Abstract
The genus Moricandia (Brassicaceae) comprises about eight species that were used in traditional medicine. Moricandia sinaica is used to alleviate certain disorders such as syphilis and exhibits analgesic, anti-inflammatory, antipyretic, antioxidant, and antigenotoxic properties. Throughout this study, we aimed to figure out the chemical composition of lipophilic extract and essential oil obtained from M. sinaica aerial parts using GC/MS analysis, as well as their cytotoxic and antioxidant activities correlated with the major detected compounds’ molecular docking. The results revealed that both the lipophilic extract and the oil were found to be rich in aliphatic hydrocarbons, accounting for 72.00% and 79.85%, respectively. Furthermore, the lipophilic extract’s major constituents are octacosanol, γ-sitosterol, α-amyrin, β-amyrin acetate, and α-tocopherol. Contrarily, monoterpenes and sesquiterpenes accounted for the majority of the essential oil. The essential oil and the lipophilic extract of M. sinaica showed cytotoxic properties towards human liver cancer cells (HepG2) with IC50 values of 126.65 and 220.21 µg/mL, respectively. The lipophilic extract revealed antioxidant activity in the DPPH assay with an IC50 value of 2679 ± 128.13 µg/mL and in the FRAP assay, moderate antioxidant potential was expressed as 44.30 ± 3.73 µM Trolox equivalent/mg sample. The molecular docking studies revealed that ꞵ-amyrin acetate, α -tocopherol, γ-sitosterol, and n-pentacosaneachieved the best docking scores for NADPH oxidase, phosphoinositide-3 kinase, and protein kinase B. Consequently, M. sinaica essential oil and lipophilic extract can be employed as a viable management strategy for oxidative stress conditions and the formulation of improved cytotoxic treatment regimens.
1. Introduction
For millennia, the spotlight has been directed toward medicinal plants as a plentiful supply of bioactive compounds, and many of the therapeutic medications currently in use are natural products or compounds derived from plants [1,2,3,4,5]. According to the World Health Organization (WHO), about 80% of the world’s population lives in developing and underdeveloped countries and relies on medicines of natural origin as a remedy for medical ailments [6]. Plants are considered a potential source for the finding of new candidate compounds. Natural plants and their isolated chemicals provide us with a great source of biologically active leads for the development of drugs with benefits both in terms of cost and fewer side effects [1,7,8,9,10,11].
The mustard family, Brassicaceae, comprises approximately 338 genera and 3709 species, which are cultivated presently worldwide with high economic importance since they are widely consumed in human diets all over the world. Moreover, they are used to produce food and oilseed crops, while a number of other varieties are used as ornamental plants (with violet, purple, and white flowers) and noxious weeds [12,13]. The genus Moricandia (family: Brassicaceae) has eight species, namely arvensis, foetida, foleyii, oricandioides, nitens, sinaica, spinosa, and suffruticosa, some of which are used in traditional medicine [14]. These species are distributed all over North Africa, the Mediterranean basin, West Asia, and Southeast Asia [15]. Moricandia plants have revealed some significant health properties. For instance, M. arvensis leaves, which are frequently used in Tunisian traditional dishes, exhibit beneficial effects in the management of syphilis [16,17]. Additionally, some M. arvensis extracts have been shown to have antioxidant and antigenotoxic properties, and they effectively slow the spread of human cancer cells [18,19].
One of these species, Moricandia sinaica, is native to Saudi Arabia and mainly seen in desert areas such as Western Asia, the Middle East, and Egypt [13,20]. Moricandia sinaica is a suffrutescent to suffruticose annual to perennial plant; the leaves are thick, pointed, and vary in shape from oval to oblong-ovate, with pink or white corolla flowers [13]. It is indigenous to the Mediterranean area, Europe, and America, and has therapeutic uses in traditional medicine [20]. To date, there are few investigations that focus on the therapeutic potential or phytoconstituents of M. sinaica. Crude extracts from the roots, stem, leaves, and shoots of M. sinaica were investigated and evaluated for potential antiangiogenic effects in zebrafish embryos [13]. Besides, in vivo, various fractions of M. sinaica aerial parts exhibit analgesic, anti-inflammatory, and antipyretic properties [21].
Herein, the current research is primarily focused on the phytochemical investigations of the essential oil and lipophilic extract of M.sinaica through GC/MS analysis, besides the cytotoxic and antioxidant biological investigations of M. sinaica extracts.
2. Results and Discussion
2.1. GC/MS Analysis of the Essential Oil and Lipophilic Extract
The GC/MS analysis investigation of the lipophilic extract and essential oil of M. sinaica is shown in Table 1 (Supplementary Figures S1 and S2). The evaluation of the lipophilic extract and essential oil contents revealed the identification of 15 and 22 chemicals, representing 97.80% and 99.90%, respectively. Both the lipophilic extract and the essential oil were found to be rich in aliphatic hydrocarbons, accounting for 72.00% and 79.85%, respectively, where n-pentacosane (78.01%) is the major hydrocarbon in the essential oil and n-nonacosane (65.66%) is the major hydrocarbon in the lipophilic extract. In the lipophilic extract, octacosanol (12.67%), γ-sitosterol (6.60%), α-amyrin (3.51%), β-amyrin acetate (0.81%), and α-tocopherol (0.54%) are the major constituents. In contrast, monoterpenes in the essential oil (8.10%) are represented as tricyclene, camphene, ꞵ-citronellene, octanal, linalool, and α-terpineol, in addition to sesquiterpenes (2.82%) such as α-cadinene, caryophylla-4(12),8(13)-dien-5α-ol, and ꞵ-eudesmol. The chemical structures of the major compounds as well as the percentage distribution of volatile components of the lipophilic extract and essential oil of M. sinaica are displayed in Figure 1 and Figure 2.

