Fabrication of Porous Carbon Nanofibers from Polymer Blends Using Template Method for Electrode-Active Materials in Supercapacitor
Abstract
:1. Introduction
2. Results and Discussion
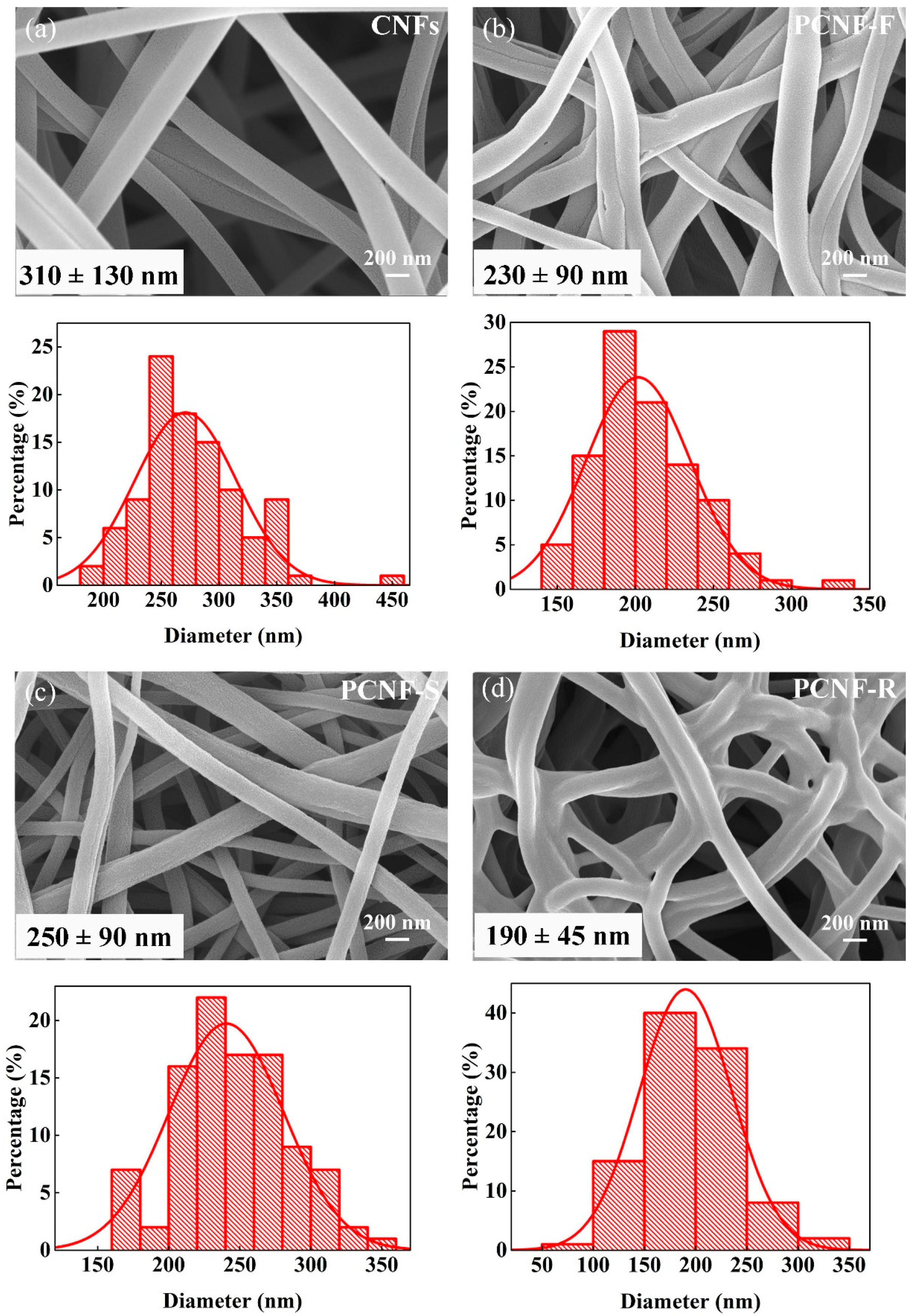
2.1. Surface Morphology
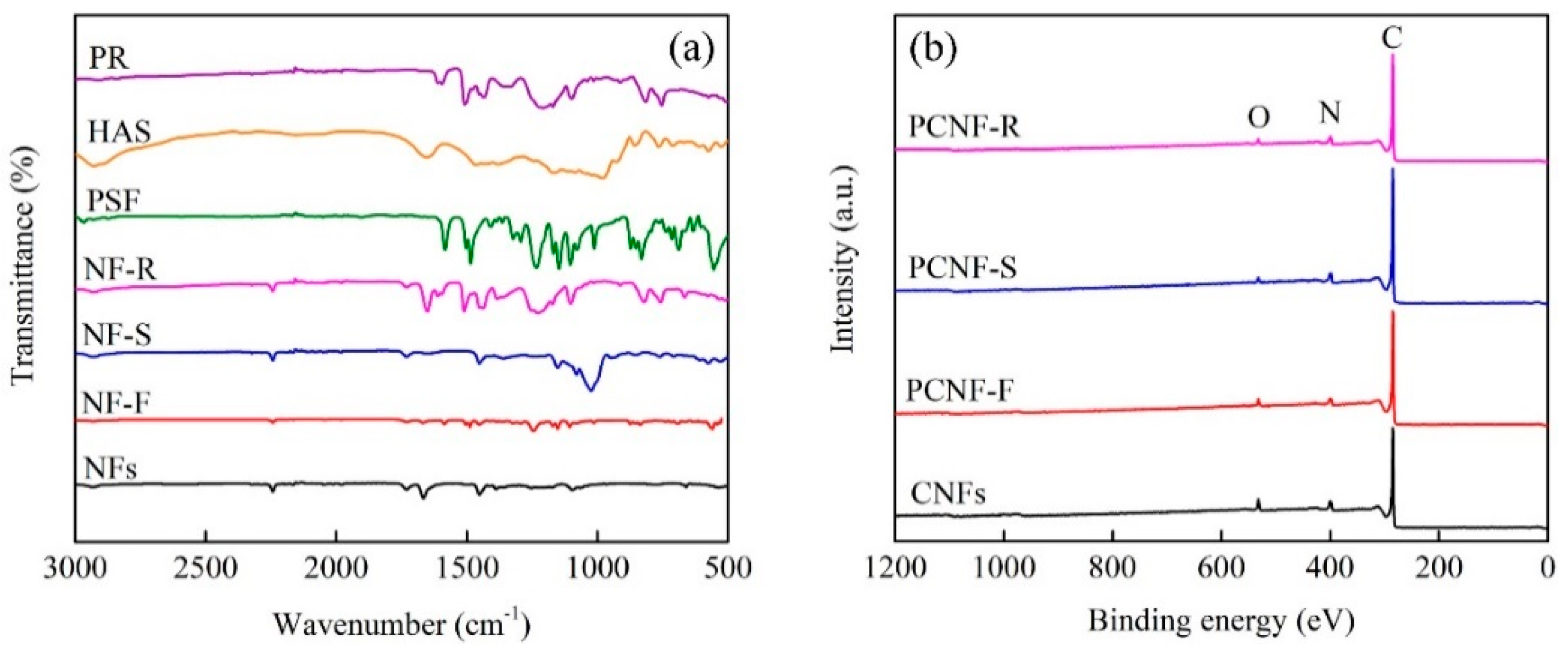
2.2. Chemical Components
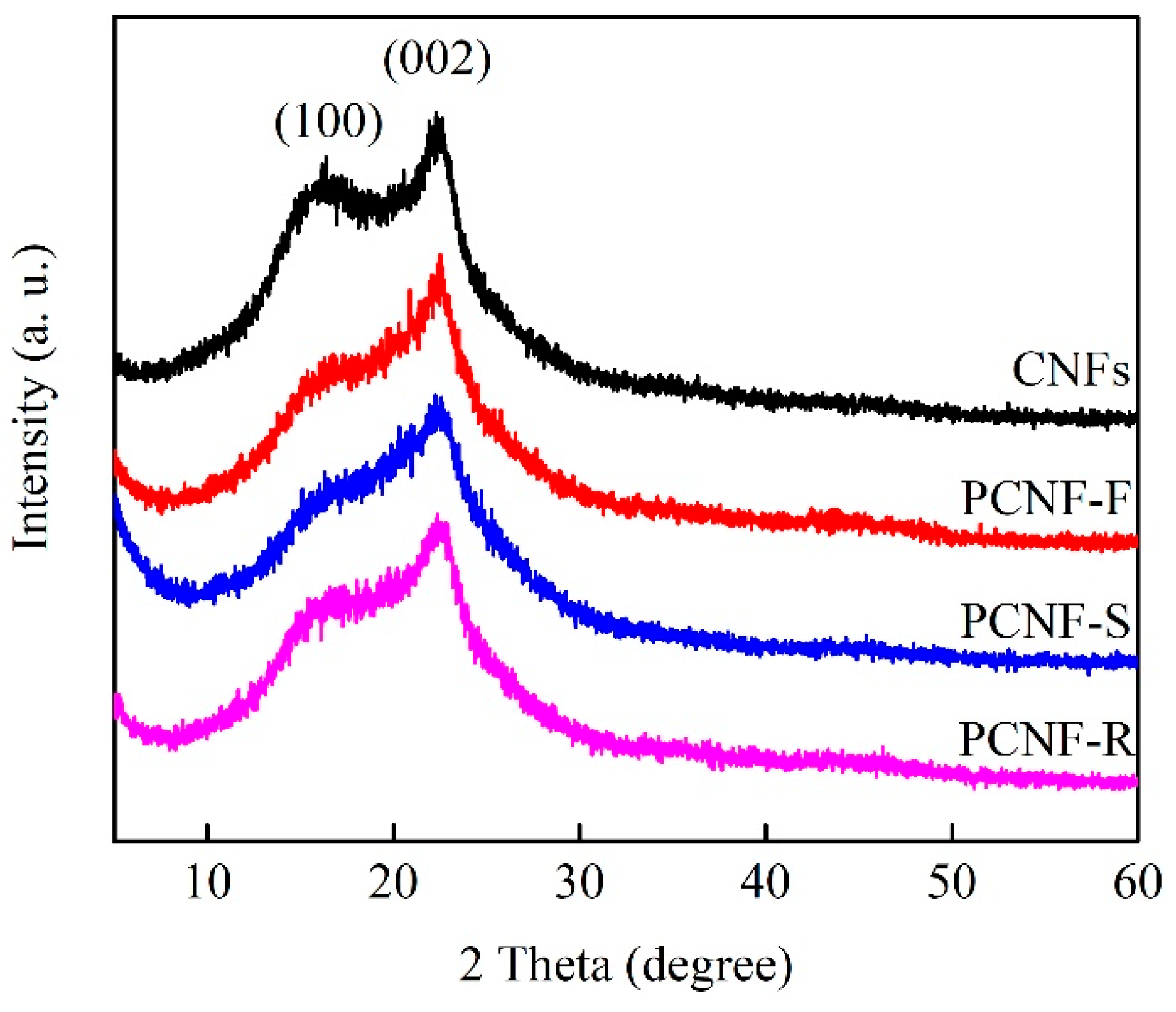
2.3. Graphitized Crystallization
2.4. Pore Characteristics
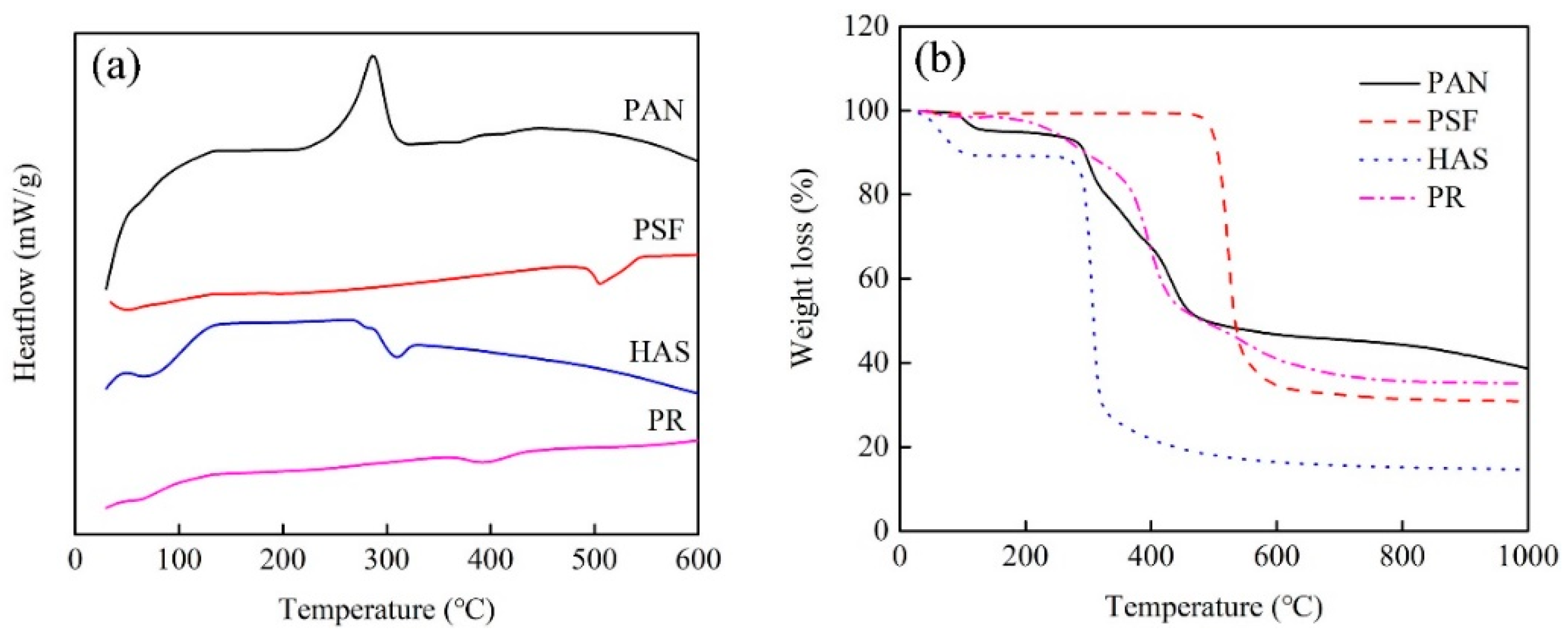
2.5. Pore-Forming Mechanism
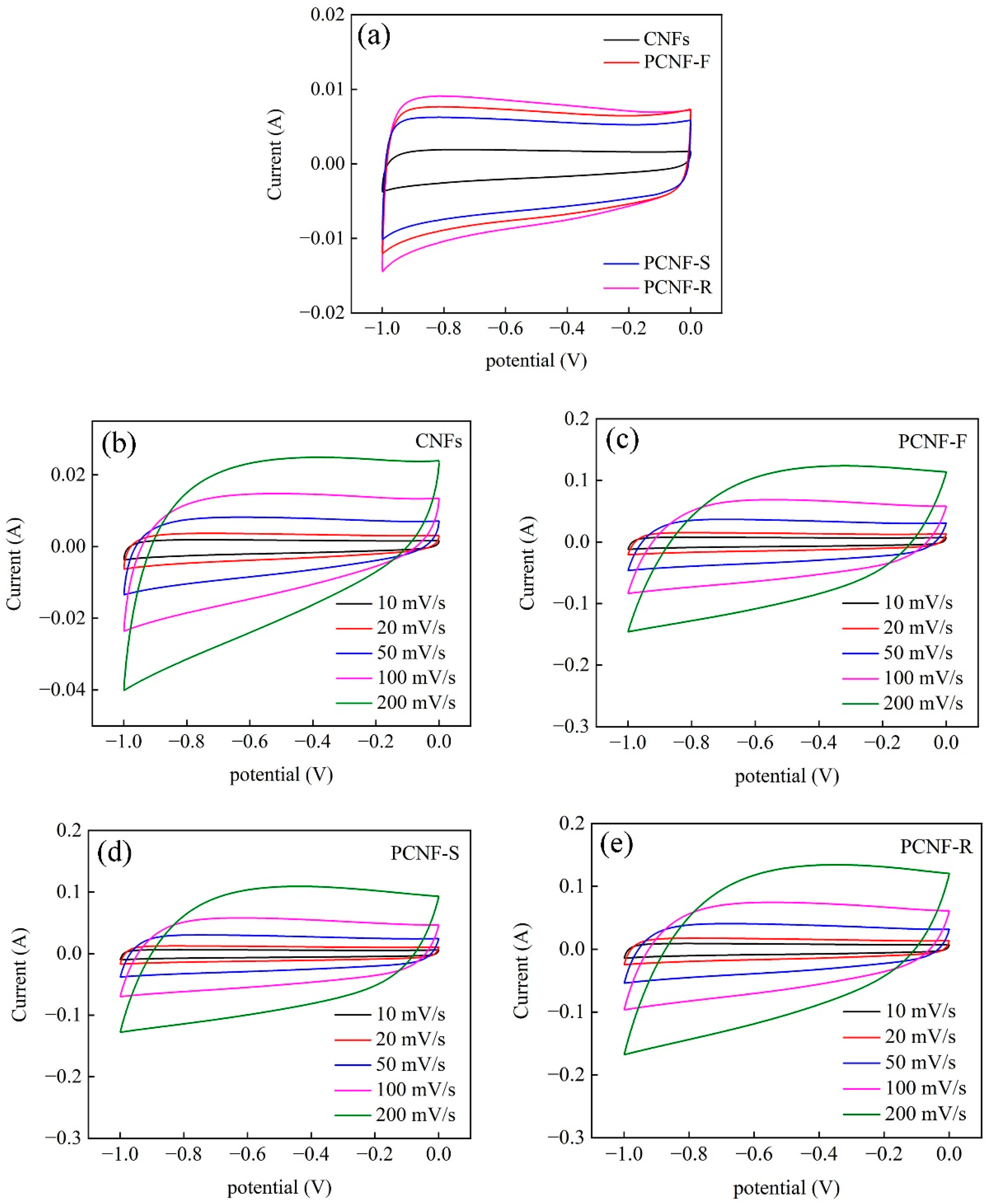
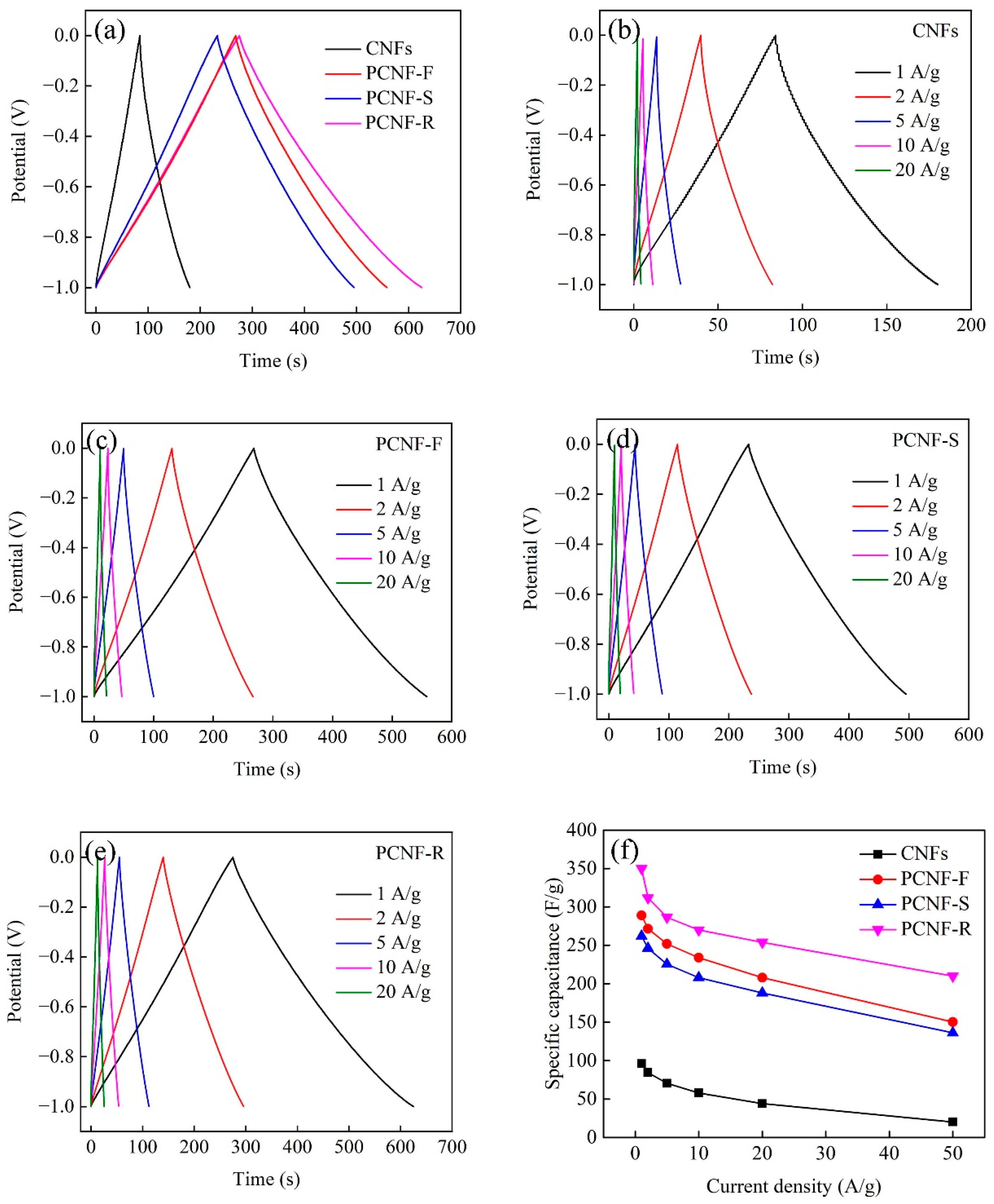
2.6. Electrochemical Performances
3. Experimental Section
3.1. Materials
3.2. Fabrication of PCNFs
3.3. Fabrication of Electrodes
3.4. Material Characterization
3.5. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yoon, J.H.; Jinsoo, B.; Cho, I.; Vinodh, R.; Pollet, B.G.; Babu, R.S.; Kim, H.-J.; Kim, S. Novel Supercapacitor Electrode Derived from One Dimensional Cerium Hydrogen Phosphate (1D-Ce(HPO4)2.xH2O). Molecules 2022, 27, 7691. [Google Scholar] [CrossRef]
- Vinodh, R.; Babu, R.S.; Sambasivam, S.; Gopi, C.V.V.M.; Alzahmi, S.; Kim, H.-J.; Barros, A.L.F.; Obaidat, I.M. Recent Advancements of Polyaniline/Metal Organic Framework (PANI/MOF) Composite Electrodes for Supercapacitor Applications: A Critical Review. Nanomaterials 2022, 12, 1511. [Google Scholar] [CrossRef]
- Harputlu, E. Nanoarchitectonics of the supercapacitor performance of LaNiO3 perovskite on the graphitic-C3N4 doped reduced graphene oxide hydrogel. Colloids Surf. A 2022, 632, 127787. [Google Scholar] [CrossRef]
