A Comprehensive Mechanistic Antibacterial and Antibiofilm Study of Potential Bioactive ((BpA)2bp)Cu/Zn Complexes via Bactericidal Mechanisms against Escherichia coli
Abstract
1. Introduction
2. Results and Discussion
2.1. Antibacterial Applicability of the Complexes
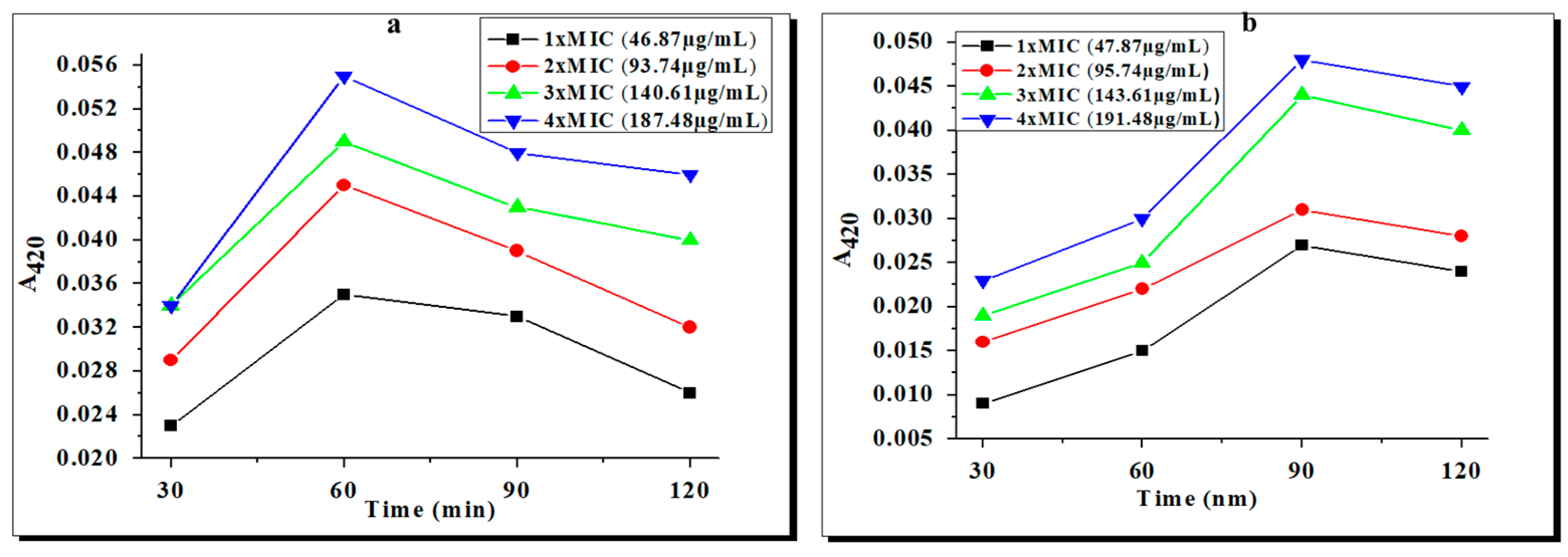
2.2. Effects of the Complexes on Membrane Integrity
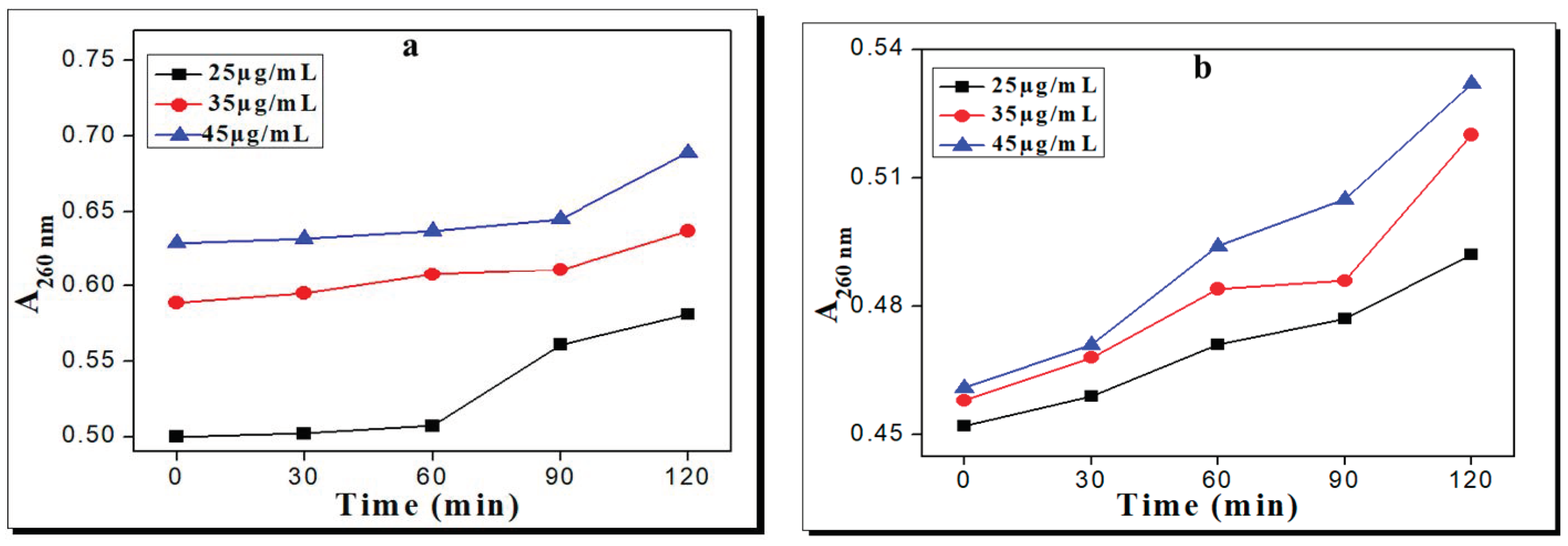
2.3. Effects of the Complexes on Inner Membrane Permeability
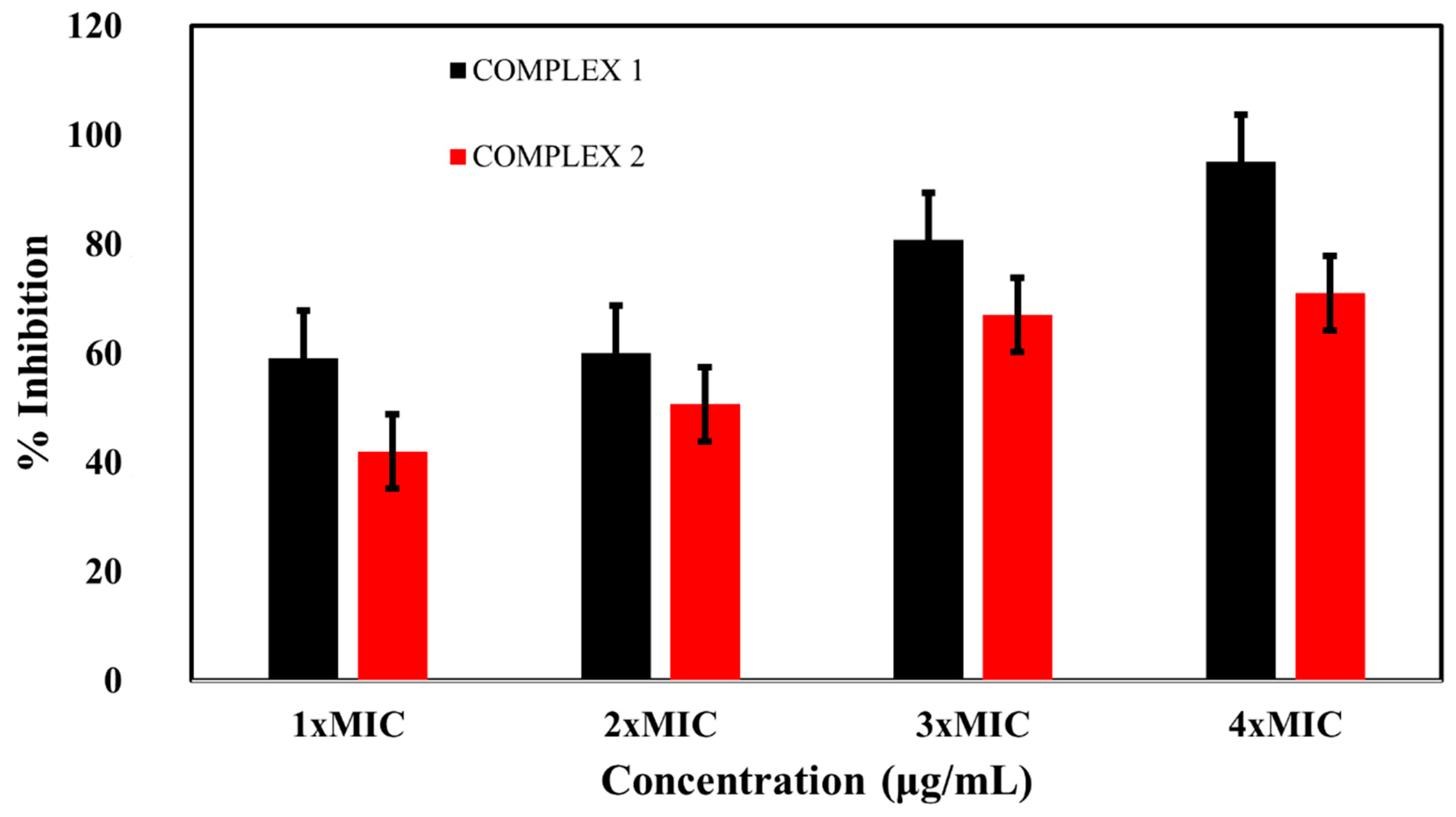
2.4. Effects of the Complexes on Biofilm Eradication
2.5. Biofilm Inhibition Activity
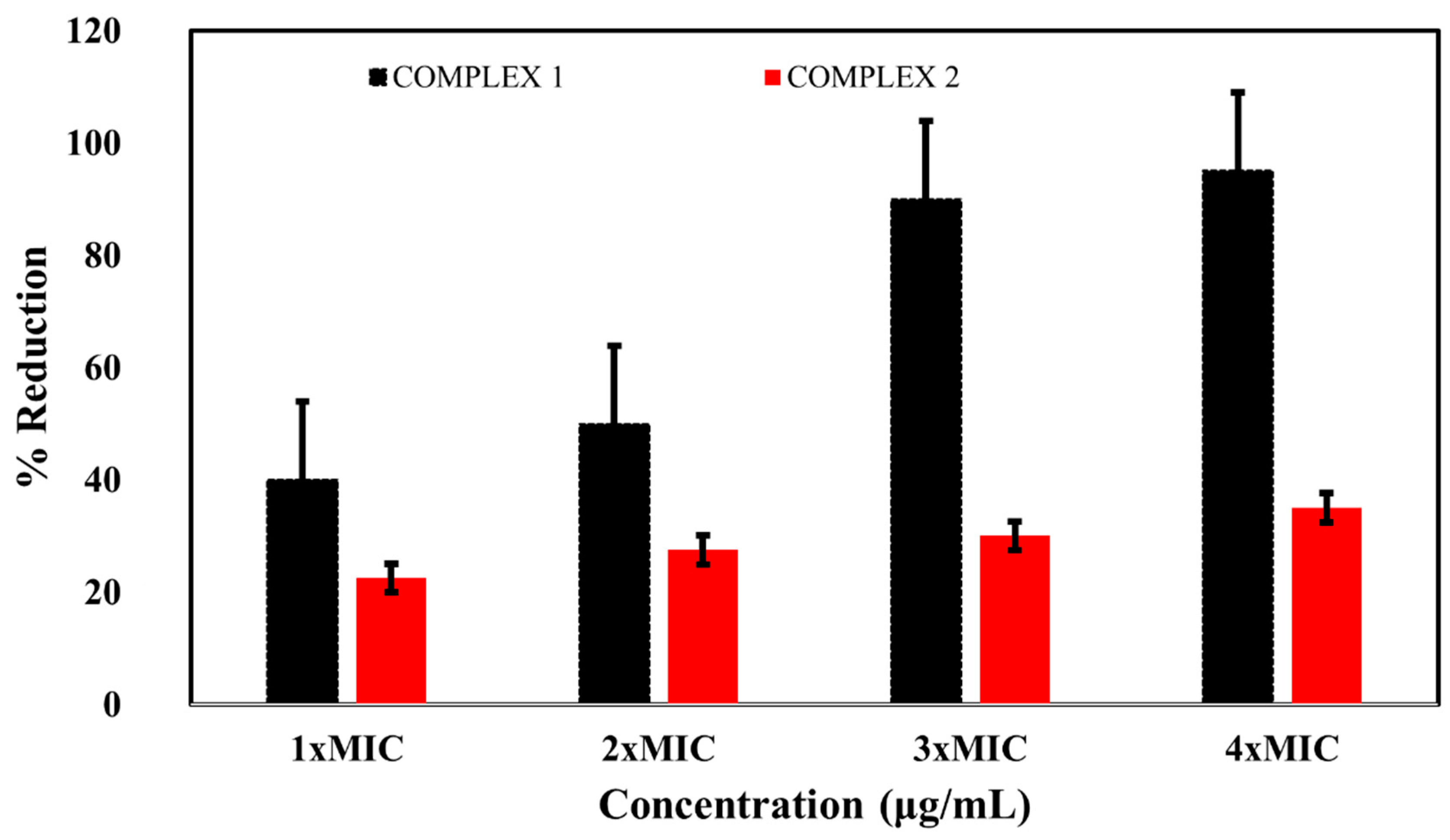
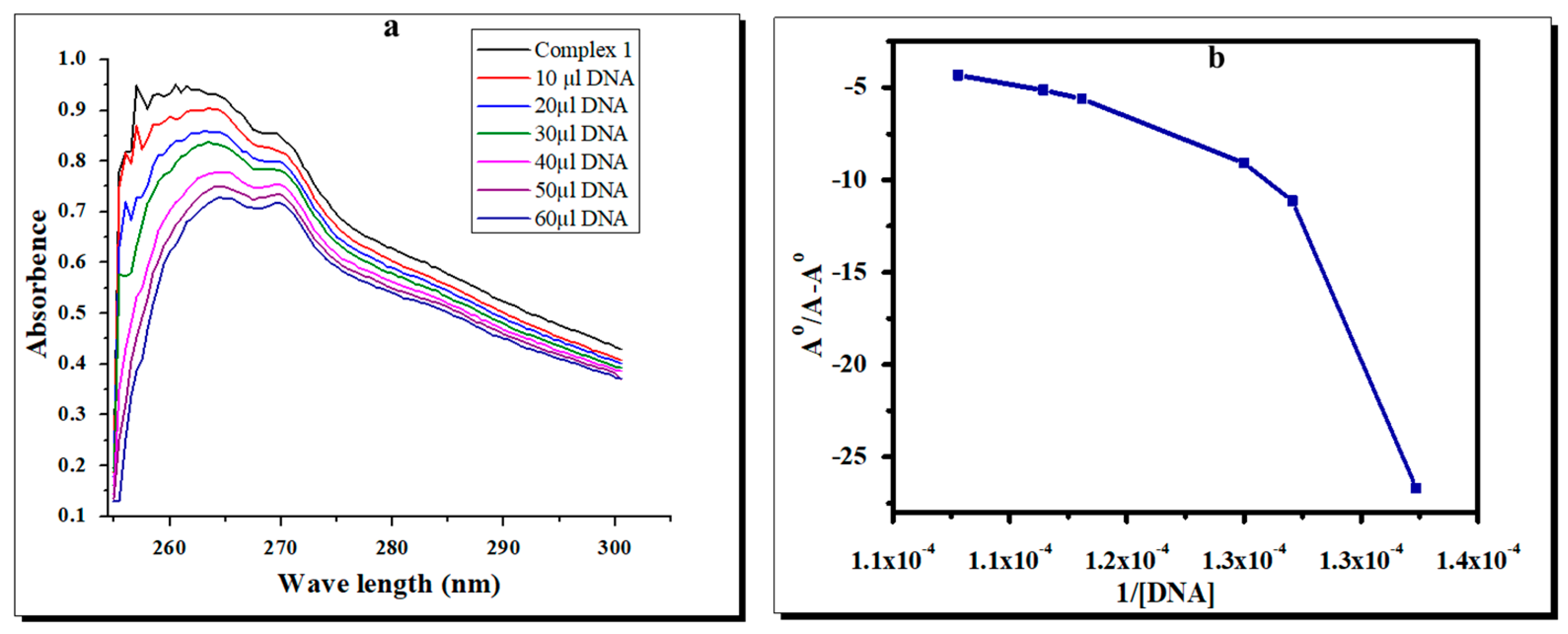
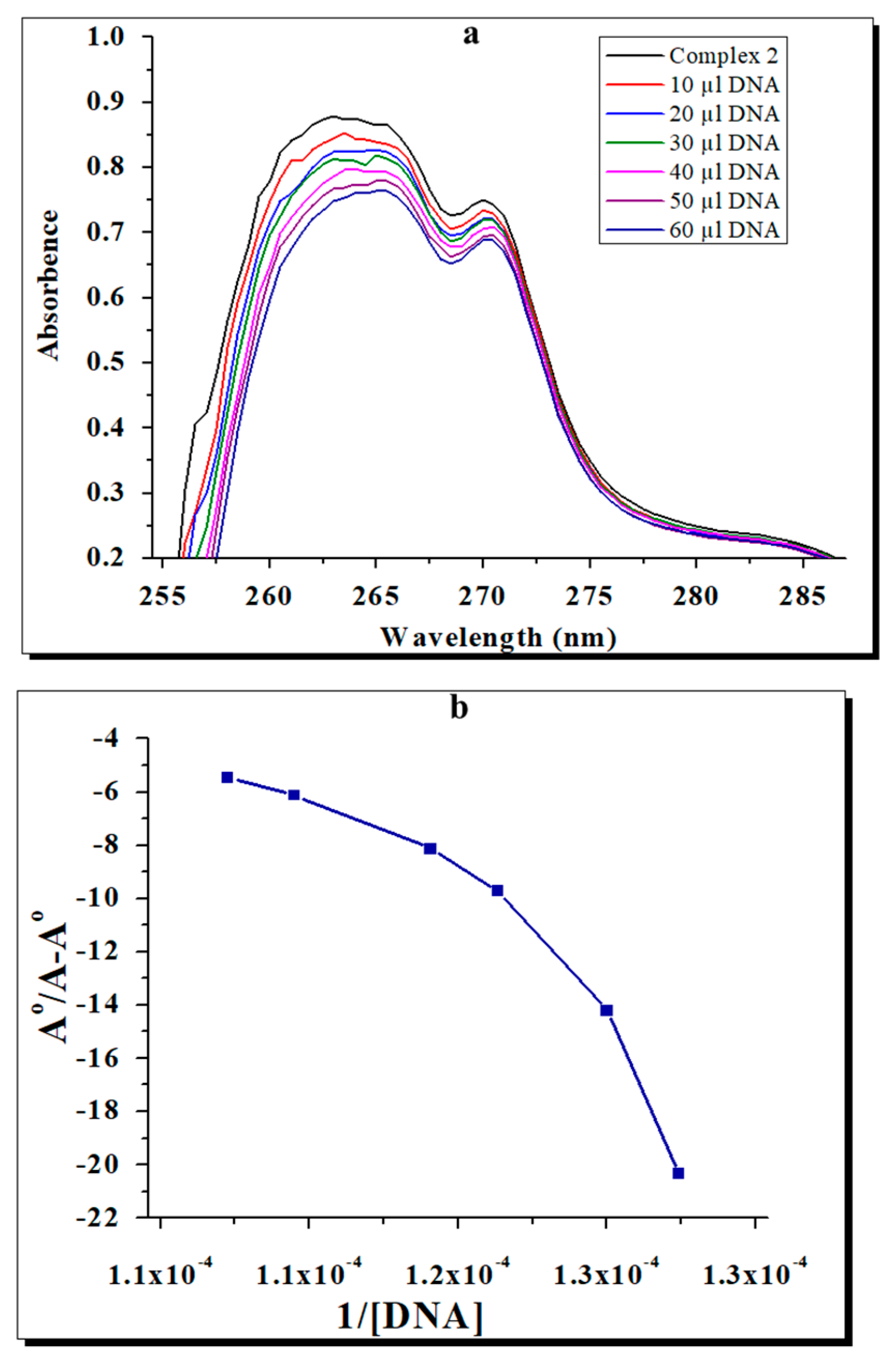
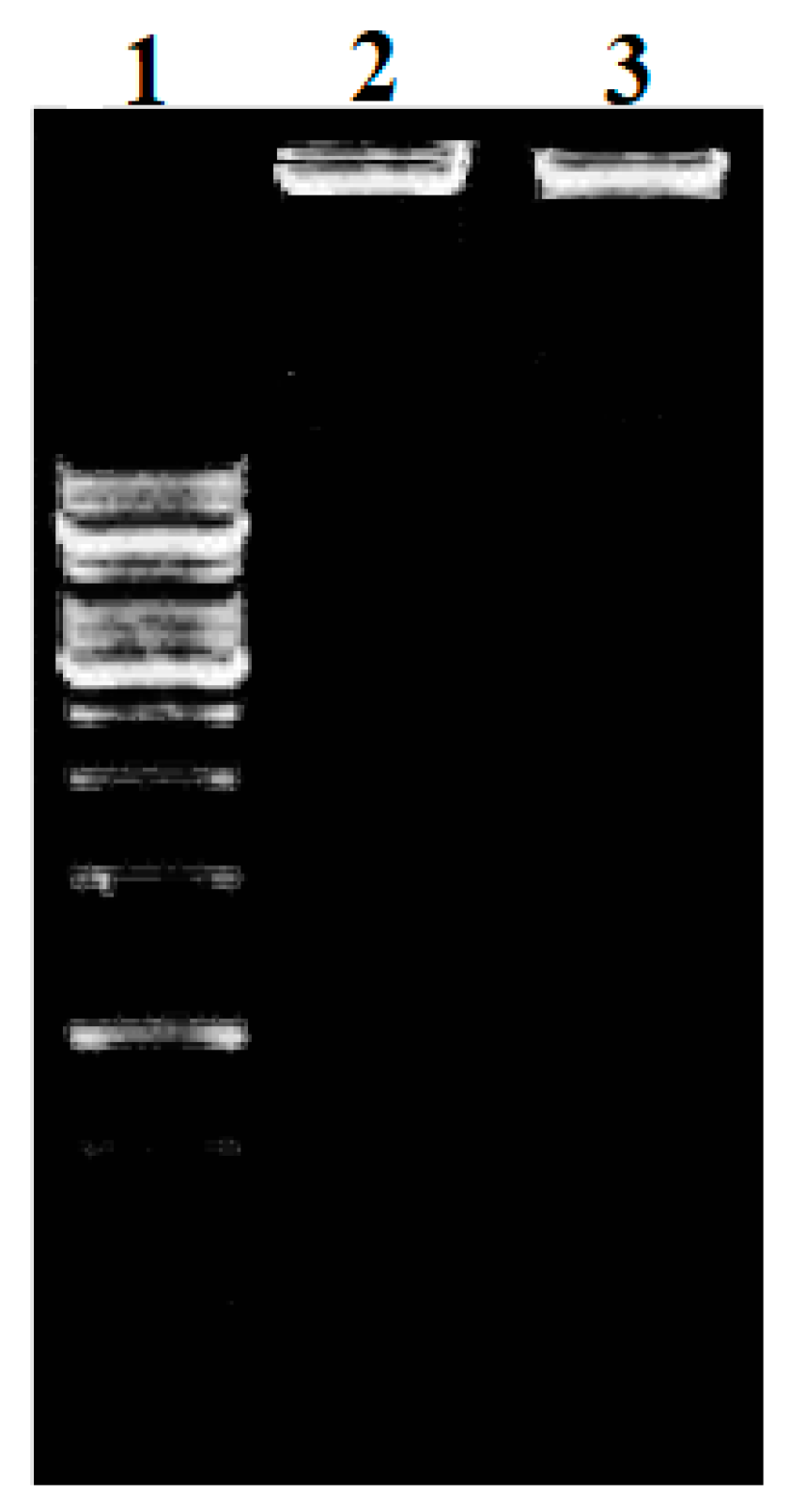
2.6. Effects of the Complexes on Bacterial DNA
2.7. Electron Microscopic Analysis
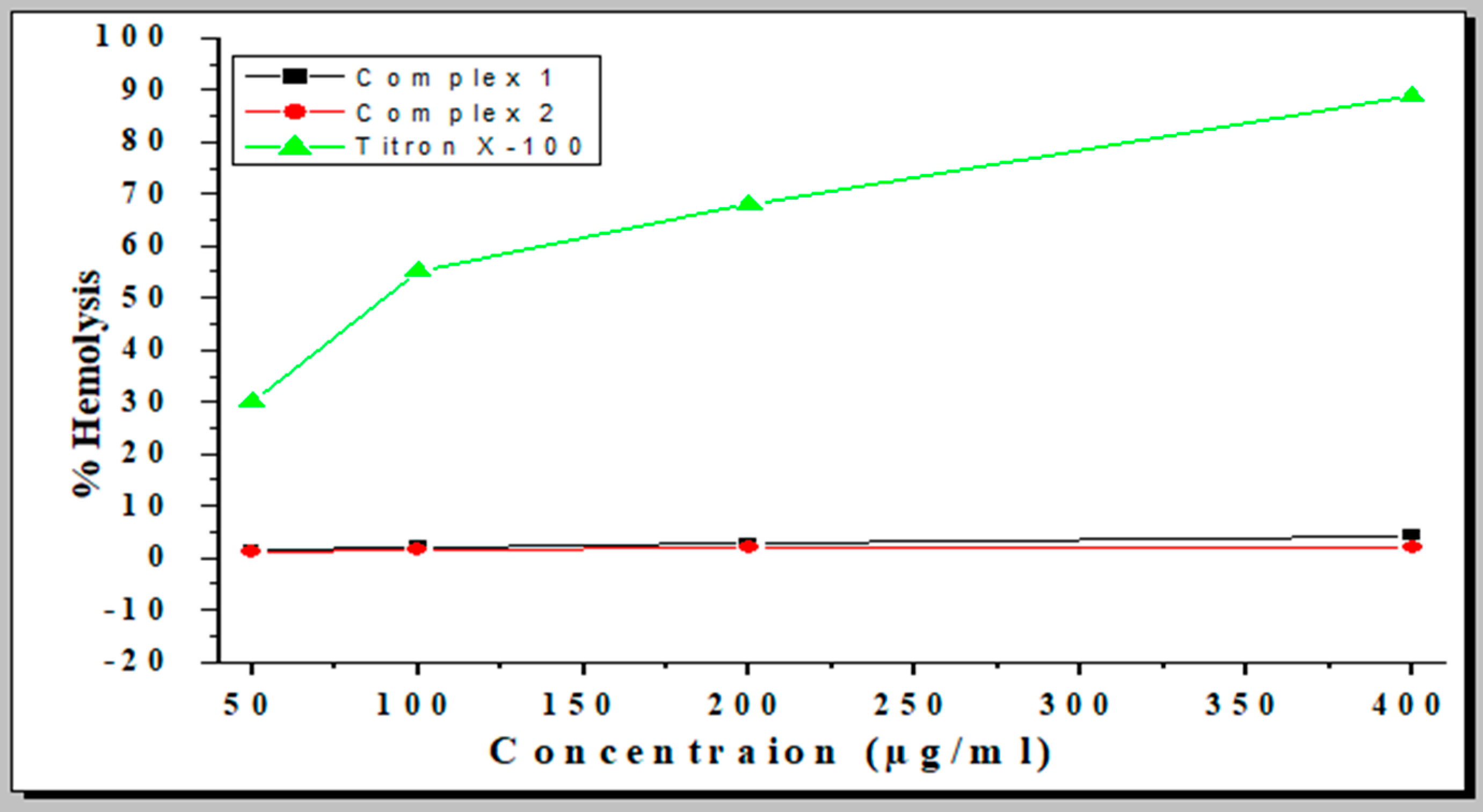
2.8. Hemolytic Activity
3. Materials and Methods
3.1. Culture Collection
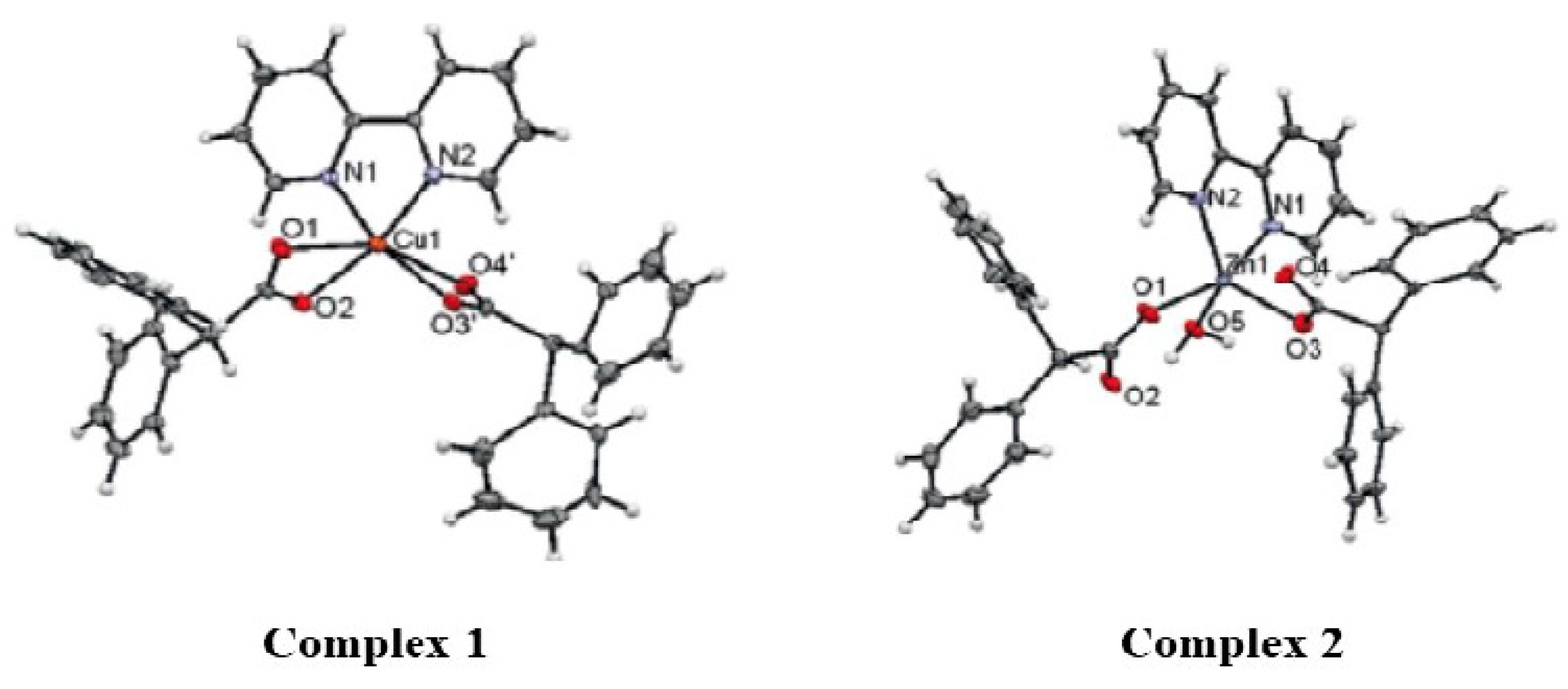
3.2. Synthesis of Bis (Biphenyl Acetate) Bipyridine Copper (II)
3.3. Synthesis of Bis (Biphenyl Acetate) Bipyridine Zinc (II)
3.4. Culture Conditions and Antibacterial Assay
3.5. Cell Membrane Spoil Assay