Table 1.
Chemical composition (%) of the essential oil and the lipophilic extract identified in Moricandia sinaica aerial parts using GC/MS analysis.
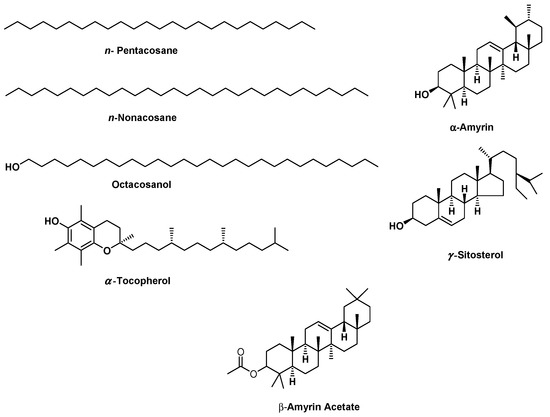
Figure 1.
The chemical structures of the major components found in the essential oil and lipophilic extract of Moricandia sinaica aerial parts using GC/MS analysis.
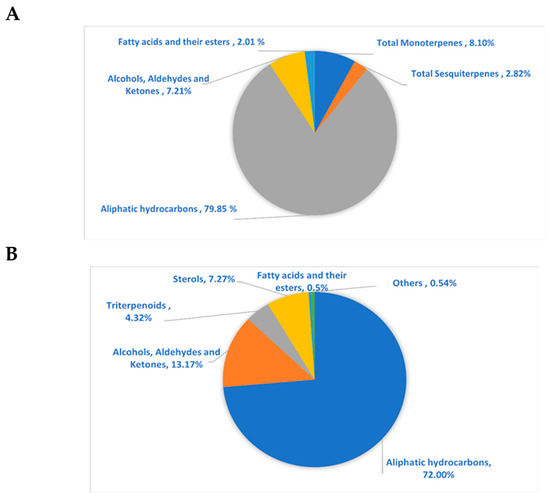
Figure 2.
The pie charts show the percentage distribution of volatile components within (A) essential oil and (B) lipophilic extract of M. sinaica aerial parts.
Prior studies on the chemical characteristics of M. arvensis aerial parts essential oil obtained from two areas in Algeria revealed the richness of the oil of the Southern Setif population with palmitic acid (13.2–12.9%) and phytol (7.9–10.5%). In comparison, the oil of the Northern population is rich with 3-butenylisothiocyanate and octadecanoic acid, 2-hydroxy-1,3-p [27]. Another report by Marrelli et al. revealed the n-hexane extract’s chemical composition of M. arvensis using GC/MS analysis. The findings revealed the existence of different fatty acids such as palmitic, stearic, and myristic acids, together with phytosterols such as ꞵ-Sitosterol, 22,24-dimethylcholesterol, and stigmasta-3,5-dien-7-one [28]. Comparing the current findings to the previously published findings, both the essential oil and the lipophilic extract revealed variations in the components and their relative quantities that might be utilized as a chemical fingerprint to assess the validity and to discriminate between the given oils or extracts.
2.2. Cytotoxic Activity Using SRB Assay
Previous studies on essential oils and lipophilic extracts of M. sinaica revealed their richness with potent secondary metabolites that have an effect on numerous cancer cells [23,29]. The cytotoxicity results of the essential oil and the lipophilic extract of M. sinaica aerial parts revealed their inhibitory activities toward human liver cancer cells (HepG2) with IC50 values of 126.65 and 220.21 µg/mL, respectively (Figure 3). In agreement with our results, GC/MS evaluation demonstrated the abundance of n-pentacosane in the essential oil of M. sinaica aerial parts (78.01%); it is a 25-carbon unbranched chain that was discovered in several essential oils with antimicrobial properties [30]. Additionally, it was reported to contain anticancer, antifungal, anti-inflammatory, antioxidant, and antiviral agents [31,32]. α-amyrin (3.51%) and β-amyrin acetate (0.81%) are also found to have antioxidant, antimicrobial, anti-inflammatory, and anticancer properties [23]. Octacosanol and 1-Octacosanol were also found to account for 12.67% and 0.50%, respectively. Studies have shown that octacosanol is an antiangiogenic substance that inhibits angiogenesis. Octacosanol prevents neovascularization and the proliferation of endothelial cells [33].
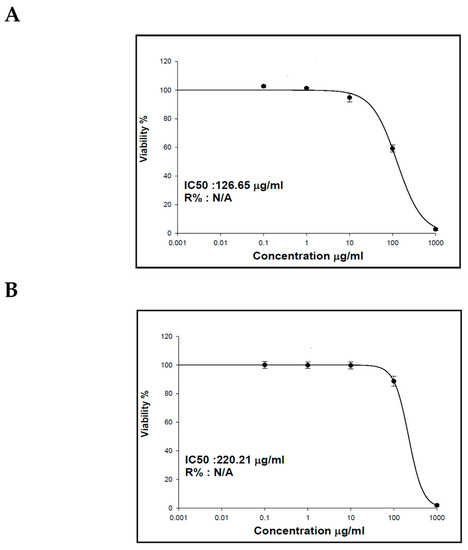
Figure 3.