- Harputlu, E.; Gecgel, C. Fabrication of LaFeO3/g-C3N4@reduced graphene oxide 3-dimensional nanostructure supercapacitor. J. Mater. Sci. 2022, 33, 25687–25703. [Google Scholar] [CrossRef]
- Sahin, M.E.; Blaabjerg, F.; Sangwongwanich, A. A Comprehensive Review on Supercapacitor Applications and Developments. Energies 2022, 15, 674. [Google Scholar] [CrossRef]
- Reece, R.; Lekakou, C.; Smith, P.A. A High-Performance Structural Supercapacitor. ACS Appl. Mater. Interfaces 2020, 12, 25683–25692. [Google Scholar] [CrossRef]
- Liu, X.; Jin, X.; Li, L.; Wang, J.; Yang, Y.; Cao, Y.; Wang, W. Air-permeable, multifunctional, dual-energy-driven MXene-decorated polymeric textile-based wearable heater with exceptional electrothermal and photothermal conversion performance. J. Mater. Chem. A 2020, 8, 12526–12537. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Lei, Z.; Wang, L.; Tian, M.; Zhu, S.; Xiao, H.; Tang, X.; Qu, L. Flexible MXene-Decorated Fabric with Interwoven Conductive Networks for Integrated Joule Heating, Electromagnetic Interference Shielding, and Strain Sensing Performances. ACS Appl. Mater. Interfaces 2020, 12, 14459–14467. [Google Scholar] [CrossRef]
- Liang, C.; Liu, Y.; Ruan, Y.; Qiu, H.; Song, P.; Kong, J.; Zhang, H.; Gu, J. Multifunctional sponges with flexible motion sensing and outstanding thermal insulation for superior electromagnetic interference shielding. Compos. Part A 2020, 139, 106143. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, C.; Chen, T.; Li, W.; Zheng, S.; Pi, Y.; Luo, Y.; Pang, H. MXene-Copper/Cobalt Hybrids via Lewis Acidic Molten Salts Etching for High Performance Symmetric Supercapacitors. Angew. Chem. 2021, 133, 25522–25526. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Cui, M.; Meng, X. Overview of transition metal-based composite materials for supercapacitor electrodes. Nanoscale Adv. 2020, 2, 5516–5528. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, J.; Cheng, H.; Li, G.; Cho, H.; Jiang, M.; Gao, Q.; Zhang, X. Developments of Advanced Electrospinning Techniques: A Critical Review. Adv. Mater. Technol. 2021, 6, 2100410. [Google Scholar] [CrossRef]
- Li, X.; Chen, W.; Qian, Q.; Huang, H.; Chen, Y.; Wang, Z.; Chen, Q.; Yang, J.; Li, J.; Mai, Y.W. Electrospinning-Based Strategies for Battery Materials. Adv. Energy Mater. 2021, 11, 2000845. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, J.; Zhang, L.; Qiu, Y. Recent advances in energy materials by electrospinning. Renew. Sustain. Energy Rev. 2018, 81, 1825–1858. [Google Scholar] [CrossRef]
- Yadav, D.; Amini, F.; Ehrmann, A. Recent advances in carbon nanofibers and their applications—A review. Eur. Polym. J. 2020, 138, 109963. [Google Scholar] [CrossRef]
- Chen, L.F.; Zhang, X.D.; Liang, H.W.; Kong, M.; Guan, Q.F.; Chen, P.; Wu, Z.Y.; Yu, S.H. Synthesis of Nitrogen-Doped Porous Carbon Nanofibers as an Efficient Electrode Material for Supercapacitors. ACS Nano 2012, 6, 7092–7102. [Google Scholar] [CrossRef]
