Molecular Pathways for Polymer Degradation during Conventional Processing, Additive Manufacturing, and Mechanical Recycling
Abstract
1. Introduction
2. Overview of Common Degradation Reactions
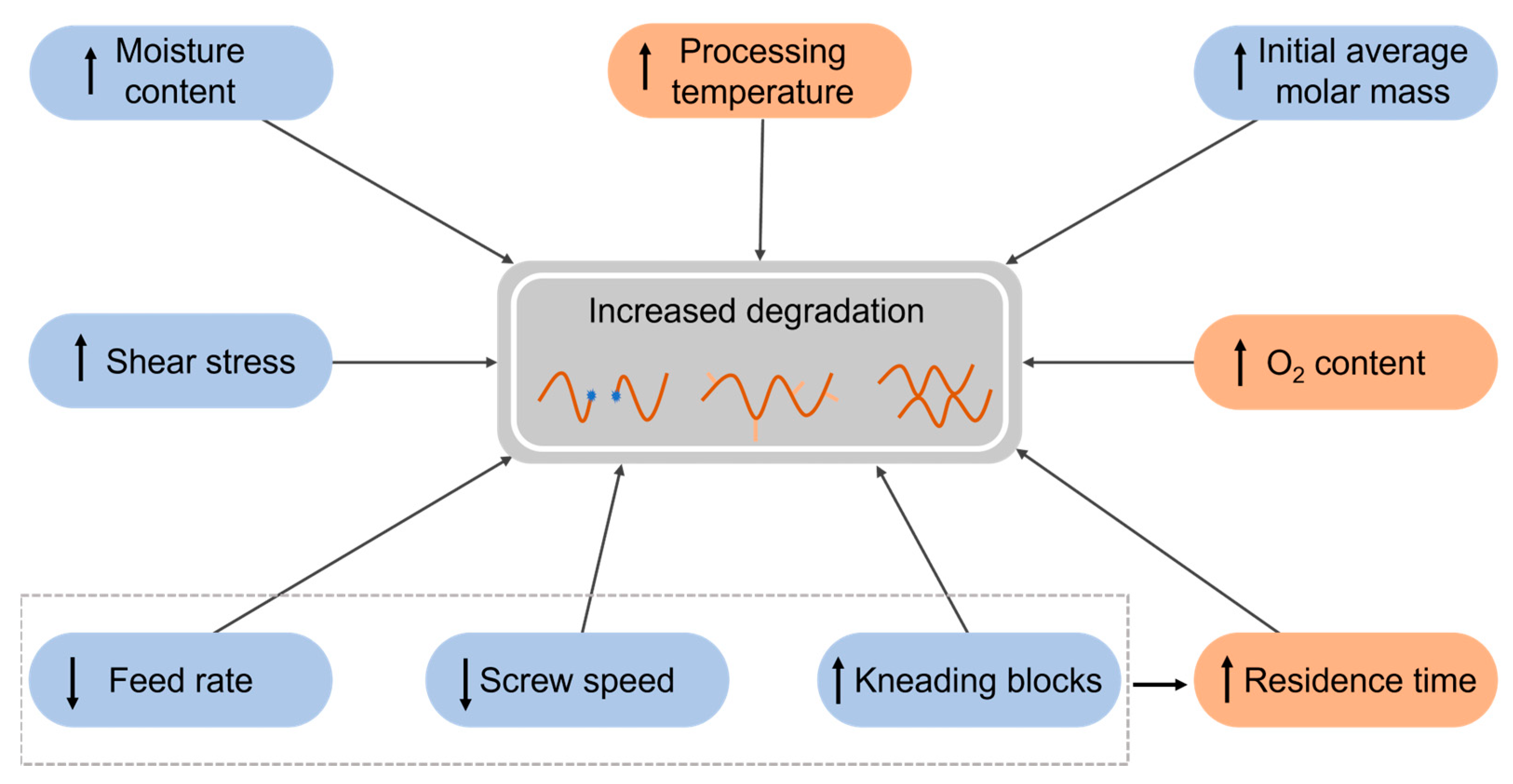
3. Dominant Degradation Reactions Illustrated for Extrusion-Based Additive Manufacturing
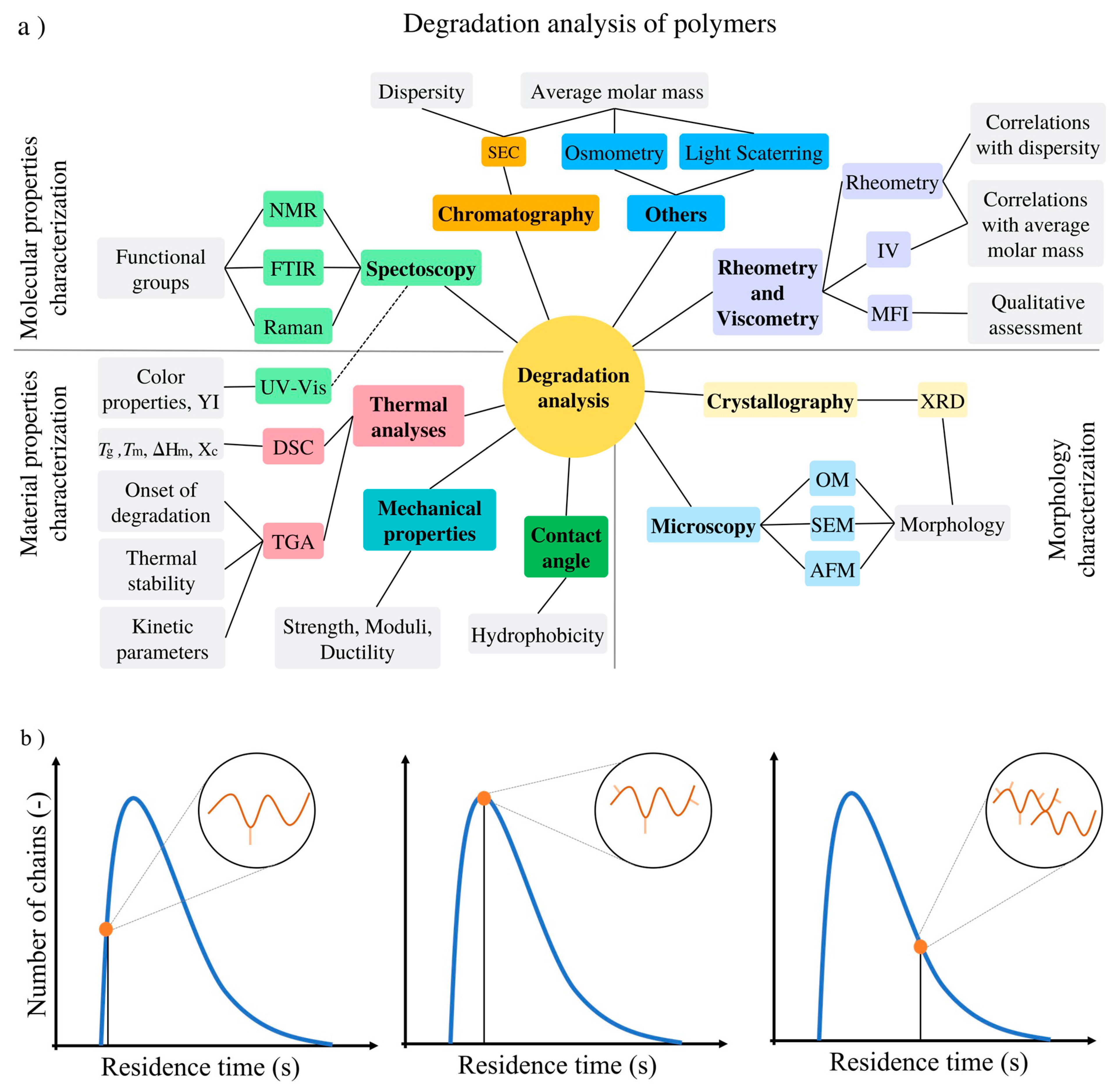
4. Experimental and Modeling Techniques to Assess and Quantify Polymer Degradation
4.1. Molecular Properties Characterization
4.2. Morphology Characterization
4.3. Material Properties Characterization
5. Manufacturing Case Studies to Assess Degradability
5.1. Manufacturing of Polyesters
5.2. Manufacturing of Styrene-Based Materials
5.3. Manufacturing of Polyolefins
5.4. Additive Manufacturing
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jones, R.G.; Wilks, E.S.; Metanomski, W.V.; Kahovec, J.; Hess, M.; Stepto, R.; Kitayama, T. (Eds.) Compendium of Polymer Terminology and Nomenclature; Royal Society of Chemistry: Cambridge, UK, 2009; ISBN 978-0-85404-491-7. [Google Scholar]
- Moens, E.; De Smit, K.; Marien, Y.; Trigilio, A.; Van Steenberge, P.; Van Geem, K.; Dubois, J.-L.; D’hooge, D. Progress in Reaction Mechanisms and Reactor Technologies for Thermochemical Recycling of Poly(methyl methacrylate). Polymers 2020, 12, 1667. [Google Scholar] [CrossRef]
- De Smit, K.; Wieme, T.; Marien, Y.W.; Van Steenberge, P.H.M.; D’hooge, D.R.; Edeleva, M. Multi-scale reactive extrusion modelling approaches to design polymer synthesis, modification and mechanical recycling. React. Chem. Eng. 2022, 7, 245–263. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Sutera, F.; Gulino, E.; Morreale, M. Degradation and Recycling of Films Based on Biodegradable Polymers: A Short Review. Polymers 2019, 11, 651. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Nagai, K. Degradation issues of polymer materials used in railway field. Polym. Degrad. Stab. 2008, 93, 1723–1735. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef] [PubMed]
- Ceretti, D.V.A.; Marien, Y.W.; Edeleva, M.; La Gala, A.; Cardon, L.; D’hooge, D.R. Thermal and Thermal-Oxidative Molecular Degradation of Polystyrene and Acrylonitrile Butadiene Styrene during 3D Printing Starting from Filaments and Pellets. Sustainability 2022, 14, 15488. [Google Scholar] [CrossRef]
- Vilaplana, F.; Karlsson, S.; Ribes-Greus, A. Changes in the microstructure and morphology of high-impact polystyrene subjected to multiple processing and thermo-oxidative degradation. Eur. Polym. J. 2007, 43, 4371–4381. [Google Scholar] [CrossRef]
- de Andrade, M.F.C.; Fonseca, G.; Morales, A.R.; Mei, L.H.I. Mechanical recycling simulation of polylactide using a chain extender. Adv. Polym. Technol. 2018, 37, 2053–2060. [Google Scholar] [CrossRef]
- ISO/ASTM52900-15; Standard Terminology for Additive Manufacturing—General Principles—Terminology (ASTM52900). International Organization for Standardization: Geneva, Switzerland, 2015.
