Abstract
Glyphosate is a widely used herbicide, and it is an important environmental pollutant that can have adverse effects on human health. Therefore, remediation and reclamation of contaminated streams and aqueous environments polluted by glyphosate is currently a worldwide priority. Here, we show that the heterogeneous nZVI–Fenton process (nZVI + H2O2; nZVI: nanoscale zero-valent iron) can achieve the effective removal of glyphosate under different operational conditions. Removal of glyphosate can also take place in the presence of excess nZVI, without H2O2, but the high amount of nZVI needed to remove glyphosate from water matrices on its own would make the process very costly. Glyphosate removal via nZVI–-Fenton was investigated in the pH range of 3–6, with different H2O2 concentrations and nZVI loadings. We observed significant removal of glyphosate at pH values of 3 and 4; however, due to a loss in efficiency of Fenton systems with increasing pH values, glyphosate removal was no longer effective at pH values of 5 or 6. Glyphosate removal also occurred at pH values of 3 and 4 in tap water, despite the occurrence of several potentially interfering inorganic ions. Relatively low reagent costs, a limited increase in water conductivity (mostly due to pH adjustments before and after treatment), and low iron leaching make nZVI–Fenton treatment at pH 4 a promising technique for eliminating glyphosate from environmental aqueous matrices.
1. Introduction
Glyphosate (GLY: N-(phosphonomethyl)glycine) is the world’s most-used herbicide, and it is employed to control weeds in both agricultural (herbaceous crops and trees) and non-agricultural areas [1]. GLY is so widely used owing to its broad spectrum (effectively killing any plant, with the exception of some genetically modified ones), high efficacy, good toxicological profile, and low cost [2,3]. Since its first commercialization in the 1970s [4], the use of GLY has steadily increased, with almost 600–700 thousand tons used annually, and an expected 740–920 thousand tons to be used by 2025 [5]. In the meanwhile, many harmful effects of GLY on plants, humans, and animals have been discovered, including the weakening of plant systems, endocrine disorders, and the disruption of animal metabolism [6,7,8]. GLY is potentially carcinogenic to humans, even if present at trace levels (<0.02 mg/L) in the environment [9]. Up to now, about 750 GLY-containing products have been used in 130 countries [1,10], but several countries have banned its use. The EU regulations have set a maximum permissible limit of 0.1 μg/L for GLY in water [11], but in some areas, its concentration is as high as 0.7 mg/L [12]. Although relatively low GLY concentrations have been detected in surface waters in Germany, Hungary, Switzerland, and northeastern Spain (0.1–2.5 μg/L, which is still often above the EU limit), high levels (up to 165 μg/L) have often been found in Denmark and France [8]. Eventually, an efficient strategy will be required to treat wastewaters and other streams that are contaminated with GLY before concentration of this compound reaches alarming levels and GLY diffuses into other compartments, such as the food chain.
Conventional water treatment methods, including adsorption, biological oxidation, and chemical oxidation, have all been used to remove GLY from water [13,14,15,16,17,18]. In recent decades, advanced oxidation processes (AOPs) have attracted much attention as promising methods for the abatement of organic pollutants [16]. The AOPs include numerous techniques based on the in situ formation of strong oxidants. Among those species, the hydroxyl radical (HO•) plays a central role in AOPs due to its high standard reduction potential (2.8 V vs. NHE), especially in acidic media. Being highly reactive and nonselective, HO• can oxidize several organic compounds [19]. Main AOPs include ultraviolet (UV) irradiation, H2O2/UV, photocatalysis, ozonation, electrochemical oxidation, Fenton processes, and Fenton-like processes. Many of these have been used to remove GLY from wastewater [20,21,22,23,24,25].
Unfortunately, the majority of AOPs are too costly to be applied to the treatment of environmental water matrices, which are much more likely than wastewater to be contaminated by GLY. Oxidation with Fenton’s reagent is an AOP based on ferrous ions reacting with hydrogen peroxide to produce hydroxyl radicals and/or other oxidizing species (e.g., ferryl). Fenton is an effective and proven method for the abatement of several hazardous organic pollutants [16,22]. Over the past few years, heterogeneous Fenton-like processes using nanoscale zero-valent iron (nZVI) have attracted much attention due to their ability to efficiently remove heavy metals and organic contaminants [26,27,28,29,30,31,32,33]. Under acidic conditions, slowly released Fe2+ (or FeII occurring on the surface of nZVI) activates H2O2 to promote the production of HO• (and/or ferryl), as described in reactions (1–2) [34,35]. The most important advantage of using nZVI, rather than conventional ferrous ions, is the rapid recycling of Fe3+ into Fe2+ at the surface of the nZVI, which can proceed as shown in reaction (3) [35]. Moreover, magnetic nZVI can be easily separated from the aqueous phase at the end of treatment.
Fe0 + H2O2 + 2 H+ → Fe2+ + 2 H2O
Fe2+ + H2O2 → Fe3+ + OH− + HO•
2 Fe3+ + Fe0 → 3 Fe2+
It has been reported that, by using nZVI (22.4 mg/L) and H2O2 (0.1 mmol/L) at pH 3, chlorpheniramine (10 mg/L) can be completely removed at room temperature [36]. Moreover, the efficiency of the degradation of polyvinyl alcohol (PVA) reached 94% in 1 min at pH 3 with a nZVI dosage of 5 mg/L and a H2O2 concentration of 1 × 10−4 mol/L [34]. Many approaches have been attempted to remove different contaminants from water using Fenton-related processes [37,38,39,40,41,42,43,44,45]. However, GLY removal from water using nZVI, through either adsorption or degradation (nZVI/H2O2 Fenton-like system), has not yet been reported.