- Tran, C.; Kalra, V. Fabrication of porous carbon nanofibers with adjustable pore sizes as electrodes for supercapacitors. J. Power Sources 2013, 235, 289–296. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, J.; Chen, L.; Zhang, P.; Fu, W.; Zhao, H.; Ma, Y.; Pan, X.; Zhang, Z.; Han, W.; et al. Highly Flexible Freestanding Porous Carbon Nanofibers for Electrodes Materials of High-Performance All-Carbon Supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 23515–23520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, Y.; Wang, L.; Zhang, C.; Liu, S. Hierarchical porous carbon nanofibers as binder-free electrode for high-performance supercapacitor. Electrochim. Acta 2016, 196, 189–196. [Google Scholar] [CrossRef]
- Tavanai, H.; Jalili, R.; Morshed, M. Effects of fiber diameter and CO2 activation temperature on the pore characteristics of polyacrylonitrile based activated carbon nanofibers. Surf. Interface Anal. 2009, 41, 814–819. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Wang, Y.H.; Dai, Z.; Lu, Z.W.; Gu, X.Y.; Zhao, H.; Sun, G.Z.; Lan, W.; Zhang, Z.X.; Pan, X.J.; et al. Nature of improved double-layer capacitance by KOH activation on carbon nanotube-carbon nanofiber hierarchical hybrids. Carbon 2019, 146, 610–617. [Google Scholar] [CrossRef]
- Zhi, M.; Liu, S.; Hong, Z.; Wu, N. Electrospun activated carbon nanofibers for supercapacitor electrodes. RSC Adv. 2014, 4, 43619–43623. [Google Scholar] [CrossRef]