- Bikas, H.; Stavropoulos, P.; Chryssolouris, G. Additive manufacturing methods and modeling approaches: A critical review. Int. J. Adv. Manuf. Technol. 2016, 83, 389–405. [Google Scholar] [CrossRef]
- Tiganis, B.E.; Burn, L.S.; Davis, P.; Hill, A.J. Thermal degradation of acrylonitrile-butadiene-styrene (ABS) blends. Polym. Degrad. Stab. 2002, 76, 425–434. [Google Scholar] [CrossRef]
- Konarzewski, M.; Durejko, T.; Łazińska, M.; Czerwińska, M.; Prasuła, P.; Panowicz, R. Thermo-oxidative aging of the polyoxymethylene (POM), acrylonitrile–butadiene–styrene (ABS) and polycarbonate (PC) polymers—A comparative study. J. Polym. Res. 2022, 29, 236. [Google Scholar] [CrossRef]
- Scott, G. Mechano-chemical degradation and stabilization of polymers. Polym. Eng. Sci. 1984, 24, 1007–1020. [Google Scholar] [CrossRef]
- Hawkins, W.L. Polymer Degradation and Stabilization; Polymers Properties and Applications; Springer: Berlin/Heidelberg, Germany, 1984; ISBN 978-3-642-69378-6. [Google Scholar]
- Gensler, R.; Plummer, C.J.G.; Kausch, H.H.; Kramer, E.; Pauquet, J.R.; Zweifel, H. Thermo-oxidative degradation of isotactic polypropylene at high temperatures: Phenolic antioxidants versus HAS. Polym. Degrad. Stab. 2000, 67, 195–208. [Google Scholar] [CrossRef]
- Gijsman, P. Polymer Stabilization. In Applied Plastics Engineering Handbook; Elsevier: Oxford, UK, 2017; pp. 395–421. ISBN 9780323390408. [Google Scholar]
- Kumar, A.P.; Depan, D.; Tomer, N.S.; Singh, R.P. Nanoscale particles for polymer degradation and stabilization—Trends and future perspectives. Prog. Polym. Sci. 2009, 34, 479–515. [Google Scholar] [CrossRef]
- Chen, J.; Xia, L.; Kong, M.; He, Y.; Lv, Y.; Huang, Y.; Li, G. Optimized design of environmentally-friendly polydopamine nanoparticles for the stabilization of both thermo- and photo-oxidation of polypropylene: Size effects. Polym. Test. 2022, 116, 107795. [Google Scholar] [CrossRef]
- Takacs, K.; Tatraaljai, D.; Pregi, E.; Huszthy, P.; Pukanszky, B. Synthesis and evaluation of a novel natural-based phosphine antioxidant for the thermal stabilization of polyethylene. J. Therm. Anal. Calorim. 2022, 147, 12513–12522. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; Al-Malaika, S.; Arrigo, R.; Morici, E. Novel strategic approach for the thermo- and photo- oxidative stabilization of polyolefin/clay nanocomposites. Polym. Degrad. Stab. 2017, 145, 41–51. [Google Scholar] [CrossRef]
- Shamsuyeva, M.; Endres, H.J. Plastics in the context of the circular economy and sustainable plastics recycling: Comprehensive review on research development, standardization and market. Compos. Part C Open Access 2021, 6, 100168. [Google Scholar] [CrossRef]
- Ghosh, S.K. Circular Economy: Global Perspective; Ghosh, S.K., Ed.; Springer: Singapore, 2020; ISBN 978-981-15-1051-9. [Google Scholar]
- Van Waeleghem, T.; Marchesini, F.H.; Cardon, L.; D’hooge, D.R. Melt exit flow modelling and experimental validation for fused filament fabrication: From Newtonian to non-Newtonian effects. J. Manuf. Process. 2022, 77, 138–150. [Google Scholar] [CrossRef]
- Penumakala, P.K.; Santo, J.; Thomas, A. A critical review on the fused deposition modeling of thermoplastic polymer composites. Compos. Part B Eng. 2020, 201, 108336. [Google Scholar] [CrossRef]
- Turner, B.N.; Strong, R.; Gold, S.A. A review of melt extrusion additive manufacturing processes: I. Process design and modeling. Rapid Prototyp. J. 2014, 20, 192–204. [Google Scholar] [CrossRef]
- Vyavahare, S.; Teraiya, S.; Panghal, D.; Kumar, S. Fused deposition modelling: A review. Rapid Prototyp. J. 2020, 26, 176–201. [Google Scholar] [CrossRef]
- Dezaki, M.L.; Ariffin, M.K.A.M.; Hatami, S. An overview of fused deposition modelling (FDM): Research, development and process optimisation. Rapid Prototyp. J. 2021, 27, 562–582. [Google Scholar] [CrossRef]
- Salifu, S.; Desai, D.; Ogunbiyi, O.; Mwale, K. Recent development in the additive manufacturing of polymer-based composites for automotive structures—A review. Int. J. Adv. Manuf. Technol. 2022, 119, 6877–6891. [Google Scholar] [CrossRef]
- Najmon, J.C.; Raeisi, S.; Tovar, A. Review of additive manufacturing technologies and applications in the aerospace industry. In Additive Manufacturing for the Aerospace Industry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 7–31. ISBN 9780128140635. [Google Scholar]
- Salmi, M. Additive manufacturing processes in medical applications. Materials 2021, 14, 191. [Google Scholar] [CrossRef] [PubMed]
- Paolini, A.; Kollmannsberger, S.; Rank, E. Additive manufacturing in construction: A review on processes, applications, and digital planning methods. Addit. Manuf. 2019, 30, 100894. [Google Scholar] [CrossRef]
- Gonzalez-Gutierrez, J.; Cano, S.; Schuschnigg, S.; Kukla, C.; Sapkota, J.; Holzer, C. Additive manufacturing of metallic and ceramic components by the material extrusion of highly-filled polymers: A review and future perspectives. Materials 2018, 11, 840. [Google Scholar] [CrossRef]
- Song, D.; Xu, Y.; Liu, S.; Wen, L.; Wang, X. Progress of 3D Bioprinting in Organ Manufacturing. Polymers 2021, 13, 3178. [Google Scholar] [CrossRef]
- Janarthanan, G.; Thavasyappan, T.; Hong, S.; Noh, I. Chapter 2. Introduction to Hydrogel Synthesis and Crosslinking Methods for Developing Bioinks for 3D Bioprinting. In Injectable Hydrogels for 3D Bioprinting; Royal Society of Chemistry: Cambridge, UK, 2021; pp. 21–47. [Google Scholar]
- Gijsman, P. Review on the thermo-oxidative degradation of polymers during processing and in service. E-Polymers 2008, 8, 727–760. [Google Scholar] [CrossRef]
- Pickett, J.E.; Coyle, D.J. Hydrolysis kinetics of condensation polymers under humidity aging conditions. Polym. Degrad. Stab. 2013, 98, 1311–1320. [Google Scholar] [CrossRef]
- Kholodovych, V.; Welsh, W.J. Thermal-Oxidative Stability and Degradation of Polymers. In Physical Properties of Polymers Handbook; Mark, J.E., Ed.; Springer: New York, NY, USA, 2007; pp. 927–938. ISBN 978-0-387-31235-4. [Google Scholar]
- Pielichowski, K.; Njuguna, J. Thermal Degradation of Polymeric Materials; Smithers Rapra: Shawbury, UK, 2005; ISBN 9781859574980. [Google Scholar]
- De Smit, K.; Marien, Y.W.; Van Geem, K.M.; Van Steenberge, P.H.M.; D’hooge, D.R. Connecting polymer synthesis and chemical recycling on a chain-by-chain basis: A unified matrix-based kinetic Monte Carlo strategy. React. Chem. Eng. 2020, 5, 1909–1928. [Google Scholar] [CrossRef]