This is the first work, to our knowledge, in which GLY removal was investigated by using nZVI + H2O2 at different pH values (a heterogeneous Fenton-like process). The removal efficiency was here maximized by optimizing the concentrations/loadings of both nZVI and H2O2. The comparative removal of GLY via either nZVI alone or nZVI + H2O2 was also studied so as to highlight the role of the nZVI–Fenton reaction in the process. Finally, nZVI–Fenton degradation of GLY was also investigated using tap water.
2. Results and Discussion
To optimize the concentrations/loadings of nZVI and H2O2, a number of experiments were performed at different pH values to investigate the efficiency of GLY degradation. Preliminary experiments with nZVI + H2O2 at pH 3 were carried out to highlight the Fenton degradation (Figure 1).
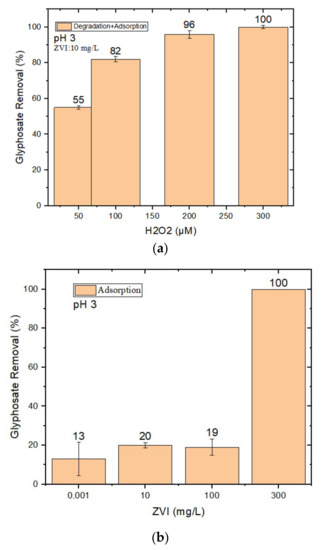
Figure 1.
(a) Effect of H2O2 concentration (50–300 μM) on GLY removal via nZVI–Fenton at pH 3 at 10 mg/L nZVI loading, optimized to minimize adsorption; (b) GLY removal using different loadings of nZVI alone, without H2O2, at pH 3. The error bounds represent the standard deviation of triplicate experiments.
The loading of nZVI (varying in the range of 0.001–300 mg/L) was optimized while minimizing GLY removal by nZVI alone (which could involve adsorption, at least in part; see Figure 1b). Indeed, although complete GLY removal could be achieved by using 300 mg/L nZVI without H2O2 (Figure 1b), such a high nZVI loading would be impractical in water treatment applications due to the elevated costs of chemical reagents. (A quantity of 300 mg/L nZVI would cost approximately USD 0.24/m3 [46]).
Note that adsorption is not the only phenomenon that could account for the removal of GLY by nZVI alone, as shown in Figure 1b. Indeed, the nZVI–Fenton process can be activated by O2 alone, without H2O2, with the generation of both Fenton reactants [47]:
Fe0 + O2 + 2H+ → Fe2+ + H2O2
The minimum tested loading of nZVI (0.001 mg/L) was effective at minimizing adsorption, but it did not induce effective GLY degradation in the presence of H2O2. Moreover, such a low level of loading could not be obtained by direct weighting, but rather by the dilution of a stock nZVI suspension. Presumably due to the difficulty of fully homogenizing the stock suspension, the system lacked reproducibility in results.
Therefore, compromise loadings (10 mg/L, i.e., the lowest loading that could be achieved via direct weighting, or 20 mg/L) were chosen for the nZVI in subsequent experiments.
2.1. GLY Removal at pH 3
As shown in Figure 1a, complete GLY removal could be achieved at pH 3 by using 10 mg/L nZVI and 300 μM H2O2. Lower H2O2 concentrations were less effective, most likely because of lower hydroxyl radical (HO•) generation when H2O2 is low. Higher H2O2 concentrations were not tested because their use would increase treatment costs (300 μM H2O2 would cost approximately USD 0.009/m3 [46]) and because effectiveness would decrease at a certain point due to HO• scavenging by the H2O2 itself [48]:
H2O2 + HO• → HO2• + H2O
2.2. GLY Removal at pH 4
It was not possible to achieve satisfactory GLY removal at pH 4 in the presence of 10 mg/L nZVI + H2O2. For this reason, higher loadings of nZVI (20–40 mg/L) were tested, but the adsorption of GLY by nZVI alone (i.e., without H2O2) was also checked. Limited adsorption was observed with 20 mg/L nZVI, which was then used to perform further degradation experiments at different concentrations of H2O2 (200–400 μM). However, as shown in Figure 2a, the maximum GLY removal by nZVI + H2O2 was, at most, only 72% when using 400 μM H2O2 at pH 4.
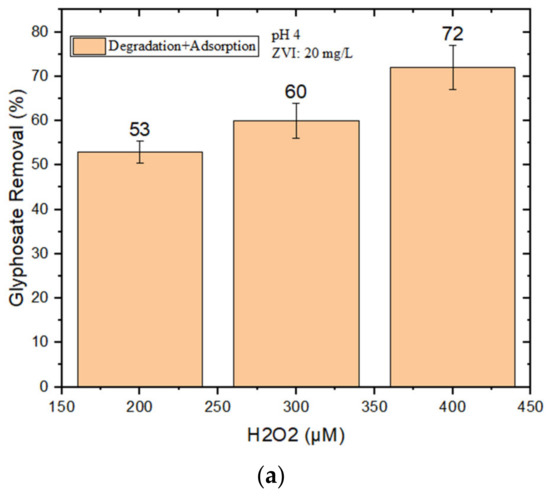
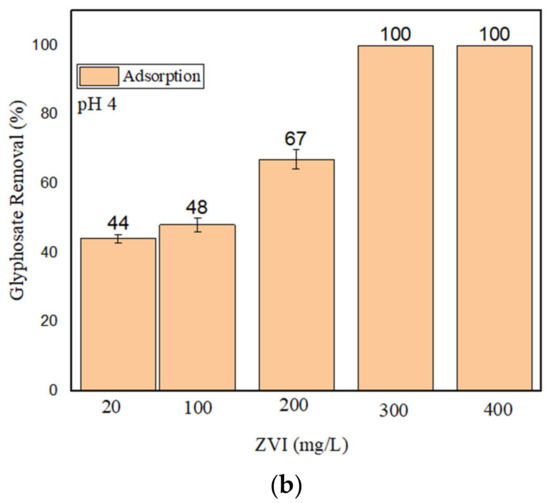
Figure 2.