The effect of the (A) essential oil and (B) lipophilic extract of the aerial parts of M. sinaica on human liver cancer cells’ (HepG2) viability.
2.3. Antioxidant Activity
Natural polyphenols from the Brassicaceae family are abundant, provide a wide range of health implications, and are known for their antioxidant effects [34]. That gives us an interest in exploring the essential oils’ and the lipophilic extract’s potential as antioxidants using DPPH and FRAP assays. The lipophilic extract in the DPPH assay showed an IC50 value of 2679 ± 128.13 µg/mL as compared to the Trolox reference drug (IC50 = 4.94 ± 0.263 µg/mL). On the other hand, the lipophilic extract showed considerable antioxidant activity in the FRAP assay, expressed as 44.30 ± 3.73 µM Trolox equivalent/mg sample. The essential oil of M. sinaica aerial parts did not show any significant results in both assays. Previous reports regarding the biological activity of different Moricandia species revealed that M. nitens green synthesized GNPs showed substantial anti-Helicobacter pylori and anticancer activity against HepG2 and HCT-116 and were reported to be an effective α-glucosidase inhibitor [35]. Moreover, the methanolic extract of M. arvensis exhibited potent lipase-inhibitory activity and antioxidant activity [28].
2.4. Molecular Docking
The essential oil and lipophilic extract of M. sinaica, which has potential cytotoxic and antioxidant properties, led us to perform a docking investigation of the major components against the enzymes NADPH oxidase, phosphoinositide-3 kinase (PI3K), and Akt, also known as protein kinase B (PKB). This research aimed to determine the probable binding mechanisms by which the major investigated metabolites may function. Therefore, the major components were docked into the NADPH oxidase, phosphoinositide-3 kinase (PI3K), and Akt 3D coordinates that were downloaded from the protein data bank using the following PDB IDs: 2cdu, 1E90, and 3cwq, respectively. Re-docking each co-crystallized ligand into its associated active site enabled us to confirm the docking parameters applied. The estimated RMSD values between the co-crystallized pose and the docked pose were 1.02, 0.97, and 1.03 Å NADPH oxidase, phosphoinositide-3 kinase (PI3K), and Akt, respectively, helping to ensure the docking technique is valid. The co-crystallized ligand’s re-docking resulted in docking scores of −11.3, −12.1, and −13.4 Kcal/mole for NADPH oxidase, phosphoinositide-3 kinase (PI3K), and Akt, respectively. The docking of the main metabolites to the three enzymes showed acceptable results corresponding to those of the reference compounds. Interestingly, in the docking with NADPH oxidase, ꞵ-amyrin acetate, α-tocopherol and γ-sitosterol were the best compounds, achieving docking scores of −14.19, −13.12, and −12.65 Kcal/Mol, respectively (Figure 4). Similarly, in the docking with PI3K, n-pentacosane, α-tocopherol and ꞵ-amyrin acetate were the best compounds, achieving docking scores of −13. 91, −14.15, and −14.05 Kcal/Mol, respectively (Figure 5). Worth noting, in the docking with AKT, n-pentacosane, α-tocopherol, and γ-sitosterol were the best compounds, achieving docking scores of −11. 9, −13.19, and −12.05 Kcal/Mol, respectively (Figure 6). The major compounds’ docking scores against the three proposed target enzymes are represented in Table 2. The major compounds’ interactions with the three enzymes are represented in Table 3, Table 4 and Table 5. The results of docking correlated with the results of the cytotoxic and antioxidant activities of the lipophilic extract of M. sinaica.
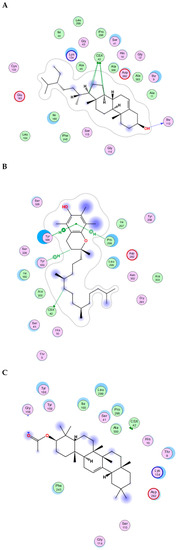
Figure 4.
2D binding modes of γ-Sitosterol (A), α-Tocopherol (B), ꞵ-Amyrin Acetate (C) to the active binding sites of NADPH oxidase.
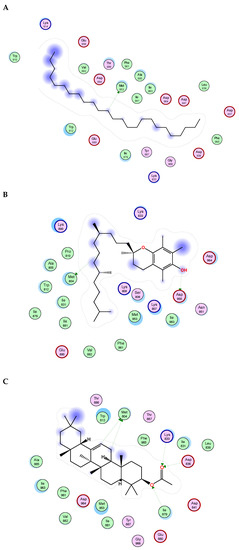
Figure 5.
2D binding modes of n- pentacosane (A), α-Tocopherol (B), ꞵ-Amyrin Acetate (C) to the active binding sites of Phosphoinositide-3 kinase (PI3K).
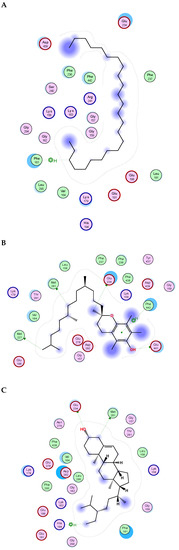
Figure 6.