- Gaitanelis, D.; Worrall, C.; Kazilas, M. Detecting, characterising and assessing PEEK’s and CF-PEEK’s thermal degradation in rapid high-temperature processing. Polym. Degrad. Stab. 2022, 204, 110096. [Google Scholar] [CrossRef]
- Carrasco, F.; Santana, O.; Cailloux, J.; Maspoch, M.L. Kinetics of the thermal degradation of poly(lactic acid) obtained by reactive extrusion: Influence of the addition of montmorillonite nanoparticles. Polym. Test. 2015, 48, 69–81. [Google Scholar] [CrossRef]
- Capone, C.; Di Landro, L.; Inzoli, F.; Penco, M.; Sartore, L. Thermal and mechanical degradation during polymer extrusion processing. Polym. Eng. Sci. 2007, 47, 1813–1819. [Google Scholar] [CrossRef]
- Van Steenberge, P.H.M.; Vandenbergh, J.; Reyniers, M.-F.; Junkers, T.; D’hooge, D.R.; Marin, G.B. Kinetic Monte Carlo Generation of Complete Electron Spray Ionization Mass Spectra for Acrylate Macromonomer Synthesis. Macromolecules 2017, 50, 2625–2636. [Google Scholar] [CrossRef]
- Huang, J.B.; Zeng, G.S.; Li, X.S.; Cheng, X.C.; Tong, H. Theoretical studies on bond dissociation enthalpies for model compounds of typical plastic polymers. IOP Conf. Ser. Earth Environ. Sci. 2018, 167, 012029. [Google Scholar] [CrossRef]
- Grunenberg, J. Ill-defined chemical concepts: The problem of quantification. Int. J. Quantum Chem. 2017, 117, e25359. [Google Scholar] [CrossRef]
- Kruse, T.M.; Wong, H.-W.; Broadbelt, L.J. Modeling the Evolution of the Full Polystyrene Molecular Weight Distribution during Polystyrene Pyrolysis. Ind. Eng. Chem. Res. 2003, 42, 2722–2735. [Google Scholar] [CrossRef]
- Sivalingam, G.; Madras, G. Thermal degradation of poly (ε-caprolactone). Polym. Degrad. Stab. 2003, 80, 11–16. [Google Scholar] [CrossRef]
- Ueno, T.; Nakashima, E.; Takeda, K. Quantitative analysis of random scission and chain-end scission in the thermal degradation of polyethylene. Polym. Degrad. Stab. 2010, 95, 1862–1869. [Google Scholar] [CrossRef]
- McNeill, I.C. Thermal degradation mechanisms of some addition polymers and copolymers. J. Anal. Appl. Pyrolysis 1997, 40–41, 21–41. [Google Scholar] [CrossRef]
- Aoyagi, Y.; Yamashita, K.; Doi, Y. Thermal degradation of poly[(R)-3-hydroxybutyrate], poly[ε-caprolactone], and poly[(S)-lactide]. Polym. Degrad. Stab. 2002, 76, 53–59. [Google Scholar] [CrossRef]
- Manring, L.E. Thermal degradation of poly(methyl methacrylate). 4. Random side-group scission. Macromolecules 1991, 24, 3304–3309. [Google Scholar] [CrossRef]
- Ray, S.; Cooney, R.P. Thermal Degradation of Polymer and Polymer Composites. In Handbook of Environmental Degradation of Materials; Elsevier: Oxford, UK, 2018; pp. 185–206. ISBN 9780323524735. [Google Scholar]
- David, C. Thermal Degradation of Polymers. In Comprehensive Chemical Kinetics; Elsevier: Amsterdam, The Netherlands, 1975; pp. 1–173. [Google Scholar]
- Sohma, J. Mechano-radical formation in polypropylene by an extruder action and its after-effects. Colloid Polym. Sci. 1992, 270, 1060–1065. [Google Scholar] [CrossRef]
- Bamford, C.H.; Tipper, C.F.H. (Eds.) Chemical Kinetics: Polymer Degradation; Elsevier: Amsterdam, The Netherlands, 1975; Volume 14, ISBN 3904144987. [Google Scholar]
- Gol’dberg, V.M.; Zaikov, G.E. Kinetics of mechanical degradation in melts under model conditions and during processing of polymers-A review. Polym. Degrad. Stab. 1987, 19, 221–250. [Google Scholar] [CrossRef]
- Edeleva, M.; Zafeiris, K.; Fiorio, R.; D’hooge, D.R.; Chariditis, C.A.; Cardon, L. Increasing sustainability of additive manufacturing techniques: Minimization of degradation and optimization of the printing parameters, Accepted. In Handbook of Circular Plastics Economy; Springer: Berlin/Heidelberg, Germany, 2023; in press. [Google Scholar]
- Zweifel, H. Stabilization of Polymeric Materials; Springer: Berlin/Heidelberg, Germany, 1998; Volume 33, ISBN 978-3-642-80307-9. [Google Scholar]
- Bolland, J.L. Kinetic studies in the chemistry of rubber and related materials. I. The thermal oxidation of ethyl linoleate. Proc. R. Soc. London. Ser. A. Math. Phys. Sci. 1946, 186, 218–236. [Google Scholar] [CrossRef]
- Bolland, J.L.; Gee, G. Kinetic studies in the chemistry of rubber and related materials. III. Thermochemistry and mechanisms of olefin oxidation. Trans. Faraday Soc. 1946, 42, 244. [Google Scholar] [CrossRef]
- Bolland, J.L. Kinetic studies in the chemistry of rubber and related materials. VI. The benzoyl peroxide-catalysed oxidation of ethyl linoleate. Trans. Faraday Soc. 1948, 44, 669–677. [Google Scholar] [CrossRef]
- Gryn’ova, G.; Hodgson, J.L.; Coote, M.L. Revising the mechanism of polymer autooxidation. Org. Biomol. Chem. 2011, 9, 480–490. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, N. Mechanistic implications of plastic degradation. Polym. Degrad. Stab. 2008, 93, 561–584. [Google Scholar] [CrossRef]
- Nguyen, T.Q. Polymer Degradation and Stabilization. In Handbook of Polymer Reaction Engineering; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2008; pp. 757–831. ISBN 9783527310142. [Google Scholar]
- Bracco, P.; Costa, L.; Luda, M.P.; Billingham, N. A review of experimental studies of the role of free-radicals in polyethylene oxidation. Polym. Degrad. Stab. 2018, 155, 67–83. [Google Scholar] [CrossRef]
- Homstrom, A.; Sorvik, E.M. Thermooxidative Degradation of Polyethylene—1 and 2. Structural Changes Occurring in Low-Density Polyethylene, High-Density Polyethylene, and Tetratetracontane Heated in Air. J. Polym. Sci. Polym. Chem. Ed. 1978, 16, 2555–2586. [Google Scholar] [CrossRef]
- Adeniyi, J.B.; Kolawole, E.G. Thermal and photo-degradation of unstabilized ABS. Eur. Polym. J. 1984, 20, 43–47. [Google Scholar] [CrossRef]
- Vert, M.; Li, S.; Garreau, H.; Mauduit, J.; Boustta, M.; Schwach, G.; Engel, R.; Coudane, J. Complexity of the hydrolytic degradation of aliphatic polyesters. Angew. Makromol. Chem. 1997, 247, 239–253. [Google Scholar] [CrossRef]
- Algarni, M.; Ghazali, S. Comparative Study of the Sensitivity of PLA, ABS, PEEK, and PETG’s Mechanical Properties to FDM Printing Process Parameters. Crystals 2021, 11, 995. [Google Scholar] [CrossRef]
- Peterson, A.M. Review of acrylonitrile butadiene styrene in fused filament fabrication: A plastics engineering-focused perspective. Addit. Manuf. 2019, 27, 363–371. [Google Scholar] [CrossRef]