(a) GLY removal (%) by nZVI (20 mg/L) and H2O2 (200–400 μM) at pH 4; (b) GLY removal at pH 4 by nZVI alone (with different loadings).
An alternative strategy, proposed by Minella et al. (2019), consists in the stepwise addition of H2O2. This procedure allows for the use of a relatively high overall amount of H2O2 (thereby providing a sufficiently high total amount of generated HO•), while simultaneously minimizing HO• scavenging by the H2O2 itself. The rationale is that the H2O2 becomes progressively degraded as the reaction goes on and, if H2O2 is added stepwise, it never reaches excessive concentration values at any time point [31]. By adding 150 μM of H2O2 at the beginning, plus an additional 50 μM at 30 min after the start of the reaction, we obtained 87% GLY removal with 20 mg/L nZVI at pH 4 (Figure 3).
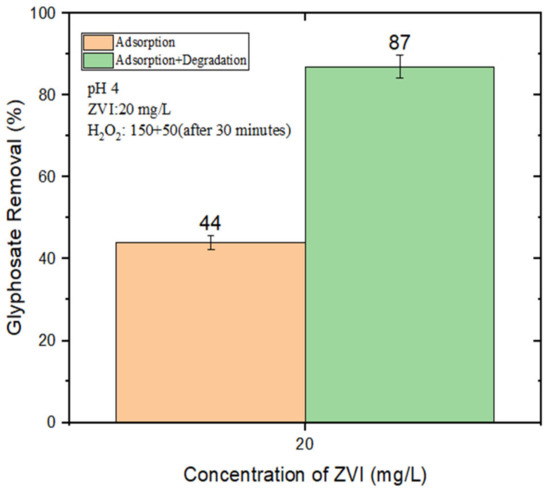
Figure 3.
GLY removal (%) by nZVI (20 mg/L) and, where applicable (“Adsorption + Degradation”), H2O2 (150 μM at t = 0 + 50 μM at t = 30 min) at pH 4.
2.3. GLY Removal at pH Values of 5 and 6
Several combinations of nZVI loadings (10–30 mg/L) and H2O2 concentrations, including multiple additions of different amounts of the latter, were attempted for the removal of GLY at pH values of 5 and 6 via nZVI–Fenton. However, no GLY degradation at all was observed. In particular, the tested conditions at both pH 5 and 6 were as follows: (i) 10 mg/L nZVI, 150 μM H2O2 at the beginning of the reaction, plus an additional 50 μM H2O2 at 30 min reaction time; (ii) 20 mg/L nZVI, 200 μM H2O2 at the beginning of the reaction, plus an additional 50 μM H2O2 at 30 min reaction time; (iii) 30 mg/L nZVI, 300 μM H2O2 at the beginning of the reaction, plus an additional 50 μM H2O2 at 30 min reaction time.
The most likely explanation for the lack of GLY degradation at pH values of 5 and 6 is the decrease in efficiency of the Fenton reaction with the increase in the pH value (and of the nZVI–Fenton reaction as well [31,46]). Actually, a major issue in the Fenton process is Fe(III) recycling to Fe(II) [46,49]. As the pH increases, the decreasing solubility of the Fe(III) hampers its reduction to Fe(II), slowing down the Fenton degradation [49].
2.4. GLY Removal from Tap Water
GLY removal was then studied using GLY-spiked tap water at pH values of 3 and 4 (the pH was adjusted by H2SO4; the original tap water pH was 7.6–7.7), starting with the loadings/concentrations of nZVI and H2O2 already optimized in ultra-pure water. At pH 3, with 10 mg/L nZVI and 300 μM H2O2, the GLY removal efficiency reached 80%. Unfortunately, it was not possible to improve the removal percentage, even by employing the stepwise addition method. In particular, the achieved removal percentages at 1 h were as follows: (i) 54% with 10 mg/L nZVI, 150 μM H2O2 at the beginning of the reaction, plus an additional 50 μM H2O2 at 30 min reaction time; (ii) 72% with 10 mg/L nZVI, 200 μM H2O2 at the beginning of the reaction, plus an additional 50 μM H2O2 at 30 min reaction time; (iii) 75% with 10 mg/L nZVI, 350 μM H2O2 at the beginning of the reaction, plus an additional 50 μM H2O2 at 30 min reaction time; (iv) 76% with 10 mg/L nZVI, 400 μM H2O2 at the beginning of the reaction, plus an additional 50 μM H2O2 at 30 min reaction time.
The lower level of GLY removal from tap water, compared to ultra-pure water, might be tentatively ascribed to the presence of common inorganic anions in tap water (Cl−, SO42−, and NO3−) (see Table 1). Some anions, and especially chloride, are able to scavenge HO• at pH 3 to produce different reactive transient species that are, however, significantly less reactive than HO• itself [48], thereby lowering the nZVI–Fenton efficiency [33]. Furthermore, the scavenging of HO• by H2O2 would become comparatively less important in tap water that already contains other HO• scavengers, thereby decreasing the effectiveness of the stepwise H2O2 addition method.

Table 1.
Concentration values of different anions in tap water.
Very interestingly, it was possible to achieve 100% GLY removal from tap water at pH 4 by using 20 mg/L nZVI, 150 μM of H2O2 at the beginning of the reaction, plus an additional 50 μM at 30 min reaction time. Complete removal of GLY was achieved in 40 min, and degradation at 30 min was around 66% (Figure 4). A possible reason for the better performance of nZVI–Fenton at pH 4, as compared to pH 3, is that the reaction rate between chloride and HO• decreases with increases in the pH value, because the HO• + Cl− reaction step becomes partially reversible [48].
