.; Adams, J.P.; Snajdrova, R.; Li, Z. Amide synthesis via aminolysis of ester or acid with an intracellular lipase. ACS Catal. 2018, 8, 8856–8865. [Google Scholar] [CrossRef]
- Serdiuk, I.E.; Roshal, A.D. Single and double intramolecular proton transfers in the electronically excited state of flavone derivatives. RSC Adv. 2015, 5, 102191–102203. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Liu, J.; Taylor, S.F.; Dupart, P.S.; Arnold, C.L.; Sridhar, J.; Jiang, Q.; Wang, Y.; Skripnikova, E.V.; Zhao, M.; Maryam Foroozesh, M. Pyranoflavones: A group of small-molecule probes for exploring the active site cavities of cytochrome P450 enzymes 1A1, 1A2, and 1B1. J. Med. Chem. 2013, 56, 4082–4092. [Google Scholar] [CrossRef]
- Du, X.-J.; Bian, Q.; Wang, H.-X.; Yu, S.-J.; Kou, J.-J.; Wang, Z.-P.; Li, Z.-M.; Zhao, W.-G. Design, synthesis, and fungicidal activity of novel carboxylic acid amides represented by N-benzhydryl valinamode carbamates. Org. Biomol. Chem. 2014, 12, 5427–5434. [Google Scholar] [CrossRef]
- Sun, M.; Yang, H.-H.; Tian, L.; Li, J.-Q.; Zhao, W.-G. Design, synthesis, and fungicidal activities of imino diacid analogs of valine amide fungicides. Bioorganic Med. Chem. Lett. 2015, 25, 5729–5731. [Google Scholar] [CrossRef]
- Atalay, S.S.; Assad, M.Y.; Amagata, T.; Wu, W. Mild, efficient, and solvent-free synthesis of 4-hydroxy-2-quinolinones. Tetrahedron Lett. 2020, 61, 151778. [Google Scholar] [CrossRef]
- Lamberth, C. Amino acid chemistry in crop protection. Tetrahedron 2010, 66, 7239–7256. [Google Scholar] [CrossRef]
- Ryzhkov, V.L. Effect of amino acids and related substances on reproduction of the tobacco mosaic virus. Dokl. Akad. Nauk SSSR 1951, 80, 677–680. [Google Scholar]
- Zhang, B.; Li, L.; Liu, Y.; Wang, Q. Antiviral mechanism study of gossypol and its Schiff base derivatives based on reactive oxygen species (ROS). RSC Adv. 2016, 6, 87637–87648. [Google Scholar] [CrossRef]
- Seyedi, S.S.; Shukri, M.; Hassandarvish, P.; Oo, A.; Shankar, E.M.; Abubakar, S.; Zandi, K. Computational approach towards exploring potential anti-chikungunya activity of selected flavonoids. Sci. Rep. 2016, 6, 24027. [Google Scholar] [CrossRef] [PubMed]
- Bhyravbhatla, B.; Watowich, S.J.; Caspar, D.L. Refined atomic model of the four-layer aggregate of the tobacco mosaic virus coat protein at 2.4-Å resolution. Biophys. J. 1998, 74, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, Y.; Sugiyama, A.; Purqon, A.; Nagao, H.; Nishikawa, K. Bingding free energy calculation and structural analysis for antigen-antibody complex. AIP Conf. Proc. 2006, 832, 566–569. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, P.; Wang, L.; Wang, Q. Design, synthesis, and anti-tobacco mosaic virus (TMV) activity of phenanthroindolizidines and their analogues. J. Agric. Food Chem. 2012, 60, 10212–10219. [Google Scholar] [CrossRef] [PubMed]
- Gooding, G.V., Jr.; Hebert, T.T. A simple technique for purification of tobacco mosaic virus in large quantities. Phytopathology 1967, 57, 1285–1290. [Google Scholar] [PubMed]
- Li, S.Z.; Wang, D.M.; Jiao, S.M. Pesticide Experiment Methods-Fungicide Sector; Li, S.Z., Ed.; Agriculture Press of China: Beijing, China, 1991; pp. 93–94. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).