- Hsueh, M.-H.; Lai, C.-J.; Wang, S.-H.; Zeng, Y.-S.; Hsieh, C.-H.; Pan, C.-Y.; Huang, W.-C. Effect of Printing Parameters on the Thermal and Mechanical Properties of 3D-Printed PLA and PETG, Using Fused Deposition Modeling. Polymers 2021, 13, 1758. [Google Scholar] [CrossRef]
- Cojocaru, V.; Frunzaverde, D.; Miclosina, C.O.; Marginean, G. The Influence of the Process Parameters on the Mechanical Properties of PLA Specimens Produced by Fused Filament Fabrication—A Review. Polymers 2022, 14, 886. [Google Scholar] [CrossRef]
- Guessasma, S.; Belhabib, S.; Nouri, H. Printability and tensile performance of 3D printed polyethylene terephthalate glycol using fused deposition modelling. Polymers 2019, 11, 1220. [Google Scholar] [CrossRef]
- Özen, A.; Auhl, D.; Völlmecke, C.; Kiendl, J.; Abali, B.E. Optimization of Manufacturing Parameters and Tensile Specimen Geometry for Fused Deposition Modeling (FDM) 3D-Printed PETG. Materials 2021, 14, 2556. [Google Scholar] [CrossRef]
- Zanjanijam, A.R.; Major, I.; Lyons, J.G.; Lafont, U.; Devine, D.M. Fused Filament Fabrication of PEEK: A Review of Process-Structure-Property Relationships. Polymers 2020, 12, 1665. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, M.; Ghidini, T.; Cecchini, F.; Brandao, A.; Nanni, F. Additive layer manufacturing of poly (ether ether ketone) via FDM. Compos. Part B 2018, 145, 162–172. [Google Scholar] [CrossRef]
- Yang, C.; Tian, X.; Li, D.; Cao, Y.; Zhao, F.; Shi, C. Influence of thermal processing conditions in 3D printing on the crystallinity and mechanical properties of PEEK material. J. Mater. Process. Technol. 2017, 248, 1–7. [Google Scholar] [CrossRef]
- Arif, M.F.; Kumar, S.; Varadarajan, K.M.; Cantwell, W.J. Performance of biocompatible PEEK processed by fused deposition additive manufacturing. Mater. Des. 2018, 146, 249–259. [Google Scholar] [CrossRef]
- Deng, X.; Zeng, Z.; Peng, B.; Yan, S.; Ke, W. Mechanical properties optimization of poly-ether-ether-ketone via fused deposition modeling. Materials 2018, 11, 216. [Google Scholar] [CrossRef]
- Geng, P.; Zhao, J.; Wu, W.; Ye, W.; Wang, Y.; Wang, S.; Zhang, S. Effects of extrusion speed and printing speed on the 3D printing stability of extruded PEEK filament. J. Manuf. Process. 2019, 37, 266–273. [Google Scholar] [CrossRef]
- Schirmeister, C.G.; Hees, T.; Licht, E.H.; Mülhaupt, R. 3D printing of high density polyethylene by fused filament fabrication. Addit. Manuf. 2019, 28, 152–159. [Google Scholar] [CrossRef]
- Carneiro, O.S.; Silva, A.F.; Gomes, R. Fused deposition modeling with polypropylene. Mater. Des. 2015, 83, 768–776. [Google Scholar] [CrossRef]
- Charlon, S.; Le Boterff, J.; Soulestin, J. Fused filament fabrication of polypropylene: Influence of the bead temperature on adhesion and porosity. Addit. Manuf. 2021, 38, 4–11. [Google Scholar] [CrossRef]
- Spoerk, M.; Gonzalez-Gutierrez, J.; Lichal, C.; Cajner, H.; Berger, G.R.; Schuschnigg, S.; Cardon, L.; Holzer, C. Optimisation of the adhesion of polypropylene-based materials during extrusion-based additive manufacturing. Polymers 2018, 10, 490. [Google Scholar] [CrossRef]
- Liao, G.; Li, Z.; Luan, C.; Wang, Z.; Yao, X.; Fu, J. Additive Manufacturing of Polyamide 66: Effect of Process Parameters on Crystallinity and Mechanical Properties. J. Mater. Eng. Perform. 2022, 31, 191–200. [Google Scholar] [CrossRef]
- Qi, S.; Gao, X.; Su, Y.; Dong, X.; Cavallo, D.; Wang, D. Correlation between welding behavior and mechanical anisotropy of long chain polyamide 12 manufactured with fused filament fabrication. Polymer 2021, 213, 123318. [Google Scholar] [CrossRef]
- Vilaplana, F.; Karlsson, S.; Ribes-Greus, A.; Schade, C.; Nestle, N. NMR relaxation reveals modifications in rubber phase dynamics during long-term degradation of high-impact polystyrene (HIPS). Polymer 2011, 52, 1410–1416. [Google Scholar] [CrossRef]
- Bai, X.; Liang, P.; Zhang, M.; Gong, S.; Zhao, L. Effects of Reprocessing on Acrylonitrile–Butadiene–Styrene and Additives. J. Polym. Environ. 2022, 30, 1803–1819. [Google Scholar] [CrossRef]
- Gonçalves, L.M.G.; Rigolin, T.R.; Frenhe, B.M.; Bettini, S.H.P. On the recycling of a biodegradable polymer: Multiple extrusion of poly (Lactic acid). Mater. Res. 2020, 23, 1–7. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Taheri, S.; Zadhoush, A.; Mehrabani-Zeinabad, A. Hydrolytic degradation of poly(ethylene terephthalate). J. Appl. Polym. Sci. 2007, 103, 2304–2309. [Google Scholar] [CrossRef]
- Saikrishnan, S.; Jubinville, D.; Tzoganakis, C.; Mekonnen, T.H. Thermo-mechanical degradation of polypropylene (PP) and low-density polyethylene (LDPE) blends exposed to simulated recycling. Polym. Degrad. Stab. 2020, 182, 109390. [Google Scholar] [CrossRef]
- González-González, V.A.; Neira-Velázquez, G.; Angulo-Sánchez, J.L. Polypropylene chain scissions and molecular weight changes in multiple extrusion. Polym. Degrad. Stab. 1998, 60, 33–42. [Google Scholar] [CrossRef]
- Andersson, T.; Stålbom, B.; Wesslén, B. Degradation of polyethylene during extrusion. II. Degradation of low-density polyethylene, linear low-density polyethylene, and high-density polyethylene in film extrusion. J. Appl. Polym. Sci. 2004, 91, 1525–1537. [Google Scholar] [CrossRef]
- Jin, H.; Gonzalez-Gutierrez, J.; Oblak, P.; Zupančič, B.; Emri, I. The effect of extensive mechanical recycling on the properties of low density polyethylene. Polym. Degrad. Stab. 2012, 97, 2262–2272. [Google Scholar] [CrossRef]
- Phillips, R.; Glauser, T.; Månson, J.A.E. Thermal stability of PEEK/carbon fiber in air and its influence on consolidation. Polym. Compos. 1997, 18, 500–508. [Google Scholar] [CrossRef]
- Pascual, A.; Toma, M.; Tsotra, P.; Grob, M.C. On the stability of PEEK for short processing cycles at high temperatures and oxygen-containing atmosphere. Polym. Degrad. Stab. 2019, 165, 161–169. [Google Scholar] [CrossRef]
- Su, K.H.; Lin, J.H.; Lin, C.C. Influence of reprocessing on the mechanical properties and structure of polyamide 6. J. Mater. Process. Technol. 2007, 192–193, 532–538. [Google Scholar] [CrossRef]
- Filippone, G.; Carroccio, S.C.; Mendichi, R.; Gioiella, L.; Dintcheva, N.T.; Gambarotti, C. Time-resolved rheology as a tool to monitor the progress of polymer degradation in the melt state—Part I: Thermal and thermo-oxidative degradation of polyamide 11. Polymer 2015, 72, 134–141. [Google Scholar] [CrossRef]
- Suzuki, M.; Wilkie, C.A. The thermal degradation of acrylonitrile-butadiene-styrene terpolymei as studied by TGA/FTIR. Polym. Degrad. Stab. 1995, 47, 217–221. [Google Scholar] [CrossRef]