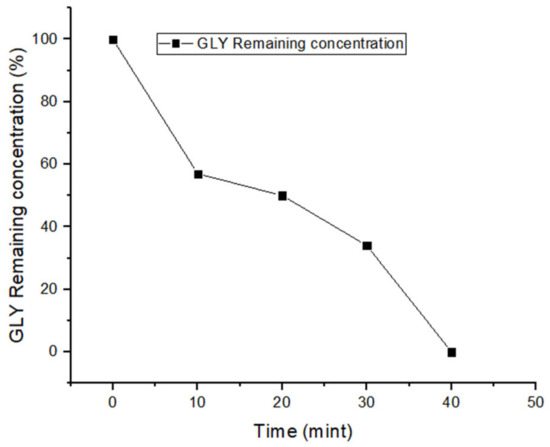
Figure 4.
Kinetics of GLY removal from tap water at pH 4.
The pH and conductivity values of the GLY-spiked tap water were monitored during the nZVI–Fenton treatment. In the case of the experiments carried out at pH 3, the pH value was quite stable and ranged between 3–3.2. Initial water conductivity was 0.53–0.58 mS/cm; it increased to around 0.9–1.1 mS/cm upon acidification to pH 3, and then it remained reasonably stable during the advancement of the reaction.
As far as the degradation experiments at a pH value of 4 are concerned, initial tap-water conductivity was around 0.5–0.55 mS/cm, and it increased to around 0.6 mS/cm upon acidification to pH 4. A further increase, by ~0.1 mS/cm units, is expected to take place in the final neutralization step, intended to bring the pH to at least 6 to allow for discharge in the environment. Despite the increase in conductivity, it would still be possible to reuse treated water in several fields, including some of the most demanding in terms of water conductivity (e.g., agriculture [50]). The pH value was reasonably stable during the reaction (4.0–4.3). Leached iron at pH 4 was quantified as well; the concentration of dissolved Fe was undetectable before treatment and amounted to 2.5 mg/L at the end of the treatment. This means that around 12% of the initially added nZVI (20 mg/L) underwent dissolution at pH 4. When compared with Fe wastewater limits (4 mg/L according to Italian law), a dissolved Fe concentration of 2.5 mg/L suggests that treated water can be safely discharged without the need for additional steps of iron removal (apart from the easy magnetic separation of nZVI).
Under optimal conditions in tap water (pH 4, 20 mg/L nZVI, 150 + 50 μM H2O2), the GLY removal capacity of nZVI–Fenton can be calculated as at least 100 mg GLY/g nZVI. This value exceeds the capacity of adsorption systems based on metals with high complexation capabilities towards P-containing ligands [51] and, to an even higher extent, the adsorption capacity of activated carbon [52], which is a widespread adsorbent used in tertiary treatments at most potabilization facilities. Therefore, the studied Fenton-like process appears to make better use of the solid reagent, as compared to adsorptive techniques.
2.5. Cost Estimates for GLY Removal from Tap Water
Based on the results reported in the previous section, a preliminary and partial estimate of the costs of the nZVI–Fenton process can be carried out within the framework of expenditures for chemical reagents per m3 of treated water. This estimate is significant, in that reagents are a major expenditure in Fenton treatments [53]. The necessary infrastructure, including basins for water treatment, also contributes to the treatment costs, but in the case of Fenton techniques, the required infrastructure is much simpler and considerably less costly, as compared to ozonation, for example. [53].
The costs of bulk chemicals that were used as reference for calculations are as follows [46]: USD 800/ton for nZVI, USD 500/ton for H2O2, USD 240/ton for H2SO4, and USD 120/ton for CaO. Note that CaO is a relatively cheap base that can be used to adjust pH after treatment. Indeed, because the environmental discharge of treated water with a pH value of 3 or 4 is not permitted, the water’s pH should be increased to at least 6 in order to avoid damaging the receiving bodies of water [50].
In the case of GLY degradation at pH 4, with 20 mg/L nZVI and 200 μM H2O2 (i.e., 150 μM + 50 μM), cost estimates are USD 0.016/m3 for nZVI and USD 0.006/m3 for H2O2. The cost of H2SO4 can be estimated by carrying out tap-water titration and then measuring the amount of H2SO4 that is required to adjust the water’s pH to any given value (pH 4 in the present case; see Figure 5). On this basis, one can conclude that the adjustment of the pH value to 4 would require 1.8 mol H2SO4 m−3, which translates to a cost of USD 0.041/m3 for H2SO4 [46]. Moreover, again based on water titration data, one would also need 1 mol CaO m−3 to finally adjust the pH value to at least 6 for eventual discharge or reuse, which entails an added cost of USD 0.0067/m3 [46]. Overall, the elimination of GLY from water using the nZVI–Fenton technique at a pH of 4 would cost USD 0.070/m3 in chemical reagents.
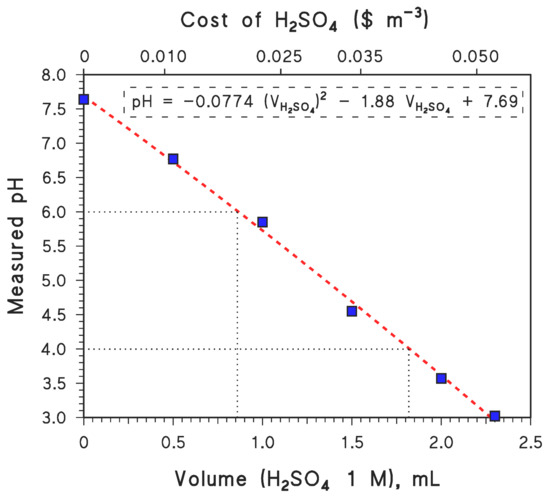
Figure 5.
Results of tap-water titration with H2SO4 (measured pH, as a function of added volume of H2SO4 1 M). The upper X-axis plots the cost of H2SO4 addition, based on an estimate of USD 240 (ton H2SO4)−1 [46]. The reported phenomenological equation is a second-order polynomial fit. The volumes of H2SO4 needed to bring the pH to values of 4 and 6 are highlighted. (The latter allows for the calculation of the amount of CaO needed to fix the pH at 6 at the end of the treatment.).