- Kim, C.; Ngoc, B.T.N.; Yang, K.S.; Kojima, M.; Kim, Y.A.; Kim, Y.J.; Endo, M.; Yang, S.C. Self-Sustained Thin Webs Consisting of Porous Carbon Nanofibers for Supercapacitors via the Electrospinning of Polyacrylonitrile Solutions Containing Zinc Chloride. Adv. Mater. 2007, 19, 2341–2346. [Google Scholar] [CrossRef]
- Le, T.H.; Yang, Y.; Huang, Z.; Kang, F. Preparation of microporous carbon nanofibers from polyimide by using polyvinyl pyrrolidone as template and their capacitive performance. J. Power Sources 2015, 278, 683–692. [Google Scholar] [CrossRef]
- Xuan, D.; Liu, J.; Wang, D.; Lu, Z.; Liu, Q.; Liu, Y.; Li, S.; Zheng, Z. Facile Preparation of Low-Cost and Cross-Linked Carbon Nanofibers Derived from PAN/PMMA/Lignin as Supercapacitor Electrodes. Energy Fuels 2021, 35, 796–805. [Google Scholar] [CrossRef]
- Wang, H.; Wang, W.; Wang, H.; Jin, X.; Niu, H.; Wang, H.; Zhou, H.; Lin, T. High Performance Supercapacitor Electrode Materials from Electrospun Carbon Nanofibers in Situ Activated by High Decomposition Temperature Polymer. ACS Appl. Energy Mater. 2018, 1, 431–439. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Ruan, F.; Feng, Q.; Wei, Y.; Fang, J. High-porosity carbon nanofibers prepared from polyacrylonitrile blended with amylose starch for application in supercapacitors. Mater. Chem. Phys. 2023, 293, 126896. [Google Scholar] [CrossRef]
- Wang, H.; Niu, H.; Wang, H.; Wang, W.; Jin, X.; Wang, H.; Zhou, H.; Lin, T. Micro-meso porous structured carbon nanofibers with ultra-high surface area and large supercapacitor electrode capacitance. J. Power Sources 2021, 482, 228986. [Google Scholar] [CrossRef]