2D binding modes of n- pentacosane (A), α-Tocopherol (B), γ-sitosterol (C) to the active binding sites of Protein kinase B (AKT).

Table 2.
Docking scores of major identified constituents in the essential oil and lipophilic extract of M. sinaica against three target enzymes: NADPH oxidase, Phosphoinositide-3 kinase, and Protein kinase B.

Table 3.
Docking results of major identified constituents in the essential oil and lipophilic extract of M. sinaica on NADPH.

Table 4.
Docking results of major identified constituents in the essential oil and lipophilic extract of M. sinaica on Phosphoinositide-3 kinase (PI3K).

Table 5.
Docking results of major identified constituents in the essential oil and lipophilic extract of M. sinaica on Protein kinase B.
3. Materials and Methods
3.1. Plant Material
Leaves of Moricandia sinaica Boiss. (Bra) were obtained in February 2022 from South Sinai, Egypt 27°57′43.2″ N and 34°16′16.7″ E. The plant was thankfully recognized and verified by Dr. Mohammed El-Gebaly (Department of Botany, National Research Centre), Giza, Egypt. Voucher specimens were provided in the Pharmacognosy Department, Faculty of Pharmacy, Badr University in Cairo (Voucher specimen number: BUC-PHG-MS-10).
3.2. The Essential Oil Isolation
The leaves were finely chopped and hydro-distilled for 5 h with a Clevenger apparatus. The produced oil is yellowish orange in color with a fragrant odor; the yield was 0.21% (21 mg/100 g). It was collected and maintained at −4 °C in a tight, dark glass vial for GC/MS analysis.
3.3. Preparation of the Lipophilic Extract
Dry leaves (100 g) of Moricandia sinaica Boiss. were extracted three times with n-hexane. To obtain the dried residue of the lipophilic extract, the filtrate was fully evaporated in vacuo at 40 °C until dryness, and (2.41 g) of the lipophilic extract was obtained. The lipophilic extract was kept in a tightly sealed container for subsequent examination.
3.4. Gas Chromatography–Mass Spectrometry (GC/MS)
A Shimadzu GCMS-QP 2010 chromatograph (Kyoto, Japan) with a DB-5 capillary column (30 m × 0.25 mm i.d. × 0.25 μm film thickness; Restek, Bellefonte, PA, USA) was used for gas chromatography/mass spectrometry (GC/MS) analysis. The oven temperature was set at 50 °C for 3 min (isothermal), programmed to 30 °C at a rate of 5 °C/min, and kept constant at 300 °C for 10 min (isothermal); the temperature of the injector was 280 °C. Helium was employed as the carrier gas, with a flow rate of 1.40 mL/min. Diluted samples (1% v/v) were injected at a split ratio of 15:1 in a volume of 1 μL. The following were the MS running specifications: 280 °C for the interface, 220 °C for the ion source, 70 eV for the EI mode, and 35–500 amu for the scan range.
3.5. Characterization of the Essential Oil and Lipophilic Extract Components
The volatile constituents were characterized based on their retention indices and fragmentation patterns matching with NIST Mass Spectral Library, Wiley library database and published in the literature [23,26,29,36,37,38,39]. Retention indices (RI) were estimated in comparison to homologous series of n-alkanes (C8-C30) injected under the same conditions.
3.6. Assessment of Cytotoxic Activity Using SRB Assay
Nawah Scientific Inc., (Mokatam, Cairo, Egypt) provided the Hepatocellular carcinoma (HepG2). Cells were cultured in DMEM media treated with 100 mg/mL of streptomycin, 100 units/mL of penicillin, and 10% heat-inactivated fetal bovine serum in a humidified, 5% (v/v) CO2 atmosphere at 37 °C. The SRB assay was employed to assess cell viability. In 96-well plates, aliquots of 100 μL cell suspension (5 × 103 cells) were incubated in complete media for 24 h. Another aliquot of 100 μL of media containing different doses of drugs was used to treat the cells. Cells were fixed by replacing media with 150 μL of 10% TCA and incubated at 4 °C for 1 h, after 72 h of drug exposure. After removing the TCA solution, the cells were washed 5 times with distilled water. Aliquots of 70 μL SRB solution (0.4% w/v) were added and incubated in a dark place at room temperature for 10 min. Plates were washed with 1% acetic acid for 3 times before being let to air dry overnight. Then, 150 μL of TRIS (10 mM) was added to dissolve protein-bound SRB stain; with the use of a BMGLABTECH®- FLUO star Omega microplate reader (Ortenberg, Germany), the absorbance was determined at 540 nm [40].
3.7. Assessment of Antioxidant Activity
3.7.1. DPPH Assay
Samples were prepared using DMSO at a concentration of 50 mg/mL. Then, from this stock, the oil was prepared at a concentration of 2 mg/mL in methanol, and the lipophilic extract was prepared at a concentration of 10 mg/mL in methanol. A stock solution of 20 μg/mL of Trolox was prepared in methanol, from which 5 concentrations were prepared, including 12.5, 7.5, 6.25, 2.5, and 1.25 μg/mL. DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) free radical assay was carried out according to the method of [41]. Briefly, at room temperature, 100 μL of freshly prepared DPPH reagent (0.1% in methanol) was added to 100 μL of the sample in 96-well plates (n = 6), which was incubated for 30 min in the dark. The resulting reduction in DPPH color intensity was measured at 540 nm after the incubation time. The data are displayed as means ± SD according to the following equation:
The microplate reader FluoStar Omega was used for recording the results. The data were processed using Microsoft Excel® and the IC50 value calculation was performed using Graph pad Prism 6® by converting the concentrations to their logarithmic value and selecting a non-linear inhibitor regression equation (log (inhibitor) vs. normalized response—variable slope equation).