3.6. Inner Cell Membrane Spoil Assay
3.7. Biofilm Eradication Assay
3.8. Biofilm Inhibition Assay
3.9. DNA Isolation and Binding
3.10. SEM Analysis
3.11. Hemolytic Assay
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Vivas, R.; Barbosa, A.A.T.; Dolabella, S.S.; Jain, S. Multidrug-resistant bacteria and alternative methods to control them. Microb. Drug. Resist. 2019, 25, 890–908. [Google Scholar] [CrossRef] [PubMed]
- Izadpanah, M.; Khalili, H. Antibiotic regimens for treatment of infections due to multidrug-resistant Gram-negative pathogens: An evidence-based literature review. J. Res. Pharm. Pract. 2015, 4, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, J.; Das, S.; Fatima, Z.; Hameed, S. Multidrug resistance: An emerging crisis. Interdiscip. Perspect. Infect. Dis. 2014, 2014, 541340. [Google Scholar] [CrossRef] [PubMed]
- Gashaw, M.; Berhane, M.; Bekele, S.; Kibru, G.; Teshager, L.; Yilma, Y.; Gudina, E.K. Emergence of high drug resistant bacterial isolates from patients with health care associated infections at Jimma University medical center: A cross sectional study. Antimicrob. Resist. Infect. Control 2018, 7, 138. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.; Pereira, V.C.; Pinheiro, L.; MoraesRiboli, D.F.; Martins, K.B.; da Cunha, R.D.S.; De Lourdes, M. Antimicrobial resistance profile of planktonic and biofilm cells of Staphylococcus aureus and coagulase-negative staphylococci. Int. J. Mol. Sci. 2016, 17, 1423. [Google Scholar] [CrossRef]
- Latif, M., Jr.; May, E.E. A multiscale agent-based model for the investigation of E. coli K12 metabolic response during biofilm formation. Bull. Math. Biol. 2018, 80, 2917–2956. [Google Scholar] [CrossRef]
- Mostafavi, S.K.S.; Najar-Peerayeh, S.; Mobarez, A.M.; Parizi, M.K. Characterization of uropathogenic E. coli O25b-B2-ST131, O15:K52:H1, and CGA: Neutrophils apoptosis, serum bactericidal assay, biofilm formation, and virulence typing. J. Cell. Physiol. 2019, 234, 18272–18282. [Google Scholar] [CrossRef]
- Dakheel, M.M.; Alkandari, F.A.H.; Mueller-Harvey, I.; Woodward, M.J.; Rymer, C. Antimicrobial in-vitro activities of condensed tannin extracts on avian pathogenic Escherichia coli. Lett. Appl. Microbiol. 2020, 70, 165–172. [Google Scholar] [CrossRef]