Treatment at pH 3 would cost USD 0.008/m3 for the nZVI, USD 0.009/m3 for the H2O2, USD 0.052/m3 for the H2SO4, and USD 0.010/m3 for the CaO, for a total of USD 0.079/m3 (i.e., a slightly higher cost, as compared to pH 4, to achieve a lower level of GLY removal). Moreover, higher conductivity increases (up to 1–1.5 mS/cm) were observed upon water treatment at pH 3, as compared to at pH 4, and 8 mg/L iron leached from 10 mg/L nZVI at pH 3 (implying 80% dissolution of initially occurring nZVI). This means that a further step of dissolved-iron removal before discharge would be required for treatment at pH 3.
By comparison, the cost of traditional wastewater treatment (including sedimentation and activated sludge, which is often unable to remove GLY) is in the range of USD 0.3–0.4/m3 [31,46]. Ozonation costs would be in the same range (about 4 times as much as the cost of chemicals in Fenton [53]), which makes nZVI–Fenton a potentially very competitive and effective technique for the removal of GLY from aqueous streams.
3. Experimental
3.1. Reagents and Materials
All chemical reagents used in the study were of analytical grade and were used without further purification. Nanoscale zero-valent iron (nZVI, ≥ 99.5% purity), hydrogen peroxide (H2O2, 30% w/w), H2SO4 (98%), glyphosate (OH)2P(O)CH2NHCH2CO2H, and NaOH (≥ 97%) were obtained from Sigma-Aldrich (Darmstadt, Germany). All solutions were prepared by using Milli-Q water (Elix-Milli Q Academic system (Millipore-Merck, Vimodrone, Italy).
3.2. Experimental Procedure
All experiments were performed at room temperature (20–22 °C), and the initial pH values were adjusted using diluted solutions of sulfuric acid (see below for the choice of H2SO4). In a typical experiment, 1 L of ultra-pure water containing 2 mg/L GLY was placed in a 1.2 L beaker. The value of the pH was monitored with a Checker pH meter (Hanna Instruments, Woonsocket, RI, US), and it was adjusted whenever required using diluted H2SO4. The required amounts of directly weighed (or pipetted) nZVI and H2O2 were added to already-prepared 2 mg/L GLY, and the whole system was then magnetically stirred for 1 h of reaction time. (Longer times would be uninteresting within the framework of water treatment.) After 1 h, a few mL of the sample was withdrawn, filtered (nylon 0.45 μm, VWR, Radnor, PE, USA), and injected into an ion chromatograph (Thermofisher-Dionex DX-100, Sunnyvale, CA, USA) equipped with a 200 μL loop, using 17 mM NaOH as the eluent. GLY was separated and detected with an IonPac AS16 column (250 × 4 mm, Thermofisher-Dionex, Sunnyvale, CA, USA), a Dionex ASRS 4 mm membrane suppressor, and a conductivity detector. The same experimental procedure was adopted to check for the adsorption efficiency of GLY using nZVI, with the exception that H2O2 was not added in this case. All experiments were conducted in triplicate.
The concentration of dissolved iron leached during the nZVI–Fenton treatment was determined via inductively coupled plasma–optical emission spectroscopy (ICP–OES, Agilent 5100, Santa Clara, CA, USA). Emission of atomic Fe was quantified at 238.204 nm, and the Fe-detection limit with this technique was at the level of μg/L. By comparison, the limit for Fe in wastewater is 4 mg/L according to Italian law [Legislative decree 152/06].
In tap-water experiments, tap water (from Turin, Italy) was spiked with GLY to achieve the desired 2 mg/L concentration. Then, the same procedure (as described earlier) was followed, but conductivity was also monitored throughout the reaction. Common inorganic anions in tap water were determined via suppressed anion chromatography.
Before deciding how to adjust the pH value, preliminary experiments were performed to minimize chromatographic interferences of acids (HClO4, HCl, and H2SO4) and buffers (phosphate and acetate) in the detection of GLY. Chromatographic interference was observed between GLY and HClO4, which was thus excluded. Although chloride did not interfere with GLY detection, HCl was not chosen because of the ability of chloride ions to scavenge HO• in acidic solutions [48,54]. Phosphate buffer was not selected, owing to the very close elution of phosphate and GLY, which is due to the similar selectivity usually exhibited by chromatographic columns for these anions. Acetate buffer was tested but excluded due to ineffectiveness. (The orange-red Fe2+-acetate complex is possibly unreactive within the framework of nZVI + H2O2.) Therefore, H2SO4 was finally selected to adjust the pH value, with consideration of the high chromatographic resolution between sulfate and GLY ions.
4. Conclusions
Significant GLY degradation was obtained in the pH range of 3–4 using the nZVI–Fenton technique after optimization, which encompassed a variety of conditions of nZVI loading and H2O2 concentration. Unfortunately, the decrease in Fenton reactivity with an increase in pH prevented effective degradation to be achieved at either pH 5 or 6. GLY removal was also obtained at pH 3 with nZVI alone, without H2O2, but the required loading (300 mg/L nZVI) was 15–30 times higher than the loading required in nZVI–Fenton. That issue would be reflected in rather high costs for water treatment with nZVI alone.
In the case of tap water, complete (100%) GLY removal was achieved at pH 4 with 20 mg/L nZVI and 150 + 50 μM H2O2 (i.e., 150 μM added at t = 0, and 50 μM added after 30 min reaction time). Overall, water treatment costs for GLY with nZVI + H2O2 at pH 4 would be very reasonable in terms of the expenditures for chemical reagents (a total of USD 0.07/m3, of which approximately 30% would be for nZVI + H2O2, and the rest would go to pH-adjustment reagents). Interestingly, these same conditions would also allow for the removal of additional emerging contaminants (pharmaceuticals) from the water matrix [29,42].