3.7.2. FRAP Assay
Trolox Standard for FRAP assay Trolox stock solution of 3 mM in methanol was prepared, and the following dilutions were prepared at the concentrations of 1200, 1000, 800, 500, 400, 300, 200, and 100 μM. Samples were prepared at a concentration of 50 mg/mL in DMSO. Then, the oil was prepared at a concentration of 2 mg/mL in methanol, and the lipophilic extract was prepared at a concentration of 10 mg/mL in methanol. The assay was carried out in accordance with the method of Benzie et al. [42] with some modifications to be carried out in microplates. A freshly prepared TPTZ reagent (300 mM Acetate Buffer (PH = 3.6), 10 mM TPTZ in 40 mM HCl, and 20 mM FeCl3, in a ratio of 10:1:1 v/v/v, respectively). About 190 uL of the freshly prepared TPTZ reagent were mixed with 10 μL of the sample in 96-well plates (n = 3), the reaction was incubated at room temperature for 30 min in the dark. The resulting blue color was measured at 593 nm at the end of the incubation period. The data are represented as means ± SD. The microplate reader FluoStar Omega was used to record the results. The ferric-reducing ability of the samples is presented as μM TE/ mg sample using the linear regression equation obtained from the following calibration curve (linear dose-response curve of Trolox) (Figure 7).
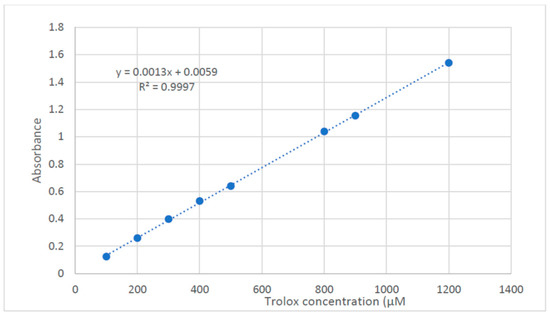
Figure 7.
Dose response line for solution of Trolox.
3.8. Molecular Docking
Applying the software Molecular Operating Environment (MOE 2019.02), docking investigations were carried out [43,44]. The X-ray crystal structures of NADPH oxidase, phosphoinositide-3 kinase (PI3K), and Akt, known as protein kinase B (PKB) were obtained from the protein data bank www.pdb.org (accessed on 10 December 2022) using the following PDB IDs: 2CDU, 1E90, and 3CWQ, respectively [45,46,47]. Hydrogens and charges of the receptors were adjusted through AMBER10: EHT implanted in MOE software. The relevant co-crystallized ligand is bound at the established binding sites of three enzymes. Five major compounds identified in the essential oil (n-pentacosane, camphene, ꞵ-citronellene, linalool, and α-terpineol) and six compounds from the lipophilic extract (n-nonacosane (65.66%), octacosanol, γ-sitosterol, α-amyrin and β-amyrin acetate, and α-tocopherol) were produced using the 2D builder of MOE2019 and transformed into 3D structures with the same software. Utilizing the triangular matcher and London dg, the compounds were docked onto the three enzyme binding sites as placement and scoring methods, respectively. Finally, MOE produced 2D interaction diagrams to analyze the docking observations.
4. Conclusions
In the present research, the chemical investigation of Moricandia sinaica lipophilic extract and essential oil was analyzed using the GC/MS technique. The results revealed that both are rich in aliphatic hydrocarbons, where n-pentacosane (78.01%), and n-nonacosane (65.66%) are the major hydrocarbons in the essential oil and the lipophilic extract. It is worth noting that γ-sitosterol, α-amyrin, β-amyrin acetate, and α-tocopherol are the main important constituents in the lipophilic extract that correlate with its antioxidant activity using DPPH and FRAP assays. In addition, monoterpenes and sesquiterpenes are present in the essential oil, which correlates with its cytotoxic activity against HEPG-2 cells. The biological investigations were supported by the molecular docking study and interactions of the major constituents with NADPH oxidase, phosphoinositide-3 kinase (PI3K), and Akt, known as protein kinase B (PKB) enzymes. Accordingly, the lipophilic extract and essential oil of M. sinaica would be a promising alternative for the production of cytotoxic and antioxidant agents, supported by further in vivo investigations as well as pharmacodynamic and pharmacokinetic studies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28052193/s1, Figure S1. GC-chromatogram of Moricandia sinaica lipophilic extract; Figure S2. GC-chromatogram of Moricandia sinaica essential oil.