- Allen, N.S.; Edge, M.; Wilkinson, A.; Liauw, C.M.; Mourelatou, D.; Barrio, J.; Martínez-Zaporta, M.A. Degradation and stabilization of styrene-ethylene-butadiene-styrene (SEBS) block copolymer. Polym. Degrad. Stab. 2000, 71, 113–122. [Google Scholar] [CrossRef]
- Boyle, D.J.; Gesner, B.D. Aging of polyblends. J. Appl. Polym. Sci. 1968, 12, 1193–1197. [Google Scholar] [CrossRef]
- Fiorio, R.; D’hooge, D.R.; Ragaert, K.; Cardon, L. A statistical analysis on the effect of antioxidants on the thermal-oxidative stability of commercial massand emulsion-polymerized ABS. Polymers 2018, 11, 25. [Google Scholar] [CrossRef]
- Casale, A.; Salvatore, O.; Pizzigoni, G. Measurement of aging effects of ABS polymers. Polym. Eng. Sci. 1975, 15, 286–293. [Google Scholar] [CrossRef]
- Vadori, R.; Misra, M.; Mohanty, A.K. Studies on the reaction of acrylonitrile butadiene styrene to melt processing conditions. Macromol. Mater. Eng. 2015, 300, 750–757. [Google Scholar] [CrossRef]
- Blom, H.; Yeh, R.; Wojnarowski, R.; Ling, M. Detection of degradation of ABS materials via DSC. Thermochim. Acta 2006, 442, 64–66. [Google Scholar] [CrossRef]
- Shimada, J.; Kabuki, K. The mechanism of oxidative degradation of ABS resin. Part I. The mechanism of thermooxidative degradation. J. Appl. Polym. Sci. 1968, 12, 655–669. [Google Scholar] [CrossRef]
- Adeniyi, J.B. Clarification and discussion of chemical transformations involved in thermal and photo-oxidative degradation of ABS. Eur. Polym. J. 1984, 20, 291–299. [Google Scholar] [CrossRef]
- Scaffaro, R.; Botta, L.; Di Benedetto, G. Physical properties of virgin-recycled ABS blends: Effect of post-consumer content and of reprocessing cycles. Eur. Polym. J. 2012, 48, 637–648. [Google Scholar] [CrossRef]
- Charles, A.; Bassan, P.M.; Mueller, T.; Elkaseer, A.; Scholz, S.G. On the Assessment of Thermo-mechanical Degradability of Multi-recycled ABS Polymer for 3D Printing Applications. In Smart Innovation, Systems and Technologies; Springer: Singapore, 2019; Volume 155, pp. 363–373. ISBN 9789811392702. [Google Scholar]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Improvement of thermal stability, rheological and mechanical properties of PLA, PBAT and their blends by reactive extrusion with functionalized epoxy. Polym. Degrad. Stab. 2012, 97, 1898–1914. [Google Scholar] [CrossRef]
- Speranza, V.; De Meo, A.; Pantani, R. Thermal and hydrolytic degradation kinetics of PLA in the molten state. Polym. Degrad. Stab. 2014, 100, 37–41. [Google Scholar] [CrossRef]
- Balani, S.B.; Chabert, F.; Nassiet, V.; Cantarel, A. Influence of printing parameters on the stability of deposited beads in fused filament fabrication of poly(lactic) acid. Addit. Manuf. 2019, 25, 112–121. [Google Scholar] [CrossRef]
- Wang, S.; Daelemans, L.; D’hooge, D.R.; Couck, L.; Van Den Broeck, W.; Cornillie, P.; Gou, M.; De Clerck, K.; Cardon, L. Lifting the quality of fused filament fabrication of polylactic acid based composites. Compos. Part B Eng. 2021, 210, 108613. [Google Scholar] [CrossRef]
- Zhao, P.; Rao, C.; Gu, F.; Sharmin, N.; Fu, J. Close-looped recycling of polylactic acid used in 3D printing: An experimental investigation and life cycle assessment. J. Clean. Prod. 2018, 197, 1046–1055. [Google Scholar] [CrossRef]
- McNeill, I.C.; Leiper, H.A. Degradation studies of some polyesters and polycarbonates-2. Polylactide: Degradation under isothermal conditions, thermal degradation mechanism and photolysis of the polymer. Polym. Degrad. Stab. 1985, 11, 309–326. [Google Scholar] [CrossRef]
- Kopinke, F.D.; Remmler, M.; Mackenzie, K.; Möder, M.; Wachsen, O. Thermal decomposition of biodegradable polyesters—II. Poly(lactic acid). Polym. Degrad. Stab. 1996, 53, 329–342. [Google Scholar] [CrossRef]
- Amorin, N.S.Q.S.; Rosa, G.; Alves, J.F.; Gonçalves, S.P.C.; Franchetti, S.M.M.; Fechine, G.J.M. Study of thermodegradation and thermostabilization of poly(lactide acid) using subsequent extrusion cycles. J. Appl. Polym. Sci. 2014, 131, 1–8. [Google Scholar] [CrossRef]
- Schyns, Z.O.G.; Shaver, M.P. Mechanical Recycling of Packaging Plastics: A Review. Macromol. Rapid Commun. 2021, 42, e2000415. [Google Scholar] [CrossRef] [PubMed]
- Holmström, A.; Sörvik, E. Thermal degradation of polyethylene in a nitrogen atmosphere of low oxygen content. I. Changes in molecular weight distribution. J. Chromatogr. A 1970, 53, 95–108. [Google Scholar] [CrossRef]
- Holmström, A.; Sörvik, E.M. Thermal degradation of polyethylene in a nitrogen atmosphere of low oxygen content. II. Structural changes occuring in low-density polyethylene at an oxygen content less than 0.0005%. J. Appl. Polym. Sci. 1974, 18, 761–778. [Google Scholar] [CrossRef]
- Holmström, A.; Sörvik, E.M. Thermal degradation of polyethylene in a nitrogen atmosphere of low oxygen content. III. Structural changes occuring in low-density polyethylene at oxygen contents below 1.2%. J. Appl. Polym. Sci. 1974, 18, 779–804. [Google Scholar] [CrossRef]
- Adams, J.H. Analysis of the nonvolatile oxidation products of polypropylene I. Thermal oxidation. J. Polym. Sci. Part A-1 Polym. Chem. 1970, 8, 1077–1090. [Google Scholar] [CrossRef]
- Adams, J.H.; Goodrich, J.E. Analysis of nonvolatile oxidation products of polypropylene. II. Process degradation. J. Polym. Sci. Part A-1 Polym. Chem. 1970, 8, 1269–1277. [Google Scholar] [CrossRef]
- De Keer, L.; Kilic, K.I.; Van Steenberge, P.H.M.; Daelemans, L.; Kodura, D.; Frisch, H.; De Clerck, K.; Reyniers, M.-F.; Barner-Kowollik, C.; Dauskardt, R.H.; et al. Computational prediction of the molecular configuration of three-dimensional network polymers. Nat. Mater. 2021, 20, 1422–1430. [Google Scholar] [CrossRef]
- Figueira, F.L.; Wu, Y.-Y.; Zhou, Y.-N.; Luo, Z.-H.; Van Steenberge, P.H.M.; D’hooge, D.R. Coupled matrix kinetic Monte Carlo simulations applied for advanced understanding of polymer grafting kinetics. React. Chem. Eng. 2021, 6, 640–661. [Google Scholar] [CrossRef]
- Wang, L.; Broadbelt, L.J. Kinetics of segment formation in nitroxide-mediated controlled radical polymerization: Comparison with classic theory. Macromolecules 2010, 43, 2228–2235. [Google Scholar] [CrossRef]
- Ali Parsa, M.; Kozhan, I.; Wulkow, M.; Hutchinson, R.A. Modeling of functional group distribution in copolymerization: A comparison of deterministic and stochastic approaches. Macromol. Theory Simul. 2014, 23, 207–217. [Google Scholar] [CrossRef]