Potential drawbacks of water treatment in acidic conditions are the elevated costs for pH adjustment, the increase in water conductivity following the acidification necessary for the Fenton process to work, the basification at the end of treatment to allow for water discharge, as well as the leaching of iron from the nZVI due to increased iron solubility at an acidic pH. As far as pH adjustment costs are concerned, we show here that they make up ~70% of the total reagent costs in the case of treatment at pH 4, and even ~80% for treatment at pH 3. With these limitations in mind, in this work, nZVI–Fenton was also tested at pH values of 5 and 6 but, unfortunately, no GLY degradation was achieved under these conditions. Water treatment at pH 4 would be quite satisfactory, however, because overall reagent costs would still be low, and the increase in conductivity was quite small, as compared, for instance, to treatment at pH 3. (Tap-water conductivity more than doubled upon treatment at pH 3, while a 40% increase is expected to occur at pH 4). Therefore, final water conductivity would still allow for water reuse in agriculture, which is a very demanding field as far as conductivity requirements are concerned. Furthermore, iron leaching from nZVI at pH 4 was low enough for treated water to meet the legal limits for safe discharge into the environment, while the same would not occur in the case of treatment at pH 3.
Author Contributions
N.A., study design, experimentation, writing—original draft preparation, writing—editing, and interpretation; D.V., writing, conception, study design, manuscript revision, critical revision, and supervision; L.R., visualization and laboratory assistance; M.C., visualization and laboratory assistance; M.S.B.-G., visualization; M.C.B., conception, study design, data revision, manuscript revision, critical revision, and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the used reagents are available from the authors.
References
- Fogliatto, S.; Ferrero, A.; Vidotto, F. Current and Future Scenarios of Glyphosate Use in Europe: Are There Alternatives? Adv. Agron. 2020, 163, 219–278. [Google Scholar] [CrossRef]
- Cuhra, M.; Bøhn, T.; Cuhra, P. Glyphosate: Too Much of a Good Thing? Front. Environ. Sci. 2016, 4, 28. [Google Scholar] [CrossRef]
- Duke, S.O.; Powles, S.B. Glyphosate: A Once-in-a-Century Herbicide. Pest Manag. Sci. 2008, 64, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Maggi, F.; la Cecilia, D.; Tang, F.H.M.; McBratney, A. The Global Environmental Hazard of Glyphosate Use. Sci. Total Environ. 2020, 717, 137167. [Google Scholar] [CrossRef]
- Maggi, F.; Tang, F.H.M.; la Cecilia, D.; McBratney, A. PEST-CHEMGRIDS, Global Gridded Maps of the Top 20 Crop-Specific Pesticide Application Rates from 2015 to 2025. Sci. Data 2019, 6, 170. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, R.; Defarge, N.; Spiroux de Vendômois, J.; Séralini, G.E. Potential Toxic Effects of Glyphosate and Its Commercial Formulations below Regulatory Limits. Food Chem. Toxicol. 2015, 84, 133–153. [Google Scholar] [CrossRef]
- Tarazona, J.V.; Court-Marques, D.; Tiramani, M.; Reich, H.; Pfeil, R.; Istace, F.; Crivellente, F. Glyphosate Toxicity and Carcinogenicity: A Review of the Scientific Basis of the European Union Assessment and Its Differences with IARC. Arch. Toxicol. 2017, 91, 2723–2743. [Google Scholar] [CrossRef] [PubMed]
- Van Bruggen, A.H.C.; He, M.M.; Shin, K.; Mai, V.; Jeong, K.C.; Finckh, M.R.; Morris, J.G. Environmental and Health Effects of the Herbicide Glyphosate. Sci. Total Environ. 2018, 616–617, 255–268. [Google Scholar] [CrossRef]
- Gupta, P.; Verma, N. Evaluation of Degradation and Mineralization of Glyphosate Pollutant in Wastewater Using Catalytic Wet Air Oxidation over Fe-Dispersed Carbon Nanofibrous Beads. Chem. Eng. J. 2021, 417, 128029. [Google Scholar] [CrossRef]
- Székács, A.; Darvas, B. Re-Registration Challenges of Glyphosate in the European Union. Front. Environ. Sci. 2018, 6, 78. [Google Scholar] [CrossRef]
- Botta, F.; Lavison, G.; Couturier, G.; Alliot, F.; Moreau-Guigon, E.; Fauchon, N.; Guery, B.; Chevreuil, M.; Blanchoud, H. Transfer of Glyphosate and Its Degradate AMPA to Surface Waters through Urban Sewerage Systems. Chemosphere 2009, 77, 133–139. [Google Scholar] [CrossRef]
- Peruzzo, P.J.; Porta, A.A.; Ronco, A.E. Levels of Glyphosate in Surface Waters, Sediments and Soils Associated with Direct Sowing Soybean Cultivation in North Pampasic Region of Argentina. Environ. Pollut. 2008, 156, 61–66. [Google Scholar] [CrossRef]
- Mayakaduwa, S.S.; Kumarathilaka, P.; Herath, I.; Ahmad, M.; Al-Wabel, M.; Ok, Y.S.; Usman, A.; Abduljabbar, A.; Vithanage, M. Equilibrium and Kinetic Mechanisms of Woody Biochar on Aqueous Glyphosate Removal. Chemosphere 2016, 144, 2516–2521. [Google Scholar] [CrossRef]