Author Contributions
Conceptualization, S.H.A.; methodology, S.H.A., N.H.K., S.S.K., M.M.M.A., R.M.H., S.S.Z., O.M.A., M.A.E.H. and W.M.E.; software, S.H.A., S.S.K., M.A.E.H., H.A., S.T.A.-R., F.A.B. and W.M.E.; validation, S.H.A., N.H.K., S.S.K. and M.M.M.A.; investigation, S.H.A., N.H.K., S.S.K., M.M.M.A., R.M.H., S.S.Z. and O.M.A.; data curation, S.H.A., N.H.K., S.S.K., M.M.M.A., R.M.H., S.S.Z., O.M.A. and M.A.E.H.; writing—original draft preparation, S.H.A., N.H.K., S.S.K., R.M.H., S.S.Z. and O.M.A.; writing—review and editing, S.H.A. and W.M.E.; funding acquisition, H.A., S.T.A.-R. and F.A.B. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge financial support from the Researchers Supporting Project number (RSP-2023/103), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request from the first author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kinghorn, A.D.; Pan, L.; Fletcher, J.N.; Chai, H. The Relevance of Higher Plants in Lead Compound Discovery Programs. J. Nat. Prod. 2011, 74, 1539–1555. [Google Scholar] [CrossRef] [PubMed]
- Aly, S.H.; Elissawy, A.M.; Fayez, A.M.; Eldahshan, O.A.; Elshanawany, M.A.; Singab, A.N.B. Neuroprotective Effects of Sophora Secundiflora, Sophora Tomentosa Leaves and Formononetin on Scopolamine-Induced Dementia. Nat. Prod. Res. 2020, 35, 5848–5852. [Google Scholar] [CrossRef] [PubMed]
- El-Nashar, H.A.S.; Aly, S.H.; Ahmadi, A.; El-Shazly, M. The Impact of Polyphenolics in the Management of Breast Cancer: Mechanistic Aspects and Recent Patents. Recent Pat. Anticancer. Drug Discov. 2021, 17, 358–379. [Google Scholar] [CrossRef] [PubMed]
- Ads, E.N.; Hassan, S.I.; Rajendrasozhan, S.; Hetta, M.H.; Aly, S.H.; Ali, M.A. Isolation, Structure Elucidation and Antimicrobial Evaluation of Natural Pentacyclic Triterpenoids and Phytochemical Investigation of Different Fractions of Ziziphus spina-christi (L.) Stem Bark Using LCHRMS Analysis. Molecules 2022, 27, 1805. [Google Scholar] [CrossRef]
- Aly, S.H.; Elissawy, A.M.; Allam, A.E.; Farag, S.M.; Eldahshan, O.A.; Elshanawany, M.A.; Singab, A.N.B. New Quinolizidine Alkaloid and Insecticidal Activity of Sophora secundiflora and Sophora tomentosa against Culex pipiens (Diptera: Culicidae). Nat. Prod. Res. 2021, 36, 2722–2734. [Google Scholar] [CrossRef]
- Farnsworth, N.R.; Akerele, O.; Bingel, A.S. Medicinal Plants in Therapy. J. Ethnopharmacol. 1987, 19, 336. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Natural Products: A Continuing Source of Novel Drug Leads. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H. Discovery and Resupply of Pharmacologically Active Plant-Derived Natural Products: A Review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Saber, F.R.; Munekata, P.E.S.; Rizwan, K.; El-nashar, H.A.S.; Fahmy, N.M.; Aly, S.H.; El-shazly, M.; Bouyahya, A.; Lorenzo, J.M. Family Myrtaceae: The Treasure Hidden in the Complex/Diverse Composition. Crit. Rev. Food Sci. Nutr. 2023, 1–19. [Google Scholar] [CrossRef]
- Aly, S.H.; Elissawy, A.M.; Salah, D.; Alfuhaid, N.A.; Zyaan, O.H.; Mohamed, H.I.; Singab, A.N.B.; Farag, S.M. Phytochemical Investigation of Three Cystoseira Species and Their Larvicidal Activity Supported with In Silico Studies. Mar. Drugs 2023, 21, 117. [Google Scholar] [CrossRef]
- Abdel Razek, M.M.M.; Moussa, A.Y.; El-Shanawany, M.A.; Singab, A.N.B. A New Phenolic Alkaloid from Halocnemum Strobilaceum Endophytes: Antimicrobial, Antioxidant and Biofilm Inhibitory Activities. Chem. Biodivers. 2020, 17, e2000496. [Google Scholar] [CrossRef]
- Vaughn, S.F.; Berhow, M.A. Glucosinolate Hydrolysis Products from Various Plant Sources: PH Effects, Isolation, and Purification. Ind. Crop. Prod. 2005, 21, 193–202. [Google Scholar] [CrossRef]
- Warwick, S.I.; Francis, A.; Gugel, R.K. Guide to Wild Germplasm of Brassica and Allied Crops (Tribe Brassiceae, Brassicaceae). Canada Agric. Agri-Food Canada 2009, 1, 19–36. [Google Scholar]
- Perfectti, F.; Gómez, J.M.; González-Megías, A.; Abdelaziz, M.; Lorite, J. Molecular Phylogeny and Evolutionary History of Moricandia DC (Brassicaceae). PeerJ 2017, 5, e3964. [Google Scholar] [CrossRef]