- Zhou, Y.N.; Luo, Z.H. State-of-the-Art and Progress in Method of Moments for the Model-Based Reversible-Deactivation Radical Polymerization. Macromol. React. Eng. 2016, 10, 516–534. [Google Scholar] [CrossRef]
- Mastan, E.; Li, X.; Zhu, S. Modeling and theoretical development in controlled radical polymerization. Prog. Polym. Sci. 2015, 45, 71–101. [Google Scholar] [CrossRef]
- Van Steenberge, P.H.M.; Sedlacek, O.; Hernández-Ortiz, J.C.; Verbraeken, B.; Reyniers, M.F.; Hoogenboom, R.; D’hooge, D.R. Visualization and design of the functional group distribution during statistical copolymerization. Nat. Commun. 2019, 10, 3641. [Google Scholar] [CrossRef]
- Al-Harthi, M.A.; Masihullah, J.K.; Abbasi, S.H.; Soares, J.B.P. Dynamic Monte Carlo simulation of ATRP in a batch reactor. Macromol. Theory Simul. 2009, 18, 307–316. [Google Scholar] [CrossRef]
- Ballard, N.; Hamzehlou, S.; Ruipérez, F.; Asua, J.M. On the Termination Mechanism in the Radical Polymerization of Acrylates. Macromol. Rapid Commun. 2016, 37, 1364–1368. [Google Scholar] [CrossRef]
- Van Steenberge, P.H.M.; Hutchinson, R.A. Design of 2-hydroxyethyl methacrylate-functional macromonomer dispersants by semi-batch cobalt chain transfer polymerization. AIChE J. 2019, 65, e16723. [Google Scholar] [CrossRef]
- D’hooge, D.R. In Silico Tracking of Individual Species Accelerating Progress in Macromolecular Engineering and Design. Macromol. Rapid Commun. 2018, 39, e1800057. [Google Scholar] [CrossRef]
- Pladis, P.; Baltsas, A.; Kiparissides, C. A Comprehensive Model for the Simulation of Ethylene Decomposition in High-Pressure LDPE Autoclaves. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2017; Volume 40, pp. 337–342. ISBN 9780444639653. [Google Scholar]
- Edeleva, M.; Van Steenberge, P.H.M.; Sabbe, M.K.; D’hooge, D.R. Connecting gas-phase computational chemistry to condensed phase kinetic modeling: The state-of-the-art. Polymers 2021, 13, 3027. [Google Scholar] [CrossRef]
- De Smit, K.; Edeleva, M.; Trigilio, A.D.; Marien, Y.W.; Van Steenberge, P.H.M.; D’hooge, D.R. Kinetic Monte Carlo residence time distributions and kinetics in view of extrusion-based polymer modification and recycling. React. Chem. Eng. 2023, 8, 563–576. [Google Scholar] [CrossRef]
- Rex, I.; Graham, B.A.; Thompson, M.R. Studying single-pass degradation of a high-density polyethylene in an injection molding process. Polym. Degrad. Stab. 2005, 90, 136–146. [Google Scholar] [CrossRef]
- Zatloukal, M.; Muras, J.; Šimek, L. Influence of the repeated extrusion on the degradation of polyethylene. Structural changes in low density polyethylene. Eur. Polym. J. 2008, 44, 2652–2658. [Google Scholar] [CrossRef]
- Dealy, J.M.; Larson, R.G. Structure and Rheology of Molten Polymers; Carl Hanser Verlag GmbH & Co. KG: München, Germany, 2006; ISBN 978-3-446-21771-3. [Google Scholar]
- Malkin, A.Y.; Kulichikhin, S.G. Rheokinetics of free-radical polymerization. Polymer 1984, 25, 778–784. [Google Scholar] [CrossRef]
- Malkin, A.Y. Rheology in polymerization processes. Polym. Eng. Sci. 1980, 20, 1035–1044. [Google Scholar] [CrossRef]
- Aulagner, P.; Ainser, A.; Carrot, C.; Guillet, J. Free radical degradation of polypropylene in the bulk: Coupling of kinetic and rheological models. J. Polym. Eng. 2000, 20, 381–401. [Google Scholar] [CrossRef]
- Berzin, F.; Vergnes, B.; Delamare, L. Rheological behavior of controlled-rheology polypropylenes obtained by peroxide-promoted degradation during extrusion: Comparison between homopolymer and copolymer. J. Appl. Polym. Sci. 2001, 80, 1243–1252. [Google Scholar] [CrossRef]
- Tuminello, W.H.; Treat, T.A.; English, A.D. Poly(tetrafluoroethylene): Molecular Weight Distributions and Chain Stiffness. Macromolecules 1988, 21, 2606–2610. [Google Scholar] [CrossRef]
- Tuminello, W.H. Molecular weight distributions of tetrafluoroethylene-hexafluoropropylene copolymers. Polym. Eng. Sci. 1989, 29, 645–653. [Google Scholar] [CrossRef]
- Ponnamma, D.; Rouxel, D.; Thomas, S. Spectroscopy—Introducing the advantages and application areas in polymer nanocomposites. In Spectroscopy of Polymer Nanocomposites; Elsevier: Oxford, UK, 2016; pp. 1–14. ISBN 9780323401838. [Google Scholar]
- Mylläri, V.; Ruoko, T.P.; Syrjälä, S. A comparison of rheology and FTIR in the study of polypropylene and polystyrene photodegradation. J. Appl. Polym. Sci. 2015, 132, 1–6. [Google Scholar] [CrossRef]
- Scheirs, J. Compositional and Failure Analysis of Polymers: A Practical Approach; John Wiley & Sons: Chichester, UK, 2000. [Google Scholar]
- Guo, X.; Lin, Z.; Wang, Y.; He, Z.; Wang, M.; Jin, G. In-line monitoring the degradation of polypropylene under multiple extrusions based on Raman spectroscopy. Polymers 2019, 11, 1698. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yang, R.; Iervolino, R.; Barbera, S. Changes of chemical structure and mechanical property levels during thermo-oxidative aging of NBR. Rubber Chem. Technol. 2013, 86, 591–603. [Google Scholar] [CrossRef]
- Harris, D.J.; Assink, R.A.; Gillen, K.T. 1H T2-NMR monitoring of crosslinked polyolefin aging. J. Appl. Polym. Sci. 2003, 90, 2578–2582. [Google Scholar] [CrossRef]
- Hoshino, A.; Tsuji, M.; Fukuda, K.; Nonagase, M.; Sawada, H.; Kimura, M. Changes in molecular structure of biodegradable plastics during degradation in soils estimated by FT-IR and NMR. Soil Sci. Plant Nutr. 2002, 48, 469–473. [Google Scholar] [CrossRef]
- Vaillant, D.; Lacoste, J.; Dauphin, G. The oxidation mechanism of polypropylene: Contribution of 13C-NMR spectroscopy. Polym. Degrad. Stab. 1994, 45, 355–360. [Google Scholar] [CrossRef]
- Celina, M.C.; Linde, E.; Martinez, E. Carbonyl Identification and Quantification Uncertainties for Oxidative Polymer Degradation. Polym. Degrad. Stab. 2021, 188, 109550. [Google Scholar] [CrossRef]
- Cheng, H.N.; English, A.D. Advances in the NMR Spectroscopy of Polymers: An Overview. In NMR Spectroscopy of Polymers in Solution and in the Solid State; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2002; Volume 834, pp. 3–20. [Google Scholar]
- Miyoshi, T. Characterization of polymers by NMR. In Molecular Characterization of Polymers; Elsevier: Amsterdam, The Netherlands, 2021; pp. 409–440. ISBN 9780128197684. [Google Scholar]
- Cáceres, C.A.; Zborowski, L.; Canevarolo, S.V. Thermo-mechanical degradation and VOC emission of unstabilized and stabilized polypropylene copolymer during multiple extrusions. Mater. Res. 2011, 14, 569–575. [Google Scholar] [CrossRef]