- Li, J.; Zhang, W.; Zhang, X.; Huo, L.; Liang, J.; Wu, L.; Liu, Y.; Gao, J.; Pang, H.; Xue, H. Copolymer derived micro/meso-porous carbon nanofibers with vacancy-type defects for high-performance supercapacitors. J. Mater. Chem. A 2020, 8, 2463–2471. [Google Scholar] [CrossRef]
- Ma, Z.; Fan, Q.; Dirican, M.; Subjalearndee, N.; Cheng, H.; Li, J.; Song, Y.; Shi, J.; Zhang, X. Rational design of meso-/micro-pores for enhancing ion transportation in highly-porous carbon nanofibers used as electrode for supercapacitors. Appl. Surf. Sci. 2021, 545, 148933. [Google Scholar] [CrossRef]
- Wang, H.; Wang, W.; Wang, H.; Li, Y.; Jin, X.; Niu, H.; Wang, H.; Zhou, H.; Lin, T. Improving Supercapacitance of Electrospun Carbon Nanofibers through Increasing Micropores and Microporous Surface Area. Adv. Mater. Interfaces 2019, 6, 1801900. [Google Scholar] [CrossRef]
- Ma, C.; Li, Z.; Li, J.; Fan, Q.; Wu, L.; Shi, J.; Song, Y. Lignin-based hierarchical porous carbon nanofiber films with superior performance in supercapacitors. Appl. Surf. Sci. 2018, 456, 568–576. [Google Scholar] [CrossRef]
- Ju, J.; Kang, W.; Deng, N.; Li, L.; Zhao, Y.; Ma, X.; Fan, L.; Cheng, B. Preparation and characterization of PVA-based carbon nanofibers with honeycomb-like porous structure via electro-blown spinning method. Microporous Mesoporous Mater. 2017, 239, 416–425. [Google Scholar] [CrossRef]
- Jiang, Q.; Pang, X.; Geng, S.; Zhao, Y.; Wang, X.; Qin, H.; Liu, B.; Zhou, J.; Zhou, T. Simultaneous cross-linking and pore-forming electrospun carbon nanofibers towards high capacitive performance. Appl. Surf. Sci. 2019, 479, 128–136. [Google Scholar] [CrossRef]