- Kilian, B.; Mammen, K.; Millet, E.; Sharma, R.; Graner, A.; Salamini, F.; Hammer, K.; Ozkan, H. Aegilops. In Wild Crop Relatives, Genomic and Breeding Resources Cereals; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1–76. [Google Scholar] [CrossRef]
- Skandrani, I.; Bouhlel, I.; Limem, I.; Boubaker, J.; Bhouri, W.; Neffati, A.; Sghaier, M.B.; Kilani, S.; Ghedira, K.; Ghedira-Chekir, L. Moricandia Arvensis Extracts Protect against DNA Damage, Mutagenesis in Bacteria System and Scavenge the Superoxide Anion. Toxicol. Vitr. 2009, 23, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Le Floc’h, E. Contribution à Une Étude Ethnobotanique de La Flore Tunisienne; Ministère de l’Enseignement Supériur et de la Recherche Scientifique: Tunis, Tunisia, 1983. [Google Scholar]
- Skandrani, I.; Ben Sghaier, M.; Neffati, A.; Boubaker, J.; Bouhlel, I.; Kilani, S.; Mahmoud, A.; Ghedira, K.; Chekir-Ghedira, L. Antigenotoxic and Free Radical Scavenging Activities of Extracts from Moricandia Arvensis. Drug Chem. Toxicol. 2007, 30, 361–382. [Google Scholar] [CrossRef] [PubMed]
- Skandrani, I.; Limem, I.; Neffati, A.; Boubaker, J.; Sghaier, M.B.; Bhouri, W.; Bouhlel, I.; Kilani, S.; Ghedira, K.; Chekir-Ghedira, L. Assessment of Phenolic Content, Free-Radical-Scavenging Capacity Genotoxic and Anti-Genotoxic Effect of Aqueous Extract Prepared from Moricandia Arvensis Leaves. Food Chem. Toxicol. 2010, 48, 710–715. [Google Scholar] [CrossRef]
- Arif, I.A.; Bakir, M.A.; Khan, H.A.; Al Farhan, A.H.; Al Homaidan, A.A.; Bahkali, A.H.; Al Sadoon, M.; Shobrak, M. Application of RAPD for Molecular Characterization of Plant Species of Medicinal Value from an Arid Environment. Genet. Mol. Res. 2010, 9, 2191–2198. [Google Scholar] [CrossRef]
- El-Mekkawy, S.; Shahat, A.A.; Alqahtani, A.S.; Alsaid, M.S.; Abdelfattah, M.A.O.; Ullah, R.; Emam, M.; Yasri, A.; Sobeh, M. A Polyphenols-Rich Extract from Moricandia Sinaica Boiss. Exhibits Analgesic, Anti-Inflammatory and Antipyretic Activities in Vivo. Molecules 2020, 25, 5049. [Google Scholar] [CrossRef]
- Radulović, N.S.; Dordević, N.D. Steroids from Poison Hemlock (Conium maculatum L.): A GC-MS Analysis. J. Serbian Chem. Soc. 2011, 76, 1471–1483. [Google Scholar] [CrossRef]
- Aly, S.H.; Elissawy, A.M.; Eldahshan, O.A.; Elshanawany, M.A.; Singab, A.N.B. Phytochemical Investigation Using GC/MS Analysis and Evaluation of Antimicrobial and Cytotoxic Activities of the Lipoidal Matter of Leaves of Sophora Secundiflora and Sophora Tomentosa. Arch. Pharm. Sci. Ain Shams Univ. 2020, 4, 207–214. [Google Scholar] [CrossRef]
- Jamalova, D.N.; Gad, H.A.; Akramov, D.K.; Tojibaev, K.S.; Al Musayeib, N.M.; Ashour, M.L.; Mamadalieva, N.Z. Discrimination of the Essential Oils Obtained from Four Apiaceae Species Using Multivariate Analysis Based on the Chemical Compositions and Their Biological Activity. Plants 2021, 10, 1529. [Google Scholar] [CrossRef] [PubMed]
- Al-Sayed, E.; Gad, H.A.; El-Kersh, D.M. Characterization of Four Piper Essential Oils (GC/MS and ATR-IR) Coupled to Chemometrics and Their Anti- Helicobacter Pylori Activity. ACS Omega 2021, 6, 25652–25663. [Google Scholar] [CrossRef] [PubMed]
- Aly, S.H.; Eldahshan, O.A.; Al-rashood, S.T.; Binjubair, F.A.; El Hassab, M.A.; Eldehna, W.M.; Acqua, S.D.; Zengin, G. Chemical Constituents, Antioxidant, and Enzyme Inhibitory Activities Supported by In-Silico Study of n-Hexane Extract and Essential Oil of Guava Leaves. Molecules 2022, 27, 8979. [Google Scholar] [CrossRef] [PubMed]
- Zeraib, A.; Ramdani, M.; Lograda, T.; Chalard, P.; Figueredo, G. Chemical Composition and Antimicrobial Activity of Essential Oil of Moricandia Arvensis L. (DC.). Asian J. Plant Sci. 2011, 10, 342–346. [Google Scholar] [CrossRef]
- Marrelli, M.; Morrone, F.; Gambacorta, L.; Argentieri, M.P.; Conforti, F.; Avato, P. Phytochemical and Biological Profile of Moricandia arvensis (L.) DC.: An Inhibitor of Pancreatic Lipase. Molecules 2018, 23, 2829. [Google Scholar] [CrossRef]
- El-Nashar, H.A.S.; Eldehna, W.M.; Al-Rashood, S.T.; Alharbi, A.; Eskandrani, R.O.; Aly, S.H. GC/MS Analysis of Essential Oil and Enzyme Inhibitory Activities of Syzygium Cumini ( Pamposia ) Grown in Docking Studies. Molecules 2021, 26, 6984. [Google Scholar] [CrossRef]