- Efthimiadou, E.K.; Thomadaki, H.; Sanakis, Y.; Raptopoulou, C.P.; Katsaros, N.; Scorilas, A.; Karaliota, A.; Psomas, G. Structure and biological properties of the copper (II) complex with the quinolone antibacterial drug N-propyl-norfloxacin and 2,2′-bipyridine. J. Inorg. Biochem. 2007, 101, 64–73. [Google Scholar] [CrossRef]
- Goker, H.; Alp, M.; Yildiz, S. Synthesis and Potent Antimicrobial Activity of Some Novel N-(Alkyl)-2-Phenyl-1H-Benzimidazole-5-Carboxamidines. Molecules 2005, 10, 1377–1386. [Google Scholar] [CrossRef]
- Shah, M.A.; Uddin, A.; Shah, M.R.; Ali, I.; Ullah, R.; Hannan, P.A.; Hussain, H. Synthesis and Characterization of Novel Hydrazone Derivatives of Isonicotinic Hydrazide and Their Evaluation for Antibacterial and Cytotoxic Potential. Molecules 2022, 27, 6770. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Janda, K.D. Traceless Solid-Phase Synthesis of 5-Benzoylbenzimidazoles. Can. J. Chem. 2001, 79, 1556–1561. [Google Scholar] [CrossRef]
- Chikashita, H.; Nishida, S.; Miyazaki, M.; Morita, Y.; Itoh, K. In Situ Generation and Synthetic Application of 2-Phenylbenzimidazoline to the Selective Reduction of Carbon–Carbon Double Bonds of Electron-Deficient Olefins. Bull. Chem. Soc. Jpn. 1987, 60, 737–746. [Google Scholar] [CrossRef]