- Herath, I.; Kumarathilaka, P.; Al-Wabel, M.I.; Abduljabbar, A.; Ahmad, M.; Usman, A.R.A.; Vithanage, M. Mechanistic Modeling of Glyphosate Interaction with Rice Husk Derived Engineered Biochar. Microporous Mesoporous Mater. 2016, 225, 280–288. [Google Scholar] [CrossRef]
- Feng, D.; Soric, A.; Boutin, O. Treatment Technologies and Degradation Pathways of Glyphosate: A Critical Review. Sci. Total Environ. 2020, 742, 140559. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, X.; Li, W.; Li, J.; Wu, T.; Wang, H.; Huang, J.; Xu, F. Enhanced Removal of Glyphosate from Aqueous Solution by Nano-CaO2/AS Composite: Oxidation and Precipitation. Sep. Purif. Technol. 2022, 288, 120349. [Google Scholar] [CrossRef]
- Fiorilli, S.; Rivoira, L.; Calì, G.; Appendini, M.; Bruzzoniti, M.C.; Coïsson, M.; Onida, B. Iron Oxide inside SBA-15 Modified with Amino Groups as Reusable Adsorbent for Highly Efficient Removal of Glyphosate from Water. Appl. Surf. Sci. 2017, 411, 457–465. [Google Scholar] [CrossRef]
- Rivoira, L.; Appendini, M.; Fiorilli, S.; Onida, B.; Del Bubba, M.; Bruzzoniti, M.C. Functionalized Iron Oxide/SBA-15 Sorbent: Investigation of Adsorption Performance towards Glyphosate Herbicide. Environ. Sci. Pollut. Res. 2016, 23, 21682–21691. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Montero, P.J.; Vega-Verduga, C.; Alulema-Pullupaxi, P.; Fernández, L.; Paz, J.L. Technologies Employed in the Treatment of Water Contaminated with Glyphosate: A Review. Molecules 2020, 25, 5550. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, Y. Study on the Photocatalytic Degradation of Glyphosate by TiO2 Photocatalyst. Chemosphere 2007, 67, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Assalin, M.R.; De Moraes, S.G.; Queiroz, S.C.N.; Ferracini, V.L.; Duran, N. Studies on Degradation of Glyphosate by Several Oxidative Chemical Processes: Ozonation, Photolysis and Heterogeneous Photocatalysis. J. Environ. Sci. Health Part B 2009, 45, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Bokare, A.D.; Choi, W. Review of Iron-Free Fenton-like Systems for Activating H2O2 in Advanced Oxidation Processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.H.; Nguyen, H.C.; Le, T.S.; Dang, V.A.D.; Cao, T.H.; Le, C.K.; Dang, T.D. Degradation of Glyphosate Herbicide by an Electro-Fenton Process Using Carbon Felt Cathode. Environ. Technol. 2019, 42, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Deng, Q.; Yan, W.; Jing, C.; Zhang, Y. Comparative Study of Glyphosate Removal on Goethite and Magnetite: Adsorption and Photo-Degradation. Chem. Eng. J. 2018, 352, 581–589. [Google Scholar] [CrossRef]
- Cao, L.; Ma, D.; Zhou, Z.; Xu, C.; Cao, C.; Zhao, P.; Huang, Q. Efficient Photocatalytic Degradation of Herbicide Glyphosate in Water by Magnetically Separable and Recyclable BiOBr/Fe3O4 Nanocomposites under Visible Light Irradiation. Chem. Eng. J. 2019, 368, 212–222. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, T.; Zhi, D.; Guo, B.; Zhou, Y.; Nie, J.; Huang, A.; Yang, Y.; Huang, H.; Luo, L. Applications of Nanoscale Zero-Valent Iron and Its Composites to the Removal of Antibiotics: A Review. J. Mater. Sci. 2019, 54, 12171–12188. [Google Scholar] [CrossRef]
- Lopez-Tejedor, D.; Benavente, R.; Palomo, J.M. Iron Nanostructured Catalysts: Design and Applications. Catal. Sci. Technol. 2018, 8, 1754–1776. [Google Scholar] [CrossRef]
- Adusei-Gyamfi, J.; Acha, V. Carriers for Nano Zerovalent Iron (NZVI): Synthesis, Application and Efficiency. RSC Adv. 2016, 6, 91025–91044. [Google Scholar] [CrossRef]
- Lu, H.J.; Wang, J.K.; Ferguson, S.; Wang, T.; Bao, Y.; Hao, H.X. Mechanism, Synthesis and Modification of Nano Zerovalent Iron in Water Treatment. Nanoscale 2016, 8, 9962–9975. [Google Scholar] [CrossRef]
- Stefaniuk, M.; Oleszczuk, P.; Ok, Y.S. Review on Nano Zerovalent Iron (NZVI): From Synthesis to Environmental Applications. Chem. Eng. J. 2016, 287, 618–632. [Google Scholar] [CrossRef]
- Minella, M.; Bertinetti, S.; Hanna, K.; Minero, C.; Vione, D. Degradation of Ibuprofen and Phenol with a Fenton-like Process Triggered by Zero-Valent Iron (ZVI-Fenton). Environ. Res. 2019, 179, 108750. [Google Scholar] [CrossRef] [PubMed]
- Bruzzoniti, M.C.; Fiore, S. Removal of Inorganic Contaminants from Aqueous Solutions: Evaluation of the Remediation Efficiency and of the Environmental Impact of a Zero-Valent Iron Substrate. Water. Air. Soil Pollut. 2014, 225, 2098 . [Google Scholar] [CrossRef]
- Ahmed, N.; Vione, D.; Rivoira, L.; Carena, L.; Castiglioni, M.; Bruzzoniti, M.C. A Review on the Degradation of Pollutants by Fenton-Like Systems Based on Zero-Valent Iron and Persulfate: Effects of Reduction Potentials, PH, and Anions Occurring in Waste Waters. Molecules 2021, 26, 4584. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Hsu, S.T. Performance of NZVI/H2O2 Process in Degrading Polyvinyl Alcohol in Aqueous Solutions. Sep. Purif. Technol. 2018, 203, 111–116. [Google Scholar] [CrossRef]