- Turgumbayeva, A.; Ustenova, G.; Datkhayev, U.; Rahimov, K.; Abramavicius, S.; Tunaityte, A.; Zhakipbekov, K.; Kozhanova, K.; Tulemissov, S.; Ustenova, O.; et al. Safflower (Carthamus tinctorius L.) a Potential Source of Drugs against Cryptococcal Infections, Malaria and Leishmaniasis. Phyton 2020, 89, 137–146. [Google Scholar] [CrossRef]
- Alamery, S.F.; Algaraawi, N.L. Phytochemical Profile and Antifungal Activity of Stems and Leaves Methanol Extract from the Juncus Maritimus Linn. Juncaceae Family against Some Dermatophytes Fungi. AIP Conf. Proc. 2020, 2290, 020034. [Google Scholar] [CrossRef]
- Gad, H.A.; Mukhammadiev, E.A.; Zengen, G.; Musayeib, N.M.A.; Hussain, H.; Ware, I.B.; Ashour, M.L.; Mamadalieva, N.Z. Chemometric Analysis Based on GC-MS Chemical Profiles of Three Stachys Species from Uzbekistan and Their Biological Activity. Plants 2022, 11, 1215. [Google Scholar] [CrossRef]
- Thippeswamy, G.; Sheela, M.L.; Salimath, B.P. Octacosanol Isolated from Tinospora Cordifolia Downregulates VEGF Gene Expression by Inhibiting Nuclear Translocation of NF-<kappa>B and Its DNA Binding Activity. Eur. J. Pharmacol. 2008, 588, 141–150. [Google Scholar] [CrossRef]
- Braham, H.; Mighri, Z.; Jannet, H.B.; Matthew, S.; Abreu, P.M. Antioxidant Phenolic Glycosides from Moricandia Arvensis. J. Nat. Prod. 2005, 68, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Soliman, N.A.; Ismail, E.H.; Abd El-Moaty, H.I.; Sabry, D.Y.; Khalil, M.M.H. Anti-Helicobacter Pylori, Anti-Diabetic and Cytotoxicity Activity of Biosynthesized Gold Nanoparticles Using Moricandia Nitens Water Extract. Egypt. J. Chem. 2018, 61, 691–703. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 9781932633214. [Google Scholar]
- Aly, S.H.; Elissawy, A.M.; Eldahshan, O.A.; Elshanawany, M.A.; Singab, A.N.B. Variability of the Chemical Composition of the Essential Oils of Flowers and the Alkaloid Contents of Leaves of Sophora Secundiflora and Sophora Tomentosa. J. Essent. Oil-Bearing Plants 2020, 23, 442–452. [Google Scholar] [CrossRef]
- NIST The National Institute of Standards and Technology (NIST) Chemistry WebBook. NIST Standard Reference Database Number 69. Available online: http://Webbook.Nist.Gov/Chemistry/ (accessed on 5 July 2022).
- Aly, S.H.; El-hassab, M.A.; Elhady, S.S.; Gad, H.A. Comparative Metabolic Study of Tamarindus Indica L.’s Various Organs Based on GC/MS Analysis, In Silico and In Vitro Anti-Inflammatory and Wound Healing Activities. Plants 2022, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Boly, R.; Lamkami, T.; Lompo, M.; Dubois, J.; Guissou, I.P. DPPH Free Radical Scavenging Activity of Two Extracts From. Int. J. Toxicol. Pharmacol. Res. 2016, 8, 29–34. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- El Hassab, M.A.; Fares, M.; Amin, M.K.A.; Al-rashood, S.T.; Alharbi, A.; Eskandrani, R.O.; Alkahtani, H.M.; Eldehna, W.M. Toward the Identification of Potential α-Ketoamide Covalent Inhibitors for SARS-CoV-2 Main Protease: Fragment-Based Drug Design and MM-PBSA Calculations. Processes 2021, 9, 1004. [Google Scholar] [CrossRef]
- Vilar, S.; Cozza, G.; Moro, S. Medicinal Chemistry and the Molecular Operating Environment (MOE): Application of QSAR and Molecular Docking to Drug Discovery. Curr. Top. Med. Chem. 2008, 8, 1555–1572. [Google Scholar] [CrossRef]
- Lountos, G.T.; Jiang, R.; Wellborn, W.B.; Thaler, T.L.; Bommarius, A.S.; Orville, A.M. The Crystal Structure of NAD(P)H Oxidase from Lactobacillus Sanfranciscensis: Insights into the Conversion of O2 into Two Water Molecules by the Flavoenzyme. Biochemistry 2006, 45, 9648–9659. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.H.; Pacold, M.E.; Perisic, O.; Stephens, L.; Hawkins, P.T.; Wymann, M.P.; Williams, R.L. Structural Determinants of Phosphoinositide 3-Kinase Inhibition by Wortmannin, LY294002, Quercetin, Myricetin, and Staurosporine. Mol. Cell 2000, 6, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Forouhar, F.; Neely, H.; Seetharaman, J.; Mao, L.; Xiao, R.; Janjua, H.; Maglaqui, M.; Foote, E.L.; Lee, D.; Everett, J.K.; et al. Crystal Structure of Chromosome Partitioning Protein (ParA) in Complex with ADP from Synechocystis sp. Northeast Struct. Genom. Consort. 2008. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).