- Saha, D.K.; Sandbhor, U.; Shirisha, K.; Padhye, S.; Deobagkar, D.; Anson, C.E.; Powell, A.K. A Novel Mixed-Ligand Antimycobacterial Dimeric Copper Complex of Ciprofloxacin and Phenanthroline. Bioorganic Med. Chem. Lett. 2004, 14, 3027–3032. [Google Scholar] [CrossRef] [PubMed]
- Rajalakshmi, S.; Kiran, M.S.; Vaidyanathan, V.G.; Azhagiya Singam, E.R.; Subramaniam, V.; Nair, B.U. Investigation of Nuclease, Proteolytic and Antiproliferative Effects of Copper(II) Complexes of Thiophenylmethanamine Derivatives. Eur. J. Med. Chem. 2013, 70, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Crisponi, G.; Nurchi, V.M.; Fanni, D.; Gerosa, C.; Nemolato, S.; Faa, G. Copper-Related Diseases: From Chemistry to Molecular Pathology. Coord. Chem. Rev. 2010, 254, 876–889. [Google Scholar] [CrossRef]
- Weder, J.E.; Dillon, C.T.; Hambley, T.W.; Kennedy, B.J.; Lay, P.A.; Biffin, J.R.; Regtop, H.L.; Davies, N.M. Copper Complexes of Non-Steroidal Anti-Inflammatory Drugs: An Opportunity yet to Be Realized. Coord. Chem. Rev. 2002, 232, 95–126. [Google Scholar] [CrossRef]