- Cai, J.; Niu, H.; Wang, H.; Shao, H.; Fang, J.; He, J.; Xiong, H.; Ma, C.; Lin, T. High-performance supercapacitor electrode from cellulose-derived, inter-bonded carbon nanofibers. J. Power Sources 2016, 324, 302–308. [Google Scholar] [CrossRef]
- Chang, W.M.; Wang, C.C.; Chen, C.Y. Fabrication of ultra-thin carbon nanofibers by centrifuged-electrospinning for application in high-rate supercapacitors. Electrochim. Acta 2019, 296, 268–275. [Google Scholar] [CrossRef]




| Samples | C | N | O | D (Å) | L | Conductivity |
|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (nm) | (S/cm) | ||
| CNFs | 90.0 | 5.1 | 4.9 | 4.01 | 0.87 | 6.64 |
| PCNF-F | 95.6 | 2.7 | 1.7 | 3.95 | 1.31 | 10.85 |
| PCNF-S | 94.5 | 3.5 | 2.0 | 3.99 | 1.12 | 9.32 |
| PCNF-R | 94.2 | 4.1 | 1.7 | 3.97 | 1.06 | 7.84 |
| Samples | Specific Surface Area | Porosity | Mesopore Volume | Mesopore Content | Micropore Volume | Micropore Content |
|---|---|---|---|---|---|---|
| (m2/g) | (cm3/g) | (cm3/g) | (%) | (cm3/g) | (%) | |
| CNFs | 46 | 0.06 | 0.05 | 83 | 0.01 | 17 |
| PCNF-F | 687 | 0.52 | 0.22 | 42 | 0.30 | 58 |
| PCNF-S | 637 | 0.42 | 0.17 | 41 | 0.25 | 59 |
| PCNF-R | 994 | 0.75 | 0.20 | 27 | 0.55 | 73 |
| Precursor | Pore-Forming Agent | Precursor to Activation Agent (Mass Ration) | Optimum Capacitance | Ref. |
|---|---|---|---|---|
| (F/g) | ||||
| PAN | PSF | 4:1 | 289 (1 A/g) | This work |
| PAN | HAS | 4:1 | 262 (1 A/g) | This work |