- Zha, S.; Cheng, Y.; Gao, Y.; Chen, Z.; Megharaj, M.; Naidu, R. Nanoscale Zero-Valent Iron as a Catalyst for Heterogeneous Fenton Oxidation of Amoxicillin. Chem. Eng. J. 2014, 255, 141–148. [Google Scholar] [CrossRef]
- Wang, L.; Yang, J.; Li, Y.; Lv, J.; Zou, J. Removal of Chlorpheniramine in a Nanoscale Zero-Valent Iron Induced Heterogeneous Fenton System: Influencing Factors and Degradation Intermediates. Chem. Eng. J. 2016, 284, 1058–1067. [Google Scholar] [CrossRef]
- Rahman, M.; Kim, J.E. Remediation of Water Contaminated with Herbicide Oxadiazon Using Fenton Reagent. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 458–463. [Google Scholar] [CrossRef]
- Yang, R.; Zeng, G.; Xu, Z.; Zhou, Z.; Zhou, Z.; Ali, M.; Sun, Y.; Sun, X.; Huang, J.; Lyu, S. Insights into the Role of Nanoscale Zero-Valent Iron in Fenton Oxidation and Its Application in Naphthalene Degradation from Water and Slurry Systems. Water Environ. Res. 2022, 94, e10710. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Gao, H.; He, J.; Yang, P.; Wang, D.; Ma, T.; Xia, H.; Xu, X. Removal of Norfloxacin Using Coupled Synthesized Nanoscale Zero-Valent Iron (NZVI) with H2O2 System: Optimization of Operating Conditions and Degradation Pathway. Sep. Purif. Technol. 2017, 172, 158–167. [Google Scholar] [CrossRef]
- Rosa Barbosa, M.P.; Lima, N.S.; de Matos, D.B.; Alves Felisardo, R.J.; Santos, G.N.; Salazar-Banda, G.R.; Cavalcanti, E.B. Degradation of Pesticide Mixture by Electro-Fenton in Filter-Press Reactor. J. Water Process Eng. 2018, 25, 222–235. [Google Scholar] [CrossRef]
- Silva, M.; Baltrusaitis, J. Destruction of Emerging Organophosphate Contaminants in Wastewater Using the Heterogeneous Iron-Based Photo-Fenton-like Process. J. Hazard. Mater. Lett. 2021, 2, 100012. [Google Scholar] [CrossRef]
- Skanes, B.; Ho, J.; Warriner, K.; Prosser, R.S. Degradation of Boscalid, Pyraclostrobin, Fenbuconazole, and Glyphosate Residues by an Advanced Oxidative Process Utilizing Ultraviolet Light and Hydrogen Peroxide. J. Photochem. Photobiol. A Chem. 2021, 418, 113382. [Google Scholar] [CrossRef]
- Li, X.; Xiao, B.; Wu, M.; Wang, L.; Chen, R.; Wei, Y.; Liu, H. In-Situ Generation of Multi-Homogeneous/Heterogeneous Fe-Based Fenton Catalysts toward Rapid Degradation of Organic Pollutants at near Neutral PH. Chemosphere 2020, 245, 125663. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xiong, R.; Li, J.; Li, W.; Yang, X.; Tong, H. Insight into N-CaO2/SBC/Fe(II) Fenton-like System for Glyphosate Degradation: PH Change, Iron Conversion, and Mechanism. J. Environ. Manag. 2023, 333, 117428. [Google Scholar] [CrossRef]
- Ziembowicz, S.; Kida, M. Limitations and Future Directions of Application of the Fenton-like Process in Micropollutants Degradation in Water and Wastewater Treatment: A Critical Review. Chemosphere 2022, 296, 134041. [Google Scholar] [CrossRef]
- Furia, F.; Minella, M.; Gosetti, F.; Turci, F.; Sabatino, R.; Di Cesare, A.; Corno, G.; Vione, D. Elimination from Wastewater of Antibiotics Reserved for Hospital Settings, with a Fenton Process Based on Zero-Valent Iron. Chemosphere 2021, 283, 131170. [Google Scholar] [CrossRef]
- Keenan, C.R.; Sedlak, D.L. Ligand-Enhanced Reactive Oxidant Generation by Nanoparticulate Zero-Valent Iron and Oxygen. Environ. Sci. Technol. 2008, 42, 6936–6941. [Google Scholar] [CrossRef] [PubMed]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of Rate Constants for Reactions of Hydrated Electrons, Hydrogen Atoms and Hydroxyl Radicals (·OH/·O− in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Wang, N.; Zheng, T.; Zhang, G.; Wang, P. A Review on Fenton-like Processes for Organic Wastewater Treatment. J. Environ. Chem. Eng. 2016, 4, 762–787. [Google Scholar] [CrossRef]
- Salgot, M.; Huertas, E.; Weber, S.; Dott, W.; Hollender, J. Wastewater Reuse and Risk: Definition of Key Objectives. Desalination 2006, 187, 29–40. [Google Scholar] [CrossRef]
- Hu, Y.S.; Zhao, Y.Q.; Sorohan, B. Removal of Glyphosate from Aqueous Environment by Adsorption Using Water Industrial Residual. Desalination 2011, 271, 150–156. [Google Scholar] [CrossRef]
- Dissanayake Herath, G.A.; Poh, L.S.; Ng, W.J. Statistical Optimization of Glyphosate Adsorption by Biochar and Activated Carbon with Response Surface Methodology. Chemosphere 2019, 227, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Balabanič, D.; Hermosilla, D.; Merayo, N.; Klemenčič, A.K.; Blanco, A. Comparison of Different Wastewater Treatments for Removal of Selected Endocrine-Disruptors from Paper Mill Wastewaters. J. Environ. Sci. Health Part A 2012, 47, 1350–1363. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.H.; Kang, S.F.; Wu, F.A. Hydroxyl Radical Scavenging Role of Chloride and Bicarbonate Ions in the H2O2/UV Process. Chemosphere 2001, 44, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).