- Tolia, C.; Papadopoulos, A.N.; Raptopoulou, C.P.; Psycharis, V.; Garino, C.; Salassa, L.; Psomas, G. Copper(II) Interacting with the Non-Steroidal Antiinflammatory Drug Flufenamic Acid: Structure, Antioxidant Activity and Binding to DNA and Albumins. J. Inorg. Biochem. 2013, 123, 53–65. [Google Scholar] [CrossRef]
- Zhang, T.; Wen, T.; Zhao, Y.; Hu, H.; Xiong, B.; Zhang, Q. Antibacterial Activity of the Sediment of Copper Removal from Wastewater by Using Mechanically Activated Calcium Carbonate. J. Clean. Prod. 2018, 203, 1019–1027. [Google Scholar] [CrossRef]
- Kang, J.H.; Kim, D.J.; Choi, B.K.; Park, J.W. Inhibition of Malodorous Gas Formation by Oral Bacteria with Cetylpyridinium and Zinc Chloride. Arch. Oral Biol. 2017, 84, 133–138. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Pearce, E.I.F.; Sissons, C.H. Inhibitory Effect of ZnCl2 on Glycolysis in Human Oral Microbes. Arch. Oral Biol. 2002, 47, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Trandafilović, L.V.; Božanić, D.K.; Dimitrijević-Branković, S.; Luyt, A.S.; Djoković, V. Fabrication and Antibacterial Properties of ZnO-Alginate Nanocomposites. Carbohydr. Polym. 2012, 88, 263–269. [Google Scholar] [CrossRef]