- Bai, X.; Isaac, D.H.; Smith, K. Reprocessing acrylonitrile–butadiene–styrene plastics: Structure–property relationships. Polym. Eng. Sci. 2007, 47, 120–130. [Google Scholar] [CrossRef]
- Chatterjee, N.; Basu, S.; Palit, S.K.; Maiti, M.M. An XRD characterization of the thermal degradation of polyacrylonitrile. J. Polym. Sci. Part B Polym. Phys. 1995, 33, 1705–1712. [Google Scholar] [CrossRef]
- Vonka, M.; Kosek, J. Modelling the morphology evolution of polymer materials undergoing phase separation. Chem. Eng. J. 2012, 207–208, 895–905. [Google Scholar] [CrossRef]
- Rimez, B.; Rahier, H.; Van Assche, G.; Artoos, T.; Biesemans, M.; Van Mele, B. The thermal degradation of poly (vinyl acetate) and poly (ethylene- co -vinyl acetate), Part I: Experimental study of the degradation mechanism. Polym. Degrad. Stab. 2008, 93, 800–810. [Google Scholar] [CrossRef]
- Thébault, M.; Pizzi, A.; Essawy, H.A.; Barhoum, A.; Van Assche, G. Isocyanate free condensed tannin-based polyurethanes. Eur. Polym. J. 2015, 67, 513–526. [Google Scholar] [CrossRef]
- Howell, B.A.; Cui, Y.; Priddy, D.B. Assessment of the thermal degradation characteristics of isomeric poly(styrene)s using TG, TG/MS and TG/GC/MS. Thermochim. Acta 2003, 396, 167–177. [Google Scholar] [CrossRef]
- Jouan, X.; Gardette, J.L. Photo-oxidation of ABS: Part 2-Origin of the photodiscoloration on irradiation at long wavelengths. Polym. Degrad. Stab. 1992, 36, 91–96. [Google Scholar] [CrossRef]
- Wang, Y.; Steinhoff, B.; Brinkmann, C.; Alig, I. In-line monitoring of the thermal degradation of poly(l-lactic acid) during melt extrusion by UV-vis spectroscopy. Polymer 2008, 49, 1257–1265. [Google Scholar] [CrossRef]
- Fiorio, R.; Díez, S.V.; Sánchez, A.; D’hooge, D.R.; Cardon, L. Influence of different stabilization systems and multiple ultraviolet a (UVA) aging/recycling steps on physicochemical, mechanical, colorimetric, and thermal-oxidative properties of ABS. Materials 2020, 13, 212. [Google Scholar] [CrossRef]
- Diaz, C.M.; Gao, X.; Robisson, A.; Amarante, M.; Zhu, S.S. Effect of hydrolytic degradation on the mechanical property of a thermoplastic polyether ester elastomer. Polym. Degrad. Stab. 2018, 155, 35–42. [Google Scholar] [CrossRef]
- Maïza, S.; Lefebvre, X.; Brusselle-Dupend, N.; Klopffer, M.H.; Cangémi, L.; Castagnet, S.; Grandidier, J.C. Physicochemical and mechanical degradation of polyamide 11 induced by hydrolysis and thermal aging. J. Appl. Polym. Sci. 2019, 136, 47628. [Google Scholar] [CrossRef]
- Hurley, C.R.; Leggett, G.J. Quantitative investigation of the photodegradation of polyethylene terephthalate film by friction force microscopy, contact-angle goniometry, and X-ray photoelectron spectroscopy. ACS Appl. Mater. Interfaces 2009, 1, 1688–1697. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Rayón, E.; López, J.; Arrieta, M.P. Enhancing the Thermal Stability of Polypropylene by Blending with Low Amounts of Natural Antioxidants. Macromol. Mater. Eng. 2019, 304, 1900379. [Google Scholar] [CrossRef]
- Taubner, V.; Shishoo, R. Influence of processing parameters on the degradation of poly(L-lactide) during extrusion. J. Appl. Polym. Sci. 2001, 79, 2128–2135. [Google Scholar] [CrossRef]
- Mysiukiewicz, O.; Barczewski, M.; Skórczewska, K.; Matykiewicz, D. Correlation between processing parameters and degradation of different polylactide grades during twin-screw extrusion. Polymers 2020, 12, 1333. [Google Scholar] [CrossRef]
- Aldhafeeri, T.; Alotaibi, M.; Barry, C.F. Impact of Melt Processing Conditions on the Degradation of Polylactic Acid. Polymers 2022, 14, 2790. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Santos, E.; Araújo, A.; Fechine, G.J.M.; Machado, A.V.; Botelho, G. The role of shear and stabilizer on PLA degradation. Polym. Test. 2016, 51, 109–116. [Google Scholar] [CrossRef]
- Spinacé, M.A.S.; De Paoli, M.A. Characterization of poly(ethylene terephthalate) after multiple processing cycles. J. Appl. Polym. Sci. 2001, 80, 20–25. [Google Scholar] [CrossRef]
- Al-AbdulRazzak, S.; Jabarin, S.A. Processing characteristics of poly(ethylene terephthalate): Hydrolytic and thermal degradation. Polym. Int. 2002, 51, 164–173. [Google Scholar] [CrossRef]
- Whitlock, L.R.; Porter, R.S. The source of degradation during extrusion of polystyrene. J. Appl. Polym. Sci. 1973, 17, 2761–2770. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Valenza, A. Long-term thermomechanical degradation of molten polystyrene. Polym. Degrad. Stab. 1985, 13, 105–111. [Google Scholar] [CrossRef]
- Farahanchi, A.; Malloy, R.; Sobkowicz, M.J. Effects of ultrahigh speed twin screw extrusion on the thermal and mechanical degradation of polystyrene. Polym. Eng. Sci. 2016, 56, 743–751. [Google Scholar] [CrossRef]
- Karahaliou, E.-K.; Tarantili, P.A. Stability of ABS compounds subjected to repeated cycles of extrusion processing. Polym. Eng. Sci. 2009, 49, 2269–2275. [Google Scholar] [CrossRef]
- Boldizar, A.; Möller, K. Degradation of ABS during repeated processing and accelerated ageing. Polym. Degrad. Stab. 2003, 81, 359–366. [Google Scholar] [CrossRef]
- Salari, D.; Ranjbar, H. Study on the Recycling of ABS Resins: Simulation of Reprocessing and Thermo-oxidation. Iran. Polym. J. 2008, 17, 599–610. [Google Scholar]
- Kalfoglou, N.K.; Chaffey, C.E. Effects of extrusion on the structure and properties of high-impact polystyrene. Polym. Eng. Sci. 1979, 19, 552–557. [Google Scholar] [CrossRef]
- Vilaplana, F.; Ribes-Greus, A.; Karlsson, S. Degradation of recycled high-impact polystyrene. Simulation by reprocessing and thermo-oxidation. Polym. Degrad. Stab. 2006, 91, 2163–2170. [Google Scholar] [CrossRef]
- Parres, F.; Crespo, J.E. Degradation of high-impact polystyrene with processing and its recovery via the addition of styrene-butadiene rubber and styrene-ethylene-butylene-styrene block copolymer. J. Appl. Polym. Sci. 2011, 121, 574–581. [Google Scholar] [CrossRef]
- Hinsken, H.; Moss, S.; Pauquet, J.; Zweifela, H. Degradation of Polyolefins during Melt Processing. Polym. Degrad. Stab. 1991, 34, 279–293. [Google Scholar] [CrossRef]
- Da Costa, H.M.; Ramos, V.D.; Rocha, M.C.G. Rheological properties of polypropylene during multiple extrusion. Polym. Test. 2005, 24, 86–93. [Google Scholar] [CrossRef]
- Canevarolo, S.V.; Babetto, A.C. Effect of screw element type in degradation of polypropylene upon multiple extrusions. Adv. Polym. Technol. 2002, 21, 243–249. [Google Scholar] [CrossRef]