| PAN | PR | 4:1 | 350 (1 A/g) | This work |
| PAN | PMMA | 4:1 | 260 (1 A/g) | [34] |
| PAN | PVP | 4:1 | 225 (1 A/g) | [34] |
| PAN | Nafion | 1:4 | 210 (1 A/g) | [20] |
| Lignin | Mg(NO3)2 | 1:2 | 248 (0.2 A/g) | [35] |
| PAN | CaCO3 | 3:8 | 251 (0.5 A/g) | [22] |
| PVA | PTFE | 1:15 | 177 (1 A/g) | [36] |
| PAN | ZnCl2 | 9:5 | 214 (1 A/g) | [37] |
| Cellulose | CO2 | - | 241.4 (1 A/g) | [38] |
| PAN | PMMA | 1:9 | 243 (1 A/g) | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Yao, L.; Zuo, H.; Ruan, F.; Wang, H. Fabrication of Porous Carbon Nanofibers from Polymer Blends Using Template Method for Electrode-Active Materials in Supercapacitor. Molecules 2023, 28, 2228. https://doi.org/10.3390/molecules28052228
Wang H, Yao L, Zuo H, Ruan F, Wang H. Fabrication of Porous Carbon Nanofibers from Polymer Blends Using Template Method for Electrode-Active Materials in Supercapacitor. Molecules. 2023; 28(5):2228. https://doi.org/10.3390/molecules28052228
Chicago/Turabian StyleWang, He, Lan Yao, Hongmei Zuo, Fangtao Ruan, and Hongjie Wang. 2023. "Fabrication of Porous Carbon Nanofibers from Polymer Blends Using Template Method for Electrode-Active Materials in Supercapacitor" Molecules 28, no. 5: 2228. https://doi.org/10.3390/molecules28052228
APA StyleWang, H., Yao, L., Zuo, H., Ruan, F., & Wang, H. (2023). Fabrication of Porous Carbon Nanofibers from Polymer Blends Using Template Method for Electrode-Active Materials in Supercapacitor. Molecules, 28(5), 2228. https://doi.org/10.3390/molecules28052228