- Lansdown, A.B.G.; Mirastschijski, U.; Stubbs, N.; Scanlon, E.; Ågren, M.S. Zinc in Wound Healing: Theoretical, Experimental, and Clinical Aspects. Wound Repair Regen. 2007, 15, 2–16. [Google Scholar] [CrossRef]
- Agren, M.S. Zinc in Wound Repair. Arch. Dermatol. 1999, 135, 1273–1274. [Google Scholar] [CrossRef]
- Alswat, A.A.; Ahmad, M.B.; Saleh, T.A.; Hussein, M.Z.B.; Ibrahim, N.A. Effect of Zinc Oxide Amounts on the Properties and Antibacterial Activities of Zeolite/Zinc Oxide Nanocomposite. Mater. Sci. Eng. C 2016, 68, 505–511. [Google Scholar] [CrossRef]
- Villanueva, M.E.; Cuestas, M.L.; Pérez, C.J.; Campo Dall′ Orto, V.; Copello, G.J. Smart Release of Antimicrobial ZnO Nanoplates from a PH-Responsive Keratin Hydrogel. J. Colloid Interface Sci. 2019, 536, 372–380. [Google Scholar] [CrossRef]
- Atta, U.K.; Iqbal, H.; Ullah, R. Synthesis, Characterization, Antimicrobial, Antioxidant Activities of Schiff Base and its Transition Metal Complexes. Chiang Mai J. Sci. 2020, 47, 1241–1254. [Google Scholar]
- Umadevi, K.J.; Vanitha, V.; Vijayalakshmi, K. Antimicrobial Activity of Three Indian Medicinal Plants—An in Vitro Study. Bot. Elixir Appl. Bot. 2011, 6, 25–28. Available online: http://indianmedicine.eldoc.ub.rug.nl/id/eprint/65520 (accessed on 22 February 2023).