- da Costa, H.M.; Ramos, V.D.; de Oliveira, M.G. Degradation of polypropylene (PP) during multiple extrusions: Thermal analysis, mechanical properties and analysis of variance. Polym. Test. 2007, 26, 676–684. [Google Scholar] [CrossRef]
- Moss, S.; Zweifel, H. Degradation and stabilization of high density polyethylene during multiple extrusions. Polym. Degrad. Stab. 1989, 25, 217–245. [Google Scholar] [CrossRef]
- Jagenteufel, R.; Hofstaetter, T.; Kamleitner, F.; Pedersen, D.B.; Tosello, G.; Hansen, H.N. Rheology of high melt strength polypropylene for additive manufacturing. Adv. Mater. Lett. 2017, 8, 712–716. [Google Scholar] [CrossRef]
- Wojtyła, S.; Klama, P.; Baran, T. Is 3D printing safe? Analysis of the thermal treatment of thermoplastics: ABS, PLA, PET, and nylon. J. Occup. Environ. Hyg. 2017, 14, D80–D85. [Google Scholar] [CrossRef]
- Fernandez, E.; Ceretti, D.A.; Wang, S.; Jiang, Y.; Zhang, J.; D’hooge, D.R.; Cardon, L. Fused filament fabrication of copolyesters by understanding the balance of inter- and intra-layer welding. Plast. Rubber Compos. 2020, 51, 126–132. [Google Scholar] [CrossRef]
- Gao, X.; Qi, S.; Kuang, X.; Su, Y.; Li, J.; Wang, D. Fused filament fabrication of polymer materials: A review of interlayer bond. Addit. Manuf. 2021, 37, 101658. [Google Scholar] [CrossRef]
- Costa, A.E.; da Silva, A.F.; Carneiro, O.S. A study on extruded filament bonding in fused filament fabrication. Rapid Prototyp. J. 2019, 25, 555–565. [Google Scholar] [CrossRef]
- Spoerk, M.; Arbeiter, F.; Raguž, I.; Holzer, C.; Gonzalez-Gutierrez, J. Mechanical recyclability of polypropylene composites produced by material extrusion-based additive manufacturing. Polymers 2019, 11, 1318. [Google Scholar] [CrossRef]
- Ahlinder, A.; Fuoco, T.; Morales-López, Á.; Yassin, M.A.; Mustafa, K.; Finne-Wistrand, A. Nondegradative additive manufacturing of medical grade copolyesters of high molecular weight and with varied elastic response. J. Appl. Polym. Sci. 2020, 137, 18–20. [Google Scholar] [CrossRef]
- Gradwohl, M.; Chai, F.; Payen, J.; Guerreschi, P.; Marchetti, P.; Blanchemain, N. Effects of two melt extrusion based additive manufacturing technologies and common sterilization methods on the properties of a medical grade PLGA copolymer. Polymers 2021, 13, 572. [Google Scholar] [CrossRef]
- Lee, H.; Yoo, J.J.; Kang, H.W.; Cho, D.W. Investigation of thermal degradation with extrusion-based dispensing modules for 3D bioprinting technology. Biofabrication 2016, 8, 015011. [Google Scholar] [CrossRef]
- La Gala, A.; Ceretti, D.V.A.; Fiorio, R.; Cardon, L.; D’hooge, D.R. Comparing pellet- and filament-based additive manufacturing with conventional processing for ABS and PLA parts. J. Appl. Polym. Sci. 2022, 139, e53089. [Google Scholar] [CrossRef]
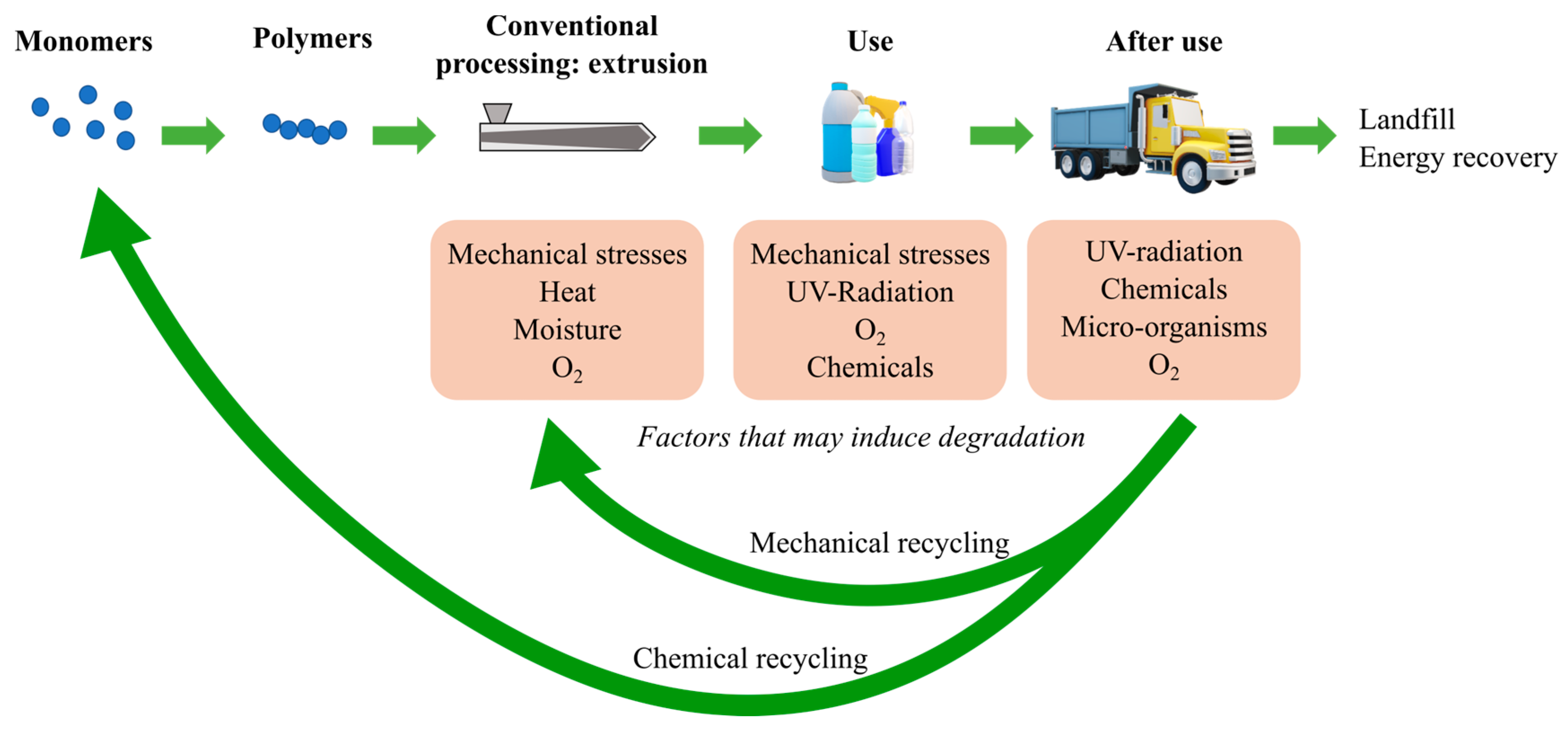
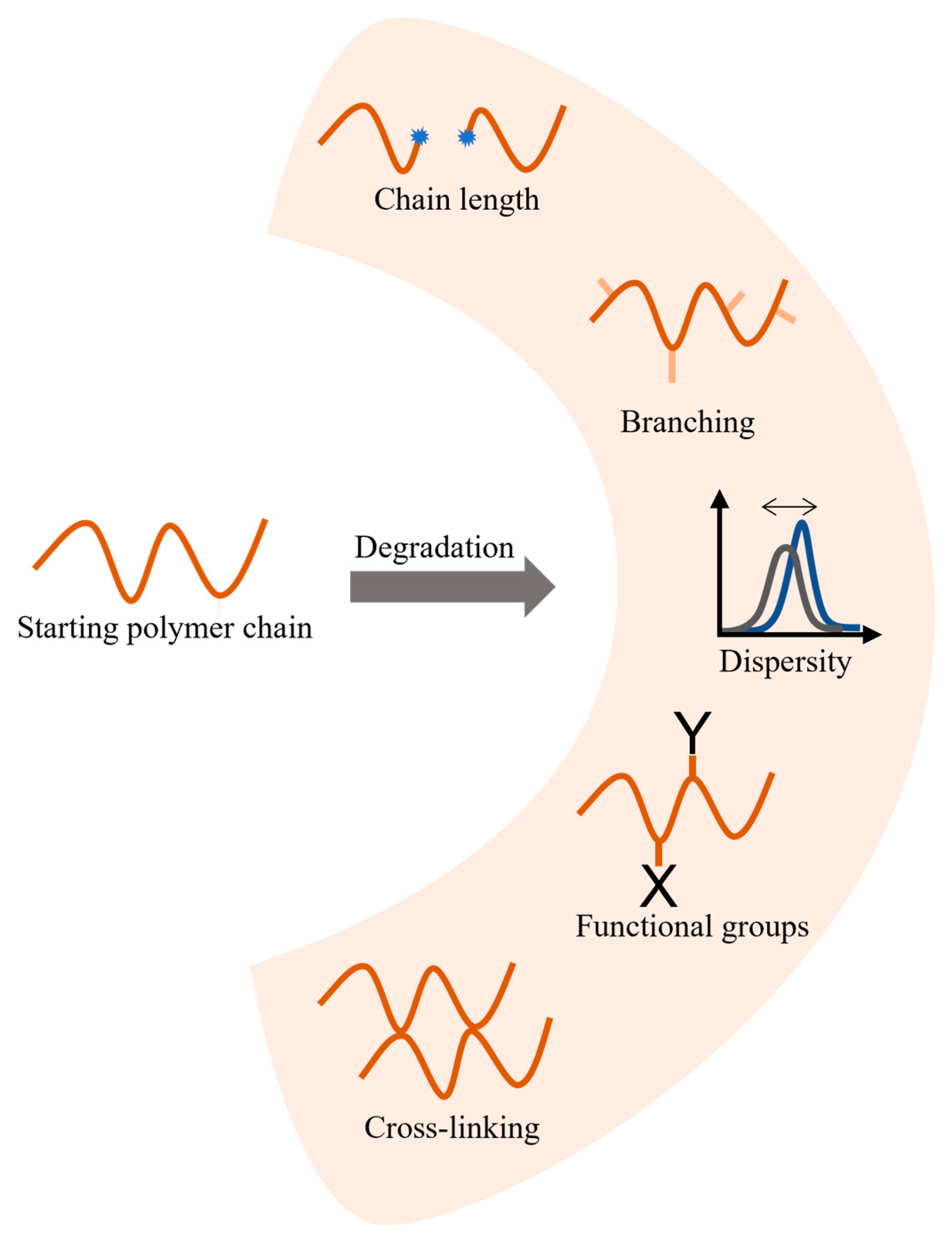
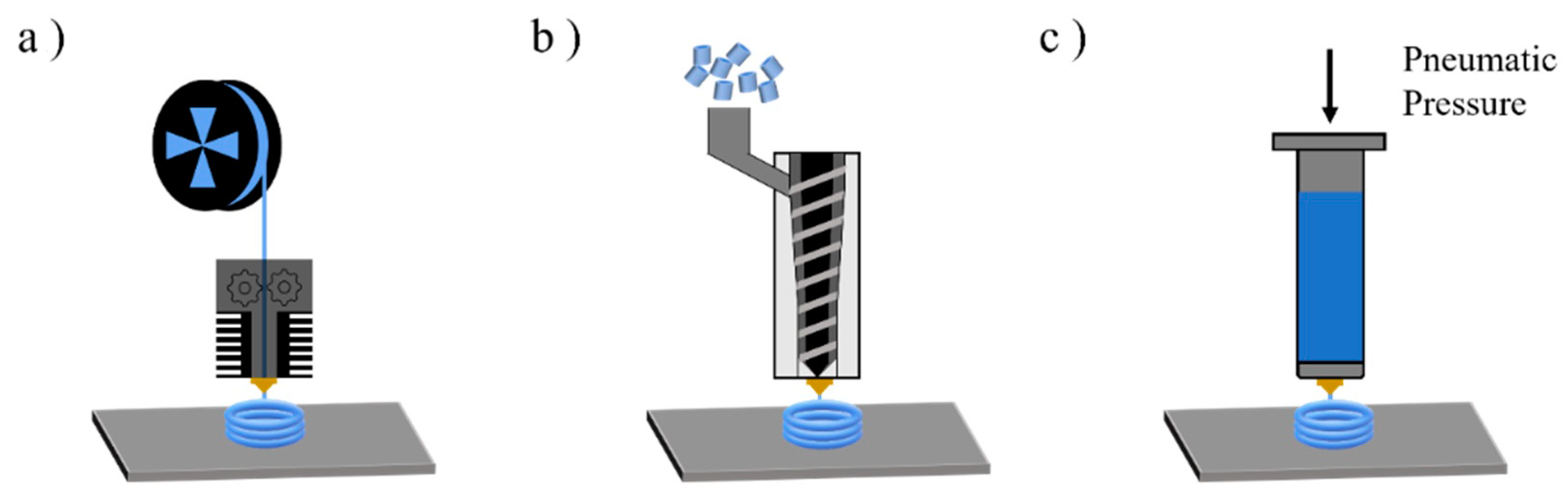
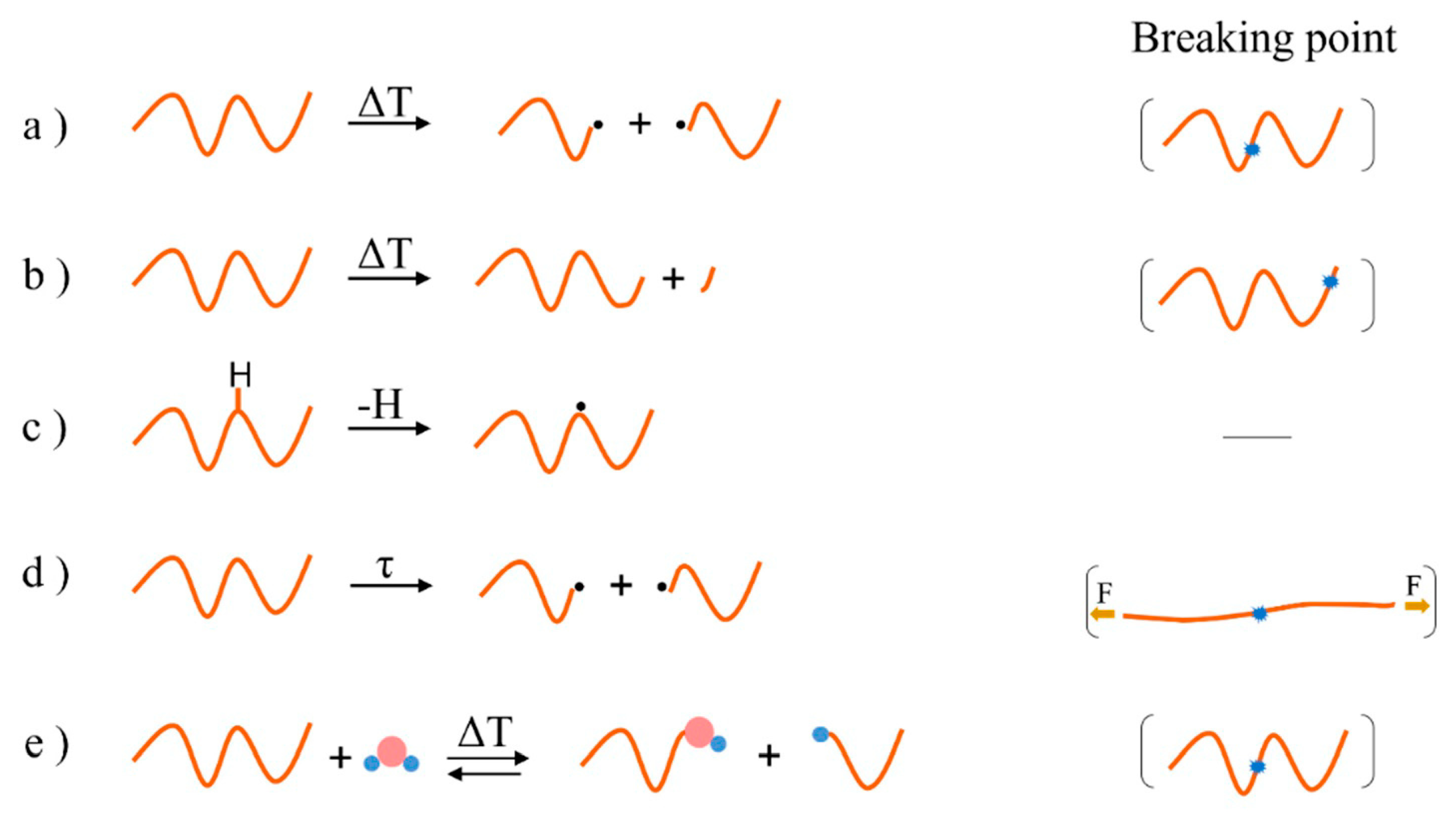
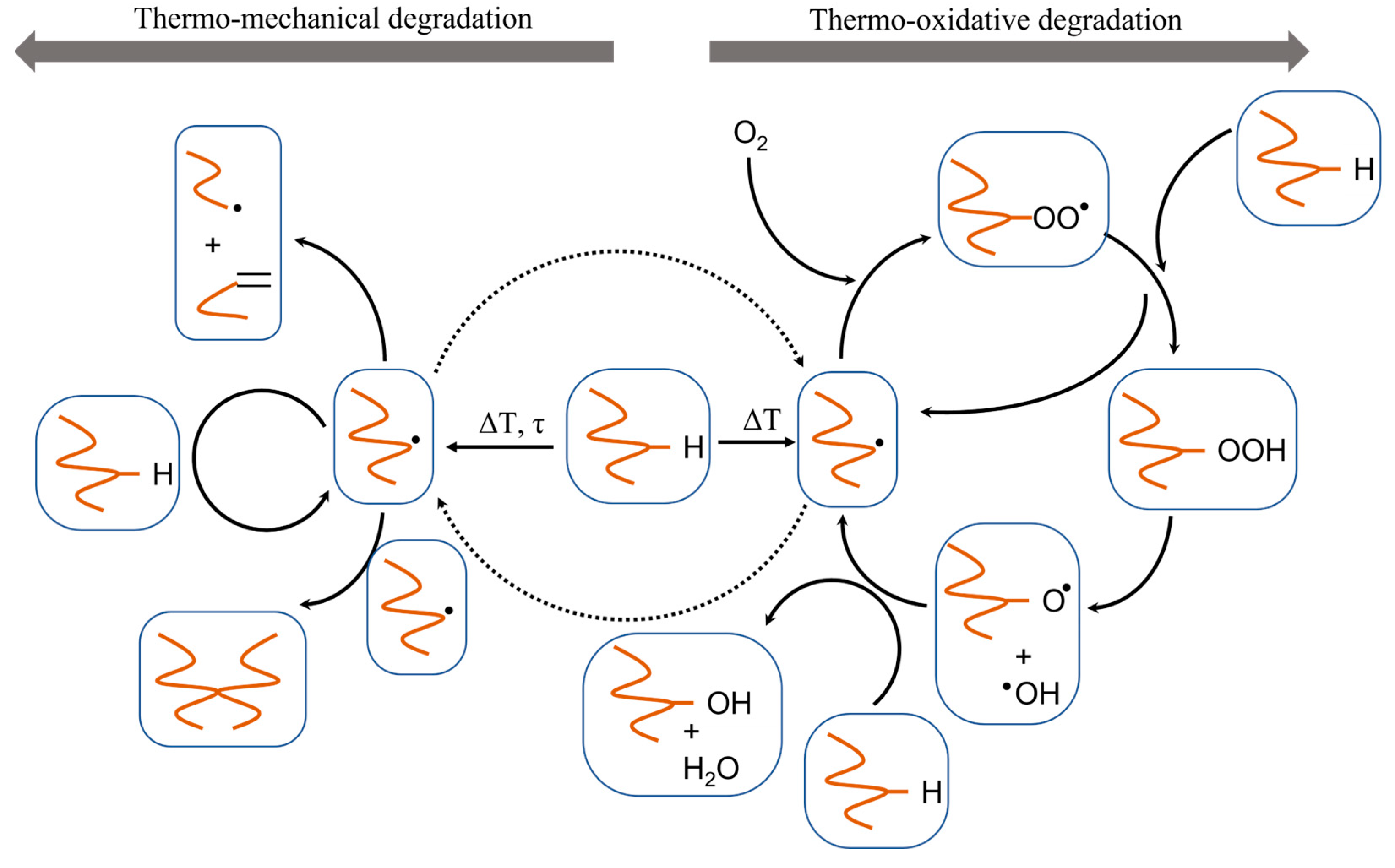
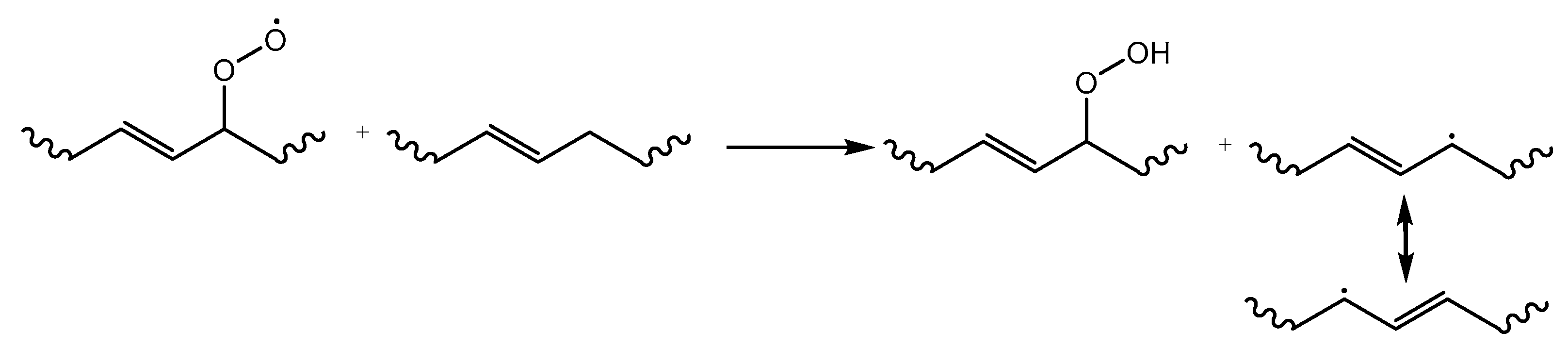

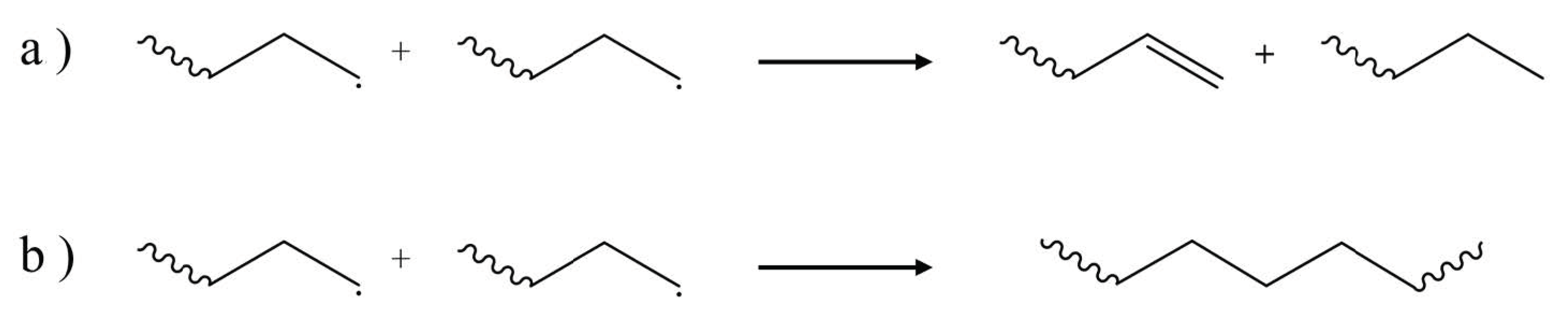
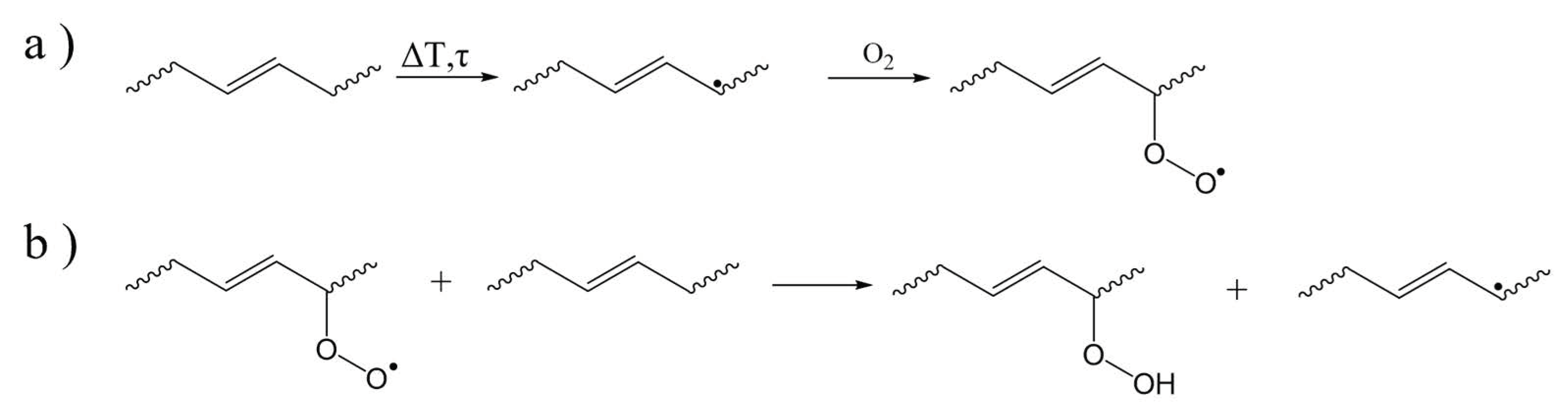
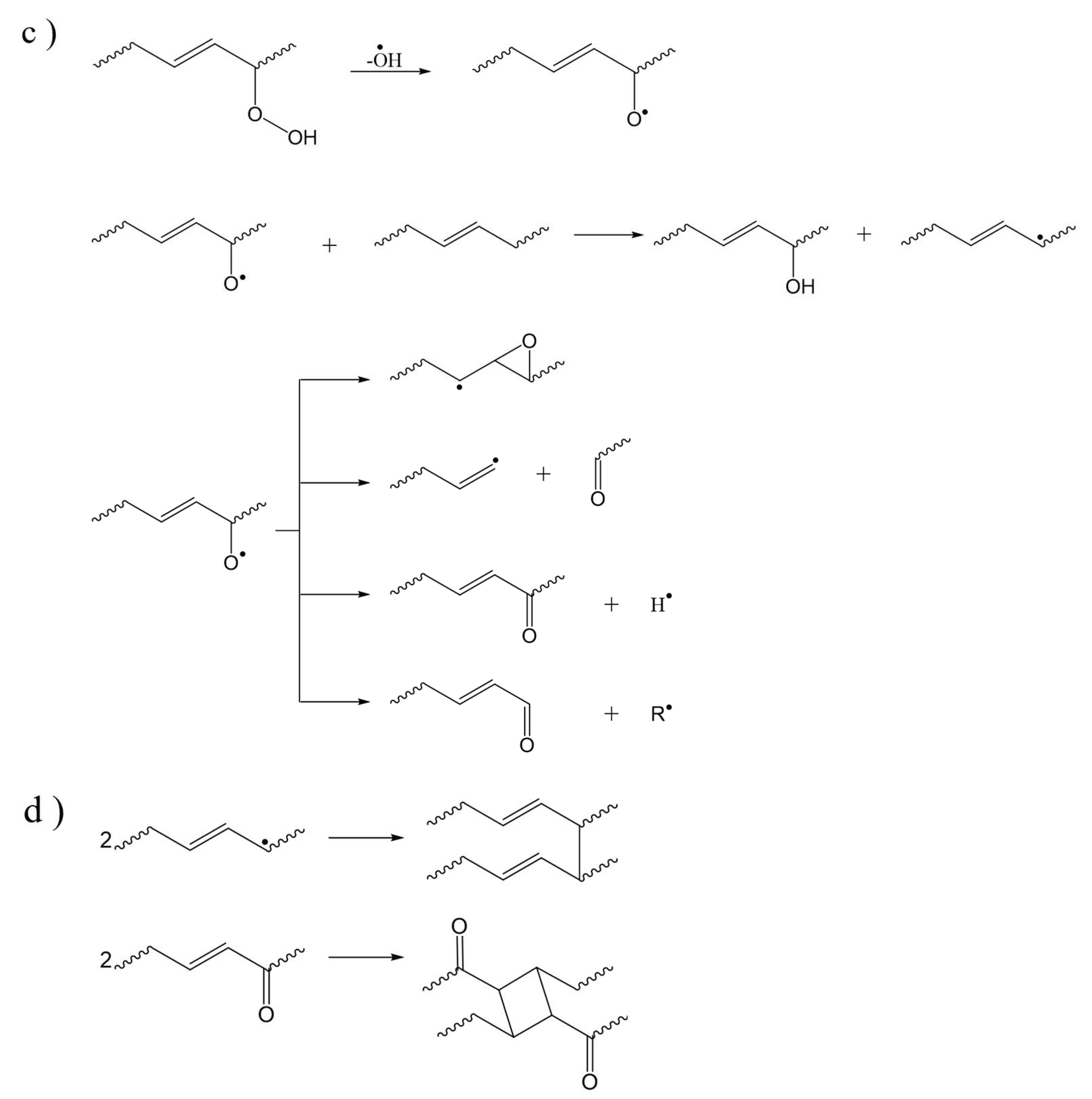
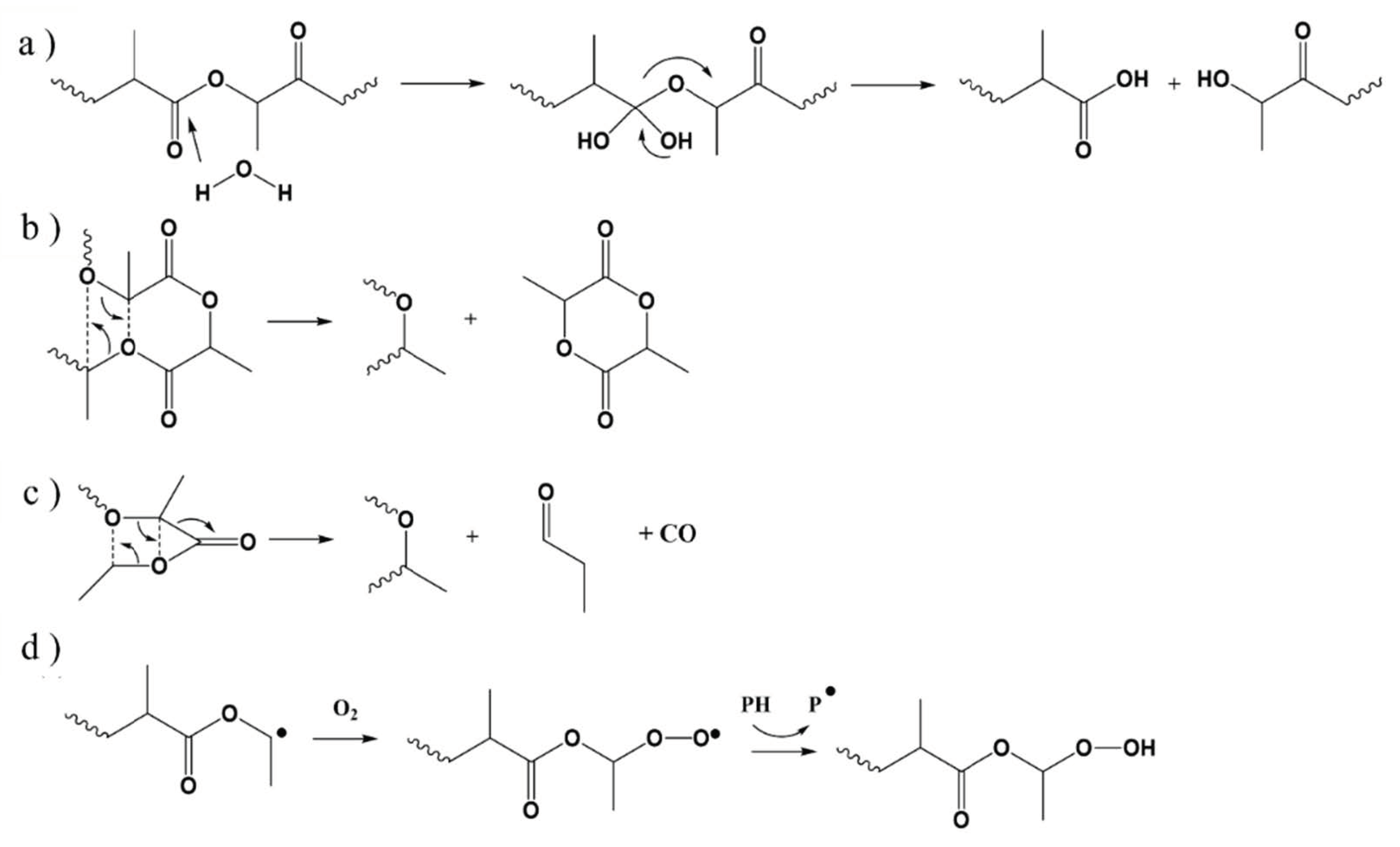
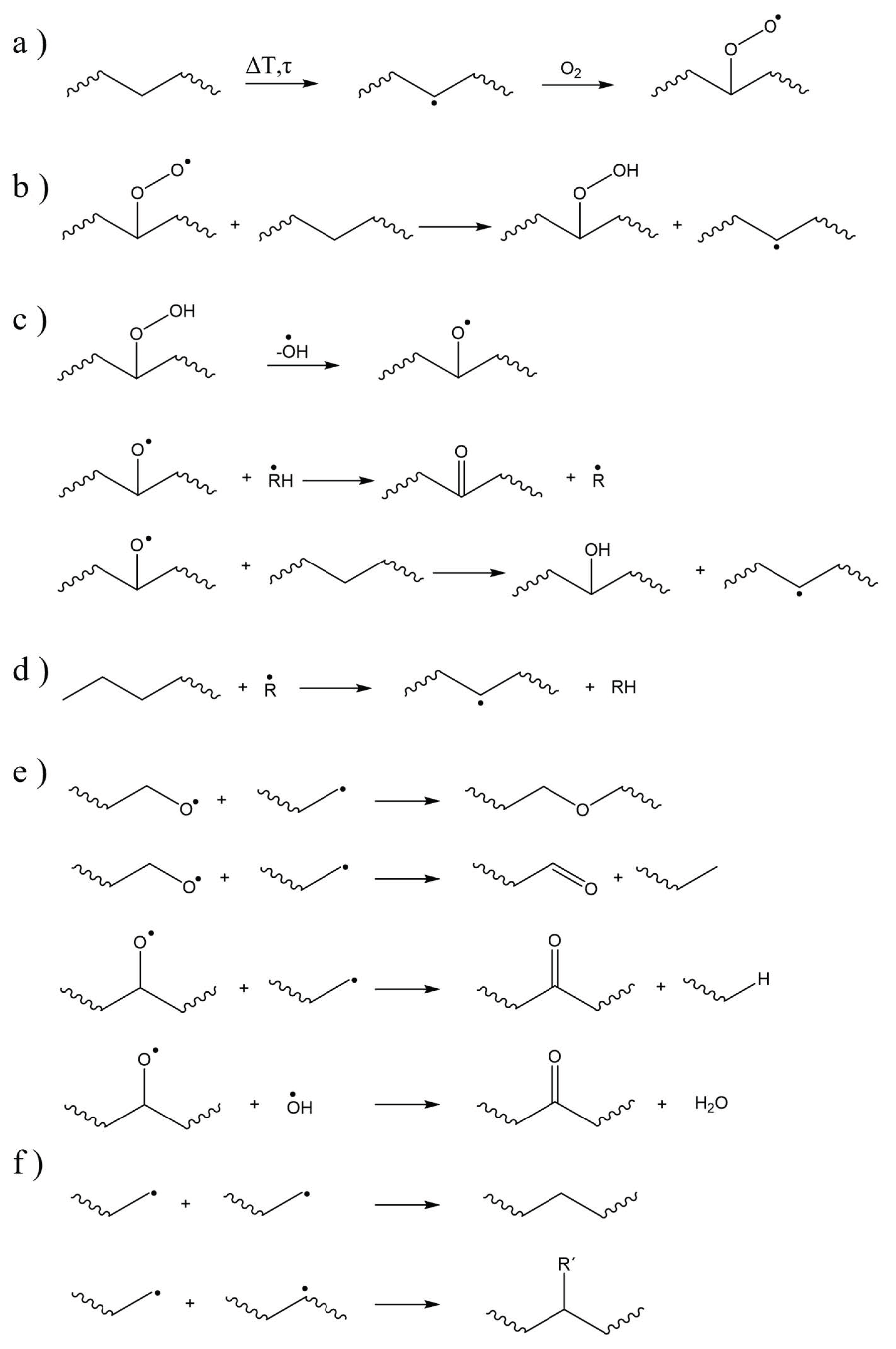
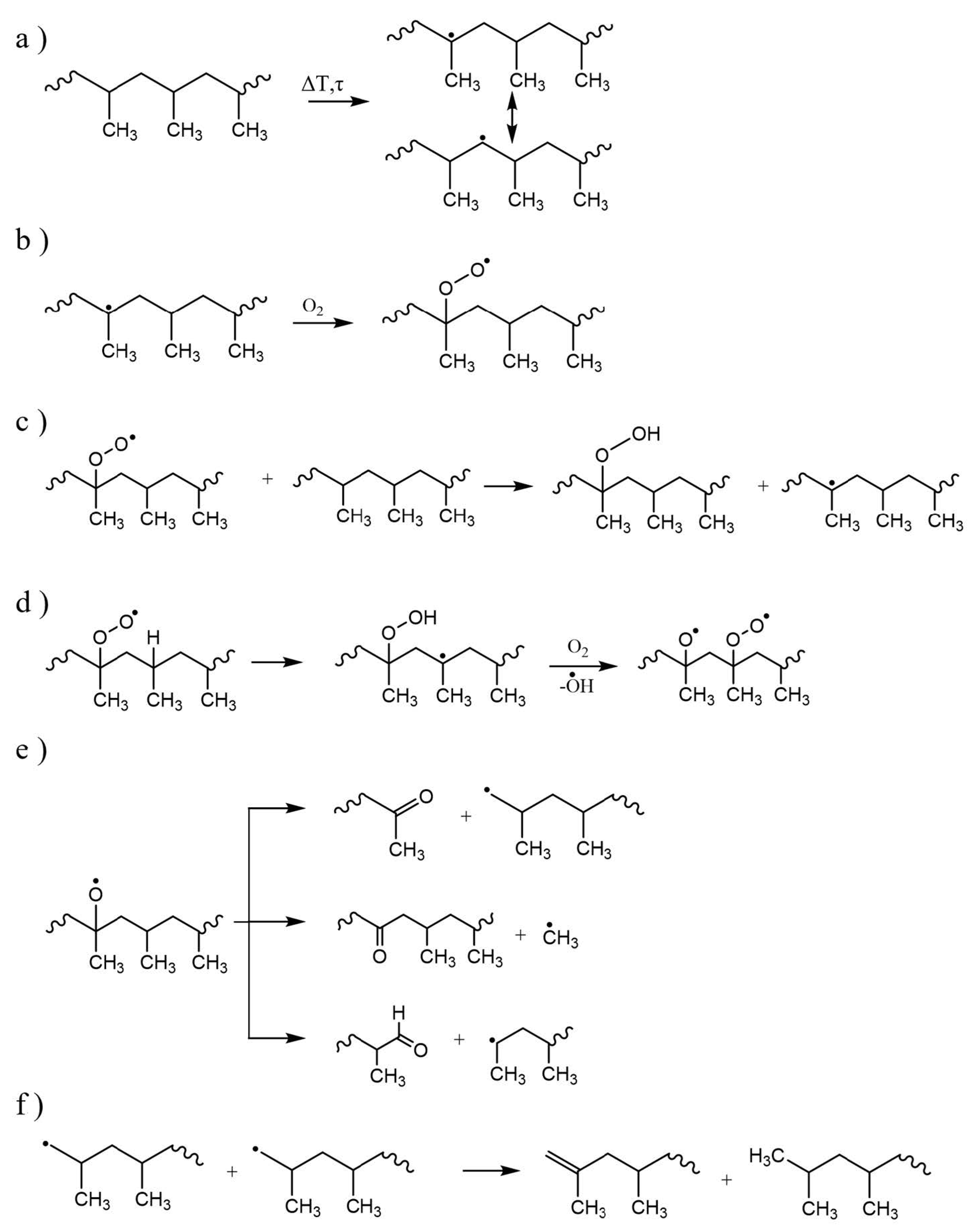
| Main Factor (s) | Degradation Mechanism |
|---|---|
| Temperature | Thermal degradation |
| Temperature and mechanical stresses | Thermo-mechanical degradation |
| Temperature and oxygen | Thermal-oxidative degradation |
| Water and temperature | Hydrolysis |
| UV radiation and oxygen | Photo-oxidative degradation |
| Chemicals | Chemical degradation |
| Micro-organisms | Biodegradation |
| Bond | Aromatic or Heterocyclic | Aliphatic |
|---|---|---|
| C-C | 410 | 284–368 |
| C=C | - | 615 |
| C-H | 427–435 | 381–410 |
| C-Cl | - | 326 |
| C-F | - | 452 |
| C-O | 448 | 350–389 |
| C-N | 460 | 293–343 |
| C=N | - | 615 |
| Group | Name and Main Chemical Structure | Degradation Mechanism | Main Outcome of Degradation | |
|---|---|---|---|---|
| Styrene-based materials (Rubber-modified) | Acrylonitrile butadiene styrene (ABS) | Thermal oxidation of butadiene units and Thermo-mechanical |
| |
 |  | |||
| Styrene-Acrylonitrile (SAN) | (trans-1,4) Polybutadiene (PB) | |||
| High-impact polystyrene (HIPS) | ||||
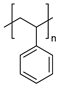 | 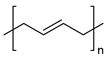 | |||
| Polystyrene (PS) | (trans-1,4) Polybutadiene (PB) | |||
| Polyesters | Polylactic acid (PLA) | Hydrolysis of ester groups and Thermo-mechanical |
| |
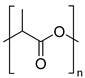 | ||||
| Polyethylene terephthalate glycol (PETG) | ||||
 | ||||
| Polyolefins | Polypropylene (PP) | Thermal oxidation and Thermo-mechanical |
| |
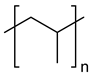 | ||||
| High-density polyethylene (linear) Low-density polyethylene (branched) |
| |||
 | ||||
| Polyaryletherketones | Polyether ether ketone (PEEK) | Thermal oxidation and Thermo-mechanical |
| |
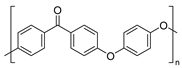 | ||||
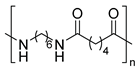
| Polyamides | Polyamide 12 | Thermal oxidation of C-H bonds adjacent to N-H groups and Thermo-mechanical | ||
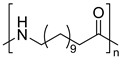 | ||||
| Polyamide 6,6 | ||||
 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceretti, D.V.A.; Edeleva, M.; Cardon, L.; D’hooge, D.R. Molecular Pathways for Polymer Degradation during Conventional Processing, Additive Manufacturing, and Mechanical Recycling. Molecules 2023, 28, 2344. https://doi.org/10.3390/molecules28052344
Ceretti DVA, Edeleva M, Cardon L, D’hooge DR. Molecular Pathways for Polymer Degradation during Conventional Processing, Additive Manufacturing, and Mechanical Recycling. Molecules. 2023; 28(5):2344. https://doi.org/10.3390/molecules28052344
Chicago/Turabian StyleCeretti, Daniel V. A., Mariya Edeleva, Ludwig Cardon, and Dagmar R. D’hooge. 2023. "Molecular Pathways for Polymer Degradation during Conventional Processing, Additive Manufacturing, and Mechanical Recycling" Molecules 28, no. 5: 2344. https://doi.org/10.3390/molecules28052344
APA StyleCeretti, D. V. A., Edeleva, M., Cardon, L., & D’hooge, D. R. (2023). Molecular Pathways for Polymer Degradation during Conventional Processing, Additive Manufacturing, and Mechanical Recycling. Molecules, 28(5), 2344. https://doi.org/10.3390/molecules28052344