- Iqbal, M.; Karim, A.; Ali, S.; Bilal, H.; Rehman, A.U. Synthesis, Characterization, Structural Description, Micellization Behavior, DNA binding Study and Antioxidant Activity of 4, 5 and 6-Coordinated Copper(II) and Zinc(II) Complexes. Z. Anorg. Allg. Chem. 2020, 646, 895–903. [Google Scholar] [CrossRef]
- Ridgway, Z.; Picciano, A.L.; Gosavi, P.M.; Moroz, Y.S.; Angevine, C.E.; Chavis, A.E.; Reiner, J.E.; Korendovych, I.V.; Caputo, G.A. Functional Characterization of a Melittin Analog Containing a Non-Natural Tryptophan Analog. Biopolymers 2015, 104, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Sirajuddin, M.; Ali, S.; Badshah, A. Drug-DNA Interactions and Their Study by UV-Visible, Fluorescence Spectroscopies and Cyclic Voltametry. J. Photochem. Photobiol. B Biol. 2013, 124, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kamran, A.W.; Ali, S.; Tahir, M.N.; Zahoor, M.; Wadood, A.; Iqbal, M. Binuclear Copper(II) Complexes: Synthesis, Structural Characterization, DNA Binding and in Silico Studies. J. Serbian Chem. Soc. 2020, 85, 751–764. [Google Scholar] [CrossRef]
- Carter, M.T.; Rodriguez, M.; Bard, A.J. Voltammetric Studies of the Interaction of Metal Chelates with DNA. 2. Tris-Chelated Complexes of Cobalt(III) and Iron(II) with 1, 10-Phenanthroline and 2,2′-Bipyridine. J. Am. Chem. Soc. 1989, 111, 8901–8911. [Google Scholar] [CrossRef]
- Feng, Q.; Li, N.Q.; Jiang, Y.Y. Electrochemical Studies of Porphyrin Interacting with DNA and Determination of DNA. Anal. Chim. Acta 1997, 344, 97–104. [Google Scholar] [CrossRef]
- Rajalakshmi, S.; Fathima, A.; Rao, J.R.; Nair, B.U. Antibacterial Activity of Copper(Ii) Complexes against Staphylococcus aureus. RSC Adv. 2014, 4, 32004–32012. [Google Scholar] [CrossRef]
- Mohamed, M.F.; Abdelkhalek, A.; Seleem, M.N. Evaluation of Short Synthetic Antimicrobial Peptides for Treatment of Drug-Resistant and Intracellular Staphylococcus aureus. Sci. Rep. 2016, 11, 29707. [Google Scholar] [CrossRef]
- Macià, M.D.; Rojo-Molinero, E.; Oliver, A. Antimicrobial Susceptibility Testing in Biofilm-Growing Bacteria. Clin. Microbiol. Infect. 2014, 20, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Char, C.D.; Guerrero, S.N.; Alzamora, S.M. Mild Thermal Process Combined with Vanillin plus Citral to Help Shorten the Inactivation Time for Listeria Innocua in Orange Juice. Food Bioprocess Technol. 2010, 3, 752–761. [Google Scholar] [CrossRef]
- Perez, C.; Pauli, M.; Bazerque, P. An Antibiotic Assay by the Agar Well Diffusion Method. Acta Biol. Med. Exp. 1990, 15, 113–115. [Google Scholar]
- Pithayanukul, P.; Tubprasert, J.; Wuthi-Udomlert, M. In Vitro Antimicrobial Activity of Zingiber Cassumunar (Plai) Oil and a 5% Plai Oil Gel. Phyther. Res. 2007, 21, 164–169. [Google Scholar] [CrossRef]
- Chen, C.Z.; Cooper, S.L. Interactions between Dendrimer Biocides and Bacterial Membranes. Biomaterials 2002, 23, 3359–3368. [Google Scholar] [CrossRef]
- Liu, H.; Du, Y.; Wang, X.; Sun, L. Chitosan Kills Bacteria through Cell Membrane Damage. Int. J. Food Microbiol. 2004, 95, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Christensen, G.D.; Simpson, W.A.; Younger, J.J.; Baddour, L.M.; Barrett, F.F.; Melton, D.M.; Beachey, E.H. Adherence of Coagulase-Negative Staphylococci to Plastic Tissue Culture Plates: A Quantitative Model for the Adherence of Staphylococci to Medical Devices. J. Clin. Microbiol. 1985, 22, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Park, S.C.; Hahm, K.S.; Park, Y. A Helix-PXXP-Helix Peptide with Antibacterial Activity without Cytotoxicity against MDRPA-Infected Mice. Biomaterials 2014, 35, 1025–1039. [Google Scholar] [CrossRef] [PubMed]


| S.NO | Sample | MIC (μg/mL) | MBC (μg/mL) |
|---|---|---|---|
| 1 | Complex 1 | 46.87 ± 1.822 | 93.75 ± 1.345 |
| 2 | Complex 2 | 47.87 ± 1.345 | 94.85 ± 1.466 |
| 3 | Levofloxacin | 11.71 ± 1.334 | 23.43 ± 1.345 |
| 4 | Ciprofloxacin | 46.77 ± 1.456 | 98.75 ± 1.765 |
| 5 | Norfloxacin | 48.87 ± 1.765 | 99.15 ± 1.433 |
| 6 | Amoxicillin | 49.97 ± 1.623 | 99.75 ± 1.222 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, S.; Faqir, N.; Naz, F.; Jan, M.I.; Khan, N.; Alotaibi, A.; Ullah, R. A Comprehensive Mechanistic Antibacterial and Antibiofilm Study of Potential Bioactive ((BpA)2bp)Cu/Zn Complexes via Bactericidal Mechanisms against Escherichia coli. Molecules 2023, 28, 2215. https://doi.org/10.3390/molecules28052215
Ali S, Faqir N, Naz F, Jan MI, Khan N, Alotaibi A, Ullah R. A Comprehensive Mechanistic Antibacterial and Antibiofilm Study of Potential Bioactive ((BpA)2bp)Cu/Zn Complexes via Bactericidal Mechanisms against Escherichia coli. Molecules. 2023; 28(5):2215. https://doi.org/10.3390/molecules28052215
Chicago/Turabian StyleAli, Sajid, Nazma Faqir, Falak Naz, Muhammad Ishtiaq Jan, Naeem Khan, Amal Alotaibi, and Riaz Ullah. 2023. "A Comprehensive Mechanistic Antibacterial and Antibiofilm Study of Potential Bioactive ((BpA)2bp)Cu/Zn Complexes via Bactericidal Mechanisms against Escherichia coli" Molecules 28, no. 5: 2215. https://doi.org/10.3390/molecules28052215
APA StyleAli, S., Faqir, N., Naz, F., Jan, M. I., Khan, N., Alotaibi, A., & Ullah, R. (2023). A Comprehensive Mechanistic Antibacterial and Antibiofilm Study of Potential Bioactive ((BpA)2bp)Cu/Zn Complexes via Bactericidal Mechanisms against Escherichia coli. Molecules, 28(5), 2215. https://doi.org/10.3390/molecules28052215







